Abstract
Studies of language representation in development have shown a bilateral distributed pattern of activation that becomes increasingly left‐lateralized and focal from young childhood to adulthood. However, the level by which canonical and extra‐canonical regions, including subcortical and cerebellar regions, contribute to language during development has not been well‐characterized. In this study, we employed fMRI connectivity analyses (fcMRI) to characterize the distributed network supporting expressive language in a group of young children (age 4–6) and adolescents (age 16–18). We conducted an fcMRI analysis using seed‐to‐voxel and seed‐to‐ROI (region of interest) strategies to investigate interactions of left pars triangularis with other brain areas. The analyses showed significant interhemispheric connectivity in young children, with a minimal connectivity of the left pars triangularis to subcortical and cerebellar regions. In contrast, adolescents showed significant connectivity between the left IFG seed and left perisylvian cortex, left caudate and putamen, and regions of the right cerebellum. Importantly, fcMRI analyses indicated significant differences between groups at 3 anatomical clusters, including left IFG, left supramarginal gyrus, and right cerebellar crura, suggesting a role in the functional development of language.
Keywords: adolescents, children, expressive language, functional connectivity magnetic resonance imaging, functional development
1. INTRODUCTION
The left perisylvian cortex, including canonical Broca's and Wernicke's areas, is known to support gross language processes in healthy adults (Binder et al., 1997; Friederici, 2011, 2012; Gabrieli, Poldrack, & Desmond, 1998; Hirata et al., 2004; Kadis et al., 2011; Lohmann et al., 2010; Pei et al., 2011; Price, 2000, 2012; Purves et al., 2004; Toga and Thompson, 2003; Turken and Dronkers, 2011). However, participation of right perisylvian cortex and subcortical and cerebellar regions in language have also been documented (Berl et al., 2014; Booth, Wood, Lu, Houk, & Bitan, 2007; Desmond, Gabrieli, & Glover, 1998; Ferstl, Neumann, Bogler, & Von Cramon, 2008; Frings et al., 2006; Gabrieli et al., 1998; Houk et al., 2007; Kellett, Kellett, Stevenson, & Gernsbacher, 2012; Middleton and Strick, 2000; Muller and Meyer, 2014; Murdoch, 2010; Schmahmann, 1997; Stoodley, Valera, & Schmahmann, 2012; Stoodley and Schmahmann, 2009; Verly et al., 2014). In particular, seminal anatomical and behavioral studies have shown nonmotor contributions of the (right) cerebellum in a range of cognitive processes, including language (Leiner, Leiner, & Dow, 1991; Petersen, Fox, Snyder, & Raichle, 1990; Raichle et al., 1994; Schmahmann, 1997; see Price, 2012, for review). While the left cerebellar and a medial cerebellar regions reflect motor‐related processes, the right cerebellum is believed to be involved in modulation of cognitive nonmotor functioning (Booth et al., 2007; Keren‐Happuch, Annabel, Ho, & Desmond, 2014; Murdoch, 2010; Shulman et al., 1997). However, the level of participation of subcortical and cerebellar regions in normal language development has not been well‐characterized.
Previous neuroimaging studies of language representation in development have shown a bilateral distributed pattern of activation that typically becomes more left‐lateralized and focal from early childhood to adulthood (Holland et al., 2007; Kadis et al., 2011). Sophisticated analytic strategies such as functional/effective connectivity have supported this developmental pattern (Bitan et al., 2006; Kadis, Dimitrijevic, Toro‐Serey, Smith, & Holland, 2016; Xiao, Friederici, Margulies, & Brauer, 2016; Youssofzadeh, Williamson, & Kadis, 2017; Yu et al., 2014). For example, in a resting‐state functional magnetic resonance imaging connectivity (fcMRI) analysis, intrahemispheric correlations were found in left perisylvian regions in adolescents. In contrast, interhemispheric correlations between left and right IFG were predominant in children (Xiao et al., 2016). Similarly, an MEG connectivity study of verb generation in children demonstrated bilateral patterns of connectivity, together with a network whose suprathreshold directed connections increased in canonical Broca's area, with age (Kadis et al., 2011). However, these studies have focused primarily on perisylvian regions, with little examination of the role of subcortical and cerebellar connectivity. Recently, we compared whole brain MEG connectivity of 4–6‐years‐old children and 16–18‐years‐old adolescents during a verb generation task (Youssofzadeh et al., 2017). In children, there were contributions by bilateral (with stronger effects on the right side) cortical language regions, whereas the pattern was left‐focal in the cortex in adolescents, and subcortical and (right) cerebellar regions were also engaged. Findings suggested that extracortical regions may change in their role in language processing across development. A functional connectivity approach is an effective way to reveal a broader network supporting language processing, developmental changes, and brain maturation.
In this work, by means of a task‐based fcMRI analysis, we investigated patterns of whole‐brain connectivity in typically developing young children and adolescents participating in verb generation. We used seed‐to‐voxel and seed‐to‐ROI (region of interest) fcMRI strategies. The two analyses complement one another; the seed‐to‐voxel analysis is more exploratory as it examines the whole brain, whereas seed‐to‐ROI analyses typically lead to a better sensitivity, and hence, more appropriate for hypothesis testing. Using the two fcMRI strategies, we demonstrate the importance of interactions between the cerebral cortex and subcortical regions, and the cerebellum in language production abilities of typically developing children.
2. MATERIAL AND METHODS
2.1. Participants and experiment design
fMRI scans were acquired in two groups of participants: 10 children (5 females, aged 4–6, mean ± standard deviation: 5.6 ± 0.99) and 13 adolescents (7 females, aged 16–18, 17.18 ± 0.79). All participants were native English speakers without a history of neurological insult, speech or language disorder, or learning disability. Participants were all right‐handed as determined by Edinburgh Handedness Inventory (EHI), and a threshold of EHI ≥ 48 (Oldfield, 1971). Written informed consent and assent were obtained for participation; the study was approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center. Participants received compensation for travel and participation.
The fMRI task consisted of 6 verb and 6 control blocks, each containing 10 stimuli (35 seconds per block). During verb blocks, participants were instructed to rapidly think of an action word that corresponded to the aurally presented nouns (names of everyday items familiar to children aged 5 years and older, e.g., book, dog, pencil). This task involves comprehension, semantic access (of the noun and associated verb), and language production (covert verb generation). During control blocks, participants passively listened to a speech‐shaped noise (contoured noise matched for spectral content and amplitude envelope to the noun stimuli used for verb generation), without responding. Development of the stimulus set, including the speech‐shaped noise items, has been described previously (Kadis et al., 2011; Youssofzadeh et al., 2017). Prior to the recording, participants were trained on an overt version of the task to establish sufficient ability and promote compliance during subsequent acquisition. They had to answer a minimum of 8 of 10 noun challenges correctly, prior to scanning.
