Abstract
The distinction between nouns and verbs is a language universal. Yet, functional neuroimaging studies comparing noun and verb processing have yielded inconsistent findings, ranging from a complete frontal(verb)–temporal(noun) dichotomy to a complete overlap in activation patterns. The current study addressed the debate about neural distinctions between nouns and verbs by conducting an activation likelihood estimation (ALE) meta‐analysis of probabilistic cytoarchitectonic maps. Two levels of analysis were conducted: simple effects (Verbs vs. Baseline, Nouns vs. Baseline), and direct comparisons (Verbs vs. Nouns, Nouns vs. Verbs). Nouns were uniquely associated with a left medial temporal cluster (BA37). Activation foci for verbs included extensive inferior frontal (BA44–47) and mid‐temporal (BA22, 21) regions in the left hemisphere. These findings confirm that the two grammatical classes have distinct neural architecture in supra‐modal brain regions. Further, nouns and verbs overlapped in a small left lateral inferior temporal activation cluster (BA37), which is a region for modality‐independent, grammatical class‐independent lexical representations. These findings are most consistent with the view that as one acquires language, linguistic representations for a lexical category shift from the modality specific cortices which represent prototypical members of that category (e.g., motion for verbs) to abstract amodal representations in close proximity to modality specific cortices.
Keywords: Broca's area, fusiform gyrus, middle temporal, nouns, semantics, verbs
1. INTRODUCTION
One well identified language universal is the existence of expressions for events and actions on the one hand (verbs), and expressions for things and objects on the other hand (nouns) (Robins, 1952). Not surprisingly, the grammatical categories of nouns and verbs have been contrasted in multiple disciplines including (psycho)linguistics, language acquisition, neuropsychology, and cognitive neuroscience in order to understand if nouns and verbs are served by distinct neurocognitive architecture. Uncovering the neural architecture of nouns and verbs is crucial in resolving the debate of whether grammatical class is a fundamental organizing principle of mental lexicon, and if this entails distinct neural architecture for each grammatical category. This debate is particularly relevant given various embodied accounts of language, which propose that words’ meanings are grounded in their perceptual and motor representations (Barsalou, 1999; Binder, 2016; Binder & Desai, 2011; Martin, 2016; Moseley & Pulvermuller, 2014; Watson & Chatterjee, 2011). For example, embodied accounts propose that tools (denoted by nouns, such as pen and shovel) and the actions that one may perform with these tools (denoted by verbs, such as writing and digging) both have similar neural representations. Despite two decades of investigations on the neural distinctness of nouns and verbs, we are far from a resolution to this question. The purpose of this study is to elucidate the neurocognitive architecture of language by determining if the processing of nouns and verbs yields distinct or overlapping neural activation. We frame our research questions by briefly reviewing relevant empirical findings and theoretical perspectives on the neurocognitive architecture of words.
1.1. Neuropsychological investigations of verbs and nouns
Early clues into the neural separation of nouns and verbs emerged from persons who developed aphasia with left hemisphere damage. For example, Damasio and Tranel (1993) described a double dissociation in which two persons with left temporal lesions had difficulty with noun retrieval and one person with a left frontal lesion found verb naming more difficult. Generally, verb retrieval deficits have been associated with grammatical difficulties and frontal lesions (Daniele, Guistolisi, Silveri, Colosimo, & Gainotti, 1994; Miceli, Silveri, Villa, & Caramazza, 1984; Williams & Canter, 1987; Zingeser & Berndt, 1990; but see Aggujaro et al., 2006; Silveri, Perri, & Cappa, 2003). In contrast, persons with left temporal lesions and anomic aphasia are more disadvantaged in the production of nouns relative to verbs. This early research is frequently cited, but is not without confounds. Most of these studies elicited naming responses with (often un‐normed) picture stimuli, and verbs are less imageable than nouns. Action pictures require more elements in the picture than object pictures. For example, the verb eating may be elicited with a picture of someone eating and something being eaten, while the noun banana may be depicted with just one entity in the picture. Not surprisingly, some verb–noun dissociations were eliminated by changing the elicitation task to sentence production without picture support or by matching verbs and nouns for properties such as imageability, manipulability, and lexical frequency (Arevalo et al., 2007; Bird, Howard, & Franklin, 2000; Crepaldi et al., 2006; Luzzatti, Raggi, Zonca, Pistarini, Contardi, & Pinna, 2002).
Another problem with grammatical category dissociations is the relatively underspecified neuroanatomical lesion evidence. Lesions were described in generalities such as left temporal or temporo‐parietal, given the typically large lesions that result in aphasia. Voxel‐based lesion symptom mapping studies of verb and noun naming deficits have thus far been underpowered with small sample sizes (Piras & Marangolo, 2007, 2010).
Several systematic reviews of grammatical category retrieval in persons with aphasia have been published. Generally, these meta‐analyses have made two conclusions: grammatical category dissociations do exist in persons with aphasia even after task‐ and stimulus‐related variables are matched, and true neuropsychological double dissociations with a distinct lesion for each grammatical category are rare (Black & Chiat, 2003; Crepaldi, Berlingeri, Paulesu, & Luzzatti, 2011; Druks, 2003; Mätzig, Druks, Masterson, & Vigliocco, 2009; Pillon & d'Honincthun, 2011; Vigliocco, Vinson, Druks, Barber, & Cappa, 2011). Mätzig et al.’s (2009) review found that verb deficits were generally more prevalent following left hemisphere lesions, are associated with a greater heterogeneity of lesions, and occur irrespective of aphasia subtype (fluent vs. nonfluent). Thus, associations between grammatical class deficits and neural regions have been imprecise in focal brain damage.
Neuropsychological investigations of grammatical category deficits have also focused on degenerative disorders. This research has examined the purported relationship between motoric impairments and action naming, particularly in Parkinson's disease, which is associated with neurodegeneration of the dopamine‐mediated fronto‐striatal network. Several studies have found impaired action naming in persons with Parkinson's disease in tasks such as picture naming (Cotelli et al., 2006; Rodríguez‐Ferreiro, Menendez, Ribacoba, & Cuetos, 2009), word association (Herrera & Cuetos, 2013), verb generation (Castner et al., 2008), and verbal fluency (Herrera, Cuetos, & Ribacoba, 2012; Piatt, Fields, Paolo, Koller, & Troster, 1999). However, in one study of semantic similarity judgments, persons with Parkinson's Disease performed with the same accuracy but slower speed as healthy controls (Kemmerer, Miller, MacPherson, Huber & Tranel, 2013). Findings about verb retrieval in Alzheimer's disease, which is characterized by temporal‐hippocampal atrophy, have been mixed. While some studies found worse verb naming (Bushell & Martin, 1997; Robinson, Grossman, White‐Devine, & D'Esposito, 1996), others have found no noun–verb dissociation (Almor et al., 2009). To summarize, both focal‐onset and degenerative neuropsychological evidence has been equivocal about neuroanatomical separability of nouns and verbs.
1.2. Neuroimaging findings of verbs and nouns in healthy individuals
Numerous functional neuroimaging studies have investigated if there is a neuroanatomical dichotomy between nouns and verbs, with vastly mixed findings. Some studies found neural differences (e.g., Peterson, Fox, Posner, Mintun, & Raichle, 1988; Shapiro, Moo, & Caramazza, 2006; Wise et al., 1991). However, these neural differences between verbs and nouns did not support a strong frontal–temporal dichotomy; in addition to the predicted left frontal activations, verbs elicited left middle temporal (Damasio et al., 2001; Martin, Haxby, Lalonde, Wiggs, & Ungerleider, 1995; Momenian, Nilipour, Samar, Oghabian, & Cappa, 2016; Peterson et al., 1988; Yokoyama et al., 2006) or left parietal activations (Berlingeri et al., 2008). In contrast, several studies have reported overlapping activations for nouns and verbs using Positron Emission Tomography (PET)/Functional magnetic resonance imaging (fMRI) (Li, Jin, & Tan, 2004; Liljestrom et al., 2008; Longe, Randall, Stamatakis, & Tyler, 2007; Perani et al., 1999; Saccuman et al., 2006; Siri et al., 2008; Tyler, Russell, Fadili, & Moss, 2001; Tyler et al., 2003; Warburton et al., 1996) and magnetoencephalography (Soros, Cornelissen, Laine, & Salmelin, 2003). A meta‐analysis of functional neuroimaging findings using a hierarchical clustering algorithm found no consistent neural difference between nouns and verbs (Crepaldi et al., 2013). The authors concluded that “the neural circuits responsible for verb and noun processing are not spatially segregated in different brain areas, but are strictly interleaved with each other in a mainly left‐lateralized fronto‐temporo‐parietal network” (p. 12).
Discussions about the disparate findings across studies have typically focused on methodological differences: tasks such as picture naming emphasize perceptually salient features, and speeded processing tasks such as lexical decision may not definitively access conceptual semantic representations (e.g., Damasio et al., 2001; Li et al., 2004; Perani et al., 1999; see McNorgan, Chabal, O'Young, Lukic, & Booth, 2015 for a discussion of task effects). Hence, some studies have utilized morphological transformations or semantic priming to tap a deeper level of word processing (e.g., Longe et al., 2007; Shapiro et al., 2006; Tyler et al., 2003). There are also differences in stimulus specific characteristics such as imageability and manipulability of the words which confound verb–noun neural distinctions (Saccuman et al., 2006; Siri et al., 2008). In other words, the discrepant neuroimaging findings could be attributed to a complex interplay between conceptual‐semantic and morphosyntactic differences between verbs and nouns, as well as methodological differences across studies. The most notable methodological confound is when the verb and noun conditions do not allow one to isolate activation associated with a grammatical category. For instance, the verb generation task involves presentation of a noun cue (e.g., scissors) to which a verb has to be produced (e.g., cut). This so‐called “verb condition” engages both noun and verb processing (e.g., Crescentini, Shallice, & Macaluso, 2010). A similar confound occurs when nouns have to be derived from verbs and vice versa (e.g., Marangolo, Piras, Galati, & Burani, 2006). The neuroimaging meta‐analysis of Crepaldi et al. (2013) included such studies, and hence it remains to be seen if clearer noun–verb distinctions might emerge when such studies are excluded from the analysis.
Several authors have highlighted another confound of verb–noun neuroimaging studies. The neural difference in grammatical categories could be conflated with conceptual differences because prototypical verbs and nouns refer to actions and objects respectively (Fargier & Laganaro, 2015; Kemmerer, 2014; Shapiro et al., 2006). This confound has been addressed in a few ways. Conceptual differences are minimized by comparing neural activity of action verbs (e.g., to drill) and action nouns (e.g., drilling) (Aravena et al., 2014; Fargier & Laganaro, 2015), by manipulating the abstract‐concrete dimension (Moseley & Pulvermuller, 2014), or by using pseudoverbs (e.g., to wug) and pseudonouns (e.g., the wug) (Shapiro, Pascual‐Leone, Mottaghy, Gangitano, & Caramazza, 2001). Another workaround has been to engage verbs and nouns in morphosyntactic processes, hence emphasizing the grammatical attributes of each word category (Shapiro et al., 2005, 2006). While some of these studies found distinct neural activity for verbs and nouns (e.g., Shapiro et al., 2005, 2006), others found no differences, or that the differences were mediated by other factors such as sensorimotor representations rather than grammatical class (Fargier & Laganaro, 2015; Moseley & Pulvermuller, 2014; Tyler et al., 2001). In sum, empirical findings about neural distinctions between grammatical categories are mixed and offer no clear resolution among the differing theoretical views on neural representation of grammatical categories.
