Abstract
Post‐traumatic stress disorder (PTSD) is a debilitating condition which can develop after exposure to traumatic stressors. Seventy‐five adults were recruited from the community, 25 diagnosed with PTSD along with 25 healthy and 25 trauma‐exposed age‐ and gender‐matched controls. Participants underwent clinical assessment and magnetic resonance imaging. A previous voxel based morphometry (VBM) study using the same subject cohort identified decreased grey matter (GM) volumes within frontal/subcortical brain regions including the hippocampus, amygdala, and anterior cingulate cortex (ACC). This study examines the microstructural integrity of white matter (WM) tracts connecting the aforementioned regions/structures. Using diffusion tensor imaging, we investigated the integrity of frontal/subcortical WM tracts between all three subject groups. Trauma exposed subjects with and without PTSD diagnosis were identified to have significant disruption in WM integrity as indexed by decreased fractional anisotropy (FA) in the uncinate fasciculus (UF), cingulum cingulate gyrus (CCG), and corpus callosum (CC), when compared with healthy non‐trauma‐exposed controls. Significant negative correlations were found between total Clinician Administered PTSD scale (CAPS) lifetime clinical subscores and FA values of PTSD subjects in the right UF, CCG, CC body, and right superior longitudinal fasciculus (SLF). An analysis between UF and SLF FA values and VBM determined rostral ACC GM values found a negative correlation in PTSD subjects. Findings suggest that compromised WM integrity in important tracts connecting limbic structures such as the amygdala to frontal regions including the ACC (i.e., the UF and CCG) may contribute to impairments in threat/fear processing associated with PTSD.
Keywords: DTI, MRI, post‐traumatic stress disorder, PTSD, TBSS, white matter
1. INTRODUCTION
For some individuals, the experience of traumatic events can trigger the development of post‐traumatic stress disorder (PTSD). Symptoms include intrusive recollections, avoidance of stimuli related to trauma, withdrawal from social networks, and hyperarousal (American Psychiatric Association, 2013). People suffering from PTSD demonstrate reduced capacity to inhibit fear and negative emotional responses. They may also exhibit heightened responses to stimuli perceived as threatening, and subsequent inability to extinguish fear (Garfinkel and Liberzon, 2009). Some individuals are afflicted with a dissociative subtype of PTSD. In addition to neurological and cognitive impairments, these individuals may also experience depersonalization, derealization, and a sense of disconnection from their self or environment (Wolf et al., 2012). The debilitating symptoms of PTSD can have long‐term, psychological, physical, and cognitive effects that adversely affect sufferer's quality of life (Bremner et al., 1993; Yehuda et al., 1995). In Western society, PTSD has a lifetime prevalence of 1.9%–6.8% (Australian Bureau of Statistics, 2007; Kessler et al., 2005). War veterans are more susceptible to PTSD, with a lifetime prevalence of 19%–22% (Dohrenwend et al., 2006; Seal et al., 2009). However, the majority of people suffering from PTSD are civilians who have experienced or witnessed distressing incidences arising from violence and trauma, for example, physical/sexual assault, motor vehicle accident.
As a relatively recent complement to the suite of neuroimaging modalities investigating PTSD, diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique that can be used to assess microstructural abnormalities across white matter (WM) tracts in a range of psychiatric disorders such as depression, schizophrenia, ADHD, and PTSD (Bessette, Nave, Caprihan, & Stevens, 2014; Manoach et al., 2007; Ota et al., 2015; Pavuluri et al., 2009). WM tracts, such as the uncinate fasciculus, cingulum bundle, and superior longitudinal fasciculus, comprise the main paths of communication between cortical and subcortical structures (Schmahmann and Pandya, 2009). Impairment of these structures, whether through injury or disease, can adversely affect cognition and behavior and may reveal vulnerabilities/existing susceptibility that could lead to PTSD symptom development. Estimation of fractional anisotropy (FA) is the most common method of analyzing DTI data, whereby the directionality of water molecules diffusion is quantified. FA is measured as an output value ranging between 0 (fully isotropic), and 1 (fully anisotropic). Reductions in FA values are indicative of decreased myelination, axon density and microstructural WM organization (Le Bihan et al., 2001; Pierpaoli and Basser, 1996). Additionally, radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD) are used as a descriptors of microstructural architecture integrity, and have been used to further identify neuropathology (Alexander, Lee, Lazar, & Field, 2007; Fox et al., 2012; Song et al., 2002).
Extant adult PTSD DTI studies have examined the microstructural integrity of WM tracts connecting the frontal and limbic regions, including the cingulum (Daniels, Lamke, Gaebler, Walter, & Scheel, 2013). However, these studies are of limited generalizability due to a lack of trauma‐exposed controls (TC), making it problematic to identify alternations to WM tracts caused by trauma versus PTSD diagnosed subjects. In a study of trauma survivors without PTSD, Chen and Shi (2011) reported lower FA values from the WM tracts adjacent to the hippocampus. Existing studies involving between group comparisons have yielded inconsistent findings with regards to FA values. Lower FA values in PTSD subjects were reported adjacent to the anterior cingulate cortex (ACC), prefrontal gyrus and posterior angular gyrus (Schuff et al., 2011), and also in the left rostral, subgenual, and dorsal aspects of the cingulate bundle in trauma exposed subjects versus healthy controls (HC) (Kim et al., 2006). In comparing PTSD and trauma exposed subjects who experienced comparable levels of trauma, Fani et al. (2012) reported significantly lower FA in the posterior cingulum bundle when compared to TC. A global brain comparison of PTSD versus HC by Kim et al. (2005) found lower FA values in the anterior cingulate gyrus and mesencephalon. Higher FA values from WM tracts in PTSD subjects have also been observed in the anterior cingulate bundle (Abe et al., 2006) and in the superior frontal gyrus, compared to HC (Zhang et al., 2011). In the current study we use tract‐based spatial statistics (TBSS) to determine and examine WM tract FA differences in PTSD diagnosed subjects compared to trauma exposed and non‐trauma‐exposed healthy controls. We hypothesize that FA reductions will be isolated to the cingulum cingulate gyrus and uncinate fasciculus WM tracts, connecting key grey matter structures associated with PTSD, specifically the hippocampus, amygdala, and ACC. We predict that more extensive WM tract FA deficits will be evident in PTSD diagnosed subjects compared to non‐trauma‐exposed healthy controls, than trauma exposed controls compared to non‐trauma‐exposed healthy controls. Further, we predict that FA, AD, RD, and MD measures of cingulum cingulate gyrus and uncinate fasciculus WM integrity will be correlated with PTSD symptom severity scores. Additionally, using results of a previous voxel based morphometry (VBM) investigation utilizing the identical subject cohort as the current study (for details see O'Doherty et al. (2017)), we hypothesize that FA values of the uncinate fasciculus WM tract will be correlated to rostral ACC (rACC) grey matter (GM) values, as determined by the results of a prior VBM analysis, in PTSD diagnosed subjects.
2. METHODS
2.1. Participant recruitment and clinical assessment
Seventy‐five participants, aged 18–50 years, were recruited from the general community through print and electronic media. Twenty‐five participants were diagnosed with post‐traumatic stress disorder (PTSD), 25 participants were healthy control subjects (HC), and 25 participants were trauma‐exposed controls subjects (TC).
A psychologically trained health professional conducted clinical assessments on the trauma‐exposed participants via two clinician administered scales: Clinician Administered PTSD scale (CAPS) (Weathers, Keane, & Davidson, 2001) and Structured Clinical Interview for DSM‐IV for comorbid disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2007); and two subject self‐report scales: Depression, Anxiety and Stress Scale (DASS) (Lovibond and Lovibond, 1995), and Impact of Event Scale ‐ Revised [IES‐R] (Weiss, 2007).
