Abstract
Background
Previous diffusion tensor imaging (DTI) studies of obsessive–compulsive disorder (OCD) have primarily used voxel‐ or tract‐based methods to assess white matter microstructure in medicated patients. This is the first probabilistic tractography study to assess the structural connectivity of all major white matter tracts in unmedicated adults with OCD without comorbid psychopathology. We hypothesized that OCD compared to healthy participants would show reduced integrity in frontal interhemispheric and fronto‐limbic tracts.
Methods
DTI data from 29 unmedicated adults with OCD were compared to that of 27 matched healthy control (HC) participants. TRACULA was used to assess probabilistic tractography and compare groups in the average fractional anisotropy (FA) of 8 bilateral tracts plus forceps minor and major, and explore group differences in axial (AD), radial (RD), and mean (MD) diffusivities in tracts where FA differed across groups.
Results
Significantly less FA was detected in OCD compared to HC participants in forceps minor, interhemispheric fibers of the frontal cortex, and right uncinate fasciculus (UNC), association fibers connecting frontal and limbic regions (p's < .05). FA in forceps minor was inversely associated with symptom severity in the OCD participants. Exploratory analyses revealed less AD in right UNC was inversely associated with OCD symptoms.
Conclusions
Structural connectivity of frontal interhemispheric and fronto‐limbic circuits may be altered in unmedicated adults with OCD, especially those with the most severe symptoms. These findings suggest a microstructural basis for the abnormal function and reduced resting‐state connectivity of frontal regions and fronto‐limbic circuits in OCD.
Keywords: diffusion tensor imaging, forceps minor, obsessive–compulsive disorder, structural connectivity, tractography, white matter, uncinate fasciculus
1. INTRODUCTION
Obsessive–compulsive disorder (OCD) is characterized by intrusive thoughts, images, or impulses (i.e., obsessions) and repetitive behaviors aimed at preventing or reducing distress (i.e., compulsions). Neuroimaging findings from individuals with OCD suggest functional abnormalities in the cortico‐striatal circuits that underlie inhibitory control processes (Marsh et al., 2014) and the cortico‐limbic circuits that support reward (Marsh et al., 2015) and threat (Admon et al., 2012) processing, fear expression, and regulation (Milad & Rauch, 2012). Our previous findings also suggest reduced resting‐state functional connectivity within the limbic cortico‐striato‐thalamo‐cortical (CSTC) loop in unmedicated OCD compared to healthy participants (Posner et al., 2014). Other data suggest increased functional connectivity across frontal cortices in OCD patients, from right orbitofrontal to left ventrolateral prefrontal cortex (Chen et al., 2016). Few studies have assessed alterations in the structural connectivity of frontal cortices or fronto‐limbic circuits, and none, to our knowledge, have assessed such alterations in unmedicated individuals with OCD in the absence of comorbid psychopathology. Given that medication can impact the integrity of white matter (Benedetti et al., 2013), we sought to investigate structural connectivity in unmedicated individuals with OCD.
The vast majority of DTI studies of OCD have used voxel‐ or tract‐based methods to assess white matter microstructure (Koch, Reeß, Rus, Zimmer, & Zaudig, 2014), quantifying parameters that measure the diffusion of water along white matter tracts in the brain. Meta‐analytic findings from voxel‐based studies suggest reduced fractional anisotropy (FA) in OCD compared to healthy participants in midline tracts including corpus callosum, cingulum, and in inferior longitudinal, fronto‐occipital, and superior longitudinal fasciculi (Radua et al., 2014). These FA reductions were most robust in midline areas in samples that included medicated participants, suggesting that medications, particularly selective serotonin reuptake inhibitors (SSRIs), likely affect white matter microstructure in OCD. Findings from studies that have employed tract‐based spatial statistics (TBSS), the most commonly used tract‐based method, also suggest widespread FA reductions in OCD compared to healthy participants (Benedetti et al., 2013; Bora et al., 2011; Fontenelle et al., 2011; Gan et al., 2017; Nakamae et al., 2011). FA reductions in corpus callosum (forceps minor), anterior corona radiate, inferior longitudinal and uncinate fasciculi have been detected in SSRI‐treated, but not drug‐naïve, OCD compared to healthy participants, suggesting that these reductions may also be a consequence of SSRI treatment (Benedetti et al., 2013).
Fiber tracking (i.e., tractography) allows for more accurate quantitative assessment of tract‐specific abnormalities than TBSS, as it is less sensitive to subtle group differences in diffusion metrics that can be obscured by normalization to standard space. In contrast to TBSS, tractography measures tract‐specific differences of anisotropy and diffusivity in local diffusion space and can additionally be used as a measure of structural connectivity, allowing identification of axonal projections (Mori & van Zijl, 2002; Mukherjee, Berman, Chung, Hess, & Henry, 2008). Previous tractography studies of adults with OCD have focused on specific tracts (Chiu et al., 2011; Oh et al., 2011) using deterministic tractography, a method that depends on the positioning of regions of interest and other factors (e.g., FA and angle threshold), leading to findings that might be difficult to replicate(Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007). Other studies of OCD have limited their fiber tracking to regions that appeared to differ in FA across OCD and healthy participants in voxel‐wise or TBSS analyses (Gan et al., 2017; Peng et al., 2014). Such findings preclude our understanding of the integrity or connectivity of other fiber tracks in the brain. Thus, to foster a better understanding of white matter microstructure in OCD, we employed TrActs Constrained by Underlying Anatomy (TRACULA), an extension of a global probabilistic tractography approach (Jbabdi, Woolrich, Andersson, & Behrens, 2007), to assess the integrity of 18 major white matter tracts in unmedicated adults with OCD.
