Abstract
Cognitive control mechanisms support the deliberate regulation of thought and behavior based on current goals. Recent work suggests that motivational incentives improve cognitive control and has begun to elucidate critical neural substrates. We conducted a quantitative meta‐analysis of neuroimaging studies of motivated cognitive control using activation likelihood estimation (ALE) and Neurosynth to delineate the brain regions that are consistently activated across studies. The analysis included studies that investigated changes in brain activation during cognitive control tasks when reward incentives were present versus absent. The ALE analysis revealed consistent recruitment in regions associated with the frontoparietal control network including the inferior frontal sulcus and intraparietal sulcus, as well as regions associated with the salience network including the anterior insula and anterior mid‐cingulate cortex. As a complementary analysis, we performed a large‐scale exploratory meta‐analysis using Neurosynth to identify regions that are recruited in studies using of the terms cognitive control and incentive. This analysis replicated the ALE results and also identified the rostrolateral prefrontal cortex, caudate nucleus, nucleus accumbens, medial thalamus, inferior frontal junction, premotor cortex, and hippocampus. Finally, we separately compared recruitment during cue and target periods, which tap into proactive engagement of rule‐outcome associations, and the mobilization of appropriate viscero‐motor states to execute a response, respectively. We found that largely distinct sets of brain regions are recruited during cue and target periods. Altogether, these findings suggest that flexible interactions between frontoparietal, salience, and dopaminergic midbrain‐striatal networks may allow control demands to be precisely tailored based on expected value.
Keywords: cognitive control, control network, frontoparietal, reward
1. INTRODUCTION
The ability to maintain attention during a lecture, or flexibly shift between writing a report and answering emails, or plan several steps ahead during a chess match all require cognitive control—the capacity to deliberately guide thought and behavior based on goals, especially in the presence of distraction or competing responses (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Desimone & Duncan, 1995; Duncan, 2013; Gollwitzer, 1999; Miller & Cohen, 2001; Miyake et al., 2000; Posner & Dehaene, 1994; Posner & DiGirolamo, 1998; Stuss & Knight, 2002). Cognitive control involves several related, yet dissociable abilities (Miyake et al., 2000), including working memory (D'Esposito & Postle, 2015; Funahashi, Chafee, & Goldman‐Rakic, 1993; Fuster & Alexander, 1971; Goldman‐Rakic, 1987), representation of rules and context (Asaad, Rainer, & Miller, 2000; Bunge, 2004; Cohen & Servan‐Schreiber, 1992; Dixon & Christoff, 2012; Koechlin, Ody, & Kouneiher, 2003; Miller & Cohen, 2001; Munakata et al., 2011), conflict and error detection (Botvinick et al., 2001; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Ullsperger, Danielmeier, & Jocham, 2014), inhibition of prepotent responses (Aron, Robbins, & Poldrack, 2004), abstract thought and reasoning (Christoff et al., 2001; Christoff, Keramatian, Gordon, Smith, & Madler, 2009), and set‐shifting (Crone, Wendelken, Donohue, & Bunge, 2006; Dias, Robbins, & Roberts, 1996; Meiran, 1996; Meiran, 2000; Rushworth, Passingham, & Nobre, 2002).
While early work identified the prefrontal cortex (PFC) as a critical neural substrate (Desimone & Duncan, 1995; Duncan, 2001; Fuster, 1989; Miller & Cohen, 2001; Passingham & Wise, 2012; Stuss & Knight, 2002; Watanabe, 2017), it soon became clear that a much broader network of regions support cognitive control, including posterior parietal, lateral temporal, insular, and mid‐cingulate cortices, as well as parts of the basal ganglia. Together, these regions are often referred to as the multiple demand (MD) system, although recent network neuroscience approaches suggest that they may form at least two distinct functional networks, known as the frontoparietal control network (FPCN), and the salience/cingulo‐opercular network (Cole et al., 2013; Cole, Repovs, & Anticevic, 2014; Cole & Schneider, 2007; Crittenden, Mitchell, & Duncan, 2016; Dixon et al., 2018; Dosenbach et al., 2007; Duncan, 2010; Mitchell et al., 2016; Seeley et al., 2007; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). Cognitive control regions flexibly represent a variety of task‐relevant information and exert a top‐down influence on other regions, guiding activation in accordance with current task demands (Badre & D'Esposito, 2009; Braver, 2012; Buschman & Miller, 2007; Crowe et al., 2013; Desimone & Duncan, 1995; Dixon, Fox, & Christoff, 2014b; Duncan, 2010; Egner & Hirsch, 2005; Miller & Cohen, 2001; Tomita, Ohbayashi, Nakahara, Hasegawa, & Miyashita, 1999).
2. THE EFFECTS OF MOTIVATION ON COGNITIVE CONTROL
As research progressed in delineating the components of cognitive control, a separate stream of inquiry focused on the neural mechanisms of assigning value to stimuli and value‐guided decision making (Daw, Niv, & Dayan, 2005; Dixon & Christoff, 2014; Dixon, Thiruchselvam, Todd, & Christoff, 2017; Levy & Glimcher, 2012; O'Doherty et al., 2004; Rangel, Camerer, & Montague, 2008; Rangel & Hare, 2010; Rushworth, Noonan, Boorman, Walton, & Behrens, 2011; Schoenbaum & Esber, 2010). The past decade has seen a synthesis of these fields with a surge of interest in understanding how value influences the decision of whether or not to engage cognitive control and the efficacy of implementing control (Botvinick & Braver, 2015; Braver et al., 2014; Cohen, Braver, & Brown, 2002; Cools, 2016; Dixon, 2015; Dixon & Christoff, 2012; Hazy, Frank, & O'Reilly, 2007; McGuire & Botvinick, 2010; O'Reilly, Herd, & Pauli, 2010). This line of inquiry is yielding new insights into mechanisms that allow the desire to achieve a specific outcome to interact with the cognitive processes that are necessary to realize that outcome and may ultimately provide critical information about pathological conditions that involve altered motivation–cognition interactions including depression, schizophrenia, ADHD, and anxiety (Barkley, 1997; Bishop, Duncan, Brett, & Lawrence, 2004; Chung & Barch, 2015; Davidson, 2000; Heller et al., 2009; Kaiser et al., 2015a; Kaiser, Andrews‐Hanna, Wager, & Pizzagalli, 2015b; Nigg & Casey, 2005; Pessoa, 2008; Shackman et al., 2011; Shackman et al., 2016).
Recent studies have shown that individuals are strongly biased toward choosing habits and simple tasks over more complex or demanding tasks that require cognitive control (Botvinick & Braver, 2015; Dixon & Christoff, 2012; Kool, McGuire, Rosen, & Botvinick, 2010; McGuire & Botvinick, 2010). This has led to notion that cognitive control carries an intrinsic effort cost. This effort cost can be offset by the opportunity to acquire a rewarding outcome. Studies have shown that participants are considerably more likely to engage cognitive control if doing so will result in a larger reward than if they chose a habitual action (Dixon & Christoff, 2012; Westbrook, Kester, & Braver, 2013). Thus, cognitive control engagement can be understood as a special case of cost/benefit decision‐making whereby the expected value of the outcome that will result from engaging cognitive control is weighed against the effort cost of its implementation (Botvinick & Braver, 2015; Dixon & Christoff, 2012; Shenhav, Botvinick, & Cohen, 2013).
Following the decision to engage cognitive control, the opportunity to earn a reward can also influence the efficacy of implementing control processes. In one study, participants performed a modified Stroop task during which they decided whether an image was a building or a house and had to ignore letters overlaid on the images (Padmala & Pessoa, 2011). The letters could be neutral (XXXXX), congruent with the image (e.g., HOUSE printed over a house image), or incongruent (e.g., BLDNG printed over a house image). Pretrial cues indicated whether monetary rewards were available or not available, and participants could only earn rewards if performance was fast and accurate. The results demonstrated enhanced implementation of cognitive control, manifested as reduced interference effects on incongruent trials when rewards were available (Padmala & Pessoa, 2011). This incentive effect may reflect a sharpening of the representation of task‐relevant information (Etzel, Cole, Zacks, Kay, & Braver, 2015; Histed, Pasupathy, & Miller, 2009), thus providing more effective modulation of sensorimotor processes that support performance. Incentive‐based facilitation of behavioral performance has been reported across numerous studies using a range of cognitive control paradigms (Chiew & Braver, 2013, 2014; Chiew, Stanek, & Adcock, 2016; Dixon & Christoff, 2012; Etzel et al., 2015; Ivanov et al., 2012; Jimura, Locke, & Braver, 2010; Krebs, Boehler, Roberts, Song, & Woldorff, 2012; Locke & Braver, 2008; Padmala & Pessoa, 2011; Taylor et al., 2004).
3. THE NEURAL BASIS OF MOTIVATIONAL EFFECTS ON COGNITIVE CONTROL
Functional neuroimaging studies have identified brain regions associated with the influence of motivation on the implementation of cognitive control (Bahlmann, Aarts, & D'Esposito, 2015; Beck, Locke, Savine, Jimura, & Braver, 2010; Engelmann, Damaraju, Padmala, & Pessoa, 2009; Gilbert & Fiez, 2004; Ivanov et al., 2012; Kouneiher, Charron, & Koechlin, 2009; Locke & Braver, 2008; Padmala & Pessoa, 2011; Pochon et al., 2002; Rowe, Eckstein, Braver, & Owen, 2008; Taylor et al., 2004). In one study, Jimura et al. (2010) used a Sternberg task with two types of task blocks. One block consisted of only nonreward trials, whereas the other block consisted of trials with varying outcomes: no reward, low reward ($0.25), or high reward ($0.75). On each trial, participants were presented with a five‐word memory set and then had to indicate whether a subsequent probe word matched one of the items in the memory set. The results demonstrated a shift from transient to sustained activation in lateral prefrontal and parietal cortices during reward versus no reward blocks, and individual differences in reward sensitivity correlated with the magnitude of sustained activation in reward contexts (Jimura et al., 2010).
These results can be interpreted in terms of the dual mechanisms of control (DMC) framework, which suggests that reward incentives shift the type and timing of cognitive control (Braver, 2012; Chiew & Braver, 2013; Jimura et al., 2010). This theory posits two temporally defined cognitive control mechanisms: (a) a proactive mechanism consisting of sustained activation of task‐relevant information (e.g., task rules) across trials, which facilitates the encoding of new information on each trial and the preparation of a target response and (b) a reactive mechanism consisting of the stimulus‐triggered transient re‐activation of rule information on a trial‐by‐trial basis. Frontoparietal activation dynamics support the idea that reward incentives lead to greater reliance on proactive control, consistent with the DMC model.
