Abstract
Obese individuals exhibit brain alterations of resting‐state functional connectivity (RSFC) integrity of resting‐state networks (RSNs) related to food intake. Bariatric surgery is currently the most effective treatment for combating morbid obesity. How bariatric surgery influences neurocircuitry is mostly unknown. Functional connectivity density (FCD) mapping was employed to calculate local (lFCD)/global (gFCD) voxelwise connectivity metrics in 22 obese participants who underwent functional magnetic resonance imaging before and 1 month after sleeve gastrectomy (SG), and in 19 obese controls (Ctr) without surgery but tested twice (baseline and 1‐month later). Two factor (group, time) repeated measures ANOVA was used to assess main and interaction effects in lFCD/gFCD; regions of interest were identified for subsequent seed to voxel connectivity analyses to assess resting‐state functional connectivity and to examine association with weight loss. Bariatric surgery significantly decreased lFCD in VMPFC, posterior cingulate cortex (PCC)/precuneus, and dorsal anterior cingulate cortex (dACC)/dorsomedial prefrontal cortex (DMPFC) and decreased gFCD in VMPFC, right dorsolateral prefrontal cortex (DLPFC) and right insula (p FWE < .05). lFCD decreased in VMPFC and PCC/precuneus correlated with reduction in BMI after surgery. Seed to voxel connectivity analyses showed the VMPFC had stronger connectivity with left DLPFC and weaker connectivity with hippocampus/parahippocampus, and PCC/precuneus had stronger connectivity with right caudate and left DLPFC after surgery. Bariatric surgery significantly decreased FCD in regions involved in self‐referential processing (VMPFC, DMPFC, dACC, and precuneus), and interoception (insula), and changes in VMPFC/precuneus were associated with reduction in BMI suggesting a role in improving control of eating behaviors following surgery.
Keywords: bariatric surgery, executive control, fMRI, obesity, self‐referential, sensory‐emotional‐memory
1. INTRODUCTION
Overeating is a major contributor to obesity. Growing evidence indicates impaired homeostatic regulation of food intake and altered food reward‐related processes in obese subjects (Berthoud & Morrison, 2008; Zhang et al., 2014). Bariatric surgery is an effective intervention that exerts immediate effects on homeostatic regulation (Diamantis et al., 2014; Gloy et al., 2013; Sjostrom et al., 2007), but its contribution to the rewiring of obesity‐related neural circuitry remains unknown.
Resting‐state fMRI (RS‐fMRI) has been employed to investigate alterations of resting‐state functional connectivity (RSFC) integrity of resting‐state networks (RSNs) related to food intake in obese subjects (Coveleskie et al., 2015; Garcia‐Garcia et al., 2013; Kullmann et al., 2012; Kullmann et al., 2014; Moreno‐Lopez, Contreras‐Rodriguez, Soriano‐Mas, Stamatakis, & Verdejo‐Garcia, 2016; Wijngaarden et al., 2015). Obese individuals showed increased RSFC between regions involved in metabolic sensing/interoception (hypothalamus, insula) and regions involved in reward processing (striatum, orbitofrontal cortex) (Coveleskie et al., 2015; Garcia‐Garcia et al., 2013; Kullmann et al., 2014; Wijngaarden et al., 2015) and decreased RSFC in regions involved in interoceptive processing/cognitive control (Kullmann et al., 2012; Moreno‐Lopez et al., 2016). Those studies indicated abnormal communication between multiple brain regions/circuitry in obese patients during resting‐state.
Bariatric surgery, such as laparoscopic sleeve gastrectomy (LSG) (Diamantis et al., 2014) can cause profound changes in gastrointestinal microbiota, appetite‐regulating peptides, and neuroendocrine function (Diamantis et al., 2014; Sjostrom et al., 2007). Molecular/functional brain imaging revealed changes in obese patients post‐surgery including increased dopamine D2 receptor (Steele et al., 2010) and attenuated brain response to food cues in mesolimbic/mesostriatal reward circuits (Ochner et al., 2011). An association between lessened postoperative craving for high‐caloric food and diminished activity within prefrontal region (dorsolateral prefrontal cortex‐DLPFC) has also been observed 1 month post‐surgery (Bruce et al., 2012; Ochner et al., 2012). Particularly, RS‐fMRI studies have also revealed a partial reversal of hypothalamic dysfunction and altered brain activity (insula) following body mass index (BMI) reduction (Van de Sande‐Lee et al., 2011; Wiemerslage et al., 2017). Bariatric surgery also decreased RSFC within the DMN comprising the anterior cingulate cortex (ACC), frontal superior gyrus, and orbitofrontal cortex (Frank et al., 2014) and RSFC between insula/left precuneus (Lepping et al., 2015).
In general, obese individuals exhibited brain functional abnormalities implicated in reward/motivation, emotion/memory, homeostatic regulation of food intake, and executive function including inhibitory control of feeding behavior. However, it remains unclear how resting‐state brain activity and RSFC change following LSG surgery. We assessed local/global functional connectivity tissue properties from RS‐fMRI datasets using functional connectivity density (FCD) mapping (Tomasi & Volkow, 2010, 2011a). FCD mapping is a powerful graph theory tool for exploring the topology of human brain function using magnetic resonance imaging (MRI) datasets collected at rest and also during task performance (Tomasi & Volkow, 2010). Whereas local FCD (lFCD) quantifies the number of voxels in the local functional connectivity cluster (local degree), and global FCD (gFCD) quantifies the number of whole‐brain connections to each voxel (Tomasi, Shokri‐Kojori, & Volkow, 2016a). Data‐driven FCD reflects the amplitude of the BOLD signal fluctuations (Tomasi & Volkow, 2018) and quantifies hubness and energy demand of brain tissue (Tomasi & Volkow, 2011b; Tomasi, Wang, & Volkow, 2013). FCD has high gray matter sensitivity/specificity and is the most resilient RSFC metrics (Tomasi et al., 2016b). Unlike the popular hypothesis‐driven seed‐voxel correlation approach, FCD does not require regional hypotheses on seed locations and is ideal for exploratory studies. Prior studied have documented FCD disruption in autism (Tomasi & Volkow, 2017) attention deficit hyperactivity disorder (Tomasi & Volkow, 2012), schizophrenia (Liu et al., 2015; Tomasi & Volkow, 2014; Zhuo et al., 2014), cocaine addiction (Konova, Moeller, Tomasi, & Goldstein, 2015), nonepileptic seizures (Ding et al., 2014), and other brain disorders and assessing efficacy of drug treatment in obesity (Wang et al., 2018).
Here, we studied FCD in 22 obese patients prior to and at 1 month after surgery, and in 19 obese control individuals. We predicted that LSG surgery would result in decreased FCD in regions, implicated in impaired self‐control and self‐referential processing (Northoff et al., 2006; Striepens et al., 2016) (VMPFC, DMPFC, precuneus), memory (HIPP), and interoception (insula), increasing connectivity of self‐referential processing regions with regions involved with executive function/self‐regulation (DLPFC, caudate).
