Abstract
In the research field of anxiety, previous studies generally focus on emotional responses following threat. A recent model of anxiety proposes that altered anticipation prior to uncertain threat is related with the development of anxiety. Behavioral findings have built the relationship between anxiety and distinct anticipatory processes including attention, estimation of threat, and emotional responses. However, few studies have characterized the brain organization underlying anticipation of uncertain threat and its role in anxiety. In the present study, we used an emotional anticipation paradigm with functional magnetic resonance imaging (fMRI) to examine the aforementioned topics by employing brain activation and general psychophysiological interactions (gPPI) analysis. In the activation analysis, we found that high trait anxious individuals showed significantly increased activation in the thalamus, middle temporal gyrus (MTG), and dorsomedial prefrontal cortex (dmPFC), as well as decreased activation in the precuneus, during anticipation of uncertain threat compared to the certain condition. In the gPPI analysis, the key regions including the amygdala, dmPFC, and precuneus showed altered connections with distributed brain areas including the ventromedial prefrontal cortex (vmPFC), dorsolateral prefrontal cortex (dlPFC), inferior parietal sulcus (IPS), insula, para‐hippocampus gyrus (PHA), thalamus, and MTG involved in anticipation of uncertain threat in anxious individuals. Taken together, our findings indicate that during the anticipation of uncertain threat, anxious individuals showed altered activations and functional connectivity in widely distributed brain areas, which may be critical for abnormal perception, estimation, and emotion reactions during the anticipation of uncertain threat.
Keywords: trait anxiety, uncertainty and anticipation model of anxiety (UAMA), functional connectivity, amygdala, dmPFC, precuneus
1. INTRODUCTION
The knowledge of uncertain threat in the future impacts people's anticipation and preparation for that threat. Alterations of this process contribute to the ontogeny and development of anxiety (Nitschke et al., 2006; Sarinopoulos et al., 2010). Previous studies about anxiety mainly focused on emotional reactions following threatening stimuli, but one recent influential theory (i.e., uncertainty and anticipation model of anxiety [UAMA]) has proposed that abnormal anticipatory cognitive and affective processes prior to potential threat serve as the fundamental mechanism of anxious pathology (Grupe & Nitschke, 2013; Nitschke et al., 2009). The aberrant and excessive anticipatory processes involved in anxiety is proposed to include increased attention to, intense emotional responses to, and inflated estimation of uncertain threat (Grupe & Nitschke, 2013), which engage multiple brain regions including the temporal gyrus, emotional circuit (such as the amygdala, bed nucleus of the stria terminalis (BNST), and insula), and medial prefrontal cortex (mPFC). However, to our knowledge, few studies have built a direct association between anxiety levels and altered brain organization including local activation and inter‐region connectivity underlying the anticipation of uncertain threat.
Anxiety is distinguished from fear regarding its association with the anticipation of uncertain threat. Compared to fear, anxiety is more likely to be triggered by distal and unpredictable threat that continues until the state of uncertainty is resolved, which engages multiple sustained and diffuse anticipatory cognitive and emotional responses. Many behavioral studies have found that participants showed stronger anticipatory responses for cues predicting uncertain threat than those predicting certain events (Grillon et al., 2004; Shankman et al., 2011). These effects were more prominent in anxious patients who suffer from anxiety disorders than healthy controls (Grillon et al., 2008; Grillon et al., 2009). Together, these behavioral findings indicate that the anticipation of uncertain threat contains distinct processes from the anticipation of certain events. Furthermore, alteration of this difference plays a central role in the pathology of anxiety. However, few imaging studies have examined the direct relationship between anxiety and abnormal uncertain threat processing from the perspective of brain activation and connectivity, which has been proposed in this UAMA theory.
1.1. Brain activation
Many studies have showed that anxiety is strongly related with hypevigilance to (Bar‐Haim et al., 2007), heighted emotional reaction to (Butler & Mathews, 1983; Stöber, 1997; Mitte, 2007), and inflated computation of uncertain threat on the behavioral level (Grillon et al., 2004; Shankman et al., 2011). These processes are commonly integrated in the anticipation of uncertain threat (Grupe & Nitschke, 2013). Regarding the neural mechanisms underlying uncertain threat anticipation, previous imaging studies have showed that the temporal gyrus is involved in attentional and perceptual processing in the context of uncertain threat (Bjork & Hommer, 2007; Critchley, Mathias, & Dolan, 2001; Kayser, Buchsbaum, Erickson, & D'Esposito, 2010). Additionally, the UAMA model emphasizes that there are different neural systems consisting of the amygdala, insula, and medial prefrontal cortex (mPFC), which are important for various anticipation processes of uncertain threat (Grupe & Nitschke, 2013). Among these systems, the amygdala is proposed as a key region for attentional and emotional responses to potential threat (Blanchard & Blanchard, 1988; Grillon, 2002; Lissek, Pine, & Grillon, 2006). As another key region in emotional processing, the insula is also important for anticipatory emotional responses during uncertain threat processing (Kuhnen & Knutson, 2005; Preuschoff, Quartz, & Bossaerts, 2008; Simmons et al., 2006). Regarding the estimation of uncertainty, the dorsomedial prefrontal cortex (dmPFC) plays a key role in computing uncertainty when participants predicted the probability of cues (Volz et al., 2003), and the ventromedial prefrontal cortex (vmPFC) overlapping with the orbitofrontal cortex (OFC) represents multiple levels of uncertainty spanning across different types of value (Peters & Büchel, 2010). Briefly, the UAMA proposes that anxiety is associated with altered anticipatory cognitive and emotional processing prior to uncertain threat. However, it remains unknown whether abnormal activation in these regions critical for the anticipation of uncertain threat would manifest in high anxious individuals.
1.2. Functional connectivity
The system neuroscience framework proposes that, aside from the local engagement (i.e., activation) of isolated regions, the communications (i.e., connectivity) between key brain areas are also essential for cognitive functions such as the anticipation of uncertain threat (Dosenbach et al., 2008; Mesulam, 1998). Indeed, the amygdala‐centered connectivity with cortical and subcortical regions are found to be involved in learning the relationship between cues and outcomes, initializing the emotional response to the cue before potential threatening stimulus (Grupe and Nitschke, 2013; and LeDoux, 2000). Altered connections within the amygdala‐centered network are associated with deficient fear extinction and over‐generalization of fear in individuals with anxiety disorders (Rauch et al., 2006). Meanwhile, the dmPFC‐related connectivity is important for value estimation and computation of uncertain threat (Kuhnen & Knutson, 2005; Padoa‐Schioppa, & Assad, 2006; Peters, & Büchel, 2010; Volz, Schubotz, & von Cramon, 2003), and it also plays a significant role in the appraisal and regulation of anxiety (Etkin et al., 2011). Another crucial hub region is the precuneus, which has wide‐spread connections with a number of functional regions involved in self‐referential processes (Raichle et al., 2001). Some studies have found that high trait anxiety is linked to more negative appraisal of threat, which strongly involves aversive self‐related memory (Ozawa et al., 2017). As a hub of self‐referential and emotional processes, the precuneus may integrate different information from other regions involved in uncertain threat processing. In the context of anticipating uncertain threat, it would be theoretically important to examine the mechanism of interactions between the amygdala, dmPFC, precuneus, and other cortical and subcortical regions. Investigating this issue may help us to detect neural circuits which bridges the anticipation of uncertain threat and emotion regulation, which are both core mechanisms underlying the pathology of anxiety. However, to our knowledge, few studies have examined whether the functional connectivity of key regions involved in the anticipation of uncertain threat would show abnormalities in anxious individuals.
The current study examined the anticipation of uncertain threat in anxious individuals by combining an emotional anticipation paradigm with functional magnetic resonance imaging (fMRI). Our aim is to investigate whether individuals with high levels of trait anxiety (as a vulnerability factor for anxiety disorders (see Bishop, 2009; Etkin et al., 2004) would show dysfunction of brain systems involved in the anticipation of uncertain threat, including attention, uncertainty estimation, and anticipatory emotional feelings. To further examine the interaction between key regions involved in altered anticipation of uncertain threat in anxious individuals, we employed general psychophysiological interactions (gPPI) analysis to detect brain circuits including amygdala‐related, dmPFC‐related, and precuneus‐related connectivity, all of which play important roles in anticipation of uncertain threat. According to previous findings, we hypothesized that anxious individuals would show altered functions of brain areas (e.g., activation) involved in uncertain threat processing including the temporal gyrus, amygdala, insula, dmPFC, and vmPFC. Moreover, we also hypothesized that altered interaction (e.g., connectivity) between the amygdala, dmPFC, and precuneus, as well as other regions in the brain would be involved in uncertain threat processing in high anxious individuals.
