Abstract
Specialization of the auditory cortices for pure tone listening may develop with age. In adults, the right hemisphere dominates when listening to pure tones and music; we thus hypothesized that (a) asymmetric function between auditory cortices increases with age and (b) this development is specific to tonal rather than broadband/non‐tonal stimuli. Cortical responses to tone‐bursts and broadband click‐trains were recorded by multichannel electroencephalography in young children (5.1 ± 0.8 years old) and adolescents (15.2 ± 1.7 years old) with normal hearing. Peak dipole moments indicating activity strength in right and left auditory cortices were calculated using the Time Restricted, Artefact and Coherence source Suppression (TRACS) beamformer. Monaural click‐trains and tone‐bursts in young children evoked a dominant response in the contralateral right cortex by left ear stimulation and, similarly, a contralateral left cortex response to click‐trains in the right ear. Responses to tone‐bursts in the right ear were more bilateral. In adolescents, peak activity dominated in the right cortex in most conditions (tone‐bursts from either ear and to clicks from the left ear). Bilateral activity was evoked by right ear click stimulation. Thus, right hemispheric specialization for monaural tonal stimuli begins in children as young as 5 years of age and becomes more prominent by adolescence. These changes were marked by consistent dipole moments in the right auditory cortex with age in contrast to decreases in dipole activity in all other stimulus conditions. Together, the findings reveal increasingly asymmetric function for the two auditory cortices, potentially to support greater cortical specialization with development into adolescence.
Keywords: beamformer, development, EEG, evoked response, hemispheric specialization, right dominance, source localization, tonal sound
1. INTRODUCTION
The aim of this study was to examine the development of functional asymmetry between the two hemispheres of the human auditory brain. In normal hearing adults, specialization of both anatomy and function in each of the two hemispheres supports listening to language and other complex sounds (Bishop, 2013; Toga & Thompson, 2003). Different roles for each hemisphere are evidenced by the predominant processing of speech and music recognition in the left and right auditory cortices, respectively (Johnsrude, Penhune, & Zatorre, 2000; Zatorre & Binder, 2000; Zatorre & Halpern, 1993). This organization appears to facilitate processing of complex auditory input so that it is detected and understood efficiently for rapid response (Zatorre, Belin, & Penhune, 2002). Complex sound processing, such as music perception using tonal information, is not fully developed until adolescence. For example, sensitivity to harmonic sounds improves from childhood through until adolescence (Costa‐Giomi, 2003), and older children increasingly rely on pitch rather than tempo cues to judge happy versus sad music (Giannantonio, Polonenko, Papsin, Paludetti, & Gordon, 2015; Peretz, Gagnon, & Bouchard, 1998). Thus, it is possible that hemispheric asymmetry of auditory cortices also undergoes development with age.
Different computations of the left versus right auditory cortices for temporal versus spectral features of sound are thought to underlie functional hemispheric asymmetry in the auditory cortices. Temporal acoustic cues of the speech envelope are essential for perception of speech (Shannon, Zeng, Kamath, Wygonski, & Ekelid, 1995), while spectral information of music is important for tonal pitch perception (Zatorre et al., 2002). Different processing of these temporal and spectral sound features, which are the basic properties of sound, are first observed in the auditory brainstem (Escabı & Schreiner, 2002; Van Gisbergen, Grashuis, Johannesma, & Vendrik, 1975). Temporal information is maintained by high fidelity afferent synapses from the anteroventral cochlear nucleus (Griffiths, Uppenkamp, Johnsrude, Josephs, & Patterson, 2001), whereas spectral information can be coded by slower responding neurons such as in the dorsal cochlear nucleus (Reiss, Bandyopadhyay, & Young, 2007). This early stage of preferential processing of temporal versus spectral features of sound continues in the cortex, as temporal features evoke stronger responses in the left auditory cortex whereas spectral features more strongly activate the right auditory cortex. This has been called the spectral‐temporal trade‐off model (Jamison, Watkins, Bishop, & Matthews, 2006; Okamoto, Stracke, Draganova, & Pantev, 2009; Schonwiesner, Rubsamen, & von Cramon, 2005; Zatorre & Belin, 2001). Considering that Fourier transforms require short time windows for high temporal resolution but long time windows for fine spectral resolutions, it is plausible that cortical processing optimized for the temporal domain may be achieved at the expense of the spectral domain and vice versa. Therefore, the left and right auditory cortices may adopt short and long time windows for processing complex sounds to enhance temporal and spectral resolutions, respectively (Zatorre et al., 2002). Focusing on cortical processing of temporal envelopes in speech, the multi‐resolution model postulates that similar short and long temporal integration windows are used to extract fast phoneme rate modulations around 40 Hz in the left hemisphere and slow syllable rate modulations around 4 Hz in the right hemisphere, respectively (Boemio, Fromm, Braun, & Poeppel, 2005; Poeppel, 2003; Poeppel, Idsardi, & van Wassenhove, 2008). Both phonemes and syllables are important factors for speech perception and in this model, the left and right auditory cortices concurrently analyze temporal cues related to phonemes and syllables, respectively.
Some degree of hemispheric asymmetry likely already exists in infancy. Early left hemispheric specialization for meaningful speech sounds versus backward speech (i.e., non‐meaningful) sounds has been observed in infants (Dehaene‐Lambertz, Dehaene, & Hertz‐Pannier, 2002; Peña et al., 2003). Moreover, cortical processing of prosodic information in speech sounds was found to lateralize to the right hemisphere in infants (Homae, Watanabe, Nakano, Asakawa, & Taga, 2006). Consistent with the multi‐time‐resolution model, the right hemisphere in infants appears to preferentially process slow syllable rate modulations (Telkemeyer et al., 2009; Telkemeyer et al., 2011). In combination, these findings are consistent with the high capacity of native language acquisition within the first year of life (Jusczyk, 2000; Kuhl, 2004). On the other hand, cortical processing for fast phoneme rate modulations becomes increasingly left‐lateralized (Bishop, 2013; Minagawa‐Kawai, Cristià, & Dupoux, 2011), which suggests immaturity relative to the well‐established adult pattern of hemispheric asymmetries to auditory input.
Most previous studies used binaural sound stimuli to investigate hemispheric specialization; however, in this study, we evaluated hemispheric specialization using monaural stimuli. Recently, we reported that monaural tone‐burst stimuli evoked right hemisphere dominant activation for both left and right ear stimulation in 16 adolescents with normal hearing (average age of 15.9 ± 6.4 years old) (Jiwani, Papsin, & Gordon, 2016), which indicates that monaural stimulation reveals cortical asymmetries in normal hearing adolescents. By contrast, the same monaural tone‐burst stimuli evoked larger responses in the auditory cortex contralateral to the ear of stimulation in a cohort of seven younger children with normal hearing (average age of 11.0 ± 2.2 years old) (Gordon, Wong, & Papsin, 2013). These cohorts were control groups in studies evaluating cortical development in deaf children after cochlear implantation in response to tone‐bursts. A more robust study was therefore warranted to investigate development of hemispheric lateralization.
In this study, development of right hemispheric specialization to tone‐bursts was compared to broadband click‐train stimuli in a group of young children (3–6 years) and adolescents (12–17 years old). We focused on these age groups because previous histological (Harris & Shepherd, 2015; Moore & Guan, 2001; Moore & Linthicum, 2007) and electrophysiological studies (Moore & Linthicum, 2007; Pang & Taylor, 2000; Ponton, Eggermont, Khosla, Kwong, & Don, 2002) provide evidence for maturation of cortical networks important for hemispheric specialization between 6 and 12 years of age in the human auditory cortex. Auditory thalamo‐cortical afferents included in this network are structurally mature between 3 and 5 years of age, while intrahemispheric and trans‐callosal interhemispheric networks, mediated by pyramidal neurons in the superficial layers II and III, develop between 6 and 12 years of age (Harris & Shepherd, 2015; Moore & Guan, 2001; Moore & Linthicum, 2007). Functional maturation of the intra‐ and interhemispheric networks in the auditory cortex results in an emergence of a negative reflection at around 100 ms (an N1 wave) in cortical auditory evoked potentials (CAEP) between 7 and 12 years of age (Moore & Linthicum, 2007; Pang & Taylor, 2000; Ponton et al., 2002). It is plausible that these changes in cortical circuits underlie the aforementioned development of sensitivity to sounds with harmonic and tonal information between young children and adolescents (Costa‐Giomi, 2003; Giannantonio et al., 2015; Peretz et al., 1998).
We hypothesized that (a) asymmetric function between auditory cortices would increase with age and (b) this development would be specific to tonal rather than broadband/non‐tonal stimuli. Results revealed (a) an early (by 5 years of age) dominance of the right auditory cortex to monaural 500 Hz tone‐bursts but not broadband clicks and (b) development of hemispheric asymmetry into adolescence driven by activity which remains consistent in the right auditory cortex.