1.3. Theoretical perspectives on verb–noun differences
There are three main theoretical perspectives on the neural organization of nouns and verbs, all of which are based on neuropsychological and/or neuroimaging evidence. The first view, the fronto temporal dichotomy hypothesis (FTDH), proposes that verbs and nouns are mediated by left hemisphere frontal and temporal networks respectively. Support for the FTDH is drawn from a variety of arguments. For instance, several studies have reported double dissociations between noun and verb retrieval which have been associated with distinct neural lesions: left temporal for noun deficits and left frontal damage for verb impairment (Caramazza & Hillis, 1991; Damasio & Tranel, 1993; Daniele et al., 1994). Another argument in favor of the FTDH is based on grammatical differences between nouns and verbs (Damasio & Tranel, 1993). That is, verbs are associated with left frontal networks due their increased syntactic (e.g., thematic roles) and morphological (e.g., tense/mood marking) properties compared with nouns. A semantic argument of FTDH is that verbs and nouns are conceptually very distinct because of their sensorimotor attributes: verbs symbolize action features and the more imageable nouns are distinguished by visual features (Bird et al., 2000; Vigliocco, Vinson, Lewis, & Garrett, 2004). Action and visual concepts activate frontal and inferior temporal modality specific cortices, respectively, thus resulting in a verb–frontal and noun–temporal association (Pulvermuller, 1999; Vigliocco et al., 2004, 2011).
In contrast to the FTDH, another view of lexical organization is the spatial‐contiguity hypothesis (Crepaldi et al., 2011, 2013). This view proposes no unique neural architecture for nouns and verbs, suggesting instead that these are represented in overlapping or contiguous neural regions. Crepaldi et al. (2013) use the term spatial contiguity to reconcile empirical findings in which nouns and verbs had overlapping neural correlates. Empirical support for spatial contiguity is drawn from lesion correlates of aphasic persons with noun–verb dissociations, particularly those with verb deficits. Two relatively large group lesion studies of 16 and 20 persons with verb deficits found widely varying left hemisphere lesions, ranging from medial‐superior temporal, posterior‐occipitotemporal, inferior parietal, insular/subcortical to the entire perisylvian region (Aggujaro et al., 2006; Tranel, Mazer, Asp, & Kemmerer, 2008). Only four of these 36 verb impaired persons had frontal lesions. A large meta‐analysis of noun–verb dissociations also found no consistent lesion correlate for verb deficits, particularly in the frontal lobe (Mätzig et al., 2009). Reviews and data meta‐analyses of brain imaging studies in neurologically healthy individuals by Crepaldi et al. (2011, 2013) also found no consistent activation differences for nouns and verbs.
Proponents of spatial contiguity assume that noun–verb activation differences found in some studies, particularly in the left inferior frontal region, are due to differences in cognitive demands, which are typically higher for verbs (e.g., Siri et al., 2008; Yokoyama et al., 2006). For example, children of most languages typically acquire verbs later than nouns (Haman et al., 2017; Gentner, 1982). Verbs are also named and processed slower than nouns in picture naming or lexical decision (Kauschke & von Frankenberg, 2008; Szekely et al., 2005). A rather straightforward interpretation of these verb disadvantages is that producing and processing verbs is cognitively more demanding than nouns. That is, the same neurocognitive networks are recruited for nouns and verbs, but to a larger extent for verbs (Bedny & Thompson‐Schill, 2006; Berlingeri et al., 2008; Crescentini et al., 2010). However, this interpretation does not account for noun–verb double dissociations following left hemisphere damage in which noun naming is more impaired than verb naming (Caramazza & Hillis, 1991; Damasio & Tranel, 1993; reviews by Crepaldi et al., 2011 and Mätzig et al., 2009).
While the spatial contiguity hypothesis was proposed mainly to reconcile empirical findings (Crepaldi et al., 2013), a theoretical argument that could be made in favor of spatial contiguity is that nouns and verbs often have conceptual similarities and may be used to describe the same event, as in pen/writing or shovel/digging. If the mental lexicon is organized by conceptual relatedness, there is no need for an additional layer of representation based on grammatical categories (Bird et al., 2000; Moseley & Pulvermuller, 2014; Moss, De Mornay Davies, Jeppeson, McLellan, & Tyler, 1998). Spatial contiguity of nouns and verbs is consistent with the Featural and Unitary Semantic Space (FUSS, Vigliocco et al., 2004) model of word meaning, which is a statistical model that recognizes that objects and actions may share the same conceptual “features” and are organized according to similar principles. The FUSS model acknowledges that words may additionally differ based on sensorimotor, phonological, and syntactic differences, but makes not direct claims about specific neural regions.
A third view of the neural organization of nouns and verbs is a middle ground between FTDH and spatial contiguity, the point‐of‐entry hypothesis (Barsalou, Simmons, Barbey, & Wilson, 2003; Watson & Chatterjee, 2011). It suggests that during language acquisition, perceptual, and motoric differences between objects and actions (i.e., prototypical nouns and verbs) may have steered the (grammatical) representation of nouns and verbs to adjoining supramodal neural tissue (Barsalou et al., 2003; Watson & Chatterjee, 2011). For instance, higher order visual areas such as color perception are crucial for identification of objects, while visual motion regions are involved for processing actions. For a child developing language, these regions become associated with the corresponding object and action labels (which are nouns and verbs). And eventually, these perceptual regions generalize to represent other (nonperceptual) words of the same grammatical category (Watson & Chatterjee, 2011). By the same logic, the frontal motor system is associated with verb naming. Other authors have also proposed the view that semantic representations are an extension of perceptuo‐motor attributes of prototypical category members (e.g., Shapiro et al., 2006). The Anterior Shift Hypothesis is a similar but more directional proposal, according to which semantic regions lie anterior to perceptual regions that are crucial for acquiring category knowledge (Thompson‐Schill, 2003). Binder and Desai (2011) used the term embodied abstraction to describe a conceptual representation that is embodied in multiple levels of abstraction from perceptuo‐motor processing.
1.4. The present study
As discussed earlier, neuropsychological and neuroimaging evidence for the neural separability of nouns and verbs is currently inconclusive and hence cannot adjudicate between these different theoretical perspectives. This study aimed to resolve the mixed findings and inform our theoretical understanding of language representation by determining if there is a common substrate of brain regions for each word category that is not an artifact of differences in stimulus properties or experimental task. We examined the neural segregation of grammatical categories by conducting a voxel‐wise meta‐analysis of published neuroimaging studies using activation likelihood estimation (ALE, Eickhoff et al., 2009; Laird, Lancaster, & Fox, 2005; Turkeltaub et al., 2012). ALE is a statistical technique which extracts voxels that are consistently activated across studies by modeling activation foci reported by each study as a probability distribution. Based on the cross‐study probability of activations across specific voxels, a brain map is created, which is corrected for multiple comparisons. In other words, ALE reveals voxels that are consistently activated in spite of differences in methodology, analyses procedures, scanner types, and participant characteristics across studies. ALE is therefore ideally suited to resolve the current lack of consensus on the neural instantiation of grammatical categories. We deliberately included all studies that met inclusionary criteria (described later), irrespective of methodological differences in order to delineate (any) neural regions specific to each word category.
The present study differs from, and adds to, prior meta‐analyses of grammatical categories in several ways (Crepladi et al., 2013; Yang et al., 2017). First, we excluded studies in which the experimental task induced activation of both grammatical categories within a single trial. Tasks which conflate grammatical categories are more likely to result in overlapping activations. Examples of such tasks are generating a verb when presented with a noun (e.g., Warburton et al., 1996), morphological derivation (e.g., Crescentini et al., 2010), or presentation of an entire sentence. Yang et al. (2017) and Crepaldi et al. (2013) meta‐analyses included studies published until 2012 and 2013 respectively, and the present ALE analysis includes studies until 2016. And, while Crepaldi et al. (2013) used a hierarchical clustering approach in their meta‐analysis, the present study used ALE. In hierarchical clustering, activations in close proximity are grouped together.
We conducted a literature search of functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) investigations of verbs and nouns and identified eligible studies. A crucial exclusionary criterion was the use of experimental tasks that conflated grammatical categories, the classic example of which is the verb generation task (generate a verb in response to a noun cue). Thus, the resulting brain activation muddles processing of nouns and verbs. After identifying eligible studies, we conducted two main levels of analysis using ALE. The first level was a simple effects analysis, which examined neural activity for a word category relative to a baseline contrast (Nouns‐vs.‐Baseline, Verbs‐vs.‐Baseline). At the second level of analysis, direct comparisons between grammatical categories were conducted (Nouns‐vs.‐Verbs, Verbs‐vs.‐Nouns). A third analysis examined the conjunction or similarity between verbs and nouns by comparing the outputs of the two simple effects analyses. The direct comparisons and conjunction analyses are the most crucial in distinguishing between the theoretical accounts. The FTDH would be supported if the direct comparisons revealed frontal‐only and temporal‐only activation foci for verbs and nouns, respectively. Additionally, the pure dichotomy of FTDH predicts that the conjunction analysis will not identify any activation foci. If the direct effects analyses identify large activation foci, while conjunction analysis does not, this will support theoretical accounts that propose neural distinctions between verbs and nouns, but without a clear frontal or temporal dichotomy. In contrast, large activation clusters in the conjunction analysis, but no clusters in the direct comparisons would support spatial contiguity (Crepaldi et al., 2013). If the neural network differences between grammatical categories arise from perceptuo‐motor differences between prototypical verbs and nouns, then direct comparisons would identify regions that lie at, or in close proximity to, perceptuo‐motor regions. This would include frontal pre‐and temporal visual motion regions for verbs, and the inferior temporal object processing stream for nouns (Moseley & Pulvermuller, 2014; Thompson‐Schill, 2003; Wu, Morganti, & Chatterjee, 2008).
