Abstract
This study aimed to characterize the clinical features and the related cerebral glucose metabolism pattern of cognitive impairments in Parkinson's disease (PD) with positron emission tomography (PET) imaging. We recruited 168 PD patients and 100 age‐matched healthy controls of similar education and gender distribution. All of those enrolled underwent clinical assessment including the unified Parkinson's disease rating scale motor score, Hoehn and Yahr scale, and comprehensive neuropsychological tests including domains of executive function, attention, memory, visuospatial function, and language. Demographics and the results of cognitive measures were compared between patients and healthy controls. Cognition status was classified as PD patients with dementia (PD‐D), PD patients with mild cognitive impairment (PD‐MCI), or PD patients with normal cognition (PD‐NC). In 53 PD patients who underwent 18F‐fluorodeoxyglucose (18F‐FDG) PET imaging, correlations between Z‐score values of the different cognitive domains and cerebral 18F‐FDG uptake were assessed using statistical parametric mapping (SPM8) corrected for age and motor severity. A total of 23.2% of PD patients were PD‐MCI and 8.9% were PD‐D. In the group of PD‐MCI, 96.3% showed multiple‐domain deficits, with executive function and attention impairment most predominantly involved. All the cognitive domain scores with the exception of language correlated with 18F‐FDG metabolisms, primarily in posterior temporo‐parieto‐occipital association cortical areas. This study found that cognitive impairment in PD particularly encompasses frontal/executive deficits. Posterior cortical areas, containing multiple neurotransmitters and neural circuits, may play an important role in the pathogenesis of cognitive impairment in PD.
Keywords: 18F‐FDG PET, cognitive impairment, neuropsychology, Parkinson's disease
1. INTRODUCTION
Parkinson's disease (PD) is primarily a movement disorder, but a wide variety of non‐motor symptoms are also commonly experienced. Cognitive impairment is a frequent comorbidity in PD, with the point prevalence of dementia (PD patients with dementia [PD‐D]) up to 30%, and more than three quarters of PD patients will eventually develop dementia (Aarsland, Andersen, Larsen, Lolk, & Kragh‐Sorensen, 2003; Aarsland, Zaccai, & Brayne, 2005). PD patients with mild cognitive impairment (PD‐MCI), as a harbinger of dementia in PD (Caviness et al., 2007), is common and occurs in about 20–40% of non‐demented PD patients (Aarsland et al., 2010).
Cognitive impairment in PD is commonly characterized by predominant frontal/executive deficits, while impairments of memory, language, and visuospatial functions are less frequently reported (Foltynie, Brayne, Robbins, & Barker, 2004). However, more recent reports implicate impairment in variable cognitive domains. This inconsistency in the literature may emanate from the variation in the assessment tools used. For example, earlier studies on cognitive impairment in PD used the Diagnostic and Statistical Manual of Mental Disorders, which focuses more on memory function than other cognitive subdomains. In more recent years, the Movement Disorder Society (MDS) Task Force published criteria for both PD‐D and PD‐MCI with a comprehensive neuropsychological test battery covering all cognitive subdomains simultaneously to allow more accurate studies (Emre et al., 2007; Litvan et al., 2011). However, studies on the clinical profiles, of cognitive impairment in PD in the Chinese population are limited, and the results of those using a comprehensive neuropsychological test battery are substantially inconsistent (Wang et al., 2015; Yu et al., 2012).
The neurobiological basis of the clinically heterogeneous cognitive deficits in PD remains controversial. Functional neuroimaging may prove useful to explore the underlying mechanisms and provide potentially objective biomarkers. Previously, we reported on regional cerebral glucose metabolism differences between PD patients with different cognitive status, particularly PD‐D and PD‐MCI (Tang et al., 2016). However, the more detailed exploration of cerebral metabolism changes relating to performance in the different cognitive domains has yet to be elucidated. We therefore aimed to further investigate the characteristics of cognitive impairment in PD patients across the major cognitive domains, and explore the associated cerebral glucose metabolism with 18F‐fluorodeoxyglucose (18F‐FDG) positron emission tomography (PET) imaging.