2. MATERIALS AND METHODS
2.1. Participants
Eleven children (Child Group; mean age ± SD: 5.1 ± 0.8 years old, 4 girls and 7 boys) and 21 adolescents (Adolescent Groups; 15.2 ± 1.7 years old, 13 girls and 8 boys) participated in the study. Cortical responses were evoked by both click‐train and tone‐burst stimuli in all 11 children of the Child Group and in six of the Adolescent Groups (Table 1).
Table 1.
Participants
| Click‐trains | Tone‐bursts | Total | ||||
|---|---|---|---|---|---|---|
| Number (F:M) | Age (years old) | Number (F:M) | Age (years old) | Number (F:M) | Age (years old) | |
| Child Group | 11 (4:7) | 5.1 ± 0.8 | 11 (4:7) | 5.1 ± 0.8 | 11 (4:7) | 5.1 ± 0.8 |
| Adolescent Groups | 15 (11:4) | 15.4 ± 1.8 | 12 (6:6) | 14.9 ± 1.7 | 21 (13:8) | 15.2 ± 1.7 |
| (Adolescent‐click Group) | (Adolescent‐tone Group) | |||||
Note. F = female; M = male.
Cortical responses were recorded in another nine adolescents in response to click‐trains alone and in six adolescents in response to tone‐bursts alone. Data from the latter 6 adolescents were included in a previous study (Jiwani et al., 2016). In total, there were 15 adolescent participants in the click‐train condition (Adolescent‐click Group) and 12 adolescent participants in the tone‐burst condition (Adolescent‐tone Group). None of the participants reported past history of otological or neurological diseases. All participants were right‐handed except for one in the Child Group and one in the Adolescent‐tone Group. The study protocol was approved by the Hospital for Sick Children's Research Ethics Board.
2.2. Multichannel recording of cortical responses
Cortical responses were evoked by 36 ms long tone‐bursts (500 Hz) and click‐trains (100 µs clicks presented at a rate of 250 clicks/s) delivered at 1 Hz by ER3–14A insert earphones (Etymotic Research, Elk Grove Village, IL) to each ear separately. Stimuli were presented at 50 dB above behavioral threshold (dB SL) and the order of click and tone‐burst presentation was counter balanced. As in previous studies (Easwar, Yamazaki, Deighton, Papsin, & Gordon, 2017a, 2017b), EEG responses from 62 cephalic electrodes referenced to the right earlobe were recorded using NeuroScan v4.5 with a Synamps‐II amplifier during passive listening (Compumedics USA, Inc., Charlotte, NC). Participants watched a silent subtitled movie, read a book, or played games on a tablet with minimal movement. The sampling rate for cortical recording was 1,000 Hz and an online bandpass filter between 0.15 and 100 Hz was used. Epochs with amplitudes over ±100 μV at a vertex cephalic electrode (Cz) were rejected. For each condition, a minimum of 250 accepted sweeps with at least two visually replicable response traces were obtained.
2.3. Source localization of cortical evoked peaks
The Time Restricted, Artefact and Coherence source Suppression (TRACS) beamformer (Wong & Gordon, 2009) was used to estimate and localize dipole activity underlying each peak in the cortical responses (Figure 1). The TRACS beamformer was developed from Linearly Constrained Minimum Variance (LCMV) beamformers, a class of adaptive spatial filters that localize sources of interest by minimizing the contributions of other uncorrelated sources. Coherent suppression methods were applied to analyze auditory evoked cortical responses. The EEG waveforms at Cz (earlobe reference) and the corresponding global field power (GFP; standard deviation of the potentials at all electrodes) were used to identify each peak. The near‐baseline amplitude before and after each GFP peak was used to define the beginning and ending boundaries, respectively, of the time window used to locate cortical activity underlying each peak. As the immature first peak, P1, which we call iP1 in this study, gradually changes to a P1–N1–P2 complex during childhood and adolescence (Pang & Taylor, 2000), time windows were determined to contain the iP1 peak in the Child Group and one of P1, N1, and P2 peaks in the Adolescent Groups. Beginning and end times and length of each iP1, P1, N1, and P2 time window are shown in Supporting Information, Table 1.
Figure 1.
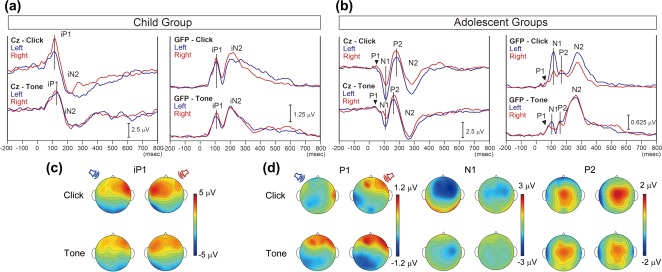
Mean cortical auditory evoked potentials (CAEP) at the vertex (Cz) and global field power (GFP) for the Child Group and across the two Adolescent Groups are shown in (a) and (b), respectively. CAEP and GFP waveforms evoked by stimulation of the left or right ear are depicted in blue and red lines, respectively. The waveforms evoked by click‐trains are shown in the top row. In the Child Group, monaural click‐trains and tone‐bursts evoked immature CAEP waveforms at Cz consisting of iP1 and iN2 peaks. Across the Adolescent Groups, mean Cz waveforms evoked by click‐trains and tone‐bursts exhibited mature CAEP waveforms containing 2 positive (P) and 2 negative (N) peaks: P1, N1, P2, and N2. In all groups, GFP waveforms showed peaks corresponding to the each peak in the Cz waveform. (c) Topographic maps at the iP1 peak latency. (d) Topographic maps at P1, N1, and P2 peak latencies in the Adolescent Groups. Tone‐evoked surface potentials are similar between the stimulated ears at all peak latencies, while click‐trains evoked different surface potential patterns between the stimulated ears
Details of the TRACS beamformer analysis have been described previously (Gordon et al., 2013; Jiwani et al., 2016; Wong & Gordon, 2009). In brief, the TRACS beamformer estimates the neuronal activity (dipole moments and latencies) in each of ∼64,000 brain spaces (3 × 3 × 3 mm voxels) from the multichannel surface EEG data with an averaged electrode‐reference. To increase accuracy of the computation of lead potentials for each dipole, a three‐layer boundary element model mesh was created to simulate the geometry and conductivities of the brain, skull, and scalp. Age‐appropriate head model template MRIs were made using the template‐O‐matic toolbox (Wilke, Holland, Altaye, & Gaser, 2008). To normalize the signal‐to‐noise ratio of each voxel, a pseudo‐Z statistic was obtained by dividing the sample signal mean by the standard deviation of the prestimulus activity between −200 and −80 ms (Vrba & Robinson, 2001). Then, a one‐tailed omnibus t test (Petersson, Nichols, Poline, & Holmes, 1999) was used to calculate a statistical threshold pseudo‐Z value (p ≤ .0005), the omnibus value, which indicated baseline brain activity and residual noise. Omnibus values were not significantly different across ears and conditions between young children and adolescents for either stimulus condition (click‐trains: F(1, 24) = 0.28, p = .60, partial η2 = 0.01; tone‐bursts: F(1, 21) = 0.87, p = .36, partial η2 = 0.04).
To focus on the brain responses above baseline activity, we used post‐omnibus pseudo‐Z values (POPZs) calculated by subtracting the omnibus value from the corresponding pseudo‐Z value in each voxel. When the pseudo‐Z value was smaller than the omnibus value, the POPZ was defined as zero. In a pseudo‐Z map, POPZs from all voxels were plotted into an axial image on the age‐appropriate head model templates to visualize cortical activity over the whole brain, with dark red indicating highest signal‐to‐noise ratios and blue indicating baseline cortical activity (POPZ of zero). The range of peak POPZ was similar in the young group of children as in adolescents (peak POPZ range = 0.28–0.39 in the young group (Figure 2a) and 0.10–0.30 in the adolescents (Figure 3a)).
Figure 2.
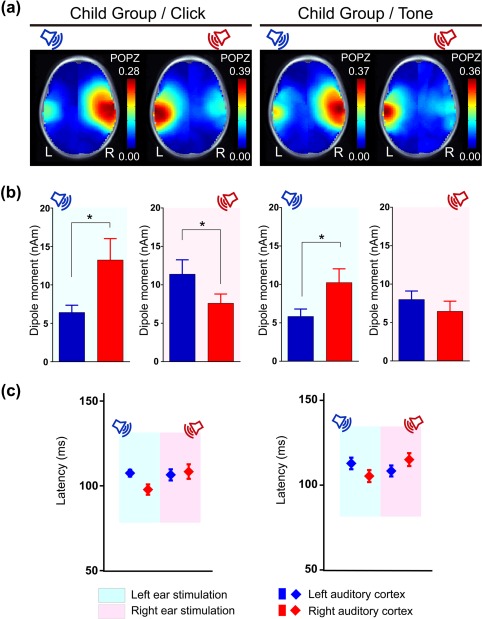
(a) Grand average pseudo‐Z maps of the 11 participants in the Child Group for the iP1 time window indicate the strongest cortical activity in the auditory cortex contralateral to the stimulated ear for left and right monaural click‐trains and tone‐bursts. (b) Significantly larger dipole moments were measured in the auditory cortex contralateral to the stimulated ear for click‐trains (left ear stimulation: p = .03; right ear stimulation: p = .035). For tone‐bursts, contralateral activity was higher than ipsilateral for left ear stimulation (p = .03) but activity was bilateral (not significantly different between left and right) for right ear presentations (p = .283) (*p < .05). (c) Peak dipoles occurred at earlier latencies in the right than left hemisphere for stimuli (collapsed) presented from the left ear (p = .04). By contrast, latency was similar between hemispheres for right ear stimuli (p = .298)
Figure 3.
