Abstract
Multiple sclerosis (MS) patients present several alterations related to sensing of bodily signals. However, no specific neurocognitive impairment has yet been proposed as a core deficit underlying such symptoms. We aimed to determine whether MS patients present changes in interoception—that is, the monitoring of autonomic bodily information—a process that might be related to various bodily dysfunctions. We performed two studies in 34 relapsing–remitting, early‐stage MS patients and 46 controls matched for gender, age, and education. In Study 1, we evaluated the heartbeat‐evoked potential (HEP), a cortical signature of interoception, via a 128‐channel EEG system during a heartbeat detection task including an exteroceptive and an interoceptive condition. Then, we obtained whole‐brain MRI recordings. In Study 2, participants underwent fMRI recordings during two resting‐state conditions: mind wandering and interoception. In Study 1, controls exhibited greater HEP modulation during the interoceptive condition than the exteroceptive one, but no systematic differences between conditions emerged in MS patients. Patients presented atrophy in the left insula, the posterior part of the right insula, and the right anterior cingulate cortex, with abnormal associations between neurophysiological and neuroanatomical patterns. In Study 2, controls showed higher functional connectivity and degree for the interoceptive state compared with mind wandering; however, this pattern was absent in patients, who nonetheless presented greater connectivity and degree than controls during mind wandering. MS patients were characterized by atypical multimodal brain signatures of interoception. This finding opens a new agenda to examine the role of inner‐signal monitoring in the body symptomatology of MS.
Keywords: body perception, functional connectivity, heartbeat detection task, heartbeat evoked potential, interoceptive processing, multiple sclerosis
1. INTRODUCTION
Patients with multiple sclerosis (MS) present alterations related to the sensing of physiological bodily signals (Rocca et al., 2015), including subclinical deficits in olfaction (Silva et al., 2012) and taste (Doty et al., 2016), and clinical symptoms such as abnormal temperature processing (Davis, Wilson, White, & Frohman, 2010), chronic pain (Michalski, Liebig, Thomae, Hinz, & Bergh, 2011), and fatigue (Krupp, 2006). These dysfunctions are related with various mechanisms implicated in interoceptive processing (Couto, Aldofi, Velasquez, et al., 2015; Craig, 2002; Hanken, Eling, & Hildebrandt, 2014; Tsakiris, Tajadura‐Jimenez, & Costantini, 2011), namely, the monitoring of autonomic bodily information (Couto, Adolfi, Sedeno, et al., 2015; Craig, 2002; Khalsa, Rudrauf, Feinstein, & Tranel, 2009). Also, the presence of these symptoms in other neurological diseases has been associated with damage in regions subserving interoceptive processes (Couto, Adolfi, Sedeno, et al., 2015). However, so far no model of MS has proposed a specific neurocognitive mechanism that might underlie such diverse body‐related symptoms. We posit that these manifestations may be related to changes in interoception—that is, the monitoring of autonomic bodily information (Couto, Adolfi, Sedeno, et al., 2015; Craig, 2002; Khalsa et al., 2009). As an initial step toward the exploration of this notion, we conducted the first systematic, multidimensional assessment of interoception in MS, integrating electrophysiological, and neuroimaging evidence.
Signals proceeding from specific senses—for example, smell, taste, and touch—have been directly related to interoception, as they result from the activity of brain areas that represent an external pathway of body‐mapped sensations (Couto, Adolfi, Sedeno, et al., 2015; Kurth, Zilles, Fox, Laird, & Eickhoff, 2010). Furthermore, smell and taste rely on common brain areas related to interoceptive processing, such as the anterior insula (Craig, 2002). This region has actually been proposed as a main hub integrating internally and externally triggered signals (Couto, Adolfi, Sedeno, et al., 2015; Kurth et al., 2010). Indeed, its alterations correlate with deficits in processing specific bodily‐sensations such as smell, taste, and thermal pain (Couto, Adolfi, Sedeno, et al., 2015). More particularly, research on MS patients and neurotypicals shows that inflammatory processes underlying fatigue (Dantzer, Heijnen, Kavelaars, Laye, & Capuron, 2014; Hanken et al., 2014) act specifically on regions subserving interoception, such as the insula and the anterior cingulate cortex (ACC) (Craig, 2002). Compatibly, focusing on bodily‐signals has been proposed as an intervention to reduce fatigue in MS (Vercoulen et al., 1996).
Despite the absence of a systematic pattern of cortical damage in MS (Kluckow, Rehbein, Schwab, Witte, & Bublak, 2016), significant atrophy has been repeatedly observed in interoceptive regions, such as the insula and the ACC (Lansley, Mataix‐Cols, Grau, Radua, & Sastre‐Garriga, 2013). Moreover, the heterogeneous patterns of white matter lesions in this condition include alterations in pathways of interoceptive areas, such as the ACC white matter bundle within the left cingulate fasciculus (Pardini et al., 2015). In the same vein, MS involves functional connectivity alterations across several regions (Faivre et al., 2012; Rocca et al., 2012, 2015; Roosendaal et al., 2010), crucially including both hypo‐connectivity in the ACC (Rocca et al., 2015) and the insula (He et al., 2009), and hyper‐connectivity in networks including these areas and the somatosensory cortex (SSC) (Faivre et al., 2012; Rocca et al., 2012; Roosendaal et al., 2010). Nevertheless, none of these reports evaluated the direct association between structural or functional connectivity data with interoceptive processes in this condition.
In sum, clinical findings converge with structural and functional imaging evidence to suggest that interoception—as an altered mechanism potentially underlying body‐related symptoms—may be distinctively impaired in MS. To bridge such a gap, we performed two complementary studies offering an unprecedented characterization of this domain in MS. First, we assessed the heartbeat‐evoked potential (HEP), a neural marker of cardiac monitoring (Pollatos & Schandry, 2004). Building on our hypothesis that body manifestations are related to changes in interoception, we expected to find HEP alterations in the patients compared with controls. Also, we measured the patients’ atrophy level in interoceptive regions to test the prediction (Lansley et al., 2013) that they would exhibit significant alterations in these areas. Moreover, we examined the association between the temporal dynamics of the HEP and gray matter volume. Then, in a second study, we used fMRI to evaluate the functional connectivity and related network properties of these areas when an interoceptive resting‐condition (ISt) state was compared with a mind‐wandering (MW) one. We predicted that these features would present different patterns of co‐activation between conditions in patients relative to controls.
2. MATERIALS AND METHODS
2.1. Participants
The study comprised 34 relapsing–remitting MS patients in early disease stages (fulfilling the McDonald's criteria [Polman et al., 2011]) and 46 healthy controls. Diagnoses were made by two MS experts (VS and FP) and complemented with a clinical standard examination, magnetic resonance imaging, and lumbar puncture, when necessary. Additionally, they were assessed with the Expanded Disability Status Scale (EDSS) (Kurtzke, 1983) and the Multiple Sclerosis Severity Score (MSSS) (Roxburgh et al., 2005) (Table 1). Both groups were matched for age, sex, and education (Table 1). The study was approved by the institutional ethics committee. All participants signed an informed consent in accordance with the Declaration of Helsinki.
Table 1.
Demographic and clinical results
| Patients | Controls | χ2 | p values | |||
|---|---|---|---|---|---|---|
| Gender | 0.45 | .83 | ||||
| Female | 28 | 37 | ||||
| Male | 6 | 9 | ||||
| M | SD | M | SD | F | ||
| Age (years) | 38.58 | 10.03 | 37.48 | 12.46 | 0.17 | .68 |
| Formal education (years) | 16.50 | 3.30 | 17.34 | 2.58 | 1.65 | .20 |
| EDSS | 1.67 | 1.67 | ||||
| MSSS | 1.59 | 2.35 | ||||
| Years since diagnosis | 12.66 | 7.42 | ||||
Demographic data was assessed using ANOVA tests to compare groups. The gender comparison was done with Pearson's chi‐square (χ2) test. Patients were assessed with the Expanded Disability Status Scale (EDSS), which quantifies disability in terms of eight functional systems (on a range from 1 to 10, where scores below 3 indicate minimal disability in two functional systems and scores above 6 indicate constant assistance to walk) (Kurtzke, 1983); and the Multiple Sclerosis Severity Score [MSSS; (severity assessed by the relationship between EDSS and disease duration; Roxburgh et al., 2005].