Numerous studies have now observed elevated frontoparietal activation when cognitive control is performed in the service of obtaining rewarding outcomes (Boehler, Schevernels, Hopf, Stoppel, & Krebs, 2014; Engelmann et al., 2009; Gilbert & Fiez, 2004; Ivanov et al., 2012; Kouneiher et al., 2009; Locke & Braver, 2008; Padmala & Pessoa, 2011; Paschke et al., 2015; Pochon et al., 2002; Rowe et al., 2008; Soutschek, Stelzel, Paschke, Walter, & Schubert, 2015; Taylor et al., 2004). In addition, frontoparietal regions encode associations between specific rules and expected reward outcomes (Dixon & Christoff, 2012), exhibit more differentiated coding of task rules on incentivized trials (Etzel et al., 2015), and are sensitive to the interaction between control level and reward availability (Bahlmann et al., 2015; Ivanov et al., 2012; Padmala & Pessoa, 2011; Soutschek et al., 2015). These regions are also recruited during value‐based decision‐making, and when participants plan and monitor progress toward future desired outcomes (Crockett et al., 2013; Dixon, Fox, & Christoff, 2014a; Gerlach, Spreng, Madore, & Schacter, 2014; Jimura, Chushak, & Braver, 2013; McClure, Laibson, Loewenstein, & Cohen, 2004). Finally, single‐cell recordings in nonhuman primates have revealed reward‐contingent enhancement of lateral PFC neural firing related to working memory and task rules (Histed et al., 2009; Leon & Shadlen, 1999; Watanabe, 1996; Watanabe & Sakagami, 2007). Thus, frontoparietal regions may integrate task‐relevant information and expected motivational outcomes (Dixon & Christoff, 2014; Pessoa, 2008; Watanabe, 2017; Watanabe & Sakagami, 2007).
4. THE CURRENT META‐ANALYSIS
Although numerous studies of motivated cognitive control have reported activation in frontoparietal regions, the consistency of activations across these studies has yet to be systematically examined. The present study sought to characterize the network of brain regions that are consistently recruited during motivated cognitive control. To this end, we used a quantitative approach, activation likelihood estimation (ALE), to identify regions that show consistent recruitment in human neuroimaging studies of cognitive control that included a manipulation of reward incentive availability. Meta‐analyses are critical for identifying generalizable associations between cognitive processes and brain activation patterns, because it is not possible to control for all extraneous variables and isolate a specific cognitive process in a single study (Yarkoni, Poldrack, Van Essen, & Wager, 2010). In addition, the small sample sizes of typical functional magnetic resonance imaging (fMRI) experiments mean that statistical analyses are susceptible to false positives (Yarkoni et al., 2010). A meta‐analysis combines a set of studies that ostensibly tap a common cognitive process yet differ in specific task details and thereby provides strong inferences about brain‐cognition mappings. We additionally used Neurosynth to identify regions that are consistently recruited in studies that use the term “cognitive control” and in studies that use the term “incentive.” Regions that are recruited by both domains may bridge cognitive and motivational functions. While the ALE analysis provided a conservative and rigorous analysis based on a set of carefully selected studies, the Neurosynth analysis provided a complementary perspective based on a liberal exploration of a much wider literature. In addition, this analysis allowed us to compare the overall collection of regions activated by cognitive control tasks to the subset of regions that are sensitive to both domains. This allows for some degree of specificity by distinguishing the regions that are important for incentive‐based modulation of cognitive control from those that play a more general role in control‐related functions. Finally, we performed two additional exploratory ALE analyses to examine activations separately during cue and target periods. During cue periods, participants are presented with information about task rules for responding to stimuli and expected payoffs. This period thus allows for preparatory construction rule‐outcome associations in service of proactive control engagement. During target periods, participants respond to stimuli and must mobilize appropriate viscero‐motor states to facilitate faster and more accurate actions when a reward is on the line. This analysis allowed us to examine the extent to which cue and target periods rely on similar versus distinct brain systems.
5. MATERIALS AND METHODS
5.1. Search strategy
We conducted a literature search through PubMed and Google Scholar to identify peer‐reviewed neuroimaging studies that have investigated motivated cognitive control. We began by searching the key terms “fMRI” AND (“reward” OR “motivation”) AND (“cognitive control” OR “executive function” OR “working memory”). We then read the abstract of each paper to confirm or reject it as a candidate study for inclusion in the meta‐analysis. We only focused on activations, because there are very few deactivations reported in the literature. In addition we focused on the effect of reward, because only a few studies have looked at the effect of punishment. To be included in the analysis, studies had to fulfill the following criteria: (a) employ fMRI and report resulting activation coordinates; (b) include a cognitive control task (e.g., Stroop) with a manipulation of motivational incentive (i.e., reward versus no reward, or high versus low reward conditions); (c) include healthy adult human participants; and d) report results from a whole‐brain analysis. Several studies of motivated cognitive control employed ROI‐based analyses and were not included in the meta‐analysis, given that ALE requires whole‐brain analyses to provide unbiased results. Sixteen studies were found that matched the inclusion criteria (Table 1). The presence of reward was associated with significantly improved behavioral performance (decreased reaction time and/or increased accuracy) in all but one of the 16 studies.
Table 1.
Studies included in the meta‐analysis
| Study | n | Task | Behavior | Trial period | Analysis | Number of peak foci |
|---|---|---|---|---|---|---|
| Pochon et al. (2002) | 6 | Working memory | Trial | Overlap of main effects of cognitive load and reward | 10 | |
| Gilbert and Fiez (2004) | 22 | Working memory | Acc ↑ | Delay | Main effect of reward | 1 |
| Taylor et al. (2004) | 10 | Working memory | RT ↓ | Target | Main effect of reward | 5 |
| Delay | Main effect of reward | 6 | ||||
| Target | Main effect of reward | 3 | ||||
| Cognitive load x reward | 1 | |||||
| All (collapsed) | Main effect of reward | 9 | ||||
| Locke and Braver (2008) | 16 | AX‐CPT | Acc ↑, RT ↓ | Block | Main effect of reward | 20 |
| Rowe et al. (2008) | 20 | AX‐CPT | Acc ↑, RT ↓ | Block | Parametric effect of reward × trial type | 14 |
| Engelmann et al. (2009) | 20 | Attentional cueing | Acc ↑ | Cue | Main effect of reward | 20 |
| Target | Main effect of reward | 10 | ||||
| Cue validity × reward | 6 | |||||
| Block | Main effect of reward | 5 | ||||
| Kouneiher et al. (2009) | 16 | Rule‐use | Acc ↑, RT ↑ | Cue/target | Trial type × reward value | 3 |
| Block | Main effect of reward | 3 | ||||
| Jimura et al. (2010) | 31 | Sternberg | RT ↓ | Block | Main effect of reward | 2 |
| Padmala and Pessoa (2011) | 50 | Stroop | Acc ↑, RT ↓ | Target | Main effect of reward | 6 |
| Interference × reward interaction | 10 | |||||
| Dixon and Christoff (2012) | 15 | Rule use | RT ↓ | Cue | Rule × reward interaction | 9 |
| Krebs et al. (2012) | 14 | Attentional cueing | Acc ↑, RT ↓ | Cue | Main effect of reward | 13 |
| Overlap of main effects of difficulty and reward | 10 | |||||
| Difficulty × reward interaction | 7 | |||||
| Ivanov et al. (2012) | 16 | Flanker | RT ↓ | Target | Conflict × reward interaction | 6 |
| Boehler et al. (2014) | 16 | Stop signal | RT ↓ | Target | Overlap of main effects of stopping and reward | 3 |
| Paschke et al. (2015) | 115 | Flanker | RT ↓ | Target | Congruency × reward interaction | 1 |
| Block | Overlap of main effects of task and reward | 1 | ||||
| Target | Overlap of main effects of task and reward | 4 | ||||
| Soutschek et al. (2015) | 20 | Stroop | RT ↓ | Target | Main effect of reward | 3 |
| Target | Congruency × expectancy × reward interaction | 6 | ||||
| Etzel et al. (2015) | 20 | Rule‐use | Acc ↑, RT ↓ | Cue | Main effect of reward | 7 |
| Total = 16 | 407 | 204 |
Note. When specifying the trial period, “trial” indicates reward effect across entire trial period, while “block” indicates reward effect across multiple trials. The column behavior specifies the change in accuracy and/or reaction time (RT) on rewarded versus nonrewarded trials.
5.2. Data extraction
From these 16 studies, we collected data on sample size, task, type of contrast (e.g., main effect of reward during task, or reward × cognitive load interaction), task period (e.g., cue, delay, or target), and peak activation coordinates (Table 1). The meta‐analysis included studies with different types of contrasts, but each examined the neural substrates that link motivational incentives to cognitive control. In every study, participants were aware that they could earn reward incentives on certain trials contingent on their task performance. Incentive information was signaled with task cues. There were three categories of contrasts performed in these studies: (a) main effect of reward during a cognitive control task; (b) conjunction effects showing overlapping activation in relation to cognitive demands and sensitivity to reward value; and (c) interaction between cognitive control level and presence of incentive. While there are some differences in these three types of contrasts, all converge on related processes that support incentive‐based modulation of cognitive control. It should be noted that we included results from the main effect of reward during task performance (e.g., during delay or target periods) but excluded results related to a main effect of reward during cue periods that only included the expected reward incentive, as this is likely to mainly capture reward processing alone, without an interaction with cognitive processes. Notably, if the cue period signaled motivational information and cognitive task demands (e.g., rules) that could be activated in a preparatory manner, then we included these foci.
Studies have repeatedly shown that responses are faster and/or more accurate on reward versus no reward trials during cognitive control tasks. While both types of trials involve control demands, the enhanced behavioral performance on reward trials indicate that the reward incentive is processed in a manner that facilitates control operations and the top‐down modulation of action selection. Indeed, prior work suggests that on reward trials, there is a change in how task information is represented in the brain (Etzel et al., 2015; Histed et al., 2009; Watanabe, 1996). There is also documented evidence of a sustained change in activation within brain regions that are sensitive to control demands on reward versus no‐reward trials (Jimura et al., 2010; Locke & Braver, 2008). Therefore, a main effect of reward in the context of engaging cognitive control‐related functions is capturing something unique that goes beyond general reward processing in the absence of control demands. Supporting this idea, lateral frontoparietal regions are rarely engaged in simple reward tasks that do not involve control demands, yet are robustly engaged during reward trials in the context of a cognitive control task. In addition, studies that use conjunction analyses target the neural basis of incentive‐based modulation of cognitive control by identifying regions that are sensitive to task demands, and from these regions, identifying those that are also sensitive to reward value. The reported regions may contribute to the process whereby control demands become associated with expected reward outcomes.