2.2. Data acquisition
Magnetic resonance imaging was conducted on at 3 T (Philips Achieva, Philips Medical Systems). Functional scans were acquired with TR = 2,000 ms, TE = 30 ms, flip angle of 75°, 2.8 × 2.8 × 3.0 mm voxels. Whole‐brain 3D T1‐weighted MRI scans were acquired using a flip angle of 90°, TE = 3.7 ms, TR = 8.1 ms, TI = 939 ms, voxel size = 1.0 × 1.0 × 1.0 mm.
2.3. Data analysis
2.3.1. fMRI preprocessing
fMRI scans were aligned, segmented, spatially normalized to MNI space, and smoothed by an 8‐mm FWHM Gaussian filter using SPM12 (Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/). Interscan movement was quantified using ARtifact detection Tools (ART), as implemented in the CONN toolbox, ver. 17c (Whitfield‐Gabrieli and Nieto‐Castanon, 2012). A threshold of 2 mm interscan motion and a global signal threshold of Z = 9 was employed to identify volumes with excessive motion. Confounds from blood oxygen level‐dependent (BOLD) signals of white matter and CSF tissues (5 components each) were detected by a principal component analysis (aCompCor; Behzadi, Restom, Liau, & Liu, 2007). Artifactual signals were linearly regressed out. Data were band‐pass filtered in the range of 0.008–0.09 Hz to remove unwanted motion, physiological, and other artefactual effects from task‐relevant BOLD activations, followed by a linear detrending to remove possible linear, quadratic, or cubic trends within each functional session.
2.3.2. Second‐level GLM analysis
A second‐level general linear model (GLM) analysis, two‐sample (unpaired) t test, was conducted to evaluate group activation maps of verb generation responses in children and adolescents. The t‐contrast maps were extracted using a cluster‐level threshold of p < .05, family‐wise error (FWE) corrected across the whole brain. This also provided a priori evidence to select a seed region in the fcMRI analyses.
2.3.3. fcMRI: Seed‐to‐voxel and seed‐to‐ROI
Motivated by the second‐level GLM analysis, a similar seed region was selected for both fcMRI analyses to investigate verb generation BOLD responses from two groups of participants, children and adolescents. We focused on connectivity due to verb generation condition only (rather than comparing to the speech‐shaped noise), to examine the full language processing network, including those regions engaged for processing auditory input, rather than isolating the lexical‐semantic component through a contrast. To quantify the connections, Pearson's bivariate correlation coefficients were calculated between mean timeseries within a seed and the timeseries of all other voxels (for seed‐to‐voxel analysis) and ROIs (for seed‐to‐ROI analysis). Correlation coefficients were converted to normally distributed scores using Fisher's transform prior to second‐level analysis. The transform improves the normality of the data, making subsequent statistics more robust (Whitfield‐Gabrieli and Nieto‐Castanon, 2012). A second‐level analysis was performed using two seed‐to‐voxel and seed‐to‐ROI analytic strategies to make inferences about group differences.
An atlas with 132 anatomical regions was used to summarize functional connectivity of the ROIs. The parcellation results from a combination of two atlases: Harvard‐Oxford cortical and subcortical structures adopted from FSL software tool (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012), and cerebellar regions from the AAL atlas (Smith et al., 2004; Tzourio‐Mazoyer et al., 2002). For group seed‐to‐voxel fcMRI analysis, we employed parametric statistics; a combination of height (voxel‐level) threshold of p < .001, uncorrected, and extent (cluster‐level) threshold of cluster size p‐FDR < .05 (Friston, Worsley, Frackowiak, Mazziotta, & Evans, 1994). For group seed‐to‐ROI fcMRI analysis, connections (by intensity) were thresholded with a p‐FDR < .05. A two‐sided t test was utilized to investigate between‐group connectivity effects.
2.3.4. Effect size: A seed‐to‐voxel fcMRI of combined groups
A seed‐to‐voxel fcMRI analysis of combined groups (23 datasets) was employed to look for between‐group connectivity differences, that is, “effect size,” and to make inferences about functional development. To quantify the effect size, Cohen's d was calculated as the difference between two means of connectivity cluster values divided by pooled standard deviation (Cohen, 1988).
To assure stability in functional connectivity due to imbalanced data, we conducted the same analysis using balanced data with 20 datasets (10 children + 10/13 randomly selected adolescents) and reported the average of the effect size measures.
3. RESULTS
3.1. Group GLM analysis
Group GLM analysis revealed a bilateral pattern of activation in children, whereas more focal activations in the left hemisphere were found in adolescents (Figure 1a,b). Specifically, results in both groups showed significant activation in left IFG regions as well as other (extra canonical) regions including right IFG and right cerebellum. The left IFG, pars triangularis region showed greatest effects in both groups, t (12) = 9.26 (p = .003, FWE corrected) in adolescents, t (9) = 6.4 (p = .01, FWE corrected) in children. This region was selected as a seed location in the fcMRI analyses. A GLM contrast of adolescents > children revealed a global peak of t (9) = 5.1, p = .01 FWE corrected, in the left IFG, pars triangularis region (Figure 1c).
Figure 1.
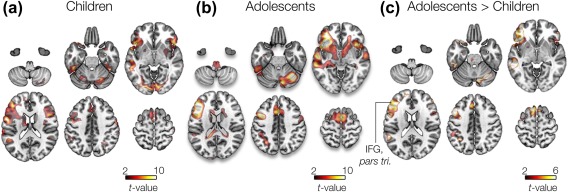
Second‐level GLM analysis. Two‐sample t‐test (unpaired) of responses from (A) children, (B) adolescent participants, and (c) contrast of adolescent > children during verb generation condition. [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Seed (left pars triangularis)‐to‐voxel fcMRI
Using a source seed selected at left pars triangularis (MNI: −50, 26, 2, the centroid of the seed region from an atlas), group seed‐to‐voxel fcMRI analysis revealed 6 significant connectivity clusters in children and 7 in adolescents. Connectivity maps are displayed in Figure 2a,b. Expectedly, in both groups, greatest connectivity to the seed location was observed in a cluster containing other regions of left IFG, including pars opercularis, canonical Broca's area. Specifically, greatest suprathreshold connections in children (t = 18.55, p < .001, corrected) were a cluster containing IFG pars opercularis, middle frontal gyrus (FG), middle temporal gyrus (TG), and inferior TG. Similarly, greatest suprathreshold cluster connections in adolescents (t = 19.08, p < .001, corrected) were a cluster containing IFG pars opercularis, middle FG, temporal pole, frontal pole, and superior FG. Statistical details of the clusters are summarized in Table 1.
Figure 2.
