Abstract
There is a long‐standing interest in exploring the factors related to student achievement. As a newly explored personality trait, grit is defined as a person's tendency to pursue long‐term goals with continual perseverance and passion, and grit plays a critical role in student achievement. Increasing evidence has shown that growth mindset, the belief that one's basic abilities are malleable and can be developed through effort, is a potential factor for cultivating grit. However, less is known about the association between grit and the brain and the role of growth mindset in this association. Here, we utilized voxel‐based morphometry to examine the neuroanatomical correlates of grit in 231 healthy adolescent students by performing structural magnetic resonance imaging. The whole‐brain regression analyses revealed that the regional gray matter volume (rGMV) in the left dorsolateral prefrontal cortex (DLPFC) negatively predicted grit. In contrast, the rGMV in the right putamen positively predicted grit. Furthermore, mediating analyses suggested that growth mindset served as a mediator in the association between left DLPFC volume and grit. Our results persisted even after controlling for the influences of self‐control and delayed gratification. Overall, our study presents novel evidence for the neuroanatomical basis of grit and highlights that growth mindset might play an essential role in cultivating a student's grit level.
Keywords: dorsolateral prefrontal cortex, grit, growth mindset, putamen, voxel‐based morphometry
1. INTRODUCTION
What makes some students more successful than others in school? Intelligence (i.e., general mental ability), motivation, and emotional intelligence are commonly regarded as the main predictors of student achievement (Duckworth and Seligman, 2005; Mcbee and Duke, 1960; Neisser et al., 1996; Perera and DiGiacomo, 2013; Richardson, Abraham, & Bond, 2012). In addition, personality factors are found to be critical for student achievement (Poropat, 2009). For the past few years, a growing body of studies has indicated that grit, a newly explored personality trait, plays a crucial role in personal achievement (Duckworth, Peterson, Matthews, & Kelly, 2007; Duckworth and Quinn, 2009; Duckworth, Quinn, & Seligman, 2009; Duckworth, Kirby, Tsukayama, Berstein, & Ericsson, 2011; Eskreis‐Winkler, Shulman, Beal, & Duckworth, 2014; Kelly, Matthews, & Bartone, 2014; Maddi, Matthews, Kelly, Villarreal, & White, 2012; Reed, Pritschet, & Cutton, 2013; Suzuki, Tamesue, Asahi, & Ishikawa, 2015). The concept of grit was first introduced by Duckworth and her colleagues, who defined it as a person's tendency to sustain continual perseverance and passion for achieving long‐term goals (Duckworth et al., 2007). Converging evidence from previous studies has suggested that grit can reliably predict distinct aspects of student achievement, such as exam performance or grade point average (GPA) in undergraduates and middle school students, drop‐out or graduation rates in public high schools, retention and performance in summer training at West Point, and final ranks in the National Spelling Bee (Bazelais, Lemay, & Doleck, 2016; Bowman, Hill, Denson, & Bronkema, 2015; Duckworth et al., 2011, 2007; Duckworth and Quinn, 2009; Kelly et al., 2014; Maddi et al., 2012).
Although the predictive ability of grit for student achievement has been established, the neurobiological basis underlying grit remains largely unknown. Conceptually, there are two key psychological processes underlying grit: self‐regulation and motivation (Duckworth et al., 2007; Duckworth and Quinn, 2009; Duckworth and Gross, 2014). Self‐regulation (e.g., conscientiousness, planning, impulsivity and self‐control) empowers individuals to persist in the same goals when facing setbacks, difficulties, and distractions. Motivation enables individuals to pursue specific goals lasting months or even years. Thus, the neural underpinnings of grit are hypothesized to primarily reside in the prefrontal cortex (PFC) and striatum (Nemmi, Nymberg, Helander, & Klingberg, 2016). Specifically, the PFC is generally considered the neural center of self‐regulation (Heatherton, 2011; Kelley, Wagner, & Heatherton, 2015), and the striatum is commonly regarded as a core brain region involved in motivation and learning (Liljeholm and O'Doherty, 2012; Shohamy, 2011). Several recent empirical neuroimaging studies have partly confirmed these neural hypotheses of grit. Through an analysis of spontaneous brain activity (i.e., low‐frequency fluctuations) via resting‐state functional magnetic resonance imaging (RS‐fMRI) (Biswal, 2012; Fox and Raichle, 2007), Wang et al. (2017e) found a negative association between individual differences in grit and spontaneous brain activity in the dorsomedial PFC (DMPFC) among adolescent students. This finding might reflect the role of the right DMPFC in self‐regulation, goal setting and maintenance, planning, and counterfactual thinking for reflecting on past failures, which are the antecedents for grit (Wang et al., 2017e). In addition, evidence from another RS‐fMRI study in children has revealed that grit is positively linked with resting‐state functional connectivity (RSFC) between the ventral striatum and PFC regions, including the dorsolateral PFC (DLPFC), medial PFC (MPFC), and rostral anterior cingulate cortex (RACC) (Myers, Wang, Black, Bugescu, & Hoeft, 2016). Given that no study has investigated the structural brain correlates of grit in adolescents, we explored the relationships between grit and brain structures in a large sample of healthy adolescent students (N = 231) through structural magnetic resonance imaging (S‐MRI).
While most investigations have focused on the role of grit in predicting outcome variables (e.g., achievement and well‐being), relatively few studies have investigated the influential factors of grit (see Eskreis‐Winkler, Gross, & Duckworth, 2016, a systematic review). If possible influential factors are established, experts in the fields of psychology and education may develop corresponding training programs to improve an individuals’ grit level by facilitating the development of these factors. Evidence from the limited literature has shown that several factors—such as belief in free will (Li et al., 2018), purpose commitment (Hill, Burrow, & Bronk, 2016), and reflecting on past failures (DiMenichi and Richmond, 2015)—might have predictive ability for grit. Among these influential factors, a psychological construct known as “mindset” has drawn considerable attention in the past several years. Mindset refers to an individual's lay beliefs about the malleability of one's basic attributes (e.g., intelligence and personality). There are two distinct mindsets: growth and fixed (Dweck, 2000; Dweck and Leggett, 1988). A growth mindset refers to the belief that one's basic attributes are malleable and can be developed or cultivated through effort; a fixed mindset refers to the belief that one's basic attributes are invariant and cannot be improved or changed. A meta‐analytic review reported that growth mindset positively predicted many aspects of self‐regulation, which, in turn, predicted goal achievements (Burnette, O'boyle, VanEpps, Pollack, & Finkel, 2013). Furthermore, evidence from several recent studies in school‐aged children and adolescents has revealed a positive and moderate association between growth mindset and grit (Kench, Hazelhurst, & Otulaja, 2016; Tucker‐Drob et al., 2016; West et al., 2016; Yeager et al., 2016). Moreover, Duckworth (2013) proposed that growth mindset may be conducive for sustained efforts and goal commitments. In summary, growth mindset might be a promising construct that can be shaped to improve the grit levels of individuals. In addition, at the neural level, some evidence has indicated that growth mindset and grit share common cortico‐striatal RSFC between the ventral striatum and the DLPFC (Myers et al., 2016). In light of these findings, growth mindset might mediate the associations between brain structures and grit.
To conduct our investigations, we employed voxel‐based morphometry (VBM) methodology and standard measurements of grit and growth mindset. VBM analysis is one of the most popular and well‐validated methods for evaluating the amount of gray matter in different regions of the brain (Mechelli, Price, Friston, & Ashburner, 2005). Considering its task‐free conditions and low‐cost characteristics, VBM analysis has been widely employed to investigate the neurobiological bases of human personalities and behaviors (Kanai and Rees, 2011; Mar, Spreng, & DeYoung, 2013). First, we performed whole‐brain regression analyses to identify the brain areas related to grit. Given previously reported brain findings regarding grit, we speculated that the structural variations in PFC regions (e.g., DLPFC, MPFC, and RACC) and striatum might predict individual differences in grit. Second, we performed mediation analyses to examine whether growth mindset plays a mediating role in the relationships between brain structures (e.g., gray matter structure in the PFC regions and striatum) and grit.