2.2. Study 1: Electrophysiological and neuroanatomical bases of Interoception
Study 1 aimed to assess the temporal dynamics of cardiac interoceptive processing and its relation with key interoceptive areas. First, we analyzed the HEP via a high‐density EEG system. Second, using voxel‐based morphometry (VBM), we examined whether MS patients presented atrophy in key hubs of the interoceptive network (bilateral insula, ACC, and SSC) (Couto, Adolfi, Sedeno, et al., 2015; Garcia‐Cordero et al., 2016). Finally, we performed correlation analyses between HEP and VBM results to explore the association between the temporal dynamics of interoception and gray matter volume, as done previously (Muller et al., 2015).
2.3. Heartbeat‐evoked potential: Preprocessing and analysis
EEG signals were recorded during two experimental conditions adapted from a validated heartbeat detection task (Couto et al., 2014; Garcia‐Cordero et al., 2016) [for a full and detailed description of these conditions, see Couto et al. (2014) and Yoris et al. (2015)]. In the first condition, named exteroceptive condition (EC), participants were binaurally presented with the same recorded heartbeat (digitally constructed from a real ECG record of a researcher) and instructed to press a key in synchrony with it. This condition included two parts (each lasting 2 min): (i) one where the heartbeats were presented at a constant and regular frequency (60 bpm), and (ii) another one that presented beats manipulated to have the same overall frequency (60 bpm) but with irregular heartbeat intervals. In the second one, named interoceptive condition (IC), they were asked to tap the same key but this time following their own heartbeats in the absence of any external feedback. The latter condition was repeated twice (each part lasting 2 min). As in previous studies, these conditions were used to induce the contrastive attentional states targeted in our HEP analyses (Fukushima, Terasawa, & Umeda, 2011; Schulz et al., 2015; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). This is in line with previous work on interoception comparing HEP modulations between healthy controls and patients, alongside their relationship with structural MRI data, without considering performance (Muller et al., 2015).
The HEP is modulated by attention to one's own heartbeats (Pollatos & Schandry, 2004) and it constitutes a robust index of interoceptive deficits in patients with other neurological disorders (e.g., fronto‐temporal dementia, Alzheimer's disease, fronto‐insular stroke) (Garcia‐Cordero et al., 2016; Yoris, Garcia, et al., 2018) as well as psychiatric conditions (e.g., borderline personality disorder, obsessive–compulsive disorder) (Muller et al., 2015; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). The HEP is also a more sensitive index of interoceptive processes than behavioral measures, which have yielded inconsistent results in several conditions, including anxiety disorders (Domschke, Stevens, Pfleiderer, & Gerlach, 2010; Yoris et al., 2015) and depersonalization–derealization syndrome (Michal et al., 2014; Sedeno et al., 2014)—with patients performing either better than or similar to controls. Here, we also tested the subjects’ behavioral performance on the two experimental conditions (EC and IC). As expected, given these previous inconsistencies, we found no differences between groups in any of them (see Supporting Information Data S1 and Supporting Information Table S1). Thus, following previous studies (Muller et al., 2015; Schulz et al., 2015), we focused our main analysis on HEP modulations, which provide critical information about the temporal dynamics of interoception. More particularly, HEP modulations have been widely shown to be autonomously reliable as a robust index of general attention to body‐inner sensations (Garcia‐Cordero et al., 2017; Garcia‐Cordero et al., 2016; Pollatos & Schandry, 2004; Yoris, Abrevaya, et al., 2018; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017) as well as other processes associated with body–brain communication, such as body awareness (Muller et al., 2015), emotional experience (Couto, Aldofi, Velasquez, et al., 2015), motivation (Weitkunat, 1990), attention (Montoya, Schandry, & Muller, 1993), and pain perception (Shao, Shen, Wilder‐Smith, & Li, 2011). These properties highlight the relevance of the HEP as a reliable and self‐sufficient nonbehavioral subclinical measure to afford critical insights of the cortical monitoring of internal signals.
EEG signals were acquired with a Biosemi Active‐two 128 channel system at 1,024 Hz, were resampled offline at 256 Hz, and filtered (0.5–30 Hz o μV). The signal was re‐referenced offline to electrodes on mastoids. Cardiac‐field artifacts and ocular movement contamination were removed from data through independent component analysis (Kim & Kim, 2012) and a visual inspection protocol (Canales‐Johnson et al., 2015), as done in previous studies (Canales‐Johnson et al., 2015; Dirlich, Vogl, Plaschke, & Strian, 1997; Garcia‐Cordero et al., 2016; Pollatos & Schandry, 2004; Schandry & Montoya, 1996; Terhaar, Viola, Bar, & Debener, 2012; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). Following findings by Terhaar et al. (2012), we explored all components that showed a high voltage aligned with the heart signal R‐wave with greater posterior positivity and greater anterior negativity. Finally, we visually compared the HEP before and after removing cardiac field artifacts to assess the effect of this procedure on the cardiac potential. Finally, noisy epochs were rejected from the analysis through a visual procedure. To detect the R‐wave‐EKG values and segment continuous EEG data for HEP analysis, we used a peakfinder function on Matlab. This allowed us to find local peaks or valleys (local extremes) in a noisy vector using the alternating nature of the derivatives and a user‐defined magnitude threshold to determine whether each peak is significantly larger (or smaller) (Kruczyk, Umer, Enroth, & Komorowski, 2013). These EEG epochs were delimited between −200 and 500 ms, and baseline‐corrected relative to a −200 to −0 ms time window.
HEP modulations were evaluated through a point‐by‐point Monte Carlo permutation test with bootstrapping (Manly, 2006). This was used to compare conditions within groups, and to assess between‐group differences upon subtraction of EC from IC modulations. To this end, the two parts of each EC and IC were merged, as done in previous reports, to avoid redundant comparisons and get a robust signal per condition (Garcia‐Cordero et al., 2017; Garcia‐Cordero et al., 2016; Yoris, Abrevaya, et al., 2018; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). This permutation analysis is a robust approach to EEG data which has been applied in several studies assessing modulations of the HEP (Canales‐Johnson et al., 2015; Couto, Adolfi, Velasquez, et al., 2015; Couto et al., 2014; Craig, 2002; Critchley & Harrison, 2013; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Garcia‐Cordero et al., 2016) and other ERPs (Amoruso et al., 2014; Chennu et al., 2013; Gonzalez‐Gadea et al., 2015; Ibanez et al., 2013; Melloni et al., 2015; Melloni et al., 2016). This method gives a straightforward solution for the multiple comparison problems and does not depend on multiple comparison corrections or Gaussian distribution assumptions. Therefore, it does not assume an a priori distribution and, given that is a point‐by‐point approach, it is not based on a time window selected a priori. Instead, it allowed us to analyze each point of the signal comprised within the typical HEP latency (100–500 ms) (Canales‐Johnson et al., 2015; Montoya et al., 1993; Pollatos & Schandry, 2004). HEP analyses were based on three fronto‐central (Couto et al., 2014; Garcia‐Cordero et al., 2016) regions of interest (ROI): a left‐frontal one (C27, C28, C31, C32), a fronto‐central one (C19, C20, C21, C22), and a right‐frontal one (C9, C10, C14, C15). Finally, cardiac parameters (e.g., interbeat interval, heart‐rate variability, and EKG amplitude) were compared within conditions and between groups to evaluate their potential influence on HEP modulations. None of these analyses yielded significant results, suggesting that our findings were not biased by cardiac events (see details in the Supporting Information Data S2 and Supporting Information Tables S2 and S3).
2.4. Image acquisition and analysis
We followed the practical guide for reporting MRI studies from the Organization for Human Brain Mapping (OHBM) (Nichols et al., 2017; Poldrack et al., 2017) to report MRI acquisition and preprocessing steps. We obtained MRI recordings from 27 patients and 28 controls from a 1.5 T Phillips Intera scanner with a standard head coil (8 channels). We acquired whole brain T1‐weighted anatomical 3D scans, spin echo volumes, parallel to the plane connecting the anterior and posterior commissures, with the following parameters: repetition time (TR) = 7,489 ms; echo time (TE) = 3,420 ms; flip angle = 8°; 196 slices, matrix dimension = 256 × 240; voxel size = 1 × 1 × 1 mm3; sequence duration = 7 min.
