Abstract
The bed nucleus of the stria terminals (BNST) is a subcortical structure involved in anticipatory and sustained reactivity to threat and is thus essential to the understanding of anxiety and stress responses. Although chronic stress and anxiety represent a hallmark of post‐traumatic stress disorder (PTSD), to date, few studies have examined the functional connectivity of the BNST in PTSD. Here, we used resting state functional Magnetic Resonance Imaging (fMRI) to investigate the functional connectivity of the BNST in PTSD (n = 70), its dissociative subtype (PTSD + DS) (n = 41), and healthy controls (n = 50). In comparison to controls, PTSD showed increased functional connectivity of the BNST with regions of the reward system (ventral and dorsal striatum), possibly underlying stress‐induced reward‐seeking behaviors in PTSD. By contrast, comparing PTSD + DS to controls, we observed increased functional connectivity of the BNST with the claustrum, a brain region implicated in consciousness and a primary site of kappa‐opioid receptors, which are critical to the dynorphin‐mediated dysphoric stress response. Moreover, PTSD + DS showed increased functional connectivity of the BNST with brain regions involved in attention and salience detection (anterior insula and caudate nucleus) as compared to PTSD and controls. Finally, BNST functional connectivity positively correlated with default‐mode network regions as a function of state identity dissociation, suggesting a role of BNST networks in the disruption of self‐relevant processing characterizing the dissociative subtype. These findings represent an important first step in elucidating the role of the BNST in aberrant functional networks underlying PTSD and its dissociative subtype.
Keywords: bed nucleus of the stria terminalis, claustrum, dissociative symptoms, kappa‐opioid receptors, PTSD, resting‐state functional connectivity
1. INTRODUCTION
The bed nucleus of the stria terminalis (BNST) is a small brain structure, considered to be part of the extended amygdala and situated in the ventral portion of the basal forebrain, surrounded by the nucleus accumbens, the caudate nucleus, the internal capsule, and the thalamus. Its central location favors structural and functional connections with cortical and subcortical structures. Here, studies in rodents and nonhuman primates have highlighted the structural and functional connections of the BNST with the basal ganglia (nucleus accumbens [NAcc], putamen, caudate, pallidum), limbic areas (amygdala, hippocampus, hypothalamus, periacqueductal grey, anterior insula, subcallosal cortex), and the thalamus (Dong, Petrovich, Watts, & Swanson, 2001; Dong & Swanson, 2006; Fox, Oler, Tromp, Fudge, & Kalin, 2015; Torrisi et al., 2015). Similar structural connections have also been reported in humans, with the addition of BNST structural connectivity with temporal pole regions (Avery et al., 2014), and connectivity to the medial prefrontal and orbitofrontal cortices through the caudate and NAcc (Krüger, Shiozawa, Kreifelts, Scheffler, & Ethofer, 2015).
Functional connectivity at rest in humans also resembles animal studies showing connectivity of the BNST with basal ganglia, limbic regions, and the thalamus, with additional connectivity between the BNST and cortical regions such as the paracingulate gyrus (Avery et al., 2014), the anterior cingulate cortex (ACC), the prefrontal cortex and the precuneus (Avery et al., 2014; Torrisi et al., 2015), and between the BNST and smaller subcortical structures (habenula, periacqueductal grey, hypothalamus, and SLEA) when assessed using ultra‐high field (7 T) (Torrisi et al., 2015). Moreover, functional connectivity in humans between the BNST and the medial prefrontal cortex (mPFC) and sensory cortices (cuneus and fusiform gyrus) was found during threat (Kinnison, Padmala, Choi, & Pessoa, 2012) and anticipation of threat (Herrmann et al., 2016), and between the BNST and the ventromedial prefrontal cortex (vmPFC) both during threat processing (Brinkmann et al., 2017) and in a study on vmPFC lesions (Motzkin et al., 2015). Taken together, these studies point toward the BNST as a relay hub for several neural networks, including networks involved in anxiety and threat processing.
To date, studies in humans have focused on the role of the BNST in fear and stress responses. Here, BNST activity has been characterized as sustained brain response occurring during anticipation of threat [e.g., in response to an electric shock or to aversive emotional pictures (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Grupe, Oathes, & Nitschke, 2013; Somerville et al., 2013)], with a highly specific increased response observed during the middle and late phases of anticipation (McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014) (for an extensive review see Avery, Clauss, & Blackford, 2015), and during anticipation of unpredictable threat (Herrmann et al., 2016). In addition, the positive correlation between both temporal (Somerville, Whalen, & Kelley, 2011) and spatial proximity (Mobbs et al., 2010) of the threat and BNST activity is thought to demonstrate the role of BNST in mediating worry about the future (Avery et al., 2015). In fact, imagery of potential threat alone was demonstrated to elicit BNST response (Coaster et al., 2011). These findings have been critical in differentiating the contributions of the BNST and the amygdala to the fear response, where the amygdala is predominantly involved in modulating fear responses that are not sustained (phasic) and need immediacy (Brinkmann et al., 2017; Herrmann et al., 2016; Lebow & Chen, 2016).
In keeping with the important emerging role of the BNST in chronic anxiety responses, a recent study involving women with post‐traumatic stress disorder (PTSD) revealed increased sustained activation of the BNST during unpredictable anticipation of threatening sounds in comparison to healthy controls (Brinkmann et al., 2017). The results of the same study also indicated increased functional connectivity of the BNST with the vmPFC and insula, and decreased functional connectivity with the dmPFC during the same condition in PTSD as compared to controls. Finally, the BNST appears involved in regulating cardiovascular responses and neuroendocrine function (such as cortisol levels through the projections to the paraventricular nucleus and HPA axis), processes shown to be altered in PTSD (Brown & Morey, 2012; Lebow & Chen, 2016; Thome et al., 2016). Hence, a deeper understanding of the role of BNST in the psychopathology of PTSD is essential to furthering our understanding of the neural circuitry underlying the chronic anxiety responses often associated with this debilitating disorder.
Anticipatory anxiety, or the prolonged feeling of an imminent threat, is a crucial component in the maintenance of the hyperarousal, re‐experiencing, and avoidance symptoms that characterize PTSD (Grupe & Nitschke, 2013; Simmons et al., 2008). The actual presence of a threat is not required to evoke feelings associated with the perception that a traumatic event is reoccurring. Instead these feelings may persist chronically in this population in the absence of an eliciting stimulus. Similar behaviors have recently been attributed to the impaired functioning of BNST during valence surveillance and threat monitoring, where the BNST mediates not only the anticipation of but also a general apprehension toward threat (Lebow & Chen, 2016). It is also important to here note that a dissociative subtype of PTSD has recently been added to the Diagnostic Statistical Manual of Mental Disorders (DSM‐5), characterized by symptoms of depersonalization and derealization (APA, 2013; Spiegel et al., 2013; Steuwe, Lanius, & Frewen, 2012). Individuals diagnosed with this subtype can experience states of defensive immobility/freezing when the fight or flight responses have not been successful (Frewen & Lanius, 2006; Nijenhuis, Vanderlinden, & Spinhoven, 1998; Schauer & Elbert, 2010). Interestingly, temporary inactivation of the BNST has been shown to reduce states of freezing in animal models, and to result in a diminished cardiovascular response to fearful stimuli in a fear conditioning paradigm (Resstel et al., 2008). Overall, these results point further to the importance of elucidating the role of the BNST as a potential neural marker of PTSD and its dissociative subtype.