2. METHODS
2.1. Participants
Two hundred and thirty‐four healthy students from several local public high schools and without a history of psychiatric or neurological diseases by self‐report participated in this study (122 females; M age = 18.60 years, SD = 0.78). These students had recently graduated in June 2015 and were recruited from a larger project with major goals of investigating the neural substrates of cognition, emotion, personality and social competence among adolescents in Chengdu, China (Wang et al., 2017a, 2017b, 2017c, 2017d, 2017e). All participants were native Mandarin Chinese speakers and were right‐handed as tested by the Edinburgh Handedness Inventory (Oldfield, 1971). Three participants were excluded due to abnormal brain structure (i.e., unusual cysts). Thus, 231 participants (121 females; M age = 18.48 years, SD = 0.54) were included in the subsequent data analyses. In accordance to the approval from the local research ethics committee at West China Hospital of Sichuan University, each student provided written informed consent prior to the experiment. We conducted the experiments from June 2015 to September 2015.
2.2. Behavioral measures
2.2.1. Short grit scale (Grit‐S)
To measure individual differences in trait grit, we administered the 8‐item Grit‐S (Duckworth and Quinn, 2009). The eight items are grouped into two factors (i.e., perseverance of effort and consistency of interest), which further load on a second‐order latent factor (i.e., grit). All of the items were rated on a 5‐point Likert scale with example items, such as “New ideas and projects sometimes distract me from previous ones” and “Setbacks don't discourage me.” Prior studies have demonstrated that the Chinese version of the Grit‐S shows adequate internal consistency (Cronbach's α = 0.80/0.81), test–retest reliability (r = .78), and criterion‐related validity with respect to academic performance, self‐control, and conscientiousness (Li et al., 2016a; Wang et al., 2017e). In our dataset, Grit‐S exhibited adequate internal consistency (Cronbach's α = 0.83). Because grit consists of two factors and there were high correlations between the Grit‐S total score and the two factors’ scores and inter‐factor correlations (Duckworth et al., 2007; Duckworth and Quinn, 2009; Duckworth and Gross, 2014), only the Grit‐S total score was used as the measure of the trait grit in the subsequent analyses, with higher scores indicating higher levels of grit.
2.2.2. Theory of intelligence scale (TIS)
We used the 3‐item TIS (Dweck, 2000; Hong, Chiu, Dweck, Lin, & Wan, 1999), which is a widely used tool for assessing the beliefs of intelligence, to measure individual differences in mindset. The participants were required to indicate the degree of agreement on each item, ranging from 1 (strongly agree) to 6 (strongly disagree). The mindset score for each participant was computed by summing the ratings across all items, and higher scores suggested a stronger belief that intelligence can be changed (i.e., growth mindset). This scale has been commonly used in Chinese adolescent students (i.e., Wang and Ng, 2012). In our dataset, Cronbach's α for TIS was 0.75, suggesting adequate internal reliability.
2.2.3. Raven's advanced progressive matrix (RAPM)
To rule out the potential impact of general intelligence on the associations between brain structures and grit (Basten, Hilger, & Fiebach, 2015), the RAPM (Raven, 2000), a popular and sound instrument for evaluating general intelligence, was administered. The RAPM consists of 36 nonverbal items in which participants are asked to choose the missing part for each graphical matrix. The general intelligence scores of the participants were indexed by the sum of the number of correct answers within 30 min (Li et al., 2015a). In our dataset, Cronbach's α for RAPM was 0.83, indicating adequate internal reliability.
2.2.4. Brief self‐control scale (BSCS)
Given the close association between grit and self‐control (Duckworth and Gross, 2014; Duckworth et al., 2007), we used the BSCS (Tangney, Baumeister, & Boone, 2004) to test the specificity of the relationship between grit and brain structure. The unidimensional BSCS contains 13 items, which are rated on a 5‐point Likert scale. Example items include “I am good at resisting temptation” and “I have trouble concentrating.” Previous evidence has revealed that the BSCS shows satisfactory reliability and validity among adolescents (Frijns, Finkenauer, Vermulst, & Engels, 2005). The Chinese version of the BSCS was established using a translation and back‐translation process and was used in our prior study (Li et al., 2016a). In this study, Cronbach's α for the BSCS was 0.78, showing acceptable internal reliability.
2.2.5. Monetary choice questionnaire (MCQ)
To evaluate the possible influence of delayed gratification on the association between grit and brain structure, the 27‐item MCQ (Kirby, Petry, & Bickel, 1999), a widely used measure for assessing delayed gratification, was administered. Based on the delayed reward magnitudes, the 27 items are grouped into three conditions (nine items per condition): small (¥25–35¥), medium (¥50–60¥), and large (¥75–85¥). The delayed time ranged from 7 to 186 days. For each item, the participants were asked to choose either a larger, delayed reward or a smaller, immediate reward. For instance, “would you prefer ¥15 today or ¥35 in 13 days?” Previous work has suggested that a hyperbolic function fits well with the participants’ responses: V = A/(1 + kD), where V is the present reward, A is the delayed reward, D is the delayed time, and k is the discount rate parameter (Mazur, 1987). According to the method developed in previous studies (Kirby, 1997; Kirby et al., 1999), we calculated the lnk value as the measure of delayed gratification, with higher lnk values representing lower delayed gratification. The Chinese version of the MCQ has adequate reliability and validity in Chinese populations (Li et al., 2016b; Liu et al., 2016). We have used the MCQ in our previous studies and found good internal consistency (Wang et al., 2017a, 2017c).
2.3. MRI data acquisition and preprocessing
2.3.1. Data acquisition
The imaging data were collected using a Siemens‐Trio Erlangen MRI scanner (3.0 T, Germany) at West China Hospital of Sichuan University, Chengdu, China. The scanner was equipped with a 12‐channel head coil. Using a magnetization‐prepared rapid gradient echo sequence, we obtained the T1‐weighted anatomical images for each participant with the following scanning parameters: voxel size, 1 × 1 × 1 mm3; flip angle, 9°; matrix size, 256 × 256; slice thickness, 1 mm; 176 slices; echo time, 2.26 ms; inversion time, 900 ms; repetition time, 1,900 ms.
2.3.2. Data preprocessing
We preprocessed the imaging data using the Statistical Parametric Mapping program (SPM8, Wellcome Department of Cognitive Neurology, London, UK). First, a medical radiologist who was blind to this study visually checked each image. Second, we manually set the origin of the images to the anterior commissure for better registration. Third, we used the new segmentation in SPM8 to segment the images into gray matter and white matter. Fourth, we performed registration, normalization and modulation analyses using Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) in SPM8 (Ashburner, 2007). Specifically, we aligned and resampled the gray matter images to 2 × 2 × 2 mm3 and then normalized the values to a study‐specific template in MNI152 space. To maintain the volume of gray matter, we employed Jacobian determinants to modulate the gray matter values in each voxel. Finally, we employed an 8‐mm full‐width at half‐maximum Gaussian kernel to smooth the modulated images. The resulting images representing the regional gray matter volume (rGMV) were used in the subsequent analyses.
2.4. Statistical analyses
2.4.1. VBM analyses
We performed whole‐brain regression analyses to explore the anatomical correlates underlying grit. In the regression model, the dependent variable was the rGMV of each voxel in the whole‐brain, the independent variable was Grit‐S score, and the controlling variables were age, gender, total gray matter volume (GMV) and RAPM score. In addition, we investigated whether there were gender differences in the association between grit and rGMV through a condition‐by‐covariate interaction analysis (Kong et al., 2014; Li et al., 2014b; Takeuchi et al., 2014; Yamasue et al., 2008; Yang et al., 2014). In this whole‐brain analysis, gender was treated as a condition, the Grit‐S score was treated as a covariate of interest, and age, total GMV and RAPM score were treated as covariates of no interest. With the exception of total GMV, all covariates were modeled to make the unique association of each covariate with rGMV evident for each gender. The t‐contrasts were used to assess the interaction effects between gender and grit on the rGMV. To exclude the edge effects between white matter and gray matter (i.e., noise voxels), we applied an absolute threshold masking of 0.2 in these analyses. The regions of significance were determined using non‐stationary cluster correction based on the random field theory (Hayasaka, Phan, Liberzon, Worsley, & Nichols, 2004) with the following settings: p < .05 at the cluster level combined with p < .001 at the underlying voxel level. The nonstationary cluster correction is a reliable and popular method to analyze VBM data (Kong, Chen, Xue, Wang, & Liu, 2015a; Li et al., 2014a; Takeuchi et al., 2015a; Wang et al., 2017a). We used nonstationary cluster correction for the following reasons. First, because this method utilizes extent and height thresholds to determine significance, it outperforms other methods that only use the height threshold to determine significance (Poline, Worsley, Evans, & Friston, 1997). Second, the structural brain data (e.g., VBM data) have been found to be non‐stationary (i.e., not uniformly smooth) (Ashburner and Friston, 2000), which could and should be corrected using the nonstationary cluster correction (Hayasaka et al., 2004; Nichols and Hayasaka, 2003). We performed these analyses using SPM8 software.