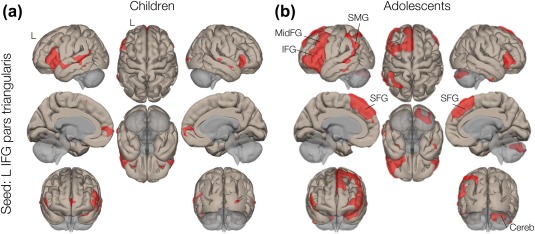
Seed‐to‐voxel connectivity analysis. Left IFG pars triangularis was selected as a seed. (A) Volume display of supra‐thresholded connectivity of children and (B) adolescents. Supramarginal gyrus (SMG), superior frontal gyrus (SAG) and middle frontal gyrus (MidFG) are specified in the figure. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Demographic characteristics of study participants
| Children (DF = 9)Seed: IFG L pars triangularis (−51, 26, 2) | |||||||
|---|---|---|---|---|---|---|---|
| # | Anatomical region (subregion voxels, % coverage) | Hemisphere (left/right) | Cluster size (voxels) | t value | Cluster p‐FDR | Cluster coordinates (MNI) | |
| 1 |
IFG pars opercularis Mid FG Mid TG Inf TG |
683, 89% 715, 24% 642, 46% 383, 55% |
L | 7,060 | 18.55 | <10 − 6 | −50, 28, 4 |
| 2 |
IFG, pars triangularis Orbital‐frontal cortex |
293, 53% 265, 18% |
R | 829 | 12.06 | <10 − 6 | 46, 26, −10 |
| 3 |
Frontal pole L Frontal pole R Paracingulate G |
2,561, 4% 144, 2% 128, 10% |
L/R | 669 | 10.92 | <10 − 5 | −8, 64, −2 |
| 4 | Supramarginal G | 134, 13% | L | 256 | 9.23 | <10 − 3 | −50, −42, 52 |
| 5 |
Amygdala Hippocampus |
138, 42% 29, 4% |
L | 244 | 7.69 | <10 − 3 | −20, −6, −14 |
| 6 |
Caudate nucleus Putamen |
100, 19% 61, 10% |
L | 163 | 6.16 | <10 − 3 | −12, 4, 16 |
| Adolescents (DF = 12) | |||||||
| 1 |
IFG pars opercularis Mid FG Temporal pole Frontal pole Sup FG |
757, 99% 2,057, 70% 1,510, 64% 2,503, 36% 1,673, 59% |
L | 14,699 | 19.08 | <10 − 6 | −46, 30, 10 |
| 2 |
Inf TG Mid TG Supramarginal G STG, posterior |
416, 60% 605, 44% 455, 43% 104, 27% |
L | 4,419 | 11.99 | <10 − 5 | −50, −42, 48 |
| 3 |
IFG pars triangularis IFG pars opercularis |
401, 72% 161, 23% |
R | 1,848 | 7.62 | <10 − 5 | 54, 42, −2 |
| 4 | Cerebellum Crus 1 | 519, 21% | R | 825 | 6.90 | <10 − 4 | 38, −62, −32 |
| 5 |
Caudate nucleus Putamen |
146, 27% 75, 9% |
L | 309 | 6.48 | <10 − 3 | −10, 16, 6 |
| 6 | Caudate nucleus | 143, 27% | R | 245 | 6.21 | <10 − 3 | −10, 10, 4 |
| 7 | Temporal fusiform cortex | 154, 49% | L | 198 | 5.49 | <10 − 2 | −36, −8, −38 |
Note. Abbreviations: DF = degree of freedom; FDR = false discovery rate; MNI = Montreal Neurological Institute.
Details of age, gender, and neuropsychological outcomes, EVT, PPVT scores are given.
#Cluster; voxel threshold (p‐uncorrected p < .001); cluster threshold (p‐FDR < .05); peak‐voxel location (in mm); statistics are one‐sided.
Significant connections were identified in left supramarginal gyrus in both groups (cluster 2 in adolescents with 455 voxels and cluster 4 in children with 134 voxels). Connectivity in two other cortical regions, (bilateral) superior frontal gyrus (SFG: 1,673 voxels), and middle frontal gyrus (MFG: 2,057 voxels) were only found in adolescents (cluster 1). In contrast, children showed prominent connections (from the seed region, left IFG pars triangularis) to right IFG pars triangularis (cluster 2 with 293 voxels). Within‐group fcMRI analysis in both groups also revealed significant connectivity in subcortical basal ganglia regions, that is, caudate nucleus and putamen (cluster 6 with 163 voxels in children, and clusters 5–6 with 309 and 245 voxels in adolescents, respectively). The fcMRI analysis revealed connectivity between left IFG and right cerebellar regions (cluster 4 with 825 voxels) in adolescents, whereas no significant connectivity was observed in young children.
3.3. Seed (IFG.tri)‐to‐ROI fcMRI
Group IFG.tri‐to‐ROI fcMRI suggested bilateral (interhemispheric) connections between left and right IFG regions in children (Figure 3a). The same analysis for adolescents suggested an intrahemispheric pattern of connectivity with significant effects at the orbitofrontal cortex, IFG pars opercularis, middle frontal gyrus and superior frontal gyrus, and right cerebellar regions, and lobules Crus 1 and 2 (Figure 3b; see also Table 2 for statistical details).
Figure 3.
