Abstract
Narcissism is one of the most fundamental personality traits in which individuals in general population exhibit a large heterogeneity. Despite a surge of interest in examining behavioral characteristics of narcissism in the past decades, the neurobiological substrates underlying narcissism remain poorly understood. Here, we addressed this issue by applying a machine learning approach to decode trait narcissism from whole‐brain resting‐state functional connectivity (RSFC). Resting‐state functional MRI (fMRI) data were acquired for a large sample comprising 155 healthy adults, each of whom was assessed for trait narcissism. Using a linear prediction model, we examined the relationship between whole‐brain RSFC and trait narcissism. We demonstrated that the machine‐learning model was able to decode individual trait narcissism from RSFC across multiple neural systems, including functional connectivity between and within limbic and prefrontal systems as well as their connectivity with other networks. Key nodes that contributed to the prediction model included the amygdala, prefrontal and anterior cingulate regions that have been linked to trait narcissism. These findings remained robust using different validation procedures. Our findings thus demonstrate that RSFC among multiple neural systems predicts trait narcissism at the individual level.
Keywords: connectome‐based predictive modeling, cross validation, narcissism, resting‐state functional connectivity
1. INTRODUCTION
Narcissism is one of the most well‐known and fundamental personality traits in which individuals in general population exhibit a large heterogeneity. Narcissistic individuals exhibit an inflated sense of importance and entitlement, believe they deserve excessive admiration and attention, disregard feelings and opinions of others, and respond with intense anger and aggression when confronted with threats to self (Campbell & Foster, 2007; Campbell & Miller, 2011; Foster, Campbell, & Twenge, 2003; Krizan & Herlache, 2018; Watson, Grisham, Trotter, & Biderman, 1984). Narcissism has garnered considerable attention in the fields of both psychology and popular culture for more than a century (Akhtar & Thomson, 1982; Freud, 1957). Especially in this new era of social networking that boosts narcissistic behaviors such as frequent selfie‐posting (Gnambs & Appel, 2017), there is a steady increase of narcissism around the world (Cai, Kwan, & Sedikides, 2012; Twenge, Konrath, Foster, Keith Campbell, & Bushman, 2008). Therefore, it is not surprising that the past decades have witnessed a surge of interest in delineating behavioral characteristics associated with narcissism (Campbell & Miller, 2011; Miller, Lynam, Hyatt, & Campbell, 2017; Zhou, Li, Zhang, & Zeng, 2012; Zhou, Zhang, Yang, & Chen, 2015). Despite widespread interest in narcissism, much less is known about the neural substrates underlying this phenomenon. In the current work, we aimed to decode trait narcissism from intrinsic whole‐brain functional connectivity with the purpose to reveal neural correlates of narcissism.
Narcissism is a complex and multidimensional construct that modulates a variety of psychological functions ranging from cognitive to affective and social domains (Clarke, Karlov, & Neale, 2015; Feng, Liang, Zhou, & Yi, 2012; Krizan & Herlache, 2018; Morf & Rhodewalt, 2001; Wink, 1991). Recent neuroscientific studies have corroborated this assertion by demonstrating the modulation of narcissism in many neuropsychological functions. For instance, among high compared to low narcissistic men, self‐relevant processing induced enhanced activation in the dorsal and ventral anterior cingulate cortex (dACC/vACC), regions associated with negative affect or emotional conflict (Jauk, Benedek, Koschutnig, Kedia, & Neubauer, 2017). Moreover, high compared to low narcissistic participants scored higher in the alexithymia, and exhibited lower deactivation of the anterior insula (AI) during empathy (Fan, et al., 2011). In addition, narcissism was associated with enhanced activity of the social pain network (e.g., dACC, AI) in response to social exclusion by peers (Cascio, Konrath, & Falk, 2015; Chester & DeWall, 2016). Likewise, aberrant amygdala and medial frontal cortex (mPFC) functioning has been thought to contribute to emotional processing deficits among narcissistic personality disorder (Ronningstam & Baskin‐Sommers, 2013). Lastly, narcissism was linked to structural changes in regions important in emotion regulation, self‐processing, empathy, and theory of mind, including the dorsolateral prefrontal cortex (dlPFC), mPFC, dACC, and AI (Mao et al., 2016; Nenadic et al., 2015; Schulze et al., 2013). Taken together, recent neuroscientific findings are in line with diverse manifestations of narcissism in multiple neuropsychological functions.
Building on previous studies, here we implemented a connectome‐based predictive modeling approach to predict trait narcissism from whole‐brain resting‐state functional connectivity (RSFC). The task‐independent neuroimaging measures are free from confounding associated with ongoing task demand as well as different experimental designs across studies (Gabrieli, Ghosh, & Whitfield‐Gabrieli, 2015). Second, the RSFC allows for examining interplay between neural systems that are associated with trait narcissism (Braun et al., 2018). The functional connectivity across large‐scale neural networks may play critical roles in maintaining trait narcissism, because narcissism is a complex and multidimensional construct and might be rooted in the functional and structural integrity of distributed networks (Chester et al., 2016; Morf & Rhodewalt, 2001; Nenadic et al., 2015; Yang et al., 2015). RSFC has emerged as a powerful network‐level approach to significantly advance our understanding of individual differences in human cognitive ability and personality traits. Many studies have revealed robust and reliable patterns of RSFC within many well‐known networks spanning the brain, which extensively overlap with coactivation patterns induced by relevant task demands (Raichle, 2011; Raichle, 2015). For instance, networks resulting from an independent component analysis of a large sample of meta‐analytic task‐dependent activation maps closely correspond to resting‐state networks (Smith et al., 2009). That is, task‐independent activity in functionally‐related brain regions are intrinsically organized, which offers a potential predictor of relevant behavioral tendencies (Harmelech & Malach, 2013). Furthermore, RSFC patterns within each individual are both unique and reliable, similarly to a fingerprint, serving to underlie individual differences in personality traits or cognitive functions (Finn et al., 2015; Hsu, Rosenberg, Scheinost, Constable, & Chun, 2018; Rosenberg et al., 2016).
Taken together, the current approach provides the following advantages: (a) the RSFC approach allows for whole‐brain measures that should provide a more holistic measure of trait narcissism than activity in individual regions and (b) the machine learning approach allowed for predicting unseen participants, offering information at the individual rather than the group level (Dubois & Adolphs, 2016; Gabrieli et al., 2015; Yarkoni & Westfall, 2016). Specifically, the machine learning approach typically implements cross‐validation procedures to estimate the model with training samples and to test the performance of the model with independent samples (i.e., test samples). Moreover, the predictive features adopted by the model reveal neural correlates of the trait narcissism. In light of previous studies (Cascio et al., 2015; Chester & DeWall, 2016; Fan et al., 2011; Jauk et al., 2017; Ronningstam & Baskin‐Sommers, 2013), we hypothesized that individual differences in trait narcissism would be predicted by a wide array of functional connectivity across distributed networks, particularly those implicated in emotion regulation (e.g., dlPFC), emotional processing (e.g., amygdala, ventral mPFC [vmPFC]), empathy (dACC, AI), and theory of mind (e.g., dorsal mPFC [dmPFC]).
2. MATERIAL AND METHODS
2.1. Participants
One hundred and sixty‐eight healthy right‐handed undergraduate or graduate students (109 males; 21.99 ± 2.38 years old, range: 18–30 years old) without history of neurological or psychiatric disorder were recruited. The study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments, and was approved by the Ethics Committee of Beijing Normal University. Written informed consents were obtained from all participants.