2. MATERIALS AND METHODS
2.1. Participants
In this study, we defined high and low anxiety groups according to the distribution of individual scores in a large sample of 220 college students (age: 22.31 ± 2.33 years; gender, 78 male/142 female; trait anxiety score, range: 21–72, mean ± SD: 43.57 ± 9.64) by using the Chinese version of the trait form of Spielberger's State and Trait Anxiety Inventory (STAI; Spielberger, 1985). Participants who scored high in trait anxiety (in the upper 20% of the distribution) were assigned to the high anxiety group (HAG), while the participants who scored low (in the lower 20% of the distribution) were assigned to the low anxiety group (LAG; Gu et al., 2010). As a result, 39 participants (mean ± SD: 23.56 ± 0.30 years, 19 male/20 female) were included in the study, such that 18 of them were in the HAG and 21 in the LAG. A t test showed that the two groups differed significantly in trait anxiety scores (53.33 vs. 32.67, p < .001), but not in age (23.78 vs. 23.38, p = .51). It turns out that the anxiety levels of the two groups in the present study were generally consistent with the standardized norm (high anxiety: 52.51; low anxiety: 34.11) proposed by Li and Qian (1995). All the participants in the current study reported no history of neurological or psychiatric disorders. This study has been approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences, and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
2.2. Stimulus
76 pictures including 72 for three conditions and 4 for square trials were chosen from the International Affective Picture System (IAPS; Lang et al. 2008), and each picture was presented only once in the whole task. The stimulus set included 36 aversive picture (valence rating [mean ± SD]: 2.77 ± 0.67, arousal rating: 6.49 ± 0.29) and 36 neutral pictures (valence rating: 5.29 ± 0.12, arousal rating: 3.34 ± 0.39). Of those 36 aversive pictures, 24 (valence rating: 2.77 ± 0.69, arousal rating: 6.49 ± 0.27) were presented on aversive trials and the remaining 12 (valence rating: 2.78 ± 0.65, arousal rating: 6.49 ± 0.33) on uncertain trials. Similarly, 24 neutral pictures (valence rating: 5.27 ± 0.11, arousal rating: 3.38 ± 0.38) were used on neutral trials and the remaining 12 (valence rating: 5.34 ± 0.13, arousal rating: 3.27 ± 0.40) on uncertain ones. There were significant difference of valence and arousal rating between aversive pictures used in the aversive trials and neutral pictures in the neutral trials (valence rating: = −17.61, p < .001; arousal rating: = 32.86, p < .001), similar difference between the aversive and neutral pictures in the uncertain trials (valence rating: = −13.31, p < .001; arousal rating: = 21.26, p < .001). There was no significant difference of valence and arousal rating between the aversive pictures used in the aversive trials and the uncertain trials (valence rating: = −0.06, p = .95; arousal rating: = 0.050, p = .96), or between the neutral pictures used in the neutral trials and the uncertain trials (valence rating: = −1.56, p = .13; arousal rating: = 0.828, p = .41).
2.3. Task procedure
Each trial started with a cue presentation for 2 s, then a fixation that presented randomly from 2 to 8 s, followed by a picture presented for 2 s and finally a fixation that presented randomly from 2 to 8 s. For the aversive anticipation condition, the cue “X” was always followed by an aversive picture. For the neutral anticipation condition, the cue “O” was always followed by a neutral picture. For the uncertain anticipation condition, the cue ‘‘?’’ was followed by either an aversive or neutral picture at exactly a 50/50 ratio (showed in Figure 1). The participants were instructed about the relationship of cue‐picture pairing before the scanning, but had no knowledge about stimulus probability in the uncertain anticipation condition. All cues were in white color and presented on the black background and had similar sizes. Trial order was pseudorandomized in order to make sure that no trial type was presented more than twice in a row. Before the fMRI task, participants finished a practice task to learn the association between cues and outcomes.
Figure 1.
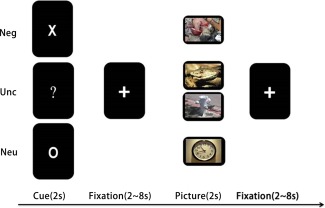
Task design. The paradigm presented to subjects during fMRI scanning. In each trial, subjects viewed an “X” (negative anticipation) or “O” (uncertain anticipation) or “?” (neutral anticipation) for 2 s, followed by a 2–8 s ISI, then, for “X” or “O”, followed by an aversive picture or neutral picture in a 100% for 2 s, for “?”, followed by either an aversive or neutral picture at exactly a 50/50 ratio for 2 s, finally a 2–8 s ISI following [Color figure can be viewed at http://wileyonlinelibrary.com]
There were a totally three functional scan runs, each of which consisting of eight aversive trials, eight neutral trials, and eight uncertain trials. Using a response box during the experiment, participants were instructed to press left button after each cue and each picture, and to press right button if they saw a square instead of the cue or picture. There were two square trials in the first functional run, two in the second run, and three in the third run. These square trials where square showed in either the position of cue (four trials) or picture (three trials) were used to make sure that participants would focus on the task. Because participants were not instructed to respond as quickly as they could, the reaction times were not analyzed.
2.4. Imaging data acquisition
Magnetic resonance images were acquired by a 3 Tesla SIEMENS MRI scanner (Erlangen, Germany). Functional images were collected with single‐shot gradient‐recalled echo planar imaging (GR‐EPI) sequences (TR = 2,000 ms, TE = 30 ms, FA = 90°, matrix = 64 × 64, FOV = 22 cm, 3 mm slice thickness, 1 mm spacing between slices, 32 transverse slices), aligned along the anterior commissure‐posterior commissure (AC‐PC) line. For spatial normalization, T1‐weighted anatomical images were collected in axial orientation using a 3D gradient‐recalled sequence (TR = 2,530 ms, TE = 3.37 ms, FA = 7°, matrix = 256 × 192, 1.33 mm slice thickness) on each subject.
2.5. Imaging data preprocessing
Data preprocessing was performed using Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging), a free and open source software written in MATLAB (The MathWorks, Inc.). All volumes were first sliced, then realigned for correction of head motion. Subsequently, the mean image was co‐registered to each participant's T1‐weighted MR image. The co‐registration parameters were used to register all aligned functional scans to the T1; images were transformed into a standard stereotaxic space with a resolution of 2 × 2 × 2 , using the Montreal Neurological Institute (MNI) echo‐planar imaging template in SPM. Functional images were spatially smoothed by convolution with an isotropic Gaussian kernel (FWHM = 6 mm).
2.6. Brain activation analysis
The smoothed volumes were used to build GLM model with regressors for anticipation conditions. To examine neural activation associated with uncertain and certain anticipation, respectively, three conditions were modeled as boxcar regressors and convolved with the canonical hemodynamic response function, fixation intervals as implicit baseline by using SPM8. Six realignment parameters were included to explain movement‐related variability. The contrast parameter images for all the three conditions (uncertain anticipation, certainty negative anticipation and certainty neutral anticipation) relative to the fixation baseline generated at the individual level were submitted to a second‐level group analysis. Previous studies using similar paradigm employed negative or neutral condition as a control condition, which is still debated (Krain et al., 2008; Sarinopoulos et al., 2010). The current study aims to examine anxiety‐related differences between uncertain threat and certainty conditions, while both negative and neutral conditions are considered as certainty conditions. Therefore, certainty negative and certainty neutral anticipation conditions were averaged as the control condition, and the contrast of interest focused on the comparison between two categories (uncertain threat/certainty condition) accordingly. A mixed analysis of variance (ANOVA) was conducted with anticipation (uncertain threat anticipation vs. certainty anticipation) as a within‐group factor and group (high vs. low anxiety) as a between‐group factor. All reported activations were thresholded at height threshold of p < .01 and an extent threshold of p < .05 with family‐wise error correction using a nonstationary suprathreshold cluster‐size approach based on Monte‐Carlo stimulations (Nichols & Hayasaka, 2003). Additionally, parameter estimates were extracted from those regions to characterize the response patterns of anticipation conditions in the two groups by using MarsBar (http://marsbar.sourceforge.net/), and the Spearman correlation analysis between parameter estimates of those regions and trait anxiety levels were performed for the uncertain and certain condition to characterize the association patterns. In order to check whether the observed experimental effects were driven by any difference between the certainty negative and neutral condition, first, we did the conjunction analysis between the difference map (negative anticipation vs. neutral anticipation; showed in Supporting Information Figure S1) and the contrast map between the uncertain threat and certain condition, and also between the difference map and the interaction map. The conjunction analysis showed that these regions did not have any contribution to the significant effect of the uncertainty versus certainty or the interaction effect. Second, we further compared the difference of activation between certain negative and neutral conditions for the significant regions in our main results. The results showed that no significant difference among these regions except IFG was found (showed in the Supporting Information Tables S2 and S3 and Figures S3 and S4), which further validated the reasonability of our approach.
