Abstract
Graphical network characteristics and nonstationary functional connectivity features, both derived from resting‐state functional magnetic resonance imaging (rsfMRI) data, have been associated with cognitive performance in healthy subjects. How these features jointly relate to cognition in diseased states has not been investigated. In this study, 46 relapsing–remitting multiple sclerosis subjects underwent rsfMRI scans and a focused cognitive battery. With a sliding window approach, we examined six dynamic network features that indicated how connectivity changed over time as well as six measures derived from graph theory to reflect static network characteristics. Multiset canonical correlation analysis (MCCA) was then carried out to investigate the relations between dynamic network features, stationary network characteristics, cognitive testing, demographic, disease severity, and mood. Multiple sclerosis (MS) subjects demonstrated weaker connectivity strength, decreased network density, reduced global changes, but increased changes in interhemispheric connectivity compared to controls. The MCCA model determined that executive functions and processing speed ability measured by Wechsler Adult Intelligence Scale IV (WAIS‐IV) Working Memory Index, WAIS‐IV Processing Speed Index, and the Verbal Fluency Test were positively correlated with education, dynamic connectivity, and static connectivity strength; while poor task switching was correlated with disease severity, psychiatric comorbidities such as depression, anxiety, and fatigue, and static network density. Taken together, our results suggest that better executive functioning in MS requires maintenance of a continued coordination between stationary and dynamic functional connectivity as well as the support of education, and dynamic functional connectivity may provide an additional cognitive biomarker of disease severity in the MS population.
Keywords: cognitive function, dynamic functional connectivity, graph theory, multiset canonical correlation analysis, network characteristics, resting‐state fMRI, sliding window approach
1. INTRODUCTION
Functional connectivity in human brain networks can be investigated with functional MRI (fMRI) approaches and is usually estimated by calculating the temporal correlation (e.g., Pearson's r correlation) or neural influence (estimated by, e.g., conditional dependence) between brain signals among anatomically segregated brain regions (Friston, 1994; Friston, 2011). These methods usually assume that the estimated functional connectivity is temporally stationary, that is, does not change over time. However, connectivity fluctuates across time from seconds to minutes even in the resting state, which can be estimated by models of dynamic functional connectivity (Allen et al., 2014; Betzel, Fukushima, He, Zuo, & Sporns, 2016; Chang & Glover, 2010; Handwerker, Roopchansingh, Gonzalez‐Castillo, & Bandettini, 2012; Hutchison, Womelsdorf, Allen, et al., 2013; Jones et al., 2012). The simplest, and perhaps most common time‐varying approach to assess dynamic functional connectivity is to estimate correlations between brain regions within a fixed‐length, sliding window, with the (possibly overlapping) windows ultimately moved over the entire data. Nevertheless, there are potential pitfalls with such an approach (Hindriks et al., 2016; Hutchison, Womelsdorf, Gati, Everling, & Menon, 2013); if the window is too long, then important dynamic changes may be missed. If the window is too short, the connectivity estimates may be unstable as too few samples are available for the statistical inferences. A window length (WL) of 30–60 s for fMRI data has been heuristically suggested (Leonardi & Van De Ville, 2015; Zalesky & Breakspear, 2015). The time‐varying approach can capture the temporal variability of functional connectivity, but the spatial network characteristics cannot be estimated with this method. A graph theoretical approach appears to be a powerful technique to obtain topological information of networks, which quantifies information flow in stationary functional connectivity globally and locally.
Graph theoretical analysis summarizes network characteristics and can represent how efficiently the networks propagate information globally and locally (Sporns, 2013). Global efficiency, a measure of integration, has been related to working memory and verbal comprehension; while modularity, a measure of segregation, correlates with motor task performance (Cohen & D'Esposito, 2016; Pamplona, Santos Neto, Rosset, Rogers, & Salmon, 2015). Hub structures are related to higher‐order cognitive functions such as executive function (Reineberg & Banich, 2016). With graphical metrics, the brain can be divided into modules, with each module related to an individual cognitive component (Bertolero, Yeo, & D'Esposito, 2015). These “cognitive brain modules” are linked by hub structures and the hubs with higher connectivity engage more cognitive modules. Alterations in graphic theoretical measures of the computed brain stationary connectivity networks have been associated with a variety of cognitive abilities and disease populations. Altered hub structure has been demonstrated in people with Parkinson's disease with attention/executive deficits, with hub nodes having reduced importance (Baggio et al., 2014). Global network measures cannot significantly distinguish depressed subjects from healthy controls; however, with a support vector machine approach, individuals have been accurately classified with network measures (Lord, Horn, Breakspear, & Walter, 2012), suggesting that a combination of machine learning methods and connectivity features is beneficial to assist diagnosis.
Dynamic functional connectivity, as also referred to network dynamics and assessed by time‐varying approaches, appears to be particularly pertinent to several cognitive processes including memory, language, attention, and executive functions (Braun et al., 2015; Bressler & Scott Kelso, 2001; Kucyi, Hove, Esterman, Hutchison, & Valera, 2016; Mattar, Cole, Thompson‐Schill, & Bassett, 2015; McIntosh, Kovacevic, & Itier, 2008; Shafto & Tyler, 2014; Thompson et al., 2013). Increased dynamical variability in the electroencephalogram was found to be correlated with better performance (i.e., shorter reaction time and higher accuracy) in a memory task, emphasizing the importance of brain complexity in cognitive development (McIntosh et al., 2008). There are associations between dynamic changes in frontoparietal/frontotemporal networks and neuropsychological measures, showing that the flexibility of neuronal activity in the frontal regions is cognitively beneficial for working memory performance and executive functioning (Braun et al., 2015). This has led to a proposed “functional cartography” of the cognitive system, based on the estimated amount of integration and recruitment of brain regions during different cognitive processes (Mattar et al., 2015). Network dynamics may also be important for language function in an aging population (Shafto & Tyler, 2014). Measures of dynamic functional connectivity have been recently applied to understand how the human brain is affected by diseases such as Parkinson's disease, Schizophrenia, Alzheimer's disease, and major depression (Damaraju et al., 2014; Kaiser et al., 2015; Madhyastha, Askren, Boord, & Grabowski, 2014; Sakoglu et al., 2010; Wee, Yang, Yap, & Shen, 2015).
A sliding window approach combined with k‐means clustering, demonstrated that patients with schizophrenia have shorter “dwell time” in metastable states (Damaraju et al., 2014), implying an unstable connectivity pattern. In schizophrenia, decreased connectivity between subcortical regions and sensory networks were only observed in dynamic networks, suggesting that static connectivity was less sensitive to functional abnormalities. Research in major depression has revealed decreased dynamic resting state connectivity between the medial prefrontal cortex and parahippocampus, and increased dynamic connectivity between the medial prefrontal cortex and dorsolateral prefrontal cortex (Kaiser et al., 2015). These distinct patterns could be the results of positive and negative correlations in activity across sliding windows, which would not have been captured in static functional connectivity analysis alone.
In Parkinson's disease, altered dynamic functional connectivity is related to attention (Madhyastha et al., 2014). Alzheimer's disease patients with mild cognitive impairment exhibit altered graphical measures such as decreased small‐world coefficients and smaller clustering coefficients in some temporal networks (Wee et al., 2015), indicating a failure to maintain a small‐world brain connectivity compared to healthy subjects.