2.4.2. Prediction analyses
We employed a machine learning approach to examine the stability of the association between brain structure and grit. In other words, we tested whether the association between brain structure and grit was affected by some factors such as the outliers and data distribution. This approach is based on balanced cross‐validation using linear regression (Kong et al., 2015a; Qin et al., 2014; Supekar et al., 2013; Wang et al., 2017a, 2017b). In this analysis, we input the rGMV of different regions as the independent variable and the grit score as the dependent variable. The predictive ability of the independent variable on the dependent variable was assessed as r (predicted, observed), which was evaluated using a fourfold balanced cross‐validation procedure. First, we divided the data by fourfold to ensure that the independent variable and dependent variable distributions across folds were balanced. Second, we used three folds to build a linear regression model, leaving out the fourth fold. Next, we employed this model to predict the fourth fold data. We repeated this procedure four times to obtain a final r (predicted, observed), which represented the association between the observed data and the data predicted by the regression model. Here, we applied a nonparametric testing method to determine the statistical significance of the model. Specifically, we generated 1,000 surrogate datasets to estimate the empirical null distribution of r (predicted, observed), where the null hypothesis corresponded to no association between brain structures and grit. Then, we permuted the labels of the observed data points to generate each surrogate dataset D i of a size equal to the observed dataset. Next, we used the predicted labels with the fourfold balanced cross‐validation procedure and the actual D i labels to calculate the r (predicted, observed) of D i [i.e., r (predicted, observed)i]. Finally, we counted the number of r (predicted, observed)i values greater than r (predicted, observed) and then divided that count by the number of Di datasets (1,000). The resulting value was considered the statistical significance (p value). Age, gender, total GMV, and general intelligence were adjusted for in these analyses.
2.4.3. Hierarchical regression analyses
We performed hierarchical regression analyses for the following two purposes. First, we evaluated the brain regions related to grit could jointly explain how much variance in grit after controlling for other factors. Second, we examined whether the brain regions related to grit could uniquely explain additional variance in grit after controlling for the other brain regions and other factors. In these analyses, the dependent variable was the Grit‐S score; the independent variables in Step 1 were age, gender, total GMV, and RAPM score; and the independent variables in Step 2 were the rGMVs of brain regions that were identified in the prior whole‐brain regression analyses. We performed these analyses using SPSS software (version 22.0).
2.4.4. Mediation analyses
We performed mediation analyses to investigate whether growth mindset could explain the effect of brain anatomy on grit. In the mediation model, the dependent variable was grit, the independent variable was the rGMV of regions detected from the whole‐brain regression analyses, and the mediator variable was growth mindset. According to the customary methodology (Baron and Kenny, 1986), the total effect (path c) is the association of the independent variable and dependent variable, the direct effect (path c′) is the association of the independent variable and dependent variable after adjusting for mediator variable, and the indirect effect is the product of path a (the association of the independent variable and mediator variable) and path b (the association of the mediator variable and dependent variable after adjusting for the independent variable) or path c–path c′. To determine the significance of the indirect effect, we employed a bootstrapping procedure (Preacher and Hayes, 2008) with 5,000 samplings to generate 95% confidence intervals (CIs). The indirect effect would be significant if a CI did not contain zero. We performed these analyses using an SPSS macro program (Preacher and Hayes, 2008) and controlled for age, gender, total GMV, and RAPM score.
3. RESULTS
3.1. Brain structures of grit
Table 1 details the descriptive statistics for all study variables. According to the conventions (Marcoulides and Hershberger, 1997), the scores of each variable were normally distributed because the skewness and kurtosis values ranged from −1 to +1, with the exception of age. Given the high correlations between the Grit‐S total score and two factors’ scores (perseverance of effort: r = .84, p < .001; consistency of interest: r = .87, p < .001), we used only the Grit‐S total score as the measure of the trait grit in the subsequent analyses. Grit was not associated with age (r = .01, p = .883) or gender [t(229) = 1.81, p = .071]. Moreover, grit was not correlated with total GMV (r = −.06, p = .333) or general intelligence (r = −.01, p = .896) after adjusting for gender and age. We subsequently examined the brain structures of grit.
Table 1.
Descriptive statistics of participant‐level variables (N = 231)
| Variable | Mean | SD | Range | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Age | 18.48 | 0.54 | 16–20 | 0.50 | 1.71 |
| Total GMV | 0.67 | 0.59 | 0.53–0.82 | 0.12 | −0.45 |
| Grit | 25.54 | 4.42 | 14–38 | 0.14 | −0.52 |
| Growth mindset | 9.98 | 2.71 | 3–18 | 0.08 | −0.09 |
| General intelligence | 24.18 | 5.68 | 6–36 | −0.26 | −0.09 |
| Self‐control | 38.14 | 6.62 | 19–56 | 0.26 | 0.12 |
| Delayed gratification (lnk) | −4.25 | 1.34 | −8.75 to −1.39 | −0.54 | 0.50 |
Note. Abbreviations: GMV = gray matter volume; N = number; SD = standard deviation.
To detect the brain regions related to grit, we performed whole‐brain regression analyses. After adjusting for age, gender, total GMV, and general intelligence, higher grit scores were related to smaller rGMV in the left DLPFC (middle frontal gyrus; r = −.27, p < .001; see Table 2 and Figure 1). In addition, higher grit scores were related to greater rGMV in the right putamen (dorsal striatum; r = .29, p < .001; see Table 2 and Figure 2). No other significant results were obtained. To examine the stability of the association between brain structures and grit, we performed prediction analyses using the machine learning approach. After adjusting for age, gender, total GMV, and general intelligence, the rGMV in the left DLPFC [r (predicted, observed) = −.24, p < .001] and right putamen [r (predicted, observed) = .26, p < .001] could significantly predict grit, respectively. In addition, to investigate whether the associations between grit and rGMV differed between males and females, we performed a condition‐by‐covariate interaction analysis with gender as a condition and grit as a covariate of interest. We found no significant regions for the interaction effect of gender by grit.
Table 2.
Brain regions where gray matter volume was related to grit
| Region | Peak MNI coordinate | Peak t score | Cluster size (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive correlation | |||||
| Right putamen | 24 | −20 | 0 | 4.97 | 1792 |
| Negative correlation | |||||
| Left DLPFC | −24 | 12 | 46 | −5.05 | 1888 |
Note. Abbreviations: DLPFC = dorsolateral prefrontal cortex; MNI = Montreal Neurological Institute.
The regions of significance were determined using nonstationary cluster correction (p < .05 at the cluster level combined with p < .001 at the underlying voxel level).
Figure 1.
