Abstract
Accumulating evidence has suggested functional interactions between prefrontal cortex (PFC) and dissociable large‐scale networks. However, how these networks interact in the human brain to enable complex behaviors is not well‐understood. Here, using a combination of behavioral, brain stimulation and neuroimaging paradigms, we tested the hypothesis that human PFC is required for successful reinforced skill formation. We additionally tested the extent to which PFC‐dependent skill formation is related to intrinsic functional communication with this region. We report that inhibitory noninvasive transcranial magnetic stimulation over lateral PFC, a hub region with a diverse connectivity profile, causally modulated effective reinforcement‐based motor skill acquisition. Furthermore, PFC‐dependent skill formation was strongly related to the strength of functional connectivity between the PFC and regions in the sensorimotor network. These results point to the involvement of lateral PFC in the neural architecture that underlies the acquisition of complex skills, and suggest that, in relation to skill acquisition, this region may be involved in functional interactions with sensorimotor networks.
Keywords: functional MRI, motor learning, prefrontal cortex, reward, sequence learning, transcranial magnetic stimulation
1. INTRODUCTION
Multiple lines of evidence suggest that lateral prefrontal cortex (lPFC) may play a significant role in modulating diverse learning and memory systems (Cieslik et al., 2012; Hasan et al., 2013; Jarbo & Verstynen, 2015; Preston & Eichenbaum, 2013). The hippocampus and its extensive connections with the prefrontal cortex (Goldman‐Rakic, Selemon, & Schwartz, 1984) have been suggested as the key circuitry necessary for declarative memory formation, while sensorimotor circuits have been linked to procedural memory formation (Dayan & Cohen, 2011; Doyon & Benali, 2005), both facilitated by reward mechanisms (Abe et al., 2011; Dayan, Hamann, Averbeck, & Cohen, 2014; Hamann, Dayan, Hummel, & Cohen, 2014; Miendlarzewska, Bavelier, & Schwartz, 2016). Altogether, accumulating evidence has pointed to functional interactions within large‐scale learning and memory systems, involving lPFC (Cieslik et al., 2012; Hasan et al., 2013; Jarbo & Verstynen, 2015; Preston & Eichenbaum, 2013; Witt et al., 2008). However, a mechanistic account on how these systems interact in the human brain to enable motor behavior is missing, forming a gap in knowledge as to how motor skill memory is formed in ecologically relevant learning environments.
Here, we used a combination of noninvasive brain stimulation and functional MRI (fMRI) to study the causal role of human lPFC in formation of reinforced skill learning, and its functional relation to large‐scale functional networks. Functional and/or structural connections exist between the lPFC and sensorimotor (Cieslik et al., 2012; Hasan et al., 2013), declarative/hippocampal (Preston & Eichenbaum, 2013), and reward systems (Haber, 2011; Jarbo & Verstynen, 2015). This region is among a set of densely inter‐connected hub regions which are believed to play a key role in information integration within large‐scale brain systems (van den Heuvel & Sporns, 2011). In addition, using different skill acquisition paradigms, previous studies have pointed to a relation between PFC and motor skill learning (Gomez Beldarrain, Gafman, Ruiz de Velasco, Pascual‐Leone, & Garcia‐Monco, 2002; Kaminski et al., 2013), suggesting that the degree of PFC involvement relies on the cognitive demands of the performed tasks (Robertson, Tormos, Maeda, & Pascual‐Leone, 2001; Witt et al., 2008). We thus hypothesized that the lPFC is causally involved in formation of reinforced skill learning, and as such, is related to connectivity in functional networks that interact with the lPFC. To address this hypothesis, we tested whether perturbing brain lPFC activity in a controlled manner, via repetitive transcranial magnetic stimulation (rTMS) (Robertson et al., 2001) would affect skill acquisition. We then tested the relation between the effects of stimulation and the strength of intrinsic functional connections with lPFC. We had subjects train on a skill learning task (Figure 1), where performance was reinforced with monetary reward. In half of the sessions, training took place immediately after inhibitory rTMS was applied to right lPFC, contralateral to the hand used in the skill learning task (consistent with previous studies, Pascual‐Leone et al., 1996; Robertson et al., 2001). In the second half of the sessions, administered in a crossover design, training took place after stimulation of the vertex, a commonly used control site (Dafotakis et al., 2008; Dayan, Censor, Buch, Sandrini, & Cohen, 2013; Ko, Monchi, Ptito, Petrides, & Strafella, 2008; Nowak et al., 2008), allowing us to quantify the influence of lPFC on learning gains. We then assessed how lPFC‐dependent learning gains related to the strength of intrinsic functional connectivity using individually defined seeds in this region (corresponding to each subject's stimulation location), based on resting‐state fMRI scans that took place independently from the training sessions. This combination of approaches allowed us to examine the casual role of lPFC in reinforced skill learning, and the degree to which it relates to intrinsic functional connections within networks that implicate lPFC.
Figure 1.
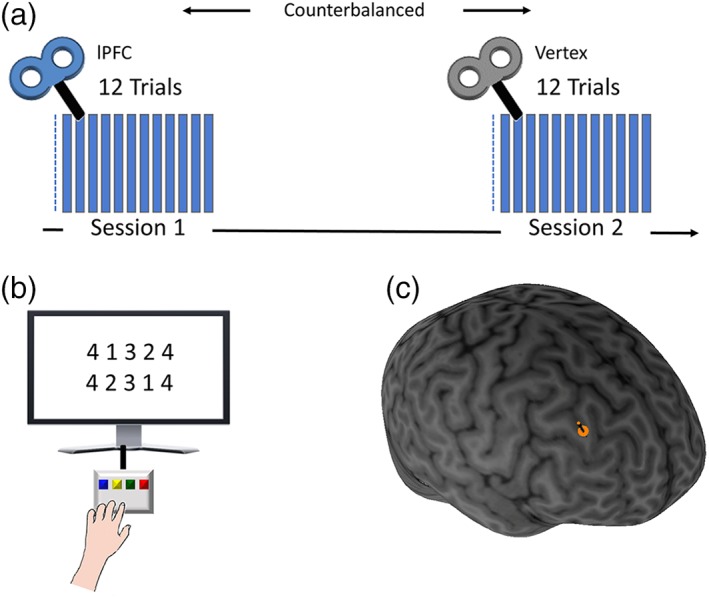
Experimental setup. Subjects practiced a sequential finger‐tapping task (a) two sessions were administered 1 week apart in a counterbalanced manner. Both sessions began with a single baseline test. Next, one session included an offline inhibitory 1 Hz rTMS of right lPFC, while the other included a control stimulation (see main text). In each of the sessions, subjects then performed 12 trials of training, lasting 30 s each, separated by 30 s breaks. (b) The task required subjects to repeatedly tap a 5‐element sequence of finger movements with their left nondominant hand as quickly and accurately as possible. Monetary reward was stochastically administered, as a strategy to facilitate learning gains (see Section 2). (c) Location of lateral PFC stimulation site, localized and maintained online using a neuronavigation system
2. MATERIALS AND METHODS
2.1. Subjects
Twenty healthy volunteers (8 males and 12 females; mean age = 26.1 ± 0.8 years) participated in the study. Subjects were all right handed. Five additional subjects were excluded from the experiment: three subjects were excluded before receiving rTMS due artifacts in the MRI scans, and two subjects stopped their participation during the TMS session due to discomfort. All subjects provided written informed consent and all procedures were in accordance with a protocol approved by the Tel Aviv Sourasky Medical Center and Tel Aviv University's Ethics committees. Musicians and video‐gamers (past or present) were excluded from the study, as well as subjects with psychiatric or neurological history. In addition, subjects were required to sleep at least 6 hr before each of the experimental sessions.
