Abstract
The glutamatergic α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor is involved in synaptic plasticity processes, and animal studies have demonstrated altered expression across the sleep wake cycle. Accordingly, glutamate levels are reduced during non‐rapid eye movement (NREM) sleep and the rate of this decrease is positively correlated with sleep EEG slow wave activity (SWA). Here, we combined proton magnetic resonance spectroscopy (1H‐MRS) and high‐density sleep EEG to assess if 1H‐MRS is sensitive to diurnal changes of glutamate + glutamine (GLX) in healthy young adults and if potential overnight changes of GLX are correlated to SWA. 1H‐MRS was measured in the parietal lobe in the evening and in the subsequent morning. High‐density sleep EEG was recorded overnight between the evening and morning scans. Our results revealed a significant overnight reduction in GLX, but no significant changes in other metabolites. The decrease in GLX positively correlated with the decrease of SWA. Our study demonstrates that quantification of diurnal changes in GLX is possible by means of 1H‐MRS and indicates that overnight changes in GLX are related to SWA, a marker that is closely linked to the restorative function of sleep. This relationship might be of particular interest in clinical populations in which sleep is disturbed.
Keywords: high‐density EEG, MRS, sleep, slow wave activity, synaptic homeostasis
1. INTRODUCTION
It is well known that sleep is essential for healthy brain function and even a moderate lack of sleep can interfere with basic neurobehavioral functions (Krause et al., 2017; Van Dongen, Maislin, Mullington, & Dinges, 2003). Although the exact mechanism underlying the restorative nature of sleep is still under debate, insights from molecular and electrophysiological studies suggest that sleep ensures efficient functioning of the brain by maintaining neuronal homeostasis. During wakefulness, we constantly interact with the environment, which leads to a progressive build‐up of synaptic strength (Tononi & Cirelli, 2014). A continuous increase in synaptic strength would lead to (a) a saturation in neural plasticity, limiting the brain's capacity to process new inputs (Turrigiano, 2008), (b) increase energy demands to an unsustainable level, necessitating increased cellular maintenance (Vyazovskiy & Harris, 2013), and (c) increase the need for removal of metabolic waste products (Xie et al., 2013). The mechanism thought to underlie the downregulation of synaptic strength has been linked to deep sleep. During non‐rapid eye movement (NREM) sleep, when large populations of cortical neurons oscillate synchronously between a phase of depolarization (on‐state) and hyperpolarization (off‐state), this oscillation becomes visible in the surface EEG in form of sleep slow waves (oscillations between 0.5 and 4.5 Hz; Vyazovskiy et al., 2009). Slow wave activity (SWA, EEG power between 1 and 4.5 Hz) increases with the time spent awake and decreases over the course of the night (Borbély & Achermann, 2005). Additionally, SWA was shown to increase in a use‐dependent manner such that cortical areas that have actively been used during the day (by practicing specific tasks) show higher SWA in the following night (Hanlon, Faraguna, Vyazovskiy, Tononi, & Cirelli, 2009; Huber, Ghilardi, Massimini, & Tononi, 2004), and areas deprived of peripheral input display a decrease in SWA (Huber et al., 2006). Thus, SWA is tightly linked to the restorative function of sleep.
A major molecular marker of changes in synaptic strength is the glutamate receptor α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptor. Changes in AMPA receptor function represent a key mechanism of synaptic plasticity (Huganir & Nicoll, 2013; Lee & Kirkwood, 2011) and it has been shown that AMPA receptor levels in the rat brain are high during wakefulness and low after sleep (Vyazovskiy, Cirelli, Pfister‐Genskow, Faraguna, & Tononi, 2008). Concordantly, an in vivo amperometry study in rats showed that glutamate (GLU) concentrations increase during wakefulness and rapid‐eye movement (REM) sleep and decrease during NREM sleep (Dash, Douglas, Vyazovskiy, Cirelli, & Tononi, 2009). Moreover, the decrease of GLU during NREM sleep positively correlated with SWA, suggesting that NREM sleep is essential to keep GLU in a homeostatic range (Dash et al., 2009). The goal of our study was to assess specifically if this relation between GLU levels and SWA is also present and measureable in the human brain.
Proton magnetic resonance spectroscopy (1H‐MRS), a noninvasive method for investigating biochemical changes in the living human brain, is widely used in both the clinical and research settings to investigate changes in a variety of metabolites, including neuronal amino acids such as gamma‐aminobutyric acid (GABA), glutamate + glutamine (GLX) or N‐acetylaspartate (NAA; Bollmann et al., 2015; Huang et al., 2015; Rowland et al., 2013). In this study, we evaluated whether 1H‐MRS is sensitive to changes in GLX from evening (after a day of being awake) to morning (after a night of sleep) in healthy young adults. Additionally, we acquired a high‐density sleep electroencephalogram (hd sleep EEG) overnight between the two 1H‐MRS sessions in order to relate potential overnight changes in GLX levels to SWA. We hypothesized that GLX concentrations in the human brain would be reduced overnight. As glutamate is widely distributed in the brain (Huganir & Nicoll, 2013; Lee & Kirkwood, 2011), we expected that GLX changes would be quantifiable in the whole cortex. As MRS only allows for the measurement of metabolite concentrations in a predefined area, the voxel of interest was placed in a standardized position in the left parietal lobe.