Multiple sclerosis (MS), a neuroinflammatory and neurodegenerative disease with demyelination in the central nervous system (Gelfand, 2014), often results in physical disabilities and cognitive impairments in information processing speed, attention, memory, and executive function domains (Wallin, Wilken, & Kane, 2006). Impaired functional integration in terms of lower mean network degree, global efficiency, and hierarchy has been reported (Rocca et al., 2016). Decreased importance in sensorimotor and ventral stream regions is related to higher disease severity and poor cognition in MS, respectively (Schoonheim et al., 2013). Studies that focused on stationary graphical measures have provided essential knowledge that is distinct from dynamic functional connectivity. Therefore, we hypothesize that both dynamic and stationary connectivity will be crucial to underline the physiology of cognitive functioning and pathology in neurological/psychiatric disorders.
In this study, the overarching purpose was to study how dynamic and static functional connectivity related to cognitive performance in MS. We applied a sliding window approach and graph theory analysis to assess dynamic functional connectivity and stationary network characteristics in MS, aiming to investigate the utility of these measures. In addition, a machine learning method, multiset canonical correlation analysis (MCCA), was applied to explore the associations between dynamic and stationary functional connectivity features, and behavioral data in order to explore models that may be beneficial for treatment development. Since previous neuroimaging studies have shown that both dynamic functional connectivity and graphical measures are beneficial to strengthen understanding of complex cognitive processes, we hypothesize that MS subjects who show cognitive dysfunction would also demonstrate abnormal dynamic functional connectivity and stationary functional connectivity. Furthermore, given that human cognition is complex and operates as a network phenomenon (Petersen & Sporns, 2015), we also hypothesize that cognitive functions in MS will be jointly affected by dynamic and stationary connectivity networks as well as disease severity.
2. MATERIALS AND METHODS
2.1. Participants
Ethics approval was issued by the University of British Columbia's (UBC) Clinical Research Ethics Board and all subjects provided written, informed consent. Forty‐six relapsing–remitting multiple sclerosis (RRMS) patients were included in the study from 2013 to 2015 and all the subjects underwent both cognitive testing and magnetic resonance imaging (MRI). Demographics are shown in Table 1. A subset of MS subjects and age‐matched normal controls (NC) with university education were included for comparison purposes (18 MS and 15 NC, mean age ± SD in MS/NC: 32.00 ± 4.93/28.93 ± 5.00) (Table 1). All patients fulfilled the McDonald 2005 criteria (Polman et al., 2005) for MS diagnosis and were recruited from the MS clinic at the University of British Columbia Hospital. Exclusion criteria included the following: (a) subjects with significant depression and/or other psychiatric illness, (b) history of drug or alcohol abuse, or (c) use of steroids in the last 3 months.
Table 1.
Demographics of MS and NC subjects
| 37 MS (mean ± SD) | 18 MS (mean ± SD) | 15 NC (mean ± SD) | |
|---|---|---|---|
| Age | 42.57 ± 11.4 | 32.00 ± 4.9 | 28.93 ± 5.0 |
| Gender | 9 M, 28 F | 3 M, 15 F | 7 M, 8 F |
| EDSS | Median: 2 | Median: 2 | No data |
| Range: 0–6 | Range: 0–6 | ||
| Education (years) | 14.70 ± 2.5 | 15.67 ± 2.5 | 18a |
| Disease duration (years) | 9.85 ± 7.7 | 5.46 ± 3.8 | No data |
Due to many missing data points, 18 is an estimated number.
2.2. Neuropsychological and clinical assessments
All patients underwent a test battery which included Digit Span, Arithmetic, Letter‐Number Sequencing, Symbol Search, and Coding subtests from the Wechsler Adult Intelligence Scale IV (WAIS‐IV), the Verbal Letter Fluency Test (FAS), Wisconsin Card Sorting Test (WCST), and Trail Making Test A and B (TMT A and B). The Working Memory Index (WMI) is a composite score of Digit Span, Arithmetic, and Letter‐Number Sequencing; while the Processing Speed Index (PSI) is another composite score of Symbol Search and Coding. These two composite scores were included in the analysis rather than the individual scores in WAIS‐IV. Clinical questionnaires were administered, which included Multiscore Depression Inventory (MDI), State–Trait Anxiety Inventory (STAI), and Fatigue Severity Scale (FSS) (Table 2). The neuropsychological battery aimed to evaluate executive skills including mental flexibility, concept formation, attentional switching, spontaneous generation of verbal information, and working memory as well as processing speed abilities including attention and visual scanning. The subject's disability severity was rated by the Kurtzke Expanded Disability Status Scale (EDSS) (Kurtzke, 1983) as scored by a neurologist at the time of recruitment.
Table 2.
Scores of cognitive tests and clinical questionnaires of 37 MS subjects in MCCA
| Neuropsychological assessment | Mean score ± SD |
|---|---|
| Working memory index standardized score | 91.76 ± 13.0 |
| Processing speed index standardized score | 98.89 ± 12.9 |
| Verbal fluency test scorea | 39.81 ± 12.4 |
| WCST number of categories completes | 4.84 ± 1.7 |
| TMT A raw score (time in seconds) | 32.30 ± 10.9 |
| TMT B raw score (time in seconds) | 75.41 ± 41.4 |
| Multiscore depression inventory total T score | 48.30 ± 8.4 |
| State anxiety inventory standardized score | 50.89 ± 10.3 |
| Trait anxiety inventory standardized score | 54.97 ± 10.8 |
| Fatigue severity scale raw score | 37.08 ± 15.5 |
Abbreviations: (TMT = trail making test; WCST = Wisconsin card sorting test).
Adjusted for age, gender, and education.
2.3. Image acquisition and processing
All subjects underwent imaging studies at the UBC MRI Research Centre with a Philips Achieva 3.0 Tesla MRI scanner. Resting‐state functional MRI (rsfMRI) data were acquired using an eiht channel head coil and an echo‐planar imaging sequence with the following parameters: 3 × 3 × 3 mm3 resolution, 36 slices, 2,000 ms TR, 30 ms TE, 90° flip angle, and 240 volumes/dynamics. Three‐dimensional (3D) T1 weighted images were acquired with 1 × 1 × 1 mm3 resolution, 60 slices, 28 ms TR, 4 ms TE and 27° flip angle.
Image preprocessing steps were performed in each subject's native space with the functions of slice timing and motion correction from Statistical Parametric Mapping 8 (SPM8, University College London, London, UK) for correcting temporal and spatial differences. For registration, the FMRIB's Linear Image Registration Tool from the FMRIB Software Library 6.0 (FSL; FMRIB, Oxford, UK) was used and a brain mask was applied to remove nonbrain areas before registration. Cortical parcellation was done on the T1‐weighted images in Freesurfer version 4.5.0 (Massachusetts General Hospital, Boston, MA) and 36 cognition‐associated regions‐of‐interest (ROIs) were selected (Table 3). These ROIs have been commonly reported in the neuropsychological literature and frequently used to investigate the relations between cognition and resting‐state functional connectivity (rsFC). Finally, the average fMRI time courses among voxels within individual ROIs were extracted using self‐programmed scripts in MATLAB (The MathWorks, Inc., Massachusetts, USA) and the data were detrended before connectivity analyses.
Table 3.
Eighteen bilateral ROIs in connectivity analyses. Some ROIs are merged as one because these ROIs are anatomically small and geographically close
| Superior frontal gyrus |
| Medial frontal gyrus |
| Inferior prefrontal cortex |
| Temporal pole, insula, and amygdala merged |
| Superior temporal cortex |
| Posterior parietal cortex |
| Post central cortex |
| Supramarginal region |
| Middle temporal lobe, hippocampus, hippocampal gyrus merged |
| Occipital‐parietal area |
| Lateral occipital lobe |
| Anterior cingulate cortex |
| Posterior cingulate cortex |
| Precuneus |
| Medial orbitofrontal cortex |
| Lateral orbitofrontal cortex |
| Fusiform gyrus |
| Superior parietal cortex |
Abbreviation: ROI = regions‐of‐interest.