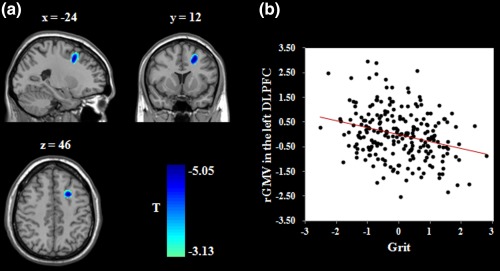
Brain regions related to grit. (a) Brain images depicting the negative association between grit and the rGMV in the left DLPFC. (b) Scatter plot showing the correlation between grit and left DLPFC volume (r = −.27, p < .001). Age, gender, total GMV, and general intelligence were adjusted for in these analyses. rGMV = regional gray matter volume; DLPFC = dorsolateral prefrontal cortex [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
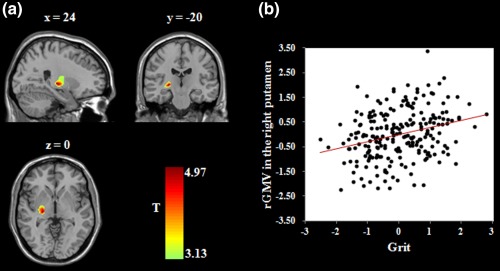
Brain regions related to grit. (a) Brain images depicting the positive association between grit and the rGMV in the right putamen. (b) Scatter plot showing the correlation between grit and right putamen volume (r = .29, p < .001). Age, gender, total GMV, and general intelligence were adjusted for in these analyses. rGMV = regional gray matter volume [Color figure can be viewed at http://wileyonlinelibrary.com]
We then extracted the rGMV in the brain regions that were identified from the whole‐brain regression analyses and performed a hierarchical regression analysis. The rGMV in the left DLPFC (β = −0.28, p < .001) and right putamen (β = 0.36, p < .001) jointly explained 14.0% of variance in grit (△R 2 = .140, p < .001) beyond the variance explained by age, gender, total GMV, and general intelligence. These results suggested that the left DLPFC and right putamen could uniquely predict individual differences in grit.
3.2. Growth mindset mediated the relationship between brain structure and grit
To investigate the role of growth mindset in the relationship between brain structures and grit, we collected data on the theory of intelligence in our sample. Behaviorally, we confirmed a significant correlation between growth mindset and grit (r = .22, p < .001). This correlation remained significant (r = .23, p < .001) even after controlling for age, gender, total GMV, and general intelligence. Then, we explored whether the brain structures associated with grit were related to growth mindset. We observed that the rGMV in the left DLPFC could significantly predict individual differences in growth mindset (r = −0.22, p < .001). The predictive ability of the rGMV in the left DLPFC on growth mindset was significant (r = −.19, p = .003) even after adjusting for age, gender, total GMV, and general intelligence. However, there was no significant association between the rGMV in the right putamen and growth mindset (r = −.09, p = .162).
The results above revealed close associations between the rGMV in the left DLPFC, growth mindset, and grit, but the nature of the associations among these factors remained unknown. To examine whether growth mindset could mediate the relation between brain structure and grit, we performed mediation analyses with age, gender, total GMV, and general intelligence as covariates. After including growth mindset as an intermediate variable, the relation between the rGMV in left DLPFC and grit was reduced, though still significant (Figure 3). The 5,000 bootstrap simulations further demonstrated that growth mindset played a mediating role in the relationship between the rGMV in the left DLPFC and grit (95% CI = [−0.01, −0.09], p < .05). However, growth mindset did not mediate the association between the rGMV in the right putamen and grit (95% CI = [−0.05, 0.02], p > .05). In summary, growth mindset partly mediated the relationship between the rGMV in the left DLPFC and grit.
Figure 3.
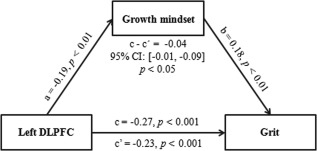
Growth mindset mediates the effect of left DLPFC on grit. The depicted diagram demonstrates that the left DLPFC affects grit through growth mindset. The standardized regression coefficients were employed to represent the paths between variables. All paths (a, b, c, and c′) and the indirect effect (c–c′ or a × b) were significant. Age, gender, total GMV, and general intelligence were adjusted for in the model. DLPFC = dorsolateral prefrontal cortex
3.3. The grit‐specific nature of the findings
To examine whether our results were specific to grit, we excluded two possible confounding factors: self‐control and delayed gratification. First, we tested the effect of self‐control and delayed gratification on the neuroanatomical correlates of grit. At the whole‐brain level, after controlling for self‐control and delayed gratification as well as age, gender, total GMV, and general intelligence, multiple regression analyses revealed that no regions were significantly correlated with grit (non‐stationary cluster corrected). We then extracted the mean rGMV values in the left DLPFC (cluster size: 1,888 mm3) and right putamen (cluster size: 1,792 mm3) related to grit and performed correlation analyses. After controlling for self‐control and delayed gratification as well as age, gender, total GMV and general intelligence, grit was still significantly correlated with the rGMV in the left DLPFC (r = −.25, p < .001) and right putamen (r = .21, p < .001). Therefore, controlling for self‐control and delayed gratification reduced but did not remove the associations between brain structures and grit.
Next, to test whether the brain structures could explain the additional variance in grit beyond the variance explained by self‐control and delayed gratification as well as age, gender, total GMV, and general intelligence, we extracted the rGMV in the brain regions that were identified from the initial whole‐brain regression analyses and then performed a hierarchical regression analysis. The rGMV in the left DLPFC (β = −0.23, p < .001) and right putamen (β = 0.22, p < .001) jointly explained 6.5% of the variance in grit (△R 2 = .065, p < .001) beyond the variance explained by self‐control and delayed gratification as well as age, gender, total GMV, and general intelligence. These results suggested that the left DLPFC and right putamen could uniquely predict individual differences in grit beyond self‐control and delayed gratification.
Finally, we checked whether self‐control and delayed gratification could affect the mediating effect of growth mindset on the association between left DLPFC volume and grit. After adjusting for self‐control and delayed gratification as well as age, gender, total GMV, and general intelligence, growth mindset still mediated the impact of the rGMV in the left DLPFC on grit (indirect effect = −0.037, 95% CI = [−0.01, −0.08], p < .05). Thus, the mediating role of growth mindset in the relation between left DLPFC volume and grit might not be affected by self‐control and delayed gratification.
Taken together, these results suggested that our findings were specific to grit to some degree, although self‐control and delayed gratification have some impacts on the grit–brain associations.
4. DISCUSSION
In this study, we investigated the association between brain structure and grit and the role of growth mindset in this association among a large sample of adolescents. Two main findings were observed. First, we showed an association between higher grit and smaller rGMV in the left DLPFC and greater rGMV in the right putamen. Second, growth mindset acted as a mediator in the association between left DLPFC volume and grit. Importantly, our results persisted even after adjusting for self‐control and delayed gratification. Taken together, our findings provide novel evidence for the neuroanatomical basis underlying grit and reveal a potential contributing mechanism of grit in which growth mindset explains the covariance between brain structure and grit.
Confirming our first hypothesis, we found that individual differences in grit were negatively related to the rGMV in the left DLPFC. This negative association might reflect the mechanism of structural maturation in the brain, in which synaptic pruning and myelination cause the developmental reductions of rGMV in certain brain regions and improve efficiency in corresponding psychological processes (Blakemore and Robbins, 2012; Kanai and Rees, 2011; Konrad, Firk, & Uhlhaas, 2013). Empirical evidence from several studies has suggested that the gray matter structures of DLPFC are negatively associated with many cognitive–emotional functions, such as creative cognitive ability (Chen et al., 2016), social well‐being (Kong, Hu, Xue, Song, & Liu, 2015b), and internet in‐tendency (Li et al., 2015b). Furthermore, the DLPFC is generally regarded as a neural center for self‐regulation (Heatherton, 2011; Kelley et al., 2015). A large number of studies have indicated that the structure and function of the DLPFC provide the neural bases for many aspects of self‐regulation, including conscientiousness (DeYoung et al., 2010; Kunisato et al., 2011), planning (Kaller, Rahm, Spreer, Weiller, & Unterrainer, 2011; Tanji, Shima, & Mushiake, 2007), cognitive control (MacDonald, Cohen, Stenger, & Carter, 2000; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004), delay discounting (Peters and Büchel, 2011; Scheres, de Water, & Mies, 2013), impulsivity (Asahi, Okamoto, Okada, Yamawaki, & Yokota, 2004; Cho et al., 2013; Schilling et al., 2013, 2012), and emotional regulation (Golkar et al., 2012; Kanske, Heissler, Schonfelder, Bongers, & Wessa, 2011). In addition, our study revealed that grit was correlated with only left DLPFC volume, but not right DLPFC volume. This is consistent with the increasing evidence showing that structural variations in the left DLPFC, but not the right DLPFC, provide the neuroanatomical basis for self‐regulation, which includes conscientiousness (DeYoung et al., 2010), impulsivity (Cho et al., 2013; Schilling et al., 2013, 2012), and delay discounting (Drobetz et al., 2014). Furthermore, evidence from a repetitive transcranial magnetic stimulation (rTMS) study has revealed that transient disruption of the left DLPFC, but not the right DLPFC, led participants to select more immediate rewards, which provides the causal role of the left DLPFC in the self‐regulation processes of intertemporal choice (Figner et al., 2010). Therefore, synaptic pruning and myelination in the processing of structural maturation in the brain might reduce left DLPFC volume and enable greater efficiency in self‐regulation, both of which are further linked with higher levels of grit. In summary, our results regarding the relation between left DLPFC volume and grit might support the self‐regulation account of grit to a certain degree (Nemmi et al., 2016).