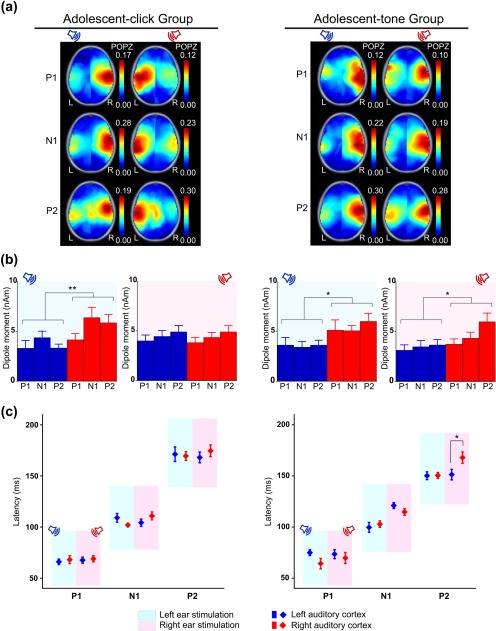
(a) Grand average pseudo‐Z maps of cortical activity indicate that monaural click‐trains evoked contralateral dominant activation in adolescents whereas tone‐bursts evoked right hemispheric dominant activation, regardless of ear stimulated in a similar aged cohort. (b) Click‐train stimulation of the left ear evoked significantly increased activity in the right than left auditory cortex (p = .002), whereas right ear stimulation evoked bilaterally symmetric cortical activation (p = .332). Averaged across three time windows, dipole moments were significantly larger in the right auditory cortex than the left auditory cortex in response to tone‐bursts to either ear (left ear stimulation: p = .014, right ear stimulation: p = .026). (c) No significant effects on latencies were found bar longer latencies in the right cortex ipsilateral to right tone‐burst stimulation during the P2 time window (p = .018) (*p < .05, **p < .01)
Similar to our previous studies using the TRACS beamformer (Gordon et al., 2013; Jiwani et al., 2016), voxels with the highest POPZ were selected from the left and right auditory cortices for each child and the dipole moments and latency of these voxels were used for further analyses. This procedure is consistent with previous EEG and magnetoencephalography (MEG) studies (Ackermann, Hertrich, Mathiak, & Lutzenberger, 2001; Jin, Ozaki, Suzuki, Baba, & Hashimoto, 2008; Mäkelä et al., 1993; Ponton et al., 2002; Woldorff et al., 1999).
2.4. Comparison of peak dipole moments and latencies between left and right hemispheres
Peak dipole moments and corresponding latencies were identified in left and right auditory cortices as defined by MNI coordinates: X < −55, −35 < Y < 5, −10 < Z < 20 mm (left) and 55 < X, −35 < Y < 5, −10 < Z < 20 mm (right) (Jiwani et al., 2016). Ten voxels within each area with the highest POPZ from each participant were averaged for analyses.
Lateralization of the evoked cortical activity in each participant was quantified using cortical lateralization scores (CL scores) for each peak evoked by either click‐trains or tone‐bursts. CL scores, which range from −100 to 100%, were calculated using the following formula: [(RH‐DipM − LH‐DipM)/(RH‐DipM + LH‐DipM)] × 100, where RH = right hemisphere, LH = left hemisphere, DipM = mean of top ten peak dipole moment. Positive and negative CL scores indicate rightward and leftward lateralization of cortical activity, respectively. CL scores were not calculated for the P1 time window for 4 and 2 participants in the Adolescent‐click and Adolescent‐tone Groups, respectively, who had no voxels in either left or right auditory cortices containing POPZs >0. Dipole moments and dipole latencies obtained in these conditions were not included for statistical analysis. On the other hand, CL scores were calculated if activity above the omnibus value was detected in either hemisphere. In this latter case, the averaged peak dipole moment and latency from the 10 highest pseudo‐Z values (although below omnibus) were chosen in the hemisphere with weaker activity to calculate CL scores.
2.5. Statistics
Peak dipole moments and dipole latencies were evaluated using three‐way repeated measures analyses of variance (ANOVA) to determine the within‐subject effects of (a) stimulus type, stimulated ear and hemisphere side for the Child Group and (b) time window, stimulated ear and hemisphere for the two Adolescent Groups (listening to click‐train or tone‐burst stimuli). Post‐hoc analyses included paired t tests corrected for multiple comparison bias using false discovery rate (FDR) (Benjamini & Hochberg, 1995). CL scores were analysed using (a) two‐way repeated measures ANOVA to evaluate the effects of stimulus type and stimulated ear in the Child Group and (b) three‐way mixed ANOVA to assess the within‐subject factors of response peak and stimulated ear and the between‐subject factor of the two Adolescent Groups. A one sample t test was conducted between 0 and CL scores in each condition to evaluate whether cortical activity significantly lateralized to either hemisphere (p < .05). To assess chronological changes in dipole moments in the left and right auditory cortices for each stimulus condition, an exponential regression analysis was performed using Y = A × e B*X, where Y was the dipole moment and X was the participant age in years. Exponential regression was used because the auditory cortex should continue to be active to sound with age (i.e., dipole moment would never reach nil). In total, there were 24 regression analyses (2 stimulus types × 3 combinations of time windows [iP1‐P1, iP1‐N1, and iP1‐P2] × 2 stimulated ears × 2 hemisphere sides). FDR‐corrected p values <.05 were considered significant. SPSS Statistics v.23 was used for statistical analyses (IBM Corp, Somers, NY, USA).
3. RESULTS
3.1. Cortical immaturity in the Child Group is evident in surface potentials
In the Child Group, monaural click‐trains and tone‐bursts evoked immature cortical waveforms at Cz, characterized by iP1 and immature N2 (iN2) peaks (Figure 1a). In the two Adolescent Groups, mean cortical responses evoked by click‐trains and tone‐bursts exhibited a mature pattern similar to adults (Pang & Taylor, 2000) at Cz with 2 positive and 2 negative peaks: P1, N1, P2, and N2 (Jiwani et al., 2016) (Figure 1b). In both Child and Adolescent Groups, the GFP waveforms showed peaks corresponding to each of the peaks in the Cz waveform. In the Child Group, iP1 activity was largest in the contralateral fronto‐temporal electrodes for all conditions bar the tone‐bursts presented from the right ear, which exhibited more bilateral fronto‐temporal activity (Figure 1c). By contrast, in the Adolescent Groups, responses were less distinct between the two monaural conditions for either click‐train or tone‐burst stimuli (upper vs lower plots of Figure 1d, respectively). In the Adolescent Groups, topographic maps showed different activation patterns between P1, N1, and P2 peaks. Tone‐evoked surface potential patterns were similar between the stimulated ears at all peak latencies, while click‐trains evoked different surface potential patterns between the stimulated ears. At the P1 peak, positive potential at the right frontal region and negative potential at the left temporal‐occipital region was evoked in all conditions except for left click‐trains, which evoked positive potentials at right temporal recording electrodes. At the N1 and P2 peaks, activity (negative and positive, respectively) was evident at central electrodes in all conditions. Click‐evoked surface potential patterns, however, indicated that N1 and P2 potentials showed a larger spread of activity for left and right ear stimulation, respectively, compared to the opposite ear stimulation (Figure 1d).
3.2. Tone‐bursts from the right ear show bilateral rather than contralateral activation of auditory cortices in young children
Figure 2a shows the grand mean of POPZs evoked by monaurally presented click‐trains (left column) and tone‐bursts (right column) in the Child Group of 11 children. During the iP1 time window, distributions of hot spots (i.e., regions of high POPZs) indicate predominant contralateral activation for both click‐train and tone‐burst stimuli.