2. MATERIALS AND METHODS
2.1. Subjects
Thirty‐five morbidly obese patients were recruited for laparoscopic sleeve gastrectomy at Xijing Gastrointestinal Hospital affiliated to the Fourth Military Medical University in Xi'an, China. Patients with psychiatric/neurological diseases, previous intestinal surgery, inflammatory intestinal disease, organ dysfunction or taking any current medication that could affect the central nervous system were excluded. Individuals who had a waist circumference (WC) > interior diameter of the scanner were excluded (Zhang et al., 2016, 2018). Given these criteria, six candidates were disqualified. Twenty‐nine remaining obese candidates (SG) completed the pre‐sleeve gastrectomy MRI scan (Baseline, PreSG) and underwent surgery. The same MRI scans were performed 1 month post‐sleeve gastrectomy (1 month later, PostSG). Seven obese subjects reported having significant weight loss after surgery via their local clinics. However, these subjects could not return for follow‐up MRI assessment due to long distance travel. As a result, 22 patients (age range 18–42 years) remained in the SG group. Nineteen morbidly obese patients who did not receive LSG surgery were recruited as the control group (Ctr); they were age‐, gender‐, and education‐matched with SG (p > .05, Table 1). The Ctr completed two identical MRI scans mirroring the SG, one (CtrT) at baseline, and retest (CtrRT) 1 month later (1 month later). The experimental protocol was approved by the Institutional Review Board of Xijing Hospital and registered in the Chinese Clinical Trial Registry Center as: ChiCTR‐OOB‐15006346 (http://www.chictr.org.cn). The experiments were conducted in accordance with the Declaration of Helsinki. All participants were informed of the nature of the research and provided written informed consent.
Table 1.
Demographic and clinical information of obese subjects in both SG and Ctr groups
| PreSG (22) (mean ± SE) | PostSG (22) (mean ± SE) | CtrT (19) (mean ± SE) | CtrRT (19) (mean ± SE) | ANOVA | Post‐hoc tests | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interaction effect | PreSG vs. CtrT | PostSG vs. PreSG | CtrRT vs. CtrT | |||||||||
| F | p | T | p | T | p | T | p | |||||
| Age (years) | 26.64 ± 1.83 | 26.64 ±1.83 | 28.63 ±2.06 | 28.63 ±2.06 | 0.940 | .334 | ||||||
| Gender | 10 M/12F | 10 M/12F | 11 M/8F | 11 M/8F | 0.631 | .427 | ||||||
| Duration of obesity (years) | 12.05 ± 2.19 | 12.05 ±2.19 | 12.05 ± 1.16 | 12.05 ± 1.16 | 0.004 | .997 | ||||||
| Weight (kg) | 109.92 ± 3.77 | 98.43 ±3.71 | 104.71 ± 4.02 | 103.68 ±3.91 | 43.216 | <.001 | 0.932 | .357 | −7.283 | <.001 | −1.637 | .119 |
| BMI (kg/m2) | 38.11 ± 1.32 | 34.03 ±1.31 | 35.27 ±1.01 | 35.14 ±1.04 | 43.101 | <.001 | 1.646 | .108 | −8.751 | <.001 | −1.323 | .202 |
| WC (cm) | 117.09 ± 3.08 | 108.59 ± 2.93 | 114.81 ±2.57 | 112.89 ±2.23 | 14.707 | <.001 | 0.558 | .580 | −6.603 | <.001 | −1.323 | .202 |
| Food intake (kg/meal) | 0.74 ± 0.10 | 0.16 ± 0.01 | 0.53 ±0.04 | 0.49 ±0.04 | 20.998 | <.001 | 1.742 | .089 | −6.603 | <.001 | −1.201 | .245 |
| YFAS | 4.95 ± 0.59 | 2.81 ±0.41 | 3.37 ±0.56 | 3.42 ±0.53 | 9.973 | .003 | 1.906 | .064 | −3.423 | .003 | 0.160 | .875 |
| HAMD | 12.50 ± 2.35 | 10.45 ± 1.65 | 8.05 ±1.84 | 8.16 ±2.02 | 0.202 | .650 | 1.213 | .232 | −1.039 | .310 | 0.787 | .442 |
| HAMA | 10.59 ± 1.77 | 7.73 ±1.36 | 7.74 ±1.76 | 6.21 ±1.38 | 0.570 | .454 | 1.133 | .264 | −2.322 | .030 | −1.591 | .129 |
Abbreviation: BMI, body mass index; WC, waist circumference; YFAS, Yale Food Addiction Scale; HAMD, Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Rating Scale; PreSG, obese patients who received MRI scan before surgery; PostSG, obese patients who received MRI scan at 1 month after surgery; CtrT, control subjects who received MRI scan at baseline; CtrRT, control subjects who received MRI scan 1 month after the first scan; SE, standard error.
2.2. Experimental design
All participants underwent 12‐hr overnight fasting, and fasting blood samples were taken and MRI scans were performed between 9 and 10 am. A designated clinician (HW) rated severity of subjects’ anxiety using Hamilton‐Anxiety‐Rating‐Scale (Hamilton, 1959) and depression using Hamilton‐Depression‐Rating‐Scale (Hamilton, 1960). Subjects were required to complete the Yale‐Food‐Addiction‐Scale (YFAS) evaluation (Gearhardt, Corbin, & Brownell, 2009) (Table 1), which has been validated in a bariatric surgery population (Clark & Saules, 2013). All clinical measurements were identically conducted before (baseline) and 1 month after surgery/baseline, and the same surgeon (GJ) performed all surgical procedures. Two sample t‐tests were used to examine the difference between SG and Ctr groups at baseline. A two‐way ANOVA was implemented in SPSS 22 to model the effects of group (SG, Ctr) and time (Baseline, 1 month later) on behavioral/clinical data. Paired t‐tests were utilized as post‐hoc tests where ANOVA indicated a significant main/interaction effects.
2.3. Peripheral hormone measurements
Blood samples were taken before and 1 month after surgery for SG group and stored at −80°C until assayed. Serum concentrations of ghrelin, leptin, insulin, C‐peptide, GIP (glucose‐dependent insulinotropic polypeptide), GLP‐1 (glucagon‐like peptide‐1), and glucagon were measured using a Bio‐Plex 200™ suspension array system (BIO‐RAD, Inc, Hercules, California).
2.4. MRI acquisition
The experiment was carried out using a 3.0 T GE (Signa Excite HD, Milwaukee, WI) scanner. First, a high‐resolution structural image for each subject was acquired using three‐dimensional magnetization‐prepared rapid acquisition gradient‐echo sequences with a voxel size of 1 mm3 and with an axial fast spoiled gradient‐echo sequence (TR = 7.8 ms, TE = 3.0 ms, matrix size = 256 × 256, field of view = 256 × 256 mm2, slice thickness = 1 mm and 166 slices). Then, a gradient‐echo T2*‐weighted echo planar imaging sequence was used for acquiring resting‐state functional images with the following parameters: TR = 2,000 ms, TE = 30 ms, matrix size = 64 × 64, FOV = 256 × 256 mm2, flip angle = 90°, in‐plane resolution of 4 mm2, slice thickness = 4 mm and 32 axial slices. The scan for RS‐fMRI lasted 360 s. Subjects were instructed to close their eyes but remain awake during the entire scanning procedure. A radiologist (GC) examined the imaging data to rule out abnormalities in brain structure.