In addition, we observed a positive association between the rGMV in the right putamen and individual differences in grit. The putamen is well known to be involved in motivation and learning related to reward processing (Liljeholm and O'Doherty, 2012; Shohamy, 2011). For example, many prior investigations have reported that activities in the putamen are related to distinct aspects of reward processing (e.g., expectation, magnitude, and predictability) (Cromwell and Schultz, 2003; O'Doherty, Deichmann, Critchley, & Dolan, 2002; O'Doherty, Dayan, Friston, Critchley, & Dolan, 2003). In particular, putamen activities are related to motivation processing, and there are greater activities in the right putamen when monetary rewards are given and when participants are highly motivated to learn (O'Doherty et al., 2003, 2002). Furthermore, evidence from an fMRI study has shown that compared with left putamen activities, right putamen activities show a greater correlation with academic achievement motivation (Mizuno et al., 2008). Similarly, using the VBM method, Takeuchi et al. (2014) found a positive association between individual differences in competitive achievement motivation and regional gray matter density of the right but not the left putamen. These findings suggest that the right putamen might play a more important role in achievement motivation and learning, fitting well with our result that the right but not the left putamen was associated with grit. The putamen was also found to be linked with action planning associated with emotional and reward information (Haruno and Kawato, 2006; Zeki and Romaya, 2008) and individual differences in trait persistence (Takeuchi et al., 2015b). In summary, the finding regarding the association between grit and the right putamen might reflect the role of the putamen in motivation and learning, action planning, and tendency of persistence, which are the prerequisites for grit (Duckworth et al., 2007; Duckworth and Quinn, 2009; Duckworth and Gross, 2014).
Confirming our second hypothesis, growth mindset played a mediating role in the association between left DLPFC volume and grit. Behaviorally, we found a moderate but statistically significant association between growth mindset and grit, which was consistent with previous findings (Kench et al., 2016; Tucker‐Drob et al., 2016; West et al., 2016; Yeager et al., 2016). Moreover, in our sample, growth mindset explained the additional variance in grit beyond that explained by self‐control and delayed gratification as well as age, gender, general intelligence and total GMV (△R 2 = .017, p < .05). Thus, our results provide additional evidence that growth mindset serves as a potential factor for cultivating grit among adolescent students. In addition, the association between growth mindset and the left DLPFC volume is consistent with previous findings demonstrating an association between growth mindset and RSFC of DLPFC and striatum (Myers et al., 2016). Using event‐related potentials (ERPs), Mangels, Butterfield, Lamb, Good, and Dweck (2006) revealed that during a test of general knowledge, participants with growth mindsets exhibited lower P3 activity in the anterior prefrontal cortex, which is associated with error correction and conflict detection. Generally, the DLPFC is implicated in error detection and monitoring during behavior adaptation (Stevens, Kiehl, Pearlson, & Calhoun, 2009). Behaviorally, evidence has suggested that individuals with growth mindsets demonstrate an enhanced ability in error monitoring and receive corrective feedbacks more easily (Moser, Schroder, Heeter, Moran, & Lee, 2011). Moreover, as reviewed above, the DLPFC plays a pivotal role in many aspects of self‐regulation, which were found to be related to growth mindset (Burnette et al., 2013). Taken together, our findings provide novel evidence for the underlying contributing mechanism of grit, in which growth mindset mediates the influence of DLPFC volume on grit.
However, growth mindset did not mediate the association between the right putamen and grit because growth mindset was not associated with the right putamen. One possible explanation for these results is that growth mindset might mainly rely on psychological processes related to self‐regulation but not to motivation (Burnette et al., 2013; Moser et al., 2011; Schroder et al., 2017). For instance, evidence from a neuroimaging study showed that growth mindset was associated with activities in the PFC (Mangels et al., 2006), which is considered the neural center of self‐regulation (Heatherton, 2011; Kelley et al., 2015). Moreover, given that this study used only rGMV as the measure of brain structure, future investigations using other measures of brain function (e.g., task‐based fMRI and RS‐fMRI) and structure (e.g., cortical surface area and cortical thickness) are necessary to validate our findings.
Several limitations of this research should be noted and considered in future investigations. First, the levels of grit and mindset were evaluated using self‐reported scales that are vulnerable to response bias, although they were selected for their adequate validity and reliability. Future researchers should consider using multiple methods to assess these constructs, which may reduce the measurement errors and improve psychometric properties. Second, the participants in our study included a group of healthy adolescent students within a narrow age range. Although our method had the advantage of obtaining sufficient statistical power for whole‐brain analyses, it may limit the generalizability of the findings. Future studies are necessary to extend our study to include more diverse populations, such as children, adults and individuals with mental disorders. Third, given the cross‐sectional design used in the current study, we could not determine the direction of causality regarding the associations among growth mindset, grit and brain structure. The findings of the mediating model used in this study suggested that experimentally modulating the left DLPFC (e.g., rTMS; Figner et al., 2010) might change growth mindset and further improve grit. However, in our dataset, left DLPFC volume could also mediate the influence of growth mindset on grit (indirect effect = 0.045, 95% CI = [0.02, 0.09], p < .05), even after controlling for age, gender, total GMV, and general intelligence. This result indicated that experimentally modulating growth mindset (e.g., behavioral interventions of growth mindset; Yeager et al., 2016) might change the brain and grit. Thus, future studies with longitudinal or experimental designs are needed to explore the causal direction of the associations among growth mindset, grit, and brain structure.
In conclusion, this study provides evidence indicating that higher levels of grit are linked with smaller rGMV in the left DLPFC and greater rGMV in the right putamen, which sheds light on the underlying structural brain basis of grit. Furthermore, growth mindset mediates the influence of left DLPFC volume on grit, revealing a potential cultivating mechanism for grit. Finally, our study might have educational implications, as we provide potential behavioral and neural markers that can be used by education experts to develop corresponding curriculums and training programs to improve grit levels in adolescent students. Our study may also provide the evidence to the developing psychoradiology (https://radiopaedia.org/articles/psychoradiology), a new field of radiology aiming to understand the neural mechanism of neurocogntive dysfunction in the patients with psychiatric disorder (Kressel, 2017; Lui et al., 2016).
CONFLICT OF INTEREST
The authors declare that they have no competing interests with the content of this article.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation (Grant Nos. 81621003, 81220108013, 81227002, 81030027 and 81401398), the National Key Technologies R&D Program (Program No. 2012BAI01B03) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of China (Grant No. IRT16R52). Dr Gong would also like to acknowledge the support from his Changjiang Scholar Professorship Award of China (Award No. T2014190) and the American CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education, USA.