The study inclusion criteria were as follows: (a) Participants with PTSD met the DSM‐IV criteria for primary diagnosis of PTSD after a Criterion‐A traumatic event/stressor, not longer than 10 years previously and not less than three months prior to participating in the study; (b) TC participants had exposure to Criterion A trauma (not longer than 10 years previously and not less than three months prior to participating in the study), but did not have a psychiatric diagnosis or a history of psychiatric disorder including PTSD, (c) HC participants had no exposure to criterion‐A trauma and no diagnosis or history of psychiatric disorder including PTSD; (d) all participants were 18–50 years of age; (e) all participants were fluent in English language in order to enhance accuracy and validity of clinical diagnoses; and (f) all participants provided written informed consent.
The study exclusion criteria were as follows: (a) participants with significant psychiatric diagnosis other than PTSD, such as bipolar disorder, schizophrenia; (b) participants who were pregnant or breastfeeding; (c) participants who suffered significant medical or neurological condition including, but not limited to, congestive heart failure, stroke, hypertension, chronic liver disease, autoimmune or connective tissue disease, blood clotting disorder; (d) participants with a history of brain injury or concussion that resulted in loss of consciousness for a duration of more than 10 min; (e) participants with a history of/or current substance dependence; and (f) any participants who manifested contraindication to having an MRI scan such as metallic implants and claustrophobia.
2.2. MRI protocol
All imaging was conducted at the Brain and Mind Centre imaging facility using a 3 T GE Discovery MR750 scanner (GE Medical Systems, Milwaukee, WI) equipped with an eight‐channel phased array head coil (InVivo, Fl, US). Whole brain diffusion‐weighted images where acquired using an echo planar imaging sequence with the following image parameters: repetition time (TR) = 8250 ms; echo time (TE) = 88 ms; Slice thickness = 2.0 mm; field of view (FOV) = 230 × 230 mm; acquisition matrix =256 × 256; 69 gradient directions. Two images without gradient loading (b0 s/mm2) were acquired prior to the acquisition of 75 images (each containing 55 slices) with uniform gradient loading (b0 = 1000 s/mm2). For purposes of anatomical localization, T1‐weighted structural images were also acquired.
For each subject, two structural images were acquired in the same session using a T1‐weighted‐magnetization prepared rapid gradient‐echo (MP‐RAGE) sequence producing 196 sagittal slices (TR = 7.2 ms; TE = 2.8 ms; flip angle = 12°; matrix 256 × 256; 0.9 mm isotropic voxels).
2.3. VBM analysis
For each subject, two individual T1‐weighted MRI scans were combined and averaged using the FMRIB Software Library (FSL) software tool (Smith, 2002), to increase signal‐to‐noise ratio (SNR). An unbiased optimized VBM protocol using FSL‐VBM (v1.1) was then carried out using the following procedure. Firstly, FSL Brain Extraction Tool (BET) (Jenkinson et al., 2005) was applied to remove non‐brain material, before all T1‐weighted images were transformed into standard space using a limited degrees‐of‐freedom nonlinear model to ensure spatial alignment and images were corrected for nonuniformity/intensity inhomogeneities (Andersson, Jenkinson, & Smith, 2007). The FAST4 tool (Zhang, Brady, & Smith, 2001) was then applied to carry out tissue‐type segmentation. The segmented grey matter partial volume images were aligned into MNI standard space by applying the affine registration tool FLIRT (Greve and Fischl, 2009) and nonlinear registration FNIRT methods (Woolrich et al., 2009). A study‐specific averaged template was created, to which grey matter partial volume images were reregistered, and these images were then modulated to correct for Jacobian warping. Visual inspection was used to ensure the quality of brain image extraction, segmentation and registration for each structural image. Segmented images were smoothed using sigma = 3; Gaussian Full width at half maximum (FWHM) kernel of 7.06 mm. A customized randomizer and study‐blinding program allowed for unbiased assessment and the clean‐up of MRI data during VBM pipeline.
2.4. Tract‐based spatial statistics (TBSS)
Diffusion‐weighted images were analyzed using FMRIB Software Library (FSL) tract based spatial statistics (TBSS) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2006) via the following routine. Firstly, the FDT toolbox was used to correct all data for spurious eddy current distortions and motion artefacts by applying affine alignment of each diffusion‐weighted image to the first volume of the diffusion data without gradient (i.e., the b = 0 image). The Brain Extraction Tool (BET) was then used to generate a binary brain mask from the b = 0 image. Next DTIfit was used to independently fit the diffusion tensor to each voxel which yielded voxel‐wise maps of FA values. A mean FA skeleton image representative of all tracts with a common center was created from all subjects and individual subject FA images were projected onto this skeleton. Finally, voxel‐wise statistics across subjects were run for each point on the mean FA skeleton using permutation‐based nonparametric testing.
2.5. Statistical analysis
Whole‐brain permutation‐based nonparametric testing was carried out via a voxel‐wise GLM (Nichols and Holmes, 2002) using a 5,000 permutation set contrasting differences between each of the three participant groups (i.e., PTSD vs HC; TC vs HC; PTSD vs TC). Family‐wise error (FWE) correction (Nichols and Hayasaka, 2003) was used to adjust the threshold for multiple comparisons across space and threshold‐free cluster enhancement (TFCE) was employed to assess cluster significance (Smith and Nichols, 2009). A FWE corrected threshold significant p value of p < .05 was selected, with a significant cluster size minimum of 10 voxels. For reporting purposes, masks of significant regions of interest (ROI) were generated via the JHU (Johns Hopkins University) white‐matter tractography atlas (Desikan et al., 2006; Frazier et al., 2005; Goldstein et al., 2007; Makris et al., 2006). Transformation matrices used to create and register FA maps were applied to eigenvalues λ1, λ2, and λ3 to determine and generate WM skeletons for radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD). ROI masks of significant FA differences were applied to RD, AD, and MD skeletons to determine values for each subject. SPSS version 20.0 for Windows (SPSS, 2013) was used to perform comparisons between group demographic and clinical variables using one‐way ANOVA, Chi‐square and independent t test. A two‐tailed Pearson correlations analysis was performed to identify correlations between diffusivity values (FA, RD, AD, MD) of ROI WM tracts and symptom severity (as measured by CAPS clinical scores) and between ROI GM volumes obtained from prior study using identical subject cohort (O'Doherty et al., 2017).
3. RESULTS
3.1. Participant characteristics
The three participant groups—PTSD, TC, and HC—did not differ in terms of age (F(2, 72) = 2.44, p = .095), or years of education (F(2, 72) = 2.76, p = .07) and gender ratio. There were differences in DASS scores across all subject groups (F(2, 66) = 24.22, p < .001). PTSD and TC groups differed in terms of IES‐R scores t(48) = −5.30, p < .001, and CAPS scores; [week t(48) = −12.20, p < .001], [month t(35.4) = −8.38, p < .001], [lifetime t(32.9) = −7.93, p < .001] (Table 1), but did not differ in time since trauma (years) (t(48) = −0.493, p = .805). No participant diagnosed with PTSD reported above threshold depersonalization or derealization (Lanius et al., 2010). The types of trauma experienced by PTSD and TC groups are listed in Supporting Information, Table 1. The PTSD group consisted of a greater number of sexual assault survivors compared to the TC group (PTSD = 12, TC = 2). The TC group consisted of a greater number of witnesses to scenes of death or severe injury (PTSD = 12, TC = 18). Subjects in the PTSD group reported a greater use of psychotropic medications than TC subjects (Supporting Information, Table 2).
Table 1.