2.7. General psychological physiological interaction analysis
To examine the modulation effect of trait anxiety on functional connectivity of key regions involved in the anticipation of uncertain threat with the rest parts of the brain during the task, we performed the general psychophysiological interaction (gPPI) analysis by using pre‐defined regions of interest including the amygdala in two sides and the significant clusters including dmPFC and precuneus as seeds which were achieved from the interaction between anticipation and group. dmPFC and precuneus are the hub regions in the anticipation of uncertain threat, which have multiple connections with other regions in the brain, therefore, we only included these two regions in the main PPI analysis. Connectivity results of other regions including the thalamus and middle temporal gyrus (no significant interaction effect for middle temporal gyrus) were showed in the Supporting Information for the readers who may be interested in (Supporting Information Figure S6). To exclude the possibility of bias effect driven by the selection of ROIs, we compared these ROIs and the representative clusters of these regions in the Neurosynth, a large‐scale coordinates‐based meta‐analysis toolbox (by using the keywords “dmPFC” and “precuneus”). It turns out that our ROIs largely overlapped with the representative clusters in the Neurosynth (showed in the Supporting Information Figures S7 and S8), which indicates these ROIs in the present study can properly represent a common definition of interest regions and specific functional areas involved in the present task. Finally, the seeds of amygdala, dmPFC and precuneus were used to extract the first eigenvariate of the individual voxel time‐series within the ROI. This representative time‐series was deconvolved from the HRF to generate an estimated neuronal time‐series. The product of this estimated neuronal time‐series and vectors representing each of the onsets for uncertain anticipation and certainty anticipation of threat were computed. These interaction terms were then reconvolved with the HRF and entered into a new GLM along with the vectors for the onsets for uncertain threat and certainty anticipation (i.e., the psychological vectors), the original eigenvariate time‐series and covariates of no interest (i.e., six movement parameters derived from realignment corrections). This “generalized” form of PPI analysis differs from standard PPI analyses, such that it allows simultaneous modeling of context‐dependent connectivity for all conditions and also shows increased sensitivity and specificity compared to traditional PPI analyses (http://www.nitrc.org/projects/gppi). Furthermore, in order to examine the interaction effect of functional connectivity of seeds, we followed the same analysis procedure as with the analysis of BOLD responses. Specifically, the contrast images of the PPI interaction term for anticipations (uncertain threat vs. certainty) in each participant were submitted to a mixed ANOVA model which resulted in an interaction effect map between anticipation (uncertain threat vs. certainty condition) and group to identify regions showing differential connectivity with seeds. Statistical thresholding and subsequent ROI analysis of this statistical map was carried out using the same criteria with the aforementioned activation analysis. Parameter estimates were also extracted from those regions to characterize the connectivity patterns of anticipation conditions of the two groups using MarsBar, and the correlation analysis between PPI parameter estimates of those regions and individual trait anxiety levels were performed for uncertain and uncertain condition to characterize the association patterns. In order to check whether the observed experimental effects were driven by any difference between the certainty negative and neutral condition, we performed the same validation analysis used in the activation analysis. Frist, the results of conjunction analysis showed that these regions did not have any contribution to the significant effect of the uncertainty versus certainty or the interaction effect. Second, the t test results showed that no significant difference among these regions was found (showed in the Supporting Information Tables S4 and S5 and Figure S5), which validated the reasonability of our approach.
3. RESULTS
3.1. Brain activation
By contrasting the uncertain threat and certainty anticipation conditions irrespective of the group factor, we found robust activations in the middle temporal gyrus (MTG), middle occipital gyrus (MOG), insula, hippocampus, dmPFC, inferior frontal gyrus (IFG), and precuneus (showed in Table 1 and Figure 2, p < .05 FWE‐corrected). By contrasting the HAG and LAG while collapsing across experimental conditions, we identified activations in (a) the superior frontal cortex and inferior parietal lobule in the fronto‐parietal network (FPN); (b) the parahippocampal gyrus and thalamus in subcortical emotion areas (showed in Table 1 and Figure 3, p < .05 FWE‐corrected). To better illustrate the engagement of these regions as a function of anxiety levels, a Spearman correlation analysis was performed for regions showing the group effects. The results showed that neural activations in all of these regions during the both conditions were positively related with trait anxiety levels (showed in Figure 4). For the interaction effect, we identified significant activations in the dmPFC, precuneus, thalamus, and middle temporal gyrus (showed in Table 1 and Figure 5, p < .05 FWE‐corrected), when contrasting neural activity of uncertain threat anticipation vs. certainty anticipation in the LAG with that in the HAG (Figure 5). These results indicate that neural activations in the dmPFC, thalamus, and middle temporal gyrus increased in the high anxious individuals when they were engaged in uncertainty anticipation compared to their low anxious counterparts. Furthermore, the results of the correlation analysis showed that neural activations of these three regions were positively related with trait anxiety levels during the uncertain condition but not the certain condition (showed in Figure 6). In contrast, the precuneus show weaker activations in high anxious individuals compared to the LAG, however, no significant correlation between activation of precuneus and trait anxiety levels was found in the two condition (ps > .13).
Table 1.
Brain regions showing main effect of uncertainty, anxiety and interaction effect between them
| Regions | Cluster size (voxel) | Peak T | Peak coordinates (x, y, z) | ||
|---|---|---|---|---|---|
| Uncertainty > Certainty | |||||
| L Superior Frontal Gyrus | 359 | 3.70 | −12 | 46 | 48 |
| R Superior Frontal Gyrus | 231 | 2.77 | 16 | 44 | 34 |
| R Middle Frontal Gyrus | 119 | 3.78 | 52 | 46 | 6 |
| R Precuneus | 880 | 4.08 | 6 | −56 | 38 |
| R Insula | 108 | 4.38 | 38 | −10 | 2 |
| R Hippocampus | 153 | 3.52 | 34 | −20 | −16 |
| L Middle Temporal Gyrus | 256 | 3.39 | −48 | −8 | −14 |
| L Middle Temporal Gyrus | 1444 | 4.87 | −54 | −34 | −4 |
| HAG > LAG | |||||
| L Middle Frontal Gyrus | 243 | 3.54 | −36 | 36 | 20 |
| L Inferior Parietal Lobule | 760 | 3.60 | −50 | −40 | 40 |
| R Inferior Parietal Lobule | 282 | 3.31 | 46 | −38 | 50 |
| L Thalamus | 276 | 3.17 | −10 | −16 | 16 |
| L Parahippocampal Gyrus | 730 | 4.07 | −30 | −50 | −2 |
| R Parahippocampal Gyrus | 664 | 4.29 | 32 | −50 | 0 |
| L Inferior Temporal Gyrus | 432 | 4.06 | −44 | −54 | −4 |
| Interaction | |||||
| L Superior Frontal Gyrus | 630 | 3.19 | −6 | 34 | 56 |
| L Precuneus | 615 | 2.64 | −10 | −24 | 60 |
| L Thalamus | 461 | 2.96 | −10 | −12 | 4 |
| R Middle Temporal Gyrus | 430 | 2.59 | 54 | −50 | 22 |
Notes: the coordinates are in the MNI system.
Abbreviations: HAG, high anxiety group; LAG, low anxiety group; L, left; R, right.
Figure 2.