2. MATERIALS AND METHODS
2.1. Participants
Eighteen adults between 18 and 24 years of age were recruited via advertisements placed at the University. Two subjects were excluded due to low sleep quality (sleep efficiency < 80%). The remaining 16 subjects (mean ± SEM, 21.3 ± 2.2 years, 6 females), fulfilled the following inclusion criteria: No personal or family history of psychopathology, no sleep disorders, good sleepers (sleep efficiency > 80%), no chronic diseases, no current use of psychoactive agents or other medications, no travelling across the time zone during the month before the study, no high caffeine (>160 mg caffeine/day) or alcohol (>14 mg alcohol/day) consumption, nonsmoker. Subjects had to refrain from alcohol starting 48 h prior to the experiment and extensive exercise or visits to a sauna were not allowed on the day of the recordings. The study was approved by the local ethics committee and subjects gave written informed consent before participating. Participants were compensated monetarily.
2.2. Experimental procedure
One week prior to the assessment, participants were instructed to keep a regular sleep–wake schedule according to their habitual bed time. Compliance was verified with self‐reported sleep logs and wrist motor actigraphy (GENEActiv, activinsights Ltd., Kimbolton, Huntingdon, UK). On the night of the sleep recording, subjects arrived ∼3 h before their usual bedtime at the sleep laboratory of the University Children's Hospital Zurich. The experimental procedures started with the magnetic resonance imaging (MRI) around 2 h before the subject's habitual bedtime (mean time of the day ± SEM = 8:21 pm ± 8.2 min). Afterward, participants were equipped with the hd EEG net and sleep was recorded all night. The following morning, after removal of the EEG net, the MRI was repeated (∼1 h after lights on; mean time of the day ± SEM = 8:08 am ± 9.4 min).
2.3. High‐density sleep electroencephalography
All‐night sleep EEG was recorded with a high‐density EEG net (128 channels, Sensor Net for long‐term monitoring; Electrical Geodesic Inc., EGI, Eugene, OR). In addition, two submental EMG electrodes for visual scoring and two electrodes at the earlobes were applied (gold, Grass Technologies, West Warwick, RI). The nets were adjusted to the vertex and mastoids and subsequently filled with electrolyte gel. Impedances were kept below 50 kΩ. EEG recordings were sampled at 500 Hz (filtered between .01 and 200 Hz) and referenced to the vertex (Cz). Subsequently, data were band‐pass filtered (0.5–50 Hz) and down sampled to 128 Hz. Sleep stages were scored for 20 s epochs according to standard criteria (Iber, Ancoli‐Israel, Chesson, & Quan, 2007) by a sleep expert and verified by a second sleep expert. Artefacts in the 20 s epochs were removed by visual inspection and if power exceeded a threshold based on a mean power value in the 0.75–4.5 or 20–30 Hz band. Channels with bad quality and channels below the ears (due to common contamination by muscle artefacts) were removed and data was re‐referenced to the average value of all good quality channels. For further analyses, sleep cycles were defined according to the criteria of Feinberg and Floyd (Feinberg & Floyd, 1979) and spectral analysis of consecutive 20 s epochs for each sleep cycle (fast Fourier transformation, Hanning window, average of five 4 s epochs, frequency resolution of 0.25 Hz) was performed. Missing data from excluded electrodes were interpolated using spherical linear interpolation (Delorme & Makeig, 2004) resulting in 109 channels per subject. SWA was calculated as the mean power in the frequency band between 1 and 4.5 Hz for every NREM sleep episode (stage N2 and stage N3).
2.4. Magnetic resonance imaging
MR imaging and spectroscopy scans were performed with a 3T GE MR 750 scanner, using an 8 channel receive‐only head coil. Subjects were instructed to stay awake during the scanning session and were asked before and after each scan if they were awake and if they had fallen asleep during the previous scan. Single voxel Point RESolved (PRESS) 1H MR spectra were acquired from a 20 × 20 × 20 mm3 voxel of interest positioned in the left parietal lobe (Figure 1). The parietal lobe was chosen because it is one of a standard set of MRS regions (Bai et al., 2015; Hallahan et al. 2012; Robertson et al., 2001), where the data quality is usually high and the position can be calculated precisely by a standard set of measurements. On a midline sagittal localizer image, the voxel was located one third of the distance between the posterior commissure and the back of the brain (along a line defined by the angle of the corpus callosum), and halfway between the vertical distance from this line to the vertex. Subsequently, the voxel position was adjusted in the lateral (right‐left) direction and visually inspected by overlying the voxel coordinates over the T1 image. To further reduce the possibility of systematic variations, the voxel position was always set by the same experimenter. Spectra were acquired with an echo time (TE) of 35 ms, a repetition time (TR) of 3 s, and 96 spectral averages. The scanning protocol also included a 3D high‐resolution T1‐weighted IR‐SPGR scan (TE = 3 ms, TR = 8 ms, inversion time = 600 ms, flip angle = 8°, voxel resolution = 1 mm3), used for correction for partial volume cerebrospinal fluid (CSF) effects. Water‐scaled concentrations and ratios to the composite creatine peak (Cr + PCr) were calculated with LCModel version 6.3‐1k. Water‐scaled concentrations were corrected for partial volume CSF contamination after segmentation of the 3D T1‐weighted images into gray matter (GM), white matter (WM), and CSF maps in SPM8 (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London) based on a unified segmentation model (Ashburner & Friston, 2005). The correction for partial volume CSF contamination was performed as described by Chowdhury (Chowdhury et al., 2015) using the formula Mcorr = M/(1 − CSF), where M is the uncorrected metabolite concentration, Mcorr is the corrected concentration, and CSF is the CSF fraction within the spectroscopy voxel. Each spectrum was visually inspected for the presence of artefacts or fitting errors, and spectra with Cramer‐Rao variance bounds of more than 10% for creatine + phosphocreatine or N‐acetyl‐aspartate, or more than 20% for glutamate were excluded from further analysis. As glutamate and glutamine show overlapping resonance frequencies (chemical shifts) at 3 T, we quantified the combined levels of glutamate and glutamine (GLX), as frequently done (Dlabac‐de Lange et al., 2017).