Wang S, Dai J, Li J, et al. Neuroanatomical correlates of grit: Growth mindset mediates the association between gray matter structure and trait grit in late adolescence. Hum Brain Mapp. 2018;39:1688–1699. 10.1002/hbm.23944
Funding information National Natural Science Foundation, Grant/Award Numbers: 81401398, 81621003, 81220108013, 81227002, 81030027; National Key Technologies R&D Program, Grant/Award Number: 2012BAI01B03; Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of China, Grant/Award Number: IRT1272; Changjiang Scholar Professorship Award of China, Grant/Award Number: T2014190; Institute of International Education, USA, Grant/Award Number: F510000/G16916411
REFERENCES
- Asahi, S. , Okamoto, Y. , Okada, G. , Yamawaki, S. , & Yokota, N. (2004). Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neurosciences, 254, 245–251. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2000). Voxel‐based morphometry ‐ The methods. NeuroImage, 11, 805–821. [DOI] [PubMed] [Google Scholar]
- Baron, R. M. , & Kenny, D. A. (1986). The moderator‐mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Basten, U. , Hilger, K. , & Fiebach, C. J. (2015). Where smart brains are different: A quantitative meta‐analysis of functional and structural brain imaging studies on intelligence. Intelligence, 51, 10–27. [Google Scholar]
- Bazelais, P. , Lemay, D. J. , & Doleck, T. (2016). How does grit impact college students’ academic achievement in science? European Journal of Science and Mathematics Education, 4, 33–43. [Google Scholar]
- Biswal, B. B. (2012). Resting state fMRI: A personal history. NeuroImage, 62, 938–944. [DOI] [PubMed] [Google Scholar]
- Blakemore, S. J. , & Robbins, T. W. (2012). Decision‐making in the adolescent brain. Nature Neuroscience, 15, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Bowman, N. A. , Hill, P. L. , Denson, N. , & Bronkema, R. (2015). Keep on truckin' or stay the course? Exploring grit dimensions as differential predictors of educational achievement, satisfaction, and intentions. Social Psychological and Personality Science, 6, 639–645. [Google Scholar]
- Burnette, J. L. , O'boyle, E. H. , VanEpps, E. M. , Pollack, J. M. , & Finkel, E. J. (2013). Mind‐sets matter: A meta‐analytic review of implicit theories and self‐regulation. Psychological Bulletin, 139, 655–701. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Beaty, R. E. , Wei, D. , Yang, J. , Sun, J. , Liu, W. , … Qiu, J. (2016). Longitudinal alterations of frontoparietal and frontotemporal networks predict future creative cognitive ability. Cerebral Cortex (New York, N.Y. : 1991), 10.1093/cercor/bhw353. [DOI] [PubMed] [Google Scholar]
- Cho, S. S. , Pellecchia, G. , Aminian, K. , Ray, N. , Segura, B. , Obeso, I. , & Strafella, A. P. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell, H. C. , & Schultz, W. (2003). Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. Journal of Neurophysiology, 89, 2823–2838. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Hirsh, J. B. , Shane, M. S. , Papademetris, X. , Rajeevan, N. , & Gray, J. R. (2010). Testing predictions from personality neuroscience: Brain structure and the big five. Psychological Science, 21, 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMenichi, B. C. , & Richmond, L. L. (2015). Reflecting on past failures leads to increased perseverance and sustained attention. Journal of Cognitive Psychology, 27, 180–193. [Google Scholar]
- Drobetz, R. , Hanggi, J. , Maercker, A. , Kaufmann, K. , Jancke, L. , & Forstmeier, S. (2014). Structural brain correlates of delay of gratification in the elderly. Behavioral Neuroscience, 128, 134–145. [DOI] [PubMed] [Google Scholar]
- Duckworth, A. , & Gross, J. J. (2014). Self‐control and grit: Related but separable determinants of success. Current Directions in Psychological Science, 23, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth, A. L. (2013). True grit. The Observer, 26, 1–3. [Google Scholar]
- Duckworth, A. L. , Kirby, T. A. , Tsukayama, E. , Berstein, H. , & Ericsson, K. A. (2011). Deliberate practice spells success: Why grittier competitors triumph at the national spelling bee. Social Psychological and Personality Science, 2, 174–181. [Google Scholar]
- Duckworth, A. L. , Peterson, C. , Matthews, M. D. , & Kelly, D. R. (2007). Grit: Perseverance and passion for long‐term goals. Journal of Personality and Social Psychology, 92, 1087–1101. [DOI] [PubMed] [Google Scholar]
- Duckworth, A. L. , & Quinn, P. D. (2009). Development and validation of the short grit scale (Grit‐S). Journal of Personality Assessment, 91, 166–174. [DOI] [PubMed] [Google Scholar]
- Duckworth, A. L. , Quinn, P. D. , & Seligman, M. E. P. (2009). Positive predictors of teacher effectiveness. Journal of Positive Psychology, 4, 540–547. [Google Scholar]
- Duckworth, A. L. , & Seligman, M. E. P. (2005). Self‐discipline outdoes IQ in predicting academic performance of adolescents. Psychological Science, 16, 939–944. [DOI] [PubMed] [Google Scholar]
- Dweck, C. S. (2000). Self‐theories: Their role in motivation, personality, and development. Philadelphia: Psychology Press. [Google Scholar]
- Dweck, C. S. , & Leggett, E. L. (1988). A social cognitive approach to motivation and personality. Psychological Review, 95, 256–273. [Google Scholar]
- Eskreis‐Winkler, L. , Gross, J. J. , & Duckworth, A. L. (2016). Grit: Sustained self‐regulation in the service of superordinate goals In Vohs K. D. & Baumeister R. F. (Eds.), Handbook of self‐regulation: Research, theory, and applications (pp. 380–395). New York, NY: Guilford Press. [Google Scholar]
- Eskreis‐Winkler, L. , Shulman, E. P. , Beal, S. A. , & Duckworth, A. L. (2014). The grit effect: Predicting retention in the military, the workplace, school and marriage. Frontiers in Psychology, 5, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner, B. , Knoch, D. , Johnson, E. J. , Krosch, A. R. , Lisanby, S. H. , Fehr, E. , & Weber, E. U. (2010). Lateral prefrontal cortex and self‐control in intertemporal choice. Nature Neuroscience, 13, 538–539. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Frijns, T. , Finkenauer, C. , Vermulst, A. A. , & Engels, R. C. M. E. (2005). Keeping secrets from parents: Longitudinal associations of secrecy in adolescence. Journal of Youth and Adolescence, 34, 137–148. [Google Scholar]
- Golkar, A. , Lonsdorf, T. B. , Olsson, A. , Lindstrom, K. M. , Berrebi, J. , Fransson, P. , … Ohman, A. (2012). Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One, 7, e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno, M. , & Kawato, M. (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus‐action‐reward association learning. Journal of Neurophysiology, 95, 948–959. [DOI] [PubMed] [Google Scholar]
- Hayasaka, S. , Phan, K. L. , Liberzon, I. , Worsley, K. J. , & Nichols, T. E. (2004). Nonstationary cluster‐size inference with random field and permutation methods. NeuroImage, 22, 676–687. [DOI] [PubMed] [Google Scholar]
- Heatherton, T. F. (2011). Neuroscience of self and self‐regulation. Annual Review of Psychology, 62, 363–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, P. L. , Burrow, A. L. , & Bronk, K. C. (2016). Persevering with positivity and purpose: An examination of purpose commitment and positive affect as predictors of grit. Journal of Happiness Studies, 17, 257–269. [Google Scholar]
- Hong, Y. Y. , Chiu, C. Y. , Dweck, C. S. , Lin, D. M. S. , & Wan, W. (1999). Implicit theories, attributions, and coping: A meaning system approach. Journal of Personality and Social Psychology, 77, 588–599. [Google Scholar]
- Kaller, C. P. , Rahm, B. , Spreer, J. , Weiller, C. , & Unterrainer, J. M. (2011). Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cerebral Cortex (New York, N.Y. : 1991), 21, 307–317. [DOI] [PubMed] [Google Scholar]
- Kanai, R. , & Rees, G. (2011). The structural basis of inter‐individual differences in human behaviour and cognition. Nature Reviews Neuroscience, 12, 231–242. [DOI] [PubMed] [Google Scholar]
- Kanske, P. , Heissler, J. , Schonfelder, S. , Bongers, A. , & Wessa, M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex (New York, N.Y. : 1991), 21, 1379–1388. [DOI] [PubMed] [Google Scholar]