2.4. Connectivity analyses
A sliding window approach (with a WL of 20 time points) and the inverse covariance matrix of the ROI time courses was used to estimate connectivity with in‐house programmed scripts in MATLAB. Such a sliding window and inverse covariance matrix approach has been applied to capture dynamic functional connectivity and accurately estimate direct connections between brain regions in fMRI (Hutchison, Womelsdorf, Allen, et al., 2013; Smith et al., 2013). Since the TR was 2 s, the WL was 40 s, consistent with previous recommendations (Zalesky & Breakspear, 2015). The window was shifted one time point at a time, resulting in 221 windowed inverse covariance matrices in total for each subject. Afterward, six network features were acquired based on the learned dynamic functional connectivity. Network power (NP) measured the summed values of the dynamic functional connectivity pairs in all the windows and the values were divided by the number of nonzero elements in each window. Density (DEN) estimated how dense the connections were by taking all nonzero connectivity values and divided by all possible connections. Network variation (NV) calculated the differences of connectivity values between two adjacent windows and the differences of each connectivity pairs were summed up and divided by the number of nonzero elements in each window. As NV and NP were global measures, the sparsity of connectivity matrix had to be taken into account. However, the following features measured connectivity in specific connections so sparsity did not significantly impact feature calculation. Flexibility of interhemispheric connections (FOCcs) calculated the connectivity differences of homologous connections only between two windows and then the values were summed up to form one measure. Flexibility of cross‐hemispheric connections (nonhomologous regions, FOCcns) was the measure of summed connectivity differences of nonhomologous connections between two windows. Flexibility of intrahemispheric connections (within hemisphere, FOCw) measured the connectivity differences of connections within left and right hemisphere between two windows and all the values were then summed up to form one value. In short, DEN and NP measured how dense and strong the overall connectivity was, while NV and flexibility measures calculated global and specific network dynamics, respectively. All the connectivity values in every measure (except DEN, as DEN focused on quality rather than quantity) were squared first, summed and then the square root of the sum was taken. All values (the square root of the sum) across windows were summed and then divided by the total number of windows. Therefore we did not take into account the effects of positive and negative correlation between two ROIs in our analyses. Instead, we considered how connectivity strength changed. Although these network features were all calculated based on learned dynamic connectivity matrices (i.e., the connectivity matrices derived from applied methods), we considered DEN and NP as stationary network features as they did not calculate the differences between two matrices. Rather, they represented the average values across the scanning time. Figure 1 demonstrates these network features in a graph fashion. Table 4 describes the mathematical definitions of each measure.
Figure 1.
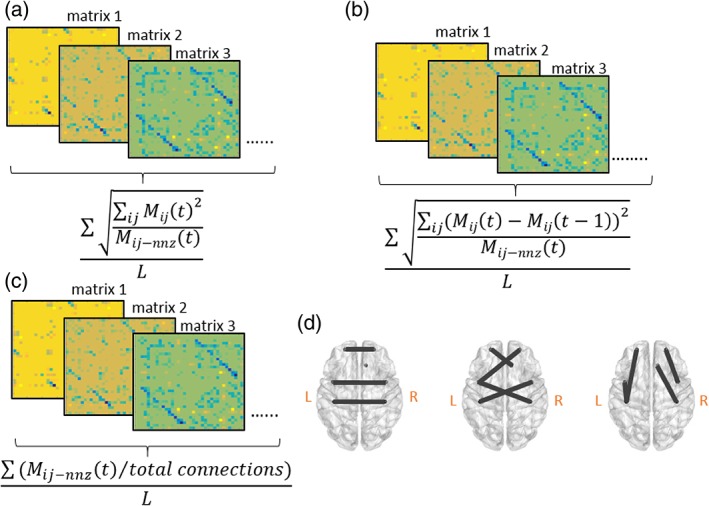
(a) NP calculates the average connectivity strength across windows. (b) Density (DEN) computes how dense the existing connections are across windows. (c) NV) estimates the average global connectivity changes between two adjacent windows across time. (d) FOCcs, (e) FOCcns, and (f) FOCw measure the average connectivity changes in interhemispheric, cross‐hemispheric, and intrahemispheric connections between two adjacent windows across time, respectively. These features are illustrated in a graph fashion as the mathematical definitions are similar to (c) (details in Table 4). M ij (t) is the i‐by‐j inverse covariance matrix containing every element at time t, M ij (t‐1) is the i‐by‐j inverse covariance matrix containing every element at time t‐1. M ij‐nnz (t) is the i‐by‐j inverse covariance matrix containing nonzero values at time t. L represents the total number of windowed matrices [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Mathematical definitions of network features learned in the sliding window approach
| Stationary network features | Definitions | |
|---|---|---|
| DEN |
|
|
| NP |
|
|
| Dynamic network features | Definitions | |
| NV |
|
|
| FOCcs |
|
|
| FOCcns |
|
|
| FOCw |
|
Abbreviations: DEN = density; NP = network power; NV = network variation; FOCcs = flexibility of interhemispheric connections; FOCcns = flexibility of cross‐hemispheric connections; FOCw = flexibility of intrahemispheric connections; ROI = regions‐of‐interest.
Note. (M ij (t) is the i‐by‐j connectivity matrix containing every element at time t, M ij‐nnz(t) contains all the nonzero connectivity values in the matrix M ij (t), totcon is the total possible connections in the matrix, which is the number of ROI times the same number, M ij (t‐1) is the i‐by‐j connectivity matrix containing every element at time t‐1, Mhij represents all homologous connections in matrix M ij, Mnij represents all interhemispheric connections except homologous elements in matrix M ij, Mwij represents all intrahemispheric connections in matrix M ij, and L represents the total number of windowed correlation matrices).
Graph theoretical values were also calculated as the network features for stationary connectivity. Partial correlation was conducted and the matrices were then proportionally thresholded (with the density of 15%) before binarization, which ensured equal density of connectivity across subjects. Global efficiency, transitivity, modularity, assortativity, characteristic path length, and rich club coefficient (at Level 6, which was the highest degree that did not give a not‐a‐number across subjects due to matrix sparsity) were computed to summarize network characteristics with the Brain Connectivity Toolbox (Rubinov & Sporns, 2010). Global efficiency calculated the average inverse shortest path length in the network, which is an index of integration. Transitivity computed the ratio of triangles to triplets in the network as a measure of segregation. Modularity also measured segregation and it quantified the degree to which the network may be subdivided into different groups/systems. Assortativity estimated the correlation coefficient between the degrees of all nodes on two opposite ends of a link; in other words, this was the tendency that nodes linked to other similar nodes. Characteristic path length was the average shortest path length in the network as well as a measure of integration. In this study, rich club coefficient assessed the fraction of edges that connected nodes of degree 6 or higher out of the maximum number of edges that such nodes might share.
The two‐sample t test was conducted to explore whether NC and MS demonstrated different dynamic and stationary network characteristics in 18 MS and 15 NC subjects.