Figure 1.
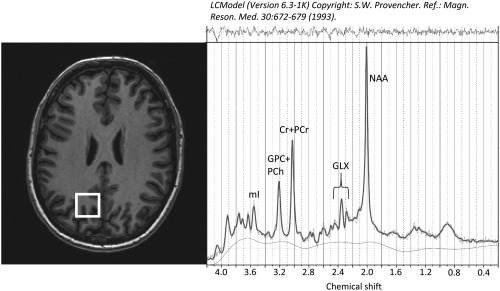
Voxel position in the left parietal lobe (white rectangle on axial T1 image) and representative magnetic resononance spectrum. mI = myo‐inositol; GPC + PCh = glycerophosphocholine + phosphocholine; Cr + PCr = creatine + phosphocreatine; GLX = glutamate + glutamine; NAA = N‐acetylaspartate
2.5. Statistics
Changes in metabolite concentrations and SWA were evaluated using Wilcoxon signed‐rank tests. To correct for multiple comparisons, a false discovery rate (FDR) correction was applied. To correct for individual differences in absolute power values, the decrease in SWA was calculated as the percentage reduction from the NREM sleep episode with maximum SWA (= 100%) to the last NREM sleep episode. Overnight changes in metabolite concentrations were calculated in the same way, with evening levels set to 100% and overnight changes calculated as a percentage change. Additionally, changes in EEG power were calculated for 4 additional classical frequency bands (5–8 Hz, 8–10 Hz, 10–12 Hz, and 12–15 Hz) in the same cycles as selected for SWA.
To assess the association between changes in GLX levels and SWA, Spearman's rank correlation coefficients between the decrease in SWA at every electrode and the overnight changes in GLX levels (and the other metabolite levels) were calculated. To correct for multiple comparisons, statistical nonparametric mapping (SnPM) using a suprathreshold cluster analysis was applied (Huber et al., 2004; Nichols and Holmes, 2002). For each permutation, the maximal cluster size of neighboring electrodes reaching an r value above the critical value was counted and used to build a cluster size distribution. From this cluster size distribution the 95th percentile was defined as the critical cluster size threshold. To assess whether the overnight change in GLX is specifically associated with SWA, the percentage change in GLX was correlated to the percentage change in the 4 additional frequency bands (5–8 Hz, 8–10 Hz, 10–12 Hz, 12–15 Hz). All analyses were performed with the software package MATLAB (MathWorks). The significance level was set at p < .05 (two‐tailed).
3. RESULTS
We obtained good‐quality MR spectra from all subjects at both time points (see Figure 1 for an example). In a first step, we compared evening and morning levels of GLX and 5 other metabolites with robust peak detection [N‐acetylaspartate (NAA), N‐acetyl aspartate + N‐acetyl aspartylglutamate (NAA + NAAG), glycerophosphocholine + phosphocholine (GPC + PCh), myo‐Inositol (mI), and creatine + phosphocreatine (Cr + PCr)]. Water‐scaled concentrations and ratios to the composite creatine peak (Cr + PCr) for these metabolites are also presented in Table 1. Significant overnight changes were only observed for GLX, where 13 out of 16 subjects showed an overnight reduction (GLX: −6.63 ± 1.59%, p = .02, FDR corrected, effect size = 1.0, GLX/Cr + PCr: −7.06 ± 1.58%, p = .01, FDR corrected, effect size = 1.0; Figure 2 and Table 1). No significant changes were observed in any other metabolite (all p > .4, FDR corrected, effect size ≤ .3). All further analyses reported in the results are based on the water‐scaled metabolite levels.
Table 1.