2.5. Image processing
Imaging data were preprocessed using Statistical Parametric Mapping 12 (SPM12, http://www.fil.ion.uclac.uk/spm). Specifically, the first 10 time points were removed to minimize nonequilibrium effects in fMRI signal, and then slice‐timing, head movement correction, and spatial normalization (voxel size of 3 × 3 × 3 mm3) were performed (Zhang et al., 2015). There was no significant interaction/main effect of Group/Time on estimates of the subjects' motion (p > .05) for mean/maximum frame‐wise displacement calculated from six translation/rotation parameters obtained from the realignment process. Demeaning/detrending were performed and head‐motion parameters, white‐matter signals, cerebrospinal‐fluid signals, and global signals were regressed out as nuisance covariates (Power et al., 2014). fMRI time points that were severely affected by motion were removed using a “scrubbing method” (FD value >0.5 mm, and ΔBOLD of DVARS >0.5%) (Power et al., 2014) (Supporting Information, SI), and <5% of time points were scrubbed per subject. Finally, band‐pass temporal filtering (0.01–0.08 Hz) was used to remove effects of very low‐frequency drift/high‐frequency noise using REST toolkit (http://resting-fmri.sourceforge.net).
2.6. FCD mapping
FCD mapping was used to compute the strength of local (lFCD) and global FCD (gFCD) based on the growing algorithm as implemented in IDL (Harris Geospatial, Broomfield, CO) (Shokri‐Kojori, Tomasi, Wiers, Wang, & Volkow, 2016; Tomasi & Volkow, 2010, 2011a; 2012). The growing algorithm for lFCD includes calculating Pearson correlation between voxel (i) and its immediate neighbors (with a surface connectivity criterion). For an edge to be considered significant, a correlation threshold of (r = .6) was selected (Tomasi & Volkow, 2010, 2011a) was applied to calculate binary connectivity coefficients, a ij = 1 (r > .6)/a ij = 0 (r < .6). For voxel, the lFCD at voxel (i) was calculated as the size of the local cluster, that is, that include voxel (i), which were functionally connected (voxels with a ij = 1) by surface. In the next step, voxels connected (with a surface connectivity criterion and r = .6) to voxels identified in the last step were calculated. This step was repeated until no new voxels were found. The total number of voxels identified in this process is lFCD. gFCD was calculated as the total number of edges for voxel (i) across the whole brain (Tomasi & Volkow, 2011a) see http://fcp-indi.github.io/docs/user/centrality.html for FCD implementation). lFCD/gFCD maps were smoothed with a Gaussian kernel of 6‐mm full‐width at half‐maximum.
2.7. Regions of interest (ROIs) identification
SPM 12 was employed to perform the voxel‐wise analysis on FCD indices, as age/gender were entered as covariates to control for differences between groups in these variables. A two‐way ANOVA was implemented to model the effects of group (SG, Ctr) and time (Baseline, 1 month later) on lFCD/gFCD, respectively. ROIs were identified after family‐wise error (FWE) correction for multiple comparisons at cluster‐level correction approach (p FWE < .05) with a minimum cluster size of 100 and a cluster‐forming threshold of p < .001 (Eklund, Nichols, & Knutsson, 2016).
2.8. RSFC analysis
After obtaining FCD ROIs with significant interaction effects, a seed‐region‐based (centered at the coordinates of peak value with a 6 mm radius) RSFC analysis was carried out. Mean time series of each ROI from the resting‐state scan was extracted, and then strength of RSFC for each voxel was estimated using Pearson correlation coefficient between average time‐varying signal in the seed and voxel in the brain. Fisher transform was used to convert correlation maps into normally distributed coefficient maps. A two‐way ANOVA was implemented in SPM 12 to model effects of group (SG, Ctr) × time (Baseline, 1 month later) seed‐based RSFC with an identical threshold as for the ROIs identification (p FWE < .05, cluster size of 100, cluster‐forming threshold of p < .001).
2.9. Association between behaviors and FCD/RSFC
A partial correlation analysis with age and gender as covariates was performed to assess the association between FCD/RSFC ROIs with significant group × interaction effects and clinical measurements with significant interaction effects (BMI, YFAS). We also performed correlation analysis between changes in FCD in 6 ROIs with significant effects and changes in clinical measurements (BMI, YFAS). Bonferroni‐correction was applied for multiple‐comparisons, all tests were 2‐tailed and level of significance was p < .004 (0.05/12).
3. RESULTS
3.1. Demographic characteristics
At baseline, there were no significant differences in age, gender, duration of obesity, weight, BMI, WC, food intake, YFAS, HAMD, and HAMA between SG and Ctr groups (p > .05, Table 1). There were significant group × time interaction effects for weight (F[1, 39] = 43.216, p < .001), BMI (F[1, 39] = 43.101, p < .001), and WC (F[1, 39] = 14.707, p < .001) due to significant weight‐loss (t = −7.283, p < .001), reduction of BMI (t = −8.751, p < .001) and WC (t = −6.603, p < .001) in SG group but not in Ctr group. There were significant interaction effects in food intake (F[1, 39] = 20.998, p < .001) and YFAS (F[1, 39] = 9.973, p = .003) due to significantly decreased food intake (t = −6.603, p < .001) and less compulsive food intake (t = −3.423, p = .003) in SG group post‐surgery, but not in Ctr group (Table 1).
3.2. Peripheral hormone measurements
Insulin, leptin, and ghrelin levels were lower post‐surgery than pre‐surgery (p < .001, Supporting Information Figure S1). There were no significant changes in C‐peptide, GIP, GLP‐1, and glucagon.
3.3. FCD
There were no differences at baseline in FCD between SG and Ctr groups. There were significant interaction effects (Group × Time) on lFCD in ventromedial prefrontal cortex (VMPFC), posterior cingulate cortex (PCC)/precuneus, dorsal anterior cingulate cortex (dACC), and dorsomedial prefrontal cortex (DMPFC) (p FWE < .05, Figure 1a, Table 2). Specifically, surgery decreased lFCD in VMPFC, PCC/precuneus, and dACC/DMPFC in PostSG. Conversely, Ctr group did not show significant lFCD changes between measures at baseline and 1 month after baseline (p > .05) (Figure 1b, Table 2). In SG group before surgery (PreSG), BMI was significantly correlated with lFCD in VMPFC (r[20] = .588, p = .004, Figure 1c) and similar association between BMI and lFCD in VMPFC was corroborated in Ctr group (r[17] = .505, p = .027, Figure 1c). Change in BMI was positively correlated with change in lFCD in VMPFC (r[20] = .466, p = .024, Figure 1c) and PCC/precuneus (r[20] = .601, p = .002, Figure 1c). ANOVA analysis also revealed a significant main effect of time for lFCD in VMPFC and PCC/precuneus (p FWE < .05, Supporting Information Figure S2A).
Figure 1.