One previous tractography study reported less FA in frontal callosal fibers in a modest sample of 20 unmedicated adults with OCD compared to 19 control participants (Oh et al., 2011). Such disrupted integrity of callosal fibers that travel through medial and lateral frontal cortices may contribute to the abnormal functioning of these cortices in OCD (Menzies, Chamberlain, Laird, Thelen, Sahakian, & Bullmore, 2008). Together with findings of reduced resting‐state functional connectivity within limbic circuits (Göttlich, Krämer, Kordon, Hohagen, & Zurowski, 2014; Posner et al., 2014), these previous data lead to our hypothesis that OCD compared to healthy participants would have reduced fractional anisotropy (FA) in forceps minor, frontal fibers that extend through the genu of the corpus callosum, and uncinate fasciculus (UNC), a tract connecting orbitofrontal cortex to amygdala and other limbic regions. We further hypothesized that FA reductions would be most prominent in the OCD participants with the most severe symptoms. We also explored group differences in other white matter indices, including radial, axial, and mean diffusivity (i.e., MD, AD, and RD, respectively).
2. METHODS AND MATERIALS
2.1. Participants
Diffusion data were acquired from 31 unmedicated adults with OCD and 32 age‐, sex‐, and IQ‐ matched HC adults. Twenty‐eight OCD and 23 HC participants had participated in our previous fMRI studies (Marsh et al., 2014, 2015). OCD and HC participants were recruited through flyers, online advertisements (i.e., Craigslist), and word‐of‐mouth. Those with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, pervasive developmental disorder, or current Axis I disorders (other than OCD for the OCD participants) were excluded. Controls had no lifetime Axis I disorders. Diagnoses of OCD and the presence of comorbid Axis I disorders were established by psychiatric evaluations and confirmed with the Structured Clinical Interview for DSM‐IV (First, Spitzer, Gibbon, & Williams, 2002). A trained rater assessed OCD severity using the Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS) (Goodman et al., 1989) and depressive severity using the Hamilton Depression Scale (Hamilton, 1967) on the day of the MRI scan. The Y‐BOCS Symptom Checklist was administered to assess the presence and severity of five different symptom dimensions (Pinto et al., 2007, 2008). Full‐scale IQs were estimated using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1981). The Institutional Review Board of the New York State Psychiatric Institute approved this study, and participants provided written informed consent before entering.
2.2. DTI data acquisition
MRI data were acquired on a GE Signa 3.0 Tesla whole body scanner (GE Medical Systems, Waukesha, WI). Diffusion‐weighted data were acquired along 25 noncollinear spatial directions using a single‐shot spin echo planar imaging sequence (TR/TE = 17000 ms/90 ms, flip angle = 90, field of view = 24 cm, matrix size = 132 × 128 (machine‐interpolated to 256 × 256 for postprocessing), b value = 1000 s/mm2, and three baseline images at b = 0 s/mm2, slice thickness = 2.5 mm without gap, and an in‐plane resolution of 0.94 mm). Scan time for each excitation was 7 min 56 s and 2 separate excitations were averaged (NEX = 2) for each participant. High‐resolution, T1‐weighted images were acquired using a fast spoiled gradient‐recall three‐dimensional pulse sequence: inversion time = 500 ms, echo time = 1.3 ms, repetition time = 4.7 ms, 1 excitation matrix size = 256 × 256, with axial slices parallel to the AC‐PC line, field of view = 25 cm, flip angle = 11, number of slices = 164, slice thickness = 1 mm encoded for sagittal slice reconstruction, providing voxel dimensions of.976 × .976 × 1.0 mm3.