Water‐scaled metabolite concentrations and ratios to creatine + phosphocreatine in the evening, the morning, and their change across the night (relative to evening levels)
| Evening | Morning | Overnight change | ||||
|---|---|---|---|---|---|---|
| (n = 16) | Mean | SEM | Mean | SEM | (%) | SEM (%) |
| Water‐scaled concentrations | ||||||
| GLX | 12.55 | ±0.22 | 11.69 | ±0.20 | −6.63a | ±1.59 |
| NAA | 11.59 | ±0.13 | 11.53 | ±0.12 | −0.33 | ±1.21 |
| NAA + NAAG | 12.64 | ±0.15 | 12.50 | ±0.12 | −0.88 | ±1.51 |
| GPC + PCh | 1.65 | ±0.05 | 1.62 | ±0.06 | −1.99 | ±1.55 |
| mI | 3.70 | ±0.08 | 3.58 | ±0.11 | −3.22 | ±2.46 |
| Cr + PCr | 7.54 | ±0.14 | 7.48 | ±0.14 | −0.66 | ±1.21 |
| Ratios to Creatine + Phosphocreatine | ||||||
| GLX | 1.72 | ±0.05 | 1.59 | ±0.04 | −7.06a | ±1.58 |
| NAA | 1.54 | ±0.03 | 1.54 | ±0.03 | 0.00 | ±1.16 |
| NAA + NAAG | 1.68 | ±0.04 | 1.68 | ±0.04 | 0.08 | ±1.25 |
| GPC + PCh | 0.22 | ±0.01 | 0.22 | ±0.01 | −0.94 | ±1.72 |
| mI | 0.50 | ±0.02 | 0.48 | ±0.02 | −5.10 | ±2.09 |
Note. GLX = glutamate + glutamine; NAA = N‐acetylaspartate; NAA + NAAG = N‐acetyl aspartate + N‐acetyl aspartylglutamate; GPC + PCh = glycerophosphocholine + phosphocholine; mI = myo Inositol; Cr + PCr = creatine + phosphocreatine.
p < .05, FDR corrected.
Figure 2.
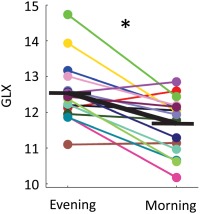
Changes in water‐scaled glutamate + glutamine (GLX) levels from evening to morning for individual subjects (colored lines) and mean (black bold line). Mean percentage change (±SEM) reduction from evening to morning was 6.63 (± 1.59%, *p = .02, Wilcoxon signed‐rank test, FDR corrected) [Color figure can be viewed at http://wileyonlinelibrary.com]
Overnight changes in metabolite levels were not driven by variations in voxel composition, as the fractional gray matter, white matter, and CSF content of the evening and morning voxels did not differ (all p ≥ .6, FDR corrected; Table 2). In a next step, we tested whether metabolite concentrations were related to sleep stages. We found no significant correlations between any of the sleep stages and metabolite concentrations in the evening, the morning or the overnight change (all p > .8, FDR corrected; Table 3). Because the gradual decrease of SWA over the night is thought to reflect the restorative function of sleep, we tested for an association between the decrease in SWA and the overnight reduction in GLX. First, we confirmed that SWA displayed a highly significant decrease in all 109 electrodes from the maximal to last NREM sleep episode (mean reduction of 70.1 ± 3.1%, p ≤ .001, FDR corrected; Figure 3). Electrode‐wise Spearman correlations between the decrease in SWA and the overnight reduction in GLX revealed a significant cluster of 69 electrodes. This cluster encompasses 32 electrodes over the frontal lobe (including the precentral gyrus, inferior, medial, middle, and medial frontal gyrus), 18 electrodes over the parietal lobe (including superior and inferior parietal lobule, postcentral gyrus, and precuneus), 12 electrodes over the temporal lobe (fusiform gyrus, inferior, middle, and superior temporal gyrus), and 6 electrodes over the occipital lobe (cuneus and middle occipital gyrus; Figure 4a). Apart from the significant correlation between SWA and the change in GLX, all other correlations between overnight changes in metabolite levels and the decrease in SWA did not survive the critical cluster threshold. In the next step, the mean decrease of SWA within the significant cluster of 69 electrodes was calculated and correlated with the decrease in GLX (r = .62, p = .01; Figure 4b).
Table 2.
Voxel composition in the evening and morning
| Evening | Morning | |||
|---|---|---|---|---|
| Mean (%) | SEM (%) | Mean (%) | SEM (%) | |
| Gray matter | 47.94 | ±0.99 | 48.31 | ±1.24 |
| White matter | 44.19 | ±1.05 | 44.31 | ±1.53 |
| CSF | 7.88 | ±0.54 | 7.44 | ±0.63 |
Note. All p ≥ .6, Wilcoxon signed‐rank test, FDR corrected.
Table 3.