Demographic and clinical variables
| PTSD subjects (n = 25) | Trauma‐exposed controls (n = 25) | Healthy controls (n = 25) | Significance test (df) [p value] | |
|---|---|---|---|---|
| Gender (F/M) | 13/12 | 13/12 | 13/12 | χ2 (2) = 0.00 [1] |
| Age (years) | 34.0 ± 8.4 | 36.4 ± 8.1 | 31.7 ± 6.0 | F (2, 72) = 2.44 [.095] |
| Time since trauma (years) | 4.61 ± 2.57 | 4.26 ± 2.45 | ‐ | t (48) = −0.493 [.805] |
| Years of education | 16.16 ± 1.3 | 15.04 ± 1.5 | 15.44 ± 2.24 | F (2, 72) = 2.76 [.07] |
| DASS | 57.2 ± 32.6 | 16.2 ± 25.1 | 9.5 ± 10.3a | F (2, 66) = 24.22 [<.001] |
| CAPS | ||||
| Lifetime | 95.6 ± 25.9 | 13.0 ± 21.8 | ‐ | t (48) = −12.20 [<.001] |
| Month | 57.0 ± 26.8 | 6.7 ± 13.5 | ‐ | t (35.4) = −8.38 [<.001] |
| Week | 53.6 ± 27.6 | 5.8 ± 12.1 | ‐ | t (32.9) = −7.93 [<.001] |
| IES‐R | 45.1 ± 18.5 | 15.0 ± 21.6 | ‐ | t (48) = −5.30 [<.001] |
Note. Mean scores (±standard deviation) for age and DASS clinical score across groups.
CAPS/IES‐R group differences were tested using two‐tailed continuous data significant at p < .05.
DASS scores available for only 19 healthy control participants.
3.2. Imaging data
3.2.1. PTSD versus HC analysis
Individuals with PTSD had significantly decreased FA across bilateral anterior thalamic radiation, cingulum cingulate gyrus, superior longitudinal fasciculus, uncinate fasciculus, and in the corpus callosum body and genu. With the exception of the superior longitudinal fasciculus, larger voxel clusters of FA reduction were observed in left hemisphere tracts of the affected WM regions (Table 2 and Figure 1).
Table 2.
Reduced FA in PTSD versus healthy controls (whole‐brain TBSS analysis with FWE correction)
| Anatomic region | Hemisphere | MNI coordinates | Cluster size (voxels) | PTSD, mean ± SD | HC, mean ± SD | FWE corrected, p value (max) |
|---|---|---|---|---|---|---|
| Anterior thalamic radiation | Left | −18, 32, 28 | 393 | 0.3999 ± 0.0188 | 0.4370 ± 0.0224 | .044 |
| Anterior thalamic radiation | Right | 21, 32, 27 | 74 | 0.4397 ± 0.0300 | 0.4549 ± 0.0330 | .046 |
| Cingulum cingulate gyrus | Left | −8, 19, 24 | 229 | 0.6138 ± 0.0430 | 0.6563 ± 0.0323 | .036 |
| Cingulum cingulate gyrus | Right | 21, 30, 30 | 24 | 0.4348 0.0352 | 0.4696 0.0445 | .026 |
| Corpus Callosum Body | Midline | −5, 19, 17 | 669 | 0.7354 ± 0.0221 | 0.7629 ± 0.0187 | .026 |
| Corpus Callosum Genu | Midline | −3, 29, 9 | 895 | 0.7673 ± 0.0265 | 0.7962 ± 0.0188 | .017 |
| Superior longitudinal fasciculus | Left | −44, 3, 17 | 156 | 0.4706 ± 0.0221 | 0.5042 ± 0.0196 | .047 |
| Superior longitudinal fasciculus | Right | 46, −2, 24 | 488 | 0.4815 ± 0.0215 | 0.5106 ± 0.0176 | .043 |
| Uncinate fasciculus | Left | −25, 18, 14 | 664 | 0.4816 ± 0.0198 | 0.5128 ± 0.0188 | .035 |
| Uncinate fasciculus | Right | 15, 34, −10 | 202 | 0.5394 ± 0.0284 | 0.5698 ± 0.0268 | .044 |
Figure 1.
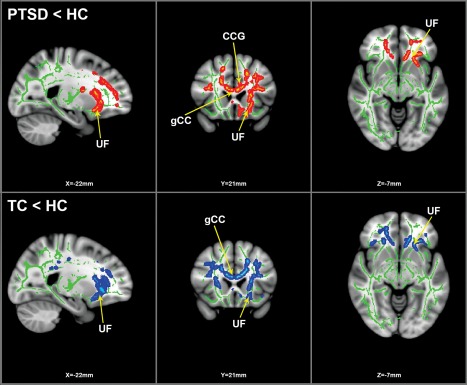
TBSS analysis results: group difference depicting FA reductions. Statistically significant clusters of FA reductions calculated using 25 (13F/12M) PTSD subjects, 25 (13F/12M) HC and 25 (13F/12M) TC. Top panel depicts PTSD versus HC whole‐brain analysis; red/orange indicates significant clusters of voxels (FWE corrected for multiple comparisons at p < .05, k > 10 voxels) with reduced FA values in PTSD compared to HC. Bottom panel depicts TC versus HC whole‐brain analysis; blue indicates significant clusters of voxels (FWE corrected, p < .05, k > 10 voxels) with reduced FA values in TC compared to HC. Green indicates FA skeleton. FA reduction clusters rendered on standard MNI152 structural template. Images are radiologically oriented. Abbreviations are as follows: HC = healthy controls; TC = trauma controls; FA = fractional anisotropy; CCG = cingulum cingulate gyrus; gCC = genu corpus callosum; UF = uncinate fasciculus
3.2.2. TC versus HC analysis
A whole‐brain TBSS analysis of the TC and HC groups found significant FA decreases in TC subjects across the same WM regions as the PTSD versus HC analysis, albeit with smaller cluster sizes and addition of the corpus callosum splenium. As with the PTSD versus HC analysis, FA reductions were found to have larger voxel clusters in left hemisphere tracts of the affected WM regions compared to the right hemisphere, with the only difference being that in this analysis the superior longitudinal fasciculus maintained this left hemisphere bias of FA reductions (Table 3 and Figure 1).
Table 3.
Reduced FA in trauma exposed controls versus healthy controls (whole‐brain TBSS analysis with FWE correction)
| Anatomic region | Hemisphere | MNI coordinates | Cluster size (voxels) | PTSD, mean ± SD | HC, mean ± SD | FWE corrected, p value (max) |
|---|---|---|---|---|---|---|
| Anterior thalamic radiation | Left | −26, 26, 16 | 219 | 0.4041 ± 0.0316 | 0.4411 ± 0.0231 | .017 |
| Anterior thalamic radiation | Right | 28, 30, 18 | 102 | 0.3659 ± 0.0277 | 0.3947 ± 0.0289 | .032 |
| Cingulum cingulate gyrus | Left | −18, −36, 33 | 58 | 0.5133 ± 0.0371 | 0.5465 ± 0.0292 | .035 |
| Cingulum cingulate gyrus | Right | 20, 30, 31 | 10 | 0.4711 ± 0.0562 | 0.4988 ± 0.0651 | .036 |
| Corpus Callosum Body | Midline | −12, 19, 22 | 1499 | 0.7146 ± 0.0282 | 0.7443 ± 0.0208 | .030 |
| Corpus Callosum Genu | Midline | −6, 28, −1 | 1160 | 0.7479 ± 0.0300 | 0.7811 ± 0.0177 | .008 |
| Corpus Callosum Splenium | Midline | −18, −41, 28 | 68 | 0.6691 ± 0.0264 | 0.6975 ± 0.0313 | .034 |
| Superior longitudinal fasciculus | Left | −34, 13, 12 | 221 | 0.4473 ± 0.0353 | 0.4877 ± 0.0259 | .015 |
| Superior longitudinal fasciculus | Right | 34, 19, 21 | 22 | 0.4665 ± 0.0433 | 0.5129 ± 0.0224 | .032 |
| Uncinate fasciculus | Left | −19, 22, −10 | 647 | 0.4902 ± 0.0228 | 0.5245 ± 0.0160 | .016 |
| Uncinate fasciculus | Right | 15, 34, −6 | 402 | 0.5000 ± 0.0277 | 0.5305 ± 0.0229 | .014 |
3.2.3. PTSD vs TC analysis
No significant differences in FA were observed in a whole‐brain TBSS analysis between the PTSD and TC groups after FWE corrections performed.