Figure 2b indicates mean ± 1 standard error (SE) of dipole moments, which differed according to stimulus type, stimulated ear and hemisphere (3‐way interaction: F(1, 10) = 5.73, p = .038, partial η2 = 0.364). Specifically, peak dipole moments showed dominant contralateral activation for either ear stimulation in response to click‐trains (mean right‐left hemispheric difference: left ear = 6.83 nAm, 95% CI [2.03, 11.62], t(10)= 10.07, p = .03; right ear = −3.81 nAm, 95% CI [−7.07 −0.55,], t(10)= 6.76, p = .035). This was found for left ear stimulation in response to tone‐bursts but, by contrast, not for the right ear stimulation (mean right–left hemispheric difference: left ear = 4.41 nAm, 95% CI [1.07, 7.75], t(10)= 8.65, p = .03; right ear = −1.54 nAm, 95% CI [−4.55, 1.48], t(10)= 1.29, p = .283).
Peak dipole latencies, plotted in Figure 2c, differed by stimulus (F(1, 10) = 8.16, p = .017, partial η2 = 0.449), and by stimulated ear and hemisphere (2‐way interaction: F(1, 10) = 5.17, p = .046, partial η2 = 0.341). Specifically, dipole latencies were significantly shorter for click‐trains than for tone‐bursts (mean difference = −5.42 ms, 95% CI [−9.65, −1.92], t(10) = 8.16, p = .017). Averaged across stimulus type, contralateral dipole latencies were significantly shorter relative to ipsilateral peak latencies for left stimulation (mean right‐left hemispheric difference = −8.58 ms, 95% CI [−15.49, −1.67], t(10)= 7.65, p = .04) but not for right stimulation (mean right–left hemispheric difference = 4.31 ms, 95% CI [−4.44, 13.05], t(10)= 1.21, p = .298).
In summary, contralateral activity showed stronger activity at shorter latencies when evoked by either click‐trains or tone‐bursts from the left ear of young children. While the same contralateral dominant pattern was evoked by click‐trains presented to the right ear, the contralateral dominance was not evident for tone‐bursts presented to the right ear.
3.3. Dominance of right auditory cortex continues to develop into adolescence
Pseudo‐Z maps derived from responses evoked by both click‐trains and tone‐bursts are plotted for both Adolescent Groups (Figure 3a). Higher POPZs in the contralateral temporal cortices were evident in adolescents during all 3 time windows listening to click‐trains (similar to results in the Child Group shown in Figure 2a). By contrast, activity was greater in the right temporal area for tone‐bursts presented to either ear during all 3 time windows. This was confirmed by mean ± 1 SE dipole moments in these groups of adolescents (Figure 3b). In the group listening to click‐trains, dipole moments differed by time window and stimulated ear (2‐way interaction: F(2, 22) = 7.60, p = .040, partial η2 = .254) and by stimulated ear and hemisphere (2‐way interaction: F(1, 11) = 29.70, p = .002, partial η2 = .613). We focused on the latter 2‐way interaction to evaluate hemispheric differences in peak dipole moment. Averaged across time windows, a significant increase in contralateral dipole moments was found for left ear stimulation (mean right–left hemispheric difference = 1.47 nAm, 95% CI [0.71, 2.23], t(11) = 18.10, p = .002), but not right ear stimulation (mean right–left hemispheric difference = −0.35 nAm, 95% CI [−1.11, 0.41], t(11) = 1.03, p = .332) (Figure 3b).
Dipole moments in the Adolescent Group listening to tone‐bursts were also affected by stimulated ear and hemisphere (2‐way interaction: F(1, 9) = 5.69, p = .041, partial η2 = 0.387), and by time window (F(2, 18) = 4.45, p = .027, partial η2 = 0.331). Dipole moments were assessed across time windows to focus on hemispheric differences, revealing a contralateral dominance for left stimulation (mean right–left hemispheric difference = 1.94 nAm, 95% CI [0.69, 3.19], t(9) = 12.29, p = .014) similar to adolescents listening to click‐trains. However, this group showed an ipsilateral dominance for right tone‐burst stimulation (mean right–left hemispheric difference = 1.38 nAm, 95% CI [0.21, 2.54], t(9) = 7.11, p = .026) (Figure 3b).
Figure 3c shows the mean ± 1 SE dipole latencies during each P1, N1, and P2 time windows in the Adolescent Groups listening to click‐trains (left panel) and tone‐bursts (right panel). For click‐trains, latencies differed in each time window as expected (F(2, 22) = 297.50, p < .001, partial η2 = 0.964), but did not change with stimulated ear (F(1, 11) = 0.096, p = .762, partial η2 = 0.009) or show hemispheric asymmetries (F(1, 11) = 1.88, p = .198, partial η2 = 0.146) (Figure 3c). Latencies to tone‐bursts, however, differed between time window, stimulated ear and hemisphere (3‐way interaction: F(2, 18) = 4.75, p = .022, partial η2 = 0.345). For most time windows, hemispheric differences in dipole latencies were minimal, with the exception of one significantly later ipsilateral peak dipole in the right versus left hemisphere during the P2 time window for right ear tone‐burst stimulation (mean right–left hemispheric difference = 16.98 ms, 95% CI [7.21, 26.75], t(10)= 15.46, p = .018). Details of post‐hoc testing are provided in Supporting Information, Table 2.
In summary, click‐trains and tone‐bursts presented to the left ears of adolescents evoked stronger dipole activity in the contralateral right than ipsilateral left auditory cortex, as also measured in the Child Group. In contrast to the Child Group, however, dipoles evoked by right ear tone‐burst stimulation were greater in magnitude (averaged across P1, N1, and P2 time windows) and later (during the P2 time window) in the ipsilateral right auditory cortex than the contralateral left auditory cortex in adolescents. In adolescents listening to click‐trains, more symmetric activity was detected in response to right than left ear stimulation.
3.4. Developmental changes in hemispheric asymmetry
CL scores were calculated for both left and right ear stimulation for each participant, as shown in Figure 4a. In the Child Group, CL scores evoked by left ear stimulation (blue triangles) were more positive (i.e., toward the contralateral right hemisphere) than when evoked by right ear stimulation (red circles) during the iP1 time window. There was only one exception (1 child in the tone‐burst condition only). Thus, there was a strongly consistent pattern for opposite lateralization of cortical activity evoked by the two ears. Moreover, most of the 11 young children (9 in the click‐train condition and 7 in the tone‐burst condition) showed contralateral dominant responses from both ears. Some children had slightly ipsilateral lateralization from the right ear along with more exaggerated lateralization to the right cortex from the left ear (2 children in response to click‐trains and tone‐bursts, 1 child in response to tone‐bursts only). A more varied pattern of cortical lateralization from each ear was found in the adolescents. Often tone‐bursts from both ears showed right hemispheric dominance (positive values) (4 of 10 (40%), 8 of 12 (66.7%), and 9 of 12 participants (75.0%) during P1, N1, and P2 time windows, respectively).
Figure 4.
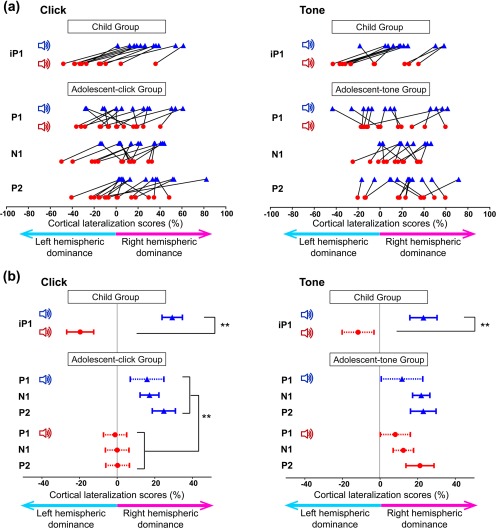
(a) In all participants (Bar 1) in the Child Group, cortical lateralization (CL) scores (100 × (dipole moment in right auditory cortex − dipole moment in left auditory cortex)/sum of right and left dipole moments) evoked by left ear stimulation are more positive (increased activity in the right auditory cortex) than by right ear stimulation. This is true for both click‐trains and tone‐bursts. This pattern is more variable in the Adolescent Groups. (b) Mean ± 1 SE CL values are shown for all time windows in the Child Group and both Adolescent Groups; solid lines are significantly different from 0 whereas dashed lines are not significant. In the Child Group, the CL score was significantly more positive (increased activity in right hemisphere) when evoked by the left than right ear stimulation during the iP1 time window (**p < .01). In adolescents listening to tone‐bursts, CL scores were significantly more positive when evoked by stimulation to the left than right ear (**p < .01), but this difference was not significant in the adolescents listening to tone‐bursts
Mean ± 1 SE CL values are shown for each time window in Figure 4b. CL values in the Child Group (iP1 time window) differed by stimulus and ear stimulated (2‐way interaction: F(1, 10) = 5.28, p = .044, partial η2 = 0.346); CL scores were significantly more positive (rightward lateralization) when stimulated from the left ear than the right ear in both click‐train and tone‐burst conditions (click‐trains: mean right–left difference = 48.91, 95% CI [38.18, 59.64], t(10) = 103.19, p < .001; tone‐bursts: mean right–left difference = 36.17, 95% CI [22.25, 50.09], t(10) = 33.53, p < .001). There was no significant difference between CL scores evoked by click‐trains and tone‐bursts in the Child Group (left stimuli: mean click‐tone difference = 4.80, 95% CI [−3.891, 13.49], t(10) = 1.51, p = .247; right stimuli: mean click‐tone difference = −7.94, 95% CI [−20.43, 4.56], t(10) = 2.00, p = .187). In the Adolescent Groups, CL scores differed between group and stimulated ear (2‐way interaction: F(1, 20) = 7.35, p = .013, partial η2 = 0.269), but not by time window (F(2, 40) = 0.821, p = .447, partial η2 = 0.113). Averaged across time windows, right hemispheric dominance (positive CL values) was greater for left than right click‐train stimulation (mean right‐left difference = 18.84, 95% CI [11.42, 6.26], t(20) = 28.08, p < .001), but similar for tone‐bursts presented to either ear (mean difference between ears = 4.54, 95% CI [−3.59, 12.66], t(20) = 1.36, p = .257). Also across time windows, there were no significant stimulus (click‐train group versus tone‐burst group) differences for left ear stimulation (mean click‐tone difference = −1.29, 95% CI [−14.87, 12.30], t(20) = 0.39, p = .845) but there was a trend for more ipsilaterally lateralized responses from the right ear for tone‐bursts versus click‐trains (mean click‐tone difference = −15.59, 95% CI [−30.12, −1.05], t(20) = 5.00, p = .074).