2.2. Narcissistic personality inventory (NPI)
A Chinese version of the NPI was administered to assess trait narcissism for each participant. The NPI for Chinese (Zhou, Zhang, Chen, & Ye, 2009) was developed based on the classical Narcissistic Personality Inventory, which is the most commonly employed measure of narcissism in the field of personality and social psychology (Emmons, 1984; Miller, Price, & Campbell, 2012; Raskin & Terry, 1988). The adapted inventory exhibited excellent reliability and validity among Chinese (Zhou et al., 2012; Zhou et al., 2009; Zhou et al., 2015). The NPI for Chinese consists of 34 items, and each item is scored on a 6‐point likert scale ranging from 1 (not at all true) to 6 (complete true). NPI scores were calculated by summing scores on the 34 items, and the higher scores on the inventory indicate higher levels of narcissism. The Cronbach's alpha coefficient of the inventory is 0.94 in the current sample.
2.3. Image acquisition
Images were acquired with a Siemens TRIO 3‐Tesla scanner at the Beijing Normal University Imaging Center for Brain Research. All participants underwent a 5‐min resting‐state fMRI scanning, during which they were instructed to close their eyes, keep still, remain awake, and not to think about anything systematically. The resting state scanning consisted of 150 contiguous echo‐planar imaging (EPI) volumes using the following parameters: axial slices, 33; slice thickness, 3.5 mm; gap, 0.7 mm; TR, 2,000 ms; TE, 30 ms; flip angle, 90°; voxel size, 3.5 × 3.5 × 3.5 mm3; and FOV, 244 × 244 mm2. In addition, high‐resolution structural images were acquired through a 3D sagittal T1‐weighted magnetization‐prepared rapid acquisition with gradient‐echo (MPRAGE) sequence, using the following parameters: sagittal slices, 144; TR, 2,530 ms; TE, 3.39 ms; slice thickness, 1.33 mm; voxel size, 1 × 1 × 1.33 mm3; flip angle, 7°; inversion time, 1,100 ms; and FOV, 256 × 256 mm2. Notably, the images of all subjects cover the whole brain, including the cerebellum.
2.4. Image preprocessing
Neuroimaging data analyses were performed with the DPABI software package (http://rfmri.org/dpabi; Yan, Wang, Zuo, & Zang, 2016), which is a convenient software plug‐in based on SPM (http://www.fil.ion.ucl.ac.uk/spm). The first 10 volumes of the functional images were discarded for signal equilibrium and participants’ adaptation to scanning noise. The images were then realigned for head movement correction. Thirteen participants (nine males) were excluded from further analysis under the criteria of head motion exceeding 2.5 mm maximum translation, 2.5° rotation or mean frame‐wise displacement (FD) exceeding 0.2 mm throughout the course of scans (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Yan, et al., 2013). To normalize functional images, participants’ structural brain images were first co‐registered to their own mean functional images and were subsequently segmented. The parameters derived from segmentation were used to normalize each participant's functional images into the standard Montreal Neurological Institute space (MNI template, resampling voxel size was 3 × 3 × 3 mm3). Afterwards, the linear trends of time courses were removed, and a band‐pass filtering (0.01–0.1 Hz) was applied to the time series of each voxel to reduce the effect of low‐frequency drifts and high‐frequency physiological noise (Biswal, Zerrin Yetkin, Haughton, & Hyde, 1995; Zuo, et al., 2010). Subsequently, the images were spatially smoothed using a Gaussian filter to decrease spatial noise (4 × 4 × 4 mm3 full width at half maximum). Finally, three common nuisance variables were regressed out, including the white matter signal, the cerebrospinal fluid signal (Fox, et al., 2005; Snyder & Raichle, 2012), and 24 movement regressors including autoregressive models of motion incorporating six head motion parameters, six head motion parameters one time point before, and the 12 corresponding squared items (Friston, Williams, Howard, Frackowiak, & Turner, 1996). Please note that global signal regression was not implemented in the preprocessing, due to the reason that it might cause negative connections that are hard to interpret (see also Murphy, Birn, Handwerker, Jones, & Bandettini, 2009).
2.5. RSFC feature extraction
In the current study (Figure 1), network nodes were first defined using a functional brain atlas, which was derived from a graph‐theory based parcellation algorithm that maximized the similarity of the voxel‐wise time series within each node (Shen, Papademetris, & Constable, 2010; Shen, Tokoglu, Papademetris, & Constable, 2013). The atlas includes 268 nodes spanning the whole brain including cerebellum and brainstem. The 268‐node atlas comprises nodes with more coherent time series than those defined by the automatic anatomic labeling atlas (Shen et al., 2013).
Figure 1.
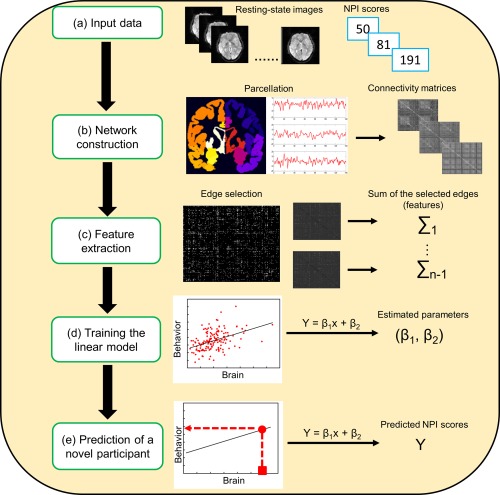
The prediction schematic flow using patterns of resting‐state brain connectivity. NPI, narcissistic personality inventory [Color figure can be viewed at http://wileyonlinelibrary.com]
For each participant, a time course was computed for each node by averaging the BOLD signal of all of its constituent voxels at each time point. Second, network edges were defined as functional connectivity between each pair of nodes, calculating as the correlation (Pearson's r) between the time courses of each pair of nodes. Fisher's r‐to‐z transformation was then implemented to improve the normality of the correlation coefficients, resulting in a 268 × 268 symmetric connectivity matrix that represented the set of edges/connections in each participant's resting‐state connectivity profile.
2.6. Exploratory correlation analysis
An exploratory correlation analysis was implemented across all participants to examine the relevance of RSFC to narcissism trait. In particular, Pearson correlation between each edge in the connectivity matrices and NPI scores was computed across participants. The resultant r values were forward to a threshold of p < .005 and separated into a positive tail (i.e., positive correlation between the strength of edge and NPI scores) and a negative tail (i.e., negative correlation between the strength of edge and NPI scores). Therefore, connections in the positive tail (hereafter referred to as “positive network”) and negative tail (hereafter referred to as “negative network”) were selected by correlations with NPI scores rather than positive or negative functional connections themselves (see also Beaty et al., 2018; Hsu et al., 2018; Rosenberg et al., 2016). Notably, a relatively conservative threshold of p < .005 was chosen to select comparable number of edges used in previous studies (e.g., Rosenberg et al., 2016) and to alleviate the complexity of contributing features. Afterwards, a single aggregate metric of network strength was employed to characterize degree of connectivity in the positive and negative tails for each participant. That is, positive network strength was computed by summing the edge strengths (i.e., Z scores) for all the edges in the positive tail. Similarly, negative network strength was computed by summing the Z scores of all the edges in the negative tail. Lastly, the positive and negative network strengths were correlated with NPI scores. Please note that results of this analysis were for display purpose, and no statistical tests were performed, as this would be considered double dipping (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009; Kristensen & Sandberg, 2017). Furthermore, conclusions on the relationship between positive/negative network strengths and narcissism were not derived from this analysis, but instead were based on results from cross‐validation detailed below.