3.2.4. Correlational analysis
There was a strong negative correlation between CAPS lifetime hyperarousal scores and FA values of the PTSD subject group in the right uncinate fasciculus (r(23) = −.625, p = .001). A moderate negative correlation was also observed in the corpus callosum body (r(23) = −.438, p = .028), and right superior longitudinal fasciculus (r(23) = −.416, p = .039). A moderate negative correlation between CAPS lifetime avoidance scores and FA values of the PTSD subject group was found in the right cingulum cingulate gyrus (r(23) = −.471, p = .017) (Table 4 and Figure 2).
Table 4.
Correlations between FA values and CAPS subscores in PTSD subjects (n = 25)
| Anatomic region | Hemisphere | MNI coordinates | CAPS subscore | Cluster size (voxels) | Correlation | p value |
|---|---|---|---|---|---|---|
| Cingulum cingulate gyrus | Right | 21, 30, 30 | Lifetime avoidance | 24 | r(23) = −.471 | .017 |
| Corpus callosum body | Midline | −5, 19, 17 | Lifetime hyperarousal | 669 | r(23) = −.438 | .028 |
| Superior longitudinal fasciculus | Right | 46, −2, 24 | Lifetime hyperarousal | 488 | r(23) = −.416 | .039 |
| Uncinate fasciculus | Right | 15, 34, −10 | Lifetime hyperarousal | 202 | r(23) = −.625 | .001 |
Figure 2.
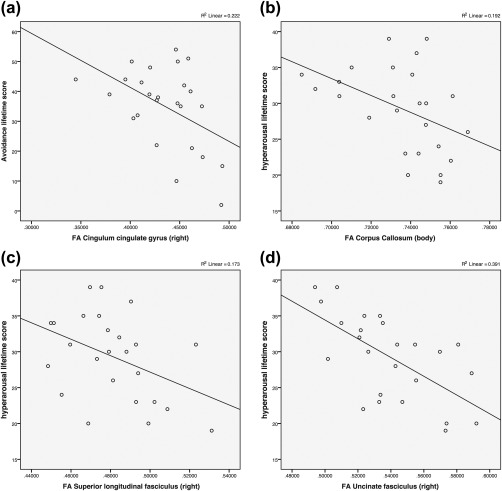
Correlations between CAPS subscores and fractional anisotropy (FA) values of the PTSD subject group. Scatterplots illustrating the association between CAPS subscore (a) lifetime avoidance and right cingulum cingulate gyrus FA (r(23) = −.471, p = .017); (b) lifetime hyperarousal and corpus callosum body FA r(23) = −.438, p = .028; (c) lifetime hyperarousal and superior longitudinal fasciculus FA (r(23) = −.416, p = .039); (d) lifetime hyperarousal and right uncinate fasciculus FA (r(23) = −.625, p = .001)
A strong positive correlation was found between CAPS lifetime hyperarousal scores and RD values of the PTSD subject group in the right uncinate fasciculus (r(23) = .739, p = .001). A moderate positive correlation between CAPS lifetime avoidance scores and RD values was found in the left uncinate fasciculus (r(23) = .458, p = .021) (see Supporting Information, Table 3 and Figure 1).
A correlational analysis of MD values found a strong association between CAPS lifetime hyperarousal scores and MD values of the PTSD subject group in the right uncinate fasciculus (r(23) = .709, p = .001) and a moderate positive correlation in the left uncinate fasciculus (r(23) = .410, p = .042).
Strong and moderate correlations between MD values and CAPS lifetime hyperarousal scores were also observed respectively in the right (r(23) = .643, p = .001), and left (r(23) = .488, p = .013) anterior thalamic radiation (Supporting Information, Table 4 and Figure 2).
A correlational analysis of AD values found a moderate association between CAPS lifetime hyperarousal scores and AD values of the PTSD subject group in the left anterior thalamic radiation (r(23) = .475, p = .016), and right anterior thalamic radiation (r(23) = .441, p = .027). A Strong correlation between AD values and CAPS lifetime hyperarousal scores was also observed in the left superior longitudinal fasciculus (r(23) = .488, p = .013) (Supporting Information, Table 5 and Figure 3).
An analysis of associations between FA values and ROI GM values in PTSD subjects found a moderate negative correlation between the left uncinate fasciculus and right rACC (r(23) = −.444, p = .026). A moderate negative correlation between the corpus callosum genu FA values and left rACC (r(23) = −.405, p = .045) was also observed, along with strong negative correlations between the left superior longitudinal fasciculus and left rACC (r(23) = −.651, p = .001) and right rACC (r(23) = −.640, p = .001) (Table 5 and Figure 3).
Table 5.
Correlations between FA values and ROI GM values in PTSD subjects (n = 25)
| Anatomic region | Hemisphere | MNI coordinates | GM ROI | Cluster size (voxels) | Correlation | p value |
|---|---|---|---|---|---|---|
| Corpus Callosum Genu | Midline | −3, 29, 9 | rACC left | 895 | r(23) = −.405 | .045 |
| Superior longitudinal fasciculus | Left | −44, 3, 17 | rACC left | 156 | r(23) = −.651 | .001 |
| Superior longitudinal fasciculus | Left | −44, 3, 17 | rACC right | 156 | r(23) = −.640 | .001 |
| Uncinate fasciculus | Left | −25, 18, 14 | rACC right | 664 | r(23) = −.444 | .026 |
Figure 3.
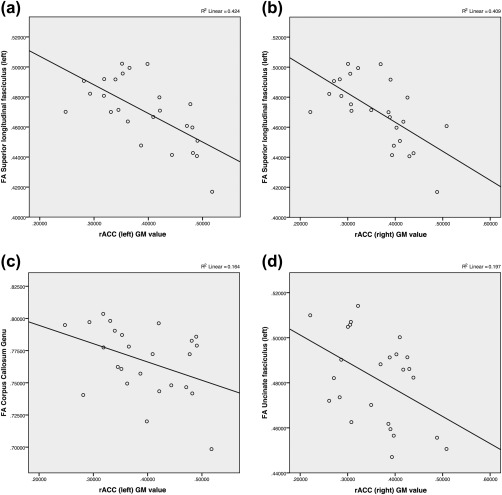
Correlations between fractional anisotropy (FA) values and rostral anterior cingulate cortex (rACC) grey matter (GM) values of the PTSD subject group. Scatterplots illustrating the association between FA values: (a) left superior longitudinal fasciculus and left rACC GM (r(23) = −.651, p = .001); (b) left superior longitudinal fasciculus and right rACC GM r(23) = −.640, p = .001; (c) corpus callosum genu and left rACC GM (r(23) = −.405, p = .045); (d) left uncinate fasciculus and right rACC GM (r(23) = −.444, p = .026)
Associations between RD values and ROI GM values in PTSD subjects found a strong positive correlation between the left uncinate fasciculus and right rACC (r(23) = .651, p = .001). A moderate positive correlation between the corpus callosum genu RD values and left rACC (r(23) = .480, p = .015) was also observed, along with moderate positive correlations between the left superior longitudinal fasciculus and left rACC (r(23) = .508, p = .010) and right rACC (r(23) = .549, p = .005) (Supporting Information, Table 6 and Figure 4).
Associations between MD values and ROI GM values in PTSD subjects revealed a strong positive correlation between the left uncinate fasciculus and right rACC (r(23) = .687, p = .001). Moderate positive correlations between the left uncinate fasciculus and left rACC (r(23) = .514, p = .009), and right uncinate fasciculus and right rACC (r(23) = .423, p = .035) were found. A strong positive correlation between the left cingulum cingulate gyrus and right rACC (r(23) = .619, p = .001) was also observed (Supporting Information, Table 7 and Figure 5).
Associations between AD values and ROI GM values in PTSD subjects revealed a strong positive correlation between the right uncinate fasciculus and right rACC (r(23) = .506, p = .010). Moderate positive correlations between the left uncinate fasciculus and left rACC (r(23) = .451, p = .024), and right uncinate fasciculus and right rACC (r(23) = .477, p = .016) were found. A strong positive correlation between the left cingulum cingulate gyrus and right rACC (r(23) = .619, p = .001) was also observed (Supporting Information, Table 8 and Figure 6).