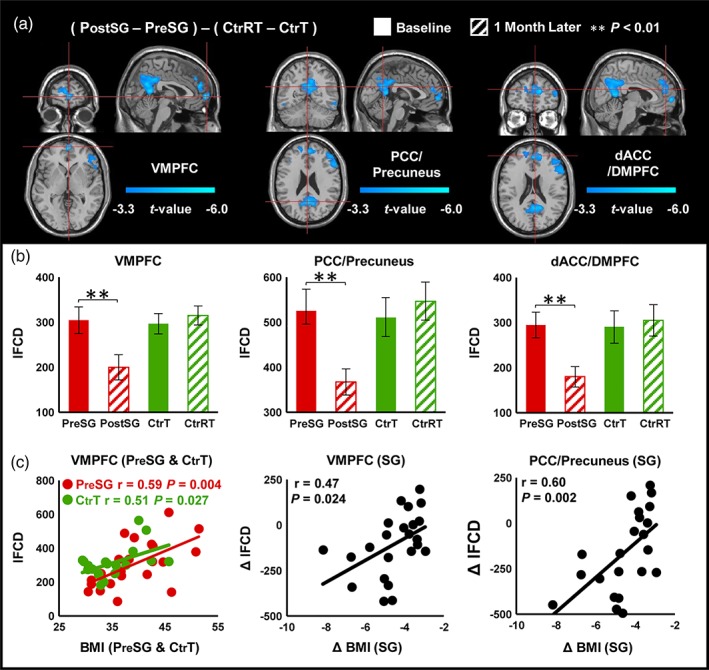
Interaction effects (group × time) for lFCD (cluster size‐corrected, p FWE < .05). (a) There were significant interaction effects (group × time) on lFCD in the VMPFC, PCC/precuneus, and dACC/DMPFC. (b) SG group after surgery had decreased lFCD in VMPFC, PCC/precuneus, and dACC/DMPFC. Ctr group did not show significant lFCD changes. (c) Correlation analysis between behavioral measurements and lFCD. The error bars indicate the standard error. Abbreviation: FCD, functional connectivity mapping; VMPFC, ventromedial prefrontal cortex; PCC, posterior cingulate cortex; dACC, dorsal anterior cingulate cortex; DMPFC, dorsomedial prefrontal cortex; BMI, body mass index [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Interaction effects (group × time) for FCD (cluster size‐corrected, p FWE < .05)
| Cluster level | Peak coordinates | Post‐hoc tests (FCD values) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PostSG vs. PreSG | CtrRT vs. CtrT | ||||||||||
| Region | BA | p FWE | Size | X | Y | Z | Peak t‐value | T | p | T | p |
| Local FCD: (PostSG − PreSG) − (CtrRT − CtrT) | |||||||||||
| VMPFC | 10 | .001 | 137 | 3 | 63 | 0 | −6.14 | −3.30 | .003 | 1.517 | .147 |
| PCC/precuneus | 23, 30 | .001 | 462 | 0 | −57 | 24 | −5.82 | −3.495 | .002 | 1.246 | .229 |
| dACC/DMPFC | 9, 32 | .001 | 112 | 6 | 51 | 21 | −5.24 | −3.160 | .002 | 0.649 | .525 |
| Global FCD: (PostSG − PreSG) − (CtrRT − CtrT) | |||||||||||
| VMPFC | 10 | .001 | 207 | 0 | 57 | 0 | −5.46 | −3.749 | <.001 | 1.243 | .230 |
| Right DLPFC | 9, 46 | .008 | 288 | 36 | 45 | 30 | −4.58 | −3.679 | <.001 | 0.541 | .595 |
| Right insula | 48 | .013 | 208 | 39 | 18 | 6 | −4.57 | −4.634 | <.001 | −1.880 | .076 |
Abbreviation: ROIs, regions of interest; BA, Brodmann area; FCD, functional connectivity mapping; VMPFC, ventromedial prefrontal cortex; PCC, posterior cingulate cortex; dACC, dorsal anterior cingulate cortex; DMPFC, dorsal medial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex.
Similarly, there were significant interaction effects on gFCD in VMPFC, right DLPFC, and right insula (p FWE < .05, Figure 2a, Table 2). Post‐hoc tests showed a significant reduction of gFCD in those regions after surgery in SG group but no changes in Ctr group 1 month after baseline (Figure 2b, Table 2). Change in YFAS was positively correlated with change in gFCD in VMPFC (r[20] = .586, p = .004, Figure 2c). In addition, there was also a main effect of time for gFCD in VMPFC and right insula (p FWE < .05, Supporting Information Figure 2B).
Figure 2.
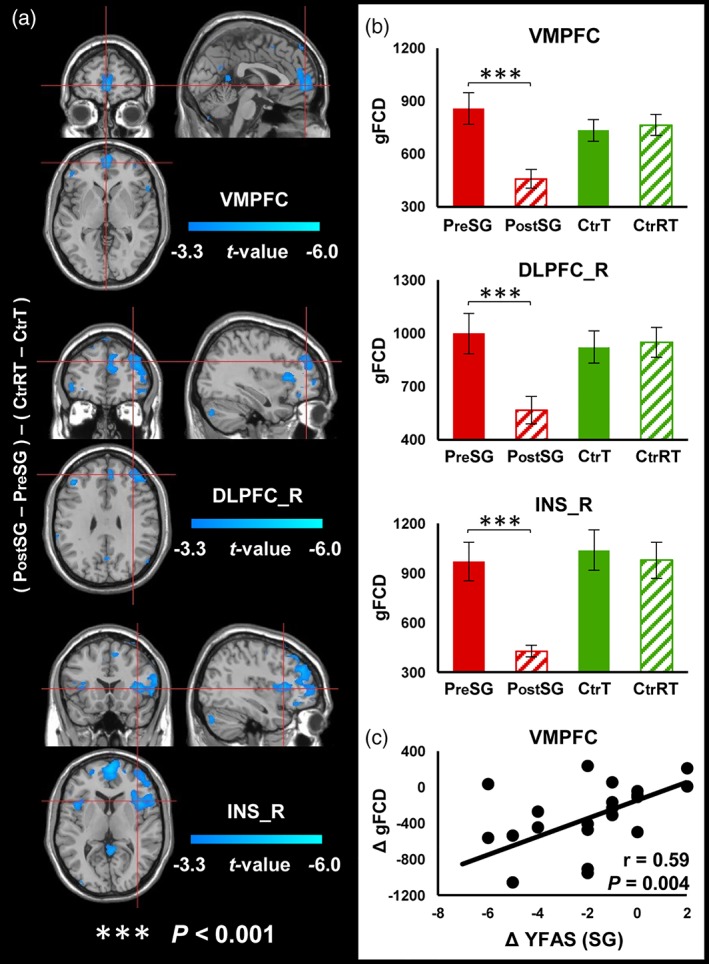
Interaction effects (group × time) for gFCD (cluster size‐corrected, p FWE < .05). (a) There were significant interaction effects (group × time) on gFCD in the VMPFC, right DLPFC and right insula. (b) SG group after surgery had decreased gFCD in those brain regions. Ctr group did not show significant gFCD changes. (c) Correlation analysis between behavioral measurements and gFCD. The error bars indicate the standard error. Abbreviation: FCD, functional connectivity mapping; VMPFC, ventromedial prefrontal cortex; DLPFC_R, right dorsolateral prefrontal cortex; INS_R, right insula; BMI, body mass index; YFAS, Yale food addiction scale [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Altered RSFC
There were significant interaction effects (Group × Time) on RSFC between PCC/precuneus seed (extracted from lFCD analysis) and right caudate/left DLPFC, and between VMPFC seed (extracted from lFCD analysis) and left DLPFC (p FWE < .05, Figure 3). Post‐hoc tests showed that LSG surgery increased RSFC strengths of these functional connections in SG group after surgery (VMPFC‐left DLPFC: t = 3.411, p = .003; PCC/precuneus‐right caudate: t = 4.119, p < .001; PCC/precuneus‐left DLPFC: t = 4.477, p < .001) but not in Ctr group (VMPFC‐left DLPFC: t = −0.866, p = .398; PCC/precuneus‐right caudate: t = −1.733, p = .100; PCC/precuneus‐left DLPFC: t = −1.494, p = .153) (Figure 3a,c). There were significant interaction effects (Group × Time) on RSFC between VMPFC seed (extracted from gFCD analysis) and right HIPP/PHIPP, wherein surgery decreased RSFC strength (t = −4.698, p < .001) and there were no significant changes in Ctr group (t = 1.205, p = .244) (Figure 3d). Change in BMI was negatively correlated with change in RSFC strength between PCC/precuneus and left DLPFC (r[20] = −.573, p = .005, Figure 3e) that did not survive correction for multiple comparison.