2.7. Prediction analysis using cross‐validation
To determine whether network strength predicted narcissism trait in unseen individuals, a leave‐one‐out cross validation (LOOCV) was used to evaluate the out‐of‐sample prediction performance. Specially, N‐1 subjects were used as the training set and the remaining one was used as the testing sample, where N is the total number of subjects. During the training procedure, each edge was normalized across training set. That is, the mean and standard deviation on each edge were first calculated across subjects in the training set; afterwards, normalization on each edge (for a given subject) was computed as the difference between the edge value and the mean divided by the standard deviation. Predictive networks were then defined and employed by calculating positive and negative network strengths as described in the exploratory correlation analysis. Afterwards, simple linear models were constructed to respectively relate positive and negative network strengths to NPI scores in the training set. During the testing procedure, each testing participant's strengths of positive and negative network was normalized using the parameters acquired during training procedure, and then the trained models were used to predict the testing participant's NPI score (Finn et al., 2015; Rosenberg et al., 2016; Shen, et al., 2017). The training and testing procedures were repeated N times such that each participant was used once as the testing participant.
Pearson correlation coefficient (r) and mean squared error (MSE) between actual and predicted NPI scores were used to evaluate the accuracy of prediction (Kristensen & Sandberg, 2017). The permutation test was applied to determine whether the obtained metrics were significantly better than expected by chance. Specially, we permuted the NPI scores across participants without replacement 1,000 times, and each time re‐applied the above LOOOCV prediction procedure. This resulted in a distribution of correlation (r) and MSE values reflecting the null hypothesis that the model did not exceed chance. The number of times the permuted value was greater than (or with respect to MSE values, less than) the true value was then divided by 1,000 providing an estimated p‐value for both the correlation coefficient (r) and observed MSE.
2.8. Contributing network in the prediction of NPI scores
To characterize the neural substrates of the contributing network, the network was first defined as the set of edges that were present in every iteration of the LOOCV described above. Afterwards, the 268 nodes were grouped into 10 macroscale brain regions, including the prefrontal lobe (46 nodes), motor lobe (21 nodes), insula lobe (7 nodes), parietal lobe (27 nodes), temporal lobe (39 nodes), occipital lobe (25 nodes), limbic lobe (36 nodes), cerebellum lobe (41 nodes), subcortical lobe (17 nodes), and brainstem lobe (9 nodes; Finn et al., 2015; Rosenberg et al., 2016). Afterwards, the number of edges between each pair of macroscale regions or canonical networks was calculated. To examine the importance of individual lobes in the prediction of narcissism trait, edges from each lobe were computationally “lesioned” in the prediction analysis. For instance, after excluding edges in the prefrontal lobe, which consisted of 46 nodes, 222 × 222 connectivity matrices rather than 268 × 268 matrices were employed for prediction analysis.
Lastly, the importance of individual nodes was measured as the number of their connections (Beaty et al., 2018; Rosenberg et al., 2016). The connectivity patterns of the top ten most highly connected nodes were illustrated.
2.9. Validation
Main results were further validated with a range of different smoothing kernels used in the preprocessing (0, 6, and 8 mm) and feature‐selection thresholds (p < .05, .01, .001). Furthermore, prediction results were validated using different cross‐validation schemes (i.e., two‐fold, five‐fold, and 10‐fold). Taken the two‐fold cross‐validation as an example, all subjects were divided into two subsets, in which one subset was used as the training set, and the remaining one was used as the testing set. Training set was normalized and used to train a linear prediction model, which then was used to predict the scores of the normalized testing data. The normalization of testing data used the normalizing parameters acquired from training data. This procedure was repeated twice, so that each subset was used as testing set once. Finally, the correlation r between the true and predicted scores was calculated across all subjects. As the full dataset were randomly divided into two subsets, the performance might depend on the data division. Therefore, the two‐fold cross‐validation was repeated 100 times, and the results were averaged to produce a final prediction performance. A 1,000 times permutation test was applied to test the significance of the prediction performance.
2.10. Control analyses
Several control analyses were implemented to further examine the significance of predictions of our models despite potential confounds of motion, age and gender. In these analyses, new predictive networks were constructed by employing those edges whose partial Pearson correlation with NPI scores while controlling for confounding variables (e.g., motion) passed the p < .005 threshold (see also Hsu et al., 2018; Shen et al., 2017). Finally, head motion was further controlled for in the data preprocessing, such that volumes with an FD > 0.5 mm, along with the immediately preceding volume and two subsequent volumes, were considered micromovement‐containing volumes, and each of these volumes were modeled as a separate regressor in nuisance covariates regression (Power et al., 2014; Yan et al., 2013).
3. RESULTS
3.1. Exploratory correlation analysis
Participants differed widely with respect to the degree of dispositional narcissism (Figure 2a). NPI scores were not significantly correlated with mean frame‐to‐frame head motion (r = −.05, p = .55) or age (r = .06, p = .46), and did not differ between genders (t 153 = 1.08, p = .28). Regarding the correlation between RSFC and NPI scores, across all participants, the average r value was r = .26 (range: 0.22 ∼ 0.41) in the positive tail that comprised 3,447 edges. The average r value was r = −0.24 (range: −0.25 ∼ −0.23) in the negative tail that comprised three edges. Because the limited number of edges in the negative tail would not allow for a reliable prediction analysis, the following analyses focused on the positive network.
Figure 2.
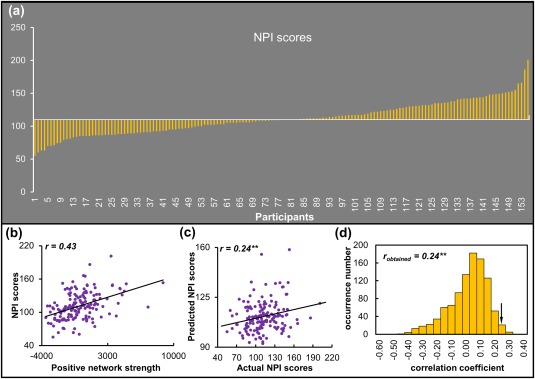
NPI scores and performance of the prediction model. (a) NPI scores across all participants. (b) Exploratory correlational analyses on the positive network strength and NPI scores. (c) Correlation between actual and predicted NPI scores. (d) Permutation distribution of the correlation coefficient (r) for the prediction analysis. NPI, narcissistic personality inventory. **p < .01 [Color figure can be viewed at http://wileyonlinelibrary.com]
The edges in the positive network represented <10% of the whole‐brain 35,778 total edges defined in the current atlas. The correlation coefficient between positive network strength, computed by summing the edge strengths for all the edges in the positive tail, and NPI scores was r = .43 (Figure 2b).
3.2. Prediction analysis using cross validation
A LOOCV approach was implemented to examine whether the relevance between positive network strength and NPI scores generalized to novel individuals. It was demonstrated that RSFC in the positive network was able to predict NPI scores in novel individuals (r = .24, p = .007; MSE = 606.70, p = .012, permutation test, Figure 2c,d).
3.3. Contributing networks in the prediction of NPI scores
Across all folds of LOOCV, the numbers of edges that contributed to the prediction ranged from 1,907 to 4,333. Notably, 1,605 of these edges appeared in every iteration of the LOOCV and were defined as the contributing network.
Based on macroscale regions (Figure 3), it was revealed that connections within prefrontal and limbic lobes, connections between prefrontal and parietal, temporal, limbic, subcortical, and brainstem lobes, and connections between limbic and motor and temporal lobes were primary predictors of NPI scores (Figure 4a,b). Notably, the lesion analysis revealed that models excluding anyone of the ten lobes were still able to predict NPI scores (Table 1). Furthermore, Steiger's z tests revealed that predictions (i.e., r) of all lesioned matrixes were not significantly different from that of the whole‐brain matrix (all p > .05). These findings suggested that prediction models did not rely on the strength in a single lobe, but instead incorporate information from widely distributed systems across the brain.
Figure 3.