For studies that had multiple periods (e.g., delay, probe), we included foci from each period; however, if a given brain region was activated in multiple periods, it was only included once in the meta‐analysis. However, for the additional analyses that separately examined cue period and target period activations, we used all available foci. We based target‐ and cue‐specific analyses on the results reported in each study. If a study reported that their task design allowed for separation of cue and target effects and reported results for one or both task phases then the foci were included in our meta‐analysis. Given that not all studies had separate cue and target periods, this resulted in a smaller sample size for these analyses.
5.3. ALE meta‐analytic data analysis
We analyzed the activation coordinates using a random effects meta‐analysis, ALE (Eickhoff et al., 2009; Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012; Laird et al., 2005; Turkeltaub et al., 2012) implemented with GingerALE 2.3.6 software (UT Health Science Center Research Imaging Institute, San Antonio, TX). This is the updated version of GingerALE that has fixed the error related to cluster‐level FWE correction (Eickhoff, Laird, Fox, Lancaster, & Fox, 2017). Coordinates reported in Talairach space were first converted to MNI space using Ginger ALE's foci converter function: Talairach to MNI (SPM). ALE models the uncertainty in localization of activation foci across studies using Gaussian probability density distributions. The voxel‐wise union of these distributions yields the ALE value, a voxel‐wise estimate of the likelihood of activation, given the input data. The algorithm aims at identifying significantly overlapping clusters of activation between studies. ALE treats activation foci from single studies as 3D Gaussian probability distributions to compensate for spatial uncertainty. The width of these distributions was statistically determined based on empirical data for between subject and between template variability (Eickhoff et al., 2009). In addition, studies were weighted according to sample size, reflecting the idea that large sample sizes more likely reflect a true localization. This is implemented in terms of a widening Gaussian distribution with lower sample sizes and a smaller Gaussian distribution (and thus a stronger impact on ALE scores) with larger sample sizes (Eickhoff et al., 2009). Modeled activation maps for each study were generated by combining the probabilities of all activation foci for each voxel (Turkeltaub et al., 2012). These ALE scores were then compared to an ALE null distribution (Eickhoff et al., 2012) in which the same number of activation foci was randomly relocated and restricted by a gray matter probability map (Evans, Kamber, Collins, & MacDonald, 1994). Spatial associations between experiments were treated as random while the distribution of foci within an experiment was treated as fixed. Thereby random effects inference focuses on significant convergence of foci between studies rather than convergence within one study. The ALE scores from the actual meta‐analysis were then tested against the ALE scores obtained under this null‐distribution yielding a p‐value based on the proportion of equal or higher random values. For the main ALE analysis, we used a cluster‐forming threshold at the voxel level of p < .001 and a cluster‐level threshold of p < .05 FWE corrected for multiple comparisons. We also ran separate analyses on foci from the cue period and foci from the target period. Given that fewer studies were included in each analysis, we used a more liberal p < .001, uncorrected threshold, with a minimum cluster size of 200 mm3. As such, the results should be interpreted with caution. Results were visualized with MRIcron software (Rorden, Karnath, & Bonilha, 2007).
5.4. Neurosynth meta‐analyses
The main ALE analysis was based on a carefully selected group of studies and used a strict statistical threshold to control for false positives. Accordingly, it likely captures true regions underlying motivated cognitive control. On the other hand, it may overlook some regions that are below threshold, but nevertheless play a role in functions that contribute to motivated cognitive control. Thus, we also utilized an alternative, complementary approach: Neurosynth forward inference meta‐analyses (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). Neurosynth makes it possible to examine consistent activation patterns across a massive database of studies, providing strong power to detect regions that are activated in the studies of interest. In this way, Neurosynth is less likely to miss relevant regions. However, Neurosynth performs an automated selection of studies based on certain key words (e.g., “cognitive control”) and therefore has less specificity in terms of delineating a select group of studies. In this way, Neurosynth analyses provide a complementary approach to ALE analyses. To perform such automated meta‐analyses, Neurosynth divides the entire database of coordinates into two sets: those that occur in articles containing a particular term and those that do not. A large‐scale meta‐analysis is then performed comparing the coordinates reported for studies with and without the term of interest. Forward inference maps reflect z‐scores corresponding to the likelihood that each voxel will activate if a study uses a particular term (P[Activation|Term]) and are corrected for multiple comparisons using a false discovery rate (FDR) of q = .01. Here, we conducted forward inference meta‐analyses using the terms “cognitive control” and “incentive” and looked for brain areas demonstrating overlapping recruitment across both domains. We reasoned that if a brain region is activated in studies of cognitive control and is activated in studies of incentive processing, then it is a good candidate for bridging cognitive and motivational functions. We used forward inference rather than reverse inference analyses because we were not looking for regions that are selective to incentive processing or cognitive control, but rather, are just involved in these domains. In other words, we were not looking to identify functional specialization in any regions. Our aim was to identify the constellation of regions that together support motivated cognitive control. Given this aim, forward inference analyses were ideal.
6. RESULTS
6.1. ALE meta‐analysis: All foci
We first performed an analysis on all foci to identify regions that consistently demonstrate increased activation during cognitive control when rewards are available versus not available. The ALE analysis revealed four large clusters (Figure 1; Table 2). These right‐lateralized regions included the inferior frontal sulcus (IFS) extending into the mid‐dorsolateral PFC (mid‐DLPFC), mid‐intraparietal sulcus (mid‐IPS) extending into the anterior inferior parietal lobule (aIPL), anterior insula, and the anterior mid‐cingulate cortex (aMCC) extending into the adjacent pre‐supplementary motor area (pre‐SMA).
Figure 1.
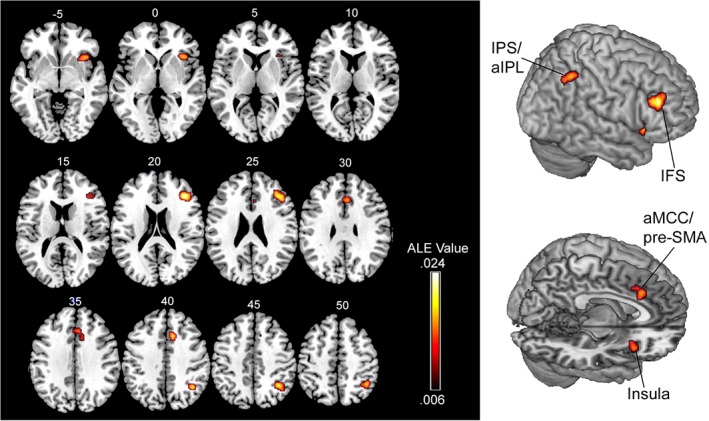
Whole‐brain ALE meta‐analytic results (p < .05 FWE corrected) showing brain regions that are consistently recruited across studies (N = 16) of motivated cognitive control. These results indicate that regions associated with the frontoparietal control network (IFS and IPS/aIPL) and regions associated with the salience network (insula and aMCC/pre‐SMA) show greater activation during cognitive control tasks on trials in which a reward incentive can be earned based on performance, versus trials in which no incentive is presented. Numbers denote z‐coordinates in MNI space. IFS, inferior frontal sulcus; IPS/aIPL, intraparietal sulcus/anterior inferior parietal lobule; aMCC/pre‐SMA, anterior mid‐cingulate cortex/pre‐supplementary motor area [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Significant ALE clusters showing consistent recruitment in studies of motivated cognitive control
| Region | Cluster size (mm3) | Peak ALE value | Weighted peak foci in MNI space (x,y,z) |
|---|---|---|---|
| R IFS/mid‐dorsolateral prefrontal cortex | 2032 | 0.0252 | 40, 32, 22 |
| R anterior insula | 1,560 | 0.0168 | 38, 20, −2 |
| R intraparietal sulcus/inferior parietal lobule | 1,544 | 0.0213 | 39, −53, 45 |
| R anterior mid‐cingulate cortex/pre‐supplementary motor area | 1,288 | 0.0172 | 10, 20, 40 |
Note. L, left; R, right; DLPFC, dorsolateral prefrontal cortex.
6.2. Neurosynth meta‐analyses
Although the strict inclusion criteria in the ALE analysis offer confidence that the identified regions do play a key role in motivated cognitive control, it is possible that this conservative analysis may overlook other relevant regions. Thus, as a complementary analysis, we used Neurosynth to identify regions that are consistently activated in studies that use the term “cognitive control” (N = 428 studies) and in studies that use the term “incentive” (N = 109 studies). We focused on brain areas demonstrating overlapping recruitment across these domains and may play a role in linking control demands to motivational outcomes. Notably, the regions demonstrating this pattern included all of the regions identified in the ALE meta‐analysis (Figure 2). This analysis additionally identified homologous regions in the left hemisphere, as well as the right rostrolateral prefrontal cortex (RLPFC), bilateral inferior frontal junction (IFJ) and pre‐motor cortex (PMC), bilateral caudate nucleus extending into the nucleus accumbens (NAcc), bilateral medial thalamus, and bilateral hippocampus (Figure 2). Interestingly, the regions that were sensitive to both cognitive control and incentive were a select subset of the regions engaged during cognitive control. This finding provides some preliminary evidence regarding specificity and functional differences across the cognitive control network. It suggests that only some regions within this broader network may facilitate the effect of reward‐incentives on cognitive control performance.
Figure 2.