Figure 3.
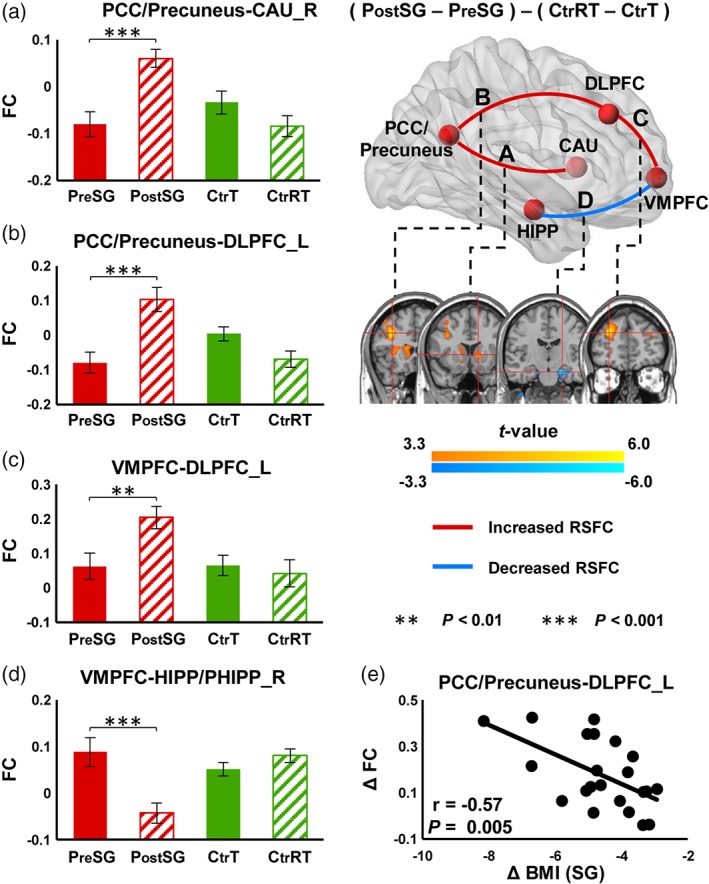
Interaction effects (group × time) on RSFC for both local and global FCD (p FWE < .05). There were significant interaction effects (group × time) on RSFC between the VMPFC seed (extracted from the lFCD analysis) and the left DLPFC, and between the PCC/precuneus seed (extracted from the lFCD analysis) and the right caudate and left DLPFC. There were also significant interaction effects (group × time) on RSFC between the VMPFC seed (extracted from the gFCD analysis) and the right HIPP/PHIPP. The error bars indicate the standard error. Abbreviation: FCD, functional connectivity mapping; VMPFC, ventromedial prefrontal cortex; DLPFC_L, left dorsolateral prefrontal cortex; CAU_R, right caudate; BMI, body mass index [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
Here we showed that sleeve gastrectomy significantly decreased FCD in midline cortical regions associated with self‐referential processing, including VMPFC, DMPFC, dACC, and precuneus and decreased gFCD in VMPFC, right insula (region involved with interoception), right DLPFC (region involved with executive control), and memory (HIPP). Additionally we showed that after surgery connectivity of the seed in VMPFC had stronger connectivity to left DLPFC and weaker connectivity to HIPP/PHIPP and the seed in PCC/precuneus had stronger connectivity with right caudate and left DLPFC. Although self‐referential processing has not been a focus in obesity, regions that are part of this midline cortical network (Northoff et al., 2006) have been consistently implicated in various aspects of food reward including the desire for food (Hollmann et al., 2012) and loss of control in food intake (Volkow et al., 2008). Enhanced connectivity of VMPFC/precuneus (key midline cortical regions involved in self‐referential processing) with DLPFC/caudate (circuits engaged in executive function/self‐regulation after sleeve gastrectomy, as well as their correlation with BMI changes) might reflect the association between the gastrectomy‐induced weight loss and the brain regions involved in the control of food craving. Attenuated connectivity between VMPFC and HIPP/PHIPP, which has been associated with memory processes related to food intake including awareness of hunger (Coppin, 2016) might also contribute to improved regulation of eating behaviors.
4.1. Alterations in self‐referential regions
Our results showed decreased FCD (both lFCD/gFCD) in VMPFC and decreased.
lFCD in dACC, DMPFC, and precuneus post‐surgery. These regions are associated with self‐referential processing involved with diverse functions linked with food reward, eating behaviors, and obesity. Specifically, VMPFC plays a critical role in emotional/behavioral functions that may affect appetitive behavior, and it is a core brain region for value‐based decision‐making (Killgore et al., 2013; Weilbächer & Gluth, 2017). VMPFC also monitors visceral signals and guides reward‐related behaviors (Vogt, 2005). DMPFC plays an important role in central regulation of eating behavior (Tataranni et al., 1999). Decreased FCD after sleeve gastrectomy in these brain regions reflects gastrectomy‐induced brain connectivity changes in regions involving self‐referential processing.
VMPFC is bidirectionally connected to entorhinal/perirhinal cortices connected to HIPP (Hernandez et al., 2017). VMPFC‐HIPP communication should underlie value‐based decision‐making, even when choice options are directly visible and do not require memory retrieval (Weilbächer & Gluth, 2017). Coupling from HIPP to VMPFC is not only important for processing memory‐based decisions but also for mediating memory bias (Weilbächer & Gluth, 2017). VMPFC‐HIPP interactions enable complex inferential memory associations (Zeithamova, Dominick, & Preston, 2012) and mediate value‐based decision from memory (Gluth, Sommer, Rieskamp, & Buchel, 2015).
For the obese individuals reported in this study, a positive correlation between BMI and baseline lFCD in VMPFC suggested the higher BMI the greater lFCD values in VMPFC (Figure 3c). Positive linear association between surgery‐related changes in BMI and lFCD and between changes in YFAS and gFCD in VMPFC suggest beneficial effects of sleeve gastrectomy in normalization of VMPFC hyperactivity. Increased RSFC between VMPFC and left DLPFC after the surgery suggest enhanced ability of obese individuals to exercise top‐down control of behavioral response/decision‐making on food choices (Hare, Camerer, & Rangel, 2009; Hare, Malmaud, & Rangel, 2011; Lopez, Hofmann, Wagner, Kelley, & Heatherton, 2014). This finding is consistent with prior studies that reported increased functional connectivity between DLPFC and VMPFC in obese subjects who achieved weight‐loss (Weygandt et al., 2013), and increased DLPFC‐VMPFC functional connectivity orchestrating top‐down control of appetite for high‐calorie foods in overweight/obese subjects after neurofeedback training (Spetter et al., 2017). SG group also showed reduced RSFC between VMPFC and HIPP/PHIPP post‐surgery. VMPFC receives visceral sensory information (memory for desired food). Alterations of RSFC between VMPFC and HIPP/PHIPP post‐surgery might allow inputs from regions involved with sensory‐emotional‐memory processing to modulate motivation drive from VMPFC for better control over stronger urges to eat.