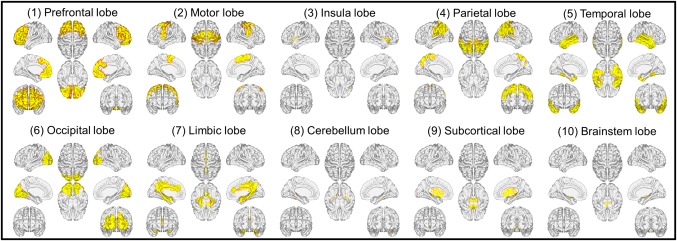
Macroscale regions used for characterizing contributing connectivity [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
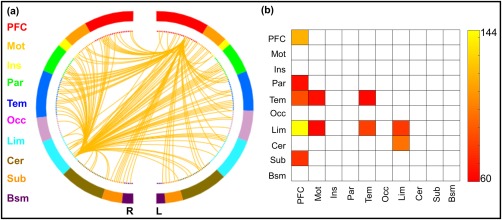
Functional connections predicting NPI scores. (a) The connectivity patterns selected by the prediction model, plotted as the number of connections within each marcoscale regions. (b) Connections plotted as the number of edges within and between each pair of marcoscale regions. L, left; R, right; PFC, prefrontal; Mot, motor; Ins, insula; Par, parietal; Tem, temporal; Occ, occipital; Lim, limbic; Cer, cerebellum; Sub, subcortical; Bsm, brainstem [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Performance of the prediction model using whole‐brain matrices or “lesioned” matrices
| r | MSE | |||
|---|---|---|---|---|
| r‐value | p‐value | MSE‐value | p‐value | |
| Whole brain | .240 | 0.007 | 606.7 | .012 |
| ‐PFC | .233 | 0.012 | 608.2 | .016 |
| ‐Mot | .234 | 0.007 | 609.6 | .008 |
| ‐Ins | .240 | 0.006 | 606.1 | .01 |
| ‐Par | .242 | 0.008 | 605.5 | .01 |
| ‐Tem | .248 | 0.004 | 603.2 | .007 |
| ‐Occ | .242 | 0.008 | 604.5 | .007 |
| ‐Lim | .225 | 0.009 | 613.7 | .019 |
| ‐Cer | .244 | 0.008 | 601.9 | .007 |
| ‐Sub | .232 | 0.01 | 608.8 | .008 |
| ‐Bsm | .235 | 0.01 | 607.7 | .006 |
Abbreviations: PFC, prefrontal; Mot, motor; Ins, insula; Par, parietal; Tem, temporal; Occ, occipital; Lim, limbic; Cer, cerebellum; Sub, subcortical; Bsm, brainstem.
Furthermore, the top ten most highly connected nodes were located in the amygdala, dmPFC, vmPFC, dACC, and ventral lPFC (vlPFC), implicating the critical roles of these regions in trait narcissism (Figure 5 and Table 2). However, it should be noted that individual levels of narcissism were best characterized by connectivity patterns of these regions across the entire brain, and one may not oversimplify predictive networks to these regions.
Figure 5.
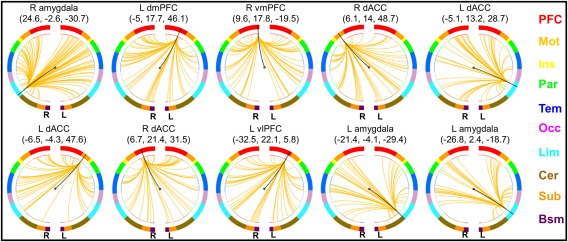
Connectivity patterns of the top ten nodes with the most connections. L, left; R, right; dmPFC, dorsomedial prefrontal cortex; vmPFC, ventromedial prefrontal cortex; dACC, dorsal anterior cingulate cortex; vlPFC, ventrolateral prefrontal cortex; PFC, prefrontal; Mot, motor; Ins, insula; Par, parietal; Tem, temporal; Occ, occipital; Lim, limbic; Cer, cerebellum; Sub, subcortical; Bsm, brainstem [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Ten nodes with the most connections selected by the prediction model
| Node | MNI coordinates (mm) | Lobe | Degree | ||
|---|---|---|---|---|---|
| R amygdala | 24.6 | −2.6 | −30.7 | Limbic | 86 |
| L dmPFC | −5 | 17.7 | 46.1 | Prefrontal | 63 |
| R vmPFC | 9.6 | 17.8 | −19.5 | Prefrontal | 53 |
| R SMA | 6.1 | 14 | 48.7 | Motor | 52 |
| L dACC | −5.1 | 13.2 | 28.7 | Limbic | 51 |
| L SMA | −6.5 | −4.3 | 47.6 | Motor | 50 |
| R dmPFC | 6.7 | 21.4 | 31.5 | Prefrontal | 49 |
| L vlPFC | −32.5 | 22.1 | 5.8 | Prefrontal | 48 |
| L amygdala | −21.4 | −4.1 | −29.4 | Limbic | 43 |
| L amygdala | −26.8 | 2.4 | −18.7 | Limbic | 41 |
Abbreviation: L, left; R, right; dmPFC, dorsomedial prefrontal cortex; vmPFC, ventromedial prefrontal cortex; SMA, supplementary motor area; dACC, dorsal anterior cingulate cortex; vlPFC, ventrolateral prefrontal cortex.
3.4. Validation
Using different smooth kernels, feature‐selection thresholds, and cross‐validation schemes, the performance of predication was re‐estimated. The resultant correlation coefficients remained significant, thus validating the reliability of main findings (Table 3). Furthermore, contributing features selected in different validations were similar to those in the primary findings (Supporting Information Figure S1). For instance, regions from limbic and prefrontal lobes constitute the most connecting nodes in all analyses (Supporting Information Table S1). Regarding the feature‐selection thresholds, it should be noted that negative network strengths could not reliably predict NPI scores in any thresholds, even if a liberal threshold (p < .05) was used to increase the number of edges.
Table 3.
Results of validation and control analyses
| r | MSE | |||
|---|---|---|---|---|
| r‐value | p‐value | MSE‐value | p‐value | |
| Smooth kernels | ||||
| 0 mm | .238 | .011 | 608.6 | .02 |
| 6 mm | .237 | .007 | 606 | .01 |
| 8 mm | .240 | .005 | 603.2 | .005 |
| feature‐selection thresholds | ||||
| 0.05 | .236 | .001 | 595.7 | .001 |
| 0.01 | .240 | .003 | 602.1 | .003 |
| 0.001 | .247 | .032 | 610.3 | .036 |
| Control analyses | ||||
| Group motion regression | .233 | .006 | 603.69 | .007 |
| Individual motion scrubbing | .236 | .006 | 607.61 | .008 |
| Age | .238 | .009 | 608.25 | .009 |
| Gender | .241 | .007 | 608.18 | .008 |
3.5. Control analyses
After controlling for the potential confounds of head motion, age, or gender, new predictive networks were constructed and used in the cross‐validation schemes. These analyses indicated that predictive models could still significantly predict NPI scores (Table 3). Contributing features to the model in these control analyses were similar to those in the primary findings (Supporting Information Figure S2 and Table S1).