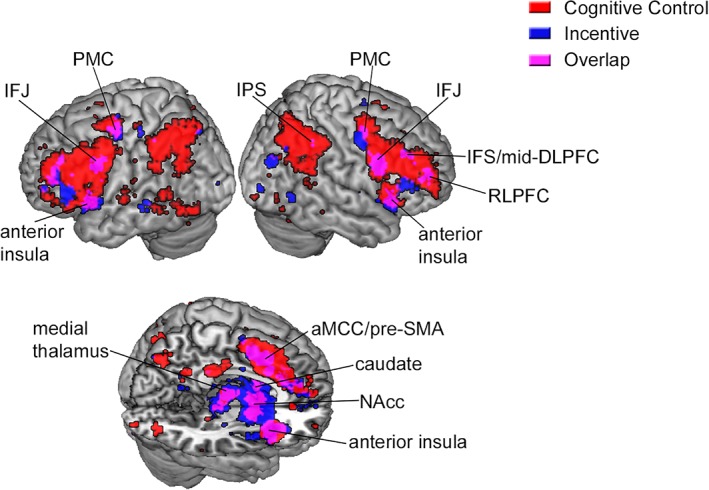
Rendered surface display of Neurosynth forward inference meta‐analyses using the terms “cognitive control” and “incentive,” corrected for multiple comparisons using a false discovery rate of q = .01. While a broad network of regions is consistently activated in studies of cognitive control, only a subset of these regions are also recruited in studies of incentive processing, and may play a role in motivated cognitive control. This finding corroborates the ALE analysis, but also reveals a number of additional regions in the left hemisphere and subcortical structures. IFS, inferior frontal sulcus; IFJ, inferior frontal junction; PMC, premotor cortex; IPS, intraparietal sulcus; DLPFC, dorsolateral prefrontal cortex; aMCC/pre‐SMA, anterior mid‐cingulate cortex/pre‐supplementary motor area; NAcc, nucleus accumbens [Color figure can be viewed at http://wileyonlinelibrary.com]
6.3. ALE meta‐analyses: Cue and target period foci
In our final analysis, we examined similarities and differences in neural recruitment during cue and target periods. Given that these analyses were based on a limited number of studies and a more lenient statistical threshold, these results should be viewed as exploratory. The brain regions that were consistently recruited during the cue period and may contribute to the proactive engagement of value‐modulated control signals included the right IFJ/PMC, left ventral IPS, bilateral caudate, right dorsal posterior cingulate cortex (PCC), right midbrain near the ventral tegmental area (VTA), and left medial thalamus (Figure 3). On the other hand, the brain regions that were consistently recruited during the target period and may contribute to the mobilization of viscero‐motor processes during action selection, included the right anterior insula, right aMCC, right IPS/aIPL, right medial thalamus, left ventral IPS, and left IFJ (Figure 3 ). The only region common to both trial events was the left ventral IPS, suggesting that value‐based modulation of control processes during cue and target periods may rely on largely distinct neural systems. However, this is a tentative conclusion, tempered by the low power of these analyses.
Figure 3.
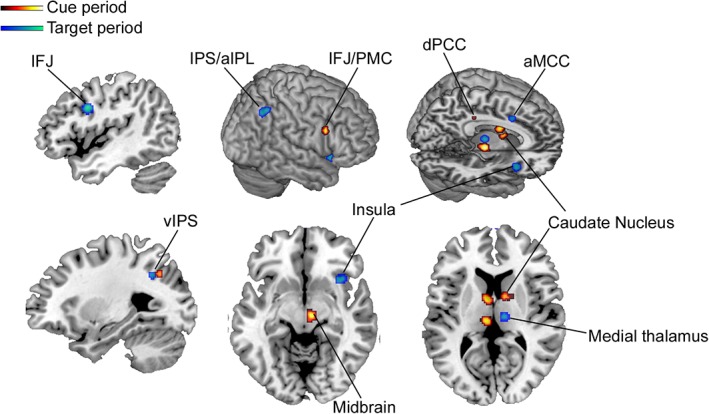
Significant ALE meta‐analytic clusters associated with different trial periods during motivated cognitive control tasks (p < .001 uncorrected). The warm colors demonstrate regions that are consistently recruited during the cue period of motivated cognitive control tasks and may contribute to proactive control functions, whereas the cool colors demonstrate regions that are consistently recruited during the target (response) period of motivated cognitive control tasks and may contribute to reactive control functions. IPS/aIPL, intraparietal sulcus/anterior inferior parietal lobule; vIPS, ventral intraparietal sulcus; dPCC, dorsal posterior cingulate cortex; aMCC, anterior mid‐cingulate cortex; IFJ, inferior frontal junction; PMC, pre‐motor cortex [Color figure can be viewed at http://wileyonlinelibrary.com]
7. DISCUSSION
Cognitive control is often enhanced when rewards are contingent on performance. This enhancement manifests as faster and more accurate responses and is often accompanied by elevated brain activation in numerous cortical regions. Here, we sought to characterize the brain regions that reliably demonstrate this pattern and may support incentive‐based behavioral improvements in cognitive control. Using quantitative ALE and Neurosynth meta‐analyses, we identified a select constellation of multimodal association cortices and subcortical regions known to play key roles in cognitive and motivational processing. An exploratory analysis also revealed differences in recruitment during cue versus target periods, suggesting partially distinct systems may underlie the proactive engagement of control versus the mobilization of viscero‐motor states that support action execution.
Several regions were implicated in both the ALE and Neurosynth analyses including the IFS, intraparietal sulcus (IPS)/anterior inferior parietal lobule (aIPL), anterior mid‐cingulate cortex (aMCC)/pre‐supplementary motor area (pre‐SMA), and anterior insula. The fact that similar results were obtained with different analysis criteria provides strong evidence that these regions are centrally involved in value‐based modulation of cognitive control. Interestingly, the Neurosynth analysis revealed that only a subset of regions engaged during cognitive control are also engaged during reward processing. For example, the posterior middle temporal gyrus and parts of the lateral prefrontal and parietal cortices only demonstrated consistent recruitment during cognitive control. This suggests that there may be a select group of regions including the IFS, IPS/aIPL, aMCC/pre‐SMA, and insula that integrate control demands and expected outcomes.
The aforementioned regions have well‐established roles in supporting cognitive control and adaptive behavior via top‐down modulation of sensory and motor processing (Cole et al., 2014; Dosenbach et al., 2006; Duncan, 2010; Miller & Cohen, 2001). The IFS and IPS/aIPL are part of the FPCN (Dixon et al., 2018; Power et al., 2011; Spreng et al., 2010; Vincent et al., 2008; Yeo et al., 2011) and contribute to working memory and the flexible representation of task rules (Badre & D'Esposito, 2009; Brass, Derrfuss, Forstmann, & von Cramon, 2005; Bunge, 2004; De Baene, Kuhn, & Brass, 2011; Derrfuss, Brass, Neumann, & von Cramon, 2005; Dixon & Christoff, 2012; Dumontheil, Thompson, & Duncan, 2011; Koechlin et al., 2003; Wallis, Anderson, & Miller, 2001). Neurons in these regions exhibit dynamic coding properties, signaling any currently relevant information (Duncan, 2010; Stokes et al., 2013), and rapidly updating their pattern of global functional connectivity according to task demands (Cole et al., 2013; Fornito, Harrison, Zalesky, & Simons, 2012; Gao & Lin, 2012; Spreng et al., 2010). One possibility is that elevated activation during motivated cognitive control reflects an amplification and sharpening of task information (e.g., rules) as a result of modulatory inputs from reward processing regions (Cohen et al., 2002; Etzel et al., 2015; Histed et al., 2009; Kouneiher et al., 2009). It could also reflect a shift in the temporal dynamics of cognitive control, toward a proactive mode of control (Braver, 2012; Jimura et al., 2010). When performance needs to be fast and accurate to procure a reward, FPCN regions exhibit greater sustained activation and reduced transient/reactive activation, ostensibly reflecting the active maintenance of task rules across trials (Braver, 2012; Jimura et al., 2010).
Several lines of evidence suggest that FPCN regions may play an integrative role, directly representing control demands in relation to expected outcomes. First, Dixon and Christoff (2012) found that the FPCN flexibly represented trial‐to‐trial shifts in the association between specific task rules and expected reward outcomes (Dixon & Christoff, 2012). This finding is consistent with the fact that FPCN neurons encode not only rule information but also experienced and expected reward and punishment (Matsumoto, Suzuki, & Tanaka, 2003; Pan, Sawa, Tsuda, Tsukada, & Sakagami, 2008; Wallis & Miller, 2003; Watanabe, Hikosaka, Sakagami, & Shirakawa, 2002)(Abe & Lee, 2011; Asaad & Eskandar, 2011; Hikosaka et al., 2000; Histed et al., 2009; Hosokawa & Watanabe, 2012; Kennerley & Wallis, 2009; Kim, Hwang, & Lee, 2008; Klein, Deaner, & Platt, 2008; Kobayashi et al., 2006; Platt & Glimcher, 1999; Seo, Barraclough, & Lee, 2007; Watanabe, 1996; Watanabe et al., 2002). Second, McGuire and Botvinick (2010) found that the lateral PFC signaled the cost of exerting cognitive effort, suggesting that the FPCN plays a role in linking control demands to other parameters that are important for deciding when to implement control. In fact, numerous studies have demonstrated FPCN activation during value‐based decision‐making (Christopoulos, Tobler, Bossaerts, Dolan, & Schultz, 2009; Diekhof & Gruber, 2010; Gianotti et al., 2009; Huettel, Song, & McCarthy, 2005; Hutcherson, Plassmann, Gross, & Rangel, 2012; Jimura et al., 2013; Jimura & Poldrack, 2012; Lebreton et al., 2013; McClure et al., 2004; Plassmann, O'Doherty, & Rangel, 2007; Plassmann, O'Doherty, & Rangel, 2010; Tanaka et al., 2004; Tobler, Christopoulos, O'Doherty, Dolan, & Schultz, 2009; Vickery, Chun, & Lee, 2011; Weber & Huettel, 2008). Third, several studies have shown an interaction between control level and reward expectancy in the FPCN (Bahlmann et al., 2015; Ivanov et al., 2012; Padmala & Pessoa, 2011). Finally, disruption of the FPCN—via transcranial magnetic stimulation or due to a lesion—disrupts value processing and leads to altered motivation (Camus et al., 2009; Essex, Clinton, Wonderley, & Zald, 2012; Paradiso, Chemerinski, Yazici, Tartaro, & Robinson, 1999; Zamboni, Huey, Krueger, Nichelli, & Grafman, 2008). Together, these findings suggest that the FPCN may play an integrative role, serving as a bridge between control demands and motivational outcomes (Dixon et al., 2017; Dixon & Christoff, 2014; Pessoa, 2008; Watanabe, 2017; Watanabe & Sakagami, 2007). In particular the FPCN may encode rule‐outcome associations, specifying the likely reward or punishment to expect if a given set of task rules are employed or not employed.