4. DISCUSSION
This study sought to identify white matter microstructural changes between three groups of subjects; those exposed to trauma who subsequently meet the diagnostic criteria for PTSD, those exposed to trauma who did not meet the diagnostic criteria for PTSD, and healthy controls who were not exposed to trauma. To our knowledge, this is the first PTSD study to analyze multiple DTI scalars measures (FA, RD, AD, and MD) via a TBSS protocol applied to three groups of adult participants. WM tract scalar value results of PTSD diagnosed subjects were correlated to PTSD symptom severity scores (as measured by CAPS), and rACC GM ROI data from a previous study (O'Doherty et al., 2017), which utilized a VBM analysis protocol on the identical subject cohort examined in this study.
Significant reductions in FA values between the trauma exposed groups and healthy controls were observed in the body and genu of the corpus callosum along with tracts associated with fear conditioning, namely, the uncinate fasciculus and cingulum cingulate. Providing connection between limbic structures in the anterior temporal lobe and the frontal lobe (Ebeling and Cramon, 1992; Schmahmann and Pandya, 2009), the uncinate fasciculus is the primary tract coupling the amygdala and ACC. Reductions of FA values within these tracts were underpinned by increases in RD, AD and MD values. RD is representative of perpendicular diffusion across the WM tract, with increased RD being indicative of demyelination, while AD measures parallel diffusivity and has been shown to be sensitive to changes of axon numbers (Alexander et al., 2011; Freund et al., 2012; Klawiter et al., 2012). While decreased AD values are often considered a marker of axonal damage, this is not always the case. Increased AD values when observed with concordant increases in RD and decreased FA values, have been associated with WM microstructural abnormalities (Le Bihan et al., 2001; Song et al., 2002; Wheeler‐Kingshott & Cercignani, 2009). As a mean of all three diffusivity axes independent of directionality, MD is a useful complement to FA, RD, and AD tensor analyses (Vos, Jones, Jeurissen, Viergever, & Leemans, 2012).
Uncinate fasciculus FA reductions have previously been reported to be associated with increased anxiety symptoms and fear extinction dysfunction (Fani et al., 2015; Phan et al., 2009). The results of the analyses between both PTSD and TC compared to the HC group showed a reduction in uncinate fasciculus FA values, with larger cluster of reduction in the left uncinate. Previous DTI studies have found a negative correlation between FA values of the left uncinate fasciculus and symptoms such as anxiety, associative and episodic memory and social‐emotional function (Baur, Hänggi, & Jäncke, 2012; Von Der Heide, Skipper, Klobusicky, & Olson, 2013). These findings are supported by results from this study, which showed a strong negative correlation between CAPS lifetime hyperarousal scores and FA values within the PTSD subject group in the right uncinate fasciculus. Interestingly, while both PTSD and TC groups showed greater FA reductions in the left uncinate fasciculus compared to HC, the TC group had a larger cluster of FA reduction in the right uncinate fasciculus than the PTSD group.
Reductions were also found in the cingulum. As a prominent tract of the limbic lobe, diminished WM integrity of the cingulum has the potential to affect communication between the ACC and the hippocampus, regions which have demonstrated impaired functioning in PTSD (Hamner, Lorberbaum, & George, 1999; Liberzon and Martis, 2006; Shin, Rauch, & Pitman, 2006). The current study identified reductions in cingulum FA values of both PTSD and TC groups when compared to healthy controls, with greater reductions observed in the PTSD group. Further to this, a moderate negative correlation between CAPS lifetime avoidance scores and FA values of the PTSD subject group was found in the right cingulum cingulate gyrus r(23) = −.471, p = .017.
Functional neuroimaging studies have found heightened activation of the amygdala in response to trauma‐related stimuli, and also in fear‐conditioning acquisition in PTSD subjects (Brunetti et al., 2010; Rauch et al., 2000; Shin et al., 2006). In general, it is found that amygdala response is exaggerated in PTSD subjects, and is more pronounced when PTSD symptoms are more severe (Brunetti et al., 2010; Dickie, Brunet, Akerib, & Armony, 2008, 2011; Shin et al., 2006; Zhong et al., 2015). Modulation/extinction of the fear response is postulated to involve an inhibitory role of medial prefrontal structures, particularly the ACC, over the amygdala (Clausen et al., 2017; Shin et al., 2001; Stevens et al., 2016). In PTSD diagnosed subjects, correlational analyses from the current study identified associations between FA/RD/AD/MD values and PTSD symptoms (as measured by CAPS subscores) in WM tracts connecting the amygdala and ACC. A strong negative correlation between CAPS lifetime hyperarousal scores and FA values was found in the right uncinate fasciculus (with concordant strong positive RD/AD/MD correlations, see Supporting Information, Tables 3–5). Additionally, a moderate negative correlation between CAPS lifetime avoidance scores and FA values was found in the right cingulum cingulate gyrus. These associations along WM tracts connecting the ACC and amygdala support the suggestion that PTSD symptoms of increased arousal and avoidance are linked to dysfunction of prefrontal inhibition of amygdala fear‐conditioning acquisition.
This study's findings of reduced WM tract integrity and association with PTSD severity scores are congruous with results of the previous VBM study using the same subjects. We found bilateral GM reductions in the rACC of PTSD subjects compared to the HC group. Further to this, it also found a moderate negative correlation between the left rACC and right amygdala GM values (r(23)= −.449, p = .024) in PTSD diagnosed subjects. Using GM data from this previous study, a correlational analysis of rACC GM and FA values found a moderate negative association between left uncinate fasciculus FA and right rACC GM values in PTSD diagnosed subjects (r(23) = −.444, p = .026) (concordant moderate positive RD/AD/MD correlations, see Supporting Information, Tables 6–8).
Interhemispheric results of correlational analyses findings may be explained by reductions in WM integrity of the corpus callosum. Consisting of the brain's principle WM fiber bundle, the corpus callosum has projections along neocortical areas, providing interhemispheric connects for the prefrontal, temporal, and parietal cortices (Gazzaniga, 2000; Schmahmann and Pandya, 2009). Both PTSD and TC subjects in the current study had lower FA values compared to the HC group (Tables 2 and 3). This observed loss of WM integrity within the body and genu of the corpus callosum, together with bilateral reductions in the anterior thalamic radiation, cingulum cingulate gyrus, superior longitudinal fasciculus, and uncinate fasciculus would likely have an effect on interhemispheric information transfer across anatomical regions associated with PTSD. Previous studies of adult onset PTSD have found reductions in corpus callosum WM integrity (Saar‐Ashkenazy et al., 2014; Villarreal et al., 2004) and correlations of FA values with PTSD symptom severity (Saar‐Ashkenazy et al., 2016). The current study observed a moderate negative correlation between FA values of the corpus callosum body and CAPS lifetime hyperarousal scores, (r(23) = −.438, p = .028) (concordant moderate positive RD/AD/MD correlations, see Supporting Information, Tables 3–5) in PTSD diagnosed subjects. Additionally, a moderate negative correlation was found between FA values of the corpus callosum genu and left rACC GM values (concordant moderate positive RD/AD/MD correlations; Tables 4–6).
The decreased FA values observed in PTSD subjects compared to HC across the uncinate fasciculus, cingulum cingulate gyrus, and corpus callosum, is consistent with a neurocircuitry model of PTSD characterized by heightened amygdala responsiveness and impaired inhibitory modulation via the ACC (Rauch, Shin, & Phelps, 2006). These FA decreases combined with positive correlations between RD/AD/MD values and PTSD symptom severity scores suggest myelin and axonal abnormalities of these WM tracts. These abnormalities may be the result of proinflammatory cytokines, such as interleukin‐12, overstimulating the hypothalamic–pituitary–adrenal (HPA) axis’ production of cortisol (Baker, Nievergelt, & O'connor, 2012; Gola et al., 2013; Passos et al., 2015). While regular cortisol production serves a protective function against excessive inflammation (Silverman, Pearce, Biron, & Miller, 2005), exposure to excessive stress or trauma can result in dysregulated HPA axis function (Charmandari, Tsigos, & Chrousos, 2005; Elenkov, Iezzoni, Daly, Harris, & Chrousos, 2005). A recent study has found evidence suggesting epigenetic mechanisms of the histone and DNA methylation regulation of genes involved in proinflammatory cytokine expression is upregulated in PTSD subjects compared to controls (Bam et al., 2016). Epigenetic mechanisms have also been linked to brain‐derived neurotrophic factor (BDNF) expression involved in the extinction of conditioned fear (Blouin, Sillivan, Joseph, & Miller, 2016; Bredy et al., 2007). BDNF, while more often linked to GM alterations, has been implicated in WM morphology and microstructure through variations in oligodendrocyte expression of myelin (Du, Fischer, Lee, Lercher, & Dreyfus, 2003; Tost et al., 2013). Further studies utilizing both genome‐wide epigenetic analyses and MRI structural/diffusion modalities are required to confirm the role of BDNF and proinflammatory cytokines in PTSD related WM alterations, and the subsequent impact on the behavioral profiles associated with PTSD such as increased hyperarousal.