4. DISCUSSION
Narcissism is one of the most important personality constructs that have drawn considerable attention from psychologists in the past decades (Campbell & Miller, 2011; Miller et al., 2017), but the neurobiological correlates of trait narcissism remain largely unknown. In the current study, we employed the intrinsic whole‐brain functional connectivity in a machine‐learning framework to predict trait narcissism, with the aim to establish neural correlates that are predictive of narcissism at the individual level. We demonstrated that intrinsic functional connectivity across multiple neural systems allowed for predicting individual trait narcissism. In particular, inter‐individual differences in narcissism were primarily accounted for by intrinsic functional connectivity between and within limbic and prefrontal systems as well as their connectivity with other networks (e.g., temporal regions). Key nodes that contributed to the prediction model included the amygdala, lateral PFC, medial PFC, and dACC, in which neural activity has been previously associated with multiple cognitive and emotional components in trait narcissism (Jankowiak‐Siuda & Zajkowski, 2013; Mao et al., 2016). Taken together, in addition to demonstrating that functional connectivity of resting‐state brain network predicts trait narcissism, our findings bolster the assertion that narcissism is a multidimensional construct rooted in interactions among multiple neural systems.
The multiple neural systems underlying narcissism fit with diverse manifestations of narcissism in a variety of cognitive, affective and social functions. For instance, narcissism involves deficiencies in behavioral inhibition and aversive information processing, such that individuals scoring high in narcissism exhibit diminished electro‐dermal reactivity to aversive stimuli (Kelsey, Ornduff, McCann, & Reiff, 2001). Furthermore, narcissism is associated with affective extremity and variability (Emmons, 1987) as well as exaggerated anger during negative interpersonal interactions (Meier & Semmer, 2013; Twenge & Campbell, 2003), implicating emotion regulation deficits among those with high levels of narcissism. Lastly, narcissism is characterized by excessive self‐occupation (Kanske, Sharifi, Smallwood, Dziobek, & Singer, 2017) as well as lack of empathy for others (Czarna, Wróbel, Dufner, & Zeigler‐Hill, 2015; Hepper, Hart, & Sedikides, 2014; Watson et al., 1984; Watson & Morris, 1991). The complex narcissistic functioning suggests that narcissism is rooted in interactions between multiple brain systems, and that a whole‐brain functional connectivity approach could provide more holistic measure of narcissism than neural activity at the regional level.
In line with behavioral characteristics of narcissism, predictive network of narcissism primarily comprises of connections within and between prefrontal and limbic lobes that are important in emotional regulation and processing. On the one hand, the prefrontal lobe has been generally involved a variety of high‐order control processes, ranging from task‐set maintaining to long‐term planning and response suppression and selection (Cole & Schneider, 2007; Menon, 2011; Miller & Cohen, 2001; Seeley, et al., 2007). On the other hand, limbic lobe has long been thought to contribute emotional processing, such as fear and empathy (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; LeDoux, 2003). However, an increasing body of evidence has indicated that these systems exhibit extensive interactions as neural network to support complex cognitive‐emotional behaviors (e.g., narcissism; Pessoa, 2008). The current findings corroborate this assertion by demonstrating the predictive roles of intrinsic functional connectivity of these systems in trait narcissism.
In the prefrontal and limbic lobes, the mPFC, lateral PFC, amygdala, and dACC are key nodes that contribute to the predictive network of narcissism. The amygdala is a key region in the limbic system associated with the encoding of aversive stimuli (Adolphs, 2008; Adolphs, et al., 2005; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998) and has been suggested to contribute to impaired fear processing among narcissistic individuals (Ronningstam & Baskin‐Sommers, 2013). Furthermore, both the vlPFC and dmPFC have been implicated in emotion regulation (Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner & Gross, 2005), such that the vlPFC is associated with reappraisal of affective events (Buhle et al., 2014), and the dmPFC is associated with mental state attribution (Ochsner et al., 2004). In addition, the vmPFC is frequently engaged by self‐ and reward‐related processing (Chavez, Heatherton, & Wagner, 2016; de Greck, et al., 2008). Lastly, the dACC has been recruited by a variety of social functions, particularly the empathy and pain processing (Lamm, Decety, & Singer, 2011; Singer, et al., 2004).
In line with the current findings, previous task‐based brain imagining studies have related narcissism to the activity of these regions in specific tasks (Cascio et al., 2015; Chester & DeWall, 2016; Fan et al., 2011; Jauk et al., 2017). Notably, important features (e.g., amygdala) contributing to predictive network of narcissism have also been previously implicated in narcissistic personality functioning of narcissistic personality disorder (Ronningstam & Baskin‐Sommers, 2013). For instance, the amygdala and prefrontal regions have been thought to play crucial roles in mediating fear and self‐regulatory deficits among narcissism personality disorders (Ronningstam & Baskin‐Sommers, 2013). Moreover, narcissistic personality disorder patients compared to healthy controls exhibited grey and white matter deficits in key nodes observed in the current study, including lateral PFC and mPFC/dACC (Nenadic et al., 2015). Lastly, narcissistic personality disorder patients often exhibit deficiencies in emotional processing and empathic functioning that are associated with brain networks identified in the current study (Ronningstam, 2016; Ronningstam, 2017). In short, deficiencies in self‐regulation, emotional processing, and empathic ability, which often engage networks highlighted in the current study, are considered as key characteristics of narcissism and narcissistic personality disorder (Ronningstam, 2010).
Taken together, the current findings provided the first evidence showing that intrinsic functional couplings of these regions with multiple systems across the brain play crucial roles in characterizing narcissism. Specifically, our findings suggest that those neural networks (e.g., the limbic and prefrontal systems) important in behavioral characteristics of narcissism exhibit intrinsic functional organization in the absence of an explicit task, the functional brain connectomics of which effectively predict individual trait narcissism. These findings corroborate the intrinsic perspective of brain function asserting that intrinsic brain functional connectivity maintains important information for interpreting, responding to and even predicting environmental demands (Raichle, 2006). Indeed, recent studies have demonstrated that RSFC shapes brain activity across a variety of cognitive tasks (Cole, Ito, Bassett, & Schultz, 2016; Ito et al., 2017). In line with the account and the current findings, recent studies have related RSFC patterns to spatial navigation performance (Kong et al., 2017), social preference (Hahn et al., 2015a; Hahn et al., 2015b), and creativity (Beaty et al., 2018) as well as personality attributes (Hsu et al., 2018).
Finally, the current work reflects the advances in the field of neuroscience advocating the applications of task‐independent neuroimaging measures to uncover sources of individual differences in social preferences and personality attributes (Nash, Gianotti, & Knoch, 2015; Shen et al., 2017). Within this framework, an accumulating body of research has revealed that brain structure and intrinsic functions account for inter‐individual variation in many social preferences and personality traits, such as altruistic behaviors and cooperation tendencies (e.g., Baumgartner, Nash, Hill, & Knoch, 2015; Baumgartner, Schiller, Hill, & Knoch, 2013; Hahn et al., 2015a; Morishima, Schunk, Bruhin, Ruff, & Fehr, 2012). Importantly, the machine‐learning approach adopted in the current study took a further step to demonstrate that brain‐imaging measures could account for personality attributes at the individual level. Indeed, the machine‐learning approach has been proposed as a promising tool in the neuroscience and psychology to (a) provide the advantage of out‐of‐sample generalizations and (b) aid the clinical assessment of individual patients (Cui, Su, Li, Shu, & Gong, 2017; Dubois & Adolphs, 2016; Gabrieli et al., 2015; Gong, et al., 2014; Rosenberg, Finn, Scheinost, Constable, & Chun, 2017; Shen et al., 2017; Yarkoni & Westfall, 2016). Therefore, a potential application of the current approach would be the use of RSFC measures in predicting symptom severity of narcissistic personality disorder. That being said, it should be noted that the current prediction model was based on a sample with large age range (19–30), whereas narcissistic personality disorder often begins during adolescence and emerging adulthood (Campbell & Miller, 2011).