Our meta‐analytic results also revealed that the anterior insula and anterior mid‐cingulate cortex play key roles in motivated cognitive control. These regions are part of the “salience network” (Menon & Uddin, 2010; Seeley et al., 2007). The insula has a well‐established role in interoception—the representation of internal bodily signals including pain, temperature, respiratory and cardiac sensations (Craig, 2002; Critchley & Harrison, 2013; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Farb, Segal, & Anderson, 2012). This region is also activated during a variety of goal‐directed tasks (Dixon et al., 2014a; Dosenbach et al., 2006; Duncan, 2010; Farb et al., 2012), suggesting that it may serve as a nexus between the FPCN and other interoceptive regions, allowing viscero‐somatic signals to become integrated with task goals (Dixon et al., 2014a; Farb et al., 2012; Jezzini, Caruana, Stoianov, Gallese, & Rizzolatti, 2012). While the aMCC has long been associated cognitive control, there is also a growing understanding of its role in motivational processes. One proposal is that the aMCC plays a role in determining the value of implementing control (Shenhav et al., 2013). The idea is that the aMCC contributes to a cost–benefit analysis based on an integration of various signals (e.g., effort, expected reward magnitude) which then influences the decision of whether it is worth engaging control during a given task, as well as just how much control to exert. Another perspective is that the aMCC plays a role in linking reinforcement to specific actions (i.e., represents action‐outcome associations) (Camille, Tsuchida, & Fellows, 2011; Dixon et al., 2017; Rushworth, Behrens, Rudebeck, & Walton, 2007). Both proposals align with evidence that the aMCC is well connected to the motor system (Morecraft & Tanji, 2009), is sensitive to the effort costs of actions (Croxson, Walton, O'Reilly, Behrens, & Rushworth, 2009; Kurniawan, Guitart‐Masip, Dayan, & Dolan, 2013; Shidara & Richmond, 2002), and links actions to expected rewards and punishment (Alexander & Brown, 2011; Procyk et al., 2014; Rushworth et al., 2007; Shackman et al., 2011). The aMCC and insula may represent information about expected outcomes and task‐related signals throughout different phases of a trial and contribute to anticipatory control processes including the preparation of rules (Denny, Ochsner, Weber, & Wager, 2014; Dosenbach et al., 2006; Knutson & Greer, 2008; Sridharan, Levitin, & Menon, 2008). Interestingly, we found that the anterior insula and aMCC were specifically recruited during the target rather than cue period of motivated cognitive control tasks, which suggests that they may be especially important for target‐period control processes that allow for the efficient selection of task‐relevant actions. One possibility is that these regions provide an interface that supports the translation of abstract task representations into more concrete body states (autonomic activity and action plans) related to the initiation of goal‐directed behavior (Dixon et al., 2017; Farb et al., 2012; Shackman et al., 2011). This idea aligns with prior work demonstrating the involvement of these regions in autonomic arousal (Medford & Critchley, 2010), response competition (Botvinick et al., 2001; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; MacDonald 3rd, Cohen, Stenger, & Carter, 2000) and the activation of motor programs (Gaymard et al., 1998; Jezzini et al., 2012; Stuss et al., 2005; Turken & Swick, 1999).
The Neurosynth analysis also highlighted the caudate nucleus extending into the NAcc, while the cue period ALE analysis highlighted the caudate and the midbrain near the VTA. These regions are part of a dopaminergic midbrain‐striatal circuit that signals prediction errors when there is a discrepancy between expected and actual rewards (Hare, O'Doherty, Camerer, Schultz, & Rangel, 2008; Montague, Dayan, & Sejnowski, 1996; O'Doherty et al., 2004; Schultz, Dayan, & Montague, 1997). Moreover, neurons in this circuit gradually shift the timing of maximal firing from actual outcomes to reward‐predictive cues (Schultz et al., 1997). These regions play a fundamental role in goal‐directed behavior via biasing action selection based on the anticipation of imminent rewards and the opportunity to exercise choice (Knutson, Adams, Fong, & Hommer, 2001; Leotti & Delgado, 2014). Accordingly, this circuit may play a role in broadcasting predicted cue values to other systems involved in constructing rule‐outcome associations, and modulating viscero‐motor processing. Indeed, prior work has outlined detailed models of how the dopaminergic midbrain‐striatal circuit serves a gating function that strengthens or destabilizes current working memory contents depending on task demands (Cohen et al., 2002; Cools, 2016; Hazy, Frank, & O'Reilly, 2006). Specifically, tonic dopamine in the PFC is thought to enhance the stability of working memory content via increased signal to noise ratio (that is, boosting the strength of local recurrent activity versus stimulus‐evoked activity). On the other hand, phasic dopamine is thought to serve as a gating signal, allowing working memory to be updated based on reward‐predicting events (Cohen et al., 2002; Cools, 2016; Hazy et al., 2006).
While the present work focused on identifying regions that are consistently activated during motivated cognitive control, it will be important for future work to characterize the functional interactions between these regions, and modulatory influences on other brain systems (e.g., sensory and motor systems). Functional connectivity and effective connectivity refer to the identification of undirected and directed functional coupling patterns, respectively (Friston, 2011), and provide a method for probing the nature of interactions between brain regions and making inferences about information flow across the network, and how that shapes task‐related activation patterns (Cole, Ito, Bassett, & Schultz, 2016). In addition, it is possible to examine how regional interactions change as a function of task context using psychophysiological interactions among other methods (Smith, Gseir, Speer, & Delgado, 2016). This is particularly relevant for understanding the neural basis of cognitive control, which is thought to rely on distributed interactions among brain systems and the top‐down modulation of task‐specific processing via frontoparietal regions (Egner & Hirsch, 2005; Miller & Cohen, 2001). Indeed, recent work has shown that lateral frontal and parietal regions exhibit flexible, task‐dependent coupling patterns with other regions (Braun et al., 2015; Fornito et al., 2012; Smith et al., 2016; Spreng et al., 2010). To understand motivated cognitive control, it will be critical to understand the nature of interactions between the FPCN, salience network, and midbrain‐striatal circuits.
A few limitations of the current findings should be noted. First, our meta‐analyses were based on peak coordinates and therefore do not include the full extent of data present in raw statistical maps. Image‐based meta‐analyses that combine raw (unthresholded) statistical maps may provide additional information, particularly concerning small but reliable effects and relative deactivation patterns (Gorgolewski et al., 2015). A second limitation is that our analysis was based on studies that employed a number of different cognitive control tasks and functions. One the one hand, this suggests that the identified brain regions support motivated cognitive control in a general sense and are not tied to any particular task. On the other hand, this may obscure the delineation of neural systems that link expected outcomes to different types of executive control. As more studies examine this topic, future work may be able to discern whether incentive effects on different aspects of cognitive control (e.g., response inhibition versus working memory updating) have similar or distinct neural substrates. We were also unable to examine the effect of punishment on cognitive control given the small number of fMRI studies on this topic. Given that the observed frontoparietal regions have been shown to encode information about aversive outcomes in addition to rewarding outcomes (Asaad & Eskandar, 2011; Kobayashi et al., 2006), it is possible that substantial overlap with the current findings would be observed. However, there is some indication in prior work that differences may also appear (Paschke et al., 2015). Future studies may also be able to provide a more in‐depth analysis of brain regions showing incentive effects during specific trial periods (e.g., cue versus delay and target processing). Our results were based on a small number of studies and should be seen as preliminary. Given that we found evidence of distinct brain regions involved in cue versus target periods, this may be a key area for future inquiry to investigate. Another important dimension of motivated cognitive control is incentive type (i.e., primary versus secondary). However, all studies included in this review operationalized motivation with monetary (i.e., secondary) incentives with the exception of Beck et al. (2010). This study compared the effects of primary (juice) and secondary (money) rewards on performance in a Sternberg task. The authors found no significant differences in behavioral improvement between the reward types but did find both regional and temporal differences in brain activation patterns. This underscores the importance of studying the different types of incentive effects separately. Another limitation is that the Neurosynth analysis may have identified regions that play a general role in processing reward information as opposed to incentive processing more specifically. However, the primary ALE analysis was based on studies in which reward was contingent on performance, suggesting that the regions identified in both analyses are likely to be specifically involved in mechanisms that underlie motivated cognitive control. With a growing number of studies in this area, it may be possible for future work to disentangle general and specific reward effects in a large sample of studies that can be meta‐analyzed with Neurosynth. Finally, our analyses were based on regions that were recruited in the relevant studies as a function of BOLD signal magnitude. It remains possible that we missed regions that would be identified using other analysis approaches (e.g., multivariate pattern analysis, or the effect of incentives on the sharpness of rule encoding as in the study by Etzel et al., 2015).
To summarize, the current findings reveal a select constellation of brain regions that are consistently recruited in studies of motivated cognitive control. Flexible interactions between frontoparietal, salience, and dopaminergic midbrain‐striatal networks may underlie the dynamic process by which control signals are precisely tailored based on expected outcomes.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
Parro C, Dixon ML, Christoff K. The neural basis of motivational influences on cognitive control. Hum Brain Mapp. 2018;39:5097–5111. 10.1002/hbm.24348
Contributor Information
Matthew L. Dixon, Email: mattdixon@psych.ubc.ca.
Kalina Christoff, Email: kchristoff@psych.ubc.ca.