2. METHODS
2.1. Identification of studies
A PubMed literature search (http://www.pubmed.com) of studies that investigated noun and/or verb processing was conducted using the key words noun, verb, brain imaging, neuroimaging, fMRI, PET, naming, speech production, semantics, synta*, gramma*, motor, and neural. In addition, citation lists of identified articles and literature searches with prominent researcher names were used. This identified 68 potential articles, of which 35 neuroimaging studies were excluded from the meta‐analysis based on the following pre‐determined criteria: (a) studies that did not report activation foci as 3D coordinates (x, y, z) in stereotactic space, (b) studies that reported results that were based on regions of interest analysis, (c) studies that did not present contrasts of interest for this study (Noun vs. Baseline, Verb vs. Baseline, Noun vs. Verb, and Verb vs. Noun), (d) the study did not recruit neurologically typical adult participants, and (e) the experimental conditions confounded noun and verb processing. Examples of paradigms that violate the latter criterion are the verb generation task, in which participants are required to generate a verb in response to a noun (e.g., ride for bicycle), and the grammatical category switching task (Berlingeri et al., 2008) in which participants derive a verb from a noun and vice versa. These paradigms require noun and verb processing in a single experimental trial. The excluded studies with reasons for exclusion are given in the online Supporting Information table. Application of exclusionary criteria resulting in a final set of 33 studies, which exceeds the minimum number of 20 experiments recommended by Eickhoff et al. (2016) for sufficient power of ALE analysis. All the studies included single word experimental tasks and are summarized in Table 1. All studies reported data for several contrasts. Of these, only contrasts examining Nouns vs. Baseline, Verbs vs. Baseline, Nouns vs. Verbs, and Verbs vs. Nouns were included in the analysis.
Table 1.
Details of the studies included in the activation likelihood estimation analysis
| Study and Technique | Participants | Language | Tasks | Contrasts for ALE | ||||
|---|---|---|---|---|---|---|---|---|
| Experimental | Baseline | NvsB | VvsB | NvsV | VvsN | |||
| Bedny & Thompson‐Schill (2006) fMRI | 13 | English | Semantic judgment | Fixation, nonword matching | √ | |||
| Bedny et al. (2008) fMRI | 12 | English | Semantic judgment | Motion perception | √ | |||
| Berlingeri et al. (2008) fMRI | 12 | Italian | Picture naming | Figure naming | √ | √ | √ | |
| Damasio et al. (1996) PET | 9 | English | Picture naming | Face processing‐judging orientation of faces | √ | |||
| Damasio et al. (2001) PET | 20 | English | Picture naming | Face processing‐judging orientation of faces | √ | √ | √ | |
| Davis et al. (2004) fMRI | 12 | English | Semantic judgment | Letter string matching | √ | √ | ||
| de Diego et al. (2006) fMRI | 12 | Spanish | Morphological transformation | Repeat verbs & pseudowords | √ | |||
| Garbin et al. (2012) fMRI | 12 | Italian | Lexical decision | √ | √ | √ | √ | |
| Hernandez et al. (2014) fMRI | 14 | Italian | Lexical decision | Fixation | √ | |||
| Kemmerer et al. (2008) fMRI | 16 | English | Semantic judgment | Symbol judgment | √ | |||
| Li et al. (2004) fMRI | 8 | Chinese | Lexical decision | Fixation | √ | √ | ||
| Liljeström et al. (2008) fMRI | 15 | Finnish | Picture naming | Fixation | √ | √ | √ | |
| Longe et al. (2007) fMRI | 12 | English | Semantic judgment | Fixation | √ | √ | ||
| Marangolo et al. (2006) fMRI | 10 | Italian | Morphological transformation | Word repetition | √ | √ | ||
| Momenian et al. (2016) | 14 | Persian | Picture naming with sentence prompts | Rest | √ | √ | √ | |
| Peelen et al. (2012) fMRI | 24 | Italian | Cued explicit recognition/recall | Fixation | √ | √ | ||
| Perani et al. (1999) PET | 14 | Italian | Lexical decision | Letter detection | √ | |||
| Saccuman et al. (2006) fMRI | 13 | Italian | Picture naming | Not mentioned | √ | √ | √ | √ |
| Shapiro et al. (2005) PET | 12 | German | Morphological transformation | Read and view pseudowords | √ | √ | ||
| Shapiro, Moo, & Caramazza (2006) fMRI | 10 | English | Morphological transformation | Fixation | √ | √ | ||
| Shapiro, moo, & Caramazza (2012) fMRI | 22 | English | Morphological transformation | Fixation | √ | √ | ||
| Siri et al. (2008) fMRI | 12 | Italian | Picture naming | Not mentioned | √ | √ | ||
| Thompson et al. (2007) fMRI | 17 | English | Lexical decision | Fixation | √ | √ | ||
| Tranel et al. (2005) PET | 10 | English | Naming from pictures and sounds | √ | √ | √ | ||
| Tyler et al. (2001) PET | 9 | English | Lexical decision (Expt. 1), semantic judgment (Expt. 2) | Letter detection (Expt.1), letter categorization (Expt.2) | √ | |||
| Tyler et al. (2003) fMRI | 12 | English | Semantic judgment | Letter matching | √ | √ | √ | |
| Tyler, Bright, Fletcher, & Stamatakis (2004) fMRI | 12 | English | Semantic judgment | Letter matching | √ | |||
| Tyler et al. (2004) fMRI | 4 | English | Picture naming | Fixation | √ | |||
| Tyler, Marslen‐Wilson, & Stamatakis, (2005) fMRI | 18 | English | Phonological judgment | Auditory judgment | √ | |||
| Tyler et al. (2008) fMRI | 15 | English | Semantic judgment | Fixation | √ | √ | √ | |
| Willms et al. (2011) fMRI | 16 | Spanish–English bilinguals | Morphological transformation | Fixation | √ | |||
| Yokoyama et al. (2006) fMRI | 28 | Japanese | Lexical decision | Letter (character) counting for pseudowords | √ | √ | ||
| Yu et al. (2011) fMRI | 21 | Chinese | Semantic judgment | Fixation | √ | √ | ||
NvsB = nouns minus baseline; VvsB = verbs minus baseline; NvsV = nouns minus verbs; VvsN = verbs minus nouns. Studies that contributed to each contrast are indicated by √.
2.2. Meta‐analysis procedures
Four separate meta‐analyses were conducted: (1) Noun versus Baseline, (2) Verb versus Baseline, (3) Noun versus Verb, and (4) Verb versus Noun. A fifth analysis, which computed the overlap between noun and verb processing, was computed using data pooled from the first two analyses. All meta‐analyses were carried out using the activation likelihood estimation (ALE) technique (Turkeltaub et al., 2012) implemented in BrainMap using GingerAle, Version 2.3.5 (Eickhoff et al., 2009; Laird et al., 2005) (http://www.brainmap.org/ale) in MNI coordinate space. In cases where coordinates were reported in Talairach space, these were transformed to MNI space using icbm2tal (Lancaster et al., 2007). Using ALE, the coordinates identified in the literature search were modeled with a three‐dimensional Gaussian distribution, and their convergence across experiments was quantitatively assessed using forward inferencing. An ALE algorithm was used to model the spatial uncertainty of each focus using an estimation of the intersubject and interlaboratory variability as well as limiting the influence of experiments that report multiple activation foci (Turkeltaub et al., 2012). This algorithm limits the meta‐analysis to an anatomically constrained space specified by a gray matter mask and includes a method that calculates the above‐chance clustering between experiments (i.e., random‐effects analysis), rather than between foci (i.e., fixed‐effects analysis). The test was corrected for multiple comparisons using the false discovery rate (FDR) algorithm (Genovese, Lazar, & Nichols, 2002). Finally, the thresholded ALE map was created making no assumptions about correlations between data (FDR pN, p corrected < .05) by applying a minimum cluster extent of 200 mm3. The thresholded ALE maps were overlaid onto a MNI anatomical template using Multi Image Analysis GUI (Lancaster & Martinez, 2015; http://rii.uthscsa.edu/mango). The coordinates and their anatomical labeling were independently verified with the Harvard–Oxford Atlas (Desikan et al., 2006) and the Yale BioImage Suite (Lacadie, Fulbright, Constable, & Papademetris, 2008).
3. RESULTS
About 33 studies with a total of 460 participants were included in the ALE analysis. In these 33 studies, the most common experimental task was semantic judgment (N = 10), followed by lexical decision (N = 7), picture naming (N = 8), and inflectional morphology transformation (N = 6). In this cohort of studies, a majority were conducted in English (N = 20), followed by Italian (N = 8). There were no consistent task differences across the different languages, particularly between the English and Italian studies.
The 15, 20, 9, and 23 studies, respectively, were included in the Noun vs. Baseline, Verb vs. Baseline, Noun vs. Verb, and Verb vs. Noun ALE analyses (Table 1). For both simple effects and direct comparisons, the ALE analysis identified a left hemisphere network, with the exception of Verb vs. Noun, which included a right temporal cluster. Details of the activation clusters for each contrast are given in Table 2. The simple and direct effects are shown in Figure 1.
Table 2.
Results of the ALE analyses
| Region | Vol. (mm3) | Gyrus, Brodmanns area (BA) | x | y | z | Extrema value | Total vol. (mm3) | |
|---|---|---|---|---|---|---|---|---|
| Nouns vs. baseline | 672 | |||||||
| Left temporal | 672 | Fusiform gyrus (BA37) | −50.7 | −46 | −13 | 0.035 | ||
| Verbs vs. baseline | 3,328 | |||||||
| Left frontal | 1,624 | Inferior frontal gyrus (BA9) | −49 | 24.2 | 18.6 | 0.030 | ||
| Inferior frontal gyrus (BA46) | 0.022 | |||||||
| Inferior frontal gyrus (BA46) | 0.022 | |||||||
| Left temporal | 624 | Superior temporal gyrus (BA22) | −54.7 | −39 | 1.1 | 0.034 | ||
| Left temporal | 352 | Fusiform gyrus (BA37) | −50 | −47 | −11 | 0.028 | ||
| Left frontal | 272 | Inferior frontal gyrus (BA47) | −44.4 | 30.6 | −13 | 0.021 | ||
| Left frontal | 232 | Middle frontal gyrus (BA6) | −48.3 | 2.3 | 43.6 | 0.024 | ||
| Left temporal | 224 | Middle temporal gyrus (BA21) | −59 | −4.5 | −13 | 0.026 | ||
| Nouns vs. verbs | 244 | |||||||
| Left temporal | 224 | Fusiform gyrus (BA37) | −29.2 | −34 | −18 | 0.021 | ||
| Verbs vs. nouns | 5,792 | |||||||
| Left temporal | 2,752 | Middle temporal gyrus (BA21) | −55.9 | −46 | 6.8 | 0.034 | ||
| Left frontal | 2,544 | Precentral gyrus (BA44) | −42.1 | 21.1 | 4.5 | 0.022 | ||
| Inferior frontal gyrus (BA45) | 0.024 | |||||||
| Insular gray matter (BA13) | 0.027 | |||||||
| Right temporal | 280 | Middle temporal gyrus (BA 21) | 64 | −28 | −2 | 0.024 | ||
| Left frontal | 216 | Middle frontal gyrus (BA6) | −48 | 6 | 40 | 0.021 | ||
| Nouns and verbs | ||||||||
| Left temporal | 344 | Fusiform gyrus (BA 37) | −50 | −46 | −12 | 0.028 | 344 |
The columns labeled x, y, z are in MNI coordinates for the weighted center of each activation cluster.