For the VBM analysis, data were preprocessed on the DARTEL Toolbox following validated procedures (Ashburner & Friston, 2000; Couto et al., 2013) on Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12). T1‐weighted images in native space were first segmented using the default parameters of the SPM12 (bias regularization was set to 0.001 and bias FWHM was set to 60‐mm cut‐off) into white matter (WM), gray matter (GM), and cerebrospinal fluid (CFS) (these three tissues were used to estimate the total intracranial volume [TIV]). Then, we ran the “DARTEL (create template) module” using the GM and WM segmented images—with the default parameters indicated by the SPM12—to create a template that is generated from the complete data set (to increase the accuracy of inter‐subject alignment [Ashburner, 2007]). Next, we used the “Normalize to MNI Space module” from DARTEL Tools to affine register the last template from the previous step into the MNI Space. This transformation was then applied to all the individual GM segmented scans to also be brought into standard space. Subsequently, all images were modulated to correct volume changes by Jacobian determinants, and avoid a bias in the intensity of an area due to its expansion during warping. Finally, an isotropic Gaussian kernel of 12‐mm full width at half maximum was applied to all images. The size of the kernel was selected based on previous recommendations (Good et al., 2001). A two sample t‐test was performed via SPM12 to establish the atrophy pattern. As in previous studies (Melloni et al., 2015; Muller et al., 2015), this statistical analysis was based on (six) specific ROIs coinciding with the main interoceptive structures: the insula, the ACC, and the SSC, each in the right and the left hemisphere. We also included the bilateral superior occipital cortex as a control region to test the specificity of our results regarding the interoceptive network‐this area was selected based on previous reports showing its lack of association with interoception (Adolfi et al., 2017; Farb, Segal, & Anderson, 2013; Schulz, 2016). Localization was derived from the Automated Anatomical Labeling Atlas (Tzourio‐Mazoyer et al., 2002). TIV was used as a covariate to discard the influence of brain‐size differences [p < .001 uncorrected (Garcia‐Cordero et al., 2016; Irish, Piguet, Hodges, & Hornberger, 2014; Melloni et al., 2016), extent threshold = 50 voxels].
Then, using Spearman's correlations (p < .05), we evaluated the association between HEP modulations and the resulting atrophy pattern of each interoceptive area (as no differences were found in the SCC and the superior occipital cortex, we framed the corresponding ROIs as a control area for this analysis). HEP data was based on the average differences between IC and EC during the 200–250 ms (Craig, 2002; Pollatos & Schandry, 2004; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017) from the left‐frontal ROI, which presented the greatest modulation in the control sample (Supporting Information Table S4).
2.5. Study 2: Functional connectivity
In Study 2, we evaluated the patterns of fMRI co‐activation and network properties of the same interoceptive hubs targeted in Study 1. We obtained fMRI recordings during two resting‐state conditions of 10 min each, namely, mind‐wandering (MW) and interoceptive state (ISt) (Sedeno et al., 2014). We compared connectivity and network properties between and within groups.
2.6. Functional image acquisition and analysis
We obtained fMRI resting‐state recordings from 27 patients and 28 controls from the same 1.5 T Phillips Intera scanner. However, some participants had to be excluded from analysis due to image artifacts or excessive movement, so that the final sample comprised 25 patients and 23 controls for the MW condition, and 23 patients and 22 controls for the interoceptive one. Functional spin echo volumes, parallel to the anterior–posterior commissures, covering the whole brain were sequential ascending acquired with the following parameters: TR = 2,777 ms; TE = 50 ms; flip angle = 90°; 33 slices, matrix dimension = 64 × 64; voxel size in plane = 3.6 mm × 3.6 mm; slice thickness = 4 mm; sequence duration = 10 min; number of volumes = 209. Participants were asked to keep eyes closed, and to avoid moving or falling asleep. Each subject underwent two fMRI resting‐state conditions of 10 min each, namely, MW and ISt. In the former, participants were told to think about their routine as waking and for the rest of the day (Barttfeld et al., 2012; Sedeno et al., 2014); in the latter, participants were instructed to focus on their heartbeats and respiration (Barttfeld et al., 2012; Sedeno et al., 2014). During all recordings, subjects were requested to keep their eyes closed and to avoid moving and falling sleep.
Resting‐state fMRI scans were preprocessed using the Data Processing Assistant for Resting‐State fMRI (DPARSF V2.3) (Chao‐Gan & Yu‐Feng, 2010), which is an open access toolbox that generates automatic pipeline analysis of imaging data. For each preprocessing step, DPARFS called the Statistical Parametric Mapping (SPM) and Resting‐Strate fMRI Data Analysis Toolkit (REST V.1.7) to process the data. Before preprocessing, the first five volumes of each subject's resting‐state session were discarded to ensure that magnetization achieved a steady state. Then, images were slice‐time corrected (using as reference the middle slice of each volume), and aligned to the first scan of the session to correct head movement (SPM functions). To reduce the effect of motion and physiological artifacts (as cardiac and respiration effects), six motion parameters, cerebrospinal fluid (CFS) and white matter (WM) signals were removed as nuisance variables (REST default functions). CFS and WM masks for this procedure were derived from the tissue segmentation of each subject's T1 scan in native space with the SPM12 software (after the co‐registration of each subject's structural image with the fMRI one). Next, functional images were normalized to the MNI space using the echo‐planar imaging (EPI) template from SPM (Ashburner & Friston, 1999), and then they were smoothed using an 8‐mm full‐width‐at‐half‐maximum isotropic Gaussian kernel (SPM functions). Finally, data was band‐pass filtered between 0.01 and 0.08 Hz given the relevance of slow frequency in the analysis of resting‐state networks (Fox et al., 2005; Raichle, 2009) (REST functions). The participants included had no movements greater than 3 mm and/or rotations higher than 3°. No between‐group differences were found in the translational and rotational parameters (Supporting Information Table S5).
Then, to analyze the connectivity and network properties of key interoceptive areas, we used the six ROIs involving the bilateral regions of the insula, the ACC, and the SSC. First, to assess whether interoceptive processing was associated with variability of inter‐regional connectivity and network properties, we compared both variables between conditions, within each group. The six interoceptive ROIs were derived from the Automated Anatomical Labeling Atlas (Tzourio‐Mazoyer et al., 2002). For this, we extracted mean time‐courses by averaging the BOLD signal of all voxels from each of the six ROIs. Pearson's correlation coefficient was used to define the strength of association between ROIs. In addition, we relied on graph‐theory metrics to explore network properties of this interoceptive network [negative correlations were discarded for graph‐theory assessment given that their analysis is still controversial and less systematic in resting‐state conditions (Rubinov & Sporns, 2010)]. Using the BCT toolbox (Bullmore & Sporns, 2009), we derived the weighted degree (K), defined as the sum of all the weighted connections of a node (Rubinov & Sporns, 2010)—in this case, the nodes were each of the six ROIs defined for the interoceptive network. K is a centrality measure that describes the relevance of a node in a network based on the total strength of its connections (Rubinov & Sporns, 2010). We selected this measure because it indicates the level of association of each ROI with the others in the interoceptive network and, hence, its involvement in the activity of such a network. Given that this is a small network (comprising only six ROIs) and that our hypothesis was not related to whether a specific hub possess a central position in its connectivity, we did not consider other centrality measures, such as betweenness or closeness centrality.
Next, we assessed inter‐regional connectivity and network differences between patients and controls for each resting‐state condition. To estimate inter‐regional connectivity, we used the same association strength values of the six ROIs based on the Pearson's correlation coefficient. For the network analysis, our aim was to evaluate whether the relevance of the six interoceptive ROIs was different between groups in each condition when considering their integration in whole‐brain dynamics. To this end, we first constructed a 90‐node, whole‐brain functional connectivity adjacency matrix (Tzourio‐Mazoyer et al., 2002) for each participant. Then, based on the abovementioned toolbox, we calculated the K of each of the six ROIs in the context of the whole brain network.
Inter‐regional connectivity and network differences were assessed via nonparametric analysis, as previously recommended (Bullmore & Bassett, 2011). Between‐group and within‐group analyses were based on the Wilcoxon Matched Pairs Test and the Mann–Whitney U Test (p < .05), respectively.
3. RESULTS
3.1. Study 1
3.1.1. HEP results
Controls exhibited greater HEP modulation in IC than the EC (Figure 1a), across all frontal ROIs and within the expected window (200–500 ms) (Couto, Adolfi, Sedeno, et al., 2015; Pollatos & Schandry, 2004; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). Conversely, no systematic differences between conditions emerged in MS patients, who showed only small differences in later windows and with an opposite direction relative to controls (in the left ROI) (Figure 1a). Cohen's d was calculated to establish the effect size of the average of each significant window for every ROI. The controls showed effect sizes of 0.27, 0.26, and 0.30 for the left, central, and right frontal ROI, respectively. The patients presented an effect size of 0.26 in the left frontal ROI (the only one that yielded significant differences).