The extent of lateralization was assessed using one sample t‐tests between 0 and CL scores for each time window. Solid and dotted error bars in Figure 4b indicate CL scores as significantly or not significantly different from 0, respectively. During the iP1 time window in the immature response of the Child Group, significant contralateral hemispheric lateralization was detected in all conditions except for right tone‐burst stimulation (mean difference from 0 = −11.72, 95% CI [−30.59, 7.15], t(10) = −1.38, p = .287). In the Adolescent Groups, significant right hemispheric lateralization was revealed during N1 and P2 in response to left click‐train and tone‐burst stimulations, and during P2 upon right tone‐burst stimulation. Statistical details of the other conditions are provided in Supporting Information, Table 3.
In summary, left ear stimulation evoked a contralateral dominant pattern of cortical responses to both click‐trains and tone‐bursts regardless of age. By contrast, cortical responses evoked by the right ear stimulation differed across stimuli and age, and dominance of ipsilateral right hemispheric activity was evident only in the tone‐burst condition in adolescents.
3.5. Sustained use of right auditory cortex occurs with age despite developmental decreases in dipole moment
To address mechanisms underlying developmental changes in cortical responses to monaural sounds, the chronological change in dipole magnitude in each auditory cortex (blue triangles and red circles for the left and right auditory cortices, respectively) was examined for changes with age (Figure 5). Because the immature first peak, iP1, in young children gradually changes into the P1–N1–P2 complex evident in adolescents, dipole moments underlying iP1 were compared with dipole moments for P1, N1, and P2 peaks individually. Nonlinear exponential regression analyses revealed a best fit to log‐transformed dipole moments and age across conditions (details in Table 2).
Figure 5.
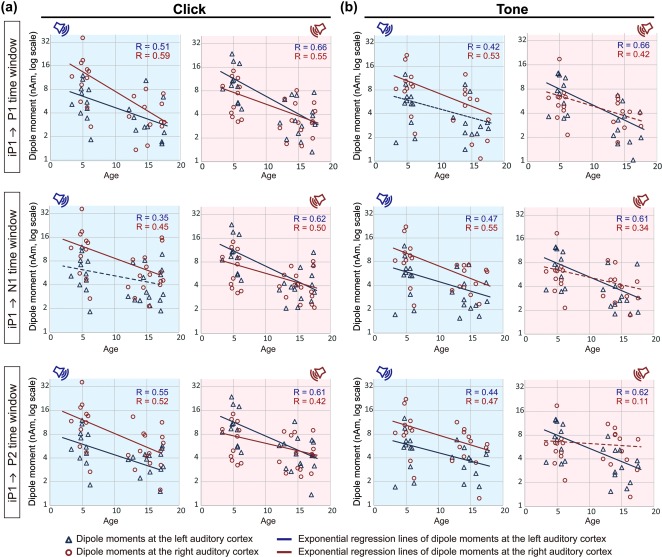
Peak dipole moments are plotted as a function of chronological age in each condition ((a) click‐trains; (b) tone‐bursts). Dipole moments during the iP1 in Child Group were compared with those at each P1, N1, and P2 time windows in the Adolescent Groups (top, middle, and bottom columns, respectively). An exponential regression line using log‐transformed dipole moments in the left and right auditory cortices (visualized by blue triangles and red circles, respectively) are shown by blue and red lines, respectively. Solid and dotted lines indicate significant (p < .05) and non‐significant correlations, respectively. Dipole moments decrease with age for most parameters evoked by left ear stimuli. On the other hand, age‐dependent decreases in amplitude upon right ear stimulation are evoked by click‐trains but not by tone‐bursts in the right auditory cortex
Table 2.
Regression analyses between peak dipole moment amplitudes and ages of the subjects
| Stimulus type | Click‐trains | Tone‐bursts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulated ear | Left ear | Right ear | Left ear | Right ear | |||||
| Hemisphere | Left | Right | Left | Right | Left | Right | Left | Right | |
| iP1 vs P1 | R | 0.51 | 0.59 | 0.66 | 0.55 | 0.42 | 0.53 | 0.66 | 0.42 |
| β | 1.01 | 0.99 | 0.99 | 0.99 | 1.00 | 1.01 | 1.02 | 1.00 | |
| SE | 0.37 | 0.29 | 0.24 | 0.31 | 0.49 | 0.36 | 0.26 | 0.49 | |
| t value | 2.75 | 3.37 | 4.23 | 3.17 | 2.05 | 2.80 | 3.90 | 2.05 | |
| p value | .021* | .012* | <.001* | .012* | .062 | .020* | .006* | .062 | |
| iP1 vs N1 | R | 0.35 | 0.45 | 0.62 | 0.50 | 0.47 | 0.55 | 0.61 | 0.34 |
| β | 0.98 | 0.96 | 0.97 | 0.99 | 1.00 | 0.98 | 1.00 | 0.99 | |
| SE | 0.55 | 0.39 | 0.25 | 0.35 | 0.41 | 0.33 | 0.29 | 0.61 | |
| t value | 1.80 | 2.45 | 3.83 | 2.82 | 2.41 | 2.99 | 3.49 | 1.65 | |
| p value | .096 | .036* | .020* | .020* | .036* | .019* | .011* | .122 | |
| iP1 vs P2 | R | 0.55 | 0.52 | 0.61 | 0.42 | 0.44 | 0.47 | 0.62 | 0.11 |
| β | 0.97 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.01 | 1.05 | |
| SE | 0.30 | 0.33 | 0.26 | 0.44 | 0.44 | 0.41 | 0.28 | 2.07 | |
| t value | 3.20 | 2.98 | 3.81 | 2.24 | 2.25 | 2.43 | 3.66 | 0.51 | |
| p value | .016* | .019* | .008* | .044* | .044* | .036* | .008* | .617 | |
The p values are FDR‐corrected p values and values ≤0.05 are to be interpreted as significant (*means p < .05 and bold p values indicate p > .05).
Dipole moments evoked by click‐trains (Figure 5a) significantly decreased with age regardless of the immature to mature peak comparison (bar iP1–N1 dipoles in the left auditory cortex evoked by the left ear). Similarly, dipoles evoked by tone‐bursts from the left ear, except for iP1–P1 dipoles in the left auditory cortex, significantly decreased with age in both auditory cortices. On the other hand, tone‐bursts from the right ear evoked decreasing dipoles with age in the left auditory cortex but dipoles in the right auditory cortex remained unchanged (Figure 5b). The consistency of dipole activity in the right auditory cortex with age, in the presence of overall developmental decreasing dipoles, suggests a relative strengthening of right hemispheric activity specific to tone stimulation.
4. DISCUSSION
Data from this study demonstrated that hemispheric dominance in cortical activity is revealed by type of monaurally presented stimulus and changes with age. These findings suggest that cortical specialization for tonal sounds develops throughout childhood into adolescence.