Figure 1.
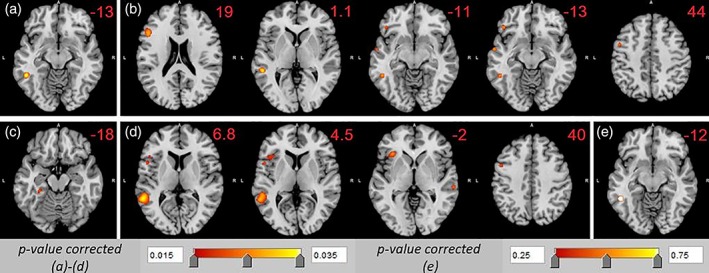
ALE foci for the five analyses at a threshold of p < .01. (a) Nouns minus baseline, (b) verbs minus baseline, (c) nouns minus verbs, (d) verbs minus nouns, and (e) conjunction of nouns and verbs. The MNI coordinates for each analysis are given in Table 1 and the z coordinates are noted on each figure panel. The figure was generated using mango image viewer (Lancaster & Martinez, 2015) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.1. Simple effects
The Noun‐versus‐Baseline (NvsB) analysis was based on 257 activation foci from 31 contrasts with a total of 183 participants. This yielded one activation cluster in the left fusiform gyrus (BA37).
The Verbs‐versus‐Baseline (VvsB) analysis was based on 352 foci from 33 contrasts with 270 participants and resulted in six clusters: three separate clusters in left frontal lobe (BA9 plus 46, BA 47, and BA 6) and three separate clusters in the left temporal lobe (BA22, BA37, and BA 21). The combined volume of the three left frontal clusters (2,128 mm3) was larger than the combined volume of the three left temporal clusters (1,200 mm3).
A comparison of these noun and verb simple effects ALE maps reveals one similarity: a cluster in the left fusiform gyrus (BA37), which was confirmed in a conjunction analysis (see Section 2.3 and Table 1). Two key differences are notable between the noun and verb ALE maps: verbs produced a larger and more widespread activation, totaling 3,328 mm3, compared with only 672 mm3 for nouns. Second, verbs activated left frontal regions while nouns did not.
3.2. Direct comparisons between nouns and verbs
The Noun‐versus‐Verb (NvsV) analysis is based on 92 activation foci from 15 contrasts and 222 participants. There was one small (244 mm3) activation cluster in the left medial fusiform gyrus (BA37) almost bordering on the parahippocampal gyrus.
About 30 contrasts contributed to Verb‐versus‐Noun (VvsN) analysis, with 190 foci, 33 contrasts, and 382 participants. This ALE identified two large activation clusters: in the left middle temporal gyrus (MTG, BA21), left postero‐inferior frontal regions (BAs 44, 46, 45) extending into the insula (BA13). There were two small clusters, in the right MTG (BA21) and left middle frontal gyrus (MFG, BA6).
The direct comparisons between nouns and verbs revealed remarkably different results. The VvsN probability map is over 20 times larger than the NvsV map (5,972 mm3 and 244 mm3, respectively). The NvsV ALE map is restricted to the left inferior temporal region almost on the medial surface (BA 37). In contrast, the VvsN ALE map includes a large left frontal and a middle temporal cluster that is lateral to the NvsV cluster.
3.3. Conjunction of nouns and verbs
This identified a single activation cluster with a volume of 344 mm3. This cluster was located in the lateral aspect of the left fusiform gyrus (BA37).
4. DISCUSSION
Neuroanatomically, the verb–noun distinction has been examined for nearly three decades using lesion data from brain damaged individuals and functional neuroimaging techniques in healthy adults. To resolve the mixed findings of past studies, this study asked whether processing of nouns and verbs recruits unique nonoverlapping brain regions when the analysis is merged across experimental tasks, languages, modalities and stimulus variables such as imageability and morphological complexity. The ALE analysis revealed mostly left hemisphere activations in which (1) there was a single area of overlap for nouns and verbs in the left lateral fusiform gyrus, (2) verbs activated left frontal and bilateral middle‐to‐inferior temporal regions while noun‐specific activity was restricted to a small inferior temporal cluster in the left medial fusiform gyrus, and (3) verb processing involved a significantly larger volume of activation compared with nouns. These findings are illustrated in Figure 2 and their implications for current understanding of lexical representation are discussed next.
Figure 2.
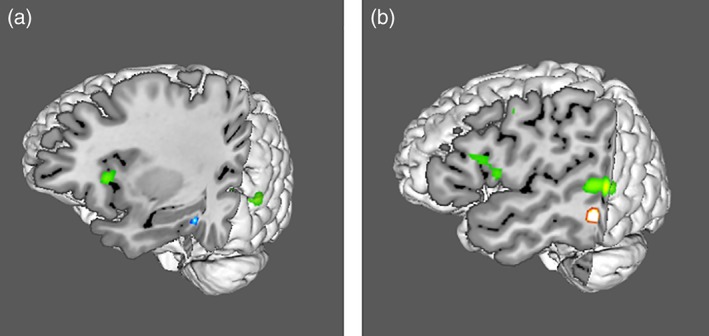
A three‐dimensional view of the left hemisphere showing the relative location of the activation clusters of the direct effects (a) and conjunction (b) analyses. Green = verbs vs. nouns, clusters are in BA44/45 and BA 21, blue = nouns vs. verbs, showing cluster in BA 37, red/yellow = conjunction of verbs and nouns, showing a cluster in BA 37. A comparison of noun (a) and conjunction (b) clusters shows that noun cluster (x = −50) is medial to the conjunction cluster (x = −29.2). The figure was generated using mango image viewer (Lancaster & Martinez, 2015) [Color figure can be viewed at http://wileyonlinelibrary.com]
4.1. Verbs and nouns overlap in the lateral fusiform gyrus (BA37)
Small‐sized activation foci in BA37 were found for both simple effects analyses (NvsB and VvsB) and the conjunction analysis. Involvement of the left lateral fusiform gyrus has been reported for a variety of lexical tasks such as semantic knowledge (Thioux, Pesenti, Costes, De Volder, & Seron, 2005), confrontation naming (Saccuman et al., 2006), orthographic and phonological processing of visually and auditorily presented words (Cohen, Jobert, Le Bihan, & Dehaene, 2004), and processing argument structure of verbs in isolation (Assadollahi, Meinzer, Flaisch, Obleser, & Rockstroh, 2009). In a series of lesion studies, Hillis et al. demonstrated that left lateral fusiform damage or hypoperfusion is associated with modality independent lexical access difficulties. For example, BA37 lesion or hypoperfusion was associated with impaired picture naming, reading, spelling, and object naming with eyes closed (i.e., tactile naming) with preserved word recognition (DeLeon et al., 2007; Sebastian et al., 2014). Further, BA37 hypoperfusion was associated with semantic errors in naming with preserved word recognition, clarifying this area's role in lexical (but not semantic) access (Cloutman et al., 2009). With the exception of Assadollahi et al.’s (2009) finding of BA37 activity for representation of verb argument structure, all the studies mentioned above investigated noun processing. Importantly, Assadollahi et al. found BA37 activity for isolated verbs when comparing one‐ versus three‐argument verbs (e.g., snore vs. give), but not when verbs were presented in context (e.g., Peter snores). This suggests BA37 does not play in role in syntactic processing, but in verb representations.
Given its involvement in lexical tasks of different modalities, Cohen et al. (2004) identified BA37 as the lateral inferotemporal multimodality area (LIMA). The current meta‐analysis establishes BA37’s involvement in both verb and noun processing, making it a convergent region for both grammatical categories. It is involved when single words are accessed. In other words, the left lateral fusiform is a modality independent, grammatical class independent, and lexical processing region. This region is lateral to the unimodal mid‐fusiform visual word form area (Cohen et al., 2004) and lies at the intersection between a verb specific (BA21) and a noun specific (BA37) region.
4.2. Verbs and nouns have unique representations
A single cluster was uniquely associated with nouns in the direct analysis (NvsV) and was located in the left medial fusiform gyrus bordering on the parahippocampal gyrus (BA37). This perirhinal region receives extensive multimodality sensory input through its interconnections with auditory association areas of the superior temporal gyrus, heteromodal regions such as retrosplenial cortex, lateral inferior parietal lobule, and the dorsal bank of the superior temporal sulcus (Suzuki, 1996). It is considered the apex of the occipitotemporal visual processing stream because of its activity for fine‐grained visual discrimination between objects, such as between a horse and donkey (Clarke & Tyler, 2014), and for crossmodal binding of perceptual features of objects into higher level conceptual representations (Taylor, Moss, Stamatakis, & Tyler, 2006). Although this region has been associated with complex visual processing in prior research, studies reporting NvsV activation foci in the present meta‐analysis included a variety of experimental tasks (lexical decision, semantic judgments, cued recall, picture naming, and morphological transformation) and imageable as well as abstract nouns (Davis et al., 2004; Garbin et al., 2012; Peelen et al., 2012; Saccuman et al., 2006; Shapiro et al., 2005, 2006; Siri et al., 2008; Yu, Bi, Han, Zhu, & Law, 2012). Thus, we can conclude that this region's association with nouns goes beyond visual processing. In fact, the vicinity of this region, the parahippocampal cortex, is implicated for contextual associations (Aminoff, Kveraga, & Bar, 2013), particularly in the left hemisphere (Li, Lu, & Zhong, 2016). Contextual processing refers to associations such as those between a sink and bathtub, or surfboard and beach. Support for BA37’s role in noun processing also comes from case studies of patients with focal and degenerative damage to this region who had a category specific noun deficit (Bi, Han, Shu, & Caramazza, 2007; Lin, Guo, Han, & Bi, 2011).
As for verbs, the inferior frontal and mid‐inferior postero‐lateral temporal network of verbs identified in this ALE analysis is consistent with verb deficits observed in patients with left inferior frontal and temporal lesions (Aggujaro et al., 2006; Mätzig et al., 2009). Aggujaro et al. (2006) also found temporo‐parietal lesions, which was not found in the current study. The largest cluster in direct comparisons (VvsN) was located in the left mid‐temporal region (BA21) with a smaller cluster in right BA21. Although BA21 has been associated with the visual motion system (e.g., Bedny & Caramazza, 2011), evidence indicates a crucial role in semantic processing of action concepts across multiple modalities. For example, it is activated for action verb processing in persons who are congenitally blind (Bedny, Caramazza, Pascual‐Leone, & Saxe, 2012), and was also identified in ALE meta‐analyses of verbal and nonverbal action concepts (Watson, Cardillo, Ianni, & Chatterjee, 2013) and action observation (Caspers, Zilles, Laird, & Eickhoff, 2010). Lesions of this region are associated with impaired verb semantics (Kemmerer, Rudrauf, Manzel, & Tranel, 2012). It is noteworthy that less than half the studies in the present meta‐analysis used purely action verbs or action pictures (e.g., Damasio et al., 2001), and several studies matched verbs and nouns for imageability (along with frequency, letter length, and age of acquisition; e.g., Berlingeri et al., 2008; Yu et al., 2011). As an illustration, Bedny et al. (2012) compared high, medium, and low motion verbs (e.g., kick, bleed, and think) with high, medium, and low motion nouns (e.g., tiger, drill, and rock) in a semantic judgment task. All verbs activated the left posterior temporal cortex relative to nouns, irrespective of the extent of motion. Hence this BA21 cluster is unlikely to be an artifact of stimulus characteristics associated with visual motion. Rather, it appears to be a verb‐specific region, consistent with the finding that it is activated for abstract and concrete verbs for grammatical and semantic tasks (Yu et al., 2012). This region is also sensitive to grammatical properties of verbs, such as first versus third person information conveyed by verbs (Papeo & Lingnau, 2015) and transitivity (Hernandez, Fairhall, Lenci, Baroni, & Caramazza, 2014).