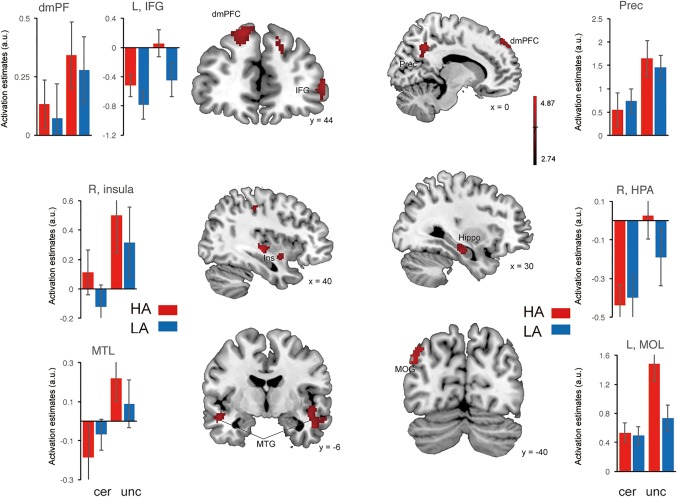
Brain systems showing significant main effect of uncertainty. Representative clusters show significant activation (coded in red) during contrasting between uncertain condition and certain condition, color bar represents T values. Parameter estimates of uncertain condition and certain condition for the high anxiety (coded in red) and low anxiety (coded in blue) group. Notes: HA, high anxiety; LA, low anxiety; a.u., arbitrary units; dmPFC, dorso‐medial prefrontal cortex; IFG, inferior frontal gyrus; Prec, precuneus; Ins, insula; Hippo, hippocampus; MTG, middle temporal gyrus; MOG, middle occipital gyrus. Error bars, SEM [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
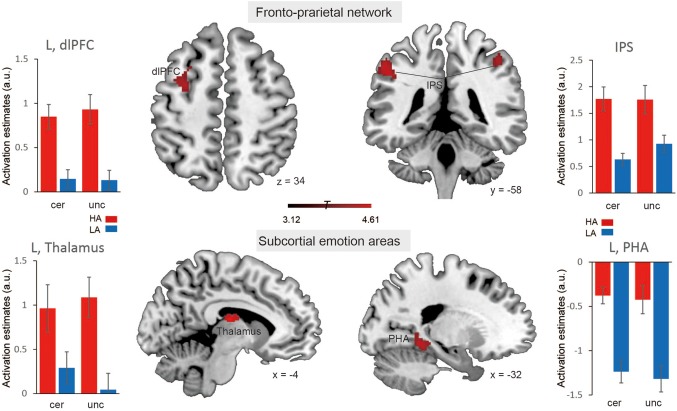
Brain systems showing significant main effects of trait anxiety. Representative clusters show significant activation (coded in red) during contrasting between the high anxiety group and low anxiety group across anticipation conditions, color bar represents T values. Parameter estimates of uncertain condition and certain condition for the high anxiety (coded in red) and low anxiety (coded in blue) group. Notes: HA, high anxiety; LA, low anxiety; a.u., arbitrary units; dlPFC, dorso‐lateral prefrontal cortex; IPS, inferior parietal sulcus; PHA, para‐hippocampus; L, left; R, right. Error bars, SEM [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
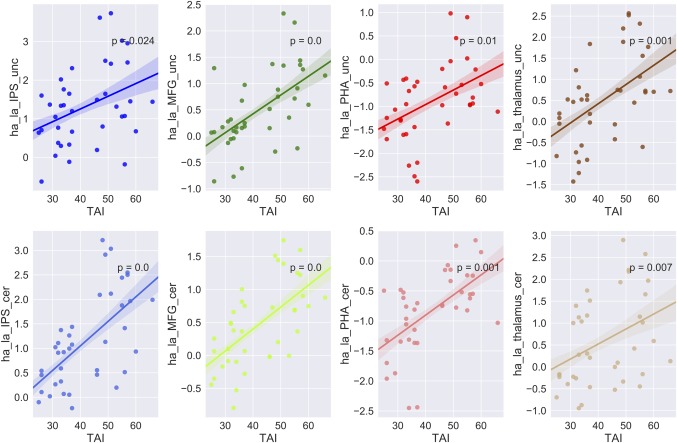
Correlation between activation of regions in the main effects of group and trait anxiety levels. Brain activation in the main effects of group versus trait anxiety scores. For each column, correlations were showed for uncertain condition (upper) and certain condition (lower). In each panel, the solid lines represent linear fitting of the correlation, the shadows indicate one standard error. Notes: MFG, middle frontal gyrus (dorso‐lateral prefrontal cortex); IPS, inferior parietal sulcus; PHA, para‐hippocampus; L, left; R, right [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
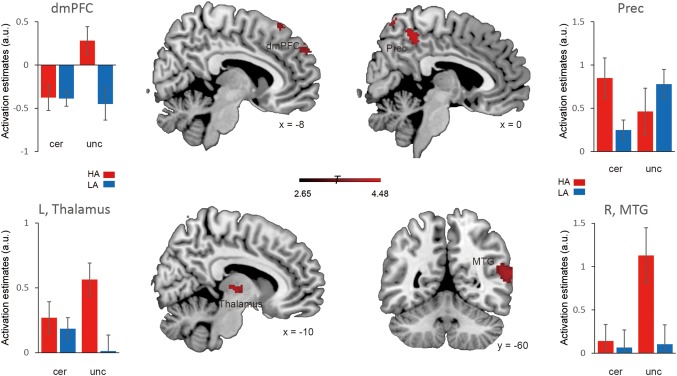
Brain regions showing significant interaction effect between anticipation conditions and anxiety groups. Representative clusters show significant activation (coded in red) in interaction contrast, color bar represents T values. Parameter estimates of uncertain condition and certain condition for the high anxiety (coded in red) and low anxiety (coded in blue) group. Notes: HA, high anxiety; LA, low anxiety; a.u., arbitrary units; dmPFC, dorso‐medial prefrontal cortex; Prec, precuneus; MTG, middle temporal gyrus; L, left; R, right. Error bars, SEM [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
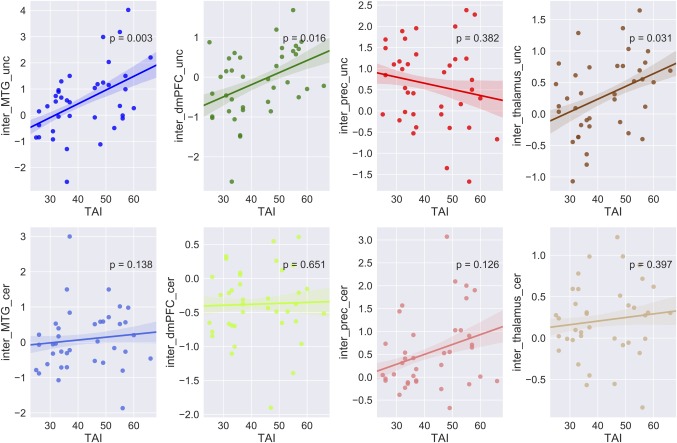
Correlation between activation of regions in the interaction effects and trait anxiety levels. Brain activation in the interaction effects versus trait anxiety scores. For each column, correlations were showed for uncertain condition (upper) and certain condition (lower). The solid lines represent linear fitting of the correlation, the shadows indicate one standard error. Notes: Notes: HA, high anxiety; LA, low anxiety; dmPFC, dorso‐medial prefrontal cortex; Prec, precuneus; MTG, middle temporal gyrus; L, left; R, right [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Functional connectivity
To further examine altered neural circuits in anxious individuals during uncertain threat anticipation, we performed the gPPI analysis by using the pre‐defined amygdala and the significant clusters in the whole‐brain contrast (including the dmPFC and precuneus) as seeds. Two‐way mixed ANOVAs of functional connectivity of seeds with group as the between‐subject factor and anticipation condition as the within‐subject factor were performed. For left amygdala, we found significant interaction effect in the middle frontal cortex (MFC), superior temporal gyrus (STG) and thalamus. For right amygdala, a significant effect in inferior occipital lobe (IOL; showed in Figure 7 and Table 2). For the dmPFC, the results showed significant interaction between anticipation and groups in the vmPFC (showed in Figure 9 and Table 2). For the precuneus, significant interactions were showed in several systems including the frontal‐parietal network consisting of the dlPFC and IPS, as well as emotion‐ and uncertainty‐related areas consisting of the insula, para‐hippocampus gyrus (PHA), and MTG (showed in Figure 10 and Table 2).
Figure 7.
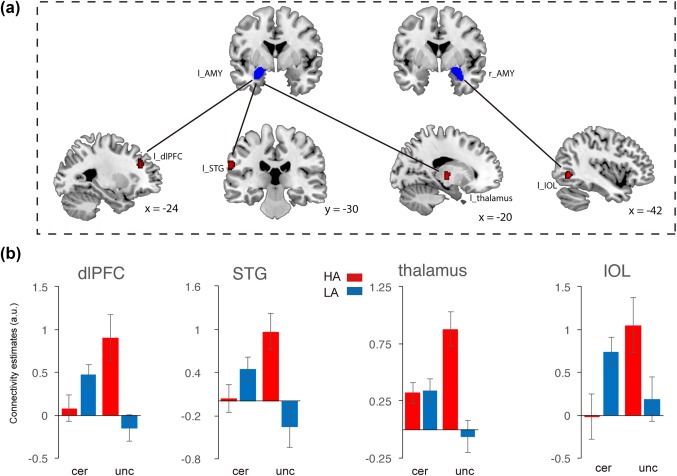
Brain region showing significant functional connectivity with amygdala in interaction effect between anticipation conditions and anxiety groups. (a) Representative clusters show significant connection with amygdala. (b) Parameter estimates of uncertain condition and certain condition for the high anxiety (coded in red) and low anxiety (coded in blue) group. Notes: HA, high anxiety; LA, low anxiety; dlPFC, dorso‐lateral prefrontal cortex; STG, superior temporal gyrus; IOL, inferior occipital lobe. Error bars, SEM [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Brain regions showing interaction effect between uncertainty and anxiety for connectivity analysis
| Regions | Cluster size (voxel) | Peak T | Peak coordinates (x, y, z) | ||
|---|---|---|---|---|---|
| L Amygdala as seed | |||||
| L Dorso‐lateral prefrontal cortex | 130 | 4.32 | −24 34 26 | ||
| L Superior Temporal Gyrus | 99 | 3.86 | −62 −30 32 | ||
| L Thalamus | 68 | 4.81 | −20 −16 2 | ||
| R Amygdala as seed | |||||
| L IOL | 61 | 4.13 | −42 −66 −2 | ||
| dmPFC as seed | |||||
| R Medial Frontal Gyrus | 379 | 3.22 | 4 | 50 | 4 |
| Precuneus as seed | |||||
| R Middle Frontal Gyrus | 134 | 3.13 | 40 | 12 | 46 |
| R Inferior Parietal Lobule | 255 | 2.89 | 60 | −52 | 40 |
| R Insula | 391 | 3.29 | 34 | −20 | 20 |
| R Parahippocampal Gyrus | 117 | 2.88 | 22 | −42 | 2 |
| L Superior Temporal Gyrus | 130 | 3.18 | −58 | −26 | 14 |
Notes: the coordinates are in the MNI system.