2.2. Procedure and task
The study comprised three sessions, with the main (stimulation) sessions following a crossover design. Subjects first underwent an imaging session where resting‐state scans were acquired, in which they were instructed to keep their eyes closed and not fall asleep. Then, subjects were randomized and performed two training sessions of the sequential finger‐tapping task in a within‐subject crossover design: half of the subjects performed a training session with one of the two sequences right after receiving rTMS to right lPFC (see more details bellow) and then returned 7 days later to perform the untrained sequence right after receiving rTMS to the vertex, as a control site (Dafotakis et al., 2008; Dayan et al., 2013; Ko et al., 2008; Nowak et al., 2008). In the remaining subjects, the order was reversed, with the first training session administered right after vertex stimulation, and the second after lPFC stimulation. As in previous studies, subjects executed a sequential finger‐tapping task with their left nondominant hand, to eliminate a possible ceiling effect on learning (Censor, Dimyan, & Cohen, 2010; Censor, Horovitz, & Cohen, 2014; Censor, Dimyan, & Cohen, 2014; de Beukelaar, Woolley, & Wenderoth, 2014; Korman et al., 2007; Walker, Brakefield, Allan Hobson, & Stickgold, 2003; Karni et al., 1998). During the task, subjects were required to repeatedly tap a 5‐element sequence of finger movements as quickly and accurately as possible (Censor et al., 2010; Karni et al., 1995, 1998) (Figure 1). The task included one 30 s baseline trial (before TMS, see below), and 12 additional trials (after TMS), lasting 30 s each, interleaved with 30 s breaks. Tapping movements were performed using a 4‐key response box (Cedrus Lumina LU440) which was placed in front of the subjects at a comfortable distance and height. The order of sessions was counterbalanced across participants, to minimize carry‐over effects, and different sequences were used in each session (4‐1‐3‐2‐4 and 4‐2‐3‐1‐4), to minimize carry‐over effects. “4,” “3,” “2,” and “1” corresponded to the index, middle, ring, and little fingers, respectively. In each training session, half of the trials were rewarded, in which subjects could win 1 Shekel per every five correct sequences (3.5 Shekels roughly equal 1 USD). Prior to each rewarded trial the instructions on the screen explicitly indicated that in the next trial subjects could win 1 Israeli Shekel for every five correct sequences they tapped. A short (5 s) feedback phase was provided after each rewarded trial indicating how much money they have won in the trial (for instance if the subject tapped 22 sequences she/he was given a “You won 4 Shekels!” feedback). Rewarded and unrewarded trials were stochastically administered, as a strategy to facilitate learning gains (Dayan, Averbeck, Richmond, & Cohen, 2014; Dayan, Hamann, et al., 2014), limiting the presentation of consecutive rewarded or unrewarded trials to not more than two in succession. Response data were collected for offline analysis using Psychtoolbox (MATLAB 8.4). During each trial, the sequence was displayed at the middle of the screen and remained in this position throughout the trial. The number of correct sequences tapped during each trial served as our primary behavioral outcome measure, a common and highly replicable end‐point measure in sequence learning (Censor et al., 2010; Censor, Dayan, et al., 2014; Censor, Horovitz, et al., 2014; de Beukelaar et al., 2014; Karni et al., 1995; Korman et al., 2007; Walker et al., 2003).
2.3. Noninvasive brain stimulation
On each of the two experimental sessions, the main task (12 trials) was performed immediately following application of rTMS at 1 Hz for 15 min, delivered to either lPFC or the vertex as a control site (Dafotakis et al., 2008; Dayan et al., 2013; Ko et al., 2008; Nowak et al., 2008). Of note, rTMS over the vertex has been a reliable control condition, as the auditory and somatosensory activations caused by vertex TMS are similar to those of real TMS (Sandrini, Cappa, Rossi, Rossini, & Miniussi, 2003; Sandrini, Censor, Mishoe, & Cohen, 2013; Sandrini, Umiltà, & Rusconi, 2011). LPFC was localized based on the international 10–20 system for EEG electrode placement. Based on studies (Griškova, Rukšėnas, Dapšys, Herpertz, & Höppner, 2007; Herwig, Satrapi, & Schönfeldt‐Lecuona, 2003) locating the lPFC at the F3 electrode placement, we used the homolog F4 location, to target the lPFC contralateral to the nondominant hand performing the behavioral task. This approach has been common for PFC stimulation (Rossi et al., 2001; Sandrini et al., 2003, 2013). Following the identification of the stimulation target, a neuro‐navigation system (Brainsight 2, Rogue Research, Montreal, Canada, http://www.rogue-research.com) was used to maintain coil stability. In addition, the neuro‐navigation system was used to coregister participants’ heads and to mark stimulation sites prior to rTMS administration. Four landmarks were used for coregistering the participants head to their MRI anatomic scan (nasion, tip of the nose, left and right crus of helix).
Stimulation was given at 100% of resting motor threshold measured over the same hemisphere, for 15 min, at a frequency of 1 Hz, a common protocol that has been shown to affect cortical excitability beyond the duration of the rTMS application itself (Chen et al., 1997; Sandrini et al., 2011), and more specifically applied for PFC offline stimulation (Sandrini et al., 2013). With this common protocol, we could expect the decrease in cortical excitability to last at least 15 min post stimulation (Chen et al., 1997). The resting motor threshold was defined as the minimal primary motor cortex (M1) stimulation intensity yielding 5 out of 10 motor‐evoked potentials (MEPs) greater than 0.05 mV in the left first dorsal interosseous (FDI) muscle (Rossini et al., 1994).