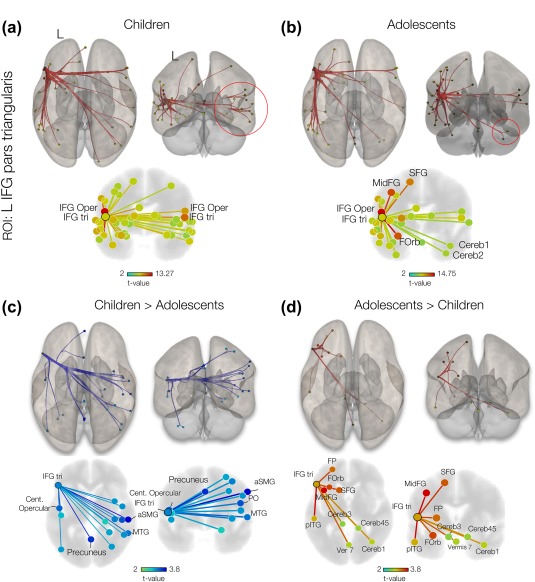
Seed (IFG.tri)‐to‐ROI connectivity analysis. (A) 3D displays of supra‐thresholded (p‐FDR < 0.05) one‐sided (positive) connectivity of children and, (B) adolescents. The left IFG pars triangularis was used as sources in the seed‐to‐ROI analyses. Red circles indicate the regions with different connectivity pattern in two groups. 3D displays of supra‐thresholded (p‐FDR < 0.05) connectivity contrast with ROI source selected at IFG pars triangularis for young children (C) and (D) adolescents. Anterior supramarginal gyrus (aSTG), parietal operculum cortex (PO), insular cortex (IC), lateral occipital cortex (LOC), superior frontal gyrus (SAG) and middle frontal gyrus (MidFG), frontal pole (FP) and Inferior temporal gyrus (IT) are specified in the figure. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Summary of seed‐to‐voxels fcMRI analysis of young children and adolescents with the selected seed at left IFG pars triangularis region
| ROI: IFG pars triangularis L | |||||||
|---|---|---|---|---|---|---|---|
| Children (n = 10) | Adolescents (n = 13) | ||||||
| ROI | L/R | t (9) | p‐FDR | ROI | L/R | t (12) | p‐FDR |
| IFG pars opercularis | L | 10.28 | 0.0004 | Orbital‐frontal cortex | L | 14.75 | <10−4 |
| IT, temporooccipital | L | 9.19 | 0.0005 | IFG pars opercularis | L | 14.34 | <10−4 |
| Supramarginal gyrus, posterior | L | 6.95 | 0.0029 | Middle FIG | L | 12.07 | <10−4 |
| IFG pars triangularis | R | 6.14 | 0.0056 | FIG, superior | L | 9.64 | <10−4 |
| Frontal operculum | L | 5.90 | 0.006 | IT, temporooccipital | L | 8.51 | <10−4 |
| MT, posterior | L | 5.49 | 0.0084 | Supramarginal gyrus, posterior | L | 7.15 | .0001 |
| Paracingulate gyrus | L | 5.17 | 0.01 | Frontal Pole | L | 7.03 | .0001 |
| Amygdala | L | 4.69 | 0.01 | IT, posterior | L | 5.48 | .0011 |
| Insular cortex | L | 4.61 | 0.01 | MT, posterior | L | 5.15 | .0018 |
| Caudate | L | 4.57 | 0.01 | Fus C, temporal, anterior | L | 4.99 | .0021 |
| Orbital‐frontal cortex | L | 4.56 | 0.01 | IFG pars opercularis | R | 4.88 | .0022 |
| Orbital‐frontal cortex | R | 4.50 | 0.01 | Frontal operculum cortex | L | 4.71 | .0027 |
| Fus C, temporooccipital | L | 4.43 | 0.01 | Temporal pole | L | 4.68 | .0027 |
| IFG pars opercularis | R | 4.22 | 0.02 | Angular gyrus | L | 4.6 | .0029 |
| MT, anterior | L | 4.15 | 0.02 | Caudate | L | 4.56 | .0029 |
| MT, temporooccipital | L | 4.01 | 0.02 | LOC, superior | L | 4.52 | .0029 |
| STG, posterior | R | 3.89 | 0.02 | MT, temporooccipital part | L | 4.07 | .0059 |
| Angular gyrus | L | 3.85 | 0.02 | Caudate | R | 3.94 | .0071 |
| FIG, superior | L | 3.75 | 0.03 | Paracingulate gyrus | L | 3.76 | .009 |
| LOC, inferior | R | 3.63 | 0.03 | IFG Oper | R | 3.75 | .009 |
| LOC, superior | L | 3.57 | 0.03 | Temporal Fus C, posterior | L | 3.31 | .0195 |
| STG, anterior | L | 3.48 | 0.04 | STG, posterior | L | 3.16 | .0245 |
| MT, temporooccipital | R | 3.47 | 0.04 | Cerebellum Crus 2 | R | 3.09 | .0262 |
| Fus C, temporooccipital | R | 3.42 | 0.04 | Putamen | L | 3.08 | .0262 |
| STG, posterior | L | 3.37 | 0.04 | Cerebellum crus 1 | R | 2.86 | .0377 |
| Occipital pole | R | 3.37 | 0.04 | STG, posterior | R | 2.82 | .0387 |
| Putamen | L | 3.37 | 0.04 | Vermis 7 | ‐ | 2.75 | .0428 |
| STG, anterior | R | 3.34 | 0.04 | STG, anterior | L | 2.68 | .047 |
Note. Abbreviations: IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; PCG = paracingulate gyrus; Fus C = fusiform cortex; MTG = middle temporal gyrus; STG = superior temporal gyrus; SFG = superior frontal gyrus; LOC = lateral occipital cortex.
Coordinates are reported as the centroids of ROIs, derived from an atlas with 132 anatomical regions.
In addition to within‐group effects, we investigated between‐group differences. A contrast of children > adolescents showed significant (p < .05) connectivity differences, mainly at right hemispheric regions, e.g. from the IFG pars triangularis to supramarginal gyrus with t (22) = 3.66, middle temporal gyrus with t (22) = 2.54, p < .05, and parietal operculum with t (22) = 2.37, as in Figure 3c. Connectivity contrast in adolescents > children suggested significant ipsilateral (left) connections from the IFG.tri to middle frontal gyrus with t (22) = 3.04, inferior temporal gyrus with t (22) = 3.00, superior frontal gyrus with t (22) = 2.53, and frontal pole with t (22) = 2.35. Contralateral connections were observed from the IFG.tri to cerebellar regions, lobule Crus 1 with t (22) = 1.62, as in Figure 3d. Details of a two‐sided statistical analysis are summarized in Table 3.
Table 3.
Summary of IFG.tri‐to‐ROI fcMRI contrast analysis of adolescents versus young children (under a two‐sided t test)
| Adolescents–childrenROI: IFG pars triangularis L | |||||||
|---|---|---|---|---|---|---|---|
| Children < adolescents | Children > adolescents | ||||||
| ROI | L/R | t (22) | p‐FDR | ROI | L/R | t (22) | p‐FDR |
| Anterior supramarginal gyrus (aSMG) | R | 3.88 | .01 | Middle FG | L | 3.04 | .01 |
| Central opercular | L | 2.26 | .01 | Inferior temporal gyrus, posterior | L | 3.00 | .02 |
| Precuneus | ‐ | 2.24 | .02 | Superior frontal gyrus (SFG) | L | 2.53 | .031 |
| Middle temporal gyrus (MTG) | R | 2.54 | .02 | Frontal pole | L | 2.35 | .031 |
| Parietal operculum cortex | R | 2.37 | .02 | Orbital‐frontal cortex | L | 2.19 | .04 |
| Insular cortex | R | 2.35 | .03 | Cerebellar Vermis 7 | ‐ | 2.12 | .04 |
| Postcentral gyrus | R | 2.12 | .04 | Cerebellum lobule 4–5 | R | 1.65 | .04 |
| Heschl's gyrus | L | 2.05 | .04 | Cerebellum Crus 1 | R | 1.62 | .049 |
| Lateral occipital cortex | L | 1.97 | .04 | ||||
3.4. Effect size: A seed‐to‐voxel fcMRI of combined groups
Effect size representing correlation coefficients (or beta coefficients) associated with the task in 2 groups of participants was examined and quantified by Cohen's d for each connectivity cluster. A seed‐to‐voxel fcMRI analysis of all participants using a seed within left pars triangularis revealed 6 clusters of connections (Figure 4a). However, only 3 clusters showed significant (p < .01) increased connectivity in adolescents with respect to children, and considerable effect size effects (Figure 4b,c). In the order of Cohen's d values, they were cluster 1 (left prefrontal: left IFG regions pars opercularis and pars triangularis, superior frontal and, middle frontal gyrus) with Cohen's d = 2.47, cluster 4 (supramarginal gyrus) with Cohen's d = 1.52, and cluster 6 (right cerebellar regions Crus 1 and 2) with Cohen's d = 1.02, as summarized in Table 4.