2.3. Preprocessing
Diffusion‐weighted (DW) data were processed using the FMRIB Software Library (FSL) 5.0.6 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) (Smith & Nichols, 2009). Eddy current induced distortions and gross subject motion were corrected using FSL's eddy correction toolbox by linearly registering the DW volumes to the first b = 0 volume. The diffusion gradient vectors were also rotated accordingly (Jones, Knosche, & Turner, 2013). Then FSL's brain extraction tool (Nichols & Holmes, 2002) was used to remove skull and nonbrain tissue. A diffusion tensor model was fitted at each voxel of the eddy corrected data using FSL's dtifit and FA, AD, RD, and MD images were derived from the fitted diffusion tensors. A ball and stick model (setting the maximum number of crossing fibers to 2) was estimated using FSL's bedpostx (Behrens et al., 2007) in order to conduct probabilistic tractography. Before running FSL's probtrackx to perform probabilistic tractography, data quality was assessed by visually checking the raw DW images, eddy current corrected DW images, and the color‐encoded FA images which were also evaluated automatically based on their color cast, calculated on the distribution statistics of the 2D histogram in the color space (He et al., 2014). Using these visual and automated methods, any participants who had motion‐corrupted DTI were excluded from analyses. Those with chemical shifts (i.e., fat artifacts) based on visual inspection were also excluded. T1‐weighted images were automatically processed using Freesurfer (http://freesurfer.net/) and visually inspected. For each participant, the b = 0 image was linearly registered to its T1‐weighted image using FSL's flirt boundary‐based registration, a method that maximizes the intensity gradient across the surface boundary obtained from the T1‐weighted image (Greve & Fischl, 2009).
2.4. Tract reconstruction
TRACULA (Yendiki et al., 2011) was used to automatically reconstruct 18 major white matter pathways: corticospinal tracts (CST), inferior longitudinal fasciculus (ILF), uncinate fasciculus (UNC), anterior thalamic radiations (ATR), cingulum‐cingulate gyrus (supracallosal) bundle (CCG), cingulum‐angular (infracallosal) bundle (CAB), superior longitudinal fasciculus‐parietal bundle (SLFP), superior longitudinal fasciculus‐temporal bundle (SLFT), all bilaterally, and corpus callosum's forceps major and minor.
TRACULA extends a global probabilistic tractography algorithm (Jbabdi et al., 2007) that uses a Bayesian framework to estimate 18 white matter tracts in a test subject from the preprocessed diffusion data. The posterior probability distribution of each white matter tract was estimated from a likelihood which utilizes the ball and stick model of diffusion (Behrens et al., 2007), and a prior distribution which combines anatomical information on that tract from a training dataset (included in the TRACULA package). Specifically, a cubic spline is used to model each streamline of white matter tract with a certain number of control points (using default settings in TRACULA), which are initialized by fitting a spline to the median of the streamlines of that tract in the training datasets, and constrained by the end region‐of‐interests which are initialized by dilating the end points of the streamlines of that tract in the training dataset and locating the intersection with the cortex (Freesurfer parcellations/segmentations) of that test subject (Yendiki et al., 2011).
Two trained image analysts (M.S. and M.F.) blind to diagnosis visually inspected each participant's tract reconstruction to ensure the quality of the data processing. For tracts with a single curve, branches into other tracts, or unusual small bundles, we first tried reinitializing those tracts automatically in TRACULA. If reinitialization failed (Supporting Information, Table S1), we manually edited the control points for those tracts in Freesurfer's Freeview tool based on the coordinates for those points in the contralateral hemisphere before rerunning TRACULA. An example of a tract before and after editing the control points is available in Supporting Information, Figure S1.
2.5. Statistical analyses
To assess potential alterations in white matter microstructure in unmedicated participants with OCD, we compared OCD and HC participants on diffusion measurements extracted from TRACULA for all 18 tracts (Figure 1). Group differences in FA were assessed via regression analyses, controlling for age; group differences in MD, AD, and RD were assessed via the same regression models in tracts where FA differed across groups (p's < .05). Regression analyses controlling for age were used to assess in the OCD group associations of OCD symptoms (YBOC scores) and the average FA of tracts in which group differences were detected. Associations of FA in these tracts with OCD age of onset and duration were also explored, and the potential confounding effects of prior medication on white matter microstructure in unmedicated participants with OCD.
Figure 1.
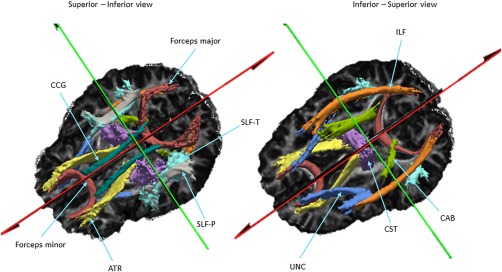
Visualization of reconstructed white matter pathways. Shown here are the 18 white matter pathways from which DTI measurements were extracted via TRACULA. These pathways are overlaid on the FA image of one subject in local space. Abbreviations: ATR, interior thalamic radiation; CAB, cingulum‐angular bundle; CCG, cingulum‐cingulate gyrus; CST, corticospinal tract; ILF, inferior longitudinal fasciculus; SLF‐P, superior longitudinal fasciculus‐parietal; SLF‐T, SLF‐temporal; UNC, uncinate fasciculus
3. RESULTS
3.1. Participants
Data from 2 OCD and 5 HC participants were excluded from analyses due to excessive motion (1 OCD and 1 HC) and chemical shift (i.e., fat) artifacts (1 OCD and 4 HC), thus DTI analyses included data from 29 unmedicated OCD participants and 27 age‐ and IQ‐matched HCs. The majority of participants (24 OCD and 25 HC) were right‐handed. All OCD participants were free of psychotropic medications at the time of imaging; 17 were treatment‐naïve and 12 had been free of any psychotropic medication for 132 weeks (SD 147 weeks, range 12–468 weeks, see Supporting Information, Table S2). None had a current comorbid axis I disorder. Table 1 shows additional demographic and clinical characteristics of both groups. The total motion index (Yendiki, 2014) did not differ across OCD and HC groups (p = .33).