Sleep architecture and Spearman's rank correlation coefficients between sleep architecture and water‐scaled metabolite concentrations
| Time in bed | Sleep latency | Total sleep time | Sleep efficiency | Wake after sleep onset | NREM sleep | N1 | N2 | N3 | REM sleep | |
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 16) | [min] | [min] | [min] | [%] | [min] | [min] | [min] | [min] | [min] | [min] |
| Mean ± SEM | 458.56 ± 6.30 | 17.12 ± 2.68 | 421.40± 7.09 | 91.94 ± 1.14 | 23.96 ± 3.51 | 321.10 ± 5.15 | 25.77 ± 2.96 | 207.75 ± 8.71 | 87.58 ± 8.61 | 100.29 ± 6.03 |
| GLX % change | −0.09 | 0.04 | −0.07 | −0.10 | 0.17 | 0.13 | −0.01 | −0.09 | 0.16 | −0.16 |
| GLX evening | −0.12 | 0.29 | −0.22 | −0.23 | 0.21 | 0.02 | 0.19 | −0.25 | 0.25 | −0.36 |
| GLX morning | 0.25 | 0.24 | 0.08 | −0.06 | 0.00 | 0.00 | 0.20 | −0.08 | 0.00 | −0.01 |
| NAA % change | 0.24 | 0.14 | 0.14 | −0.12 | 0.12 | 0.46 | 0.18 | −0.35 | 0.54 | −0.26 |
| NAA evening | 0.04 | 0.18 | 0.02 | −0.17 | 0.12 | 0.52 | 0.30 | −0.21 | 0.51 | −0.47 |
| NAA morning | −0.41 | 0.04 | −0.35 | −0.01 | −0.09 | −0.18 | −0.12 | 0.08 | −0.06 | −0.26 |
| NAA + NAAG % change | −0.19 | −0.30 | −0.10 | −0.01 | 0.11 | 0.10 | 0.03 | −0.11 | 0.14 | −0.11 |
| NAA + NAAG evening | −0.23 | −0.33 | −0.03 | 0.08 | 0.01 | 0.28 | 0.07 | −0.39 | 0.54 | −0.19 |
| NAA + NAAG morning | −0.38 | 0.08 | −0.32 | −0.19 | 0.13 | 0.06 | 0.02 | −0.14 | 0.24 | −0.43 |
| GPC + PCh % change | −0.15 | −0.42 | 0.13 | 0.31 | −0.16 | −0.22 | −0.26 | −0.12 | 0.11 | 0.41 |
| GPC + PCh evening | 0.52 | −0.18 | 0.53 | 0.35 | −0.27 | 0.25 | −0.03 | −0.24 | 0.31 | 0.39 |
| GPC + PCh morning | 0.61 | 0.09 | 0.47 | 0.12 | −0.09 | 0.32 | 0.16 | −0.06 | 0.12 | 0.21 |
| mI % change | 0.28 | 0.32 | 0.08 | −0.21 | 0.12 | 0.12 | 0.08 | 0.14 | −0.16 | −0.08 |
| mI evening | 0.36 | 0.14 | 0.04 | −0.42 | 0.54 | 0.26 | 0.31 | −0.22 | 0.19 | −0.16 |
| mI morning | 0.02 | −0.27 | 0.02 | −0.03 | 0.21 | 0.09 | 0.10 | −0.13 | 0.15 | 0.01 |
| Cr + PCr % change | 0.17 | 0.06 | 0.08 | −0.18 | 0.26 | 0.38 | 0.15 | −0.02 | 0.23 | −0.21 |
| Cr + PCr evening | −0.11 | −0.07 | 0.05 | 0.42 | −0.46 | −0.27 | −0.62 | −0.01 | 0.01 | 0.31 |
| Cr + PCr morning | −0.35 | 0.10 | −0.16 | 0.31 | −0.48 | −0.33 | −0.45 | 0.12 | −0.17 | 0.09 |
Note. Abbreviations: NREM sleep = non‐rapid eye movement sleep; REM sleep = rapid eye movement sleep; sleep efficiency = time in bed divided by total sleep time (NREM + REM).
All p > .8, FDR corrected.
Figure 3.
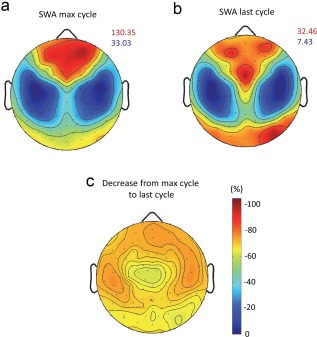
Topographical distribution of EEG slow wave activity (SWA). (a) Topographical map of SWA during the NREM sleep episode with maximum SWA and (b) during the last NREM sleep episode, respectively, scaled to their maximum (red) and minimum (blue) power values (µV2/Hz). (c) Electrode wise reduction in SWA from NREM sleep episode with maximal SWA to last NREM sleep episode (%), minimal reduction—62%; maximal reduction—76%. Reduction was significant at all electrodes (all p ≤ .001, Wilcoxon signed‐rank test, FDR corrected) [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
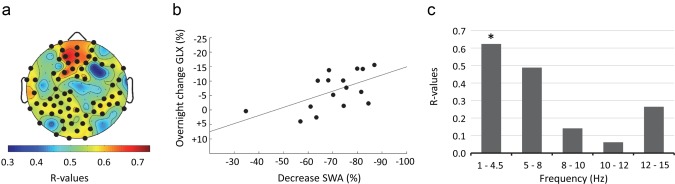
Correlation between the decrease in SWA and overnight changes in GLX. (a) Results of the electrode‐wise Spearman's signed‐rank correlations between the decrease of SWA (%) and the overnight change in GLX (%) plotted on the planar projection of the hemispheric scalp model. Bold black dots indicate electrodes showing significant correlations [defined by statistical nonparametric mapping (SnPM); suprathreshold cluster analysis to control for multiple comparison (Nichols & Holmes, 2002)]. (b) Association between the average decrease of SWA (%) within the significant cluster of 69 electrodes and overnight changes in GLX (%), r = 0.62, p = .01. (c) Spearman's rank correlation coefficients of changes in GLX (%) and changes (%) in power in the 4 additional frequency bands. *p < .05 [Color figure can be viewed at http://wileyonlinelibrary.com]
In contrast to the significant correlation between the overnight decrease in SWA and the decrease in GLX, there was no significant correlation between average SWA (for the whole night, averaged across all electrodes) and changes in GLX (p = .1). We then calculated the percentage change of EEG power in the 4 other frequency bands within the cluster of 69 electrodes and correlated it with the reduction in GLX. Results revealed that there was no significant association between GLX and power in other frequency bands. However, there was a trend in the theta frequency band (5–8 Hz; r = .49, p = .06; all other bands p ≥ .3; Figure 4c).