4.1. Limitations
There are several limitations to consider when interpreting results from the current study. Firstly, sample sizes for each subject cohort are relatively small and may have contributed to a lack of significant findings in a whole brain analysis between the PTSD and TC groups. Additionally, while the subjects within each group were age and gender matched, the age range was large. Some PTSD diagnosed subjects were currently being treated, or had previously received treatment in the form of psychosocial (e.g., cognitive behavioral therapy) and/or pharmaceutical interventions (e.g., selective serotonin reuptake inhibitors). As this is a cross‐sectional study, it is not possible to identify if WM fiber tract FA reductions are the result of PTSD progression, or if such structural abnormalities were already present in the study participants prior to exposure to trauma. Pre‐existing WM tract abnormalities in certain individuals may predispose them to developing PTSD after exposure to trauma, and additionally exacerbate PTSD severity due to further reductions in WM integrity (Chetty et al., 2014). GM data from a prior study using an identical subject cohort was acquired using a VBM protocol, which, despite careful application of tissue classification and segmentation procedures, can suffer from GM/WM partial tissue volume estimates. As such, the current study's correlational analyses between WM tract scalar values and VBM determined ROI GM values should be interpreted with caution. While subjects were screened to exclude anyone who had experienced concussion which resulted in loss of consciousness >10 min, recent research has suggested that subconcussive impacts acquired during sports or accidents can result in measureable brain changes (Davenport et al., 2016). The effect of trauma on the brain has been shown to differ depending on previous exposure to childhood maltreatment (Paquola, Bennett, Hatton, Hermens, & Lagopoulos, 2017). This study does not use a measure such as the childhood trauma questionnaire (CTQ) to screen for early life trauma. Given childhood maltreatment significantly increases the risk of PTSD later in life (Yehuda, Halligan, & Grossman, 2001), future studies would benefit from consideration of its effect.
5. CONCLUSION
Findings from this study help extend prior observations of WM integrity differences in PTSD, as measured by DTI FA values. FA reductions within key tracts associated with PTSD symptomology were found in PTSD and TC subject groups compared to healthy controls. The WM integrity of tracts connecting limbic structures to the ACC, uncinate fasciculus and cingulum cingulate, was found to have a negative correlation with PTSD symptom severity. Moreover, using GM results from a previous VBM study containing identical subjects, negative correlations between the left uncinate fasciculus WM integrity and GM values of the right rACC was observed in PTSD diagnosed subjects. These findings add support to a model where reduced WM connectivity may contribute to dysfunction of the inhibitory and extinction roles of the rACC on fear conditioning in PTSD. While the current study assists in the understanding of WM tract differences in trauma exposed individuals who develop PTSD, compared to those who do not and healthy controls, it is not able to determine if these represent a pre‐existing vulnerability to, or consequence of, PTSD acquisition. Further studies are required to explore the mechanisms underpinning WM alterations in PTSD, preferably using longitudinal datasets.
The findings from this study contribute to the established literature elucidating brain differences in those who do and do not develop PTSD after major stress. Of interest, the observation of FA abnormalities suggests that alterations in the prefrontal cortex may play a significant role in PSTD symptomology. As this was a cross‐sectional study, however, the role of WM alterations in this region over the course of a longer term illness would be more appropriately determined through longitudinal studies. Future comparative studies of nonexposed controls within the first year after stress would also be informative for understanding illness consequences and perhaps for identifying general stress effects that do not lead to PTSD.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Figure legends
Supporting Figure 01
Supporting Figure 02
Supporting Figure 03
Supporting Figure 04
Supporting Figure 05
Supporting Figure 06
Supporting Table 01
Supporting Table 02
Supporting Table 03
Supporting Table 04
Supporting Table 05
Supporting Table 06
Supporting Table 07
Supporting Table 08
ACKNOWLEDGMENTS
The authors report no conflict of interest.
They thank the research participants involved this research project for the contribution of their time.
O'Doherty DCM, Ryder W, Paquola C, et al. White matter integrity alterations in post‐traumatic stress disorder. Hum Brain Mapp. 2018;39:1327–1338. 10.1002/hbm.23920
REFERENCES
- Abe, O. , Yamasue, H. , Kasai, K. , Yamada, H. , Aoki, S. , Iwanami, A. , … Ohtomo, K. (2006). Voxel‐based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Research, 146, 231–242. [DOI] [PubMed] [Google Scholar]
- Alexander, A. L. , Hurley, S. A. , Samsonov, A. A. , Adluru, N. , Hosseinbor, A. P. , Mossahebi, P. , … Field, A. S. (2011). Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity, 1, 423–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, A. L. , Lee, J. E. , Lazar, M. , & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics: The Journal of the American Society for Experimental Neurotherapeutics, 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM‐5®). Arlington, VA: The American Psychiatric Association. [Google Scholar]
- Andersson, J. L. , Jenkinson, M. , & Smith, S. (2007). Non‐linear registration, aka spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford.
- Australian Bureau of Statistics . (2007). National Survey of Mental Health and Wellbeing: Summary of Results, 2007, cat. no. 4326.0, Canberra. In: ABS, editor. Canberra: Australian Bureau of Statistics.