Abbreviations: L, left; R, right.
Figure 9.
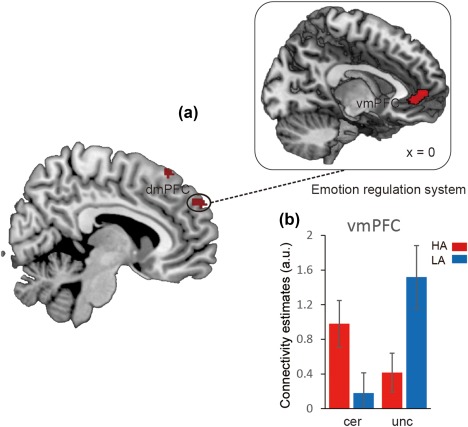
Brain region showing significant functional connectivity with dmPFC in interaction effect between anticipation conditions and anxiety groups. (a) Representative clusters show significant connection with dmPFC. (b) Parameter estimates of uncertain condition and certain condition for the high anxiety (coded in red) and low anxiety (coded in blue) group. Notes: HA, high anxiety; LA, low anxiety; a.u., arbitrary units; dmPFC, dorso‐medial prefrontal cortex; vmPFC, ventral‐medial prefrontal cortex. Error bars, SEM [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 10.
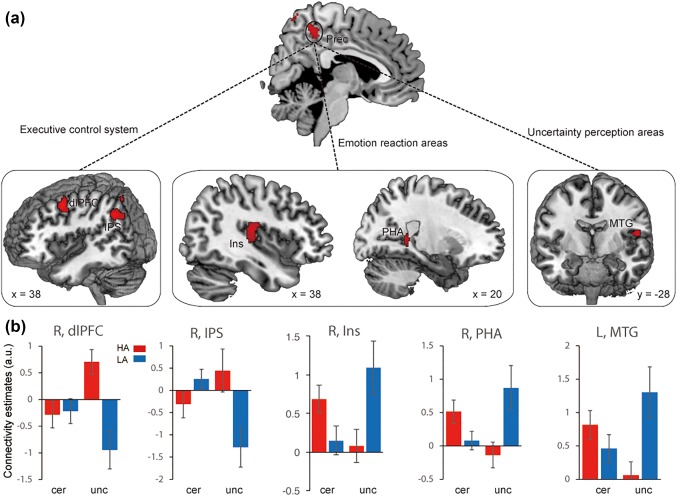
Brain systems showing significant functional connectivity with precuneus in interaction effect between anticipation conditions and anxiety groups. (a) Representative clusters show significant connection with precuneus. (b) Parameter estimates of uncertain condition and certain condition for the high anxiety (coded in red) and low anxiety (coded in blue) group. Notes: HA, high anxiety; LA, low anxiety; a.u., arbitrary units; Prec, precuneus; dlPFC, dorso‐lateral prefrontal cortex; IPS, inferior parietal sulcus; Ins, insula; PHA, para‐hippocampus; MTG, middle temporal gyrus; L, left; R, right. Error bars, SEM [Color figure can be viewed at http://wileyonlinelibrary.com]
Furthermore, connectivity patterns of anticipation conditions in the two groups were characterized (showed in Figures 7, 9, and 10b). The findings suggested that the amygdala showed increased connectivity with the MFG, STG, IOL and thalamus in the uncertain threat condition in the HAG compared to the LAG. Furthermore, the correlation analysis found that functional connectivity between the amygdala and these regions was positively related with trait anxiety levels during the uncertain condition, but showed an opposite pattern in the certain condition (except thalamus) (showed in Figure 8). Additionally, the results indicate that the dmPFC showed significantly weaker connection with the vmPFC in the HAG during the anticipation of uncertainty compared to the LAG. Additionally, functional connectivity between the precuneus and dlPFC, IPS and insula significantly increased, connectivity between precuneus and PHA and MTG significantly decreased in the HAG than the LAG. The correlation analysis showed that during the uncertain condition, dmPFC‐vmPFC, precuneus‐PHA, and precuneus‐MTG connectivity were negatively related with trait anxiety levels, while precuneus‐insula, precuneus‐dlPFC and precuneus‐IPS connectivity were positively correlated with trait anxiety levels. In the certain condition, no connections, except precuneus‐PHA, showed significant correlation with trait anxiety levels (showed in Figure 11).
Figure 8.
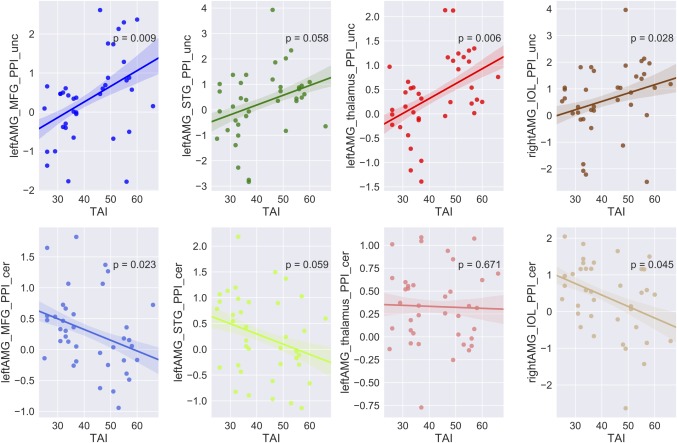
Correlation between amygdala‐related connectivity and trait anxiety levels. Brain connectivity versus trait anxiety scores. For each column, correlations were showed for uncertain condition (upper) and certain condition (lower). The solid lines represent linear fitting of the correlation, the shadows indicate one standard error. Notes: HA, high anxiety; LA, low anxiety; dlPFC, dorso‐lateral prefrontal cortex; STG, superior temporal gyrus; IOL, inferior occipital lobe [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 11.
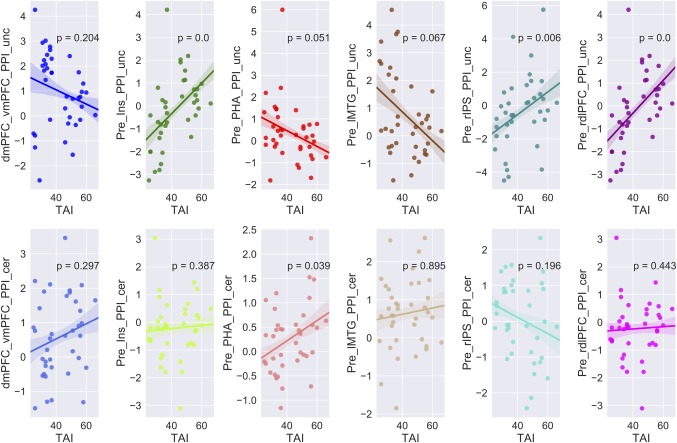
Correlation between dmPFC‐related and precuneus‐related connectivity and trait anxiety levels. Brain connectivity versus trait anxiety scores. For each column, correlations were showed for uncertain condition (upper) and certain condition (lower). The solid lines represent linear fitting of the correlation, the shadows indicate one standard error. Notes: Notes: HA, high anxiety; LA, low anxiety; dmPFC, dorso‐medial prefrontal cortex; vmPFC, ventral‐medial prefrontal cortex; Prec, precuneus; dlPFC, dorso‐lateral prefrontal cortex; IPS, inferior parietal sulcus; Ins, insula; PHA, para‐hippocampus; MTG, middle temporal gyrus; L, left; R, right [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
In the present study, our aim is to examine the brain organization underlying uncertain threat processing in anxious individuals. By integrating brain activation and functional connectivity analysis, we found that the anticipation of uncertain threat significantly engaged the dmPFC, precuneus, insula, and MTG. More critically, high anxious individuals showed stronger activations in the thalamus and PHA, as well as the dlPFC and IPS, compared to their low anxious counterparts during the anticipation process regardless of uncertainty levels. Moreover, high anxious individuals showed significantly increased activation in the thalamus, MTG, and dmPFC, as well as decreased activation in the precuneus, during the anticipation of uncertain threat compared to the certain condition. In addition, the follow‐up correlation analysis confirmed the engagement of these regions as a function of trait anxiety levels. We used gPPI functional connectivity analysis to further characterize the interactions between key regions in the anxiety circuit and other parts in the brain. We found that these regions including the amygdala, dmPFC, and precuneus involved in the anticipation of uncertain threat in anxious individuals showed altered connections with distributed brain areas (including the MFG, thalamus, vmPFC, dlPFC, IPS, insula, PHA, and MTG). The follow‐up correlation analysis further confirmed the strength of these connectivity as a function of trait anxiety levels. Taken together, our findings indicate that during the anticipation of uncertain threat, high anxious individuals showed altered activations and connectivity in widely distributed brain areas, which may be linked to abnormal perception, estimation, and emotion reactions.