Accordingly, the aim of this study was to investigate the resting‐state functional connectivity of the BNST in PTSD, its dissociative subtype, and healthy controls. To our knowledge, few studies have investigated the functional connectivity of BNST in humans at rest (Avery et al., 2014; Torrisi et al., 2015), and no such study has been conducted in PTSD. We hypothesized that a diagnosis of PTSD would differentiate resting state functional connectivity of the BNST in comparison to healthy controls, and that the presence of the dissociative subtype would further contribute to such differentiation. Here, we expected to find unique functional connections between the BNST and core brain regions characterizing PTSD psychopathology, such as the mPFC, the amygdala and hippocampus, the insula, and the PAG, when comparing PTSD to controls (Brown & Morey, 2012; Harricharan et al., 2016; Jin et al., 2013; Nicholson et al., 2015, 2016; Rabinak et al., 2011; Sripada et al., 2012; Yin et al., 2011). Moreover, we expected PTSD + DS to further differentiate BNST connectivity in brain regions previously shown to be involved in consciousness (e.g., the bilateral insula, claustrum) and emotion regulation (mPFC).
2. MATERIALS AND METHODS
2.1. Participants
The sample consisted of 161 individuals including: 70 participants with a primary diagnosis of PTSD, 41 participants who met criteria for the dissociative subtype of PTSD (PTSD + DS), and 50 nontrauma exposed healthy controls (HC). Resting‐state data of part of this sample have already been presented in previous studies investigating the functional connectivity of other seed regions of the brain (Harricharan et al., 2016; Nicholson et al., 2015, 2016; Thome et al., 2016).
PTSD was diagnosed through the Clinician Administered PTSD scale IV or 5 [cutoff ≥ 50 for CAPS‐IV (Blake et al., 1995) (n = 135); criteria met on CAPS‐5 (Weathers et al., 2013) (n = 26)]. To meet criteria for the dissociative subtype of PTSD (PTSD + DS), participants had to endorse a severity ≥4 (frequency + intensity) for CAPS‐4, or ≥2 (severity) for CAPS‐5 on the CAPS depersonalization or derealization symptoms as per standard methods (Rabellino et al., 2015; Weathers, Ruscio, & Keane, 1999). Comorbidity for other psychiatric disorders was assessed with the Structured Clinical Interview for DSM‐IV Axis I Disorders (SCID‐I (First, Spitzer, Gibbon, & Williams, 2002). Additionally, dissociative symptoms were investigated with the Multiscale Dissociation Inventory (MDI (Briere, 2002), while childhood trauma history was evaluated with the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003). Demographic and clinical characteristics are presented in Table 1. Among PTSD participants, 48 (PTSD, n = 29; PTSD + DS, n = 19) were receiving psychotropic treatment at the time of the study. Medications included antidepressants (total n = 43: SSRIs, n = 18; SNRIs, n = 9; NDRIs, n = 9; tetracyclics, n = 7), atypical antipsychotics (n = 10), sedatives (total n = 19: benzodiazepines, n = 13; cyclopyrrolone, n = 6), and anticonvulsants (n = 1). Exclusion criteria for all participants included a previous head injury with associated loss of consciousness, current or past history of neurological disorders, significant medical conditions, fMRI incompatibility, history of psychosis, bipolar disorder, substance or alcohol use disorder for 6 months prior to study entry. In addition, the control sample did not meet any current or lifetime criteria for psychiatric disorders (SCID‐I).
Table 1.
Demographic and clinical data
| Demographic and clinical characteristics | PTSD group (n = 70) | PTSD + DS (n = 41) | Control group (n = 50) | p a |
|---|---|---|---|---|
| Age (mean ± SD) years | 37.83 ± 11.7 | 41.12 ± 13.34 | 35.20 ± 11.6 | .099 |
| Gender (F) frequency | 45 | 33 | 34 | .193 |
| CAPS‐IV total (mean ± SD) | 66.6 ± 14.91 (n = 55) | 81.6 ± 12.89 (n = 30) | 0.61 ± 2.76 (n = 50) | <.0001 |
| CAPS‐5 total (mean ± SD) | 34.13 ± 9.68 (n = 15) | 39 ± 8.64 (n = 11) | n/a | .212 |
| MDI total score (mean ± SD) | 54.28 ± 15.22 | 78.77 ± 21.91 | 33.97 ± 4.02 | <.0001 |
| CTQ total score (mean ± SD) | 55.97 ± 23.7 | 68 ± 18.58 | 32.1 ± 9.11 | <.0001 |
| Postscan STAI total score (mean ± SD) | 5.79 ± 2.14 | 6.17 ± 2.43 | 3.55 ± 1.17 | <.0001 |
| Postscan CADSS total score (mean ± SD) | 3.67 ± 1.20 | 4.65 ± 2.70 | 3.18 ± 0.56 | .002 |
| Postscan RSDI total score (mean ± SD) | 3.63 ± 1.34 | 4.83 ± 2.03 | 2.74 ± 0.47 | <.0001 |
| AXIS I comorbidity, current [past] frequency | ||||
| Major depressive disorder | 12 [26] | 23 [9] | ‐ | |
| Panic disorder/agoraphobia | 10 [6] | 9 [7] | ‐ | |
| Social Phobia | 2 [2] | 6 [0] | ‐ | |
| Obsessive–compulsive disorder | 3 [2] | 0 [2] | ‐ | |
| Eating disorder | 0 [3] | 3 [4] | ‐ | |
| Somatoform/somatization disorder | 7 [3] | 11 [0] | ‐ | |
| Generalized anxiety disorder | 1 [0] | 0 [0] | ‐ | |
| Lifetime history of alcohol abuse or dependence | 0 [23] | 0 [20] | ‐ | |
| Lifetime history of substance abuse or dependence | 0 [8] | 0 [6] | ‐ |
Note. Abbreviations: CAPS = Clinical Administered PTSD Scale; CTQ = Childhood Trauma Questionnaire; MDI = Multiscale Dissociation Inventory; PTSD = post‐traumatic stress disorder; PTSD + DS = PTSD dissociative subtype; SD = standard deviation.
The last column reports p values for Kruskal–Wallis and Pearson chi‐squared tests.
All participants were recruited from the Department of Psychiatry London Health Services Center (LHSC) between 2011 and 2016 through community advertisement, mental health practitioners, and family physicians within the London (ON) community, and provided informed written consent. The study was approved by the research ethics board at Western University of Canada.
2.2. fMRI image acquisition
MRI images were collected at either Robarts Research Institute's Center for Functional and Metabolic Mapping or Lawson Health Research Institute in London, Ontario, Canada. After the initial acquisition of structural images, participants were asked to let their mind wander for 6 minutes with eyes closed during a resting fMRI scan. At the end of the scan, all subjects were administered the State‐Trait Anxiety Inventory (STAI) (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998), and the Responses to Script‐Driven Imagery Scale (RSDI) (Hopper, Frewen, & Lanius, 2007) to assess state anxiety and dissociative symptoms (see detailed items in the Supplementary Information, Materials and Methods section).
A whole‐body 3.0 T MRI scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany) was used to collect the data, with a 32‐channel phased array head coil.
High‐resolution T1‐weighted structural images were acquired with magnetization‐prepared rapid acquisition gradient echo (MPRAGE) sequence, with 1 mm isotropic resolution (192 slices). T2*‐weighted functional images were collected using a standard gradient echo planar imaging (EPI) sequence with 2 mm isotropic resolution and the following parameters: FOV = 192 mm × 192 mm × 128 mm (94 × 94 matrix, 64 slices), TR/TE = 3,000 ms/20 ms, flip angle = 90°, 120 volumes. The first four volumes were discarded automatically to allow for equilibration.
2.3. Statistical analyses
2.3.1. Demographic and psychological measures
Kruskall–Wallis H tests, followed by post‐hoc Mann–Whitney tests, were performed to assess group differences on age, CAPS‐IV, CTQ, MDI, CADSS, RSDI, and STAI measures. Pearson's chi‐square tests were used to assess for gender differences between groups.