The direct analysis of verbs (VvsN) identified a cluster in Broca's area (BA44/45). Broca's region has been traditionally associated with verb processing across tasks such as verb‐specific morphological transformation (Sahin, Pinker, & Halgren, 2006; Shapiro et al., 2005, 2006), action processing (Caspers et al., 2010), lesion studies of action picture naming (Damasio & Tranel, 1993) and verb semantics (Kemmerer et al., 2012). Hence, it is not surprising that BA45/45 emerged as the second verb selective region. Given Broca's area's role in numerous neuroimaging studies of syntactic processing (see Friederici, 2011 for a review) and patients with syntactic deficits (Faroqi‐Shah et al., 2014), it is likely that these two verb selective regions subserve different aspects of verb processing: BA21 for verb representations and BA44/45 for implementing a verb's syntactic constraints. Although Broca's area activations were specific to verbs in the present ALE analysis, it is important to point out that Broca's area is associated with numerous cognitive operations, including language selective and domain general operations, such as math, music, cognitive control and working memory (Fedorenko, Behr, & Kanwisher, 2011; Fedorenko, Duncan, & Kanwisher, 2012; Hsu, Jaeggi, & Novick, 2017). This cluster included parts of the insula (BA13), whose lateral aspect is interspersed between BA44 and BA45. The insula has been identified to play a role in language (Ardila, Bernal, & Roselli, 2014; Oh, Duerden, & Pang, 2014).
In addition to Broca's area, the simple effects analysis (VvsB) identified two other frontal regions, one dorsal (BA9/46) and the other rostral (BA47) to BA44/45. Given that these regions were not isolated in the direct analysis, it is likely that these middle prefrontal regions reflect task difficulty of verb processing compared with baseline tasks: BA9/46 is known to be recruited for cognitively demanding tasks (e.g., Volle et al., 2008), and as described earlier, verbs demonstrate numerous processing disadvantages compared with nouns. BA47 is associated with syntactic processes in oral, musical and sign language (e.g., Levitin & Menon, 2003), and this activation cluster is consistent with the central role that verbs play in syntactic processing. The VvsB clusters identify regions that are associated with, but not isomorphic with, verb processing. In sum, the left frontal activation clusters either reflect verb specific lexical and morphosyntactic processes (BA44/45), or for the VvsB clusters, additional cognitive (BA9/46) and syntactic (BA47) demands of verbs.
It is noteworthy that the present ALE did not identify any premotor or motor regions in the frontal lobe (BA6/4). This is consistent with the ALE meta‐analysis of action concepts by Watson et al. (2013). Embodied views of verb processing propose that motor aspects of verb meaning are represented in the motor/premotor cortices (Kemmerer, 2015; Pulvermuller, 1999). This prediction has been borne out in reading (Hauk et al., 2004) or listening (Raposo, Moss, Stamatakis, & Tyler, 2009) to action verbs, making semantic similarity judgments about action verbs (Kemmerer, Castillo, Talavage, Patterson, & Wiley, 2008), and distinguishing action verbs from nonwords (De Grauwe, 2014). There are several reasons why premotor/motor regions were not isolated in the present ALE. First, as pointed out earlier, fewer than half of the studies included in the present ALE analysis involved purely action verbs. Abstract (e.g., think) and state (e.g., live) verbs, which were included in several studies, are unlikely to strongly engage in sensorimotor activity. Secondly, activation of premotor/motor cortices for action verbs could depend on the extent to which the experimental task demands motor processing (Kemmerer, 2015). Finally, the current ALE included only whole‐brain analyses (as is typical of ALE analyses, Laird et al., 2005). Studies that report premotor/motor activations often do so based on regions of interest (ROI) analyses (e.g., Moseley & Pulvermuller, 2014; Wu et al., 2008).
4.3. Implications for the cognitive architecture of lexical categories
This study found two verb selective regions, posterior middle temporal (BA21) and inferior frontal (BA44/45), one (rather small) noun selective region in the inferomedial posterior temporal region (BA37), and a region of overlap in the lateral temporal/fusiform region (BA37). As discussed in the previous section, these findings are largely consistent with prior research and are in line with the cognitive functions typically associated with these regions. These activations are inconsistent with a pure interpretation of the fronto‐temporal dichotomy hypothesis (FTDH) (e.g., Damasio & Tranel, 1993) because verbs activated frontal and temporal regions, with a larger temporal activation. Neither are these findings compatible with spatial contiguity (Crepaldi et al., 2013) or single semantic space (FUSS: Featural and Unitary Semantic Space; Vigliocco et al., 2004) accounts, which propose complete neuroanatomical overlap between nouns and verbs. These accounts are not supported because of the large nonoverlapping temporal activations of nouns and verbs and the absence of noun‐related frontal activity.
We now briefly speculate on the interpretation of these findings within the broader issue of lexical representation. The key question is what these patterns of lexical category overlap and selectivity signify: do these findings pertain to conceptual representations, lexical representations, grammatical differences, or something domain general, such as mental effort? Regarding mental effort, verbs are consistently associated with longer processing times than nouns (e.g., Palti, Ben Shachar, Hendler, & Hadar, 2007; Szekely et al., 2005). Indeed, left inferior frontal activity has been associated with increased reaction times for language tasks including phonological processing (Cummine, Borowsky, Vakorin, Bird, & Sarty, 2008) and lexical decision (Liu, Liao, Fang, Chu, & Tan, 2004), but this association with processing time has not been localized consistently to Broca's area. In a study that directly compared nouns and verbs in semantic and morphological tasks, verbs had longer response times and were associated with posterior IFG (BA44) activity; however, the task with longer response times (semantic) was associated with anterior IFG (BA45–47) (Palti et al., 2007). At present, evidence is equivocal regarding the role of left inferior and prefrontal regions for verb processing: these could reflect domain general processing demands (Berlingeri et al., 2008; Cummine et al., 2008; Liu et al., 2004; Palti et al., 2007; Volle et al., 2008) or the syntactic properties of verbs (Friederici, 2011; Levitin & Menon, 2003). The other verb specific region, BA21, was unaffected by processing time differences when blind and sighted participants made semantic judgments about action verbs (Bedny et al., 2012). Thus, the limited available evidence does not raise the possibility that left MTG activity of verbs is an artifact of cognitive demands.
Verbs and nouns have been extensively contrasted based on their conceptual differences (events vs. entities) (e.g., Bird et al., 2000). Hence, it is important to consider if the present findings can be accommodated by a conceptual account. The anterior temporal lobe (ATL) bilaterally has been identified as the center of all concepts that links with modality specific (hub‐and‐spoke model of Lambon Ralph, 2014; Lambon Ralph, Jefferies, Patterson, Rogers, 2017) or supramodal convergence (Binder & Desai, 2011) regions. The left lateral fusiform region (BA37) identified in the present V‐N conjunction analysis does not coincide with this conceptual hub in the ATL, suggesting that BA37 is less likely a conceptual hub, and more likely a lexical region which is independent of response modality and grammatical class. It is noteworthy that this activation cluster lies in close proximity to the ATL.
As for the verb and noun selective regions in the left temporal lobe, there are two characteristics of these selective regions. First, these regions are connected by the left fusiform grammatical category independent LIMA area discussed earlier. Second, they each coincide (or are in close proximity) with visual processing areas that are crucial for perceiving prototypical objects (fine grained visual analysis in BA37) and actions (visual motion in BA21). It has been proposed that abstract amodal linguistic representations may be located near the modality specific cortices from which prototypical members of that category are derived (Aggujaro et al., 2006; Binder, 2016; Barsalou et al., 2003; Shapiro et al., 2006; Thompson‐Schill, 2003; Watson & Chatterjee, 2011). In the case of verbs, action/visual motion processing regions in the left temporal cortex could become the conduit (or “points of entry” per Watson & Chatterjee, 2011) for the acquisition of abstract action and event knowledge, which then generalizes to other verbs. Hence BA21, which is in the immediate vicinity of visual motion regions, could have evolved into a verb‐specific region. By that logic, BA 37 is associated with complex visual processing and is the apex of the visual processing stream (Clarke & Tyler, 2014). Activation of this region by prototypical objects could eventually generalize to other entities (Taylor et al., 2006) and become a noun‐specific region.
The evolution of BA21 and BA 37 from prototypical modality specific regions to lexical category specific regions is proposed to occur over time with increasing language exposure as shared properties of these categories are extracted through statistical learning (Binder, 2016; Barsalou et al., 2003; Shapiro et al., 2006; Thompson‐Schill, 2003; Watson & Chatterjee, 2011). For instance, depending on the speaker's language, verbs predominantly occur in specific sentence locations and with specific morphemes. This distributional property cuts across action and nonaction verbs, paving the way for strong associations among all verbs in a neural region initially associated with prototypical verbs. In other words, grammatical category differences could emerge as a consequence of conceptual semantic differences. In fact, recent models of conceptual representation recognize the role of experience in fine‐tuning category representations (Chen, Lambon Ralph, & Rogers, 2017). Chen et al. (2017), within the context of nouns, proposed that cortical specialization occurs through connectivity‐constrained cognition (C 3), which is the joint effect of (1) learning/experience, (2) perceptual, linguistic and motor structures in the environment, and (3) anatomical connectivity in the brain. Future research can test these conclusions and arrive at a better understanding of the neural functions subserved by these lexical category specific regions by direct empirical manipulations (which were not the scope of the present study).
We conclude by noting that this study is not without the limitations that are inherent to all meta‐analyses. Obviously, the analysis is based on coordinates of activation peaks from published studies and not on raw statistical brain maps, which affects the precision of the results (Radua et al., 2012). Secondly, different statistical thresholds are employed by different studies. We also had a relatively small number of studies for the noun direct effects (NvsV) analysis because most studies did not find any significant activations for this contrast. This may have impacted the false‐negative rate (Genovese et al., 2002; Radua et al., 2012). It is also important to point out that, in addition to these regions, both verbs and nouns typically activate an extensive bilateral language network (e.g., Ardila, Bernal, & Roselli, 2016).