Figure 4.
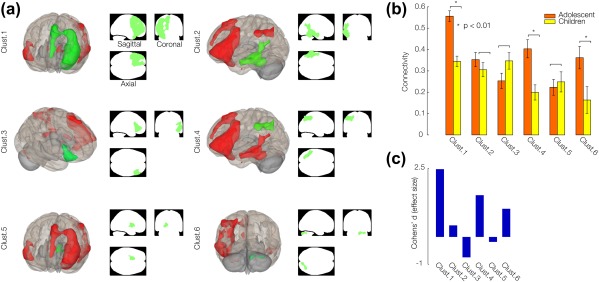
Connectivity clusters suggested by group seed‐to‐voxel analysis. (A) 3D volume displays of supra‐thresholded by a combination of height (voxel‐level) threshold of p‐FDR < 0.001 and extent (cluster‐level) threshold of p‐FDR < 0.05 with seed selected at IFG pars triangularis for all participants (young children and adolescents). Details of clusters are reported in the Table. 4. (B) between‐subjects effect size (fisher‐transformed group wise difference in connectivity) representing the mean and standard deviation of 6 connectivity clusters. Significant differences in correlations are denoted by ‘*’. (C) Cohen's d (effect size) of the comparison between the two groups evaluated for 6 connectivity clusters. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Summary of seed‐to‐voxels fcMRI analysis of all participants (young children + adolescents) with the selected seed at left IFG pars triangularis region
| # | Anatomical region subregion (voxel), atlas coverage (%) | Hemisphere (left/right) | Cluster size (voxels) | Cluster p‐FDR | Cohen’ d (effect size) | MNI coordinates | |
|---|---|---|---|---|---|---|---|
| 1 |
IFG pars opercularis IFG, pars triangularis Orbital‐frontal cortex Frontal pole Middle frontal gyrus Superior frontal gyrus |
763, 100% 650, 100% 1,154, 69% 1,700, 25% 1,573, 54% 968, 34% |
L | 10,909 | <10−6 | 2.47 | −50, 32, 8 |
| 2 |
Inferior temporal gyrus Middle temporal gyrus, posterior |
436, 62% 750, 54% |
L | 3,133 | <10−6 | 0.42 | −52, −38, −14 |
| 3 |
IFG, pars triangularis IFG pars opercularis Orbital‐frontal cortex Frontal pole |
409, 74% 194, 28% 243, 17% 242, 3% |
R | 1,476 | <10−4 | −0.74 | 50, 32, −4 |
| 4 |
Supramarginal gyrus Superior parietal lobule Lateral occipital cortex |
86, 23% 251, 17% 420, 8% |
L | 1,134 | <10−4 | 1.52 | −50, −44, 48 |
| 5 |
Caudate Putamen |
171, 32% 55, 6% |
L | 321 | <10−3 | −0.18 | −12, 8, 12 |
| 6 |
Cerebellum Crus 1 Cerebellum Crus 2 |
182, 7% 90, 4% |
R | 302 | <10−3 | 1.02 | 14, −76, −26 |
Coordinates are reported as the centroids of ROIs, derived from an atlas with 132 anatomical regions.
4. DISCUSSION
By means of fcMRI analysis, we set out to investigate developmental changes in verb generation whole‐brain connectivity patterns in young children and adolescents. Findings suggest predominately interhemispheric connectivity pattern in young children, and a strong left lateralized connectivity pattern with prominent contributions by subcortical and cerebellar regions in adolescents. Specifically, significant between‐group effects were found in unique connectivity clusters in left IFG, supramarginal gyrus (SMG), and right cerebellum regions, implying their role in functional development in regulating language production.
Second‐level fcMRI analyses suggested significant interhemispheric (bilateral) connectivity in the youngest children (Figures 2 and 3), in agreement with previous fcMEG studies (Kadis et al., 2016; Youssofzadeh et al., 2017). Other verb generation studies on children have reported activations of the frontotemporal network, with increased lateralization and engagement beyond the canonical language network in older children (Berl et al., 2014; Holland et al., 2007; Kadis et al., 2011; Karunanayaka et al., 2007). In contrast, the second‐level connectivity analysis for adolescents yielded an expected pattern of connections at left perisylvian cortex in canonical language sites. This pattern of localization has been reported in previous expressive language studies on similar age groups (Doesburg, Vinette, Cheung, & Pang, 2012; Kadis, Smith, Lou, Mills, & Pang, 2008; Kadis et al., 2011; Pang, Wang, Malone, Kadis, & Donner, 2011). Connectivity from left IFG to other left‐hemisphere regions differed significantly between adolescents and children (Figure 4). This is consistent with other studies showing increases of functional lateralization from childhood to adulthood (Berl et al., 2014; Chiron et al., 1997; Holland et al., 2007; Kadis et al., 2011; Lebel and Beaulieu, 2009; Szaflarski et al., 2006, 2012; Xiao et al., 2016).
Findings for adolescents suggested strong contributions by two other cortical regions SFG and MFG in frontal cortex and SMG in parietal cortex (Figure 2b,d). Involvement of SFG/MFG cortical regions has been reported in adolescents during semantic processing, for example, overt generation (say) versus overt repetition (repeat) (Wang, Holland, & Vannest, 2012). Other studies have reported involvements of the SFG/MFG regions during receptive and semantic processing (Binder and Desai, 2011; Gabrieli et al., 1998; Price, Wise, & Frackowiak, 1996). The SMG activations have been observed during phonetic processing in young adults (Gow, Segawa, Ahlfors, & Lin, 2008; Stoodley et al., 2012). We believe, because adolescents have larger vocabularies and more extensive word knowledge, they are likely to engage a more extended semantic network than young children. Adolescents may be considering multiple candidate verbs, leading to the greater engagement of the frontal and parietal regions.