2.5. Brain‐behavior analyses
We utilized MCCA—a machine learning method—to explore the associations between modalities/data sets (Kettenring, 1971). MCCA seeks the linear combinations of data sets that maximize the correlations between data sets (Kettenring, 1971). MCCA is a popular method for blind source separation and lately has been used to explore the associations between neuroimaging and clinical/behavioral data (Chen, Wang, & McKeown, 2016; Correa, Eichele, Adalı, Li, & Calhoun, 2010; Sui et al., 2013). In this study, we included five data sets to investigate the relations between dynamic rsFC, stationary rsFC, demographics, cognitive scores, and affective variables in RRMS. Dynamic rsFC included NV, FOCcs, FOCcns, and FOCw. Stationary rsFC included DEN, NP, global efficiency, assortativity, characteristic path length, modularity, rich club coefficient, and transitivity. The demographical set included education, EDSS, and disease duration. Cognitive scores included WMI, PSI, FAS, WCST number of categories completes, TMT A and B, in which WMI, PSI, and FAS were standardized/adjusted scores. Affective variables contained MDI, trait anxiety (STAIT), state anxiety (STAIS), and FSS, in which MDI (total score), STAIT, and STAIS were transformed/standardized scores. Subjects who showed more than one variable that was bigger/smaller than 2 SD of the mean were considered outliers. In the end, nine subjects/outliers were excluded in the MCCA analysis, resulting in 37 MS subjects in total. Age effects were regressed out in a linear regression model and all the data were whitened before performing MCCA. A permutation test with 1,000 permutations was performed to assess the significance level of each MCCA component. In order to ensure the robustness of results, we used a leave‐one‐out cross‐validation. Finally, the MCCA loadings (i.e., correlation between transformed canonical data and input scores) and SEs of each variable were reported.
2.6. Results
Table 2 shows the scores of cognitive tests and clinical questionnaires included in the MCCA analysis and supplementary materials (Table S1, Supporting Information) list full scores.
For dynamic network features, MS subjects showed lower NV and higher FOCcs then NC while controlling for a false discovery rate (FDR) (corrected p values: .02 and .04, respectively, Figure 2 upper panel). For stationary network features, MS demonstrated lower DEN and NP with FDR correction (corrected p values: .02 and .02, Figure 2 lower panel). None of the graphical measures showed significant differences between MS and NC. These results indicated that MS subjects had overall weaker connectivity and fewer connections. Overall connectivity was less dynamic and interhemispheric connections were more flexible in MS subjects. In other words, there is a loss of dynamic coordination in global connectivity and increased interhemispheric connectivity fluctuations in MS subjects compared to NC.
Figure 2.
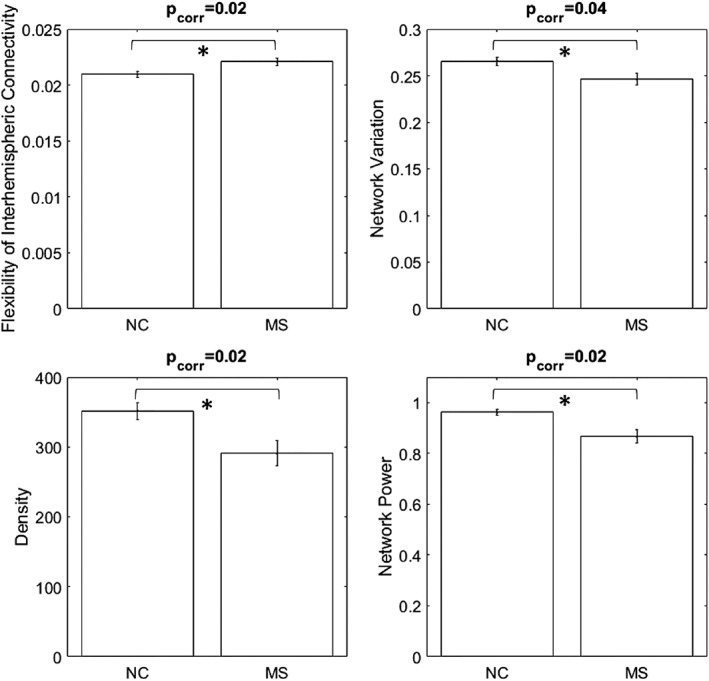
The upper panel shows the differences between NC and MS in dynamic features. MS shows higher flexibility of interhemispheric connectivity (corrected p = .02) and lower NV (corrected p = .04). The lower panel illustrates that MS presents lower network density (corrected p = .02) and NP (corrected p = .02). All p values are controlled for FDR
MCCA identified one significant component that showed moderate to strong correlations between the linear combination of almost all data sets (p = .01 in a permutation test, Figure 3). This component represented a linear combination of features in all sets that maximally correlate with each other. Dynamic features showed strong and moderate correlation to stationary features (r = 0.80) and cognitive scores (r = 0.34) as well as demographics (r = 0.22). Stationary features also demonstrated associations to cognitive scores (r = 0.24) but showed limited relations to other behavioral measures (i.e., demographics and affect). Demographics were strongly associated with affective variables (r = 0.60) as well as cognition (r = 0.46) and dynamic features (r = 0.22). Affective (mood) variables were not strongly linked to rsFC features. However, affect was associated with cognitive performance tests and demographic features. Overall, cognition was more correlated to demographics and dynamic rsFC than static rsFC and affect. Affective variables mainly had strong relations to demographics, but they also showed mild associations to cognition.
Figure 3.
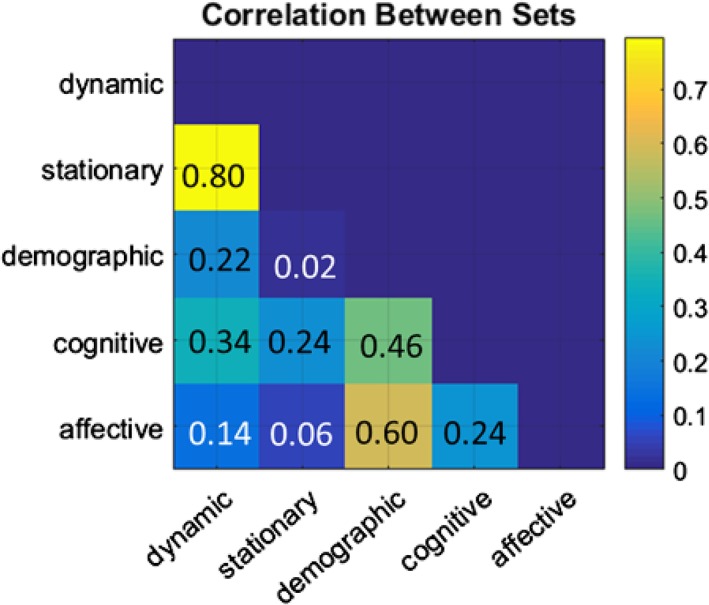
The lower triangle shows the correlation coefficients between all data sets in the significant MCCA component (p = .01 in a permutation test). Each column/row represents the linear combination of the MCCA loadings from all variables within each set. The exact correlation coefficient between two sets is illustrated in each corresponding element [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4 demonstrates the MCCA loadings of all variables in the significant component (p = .01 in a permutation test). Within each set, red stars indicated the variables that showed significant loadings and the results were mainly interpreted based on these variables. All the variables which showed positive loadings were positively correlated with each other; likewise, all the variables that demonstrated negative loadings were positively associated with each other. In short, higher dynamics, stronger static connectivity strength, longer education, and better cognitive performances were correlated with each other. On the other hand, denser static connectivity, higher EDSS and longer disease duration, worse TMTB performance, and depression/anxiety/fatigue were positively associated with each other.
Figure 4.