5. CONCLUSIONS
The present study aimed to inform current understanding of lexical organization by resolving the inconsistencies in prior neuroimaging and neuropsychological studies that compared verb and noun processing. The present study added to prior meta‐analyses of grammatical categories (Crepladi et al., 2013; Yang et al., 2017) by (1) eliminating studies that confounded verb and noun processing, (2) including studies published as the previous meta‐analyses, and using activation likelihood estimation instead of hierarchical clustering (Crepaldi et al., 2013). Three main conclusions can be drawn from this study. First, the core lexical network is mainly left lateralized. Second, there are well‐defined nonadjacent neural correlates for verbs and nouns, a left inferior frontal and bilateral middle temporal circuit for verbs and a left inferior‐medial temporal locus for nouns. These findings are most compatible with the phylogenic and ontogenic evolution of specialized grammatical category specific representations based on prototypical examplars, as proposed by views such as embodied abstraction (Binder & Desai, 2011), point‐of‐entry hypothesis (Watson & Chatterjee, 2011), and anterior shift (Thompson‐Schill, 2003). Finally, we found that both nouns and verbs essentially recruit the left lateral fusiform gyrus (BA37). This left inferotemporal multimodality area is therefore a grammatical‐class independent lexical processing region and lies at the intersection of noun specific and verb specific brain regions. These findings constrain current neural models of lexical organization by showing that there are brain regions unique to each grammatical category and that verb‐specific regions are not located in the premotor and motor cortices.
Supporting information
Supplementary Table S1
ACKNOWLEDGMENTS
The authors have no conflicts of interest to declare. This study was not funded by any agency.
Faroqi‐Shah Y, Sebastian R, Woude AV. Neural representation of word categories is distinct in the temporal lobe: An activation likelihood analysis. Hum Brain Mapp. 2018;39:4925–4938. 10.1002/hbm.24334
REFERENCES
- Aggujaro, S. , Crepaldi, D. , Pistarini, C. , Taricco, M. , & Luzzatti, C. (2006). Neuro‐anatomical correlates of impaired retrieval of verbs and nouns: Interaction of grammatical class, imageability and actionality. Journal of Neurolinguistics, 19(3), 175–194. [Google Scholar]
- Almor, A. , Aronoff, J. M. , MacDonald, M. C. , Gonnerman, L. M. , Kempler, D. , Hintiryan, H. , et al (2009). A common mechanism in verb and noun naming deficits in Alzheimer's patients. Brain and Language, 111(1), 8–19. 10.1016/j.bandl.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff, E. M. , Kveraga, K. , & Bar, M. (2013). The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences, 17(8), 379–390. 10.1016/j.tics.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravena, P. , Courson, M. , Frak, V. , Cheylus, A. , Paulignan, Y. , Deprez, V. , & Nazir, T. (2014). Action relevance in linguistic context drives word‐induced motor activity. Frontiers in Human Neuroscience, 8, 163 10.3389/fnhum.2014.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila, A. , Bernal, B. , & Rosselli, M. (2014). Participation of the insula in language revisited: A meta‐analytic connectivity study. Journal of Neurolinguistics, 29, 31–41. 10.1016/j.jneuroling.2014.02.001 [DOI] [Google Scholar]
- Ardila, A. , Bernal, B. , & Rosselli, M. (2016). How localized are language brain areas? A review of Brodmann areas involvement in oral language. Archives of Clinical Neuropsycholology, 31(1), 112–122. 10.1093/arclin/acv081 [DOI] [PubMed] [Google Scholar]
- Arévalo, A. , Perani, D. , Cappa, S. F. , Butler, A. , Bates, E. , & Dronkers, N. (2007). Action and object processing in aphasia: From nouns and verbs to the effect of manipulability. Brain and Language, 100(1), 79–94. [DOI] [PubMed] [Google Scholar]
- Assadollahi, R. , Meinzer, M. , Flaisch, T. , Obleser, J. , & Rockstroh, B. (2009). The representation of the verb's argument structure as disclosed by fMRI. BMC Neuroscience, 10, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou, L. W. (1999). Perceptual symbol systems. Behavioral & Brain Sciences, 22, 577–609. [DOI] [PubMed] [Google Scholar]
- Barsalou, L. W. , Simmons, W. K. , Barbey, A. K. , & Wilson, C. D. (2003). Grounding conceptual knowledge in modality‐specific systems. Trends in Cognitive Sciences, 7(2), 84–91. [DOI] [PubMed] [Google Scholar]
- Bedny, M. , & Caramazza, A. (2011). Perception, action, and word meanings in the human brain: The case from action verbs. Annals of the New York Academy of Sciences, 1224, 81–95. [DOI] [PubMed] [Google Scholar]
- Bedny, M. , Caramazza, A. , Pascual‐Leone, A. , & Saxe, R. (2012). Typical neural representations of action verbs develop without vision. Cerebral Cortex, 22, 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny, M. , & Thompson‐Schill, S. L. (2006). Neuroanatomically separable effects of imageability and grammatical class during single‐word comprehension. Brain & Language, 98(2), 127–139. [DOI] [PubMed] [Google Scholar]
- Berlingeri, M. , Crepaldi, D. , Roberti, R. , Scialfa, G. , Luzzatti, C. , & Paulesu, E. (2008). Nouns and verbs in the brain: Grammatical class and task specific effects as revealed by fMRI. Cognitive Neuropsychology, 25(4), 528–558. [DOI] [PubMed] [Google Scholar]
- Bi, Y. , Han, Z. , Shu, H. , & Caramazza, A. (2007). Nouns, verbs, objects, actions, and the animate / inanimate effect. Cognitive Neuropsychology, 24(5), 485–504. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. (2016). In defense of abstract conceptual representations. Psychonomic Bulletin & Review, 23(4), 1096–1108. 10.3758/s13423-015-0909-1 [DOI] [PubMed] [Google Scholar]
- Binder, J. , & Desai, R. (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, H. , Howard, D. , & Franklin, S. (2000). Why is a verb like an inanimate object? Grammatical category and semantic category deficits. Brain and Language, 72(3), 246–309. [DOI] [PubMed] [Google Scholar]
- Black, M. , & Chiat, S. (2003). Noun‐verb dissociations: A multi‐faceted phenomenon. Journal of Neurolinguistics, 16, 231–250. [Google Scholar]
- Bushell, C. M. , & Martin, A. (1997). Automatic semantic priming of nouns and verbs in patients with Alzheimer's disease. 35(8), 1059. [DOI] [PubMed] [Google Scholar]
- Caramazza, A. , & Hillis, A. E. (1991). Lexical organization of nouns and verbs in the brain. Nature, 349(6312), 788–790. [DOI] [PubMed] [Google Scholar]
- Caspers, S. , Zilles, K. , Laird, A. R. , & Eickhoff, S. B. (2010). ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage, 50, 1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Lambon Ralph, M. A. , & Rogers, T. T. (2017). A unified model of human semantic knowledge and its disorders. Nature Human Communication, 1, 0039 10.1038/s41562-016-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A. , & Tyler, L. K. (2014). Object‐specific semantic coding in human Perirhinal cortex. The Journal of Neuroscience, 34(14), 4766–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman, L. , Gottesman, R. , Chaudhry, P. , Davis, C. , Kleinman, J. T. , Pawlak, M. , … Hillis, A. E. (2009). Where (in the brain) do semantic errors come from? Cortex, 45(5), 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, L. , Jobert, A. , Le Bihan, D. , & Dehaene, S. (2004). Distinct unimodal and multimodal regions for word processing in the left temporal cortex. NeuroImage, 23(4), 1256–1270. [DOI] [PubMed] [Google Scholar]
- Cotelli, M. , Borroni, B. , Manenti, R. , Alberici, A. , Calabria, M. , Agosti, C. , … Cappa, S. F. (2006). Action and object naming in Frontotemporal dementia, progressive Supranuclear palsy, and Corticobasal degeneration. Neuropsychology, 20(5), 558–565. [DOI] [PubMed] [Google Scholar]
- Crepaldi, D. , Aggujaro, S. , Arduino, L. S. , Zonca, G. , Ghirardi, G. , Inzaghi, M. G. , … Luzzatti, C. (2006). Noun‐verb dissociation in aphasia: The role of imageability and functional locus of the lesion. Neuropsychologia, 44(1), 73–84. [DOI] [PubMed] [Google Scholar]
- Crepaldi, D. , Berlingeri, M. , Cattinelli, I. , Borghese, N. A. , Luzzatti, C. , & Paulesu, E. (2013). Clustering the lexicon in the brain: A meta‐analysis of the neurofunctional evidence on noun and verb processing. Frontiers in Human Neuroscience, 7(303), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi, D. , Berlingeri, M. , Paulesu, E. , & Luzzatti, C. (2011). A place for nouns and a place for verbs? A critical review of neurocognitive data on grammatical‐class effects. Brain and Language, 116, 33–49. [DOI] [PubMed] [Google Scholar]
- Crescentini, C. , Shallice, T. , & Macaluso, E. (2010). Item retrieval and competition in noun and verb generation: An FMRI study. Journal of Cognitive Neuroscience, 22(6), 1140–1157. 10.1162/jocn.2009.21255 [DOI] [PubMed] [Google Scholar]
- Cummine, J. , Borowsky, R. , Vakorin, V. , Bird, J. , & Sarty, G. (2008). The relationship between naming reaction time and functional MRI parameters in Broca's area. Magnetic Resonance Imaging, 26(6), 824–834. 10.1016/j.mri.2008.01.032 [DOI] [PubMed] [Google Scholar]
- Damasio, H. , Grabowski, T. J. , Tranel, D. , Ponto, L. L. , Hichwa, R. D. , & Damasio, A. R. (2001). Neural correlates of naming actions and of naming spatial relations. NeuroImage, 13(6 Pt 1), 1053–1064. [DOI] [PubMed] [Google Scholar]
- Damasio, A. R. , & Tranel, D. (1993). Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences of the United States of America, 90(11), 4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele, A. , Giustolisi, L. , Silveri, M. C. , Colosimo, C. , & Gainotti, G. (1994). Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia, 32(11), 1325–1341. [DOI] [PubMed] [Google Scholar]
- Davis, M. H. , Meunier, F. , & Marslen‐Wilson, W. D. (2004). Neural responses to morphological, syntactic and semantic properties of single words: An fMRI study. Brain and Language, 89:439–449. [DOI] [PubMed] [Google Scholar]
- De Grauwe, S. (2014). Embodied language in first‐ and second‐language speakers: Neural correlates of processing motor verbs. Neuropsychologia, 5656, 334–349. [DOI] [PubMed] [Google Scholar]