Table 1.
Demographic, clinical, and neuropsychological characteristics of participants
| Characteristic | OCD (n = 29) | HC (n = 27) | Test statistic |
|---|---|---|---|
| Age, years | 29.20 (7.19, range 20.25–51.58) | 28.75 (8.13, range 18.25–46.42) | t 52 = −0.22; p = .83 |
| WASI IQ score (Full‐4)a | 111.44 (13.47) | 110.5 (11.96) | t 47 = −0.26; p = .80 |
| Duration of Illness, months | 14.41(7.01, range 3.17–30.75) | … | |
| Age of OCD onset, years | 14.79 (6.81) | … | |
| Y‐BOCS total | 25.93 (3.41) | … | |
| Obsessions | 12.38 (1.92) | … | |
| Compulsions | 13.55 (1.97) | … | |
| HAM‐D scoresb | 5.03 (3.53) | 0.64 (1.19) | |
| Sex | |||
| Male | 14 (52%) | 13 (48%) | |
| Female | 15 (48%) | 14 (52%) | |
| Handedness | |||
| Right | 24 (83%) | 25 (93%) | |
| Left | 5 (17%) | 2 (7%) | |
| Ethnicity | |||
| Asian or Pacific Islander | 1 (3%) | 1 (4%) | |
| African‐American | 6 (21%) | 7 (26%) | |
| Caucasian | 20 (69%) | 16 (59%) | |
| Hispanic | 4(14%) | 4 (15%) | |
| Other | 2 (7%) | 3 (11%) |
Note. Abbreviations: HAM‐D, Hamilton depression; WASI, Wechsler Abbreviated Scale of Intelligence; Y‐BOCS, Yale‐Brown Obsessive Compulsive Scale.
*Values are mean (standard deviation) unless otherwise specified.
WASI data were available for 24 HC and 25 OCD participants.
HAM‐D data were available for 25 HC participants.
3.2. A priori hypothesis testing
Compared to HC participants, those with OCD had significantly less FA in forceps minor (p = .03) and right uncinate fasciculus (UNC, p = .04, Figure 2a and Table 2). These findings remained when a permutation test was performed (Supporting Information, Table S3). FA in forceps minor was inversely associated with total YBOC scores (p < .05) in the OCD group, and a scatterplot revealed that those who had the most severe symptoms had the least FA in this tract (Figure 2b). In no instance did participants with OCD exhibit significantly more FA than HC participants. Since groups did not differ in the maximum or center tract length or volume of forceps minor or right UNC (p's > .05), these variables were not included as covariates in our regression analyses.
Figure 2.
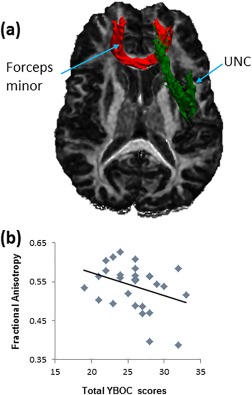
Group differences in white matter integrity and symptom severity correlates. (a) FA was decreased in the OCD compared to HC participants in forceps minor and right uncinated fasciculus (UNC), which are overlaid on the FA image of one subject in local space and displayed in neurological orientation for better visualization. (b) Significant inverse associations of FA with OCD symptoms were detected in forceps minor. Mean FA values are plotted on the y axis and total YBOC scores are plotted on the x axis
Table 2.
Group differences in FA
| Tracts | Hemisphere | t value | p value |
|---|---|---|---|
| Forceps major | 0.5978 | 0.5525 | |
| Forceps minor | −2.2167 | 0.0310* | |
| ATR | Left | 0.4565 | 0.6499 |
| CAB | −0.8499 | 0.3992 | |
| CCG | 0.7598 | 0.4507 | |
| CST | 1.2564 | 0.2145 | |
| ILF | −0.5934 | 0.5555 | |
| SLF‐P | 0.7293 | 0.469 | |
| SLF‐T | −0.1284 | 0.8983 | |
| UNC | −0.1502 | 0.8812 | |
| ATR | Right | −0.3654 | 0.7162 |
| CAB | −1.343 | 0.185 | |
| CCG | 1.0144 | 0.315 | |
| CST | 0.139 | 0.8899 | |
| ILF | −0.9744 | 0.3343 | |
| SLF‐P | 0.377 | 0.7077 | |
| SLF‐T | −0.4385 | 0.6628 | |
| UNC | −2.1444 | 0.0366* |
Note. Abbreviations: ATR, interior thalamic radiation; CAB, cingulum‐angular bundle; CCG, cingulum‐cingulate gyrus; CST, corticospinal tract; ILF, inferior longitudinal fasciculus; SLF‐P, superior longitudinal fasciculus‐parietal; SLF‐T, SLF‐temporal; UNC, uncinate fasciculus.