- Baker, D. G. , Nievergelt, C. M. , & O'connor, D. T. (2012). Biomarkers of PTSD: Neuropeptides and immune signaling. Neuropharmacology, 62, 663–673. [DOI] [PubMed] [Google Scholar]
- Bam, M. , Yang, X. , Zhou, J. , Ginsberg, J. P. , Leyden, Q. , Nagarkatti, P. S. , & Nagarkatti, M. (2016). Evidence for epigenetic regulation of pro‐inflammatory cytokines, interleukin‐12 and interferon gamma, in peripheral blood mononuclear cells from PTSD patients. Journal of Neuroimmune Pharmacology, 11, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur, V. , Hänggi, J. , & Jäncke, L. (2012). Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neuroscience, 13(1), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette, K. L. , Nave, A. M. , Caprihan, A. , & Stevens, M. C. (2014). White matter abnormalities in adolescents with major depressive disorder. Brain Imaging and Behavior, 8, 531–541. [DOI] [PubMed] [Google Scholar]
- Blouin, A. M. , Sillivan, S. E. , Joseph, N. F. , & Miller, C. A. (2016). The potential of epigenetics in stress‐enhanced fear learning models of PTSD. Learning & Memory (Cold Spring Harbor, N.Y.), 23, 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy, T. W. , Wu, H. , Crego, C. , Zellhoefer, J. , Sun, Y. E. , & Barad, M. (2007). Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning & Memory, 14, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner, J. D. , Scott, T. M. , Delaney, R. C. , Southwick, S. M. , Mason, J. W. , Johnson, D. R. , … Charney, D. S. (1993). Deficits in short‐term memory in posttraumatic stress disorder. American Journal of Psychiatry, 150, 1015–1019. [DOI] [PubMed] [Google Scholar]
- Brunetti, M. , Sepede, G. , Mingoia, G. , Catani, C. , Ferretti, A. , Merla, A. , … Babiloni, C. (2010). Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: Neural correlates of generalized emotional response. Neuroscience, 168, 670–679. [DOI] [PubMed] [Google Scholar]
- Charmandari, E. , Tsigos, C. , & Chrousos, G. (2005). Endocrinology of the stress response 1. Annual Review of Physiology, 67, 259–284. [DOI] [PubMed] [Google Scholar]
- Chen, J. , & Shi, S. (2011). A review of neuroimaging studies of anxiety disorders in China. Neuropsychiatric Disease and Treatment, 7, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty, S. , Friedman, A. R. , Taravosh‐Lahn, K. , Kirby, E. D. , Mirescu, C. , Guo, F. , … Krishnamurthy, A. (2014). Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Molecular Psychiatry, 19, 1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, A. N. , Francisco, A. J. , Thelen, J. , Bruce, J. , Martin, L. E. , McDowd, J. , … Aupperle, R. L. (2017). PTSD and cognitive symptoms relate to inhibition‐related prefrontal activation and functional connectivity. Depression and Anxiety. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, J. K. , Lamke, J. P. , Gaebler, M. , Walter, H. , & Scheel, M. (2013). White matter integrity and its relationship to ptsd and childhood trauma–a systematic review and meta‐analysis. Depression and Anxiety, 30, 207–216. [DOI] [PubMed] [Google Scholar]
- Davenport, E. M. , Urban, J. E. , Mokhtari, F. , Lowther, E. L. , Van Horn, J. D. , Vaughan, C. G. , … Maldjian, J. A. (2016). Subconcussive impacts and imaging findings over a season of contact sports. Concussion, 1, CNC19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. S. , Ségonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Hyman, B. T. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Dickie, E. W. , Brunet, A. , Akerib, V. , & Armony, J. L. (2008). An fMRI investigation of memory encoding in PTSD: Influence of symptom severity. Neuropsychologia, 46, 1522–1531. [DOI] [PubMed] [Google Scholar]
- Dickie, E. W. , Brunet, A. , Akerib, V. , & Armony, J. L. (2011). Neural correlates of recovery from post‐traumatic stress disorder: A longitudinal fMRI investigation of memory encoding. Neuropsychologia, 49, 1771–1778. [DOI] [PubMed] [Google Scholar]
- Dohrenwend, B. P. , Turner, J. B. , Turse, N. A. , Adams, B. G. , Koenen, K. C. , & Marshall, R. (2006). The psychological risks of Vietnam for U.S. veterans: A revisit with new data and methods. Science, 313, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Fischer, T. Z. , Lee, L. N. , Lercher, L. D. , & Dreyfus, C. F. (2003). Regionally specific effects of BDNF on oligodendrocytes. Developmental Neuroscience, 25, 116–126. [DOI] [PubMed] [Google Scholar]
- Ebeling, U. , & Cramon, D. V. (1992). Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochirurgica, 115, 143–148. [DOI] [PubMed] [Google Scholar]
- Elenkov, I. J. , Iezzoni, D. G. , Daly, A. , Harris, A. G. , & Chrousos, G. P. (2005). Cytokine dysregulation, inflammation and well‐being. Neuroimmunomodulation, 12, 255–269. [DOI] [PubMed] [Google Scholar]
- Fani, N. , King, T. Z. , Brewster, R. , Srivastava, A. , Stevens, J. S. , Glover, E. M. , … Jovanovic, T. (2015). Fear‐potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex, 64, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani, N. , King, T. Z. , Jovanovic, T. , Glover, E. M. , Bradley, B. , Choi, K. , … Ressler, K. J. (2012). White matter integrity in highly traumatized adults with and without post‐traumatic stress disorder. Neuropsychopharmacology, 37, 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. (2007). SCID‐I/P.
- Fox, R. J. , Sakaie, K. , Lee, J.‐C. , Debbins, J. , Liu, Y. , Arnold, D. , … Lowe, M. (2012). A validation study of multicenter diffusion tensor imaging: Reliability of fractional anisotropy and diffusivity values. American Journal of Neuroradiology, 33, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, J. A. , Chiu, S. , Breeze, J. L. , Makris, N. , Lange, N. , Kennedy, D. N. , … Dieterich, M. E. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry, 162, 1256–1265. [DOI] [PubMed] [Google Scholar]
- Freund, P. , Wheeler‐Kingshott, C. A. , Nagy, Z. , Gorgoraptis, N. , Weiskopf, N. , Friston, K. , … Hutton, C. (2012). Axonal integrity predicts cortical reorganisation following cervical injury. Journal of Neurology, Neurosurgery & Psychiatry, 83, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel, S. N. P. , & Liberzon, I. (2009). Neurobiology of PTSD: A review of neuroimaging findings. Psychiatric Annals, 39(370–372), 376–381. [Google Scholar]
- Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication. Brain, 123, 1293–1326. [DOI] [PubMed] [Google Scholar]
- Gola, H. , Engler, H. , Sommershof, A. , Adenauer, H. , Kolassa, S. , Schedlowski, M. , … Kolassa, I.‐T. (2013). Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro‐inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry, 13, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, J. M. , Seidman, L. J. , Makris, N. , Ahern, T. , O'brien, L. M. , Caviness, V. S. , … Tsuang, M. T. (2007). Hypothalamic abnormalities in schizophrenia: Sex effects and genetic vulnerability. Biological Psychiatry, 61, 935–945. [DOI] [PubMed] [Google Scholar]
- Greve, D. N. , & Fischl, B. (2009). Accurate and robust brain image alignment using boundary‐based registration. NeuroImage, 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner, M. B. , Lorberbaum, J. P. , & George, M. S. (1999). Potential role of the anterior cingulate cortex in PTSD: Review and hypothesis. Depression and Anxiety, 9, 1–14. [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. , Woolrich, M. W. , & Smith, S. M. (2012). Fsl. NeuroImage, 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Pechaud, M. , & Smith, S. (2005). BET2: MR‐based estimation of brain, skull and scalp surfaces. p 167.