To examine if baseline levels of GLX are associated with SWA, we correlated the evening levels of GLX to all night SWA and to maximal SWA (SWA during the NREM sleep episode showing maximal SWA). There was no significant association between evening levels of GLX and all night SWA (p = .21, r = .33) or maximal SWA (p = .23, r = .31).
4. DISCUSSION
In this study, we assessed whether overnight changes in levels of GLX are measureable by 1H‐MRS in healthy young adults. Indeed, we found a highly significant overnight reduction in GLX, whereas other metabolites did not display an overnight change. This observation suggests that our findings are specific to GLX and do not arise from changes in the unsuppressed water signal used for scaling of the metabolite values, as a change in the unsuppressed water signal overnight would be expected to lead to significant changes in all the water‐scaled metabolites.
Next, we tested if changes in GLX are correlated to EEG SWA, our primary electrophysiological marker of the restorative function of sleep. We found a positive correlation between the decrease in GLX and the decrease in SWA in the course of the night, suggesting that GLX might be homeostatically regulated across sleep and wake. Both of our results fit to studies in rats demonstrating changes in the level of glutamate as a function of sleep and wake times. More specifically, it has been shown that levels of glutamate decrease during NREM sleep and that this decrease is positively correlated with SWA (Dash et al., 2009).
We did not find a significant correlation between the evening levels of GLX and absolute SWA. These results suggest that GLX values in the evening are not associated with individual differences in SWA (e.g., that subjects with generally high SWA do not necessarily start with low GLX concentration). However, our participant group included only good sleepers (with sleep efficiency over 80%) who all show SWA in a similar range. Therefore, our sample might not be ideal for investigating whether individual differences in SWA levels impact on absolute levels of GLX. Further studies would need to include subjects with lower sleep quality to investigate if there is a relationship between absolute levels of GLX and SWA.
In our study, all subjects who reported sleep disorders in themselves or in their family in a subjective sleep questionnaire were excluded from the study. However, we did not screen objectively for certain specific sleep disorders, such as sleep disordered breathing. Severe forms of sleep disordered breathing are associated with hypoxia, which can trigger glutamate release and is linked to excitotoxic processes (Meldrum & Garthwaite, 1990; Nicholls & Attwell, 1990). It is, however, unlikely that subjects with sleep‐disordered breathing were included in this study because all subjects show a normal distribution of sleep stages with high sleep efficiency.
Another limitation of our study might be sleeping in the scanner. Although we endeavored to ensure that subjects did not fall asleep during the scan, we cannot fully ensure that all subjects stayed awake during the entire MRS measurements. Although all subjects were responsive before and after the MRS sequence, two subjects reported afterwards that they might have been fallen asleep shortly at some point in the scanner. However, the main result stays unaltered with a highly significant overnight reduction in GLX when these two subjects were excluded from the analysis (data not shown). However, subjective reporting might be unreliable and we therefore cannot exclude that other subjects might have slept in the scanner. Yet, if subjects were likely to fall asleep, it would be more probable during the evening MRS session, as sleep pressure is higher in the evening compared to the morning. Moreover, sleep in the scanner would be expected to lead to a reduction in evening GLX levels according to the literature (Dash et al., 2009), leading in turn to a smaller overnight decrease in GLX, which would reduce the significance of our results. Our observation of a highly significant overnight decrease in GLX levels, therefore, seems unlikely to be confounded by participants falling asleep in the scanner.
A potential problem, particularly in repetitive measurements, is the voxel positioning. While every effort was made to standardize the voxel position according to a set of anatomical landmarks (scaled to the head angle and size, always performed by the same experimenter), it is possible that the voxel was positioned in a slightly different position between subjects or between sessions in the same subject. However, post‐hoc examination of voxel positions overlayed on the individual T1 images did not show an obvious variation in the position between sessions or between subjects.
In the clinical setting, MRS measured GLX levels are often used as a stress marker. Thus, elevated GLX levels have been reported following severe traumatic brain injury (TBI; Ashwal et al., 2004; Shutter, Tong, & Holshouser, 2004) and were shown to be a predictive marker for long‐term outcome after severe TBI, potentially reflecting excitotoxicity (Shutter, Tong & Holshouser, 2004). For example, in this latter study, GLX levels were increased by 30%–40% in patients with poor outcome. Thus, these increased levels found in the clinical population exceed the magnitude of GLX changes we observe in our healthy young cohort.
An obvious limitation of our study is that we were not able to robustly differentiate between glutamine and glutamate for technical reasons. Examining if glutamate levels go in the same direction as GLX would hold valuable information. Scanning at stronger magnetic fields (e.g., 7 T) might overcome this limitation but even with improved differentiation, 1H‐MRS does not allow the separation of intracellular and extracellular glutamate. Differentiation between extracellular and intracellular glutamate (e.g., between the synaptic, perisynaptic, or nonsynaptic space) might provide more information about whether changes in glutamate are predominantly related to changes in metabolism, storage capacity, or changes in glutamatergic signaling.