2.4. Behavioral data analysis
Behavioral data were analyzed with SPSS 23. A standard behavioral outcome measure was used, the number of correct sequences per trial, which accounts for both speed and accuracy (Censor et al., 2010; Censor, Dayan, et al., 2014; de Beukelaar et al., 2014; Korman et al., 2007; Walker et al., 2003). Learning gains were then measured as the difference between performance at baseline and peak performance at the last three trials of the session (Censor et al., 2010; Censor, Horovitz, et al., 2014; de Beukelaar et al., 2014; Walker et al., 2003). Paired t‐tests were used to test for between session differences in baseline performance, and for differences in stimulation‐dependent learning gains in rewarded and unrewarded trials. Finally, we tested stimulation‐dependent differences in learning gains using a repeated measures Analysis of Variance (ANOVA) with stimulation type (lPFC vs. control) and performance (baseline vs. peak) as within‐subjects factors.
2.5. Imaging data acquisition
Imaging data were acquired with a 3 T SIEMENS MAGNETOM Prisma scanner equipped with a 20‐channel head coil at the Wohl Institute for Advanced Imaging, Tel Aviv Sourasky Medical Center. Structural images were acquired with an MPRAGE sequence (repetition time/echo time [TR/TE] = 1860/2.74 ms; flip angle = 8°; field of view [FOV] = 256 × 256 mm; slice thickness = 1 mm; 208 axial slices). Resting state fMRI images were acquired with a gradient echo‐planar imaging (EPI) sequence of functional T2*‐weighted images (TR/TE = 2000/35 ms; flip angle = 90°; FOV = 384 × 384 mm; slice thickness = 4 mm; 34 interleaved axial slices per volume). The functional scans comprised a total of 240 volumes which lasted 8 min. The first three volumes were discarded to account of T1‐equilibrium effects. Two subjects (of the total 20) were scanned with different functional parameters (TR/TE = 3000/35 ms; flip angle = 90°; FOV = 672 × 672 mm; slice thickness = 3 mm; 46 interleaved axial slices per volume). We confirmed that all imaging results were retained when excluding these two subjects.
2.6. Imaging data analysis
Imaging data analysis was based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12) and the Conn toolbox (Whitfield‐Gabrieli & Nieto‐Castanon, 2012) version 16b. Preprocessing of functional images included realignment, unwarping and slice‐time correction, segmentation of gray‐matter, white matter, and cerebrospinal fluid (CSF) and normalization to the MNI template. The data were additionally spatially smoothed with a Gaussian kernel set at 8 mm full width at half maximum. Signals from the segmented white matter and CSF, and the six motion realignment parameters and their first‐order derivatives were regressed out of the signal. We have additionally regressed out outlier volumes (and the single volumes preceding the outliers) detected using the ART‐based scrubbing method (based on a threshold of 2 mm subject motion and a global signal threshold of Z = 9). Subsequently, the data were linearly detrended and band‐pass filtered (0.01 to 0.1 Hz). Two‐levels of analysis were included. On the first level of analysis, seed‐to‐voxel maps were computed for each subject based on individually localized seeds in each subject's location of stimulation, where a spherical seed (6 mm radius) was defined using MarsBaR (http://marsbar.sourceforge.net/). Individualized seed localization was done in 19/20 of the subjects. In one additional subject, storage of stimulation location was not saved due to technical malfunction. In this subject, the lPFC seed was based on the average lPFC coordinates of all other subjects. The maps express the correlation (Fisher Z‐transformed Pearson's correlation values) of each voxel with the mean time series extracted from each seed region of interest. Then, on the second level of analysis, the maps were subjected to a random effects second‐level general linear model analysis, testing how connectivity correlated with a continuous covariate that expressed stimulation‐dependent learning gains. The covariate was defined as learning gains in the lPFC session subtracted from gains in the control session (Δ [trainingmax_control − baseline control] − [trainingmax_lPFC − baseline lPFC]). Two additional covariates were tested independently to examine how lPFC connectivity related to learning gains in rewarded and unrewarded trials. A covariate for learning gains in rewarded trials was defined as: (Δ [trainingmaxRewarded_control − baselinecontrol] − [trainingmaxRewarded_lPFC − baseline lPFC]). Similarly, a covariate for learning gains in unrewarded trials was defined as: (Δ [trainingmaxUnRewarded_control − baseline control] − [trainingmaxUnRewarded_lPFC − baseline lPFC]). An intercept term was included in the model as well. A voxel‐level threshold of p < .001 was used in all analyses, qFDR (<0.05) corrected for multiple comparisons at the cluster level.
3. RESULTS
3.1. Effects of stimulation on learning
Our first objective was to probe for the role of human PFC in successful reinforced skill formation. We fitted the data with a Repeated measures ANOVA, with stimulation type (lPFC vs. control) and performance (baseline vs. peak) as within‐subject factors. There was a significant main effect for performance (F 1,19 = 233.916, p < .001; ηp 2 = 0.925), and a nonsignificant main effect for stimulation type (F 1,19 = 0.325, p = .575; ηp 2 = 0.017). The interaction between the two factors was statistically significant (F 1,19 = 4.505, p = .047, ηp 2 = 0.192; see Figure 2c; for single‐subject data see Figure 2b), showing that maximal gains in performance were reduced following lPFC stimulation. These results suggest that in comparison to control stimulation, 1 Hz rTMS applied over the lPFC decreased reinforced skill learning gains (Figure 2c).
Figure 2.