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kim, M. J. , Lyoo, I. K. , Kim, S. J. , Sim, M. , Kim, N. , Choi, N. , … Renshaw, P. F. (2005). Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport, 16, 1049–1053. [DOI] [PubMed] [Google Scholar]
- Kim, S. J. , Jeong, D. U. , Sim, M. E. , Bae, S. C. , Chung, A. , Kim, M. J. , … Lyoo, I. K. (2006). Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology, 54, 120–125. [DOI] [PubMed] [Google Scholar]
- Klawiter, E. C. , Xu, J. , Naismith, R. T. , Benzinger, T. L. , Shimony, J. S. , Lancia, S. , … Cross, A. H. (2012). Increased radial diffusivity in spinal cord lesions in neuromyelitis optica compared with multiple sclerosis. Multiple Sclerosis Journal, 18, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Vermetten, E. , Loewenstein, R. J. , Brand, B. , Schmahl, C. , Bremner, J. D. , & Spiegel, D. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. The American Journal of Psychiatry, 167, 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan, D. , Mangin, J. F. , Poupon, C. , Clark, C. A. , Pappata, S. , Molko, N. , & Chabriat, H. (2001). Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging, 13, 534–546. [DOI] [PubMed] [Google Scholar]
- Liberzon, I. , & Martis, B. (2006). Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences, 1071, 87–109. [DOI] [PubMed] [Google Scholar]
- Lovibond, P. F. , & Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33, 335–343. [DOI] [PubMed] [Google Scholar]
- Makris, N. , Goldstein, J. M. , Kennedy, D. , Hodge, S. M. , Caviness, V. S. , Faraone, S. V. , … Seidman, L. J. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research, 83, 155–171. [DOI] [PubMed] [Google Scholar]
- Manoach, D. S. , Ketwaroo, G. A. , Polli, F. E. , Thakkar, K. N. , Barton, J. J. , Goff, D. C. , … Tuch, D. S. (2007). Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. NeuroImage, 37, 599–610. [DOI] [PubMed] [Google Scholar]
- Nichols, T. , & Hayasaka, S. (2003). Controlling the familywise error rate in functional neuroimaging: A comparative review. Statistical Methods in Medical Research, 12, 419–446. [DOI] [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, D. C. , Tickell, A. , Ryder, W. , Chan, C. , Hermens, D. F. , Bennett, M. R. , & Lagopoulos, J. (2017). Frontal and subcortical grey matter reductions in PTSD. Psychiatry Research, 266, 1–9. [DOI] [PubMed] [Google Scholar]
- Ota, M. , Noda, T. , Sato, N. , Hattori, K. , Hori, H. , Sasayama, D. , … Higuchi, T. (2015). White matter abnormalities in major depressive disorder with melancholic and atypical features: A diffusion tensor imaging study. Psychiatry and Clinical Neurosciences, 69, 360–368. [DOI] [PubMed] [Google Scholar]
- Paquola, C. , Bennett, M. R. , Hatton, S. N. , Hermens, D. F. , & Lagopoulos, J. (2017). Utility of the cumulative stress and mismatch hypotheses in understanding the neurobiological impacts of childhood abuse and recent stress in youth with emerging mental disorder. Human Brain Mapping, 38, 2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos, I. C. , Vasconcelos‐Moreno, M. P. , Costa, L. G. , Kunz, M. , Brietzke, E. , Quevedo, J. , … Kauer‐Sant'anna, M. (2015). Inflammatory markers in post‐traumatic stress disorder: A systematic review, meta‐analysis, and meta‐regression. The Lancet Psychiatry, 2, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Pavuluri, M. N. , Yang, S. , Kamineni, K. , Passarotti, A. M. , Srinivasan, G. , Harral, E. M. , … Zhou, X. J. (2009). Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention‐deficit/hyperactivity disorder. Biological Psychiatry, 65, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, K. L. , Orlichenko, A. , Boyd, E. , Angstadt, M. , Coccaro, E. F. , Liberzon, I. , & Arfanakis, K. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry, 66, 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli, C. , & Basser, P. J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine, 36, 893–906. [DOI] [PubMed] [Google Scholar]
- Rauch, S. L. , Shin, L. M. , & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research–past, present, and future. Biological Psychiatry, 60, 376–382. [DOI] [PubMed] [Google Scholar]
- Rauch, S. L. , Whalen, P. J. , Shin, L. M. , McInerney, S. C. , Macklin, M. L. , Lasko, N. B. , … Pitman, R. K. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry, 47, 769–776. [DOI] [PubMed] [Google Scholar]
- Saar‐Ashkenazy, R. , Veksler, R. , Guez, J. , Jacob, Y. , Shelef, I. , Shalev, H. , … Cohen, J. E. (2016). Breakdown of inter‐hemispheric connectivity is associated with posttraumatic symptomatology and memory impairment. PLoS One, 11, e0144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar‐Ashkenazy, R. , Cohen, J. E. , Guez, J. , Gasho, C. , Shelef, I. , Friedman, A. , & Shalev, H. (2014). Reduced corpus‐callosum volume in posttraumatic stress disorder highlights the importance of interhemispheric connectivity for associative memory. Journal of Traumatic Stress, 27, 18–26. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , & Pandya, D. (2009). Fiber pathways of the brain. OUP USA. [Google Scholar]
- Schuff, N. , Zhang, Y. , Zhan, W. , Lenoci, M. , Ching, C. , Boreta, L. , … Neylan, T. C. (2011). Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. NeuroImage, 54(Suppl 1), S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal, K. H. , Metzler, T. J. , Gima, K. S. , Bertenthal, D. , Maguen, S. , & Marmar, C. R. (2009). Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. American Journal of Public Health, 99, 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, L. M. , Rauch, S. L. , & Pitman, R. K. (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences, 1071, 67–79. [DOI] [PubMed] [Google Scholar]
- Shin, L. M. , Whalen, P. J. , Pitman, R. K. , Bush, G. , Macklin, M. L. , Lasko, N. B. , … Rauch, S. L. (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50, 932–942. [DOI] [PubMed] [Google Scholar]
- Silverman, M. N. , Pearce, B. D. , Biron, C. A. , & Miller, A. H. (2005). Immune modulation of the hypothalamic‐pituitary‐adrenal (HPA) axis during viral infection. Viral Immunology, 18, 41–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , … Matthews, P. M. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , & Nichols, T. E. (2009). Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Song, S.‐K. , Sun, S.‐W. , Ramsbottom, M. J. , Chang, C. , Russell, J. , & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17, 1429–1436. [DOI] [PubMed] [Google Scholar]
- SPSS (2013). IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. [Google Scholar]
- Stevens, J. S. , Kim, Y. J. , Galatzer‐Levy, I. R. , Reddy, R. , Ely, T. D. , Nemeroff, C. B. , … Ressler, K. J. (2016). Amygdala reactivity and anterior cingulate habituation predict PTSD symptom maintenance after acute civilian trauma. Biological Psychiatry, 81, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost, H. , Alam, T. , Geramita, M. , Rebsch, C. , Kolachana, B. , Dickinson, D. , … Trampush, J. W. (2013). Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology, 38, 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal, G. , Hamilton, D. A. , Graham, D. P. , Driscoll, I. , Qualls, C. , Petropoulos, H. , & Brooks, W. M. (2004). Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Research, 131, 227–235. [DOI] [PubMed] [Google Scholar]
- Von Der Heide, R. J. , Skipper, L. M. , Klobusicky, E. , & Olson, I. R. (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136, 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, S. B. , Jones, D. K. , Jeurissen, B. , Viergever, M. A. , & Leemans, A. (2012). The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. NeuroImage, 59, 2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F. W. , Keane, T. M. , & Davidson, J. R. (2001). Clinician‐Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety, 13, 132–156. [DOI] [PubMed] [Google Scholar]
- Weiss, D. S. (2007). The impact of event scale: revised. Cross‐cultural assessment of psychological trauma and PTSD (pp. 219–238). Springer. [Google Scholar]
- Wheeler‐Kingshott, C. A. , & Cercignani, M. (2009). About “axial” and “radial” diffusivities. Magnetic Resonance in Medicine, 61, 1255–1260. [DOI] [PubMed] [Google Scholar]
- Wolf, E. J. , Lunney, C. A. , Miller, M. W. , Resick, P. A. , Friedman, M. J. , & Schnurr, P. P. (2012). The dissociative subtype of PTSD: A replication and extension. Depression and Anxiety, 29, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich, M. W. , Jbabdi, S. , Patenaude, B. , Chappell, M. , Makni, S. , Behrens, T. , … Smith, S. M. (2009). Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45, S173–S186. [DOI] [PubMed] [Google Scholar]
- Yehuda, R. , Halligan, S. L. , & Grossman, R. (2001). Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development and Psychopathology, 13, 733–753. [DOI] [PubMed] [Google Scholar]
- Yehuda, R. , Keefe, R. S. E. , Harvey, P. D. , Levengood, R. A. , Gerber, D. K. , Geni, J. , & Siever, L. J. (1995). Learning and memory in combat veterans with posttraumatic stress disorder. The American Journal of Psychiatry, 152, 137–139. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, Y. , Li, L. , Li, Z. , Li, W. , Ma, N. , … Lu, G. (2011). Different white matter abnormalities between the first‐episode, treatment‐naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. Journal of Affective Disorders, 133, 294–299. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Brady, M. , & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Transactions on Medical Imaging, 20, 45–57. [DOI] [PubMed] [Google Scholar]
- Zhong, Y. , Zhang, R. , Li, K. , Qi, R. , Zhang, Z. , Huang, Q. , & Lu, G. (2015). Altered cortical and subcortical local coherence in PTSD: Evidence from resting‐state fMRI. Acta Radiologica, 56, 746–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Figure legends
Supporting Figure 01
Supporting Figure 02
Supporting Figure 03
Supporting Figure 04
Supporting Figure 05
Supporting Figure 06
Supporting Table 01
Supporting Table 02
Supporting Table 03
Supporting Table 04
Supporting Table 05
Supporting Table 06
Supporting Table 07
Supporting Table 08