REFERENCES
- Abe, H. , & Lee, D. (2011). Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron, 70(4), 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, W. H. , & Brown, J. W. (2011). Medial prefrontal cortex as an action‐outcome predictor. Nature Neuroscience, 14(10), 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177. [DOI] [PubMed] [Google Scholar]
- Asaad, W. F. , & Eskandar, E. N. (2011). Encoding of both positive and negative reward prediction errors by neurons of the primate lateral prefrontal cortex and caudate nucleus. The Journal of Neuroscience, 31(49), 17772–17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad, W. F. , Rainer, G. , & Miller, E. K. (2000). Task‐specific neural activity in the primate prefrontal cortex. Journal of Neurophysiology, 84(1), 451–459. [DOI] [PubMed] [Google Scholar]
- Badre, D. , & D'Esposito, M. (2009). Is the rostro‐caudal axis of the frontal lobe hierarchical? Nature Reviews. Neuroscience, 10(9), 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlmann, J. , Aarts, E. , & D'Esposito, M. (2015). Influence of motivation on control hierarchy in the human frontal cortex. The Journal of Neuroscience, 35(7), 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley, R. A. (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. [DOI] [PubMed] [Google Scholar]
- Beck, S. M. , Locke, H. S. , Savine, A. C. , Jimura, K. , & Braver, T. S. (2010). Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS One, 5(2), e9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, S. , Duncan, J. , Brett, M. , & Lawrence, A. D. (2004). Prefrontal cortical function and anxiety: Controlling attention to threat‐related stimuli. Nature Neuroscience, 7(2), 184–188. [DOI] [PubMed] [Google Scholar]
- Boehler, C. N. , Schevernels, H. , Hopf, J. M. , Stoppel, C. M. , & Krebs, R. M. (2014). Reward prospect rapidly speeds up response inhibition via reactive control. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 593–609. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. , & Braver, T. (2015). Motivation and cognitive control: From behavior to neural mechanism. Annual Review of Psychology, 66, 83–113. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. , Braver, T. S. , Barch, D. M. , Carter, C. S. , & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick, M. , Nystrom, L. E. , Fissell, K. , Carter, C. S. , & Cohen, J. D. (1999). Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature, 402(6758), 179–181. [DOI] [PubMed] [Google Scholar]
- Brass, M. , Derrfuss, J. , Forstmann, B. , & von Cramon, D. Y. (2005). The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences, 9(7), 314–316. [DOI] [PubMed] [Google Scholar]
- Braun, U. , Schafer, A. , Walter, H. , Erk, S. , Romanczuk‐Seiferth, N. , Haddad, L. , … Bassett, D. S. (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proceedings of the National Academy of Sciences of the United States of America, 112(37), 11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver, T. S. (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver, T. S. , Krug, M. K. , Chiew, K. S. , Kool, W. , Westbrook, J. A. , Clement, N. J. , … Somerville, L. H. (2014). Mechanisms of motivation‐cognition interaction: Challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 443–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge, S. A. (2004). How we use rules to select actions: A review of evidence from cognitive neuroscience. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 564–579. [DOI] [PubMed] [Google Scholar]
- Buschman, T. J. , & Miller, E. K. (2007). Top‐down versus bottom‐up control of attention in the prefrontal and posterior parietal cortices. Science, 315(5820), 1860–1862. [DOI] [PubMed] [Google Scholar]
- Camille, N. , Tsuchida, A. , & Fellows, L. K. (2011). Double dissociation of stimulus‐value and action‐value learning in humans with orbitofrontal or anterior cingulate cortex damage. The Journal of Neuroscience, 31(42), 15048–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus, M. , Halelamien, N. , Plassmann, H. , Shimojo, S. , O'Doherty, J. , & Camerer, C. (2009). Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. The European Journal of Neuroscience, 30(10), 1980–1988. [DOI] [PubMed] [Google Scholar]
- Chiew, K. S. , & Braver, T. S. (2013). Temporal dynamics of motivation‐cognitive control interactions revealed by high‐resolution pupillometry. Frontiers in Psychology, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew, K. S. , & Braver, T. S. (2014). Dissociable influences of reward motivation and positive emotion on cognitive control. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 509–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew, K. S. , Stanek, J. K. , & Adcock, R. A. (2016). Reward anticipation dynamics during cognitive control and episodic encoding: Implications for dopamine. Frontiers in Human Neuroscience, 10, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff, K. , Keramatian, K. , Gordon, A. M. , Smith, R. , & Madler, B. (2009). Prefrontal organization of cognitive control according to levels of abstraction. Brain Research, 1286, 94–105. [DOI] [PubMed] [Google Scholar]
- Christoff, K. , Prabhakaran, V. , Dorfman, J. , Zhao, Z. , Kroger, J. K. , Holyoak, K. J. , & Gabrieli, J. D. (2001). Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage, 14(5), 1136–1149. [DOI] [PubMed] [Google Scholar]
- Christopoulos, G. I. , Tobler, P. N. , Bossaerts, P. , Dolan, R. J. , & Schultz, W. (2009). Neural correlates of value, risk, and risk aversion contributing to decision making under risk. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(40), 12574–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, Y. S. , & Barch, D. (2015). Anhedonia is associated with reduced incentive cue related activation in the basal ganglia. Cognitive, Affective, & Behavioral Neuroscience, 15(4), 749–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. D. , Braver, T. S. , & Brown, J. W. (2002). Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology, 12(2), 223–229. [DOI] [PubMed] [Google Scholar]
- Cohen, J. D. , & Servan‐Schreiber, D. (1992). Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychological Review, 99(1), 45–77. [DOI] [PubMed] [Google Scholar]
- Cole, M. W. , Ito, T. , Bassett, D. S. , & Schultz, D. H. (2016). Activity flow over resting‐state networks shapes cognitive task activations. Nature Neuroscience, 19(12), 1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Repovs, G. , & Anticevic, A. (2014). The frontoparietal control system: A central role in mental health. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20(6), 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Reynolds, J. R. , Power, J. D. , Repovs, G. , Anticevic, A. , & Braver, T. S. (2013). Multi‐task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , & Schneider, W. (2007). The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage, 37(1), 343–360. [DOI] [PubMed] [Google Scholar]
- Cools, R. (2016). The costs and benefits of brain dopamine for cognitive control. Wiley Interdisciplinary Reviews Cognitive Science, 7(5), 317–329. [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews. Neuroscience, 3(8), 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Ohman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. [DOI] [PubMed] [Google Scholar]
- Crittenden, B. M. , Mitchell, D. J. , & Duncan, J. (2016). Task encoding across the multiple demand cortex is consistent with a Frontoparietal and Cingulo‐Opercular dual networks distinction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(23), 6147–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett, M. J. , Braams, B. R. , Clark, L. , Tobler, P. N. , Robbins, T. W. , & Kalenscher, T. (2013). Restricting temptations: Neural mechanisms of precommitment. Neuron, 79(2), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, E. A. , Wendelken, C. , Donohue, S. E. , & Bunge, S. A. (2006). Neural evidence for dissociable components of task‐switching. Cerebral Cortex, 16(4), 475–486. [DOI] [PubMed] [Google Scholar]
- Crowe, D. A. , Goodwin, S. J. , Blackman, R. K. , Sakellaridi, S. , Sponheim, S. R. , Macdonald, A. W., 3rd , & Chafee, M. V. (2013). Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nature Neuroscience, 16(10), 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson, P. L. , Walton, M. E. , O'Reilly, J. X. , Behrens, T. E. , & Rushworth, M. F. (2009). Effort‐based cost‐benefit valuation and the human brain. The Journal of Neuroscience, 29(14), 4531–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito, M. , & Postle, B. R. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. J. (2000). Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. The American Psychologist, 55(11), 1196–1214. [DOI] [PubMed] [Google Scholar]
- Daw, N. D. , Niv, Y. , & Dayan, P. (2005). Uncertainty‐based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience, 8(12), 1704–1711. [DOI] [PubMed] [Google Scholar]
- De Baene, W. , Kuhn, S. , & Brass, M. (2011). Challenging a decade of brain research on task switching: Brain activation in the task‐switching paradigm reflects adaptation rather than reconfiguration of task sets. Human Brain Mapping. 33(3), 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny, B. T. , Ochsner, K. N. , Weber, J. , & Wager, T. D. (2014). Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive and Affective Neuroscience, 9(4), 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss, J. , Brass, M. , Neumann, J. , & von Cramon, D. Y. (2005). Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Human Brain Mapping, 25(1), 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone, R. , & Duncan, J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Dias, R. , Robbins, T. W. , & Roberts, A. C. (1996). Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380(6569), 69–72. [DOI] [PubMed] [Google Scholar]
- Diekhof, E. K. , & Gruber, O. (2010). When desire collides with reason: Functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. The Journal of Neuroscience, 30(4), 1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. (2015). Cognitive control, emotional value, and the lateral prefrontal cortex. Frontiers in Psychology, 6. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. , & Christoff, K. (2012). The decision to engage cognitive control is driven by expected reward‐value: Neural and behavioral evidence. PLoS One, 7(12), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. , & Christoff, K. (2014). The lateral prefrontal cortex and complex value‐based learning and decision making. Neuroscience and Biobehavioral Reviews, 45, 9–18. [DOI] [PubMed] [Google Scholar]
- Dixon, M. L. , De La Vega, A. , Mills, C. , Andrews‐Hanna, J. , Spreng, R. N. , Cole, M. W. , & Christoff, K. (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences of the United States of America, 115(7), E1598–E1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, M. L. , Fox, K. C. R. , & Christoff, K. (2014a). Evidence for rostro‐caudal functional organization in multiple brain areas related to goal‐directed behavior. Brain Research, 1572, 26–39. [DOI] [PubMed] [Google Scholar]
- Dixon, M. L. , Fox, K. C. R. , & Christoff, K. (2014b). A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia, 62, 321–330. [DOI] [PubMed] [Google Scholar]
- Dixon, M. L. , Thiruchselvam, R. , Todd, R. , & Christoff, K. (2017). Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin, 143(10), 1033–1081. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Fair, D. A. , Miezin, F. M. , Cohen, A. L. , Wenger, K. K. , Dosenbach, R. A. , … Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Visscher, K. M. , Palmer, E. D. , Miezin, F. M. , Wenger, K. K. , Kang, H. C. , … Petersen, S. E. (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil, I. , Thompson, R. , & Duncan, J. (2011). Assembly and use of new task rules in fronto‐parietal cortex. Journal of Cognitive Neuroscience, 23(1), 168–182. [DOI] [PubMed] [Google Scholar]
- Duncan, J. (2001). An adaptive coding model of neural function in prefrontal cortex. Nature Reviews. Neuroscience, 2(11), 820–829. [DOI] [PubMed] [Google Scholar]
- Duncan, J. (2010). The multiple‐demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. [DOI] [PubMed] [Google Scholar]
- Duncan, J. (2013). The structure of cognition: Attentional episodes in mind and brain. Neuron, 80(1), 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner, T. , & Hirsch, J. (2005). Cognitive control mechanisms resolve conflict through cortical amplification of task‐relevant information. Nature Neuroscience, 8(12), 1784–1790. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Bzdok, D. , Laird, A. R. , Kurth, F. , & Fox, P. T. (2012). Activation likelihood estimation meta‐analysis revisited. NeuroImage, 59(3), 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. M. , Lancaster, J. L. , & Fox, P. T. (2017). Implementation errors in the GingerALE software: Description and recommendations. Human Brain Mapping, 38(1), 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann, J. B. , Damaraju, E. , Padmala, S. , & Pessoa, L. (2009). Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Frontiers in Human Neuroscience, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex, B. G. , Clinton, S. A. , Wonderley, L. R. , & Zald, D. H. (2012). The impact of the posterior parietal and dorsolateral prefrontal cortices on the optimization of long‐term versus immediate value. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(44), 15403–15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel, J. A. , Cole, M. W. , Zacks, J. M. , Kay, K. N. , & Braver, T. S. (2015). Reward motivation enhances task coding in Frontoparietal cortex. Cerebral Cortex. 26(4), 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A.C., Kamber M., Collins D.L., MacDonald D. (1994) An MRI‐Based Probabilistic Atlas of Neuroanatomy. In: Shorvon S.D., Fish D.R., Andermann F., Bydder G.M., Stefan H. (eds) Magnetic Resonance Scanning and Epilepsy. NATO ASI Series (Series A: Life Sciences), vol 264. Springer, Boston, MA
- Farb, N. A. , Segal, Z. V. , & Anderson, A. K. (2012). Attentional modulation of primary interoceptive and Exteroceptive cortices. Cerebral Cortex. 23(1), 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito, A. , Harrison, B. J. , Zalesky, A. , & Simons, J. S. (2012). Competitive and cooperative dynamics of large‐scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the United States of America, 109(31), 12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13–36. [DOI] [PubMed] [Google Scholar]
- Funahashi, S. , Chafee, M. V. , & Goldman‐Rakic, P. S. (1993). Prefrontal neuronal activity in rhesus monkeys performing a delayed anti‐saccade task. Nature, 365(6448), 753–756. [DOI] [PubMed] [Google Scholar]
- Fuster, J. M. (1989). The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe (2nd ed.). New York, NY: Raven Press. [Google Scholar]
- Fuster, J. M. , & Alexander, G. E. (1971). Neuron activity related to short‐term memory. Science, 173(3997), 652–654. [DOI] [PubMed] [Google Scholar]
- Gao, W. , & Lin, W. (2012). Frontal parietal control network regulates the anti‐correlated default and dorsal attention networks. Human Brain Mapping, 33(1), 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard, B. , Rivaud, S. , Cassarini, J. F. , Dubard, T. , Rancurel, G. , Agid, Y. , & Pierrot‐Deseilligny, C. (1998). Effects of anterior cingulate cortex lesions on ocular saccades in humans. Experimental Brain Research, 120(2), 173–183. [DOI] [PubMed] [Google Scholar]
- Gerlach, K. D. , Spreng, R. N. , Madore, K. P. , & Schacter, D. L. (2014). Future planning: Default network activity couples with frontoparietal control network and reward‐processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience. 9(12), 1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti, L. R. , Knoch, D. , Faber, P. L. , Lehmann, D. , Pascual‐Marqui, R. D. , Diezi, C. , … Fehr, E. (2009). Tonic activity level in the right prefrontal cortex predicts individuals' risk taking. Psychological Science, 20(1), 33–38. [DOI] [PubMed] [Google Scholar]
- Gilbert, A. M. , & Fiez, J. A. (2004). Integrating rewards and cognition in the frontal cortex. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 540–552. [DOI] [PubMed] [Google Scholar]
- Goldman–Rakic, P. S. (1987). Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In Handbook of Physiology: The Nervous System, Higher Functions of the Brain, Part 1. American Physiology Society, 373–417.