Figure 1.
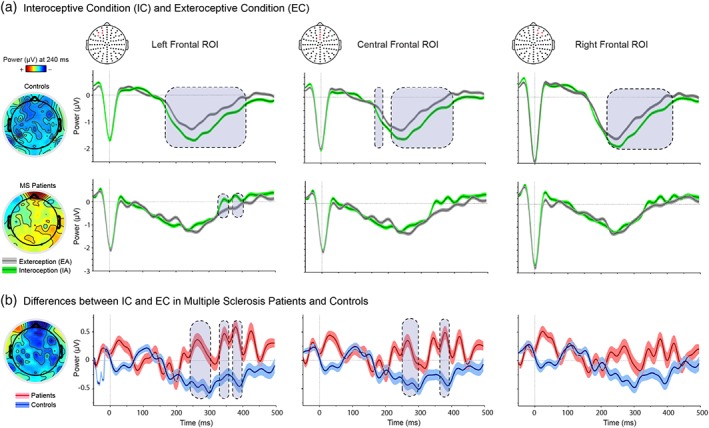
HEP results. (a) Modulations during the IC (green) and the EC (gray). The three top panels show the results of controls in both tasks, whereas the three bottom panels show the results in patients. Results for each ROI are shown separately from left to right. Scalp topography shows the differences in amplitude (microvolts) between conditions for each group. (b) Differences between IC and EC. Scalp topography shows the differences in amplitude (microvolts) between groups. For a and b: Gray boxes show statistically significant differences (at p < .05 for a minimum extension of five consecutive points of difference, following previous reports (Garcia‐Cordero et al., 2016), and shadowed bars around potentials indicate SEM statistical details in Supporting Information Tables S4 and S6
To corroborate that these main effects held their distinctiveness beyond our ROI‐based approach, we performed a spatiotemporal‐cluster‐based analysis. Results from healthy controls showed similar patterns of significant modulations between conditions in a cluster covering most fronto‐central electrodes (and including the ones reported here). On the other hand, no significant cluster emerged in MS patients (for further details see Supporting Information Data S3 and Supporting Information Figure S1). In addition, we performed a source‐reconstruction analysis to corroborate that HEP modulations were generated in interoceptive areas (Canales‐Johnson et al., 2015; Pollatos, Kirsch, & Schandry, 2005). Results revealed that HEP sources comprised the insula, the ACC, and the SSC. Moreover, compared with controls, MS patients presented significantly decreased amplitude in the right insular cortex (see Supporting Information Data S4, Supporting Information Figure S2 for further details).
The subtraction analysis, in which the EC was framed as a baseline control condition for IC, yielded significant differences between samples in a 200–500‐ms window and across left (Cohen's d = 0.60) and central‐frontal (Cohen's d = 0.53) ROIs (the highest difference emerged in the left‐frontal ROI; Figure 1b and Supporting Information Tables S6 and S7).
To test the possibility that HEP results in controls were influenced by motor‐related activity, we reran our main analysis introducing modulations of the motor potential as a covariate. Our findings remained the same. In addition, we performed a logistic regression between the trial‐by‐trial modulation of the motor potential and each condition (exteroception and interoception), which yielded negative results. Taken together, these findings further indicate that the observed HEP effects were uninfluenced by motor responses (see Supporting Information Data S5 and Supporting Information Table S8 and Supporting Information Figure S3 for the details of both analysis).
3.1.2. VBM and association with HEP
MS patients presented significant atrophy compared with controls in the left insula, the posterior part of the right insula, and the right ACC (Figure 2a and Supporting Information Table S4). To estimate the effect size (via Cohen's d), we used the average volume of these areas for each participant. The size of the between‐group effect was moderate (d = 0.60). Given the absence of significant results within the SSC and the superior occipital cortex, we used the pre‐defined (bilateral) ROIs from the selected Atlas (Tzourio‐Mazoyer et al., 2002). Analyses over the SSC and the superior occipital cortex yielded small effect sizes of 0.22 and 0.28, respectively.
Figure 2.
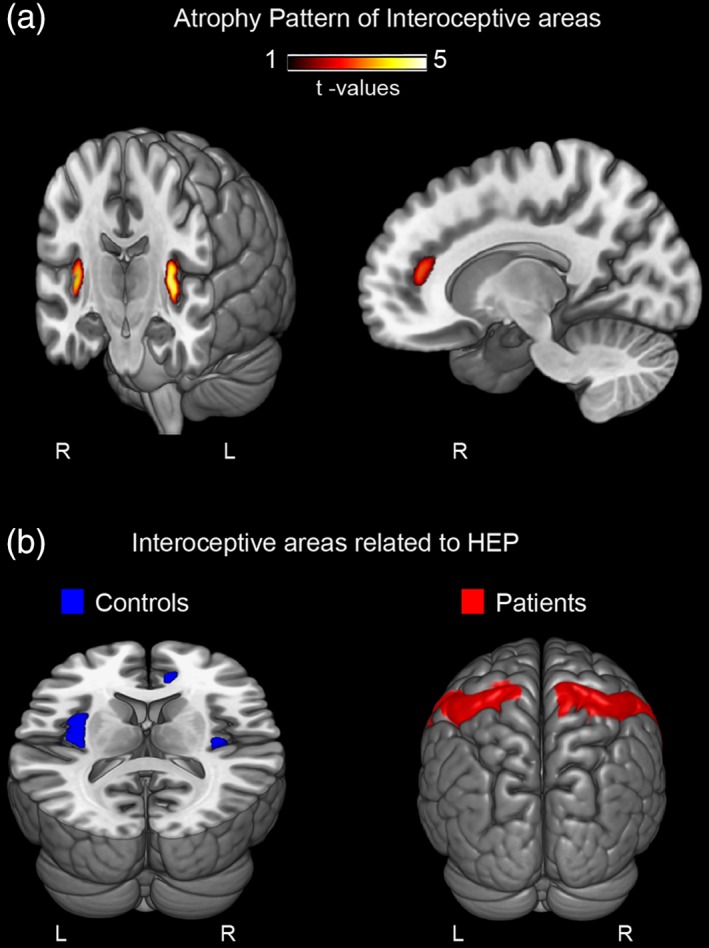
VBM: Multiple sclerosis atrophy and brain‐behavior association. (a) Atrophy pattern of multiple sclerosis patients compared with controls. VBM was performed within key interoceptive areas (namely: the insula, the ACC, and the SSC) and a control region (superior occipital cortex) (p < .001 uncorrected). Statistical details in Supporting Information Table S7. (b) Correlations between results from the VBM group comparison and HEP subtraction. The bilateral insula and the right ACC were significantly associated with HEP subtraction results in the control sample. No association was found with the SSC for this group. On the other hand, multiple sclerosis patients presented significant correlations only between HEP subtractions and the bilateral SSC. Both groups presented no association with the superior occipital cortex. L = left; R = right. See Supporting Information Figure S4 for the scatter plots of each of these correlation analyses
Correlation analyses (Figure 2b) revealed that, in controls, the volume of the bilateral insula (r = .37, p = .04) and the right ACC (r = .43, p = .01) was significantly associated with HEP interoceptive modulations (subtraction results from the 200–250 ms window of the left‐frontal ROI). Thus, the greater the participants' differences in HEP modulation between IC and EC, the larger the volume of these regions. No association was found with the SSC for this group (r = −.18, p = .34). In contrast, HEP modulations in the patients had low correlation values with the bilateral insula (r = −.06, p = .74) and the right ACC (r = −.16, p = .40), but significant ones with bilateral the SSC (r = .41, p = .03). Finally, no significant association was observed between the volume of the superior occipital cortex and the HEP modulation in either groups (r = −.03, p = .86 for controls, and r = −.15, p = .44 for MS patients).
3.2. Study 2
3.2.1. Connectivity and degree (K) between conditions
In controls, the ISt showed greater inter‐regional connectivity and greater K between ROIs compared with MW. No differences between conditions emerged in patients (Figure 3b, and Supporting Information Tables S9 and S10).
Figure 3.