The fcMRI analysis revealed contributions by subcortical basal ganglia nuclei, for example, caudate and putamen, in both children and adolescents (Table 1). The importance of subcortical left basal ganglia and right cerebellar regions for normal brain function has been noted in previous language studies (Barbas, García‐Cabezas, & Zikopoulos, 2013; Booth et al., 2007; Mestres‐Missé, Càmara, Rodriguez‐Fornells, Rotte, & Münte, 2008; Tettamanti et al., 2005, for review, see Murdoch, 2010). The left caudate and putamen showed increased activation during phonological processing (Tettamanti et al., 2005). The left putamen and bilateral caudate nuclei were activated while encoding the meaning of novel words in adults (Mestres‐Missé et al., 2008). An fMRI dynamic causal modelling (DCM) study demonstrated a pivotal role for the left putamen during reading aloud familiar words in skilled readers (Seghier and Price, 2010). In addition, in a rhyming judgment task in adults using the DCM approach, unidirectional connections were found from the left putamen to two cortical left IFG and left temporal regions, although, connections from the right cerebellum to similar cortical regions were stronger and reciprocal (Booth et al., 2007). It was hypothesized that subcortical (left putamen) regions mainly engage in cortical initiation of phonological representations whereas the (right) cerebellar regions facilitate processing complex processes, for example, semantic relationships between words. The hypothesis of greater involvement of cerebellar regions in semantic processes might be better linked to our developmental findings where connectivity in right cerebellar crura (cluster 6 in Figure 4c) was found to be greater in adolescents (who have better word knowledge) than the children. In contrast, connections in subcortical regions did not show significant differences between the two groups (cluster 5 in Figure 4c).
Adolescents showed a pattern of fMRI connectivity between left prefrontal and right cerebellar regions that were not present in young children, suggesting an emergent functional link between the two regions (Figure 2c,d). Previous expressive language studies have reported co‐activation of the cerebellum with the prefrontal cortex in healthy adults (Booth et al., 2007; Gabrieli et al., 1998; Gebhart, Petersen, & Thach, 2002; Imaizumi et al., 1997; Raichle et al., 1994; Seger, Desmond, Glover, & Gabrieli, 2000; Stoodley et al., 2012). Specifically, our fcMRI findings show increased the involvement of Crus I and II of the right cerebellum in adolescents during verb generation. Two recent meta‐analyses documented significant engagement of the cerebellar crura in connection with expressive language tasks (Keren‐Happuch et al., 2014; Stoodley and Schmahmann, 2009). In addition, in a recent verb generation language study using conventional GLM analysis, predominant activation of right‐hemisphere cerebellar lobules was evident (Stoodley et al., 2012). All these support the fcMRI network localizations that suggest interactive links between left‐frontal and right cerebellar regions.
Classic neuroimaging studies have reported coactivations of left‐frontal and right cerebellar regions during semantic search tasks, for example, a verbal selection task (saying an appropriate verb for a visually presented noun) (Gabrieli et al., 1998; Raichle et al., 1994, see De Smet, Paquier, Verhoeven, & Mariën, 2013 for review). These studies suggested that frontal‐cerebellar interactions play a role in executive control and maintenance of information in verbal working memory. We believe, because adolescents have larger vocabularies, and a wider network of word knowledge than younger children when they are presented with a noun and are asked to generate a single verb, they are likely making a selection among multiple verb responses. The increased cerebellar involvement may reflect a brief period of maintaining multiple verbs in working memory, and/or executive processes involved in selecting a single verb response. In line with this hypothesis, two classic verb generation studies have reported increased right cerebellar fMRI activations when adult participants performed a complex task (choosing an appropriate verb within a large mental lexicon) than a simple task (responding “yes” or “no” to a simple conceptual decision task) (Desmond et al., 1998), or responses to unusual verbs (that demand more semantic processing) versus repeated verbs (Seger et al., 2000). Alternatively, the cerebellum has been suggested to contribute to the automatization of linguistic processes via adaptive prediction (Sokolov, Miall, & Ivry, 2017). Because adolescents have a greater degree of experience with semantic relationships between words than children do, they may be able to more automatically generate a related verb for each noun via this predictive mechanism that engages the cerebellum, rather than completing a full semantic analysis of the noun as a means of generating a related verb.
Between‐group (effect size) comparisons suggested significant changes in 3 cluster communities of left prefrontal and supramarginal cortices, and right cerebellum (Figure 4). The interactions between the cerebellar, prefrontal, and parietal association cortices have been reported in previous language studies (Clower, Dum, & Strick, 2005; Fiez, Raichle, Balota, Tallal, & Petersen, 1996; Schlosser et al., 1998; Seger et al., 2000; de Zubicaray et al., 1998). Those findings are in agreement with Ito's (2008) hypothesis of internal model control of the mental activity. The theory posits that development and refinement of both motor and mental operations are controlled by a large‐scale network comprising 3 key regions of prefrontal, temporal‐parietal cortices, and the cerebellum. The prefrontal cortex has a role in conscious control (executive function), the temporoparietal cortex encodes relationships among concepts (noun–verb relationships), and the cerebellum interacts with cerebral regions to “regulate” the process. This theory is also consistent with cerebellar involvement in adaptive prediction, as mentioned above (Sokolov et al., 2017). As a limitation, effect‐size measures representing differences in connectivity between two groups within each cluster were estimated using limited sample size, which might affect the reliability of the findings (Lakens and Evers, 2014). We hope the future studies can replicate these findings using larger samples.
Beeman et al. (1994) suggested both left and right cerebral hemispheres process semantic information, at different levels. The left hemisphere is biased toward processing proximal associates, whereas the right hemisphere is linked to distal associates, that is, necessary for creative thought, abstraction, or complex problem‐solving. Hence, we hypothesize that the increased connectivity in left prefrontal cortical regions in adolescents reflects the development of this specialization, and in addition, cerebro–cerebellar interactions discussed above may increase with the maturation of language function. The importance of emergent connectivity between the cerebral cortex and cerebellum should be further investigated in future developmental studies of typically developing children.
ACKNOWLEDGMENTS
This research was supported by a Trustee Award Grant received by DSK from Cincinnati Children's Hospital Research Foundation. The authors wish to acknowledge Claudio A. Toro‐Serey and Cameron Laue Evans for their assistance with recruitment, assessment, and data management.