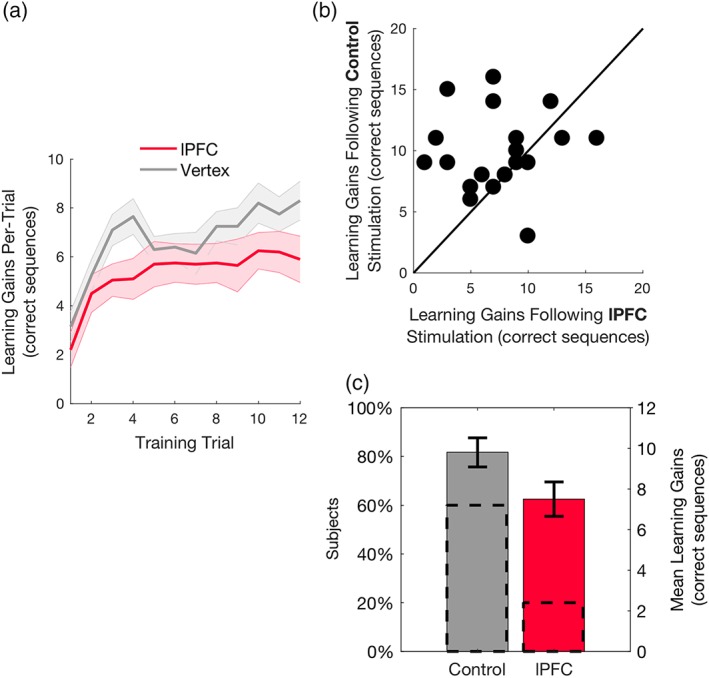
Effects of stimulation on learning gains. (a) Performance per training block following control and lPFC stimulation. Shaded area denotes standard error of the mean (SEM). (b) Single‐subject data of maximal learning gains following lPFC or control stimulation are presented in a scatterplot along the unit slope line (y = x), where each point reflects a participant (Herszage and Censor, 2017). Data accumulating above the line indicate reduced learning gains following lPFC stimulation (data accumulating exactly on the line indicate equal learning gains in both lPFC and control stimulation). (c) Mean maximal learning gains following lPFC and control stimulation. Dashed lines reflect the percentage of participants on each side of the unit slope line in (b) and the bars reflect the mean maximal learning gains at each session. Error bars represent SEM
To rule out effects on motor performance per se (rather than learning), a control analysis showed that the differences between the prestimulation trial (baseline) and initial poststimulation trial (always unrewarded) were comparable across lPFC and control locations (t 19 = −0.49, p = .63, Cohen's d = 0.109, two‐tailed). In addition, performance at the baseline trials, which were completed prior to the stimulation on each session, did not differ between the times of sessions (t 19 = 1.836, p = .082, d = 0.41, two‐tailed).
3.2. Association between baseline functional connectivity and stimulation effects
In light of lPFC's dense functional connections with sensorimotor and reward networks (Cieslik et al., 2012; Haber, 2011; Hasan et al., 2013; Jarbo & Verstynen, 2015; Preston & Eichenbaum, 2013), we next tested if the effects of stimulation on learning gains were mediated by baseline functional connectivity with the lPFC, as recorded prior to the main stimulation/training sessions.
We first defined, for each of the subjects, intrinsic functional networks based on functional connectivity between the right lPFC and the rest of the brain. This network was primarily composed of clusters in contralateral lateral, orbitofrontal and dorsomedial PFC, and supramarginal and angular gyri (Supporting Information Figure S1), largely mirroring the widely studied frontoparietal control network (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). To assess how stimulation‐dependent learning gains related to connectivity, we subjected the data to an analysis of covariance (ANCOVA) analysis (Figure 3b,c and Table 1). Stimulation‐dependent learning gains correlated with functional connectivity between lPFC and sensorimotor cortex (mainly the medial and dorsolateral parts of the precentral gyrus), postcentral and central opercular cortex, and occipitotemporal cortex (Figure 3). There were no significant hippocampal or striatal clusters where functional connectivity correlated with learning gains.
Figure 3.
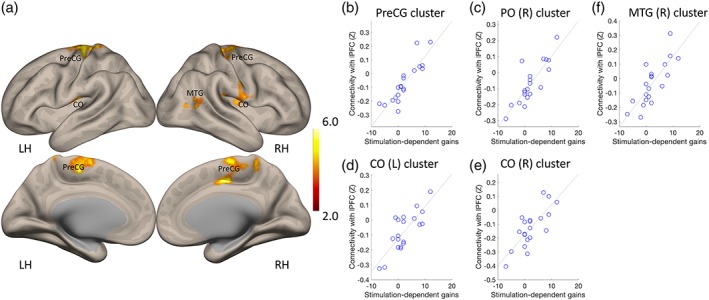
Clusters of voxels where connectivity with the lPFC was significantly correlated with stimulation‐dependent learning gains. (a) Location of clusters. (b–f) Association between connectivity (fisher Z transformed values) and stimulation‐dependent learning gains in each of the clusters reported in panel (a). LH, left hemisphere; RH, right hemisphere. Color bar represents the strength of the association between connectivity and learning gains (ANCOVA test). CO, central opercular cortex cortex; MTG, middle temporal gyrus; PreCG, precentral gyrus
Table 1.
Functional connectivity and its relation to learning gains
| Cluster X Y Z | Locations | Cluster size | Cluster q‐FDR | Peak p |
|---|---|---|---|---|
| −14 −36 +70 | PreCG (L,R), PostCG (L,R) | 2,552 | <.000001 | .000004 |
| 48 −30 14 | PO (R), IC (R) | 252 | .002548 | .000002 |
| −50 −16 +18 | CO (l), IC (L) | 214 | .004288 | .000002 |
| +64 –10 +34 | CO (R), PostCG (R) | 191 | .005785 | .000063 |
| +52 –62 +08 | iLOC (R), toMTG (R) | 180 | .006179 | .000033 |
Clusters where functional connectivity correlated significantly with stimulation‐dependent learning gains. MNI coordinates are shown for each cluster, along with its size, corrected FDR (q‐FDR) and peak voxel‐level p value. CO = central Opercular cortex; IC = insular cortex; iLOC = inferior lateral occipital cortex; PO = parietal operculum; PreCG = precentral gyrus; toMTG = temporooccipital middle temporal gyrus.
3.3. Association between functional connectivity and gains in rewarded and unrewarded trials
Our behavioral paradigm alternated between rewarded and unrewarded trials, in a within‐subject design. We thus next tested if stimulation‐dependent learning gains differed in rewarded and unrewarded trials and how these two learning gain metrics related to lPFC functional connectivity (Figure 4a). The difference between stimulation‐dependent gains in rewarded and unrewarded trials was not statistically significant (t 19 = −0.415, p = .683, d = −0.093 two‐tailed). The results however suggest a dissociation between these two learning metrics and lPFC functional connectivity. First, stimulation‐dependent gains in rewarded trials were associated across subjects with connectivity between the lPFC and sensorimotor cortex, postcentral and central opercular cortex and occipitotemporal cortex (Figure 4b). This pattern of connectivity was highly overlapping with that observed for stimulation‐dependent gains, irrespective of reinforcement (as reported in Figure 3). Conversely, stimulation‐dependent gains in unrewarded trials were associated with connectivity between lPFC and angular gyrus, extending to temporooccipital Middle Temporal Gyrus (Table 2), with little overlap with the network that correlated with stimulation‐dependent gains.