2.3.2. fMRI preprocessing
FMRI analyses were conducted using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK) implemented in MATLAB R2015 (Mathworks Inc., MA). The functional images were realigned to the first image and resliced, and the mean functional image was created and coregistered to the anatomical for each subject. Coregistered images were then segmented into each tissue type, spatially normalized to the MNI standard template and smoothed with a 6 mm full‐width at half‐maximum (FWHM) Gaussian kernel. Motion correction was applied using ART (version 2015‐ 10; Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA) at 2 mm motion threshold, as head movements have been indicated to affect resting‐state functional connectivity measurements (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). The computed motion outlier regressors were subsequently used at the first‐level analysis in addition to the standard six movement parameters. Finally, smoothed images were band‐pass filtered within a 0.012–0.1 Hz range with in‐house software by co‐author J. Théberge.
2.3.3. Seed‐based connectivity analyses
The seed regions of interest (ROIs) included the right and left bed nucleus of the stria terminalis, respectively. ROI masks were generated using WFU PickAtlas (Functional MRI Laboratory, Wake Forest University School of Medicine), drawing two 5 × 4 × 7 mm boxes centered in Talairach coordinates −5.5, 1, 2.5 (x, y, z, for the left BNST) and 5.5, 1, 2.5 (x, y, z, for the right BNST) based on an anatomical atlas of the human brain (Mai, Paxinos, & Voss, 2008) as per standard methods (McMenamin et al., 2014; Mobbs et al., 2010; Straube, Mentzel, & Miltner, 2007). The mean signal intensity was extracted from each seed region time course using in‐house software by co‐author J. Théberge and was then used as a regressor within a multiple regression model for each seed region per each subject. Final connectivity indicated positive correlations between each seed region and other voxels in the brain.
At the group level, a 3 (group: HC, PTSD, PTSD + DS) × 2 (ROI: right BNST, left BNST) full‐factorial ANOVA was performed to investigate functional connectivity. Sex was included as a nuisance covariate in order to control for differences related to the anatomical sexual dimorphism of the BNST (Lebow & Chen, 2016). Post‐hoc one and two‐sample t test were conducted to further explore significant main and interaction effects. A separate full‐factorial ANOVA was performed adding current medication treatment as a nuisance covariate to explore the influence of current medications on BNST functional connectivity.
Regression analyses were subsequently conducted to investigate the correlation of symptom severity (total CAPS‐IV score, n = 85), childhood trauma exposure (as per CTQ, n = 88), dissociative traits (as per MDI, n = 106), and comorbidity with current Major Depression Disorder (as per SCID‐I, n = 111) with the functional connectivity of left and right BNST, respectively, within the whole PTSD sample (PTSD and PTSD + DS). Further regression analyses were performed within the whole study sample to investigate the correlation of state anxiety (as per STAI, n = 144), state identity dissociative symptoms (as per CADSS, n = 144), and state depersonalization/derealization symptoms (as per RSDI depersonalization/derealization average, n = 144) with the functional connectivity of the left and right BNST, respectively.
Whole‐brain correction at alpha < .05 was ensured using an initial uncorrected threshold set at p < .001 followed by a 1000‐iteration Monte Carlo simulation procedure [AlphaSim in RESTplus Toolbox version 1.8 (http://www.restfmri.net); Song et al., 2011] that yielded minimal cluster extents to control for false‐positive rate of 5% at the whole brain level. Individual extent thresholds were determined for each T‐map investigated.
3. RESULTS
3.1. Demographic and psychological measures
There were no between‐group differences in age or sex (p ≥ .099). By contrast, group differences emerged on CAPS‐IV, CTQ, MDI, CADSS, RSDI and STAI scores (all p ≤ .002). Specifically, post‐hoc tests revealed significantly higher CAPS‐IV, CTQ, MDI, CADSS, RSDI, and STAI scores in PTSD and PTSD + DS as compared to controls (all p ≤ .011). Moreover, the PTSD + DS group showed significantly higher CAPS‐IV, CTQ, RSDI, and MDI scores as compared to the PTSD group (all p ≤ .002; Table 1).
3.2. Seed‐based connectivity
The full‐factorial ANOVA revealed a main effect of group in the vermis (lobule V) and the left lingual gyrus, and a main effect of hemisphere laterality in the right medial prefrontal cortex (mPFC) extending to the rostral anterior cingulum (ACC) and the bilateral superior frontal cortex. In addition, a significant interaction effect was found in the right middle frontal gyrus extending to the ventromedial prefrontal cortex (vmPFC), the left superior frontal gyrus, and the bilateral dorsolateral prefrontal cortex (dlPFC) (Supporting Information, Table S1). Post‐hoc within‐group results are reported in the Supporting Information (Results and Figure S1). Between‐group results are presented in the following paragraphs.
3.3. Between‐group functional connectivity
3.3.1. PTSD versus controls
The PTSD group showed increased functional connectivity between the left BNST and the right caudate head and NAcc as compared to the control group (Table 2 and Figure 1). No other significant differences emerged comparing the functional connectivity of the left and right BNST in the PTSD versus the control group.
Table 2.
Between‐group comparisons of functional connectivity of the left and right BNST
| Peak MNI coordinate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SEED REGION | Comparison | L/R | Brain region | k | Z | P | x | y | z | |
| LEFT BNST | PTSD>CNTR | R | Caudate head/nucleus accumbens | 210 | 4.41 | .000 | 12 | 20 | −4 | |
| PTSD+DS>CNTR | R | Ventral and dorsal anterior and mid insula/claustrum/caudate head | 2728 | 5.16 | .000 | 36 | 2 | 6 | ||
| R | Frontal operculum | Of2728 | 4.9 | .000 | 44 | 20 | 6 | |||
| R | Superior temporal/orbitofrontal cortex | Of2728 | 4.64 | .000 | 48 | 12 | −8 | |||
| L/R | Lingual gyrus/fusiform gyrus/vermis V–VI | 2940 | 4.75 | .000 | −20 | −70 | −6 | |||
| L/R | Cuneus/calcarine cortex | Of2940 | 4.4 | .000 | 14 | −62 | 12 | |||
| PTSD+DS>PTSD | L | Lingual gyrus/calcarine | 2252 | 4.79 | .000 | −18 | −70 | −4 | ||
| L | Cuneus | Of2252 | 4.12 | .000 | −20 | −56 | 22 | |||
| R | Dorsal anterior insula/frontal operculum | 364 | 3.99 | .000 | 46 | 14 | −6 | |||
| R | Superior temporal | Of364 | 3.88 | .000 | 50 | 14 | −18 | |||
| R | Frontal operculum | Of364 | 3.87 | .000 | 46 | 20 | 6 | |||
| RIGHT BNST | PTSD>CNTR | Ns | ||||||||
| PTSD+DS>CNTR | L | Lingual gyrus/fusiform gyrus | 842 | 4.73 | .000 | −22 | −70 | −4 | ||
| R | Lingual gyrus/fusiform gyrus | Of842 | 4.05 | .000 | 22 | −74 | −4 | |||
| L/R | Cerebellar vermis V–VI | Of842 | 4.04 | .000 | −2 | −64 | −14 | |||
| PTSD+DS>PTSD | L | Lingual gyrus/calcarine cortex | 215 | 4.48 | .000 | −20 | −70 | −4 | ||
| L | Cuneus | 515 | 3.63 | .000 | −22 | −78 | 18 | |||
Note. Abbreviations: BNST: bed nucleus of the stria terminalis; CNTR: control group; k: cluster extent; L: left hemisphere; PTSD: post‐traumatic stress disorder group; PTSD + DS: PTSD dissociative subtype group; R: right hemisphere.
All results are reported at p < .05 whole‐brain corrected. No significant results emerged when comparing CNTR > PTSD, CNTR > PTSD + DS, and PTSD > PTSD + DS with either the left or right BNST.
Figure 1.