4.1. Hemispheric dominance in cortical activity is revealed by type of monaural stimulation
A novel aspect of this study was the use of monaural stimuli in children to assess hemispheric asymmetries as compared to many other studies using binaural sound stimuli (Belin et al., 1998; Boemio et al., 2005; Howard & Poeppel, 2009; Hyde, Peretz, & Zatorre, 2008; Jamison et al., 2006; Okamoto et al., 2009; Zatorre et al., 2002). Studies using monaural sound stimuli in adults also showed trends of right hemispheric dominance for spectral sounds with less clear findings than those observed in the binaural studies (Hine & Debener, 2007; Jin et al., 2008; Mäkelä et al., 1993; Woldorff et al., 1999). This can likely be attributed to an emphasis of anatomical contralaterality of the central auditory system in response to monaural rather than binaural input. This pattern of cortical responses to monaural tonal stimulation differs slightly from our results in the Adolescent‐tone Group, who show clear right hemispheric dominance regardless of stimulated ear (Figures 3b and 4b). Perhaps this reflects an increased sensitivity for hemispheric differences in our beamforming method. By using the TRACS beamformer (Wong & Gordon, 2009), sources of evoked neural activity were evaluated with a spatial resolution of ∼64,000 3 × 3 × 3 mm voxels while taking into account coherent sources across hemispheres. By evaluating activity across the whole brain, and taking into account coherent sources, the present beamformer analysis may be particularly sensitive to detecting hemispheric specialization in the auditory cortices relative to previous methods (Hine & Debener, 2007; Jin et al., 2008; Mäkelä et al., 1993).
4.2. Early emerging specialization of the right auditory cortex for tonal stimulation
In the Child Group, click‐trains‐evoked stronger dipole moments in the contralateral auditory cortex than ipsilateral cortex, regardless of stimulated ear (Figure 2b). These results are consistent with a contralateral dominant pattern of cortical responses to monaural stimulation reported in animals (Kral, Hartmann, Tillein, Heid, & Klinke, 2002; Mrsic‐Flogel, Versnel, & King, 2006) and human adults using click (Celesia, 1976) and noise stimuli (Gutschalk & Steinmann, 2015; Langers, van Dijk, & Backes, 2005). This cortical pattern likely reflects the anatomical organization of the auditory pathways in which the majority of afferent neural project from the cochlea to the contralateral auditory cortex (Aitkin, 1990; Langers et al., 2005).
Relative to the click trains, the tone‐bursts evoked cortical responses were more weighted to right auditory cortex in the Child Group. This expands from findings of contralaterally dominant responses to tone‐bursts in a smaller group of children with a wider age range than the present cohorts (Gordon et al., 2013). In this study, children were passively listening and the order of stimulus presentation was counter balanced so the change in pattern of cortical activation likely reflects the distinct features of the two types of sound stimuli. There are both spectral differences between the broadband click (1–9000 Hz and shaped by the transducer/earphone) and frequency specific tone‐burst centered at 500 Hz and temporal differences (slower onset and offset in tone‐burst than click‐train). The increased role of the right auditory cortex for processing the slower onset tonal stimulation is consistent with the multiresolution model in which longer temporal integration windows are used to extract slow spectral modulations (Boemio et al., 2005; Poeppel, 2003; Poeppel et al., 2008; Zatorre et al., 2002). Present data indicates that specialized function of the two cortical hemispheres for sound processing emerges in children as young as 5 years of age which is consistent with reports of lateralized cortical function for speech and nonspeech sounds in infants (Dehaene‐Lambertz et al., 2002; Peña et al., 2003).
4.3. Increasing role of the right auditory cortex for processing tones in adolescents
In the Adolescent Groups, dipole moments evoked by left ear stimulation were larger in the contralateral hemisphere for both click‐train and tone‐burst conditions. Right ear stimulation, however, activated both auditory cortices in the click‐train condition while the ipsilateral right auditory cortex was predominantly activated in the tone‐burst condition (Figure 3b). These results demonstrated that (a) cortical responses to tone‐bursts are different from click‐trains in both young children and adolescents and (b) emerging right hemispheric specialization to monaural tone‐bursts in young children undergoes further development into adolescence.
Increasing hemispheric asymmetry for sound processing with age reflects continuing development of hearing into adolescence. Children have a high capacity for their native language acquisition during the first year after birth (Jusczyk, 2000; Kuhl, 2004) but do not have fully mature hearing. For example, frequency discrimination is poorer in young children (4–6 years of age) than adults (Jensen & Neff, 1993) and, consistent with this, perception of pitch in speech and music also develops with age (Costa‐Giomi, 2003; Giannantonio et al., 2015; McDermott & Oxenham, 2008; Peretz et al., 1998; Schirmer & Kotz, 2006; Trainor & Unrau, 2012). It is possible that music exposure and training can contribute to this development both in children who are typically developing (Giannantonio et al., 2015; Hannon & Trainor, 2007; Trainor, 2005) and in children with developmental challenges (Kraus & Chandrasekaran, 2010). The role of music exposure/training on auditory function in the present cohorts was not explored.
4.4. Tone‐specific asymmetric decrease in dipole moment underlies development of right hemispheric specialization
Figure 5 demonstrated an age‐related decrease in dipole moment activity for all conditions except for responses in the right auditory cortex evoked by right ear tone‐burst stimulation. This tone‐specific asymmetry in dipole moment change with age appears to underlie the development of right hemispheric specialization to monaural tone‐bursts observed between childhood and adolescent periods. The general decrease in dipole moments with age in most stimulus conditions is consistent with synaptic pruning (the elimination of naturally overproduced synapses) in the auditory cortex and other cortical regions, which is essential for maturation of the central nervous system after birth (Purves & Lichtman, 1980). In the auditory cortex, synaptic density reaches its maximum level around 3 months of age, and then decreases between 3 and 12 years of age (Huttenlocher & Dabholkar, 1997). Based on histological data, synaptic density in the auditory cortex between 12 and 15 years of age was 44%–70% of that found in 3‐year‐old children (Huttenlocher & Dabholkar, 1997). The electrophysiological responses recorded in this study by surface electrodes reflect an open field of postsynaptic potentials from pyramidal neurons that lie perpendicular to the cortical surface (Luck, 2014). A developmental decrease in synaptic density could thus explain the general age‐related decrease in dipole moment observed in this study. Another possible explanation for the age‐related decrease in dipole moments is the development of local and upstream inhibitory circuits in the auditory cortex. Activity‐dependent development of local GABAergic inhibitory circuits play important roles in refining cortical representation of sensory inputs in the visual cortex (Hensch, 2005). In the auditory cortex, development of local inhibitory circuits is essential for development of fine spectral and temporal resolution (Chang, Bao, Imaizumi, Schreiner, & Merzenich, 2005; Froemke & Jones, 2011). Maturation of local inhibitory circuits in adolescence may thus sharpen the receptive fields of auditory cortical neurons, resulting in smaller numbers of activated neurons for identical inputs, compared to young children with immature inhibitory circuits. In sum, developmental synaptic pruning and/or increased local inhibition with age could account for decreased dipole activity measured in cortical responses.
In contrast to the general age‐related decrease in dipole moment, no significant change was observed in the right auditory cortex upon right ear stimulation with tone‐bursts, consistently during P1, N1, and P2 time windows (Figure 5). Sustained dipole moments in the right auditory cortex across age cannot be explained by reduced synaptic pruning in the right auditory cortex given decreases in dipole moments in response to click‐trains (Figure 5). Rather, the unique effects of the tone‐bursts suggest a strengthening of the neural circuit involved in right ear tone‐burst stimulation to compensate for synaptic pruning with age. Such increased excitation could come from developmental strengthening of the neural circuit and/or from a decrease in local inhibition.
4.5. Tone‐burst stimulation of the right ear evoked stronger and later dipoles in the right hemisphere during P2
Sustained cortical activity in the right auditory cortex across age is also evident when comparing the immature iP1 in young children and the more mature P2 from adolescents (Figure 5b). Dipole peaks underlying iP1 were earlier in latency and with larger dipole moments in response to contralateral left ear than ipsilateral right ear tone‐bursts (Figure 2b,c), as expected given the anatomical contralaterality in the auditory system (Gutschalk & Steinmann, 2015; Langers et al., 2005). By comparison, tone‐burst stimulation of the right ear in adolescents evoked stronger and later dipoles in the ipsilateral right auditory cortex than the contralateral left hemisphere during the P2 time window (Figure 3b,c). Moreover, tone‐bursts to the left ear in adolescents evoked larger dipole moments in the contralateral right auditory cortex, but similar dipole latencies between the hemispheres during the P2 time window (Figure 3c). One possible explanation for this deviation in contralateral dominance with age is that there is an increasing role of trans‐callosal interhemispheric projections to the right auditory cortex. The right auditory cortex receives excitatory auditory input directly from the right medial geniculate nucleus and indirectly from the left medial geniculate nucleus via the left auditory cortex and corpus callosum (Carrasco et al., 2013; Reser, Fishman, Arezzo, & Steinschneider, 2000; Stephan, Fink, & Marshall, 2007). Indirect pathways could play an increasing role for sound processing with age as the corpus callosum develops (Moore & Guan, 2001; Moore & Linthicum, 2007). Latency differences can distinguish indirect from direct pathways given that interhemispheric transfer time mediated by the corpus callosum can be up to 20 ms depending on the type of task and sensory modality (Cherbuin & Brinkman, 2006; Fendrich, Hutsler, & Gazzaniga, 2004; Krumbholz, Hewson‐Stoate, & Schonwiesner, 2007). It is possible then that delayed but strong responses of the right auditory cortex to ipsilaterally presented tone‐bursts (underlying the P2 peak) reflect a relay of activity from the left auditory cortex through indirect pathways involving trans‐callosal interhemispheric projections.