Figure 4.
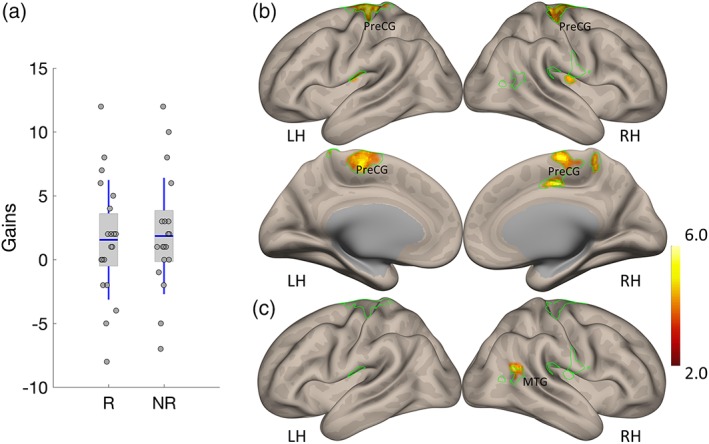
Stimulation‐dependent learning gains in rewarded and unrewarded trials, and their link to lPFC connectivity. (a) A comparison of stimulation‐dependent gains in rewarded and unrewarded trials. (b) Association between lPFC connectivity and stimulation‐dependent gains in rewarded trials. (c) Association between lPFC connectivity and stimulation‐dependent gains in unrewarded trials. Green contours depict the clusters of voxels where connectivity with the lPFC was significantly correlated with stimulation‐dependent learning gains, irrespective of reward. Color bar represents the strength of the association between connectivity and learning gains
Table 2.
Functional connectivity and its relation to learning gains in rewarded and unrewarded trials
| Cluster X Y Z | Locations | Cluster size | Cluster q‐FDR | Peak p |
|---|---|---|---|---|
| Rewarded trials | ||||
| −20 −22 +66 | PreCG (L,R), PostCG (L,R) | 2,102 | <.000001 | .000002 |
| −50 −18 +14 | CO (l), IC (L) | 221 | .005440 | .000004 |
| +44 –16 +18 | CO (R), IC (R) | 172 | .012830 | .000012 |
| +14 –14 +44 | SMA (R), ACC | 116 | .047865 | .000008 |
| Unrewarded trials | ||||
| +46 –58 +16 | AG (R), toMTG (R) | 185 | .019710 | .000008 |
The table displays the MNI, location, cluster size as well as voxel‐ and cluster‐wise statistics for each cluster where functional connectivity correlated significantly with stimulation‐dependent learning gains, calculated separately for rewarded and unrewarded trials. ACC = anterior cingulate cortex; AG = angular gyrus; SMA = supplementary motor cortex. All other abbreviations are as in Table 1.
3.4. Analysis of a control network
The results suggest a link between intrinsic lPFC functional connectivity and stimulation dependent learning gains. To test the specificity of these results, we examined the extent to which stimulation‐dependent learning gains correlated with intrinsic connectivity in networks that do not implicate lPFC. To that extent, we localized the sensorimotor network (Biswal, Zerrin Yetkin, Haughton, & Hyde, 1995) in each of our subjects, based on seed region in right precentral gyrus (Supporting Information Figure S2). Connectivity in the resulting network did not significantly correlate with stimulation‐related learning gains, suggesting that the results above were indeed specific to lPFC functional connectivity.
4. DISCUSSION
Experimental support for the role of PFC and its functional interactions in learning keep accumulating (e.g., Antzoulatos & Miller, 2014; Neubert, Mars, Sallet, & Rushworth, 2015; Qin et al., 2014). While earlier studies have examined the causal role of PFC in value‐based decision making (Smittenaar, FitzGerald, Romei, Wright, & Dolan, 2013) or in the context of implicit sequence learning (Galea, Albert, Ditye, & Miall, 2010), it remained incompletely understood how PFC and related networks are controlled in the human brain to support the acquisition of complex reinforced motor skills. Here, we designed a study to probe for the role of human PFC in successful reinforcement‐based skill formation and examine how its interactions with functional large‐scale networks contribute to skill acquisition. Our results show that a transient perturbation of lPFC significantly diminished learning gains, indicating a causal modulation of skill formation. Furthermore, we show that lPFC‐dependent learning gains related specifically to the strength of intrinsic interactions between lPFC and sensorimotor regions. We further report that connectivity between lPFC and sensorimotor regions was primarily related to learning gains in rewarded trials. Altogether, the results suggest that reinforced skill learning relies on lPFC and is related to the strength of functional interactions between lPFC and sensorimotor cortex.
Rather than solely through a local perturbation of lPFC activity, our results may suggest that lPFC stimulation affects reinforced skill learning through a distributed mechanism of operation. Distributed effects of rTMS have been extensively discussed in the literature (Chou et al., 2015; Dayan et al., 2013; Lee, Siebner, & Bestmann, 2006). Here, we show that pre‐task intrinsic connectivity, recorded before stimulation, correlated with the effects of stimulation. Similar relations between baseline connectivity and later effects of stimulation were reported before. For example, markers based on intrinsic connectivity can predict the responsiveness of patients with depression to rTMS therapy (Drysdale et al., 2016; Salomons et al., 2014). Our results join these previous studies in suggesting that intrinsic functional connectivity (van den Heuvel & Hulshoff Pol, 2010), can predict the effects of stimulation, plausibly as stimulation itself affects distributed rather localized neural systems. In this respect, the combination of rTMS with assessment of intrinsic brain connectivity relates to other recent developments in noninvasive brain stimulation approaches, particularly methods allowing for simultaneous assessment of oscillatory brain activity during frontal brain stimulation (Chander et al., 2016; Witkowski et al., 2016) or closed‐loop TMS paradigms (Zrenner, Belardinelli, Müller‐Dahlhaus, & Ziemann, 2016). Utilization of such methods, in particular simultaneous imaging and stimulation methods will allow to more directly examine the distributed mechanism of operation underlying the local perturbation of brain regions. Future studies should also consider anatomical and functional markers of lPFC and aim to co‐localize them with neuronavigation. This would encompass taking into account that F3/F4 stimulation based on the international 10–20 system may also engage frontal structures such as the frontal eye fields, implicated in processing spatial cues which may play a subsidiary role in sequence learning (Herwig et al., 2003).
