Abstract
Levels of GABA, the main inhibitory neurotransmitter in the brain, can be regionally quantified using magnetic resonance spectroscopy (MRS). Although GABA is crucial for efficient neuronal functioning, little is known about age‐related differences in GABA levels and their relationship with age‐related changes in brain structure. Here, we investigated the effect of age on GABA levels within the left sensorimotor cortex and the occipital cortex in a sample of 85 young and 85 older adults using the MEGA‐PRESS sequence. Because the distribution of GABA varies across different brain tissues, various correction methods are available to account for this variation. Considering that these correction methods are highly dependent on the tissue composition of the voxel of interest, we examined differences in voxel composition between age groups and the impact of these various correction methods on the identification of age‐related differences in GABA levels. Results indicated that, within both voxels of interest, older (as compared to young adults) exhibited smaller gray matter fraction accompanied by larger fraction of cerebrospinal fluid. Whereas uncorrected GABA levels were significantly lower in older as compared to young adults, this age effect was absent when GABA levels were corrected for voxel composition. These results suggest that age‐related differences in GABA levels are at least partly driven by the age‐related gray matter loss. However, as alterations in GABA levels might be region‐specific, further research should clarify to what extent gray matter changes may account for age‐related differences in GABA levels within other brain regions.
Keywords: aging, cerebrospinal fluid, gamma aminobutyric acid, gray matter, magnetic resonance spectroscopy
1. INTRODUCTION
The balance between excitatory and inhibitory processes in the brain is crucial to enable effective neuronal processing (Heise et al., 2013; Imbrosci & Mittmann, 2011; Rozycka & Liguz‐Lecznar, 2017). Excitation is mainly mediated by glutamate, whereas gamma aminobutyric acid (GABA) serves as the main inhibitory neurotransmitter within the brain (Lehmann, Steinecke, & Bolz, 2012; McCormick, 1989; Petroff, 2002). With an increasing number of older adults in our society, identifying age‐related changes in the excitation–inhibition balance and their role in degraded sensory, cognitive, and motor functions has gained considerable interest. Animal studies have reported a decline in GABA levels with advancing age (He, Koo, & Killiany, 2016; Liguz‐Lecznar et al., 2015), resulting in a loss of function (Leventhal et al., 2003). In humans, a direct measure of regional concentration of GABA and other endogenous metabolites in the brain can be obtained in vivo using magnetic resonance spectroscopy (MRS) (De Graaf, 2007; Mullins et al., 2014; Puts & Edden, 2012). MRS relies upon quantifying the GABA signal relative to the signal of a reference metabolite (Mullins et al., 2014). So far, only a limited number of studies have investigated aging‐induced changes in GABA levels in human by means of MRS. Aufhaus et al. (2013) investigated the effect of age on GABA levels, relative to water, within the anterior cingulate cortex in young to middle‐aged participants (age range 21–52 years), using two different editing pulse frequencies. Their findings suggested an age‐dependent increase of macromolecules rather than an age‐related decline in GABA levels (Aufhaus et al., 2013). Gao et al. (2013) explored changes in GABA levels within frontal and parietal voxels of interest (VOIs) across the lifespan (age range 20–76 years). Both creatine (Cr) and N‐acetyl aspartate (NAA) were used as reference signals and a reduction of 5% in GABA+/Cr and 4% in GABA+/NAA per decade of life was observed (Gao et al., 2013). A more recent study by Porges et al. (2017) investigated changes in GABA levels within a sample of older adults (73.12 ± 9.9 years) using water as a reference compound. In accordance with the results from Gao et al. (2013), they observed a decrease in GABA+ levels with advancing age within the same frontal and parietal VOIs. In summary, although the use of different correction methods hampers a direct comparison of results, GABA+ levels seem to decrease with advancing age.
Because the distribution of GABA differs across cerebrospinal fluid (CSF), gray matter, and white matter, it is important to account for these differences by employing a correction method on the GABA measures when referencing to water (Harris, Puts, Barker, & Edden, 2015). To this end, an individual's T1‐weighted anatomical brain scan is usually first segmented into different brain tissues and consecutively, fractions of gray matter, white matter and CSF within each VOI are calculated. As the GABA concentration within CSF is negligible, some studies (including Aufhaus et al., 2013 and Porges et al., 2017) chose to correct for CSF in the VOI. Thus, this method assumes that the estimated GABA level originates solely from the gray and white matter tissue fractions. However, application of a novel correction method that fully accounts for variations in voxel composition and tissue segmentation and relaxation values has recently been recommended (Harris et al., 2015; Mikkelsen, Singh, Brealy, Linden, & Evans, 2016). It has been suggested that this correction method most fully compensates for varying tissue composition within a VOI. However, voxel composition might also be affected by aging, as age‐related decreases in gray matter volume and increases in CSF volume have been consistently reported (Giorgio et al., 2010; Ward 2006; Hedden & Gabrieli, 2004). As the proposed correction methods are based on individual tissue fractions within the VOI, these age‐induced variations in tissue fraction may differentially impact the tissue corrected GABA levels in young compared to older adults (Mikkelsen et al., 2016). Hence, to ensure that a proper corrected value of the GABA levels is estimated, the effect of age on these correction methods needs to be established (Long et al., 2015).
In summary, initial evidence points toward an age‐related decrease in GABA concentration, although this decrease might be region‐specific. However, given the low spatial resolution of MRS, it is important to differentiate between changes in GABA level that reflect microscopic‐level cellular processes and those that simply mirror bulk tissue change. The aim of this study was to examine the effect of age on GABA measures in two VOIs using different correction methods, either employed by previous studies or proposed by Harris et al. (2015). In this regard, we hypothesized that age‐related differences in GABA levels between young and older adults might be driven by the decline in gray matter (cortical or subcortical) with advancing age (Hedden & Gabrieli, 2004; Ward 2006; van Ruitenbeek et al., 2017; Serbruyns et al., 2015; Chalavi et al., 2018), and that such effects might be altered by correcting for changes in tissue composition. Results of this study can provide new insights into aging‐induced modulations of GABA and thereby contribute to the fundamental knowledge on the interaction between neurochemistry and brain morphology within the aging population.
2. METHODS
2.1. Participants
Eighty‐five young adults (YA) (18–35 years, 23.17 ± 3.69, 41 female) and 85 older adults (OA) (60–75 years, 67.40 ± 3.92, 39 female) were included in this study. The current dataset consists of data from three different projects conducted in the context of an overarching project on aging and movement control. The general protocol and the individual protocol of each subproject were approved by the local ethics committee of KU Leuven, Belgium, and were in accordance with the Declaration of Helsinki (1964). All participants were right‐handed, as defined by the Oldfield Handedness scale (Oldfield 1971), declared to be in good physical and mental health and reported no contraindications for MRI scanning. Prior to testing, written informed consent was obtained from all participants, thereby agreeing data to be used in the context of the large overarching project. At the time of scanning, participants completed a questionnaire concerning their use of substances over the past 24 hours. Participants showing evidence of use of substances were excluded. All participants were compensated for taking part in the study.