5. CONCLUSION
In conclusion, the results of this study indicate that right cortical specialization to monaural tone‐bursts begins as early as 5 years of age and becomes increasing prominent with development by 15 years of age. This change is promoted by sustained peak activity in the right auditory cortex in response to tone‐bursts in contrast to an overall decrease in sound evoked dipole moment with age. Possible strengthening and/or reduced inhibition in direct and indirect pathways to the right auditory cortex with age which could explain the present findings, should be explored in future studies.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplemental Table 1‐3
ACKNOWLEDGMENTS
The authors recognize the support of the families, children, and adolescents who participated in our studies. They also thank Stephanie Jewell, Carmen McKnight, and Alexander Andrews for support in creating the stimuli, data collection, and analyses. Funding for this study was provided by a Canadian Institute for Health Research operating grant to KG, SickKids Restracomp award to VE, doctoral award from the Ontario Government, SickKids Restracomp award, SickKids Clinician‐Scientist Training Program to MP, and SickKids Clinician‐Scientist Training Program to SJ.
Yamazaki H, Easwar V, Polonenko MJ, et al. Cortical hemispheric asymmetries are present at young ages and further develop into adolescence. Hum Brain Mapp. 2018;39:941–954. 10.1002/hbm.23893
Funding information Canadian Institute for Health Research; SickKids Restracomp; Ontario Government, SickKids Restracomp award; SickKids Clinician‐Scientist Training Program
REFERENCES
- Ackermann, H. , Hertrich, I. , Mathiak, K. , & Lutzenberger, W. (2001). Contralaterality of cortical auditory processing at the level of the M50/M100 complex and the mismatch field: A whole‐head magnetoencephalography study. Neuroreport, 12, 1683–1687. [DOI] [PubMed] [Google Scholar]
- Aitkin, L. (1990). The auditory cortex: Structural and functional bases of auditory perception. Chapman & Hall. [Google Scholar]
- Belin, P. , Zilbovicius, M. , Crozier, S. , Thivard, L. , Fontaine, A. , Masure, M. C. , & Samson, Y. (1998). Lateralization of speech and auditory temporal processing. Journal of Cognitive Neuroscience, 10, 536–540. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Bishop, D. V. (2013). Cerebral asymmetry and language development: Cause, correlate, or consequence? Science (New York, N.Y.), 340, 1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boemio, A. , Fromm, S. , Braun, A. , & Poeppel, D. (2005). Hierarchical and asymmetric temporal sensitivity in human auditory cortices. Nature Neuroscience, 8, 389–395. [DOI] [PubMed] [Google Scholar]
- Carrasco, A. , Brown, T. A. , Kok, M. A. , Chabot, N. , Kral, A. , & Lomber, S. G. (2013). Influence of core auditory cortical areas on acoustically evoked activity in contralateral primary auditory cortex. The Journal of Neuroscience, 33, 776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesia, G. G. (1976). Organization of auditory cortical areas in man. Brain: A Journal of Neurology, 99, 403–414. [DOI] [PubMed] [Google Scholar]
- Chang, E. F. , Bao, S. , Imaizumi, K. , Schreiner, C. E. , & Merzenich, M. M. (2005). Development of spectral and temporal response selectivity in the auditory cortex. Proceedings of the National Academy of Sciences of the United States of America, 102, 16460–16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin, N. , & Brinkman, C. (2006). Efficiency of callosal transfer and hemispheric interaction. Neuropsychology, 20, 178–184. [DOI] [PubMed] [Google Scholar]
- Costa‐Giomi, E. (2003). Young children's harmonic perception. Annals of the New York Academy of Sciences, 999, 477–484. [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz, G. , Dehaene, S. , & Hertz‐Pannier, L. (2002). Functional neuroimaging of speech perception in infants. Science, 298, 2013–2015. [DOI] [PubMed] [Google Scholar]
- Easwar, V. , Yamazaki, H. , Deighton, M. , Papsin, B. , & Gordon, K. (2017a). Cortical representation of interaural time difference is impaired by deafness in development: Evidence from children with early long‐term access to sound through bilateral cochlear implants provided simultaneously. Journal of Neuroscience, 37, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwar, V. , Yamazaki, H. , Deighton, M. , Papsin, B. , & Gordon, K. (2017b). Simultaneous bilateral cochlear implants: Developmental advances do not yet achieve normal cortical processing. Brain and Behavior, 7, e00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escabı, M. A. , & Schreiner, C. E. (2002). Nonlinear spectrotemporal sound analysis by neurons in the auditory midbrain. The Journal of Neuroscience, 22, 4114–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich, R. , Hutsler, J. J. , & Gazzaniga, M. S. (2004). Visual and tactile interhemispheric transfer compared with the method of Poffenberger. Experimental Brain Research, 158, 67–74. [DOI] [PubMed] [Google Scholar]
- Froemke, R. C. , & Jones, B. J. (2011). Development of auditory cortical synaptic receptive fields. Neuroscience & Biobehavioral Reviews, 35, 2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannantonio, S. , Polonenko, M. J. , Papsin, B. C. , Paludetti, G. , & Gordon, K. A. (2015). Experience changes how emotion in music is judged: Evidence from children listening with bilateral cochlear implants, bimodal devices, and normal hearing. PLoS One, 10, e0136685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, K. A. , Wong, D. D. , & Papsin, B. C. (2013). Bilateral input protects the cortex from unilaterally‐driven reorganization in children who are deaf. Brain: A Journal of Neurology, 136, 1609–1625. [DOI] [PubMed] [Google Scholar]
- Griffiths, T. D. , Uppenkamp, S. , Johnsrude, I. , Josephs, O. , & Patterson, R. D. (2001). Encoding of the temporal regularity of sound in the human brainstem. Nature Neuroscience, 4, 633–637. [DOI] [PubMed] [Google Scholar]
- Gutschalk, A. , & Steinmann, I. (2015). Stimulus dependence of contralateral dominance in human auditory cortex. Human Brain Mapping, 36, 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, E. E. , & Trainor, L. J. (2007). Music acquisition: Effects of enculturation and formal training on development. Trends in Cognitive Sciences, 11, 466–472. [DOI] [PubMed] [Google Scholar]
- Harris, K. D. , & Shepherd, G. M. (2015). The neocortical circuit: Themes and variations. Nature Neuroscience, 18, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nature Reviews. Neuroscience, 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hine, J. , & Debener, S. (2007). Late auditory evoked potentials asymmetry revisited. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118, 1274–1285. [DOI] [PubMed] [Google Scholar]
- Homae, F. , Watanabe, H. , Nakano, T. , Asakawa, K. , & Taga, G. (2006). The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience Research, 54, 276–280. [DOI] [PubMed] [Google Scholar]
- Howard, M. F. , & Poeppel, D. (2009). Hemispheric asymmetry in mid and long latency neuromagnetic responses to single clicks. Hearing Research, 257, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher, P. R. , & Dabholkar, A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Hyde, K. L. , Peretz, I. , & Zatorre, R. J. (2008). Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia, 46, 632–639. [DOI] [PubMed] [Google Scholar]
- Jamison, H. L. , Watkins, K. E. , Bishop, D. V. , & Matthews, P. M. (2006). Hemispheric specialization for processing auditory nonspeech stimuli. Cerebral Cortex, 16, 1266–1275. [DOI] [PubMed] [Google Scholar]
- Jensen, J. K. , & Neff, D. L. (1993). Development of basic auditory discrimination in preschool children. Psychological Science, 4, 104–107. [Google Scholar]
- Jin, C. Y. , Ozaki, I. , Suzuki, Y. , Baba, M. , & Hashimoto, I. (2008). Dynamic movement of N100m current sources in auditory evoked fields: Comparison of ipsilateral versus contralateral responses in human auditory cortex. Neuroscience Research, 60, 397–405. [DOI] [PubMed] [Google Scholar]
- Jiwani, S. , Papsin, B. C. , & Gordon, K. A. (2016). Early unilateral cochlear implantation promotes mature cortical asymmetries in adolescents who are deaf. Human Brain Mapping, 37, 135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrude, I. S. , Penhune, V. B. , & Zatorre, R. J. (2000). Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain, 123, 155–163. [DOI] [PubMed] [Google Scholar]
- Jusczyk, P. W. (2000). The discovery of spoken language. MIT Press. [Google Scholar]