3.3. Exploratory analyses
Significantly less AD was also detected in right UNC (p's < .05). AD in right UNC was inversely associated with OCD symptoms in the OCD group (total YBOC scores, p's < .05), suggesting that those with the most severe symptoms had the least AD in this tract. Neither age of onset nor illness duration was associated with FA in forceps minor (p's > .05) or right UNC (p's > .05, and see supplement). To explore the potential confounding effects of prior medication, we included only the treatment‐naïve OCD participants (n = 17) in another regression analysis. No group (OCD vs HC) differences in FA were detected in either tract (forceps minor, p = .08; right UNC, p = .07). Finally, an exploratory TBSS analysis did not reveal significant group differences in FA in any brain area (Supporting Information).
4. DISCUSSION
Herein, we used probabilistic tractography to assess the microstructure of 18 major fiber tracts in the brains of unmedicated participants with OCD. Compared to their healthy counterparts, OCD participants had less FA in forceps minor and right UNC and those with the most severe symptoms had the least FA in forceps minor. Exploratory analyses also revealed less AD in right UNC in OCD participants, especially those with the most severe symptoms. These findings replicate previous findings of compromised integrity of frontal‐callosal fibers (Oh et al., 2011), but are the first to show abnormal UNC microstructure in unmedicated OCD participants. Such decreased structural connectivity across frontal cortices and fronto‐limbic regions may suggest a microstructural basis for the altered function and reduced resting‐state functional connectivity of frontal regions and fronto‐limbic circuits in OCD.
Our finding of less FA in forceps minor in OCD compared to healthy participants replicates previous TBSS (Benedetti et al., 2013; Gan et al., 2017; Nakamae et al., 2011) and tractography (Oh et al., 2011) findings of altered integrity of corpus callosum in adults with OCD. Forceps minor connects the medial and lateral surfaces of frontal cortices, crossing the midline via the genu of the corpus callosum. Altered integrity of forceps minor may lead to poor interhemispheric communication across frontal cortices and therefore underlie some of the documented functional and structural abnormalities of medial and lateral frontal cortices in OCD (Menzies et al., 2008), especially those suggesting lateralized functional abnormalities (Marsh et al., 2014; Roth et al., 2007). FA in forceps minor correlated inversely with the severity of symptoms in the OCD group. Perhaps the inability to inhibit intrusive thoughts stems not just from functional deficits in the frontal cortices that support inhibitory control processes (Aron, 2007; Aron, Robbins, & Poldrack, 2004), but also from poor interhemispheric communication across those cortices which might necessitate compensatory overengagement of right (or left) lateralized frontal regions on tasks that require the engagement of control processes.
The uncinate fasciculus (UNC) is a white matter tract that connects limbic and frontal areas and previous findings using a voxel‐based method suggest less FA in medicated OCD compared to healthy participants (Peng et al., 2014). Our finding of less FA (along with less AD) in right UNC suggests altered fronto‐limbic structural connectivity in unmedicated individuals with OCD, consistent with findings of altered functional connectivity within the limbic CSTC circuit in unmedicated OCD (Posner et al., 2014). Decreased connectivity of limbic and frontal regions may reflect diminished top–down control over limbic functions such as reward and threat processing. FMRI findings from OCD participants indeed suggest altered limbic responses to rewarding (Figee et al., 2011; Marsh et al., 2015) and threatening stimuli (Admon et al., 2012), consistent with their altered processing of rewards and heightened tendency to overestimate threat. Altered fronto‐limbic connectivity is also consistent with the purported role of the amygdala on this circuit in the pathophysiology of OCD (Wood & Ahmari, 2015). Specifically, amygdala dysregulation may contribute to compulsive behaviors by allowing undue affective influence over behavioral selection.
Our exploratory findings revealed less AD in right UNC correlated inversely with OCD symptoms. AD measures diffusion along the principal direction of a given fiber (Alexander et al., 2011). As the clinical relevance of having reduced AD is rather unknown, these exploratory findings must be interpreted with caution. We speculate that reduced AD in right UNC might reflect damage to this tract, since AD is thought to be a specific marker of axonal damage (Alexander, Lee, Lazar, & Field, 2007). Such damage to right UNC could disrupt fronto‐limbic connectivity in OCD, especially in the most severely ill patients. Future tractography studies with larger samples should assess all DTI parameters in OCD, as FA alone might not be sufficient for our understanding of white matter microstructure (Hasan, 2006).