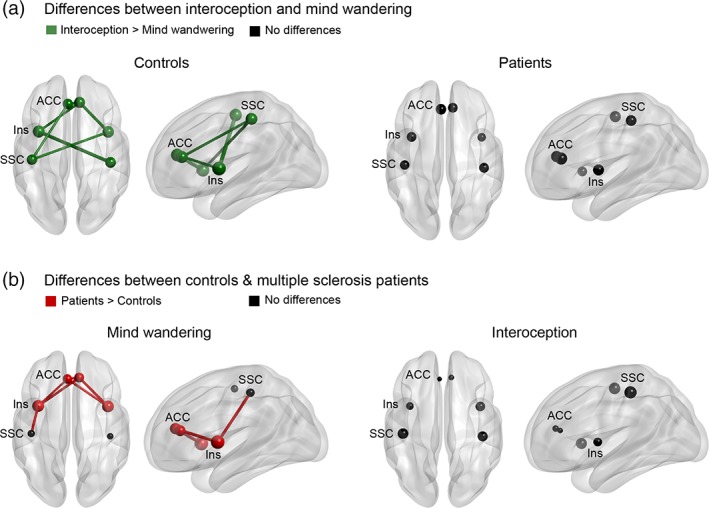
Functional connectivity results. (a) Differences between conditions. While for the control sample ISt showed greater inter‐regional connectivity and greater K compared with MW, no differences were found in multiple sclerosis patients. Figure supported by Supporting Information Tables S9 and S10. (b) Between‐group differences in the mind‐wandering and interoceptive conditions. While patients presented greater inter‐regional connectivity and K than controls for MW, no differences were found during the interoceptive condition. For (a) and (b): Links indicate interregional connectivity. Circles indicate network degree (K). Colors indicate statistically significant differences (p < .05; green = interoception > mind wandering; red = MS patients > controls; black = no significant differences). ACC: anterior cingulate cortex, INS: insula, SSC: somatosensory cortex. Figure supported by Supporting Information Tables S11 and S12
3.2.2. Connectivity and degree (K) between groups
During MW, the patients presented greater inter‐regional connectivity (mainly between insular and ACC connections) and K (all regions except for SSC) than controls. No between‐group differences were found during the interoceptive condition (Figure 3a and Supporting Information Tables S11 and S12).
4. DISCUSSION
This is the first study assessing interoception as a potential core signature of body‐signal processing deficits in MS. We found that patients feature multidimensional disruptions of interception's brain signatures, including abnormal task‐related modulations of a relevant cortical marker, reduced gray matter volume in key interoceptive areas, atypical associations between neurophysiological and neuroanatomical correlates, and selectively altered patterns of functional connectivity during interoceptive states. Our findings indicate that interoceptive alterations may constitute a cardinal marker of disrupted bodily‐signal processing in MS, opening a new research agenda on the disease.
As in previous research (Pollatos et al., 2005; Pollatos & Schandry, 2004; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017), healthy subjects evinced greater HEP modulations when monitoring internal signals compared with external stimuli. Conversely, the patients showed no such effect in the expected ROIs and time windows (from 200 to 500 ms), and they even exhibited inverse modulations in a late window. This abnormal pattern was further supported by the subtraction analysis, as differential HEP modulations between IC and EC were significantly smaller in patients than in controls (Figure 1b). Note that this effect emerged in the absence of behavioral differences, arguably due to the inconsistent relation between cardiac interoceptive accuracy and HEP modulation (Katkin, Cestaro, & Weitkunat, 1991; Montoya et al., 1993; Pollatos & Schandry, 2004; Schandry & Weitkunat, 1990; Schulz et al., 2015; Terhaar et al., 2012; Yuan, Yan, Xu, Han, & Yan, 2007)—for an extensive discussion, see Supporting Information Data S1. Therefore, our ERP results might reflect subtle deficits in the mechanisms subserving internally driven attention, which escape the sensitivity of more basic behavioral measures. Indeed, reduced HEP modulations during heartbeat monitoring is a key marker of poor interoceptive skills in healthy subjects (Pollatos et al., 2005; Pollatos & Schandry, 2004) and in patients with other neurological (Garcia‐Cordero et al., 2016) and psychiatric (Muller et al., 2015; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017) conditions. Moreover, previous findings from other neuropsychiatric conditions have also revealed robust interoceptive alterations indexed by HEP activity, without any congruent behavioral result (Schulz et al., 2015). This evidence highlights the relevance of the HEP as a reliable, nonbehavioral subclinical measure to afford critical insights on the cortical monitoring of internal signals—and, at the same time, as a distinctly sensitive index of impairments in MS and pathological populations.
Moreover, as in several previous studies (Lansley et al., 2013), MS patients presented structural damage in portions of key interoceptive hubs (left insula, right posterior insula, right ACC), without accompanying abnormalities in the SCC (Couto, Adolfi, Sedeno, et al., 2015; Khalsa et al., 2009), nor in the superior occipital cortex. This could suggest specific deficits in an autonomical‐vagal interoceptive pathway (insula and ACC), with preservation of somatosensory pathways (Couto et al., 2014; Khalsa et al., 2009). The latter might provide a compensatory mechanism for interoception. Note that compromised regions in the patients play important roles in interoception, with the insula and ACC acting as viscerosensory and visceromotor centers, respectively (Craig, 2004). Indeed, they are implicated in interoceptive processes in healthy subjects (Critchley et al., 2004; Pollatos & Schandry, 2004) and their disruption is associated with interoceptive deficits in neuropsychiatric disorders (Garcia‐Cordero et al., 2016; Muller et al., 2015; Pollatos et al., 2005; Pollatos & Schandry, 2004). By the same token, cingulo‐insular degeneration may contribute to core body‐sensing mechanisms in MS.
Previous evidence from HEP source analysis also corroborates the crucial role of cingulo‐insular regions in cardiac interoception (Pollatos et al., 2005). Such dependence of the HEP on these areas is consistent with both their association in controls and the null relationship in patients, further reinforcing the multidimensional nature of interoceptive alterations in MS. However, the patients did show associations between their HEP modulation and the volume of the SCC. Given that this structure has been proposed as a complementary pathway for cardiac processing (Couto et al., 2014; Khalsa et al., 2009), the previous result (together with the absence of atrophy in this area) may be reflecting a compensatory interoceptive mechanism providing alternative pathways following the disruption of putative circuits.
Interoceptive deficits in the patients were further corroborated by connectivity results. In line with evidence that attention to heartbeats increases functional synchronization in the interoceptive network (Garcia‐Cordero et al., 2016; Simmons et al., 2013), controls showed higher inter‐regional connectivity and centrality for its hubs during interoceptive than MW states. However, the patients failed to exhibit such a modulation, suggesting a functional disruption in the dynamics of mechanisms subserving body‐signal processing. Indeed, the heterogeneous patterns of functional connectivity alterations in MS include abnormalities between interoceptive areas, such as the ACC and the insula (Faivre et al., 2012; Rocca et al., 2015; Roosendaal et al., 2010), alongside disruptions in relevant anatomical pathways—for example, the ACC white matter bundle within the left cingulate fasciculus (Cruz Gomez, Ventura Campos, Belenguer, Avila, & Forn, 2013; Pardini et al., 2015). We surmise that such functional and structural disturbances may compromise core mechanisms engaged by body‐signal processing, which would be critically indexed by interoceptive impairments.
Further specifications on these connectivity dysfunctions come from the comparison between conditions across groups, showing greater connectivity and centrality in patients during MW only. The same pattern has been reported in previous MS research based on this or other noninteroceptive conditions (Rocca et al., 2012). Hyper‐activation patterns have been proposed to reflect compensatory mechanisms during initial disease stages (Filippi, Agosta, Spinelli, & Rocca, 2013). Indeed, while resting‐state connectivity may be reduced when structural damage becomes pervasive (Roosendaal et al., 2010), hyper‐connectivity has been reported in less advanced stages, especially between networks including interoceptive hubs, such as the ACC during resting state conditions (Rocca et al., 2012). Although more specific studies are needed, this would suggest that the patients featured elevated basal levels of connectivity in the interoceptive network, arguably reflecting compensatory mechanisms to cope with overall alterations in body‐signal processing. In fact, the patients did not present higher connectivity during interoception compared with resting‐state conditions. This indicates that whereas body‐signal monitoring in healthy subjects involves synchronization changes within interoceptive areas, such internally driven states do not elicit distinct modulations in patients. In sum, this two‐fold pattern of dissociations (between conditions and groups) strengthens the view that interoceptive alterations may constitute a core signature of altered body‐signal processing in MS.