Our experimental design allowed an MRS scan of a single voxel. We therefore cannot draw any conclusions for the whole brain. Interestingly, although we measured GLX levels in this single voxel sized 20 × 20 × 20 mm3 in the left parietal lobe, we found a rather global correlation with SWA over a widespread cluster of electrodes. Within this cluster, the correlation was strongest in the frontal region followed by correlations in the parietal and occipital lobes. One explanation for this rather global effect might be that glutamate, the most abundant excitatory neurotransmitter, is widely distributed in the brain (Huganir & Nicoll, 2013; Lee & Kirkwood, 2011) and its release strongly depends on overall neuromodulation and neuronal firing (Tzingounis & Wadiche, 2007). Moreover, as shown in Figure 3, SWA decreases consistently across the entire cortex, which may also contribute to a global relationship with GLX. To further investigate the regional specificity of the relationship between GLX and SWA, future studies should quantify glutamate in additional brain areas.
A critical limitation of our study is that GLX was assessed at two time points differing in circadian phase. As circadian rhythms exert strong influences at various levels, ranging from cognitive activity (Waterhouse, 2010) to the level of neuromodulation (Stanley, Schwartz, Hernandez, Hoebel, & Leibowitz, 1989), our observation of an overnight decrease in GLX could be explained by a difference in circadian time. However, the specific correlation with SWA speaks against such an explanation because the recovery function of sleep, reflected by SWA, is to a large extent independent of circadian rhythms (Borbély & Achermann, 2005). Future studies incorporating sleep deprived participants may be able to confirm whether the apparent decrease in GLX in the morning is due to sleep or the circadian time.
As our study is purely correlative, future studies would be needed to prove causality. Moreover, as is done in animal studies, it would be important to assess the continuous changes in glutamate in the course of sleep. Such studies could investigate whether or not our observed reduction in GLX is related to the ability of deep sleep to reduce high energy demands and/or the downregulation of synaptic strength, preventing saturation of neuronal networks, and ensuring efficient cortical functioning. If indeed sleep SWA plays a critical role in glutamate homeostasis, MRS measured GLX levels might be a promising biomarker of the recuperative function of sleep.
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (320030_153387) and the Clinical Research Priority Program (CRPP) Sleep and Health of the University of Zurich.
The authors thank Melanie Furrer, Michelle Suppiger, Simone Poli, Sara Fattinger, Natalie Heyse, Kathrin Michel, Sophie Zwick, Elena Krugliakova, and Flavia Wehrle for their support in data collection and preprocessing and Angelina Maric for her help in sleep data analysis.
Volk C, Jaramillo V, Merki R, O'Gorman Tuura R, Huber R. Diurnal changes in glutamate + glutamine levels of healthy young adults assessed by proton magnetic resonance spectroscopy. Hum Brain Mapp. 2018;39:3984–3992. 10.1002/hbm.24225
Funding information Swiss National Science Foundation, Grant/Award Number: 320030_153387; Clinical Research Priority Program (CRPP) Sleep and Health of the University of Zurich
REFERENCES
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Ashwal, S. , Holshouser, B. , Tong, K. , Serna, T. , Osterdock, R. , Gross, M. , & Kido, D. (2004). Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. Journal of Neurotrauma, 21(11), 1539–1552. [DOI] [PubMed] [Google Scholar]
- Bai, X. , Edden, R. A. E. , Gao, F. , Wang, G. , Wu, L. , Zhao, B. , … Barker, P. B. (2015). Decreased γ‐aminobutyric acid levels in the parietal region of patients with Alzheimer's disease. Journal of Magnetic Resonance Imaging, 41(5), 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann, S. , Ghisleni, C. , Poil, S. S. , Martin, E. , Ball, J. , Eich‐Hochli, D. , … O'Gorman, R. L. (2015). Developmental changes in gamma‐aminobutyric acid levels in attention‐deficit/hyperactivity disorder. Translational Psychiatry, 5(6), e589 10.1038/tp.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély, A. A. , & Achermann, P. (2005). Sleep homeostasis and models of sleep regulation In Kryger M. H., Roth T., & Dement W. C. (Eds.), Principles and practice of sleep medicine (pp. 405–417). Philadelphia: Elsevier Saunders. [Google Scholar]
- Chowdhury, F. A. , O'Gorman, R. L. , Nashef, L. , Elwes, R. D. , Edden, R. A. , Murdoch, J. B. , … Richardson, M. P. (2015). Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. Journal of Magnetic Resonance Imaging, 41(3), 694–699. 10.1002/jmri.24611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, M. B. , Douglas, C. L. , Vyazovskiy, V. V. , Cirelli, C. , & Tononi, G. (2009). Long‐term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. Journal of Neuroscience, 29(3), 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Dlabac‐de Lange, J. J. , Liemburg, E. J. , Bais, L. , van de Poel‐Mustafayeva, A. T. , de Lange‐de Klerk, E. S. , Knegtering, H. , & Aleman, A. (2017). Effect of bilateral prefrontal rTMS on left prefrontal NAA and Glx levels in schizophrenia patients with predominant negative symptoms: An exploratory study. Brain Stimulation, 10(1), 59–64. [DOI] [PubMed] [Google Scholar]