- DeLeon, J. , Gottesman, R. F. , Kleinman, J. T. , Newhart, M. , Davis, C. , Heidler‐Gary, J. , … Hillis, A. E. (2007). Neural regions essential for distinct cognitive processes underlying picture naming. Brain, 130(5), 1408–1422. [DOI] [PubMed] [Google Scholar]
- Desikan, R. S. , Segonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Druks, J. (2003). Verbs and nouns: A review of literature. Journal of Neurolinguistics, 16, 289–315. [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , … Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. 10.1016/j.neuroimage.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargier, R. , & Laganaro, M. (2015). Neural dynamics of object noun, action verb and action noun production in picture naming. Brain and Language, 150, 129–142. 10.1016/j.bandl.2015.09.004 [DOI] [PubMed] [Google Scholar]
- Faroqi‐Shah, Y. , Kling, T. , Solomon, J. , Liu, S. , Park, G. , & Braun, A. R. (2014). Lesion analysis of language production deficits in aphasia. Aphasiology, 28(3), 258–277. 10.1080/02687038.2013.853023 [DOI] [Google Scholar]
- Fedorenko, E. , Behr, M. K. , & Kanwisher, N. (2011). Functional specificity for high‐level linguistic processing in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 108(39), 16428–16433. 10.1073/pnas.1112937108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko, E. , Duncan, J. , & Kanwisher, N. (2012). Language‐selective and domain‐general regions lie side by side within Broca's area. Current Biology, 22(21), 2059–2062. 10.1016/j.cub.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici, A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. 10.1152/physrev.00006.2011 [DOI] [PubMed] [Google Scholar]
- Genovese, C. R. , Lazar, N. A. , & Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage, 15, 870–878. [DOI] [PubMed] [Google Scholar]
- Gentner, D. (1982). Why nouns are learned before verbs: Linguistic relativity versus natural partitioning In Kucszaj S. (Ed.), Language Development (Vol. 2: Language, thought and culture) (pp. 301–334). Hillsdale, N.J.: Lawrence Erlbaum. [Google Scholar]
- Haman, E. , Luniewska, M. , Hansen, P. , Simonsen, H. G. , Chiat, S. , Bjekic, J. , … Armon‐Lotem, S. (2017). Noun and verb knowledge in monolingual preschool children across 17 languages: Data from cross‐linguistic lexical tasks (LITMUS‐CLT). Clinical Linguistics & Phonetics, 31, 1–26. 10.1080/02699206.2017.1308553 [DOI] [PubMed] [Google Scholar]
- Hauk, O. , Johnsrude, I. , & Pulvermuller, F. (2004). Somatotopic representation of action words in human motor and premotor cortex. Neuron, 41(2), 301–307. [DOI] [PubMed] [Google Scholar]
- Hernandez, M. , Fairhall, S. L. , Lenci, A. , Baroni, M. , & Caramazza, A. (2014). Predication drives verb cortical signatures. Journal of Cognitive Neuroscience, 26(8), 1829–1839. [DOI] [PubMed] [Google Scholar]
- Herrera, E. , & Cuetos, F. (2013). Semantic disturbance for verbs in Parkinson's disease patients off medication. Journal of Neurolinguistics, 26(6), 737–744. 10.1016/j.jneuroling.2013.01.002. [DOI] [Google Scholar]
- Herrera, E. , Cuetos, F. , & Ribacoba, R. (2012). Verbal fluency in Parkinson's disease patients on/off dopamine medication. Neuropsychologia, 50(14), 3636–3640. 10.1016/j.neuropsychologia.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Hsu, N. S. , Jaeggi, S. M. , & Novick, J. M. (2017). A common neural hub resolves syntactic and non‐syntactic conflict through cooperation with task‐specific networks. Brain and Language, 166, 63–77. 10.1016/j.bandl.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauschke, C. , & von Frankenberg, J. (2008). The differential influence of lexical parameters on naming latencies in German. A study on noun and verb picture naming. Journal of Psycholinguistic Research, 37(4), 243–257. [DOI] [PubMed] [Google Scholar]
- Kemmerer, D. (2014). Word classes in the brain: Implications of linguistic typology for cognitive neuroscience. Cortex, 58(0), 27–51. 10.1016/j.cortex.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Kemmerer, D. (2015). Are the motor features of verb meanings represented in the precentral motor cortices? Yes, but within the context of a flexible, multilevel architecture for conceptual knowledge. Psychonomic Bulletin & Review, 22(4), 1068–1075. 10.3758/s13423-014-0784-1 [DOI] [PubMed] [Google Scholar]
- Kemmerer, D. , Castillo, J. G. , Talavage, T. , Patterson, S. , & Wiley, C. (2008). Neuroanatomical distribution of five semantic components of verbs: Evidence from fMRI. Brain and Language, 107, 16–43. [DOI] [PubMed] [Google Scholar]
- Kemmerer, D. , Miller, L. , MacPherson, M. K. , Huber, J. , & Tranel, D. (2013). An investigation of semantic similarity judgments about action and non‐action verbs in Parkinson's disease: Implications for the Embodied Cognition Framework. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer, D. , Rudrauf, D. , Manzel, K. , & Tranel, D. (2012). Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex, 48(7), 826–848. 10.1016/j.cortex.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie, C. M. , Fulbright, R. K. , Constable, R. T. , & Papademetris, X. (2008). More accurate Talairach coordinates for NeuroImaging using nonlinear registration. NeuroImage, 42(2), 717–725. 10.1016/j.neuroimage.2008.04.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Lancaster, J. L. , & Fox, P. T. (2005). BrainMap: The social evolution of a human brain mapping database. Neuroinformatics, 3(1), 65–78. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph, M. A. (2014). Neurocognitive insights on conceptual knowledge and its breakdown. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1634), 20120392 10.1098/rstb.2012.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph, M. A. , Jefferies, E. , Patterson, K. , & Rogers, T. T. (2017). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18(1), 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Lancaster, J. L. , & Martinez, M. (2015). Mango (version 3.5) [computer software]. Available from: http://rii.uthscsa.edu/
- Lancaster, J. L. , Tordesillas‐Gutierrez, D. , Martinez, M. , Salinas, F. , Evans, A. , Zilles, K. , … Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Human Brain Mapping, 28(11), 1194–1205. 10.1002/hbm.20345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin, D. J. , & Menon, V. (2003). Musical structure is processed in “language” areas of the brain: A possible role for Brodmann area 47 in temporal coherence. NeuroImage, 20(4), 2142–2152. [DOI] [PubMed] [Google Scholar]
- Li, P. , Jin, Z. , & Tan, L. H. (2004). Neural representations of nouns and verbs in Chinese: An fMRI study. NeuroImage, 21(4), 1533–1541. [DOI] [PubMed] [Google Scholar]
- Li, M. , Lu, S. , & Zhong, N. (2016). The Parahippocampal cortex mediates contextual associative memory: Evidence from an fMRI study. BioMed Research International, 2016, 9860604 10.1155/2016/9860604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljestrom, M. , Tarkiainen, A. , Parviainen, T. , Kujala, J. , Numminen, J. , Hiltunen, J. , … Salmelin, R. (2008). Perceiving and naming actions and objects. NeuroImage, 41, 1132–1141. [DOI] [PubMed] [Google Scholar]
- Lin, N. , Guo, Q. , Han, Z. , & Bi, Y. (2011). Motor knowledge is one dimension for concept organization: Further evidence from a Chinese semantic dementia case. Brain and Language, 119(2), 110–118. 10.1016/j.bandl.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Liu, H.‐L. , Liao, W.‐T. , Fang, S.‐Y. , Chu, T.‐C. , & Tan, L. H. (2004). Correlation between temporal response of fMRI and fast reaction time in a language task. Magnetic Resonance Imaging, 22(4), 451–455. 10.1016/j.mri.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Longe, O. , Randall, B. , Stamatakis, E. , & Tyler, L. (2007). Grammatical categories in the brain: The role of morphological structure. Cerebral Cortex, 17, 1812–1820. [DOI] [PubMed] [Google Scholar]
- Luzzatti, C. , Raggi, R. , Zonca, G. , Pistarini, C. , Contardi, A. , & Pinna, G.‐D. (2002). Verb‐noun double dissociation in aphasic lexical impairments: The role of word frequency and imageability. Brain and Language, 81, 432–444. [DOI] [PubMed] [Google Scholar]
- Marangolo, P. , Piras, F. , Galati, G. , & Burani, C. (2006). Functional anatomy of derivational morphology. Cortex, 42(8), 1093–1106. [DOI] [PubMed] [Google Scholar]
- Martin, A. (2016). GRAPES—Grounding representations in action, perception, and emotion systems: How object properties and categories are represented in the human brain. Psychonomic Bulletin & Review, 23(4), 979–990. 10.3758/s13423-015-0842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A. , Haxby, J. V. , Lalonde, F. M. , Wiggs, C. L. , & Ungerleider, L. G. (1995). Discrete cortical regions associated with knowledge of color and knowledge of action. Science, 270(5233), 102–105. [DOI] [PubMed] [Google Scholar]
- Mätzig, S. , Druks, J. , Masterson, J. , & Vigliocco, G. (2009). Noun and verb differences in picture naming: Past studies and new evidence. Cortex, 45, 738–758. [DOI] [PubMed] [Google Scholar]
- McNorgan, C. , Chabal, S. , O'Young, D. , Lukic, S. , & Booth, J. R. (2015). Task dependent Lexicality effects support interactive models of reading: A meta‐analytic neuroimaging review. Neuropsychologia, 67, 148–158. 10.1016/j.neuropsychologia.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli, G. , Silveri, M. C. , Villa, G. , & Caramazza, A. (1984). On the basis for the agrammatic's difficulty in producing main verbs. Cortex, 20(2), 207–220. [DOI] [PubMed] [Google Scholar]
- Momenian, M. , Nilipour, R. , Samar, R. G. , Oghabian, M. A. , & Cappa, S. (2016). Neural correlates of verb and noun processing: An Fmri study of Persian. Journal of Neurolinguistics, 37, 12–21. [Google Scholar]
- Moseley, R. L. , & Pulvermuller, F. (2014). Nouns, verbs, objects, actions, and abstractions: Local fMRI activity indexes semantics, not lexical categories. Brain and Language, 132, 28–42. 10.1016/j.bandl.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, H. E. , De Mornay Davies, P. , Jeppeson, C. , McLellan, S. , & Tyler, L. K. (1998). The relationship between knowledge of nouns and verbs in a category‐specific deficit for living things. Brain and Language, 65, 92–95. [Google Scholar]