2.2. MRS acquisition
MRS data were obtained in the university hospital of Leuven on a Phillips Achieva 3 T dstream scanner using a 32‐channel receiver head coil. For each participant, MRS data were acquired from the left primary sensorimotor cortex (SM1) and midline occipital cortex (OCC). First, a three‐dimensional T1‐weighted scan was acquired to capture the anatomical characteristics of the brain (magnetization prepared rapid gradient echo, time repetition/time echo (TR/TE) = 9.6 ms/4.6 ms; 0.98 × 0.98 × 1.2 mm voxel size; field of view (FOV) = 192 × 250 × 250 mm; 160 coronal slices). This anatomical scan was used to adequately position the SM1 and OCC VOIs for MRS acquisition, both with a size of 3 × 3 × 3 cm3. For SM1, the voxel was placed over the left hand knob (Yousry et al., 1997) and rotated in the coronal and sagittal planes to align with the cortical surface of the brain (Puts, Edden, Evans, McGlone, & McGonigle, 2011). The occipital voxel was placed posteriorly on the midline, parallel to the cerebellum (Figure 1). To examine the consistency in voxel placement per age group, heatmaps were included showing the degree of spatial overlap for both voxels (Figure 2). The MEGA‐PRESS sequence (Mescher, Merkle, Kirsch, Garwood, & Gruetter, 1998) was used to estimate GABA levels (TE = 68 ms; TR = 2 s; 320 averages; 2,048 points, 2 kHz spectral width, ∼11 min acquisition time per voxel). Although the GABA measurements refer to GABA + macromolecules (Edden et al., 2012; Rothman et al., 1993), for simplicity we refer to it as GABA. Baseline GABA levels of each subproject included in this study were acquired using identical acquisition parameters, except for the water suppression parameters. More specifically, CHESS water suppression was applied using one of two protocols: for 55 participants (32 OA and 23 YA) Philips “excitation” water suppression was applied; for the remaining cases, MOIST water suppression (Murdoch & Lampman, 1993) was applied. ON and OFF averages were acquired in an interleaved fashion by placing the 14 ms editing pulse at 1.9 and 7.46 ppm, respectively. Sixteen unsuppressed water averages were acquired from the same regions using identical acquisition parameters.
Figure 1.
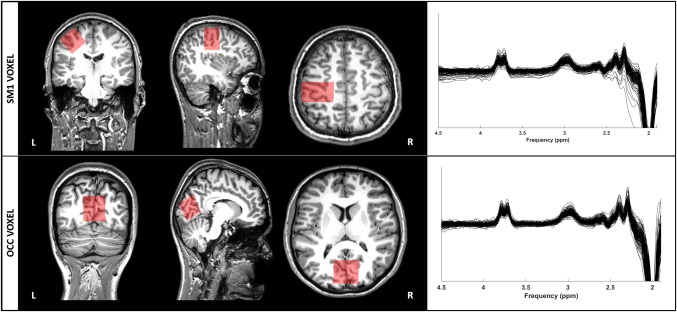
SM1 and OCC voxel positioning (left) and their corresponding edited spectra (right). On the left side, the red boxes show the positioning of the voxel: the SM1 voxel was placed over the left hand knob, whereas the OCC voxel was placed posteriorly on the midline, parallel to the cerebellum in the sagittal plane. On the right side of the figure, edited spectra from all participants are shown for the SM1 voxel (upper) and OCC voxel (lower) [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
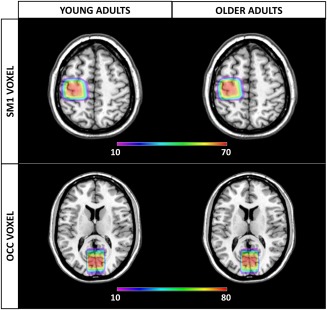
Spatial overlap of each MRS voxel overlaid on the standard template, in young and older adults. The high degree of spatial overlap indicates that, for both age groups, there was a high consistency in voxel placement. However, to minimize the fraction of CSF within the voxel, voxels of older adults tended to be positioned further from the skull as compared to voxels of young adults. Of note, for optimal illustration, only regions that displayed an overlap in more than 10 participants are shown [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. Data analysis
MRS data were analyzed using the GABA analysis Toolkit (“Gannet” (v2.0)), using water as a reference compound (Edden, Puts, Harris, Barker, & Evans, 2014) and applying Spectral Registration (Near et al., 2015) for frequency and phase correction. The GABA signal from the difference spectrum was then fit with a single Gaussian using five parameters (Edden et al., 2014). The average unsuppressed water signal was fit using a Gaussian‐Lorentzian model, from which the measured (uncorrected) GABA level referenced to water was derived (Gannet parameter name: GABAconciu, Equation 1) (Figure 3a). Data quality was assessed by examining the fit error of the GABA signal and the frequency drift. Furthermore, lipid contamination and water suppression were visually checked in the GannetLoad output file. In a second step (GannetCoRegister and GannetSegment), Statistical Parametric Mapping (SPM) version 12 was used for segmentation of the T1‐weighted image, enabling calculation of the individual voxel compositions, namely, gray matter fraction (f GM), white matter fraction (f WM), and CSF fraction (f CSF) (Hone‐Blanchet et al., 2015). CSF‐corrected GABA levels (Gannet parameter name: GABAconciuTissCorr) were calculated (Equation 2; Figure 3a). Recently, Harris et al. (2015) proposed the use of additional corrections for GABA‐edited MRS (Equations 3–5; Figure 3b). To this end, first, tissue‐specific relaxation parameters and water visibility values were added to the equation of the correction method (Gannet parameter name: QuantGABAiu, Equation 3). Whereas these tissue‐specific values are known for the portion of water within each fraction, values for GABA are assumed to be identical across all tissue fractions (Harris et al., 2015). Subsequently, voxel GM/WM composition was considered: that is, besides the aforementioned CSF correction, an α value was added to the equation to account for the higher amount of GABA within the gray as compared to the white matter (Gannet parameter name: QuantTissCorrGABAiu, Equation 4). This α was set to 0.5, indicating a GM/WM signal ratio of 2/1 (Harris et al., 2015). Although Mikkelsen et al. (2016) recommended a region‐specific estimate of the GM/WM ratio, their estimation of the GM/WM ratio in the occipital lobe of a large reference cohort also pointed toward a 2/1 for GM/WM ratio, as proposed by Harris Harris et al. (2015). Importantly, the treatment of voxel composition as illustrated in Equation 4, results in a corrected GABA level that would have been measured when the entire voxel was composed of gray matter (Harris et al., 2015). As this is not the case, a correction factor based on the tissue composition of the VOI is incorporated, resulting in the final QuantNormTissCorrGABAiu value that is considered to reflect the true GABA level within the VOI (Equation 5; Harris et al., 2015). For groups where voxel composition is thought to be drastically different, such as in young versus older participants or in healthy versus pathological conditions, at least two approaches can be taken: either an average voxel composition could be calculated across both age groups (QuantNormTissCorrGABAiu (1)); or voxel composition could be averaged per age group (QuantNormTissCorrGABAiu (2)). Here, both options were explored to investigate their impact on GABA levels in older compared to young adults. Finally, to allow comparison with the results reported by Gao et al. (2013), GABA was referenced to Cr and NAA (GABAconcCr and GABAconcNAA) and was compared across age groups.
Figure 3.