- Feinberg, I. , & Floyd, T. C. (1979). Systematic trends across the night in human sleep cycles. Psychophysiology, 16(3), 283–291. [DOI] [PubMed] [Google Scholar]
- Hallahan, B. P. , Daly, E. M. , Simmons, A. , Moore, C. J. , Murphy, K. C. , & Murphy, D. D. (2012). Fragile X syndrome: A pilot proton magnetic resonance spectroscopy study in premutation carriers. Journal of Neurodevelopmental Disorders, 4(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon, E. C. , Faraguna, U. , Vyazovskiy, V. V. , Tononi, G. , & Cirelli, C. (2009). Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep, 32(6), 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z. , Davis, H. I. , Yue, Q. , Wiebking, C. , Duncan, N. W. , Zhang, J. , … Northoff, G. (2015). Increase in glutamate/glutamine concentration in the medial prefrontal cortex during mental imagery: A combined functional mrs and fMRI study. Human Brain Mapping, 36(8), 3204–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, R. , Ghilardi, M. F. , Massimini, M. , Ferrarelli, F. , Riedner, B. A. , Peterson, M. J. , & Tononi, G. (2006). Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nature Neuroscience, 9(9), 1169–1176. [DOI] [PubMed] [Google Scholar]
- Huber, R. , Ghilardi, M. F. , Massimini, M. , & Tononi, G. (2004). Local sleep and learning. Nature, 430(6995), 78–81. [DOI] [PubMed] [Google Scholar]
- Huganir, R. L. , & Nicoll, R. A. (2013). AMPARs and synaptic plasticity: The last 25 years. Neuron, 80(3), 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber, C. , Ancoli‐Israel, S. , Chesson, A. , & Quan, S. F. (2007). The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. (ed.) A. A. o. S. Medicine, 1st edition. Westchester, Illinois.
- Krause, A. J. , Simon, E. B. , Mander, B. A. , Greer, S. M. , Saletin, J. M. , Goldstein‐Piekarski, A. N. , & Walker, M. P. (2017). The sleep‐deprived human brain. Nature Reviews Neuroscience, 18(7), 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. K. , & Kirkwood, A. (2011). AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Seminars in Cell and Developmental Biology, 22(5), 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum, B. , & Garthwaite, J. (1990). Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends in Pharmacological Sciences, 11(9), 379–387. [DOI] [PubMed] [Google Scholar]
- Nicholls, D. , & Attwell, D. (1990). The release and uptake of excitatory amino acids. Trends in Pharmacological Sciences, 11(11), 462–468. [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, D. M. W. , van Amelsvoort, T. , Daly, E. , Simmons, A. , Whitehead, M. , Morris, R. G. , … Murphy, D. G. M. (2001). Effects of estrogen replacement therapy on human brain aging: An in vivo 1H MRS study. Neurology, 57(11), 2114–2117. [DOI] [PubMed] [Google Scholar]
- Rowland, L. M. , Kontson, K. , West, J. , Edden, R. A. , Zhu, H. , Wijtenburg, S. A. , … Barker, P. B. (2013). In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophrenia Bulletin, 39(5), 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter, L. , Tong, K. A. , & Holshouser, B. A. (2004). Proton MRS in acute traumatic brain injury: Role for glutamate/glutamine and choline for outcome prediction. Journal of Neurotrauma, 21(12), 1693–1705. [DOI] [PubMed] [Google Scholar]
- Stanley, B. G. , Schwartz, D. H. , Hernandez, L. , Hoebel, B. G. , & Leibowitz, S. F. (1989). Patterns of extracellular norepinephrine in the paraventricular hypothalamus: Relationship to circadian rhythm and deprivation‐induced eating behavior. Life Sciences, 45(4), 275–282. [DOI] [PubMed] [Google Scholar]
- Tononi, G. , & Cirelli, C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81(1), 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano, G. G. (2008). The self‐tuning neuron: Synaptic scaling of excitatory synapses. Cell, 135(3), 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis, A. V. , & Wadiche, J. I. (2007). Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nature Reviews Neuroscience, 8(12), 935–947. [DOI] [PubMed] [Google Scholar]
- Van Dongen, H. P. , Maislin, G. , Mullington, J. M. , & Dinges, D. F. (2003). The cumulative cost of additional wakefulness: Dose‐response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep, 26(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy, V. V. , Cirelli, C. , Pfister‐Genskow, M. , Faraguna, U. , & Tononi, G. (2008). Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nature Neuroscience, 11(2), 200–208. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy, V. V. , & Harris, K. D. (2013). Sleep and the single neuron: The role of global slow oscillations in individual cell rest. Nature Reviews Neuroscience, 14(6), 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy, V. V. , Olcese, U. , Lazimy, Y. M. , Faraguna, U. , Esser, S. K. , Williams, J. C. , … Tononi, G. (2009). Cortical firing and sleep homeostasis. Neuron, 63(6), 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, J. (2010). Circadian rhythms and cognition. Progress in Brain Research, 185, 131–153. [DOI] [PubMed] [Google Scholar]
- Xie, L. , Kang, H. , Xu, Q. , Chen, M. J. , Liao, Y. , Thiyagarajan, M. , … Nedergaard, M. (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