- Oh, A. , Duerden, E. G. , & Pang, E. W. (2014). The role of the insula in speech and language processing. Brain and Language, 135, 96–103. 10.1016/j.bandl.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palti, D. , Ben Shachar, M. , Hendler, T. , & Hadar, U. (2007). Neural correlates of semantic and morphological processing of Hebrew nouns and verbs. Human Brain Mapping, 28(4), 303–314. 10.1002/hbm.20280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeo, L. , & Lingnau, A. (2015). First‐person and third‐person verbs in visual motion‐perception regions. Brain and Language, 141, 135–141. [DOI] [PubMed] [Google Scholar]
- Perani, D. , Cappa, S. F. , Schnur, T. , Tettamanti, M. , Collina, S. , Rosa, M. M. , & Fazio, F. (1999). The neural correlates of verb and noun processing. A PET study. Brain, 122(Pt 12), 2337–2344. [DOI] [PubMed] [Google Scholar]
- Peterson, S. E. , Fox, P. T. , Posner, M. I. , Mintun, M. , & Raichle, M. E. (1988). Positron emission tomographic studies of the anatomy of single word processing. Nature, 331, 585–589. [DOI] [PubMed] [Google Scholar]
- Piatt, A. L. , Fields, J. A. , Paolo, A. M. , & Tröster, A. I. (1999). Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia, 37(13), 1499–1503. [DOI] [PubMed] [Google Scholar]
- Pillon, A. , & d'Honincthun, P. (2011). The organization of the conceptual system: The case of the "object versus action" dimension. Cognitive Neuropsychology, 27(7), 587–613. 10.1080/02643294.2011.609652 [DOI] [PubMed] [Google Scholar]
- Piras, F. , & Marangolo, P. (2007). Noun‐verb naming in aphasia: A voxel‐based lesion‐symptom mapping study. Neuroreport, 18(14), 1455–1458. [DOI] [PubMed] [Google Scholar]
- Piras, F. , & Marangolo, P. (2010). When "crack walnuts" lies in different brain regions: Evidence from a voxel‐based lesion‐symptom mapping study. Journal of the International Neuropsychological Society, 16(3), 433–442. 10.1017/s1355617710000068 [DOI] [PubMed] [Google Scholar]
- Pulvermuller, F. (1999). Words in the brain's language. Behavioral and Brain Sciences, 22(2), 253–279. [PubMed] [Google Scholar]
- Radua, J. , Mataix‐Cols, D. , Phillips, M. L. , El‐Hage, W. , Kronhaus, D. M. , Cardoner, N. , & Surguladze, S. (2012). A new meta‐analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27(8), 605–611. 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Raposo, A. , Moss, H. E. , Stamatakis, E. A. , & Tyler, L. K. (2009). Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia, 47(2), 388–396. [DOI] [PubMed] [Google Scholar]
- Robins, R. H. (1952). Noun and verb in universal grammar. Language, 28, 289–298. [Google Scholar]
- Robinson, K. M. , Grossman, M. , White‐Devine, T. , & D'Esposito, M. (1996). Category‐specific difficulty naming with verbs in Alzheimer's disease. Neurology, 47(1), 178–182. 10.1212/WNL.47.1.178. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Ferreiro, J. , Davies, R. , González‐Nosti, M. , Barbón, A. , & Cuetos, F. (2009). Name agreement, frequency and age of acquisition, but not grammatical class, affect object and action naming in Spanish speaking participants with Alzheimer's disease. Journal of Neurolinguistics, 22(1), 37–54. [Google Scholar]
- Saccuman, M. C. , Cappa, S. F. , Bates, E. A. , Arevalo, A. , Della Rosa, P. , Danna, M. , & Perani, D. (2006). The impact of semantic reference on word class: An fMRI study of action and object naming. NeuroImage, 32, 1865–1878. [DOI] [PubMed] [Google Scholar]
- Sahin, N. T. , Pinker, S. , & Halgren, E. (2006). Abstract grammatical processing of nouns and verbs in Broca's area: Evidence from FMRI. Cortex, 42(4), 540–562. [DOI] [PubMed] [Google Scholar]
- Sebastian, R. , Gomez, Y. , Leigh, R. , Davis, C. , Newhart, M. , & Hillis, A. E. (2014). The roles of occipitotemporal cortex in reading, spelling, and naming. Cognitive Neuropsychology, 31(5–6), 511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, K. A. , Mottaghy, F. M. , Schiller, N. O. , Poeppel, T. D. , Flu, M. O. , Muller, H. W. , … Krause, B. J. (2005). Dissociating neural correlates for nouns and verbs. NeuroImage, 24, 1058–1065. [DOI] [PubMed] [Google Scholar]
- Shapiro, K. , Moo, L. R. , & Caramazza, A. (2006). Cortical signatures of noun and verb production. Proceedings of the National Academy of Sciences of the United States of America, 103, 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, K. A. , Pascual‐Leone, A. , Mottaghy, F. M. , Gangitano, M. , & Caramazza, A. (2001). Grammatical distinctions in the left frontal cortex. Journal of Cognitive Neuroscience, 13, 713–720. [DOI] [PubMed] [Google Scholar]
- Silveri, M. , Perri, R. , & Cappa, A. (2003). Grammatical class effects in brain‐damaged patients: Functional locus of noun and verb deficit. Brain and Language, 85(1), 49–66. 10.1016/S0093-934X(02)00504-7 [DOI] [PubMed] [Google Scholar]
- Siri, S. , Tettamanti, M. , Cappa, S. F. , Rosa, P. D. , Saccuman, C. , Scifo, P. , & Vigliocco, G. (2008). The neural substrate of naming events: Effects of processing demands but not of grammatical class. Cerebral Cortex, 18, 171–177. [DOI] [PubMed] [Google Scholar]
- Soros, P. , Cornelissen, K. , Laine, M. , & Salmelin, R. (2003). Naming actions and objects: Cortical dynamics in healthy adults and in an anomic patient with a dissociation in action/object naming. NeuroImage, 19(4), 1787–1801. [DOI] [PubMed] [Google Scholar]
- Suzuki, W. A. (1996). Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: Organization of cortical inputs and interconnections with amygdala and striatum. Seminars in Neuroscience, 8(1), 3–12. 10.1006/smns.1996.0002 [DOI] [Google Scholar]
- Szekely, A. , D'Amico, S. , Devescovi, A. , Federmeier, K. , Herron, D. , Iyer, G. , … Bates, E. (2005). Timed action and object naming. Cortex, 41(1), 7–25. [DOI] [PubMed] [Google Scholar]
- Taylor, K. I. , Moss, H. E. , Stamatakis, E. A. , & Tyler, L. K. (2006). Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences, 103(21), 8239–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thioux, M. , Pesenti, M. , Costes, N. , De Volder, A. , & Seron, X. (2005). Task‐independent semantic activation for numbers and animals. Cognitive Brain Research, 24(2), 284–290. 10.1016/j.cogbrainres.2005.02.009 [DOI] [PubMed] [Google Scholar]
- Tranel, D. , Manzel, K. , Asp, E. , & Kemmerer, D. (2008). Naming dynamic and static actions: Neuropsychological evidence. Journal of Physiology‐Paris, 102(1–3), 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human Brain Mapping, 33, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, L. K. , Bright, P. , Fletcher, P. , & Stamatakis, E. A. (2004). Neural processing of nouns and verbs: The role of inflectional morphology. Neuropsychologia, 42, 512–523. [DOI] [PubMed] [Google Scholar]
- Tyler, L. K. , Marslen‐Wilson, W. D. , & Stamatakis, E. A. (2005). Differentiating lexical form, meaning, and structure in the neural language system. Proceedings of the National Academy of Science of the United States America, 102, 8375–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, L. K. , Russell, R. , Fadili, J. , & Moss, H. E. (2001). The neural representation of nouns and verbs: PET studies. Brain, 124(Pt.8), 1619–1634. [DOI] [PubMed] [Google Scholar]
- Tyler, L. K. , Stamatakis, E. A. , Dick, E. , Bright, P. , Fletcher, P. , & Moss, H. (2003). Objects and their actions: Evidence for a neurally distributed semantic system. NeuroImage, 18, 542–550. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill, S. L. (2003). Neuroimaging studies of semantic memory: Inferring "how" from "where". Neuropsychologia, 41(3), 280–292. [DOI] [PubMed] [Google Scholar]
- Vigliocco, G. , Vinson, D. P. , Druks, J. , Barber, H. , & Cappa, S. F. (2011). Nouns and verbs in the brain: A review of behavioural, electrophysiological, neuropsychological and imaging studies. Neuroscience & Biobehavioral Reviews, 35(3), 407–426. [DOI] [PubMed] [Google Scholar]
- Vigliocco, G. , Vinson, D. P. , Lewis, W. , & Garrett, M. F. (2004). Representing the meanings of object and action words: The featural and unitary semantic space hypothesis. Cognitive Psychology, 48(4), 422–488. 10.1016/j.cogpsych.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Volle, E. , Kinkingnéhun, S. , Pochon, J. B. , Mondon, K. , Thiebaut de Schotten, M. , Seassau, M. , … Levy, R. (2008). The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cerebral Cortex, 18(10), 2460–2469. [DOI] [PubMed] [Google Scholar]
- Warburton, E. , Wise, R. J. S. , Price, C. J. , Weiller, C. , Hadar, U. , Ramsay, S. , & Frackowiak, R. S. J. (1996). Noun and verb retrieval by normal subjects: Studies with PET. Brain, 119, 159–179. [DOI] [PubMed] [Google Scholar]
- Watson, C. E. , Cardillo, E. R. , Ianni, G. R. , & Chatterjee, A. (2013). Action concepts in the brain: An activation likelihood estimation meta‐analysis. Journal of Cognitive Neuroscience, 25(8), 1191–1205. [DOI] [PubMed] [Google Scholar]
- Watson, C. E. , & Chatterjee, A. (2011). The functional neuroanatomy of actions. Neurology, 76(16), 1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S. E. , & Canter, G. J. (1987). Action‐naming performance in four syndromes of aphasia. Brain and Language, 32(1), 124–136. [DOI] [PubMed] [Google Scholar]
- Wise, R. , Chollet, F. , Hadar, U. , Friston, K. , Hoffner, E. , & Frackowaik, R. (1991). Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain, 114, 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wu, D. H. , Morganti, A. , & Chatterjee, A. (2008). Neural substrates of processing path and manner information of a moving event. Neuropsychologia, 46, 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Lin, Q. , Han, Z. , Li, H. , Song, L. , Chen, L. , et al (2017). Dissociable intrinsic functional networks support noun‐object and verb‐action processing. Brain Lang, 175:29–41. 10.1016/j.bandl.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Yokoyama, S. , Miyamoto, T. , Riera, J. , Kim, J. , Akitsuki, Y. , Iwata, K. , … Kawashima, R. (2006). Cortical mechanisms involved in the processing of verbs: An fMRI study. Journal of Cognitive Neuroscience, 18, 1304–1313. [DOI] [PubMed] [Google Scholar]
- Yu, X. , Bi, Y. , Han, Z. , Zhu, C. , & Law, S.‐P. (2012). Neural correlates of comprehension and production of nouns and verbs in Chinese. Brain and Language, 122, 126–131. [DOI] [PubMed] [Google Scholar]
- Zingeser, L. B. , & Berndt, R. S. (1990). Retrieval of nouns and verbs in agrammatism and anomia [see comments]. Brain & Language, 39(1), 14–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1