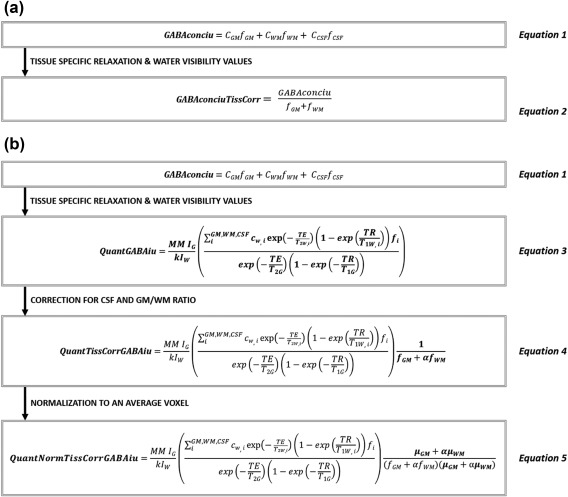
(a) CSF‐correction. Equation 1 shows the basic Equation of the uncorrected, measured GABA concentration referenced to water. As GABA levels within the CSF are negligible, the measured GABA concentration is assumed to originate from the gray and white matter fractions only. Therefore, a correction for CSF is made in Equation 2. (b) Overview of the different correction methods used in Gannet Quantify, based on Harris et al. (2015). Step by step, correction methods are added: tissue specific relaxation and water visibility values in Equation 3, correction for the CSF Fraction and 2/1 GM/WM ratio of GABA in Equation 4 and normalization to an average voxel in Equation 5. c = concentration; f = fraction; GM = gray matter; WM = white matter; CSF = cerebrospinal fluid; w = water; MM = correction factor for co‐edited macromolecules; I G/w = signal integral for GABA/water; c w = visible water concentration; k = editing efficiency of GABA (set to 0.65); TE = time echo; TR = time repetition; T 1/2 = relaxation time constants (specific relaxation parameters per tissue fraction for water, assumed to be identical across tissue fractions for GABA); α = GM/WM ratio (set to 0.5); μ = average voxel fraction
2.4. Statistics
Statistical analyses were conducted using SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY). To investigate whether there was a difference in GABA levels between age groups (using water as a reference) dependent on the correction method that was used, independent samples t tests were conducted for each correction method separately. To account for voxel composition in GABA levels referenced to Cr or NAA, GABAconcNAA and GABAconcCr were additionally assessed including the fraction of GM as a covariate. Differences in fractions of GM, WM, and CSF and quality metrics (fit error and frequency drift) across age groups were also assessed using independent samples t tests. To verify whether results were affected by data quality, a secondary analysis was performed including frequency drift as a covariate.
3. RESULTS
Prior to analysis, voxel positioning was verified using MRIcroN. In total, 5 SM1 voxels (2 YA and 3 OA) were excluded because of an erroneous positioning of the voxel. After a visual check of the Gannet output files, results of 14 SM1 voxels (7 OA and 7 YA) and 5 OCC voxels (1 OA and 4 YA) were excluded from further analysis because of poor data quality (excessive lipid or poor water suppression). Before calculating the average voxel compositions either across both groups or within each age group, outliers in (un)corrected GABA levels (mean ± 3 standard deviations) were excluded. For the SM1 voxel, 7 outliers were identified (4 OA and 3 YA) and for the OCC voxel, 6 outliers (4 OA and 2 YA) were excluded. In the final statistical analysis, 144 SM1 voxels (71 OA and 73 YA) and 159 OCC voxels (80 OA and 79 YA) were included. With respect to data quality, frequency drift was higher in older compared to young adults (M1: YA 0.425 ± 0.232 Hz, OA 0.590 ± 0.243 Hz, p < .001; OCC: YA 0.521 ± 0.282 Hz, OA 0.692 ± 0.316 Hz, p < .001), whereas the fit error was similar across age groups (M1: YA 4.660 ± 1.151, OA 4.655 ± 1.151, p = .981; OCC: YA 4.377 ± 0.873, OA 4.637 ± 1.150, p = .111). These values are in line with a recent large‐scale multi‐vendor and multisite study, confirming good data quality in the present study (Mikkelsen et al., 2017). GABA levels and voxel compositions are shown in Tables 1 and 2, respectively (for a visual representation, see Supporting Information).
Table 1.
GABA levels in the SM1 and OCC voxel after applying different corrections
| SM1 | OCC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | t | p | p* | Mean ± SD | t | p | p* | |||
| YA | OA | YA | OA | |||||||
| GABAconcCr | 0.120 ± 0.013 | 0.115 ± 0.013 | 2.69 | .008 | .015 | 0.115 ± 0.011 | 0.108 ± 0.015 | 3.17 | .002 | .003 |
| GABAconcNAA | 0.075 ± 0.008 | 0.077 ± 0.009 | −1.87 | .064 | .053 | 0.081 ± 0.008 | 0.083 ± 0.108 | −1.26 | .211 | .174 |
| GABAconciu | 1.956 ± 0.235 | 1.775 ± 0.240 | 4.57 | <.001 | <.001 | 2.204 ± 0.279 | 2.067 ± 0.336 | 2.78 | .006 | .016 |
| GABAconciuTissCorr | 2.147 ± 0.268 | 2.071 ± 0.268 | 1.70 | .092 | .230 | 2.430 ± 0.314 | 2.440 ± 0.404 | −1.75 | .861 | .560 |
| QuantGABAiu | 2.117 ± 0.259 | 1.919 ± 0.257 | 4.59 | <.001 | <.001 | 2.572 ± 0.321 | 2.402 ± 0.384 | 3.03 | .003 | .009 |
| QuantTissCorrGABAiu | 3.365 ± 0.410 | 3.386 ± 0.428 | − 0.30 | .766 | .499 | 3.338 ± 0.450 | 3.458 ± 0.593 | −1.43 | .155 | .073 |
| QuantNormTissCorrGABAiu (1) | 2.279 ± 0.278 | 2.293 ± 0.290 | − 0.30 | .766 | .499 | 2.793 ± 0.376 | 2.887 ± 0.496 | −1.34 | .183 | .091 |
| QuantNormTissCorrGABAiu (2) | 2.326 ± 0.283 | 2.240 ± 0.283 | 1.82 | .071 | .186 | 2.838 ± 0.382 | 2.840 ± 0.487 | −.02 | .982 | .658 |
Note. p*: p value after adding frequency drift as a covariate.
Table 2.