4.1. Implications of the interoceptive deficits in MS
Our results show that MS patients present multidimensional alterations in interoceptive mechanisms, supporting their potential role as core disruptions behind its body‐signal processing deficits. Sensory, gustatory, olfactory, and thermal information, as well as feelings of pain and fatigue, have been directly related to interoception, as they rely on external pathways which converge in domain‐relevant areas (Couto, Adolfi, Sedeno, et al., 2015; Gramsch et al., 2014; Kurth et al., 2010). Accordingly, we propose that alterations of the interoceptive network could lie at the root of deficits in such varied body‐sensing domains. Indeed, fatigue, a core symptom of body‐signal processing in MS, has also been related to interoceptive systems (Hanken et al., 2014). Yet, despite such multifarious links, no previous work had empirically assessed interoception in MS. Here, we did not find a significant relationship between the MS severity scores and HEP modulation (see Supporting Information Data S6 for further details of these analysis). This was expected given that both the EDSS and MSSS focus on a broad constellation of alterations, and neither include extensive and specific measures of body‐related symptoms (see Supporting Information Data S6). Our results thus open a new agenda for the study of this condition's diverse bodily symptoms and their underexplored relations with interoceptive deficits and other body‐signal alterations in MS. In this way, future research could directly evaluate whether the body‐signal deficits in MS are modulated by alterations in interoceptive processing.
4.2. Limitations and further research
Our design did not include fMRI active tasks; however, this shortcoming was offset by the contrast between validated interoceptive and noninteroceptive resting‐state conditions (Sedeno et al., 2014), which illuminates specific neural dynamics underlying our target domain. Still, future studies should extend the present design assessing active paradigms. Also, we did not measure other interoceptive dimensions, such as interoceptive sensibility and awareness (Garfinkel, Seth, Barrett, Suzuki, & Critchley, 2015; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). Future studies could refine current findings looking for specific markers of each of these (Garcia‐Cordero et al., 2016; Yoris, García, Traiber, Santamaría‐García, Esteves, et al., 2017; Yoris, García, Traiber, Santamaría‐García, Martorell, et al., 2017). Finally, as we were unable to obtain data on the patients’ bodily symptoms, no direct links could be made between our findings and overall body‐sensing impairments. Still, our study offers a promising avenue to conduct groundbreaking research on the possible relations between interoception and other forms of body‐signal processing as a new outlook on the neurocognitive impact of MS.
5. CONCLUSION
This is the first report assessing interoception in MS patients through multidimensional evidence. We found that patients presented alterations in electrophysiological markers of body‐signal processing and in the structure and network dynamics of interoceptive hubs. Our study represents a novel approach to assess the potential cardinal basis of diverse bodily alterations in this disease. Future studies should build upon these findings to promote deeper understanding of the role of interoception in the overall symptomatology of MS.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest related to this article.
Supporting information
Supporting Information.
ACKNOWLEDGMENTS
Study funded by Novartis Argentina (AR16054872569); CONICYT/FONDECYT Regular; (grant number 1170010); PICT (grant numbers 2017‐1818 and 2017‐1820); CONICET, CONICYT/FONDAP (grant number 15150012) and the INECO Foundation.
Salamone PC, Esteves S, Sinay VJ, et al. Altered neural signatures of interoception in multiple sclerosis. Hum Brain Mapp. 2018;39:4743–4754. 10.1002/hbm.24319
Paula Celeste Salamone and Sol Esteves contributed equally to this study.
Funding information CONICYT/FONDAP, Grant/Award Number: 15150012; CONICYT/FONDECYT Regular, Grant/Award Number: 1170010; Consejo Nacional de Investigaciones Científicas y Técnicas; INECO Foundation; PICT, Grant/ Award Number: 2017‐1818 and 2017‐1820; Novartis Argentina, Grant/Award Number: AR16054872569
REFERENCES
- Adolfi, F. , Couto, B. , Richter, F. , Decety, J. , Lopez, J. , Sigman, M. , & Ibanez, A. (2017). Convergence of interoception, emotion, and social cognition: A twofold fMRI meta‐analysis and lesion approach. Cortex, 88, 124–142. 10.1016/j.cortex.2016.12.019 [DOI] [PubMed] [Google Scholar]
- Amoruso, L. , Sedeno, L. , Huepe, D. , Tomio, A. , Kamienkowski, J. , Hurtado, E. , & Ibanez, A. (2014). Time to tango: Expertise and contextual anticipation during action observation. NeuroImage, 98, 366–385. 10.1016/j.neuroimage.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (1999). Nonlinear spatial normalization using basis functions. Human Brain Mapping, 7(4), 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2000). Voxel‐based morphometry‐‐the methods. NeuroImage, 11(6 Pt 1), 805–821. 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- Barttfeld, P. , Wicker, B. , Cukier, S. , Navarta, S. , Lew, S. , Leiguarda, R. , & Sigman, M. (2012). State‐dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder. Neuropsychologia, 50(14), 3653–3662. 10.1016/j.neuropsychologia.2012.09.047 [DOI] [PubMed] [Google Scholar]
- Bullmore, E. T. , & Bassett, D. S. (2011). Brain graphs: Graphical models of the human brain connectome. Annual Review of Clinical Psychology, 7, 113–140. 10.1146/annurev-clinpsy-040510-143934 [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews. Neuroscience, 10(3), 186–198. 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Canales‐Johnson, A. , Silva, C. , Huepe, D. , Rivera‐Rei, A. , Noreika, V. , Garcia Mdel, C. , & Bekinschtein, T. A. (2015). Auditory feedback differentially modulates Behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cerebral Cortex, 25(11), 4490–4503. 10.1093/cercor/bhv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao‐Gan, Y. , & Yu‐Feng, Z. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4, 13 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennu, S. , Noreika, V. , Gueorguiev, D. , Blenkmann, A. , Kochen, S. , Ibanez, A. , & Bekinschtein, T. A. (2013). Expectation and attention in hierarchical auditory prediction. The Journal of Neuroscience, 33(27), 11194–11205. 10.1523/JNEUROSCI.0114-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto, B. , Adolfi, F. , Sedeno, L. , Salles, A. , Canales‐Johnson, A. , Alvarez‐Abut, P. , & Ibanez, A. (2015). Disentangling interoception: Insights from focal strokes affecting the perception of external and internal milieus. Frontiers in Psychology, 6, 503 10.3389/fpsyg.2015.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto, B. , Adolfi, F. , Velasquez, M. , Mesow, M. , Feinstein, J. , Canales‐Johnson, A. , & Ibanez, A. (2015). Heart evoked potential triggers brain responses to natural affective scenes: A preliminary study. Autonomic Neuroscience, 193, 132–137. 10.1016/j.autneu.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Couto, B. , Manes, F. , Montanes, P. , Matallana, D. , Reyes, P. , Velasquez, M. , & Ibanez, A. (2013). Structural neuroimaging of social cognition in progressive non‐fluent aphasia and behavioral variant of frontotemporal dementia. Frontiers in Human Neuroscience, 7, 467 10.3389/fnhum.2013.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto, B. , Salles, A. , Sedeno, L. , Peradejordi, M. , Barttfeld, P. , Canales‐Johnson, A. , & Ibanez, A. (2014). The man who feels two hearts: The different pathways of interoception. Social Cognitive and Affective Neuroscience, 9(9), 1253–1260. 10.1093/scan/nst108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews. Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2004). Human feelings: Why are some more aware than others? Trends in Cognitive Sciences, 8(6), 239–241. 10.1016/j.tics.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Ohman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Cruz Gomez, A. J. , Ventura Campos, N. , Belenguer, A. , Avila, C. , & Forn, C. (2013). Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS One, 8(10), e77914 10.1371/journal.pone.0077914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer, R. , Heijnen, C. J. , Kavelaars, A. , Laye, S. , & Capuron, L. (2014). The neuroimmune basis of fatigue. Trends in Neurosciences, 37(1), 39–46. 10.1016/j.tins.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, S. L. , Wilson, T. E. , White, A. T. , & Frohman, E. M. (2010). Thermoregulation in multiple sclerosis. Journal of Applied Physiology (1985), 109(5), 1531–1537. 10.1152/japplphysiol.00460.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirlich, G. , Vogl, L. , Plaschke, M. , & Strian, F. (1997). Cardiac field effects on the EEG. Electroencephalography and Clinical Neurophysiology, 102(4), 307–315. [DOI] [PubMed] [Google Scholar]