- Kelley, W. M. , Wagner, D. D. , & Heatherton, T. F. (2015). In search of a human self‐regulation system. Annual Review of Neuroscience, 38, 389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D. R. , Matthews, M. D. , & Bartone, P. T. (2014). Grit and hardiness as predictors of performance among west point cadets. Military Psychology, 26, 327–342. [Google Scholar]
- Kench, D. , Hazelhurst, S. , & Otulaja, F. (2016). Grit and growth mindset among high school students in a computer programming project: A mixed methods study In Gruner S. (Ed.), ICT education. SACLA. Communications in computer and information science (Vol. 642, pp. 187–194). Springer. [Google Scholar]
- Kirby, K. N. (1997). Bidding on the future: Evidence against normative discounting of delayed rewards. Journal of Experimental Psychology: General, 126, 54–70. [Google Scholar]
- Kirby, K. N. , Petry, N. M. , & Bickel, W. K. (1999). Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. Journal of Experimental Psychology: General, 128, 78–87. [DOI] [PubMed] [Google Scholar]
- Kong, F. , Chen, Z. C. , Xue, S. , Wang, X. , & Liu, J. (2015a). Mother's but not father's education predicts general fluid intelligence in emerging adulthood: Behavioral and neuroanatomical evidence. Human Brain Mapping, 36, 4582–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, F. , Hu, S. Y. , Xue, S. , Song, Y. Y. , & Liu, J. (2015b). Extraversion mediates the relationship between structural variations in the dorsolateral prefrontal cortex and social well‐being. NeuroImage, 105, 269–275. [DOI] [PubMed] [Google Scholar]
- Kong, F. , Zhen, Z. L. , Li, J. G. , Huang, L. J. , Wang, X. , Song, Y. Y. , & Liu, J. (2014). Sex‐related neuroanatomical basis of emotion regulation ability. PLoS One, 9, e97071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad, K. , Firk, C. , & Uhlhaas, P. J. (2013). Brain development during adolescence: Neuroscientific insights into this developmental period. Deutsches Arzteblatt International, 110, 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressel, H. Y. (2017). Setting sail: 2017. Radiology, 282, 4–6. [DOI] [PubMed] [Google Scholar]
- Kunisato, Y. , Okamoto, Y. , Okada, G. , Aoyama, S. , Nishiyama, Y. , Onoda, K. , & Yamawaki, S. (2011). Personality traits and the amplitude of spontaneous low‐frequency oscillations during resting state. Neuroscience Letters, 492, 109–113. [DOI] [PubMed] [Google Scholar]
- Li, H. J. , Li, W. F. , Wei, D. T. , Chen, Q. L. , Jackson, T. , Zhang, Q. L. , & Qiu, J. (2014a). Examining brain structures associated with perceived stress in a large sample of young adults via voxel‐based morphometry. NeuroImage, 92, 1–7. [DOI] [PubMed] [Google Scholar]
- Li, H. J. , (2014b). Neuroanatomical differences between men and women in help‐seeking coping strategy. Scientific Reports‐UK, 4, 5700 Sun, J. Z. , Zhang, Q. L. , Wei, D. T. , Li, W. F. , Jackson, T. , Hitchman, G. , & Qiu, J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Zhao, Y. , Kong, F. , Du, S. , Yang, S. , & Wang, S. (2016a). Psychometric assessment of the short grit scale among Chinese adolescents. Journal of Psychoeducational Assessment, 10.1177/0734282916674858. [DOI] [Google Scholar]
- Li, J. , Zhao, Y. , Lin, L. , Chen, J. , & Wang, S. (2018). The freedom to persist: Belief in free will predicts perseverance for long‐term goals among Chinese adolescents. Personality & Individual Differences, 121, 7–10. [Google Scholar]
- Li, W. F. , Li, X. T. , Huang, L. J. , Kong, X. Z. , Yang, W. J. , Wei, D. T. , … Liu, J. (2015a). Brain structure links trait creativity to openness to experience. Social Cognitive and Affective Neuroscience, 10, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. W. , Li, Y. D. , Yang, W. J. , Zhang, Q. L. , Wei, D. T. , Li, W. F. , … Qiu, J. (2015b). Brain structures and functional connectivity associated with individual differences in Internet tendency in healthy young adults. Neuropsychologia, 70, 134–144. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Shi, Y. F. , Parker, G. J. , Huang, J. , Yan, C. , Lui, S. S. , … Chan, R. C. (2016b). Devaluation of rewards for the future is associated with schizotypal personality features. Australian Psychologist, 51, 481–489. [Google Scholar]
- Liljeholm, M. , & O'doherty, J. P. (2012). Contributions of the striatum to learning, motivation, and performance: An associative account. Trends in Cognitive Sciences, 16, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. L. , Chen, X. J. , Cui, J. F. , Wang, J. , Zhang, Y. B. , Neumann, D. L. , … Chan, R. C. K. (2016). Age differences in delay discounting in Chinese adults. Personality & Individual Differences, 90, 205–209. [Google Scholar]
- Lui, S. , Zhou, X. J. , Sweeney, J. A. , & Gong, Q. (2016). Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology, 281, 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, A. W. , Cohen, J. D. , Stenger, V. A. , & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Maddi, S. R. , Matthews, M. D. , Kelly, D. R. , Villarreal, B. , & White, M. (2012). The role of hardiness and grit in predicting performance and retention of USMA cadets. Military Psychology, 24, 19–28. [Google Scholar]
- Mangels, J. A. , Butterfield, B. , Lamb, J. , Good, C. , & Dweck, C. S. (2006). Why do beliefs about intelligence influence learning success? A social cognitive neuroscience model. Social Cognitive and Affective Neuroscience, 1, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar, R. A. , Spreng, R. N. , & DeYoung, C. G. (2013). How to produce personality neuroscience research with high statistical power and low additional cost. Cognitive, Affective, & Behavioral Neuroscience, 13, 674–685. [DOI] [PubMed] [Google Scholar]
- Marcoulides, G. A. , & Hershberger, S. L. (1997). Multivariate statistical methods: A first course. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Mazur, J. E. (1987). An adjusting procedure for studying delayed reinforcement In. Commons M. L., Mazur J. E., Nevin J. A., & Rachlin H. (Eds.), Quantitative analyses of behavior. The effect of delay and of intervening events on reinforcement value (Vol. 5, pp. 55–73). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Mcbee, G. , & Duke, R. L. (1960). Relationship between intelligence, scholastic motivation, and academic‐achievement. Psychological Reports, 6, 3–8. [Google Scholar]
- Mechelli, A. , Price, C. J. , Friston, K. J. , & Ashburner, J. (2005). Voxel‐based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews, 1, 105–113. [Google Scholar]
- Mizuno, K. , Tanaka, M. , Ishii, A. , Tanabe, H. C. , Onoe, H. , Sadato, N. , & Watanabe, Y. (2008). The neural basis of academic achievement motivation. NeuroImage, 42, 369–378. [DOI] [PubMed] [Google Scholar]
- Moser, J. S. , Schroder, H. S. , Heeter, C. , Moran, T. P. , & Lee, Y. H. (2011). Mind your errors: Evidence for a neural mechanism linking growth mind‐set to adaptive posterror adjustments. Psychological Science, 22, 1484–1489. [DOI] [PubMed] [Google Scholar]
- Myers, C. A. , Wang, C. , Black, J. M. , Bugescu, N. , & Hoeft, F. (2016). The matter of motivation: Striatal resting‐state connectivity is dissociable between grit and growth mindset. Social Cognitive and Affective Neuroscience, 11, 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser, U. , Boodoo, G. , Bouchard, T. J. , Boykin, A. W. , Brody, N. , Ceci, S. J. , … Urbina, S. (1996). Intelligence: Knowns and unknowns. American Psychology, 51, 77–101. [Google Scholar]
- Nemmi, F. , Nymberg, C. , Helander, E. , & Klingberg, T. (2016). Grit is associated with structure of nucleus accumbens and gains in cognitive training. J Cognitive. Journal of Cognitive Neuroscience, 28, 1688–1699. [DOI] [PubMed] [Google Scholar]
- Nichols, T. , & Hayasaka, S. (2003). Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research, 12, 419–446. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. P. , Dayan, P. , Friston, K. J. , Critchley, H. , & Dolan, R. J. (2003). Temporal difference models and reward‐related learning in the human brain. Neuron, 38, 329–337. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. P. , Deichmann, R. , Critchley, H. D. , & Dolan, R. J. (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33, 815–826. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Perera, H. N. , & DiGiacomo, M. (2013). The relationship of trait emotional intelligence with academic performance: A meta‐analytic review. Learning and Individual Differences, 28, 20–33. [Google Scholar]