Voxel composition of the SM1 and OCC voxel
| SM1 | OCC | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | t | p value | Mean ± SD | t | p value | |||
| YA | OA | YA | OA | |||||
| GM fraction | 0.346 ± 0.030 | 0.276 ± 0.031 | 13.88 | <.001 | 0.636 ± 0.037 | 0.545 ± 0.042 | 14.45 | <.001 |
| WM fraction | 0.566 ± 0.051 | 0.581 ± 0.051 | −1.76 | .080 | 0.271 ± 0.039 | 0.303 ± 0.036 | −5.47 | <.001 |
| CSF fraction | 0.088 ± 0.033 | 0.143 ± 0.041 | −8.95 | <.001 | 0.093 ± 0.021 | 0.151 ± 0.043 | −10.80 | <.001 |
3.1. Differences in GABA levels between young and older adults
3.1.1. SM1 voxel
For the SM1 voxel, a significant effect of age was observed for the uncorrected GABAconciu value, indicating a lower GABA level in older as compared to young adults (Table 1). After correcting this value for CSF fraction (GABAconciuTissCorr), only a trend toward a lower GABA level in older compared to young adults could be observed. Implementing the correction methods proposed by Harris et al. (2015) yielded mixed results. First, after adding tissue specific relaxation and water visibility values to the equation (i.e., QuantGABAiu), GABA levels remained significantly lower in older compared to young adults. However, when the corrections for voxel composition were implemented subsequently—which considers no GABA concentration in CSF and a concentration ratio of 2/1 in GM/WM (QuantTissCorrGABAiu)—the difference between age groups was no longer significant. Finally, after a normalization to an average voxel (QuantNormTissCorrGABAiu), no effect of age was observed when normalizing to an average voxel across the two age groups, whereas there was a trend toward lower GABA levels in older relative to young adults when normalizing to within‐group average voxel composition. When Cr as opposed to water was used as the reference compound, GABA levels were significantly lower in older compared to young adults. However, using NAA as the reference compound, a trend toward the opposite effect was observed: GABA/NAA levels tended to be higher in older as compared to young adults. When the GM fraction was added to the analysis as a covariate, GABA/Cr levels tended to be lower in old as compared to young adults, whereas GABA/NAA levels tended to be higher in older as compared to young adults (GABA/Cr: YA 0.120 ± 0.002, OA 0.115 ± 0.002) p = .090; GABA/NAA: YA 0.074 ± 0.001, OA 0.078 ± 0.001 p = .56). In summary, depending on the correction method being used, a decrease in GABA concentration within SM1 was shown with increasing age. To investigate whether differences in frequency drift affected our results, a secondary analysis was performed including frequency drift as a covariate. Results of the analysis of covariance indicated that differences in GABA levels are not significantly impacted by frequency drift (p ≥ .230). However, trends for lower GABA levels in older as compared to young adults after applying CSF correction (GABAconciuTissCorr) or normalization to a within‐group average voxel (QuantNormTissCorrGABAiu) were absent when frequency drift was added as a covariate.
3.1.2. OCC voxel
With respect to the OCC voxel, a significant effect of age was found for the GABAconciu, QuantGABAiu and the GABAconcCr value. However, no difference between age groups was found within any of the other corrected values referenced to water, nor the GABAconcNAA value. Adding frequency drift as a covariate in the secondary analysis did not affect our results significantly (p ≥ .124). Nonetheless, the QuantTissCorrGABAiu and QuantNormTissCorrGABAiu value did show a trend toward higher GABA levels in old as compared to young adults when correcting for variance in frequency drift. When GM fraction was added as a covariate to account for voxel composition in GABA levels referenced to Cr and NAA, GABA/Cr, nor GABA/NAA significantly differed across age groups. However, there was a trend toward lower GABA/Cr in older as compared to young adults (GABA/Cr: YA 0.115 ± 0.002, OA 0.109 ± 0.002, p = .059; GABA/NAA: YA 0.81 ± 0.001, OA 0.82 ± 0.001, p = 0.618).
3.2. Differences in voxel composition between young and older adults
In both age groups, the majority of the SM1 voxel was white matter, whereas the OCC voxel consisted predominantly of gray matter tissue (Table 2). However, there was a difference in tissue fractions between age groups. The gray matter fractions of both the SM1 and OCC VOIs were lower in older compared to young adults, while the opposite was true for the CSF fractions. With regard to WM, as compared to young adults, older adults showed a trend toward higher WM fraction in the SM1 voxel and significantly higher WM tissue fraction in the OCC voxel. As correction methods are highly affected by the tissue composition, age‐related differences in tissue compositions of the VOI are of huge importance in the interpretation of measured GABA concentration.
4. DISCUSSION
This study examined differences in GABA levels in SM1 and OCC voxels between young and older adults, as well as the relationship between GABA levels and tissue changes. The observation of an age‐related difference in GABA levels was critically dependent on the reference compound. Furthermore, results indicated a lower fraction of gray matter within the VOIs in older compared to young adults, accompanied by higher CSF and WM fractions. After correcting water‐referenced GABA levels for voxel composition, age‐related differences in GABA levels were no longer significant, suggesting that changes in GABA levels might be explained by bulk tissue changes with advancing age.
4.1. Age‐related differences in GABA levels using Cr or NAA as a reference
To allow comparison with previous studies, age‐related differences in GABA levels were assessed using Cr or NAA as a reference compound. Whereas Cr‐referenced GABA levels within both VOIs were significantly lower in older as compared to young adults, NAA‐referenced GABA levels tended to be higher in older as compared to young adults within the SM1 voxel only. These results are not fully in line with results by Gao et al. (2013), who reported declines in GABA/Cr and GABA/NAA levels within a frontal and a parietal VOI with advancing age. They used both Cr and NAA as reference compounds to verify that potential differences were due to GABA itself and not due to the choice of reference compound. However, whereas NAA levels are consistently shown to decline with advancing age, it is less clear how Cr levels are affected by age (Brooks et al., 2001; Charles et al., 1994; Christiansen, Toft, Larsson, Stubgaard, & Henriksen, 1993; Eylers et al., 2016; Grachev & Apkarian, 2001; Gruber et al., 2008; Haga, Khor, Farrall, & Wardlaw, 2009; Rae, 2014). Indeed, we found contrasting results using Cr or NAA as a reference compound. Hence, these results highlight the instability of Cr and/or NAA as reference compounds, which complicates the interpretation of the origin of the age‐related differences in that these age‐related differences might be caused by changes in both the numerator and/or the denominator. Therefore, results of studies using only one of these two reference compounds should be interpreted with caution.
4.2. Water‐referenced GABA levels corrected using various correction methods: Voxel composition is a major driver of age‐related differences in GABA levels
Correction methods applied to water‐referenced GABA levels are predominantly based on voxel composition. Therefore, it is crucial to investigate the impact of age on the relative proportion of tissue fractions within the VOIs. Our results clearly demonstrated that the well‐known age‐related decline in gray matter volume, accompanied by an increase in CSF volume, is also reflected within the composition of both VOIs. In view of these findings, and considering that the correction methods mostly rely on the tissue fractions, it needs to be clarified whether differences in GABA levels are mostly driven by age‐related changes in gray matter volume or whether they signify biochemical changes that go beyond this altered composition of brain structure with advancing age. Our results indicated lower uncorrected GABA/water levels within both VOIs in older as compared to young adults. However, after subsequent correction of the water‐referenced GABA value for the CSF fraction and/or assuming a 2/1 GABA ratio in GM/WM, age‐related differences in GABA levels were absent. Therefore, our results seem to support the former hypothesis that, although the VOIs of older adults contained more CSF and less gray matter compared to young adults, the GABA concentration within the available gray and white matter fractions did not seem to differ across age groups. This was the case for both the SM1 and OCC voxel. Interestingly, research on cerebral blood flow in the context of aging resulted in similar insights. In the context of brain perfusion, partial volume effects, that is, the presence of multiple brain tissues within one voxel, serve as a major source of error (Asllani et al., 2009). When accounting for these effects, age‐related differences in cerebral blood flow are resolved (Meltzer et al., 2000), also pointing toward an important role of bulk tissue changes.