Previous DTI studies have implicated other tracts as abnormal in OCD, specifically showing reduced FA in the cingulum bundle, ILF and SLF (Radua et al., 2014). However, the majority of those studies included medicated OCD patients and report cluster‐based findings that cannot be compared directly with tractography findings such as ours. Our exploratory TBSS analysis did not reveal significant group differences in FA in any brain area, suggesting that previous cluster‐based findings may be due, in part, to effects of medication or comorbid psychiatric illnesses. Our exploratory analysis of treatment naïve OCD participants in fact revealed that prior medication may have contributed to our findings of less FA in forceps minor and right UNC. Alternatively, that subgroup analysis was insufficiently powered to detect group differences. Findings from previous TBSS studies of unmedicated OCD participants are discrepant, with one showing reduced FA in anterior corpus callosum (Nakamae et al., 2011) (i.e., forceps minor) and the other showing reduced FA in the left cingulum (Fan et al., 2016). Over half of the OCD participants included in the latter study also met criteria for one or more comorbid current axis 1 disorder, suggesting that further study of white matter microstructure in a larger sample of unmedicated OCD participants free of psychiatric comorbidities is warranted.
This study has important strengths. First, we were able to recruit unmedicated OCD patients, more than half of whom were treatment‐naïve and all were free of current comorbidity. The result is one of the largest DTI datasets in unmedicated adults with OCD described to date. Second, we overcome the limitations of automatic reconstruction by using a manual editing method that was developed in collaboration with the TRACULA's developer (Anastasia Yendiki, personal communication, January 26–30, 2016). This allowed us to assess, for the first time, the integrity of all 18 tracts in OCD, rather than focusing only on the tracts that segmented successfully for each participant.
Several limitations are also worth noting. First, although one of the largest DTI datasets in unmedicated adults, the sample size was still small and thus warrants replication. Given the low power due this small sample size, we did not correct for multiple tests, thereby increasing the risk of type I error. We thus limited our exploratory analyses to the two tracts in which group differences in FA were detected, but still underscore the importance of interpreting these findings with caution prior to replication in a larger sample. Second, the DTI data were collected from 2012 to 2014; recent advances suggest that future studies should acquire at least thirty gradient directions and isotropic voxels (Jones et al., 2013). Third, the manual editing method used to improve reconstruction is time‐consuming and requires well‐trained staff skilled in neuroanatomy; automation of these methods will be required to permit their widespread use. Fourth, TRACULA does not permit reconstruction of the internal capsule (although the anterior limb overlaps with the ATR). The anterior limb contains white matter tracts connecting the orbito‐frontal cortex and the basal ganglia, two regions implicated in the pathophysiology of OCD (McGovern & Sheth, 2017); indeed, stereotactic lesioning of the anterior limb has shown efficacy for treating refractory OCD (McGovern & Sheth, 2017). Future probabilistic tractography studies should incorporate novel methods to interrogate this tract directly in OCD. Finally, although none of our OCD patients had comorbid anxiety disorders, we did not collect dimensional measures of anxiety and thus cannot address whether altered fronto‐limbic structural connectivity in our sample was associated with anxiety symptoms. Because FA in the UNC has been demonstrated in individuals with generalized (Tromp et al., 2012) and social (Phan et al., 2009) anxiety disorders, future studies should assess whether altered integrity of white matter within fronto‐limbic circuits is related to anxiety symptoms as a dimensional characteristic across all these disorders.
In summary, these tractography findings suggest altered integrity of frontal inter‐hemispheric and fronto‐limbic circuits in unmedicated adults with OCD in the absence of comorbid psychopathology. Future multimodal imaging studies of unmedicated adults with OCD should combine DTI with fMRI data to simultaneously probe the functional and structural connectivity and determine whether altered white matter integrity in forceps minor and UNC indeed contributes to altered functioning of frontal cortices and fronto‐limbic circuits in OCD.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
This work was supported in part by NIMH grant R21MH104648 (RM), and by a grant from the National Alliance for Research on Schizophrenia and Depression (NARSAD, XH). No authors report any biomedical financial interests or potential conflicts of interest.