PCC is also part of midline cortical regions implicated in self‐referential processing (Northoff et al., 2006). It is a prominent integrative hub in the brain (Tomasi & Volkow, 2011b) and a key region of DMN (Raichle et al., 2001). DMN is a network most active while processing internal mental status, such as self‐referential thinking/autobiographical memory, and during external unfocused attention (Andrews‐Hanna, Smallwood, & Spreng, 2014). Previous studies found that PCC activity was increased in obese/overweight individuals (Kullmann et al., 2012) and that activity in this region was reduced following chronic exercise (Legget et al., 2016). PCC activation has also been observed during presentation of visual food cues and during food tasting (DelParigi, Chen, Salbe, Reiman, & Tataranni, 2005). Precuneus plays a critical role in self‐referential processes and appetite control such as evaluating benefits of not eating compared with eating HC food (Yokum & Stice, 2013). Precuneus is involved in obesity‐inducing/preventing behavior through self‐body consciousness as well as body weight control, whose failure can be manifested as obesity/eating disorder (Nakamura & Ikuta, 2017). Reductions in outgoing connectivity from the PCC to other components of the DMN, such as the precuneus, and networks involved in various aspects of sensory processing could relate to the role of the PCC in monitoring the external environment and accordingly allocating neuronal resources to salient stimuli (Andrews‐Hanna et al., 2014).
Decreased lFCD in PCC/precuneus after gastrectomy might reflect reduced attention to internal states such as hunger/desire for food. After surgery, subjects had increased RSFC between PCC/precuneus and right caudate which is a striatal region necessary for executive function (Macfarlane et al., 2013). One latest study showed a linear association between higher caudate‐precuneus connectivity and lower obesity tendency (Nakamura & Ikuta, 2017), and our finding of increased RSFC between the PCC/precuneus and right caudate in the SG group after surgery may reflect lessened cognitive prevention of obesity (Nakamura & Ikuta, 2017). Enhanced RSFC between PCC/precuneus and left DLPFC, which is a frontal cortical region necessary for executive function (Koechlin, 2016) as well as its inverse association with change in BMI indicate that alterations of outgoing information flow could be related to improvements in cognitive function associated with weight‐loss induced by sleeve gastrectomy.
Surgery also decreased lFCD in dACC/DMPFC. ACC is implicated in executive control of internal/external stimuli‐related, context‐dependent behaviors involving emotional information, and modulation of emotional response (Bush, Luu, & Posner, 2000). ACC may contribute to imbalance between cognitive/emotional processing and increased risk to overeat (Kullmann et al., 2012). DMPFC is a potential downstream area receiving information from VMPFC selecting available choice options (Gluth, Rieskamp, & Buchel, 2012). Previous studies in obese individuals indicated a dysfunction of ACC in mediating hunger/satiety and emotional response (Bush et al., 2000; Kullmann et al., 2012), and attenuated lFCD in dACC/DMPFC suggests decreased need for executive control after surgery.
4.2. Alterations in interoceptive regions
The insular cortex is involved in the interceptive sense of the body and in emotional awareness (Craig, 2011). The anterior insula is represented in the processing of visually presented, tasted or smelled food stimuli, and also in food craving (Frank, Kullmann, & Veit, 2013). Reduced awareness of bodily state and appetite signaling may cause obese individuals to consume more food to respond to interoceptive cues (Frank et al., 2013). However, our results showed sleeve gastrectomy decreased gFCD in right insula. This was consistent with decreased activation in motivational/reward‐related regions with food picture stimulation after gastric banding (Bruce et al., 2012). There was also reduced neural response to food in insula associated with chronic exercise (Cornier, Melanson, Salzberg, Bechtell, & Tregellas, 2012).
4.3. Limitation
Due to strict exclusion criteria and difficulty in retaining patients after surgery for follow‐up scanning, we did not have a larger cohort size for SG group prior to and after surgery including Ctr group. We did not include healthy lean subjects as a control group. We assessed obese participants at two particular junctures (before and 1 month after surgery). These factors limit the generalization of our observations.
5. CONCLUSIONS
The current study investigated the effect of sleeve gastrectomy on resting brain activity of obese individuals. Our results showed significant reduction in cortical regions implicated in self‐referential processing and interoceptive awareness along with strengthening of connectivity of these regions with cortical (DLPFC) and striatal (caudate) regions implicated in executive control/self‐regulation. Thus, these changes might underlie improvement in control of eating behavior following sleeve gastrectomy. Identifying neural changes after sleeve gastrectomy may provide a neurophysiological support for the development of nonoperative treatment such as brain stimulation (Goebel, Tronnier, & Muente, 2017), and minimum invasive approach to the gastric fundus, that is, bariatric embolization (Weiss & Kathait, 2017) for the large proportion of overweight individuals whose BMI do not reach the standard of bariatric surgery and even for the morbidly obese individuals.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest related to this article.
Supporting information
Supporting information
Li G, Ji G, Hu Y, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self‐referential processing. Hum Brain Mapp. 2018;39:4755–4765. 10.1002/hbm.24320
Funding information Intramural Research Program of the United States National Institute on Alcoholism and Alcohol Abuse, Grant/Award Number: Z01AA3009 (DT, CEW, NDV, GJW); National Clinical Research Center for Digestive Diseases, Grant/Award Number: 2015BAI13B07; National Natural Science Foundation of Shaanxi Province, Grant/Award Number: 2018JM3007; National Natural Science Foundation of China, Grant/Award Number: 61431013, 81470816, 81601563, 81501543, and 817300
Contributor Information
Gang Ji, Email: jigang@fmmu.edu.cn.
Yi Zhang, Email: yizhang@xidian.edu.cn.
Gene‐Jack Wang, Email: gene-jack.wang@nih.gov.