Our result about a lack of a significant age effect on the GABA/water levels within either VOI, after accounting for the CSF fraction, appears in contrast with Porges et al. (2017). These conflicting results might indirectly point to a region‐specific decrease in GABA levels with advancing age, as Porges et al. (2017) observed the age effects on GABA/water levels within a frontal and parietal region. In accordance with this hypothesis, after correcting for voxel composition, we saw a trend toward an age‐related difference in GABA levels for SM1 but not OCC, possibly pointing toward some degree of disproportionality in GABA content within tissue fractions as a function of age in the SM1 voxel only. Considering that participants in the study by Porges et al. (2017) were generally older as compared to the group of older adults included in the present study (mean age 73 vs 67 years), it is also possible that an age‐related decline in GABA levels beyond the emerging gray matter atrophy only manifests itself at the higher end of older age. Moreover, whereas the study by Porges et al. (2017) was a correlational study, a cross‐sectional design was adopted in this study.
4.3. Analyzing MRS of GABA in the context of aging: Considerations and limitations
It should be noted that the assumption of a 2/1 ratio of GABA+ signal in GM/WM used in our water‐referenced tissue correction might not be valid for the whole brain or the full age‐range studied. Previous research demonstrated a region‐dependency of GABA+ levels (Grachev & Apkarian, 2000; Fahn & Cote, 1968), independent of the gray matter fraction of the VOI (Durst et al., 2015; Grewal et al., 2016; Harada, Kubo, Nose, Nishitani, & Matsuda, 2011). Although recent evidence supports a 2/1 ratio for the OCC voxel (Mikkelsen et al., 2016), it is currently unknown whether this assumption is valid for the SM1 voxel studied in this cohort. Furthermore, a study by Ding et al. (2016) revealed that GM/WM ratios change with age for multiple brain metabolites. Nevertheless, the study by Harris et al. (2015) did show that the estimation of a 2/1 ratio performs better than simply not including this factor in the tissue correction calculation. It is critical to further investigate this issue to allow a region‐ and age‐specific quantification of GABA levels. This demands the inclusion of a robust assessment of, for example, cortical thickness and other factors not approachable using in vivo MRI (e.g., metabolism).
Furthermore, it is important to consider the macromolecular contamination of the GABA peak in the context of aging. Macromolecules are known to be higher in old as compared to young adults (Aufhaus et al., 2013; Marjańska et al., 2018; Noworolski et al., 1999) and therefore, the observed age‐related differences in GABA+ levels might underestimate reductions in GABA with advancing age.
Moreover, age‐related differences in GABA levels could also be alternatively explained by voxel positioning. This is because placement of the MRS voxels is performed on a subject‐by‐subject basis, based on gross anatomical features. Therefore, it is possible that systematic differences in voxel placement occur in response to enlarged sulci (Giorgio et al., 2010). This matter was further investigated by including heatmaps to examine consistency in voxel placement for both age groups (Figure 2). Although a high degree of consistency in voxel placement was observed, voxels of older adults indeed seemed to be positioned somewhat further from the skull as compared to voxels of young adults in order to minimize the CSF fraction within the VOI. However, as the centers of the voxel are very similar in young and older adults, it is unlikely to be the only factor contributing to age‐related differences in GABA levels. Moreover, alterations in tissue relaxation with age might bias segmentation (Harris et al., 2015). It is well established that aging‐induced changes in brain morphology and metabolism are related to alterations in relaxation values (Bottomley, Foster, Argersinger, & Pfeifer, 1984; Ding et al., 2004; Eylers et al., 2016; Sedlacik et al., 2014). Given that age‐related changes in relaxation are well‐documented, it would not be surprising if there are age‐related biases in the segmentation of T1‐weighted structural images, which might interfere with tissue corrections (Cabezas, Oliver, Lladó, Freixenet, & Bach Cuadra, 2011). Eylers et al. (2016) demonstrated an age‐related decline in transverse relaxation time in several brain regions including the occipital grey matter and the hand area of SM1, possibly caused by changes of the free water content. Nevertheless, assessing the dependency of multiple brain metabolites on aging in various brain regions using distinct spectroscopy techniques, Gruber et al. (2008) argued that changes in relaxation time are most likely not the only factor contributing to the observed age‐related differences in metabolites.
Equally important, the choice of reference signal remains an enduring challenge in MRS, which is quantitative in relative terms only. Especially for studies on aging, the common reference signals are all problematic to some degree: water concentration, density and visibility all change with age (Gasparovic et al., 2006; Neeb, Zilles, & Shah, 2006), in addition to tissue atrophy and the need to address CSF fractions. Furthermore, there is strong evidence for NAA concentration decreases with age (Eylers et al., 2016; Haga et al., 2009), evidence for NAA relaxation changes (Eylers et al., 2016; Marjańska, Emir, Deelchand, & Terpstra, 2013); and some evidence of increases in Cr (Gruber et al., 2008; Saunders, Howe, van den Boogaart, Griffiths, & Brown, 1999). While purists tend to prefer quantification relative to water, the metabolite references are localized to tissue, so inherently less impacted by large‐scale atrophy. In our study, we present quantification relative to all references to highlight this challenge and allow fullest interpretation of the data. Future research needs to focus on the generation of age‐normed reference values to allow more precise and evidence‐based corrections of GABA levels.
Notably, with respect to data quality, frequency drift was higher in older as compared to young adults. Harris et al. (2014) demonstrated that uncorrected frequency drift results in a decrease in GABA levels, mostly explained by subtraction artefacts. This alters the relative contribution of GABA and macromolecules to the GABA signal, which possibly confounds our results. Although our data were frequency and phase corrected, differences in frequency drift between age groups might be explained by larger head movement in older compared to young adults during the MRS acquisition (Near et al., 2015; Rowland et al., 2017). However, a secondary analysis including frequency drift as a covariate did not influence our results, indicating that observed differences in GABA levels may not be due to differences in data quality.
4.4. Functional relevance of MRS‐based GABA levels
Multiple studies have linked GABA levels to different aspects of motor performance such as motor decision speed and dexterity (Boy et al., 2010; Dharmadhikari et al., 2015; Long et al., 2014; Quetscher et al., 2015; Sumner, Edden, Bompas, Evans, & Singh, 2010). Furthermore, cognitive function has been related to frontal GABA levels (Porges et al., 2017). Although the precise functional relevance of age‐related differences in GABA levels remains unclear (Bachtiar & Stagg, 2014), local GABA levels are known to modulate synaptic strength and have an impact on local inhibition, thereby impacting behavior (Stagg, 2014). Additional research is warranted to establish which specific measures of GABA level (tissue‐corrected or not) are most informative for the study of brain–behavior associations, that is, whether motor, cognitive and/or sensory function is determined by the overall amount of GABA within a brain region or by the available GABA levels within the GM fraction of that specific area.
Besides assessing GABA levels with MRS, GABAergic mediated inhibition is often quantified using transcranial magnetic stimulation (TMS) protocols. TMS studies have established the importance of GABAergic mediated inhibition in the maintenance of the inhibition‐excitation balance, contributing to the specificity of neuronal responses (Heise et al., 2013; Imbrosci & Mittmann, 2011; Liguz‐Lecznar et al., 2015). With aging, excitability and the capability to modulate excitability seems to decrease, causing motor deficits in older adults (Bhandari et al., 2016). Interestingly, current studies show that TMS measures of GABAergic inhibition are not correlated with GABA concentrations obtained by MRS (Dyke et al., 2017; Hermans et al., 2018; Mooney, Cirillo, & Byblow, 2017; Stagg et al., 2011; Tremblay et al., 2013). This appears to suggest that MRS and TMS investigate distinct mechanisms: whereas MRS is thought to reflect the inhibitory tone of a certain brain region, TMS targets the GABAergic synaptic transmission (Dyke et al., 2017; Stagg et al., 2011; Tremblay et al., 2013). It is not clear to what extent TMS measures of inhibition would be impacted by bulk tissue loss. Therefore, further research specifically examining the functional relevance of GABA within an older population is required.