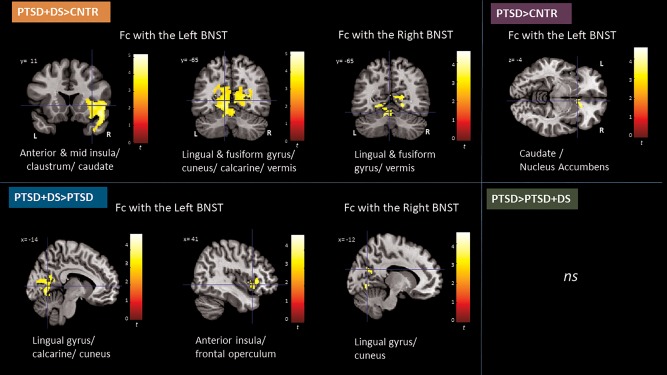
Between‐group comparisons of the functional connectivity of the left and right BNST in PTSD + DS as compared to controls (orange labeled, top left), PTSD + DS as compared to PTSD (blue labelled, bottom left), PTSD as compared to controls (purple labeled, top right), and PTSD as compared to PTSD + DS (green labeled, bottom right). Results are shown at a cluster extent threshold ensuring whole‐brain correction at α < 0.05. BNST: bed nucleus of the stria terminalis; CNTR: control group; Fc: functional connectivity; ns: nonsignificant results; PTSD: posttraumatic stress disorder group; PTSD + DS: dissociative subtype of PTSD group [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3.2. PTSD + DS versus controls
The PTSD + DS group exhibited increased functional connectivity of the left and right BNST with the bilateral lingual gyrus, the occipital cortex (calcarine), and the vermis V–VI in comparison to the control group. In addition, the left BNST showed increased functional connectivity with the right anterior/mid insula, the right frontal operculum, the right orbitofrontal cortex, caudate, claustrum, and superior temporal pole in PTSD + DS as compared to controls (Table 2 and Figure 1).
3.3.3. PTSD versus PTSD + DS
The PTSD + DS group showed increased functional connectivity of the right and left BNST with the left cuneus, calcarine cortex, and lingual gyrus as compared to the PTSD group. In addition, the left BNST showed increased functional connectivity with the right dorsal anterior insula and the right frontal operculum (Table 2 and Figure 1) in the PTSD + DS as compared to the PTSD group.
3.4. Correlation with psychological measures
No significant correlations emerged between the functional connectivity of the left or right BNST and symptom severity (CAPS), trait dissociation (MDI), childhood trauma exposure (CTQ), or comorbidity with major depression disorder (SCID‐I).
By contrast, state anxiety symptoms during the scan (STAI) correlated positively with functional connectivity between the left and right BNST and the left middle temporal pole. Furthermore, state identity dissociation, as assessed by the CADSS correlated positively with the functional connectivity between the bilateral BNST and the right middle frontal and precentral gyrus, the dlPFC, the supplementary motor area, the bilateral posterior cingulate cortex, the bilateral precuneus, and the bilateral occipital cortex (including the calcarine cortex and the lingual gyrus), the left fusiform gyrus, and the left lobules VI–VII of the cerebellum. In addition, the functional connectivity of the right BNST with the right inferior/middle temporal gyrus correlated positively with state depersonalization/derealization symptoms as assessed by the RSDI (Figure 2).
Figure 2.
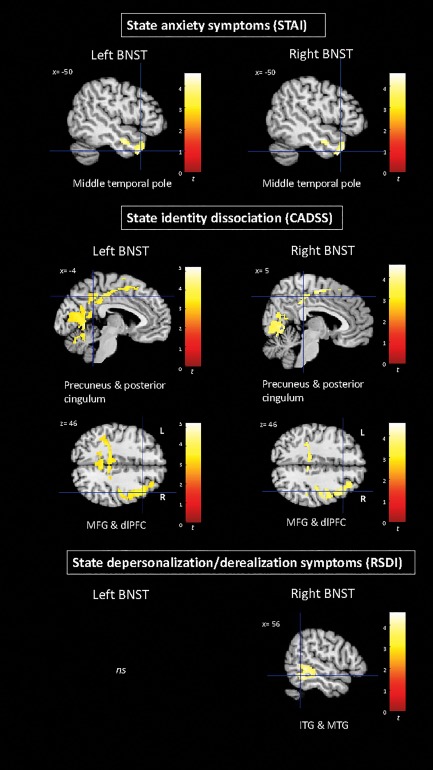
Correlation between the functional connectivity of the left and right BNST with state anxiety symptoms (STAI), state identity dissociation symptoms (CADSS), and state depersonalization/derealization symptoms (RSDI) within the whole sample. Results are shown at a cluster extent threshold ensuring whole‐brain correction at α < 0.05. BNST: bed nucleus of the stria terminalis; ITG: inferior temporal gyrus; MTG: middle temporal gyrus [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. The effects of medication treatment on BNST functional connectivity
Addition of current medication treatment to the ANOVA model as a confounding variable changed the results as follows: (a) While there was no longer a main effect of group, the main effect of hemisphere and especially the interaction effect of group × hemisphere remained statistically significant; (b) Increased functional connectivity between the right BNST and the right lingual gyrus/fusiform gyrus and vermis was no longer apparent when comparing PTSD + DS to controls; (c) When comparing PTSD to controls, increased connectivity between the left BNST and the right caudate head and NAcc only approached significance (k = 148).
All other results remained statistically significant when adding current medical treatment as a nuisance covariate (Supporting Information, Tables S2 and S3).
4. DISCUSSION
As hypothesized, individuals with PTSD and its dissociative subtype showed significant differences in functional connectivity of the right and left BNST in comparison to healthy controls. Here, it is interesting to note that, overall, functional connectivity of the BNST was found to be increased when comparing individuals with PTSD and its dissociative subtype to controls, suggesting a heightened coupling of the BNST with cortical and subcortical regions as a function of PTSD. Moreover, group comparisons better clarified the specificity of the BNST functional connectivity, demonstrating increased functional connectivity of the left BNST with reward system areas (ventral and dorsal striatum) in the PTSD as compared to the control group. By contrast, the PTSD + DS group showed stronger functional connectivity of the bilateral BNST with cortical and subcortical regions related to consciousness (claustrum), emotional awareness and salience detection (insula and caudate), the visual network (lingual gyrus and calcarine fissure), and the limbic cerebellum (vermis) as compared to the control and PTSD group. Finally, we observed a lateralized effect of BNST functional connectivity, where the left BNST exhibited a greater number of connections to other brain regions as compared to the right BNST (Table 2).
4.1. Functional connectivity of the BNST in PTSD
Individuals with PTSD demonstrated increased functional connectivity of the left BNST with the right caudate head (in PTSD + DS as well) and with the NAcc as compared to controls. These subcortical structures, part of the dorsal and ventral striatum, respectively, are highly innervated by dopaminergic, GABAergic and glutamatergic neurons and take part in the cortico–basal ganglia–thalamic loop, which is thought to drive motivation and reward (Yager, Garcia, Wunsch, & Ferguson, 2015). Previous neuroimaging studies indicate the BNST is functionally and structurally connected to the head of the caudate and the NAcc (Avery et al., 2014; Lebow & Chen, 2016); the current findings demonstrate enhanced connection of this neural circuitry in PTSD. Given the role of the BNST in addiction (Avery et al., 2015; Koob & Volkow, 2010; Stamatakis et al., 2014; Torrisi et al., 2015), our findings may shed further light on the neural underpinnings of the link between chronic anxiety and reward‐seeking behaviors, or, alternatively, negative reinforcement as previously suggested in relation to addiction and anxiety (Avery et al., 2015) in PTSD. This hypothesis is supported by a diagnosis of past alcohol and substance abuse in our PTSD and PTSD + DS sample (Table 1). Notably, when we added current medication treatment as a confounding variable, this result approached significance only. Future research examining the effects of different forms of medication treatment on this neural circuitry is therefore warranted.