Our results demonstrate that functional connections between the lPFC and sensorimotor regions were associated with stimulation‐dependent learning gains. Reciprocal connections between lPFC and ventromedial PFC (Saraiva & Marshall, 2015), and between the more dorsal parts of lPFC and the frontal pole to rostral orbitofrontal cortex (Haber & Behrens, 2014) underlie motivated value‐based processes. The contribution of the dorsal parts of lPFC to procedural learning (Dayan & Cohen, 2011) and executive motor behavior (Cieslik et al., 2012) has also been well‐established. Transient perturbation of dorsolateral PFC via rTMS disrupts performance in an implicit sequence learning task, performed with the contralateral hand (Pascual‐Leone et al., 1996). Moreover, it has been suggested that in the context of implicit sequence learning, dorsolateral PFC contributes specifically to the retention and manipulation of spatial information pertinent to the performance of the task (Robertson et al., 2001). Similarly, a meta‐analysis of finger‐tapping neuroimaging studies has found more pronounced involvement of lateral PFC in self‐paced designs, relative to cued designs (Witt et al., 2008). As self‐paced tapping tasks are likely more cognitively demanding, the involvement of lateral PFC in several sequence learning tasks may reflect heightened recruitment of cognitive control centers in the brain (Witt et al., 2008).
Interestingly, lPFC (specifically, the superior frontal gyrus) is among a set of hub regions which are not only more densely structurally connected to other regions in the brain but are also densely connected among themselves. This so called “rich club” organization, demonstrated in both human (Grayson et al., 2014; van den Heuvel & Sporns, 2011) and non‐human brains (Liang et al., 2017) is believed to allow information integration within large‐scale brain systems, and may allow memory and reward systems to interact during motor skill learning.
Several recent studies have established that procedural and motor learning are mediated by reinforcement, whether in the form of reward or punishment (Abe et al., 2011; Dayan, Averbeck, et al., 2014; Galea, Mallia, Rothwell, & Diedrichsen, 2015). While only a few studies have sought to identify the neural substrates underlying reward based motor and procedural learning, our results fit in well with previous data, suggesting that the lPFC mediates the efficacy of reward during skill learning (Dayan, Hamann, et al., 2014). More generally, through its connections with orbital and medial PFC, the lPFC plays a pivotal role in value‐based learning (Dixon & Christoff, 2014) and has been linked primarily with anticipation of reward (Bjork & Hommer, 2007) and the representation of motivationally relevant outcomes (Dixon & Christoff, 2014; Liu, Hairston, Schrier, & Fan, 2011). While our results suggest that connectivity between lPFC and sensorimotor regions was primarily related to learning gains in rewarded trials, they cannot be taken as reflecting a strict difference between rewarded and unrewarded trials. As in our paradigm learning alternated stochastically between rewarded and unrewarded trials (Dayan, Averbeck, et al., 2014; Dayan, Hamann, et al., 2014), it is plausible to assume that subjects acted under incentive motivation also in unrewarded trials. Paradigms that separate rewarded and unrewarded trials between subjects are needed to further explore the association between reward‐based learning and intrinsic interactions in large‐scale brain networks.
It has been suggested that motor skill learning likely relies on both striatal‐procedural and hippocampal‐declarative systems (Stanley & Krakauer, 2013). While the current results did not implicate fronto‐hippocampal connectivity in the effects of lPFC stimulation on learning gains, it would be of interest to test the role of declarative knowledge formation in future studies, using suitable tasks. Future work could test for memory and reward system interaction using declarative tasks which have a weaker motor component, or alternatively using interference paradigms that assess interactions among the two systems (Brown & Robertson, 2007a, 2007b).
In sum, we report here that perturbation of lPFC significantly reduced subjects’ reinforced learning gains, and further show that these effects were related to the strength of task‐free functional connectivity in intrinsic connections between lPFC and sensorimotor cortex. Our results thus suggest that lPFC may contribute to incentive‐based acquisition of procedural skills through its interactions with the sensorimotor system. As such, our results support a systems‐level characterization of this form of learning.
CONFLICT OF INTEREST
The authors have no Conflicts of Interest to disclose.
Supporting information
Supplementary Figure S1. Functional connectivity with right lateral prefrontal cortex. The functional network was defined, based on seed‐based connectivity analysis with the right lateral PFC as the seed region (as individually defined for each subject based on their location of rTMS stimulation). LH, left hemisphere; RH, right hemisphere. Color bar represents connectivity strength.
Supplementary Figure S2. The sensorimotor functional network. The functional network was defined, based on seed‐based connectivity analysis with the right precentral gyrus as the seed region. Connectivity in this network did not significantly correlate with stimulation‐dependent gains. LH, left hemisphere; RH, right hemisphere. Color bar represents connectivity strength.
ACKNOWLEDGMENTS
We thank Yehuda Zur for programming and technical support, and Nadav Stoppelman and Talma Hendler from Tel Aviv Sourasky Medical Center. The study was supported by the by the I‐CORE program of the Planning and Budgeting Committee and The ISF (grant 51/11).
Dayan E, Herszage J, Laor‐Maayany R, Sharon H, Censor N. Neuromodulation of reinforced skill learning reveals the causal function of prefrontal cortex. Hum Brain Mapp. 2018;39:4724–4732. 10.1002/hbm.24317
Eran Dayan and Jasmine Herszage contributed equally to this work.