- Kral, A. , Hartmann, R. , Tillein, J. , Heid, S. , & Klinke, R. (2002). Hearing after congenital deafness: Central auditory plasticity and sensory deprivation. Cerebral Cortex, 12, 797–807. [DOI] [PubMed] [Google Scholar]
- Kraus, N. , & Chandrasekaran, B. (2010). Music training for the development of auditory skills. Nature Reviews. Neuroscience, 11, 599–605. [DOI] [PubMed] [Google Scholar]
- Krumbholz, K. , Hewson‐Stoate, N. , & Schonwiesner, M. (2007). Cortical response to auditory motion suggests an asymmetry in the reliance on inter‐hemispheric connections between the left and right auditory cortices. Journal of Neurophysiology, 97, 1649–1655. [DOI] [PubMed] [Google Scholar]
- Kuhl, P. K. (2004). Early language acquisition: Cracking the speech code. Nature Reviews. Neuroscience, 5, 831–843. [DOI] [PubMed] [Google Scholar]
- Langers, D. R. , van Dijk, P. , & Backes, W. H. (2005). Lateralization, connectivity and plasticity in the human central auditory system. NeuroImage, 28, 490–499. [DOI] [PubMed] [Google Scholar]
- Luck, S. J. (2014). An introduction to the event‐related potential technique. MIT Press. [Google Scholar]
- Mäkelä, J. P. , Ahonen, A. , Hämäläinen, M. , Hari, R. , Llmoniemi, R. , Kajola, M. , … Salmelin, R. (1993). Functional differences between auditory cortices of the two hemispheres revealed by whole‐head neuromagnetic recordings. Human Brain Mapping, 1, 48–56. [Google Scholar]
- McDermott, J. H. , & Oxenham, A. J. (2008). Music perception, pitch, and the auditory system. Current Opinion in Neurobiology, 18, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa‐Kawai, Y. , Cristià, A. , & Dupoux, E. (2011). Cerebral lateralization and early speech acquisition: A developmental scenario. Developmental Cognitive Neuroscience, 1, 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. K. , & Guan, Y. L. (2001). Cytoarchitectural and axonal maturation in human auditory cortex. Journal of the Association for Research in Otolaryngology, 2, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. K. , & Linthicum, F. H. Jr. (2007). The human auditory system: A timeline of development. International Journal of Audiology, 46, 460–478. [DOI] [PubMed] [Google Scholar]
- Mrsic‐Flogel, T. D. , Versnel, H. , & King, A. J. (2006). Development of contralateral and ipsilateral frequency representations in ferret primary auditory cortex. European Journal of Neuroscience, 23, 780–792. [DOI] [PubMed] [Google Scholar]
- Okamoto, H. , Stracke, H. , Draganova, R. , & Pantev, C. (2009). Hemispheric asymmetry of auditory evoked fields elicited by spectral versus temporal stimulus change. Cerebral Cortex, 19, 2290–2297. [DOI] [PubMed] [Google Scholar]
- Pang, E. W. , & Taylor, M. J. (2000). Tracking the development of the N1 from age 3 to adulthood: An examination of speech and non‐speech stimuli. Clinical Neurophysiology, 111, 388–397. [DOI] [PubMed] [Google Scholar]
- Peña, M. , Maki, A. , Kovac˘ić, D. , Dehaene‐Lambertz, G. , Koizumi, H. , Bouquet, F. , & Mehler, J. (2003). Sounds and silence: An optical topography study of language recognition at birth. Proceedings of the National Academy of Sciences of the United States of America, 100, 11702–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz, I. , Gagnon, L. , & Bouchard, B. (1998). Music and emotion: Perceptual determinants, immediacy, and isolation after brain damage. Cognition, 68, 111–141. [DOI] [PubMed] [Google Scholar]
- Petersson, K. M. , Nichols, T. E. , Poline, J.‐B. , & Holmes, A. P. (1999). Statistical limitations in functional neuroimaging II. Signal detection and statistical inference. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 354, 1261–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel, D. (2003). The analysis of speech in different temporal integration windows: Cerebral lateralization as ‘asymmetric sampling in time. Speech Communication, 41, 245–255. [Google Scholar]
- Poeppel, D. , Idsardi, W. J. , & van Wassenhove, V. (2008). Speech perception at the interface of neurobiology and linguistics. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 363, 1071–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton, C. , Eggermont, J. J. , Khosla, D. , Kwong, B. , & Don, M. (2002). Maturation of human central auditory system activity: Separating auditory evoked potentials by dipole source modeling. Clinical Neurophysiology, 113, 407–420. [DOI] [PubMed] [Google Scholar]
- Purves, D. , & Lichtman, J. W. (1980). Elimination of synapses in the developing nervous system. Science, 210, 153–157. [DOI] [PubMed] [Google Scholar]
- Reiss, L. A. , Bandyopadhyay, S. , & Young, E. D. (2007). Effects of stimulus spectral contrast on receptive fields of dorsal cochlear nucleus neurons. Journal of Neurophysiology, 98, 2133–2143. [DOI] [PubMed] [Google Scholar]
- Reser, D. , Fishman, Y. , Arezzo, J. , & Steinschneider, M. (2000). Binaural interactions in primary auditory cortex of the awake macaque. Cerebral Cortex, 10, 574–584. [DOI] [PubMed] [Google Scholar]
- Schirmer, A. , & Kotz, S. A. (2006). Beyond the right hemisphere: Brain mechanisms mediating vocal emotional processing. Trends in Cognitive Sciences, 10, 24–30. [DOI] [PubMed] [Google Scholar]
- Schonwiesner, M. , Rubsamen, R. , & von Cramon, D. Y. (2005). Hemispheric asymmetry for spectral and temporal processing in the human antero‐lateral auditory belt cortex. European Journal of Neuroscience, 22, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Shannon, R. V. , Zeng, F.‐G. , Kamath, V. , Wygonski, J. , & Ekelid, M. (1995). Speech recognition with primarily temporal cues. Science (New York, N.Y.), 270, 303–304. [DOI] [PubMed] [Google Scholar]
- Stephan, K. E. , Fink, G. R. , & Marshall, J. C. (2007). Mechanisms of hemispheric specialization: Insights from analyses of connectivity. Neuropsychologia, 45, 209–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer, S. , Rossi, S. , Koch, S. P. , Nierhaus, T. , Steinbrink, J. , Poeppel, D. , … Wartenburger, I. (2009). Sensitivity of newborn auditory cortex to the temporal structure of sounds. The Journal of Neuroscience, 29, 14726–14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer, S. , Rossi, S. , Nierhaus, T. , Steinbrink, J. , Obrig, H. , & Wartenburger, I. (2011). Acoustic processing of temporally modulated sounds in infants: Evidence from a combined near‐infrared spectroscopy and EEG study. Frontiers in Psychology, 1, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga, A. W. , & Thompson, P. M. (2003). Mapping brain asymmetry. Nature Reviews. Neuroscience, 4, 37–48. [DOI] [PubMed] [Google Scholar]
- Trainor, L. J. (2005). Are there critical periods for musical development? Developmental Psychobiology, 46, 262–278. [DOI] [PubMed] [Google Scholar]
- Trainor, L. J. , & Unrau, A. (2012). Development of pitch and music perception. Human auditory development (pp. 223–254). Springer. [Google Scholar]
- Van Gisbergen, J. , Grashuis, J. , Johannesma, P. , & Vendrik, A. (1975). Spectral and temporal characteristics of activation and suppression of units in the cochlear nuclei of the anaesthetized cat. Experimental Brain Research, 23, 367–386. [DOI] [PubMed] [Google Scholar]
- Vrba, J. , & Robinson, S. E. (2001). Signal processing in magnetoencephalography. Methods (San Diego, Calif.), 25, 249–271. [DOI] [PubMed] [Google Scholar]
- Wilke, M. , Holland, S. K. , Altaye, M. , & Gaser, C. (2008). Template‐O‐Matic: A toolbox for creating customized pediatric templates. NeuroImage, 41, 903–913. [DOI] [PubMed] [Google Scholar]
- Woldorff, M. G. , Tempelmann, C. , Fell, J. , Tegeler, C. , Gaschler‐Markefski, B. , Hinrichs, H. , … Scheich, H. (1999). Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Human Brain Mapping, 7, 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, D. D. , & Gordon, K. A. (2009). Beamformer suppression of cochlear implant artifacts in an electroencephalography dataset. IEEE Transactions on Biomedical Engineering, 56, 2851–2857. [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , & Belin, P. (2001). Spectral and temporal processing in human auditory cortex. Cerebral Cortex, 11, 946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , Belin, P. , & Penhune, V. B. (2002). Structure and function of auditory cortex: Music and speech. Trends in Cognitive Sciences, 6, 37–46. [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , & Binder, J. (2000). Functional and structural imaging of the human auditory system. Brain Mapping: The Systems, 365–402. [Google Scholar]
- Zatorre, R. J. , & Halpern, A. R. (1993). Effect of unilateral temporal‐lobe excision on perception and imagery of songs. Neuropsychologia, 31, 221–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplemental Table 1‐3