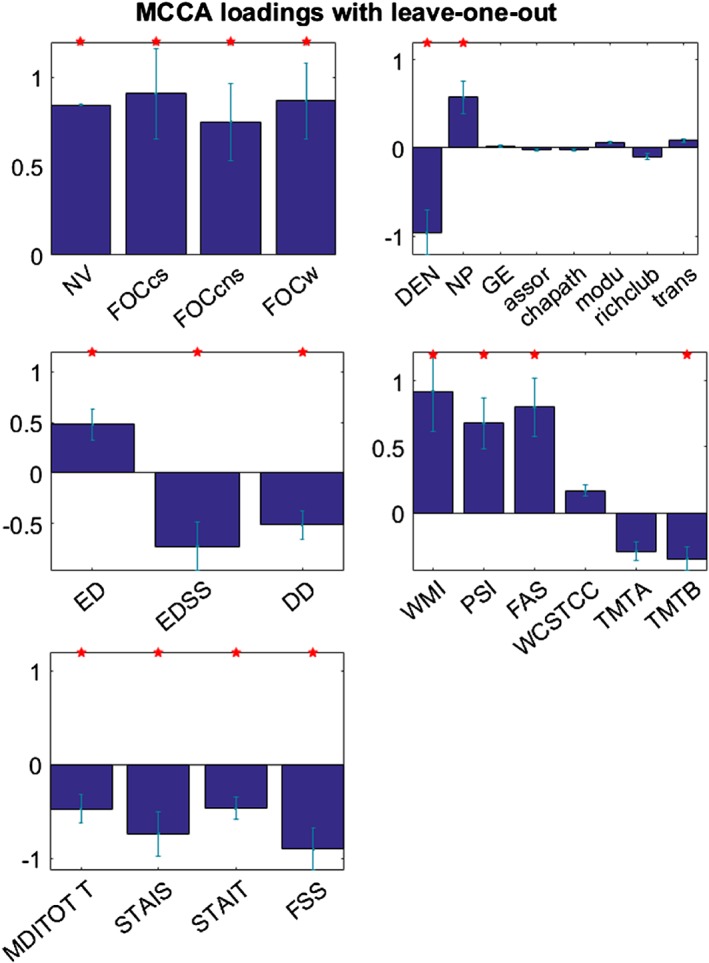
MCCA loadings of all variables in all sets are shown with error bars indicating SEs. Red stars highlight the individual variables that demonstrate significant loadings (p < .05). All the variables that show positive loadings are positively associated with each other. All the variables that present negative loadings are positively correlated with each other. (NV: Network variation, FOCcs: Flexibility of interhemispheric connections, FOCcns: Flexibility of cross‐hemispheric connections, FOCw: Flexibility of intrahemispheric connections, DEN: Density, NP: Network power, GE: Global efficiency, assor: Assortativity, chapath: Characteristic path length, modu: Modularity, richclub: Rich club coefficient, trans: Transitivity, ED: Education, EDSS: Expanded disability status scale, DD: Disease duration, WMI: Working memory index, PSI: Processing speed index, FAS: Verbal fluency test, WCSTCC: Wisconsin card sorting test number of categories completes, TMTA: Trail making test a, TMTB: Trail making test B, MDITOTT: Multiscore depression inventory Total T score, STAIS: Anxiety inventory‐state, STAIT: Anxiety inventory‐transit, FSS: Fatigue severity scale) [Color figure can be viewed at http://wileyonlinelibrary.com]
Better executive functions on cognitive testing were related to higher dynamics, stronger stationary connectivity, and higher education; while worse mental flexibility measured by TMT B test are expressed with the associations of denser static connectivity, higher disease severity, and disease comorbidity (i.e., depression, anxiety, and fatigue).
We also computed MCCA loadings with different scores (i.e., switch raw scores to standardized scores and vice versa). The results did not change.
3. DISCUSSION
3.1. Connectivity alterations indicate compensation/adaptation
We evaluated changes in both dynamic and stationary rsFC and how these network features were related to cognitive test scores, measures of emotional self‐report inventories and disease severity from patients with MS. Although graphical measures on the stationary connectivity maps did not distinguish MS and NC, average connectivity strength (i.e., NP) and density were both reduced in MS. Our results demonstrate that MS subjects had overall reduced dynamic functional connectivity. Interestingly, interhemispheric connectivity was more variable compared to NC, possibly to compensate for overall decreased global connectivity.
Results from previous fMRI studies showing patterns of activation that differ from NC have inferred compensatory effects in MS. Inferring compensatory changes in disease states can be difficult as some alterations may relate nonlinearly to disease severity (Schoonheim, Geurts, & Barkhof, 2010): compensatory changes may become apparent in early disease stages but ultimately get overwhelmed with disease progression. Nevertheless, in task‐based studies, MS patients demonstrate expanded neural activation with memory and attention tasks (Amann et al., 2011; Forn et al., 2007; Rocca et al., 2009; Staffen et al., 2002), which indicates that more brain areas are involved in such tasks in order to maintain the functions. In rsfMRI, MS subjects have stronger functional connectivity including higher connectivity strength and expanded activation maps than healthy subjects (Dogonowski et al., 2012; Hawellek, Hipp, Lewis, Corbetta, & Engel, 2011; He et al., 2009). In addition, a multimodal study concluded that stronger functional connectivity in MS over long time‐scales could be a result of rigid connectivity patterns (i.e., brain regions losing flexibility in their functional interactions) as these patterns are associated with worsening cognition (Hawellek et al., 2011). Another study, which specifically investigated dynamic connectivity with a sliding window approach and principal component analysis, found that MS subjects had reduced dynamic connectivity strength (Leonardi et al., 2013). Network communicability, a graphical metric that quantifies the efficiency of information flow, has been shown to be increased in the regions that communicate through the corpus callosum in a structural study, which was interpreted as compensation (Li et al., 2013). However, a longitudinal rsfMRI study argued against the existence of compensation as after reaching a maximum level of connectivity enhancement, as network efficiency decreased with disease progression (Faivre et al., 2016).
The altered network features that reflect compensation and adaptation in our cohort are partially in agreement with previous studies that have concluded that there is loss in dynamic coordination and flexibility of connectivity among some brain regions in MS (Hawellek et al., 2011; Leonardi et al., 2013). The interhemispheric connections, therefore, become more fluctuated overtime to compensate for global disruption as these connections still possess neuronal resources at early stages.
3.2. Relations between dynamic, stationary functional connectivity, education, and cognition
We found two major patterns of the associations between rsFC and behavioral data with the MCCA approach. Better working memory, processing speed, and verbal fluency abilities were associated with higher education, stronger stationary rsFC as well as higher network variability. The other pattern demonstrated that poor switching ability was associated with higher depression, state and trait anxiety, fatigue, higher disease severity, longer disease duration, and denser but perhaps more rigid rsFC (as density was anti‐correlated with all dynamic features).
Previous research has proposed that more variability in functional connectivity is related to better cognitive performance (Kucyi et al., 2016; Mattar et al., 2015; McIntosh et al., 2008). In our results, we also observed positive correlations between variable network features and cognitive performances. These cognitive performances not only required attention but also a variety of executive skills such as working memory (to temporary hold and manipulate information to formulate an answer), processing speed abilities (to perform focused attention, visual scanning, and discriminating visual details under timed conditions), and fluency (to spontaneously generate information according to rules and retrieve information from memory) (Kreutzer, DeLuca, & Caplan, 2011), which were all higher‐order cognitive functions and required more neuronal resources (Miller & Wallis, 2009).
We found higher education and stronger static connectivity strength were also associated with better higher‐order cognitive functions, in keeping with prior results (Alosaimi et al., 2017) and “cognitive reserve theory” which suggests that education may provide neuroprotection effects and enhance plasticity and flexibility in a variety of neural circuits imparting protection in neurodegenerative disease (Stern, 2002; Vance, Roberson, McGunness, & Fazeli, 2010). Prior studies relating functional connectivity with cognition have been variable; stronger (Smith, 2015), or weaker but more efficient connections (Santarnecchi, Galli, Polizzotto, Rossi, & Rossi, 2014) have both been proposed for supporting cognitive functions. We suggest, that, the balance between dynamic and stationary connectivity and coordination of information flow might be the key factor to facilitate cognition.