REFERENCES
- Andrews‐Hanna, J. R. , Smallwood, J. , & Spreng, R. N. (2014). The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud, H. R. , & Morrison, C. (2008). The brain, appetite, and obesity. Annual Review of Psychology, 59, 55–92. [DOI] [PubMed] [Google Scholar]
- Bruce, J. M. , Hancock, L. , Bruce, A. , Lepping, R. J. , Martin, L. , Lundgren, J. D. , … Savage, C. R. (2012). Changes in brain activation to food pictures after adjustable gastric banding. Surgery for Obesity and Related Diseases, 8(5), 602–608. [DOI] [PubMed] [Google Scholar]
- Bush, G. , Luu, P. , & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Clark, S. M. , & Saules, K. K. (2013). Validation of the Yale food addiction scale among a weight‐loss surgery population. Eating Behaviors, 14(2), 216–219. [DOI] [PubMed] [Google Scholar]
- Coppin, G. (2016). The anterior medial temporal lobes: Their role in food intake and body weight regulation. Physiology & Behavior, 167, 60–70. [DOI] [PubMed] [Google Scholar]
- Cornier, M. A. , Melanson, E. L. , Salzberg, A. K. , Bechtell, J. L. , & Tregellas, J. R. (2012). The effects of exercise on the neuronal response to food cues. Physiology & Behavior, 105(4), 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveleskie, K. , Gupta, A. , Kilpatrick, L. A. , Mayer, E. D. , Ashe‐McNalley, C. , Stains, J. , … Mayer, E. A. (2015). Altered functional connectivity within the central reward network in overweight and obese women. Nutritional Diabetes, 5, e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- DelParigi, A. , Chen, K. , Salbe, A. D. , Reiman, E. M. , & Tataranni, P. A. (2005). Sensory experience of food and obesity: A positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. NeuroImage, 24(2), 436–443. [DOI] [PubMed] [Google Scholar]
- Diamantis, T. , Apostolou, K. G. , Alexandrou, A. , Griniatsos, J. , Felekouras, E. , & Tsigris, C. (2014). Review of long‐term weight loss results after laparoscopic sleeve gastrectomy. Surgery for Obesity and Related Diseases, 10(1), 177–183. [DOI] [PubMed] [Google Scholar]
- Ding, J. , An, D. , Liao, W. , Wu, G. , Xu, Q. , Zhou, D. , & Chen, H. (2014). Abnormal functional connectivity density in psychogenic non‐epileptic seizures. Epilepsy Research, 108(7), 1184–1194. [DOI] [PubMed] [Google Scholar]
- Eklund, A. , Nichols, T. E. , & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. , Kullmann, S. , & Veit, R. (2013). Food related processes in the insular cortex. Frontiers in Human Neuroscience, 7, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. , Wilms, B. , Veit, R. , Ernst, B. , Thurnheer, M. , Kullmann, S. , … Schultes, B. (2014). Altered brain activity in severely obese women may recover after roux‐en Y gastric bypass surgery. International Journal of Obesity, 38(3), 341–348. [DOI] [PubMed] [Google Scholar]
- Garcia‐Garcia, I. , Jurado, M. A. , Garolera, M. , Segura, B. , Sala‐Llonch, R. , Marques‐Iturria, I. , … Junque, C. (2013). Alterations of the salience network in obesity: A resting‐state fMRI study. Human Brain Mapping, 34(11), 2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt, A. N. , Corbin, W. R. , & Brownell, K. D. (2009). Preliminary validation of the Yale food addiction scale. Appetite, 52(2), 430–436. [DOI] [PubMed] [Google Scholar]
- Gloy, V. L. , Briel, M. , Bhatt, D. L. , Kashyap, S. R. , Schauer, P. R. , Mingrone, G. , … Nordmann, A. J. (2013). Bariatric surgery versus non‐surgical treatment for obesity: A systematic review and meta‐analysis of randomised controlled trials. BMJ, 347(1), f5934–f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluth, S. , Rieskamp, J. , & Buchel, C. (2012). Deciding when to decide: Time‐variant sequential sampling models explain the emergence of value‐based decisions in the human brain. The Journal of Neuroscience, 32(31), 10686–10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluth, S. , Sommer, T. , Rieskamp, J. , & Buchel, C. (2015). Effective connectivity between hippocampus and ventromedial prefrontal cortex controls preferential choices from memory. Neuron, 86(4), 1078–1090. [DOI] [PubMed] [Google Scholar]
- Goebel, C. H. , Tronnier, V. M. , & Muente, T. F. (2017). Brain stimulation in obesity. International Journal of Obesity, 41(12), 1721–1727. [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1959). The assessment of anxiety states by rating. The British Journal of Medical Psychology, 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare, T. A. , Camerer, C. F. , & Rangel, A. (2009). Self‐control in decision‐making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–648. [DOI] [PubMed] [Google Scholar]
- Hare, T. A. , Malmaud, J. , & Rangel, A. (2011). Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. The Journal of Neuroscience, 31(30), 11077–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, A. R. , Reasor, J. E. , Truckenbrod, L. M. , Lubke, K. N. , Johnson, S. A. , Bizon, J. L. , … Burke, S. N. (2017). Medial prefrontal‐perirhinal cortical communication is necessary for flexible response selection. Neurobiology of Learning and Memory, 137, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann, M. , Hellrung, L. , Pleger, B. , Schlogl, H. , Kabisch, S. , Stumvoll, M. , … Horstmann, A. (2012). Neural correlates of the volitional regulation of the desire for food. International Journal of Obesity, 36(5), 648–655. [DOI] [PubMed] [Google Scholar]
- Killgore, W. D. , Weber, M. , Schwab, Z. J. , Kipman, M. , DelDonno, S. R. , Webb, C. A. , & Rauch, S. L. (2013). Cortico‐limbic responsiveness to high‐calorie food images predicts weight status among women. International Journal of Obesity, 37(11), 1435–1442. [DOI] [PubMed] [Google Scholar]
- Koechlin, E. (2016). Prefrontal executive function and adaptive behavior in complex environments. Current Opinion in Neurobiology, 37, 1–6. [DOI] [PubMed] [Google Scholar]
- Konova, A. B. , Moeller, S. J. , Tomasi, D. , & Goldstein, R. Z. (2015). Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Research, 1628, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, S. , Heni, M. , Linder, K. , Zipfel, S. , Haring, H. U. , Veit, R. , … Preissl, H. (2014). Resting‐state functional connectivity of the human hypothalamus. Human Brain Mapping, 35(12), 6088–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, S. , Heni, M. , Veit, R. , Ketterer, C. , Schick, F. , Haring, H. U. , … Preissl, H. (2012). The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Human Brain Mapping, 33(5), 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legget, K. T. , Wylie, K. P. , Cornier, M. A. , Melanson, E. L. , Paschall, C. J. , & Tregellas, J. R. (2016). Exercise‐related changes in between‐network connectivity in overweight/obese adults. Physiology & Behavior, 158, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepping, R. J. , Bruce, A. S. , Francisco, A. , Yeh, H. W. , Martin, L. E. , Powell, J. N. , … Donnelly, J. E. (2015). Resting‐state brain connectivity after surgical and behavioral weight loss. Obesity (Silver Spring), 23(7), 1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B. , Fan, L. , Cui, Y. , Zhang, X. , Hou, B. , Li, Y. , … Jiang, T. (2015). DISC1 Ser704Cys impacts thalamic‐prefrontal connectivity. Brain Structure & Function, 220(1), 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, R. B. , Hofmann, W. , Wagner, D. D. , Kelley, W. M. , & Heatherton, T. F. (2014). Neural predictors of giving in to temptation in daily life. Psychological Science, 25(7), 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane, M. D. , Looi, J. C. , Walterfang, M. , Spulber, G. , Velakoulis, D. , Crisby, M. , … Wahlund, L. O. (2013). Executive dysfunction correlates with caudate nucleus atrophy in patients with white matter changes on MRI: A subset of LADIS. Psychiatry Research, 214(1), 16–23. [DOI] [PubMed] [Google Scholar]
- Moreno‐Lopez, L. , Contreras‐Rodriguez, O. , Soriano‐Mas, C. , Stamatakis, E. A. , & Verdejo‐Garcia, A. (2016). Disrupted functional connectivity in adolescent obesity. Neuroimage Clin, 12, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. , & Ikuta, T. (2017). Caudate‐Precuneus functional connectivity is associated with obesity preventive eating tendency. Brain Connectivity, 7(3), 211–217. [DOI] [PubMed] [Google Scholar]