4.2. Functional connectivity of the BNST in PTSD + DS
The dissociative subtype of PTSD group showed increased functional connectivity of the left BNST with the right dorsal anterior and mid‐insula, and the claustrum, in comparison to controls, and with the right dorsal anterior insula and frontal operculum in comparison to the PTSD group. The dorsal anterior insula is known to play a key role in interoceptive and emotional awareness and anticipatory anxiety (Chua, Krams, Toni, Passingham, & Dolan, 1999; Craig, 2009) and represents a core region of the salience network (Menon & Uddin, 2010; Seeley et al., 2007), where it has been thought to modulate switching between the central executive and the default mode network (Goulden et al., 2014; Menon & Uddin, 2010). We hypothesize that connectivity of the left BNST with the dorsal insula may be involved in chronic states of anticipatory anxiety for a potential incoming threat, which would foster constant heightened attention for the detection of threatening cues in the environment. This notion is further supported by increased connectivity of the left BNST with the right caudate, a brain region involved in salience detection (Menon, 2011; Peters, Dunlop, & Downar, 2016). In addition, these findings may point to increased attention dedicated to interoceptive stimuli to detect whether the body is under threat, or, alternatively, may suggest a compensatory mechanism for impaired interoceptive awareness often observed in PTSD + DS (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Frewen & Lanius, 2015; Lanius, 2015).
The increased functional connectivity of the BNST with the claustrum in PTSD + DS as compared to controls represents a crucial finding, as the claustrum has been proposed to play a major role in consciousness and nociception (Chavkin, 2011). The function of the claustrum has not yet been fully elucidated (Mathur, 2014); however, promising theories point toward its role as a relay station for multisensory integration or as the site of the unification of the perceptual experience that leads to consciousness (Mathur, 2014). Lesions of the claustrum have induced a disruption in consciousness (Chau, Salazar, Krueger, Cristofori, & Grafman, 2015), while its electrical stimulation has been associated with temporary loss of consciousness (Koubeissi, Bartolomei, Beltagy, & Picard, 2014). It is also the locus of the highest density of k‐opioid receptors (KORS), proteins involved in the opioid neuromodulatory system that regulates stress response, reward, and mood processes (Bruijnzeel, 2010; Lalanne, Ayranci, Brigitte, & Lutz, 2014). KORS bind primarily to dynorphins, opioid peptides considered to be key mediators of the dysphoric response to stress (Land et al., 2008). Here, an elegant study using a mouse model suggested that the KOR/dynorphin system is activated by the corticotropin‐releasing factor (CRF) (Land et al., 2008) released under stress, eventually inducing negative affective states (Carr et al., 2010; Mague & Pliakas, 2003). Previous studies examining KORS agonists have also shown their analgesic potential (Chavkin, 2011), with side effects including dysphoria and dissociative symptoms. In this regard, it is important to note that in a recent study (Addy, Garcia‐Romeu, Metzger, & Wade, 2015) administration of a KOR agonist, Salvia divinorum, in humans resulted in experiences of depersonalization, body‐ownership distortions, and sensory alterations. These experiences mirror the phenomenology often observed in individuals with the dissociative subtype of PTSD (Spiegel et al., 2013). In vivo KOR availability has been studied in trauma‐related psychopathology through innovative neuroimaging techniques (combining a radiotracer and high‐resolution positron emission tomography). In one study, KOR availability within a limbic‐ventral striatum circuit mediated trauma‐related dysphoric symptoms (Pietrzak et al., 2014). Interestingly, KORS are distributed in the BNST, the prefrontal cortex, the dorsal and ventral striatum, and the hippocampus. Critically, these regions, among others, include several cortical and subcortical regions significantly connected to the BNST in PTSD + DS in the current study. Taken together, these findings suggest that the KOR/dynorphin system may emerge as an important biological marker for dissociative symptoms and negative affective states in PTSD + DS. The increased connectivity between the BNST and the claustrum in this group might suggest a more frequent activation of the KOR/dynorphin system in circumstances of chronic stress, where the subject perceives a continuous imminence of an incoming threat and thus seeks the analgesic and dissociative effects of kappa opioids to face potential injuries (Lanius, Paulsen, & Corrigan, 2014), with secondary negative effects including dysphoria.
Increased connectivity with the BNST in PTSD + DS as compared to the control and the PTSD group was also observed in the visual cortex, specifically in the lingual gyrus and the calcarine fissure. While previous human studies have demonstrated functional connectivity of the BNST with the visual areas (Avery et al., 2014) at rest, individuals with PTSD + DS seem to exhibit exacerbated connectivity with these brain regions. This may be due to a hyperactive visual imagery related to the traumatic event (as can occur during flashbacks and reliving symptoms) induced by chronic anxiety states, or to altered neural activity of visual areas (low‐level perceptual deficits; Shang et al., 2014) due to increased processing demands associated with intrusive memories and flashbacks in PTSD. In this regard, trauma‐exposed and PTSD individuals show reduced grey matter density within areas of the visual network (including lingual gyrus, cuneus, and calcarine fissure) (Nardo et al., 2010; Teicher, Samson, Anderson, & Ohashi, 2016; Zhang et al., 2011) and reduced neural activity of these regions at rest (Wang et al., 2016). Future research to elucidate further patterns of connectivity between limbic regions and the visual cortex in PTSD is therefore warranted.
Interestingly, the PTSD + DS group also showed increased functional connectivity of the BNST with the vermis, a critical cerebellar region of the “limbic cerebellum” (Schmahmann, 2000b). Indeed, the cerebellum has been recently rediscovered as a complex structure involved in emotion regulation, in addition to the already acknowledged motor control functions (Schmahmann, 2000a). The vermis in particular, together with the flocculonodular lobe and the fastigial nucleus, has been proposed to be part of an extended Papez circuitry dedicated to emotion processing (Schutter & van Honk, 2005). Our results may therefore suggest that the overmodulation of emotion and associated emotional detachment characterizing PTSD + DS (Lanius et al., 2010) may extend to brain circuits involving heightened connectivity between the BNST and the “emotional cerebellum.”
4.3. Correlation with psychological measures
Anxiety and depersonalization/derealization symptoms experienced during the resting‐state scan, as measured by STAI and RSDI, respectively, were found to be positively correlated with functional connectivity between the BNST and inferior/middle temporal regions. In previous studies, the middle temporal gyrus showed reduced grey matter volumes in PTSD (Kühn & Gallinat, 2013) and reduced activity associated with dissociative symptoms in PTSD (Lanius et al., 2002; Simeon et al., 2000).The inferior/middle temporal pole is also considered to be part of the ventral visual stream, enabling object recognition and allocentric representation of scenes (Norman, 2002). These results support the findings described above of an increased functional connectivity between the BNST and the visual cortex in PTSD + DS, and point towards the importance of the ventral visual pathway in association with anxiety and dissociative states at rest. Furthermore, state identity dissociation during the resting state scan as measured by CADSS was found to be positively correlated with functional connectivity between the BNST and brain regions that form the default mode network (DMN), including the precuneus, posterior cingulum, and prefrontal cortex. The DMN is an intrinsic connectivity network mainly active during idling states, thus specific to self‐referential processing (Menon, 2011), and necessary for building and maintaining an integrated sense of self (Lanius, Frewen, Tursich, Jetly, & McKinnon, 2015). The DMN has previously been found altered in PTSD at rest (Koch et al., 2016; Liu et al., 2017; Shang et al., 2014; Tursich et al., 2015). In addition, our findings indicate that the more the subjects feel confused about their identity, the more the BNST activity couples with DMN‐related regions during the scan, thereby potentially disturbing self‐referential processing, eventually leading to identity confusion. Alternatively, the increased functional connectivity between the BNST and DMN regions as a function of state identity dissociation may be indicative of an attempt to restore a more integrated sense of self during sustained threat processing.