5. CONCLUSION
Results indicated that uncorrected water‐referenced GABA levels were lower in older as compared to young adults. However, after correcting for voxel tissue composition, this age effect was absent. These results suggest that age‐related differences in GABA levels are at least partly accounted for by gray matter loss rather than altered GABA levels within the available gray and white matter tissues of the brain volumes studied. Therefore, future studies should consider the effect of advanced correction techniques on MRS‐obtained GABA levels in other brain regions to arrive at a fuller understanding of age‐related differences in brain neurochemistry. Furthermore, our data highlight the importance of using a reliable reference compound. In addition, further research should investigate the link between sensory, motor and cognitive performance and age‐related changes in GABA levels and whether and how this link might be mediated by voxel composition. Finally, standardization of measures is of vital importance to enable comparison of results across different studies.
DECLARATION OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2
ACKNOWLEDGMENTS
This work was supported by the Research Fund KU Leuven (C16/15/070) and the Research Foundation Flanders (FWO) grant (G089818N) and Excellence of Science grant (EOS 30446199, MEMODYN). CM is funded by an aspirant fellowship of the Research Foundation Flanders (FWO).
Maes C, Hermans L, Pauwels L, et al. Age‐related differences in GABA levels are driven by bulk tissue changes. Hum Brain Mapp. 2018;39:3652–3662. 10.1002/hbm.24201
Funding information Research Fund KU Leuven, Grant/Award Number: C16/15/070; Research Foundation Flanders (FWO), Grant/Award Number: G089818N; Excellence of Science, Grant/Award Numbers: EOS 30446199, MEMODYN
REFERENCES
- Asllani, I. , Habeck, C. , Borogovac, A. , Brown, T. R. , Brickman, A. M. , & Stern, Y. (2009). Separating function from structure in perfusion imaging of the aging brain. Human Brain Mapping, 30(9), 2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufhaus, E. , Weber‐Fahr, W. , Sack, M. , Tunc‐Skarka, N. , Oberthuer, G. , Hoerst, M. , … Ende, G. (2013). Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: A MEGA‐PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magnetic Resonance in Medicine, 69(2), 317–320. [DOI] [PubMed] [Google Scholar]
- Bachtiar, V. , & Stagg, C. J. (2014). The role of inhibition in human motor cortical plasticity. Neuroscience, 278, 93–104. [DOI] [PubMed] [Google Scholar]
- Bhandari, A. , Radhu, N. , Farzan, F. , Mulsant, B. H. , Rajji, T. K. , Daskalakis, Z. J. , & Blumberger, D. M. (2016). A meta‐analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clinical Neurophysiology, 127(8), 2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley, P. A. , Foster, T. H. , Argersinger, R. E. , & Pfeifer, L. M. (1984). A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1 ‐ 100 MHz: Dependence on tissue type, NMR frequency, temperature, species, excision, and age. Medical Physics, 11(4), 425–448. [DOI] [PubMed] [Google Scholar]
- Boy, F. , Evans, C. J. , Edden, R. A. E. , Singh, K. D. , Husain, M. , & Sumner, P. (2010). Individual differences in subconscious motor control predicted by GABA concentration in SMA. Current Biology, 20(19), 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, J. C. W. , Roberts, N. , Kemp, G. J. , Gosney, M. A. , Lye, M. , & Whitehouse, G. H. (2001). A proton magnetic resonance spectroscopy study of age‐related changes in frontal lobe metabolite concentrations. Cerebral Cortex, 11(7), 598–605. [DOI] [PubMed] [Google Scholar]
- Cabezas, M. , Oliver, A. , Lladó, X. , Freixenet, J. , & Bach Cuadra, M. (2011). A review of atlas‐based segmentation for magnetic resonance brain images. Computer Methods and Programs in Biomedicine, 104(3), e158–e177. [DOI] [PubMed] [Google Scholar]
- Chalavi, S. , Adab, H. Z. , Pauwels, L. , Beets, I. A. M. , van Ruitenbeek, P. , Boisgontier, M. P. , … Swinnen, S. P. (2018). Anatomy of subcortical structures predicts age‐related differences in skill acquisition. Cerebral Cortex, 28(2), 459–473. [DOI] [PubMed] [Google Scholar]
- Charles, H. C. , Lazeyras, F. , Krishnan, K. R. R. , Boyko, O. B. , Patterson, L. J. , Doraiswamy, P. M. , & Mcdonald, W. M. (1994). Proton spectroscopy of human brain: Effects of age and sex. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 18(6), 995–1004. [DOI] [PubMed] [Google Scholar]
- Christiansen, P. , Toft, P. , Larsson, H. B. W. , Stubgaard, M. , & Henriksen, O. (1993). The concentration of N‐acetyl aspartate, creatine + phosphocreatine, and choline in different parts of the brain in adulthood and senium. Magnetic Resonance Imaging, 11(6), 799–806. [DOI] [PubMed] [Google Scholar]
- Dharmadhikari, S. , Ma, R. , Yeh, C.‐L. , Stock, A.‐K. , Snyder, S. , Zauber, S. E. , … Beste, C. (2015). Striatal and thalamic GABA level concentrations play differential roles for the modulation of response selection processes by proprioceptive information. NeuroImage, 120, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X.‐Q. , Maudsley, A. A. , Sabati, M. , Sheriff, S. , Schmitz, B. , Schütze, M. , … Lanfermann, H. (2016). Physiological neuronal decline in healthy aging human brain — An in vivo study with MRI and short echo‐time whole‐brain 1 H MR spectroscopic imaging. NeuroImage, 137, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X.‐Q. , Kucinski, T. , Wittkugel, O. , Goebell, E. , Grzyska, U. , Görg, M. , … Zeumer, H. (2004). Normal brain maturation characterized with age‐related T2 relaxation times: An attempt to develop a quantitative imaging measure for clinical use. Investigative Radiology, 39(12), 740–746. [DOI] [PubMed] [Google Scholar]
- Durst, C. R. , Michael, N. , Tustison, N. J. , Patrie, J. T. , Raghavan, P. , Wintermark, M. , & Sendhil Velan, S. (2015). Noninvasive evaluation of the regional variations of GABA using magnetic resonance spectroscopy at 3 Tesla. Magnetic Resonance Imaging, 33(5), 611–617. [DOI] [PubMed] [Google Scholar]
- Dyke, K. , Pépés, S. E. , Chen, C. , Kim, S. , Sigurdsson, H. P. , Draper, A. , … Jackson, S. R. (2017). Comparg GABA‐dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurements of GABA using ultra‐high‐field MRI. NeuroImage, 152, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden, R. A. E. , Puts, N. A. J. , Harris, A. D. , Barker, P. B. , & Evans, C. J. (2014). Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid‐edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging, 40(6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden, R. A. E. , Puts, N. A. J. , & Barker, P. B. (2012). Macromolecule‐suppressed GABA‐edited magnetic resonance spectroscopy at 3T. Magnetic Resonance in Medicine, 68(3), 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylers, V. V. , Maudsley, A. A. , Bronzlik, P. , Dellani, P. R. , Lanfermann, H. , & Ding, X.‐Q. (2016). Detection of normal aging effects on human brain metabolite concentrations and microstructure with whole‐brain MR spectroscopic imaging and quantitative MR imaging. American Journal of Neuroradiology, 37(3), 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn, S. , & Cote, L. (1968). Regional distribution of gamma‐aminobutyric acid (GABA) in brain of the rhesus monkey. Journal of Neurochemistry, 15(3), 209–213. [DOI] [PubMed] [Google Scholar]
- Gao, F. , Edden, R. A. E. , Li, M. , Puts, N. A. J. , Wang, G. , Liu, C. , … Barker, P. B. (2013). Edited magnetic resonance spectroscopy detects an age‐related decline in brain GABA levels. NeuroImage, 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic, C. , Song, T. , Devier, D. , Bockholt, H. J. , Caprihan, A. , Mullins, P. G. , … Morrison, L. A. (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine, 55(6), 1219–1226. [DOI] [PubMed] [Google Scholar]