Several limitations related to the current study should be noted. First, the NPI mainly captures the grandiose dimension of narcissism (Ames, Rose, & Anderson, 2006), and future studies are needed to examine the neural signatures underlying the vulnerable dimension of narcissism (Pincus et al., 2009). Second, although the current study controlled for potential major confounds such as age, gender and motion, other demographic information (e.g., education background and years) should be collected and controlled for as potential confounding variables in future studies. Third, the current work did not reveal sex differences in narcissism, but conclusions on these findings should be drawn with cautions, since the number of males and females were not equal in the current sample. Because previous studies have identified sex differences in narcissism and neural correlates (Jauk et al., 2017; Tschanz, Morf, & Turner, 1998; Yang et al., 2015; but see also Chegeni, Pirkalani, & Dehshiri, 2018), future studies are needed to address this issue with comparable number of males and females. Fourth, we did not identify reliable prediction of narcissism from negative network strengths across different feature selection thresholds, and the reason for the null findings remains to be elucidated. Finally, one may not interpret the predictive network as a “neuromarker” of narcissism, since the definition of “neuromarker” often entails sensitivity and specificity of neural measures (Kristensen & Sandberg, 2017; Kropotov, 2016), which was not addressed in the current study.
Despite these limitations, we demonstrate for the first time that functional connectivity of distributed large‐scale networks effectively predicts trait narcissism at the individual level, corroborating psychological models that conceptualize narcissism as a complex and multidimensional construct rooted in interplay of the cognitive, affective, and social processes (Morf & Rhodewalt, 2001). Beyond this finding, the current data‐driven approach opens an exciting avenue to predict other personality traits and behavioral tendencies in a broad range of settings and to characterize neural mechanisms of them.
CONFLICT OF INTEREST
The authors are unaware of any conflicts of interest, financial or otherwise.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Postdoctoral Program for Innovative Talents (BX201600019, BX201600187), the China Postdoctoral Science Foundation (2017M610055), the National Natural Science Foundation of China (31500920, 31571124, 61571047), the foundation of the National Key Laboratory of Human Factors Engineering (HF2012‐K‐03), and the Fundamental Research Funds for the Central Universities (2017EYT36). The authors thank Yu L. L. Luo for her suggestions on manuscript revision.
Feng C, Yuan J, Geng H, et al. Individualized prediction of trait narcissism from whole‐brain resting‐state functional connectivity. Hum Brain Mapp. 2018;39:3701–3712. 10.1002/hbm.24205
Funding information National Postdoctoral Program for Innovative Talents, Grant/Award Numbers: BX201600019 and BX201600187; China Postdoctoral Science Foundation, Grant/Award Number: 2017M610055; National Natural Science Foundation of China, Grant/Award Numbers: 31500920, 31571124, and 61571047; Foundation of the National Key Laboratory of Human Factors Engineering, Grant/Award Number: HF2012‐K‐03; Fundamental Research Funds for the Central Universities, Grant/Award Number: 2017EYT36
Contributor Information
Xia Wu, Email: wuxia@bnu.edu.cn.
Yuejia Luo, Email: luoyj@szu.edu.cn.
REFERENCES
- Adolphs, R. (2008). Fear, faces, and the human amygdala. Current Opinion in Neurobiology, 18(2), 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs, R. , Gosselin, F. , Buchanan, T. W. , Tranel, D. , Schyns, P. , & Damasio, A. R. (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433(7021), 68–72. [DOI] [PubMed] [Google Scholar]
- Akhtar, S. , & Thomson, J. A. (1982). Overview: Narcissistic personality disorder. The American Journal of Psychiatry, 139(1), 12–20. [DOI] [PubMed] [Google Scholar]
- Ames, D. R. , Rose, P. , & Anderson, C. P. (2006). The NPI‐16 as a short measure of narcissism. Journal of Research in Personality, 40(4), 440–450. [Google Scholar]
- Baumgartner, T. , Nash, K. , Hill, C. , & Knoch, D. (2015). Neuroanatomy of intergroup bias: A white matter microstructure study of individual differences. NeuroImage, 122, 345–354. [DOI] [PubMed] [Google Scholar]
- Baumgartner, T. , Schiller, B. , Hill, C. , & Knoch, D. (2013). Impartiality in humans is predicted by brain structure of dorsomedial prefrontal cortex. Neuroimage, 81, 317–324. [DOI] [PubMed] [Google Scholar]
- Beaty, R. E. , Kenett, Y. N. , Christensen, A. P. , Rosenberg, M. D. , Benedek, M. , Chen, Q. , … Kane, M. J. (2018). Robust prediction of individual creative ability from brain functional connectivity. Proceedings of the National Academy of Sciences, 201713532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Zerrin Yetkin, F. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Braun, U. , Schaefer, A. , Betzel, R. F. , Tost, H. , Meyer‐Lindenberg, A. , & Bassett, D. S. (2018). From maps to multi‐dimensional network mechanisms of mental disorders. Neuron, 97(1), 14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , … Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, H. , Kwan, V. S. , & Sedikides, C. (2012). A sociocultural approach to narcissism: The case of modern China. European Journal of Personality, 26(5), 529–535. [Google Scholar]
- Campbell, W. K. , & Foster, J. D. (2007). The narcissistic self: Background, an extended agency model, and ongoing controversies. In C. Sedikides & S. J. Spencer (Eds.), Frontiers of social psychology. The self (pp. 115–138). New York, NY, US: Psychology Press. [Google Scholar]
- Campbell, W. K. , & Miller, J. D. (2011). The handbook of narcissism and narcissistic personality disorder: Theoretical approaches, empirical findings, and treatments. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Carr, L. , Iacoboni, M. , Dubeau, M.‐C. , Mazziotta, J. C. , & Lenzi, G. L. (2003). Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences, 100(9), 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio, C. N. , Konrath, S. H. , & Falk, E. B. (2015). Narcissists’ social pain seen only in the brain. Social Cognitive and Affective Neuroscience, 10(3), 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez, R. S. , Heatherton, T. F. , & Wagner, D. D. (2016). Neural population decoding reveals the intrinsic positivity of the self. Cerebral Cortex, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegeni, R. , Pirkalani, R. K. , & Dehshiri, G. (2018). On love and darkness: The Dark Triad and mate retention behaviors in a non‐Western culture. Personality and Individual Differences, 122, 43–46. [Google Scholar]