3.3. Disease effects on cognition and comorbidity in MS
The MCCA model also suggested a relation between disease severity, comorbidity, and worsening executive functions. Depression and anxiety are frequent comorbidities in MS and are often associated with more severe physical disability and disease progression, and poorer quality of life (Marrie, 2016). Although comorbidities in MS have been extensively studied in the clinical literature (e.g., (Alosaimi et al., 2017; Marrie, 2017; Rahn, Slusher, & Kaplin, 2012)), the relations between disease severity, comorbidity, cognition, and rsFC have not been previously investigated. We found affective variables were highly correlated with demographics as shown in Figure 3. Within this association, higher disease severity, reflected by higher EDSS and longer disease duration, was related to higher depression, state and transit anxiety, and fatigue. Worse TMTB performance (higher scores) was correlated with above‐mentioned associations and higher network density, which might be more rigid as DEN was anti‐correlated with all dynamic features. TMTB performance required a great deal of executive components especially task switching abilities and mental speed; therefore, this MCCA pattern indicated a co‐existence of disease severity, comorbidity, reduced shifting abilities, mental speed, and denser but rigid static rsFC in MS.
Recent studies have investigated the relations between cognitive decline and psychiatric comorbidity in neurological diseases and three theories have been proposed, whereby: (a) comorbidity is independent of cognitive decline, (b) cognitive dysfunction is qualitatively influenced by comorbidity, and (c) comorbidity manifests the existing cognitive impairments in neurological diseases (i.e., cognition is quantitatively affected) (Barone & Santangelo, 2010; Karadayi, Arisoy, Altunrende, Boztas, & Sercan, 2014; Mavandadi et al., 2009). We found that disease severity exhibited the strongest association with depression, anxiety, and fatigue; while it demonstrated the second strongest correlation to cognitive scores. In addition, the correlation between affect and cognition was relatively mild. Affective status might have relatively weaker interactions with rsFC but indirectly impact higher order cognitive functions in early stage MS. Therefore, our results are most supportive of the theory that comorbidity does not directly impact cognition, but rather, it exacerbates existing cognitive problems in MS.
3.4. Limitations and future work
There are several limitations to our study. All MS subjects were relapsing remitting in the study. Therefore, the results might not be representative enough to the whole MS population, but the connectivity profile related to cognition in the RRMS disease stage was suggested. In order to compare rsFC features between MS and NC, we selected a subset of MS subjects who were age‐matched with NC, resulting in a smaller sample size. As the original study design focused on the cognitive impairments commonly seen in the clinic, the selected ROIs covered the areas that have been identified in the traditional neuropsychological literature. However, with a greater recognition that complex cognition is a network phenomenon, complete whole brain coverage should be included in the future research. The issue of WL and the ways to report connectivity changes might be confounding factors while investigating dynamic functional connectivity. In this study, we used suggested WL parameters and selected six network features to summarize connectivity differences, which might not capture all the potential connectivity changes. Recently other approaches have been proposed to unbiasedly study network dynamics such as the Sticky Weighted Regression Model (Liu et al., 2015) and Dynamic Conditional Correlations (Lindquist et al., 2014). Furthermore, other methods have been applied to report meaningful connectivity patterns other than selecting certain features (Leonardi et al., 2013). These methods should also be tested with clinical data in the future. We included graphical measures as stationary features, but other network representations such as whole‐brain connectivity matrices are also alternatives. In addition, nine subjects were considered outliers, implying intersubject variability may still be an issue for robustness. Finally, changes in functional connectivity might be a result of compensation and adaptation for structural damages in MS. We did include brain volume and lesion load to test whether structural damages show impacts, but the MCCA model was not significant (data are not shown). Combining structural MRI data and functional connectivity in a sophisticated way is beyond the scope of this article, but it will be implemented to further investigate the disease effects structurally and functionally.
4. CONCLUSION
In this study, we performed a sliding window approach and a graph theoretical analysis on rsfMRI data to investigate dynamic functional connectivity and stationary network characteristics. MCCA was applied to explore the associations between dynamic, static network measures, and behavioral data which included demographics, clinical data, cognitive performances, and affective status in subjects with RRMS. MS subjects demonstrated higher FOCcs but decreased global connectivity strength, suggesting a compensatory effect or adaptation that certain connections became more flexible (i.e., fluctuate more across time) to maintain brain function during rest. Other decreased network measures in MS, however, indicated that the disease compromised both dynamic and static rsFC globally. Finally, with an MCCA approach that controlled for age effects, we discovered that better executive functioning was supported by higher education, stronger rsFC, and dynamic functional connectivity in MS; while disease severity was highly related to poor executive functioning and affective variables, reinforcing the strong binding of pathology and comorbidities as well as the fact that comorbidity indirectly modulated cognition. The brain‐behavior relations revealed in this study may provide influential information for the development of customized cognitive treatment and rehabilitation in MS (e.g., rehabilitation in specific domains based on disease severity), but further research is necessary to better understand the disease effects as a whole such as combining structural, functional, and behavioral data.
CONFLICT OF INTEREST
S.‐J.L., I.V., B.K., M.M., and A.M. do not have conflict of interest to declare.
Supporting information
Table S1 Full scores of neuropsychological assessments in 37 MS subjects
ACKNOWLEDGMENTS
This study was supported by an operating grant from the Multiple Sclerosis Society of Canada, which was awarded to Drs A.M. and M.J.M. The authors would like to thank Jessie Fu, Tobias R Baumeister, Aiping Liu, and Jiayue Cai for their technical help. D.L. has received research funding from the Canadian Institute of Health Research and Multiple Sclerosis Society of Canada. He is the Emeritus Director of the UBC MS/MRI Research Group which has been contracted to perform central analysis of MRI scans for therapeutic trials with Novartis, Perceptives, Roche and Sanofi‐Aventis. The UBC MS/MRI Research Group has also received grant support for investigator‐initiated independent studies from Genzyme, Merck‐Serono, Novartis, and Roche. He has acted as a consultant to Vertex Pharmaceuticals and served on the Data and Safety Advisory Board for Opexa Therapeutics and Scientific Advisory Boards for Adelphi Group, Celgene, Novartis and Roche. He has also given lectures which have been supported by nonrestricted education grants from Academy of Health Care Learning, Biogen‐Idec, Consortium of MS Centers, Novartis, Sanofi‐Genzyme and Teva. A.T. has the following competing financial interests: Research funding from Chugai, Roche, Novartis, Genzyme, Biogen. Consultancy honoraria from Genzyme, Roche, Teva, Biogen, Serono.