4.1. Engagement of brain areas during anticipation of uncertain threat
We observed stronger activations in distributed regions when contrasting uncertain threat anticipation with certain anticipation across the two groups. These regions include the MTG, insula, dmPFC, and precuneus, which are thought to be involved in several fundamental anticipatory cognitive and emotional processes. Among these regions, the increased engagement of the MTG is consistent with its roles in attentional and perceptional vigilance to uncertain threats, as suggested by previous literature (Bjork & Hommer, 2007; Critchley et al., 2001; Kayser et al., 2010). The increased activation of insula during the anticipation for viewing negative stimulus may reflect emotional recruitment of participants, which is in line with the role of insula in anticipatory emotional feelings (Simmons et al., 2006). In addition, previous studies have found increased activation in the dmPFC, which is related with uncertainty estimation when the context is ambiguous (Michael et al., 2015; Volz et al., 2003). In the present study, we found activation of the dmPFC in the uncertain threat condition, which probably plays a role in the computation of uncertainty for potential threat. Moreover, the precuneus is regarded as a functional hub connecting many brain systems, and is involved in a wide range of cognitive functions including future‐oriented self‐processing (Fox et al., 2005; Raichle et al., 2001). Seeing that the anticipation of potential threat is strongly related with self (Marchetti et al., 2012), the engagement of the precuneus may reflect future‐oriented threat related self‐processing. Briefly, the current findings point out that the anticipation of uncertain threat engages distributed brain areas including the MTG, insula, dmPFC, and precuneus, which play important roles in attention, anticipatory emotional feeling, estimation of uncertainty, as well as uncertain threat‐related self‐processing.
The amygdala is a typical brain region involved in negative emotion processing. Previous studies have found activations of the amygdala during the anticipation of potential threat (Grupe et al., 2013; Nitschke et al., 2006). However, in the present study, no activation of the amygdala was found when contrasting between the uncertain and certain conditions, which might be unexpected. Considering that the importance of brain regions not only manifest on their local activation but also on how they interact with other regions in the brain, we chose pre‐defined masks of the amygdala in the PPI analysis to examine whether the amygdala would show abnormal interaction with other regions during the anticipation of uncertain threat in anxious individuals. The findings are discussed in the PPI part of the discussion (see below).
4.2. Trait anxiety modulates brain activations during anticipation
To examine the effect of anxiety on neural processing of anticipation, we investigated brain activations by contrasting HAG and LAG across different conditions. We found that high anxious individuals showed stronger activations in the thalamus, PHA, dlPFC, and IPS, which are important for both emotional processing and cognitive control. The thalamus is a core hub in the ‘low road’ emotional path, which is critical for automatic processing of emotional stimulus (Mizuno‐Matsumoto et al., 2013; Pessoa & Adolphs, 2010). Increased activation of the thalamus in this study may indicate rapid “high‐way” emotional reaction during the anticipation of the future. In addition, the dlPFC and IPS are regarded as parts of the executive control network involved in cognitive control (Seeley et al., 2007). Increased activations in this fronto‐parietal system may reflect compensatory function of cognitive control (i.e., impaired neural efficiency) in high anxious individuals, which is in line with previous findings that individuals with high anxiety required higher activation of the dlPFC to achieve the same level of cognitive control compared to low anxious people (Basten et al., 2011). This deficit in anxious individuals happened in both threat and safe (neutral) condition (Berggren & Derakshan, 2013; Bishop, Jenkins, & Lawrence, 2007; Bishop, 2009), indicating a general alteration of cognitive control in these people. Furthermore, the correlation analysis between beta values of these regions and trait anxiety score revealed similar patterns with the main effects of anxiety group, which indicates that the activation of these regions was linearly modulated by trait anxiety levels. In short, anticipation is a complex process engaging emotional and cognitive aspects, and increased emotional response and impaired efficiency of cognitive control during anticipation may contribute to pathology of anxiety.
4.3. Trait anxiety alters brain activation of uncertain threat anticipation
Most critically, we found that high anxious individuals showed increased activation in the MTG, thalamus, and dmPFC, and decreased activation in the precuneus during the anticipation of uncertain threat, as compared with low anxious individuals. These altered engagements may be linked to impaired perception, emotion processing, and computation of uncertainty. First, stronger activations of the MTG in high anxious individuals indicate that they engaged stronger perception for uncertain threat during the anticipation, seeing that anxiety has been consistently related with heightened perceptions and attention to threat (Bar‐Haim et al., 2007; Rosen, & Schulkin, 1998). A pervious study has showed that anxious individuals estimated uncertain threat as more dangerous (Sarinopoulos et al., 2010), thus they draw more attention and stronger perceptual resources (indicated by hyper‐activation in the MTG) to cues predicting uncertain threat.
Second, high anxious individuals showed stronger thalamus activation in the uncertain threat condition. Given the aforementioned role of the thalamus in rapid emotional processing, this finding suggests that when facing uncertain threat situations, high anxious individuals engage stronger and more rapid anticipatory emotional reaction (Mizuno‐Matsumoto et al., 2013). This statement is supported by previous behavioral studies which found that anxious individuals showed intense emotional reactions (i.e., startle) during the anticipation of uncertain threat than the certain condition (Grillon et al., 2004; Shankman et al., 2011).
Third, as mentioned before, the dmPFC is very important for the computation and estimation of uncertainty of potential threat (Michael et al., 2015; Volz et al., 2003). Some studies in anxiety disorders have revealed that high anxious individuals showed an inflated estimation of outcome when facing hypothetical uncertain situations about negative outcomes (Borkovec et al., 1999; Butler, & Mathews, 1983; Mitte, 2007). Hereby, excessive activities of the dmPFC in our results may imply overly pessimistic expectations in high anxious individuals, which are highly related to the symptoms of anxiety (Shepperd et al., 2005).
Last, the precuneus serves as a functional hub with high rates of metabolism in healthy subjects at resting state, showing distributed connections with different functional systems that involved in self‐referential activities and future planning (Raichle et al., 2001). The decreased activation in the precuneus in high anxious individuals may reflect impaired uncertainty‐related, future‐oriented self‐processing. The impaired engagement of the precuneus may lead to dysfunctions of the integration of different information from various regions involved in uncertainty processing including perception, emotion processing, and estimation. Crucially, our findings of these interaction effects is supported by the findings of correlation analysis which indicate the engagements of MTG, thalamus, and dmPFC as a function of trait anxiety levels during the uncertain condition.
To sum up, our findings suggest that when anticipating uncertain threats, high anxious individuals show altered neural activation in the MTG, thalamus, dmPFC, and precuneus, which may be involved in heighted perception, dramatic emotion reaction, altered estimation, and impaired information integration. These impaired functions are interconnected and interact with each other. Increased attention and emotional reactions for a potential uncertain threat may facilitate the processing of threat‐related stimulus, which further lead to inflated estimation of uncertainty of threat in the future. Furthermore, the experience‐related estimation bias during the anticipation of uncertain threat may also in turn result in drastic anticipatory attentional and emotional responses to a world that appears to be more dangerous to anxious individuals.
4.4. Trait anxiety modulates brain connectivity during anticipation of uncertain threat
To further characterize the roles of interactions between different brain systems involved in the anticipation of uncertain threat, we performed gPPI analysis by using key regions including the pre‐defined amygdala, dmPFC, and precuneus as ROIs. We found that the amygdala showed significantly increased connectivity with the dlPFC, STG, IOL, and thalamus. Altered connectivity between the amygdala and these regions in high anxious individuals is consistent with previous studies showing the roles of amygdala in the anticipation of uncertain threat (Grupe et al., 2013; Nitschke et al., 2006). Cortical and subcortical circuits including the prefrontal cortex and thalamus showed abnormal connections with the amygdala involved in the anticipation of uncertain threat, which may allow for an interaction between emotional information processing, uncertain estimation, and executive control. Further correlation analysis showed that high trait anxiety level enhances the connectivity between the amygdala and these regions. In particular, the modulation effect of anxiety manifested in uncertain situations, which indicates that these connections are specific for uncertain‐related information processing in anxious individuals. Considering the brain activation results, although we did not find any activation of the amygdala when contrasting between the uncertain and certain conditions, the abnormal connectivity between the amygdala and distributed cortical/subcortical regions were indeed involved in the uncertain condition in anxious individuals. Our findings show that the multivariable connectivity approach is more sensitive to detect altered neural patterns associated with anxiety than the classic univariate activation approach. We therefore suggest future studies to combine various advanced imaging analysis approaches to provide a more comprehensive understanding of neural mechanism underlying cognitive functions.
The dmPFC showed significantly weaker connection with the vmPFC in high anxious individuals compared to low anxious ones during the anticipation of uncertain threat. The decreased connectivity between the dmPFC and vmPFC implies that the impaired uncertain threat anticipation might be related to abnormal emotion regulation in high anxious individuals. This interpretation is supported by the suggestion that the neural circuit between the dmPFC and vmPFC are both associated with uncertain threat processing and emotion regulation. During the uncertain threat processing, the vmPFC is suggested to estimate the value of potential threat and the dmPFC computes the probability of uncertain threat. By the same token, the vmPFC is critical for the suppression of emotion regulation and the dmPFC is highly involved in reappraisal‐based emotion regulation (Etkin et al., 2011). An influential neurocognitive model of emotion proposes that the route between the dmPFC and vmPFC may be critical for evaluating and inhibiting negative emotion (Etkin et al., 2011). Therefore, our finding may bridge the research fields of the anticipation of uncertain threat and emotion regulation, which are both involved in the mechanisms of anxiety.