- Chester, D. S. , & DeWall, C. N. (2016). Sound the alarm: The effect of narcissism on retaliatory aggression is moderated by dACC reactivity to rejection. Journal of Personality, 84(3), 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester, D. S. , Lynam, D. R. , Powell, D. K. , & DeWall, C. N. (2016). Narcissism is associated with weakened frontostriatal connectivity: A DTI study. Social Cognitive and Affective Neuroscience, 11(7), 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, I. E. , Karlov, L. , & Neale, N. J. (2015). The many faces of narcissism: Narcissism factors and their predictive utility. Personality and Individual Differences, 81, 90–95. [Google Scholar]
- Cole, M. W. , Ito, T. , Bassett, D. S. , & Schultz, D. H. (2016). Activity flow over resting‐state networks shapes cognitive task activations. Nature Neuroscience, 19(12), 1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , & Schneider, W. (2007). The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage, 37(1), 343–360. [DOI] [PubMed] [Google Scholar]
- Cui, Z. , Su, M. , Li, L. , Shu, H. , & Gong, G. (2017). Individualized prediction of reading comprehension ability using gray matter volume. Cerebral Cortex, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarna, A. Z. , Wróbel, M. , Dufner, M. , & Zeigler‐Hill, V. (2015). Narcissism and emotional contagion: Do narcissists “catch” the emotions of others? Social Psychological and Personality Science, 6(3), 318–324. [Google Scholar]
- de Greck, M. , Rotte, M. , Paus, R. , Moritz, D. , Thiemann, R. , Proesch, U. , … Bogerts, B. (2008). Is our self based on reward? Self‐relatedness recruits neural activity in the reward system. Neuroimage, 39(4), 2066–2075. [DOI] [PubMed] [Google Scholar]
- Dubois, J. , & Adolphs, R. (2016). Building a science of individual differences from fMRI. Trends in Cognitive Sciences, 20(6), 425–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons, R. A. (1984). Factor analysis and construct validity of the narcissistic personality inventory. Journal of Personality Assessment, 48, 291–300. [DOI] [PubMed] [Google Scholar]
- Emmons, R. A. (1987). Narcissism: Theory and measurement. Journal of Personality and Social Psychology, 52(1), 11. [DOI] [PubMed] [Google Scholar]
- Fan, Y. , Wonneberger, C. , Enzi, B. , De Greck, M. , Ulrich, C. , Tempelmann, C. , … Northoff, G. (2011). The narcissistic self and its psychological and neural correlates: An exploratory fMRI study. Psychological Medicine, 41(08), 1641–1650. [DOI] [PubMed] [Google Scholar]
- Feng, C. , Liang, Y. , Zhou, H. , & Yi, L. (2012). Two faces of narcissism and romantic attraction: Evidence from a collectivistic culture. Psychological Reports, 111(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Finn, E. S. , Shen, X. , Scheinost, D. , Rosenberg, M. D. , Huang, J. , Chun, M. M. , … Constable, R. T. (2015). Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, J. D. , Campbell, W. K. , & Twenge, J. M. (2003). Individual differences in narcissism: Inflated self‐views across the lifespan and around the world. Journal of Research in Personality, 37(6), 469–486. [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud, S. (1957). On narcissism: An introduction (J. Strachey, Trans.). In J. Strachey. (Ed.), The Standard Edition of the Complete Psychological Works of Sigmund Freud (Vol. 14, pp. 73–102). London, England: Hogarth Press. [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Gabrieli, J. D. , Ghosh, S. S. , & Whitfield‐Gabrieli, S. (2015). Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron, 85(1), 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnambs, T. , & Appel, M. (2017). Narcissism and social networking behavior: A meta‐analysis. Journal of Personality, 86(2), 200–212. [DOI] [PubMed] [Google Scholar]
- Gong, Q. , Li, L. , Du, M. , Pettersson‐Yeo, W. , Crossley, N. , Yang, X. , … Mechelli, A. (2014). Quantitative prediction of individual psychopathology in trauma survivors using resting‐state FMRI. Neuropsychopharmacology: Official publication of the American College of. Neuropsychopharmacology, 39(3), 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, T. , Notebaert, K. , Anderl, C. , Reicherts, P. , Wieser, M. , Kopf, J. , … Windmann, S. (2015a). Reliance on functional resting‐state network for stable task control predicts behavioral tendency for cooperation. NeuroImage, 118, 231–236. [DOI] [PubMed] [Google Scholar]
- Hahn, T. , Notebaert, K. , Anderl, C. , Teckentrup, V. , Kaßecker, A. , & Windmann, S. (2015b). How to trust a perfect stranger: Predicting initial trust behavior from resting‐state brain‐electrical connectivity. Social Cognitive and Affective Neuroscience, 10(6), 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmelech, T. , & Malach, R. (2013). Neurocognitive biases and the patterns of spontaneous correlations in the human cortex. Trends in Cognitive Sciences, 17(12), 606–615. [DOI] [PubMed] [Google Scholar]
- Hepper, E. G. , Hart, C. M. , & Sedikides, C. (2014). Moving Narcissus: Can narcissists be empathic? Personality & Social Psychology Bulletin, 40(9), 1079–1091. [DOI] [PubMed] [Google Scholar]
- Hsu, W. , Rosenberg, M. , Scheinost, D. , Constable, R. , & Chun, M. (2018). Resting‐state functional connectivity predicts neuroticism and extraversion in novel individuals. Social Cognitive and Affective Neuroscience, 13(2), 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T. , Kulkarni, K. R. , Schultz, D. H. , Mill, R. D. , Chen, R. H. , Solomyak, L. I. , & Cole, M. W. (2017). Cognitive task information is transferred between brain regions via resting‐state network topology. Nature Communications, 8(1), 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowiak‐Siuda, K. , & Zajkowski, W. (2013). A neural model of mechanisms of empathy deficits in narcissism. Medical Science Monitor, 19, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauk, E. , Benedek, M. , Koschutnig, K. , Kedia, G. , & Neubauer, A. C. (2017). Self‐viewing is associated with negative affect rather than reward in highly narcissistic men: An fMRI study. Scientific Reports, 7(1), 5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske, P. , Sharifi, M. , Smallwood, J. , Dziobek, I. , & Singer, T. (2017). Where the narcissistic mind wanders: Increased self‐related thoughts are more positive and future oriented. Journal of Personality Disorders, 31(4), 553–566. [DOI] [PubMed] [Google Scholar]
- Kelsey, R. M. , Ornduff, S. R. , McCann, C. M. , & Reiff, S. (2001). Psychophysiological characteristics of narcissism during active and passive coping. Psychophysiology, 38(2), 292–303. [PubMed] [Google Scholar]
- Kong, X.‐Z. , Pu, Y. , Wang, X. , Xu, S. , Hao, X. , Zhen, Z. , & Liu, J. (2017). Intrinsic hippocampal‐caudate interaction correlates with human navigation. bioRxiv, 116129. [Google Scholar]
- Kriegeskorte, N. , Simmons, W. K. , Bellgowan, P. S. , & Baker, C. I. (2009). Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience, 12(5), 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen, S. B. , & Sandberg, K. (2017). Is whole‐brain functional connectivity a neuromarker of sustained attention? Comment on Rosenberg et al. (2016). bioRxiv:216697.