2. SUBJECTS AND METHODS
2.1. Subjects
A cross‐sectional study was conducted in 168 patients diagnosed as idiopathic PD according to the UK Brain Bank criteria (Hughes, Daniel, Kilford, & Lees, 1992) in Huashan Hospital between March 2012 and March 2015. A total of 100 healthy controls with similar age, education, and gender distribution as the PD patients were recruited to obtain normative data. None of the healthy controls had a past history of cognitive impairment, psychiatric illness, central nervous system disease, or head injury. All participants provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Human Studies Institutional Review Board, Huashan Hospital, Fudan University.
2.2. Motor assessment
Motor status in PD patients was assessed using the Hoehn and Yahr scale and the motor section of the unified Parkinson's disease rating scale (UPDRS‐III; Fahn, Marsonden, Calne, & Goldstein, 1987) in the “OFF” state, which was defined as being off anti‐parkinsonian medications for at least 12 hr.
2.3. Cognition assessment
Global cognitive function was evaluated using the Mini Mental State Examination (MMSE). According to the MDS diagnostic criteria for PD‐MCI (Litvan et al., 2011), different cognitive domains (executive function, attention, memory, visuospatial function, and language) were evaluated using a battery of neuropsychological tests. Executive functions were assessed with the Stroop Color‐Word Test part C (Steinberg, Bieliauskas, Smith, & Ivnik, 2005) and Trail Making Test part B (TMT‐B) (Zhao et al., 2013); attention was assessed with Trail Making Test part A (TMT‐A) (Zhao et al., 2013) and the Symbol Digit Modalities Test (SDMT) (Sheridan et al., 2006); memory function was assessed using the Rey Auditory Verbal Learning Test (AVLT) (Guo, Zhao, Chen, Ding, & Hong, 2009) and delayed recall of the Rey–Osterrieth Complex Figure Test (CFT‐delayed recall) (Caffarra, Vezzadini, Dieci, Zonato, & Venneri, 2002); visuospatial function was evaluated with the Clock Drawing Test and Rey–Osterrieth Complex Figure Copy Test (CFT) (Caffarra et al., 2002); language ability was assessed with Boston Naming Test (BNT) and Verbal Fluency Test (VFT) (Lucas et al., 2005).
Cognitive assessment was performed with patients taking their usual anti‐parkinsonian medications (i.e., in the ON condition) to conform to current protocols in our clinical research studies. Individual neuropsychological test scores were transformed into Z‐scores using the mean and standard deviation of the control sample according to the following formula: (test score − median score from control sample)/standard deviation from control sample. In addition, the Z‐score for each cognition domain was the average of the Z‐scores of the two tests assessing the same domain.
2.4. Determination of cognitive status
The clinical diagnostic criteria for dementia associated with PD were applied to diagnose dementia in this study (Emre et al., 2007). MCI was diagnosed according to the Level II recommendations of the MDS Task Force 2012 whereby, deficits should be present in at least two tests, either within one single cognitive domain or across different domains (Emre et al., 2007; Litvan et al., 2012). Two SDs below the norm was considered abnormal in each test. Values used to determine test score deviations in PD patients were taken from the sample of 100 age‐ and education‐matched healthy control subjects. Patients not fulfilling criteria for MCI or dementia were considered to have normal cognition.
2.5. PET imaging and data analysis
A total of 53 patients who agreed to undergo 18F‐FDG PET imaging were consecutively recruited within 1 month (before or after) of the neuropsychological evaluation. A CT transmission scan was first performed for attenuation correction. Subjects were injected intravenously with 18F‐FDG (185 MBq), required to rest for 45 min in the supine position on the PET scanner bed with their eyes closed and then underwent a 10 min scan using a Siemens Biograph. Images were reconstructed by means of a filtered back‐projection method. As no blood samples were taken in these subjects according to our clinical imaging protocols we used PET images to measure relative glucose metabolism as described previously (Ge et al., 2015; Wu et al., 2013).