Youssofzadeh V, Vannest J, Kadis DS. fMRI connectivity of expressive language in young children and adolescents. Hum Brain Mapp. 2018;39:3586–3596. 10.1002/hbm.24196
Funding information Trustee Award Grant
REFERENCES
- Barbas, H. , García‐Cabezas, M. Á. , & Zikopoulos, B. (2013). Frontal‐thalamic circuits associated with language. Brain and Language, 126(1), 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeman, M. , Friedman, R. B. , Grafman, J. , Perez, E. , Diamond, S. , & Lindsay, M. B. (1994). Summation priming and coarse semantic coding in the right hemisphere. Journal of Cognitive Neuroscience, 6(1), 26–45. [DOI] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl, M. M. , Mayo, J. , Parks, E. N. , Rosenberger, L. R. , Vanmeter, J. , Ratner, N. B. , … Gaillard, W. D. (2014). Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping, 35(1), 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. , Frost, J. A. , Hammeke, T. A. , Cox, R. W. , Rao, S. M. , & Prieto, T. (1997). Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience, 17(1), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. , & Desai, R. H. (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan, T. , Burman, D. D. , Lu, D. , Cone, N. E. , Gitelman, D. R. , Mesulam, M. M. , & Booth, J. R. (2006). Weaker top‐down modulation from the left inferior frontal gyrus in children. NeuroImage, 33(3), 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, J. R. , Wood, L. , Lu, D. , Houk, J. C. , & Bitan, T. (2007). The role of the basal ganglia and cerebellum in language processing. Brain Research, 1133(1), 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron, C. , Jambaque, I. , Nabbout, R. , Lounes, R. , Syrota, A. , & Dulac, O. (1997). The right brain hemisphere is dominant in human infants. Brain, 120(6), 1057–1065. [DOI] [PubMed] [Google Scholar]
- Clower, D. M. , Dum, R. P. , & Strick, P. L. (2005). Basal ganglia and cerebellar inputs to “AIP. Cerebral Cortex (New York, N.Y. : 1991), 15(7), 913–920. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Statistical Power Analysis for the Behavioral Sciences, [Google Scholar]
- Desmond, J. E. , Gabrieli, J. D. , & Glover, G. H. (1998). Dissociation of frontal and cerebellar activity in a cognitive task: Evidence for a distinction between selection and search. NeuroImage, 7(4), 368–376. [DOI] [PubMed] [Google Scholar]
- Doesburg, S. M. , Vinette, S. A. , Cheung, M. J. , & Pang, E. W. (2012). Theta‐modulated gamma‐band synchronization among activated regions during a verb generation task. Frontiers in Psychology, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl, E. C. , Neumann, J. , Bogler, C. , & Von Cramon, D. Y. (2008). The extended language network: A meta‐analysis of neuroimaging studies on text comprehension. Human Brain Mapping, 29(5), 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez, J. A. , Raichle, M. E. , Balota, D. A. , Tallal, P. , & Petersen, S. E. (1996). PET activation of posterior temporal regions during auditory word presentation and verb generation. Cerebral Cortex (New York, N.Y. : 1991), 6(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. [DOI] [PubMed] [Google Scholar]
- Friederici, A. D. (2012). The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16(5), 262–268. [DOI] [PubMed] [Google Scholar]
- Frings, M. , Dimitrova, A. , Schorn, C. F. , Elles, H. G. , Hein‐Kropp, C. , Gizewski, E. R. , … Timmann, D. (2006). Cerebellar involvement in verb generation: An fMRI study. Neuroscience Letters, 409(1), 19–23. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Worsley, K. J. , Frackowiak, R. S. J. , Mazziotta, J. C. , & Evans, A. C. (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping, 1(3), 210–220. [DOI] [PubMed] [Google Scholar]
- Gabrieli, J. D. , Poldrack, R. A. , & Desmond, J. E. (1998). The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart, A. L. , Petersen, S. E. , & Thach, W. T. (2002). Role of the posterolateral cerebellum in language. Annals of the New York Academy of Sciences, 978, 318–333. [DOI] [PubMed] [Google Scholar]
- Gow, D. W. , Segawa, J. A. , Ahlfors, S. P. , & Lin, F. H. (2008). Lexical influences on speech perception: A Granger causality analysis of MEG and EEG source estimates. NeuroImage, 43(3), 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, M. , Kato, A. , Taniguchi, M. , Saitoh, Y. , Ninomiya, H. , Ihara, A. , … Yoshimine, T. (2004). Determination of language dominance with synthetic aperture magnetometry: Comparison with the Wada test. NeuroImage, 23(1), 46–53. [DOI] [PubMed] [Google Scholar]
- Holland, S. K. , Vannest, J. , Mecoli, M. , Jacola, L. M. , Tillema, J. , Karunanayaka, P. R. , … Byars, W. (2007). Functional MRI of language lateralization during development in children. International Journal of Audiology, 46(9), 533–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk, J. C. , Bastianen, C. , Fansler, D. , Fishbach, A. , Fraser, D. , Reber, P. J. , … Simo, L. S. (2007). Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 362(1485), 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi, S. , Mori, K. , Kiritani, S. , Kawashima, R. , Sugiura, M. , Fukuda, H. , … Nakamura, K. (1997). Vocal identification of speaker and emotion activates different brain regions. NeuroReport, 8(12), 2809–2812. [DOI] [PubMed] [Google Scholar]
- Ito, M. (2008). Control of mental activities by internal models in the cerebellum. Nature Reviews. Neuroscience, 9(4), 304–313. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). FSL. NeuroImage, 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Kadis, D. S. , Dimitrijevic, A. , Toro‐Serey, C. A. , Smith, M. L. , & Holland, S. K. (2016). Characterizing information flux within the distributed pediatric expressive language network: A core region mapped through fMRI‐constrained MEG effective connectivity analyses. Brain Connectivity, 6(1), 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis, D. S. , Pang, E. W. , Mills, T. , Taylor, M. J. , McAndrews, M. P. , & Smith, M. L. (2011). Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. Journal of the International Neuropsychological Society, 17(05), 896–904. [DOI] [PubMed] [Google Scholar]
- Kadis, D. S. , Smith, M. L. , Lou, Mills, T. , & Pang, E. W. (2008). Expressive language mapping in children using MEG; MEG localization of expressive language cortex in healthy children: Application to paediatric clinical populations. Down Syndrome Quarterly, 10, 5–12. [Google Scholar]
- Karunanayaka, P. R. , Holland, S. K. , Schmithorst, V. J. , Solodkin, A. , Chen, E. E. , Szaflarski, J. P. , & Plante, E. (2007). Age‐related connectivity changes in fMRI data from children listening to stories. NeuroImage, 34(1), 349–360. [DOI] [PubMed] [Google Scholar]
- Kellett, K. , Kellett, K. A. , Stevenson, J. L. , & Gernsbacher, M. A. (2012). What role does the cerebellum play in language processing? In: Faust M. (Ed.), Handbook of the neuropsychology of language (pp. 294–316). Wiley‐Blackwell. [Google Scholar]
- Keren‐Happuch, E. , Annabel, C. S.‐H. , Ho, M.‐H. R. , & Desmond, J. E. (2014). A meta‐analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Human Brain Mapping, 35(2), 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens, D. , & Evers, E. R. K. (2014). Sailing from the Seas of Chaos into the corridor of stability: Practical recommendations to increase the informational value of studies. Perspectives on Psychological Science, 9(3), 278–292. [DOI] [PubMed] [Google Scholar]