5. LIMITATIONS AND FUTURE DIRECTIONS
Some limitations of this study need to be considered. First, we drew a single left and right BNST mask based on an anatomical atlas across all participants, thus lacking sensitivity for individual anatomical differences of this complex brain region. However, this procedure is commonly used in resting state investigations, and has been successfully used in previous studies on BNST neural activity and functional connectivity (Brinkmann et al., 2017; Herrmann et al., 2016; McMenamin et al., 2014). Future research will need to examine potential structural differences associated with PTSD. This study also lacks a trauma‐exposed control group. Although we are able to show no correlation between BNST connectivity and childhood trauma, the inclusion of a second control group (trauma‐exposed) would have allowed us to exclude a possible interpretation of our findings as a consequence of trauma‐exposure alone. In addition, the cross‐sectional nature of our study did not allow us to account for premorbid neural and behavioral characteristics; longitudinal studies will therefore be required to address this matter. Moreover, seed‐based connectivity analyses do not provide data on the directionality of connectivity, which will need to be explored in future studies. Furthermore, as current medication treatment appears to affect the functional connectivity of the BNST in PTSD, further research is warranted to specifically target pharmacological treatment as a variable of interest in PTSD neural circuitry (Lanius, 2010). Finally, alterations in BNST connectivity with the KOR/dynorphin system in the dissociative subtype points toward the need for further investigation of K‐opioid‐receptors as potential target of pharmacological treatment for the dissociative subtype of PTSD (Bailey, Cordell, Sobin, & Neumeister, 2014; Naganawa et al., 2016).
6. CONCLUSIONS
The current investigation of the functional connectivity of the BNST elucidated critical early findings surrounding the neural correlates underlying PTSD and its dissociative subtype. Here, provocative results within the PTSD group revealed the unique functional connectivity of the BNST with subcortical regions involved in the reward system, providing key evidence for a connection between stress‐induced anxiety and reward‐seeking behaviors in this population. Crucially, identification of the functional connectivity characterizing the BNST within the PTSD + DS group provides an important first step towards outlining the neural basis of the relationship between BNST activity and the KOR/dynorphin system as a biological marker of dissociative symptomatology related to stress. In addition, the results showing increased functional connectivity between the BNST and brain regions associated with attention and salience detection (dorsal anterior insula and caudate nucleus) highlight the link between chronic and anticipatory anxiety and hypervigilance in PTSD. Furthermore, our findings demonstrate the involvement of the BNST with the default‐mode network during state identity dissociation suggest aberrant self‐referential processing in the dissociative subtype of PTSD. Finally, identification of the coupling of the BNST with the visual network during anxiety and dissociative states may aid in elucidating the neural pathways underlying intrusive memories and flashbacks, and of visual distortions of the self, such as allocentric self‐representation in out‐of‐body experiences, respectively. Treatments designed for PTSD will therefore need to consider the BNST as an important emerging subcortical structure in connection with cortical and subcortical regions in maintaining altered states of consciousness and reward‐seeking behaviors.
DISCLOSURE OF CONFLICTS
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2
ACKNOWLEDGMENTS
The study was supported by Canadian Institutes of Health Research (CIHR) grant n. 137150 and grant n. 148784. Rabellino D. was supported by MITACS and Homewood Research Institute.
Rabellino D, Densmore M, Harricharan S, Jean T, McKinnon MC, Lanius RA. Resting‐state functional connectivity of the bed nucleus of the stria terminalis in post‐traumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018;39:1367–1379. 10.1002/hbm.23925
Funding information Canadian Institutes of Health Research (CIHR), Grant/Award Numbers: 137150, 148784; MITACS; Homewood Research Institute
REFERENCES
- Addy, P. H. , Garcia‐Romeu, A. , Metzger, M. , & Wade, J. (2015). The subjective experience of acute, divinorum inebriation. Journal of Psychopharmacology (Oxford, England), 29, 426–435. 10.1177/0269881115570081. [DOI] [PubMed] [Google Scholar]
- Alvarez, R. P. , Chen, G. , Bodurka, J. , Kaplan, R. , & Grillon, C. (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55, 389–400. 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (2013). Diagnostic and statistical manual of mental disorders. American Journal of Psychiatry 5th ed. Washington DC: American Psychiatric Publishing; 10.1176/appi.books.9780890425596.744053. [DOI] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , & Blackford, J. U. (2015). The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology, 41, 126–141. 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N. , Clauss, J. A. , Winder, D. G. , Woodward, N. , Heckers, S. , & Blackford, J. U. (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, C. R. , Cordell, E. , Sobin, S. M. , & Neumeister, A. (2014). Recent progress in understanding the pathophysiology of post‐traumatic stress disorder. CNS Drugs, 27, 221–232. 10.1007/s40263-013-0051-4.Recent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , … Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27, 169–190. 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8, 75–90. 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner, J. D.,J. , Krystal, J. H. , Putnam, F. W. , Southwick, S. M. , Marmar, C. , Charney, D. S. , & Mazure, C. M. (1998). Measurement of dissociative states with the Clinician‐Administered Dissociative States Scale (CADSS). Journal of Traumatic Stress, 11, 125–136. [DOI] [PubMed] [Google Scholar]
- Briere, J. (2002). Multiscale dissociation inventory professional manual. Odessa, Florida: Psychological Assessment Resources. [Google Scholar]
- Brinkmann, L. , Buff, C. , Neumeister, P. , Tupak, S. V. , Becker, M. P. I. , Herrmann, M. J. , & Straube, T. (2017). Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post‐traumatic stress disorder patients. Human Brain Mapping. 10.1002/hbm.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, V. M. , & Morey, R. A. (2012). Neural systems for cognitive and emotional processing in posttraumatic stress disorder. Frontiers in Psychology, 3, 449 10.3389/fpsyg.2012.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel, A. W. (2010). Kappa‐opioid receptor signaling and brain reward function. Brain Research Reviews, 62, 127–146. 10.1016/j.brainresrev.2009.09.008.Kappa-opioid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, G. V. , Bangasser, D. A. , Bethea, T. , Young, M. , Valentino, R. J. , & Lucki, I. (2010). Antidepressant‐like effects of κ‐opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology, 35, 752–763. 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau, A. , Salazar, A. M. , Krueger, F. , Cristofori, I. , & Grafman, J. (2015). The effect of claustrum lesions on human consciousness and recovery of function. Consciousness and Cognition, 36, 256–264. 10.1016/j.concog.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Chavkin, C. (2011). The therapeutic potential of κ‐opioids for treatment of pain and addiction. Neuropsychopharmacology, 36, 369–370. 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua, P. , Krams, M. , Toni, I. , Passingham, R. , & Dolan, R. (1999). A functional anatomy of anticipatory anxiety. NeuroImage, 9, 563–571. 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Coaster, M. , Rogers, B. P. , Jones, O. D. , Viscusi, W. K. , Merkle, K. L. , Zald, D. H. , & Gore, J. C. (2011). Variables influencing the neural correlates of perceived risk of physical harm.Cognitive, Affective & Behavioral Neuroscience, 11, 494–507. 10.3758/s13415-011-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. DB. (2009). How do you feel–now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10, 59–70. 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Ohman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–195. 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dong, H. W. , Petrovich, G. D. , Watts, A. G. , & Swanson, L. W. (2001). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology, 436, 430–455. 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong, H. W. , & Swanson, L. W. (2006). Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: Implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. The Journal of Comparative Neurology, 494, 75–107. 10.1002/cne.20790.Projections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. W. (2002). Structured clinical interview for DSM‐IV‐TR Axis I disorders, patient edition (SCID‐I/P, 11/2002 revision) for DSMIV. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fox, A. S. , Oler, J. A. , Tromp, D. P. M. , Fudge, J. L. , & Kalin, N. H. (2015). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38, 319–329. 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen, P. A. , & Lanius, R. A. (2015). Healing the trauamtized self. New York: W. W. Norton and Company Inc. [Google Scholar]