- Peters, J. , & Büchel, C. (2011). The neural mechanisms of inter‐temporal decision‐making: Understanding variability. Trends in Cognitive Sciences, 15, 227–239. [DOI] [PubMed] [Google Scholar]
- Poline, J. B. , Worsley, K. J. , Evans, A. C. , & Friston, K. J. (1997). Combining spatial extent and peak intensity to test for activations in functional imaging. NeuroImage, 5, 83–96. [DOI] [PubMed] [Google Scholar]
- Poropat, A. E. (2009). A meta‐analysis of the five‐factor model of personality and academic performance. Psychological Bulletin, 135, 322–338. [DOI] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Qin, S. Z. , Young, C. B. , Duan, X. J. , Chen, T. W. , Supekar, K. , & Menon, V. (2014). Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biological Psychiatry, 75, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. (2000). The Raven's Progressive Matrices: Change and stability over culture and time. Cognitive Psychology, 41, 1–48. [DOI] [PubMed] [Google Scholar]
- Reed, J. , Pritschet, B. L. , & Cutton, D. M. (2013). Grit, conscientiousness, and the transtheoretical model of change for exercise behavior. Journal of Health Psychology, 18, 612–619. [DOI] [PubMed] [Google Scholar]
- Richardson, M. , Abraham, C. , & Bond, R. (2012). Psychological correlates of university students' academic performance: A systematic review and meta‐analysis. Psychological Bulletin, 138, 353–387. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. , van den Wildenberg, W. P. M. , Segalowitz, S. J. , & Carter, C. S. (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward‐based learning. Brain and Cognition, 56, 129–140. [DOI] [PubMed] [Google Scholar]
- Scheres, A. , de Water, E. , & Mies, G. W. (2013). The neural correlates of temporal reward discounting. Wiley Interdisciplinary Reviews. Cognitive Science, 4, 523–545. [DOI] [PubMed] [Google Scholar]
- Schilling, C. , Kuhn, S. , Paus, T. , Romanowski, A. , Banaschewski, T. , Barbot, A. , … Consortium, I. (2013). Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Molecular Psychiatry, 18, 624–630. [DOI] [PubMed] [Google Scholar]
- Schilling, C. , Kuhn, S. , Romanowski, A. , Schubert, F. , Kathmann, N. , & Gallinat, J. (2012). Cortical thickness correlates with impulsiveness in healthy adults. NeuroImage, 59, 824–830. [DOI] [PubMed] [Google Scholar]
- Schroder, H. S. , Fisher, M. E. , Lin, Y. , Lo, S. L. , Danovitch, J. H. , & Moser, J. S. (2017). Neural evidence for enhanced attention to mistakes among school‐aged children with a growth mindset. Developmental Cognitive Neuroscience, 24, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy, D. (2011). Learning and motivation in the human striatum. Current Opinion in Neurobiology, 21, 408–414. [DOI] [PubMed] [Google Scholar]
- Stevens, M. C. , Kiehl, K. A. , Pearlson, G. D. , & Calhoun, V. D. (2009). Brain network dynamics during error commission. Human Brain Mapping, 30, 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar, K. , Swigart, A. G. , Tenison, C. , Jolles, D. D. , Rosenberg‐Lee, M. , Fuchs, L. , & Menon, V. (2013). Neural predictors of individual differences in response to math tutoring in primary‐grade school children. Proceedings of the National Academy of Sciences of the United States of America, 110, 8230–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, Y. , Tamesue, D. , Asahi, K. , & Ishikawa, Y. (2015). Grit and work engagement: A cross‐sectional study. PLoS One, 10, e0137501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Hashizume, H. , Asano, K. , Asano, M. , Sassa, Y. , … Kawashima, R. (2015a). The impact of television viewing on brain structures: Cross‐sectional and longitudinal analyses. Cerebral Cortex (New York, N.Y. : 1991), 25, 1188–1197. [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Nouchi, R. , Sekiguchi, A. , Kotozaki, Y. , Miyauchi, C. M. , … Kawashima, R. (2014). Regional gray matter density is associated with achievement motivation: Evidence from voxel‐based morphometry. Brain Structure and Function, 219, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , Taki, Y. , Sekiguchi, A. , Hashizume, H. , Nouchi, R. , Sassa, Y. , … Kawashima, R. (2015b). Mean diffusivity of globus pallidus associated with verbal creativity measured by divergent thinking and creativity‐related temperaments in young healthy adults. Human Brain Mapping, 36, 1808–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney, J. P. , Baumeister, R. F. , & Boone, A. L. (2004). High self‐control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality, 72, 271–324. [DOI] [PubMed] [Google Scholar]
- Tanji, J. , Shima, K. , & Mushiake, H. (2007). Concept‐based behavioral planning and the lateral prefrontal cortex. Trends in Cognitive Sciences, 11, 528–534. [DOI] [PubMed] [Google Scholar]
- Tucker‐Drob, E. M. , Briley, D. A. , Engelhardt, L. E. , Mann, F. D. , & Harden, K. P. (2016). Genetically‐mediated associations between measures of childhood character and academic achievement. Journal of Personality and Social Psychology, 111, 790–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , & Ng, F. F. Y. (2012). Chinese students' implicit theories of intelligence and school performance: Implications for their approach to schoolwork. Personality & Individual Differences, 52, 930–935. [Google Scholar]
- Wang, S. , Kong, F. , Zhou, M. , Chen, T. , Yang, X. , Chen, G. , & Gong, Q. (2017a). Brain structure linking delay discounting and academic performance. Human Brain Mapping, 38, 3917–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Xu, X. , Zhou, M. , Chen, T. , Yang, X. , Chen, G. , & Gong, Q. (2017b). Hope and the brain: Trait hope mediates the protective role of medial orbitofrontal cortex spontaneous activity against anxiety. NeuroImage, 157, 439–447. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Zhou, M. , Chen, T. L. , Yang, X. , Chen, G. X. , & Gong, Q. Y. (2017c). Delay discounting is associated with the fractional amplitude of low‐frequency fluctuations and resting‐state functional connectivity in late adolescence. Scientific Reports‐UK, 7, 10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Zhou, M. , Chen, T. L. , Yang, X. , Chen, G. X. , Wang, M. Y. , & Gong, Q. Y. (2017d). Examining gray matter structure associated with academic performance in a large sample of Chinese high school students. Scientific Reports‐UK, 7, 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Zhou, M. , Chen, T. L. , Yang, X. , Chen, G. X. , Wang, M. Y. , & Gong, Q. Y. (2017e). Grit and the brain: Spontaneous activity of the dorsomedial prefrontal cortex mediates the relationship between the trait grit and academic performance. Social Cognitive and Affective Neuroscience, 12, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, M. R. , Kraft, M. A. , Finn, A. S. , Martin, R. E. , Duckworth, A. L. , Gabrieli, C. F. O. , & Gabrieli, J. D. E. (2016). Promise and paradox: Measuring students' non‐cognitive skills and the impact of schooling. Educational Evaluation and Policy Analysis, 38, 148–170. [Google Scholar]
- Yamasue, H. , Abe, O. , Suga, M. , Yamada, H. , Rogers, M. A. , Aoki, S. , … Kasai, K. (2008). Sex‐linked neuroanatomical basis of human altruistic cooperativeness. Cerebral Cortex (New York, N.Y. : 1991), 18, 2331–2340. [DOI] [PubMed] [Google Scholar]
- Yang, W. J. , Liu, P. D. , Wei, D. T. , Li, W. F. , Hitchman, G. , Li, X. P. , … Zhang, Q. L. (2014). Females and males rely on different cortical regions in Raven's matrices reasoning capacity: Evidence from a voxel‐based morphometry study. PLoS One, 9, e93104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager, D. S. , Romero, C. , Paunesku, D. , Hulleman, C. S. , Schneider, B. , Hinojosa, C. , … Dweck, C. S. (2016). Using design thinking to improve psychological interventions: The case of the growth mindset during the transition to high school. Journal of Educational Psychology, 108, 374–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki, S. , & Romaya, J. P. (2008). Neural correlates of hate. PLoS One, 3, e3556. [DOI] [PMC free article] [PubMed] [Google Scholar]