- Giorgio, A. , Santelli, L. , Tomassini, V. , Bosnell, R. , Smith, S. , De Stefano, N. , & Johansen‐Berg, H. (2010). Age‐related changes in grey and white matter structure throughout adulthood. NeuroImage, 51(3), 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf (2007). In vivo NMR spectroscopy (2nd ed.). Available at http://onlinelibrary.wiley.com/book/10.1002/9780470512968. [Google Scholar]
- Grachev, I. D. , & Apkarian, V. A. (2001). Aging alters regional multichemical profile of the human brain: An in vivo 1H‐MRS study of young versus middle‐aged subjects. Journal of Neurochemistry, 76(2), 582–593. [DOI] [PubMed] [Google Scholar]
- Grachev, I. D. , & Apkarian, V. A. (2000). Chemical heterogeneity of the living human brain : A proton MR spectroscopy study on the effects of sex, age, and brain region. NeuroImage, 11(5), 554–563. [DOI] [PubMed] [Google Scholar]
- Grewal, M. , Dabas, A. , Saharan, S. , Barker, P. B. , Edden, R. A. E. , & Mandal, P. K. (2016). GABA quantitation using MEGA‐PRESS: Regional and hemispheric differences. Journal of Magnetic Resonance Imaging, 44(6), 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, S. , Pinker, K. , Riederer, F. , Chmelík, M. , Stadlbauer, A. , Bittšanský, M. , … Moser, E. (2008). Metabolic changes in the normal ageing brain: Consistent findings from short and long echo time proton spectroscopy. European Journal of Radiology, 68(2), 320–327. [DOI] [PubMed] [Google Scholar]
- Haga, K. K. , Khor, Y. P. , Farrall, A. , & Wardlaw, J. M. (2009). A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiology of Aging, 30(3), 353–363. [DOI] [PubMed] [Google Scholar]
- Harada, M. , Kubo, H. , Nose, A. , Nishitani, H. , & Matsuda, T. (2011). Measurement of variation in the human cerebral GABA level by in vivo MEGA‐editing proton MR spectroscopy using a clinical 3 T instrument and its dependence on brain region and the female menstrual cycle. Human Brain Mapping, 32(5), 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. D. , Glaubitz, B. , Near, J. , John Evans, C. , Puts, N. A. J. , Schmidt‐Wilcke, T. , … Edden, R. A. E. (2014). Impact of frequency drift on gamma‐aminobutyric acid‐edited MR spectroscopy. Magnetic Resonance in Medicine, 72(4), 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. D. , Puts, N. A. J. , Barker, P. B. , & Edden, R. A. E. (2015). Spectral‐editing measurements of GABA in the human brain with and without macromolecule suppression. Magnetic Resonance in Medicine, 74(6), 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A. D. , Puts, N. A. J. , & Edden, R. A. E. (2015). Tissue correction for GABA‐edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of Magnetic Resonance Imaging, 42(5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Koo, B. , & Killiany, R. J. (2016). Edited magnetic resonance spectroscopy detects an age‐related decline in nonhuman primate brain GABA levels. BioMed Research International, 2016, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden, T. , & Gabrieli, J. D. E. (2004). Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews. Neuroscience, 5(2), 87–96. [DOI] [PubMed] [Google Scholar]
- Heise, K.‐F. , Zimerman, M. , Hoppe, J. , Gerloff, C. , Wegscheider, K. , & Hummel, F. C. (2013). The aging motor system as a model for plastic changes of GABA‐mediated intracortical inhibition and their behavioral relevance. Journal of Neuroscience, 33(21), 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, L. , Levin, O. , Maes, C. , van Ruitenbeek, P. , Heise, K.‐F. , Edden, R. A. E. , … Cuypers, K. (2018). GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults : An MRS‐TMS study. Neurobiology of Aging, 65, 168–177. [DOI] [PubMed] [Google Scholar]
- Hone‐Blanchet, A. , Salas, R. E. , Celnik, P. , Kalloo, A. , Schar, M. , Puts, N. A. J. , … Edden, R. A. (2015). Co‐registration of magnetic resonance spectroscopy and transcranial magnetic stimulation. Journal of Neuroscience Methods, 242, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbrosci, B. , & Mittmann, T. (2011). Functional consequences of the disturbances in the GABA‐mediated inhibition induced by injuriesin the cerebral cortex. Neural Plasticity, 2011, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, K. , Steinecke, A. , & Bolz, J. (2012). GABA through the ages: Regulation of cortical function and plasticity by inhibitory interneurons. Neural Plasticity, 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal, A. G. , Wang, Y. , Pu, M. , Zhou, Y. , & Ma, Y. (2003). GABA and its agonists improved visual cortical function in senescent monkeys. Science, 300(5620), 812–815. [DOI] [PubMed] [Google Scholar]
- Liguz‐Lecznar, M. , Lehner, M. , Kaliszewska, A. , Zakrzewska, R. , Sobolewska, A. , & Kossut, M. (2015). Altered glutamate/GABA equilibrium in aged mice cortex influences cortical plasticity. Brain Structure and Function, 220(3), 1681–1693. [DOI] [PubMed] [Google Scholar]
- Long, Z. , Dyke, J. P. , Ma, R. , Huang, C. C. , Louis, E. D. , & Dydak, U. (2015). Reproducibility and effect of tissue composition on cerebellar GABA MRS in an elderly population. NMR in Biomedicine, 21(18), 4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Z. , Li, X.‐R. , Xu, J. , Edden, R. A. E. , Qin, W.‐P. , Long, L.‐L. , … Dydak, U. (2014). Thalamic GABA predicts fine motor performance in manganese‐exposed smelter workers. PLoS ONE, 9(2), e88220–e88227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjańska, M. , Deelchand, D. K. , Hodges, J. S. , McCarten, J. R. , Hemmy, L. S. , Grant, A. , & Terpstra, M. (2018). Altered macromolecular pattern and content in the aging human brain. NMR in Biomedicine, 31(2), e3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjańska, M. , Emir, U. E. , Deelchand, D. K. , & Terpstra, M. (2013). Faster metabolite 1 H transverse relaxation in the elder human brain. PLoS ONE, 8(10), e77572–e77577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, D. A. (1989). GABA as an inhibitory neurotransmitter in human cerebral cortex. Journal of Neurophysiology, 62(5), 1018–1027. [DOI] [PubMed] [Google Scholar]
- Meltzer, C. C. , Cantwell, M. N. , Greer, P. J. , Ben‐Eliezer, D. , Smith, G. , Frank, G. , … Price J. (2000). Does cerebral blood flow decline in healthy aging? A PET study with partial‐volume correction. Journal of Nuclear Medicine, 41(11), 1842–1849. [PubMed] [Google Scholar]
- Mescher, M. , Merkle, H. , Kirsch, J. , Garwood, M. , & Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine, 11(6), 266–272. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, M. , Barker, P. , Bhattacharyya, P. , Brix, M. , Buur, P. , Cecil, K. , … Edden, R. A. E. (2017). Big GABA : Edited MR spectroscopy at 24 research sites. NeuroImage, 159, 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen, M. , Singh, K. D. , Brealy, J. A. , Linden, D. E. J. , & Evans, C. J. (2016). Quantification gamma‐aminobutyric acid (GABA) in 1H MRS volumes composed heterogeneously of grey and white matter. NMR in Biomedicine, 29(11), 1644–1655. [DOI] [PubMed] [Google Scholar]
- Mooney, R. A. , Cirillo, J. , & Byblow, W. D. (2017). GABA and primary motor cortex inhibition in young and older adults: A multimodal reliability study. Journal of Neurophysiology, 118(1), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, P. G. , McGonigle, D. J. , O'Gorman, R. L. , Puts, N. A. J. , Vidyasagar, R. , Evans, C. J. , & Edden, R. A. E. (2014). Current practice in the use of MEGA‐PRESS spectroscopy for the detection of GABA. NeuroImage, 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch, J. B. , & Lampman, D. A. (1993). Beyond WET and DRY: Optimized pulses for water suppression. In Proceedings of the SMRM 12th Annual Meeting p 1191.