Lin S‐J, Vavasour I, Kosaka B, et al. Education, and the balance between dynamic and stationary functional connectivity jointly support executive functions in relapsing–remitting multiple sclerosis. Hum Brain Mapp. 2018;39:5039–5049. 10.1002/hbm.24343
Funding information Multiple Sclerosis Society of Canada
REFERENCES
- Allen, E. a. , Damaraju, E. , Plis, S. M. , Erhardt, E. B. , Eichele, T. , & Calhoun, V. D. (2014). Tracking whole‐brain connectivity dynamics in the resting state. Cerebral Cortex, 24, 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosaimi, F. D. , AlMulhem, A. , Moscovici, M. , AlShalan, H. , Alqazlan, M. , Aldaif, A. , & Sockalingam, S. (2017). The relationship between psychosocial factors and cognition in multiple sclerosis. Behavioural Neurology, 2017, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann, M. , Dössegger, L. S. , Penner, I.‐K. , Hirsch, J. G. , Raselli, C. , Calabrese, P. , … Gass, A. (2011). Altered functional adaptation to attention and working memory tasks with increasing complexity in relapsing‐remitting multiple sclerosis patients. Human Brain Mapping, 32, 1704–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, H.‐C. , Sala‐Llonch, R. , Segura, B. , Marti, M.‐J. , Valldeoriola, F. , Compta, Y. , … Junqué, C. (2014). Functional brain networks and cognitive deficits in Parkinson's disease. Human Brain Mapping, 35, 4620–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone, P. , & Santangelo, G. (2010). Interaction between affect and executive functions in Parkinson's disease In Emre M. (Ed.), Cognitive impairment and dementia in Parkinson's disease (pp. 66–73). Oxford, Oxford University Press. [Google Scholar]
- Bertolero, M. A. , Yeo, B. T. T. , & D'Esposito, M. (2015). The modular and integrative functional architecture of the human brain. Proceedings of the National Academy of Sciences of the United States of America, 112, E6798–E6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel, R. F. , Fukushima, M. , He, Y. , Zuo, X. N. , & Sporns, O. (2016). Dynamic fluctuations coincide with periods of high and low modularity in resting‐state functional brain networks. NeuroImage, 127, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, U. , Schäfer, A. , Walter, H. , Erk, S. , Romanczuk‐Seiferth, N. , Haddad, L. , … Bassett, D. S. (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences of the United States of America, 112, 11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler, S. , & Scott Kelso, J. A. (2001). Cortical coordination dynamics and cognition. Trends in Cognitive Sciences, 5, 26–36. [DOI] [PubMed] [Google Scholar]
- Chang, C. , & Glover, G. H. (2010). Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. NeuroImage, 50, 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Wang, Z. J. , & McKeown, M. (2016). Joint blind source separation for neurophysiological data analysis: Multiset and multimodal methods. IEEE Signal Processing Magazine, 33, 86–107. [Google Scholar]
- Cohen, J. R. , & D'Esposito, M. (2016). The segregation and integration of distinct brain networks and their relationship to cognition. The Journal of Neuroscience, 36, 12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa, N. M. , Eichele, T. , Adalı, T. , Li, Y.‐O. , & Calhoun, V. D. (2010). Multi‐set canonical correlation analysis for the fusion of concurrent single trial ERP and functional MRI. NeuroImage, 50, 1438–1445 Retrieved from https://www.sciencedirect.com/science/article/pii/S1053811910000844#bib22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju, E. , Allen, E. A. , Belger, A. , Ford, J. M. , McEwen, S. , Mathalon, D. H. , … Calhoun, V. D. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clinical, 5, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogonowski, A.‐M. , Siebner, H. R. , Sorensen, P. S. , Wu, X. , Biswal, B. , Paulson, O. B. , … Madsen, K. H. (2012). Expanded functional coupling of subcortical nuclei with the motor resting‐state network in multiple sclerosis. Multiple Sclerosis Journal, 19, 559–566. [DOI] [PubMed] [Google Scholar]
- Faivre, A. , Robinet, E. , Guye, M. , Rousseau, C. , Maarouf, A. , Le Troter, A. , … Audoin, B. (2016). Depletion of brain functional connectivity enhancement leads to disability progression in multiple sclerosis: A longitudinal resting‐state fMRI study. Multiple Sclerosis, 22, 1695–1708. [DOI] [PubMed] [Google Scholar]
- Forn, C. , Barros‐Loscertales, A. , Escudero, J. , Benlloch, V. , Campos, S. , Parcet, M. A. , & Ávila, C. (2007). Compensatory activations in patients with multiple sclerosis during preserved performance on the auditory n‐back task. Human Brain Mapping, 28, 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. (1994). Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping, 2, 56–78. [Google Scholar]
- Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1, 13–36. [DOI] [PubMed] [Google Scholar]
- Gelfand, J. M. (2014). Multiple sclerosis: Diagnosis, differential diagnosis, and clinical presentation In Vinken, P. J., Bruyn, G. W., & Klawans, H. L. (Eds.) Handbook of clinical neurology (pp. 269–290). Amsterdam, Elsevier. [DOI] [PubMed] [Google Scholar]
- Handwerker, D. A. , Roopchansingh, V. , Gonzalez‐Castillo, J. , & Bandettini, P. A. (2012). Periodic changes in fMRI connectivity. NeuroImage, 63, 1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawellek, D. J. , Hipp, J. F. , Lewis, C. M. , Corbetta, M. , & Engel, A. K. (2011). Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proceedings of the National Academy of Sciences, 108, 19066–19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Dagher, A. , Chen, Z. , Charil, A. , Zijdenbos, A. , Worsley, K. , & Evans, A. (2009). Impaired small‐world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain, 132, 3366–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks, R. , Adhikari, M. H. , Murayama, Y. , Ganzetti, M. , Mantini, D. , Logothetis, N. K. , & Deco, G. (2016). Can sliding‐window correlations reveal dynamic functional connectivity in resting‐state fMRI? NeuroImage, 127, 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Allen, E. A. , Bandettini, P. A. , Calhoun, V. D. , Corbetta, M. , … Chang, C. (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison, R. M. , Womelsdorf, T. , Gati, J. S. , Everling, S. , & Menon, R. S. (2013). Resting‐state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Human Brain Mapping, 34, 2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. T. , Vemuri, P. , Murphy, M. C. , Gunter, J. L. , Senjem, M. L. , Machulda, M. M. , … Jack, C. R. (2012). Non‐stationarity in the “resting brain's” modular architecture. PLoS One, 7, e39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, R. H. , Whitfield‐Gabrieli, S. , Dillon, D. G. , Goer, F. , Beltzer, M. , Minkel, J. , … Pizzagalli, D. A. (2015). Dynamic resting‐state functional connectivity in major depression. Neuropsychopharmacology, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadayi, H. , Arisoy, O. , Altunrende, B. , Boztas, M. H. , & Sercan, M. (2014). The relationship of cognitive impairment with neurological and psychiatric variables in multiple sclerosis patients. International Journal of Psychiatry in Clinical Practice, 18, 45–51. [DOI] [PubMed] [Google Scholar]
- Kettenring, J. R. (1971). Canonical analysis of several sets of variables. Biometrika, 58, 433–451. [Google Scholar]
- Kreutzer, J. , DeLuca, J. , & Caplan, B. (2011). In Test F.‐A.‐S., Kreutzer J. S., DeLuca J., & Caplan B. (Eds.), Encyclopedia of clinical neuropsychology. New York, NY: Springer. [Google Scholar]
- Kucyi, A. , Hove, M. J. , Esterman, M. , Hutchison, R. M. , & Valera, E. M. (2016). Dynamic brain network correlates of spontaneous fluctuations in attention. Cerebral Cortex, 27, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke, J. (1983). Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology, 33, 1444–1452. [DOI] [PubMed] [Google Scholar]