- Gollwitzer, P. M. (1999). Implementation intentions: Strong effects of simple plans. American Psychologist, 54(7), 493–503. [Google Scholar]
- Gorgolewski, K. J. , Varoquaux, G. , Rivera, G. , Schwarz, Y. , Ghosh, S. S. , Maumet, C. , … Margulies, D. S. (2015). NeuroVault.Org: A web‐based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare, T. A. , O'Doherty, J. , Camerer, C. F. , Schultz, W. , & Rangel, A. (2008). Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. The Journal of Neuroscience, 28(22), 5623–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy, T. E. , Frank, M. J. , & O'Reilly, R. C. (2007). Towards an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London Series B: Biological sciences, 362(1485), 1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy, T. E. , Frank, M. J. , & O'Reilly, R. C. (2006). Banishing the homunculus: Making working memory work. Neuroscience, 139(1), 105–118. [DOI] [PubMed] [Google Scholar]
- Heller, A. S. , Johnstone, T. , Shackman, A. J. , Light, S. N. , Peterson, M. J. , Kolden, G. G. , … Davidson, R. J. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto‐striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka, M. , Yuasa, F. , Yuyama, R. , Motohiro, M. , Mimura, J. , Kawamura, A. , … Iwasaka, T. (2000). Effect of angiotensin‐converting enzyme inhibitor on cardiopulmonary baroreflex sensitivity in patients with acute myocardial infarction. The American Journal of Cardiology, 86(11), 1241–1244 A1246. [DOI] [PubMed] [Google Scholar]
- Histed, M. H. , Pasupathy, A. , & Miller, E. K. (2009). Learning substrates in the primate prefrontal cortex and striatum: Sustained activity related to successful actions. Neuron, 63(2), 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, T. , & Watanabe, M. (2012). Prefrontal neurons represent winning and losing during competitive video shooting games between monkeys. The Journal of Neuroscience, 32(22), 7662–7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel, S. A. , Song, A. W. , & McCarthy, G. (2005). Decisions under uncertainty: Probabilistic context influences activation of prefrontal and parietal cortices. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(13), 3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcherson, C. A. , Plassmann, H. , Gross, J. J. , & Rangel, A. (2012). Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(39), 13543–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, I. , Liu, X. , Clerkin, S. , Schulz, K. , Friston, K. , Newcorn, J. H. , & Fan, J. (2012). Effects of motivation on reward and attentional networks: An fMRI study. Brain and Behavior: A Cognitive Neuroscience Perspective, 2(6), 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzini, A. , Caruana, F. , Stoianov, I. , Gallese, V. , & Rizzolatti, G. (2012). Functional organization of the insula and inner perisylvian regions. Proceedings of the National Academy of Sciences of the United States of America, 109(25), 10077–10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura, K. , Chushak, M. S. , & Braver, T. S. (2013). Impulsivity and self‐control during intertemporal decision making linked to the neural dynamics of reward value representation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(1), 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura, K. , Locke, H. S. , & Braver, T. S. (2010). Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura, K. , & Poldrack, R. A. (2012). Analyses of regional‐average activation and multivoxel pattern information tell complementary stories. Neuropsychologia, 50(4), 544–552. [DOI] [PubMed] [Google Scholar]
- Kaiser, R. H. , Andrews‐Hanna, J. R. , Spielberg, J. M. , Warren, S. L. , Sutton, B. P. , Miller, G. A. , … Banich, M. T. (2015a). Distracted and down: Neural mechanisms of affective interference in subclinical depression. Social Cognitive and Affective Neuroscience, 10(5), 654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, R. H. , Andrews‐Hanna, J. R. , Wager, T. D. , & Pizzagalli, D. A. (2015b). Large‐scale network dysfunction in major depressive disorder: A meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry, 72(6), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley, S. W. , & Wallis, J. D. (2009). Evaluating choices by single neurons in the frontal lobe: Outcome value encoded across multiple decision variables. The European Journal of Neuroscience, 29(10), 2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Hwang, J. , & Lee, D. (2008). Prefrontal coding of temporally discounted values during intertemporal choice. Neuron, 59(1), 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, J. T. , Deaner, R. O. , & Platt, M. L. (2008). Neural correlates of social target value in macaque parietal cortex. Current Biology: CB, 18(6), 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , Adams, C. M. , Fong, G. W. , & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience, 21(16), RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , & Greer, S. M. (2008). Anticipatory affect: Neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1511), 3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, S. , Nomoto, K. , Watanabe, M. , Hikosaka, O. , Schultz, W. , & Sakagami, M. (2006). Influences of rewarding and aversive outcomes on activity in macaque lateral prefrontal cortex. Neuron, 51(6), 861–870. [DOI] [PubMed] [Google Scholar]
- Koechlin, E. , Ody, C. , & Kouneiher, F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science, 302(5648), 1181–1185. [DOI] [PubMed] [Google Scholar]
- Kool, W. , McGuire, J. T. , Rosen, Z. B. , & Botvinick, M. M. (2010). Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology. General, 139(4), 665–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher, F. , Charron, S. , & Koechlin, E. (2009). Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience, 12(7), 939–945. [DOI] [PubMed] [Google Scholar]
- Krebs, R. M. , Boehler, C. N. , Roberts, K. C. , Song, A. W. , & Woldorff, M. G. (2012). The involvement of the dopaminergic midbrain and cortico‐striatal‐thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex, 22(3), 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan, I. T. , Guitart‐Masip, M. , Dayan, P. , & Dolan, R. J. (2013). Effort and valuation in the brain: The effects of anticipation and execution. The Journal of Neuroscience, 33(14), 6160–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Price, C. J. , Glahn, D. C. , Uecker, A. M. , Lancaster, J. L. , … Fox, P. T. (2005). ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25(1), 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton, M. , Bertoux, M. , Boutet, C. , Lehericy, S. , Dubois, B. , Fossati, P. , & Pessiglione, M. (2013). A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biology, 11(10), e1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon, M. I. , & Shadlen, M. N. (1999). Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron, 24(2), 415–425. [DOI] [PubMed] [Google Scholar]
- Leotti, L. A. , & Delgado, M. R. (2014). The value of exercising control over monetary gains and losses. Psychological Science, 25(2), 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D. J. , & Glimcher, P. W. (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, H. S. , & Braver, T. S. (2008). Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience, 8(1), 99–112. [DOI] [PubMed] [Google Scholar]
- MacDonald, A. W., 3rd , Cohen, J. D. , Stenger, V. A. , & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. [DOI] [PubMed] [Google Scholar]
- Matsumoto, K. , Suzuki, W. , & Tanaka, K. (2003). Neuronal correlates of goal‐based motor selection in the prefrontal cortex. Science, 301(5630), 229–232. [DOI] [PubMed] [Google Scholar]
- McClure, S. M. , Laibson, D. I. , Loewenstein, G. , & Cohen, J. D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 306(5695), 503–507. [DOI] [PubMed] [Google Scholar]
- McGuire, J. T. , & Botvinick, M. M. (2010). Prefrontal cortex, cognitive control, and the registration of decision costs. Proceedings of the National Academy of Sciences of the United States of America, 107(17), 7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford, N. , & Critchley, H. D. (2010). Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Structure & Function, 214(5–6), 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran, N. (1996). Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology: Learning, Memory, and Cognition, 22(6), 1423. [Google Scholar]
- Meiran, N. (2000). Modeling cognitive control in task‐switching. Psychological Research, 63(3–4), 234–249. [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Mitchell, D. J. , Bell, A. H. , Buckley, M. J. , Mitchell, A. S. , Sallet, J. , & Duncan, J. (2016). A putative multiple‐demand system in the macaque brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(33), 8574–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, A. , Friedman, N. P. , Emerson, M. J. , Witzki, A. H. , Howerter, A. , & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Montague, P. R. , Dayan, P. , & Sejnowski, T. J. (1996). A framework for mesencephalic dopamine systems based on predictive Hebbian learning. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 16(5), 1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Tanji J. (2009). Cingulofrontal interactions and the cingulate motor areas. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; NY. pp. 113–144.