- Lebel, C. , & Beaulieu, C. (2009). Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping, 30(11), 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner, H. C. , Leiner, A. L. , & Dow, R. S. (1991). The human cerebro‐cerebellar system: Its computing, cognitive, and language skills. Behavioural Brain Research, 44(2), 113–128. [DOI] [PubMed] [Google Scholar]
- Lohmann, G. , Hoehl, S. , Brauer, J. , Danielmeier, C. , Bornkessel‐Schlesewsky, I. , Bahlmann, J. , … Friederici, A. (2010). Setting the frame: The human brain activates a basic low‐frequency network for language processing. Cerebral Cortex (New York, N.Y. : 1991), 20(6), 1286–1292. [DOI] [PubMed] [Google Scholar]
- Mestres‐Missé, A. , Càmara, E. , Rodriguez‐Fornells, A. , Rotte, M. , & Münte, T. F. (2008). Functional neuroanatomy of meaning acquisition from context. Journal of Cognitive Neuroscience, 20(12), 2153–2166. [DOI] [PubMed] [Google Scholar]
- Middleton, F. A. , & Strick, P. L. (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research. Brain Research Reviews, 31(2–3), 236–250. [DOI] [PubMed] [Google Scholar]
- Muller, A. M. , & Meyer, M. (2014). Language in the brain at rest: New insights from resting state data and graph theoretical analysis. Frontiers in Human Neuroscience, 8, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch, B. E. (2010). The cerebellum and language: Historical perspective and review. Cortex, 46(7), 858–868. 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Pang, E. W. , Wang, F. , Malone, M. , Kadis, D. S. , & Donner, E. J. (2011). Localization of Broca's area using verb generation tasks in the MEG: Validation against fMRI. Neuroscience Letters, 490(3), 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, X. , Leuthardt, E. C. , Gaona, C. M. , Brunner, P. , Wolpaw, J. R. , & Schalk, G. (2011). Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. NeuroImage, 54(4), 2960–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, S. E. , Fox, P. T. , Snyder, A. Z. , & Raichle, M. E. (1990). Activation of extrastriate and frontal cortical areas by visual words and word‐like stimuli. Science (80‐), 249(4972), 1041–1044. [DOI] [PubMed] [Google Scholar]
- Price, C. J. , Wise, R. J. S. , & Frackowiak, R. S. J. (1996). Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex (New York, N.Y. : 1991), 6(1), 62–70. [DOI] [PubMed] [Google Scholar]
- Price, C. J. (2000). The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy, 197(3), 335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves, D. , Augustine, G. , Fitzpatrick, D. , Katz, L. , LaMantia, A.‐S. , McNamara, J. , & Williams, M. (2004). Language and lateralization In: Purves D., Augustine G. J., Fitzpatrick D., Katz L. C., LaMantia A.‐S., McNamara J. O., and Williams S. M. (Eds.), Neuroscience (3rd ed., pp. 109–112). [Google Scholar]
- Raichle, M. E. , Fiez, J. A. , Videen, T. O. , MacLeod, A. M. , Pardo, J. V. , Fox, P. T. , & Petersen, S. E. (1994). Practice‐related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex (New York, N.Y. : 1991), 4(1), 8–26. [DOI] [PubMed] [Google Scholar]
- Schlosser, R. , Hutchinson, M. , Joseffer, S. , Rusinek, H. , Saarimaki, A. , Stevenson, J. , … Brodie, J. D. (1998). Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Journal of Neurology, Neurosurgery, and Psychiatry, 64(4), 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. (1997). Rediscovery of an early concept. International Review of Neurobiology, 41, 3–27. [DOI] [PubMed] [Google Scholar]
- Seger, C. A. , Desmond, J. E. , Glover, G. H. , & Gabrieli, J. D. (2000). Functional magnetic resonance imaging evidence for right‐hemisphere involvement in processing unusual semantic relationships. Neuropsychology, 14(3), 361–369. [DOI] [PubMed] [Google Scholar]
- Seghier, M. L. , & Price, C. J. (2010). Reading aloud boosts connectivity through the putamen. Cerebral Cortex (New York, N.Y. : 1991), 20(3), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, G. L. , Corbetta, M. , Buckner, R. L. , Fiez, J. A. , Miezin, F. M. , Raichle, M. E. , & Petersen, S. E. (1997). Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. Journal of Cognitive Neuroscience, 9(5), 648–647. [DOI] [PubMed] [Google Scholar]
- De Smet, H. J. , Paquier, P. , Verhoeven, J. , & Mariën, P. (2013). The cerebellum: Its role in language and related cognitive and affective functions. Brain and Language, 127(3), 334–342. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Sokolov, A. A. , Miall, R. C. , & Ivry, R. B. (2017). The cerebellum: Adaptive prediction for movement and cognition. Trends in Cognitive Sciences, 21(5), 313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage, 44(2), 489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , Valera, E. M. , & Schmahmann, J. D. (2012). Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage, 59(2), 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Schmithorst, V. J. , Altaye, M. , Byars, A. W. , Ret, J. , Plante, E. , & Holland, S. K. (2006). A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Annals of Neurology, 59(5), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Rajagopal, A. , Altaye, M. , Byars, A. W. , Jacola, L. , Schmithorst, V. J. , … Holland, S. K. (2012). Left‐handedness and language lateralization in children. Brain Research, 1433, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti, M. , Moro, A. , Messa, C. , Moresco, R. M. , Rizzo, G. , Carpinelli, A. , … Perani, D. (2005). Basal ganglia and language: Phonology modulates dopaminergic release. NeuroReport, 16(4), 397–401. [DOI] [PubMed] [Google Scholar]
- Toga, A. W. , & Thompson, P. M. (2003). Mapping brain asymmetry. Nature Reviews. Neuroscience, 4(1), 37–48. [DOI] [PubMed] [Google Scholar]
- Turken, A. U. , & Dronkers, N. F. (2011). The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Verly, M. , Verhoeven, J. , Zink, I. , Mantini, D. , Peeters, R. , Deprez, S. , … Sunaert, S. (2014). Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. NeuroImage. Clinical, 4, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Holland, S. K. , & Vannest, J. (2012). Concordance of MEG and fMRI patterns in adolescents during verb generation. Brain Research, 1447, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- Xiao, Y. , Friederici, A. D. , Margulies, D. S. , & Brauer, J. (2016). Development of a selective left‐hemispheric fronto‐temporal network for processing syntactic complexity in language comprehension. Neuropsychologia, 83, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssofzadeh, V. , Williamson, B. , & Kadis, D. S. (2017). Mapping critical language sites in children performing verb generation: Whole‐brain connectivity and graph theoretical analysis in MEG. Frontiers in Human Neuroscience, 11, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, V. Y. , Macdonald, M. J. , Oh, A. , Hua, G. N. , Nil, L. F. , , De Elizabeth, W. , … Ang, E. W. (2014). Age-related sex differences in language lateralization : A magnetoencephalography study in children age‐related sex differences in language lateralization. American Psychological Association, 50, 2276–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray, G. I. , Williams, S. C. R. , Wilson, S. J. , Rose, S. E. , Brammer, M. J. , Bullmore, E. T. , … Doddrell, D. M. (1998). Prefrontal cortex involvement in selective letter generation: A functional magnetic resonance imaging study. Cortex, 34(3), 389–401. [DOI] [PubMed] [Google Scholar]