- Domschke, K. , Stevens, S. , Pfleiderer, B. , & Gerlach, A. L. (2010). Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clinical Psychology Review, 30(1), 1–11. 10.1016/j.cpr.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Doty, R. L. , Tourbier, I. A. , Pham, D. L. , Cuzzocreo, J. L. , Udupa, J. K. , Karacali, B. , & Yousem, D. M. (2016). Taste dysfunction in multiple sclerosis. Journal of Neurology, 263(4), 677–688. 10.1007/s00415-016-8030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre, A. , Rico, A. , Zaaraoui, W. , Crespy, L. , Reuter, F. , Wybrecht, D. , & Audoin, B. (2012). Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Multiple Sclerosis, 18(9), 1251–1258. 10.1177/1352458511435930 [DOI] [PubMed] [Google Scholar]
- Farb, N. A. , Segal, Z. V. , & Anderson, A. K. (2013). Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral Cortex, 23(1), 114–126. 10.1093/cercor/bhr385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi, M. , Agosta, F. , Spinelli, E. G. , & Rocca, M. A. (2013). Imaging resting state brain function in multiple sclerosis. Journal of Neurology, 260(7), 1709–1713. 10.1007/s00415-012-6695-z [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima, H. , Terasawa, Y. , & Umeda, S. (2011). Association between interoception and empathy: Evidence from heartbeat‐evoked brain potential. International Journal of Psychophysiology, 79(2), 259–265. 10.1016/j.ijpsycho.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Garcia‐Cordero, I. , Esteves, S. , Mikulan, E. P. , Hesse, E. , Baglivo, F. H. , Silva, W. , & Sedeno, L. (2017). Attention, in and out: Scalp‐level and intracranial EEG correlates of Interoception and Exteroception. Frontiers in Neuroscience, 11, 411 10.3389/fnins.2017.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Cordero, I. , Sedeno, L. , de la Fuente, L. , Slachevsky, A. , Forno, G. , Klein, F. , & Ibanez, A. (2016). Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371(1708), 20160006 10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel, S. N. , Seth, A. K. , Barrett, A. B. , Suzuki, K. , & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104, 65–74. 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Gadea, M. L. , Chennu, S. , Bekinschtein, T. A. , Rattazzi, A. , Beraudi, A. , Tripicchio, P. , & Ibanez, A. (2015). Predictive coding in autism spectrum disorder and attention deficit hyperactivity disorder. Journal of Neurophysiology, 114(5), 2625–2636. 10.1152/jn.00543.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. S. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R. S. (2001). A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1 Pt 1), 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Gramsch, C. , Kattoor, J. , Icenhour, A. , Forsting, M. , Schedlowski, M. , Gizewski, E. R. , & Elsenbruch, S. (2014). Learning pain‐related fear: Neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiology of Learning and Memory, 116, 36–45. 10.1016/j.nlm.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Hanken, K. , Eling, P. , & Hildebrandt, H. (2014). The representation of inflammatory signals in the brain ‐ a model for subjective fatigue in multiple sclerosis. Frontiers in Neurology, 5, 264 10.3389/fneur.2014.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Dagher, A. , Chen, Z. , Charil, A. , Zijdenbos, A. , Worsley, K. , & Evans, A. (2009). Impaired small‐world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain, 132(Pt 12), 3366–3379. 10.1093/brain/awp089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez, A. , Cardona, J. F. , Dos Santos, Y. V. , Blenkmann, A. , Aravena, P. , Roca, M. , & Bekinschtein, T. (2013). Motor‐language coupling: Direct evidence from early Parkinson's disease and intracranial cortical recordings. Cortex, 49(4), 968–984. 10.1016/j.cortex.2012.02.014 [DOI] [PubMed] [Google Scholar]
- Irish, M. , Piguet, O. , Hodges, J. R. , & Hornberger, M. (2014). Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer's disease. Human Brain Mapping, 35(4), 1422–1435. 10.1002/hbm.22263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katkin, E. S. , Cestaro, V. L. , & Weitkunat, R. (1991). Individual differences in cortical evoked potentials as a function of heartbeat detection ability. The International Journal of Neuroscience, 61(3–4), 269–276. [DOI] [PubMed] [Google Scholar]
- Khalsa, S. S. , Rudrauf, D. , Feinstein, J. S. , & Tranel, D. (2009). The pathways of interoceptive awareness. Nature Neuroscience, 12(12), 1494–1496. 10.1038/nn.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. , & Kim, S. K. (2012). Comparing patterns of component loadings: Principal component analysis (PCA) versus independent component analysis (ICA) in analyzing multivariate non‐normal data. Behavior Research Methods, 44(4), 1239–1243. 10.3758/s13428-012-0193-1 [DOI] [PubMed] [Google Scholar]
- Kluckow, S. W. , Rehbein, J. G. , Schwab, M. , Witte, O. W. , & Bublak, P. (2016). What you get from what you see: Parametric assessment of visual processing capacity in multiple sclerosis and its relation to cognitive fatigue. Cortex, 83, 167–180. 10.1016/j.cortex.2016.07.018 [DOI] [PubMed] [Google Scholar]
- Kruczyk, M. , Umer, H. M. , Enroth, S. , & Komorowski, J. (2013). Peak finder Metaserver ‐ a novel application for finding peaks in ChIP‐seq data. BMC Bioinformatics, 14, 280 10.1186/1471-2105-14-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp, L. (2006). Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Multiple Sclerosis, 12(4), 367–368. [DOI] [PubMed] [Google Scholar]
- Kurth, F. , Zilles, K. , Fox, P. T. , Laird, A. R. , & Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Structure & Function, 214(5–6), 519–534. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke, J. F. (1983). Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology, 33(11), 1444–1452. [DOI] [PubMed] [Google Scholar]
- Lansley, J. , Mataix‐Cols, D. , Grau, M. , Radua, J. , & Sastre‐Garriga, J. (2013). Localized grey matter atrophy in multiple sclerosis: A meta‐analysis of voxel‐based morphometry studies and associations with functional disability. Neuroscience and Biobehavioral Reviews, 37(5), 819–830. 10.1016/j.neubiorev.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Manly, B. F. (2006). Randomization, bootstrap and Monte Carlo methods in biology. Boca Raton, FL: CRC Press. [Google Scholar]
- Melloni, M. , Billeke, P. , Baez, S. , Hesse, E. , de la Fuente, L. , Forno, G. , & Ibanez, A. (2016). Your perspective and my benefit: Multiple lesion models of self‐other integration strategies during social bargaining. Brain, 139, 3022–3040. 10.1093/brain/aww231 [DOI] [PubMed] [Google Scholar]
- Melloni, M. , Sedeno, L. , Hesse, E. , Garcia‐Cordero, I. , Mikulan, E. , Plastino, A. , & Ibanez, A. (2015). Cortical dynamics and subcortical signatures of motor‐language coupling in Parkinson's disease. Scientific Reports, 5, 11899 10.1038/srep11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal, M. , Reuchlein, B. , Adler, J. , Reiner, I. , Beutel, M. E. , Vogele, C. , & Schulz, A. (2014). Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization‐derealization disorder. PLoS One, 9(2), e89823 10.1371/journal.pone.0089823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski, D. , Liebig, S. , Thomae, E. , Hinz, A. , & Bergh, F. T. (2011). Pain in patients with multiple sclerosis: A complex assessment including quantitative and qualitative measurements provides for a disease‐related biopsychosocial pain model. Journal of Pain Research, 4, 219–225. 10.2147/JPR.S20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya, P. , Schandry, R. , & Muller, A. (1993). Heartbeat evoked potentials (HEP): Topography and influence of cardiac awareness and focus of attention. Electroencephalography and Clinical Neurophysiology, 88(3), 163–172. [DOI] [PubMed] [Google Scholar]