- Munakata, Y. , Herd, S. A. , Chatham, C. H. , Depue, B. E. , Banich, M. T. , & O'Reilly, R. C. (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15(10), 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, J. T. , & Casey, B. J. (2005). An integrative theory of attention‐deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology, 17(3), 785–806. [DOI] [PubMed] [Google Scholar]
- O'Doherty, J. , Dayan, P. , Schultz, J. , Deichmann, R. , Friston, K. , & Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304(5669), 452–454. [DOI] [PubMed] [Google Scholar]
- O'Reilly, R. C. , Herd, S. A. , & Pauli, W. M. (2010). Computational models of cognitive control. Current Opinion in Neurobiology, 20(2), 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala, S. , & Pessoa, L. (2011). Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience, 23(11), 3419–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X. , Sawa, K. , Tsuda, I. , Tsukada, M. , & Sakagami, M. (2008). Reward prediction based on stimulus categorization in primate lateral prefrontal cortex. Nature Neuroscience, 11(6), 703–712. [DOI] [PubMed] [Google Scholar]
- Paradiso, S. , Chemerinski, E. , Yazici, K. M. , Tartaro, A. , & Robinson, R. G. (1999). Frontal lobe syndrome reassessed: Comparison of patients with lateral or medial frontal brain damage. Journal of Neurology, Neurosurgery, and Psychiatry, 67(5), 664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschke, L. M. , Walter, H. , Steimke, R. , Ludwig, V. U. , Gaschler, R. , Schubert, T. , & Stelzel, C. (2015). Motivation by potential gains and losses affects control processes via different mechanisms in the attentional network. NeuroImage, 111, 549–561. [DOI] [PubMed] [Google Scholar]
- Passingham, R. E. , & Wise, S. P. (2012). The neurobiology of the prefrontal cortex: Anatomy, evolution, and the origin of insight (1st ed.). Oxford, UK: Oxford University Press. [Google Scholar]
- Pessoa, L. (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9(2), 148–158. [DOI] [PubMed] [Google Scholar]
- Plassmann, H. , O'Doherty, J. , & Rangel, A. (2007). Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(37), 9984–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann, H. , O'Doherty, J. P. , & Rangel, A. (2010). Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(32), 10799–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, M. L. , & Glimcher, P. W. (1999). Neural correlates of decision variables in parietal cortex. Nature, 400(6741), 233–238. [DOI] [PubMed] [Google Scholar]
- Pochon, J. B. , Levy, R. , Fossati, P. , Lehericy, S. , Poline, J. B. , Pillon, B. , … Dubois, B. (2002). The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner, M. I. , & Dehaene, S. (1994). Attentional networks. Trends in Neurosciences, 17(2), 75–79. [DOI] [PubMed] [Google Scholar]
- Posner, M.I., & DiGirolamo, G.J. (1998). Executive attention: conflict, target detection and cognitive control. In The Attentive Brain (Parasuraman, R., ed.), pp. 401–423, MIT Press
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , … Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyk, E. , Wilson, C. R. , Stoll, F. M. , Faraut, M. C. , Petrides, M. , & Amiez, C. (2014). Midcingulate motor map and feedback detection: Converging data from humans and monkeys. Cerebral Cortex, 26(2), 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel, A. , Camerer, C. , & Montague, P. R. (2008). A framework for studying the neurobiology of value‐based decision making. Nature Reviews Neuroscience, 9(7), 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel, A. , & Hare, T. (2010). Neural computations associated with goal‐directed choice. Current Opinion in Neurobiology, 20(2), 262–270. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. , Ullsperger, M. , Crone, E. A. , & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443–447. [DOI] [PubMed] [Google Scholar]
- Rorden, C. , Karnath, H. O. , & Bonilha, L. (2007). Improving lesion‐symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. [DOI] [PubMed] [Google Scholar]
- Rowe, J. B. , Eckstein, D. , Braver, T. , & Owen, A. M. (2008). How does reward expectation influence cognition in the human brain? Journal of Cognitive Neuroscience, 20(11), 1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth, M. F. , Behrens, T. E. , Rudebeck, P. H. , & Walton, M. E. (2007). Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences, 11(4), 168–176. [DOI] [PubMed] [Google Scholar]
- Rushworth, M. F. , Noonan, M. P. , Boorman, E. D. , Walton, M. E. , & Behrens, T. E. (2011). Frontal cortex and reward‐guided learning and decision‐making. Neuron, 70(6), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Rushworth, M. F. , Passingham, R. E. , & Nobre, A. C. (2002). Components of switching intentional set. Journal of Cognitive Neuroscience, 14(8), 1139–1150. [DOI] [PubMed] [Google Scholar]
- Schoenbaum, G. , & Esber, G. R. (2010). How do you (estimate you will) like them apples? Integration as a defining trait of orbitofrontal function. Current Opinion in Neurobiology, 20(2), 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, W. , Dayan, P. , & Montague, P. R. (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, H. , Barraclough, D. J. , & Lee, D. (2007). Dynamic signals related to choices and outcomes in the dorsolateral prefrontal cortex. Cerebral Cortex, 17(Suppl 1), i110–i117. [DOI] [PubMed] [Google Scholar]
- Shackman, A. J. , Salomons, T. V. , Slagter, H. A. , Fox, A. S. , Winter, J. J. , & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience, 12(3), 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman, A. J. , Tromp, D. P. , Stockbridge, M. D. , Kaplan, C. M. , Tillman, R. M. , & Fox, A. S. (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav, A. , Botvinick, M. M. , & Cohen, J. D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara, M. , & Richmond, B. J. (2002). Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science, 296(5573), 1709–1711. [DOI] [PubMed] [Google Scholar]
- Smith, D. V. , Gseir, M. , Speer, M. E. , & Delgado, M. R. (2016). Toward a cumulative science of functional integration: A meta‐analysis of psychophysiological interactions. Human Brain Mapping, 37(8), 2904–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek, A. , Stelzel, C. , Paschke, L. , Walter, H. , & Schubert, T. (2015). Dissociable effects of motivation and expectancy on conflict processing: An fMRI study. Journal of Cognitive Neuroscience, 27(2), 409–423. [DOI] [PubMed] [Google Scholar]
- Spreng, R. N. , Stevens, W. D. , Chamberlain, J. P. , Gilmore, A. W. , & Schacter, D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. NeuroImage, 53(1), 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan, D. , Levitin, D. J. , & Menon, V. (2008). A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes, M. G. , Kusunoki, M. , Sigala, N. , Nili, H. , Gaffan, D. , & Duncan, J. (2013). Dynamic coding for cognitive control in prefrontal cortex. Neuron, 78(2), 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss, D. T. , Alexander, M. P. , Shallice, T. , Picton, T. W. , Binns, M. A. , Macdonald, R. , … Katz, D. I. (2005). Multiple frontal systems controlling response speed. Neuropsychologia, 43(3), 396–417. [DOI] [PubMed] [Google Scholar]
- Stuss, D. T. , & Knight, R. T. (2002). Principles of frontal lobe function. New York, NY: Oxford University Press. [Google Scholar]
- Tanaka, S. C. , Doya, K. , Okada, G. , Ueda, K. , Okamoto, Y. , & Yamawaki, S. (2004). Prediction of immediate and future rewards differentially recruits cortico‐basal ganglia loops. Nature Neuroscience, 7(8), 887–893. [DOI] [PubMed] [Google Scholar]
- Taylor, S. F. , Welsh, R. C. , Wager, T. D. , Phan, K. L. , Fitzgerald, K. D. , & Gehring, W. J. (2004). A functional neuroimaging study of motivation and executive function. NeuroImage, 21(3), 1045–1054. [DOI] [PubMed] [Google Scholar]
- Tobler, P. N. , Christopoulos, G. I. , O'Doherty, J. P. , Dolan, R. J. , & Schultz, W. (2009). Risk‐dependent reward value signal in human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 106(17), 7185–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita, H. , Ohbayashi, M. , Nakahara, K. , Hasegawa, I. , & Miyashita, Y. (1999). Top‐down signal from prefrontal cortex in executive control of memory retrieval. Nature, 401(6754), 699–703. [DOI] [PubMed] [Google Scholar]
- Turkeltaub, P. E. , Eickhoff, S. B. , Laird, A. R. , Fox, M. , Wiener, M. , & Fox, P. (2012). Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Human Brain Mapping, 33(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken, A. U. , & Swick, D. (1999). Response selection in the human anterior cingulate cortex. Nature Neuroscience, 2(10), 920–924. [DOI] [PubMed] [Google Scholar]
- Ullsperger, M. , Danielmeier, C. , & Jocham, G. (2014). Neurophysiology of performance monitoring and adaptive behavior. Physiological Reviews, 94(1), 35–79. [DOI] [PubMed] [Google Scholar]
- Vickery, T. J. , Chun, M. M. , & Lee, D. (2011). Ubiquity and specificity of reinforcement signals throughout the human brain. Neuron, 72(1), 166–177. [DOI] [PubMed] [Google Scholar]
- Vincent, J. L. , Kahn, I. , Snyder, A. Z. , Raichle, M. E. , & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, J. D. , Anderson, K. C. , & Miller, E. K. (2001). Single neurons in prefrontal cortex encode abstract rules. Nature, 411(6840), 953–956. [DOI] [PubMed] [Google Scholar]
- Wallis, J. D. , & Miller, E. K. (2003). Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. The European Journal of Neuroscience, 18(7), 2069–2081. [DOI] [PubMed] [Google Scholar]
- Watanabe, M. (1996). Reward expectancy in primate prefrontal neurons. Nature, 382(6592), 629–632. [DOI] [PubMed] [Google Scholar]
- Watanabe, M. (2017). The prefrontal cortex as an executive, emotional, and social brain. Japan: Springer. [Google Scholar]
- Watanabe, M. , Hikosaka, K. , Sakagami, M. , & Shirakawa, S. (2002). Coding and monitoring of motivational context in the primate prefrontal cortex. The Journal of Neuroscience, 22(6), 2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M. , & Sakagami, M. (2007). Integration of cognitive and motivational context information in the primate prefrontal cortex. Cerebral Cortex, 17(Suppl 1), i101–i109. [DOI] [PubMed] [Google Scholar]
- Weber, B. J. , & Huettel, S. A. (2008). The neural substrates of probabilistic and intertemporal decision making. Brain Research, 1234, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook, A. , Kester, D. , & Braver, T. S. (2013). What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS One, 8(7), e68210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Van Essen, D. C. , & Wager, T. D. (2010). Cognitive neuroscience 2.0: Building a cumulative science of human brain function. Trends in Cognitive Sciences, 14(11), 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni, G. , Huey, E. D. , Krueger, F. , Nichelli, P. F. , & Grafman, J. (2008). Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology, 71(10), 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]