- Frewen, P. A. , & Lanius, R. A. (2006). Toward a psychobiology of posttraumatic self‐dysregulation: Reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences, 1071, 110–124. 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Goulden, N. , Khusnulina, A. , Davis, N. J. , Bracewell, R. M. , Bokde, A. L. , McNulty, J. P. , & Mullins, P. G. (2014). The Salience Network is responsible for switching between the Default Mode Network and the Central Executive Network: Replication from DCM. NeuroImage, 99, 180–190. 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Grupe, D. W. , & Nitschke, J. B. (2013). Uncertainty and Anticipation in Anxiety: An integrated neurobiological and psychological perspective. Nature Reviews. Neuroscience, 14, 488–501. 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe, D. W. , Oathes, D. J. , & Nitschke, J. B. (2013). Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex (New York, N.Y.: 1991), 23, 1874–1883. 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan, S. , Rabellino, D. , Frewen, P. , Densmore, M. , Théberge, J. , McKinnon, M. , … Lanius, R. (2016). fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain and Behavior, 1–16. 10.1002/elan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann, M. J. , Boehme, S. , Becker, M. P. I. , Tupak, S. V. , Guhn, A. , Schmidt, B. , … Straube, T. (2016). Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping, 37, 1091–1102. 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper, J. W. , Frewen, P. A. , & Lanius, R. A. (2007). Neural correlates of reexperiencing, avoidance, and dissociation in PTSD : Symptom dimensions and emotion dysregulation in responses to script‐driven trauma imagery. Journal of Traumatic Stress, 20, 713–725. 10.1002/jts. [DOI] [PubMed] [Google Scholar]
- Jin, C. , Qi, R. , Yin, Y. , Hu, X. , Duan, L. , Xu, Q. , … Li, L. (2013). Abnormalities in whole‐brain functional connectivity observed in treatment‐naive post‐traumatic stress disorder patients following an earthquake. Psychological Medicine, 1–10. 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- Kinnison, J. , Padmala, S. , Choi, J.‐M. , & Pessoa, L. (2012). Network analysis reveals increased integration during emotional and motivational processing. The Journal of Neuroscience, 32, 8361–8372. 10.1523/JNEUROSCI.0821-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, S. B. J. , van Zuiden, M. , Nawijn, L. , Frijling, J. L. , Veltman, D. J. , & Olff, M. (2016). Aberrant resting‐state brain activity in posttraumatic stress disorder: A meta‐analysis and systematic review. Depression and Anxiety, 33, 592–605. 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Koob, G. F. , & Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35, 217–238. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubeissi, M. Z. , Bartolomei, F. , Beltagy, A. , & Picard, F. (2014). Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy & Behavior, 37, C:32–35. 10.1016/j.yebeh.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Krüger, O. , Shiozawa, T. , Kreifelts, B. , Scheffler, K. , & Ethofer, T. (2015). Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex, 66, 60–68. 10.1016/j.cortex.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Kühn, S. , & Gallinat, J. (2013). Gray matter correlates of posttraumatic stress disorder: A quantitative meta‐analysis. Biological Psychiatry, 73, 70–74. 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Land, B. B. , Bruchas, M. R. , Lemos, J. C. , Xu, M. , Melief, E. J. , & Chavkin, C. (2008). The dysphoric component of stress is encoded by activation of the dynorphin κ‐opioid system. The Journal of Neuroscience, 28, 407–414. 10.1523/JNEUROSCI.4458-07.2008.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. (2010). Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication‐free patients? Journal of Psychiatry & Neuroscience: Japan, 35, 80–89. 10.1503/jpn.090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. (2015). Trauma‐related dissociation and altered states of consciousness: A call for clinical, treatment, and neuroscience research. European Journal of Psychotraumatology, 6, 1–9. 10.3402/ejpt.v6.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Frewen, P. A. , Tursich, M. , Jetly, R. , & McKinnon, M. C. (2015). Restoring large‐scale brain networks in PTSD and related disorders: A proposal for neuroscientifically‐informed treatment interventions. European Journal of Psychotraumatology, 6, 27313 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Vermetten, E. , Loewenstein, R. J. , Brand, B. , Schmahl, C. , Bremner, J. D. , & Spiegel, D. (2010). Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry, 167, 640–647. 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius, R. A. , Williamson, P. C. , Boksman, K. , Densmore, M. , Gupta, M. , Neufeld, R. W. J. , … Menon, R. S. (2002). Brain activation during script‐driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biological Psychiatry, 52, 305–311. [DOI] [PubMed] [Google Scholar]
- Lanius, U. F. , Paulsen, S. L. , & Corrigan, F. M. (2014). Neurobiology & treatment of traumatic dissociation. New York: Springer Publishing Company. [Google Scholar]
- Lalanne, L. , Ayranci, G. , Kieffer, B. K. , & Lutz, P. E. (2014). The kappa opioid receptor: From addiction to depression, and back. Frontiers in Psychiatry, 5, 1–17. 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow, M. A. , & Chen, A. (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21, 450–463. 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Li, L. , Li, B. , Feng, N. , Li, L. , Zhang, X. , … Yin, H. (2017). Decreased triple network connectivity in patients with recent onset post‐traumatic stress disorder after a single prolonged trauma exposure. Scientific Reports, 7, 12625 10.1038/s41598-017-12964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague, S. D. , & Pliakas, A. M. (2003). Antidepressant‐like effects of κ‐opioid receptor antagonists in the forced swim test in rats. The Journal of Pharmacology and Experimental Therapeutics, 305, 323–330. 10.1124/jpet.102.046433.more. [DOI] [PubMed] [Google Scholar]
- Mai, J. K. , Paxinos, G. , & Voss, T. (2008). Atlas of the human brain (3rd ed). New York: Elsevier. [Google Scholar]
- Mathur, B. N. (2014). The claustrum in review. Frontiers in Systems Neuroscience, 8, 1–11. 10.3389/fnsys.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin, B. W. , Langeslag, S. J. E. , Sirbu, M. , Padmala, S. , & Pessoa, L. (2014). Network organization unfolds over time during periods of anxious anticipation. The Journal of Neuroscience, 34, 11261–11273. 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214, 655–667. 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs, D. , Yu, R. , Rowe, J. B. , Eich, H. , FeldmanHall, O. , & Dalgleish, T. (2010). Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Academy of Sciences of the United States of America, 107, 20582–20586. 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin, J. C. , Philippi, C. L. , Oler, J. A. , Kalin, N. H. , Baskaya, M. K. , & Koenigs, M. (2015). Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex, 64, 281–288. 10.1016/j.cortex.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa, M. , Dickinson, G. L. , Zheng, M. Q. , Henry, S. , Vandenhende, F. , Witcher, J. , … Carson, R. E. (2016). Receptor occupancy of the kappa‐opioid antagonist LY2456302 measured with positron emission tomography and the novel radiotracer 11C‐LY2795050. The Journal of Pharmacology and Experimental Therapeutics, 356, 260–266. 10.1124/jpet.115.229278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo, D. , Högberg, G. , Chee, J. , Looi, L. , Larsson, S. , Hällström, T. , & Pagani, M. (2010). Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. Journal of Psychiatric Research, 44, 477–485. 10.1016/j.jpsychires.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Nicholson, A. , Densmore, M. , Frewen, P. A. , Théberge, J. , Neufeld, R. W. J. , McKinnon, M. C. & Lanius, (2015). The dissociative subtype of posttraumatic stress disorder : Unique resting‐state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology, 40, 2317–2326. 10.10138/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A. , Sapru, I. , Densmore, M. , Frewen, P. A. , Neufeld, R. W. J. , Théberge, J. , … Lanius, R. A. (2016). Unique insula subregion resting‐state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Research: Neuroimaging, 250, 61–72. 10.1016/j.pscychresns.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Nijenhuis, E. R. , Vanderlinden, J. , & Spinhoven, P. (1998). Animal defensive reactions as a model for trauma‐induced dissociative reactions. Journal of Traumatic Stress, 11, 243–260. 10.1023/A:1024447003022. [DOI] [PubMed] [Google Scholar]