- Near, J. , Edden, R. , Evans, C. J. , Paquin, R. , Harris, A. , & Jezzard, P. (2015). Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magnetic Resonance in Medicine, 73(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeb, H. , Zilles, K. , & Shah, N. J. (2006). Fully‐automated detection of cerebral water content changes : Study of age‐ and gender‐related H 2 O patterns with quantitative MRI. NeuroImage, 29(3), 910–922. [DOI] [PubMed] [Google Scholar]
- Noworolski, S. M. , Nelson, S. J. , Henry, R. G. , Day, M. R. , Wald, L. L. , Star‐Lack, J. , & Vigneron, D. B. (1999). High spatial resolution 1 H‐MRSI and segmented MRI of cortical gray matter and subcortical white matter in three regions of the human brain. Magnetic Resonance in Medicine, 41(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Petroff, O. A. C. (2002). Book review: GABA and glutamate in the human brain. Neuroscientist, 8(6), 562–573. [DOI] [PubMed] [Google Scholar]
- Porges, E. C. , Woods, A. J. , Edden, R. A. E. , Puts, N. A. J. , Harris, A. D. , Chen, H. , … Cohen, R. A. (2017). Frontal gamma‐aminobutyric acid concentrations are associated with cognitive performance in older adults. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts, N. A. J. , Edden, R. A. E. , Evans, C. J. , McGlone, F. , & McGonigle, D. J. (2011). Regionally specific human GABA concentration correlates with tactile discrimination thresholds. Journal of Neuroscience, 31(46), 16556–16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts, N. A. J. , & Edden, R. A. E. (2012). In vivo magnetic resonance spectroscopy of GABA: A methodological review. Progress in Nuclear Magnetic Resonance Spectroscopy, 60, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetscher, C. , Yildiz, A. , Dharmadhikari, S. , Glaubitz, B. , Schmidt‐Wilcke, T. , Dydak, U. , & Beste, C. (2015). Striatal GABA‐MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Structure and Function, 220(6), 3555–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae, C. D. (2014). A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochemical Research, 39(1), 1–36. [DOI] [PubMed] [Google Scholar]
- Rothman, D. L. , Petroff, O. A. , Behar, K. L. , & Mattson, R. H. (1993). Localized 1H NMR measurements of g‐aminobutyric acid in human brain in vivo. Proceedings of the National Academy of Sciences of the United States of America, 90(12), 5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, B. C. , Liao, H. , Adan, F. , Mariano, L. , Irvine, J. , & Lin, A. P. (2017). Correcting for frequency drift in clinical brain MR spectroscopy. Journal of Neuroimaging, 27(1), 23–28. [DOI] [PubMed] [Google Scholar]
- Rozycka, A. , & Liguz‐Lecznar, M. (2017). The space where aging acts: Focus on the GABAergic synapse. Aging Cell, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, D. E. , Howe, F. A. , van den Boogaart, A. , Griffiths, J. R. , & Brown, M. M. (1999). Aging of the adult human brain : In vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. Journal of Magnetic Resonance Imaging, 9(5), 711–716. [DOI] [PubMed] [Google Scholar]
- Sedlacik, J. , Boelmans, K. , Löbel, U. , Holst, B. , Siemonsen, S. , & Fiehler, J. (2014). Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. NeuroImage, 84, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Serbruyns, L. , Leunissen, I. , Huysmans, T. , Cuypers, K. , Meesen, R. L. , van Ruitenbeek, P. , … Swinnen, S. P. (2015). Subcortical volumetric changes across the adult lifespan : Subregional thalamic atrophy accounts for age‐related sensorimotor performance declines. Cortex, 65, 128–138. [DOI] [PubMed] [Google Scholar]
- Stagg, C. J. (2014). Magnetic resonance spectroscopy as a tool to study the role of GABA in motor‐cortical plasticity. NeuroImage, 86, 19–27. [DOI] [PubMed] [Google Scholar]
- Stagg, C. J. , Bestmann, S. , Constantinescu, A. O. , Moreno Moreno, L. , Allman, C. , Mekle, R. , … Rothwell, J. C. (2011). Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. Journal of Physiology, 589(23), 5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner, P. , Edden, R. A. E. , Bompas, A. , Evans, C. J. , & Singh, K. D. (2010). More GABA, less distraction: A neurochemical predictor of motor decision speed. Nature Neuroscience, 13(7), 825–827. [DOI] [PubMed] [Google Scholar]
- Tremblay, S. , Beaulé, V. , Proulx, S. , de Beaumont, L. , Marjańska, M. , Doyon, J. , … Théoret, H. (2013). Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. Journal of Neurophysiology, 109(5), 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruitenbeek, P. , Serbruyns, L. , Solesio‐Jofre, E. , Meesen, R. , Cuypers, K. , & Swinnen, S. P. (2017). Cortical grey matter content is associated with both age and bimanual performance, but is not observed to mediate age‐related behavioural decline. Brain Structure and Function, 222(1), 437–448. [DOI] [PubMed] [Google Scholar]
- Ward, N. S. (2006). Compensatory mechanisms in the aging motor system. Ageing Research Reviews, 5(3), 239–254. [DOI] [PubMed] [Google Scholar]
- Yousry, T. A. , Schmid, U. D. , Alkadhi, H. , Schmidt, D. , Peraud, A. , Beuttner, A. , & Winkler, P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain, 120(1), 141–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2