Additionally, functional connectivity between the precuneus and dlPFC as well as IPS in the executive control network significantly increased in high anxious individuals than low anxious ones when anticipating uncertain threat. The precuneus is consisted of the default mode network (DMN) that is involved in self‐processing, and also strongly interacts with the executive control network critical for cognitive and emotional regulation of anxiety (Fox et al., 2005). In this study, we found that the connectivity between the precuneus and dlPFC/IPS in the executive control network significantly increased in highly anxious individuals than low anxious ones. Increased interactions between these two networks may reflect more efforts of cognitive and emotional regulation in high anxious individuals when anticipating uncertain threat. Furthermore, the precuneus had weaker connections with the MTG (an area associated with uncertainty perception) and the PHA (involved in the emotion processing system) in high anxious individuals, which is likely to be associated with ineffective regulation of perception of uncertainty and emotional responses. To sum up, our findings are in line with many resting‐state and task‐related studies that highlight the precuneus as a functional hub in the whole brain network, and the hub region in anxiety‐related context may abnormally interact with different brain systems including uncertainty perception, emotional reaction, and executive control systems underlying uncertain threat processing in anxious individuals (Cavanna & Trimble, 2006; Zhang & Li, 2012).
Taken together, the amygdala, dmPFC, and precuneus are key nodes in the neural systems underlying emotional responses, estimating uncertain threat, and regulating emotion. All of them showed impaired connections with the neural systems responsible for uncertainty perception and emotion reaction, as well as compensatory increased connectivity with the executive control network in high anxious individuals. These altered neural interactions might originate from the dysfunction of central amygdala‐centered circuits and dmPFC‐vmPFC circuit that are critical for emotional reactions and uncertainty estimation of potential threat. One potential explanation is that these neural routes‐centered impairments contribute to the development of anxiety. People learn to associate cues with potential threats. The repeated associative learning during the early stages of lifespan contributes to estimation bias of cost or possibility of potential threat. It is possible to result in impaired amygdala‐centered circuits and dmPFC‐vmPFC connection and in turn further altering emotional reactions and computation of uncertain threat. From this perspective, an anxious individual builds up neural pathways of anxiety in a way similar with a concert pianist strengthens neural pathways of musicianship through hours of daily practice. The only difference is that pianists construct music‐related neural circuits, while anxious people establish emotional reactions and uncertain estimation related neural routes. Considering this proposition, the interventions targeting these circuits are of great importance for both mechanism‐directed and clinic‐focused studies.
5. LIMITATIONS
In the present study, we have tried to address the concern that including extremely low anxious people as controls may have biased the brain activation and functional connectivity findings. First, we followed the similar criterion used in many previous studies to divide people into the low and high groups, which showed reliable group differences in various experimental tasks (e.g., Gu, Ge, Jiang, & Luo, 2010; de Visser et al., 2010). Second, on the brain level, the scatter plots (see Figures 4, 6, 8, and 11) in our study showed that the interaction effects were not driven by outliers, supported by the phenomenon that the beta values of activation and connectivity in the low and high anxiety groups were continuously rather than separately distributed, indicating that values in these two groups were comparable. Furthermore, the correlation analysis indicated that trait anxiety level was linearly associated with brain activation and connectivity patterns, which further help resolve the concern that the group effects reported in the ANOVA analysis were due to the influence of outliers. In a word, brain activation and connectivity patterns in low anxious individuals were comparable with those in high anxious individuals, making it reasonable to conduct ANOVA analysis in the present study. However, it is still worth to note that considering other potential confounding factors, full characterization of resilience, coping style, and other related features of participants would provide a more comprehensive understanding to the mechanism of anticipating uncertain threat in follow‐up studies.
There might be another concern about combining the certain neutral and negative conditions as the control condition, which was used to compare with the “uncertain threat anticipation” condition in this study. As we mentioned in the Methods part, previous studies have used similar paradigm employing negative or neutral condition as a control condition, the reliability of which is still debated (Krain et al., 2008; Sarinopoulos et al., 2010). In the present study, we aimed to examine anxiety‐related differences between uncertain threat and certain conditions; therefore, both negative and neutral conditions were combined as a “certain” condition. Consequently, it was possible that the observed experimental effects were driven by the difference between the certain negative and neutral conditions. To address this possibility, we finished several additional analyses as follows. First, we employed two conjunction analyses (a) between the difference map (negative anticipation vs. neutral anticipation; showed in Supporting Information Figures S1 and S2) and the contrast map across the uncertain threat and certain conditions, and (b) between the difference map and the interaction map for both brain activation and connectivity. The results showed that these regions did not have any contribution to our major findings, that is, the main effect of the uncertainty versus certainty or the interaction effect. Second, we further compared the difference of brain activation and connectivity between certain negative and neutral conditions in the significant regions from our major results. The results showed no significant difference in these regions except the IFG was found (showed in the Supporting Information Tables S2–S5), which further validated the reliability of our approach. In a word, the results of these validation analyses indicate that our findings were mainly driven by the difference between uncertain and certain conditions, rather than the difference between certain negative and neutral conditions. Still, it is worth noting that our major findings were based on contrasting the anticipation of uncertain threat and the averaged anticipation of certain conditions.
Several other limitations related to the present study should be noted. First, we did not found any group effect or interaction effect on neural activations of the amygdala. Considering that the amygdala is important for emotional processing and anticipation of potential threat, this result might be out of expectation. However, functional connectivity between several key cortical and subcortical regions and the amygdala has been involved in the anticipation of potential threat, which was positively related with trait anxiety levels (see above). It is possible that high anxious individuals do not show altered local engagements of the amygdala, but abnormal global communication between the amygdala and distributed systems in the brain, which contributes to cognitive and emotional processing during the anticipation of uncertain threat. Second, the present threshold for multiple comparisons might be liberal, although it has been used in many previous studies (Qin et al., 2014a; Qin et al., 2014b). However, reducing false positives may also increase the risk of missing meaningful findings. A threshold which can balance between the probability of false alarm and that of miss would facilitate novel findings. We agree that it would be important for follow‐up studies to replicate the current findings with alternative methodologies.
6. CONCLUSION
By characterizing alterations in task‐related brain activation and functional connectivity during the anticipation of uncertain threat in individuals with high and low trait anxiety levels, our findings show altered activations in the dmPFC, precuneus, thalamus, and MTG; impaired connections of amygdala‐thalamus, amygdala‐PFC, dmPFC‐vmPFC, precuneus‐FPN, precuneus‐MTG, and precuneus‐PHA during the anticipation of uncertain threat in anxious individuals, which may be involved in emotional reactions to, estimation of, and perception of uncertain threat. All of these altered neural patterns may together contribute to the pathology of anxiety. The current study also provides a new insight for neural and behavioral treatments focusing on the neural circuits that underlies uncertainty estimation and emotion regulation in anxiety‐related disorders.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
The authors thank Chunliang Feng for his helpful comments on manuscript revisions. This research was supported by National National Natural Science Foundation of China (31671136, 31530031, 31571124, 81471376), Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning, Shenzhen Peacock Plan (KQTD2015033016104926) and the Guangdong Pearl River Talents Plan Innovative and Entrepreneurial Team grant (2016ZT06S220).