- Krizan, Z. , & Herlache, A. D. (2018). The narcissism spectrum model: A synthetic view of narcissistic personality. Personality and Social Psychology Review, 22(1), 3–31. [DOI] [PubMed] [Google Scholar]
- Kropotov, J. D. (2016). Functional neuromarkers for psychiatry: Applications for diagnosis and treatment. Cambridge, MA: Academic Press. [Google Scholar]
- LaBar, K. S. , Gatenby, J. C. , Gore, J. C. , LeDoux, J. E. , & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron, 20(5), 937–945. [DOI] [PubMed] [Google Scholar]
- Lamm, C. , Decety, J. , & Singer, T. (2011). Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54(3), 2492–2502. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. (2003). The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology, 23(4–5), 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y. , Sang, N. , Wang, Y. , Hou, X. , Huang, H. , Wei, D. , … Qiu, J. (2016). Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience, 328, 50–57. [DOI] [PubMed] [Google Scholar]
- Meier, L. L. , & Semmer, N. K. (2013). Lack of reciprocity, narcissism, anger, and instigated workplace incivility: A moderated mediation model. European Journal of Work and Organizational Psychology, 22(4), 461–475. [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. [DOI] [PubMed] [Google Scholar]
- Miller, J. D. , Lynam, D. R. , Hyatt, C. S. , & Campbell, W. K. (2017). Controversies in narcissism. Annual Review of Clinical Psychology, 13, 291–315. [DOI] [PubMed] [Google Scholar]
- Miller, J. D. , Price, J. , & Campbell, W. K. (2012). Is the Narcissistic Personality Inventory still relevant? A test of independent grandiosity and entitlement scales in the assessment of narcissism. Assessment, 19(1), 8–13. [DOI] [PubMed] [Google Scholar]
- Morf, C. C. , & Rhodewalt, F. (2001). Unraveling the paradoxes of narcissism: A dynamic self‐regulatory processing model. Psychological Inquiry, 12(4), 177–196. [Google Scholar]
- Morishima, Y. , Schunk, D. , Bruhin, A. , Ruff, C. C. , & Fehr, E. (2012). Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron, 75(1), 73–79. [DOI] [PubMed] [Google Scholar]
- Murphy, K. , Birn, R. M. , Handwerker, D. A. , Jones, T. B. , & Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage, 44(3), 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, K. , Gianotti, L. R. , & Knoch, D. (2015). A neural trait approach to exploring individual differences in social preferences. Frontiers in Behavioral Neuroscience, 8, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic, I. , Güllmar, D. , Dietzek, M. , Langbein, K. , Steinke, J. , & Gaser, C. (2015). Brain structure in narcissistic personality disorder: A VBM and DTI pilot study. Psychiatry Research: Neuroimaging, 231(2), 184–186. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Bunge, S. A. , Gross, J. J. , & Gabrieli, J. D. (2002). Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Knierim, K. , Ludlow, D. H. , Hanelin, J. , Ramachandran, T. , Glover, G. , & Mackey, S. C. (2004). Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16(10), 1746–1772. [DOI] [PubMed] [Google Scholar]
- Pessoa, L. (2008). On the relationship between emotion and cognition. Nature Reviews. Neuroscience, 9(2), 148. [DOI] [PubMed] [Google Scholar]
- Pincus, A. L. , Ansell, E. B. , Pimentel, C. A. , Cain, N. M. , Wright, A. G. , & Levy, K. N. (2009). Initial construction and validation of the Pathological Narcissism Inventory. Psychological Assessment, 21(3), 365. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. (2006). The brain's dark energy. Science (New York, N.Y.), 314(5803), 1249. [PubMed] [Google Scholar]
- Raichle, M. E. (2011). The restless brain. Brain Connectivity, 1(1), 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. (2015). The brain's default mode network. Annual Review of Neuroscience, 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Raskin, R. , & Terry, H. (1988). A principal‐components analysis of the Narcissistic Personality Inventory and further evidence of its construct validity. Journal of Personality and Social Psychology, 54(5), 890. [DOI] [PubMed] [Google Scholar]
- Ronningstam, E. (2010). Narcissistic personality disorder: A current review. Current Psychiatry Reports, 12(1), 68–75. [DOI] [PubMed] [Google Scholar]
- Ronningstam, E. (2016). Pathological narcissism and narcissistic personality disorder: Recent research and clinical implications. Current Behavioral Neuroscience Reports, 3(1), 34–42. [Google Scholar]
- Ronningstam, E. (2017). Intersect between self‐esteem and emotion regulation in narcissistic personality disorder‐implications for alliance building and treatment. Borderline Personality Disorder and Emotion Dysregulation, 4(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronningstam, E. , & Baskin‐Sommers, A. R. (2013). Fear and decision‐making in narcissistic personality disorder—a link between psychoanalysis and neuroscience. Dialogues in Clinical Neuroscience, 15, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. , Finn, E. , Scheinost, D. , Constable, R. , & Chun, M. (2017). Characterizing attention with predictive network models. Trends in Cognitive Sciences, 21(4), 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. D. , Finn, E. S. , Scheinost, D. , Papademetris, X. , Shen, X. , Constable, R. T. , & Chun, M. M. (2016). A neuromarker of sustained attention from whole‐brain functional connectivity. Nature Neuroscience, 19(1), 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze, L. , Dziobek, I. , Vater, A. , Heekeren, H. R. , Bajbouj, M. , Renneberg, B. , … Roepke, S. (2013). Gray matter abnormalities in patients with narcissistic personality disorder. Journal of Psychiatric Research, 47(10), 1363–1369. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X. , Finn, E. S. , Scheinost, D. , Rosenberg, M. D. , Chun, M. M. , Papademetris, X. , & Constable, R. T. (2017). Using connectome‐based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols, 12(3), 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X. , Papademetris, X. , & Constable, R. T. (2010). Graph‐theory based parcellation of functional subunits in the brain from resting‐state fMRI data. Neuroimage, 50(3), 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X. , Tokoglu, F. , Papademetris, X. , & Constable, R. T. (2013). Groupwise whole‐brain parcellation from resting‐state fMRI data for network node identification. Neuroimage, 82, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, T. , Seymour, B. , O'doherty, J. , Kaube, H. , Dolan, R. J. , & Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–1162. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Laird, A. R. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, A. Z. , & Raichle, M. E. (2012). A brief history of the resting state: The Washington University perspective. Neuroimage, 62(2), 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschanz, B. T. , Morf, C. C. , & Turner, C. W. (1998). Gender differences in the structure of narcissism: A multi‐sample analysis of the Narcissistic Personality Inventory. Sex Roles, 38, 863–870. [Google Scholar]
- Twenge, J. M. , & Campbell, W. K. (2003). Isn't it fun to get the respect that we're going to deserve?” Narcissism, social rejection, and aggression. Personality and Social Psychology Bulletin, 29(2), 261–272. [DOI] [PubMed] [Google Scholar]
- Twenge, J. M. , Konrath, S. , Foster, J. D. , Keith Campbell, W. , & Bushman, B. J. (2008). Egos inflating over time: A cross‐temporal meta‐analysis of the Narcissistic Personality Inventory. Journal of Personality, 76(4), 875–902. [DOI] [PubMed] [Google Scholar]
- Watson, P. , Grisham, S. O. , Trotter, M. V. , & Biderman, M. D. (1984). Narcissism and empathy: Validity evidence for the Narcissistic Personality Inventory. Journal of Personality Assessment, 48(3), 301–305. [DOI] [PubMed] [Google Scholar]
- Watson, P. , & Morris, R. J. (1991). Narcissism, empathy and social desirability. Personality and Individual Differences, 12(6), 575–579. [Google Scholar]
- Wink, P. (1991). Two faces of narcissism. Journal of Personality and Social Psychology, 61(4), 590. [DOI] [PubMed] [Google Scholar]
- Yan, C.‐G. , Cheung, B. , Kelly, C. , Colcombe, S. , Craddock, R. C. , Di Martino, A. , … Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.‐G. , Wang, X.‐D. , Zuo, X.‐N. , & Zang, Y.‐F. (2016). DPABI: Data processing & analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Yang, W. , Cun, L. , Du, X. , Yang, J. , Wang, Y. , Wei, D. , … Qiu, J. (2015). Gender differences in brain structure and resting‐state functional connectivity related to narcissistic personality. Scientific Reports, 5(1), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , & Westfall, J. (2016). Choosing prediction over explanation in psychology: Lessons from machine learning. Unpublished manuscript. Retrieved from http://jakewestfall. org/publications/Yarkoni_Westfall_choosing_prediction. pdf. [DOI] [PMC free article] [PubMed]
- Zhou, H. , Li, Y. , Zhang, B. , & Zeng, M. (2012). The relationship between narcissism and friendship qualities in adolescents: Gender as a moderator. Sex Roles, 67(7–8), 452–462. [Google Scholar]
- Zhou, H. , Zhang, B. , Chen, L. , & Ye, M. (2009). Development and validation of narcissistic personality inventory for Chinese. Chinese Journal of Clinical Psychology, 17, 5–7. [Google Scholar]
- Zhou, H. , Zhang, B. , Yang, X. , & Chen, X. (2015). Are Chinese narcissists disagreeable? Evidence from self‐ and peer‐ratings of agreeableness. Asian Journal of Social Psychology, 18(2), 163–169. [Google Scholar]
- Zuo, X.‐N. , Di Martino, A. , Kelly, C. , Shehzad, Z. E. , Gee, D. G. , Klein, D. F. , … Milham, M. P. (2010). The oscillating brain: Complex and reliable. Neuroimage, 49(2), 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