- Northoff, G. , Heinzel, A. , de Greck, M. , Bermpohl, F. , Dobrowolny, H. , & Panksepp, J. (2006). Self‐referential processing in our brain‐‐a meta‐analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. [DOI] [PubMed] [Google Scholar]
- Ochner, C. N. , Kwok, Y. , Conceicao, E. , Pantazatos, S. P. , Puma, L. M. , Carnell, S. , … Geliebter, A. (2011). Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Annals of Surgery, 253(3), 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner, C. N. , Stice, E. , Hutchins, E. , Afifi, L. , Geliebter, A. , Hirsch, J. , & Teixeira, J. (2012). Relation between changes in neural responsivity and reductions in desire to eat high‐calorie foods following gastric bypass surgery. Neuroscience, 209, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , TO, L. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri‐Kojori, E. , Tomasi, D. , Wiers, C. E. , Wang, G. J. , & Volkow, N. D. (2016). Alcohol affects brain functional connectivity and its coupling with behavior: Greater effects in male heavy drinkers. Molecular Psychiatry, 22(8), 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom, L. , Narbro, K. , Sjostrom, C. D. , Karason, K. , Larsson, B. , Wedel, H. , … Carlsson, L. M. (2007). Effects of bariatric surgery on mortality in Swedish obese subjects. The New England Journal of Medicine, 357(8), 741–752. [DOI] [PubMed] [Google Scholar]
- Spetter, M. S. , Malekshahi, R. , Birbaumer, N. , Luhrs, M. , van der Veer, A. H. , Scheffler, K. , … Hallschmid, M. (2017). Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real‐time fMRI feedback study. Appetite, 112, 188–195. [DOI] [PubMed] [Google Scholar]
- Steele, K. E. , Prokopowicz, G. P. , Schweitzer, M. A. , Magunsuon, T. H. , Lidor, A. O. , Kuwabawa, H. , … Wong, D. F. (2010). Alterations of central dopamine receptors before and after gastric bypass surgery. Obesity Surgery, 20(3), 369–374. [DOI] [PubMed] [Google Scholar]
- Striepens, N. , Schroter, F. , Stoffel‐Wagner, B. , Maier, W. , Hurlemann, R. , & Scheele, D. (2016). Oxytocin enhances cognitive control of food craving in women. Human Brain Mapping, 37(12), 4276–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni, P. A. , Gautier, J. F. , Chen, K. , Uecker, A. , Bandy, D. , Salbe, A. D. , … Ravussin, E. (1999). Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America, 96(8), 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , Shokri‐Kojori, E. , & Volkow, N. D. (2016a). High‐resolution functional connectivity density: Hub locations, sensitivity, specificity, reproducibility, and reliability. Cerebral Cortex, 26(7), 3249–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2010). Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America, 107(21), 9885–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2011a). Functional connectivity hubs in the human brain. NeuroImage, 57(3), 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2011b). Association between functional connectivity hubs and brain networks. Cerebral Cortex, 21(9), 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Abnormal functional connectivity in children with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 71(5), 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2014). Mapping small‐world properties through development in the human brain: Disruption in schizophrenia. PLoS One, 9, e961764, e96176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2017). Reduced local and increased long‐range functional connectivity of the thalamus in autism spectrum disorder. Cereb Cortex, doi: 10.1093/cercor/bhx340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2018). Association between brain activation and functional connectivity. Cerebral Cortex, doi: 10.1093/cercor/bhy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , Wang, G. J. , & Volkow, N. D. (2013). Energetic cost of brain functional connectivity. Proceedings of the National Academy of Sciences of the United States of America, 110(33), 13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. G. , Shokri‐Kojori, E. , & Volkow, N. D. (2016b). Temporal evolution of brain functional connectivity metrics: Could 7 min of rest be enough? Cerebral Cortex, 27(8), 4153–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Sande‐Lee, S. , Pereira, F. R. , Cintra, D. E. , Fernandes, P. T. , Cardoso, A. R. , Garlipp, C. R. , … Velloso, L. A. (2011). Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes, 60(6), 1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience, 6(7), 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D. , Wang, G. J. , Telang, F. , Fowler, J. S. , Thanos, P. K. , Logan, J. , … Pradhan, K. (2008). Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. NeuroImage, 42(4), 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Zhao, J. , Tomasi, D. , Kojori, E. S. , Wang, R. , Wiers, C. E. , … Volkow, N. D. (2018). Effect of combined naltrexone and bupropion therapy on the brain's functional connectivity. International Journal of Obesity, doi: 10.1038/s41366-018-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbächer, R. , & Gluth, S. (2017). The interplay of hippocampus and ventromedial prefrontal cortex in memory‐based decision making. Brain Sciences, 7(12), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C. R. , & Kathait, A. S. (2017). Bariatric embolization: A new and effective option for the obese patient? Expert Review of Gastroenterology & Hepatology, 11(4), 293–302. [DOI] [PubMed] [Google Scholar]
- Weygandt, M. , Mai, K. , Dommes, E. , Leupelt, V. , Hackmack, K. , Kahnt, T. , … Haynes, J. D. (2013). The role of neural impulse control mechanisms for dietary success in obesity. NeuroImage, 83, 669–678. [DOI] [PubMed] [Google Scholar]
- Wiemerslage, L. , Zhou, W. , Olivo, G. , Stark, J. , Hogenkamp, P. S. , Larsson, E. M. , … Schioth, H. B. (2017). A resting‐state fMRI study of obese females between pre‐ and postprandial states before and after bariatric surgery. The European Journal of Neuroscience, 45(3), 333–341. [DOI] [PubMed] [Google Scholar]
- Wijngaarden, M. A. , Veer, I. M. , Rombouts, S. A. , van Buchem, M. A. , Willems, V. D. K. , Pijl, H. , & van der Grond, J. (2015). Obesity is marked by distinct functional connectivity in brain networks involved in food reward and salience. Behavioural Brain Research, 287, 127–134. [DOI] [PubMed] [Google Scholar]
- Yokum, S. , & Stice, E. (2013). Cognitive regulation of food craving: Effects of three cognitive reappraisal strategies on neural response to palatable foods. International Journal of Obesity, 37(12), 1565–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova, D. , Dominick, A. L. , & Preston, A. R. (2012). Hippocampal and ventral medial prefrontal activation during retrieval‐mediated learning supports novel inference. Neuron, 75(1), 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Ji, G. , Li, G. , Hu, Y. , Lliu, L. , Jin, Q. , … Wang, G. J. (2018). Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. International Journal of Obesity (Lond). 10.1038/s41366-018-0126-x [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Ji, G. , Xu, M. , Cai, W. , Zhu, Q. , Qian, L. , … Wang, G. J. (2016). Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. International Journal of Obesity, 40(10), 1558–1565. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Liu, J. , Yao, J. , Ji, G. , Qian, L. , Wang, J. , … Liu, Y. (2014). Obesity: Pathophysiology and intervention. Nutrients, 6(11), 5153–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, J. , Zhang, G. , Zhu, Q. , Cai, W. , Tian, J. , … Liu, Y. (2015). The neurobiological drive for overeating implicated in Prader‐Willi syndrome. Brain Research, 1620, 72–80. [DOI] [PubMed] [Google Scholar]
- Zhuo, C. , Zhu, J. , Qin, W. , Qu, H. , Ma, X. , Tian, H. , … Yu, C. (2014). Functional connectivity density alterations in schizophrenia. Frontiers in Behavioral Neuroscience, 19(8), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