He X, Steinberg E, Stefan M, Fontaine M, Simpson HB, Marsh R. Altered frontal interhemispheric and fronto‐limbic structural connectivity in unmedicated adults with obsessive‐compulsive disorder. Hum Brain Mapp. 2018;39:803–810. 10.1002/hbm.23883
Funding information National Institute of Medical Health, Grant/Award Number: R21MH104648; National Alliance for Research on Schizophrenia and Depression (NARSAD)
REFERENCES
- Admon, R. , Bleich‐Cohen, M. , Weizmant, R. , Poyurovsky, M. , Faragian, S. , & Hendler, T. (2012). Functional and structural neural indices of risk aversion in obsessive–compulsive disorder (OCD). Psychiatry Research: Neuroimaging, 203, 207–213. [DOI] [PubMed] [Google Scholar]
- Alexander, A. L. , Hurley, S. A. , Samsonov, A. A. , Adluru, N. , Hosseinbor, A. P. , Mossahebi, P. , … Field, A. S. (2011). Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity, 1, 423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, A. L. , Lee, J. E. , Lazar, M. , & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. R. (2007). The neural basis of inhibition in cognitive control. The Neuroscientist, 13, 214–228. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends Cogn Sci, 8, 170–177. [DOI] [PubMed] [Google Scholar]
- Behrens, T. E. , Berg, H. J. , Jbabdi, S. , Rushworth, M. , & Woolrich, M. (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain?. Neuroimage, 34, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti, F. , Giacosa, C. , Radaelli, D. , Poletti, S. , Pozzi, E. , Dallaspezia, S. , … Smeraldi, E. (2013). Widespread changes of white matter microstructure in obsessive–compulsive disorder: Effect of drug status. European Neuropsychopharmacology, 23, 581–593. [DOI] [PubMed] [Google Scholar]
- Bora, E. , Pujol, J. , Fontenelle, L. F. , Velakoulis, D. , Pantelis, C. , & Murat Yücel PhD, M. (2011). White matter microstructure in patients with obsessive‐compulsive disorder. Journal of Psychiatry & Neuroscience: JPN, 36, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Meng, X. , Hu, Q. , Cui, H. , Ding, Y. , Kang, L. , … Wang, Y. (2016). Altered resting‐state functional organization within the central executive network in obsessive–compulsive disorder. Psychiatry and Clinical Neurosciences, 70, 448–456. [DOI] [PubMed] [Google Scholar]
- Chiu, C.‐H. , Lo, Y.‐C. , Tang, H.‐S. , Liu, I.‐C. , Chiang, W.‐Y. , Yeh, F.‐C. , … Tseng, W.‐Y. I. (2011). White matter abnormalities of fronto‐striato‐thalamic circuitry in obsessive–compulsive disorder: a study using diffusion spectrum imaging tractography. Psychiatry Research: Neuroimaging, 192, 176–182. [DOI] [PubMed] [Google Scholar]
- Fan, S. , van den Heuvel, O. A. , Cath, D. C. , van der Werf, Y. D. , de Wit, S. J. , de Vries, F. E. , … Pouwels, P. J. (2016). Mild white matter changes in un‐medicated obsessive‐compulsive disorder patients and their unaffected siblings. Frontiers in Neuroscience, 9, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee, M. , Vink, M. , de Geus, F. , Vulink, N. , Veltman, D. J. , Westenberg, H. , & Denys, D. (2011). Dysfunctional reward circuitry in obsessive‐compulsive disorder. Biol Psychiatry, 69, 867–874. [DOI] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (2002). Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐Patient Edition (SCID‐I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fontenelle, L. F. , Bramati, I. E. , Moll, J. , Mendlowicz, M. V. , de Oliveira‐Souza, R. , & Tovar‐Moll, F. (2011). White matter changes in OCD revealed by diffusion tensor imaging. CNS Spectrums, 16, 101–109. [DOI] [PubMed] [Google Scholar]
- Gan, J. , Zhong, M. , Fan, J. , Liu, W. , Niu, C. , Cai, S. , … Tan, C. (2017). Abnormal white matter structural connectivity in adults with obsessive‐compulsive disorder. Translational Psychiatry, 7, e1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, W. K. , Price, L. H. , Rasmusson, S. A. , Mazure, C. , Delgado, P. , Henninger, G. R. , & Charney, D. S. (1989). The Yale‐Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Archives of General Psychiatry, 46, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Göttlich, M. , Krämer, U. M. , Kordon, A. , Hohagen, F. , & Zurowski, B. (2014). Decreased limbic and increased fronto‐parietal connectivity in unmedicated patients with obsessive‐compulsive disorder. Human Brain Mapping, 35, 5617–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2009). Accurate and robust brain image alignment using boundary‐based registration. Neuroimage, 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. A. X. (1967). Development of a Rating Scale For Primary Depressive Illness. The British Journal of Social and Clinical Psychology, 6, 278–296. [DOI] [PubMed] [Google Scholar]
- Hasan, K. M. (2006). Diffusion Tensor Eigenvalues or Both Mean Diffusivity and Fractional Anisotropy Are Required in Quantitative Clinical Diffusion Tensor MR Reports: Fractional Anisotropy Alone Is Not Sufficient. Radiology, 239, 611–613. [DOI] [PubMed] [Google Scholar]
- He, X. F. , Liu, W. , Li, X. Z. , Li, Q. L. , Liu, F. , Rauh, V. A. , … Xu, D. R. (2014). Automated assessment of the quality of diffusion tensor imaging data using color cast of color‐encoded fractional anisotropy images. Magn Reson Imaging, 32, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi, S. , Woolrich, M. , Andersson, J. , & Behrens, T. (2007). A Bayesian framework for global tractography. Neuroimage, 37, 116–129. [DOI] [PubMed] [Google Scholar]