- Leonardi, N. , Richiardi, J. , Gschwind, M. , Simioni, S. , Annoni, J. M. , Schluep, M. , … Van De Ville, D. (2013). Principal components of functional connectivity: A new approach to study dynamic brain connectivity during rest. NeuroImage, 83, 937–950. [DOI] [PubMed] [Google Scholar]
- Leonardi, N. , & Van De Ville, D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage, 104, 430–436. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Jewells, V. , Kim, M. , Chen, Y. , Moon, A. , Armao, D. , … Shen, D. (2013). Diffusion tensor imaging based network analysis detects alterations of neuroconnectivity in patients with clinically early relapsing‐remitting multiple sclerosis. Human Brain Mapping, 34, 3376–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, M. A. , Xu, Y. , Nebel, M. B. , & Caffo, B. S. (2014). Evaluating dynamic bivariate correlations in resting‐state fMRI: A comparison study and a new approach. Neuroimage, 101, 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A. , Chen, X. , Mckeown, M. J. , Wang, Z. J. , & Member, S. (2015). A Sticky Weighted Regression Model for Connectivity Estimation. IEEE Trans Biomed Eng, 62, 501–510. [DOI] [PubMed] [Google Scholar]
- Lord, A. , Horn, D. , Breakspear, M. , & Walter, M. (2012). Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One, 7, e41282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha, T. M. , Askren, M. K. , Boord, P. , & Grabowski, T. J. (2014). Dynamic connectivity at rest predicts attention task performance. Brain Connectivity, 5, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie, R. A. (2016). Comorbidity in multiple sclerosis: Some answers, more questions. International Journal of MS Care, 18, 271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie, R. A. (2017). Comorbidity in multiple sclerosis: Implications for patient care. Nature Reviews. Neurology, 13, 375–382. [DOI] [PubMed] [Google Scholar]
- Mattar, M. G. , Cole, M. W. , Thompson‐Schill, S. L. , & Bassett, D. S. (2015). A functional cartography of cognitive systems. PLoS Computational Biology, 11, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavandadi, S. , Nazem, S. , Ten Have, T. R. , Siderowf, A. D. , Duda, J. E. , Stern, M. B. , & Weintraub, D. (2009). Use of latent variable modeling to delineate psychiatric and cognitive profiles in parkinson disease. The American Journal of Geriatric Psychiatry, 17, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, A. R. , Kovacevic, N. , & Itier, R. J. (2008). Increased brain signal variability accompanies lower behavioral variability in development. PLoS Computational Biology, 4, e1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. K. , & Wallis, J. D. (2009). Executive function and higher‐order cognition : Definition and neural substrates In Larry R. Squire (Ed.) Encyclopedia of neuroscience (Vol. 4, pp. 99–104). New York, Elsevier. [Google Scholar]
- Pamplona, G. S. P. , Santos Neto, G. S. G. S. , Rosset, S. R. E. , Rogers, B. P. , & Salmon, C. E. G. (2015). Analyzing the association between functional connectivity of the brain and intellectual performance. Frontiers in Human Neuroscience, 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, S. E. , & Sporns, O. (2015). Brain networks and cognitive architectures. Neuron, 88, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman, C. , Reingold, S. , Edan, G. , Filippi, M. , Hartung, H. , Kappos, L. , … Wolinsky, J. (2005). Diagnostic criteria for multiple sclerosis: 2005 revisions to the McDonald criteria. Annals of Neurology, 58, 840–846. [DOI] [PubMed] [Google Scholar]
- Rahn, K. , Slusher, B. , & Kaplin, A. (2012). Cognitive impairment in multiple sclerosis: A forgotten disability remembered. Cerebrum, 2012, Epub. [PMC free article] [PubMed] [Google Scholar]
- Reineberg, A. E. , & Banich, M. T. (2016). Functional connectivity at rest is sensitive to individual differences in executive function: A network analysis. Human Brain Mapping, 37, 2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca, M. , Valsasina, P. , Meani, A. , Falini, A. , Comi, G. , & Filippi, M. (2016). Impaired functional integration in multiple sclerosis: A graph theory study. Brain Structure & Function, 221, 115–131. [DOI] [PubMed] [Google Scholar]
- Rocca, M. A. , Absinta, M. , Valsasina, P. , Ciccarelli, O. , Marino, S. , Rovira, A. , … Filippi, M. (2009). Abnormal connectivity of the sensorimotor network in patients with MS: A multicenter fMRI study. Human Brain Mapping, 30, 2412–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Sakoglu, U. , Pearlson, G. D. , Kiehl, K. A. , Wang, Y. M. , Michael, A. M. , & Calhoun, V. D. (2010). A method for evaluating dynamic functional network connectivity and task‐modulation: Application to schizophrenia. Magnetic Resonance Materials in Physics, Biology and Medicine, 23, 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi, E. , Galli, G. , Polizzotto, N. R. , Rossi, A. , & Rossi, S. (2014). Efficiency of weak brain connections support general cognitive functioning. Human Brain Mapping, 35, 4566–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim, M. , Geurts, J. , Wiebenga, O. , De Munck, J. , Polman, C. , Stam, C. , … Wink, A. (2013). Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Multiple Sclerosis, 20, 1058–1065. [DOI] [PubMed] [Google Scholar]
- Schoonheim, M. M. , Geurts, J. J. G. , & Barkhof, F. (2010). The limits of functional reorganization in multiple sclerosis. Neurology, 74, 1246–1247. [DOI] [PubMed] [Google Scholar]
- Shafto, M. A. , & Tyler, L. K. (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346, 583–588. [DOI] [PubMed] [Google Scholar]
- Smith, S. (2015). Linking cognition to brain connectivity. Nature Neuroscience, 19, 7–9. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Vidaurre, D. , Beckmann, C. F. , Glasser, M. F. , Jenkinson, M. , Miller, K. L. , … Van Essen, D. C. (2013). Functional connectomics from resting‐state fMRI. Trends in Cognitive Sciences, 17, 666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2013). Network attributes for segregation and integration in the human brain. Current Opinion in Neurobiology, 23, 162–171. [DOI] [PubMed] [Google Scholar]
- Staffen, W. , Mair, a. , Zauner, H. , Unterrainer, J. , Niederhofer, H. , Kutzelnigg, a. , … Ladurner, G. (2002). Cognitive function and fMRI in patients with multiple sclerosis: Evidence for compensatory cortical activation during an attention task. Brain, 125, 1275–1282. [DOI] [PubMed] [Google Scholar]
- Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460. [PubMed] [Google Scholar]
- Sui, J. , He, H. , Pearlson, G. D. , Adali, T. , Kiehl, K. A. , Yu, Q. , … Calhoun, V. D. (2013). Three‐way (N‐way) fusion of brain imaging data based on mCCA+jICA and its application to discriminating schizophrenia. NeuroImage, 66, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, G. J. , Magnuson, M. E. , Merritt, M. D. , Schwarb, H. , Pan, W. J. , Mckinley, A. , … Keilholz, S. D. (2013). Short‐time windows of correlation between large‐scale functional brain networks predict vigilance intraindividually and interindividually. Human Brain Mapping, 34, 3280–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance, E. , Roberson, A. , McGunness, T. , & Fazeli, P. (2010). How neuroplasticity and cognitive reserve protect cognitive functioning. Journal of Psychosocial Nursing, 48, 23–30. [DOI] [PubMed] [Google Scholar]
- Wallin, M. T. , Wilken, J. A. , & Kane, R. (2006). Cognitive dysfunction in multiple sclerosis: Assessment, imaging, and risk factors. Journal of Rehabilitation Research and Development, 43, 63–72. [DOI] [PubMed] [Google Scholar]
- Wee C. Y., Yang S., Yap P. T., Shen D. (2015). Sparse temporally dynamic resting‐state functional connectivity networks for early MCI identification. Brain Imaging Behavior, 10, 342–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky, A. , & Breakspear, M. (2015). Towards a statistical test for functional connectivity dynamics. NeuroImage, 114, 466–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Full scores of neuropsychological assessments in 37 MS subjects