- Muller, L. E. , Schulz, A. , Andermann, M. , Gabel, A. , Gescher, D. M. , Spohn, A. , & Bertsch, K. (2015). Cortical representation of afferent bodily signals in borderline personality disorder: Neural correlates and relationship to emotional Dysregulation. JAMA Psychiatry, 72(11), 1077–1086. 10.1001/jamapsychiatry.2015.1252 [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , Das, S. , Eickhoff, S. B. , Evans, A. C. , Glatard, T. , Hanke, M. , & Yeo, B. T. (2017). Best practices in data analysis and sharing in neuroimaging using MRI. Nature Neuroscience, 20(3), 299–303. 10.1038/nn.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini, M. , Bonzano, L. , Bergamino, M. , Bommarito, G. , Feraco, P. , Murugavel, A. , & Roccatagliata, L. (2015). Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Multiple Sclerosis, 21(4), 442–447. 10.1177/1352458514546791 [DOI] [PubMed] [Google Scholar]
- Poldrack, R. A. , Baker, C. I. , Durnez, J. , Gorgolewski, K. J. , Matthews, P. M. , Munafo, M. R. , & Yarkoni, T. (2017). Scanning the horizon: Toward transparent and reproducible neuroimaging research. Nature Reviews. Neuroscience, 18(2), 115–126. 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman, C. H. , Reingold, S. C. , Banwell, B. , Clanet, M. , Cohen, J. A. , Filippi, M. , & Wolinsky, J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos, O. , Kirsch, W. , & Schandry, R. (2005). Brain structures involved in interoceptive awareness and cardioafferent signal processing: A dipole source localization study. Human Brain Mapping, 26(1), 54–64. 10.1002/hbm.20121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos, O. , & Schandry, R. (2004). Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat‐evoked brain potential. Psychophysiology, 41(3), 476–482 doi:10.111/1469‐8986.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. (2009). A paradigm shift in functional brain imaging. The Journal of Neuroscience, 29(41), 12729–12734. 10.1523/JNEUROSCI.4366-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca, M. A. , Amato, M. P. , De Stefano, N. , Enzinger, C. , Geurts, J. J. , Penner, I. K. , & Filippi, M. (2015). Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurology, 14(3), 302–317. 10.1016/S1474-4422(14)70250-9 [DOI] [PubMed] [Google Scholar]
- Rocca, M. A. , Valsasina, P. , Martinelli, V. , Misci, P. , Falini, A. , Comi, G. , & Filippi, M. (2012). Large‐scale neuronal network dysfunction in relapsing‐remitting multiple sclerosis. Neurology, 79(14), 1449–1457. 10.1212/WNL.0b013e31826d5f10 [DOI] [PubMed] [Google Scholar]
- Roosendaal, S. D. , Schoonheim, M. M. , Hulst, H. E. , Sanz‐Arigita, E. J. , Smith, S. M. , Geurts, J. J. , & Barkhof, F. (2010). Resting state networks change in clinically isolated syndrome. Brain, 133(Pt 6), 1612–1621. 10.1093/brain/awq058 [DOI] [PubMed] [Google Scholar]
- Roxburgh, R. H. , Seaman, S. R. , Masterman, T. , Hensiek, A. E. , Sawcer, S. J. , Vukusic, S. , & Compston, D. A. (2005). Multiple sclerosis severity score: Using disability and disease duration to rate disease severity. Neurology, 64(7), 1144–1151. 10.1212/01.WNL.0000156155.19270.F8 [DOI] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Schandry, R. , & Montoya, P. (1996). Event‐related brain potentials and the processing of cardiac activity. Biological Psychology, 42(1–2), 75–85. [DOI] [PubMed] [Google Scholar]
- Schandry, R. , & Weitkunat, R. (1990). Enhancement of heartbeat‐related brain potentials through cardiac awareness training. The International Journal of Neuroscience, 53(2–4), 243–253. [DOI] [PubMed] [Google Scholar]
- Schulz, A. , Koster, S. , Beutel, M. E. , Schachinger, H. , Vogele, C. , Rost, S. , & Michal, M. (2015). Altered patterns of heartbeat‐evoked potentials in depersonalization/derealization disorder: Neurophysiological evidence for impaired cortical representation of bodily signals. Psychosomatic Medicine, 77(5), 506–516. 10.1097/PSY.0000000000000195 [DOI] [PubMed] [Google Scholar]
- Schulz, S. M. (2016). Neural correlates of heart‐focused interoception: A functional magnetic resonance imaging meta‐analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371(1708), 20160018 10.1098/rstb.2016.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeno, L. , Couto, B. , Melloni, M. , Canales‐Johnson, A. , Yoris, A. , Baez, S. , & Ibanez, A. (2014). How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization‐derealization disorder. PLoS One, 9(6), e98769 10.1371/journal.pone.0098769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, S. , Shen, K. , Wilder‐Smith, E. P. , & Li, X. (2011). Effect of pain perception on the heartbeat evoked potential. Clinical Neurophysiology, 122(9), 1838–1845. 10.1016/j.clinph.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Silva, A. M. , Santos, E. , Moreira, I. , Bettencourt, A. , Coutinho, E. , Goncalves, A. , & Cavaco, S. (2012). Olfactory dysfunction in multiple sclerosis: Association with secondary progression. Multiple Sclerosis, 18(5), 616–621. 10.1177/1352458511427156 [DOI] [PubMed] [Google Scholar]
- Simmons, W. K. , Avery, J. A. , Barcalow, J. C. , Bodurka, J. , Drevets, W. C. , & Bellgowan, P. (2013). Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human Brain Mapping, 34(11), 2944–2958. 10.1002/hbm.22113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhaar, J. , Viola, F. C. , Bar, K. J. , & Debener, S. (2012). Heartbeat evoked potentials mirror altered body perception in depressed patients. Clinical Neurophysiology, 123(10), 1950–1957. 10.1016/j.clinph.2012.02.086 [DOI] [PubMed] [Google Scholar]
- Tsakiris, M. , Tajadura‐Jimenez, A. , & Costantini, M. (2011). Just a heartbeat away from one's body: Interoceptive sensitivity predicts malleability of body‐representations. Proceedings of the Biological Sciences, 278(1717), 2470–2476. 10.1098/rspb.2010.2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Vercoulen, J. H. , Hommes, O. R. , Swanink, C. M. , Jongen, P. J. , Fennis, J. F. , Galama, J. M. , & Bleijenberg, G. (1996). The measurement of fatigue in patients with multiple sclerosis. A multidimensional comparison with patients with chronic fatigue syndrome and healthy subjects. Archives of Neurology, 53(7), 642–649. [DOI] [PubMed] [Google Scholar]
- Weitkunat, R. (1990). Motivation and heartbeat evoked potentials. Psychophysiology, 4, 33–40. [Google Scholar]
- Yoris, A. , Abrevaya, S. , Esteves, S. , Salamone, P. , Lori, N. , Martorell, M. , & Ibanez, A. (2018). Multilevel convergence of interoceptive impairments in hypertension: New evidence of disrupted body‐brain interactions. Human Brain Mapping, 39(4), 1563–1581. 10.1002/hbm.23933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoris, A. , Esteves, S. , Couto, B. , Melloni, M. , Kichic, R. , Cetkovich, M. , & Sedeno, L. (2015). The roles of interoceptive sensitivity and metacognitive interoception in panic. Behavioral and Brain Functions, 11, 14 10.1186/s12993-015-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoris, A. , García, A. , Traiber, L. , Santamaría‐García, H. , Esteves, S. , Martorell, M. , … Sedeno, L. (2017). The inner world of overactive monitoring: Neural markers of interoception in OCD. Psychological Medicine, 47, 1957–1970. [DOI] [PubMed] [Google Scholar]
- Yoris, A. , García, A. M. , Salamone, P. , Sedeno, L. , García‐Cordero, I. , & Ibanez, A. (2018). Cardiac interoception in neurological conditions and its relevance for dimensional approaches In Tsakiris M. & De Preester H. E. (Eds.), The Interoceptive Basis of the Mind. Oxford, UK: Oxford University Press. [Google Scholar]
- Yoris, A. , Garcia, A. M. , Traiber, L. , Santamaria‐Garcia, H. , Martorell, M. , Alifano, F. , & Sedeno, L. (2017). The inner world of overactive monitoring: Neural markers of interoception in obsessive‐compulsive disorder. Psychological Medicine, 47(11), 1957–1970. 10.1017/S0033291717000368 [DOI] [PubMed] [Google Scholar]
- Yuan, H. , Yan, H. M. , Xu, X. G. , Han, F. , & Yan, Q. (2007). Effect of heartbeat perception on heartbeat evoked potential waves. Neuroscience Bulletin, 23(6), 357–362. 10.1007/s12264-007-0053-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