Funding information Israel Science Foundation , Grant/Award Number: grant 51/11; Planning and Budgeting Committee
REFERENCES
- Abe, M. , Schambra, H. , Wassermann, E. M. , Luckenbaugh, D. , Schweighofer, N. , & Cohen, L. G. (2011). Reward improves long‐term retention of a motor memory through induction of offline memory gains. Current Biology, 21, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzoulatos, E. G. , & Miller, E. K. (2014). Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron, 83, 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Zerrin Yetkin, F. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Bjork, J. M. , & Hommer, D. W. (2007). Anticipating instrumentally obtained and passively‐received rewards: A factorial fMRI investigation. Behavioural Brain Research, 177, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R. M. , & Robertson, E. M. (2007a). Inducing motor skill improvements with a declarative task. Nature Neuroscience, 10, 148–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R. M. , & Robertson, E. M. (2007b). Off‐line processing: Reciprocal interactions between declarative and procedural memories. The Journal of Neuroscience, 27, 10468–10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor, N. , Dayan, E. , & Cohen, L. G. (2014). Cortico‐subcortical neuronal circuitry associated with reconsolidation of human procedural memories. Cortex, 58, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor, N. , Dimyan, M. A. , & Cohen, L. G. (2010). Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Current Biology, 20, 1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor, N. , Horovitz, S. G. , & Cohen, L. G. (2014). Interference with existing memories alters offline intrinsic functional brain connectivity. Neuron, 81, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander, B. S. , Witkowski, M. , Braun, C. , Robinson, S. E. , Born, J. , Cohen, L. G. , … Soekadar, S. R. (2016). tACS phase locking of frontal midline theta oscillations disrupts working memory performance. Frontiers in Cellular Neuroscience, 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Classen, J. , Gerloff, C. , Celnik, P. , Wassermann, E. M. , Hallett, M. , & Cohen, L. G. (1997). Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology, 48, 1398–1403. [DOI] [PubMed] [Google Scholar]
- Chou, Y. , You, H. , Wang, H. , Zhao, Y.‐P. , Hou, B. , Chen, N. , & Feng, F. (2015). Effect of repetitive transcranial magnetic stimulation on fMRI resting‐state connectivity in multiple system atrophy. Brain Connectivity, 5, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik, E. C. , Zilles, K. , Caspers, S. , Roski, C. , Kellermann, T. S. , Jakobs, O. , … Eickhoff, S. B. (2012). Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cerebral Cortex, 23, 2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafotakis, M. , Grefkes, C. , Eickhoff, S. B. , Karbe, H. , Fink, G. R. , & Nowak, D. A. (2008). Effects of rTMS on grip force control following subcortical stroke. Experimental Neurology, 211, 407–412. [DOI] [PubMed] [Google Scholar]
- Dayan, E. , Averbeck, B. B. , Richmond, B. J. , & Cohen, L. G. (2014). Stochastic reinforcement benefits skill acquisition. Learning & Memory, 21, 140–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan, E. , Censor, N. , Buch, E. R. , Sandrini, M. , & Cohen, L. G. (2013). Noninvasive brain stimulation: From physiology to network dynamics and back. Nature Neuroscience, 16, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan, E. , & Cohen, L. G. (2011). Neuroplasticity subserving motor skill learning. Neuron, 72, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan, E. , Hamann, J. M. , Averbeck, B. B. , & Cohen, L. G. (2014). Brain structural substrates of reward dependence during behavioral performance. The Journal of Neuroscience, 34, 16433–16441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beukelaar, T. T. , Woolley, D. G. , & Wenderoth, N. (2014). Gone for 60 seconds: Reactivation length determines motor memory degradation during reconsolidation. Cortex, 59, 138–145. [DOI] [PubMed] [Google Scholar]
- Dixon, M. L. , & Christoff, K. (2014). The lateral prefrontal cortex and complex value‐based learning and decision making. Neuroscience and Biobehavioral Reviews, 45, 9–18. [DOI] [PubMed] [Google Scholar]
- Doyon, J. , & Benali, H. (2005). Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology, 15, 161–167. [DOI] [PubMed] [Google Scholar]
- Drysdale, A. T. , Grosenick, L. , Downar, J. , Dunlop, K. , Mansouri, F. , Meng, Y. , … Liston, C. (2016). Resting‐state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea, J. M. , Albert, N. B. , Ditye, T. , & Miall, R. C. (2010). Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. Journal of Cognitive Neuroscience, 22, 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea, J. M. , Mallia, E. , Rothwell, J. , & Diedrichsen, J. (2015). The dissociable effects of punishment and reward on motor learning. Nature Neuroscience, 18, 597–602. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic, P. S. , Selemon, L. D. , & Schwartz, M. L. (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience, 12, 719–743. [DOI] [PubMed] [Google Scholar]
- Gomez Beldarrain, M. , Gafman, J. , Ruiz de Velasco, I. , Pascual‐Leone, A. , & Garcia‐Monco, J. (2002). Prefrontal lesions impair the implicit and explicit learning of sequences on visuomotor tasks. Experimental Brain Research, 142, 529–538. [DOI] [PubMed] [Google Scholar]
- Grayson, D. S. , Ray, S. , Carpenter, S. , Iyer, S. , Dias, T. G. C. , Stevens, C. , … Fair, D. A. (2014). Structural and functional Rich Club Organization of the Brain in children and adults. PLoS One, 9, e88297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griškova, I. , Rukšėnas, O. , Dapšys, K. , Herpertz, S. , & Höppner, J. (2007). The effects of 10Hz repetitive transcranial magnetic stimulation on resting EEG power spectrum in healthy subjects. Neuroscience Letters, 419, 162–167. [DOI] [PubMed] [Google Scholar]
- Haber, S. N. (2011). Neuroanatomy of reward: A view from the ventral striatum In Gottfried J. A. (Ed.), Neurobiology of sensation and reward. Boca Raton, FL: CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Haber, S. N. , & Behrens, T. E. J. (2014). The neural network underlying incentive‐based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron, 83, 1019–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann, J. M. , Dayan, E. , Hummel, F. C. , & Cohen, L. G. (2014). Baseline frontostriatal‐limbic connectivity predicts reward‐based memory formation. Human Brain Mapping, 35, 5921–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, A. , Galea, J. M. , Casula, E. P. , Falkai, P. , Bestmann, S. , & Rothwell, J. C. (2013). Muscle and timing‐specific functional connectivity between the dorsolateral prefrontal cortex and the primary motor cortex. Journal of Cognitive Neuroscience, 25, 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herszage, J. , & Censor, N. (2017). Memory Reactivation Enables Long‐Term Prevention of Interference. Current Biology, 27, 1529–1534.e2. [DOI] [PubMed] [Google Scholar]
- Herwig, U. , Satrapi, P. , & Schönfeldt‐Lecuona, C. (2003). Using the international 10‐20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography, 16, 95–99. [DOI] [PubMed] [Google Scholar]
- Jarbo, K. , & Verstynen, T. D. (2015). Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. The Journal of Neuroscience, 35, 3865–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski, E. , Hoff, M. , Sehm, B. , Taubert, M. , Conde, V. , Steele, C. J. , … Ragert, P. (2013). Effect of transcranial direct current stimulation (tDCS) during complex whole body motor skill learning. Neuroscience Letters, 552, 76–80. [DOI] [PubMed] [Google Scholar]