- Jones, D. K. , Knosche, T. R. , & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage, 73, 239–254. [DOI] [PubMed] [Google Scholar]
- Koch, K. , Reeß, T. J. , Rus, O. G. , Zimmer, C. , & Zaudig, M. (2014). Diffusion tensor imaging (DTI) studies in patients with obsessive‐compulsive disorder (OCD): a review. Journal of Psychiatric Research, 54, 26–35. [DOI] [PubMed] [Google Scholar]
- Marsh, R. , Horga, G. , Parashar, N. , Wang, Z. , Peterson, B. S. , & Simpson, H. B. (2014). Altered activation in fronto‐striatal circuits during sequential processing of conflict in unmedicated adults with obsessive‐compulsive disorder. Biol Psychiatry, 75, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, R. , Tau, G. Z. , Wang, Z. , Huo, Y. , Liu, G. , Hao, X. , … Simpson, H. B. (2015). Reward‐based spatial learning in unmedicated adults with obsessive‐compulsive disorder. American Journal of Psychiatry, 172, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern, R. A. , & Sheth, S. A. (2017). Role of the dorsal anterior cingulate cortex in obsessive‐compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. Journal of Neurosurgery, 126, 132–147. [DOI] [PubMed] [Google Scholar]
- Menzies, L. , Chamberlain, S. R. , Laird, A. R. , Thelen, S. M. , Sahakian, B. J. , & Bullmore, E. T. (2008). Integrating evidence from neuroimaging and neuropsychological studies of obsessive‐compulsive disorder: the orbitofronto‐striatal model revisited. Neurosci Biobehav Rev, 32, 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R. , & Rauch, S. L. (2012). Obsessive‐compulsive disorder: beyond segregated cortico‐striatal pathways. Trends Cogn Sci, 16, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S. , & van Zijl, P. (2002). Fiber tracking: principles and strategies–a technical review. NMR in Biomedicine, 15, 468–480. [DOI] [PubMed] [Google Scholar]
- Mukherjee, P. , Berman, J. , Chung, S. , Hess, C. , & Henry, R. (2008). Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. American Journal of Neuroradiology, 29, 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamae, T. , Narumoto, J. , Sakai, Y. , Nishida, S. , Yamada, K. , Nishimura, T. , & Fukui, K. (2011). Diffusion tensor imaging and tract‐based spatial statistics in obsessive‐compulsive disorder. Journal of Psychiatric Research, 45, 687–690. [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J. S. , Jang, J. H. , Jung, W. H. , Kang, D. H. , Choi, J. S. , Choi, C. H. , … Kwon, J. S. (2011). Reduced fronto‐callosal fiber integrity in unmedicated OCD patients: A diffusion tractography study. Hum Brain Mapp, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Z. , Shi, F. , Shi, C. , Miao, G. , Yang, Q. , Gao, W. , … Shen, D. (2014). Structural and diffusion property alterations in unaffected siblings of patients with obsessive‐compulsive disorder. PloS One, 9, e85663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, K. L. , Orlichenko, A. , Boyd, E. , Angstadt, M. , Coccaro, E. F. , Liberzon, I. , & Arfanakis, K. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry, 66, 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, A. , Eisen, J. L. , Mancebo, M. C. , Greenberg, B. D. , Stout, R. L. , & Rasmussen, S. A. (2007). Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive‐compulsive disorder symptoms. Psychiatry Res, 151, 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, A. , Greenberg, B. D. , Grados, M. A. , Bienvenu, O. J. , 3rd, Samuels, J. F. , Murphy, D. L. , … Nestadt, G. (2008). Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res, 160, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner, J. , Marsh, R. , Maia, T. V. , Peterson, B. S. , Gruber, A. , & Simpson, H. B. (2014). Reduced functional connectivity within the limbic cortico‐striato‐thalamo‐cortical loop in unmedicated adults with obsessive‐compulsive disorder. Human Brain Mapping, 35, 2852–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J. , Grau, M. , Van Den Heuvel, O. A. , De Schotten, M. T. , Stein, D. J. , Canales‐Rodríguez, E. J. , … Mataix‐Cols, D. (2014). Multimodal voxel‐based meta‐analysis of white matter abnormalities in obsessive–compulsive disorder. Neuropsychopharmacology, 39, 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, R. M. , Saykin, A. J. , Flashman, L. A. , Pixley, H. S. , West, J. D. , & Mamourian, A. C. (2007). Event‐related functional magnetic resonance imaging of response inhibition in obsessive‐compulsive disorder. Biol Psychiatry, 62, 901–909. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Tromp, D. P. , Grupe, D. W. , Oathes, D. J. , McFarlin, D. R. , Hernandez, P. J. , Kral, T. R. , … Nitschke, J. B. (2012). Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Archives of General Psychiatry, 69, 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1981). WAIS‐R Manual. Wechsler Adult Intelligence Scale‐Revised. San Antonio, TX: The Psychological Corporation. Harcourt Brace Jovanovich, Inc. [Google Scholar]
- Wood, J. , & Ahmari, S. E. (2015). A framework for understanding the emerging role of corticolimbic‐ventral striatal networks in OCD‐associated repetitive behaviors. Frontiers in Systems Neuroscience, 9, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki, A. (2014). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki, A. , Panneck, P. , Srinivasan, P. , Stevens, A. , Zöllei, L. , Augustinack, J. , … Behrens, T. (2011). Automated probabilistic reconstruction of white‐matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform, 5, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