- Norman, J. (2002). Two visual systems and two theories of perception : An attempt to reconcile the constructivist and ecological approaches. The Behavioral and Brain Sciences, 25, 73–144. [DOI] [PubMed] [Google Scholar]
- Peters, S. K. , Dunlop, K. , & Downar, J. (2016). Cortico‐striatal‐thalamic loop circuits of the salience network : A central pathway in psychiatric disease and treatment. Frontiers in Systems Neuroscience, 10, 1–23. 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak, R. H. , Naganawa, M. , Huang, Y. , Corsi‐Travali, S. , Zheng, M.‐Q. , Stein, M. B. , … Neumeister, A. (2014). Association of in vivo κ‐opioid receptor availability and the transdiagnostic dimensional expression of trauma‐related psychopathology. JAMA Psychiatry, 71, 1262 10.1001/jamapsychiatry.2014.1221. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , Petersen, S. E. . (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino, D. , Tursich, M. , Frewen, P. A. , Daniels, J. K. , Densmore, M. , Théberge, J. , & Lanius, R. A. (2015). Intrinsic connectivity networks in post‐traumatic stress disorder during sub‐ and supraliminal processing of threat‐related stimuli. Acta Psychiatrica Scandinavica, 132, 365–378. 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Rabinak, C. A. , Angstadt, M. , Welsh, R. C. , Kenndy, A. E. , Lyubkin, M. , Martis, B. , & Phan, K. L. (2011). Altered amygdala resting‐state functional connectivity in post‐traumatic stress disorder. Frontiers in Psychiatry, 2, 62 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel, L. B. M. , Alves, F. H. F. , Reis, D. G. , Crestani, C. C. , Corrêa, F. M. A. , & Guimarães, F. S. (2008). Anxiolytic‐like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience, 154, 869–876. 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Schauer, M. , & Elbert, T. (2010). Dissociation following traumatic stress. Zeitschrift Für Psychol/Journal of Psychology, 218, 109–127. 10.1027/0044-3409/a000018. [DOI] [Google Scholar]
- Schmahmann, J. D. (2000a). The role of the cerebellum in a affect and psychosis. Journal of Neurolinguistics, 13, 189–214. [Google Scholar]
- Schmahmann, J. D. (2000b). The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics, 13, 189–214. 10.1016/S0911-6044(00)00011-7. [DOI] [Google Scholar]
- Schutter, D. J. L. G. , & van Honk, J. (2005). The cerebellum on the rise in human emotion. Cerebellum (London, England), 4, 290–294. 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J. , Lui, S. , Meng, Y. , Zhu, H. , Qiu, C. , Gong, Q. , & Liao, W. (2014). Alterations in low‐level perceptual networks related to clinical severity in PTSD after an earthquake : A resting‐state fMRI study. PLoS One, 9, 1–8. 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon, D. , Guralnik, O. , Hazlett, E. A. , Spiegel‐Cohen, J. , Hollander, E. , & Buchsbaum, M. S. (2000). Feeling unreal: A PET study of depersonalization disorder. The American Journal of Psychiatry, 157, 1782–1788. 10.1176/appi.ajp.157.11.1782. [DOI] [PubMed] [Google Scholar]
- Simmons, A. , Paulus, M. P. , Thorp, S. R. , Matthews, S. C. , Norman, S. B. , & Stein, M. B. (2008). Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry, 64, 681–690. 10.1016/j.biopsych.2008.05.027.Functional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. , Wagner, D. D. , Wig, G. S. , Moran, J. M. , Whalen, P. J. , & Kelley, W. M. (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex (New York, N.Y.: 1991), 23, 49–60. 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, L. H. , Whalen, P. J. , & Kelley, W. M. (2011). Human bed nucleus of the stria terminalis indexed hypervigilant threat monitoring. Biological Psychiatry, 68, 416–424. 10.1016/j.biopsych.2010.04.002.Human. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X. W. , Dong, Z. Y. , Long, X. Y. , Li, S. F. , Zuo, X. N. , Zhu, C. Z. , … Zang, Y. F. (2011). REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One, 6 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel, D. , Lewis‐Fernández, R. , Lanius, R. , Vermetten, E. , Simeon, D. , & Friedman, M. (2013). Dissociative disorders in DSM‐5. Annual Review of Clinical Psychology, 9, 299–326. 10.1146/annurev-clinpsy-050212-185531. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, California: Consulting Psychologists Press. [Google Scholar]
- Sripada, R. K. , King, A. P. , Garfinkel, S. N. , Wang, X. , Sripada, C. S. , Welsh, R. C. , & Liberzon, I. (2012). Altered resting‐state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry & Neuroscience: Japan, 37, 241–249. 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis, A. M. , Sparta, D. R. , Jennings, J. H. , Mcelligott, Z. A. , Decot, H. , & Stuber, G. D. (2014). Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction‐related behaviors. Neuropharmacology, 76, 320–328. 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe, C. , Lanius, R. A. , & Frewen, P. A. (2012). Evidence for a dissociative subtype of PTSD by latent profile and confirmatory factor analyses in a civilian sample. Depression and Anxiety, 29, 689–700. 10.1002/da.21944. [DOI] [PubMed] [Google Scholar]
- Straube, T. , Mentzel, H. J. , & Miltner, W. H. R. (2007). Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. NeuroImage, 37, 1427–1436. 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , Samson, J. A. , Anderson, C. M. , & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews. Neuroscience, 17, 652–666. 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thome, J. , Densmore, M. , Frewen, P. A. , Mckinnon, M. C. , Théberge, J. , Nicholson, A. A. , … Lanius, R. A. (2016). Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder. Human Brain Mapping, 10.1002/hbm.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi, S. , O'connell, K. , Davis, A. , Reynolds, R. , Balderston, N. , Fudge, J. L. , … Ernst, M. (2015). Resting state connectivity of the bed nucleus of the stria terminalis at ultra‐high field. Human Brain Mapping, 36, 4076–4088. 10.1002/hbm.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich, M. , Ros, T. , Frewen, P. A. , Kluetsch, R. C. , Calhoun, V. D. , & Lanius, R. A. (2015). Distinct intrinsic network connectivity patterns of post‐traumatic stress disorder symptom clusters. Acta Psychiatrica Scandinavica, 132, 29–38. 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- Wang, T. , Liu, J. , Zhang, J. , Zhan, W. , Li, L. , Wu, M. , & Huang, H. (2016). Altered resting‐state functional activity in posttraumatic stress disorder : A quantitative meta‐analysis. Scientific Reports, 6, 1–14. 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F. W. , Blake, D. D. , Schnurr, P. P. , Kaloupek, D. G. , Marx, B. P. , & Keane, T. M. (2013). The clinician‐administered PTSD scale for DSM‐5 (CAPS‐5). The CAPS‐5 is available from the National Center for PTSD at http://www.ptsd.va.gov.
- Weathers, F. W. , Ruscio, A. M. , & Keane, T. M. (1999). Psychometric properties of nine scoring rules for the clinician‐administered postrtraumatic stress disorder scale. Psychological Assessment, 11, 124–133. [Google Scholar]
- Yager, L. M. , Garcia, A. F. , Wunsch, A. M. , & Ferguson, S. M. (2015). The ins and outs of the striatum: Role in drug addiction. Neuroscience, 301, 529–541. 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y. , Li, L. , Jin, C. , Hu, X. , Duan, L. , Eyler, L. T. , … Li, W. (2011). Abnormal baseline brain activity in posttraumatic stress disorder: A resting‐state functional magnetic resonance imaging study. Neuroscience Letters, 498, 185–189. 10.1016/j.neulet.2011.02.069. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, Y. , Li, L. , Li, Z. , Li, W. , Ma, N. , … Lu, G. (2011). Different white matter abnormalities between the first‐episode, treatment‐naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. Journal of Affective Disorders, 133, 294–299. 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
Supporting Information 2