- Karni, A. , Meyer, G. , Jezzard, P. , Adams, M. M. , Turner, R. , & Ungerleider, L. G. (1995). Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature, 377, 155–158. [DOI] [PubMed] [Google Scholar]
- Karni, A. , Meyer, G. , Rey‐Hipolito, C. , Jezzard, P. , Adams, M. M. , Turner, R. , & Ungerleider, L. G. (1998). The acquisition of skilled motor performance: Fast and slow experience‐driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America, 95, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, J. H. , Monchi, O. , Ptito, A. , Petrides, M. , & Strafella, A. P. (2008). Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex affects performance of the Wisconsin card sorting task during provision of feedback. International Journal of Biomedical Imaging, 2008, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman, M. , Doyon, J. , Doljansky, J. , Carrier, J. , Dagan, Y. , & Karni, A. (2007). Daytime sleep condenses the time course of motor memory consolidation. Nature Neuroscience, 10, 1206–1213. [DOI] [PubMed] [Google Scholar]
- Lee, L. , Siebner, H. , & Bestmann, S. (2006). Rapid modulation of distributed brain activity by transcranial magnetic stimulation of human motor cortex. Behavioural Neurology, 17, 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X. , Hsu, L.‐M. , Lu, H. , Sumiyoshi, A. , He, Y. , & Yang, Y. (2017). The Rich‐Club organization in rat functional brain network to balance between communication cost and efficiency. Cerebral Cortex, 28, 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Hairston, J. , Schrier, M. , & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35, 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miendlarzewska, E. A. , Bavelier, D. , & Schwartz, S. (2016). Influence of reward motivation on human declarative memory. Neuroscience and Biobehavioral Reviews, 61, 156–176. [DOI] [PubMed] [Google Scholar]
- Neubert, F.‐X. , Mars, R. B. , Sallet, J. , & Rushworth, M. F. S. (2015). Connectivity reveals relationship of brain areas for reward‐guided learning and decision making in human and monkey frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 112, E2695–E2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak, D. A. , Grefkes, C. , Dafotakis, M. , Eickhoff, S. , Küst, J. , Karbe, H. , & Fink, G. R. (2008). Effects of low‐frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Archives of Neurology, 65, 741–747. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Wassermann, E. M. , Grafman, J. , & Hallett, M. (1996). The role of the dorsolateral prefrontal cortex in implicit procedural learning. Experimental Brain Research, 107, 479–485. [DOI] [PubMed] [Google Scholar]
- Preston, A. R. , & Eichenbaum, H. (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23, R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, S. , Cho, S. , Chen, T. , Rosenberg‐Lee, M. , Geary, D. C. , & Menon, V. (2014). Hippocampal‐neocortical functional reorganization underlies children's cognitive development. Nature Neuroscience, 17, 1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, E. M. , Tormos, J. M. , Maeda, F. , & Pascual‐Leone, A. (2001). The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cerebral Cortex, 11, 628–635. [DOI] [PubMed] [Google Scholar]
- Rossi, S. , Cappa, S. F. , Babiloni, C. , Pasqualetti, P. , Miniussi, C. , Carducci, F. , … Rossini, P. M. (2001). Prefontal cortex in long‐term memory: An “interference” approach using magnetic stimulation. Nature Neuroscience, 4, 948–952. [DOI] [PubMed] [Google Scholar]
- Rossini, P. M. , Barker, A. T. , Berardelli, A. , Caramia, M. D. , Caruso, G. , Cracco, R. Q. , … Lücking, C. H. (1994). Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91, 79–92. [DOI] [PubMed] [Google Scholar]
- Salomons, T. V. , Dunlop, K. , Kennedy, S. H. , Flint, A. , Geraci, J. , Giacobbe, P. , & Downar, J. (2014). Resting‐state Cortico‐thalamic‐striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology, 39, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini, M. , Cappa, S. F. , Rossi, S. , Rossini, P. M. , & Miniussi, C. (2003). The role of prefrontal cortex in verbal episodic memory: rTMS evidence. Journal of Cognitive Neuroscience, 15, 855–861. [DOI] [PubMed] [Google Scholar]
- Sandrini, M. , Censor, N. , Mishoe, J. , & Cohen, L. G. (2013). Causal role of prefrontal cortex in strengthening of episodic memories through reconsolidation. Current Biology, 23, 2181–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini, M. , Umiltà, C. , & Rusconi, E. (2011). The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neuroscience and Biobehavioral Reviews, 35, 516–536. [DOI] [PubMed] [Google Scholar]
- Saraiva, A. C. , & Marshall, L. (2015). Dorsolateral‐ventromedial prefrontal cortex interactions during value‐guided choice: A function of context or difficulty? The Journal of Neuroscience, 35, 5087–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smittenaar, P. , FitzGerald, T. H. B. , Romei, V. , Wright, N. D. , & Dolan, R. J. (2013). Disruption of dorsolateral prefrontal cortex decreases model‐based in favor of model‐free control in humans. Neuron, 80, 914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, J. , & Krakauer, J. W. (2013). Motor skill depends on knowledge of facts. Frontiers in Human Neuroscience, 7, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting‐state fMRI functional connectivity. European Neuropsychopharmacology, 20, 519–534. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , & Sporns, O. (2011). Rich‐club organization of the human connectome. The Journal of Neuroscience, 31, 15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, J. L. , Kahn, I. , Snyder, A. Z. , Raichle, M. E. , & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100, 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, M. P. , Brakefield, T. , Allan Hobson, J. , & Stickgold, R. (2003). Dissociable stages of human memory consolidation and reconsolidation. Nature, 425, 616–620. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Witt, S. T. , Meyerand, M. E. , & Laird, A. R. (2008). Functional neuroimaging correlates of finger tapping task variations: An ALE meta‐analysis. NeuroImage 42, 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski, M. , Garcia‐Cossio, E. , Chander, B. S. , Braun, C. , Birbaumer, N. , Robinson, S. E. , & Soekadar, S. R. (2016). Mapping entrained brain oscillations during transcranial alternating current stimulation (tACS). NeuroImage, 140, 89–98. [DOI] [PubMed] [Google Scholar]
- Zrenner, C. , Belardinelli, P. , Müller‐Dahlhaus, F. , & Ziemann, U. (2016). Closed‐loop neuroscience and non‐invasive brain stimulation: A tale of two loops. Frontiers in Cellular Neuroscience, 10, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Functional connectivity with right lateral prefrontal cortex. The functional network was defined, based on seed‐based connectivity analysis with the right lateral PFC as the seed region (as individually defined for each subject based on their location of rTMS stimulation). LH, left hemisphere; RH, right hemisphere. Color bar represents connectivity strength.
Supplementary Figure S2. The sensorimotor functional network. The functional network was defined, based on seed‐based connectivity analysis with the right precentral gyrus as the seed region. Connectivity in this network did not significantly correlate with stimulation‐dependent gains. LH, left hemisphere; RH, right hemisphere. Color bar represents connectivity strength.