Geng H, Wang Y, Gu R, et al. Altered brain activation and connectivity during anticipation of uncertain threat in trait anxiety. Hum Brain Mapp. 2018;39:3898–3914. 10.1002/hbm.24219
Funding information National Natural Science Foundation of China, Grant/Award Numbers: 31671136, 31530031, 31571124, and 81471376; Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning, Shenzhen Peacock Plan; Grant/Award Number: KQTD2015033016104926; Guangdong Pearl River Talents Plan Innovative and Entrepreneurial Team grant; Grant/Award Number: 2016ZT06S220
REFERENCES
- Bar‐Haim, Y. , Lamy, D. , Pergamin, L. , Bakermans‐Kranenburg, M. J. , & van IJzendoorn, M. H. (2007). Threat‐related attentional bias in anxious and nonanxious individuals: A meta‐analytic study. Psychological Bulletin, 133(1), 1–24. [DOI] [PubMed] [Google Scholar]
- Basten, U. , Stelzel, C. , & Fiebach, C. J. (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 23(10), 3132–3145. [DOI] [PubMed] [Google Scholar]
- Berggren, N. , & Derakshan, N. (2013). Attentional control deficits in trait anxiety: Why you see them and why you don't. Biological Psychology, 92(3), 440–446. [DOI] [PubMed] [Google Scholar]
- Bishop, S. J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12(1), 92–98. [DOI] [PubMed] [Google Scholar]
- Bishop, S. J. , Jenkins, R. , & Lawrence, A. D. (2007). Neural processing of fearful faces: Effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex, 17(7), 1595–1603. [DOI] [PubMed] [Google Scholar]
- Bjork, J. M. , & Hommer, D. W. (2007). Anticipating instrumentally obtained and passively‐received rewards: A factorial fMRI investigation. Behavioural Brain Research, 177(1), 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec, T. D. , Hazlett‐Stevens, H. , & Diaz, M. L. (1999). The role of positive beliefs about worry in generalized anxiety disorder and its treatment. Clinical Psychology & Psychotherapy, 6(2), 126–138. [Google Scholar]
- Butler, G. , & Mathews, A. (1983). Cognitive processes in anxiety. Adv Behav Res Ther 5. Cognitions and Mood: Clinical Aspects and Applications, 5(1), 51–62. [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain: A Journal of Neurology, 129(Pt 3), 564–583. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Mathias, C. J. , & Dolan, R. J. (2001). Neuroanatomical basis for first‐and second‐order representations of bodily states. Nature Neuroscience, 4(2), 207–212. [DOI] [PubMed] [Google Scholar]
- Blanchard, D. C. , & Blanchard, R. J. (1988). Ethoexperimental approaches to the biology of emotion. Annual Review of Psychology, 39, 43–68. [DOI] [PubMed] [Google Scholar]
- de Visser, L. , van der Knaap, L. J. , van de Loo, A. J. A. E. , van der Weerd, C. M. M. , Ohl, F. , & van den Bos, R. (2010). Trait anxiety affects decision‐making differently in healthy men and women: Towards gender‐specific endophenotypes of anxiety. Neuropsychologia, 48(6), 1598–1606. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. F. , Fair, D. A. , Cohen, A. L. , Schlaggar, B. L. , & Petersen, S. E. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12(3), 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Klemenhagen, K. C. , Dudman, J. T. , Rogan, M. T. , Hen, R. , Kandel, E. R. , & Hirsch, J. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–1055. [DOI] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon, C. (2002). Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry, 52(10), 958–975. [DOI] [PubMed] [Google Scholar]
- Grillon, C. , Baas, J. P. , Lissek, S. , Smith, K. , & Milstein, J. (2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118(5), 916–924. [DOI] [PubMed] [Google Scholar]
- Grillon, C. , Lissek, S. , Rabin, S. , McDowell, D. , Dvir, S. , & Pine, D. S. (2008). Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry, 165(7), 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon, C. , Pine, D. S. , Lissek, S. , Rabin, S. , Bonne, O. , & Vythilingam, M. (2009). Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry, 66(1), 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D. W. , & Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews. Neuroscience, 14(7), 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D. W. , Oathes, D. J. , & Nitschke, J. B. (2013). Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex, 23(8), 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, R. , Ge, Y. , Jiang, Y. , & Luo, Y. (2010). Anxiety and outcome evaluation: The good, the bad and the ambiguous. Biological Psychology, 85(2), 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser, A. S. , Buchsbaum, B. R. , Erickson, D. T. , & D'Esposito, M. (2010). The functional anatomy of a perceptual decision in the human brain. Journal of Neurophysiology, 103(3), 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain, A. L. , Gotimer, K. , Hefton, S. , Ernst, M. , Castellanos, F. X. , Pine, D. S. , & Milham, M. P. (2008). A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biological Psychiatry, 63(6), 563–568. [DOI] [PubMed] [Google Scholar]
- Kuhnen, C. M. , & Knutson, B. (2005). The neural basis of financial risk taking. Neuron, 47(5), 763–770. [DOI] [PubMed] [Google Scholar]
- Lang, P. J. , Bradley, M. M. , & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐8. Gainesville (FL): University of Florida
- LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Li, W. , & Qian, M. (1995). Revised norm of State‐aTrait Anxiety Inventory in Chinese college students. Acta Scientiarum Naturalium Universitatis Pekinensis, 31(1), 108–112. [Google Scholar]
- Lissek, S. , Pine, D. S. , & Grillon, C. (2006). The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology, 72(3), 265–270. [DOI] [PubMed] [Google Scholar]
- Marchetti, I. , Koster, E. H. W. , Sonuga‐Barke, E. J. , & Raedt, R. D. (2012). The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychology Review, 22(3), 229–251. [DOI] [PubMed] [Google Scholar]
- Mesulam, M. M. (1998). From sensation to cognition. Brain, 121(6), 1013–1052. [DOI] [PubMed] [Google Scholar]
- Michael, E. , Gardelle, V. , de, Nevado‐Holgado, A. , & Summerfield, C. (2015). Unreliable evidence: 2 sources of uncertainty during perceptual choice. Cerebral Cortex (New York, N.Y. : 1991), 25(4), 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitte, K. (2007). Anxiety and risky decision‐making: The role of subjective probability and subjective costs of negative events. Personality and Individual Differences, 43(2), 243–253. [Google Scholar]
- Mizuno‐Matsumoto, Y. , Hayashi, T. , Okamoto, E. , Miwa, D. , Asakawa, T. , Muramatsu, A. , … Murata, T. (2013). Human‐related emotional stimuli can cause a hippocampal and thalamic over‐response in people with unstable personalities. Journal of Behavioral and Brain Science, 03(07), 509–517. [Google Scholar]
- Nichols, T. , & Hayasaka, S. (2003). Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research, 12(5), 419–446. [DOI] [PubMed] [Google Scholar]
- Nitschke, J. B. , Sarinopoulos, I. , Mackiewicz, K. L. , Schaefer, H. S. , & Davidson, R. J. (2006). Functional neuroanatomy of aversion and its anticipation. NeuroImage, 29(1), 106–116. [DOI] [PubMed] [Google Scholar]
- Nitschke, J. B. , Sarinopoulos, I. , Oathes, D. J. , Johnstone, T. , Whalen, P. J. , Davidson, R. J. , & Kalin, N. H. (2009). Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. The American Journal of Psychiatry, 166(3), 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa‐Schioppa, C. , & Assad, J. A. (2006). Neurons in the orbitofrontal cortex encode economic value. Nature, 441(7090), 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa, L. , & Adolphs, R. (2010). Emotion processing and the amygdala: From a'low road'to'many roads’ of evaluating biological significance. Nature Reviews. Neuroscience, 11(11), 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, J. , & Büchel, C. (2010). Neural representations of subjective reward value. Behavioural Brain Research, 213(2), 135–141. [DOI] [PubMed] [Google Scholar]
- Preuschoff, K. , Quartz, S. R. , & Bossaerts, P. (2008). Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience, 28(11), 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, S. , Cho, S. , Chen, T. , Rosenberg‐Lee, M. , Geary, D. C. , & Menon, V. (2014). Hippocampal‐neocortical functional reorganization underlies children's cognitive development. Nature Neuroscience, 17(9), 1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, S. , Young, C. B. , Duan, X. , Chen, T. , Supekar, K. , & Menon, V. (2014). Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biological Psychiatry, 75(11), 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, S. L. , Shin, L. M. , & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biological Psychiatry, 60(4), 376–382. [DOI] [PubMed] [Google Scholar]
- Rosen, J. B. , & Schulkin, J. (1998). From normal fear to pathological anxiety. Psychological Review, 105(2), 325–350. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos, I. , Grupe, D. W. , Mackiewicz, K. L. , Herrington, J. D. , Lor, M. , Steege, E. E. , & Nitschke, J. B. (2010). Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex, 20(4), 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman, S. A. , Robison‐Andrew, E. J. , Nelson, B. D. , Altman, S. E. , & Campbell, M. L. (2011). Effects of predictability of shock timing and intensity on aversive responses. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 80(2), 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd, J. A. , Grace, J. , Cole, L. J. , & Klein, C. (2005). Anxiety and outcome predictions. Personality & Social Psychology Bulletin, 31(2), 267–275. [DOI] [PubMed] [Google Scholar]
- Simmons, A. , Strigo, I. , Matthews, S. C. , Paulus, M. P. , & Stein, M. B. (2006). Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety‐prone subjects. Biological Psychiatry, 60(4), 402–409. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. (1985). Assessment of state and trait anxiety: Conceptual and methodological issues. South African Journal of Psychology, 2, 6–16. [Google Scholar]
- Stöber, J. (1997). Trait anxiety and pessimistic appraisal of risk and chance. Personality and Individual Differences, 22(4), 465–476. [Google Scholar]
- Volz, K. G. , Schubotz, R. I. , & von Cramon, D. Y. (2003). Predicting events of varying probability: Uncertainty investigated by fMRI. NeuroImage, 19(2), 271–280. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , & Li, C. R. (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage, 59(4), 3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa, T. , Ycu, E. A. , Kumar, A. , Yeh, L.‐F. , Ahmed, T. , Koivumaa, J. , & Johansen, J. P. (2017). A feedback neural circuit for calibrating aversive memory strength. Nature Neuroscience, 20(1), 90–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