Data were processed using statistical parametric mapping (SPM) software implemented in MATLAB6.5.1 (Math Works Inc., Sherborn, MA). First, we spatially normalized all 18F‐FDG PET images into a standard stereotactic Montreal Neurological Institute space using the default PET template in SPM and then smoothed the resulting PET scans with an isotropic three‐dimensional Gaussian filter with a full‐width at half‐maximum of 10 mm. The relationships between metabolic images and behavioral measures were assessed according to brain mapping procedures we established previously (Liu et al., 2018; Ge et al., 2015; Zuo et al., 2013). In brief, the correlation between PET images and the mean Z‐scores of each cognitive domain was analyzed separately, all with two variables (age and UPDRS‐III scores) entered as covariates into the model to eliminate the interaction caused by these two factors. Differences in global metabolic values were modeled by analysis of covariance (ANCOVA) to minimize inter‐subject variability and improve signal‐noise ratios for SPM. We reported all the clusters that satisfied three criteria: (1) significant with a voxel‐wise threshold at p < .001 (uncorrected) over whole‐brain regions; (2) had an extent threshold exceeding several times the average cluster size determined by the model; and (3) survived a false discovery rate (FDR) correction at p < .05. The coordinates of the voxel peaks were extracted and their anatomical locations determined using the Talairach Daemon program (Research Imaging Center, University of Texas Health Science Center, San Antonio, TX).
To quantify the metabolic values in specific regions, we constructed a 4‐mm radius spherical volume of interest (VOI) in the image space, centered at the peak voxel of clusters that were significant in each SPM analysis. To enable post hoc correlations of imaging measures with cognitive scores, we calculated the corresponding VOI values in 18F‐FDG images of all patients with ScAnVP software (Version 5.9.1; Center for Neuroscience, the Feinstein Institute for Medical Research, Manhasset, NY). The individual metabolic values of each region were then expressed as percentage of whole‐brain metabolism (metabolic value = [VOI value/whole‐brain metabolism] × 50 × 100%).
2.6. Statistical analysis
Differences in the demographic and clinical characteristics between the PD groups and controls were analyzed using Pearson's chi‐square test in cases of categorical variables, analysis of variance with post hoc Bonferroni's multiple comparison in cases of continuous, normally distributed variables, and the Kruskal–Wallis and Mann–Whitney U tests for continuous, nonparametric variables. In addition, relationships between global metabolic values and each of clinical measures were assessed by nonparametric Spearmen correlation analysis. A value of p < .05 was considered to indicate statistical significance.
3. RESULTS
The demographic profiles and clinical information for the 168 PD patients and 100 healthy controls are provided in Table 1. No significant differences were identified between the two groups.
Table 1.
Demographic and clinical characteristics in PD patients and healthy controls
| Variable | Controls (n = 100) | Total PD (n = 168) | PD with PET (n = 53) | p a | p b |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 62.12 ± 8.84 | 61.49 ± 8.32 | 61.49 ± 9.07 | .433 | .932 |
| (Min, Max) | (39, 77) | (40, 84) | (42, 81) | ||
| Sex | |||||
| Male | 57 (57%) | 103 (61%) | 29 (55%) | .487 | .394 |
| Female | 43 (43%) | 65 (39%) | 24 (45%) | ||
| Education (years) | |||||
| Mean ± SD | 12.39 ± 3.09 | 11.65 ± 4.02 | 13.15 ± 3.05 | .403 | .063 |
| (Min, Max) | (5, 21) | (0, 19) | (5, 19) | ||
| PD duration (months) | |||||
| Mean | NA | 62.3 | 48.28 | NA | .422 |
| (Min, Max) | NA | (1, 333) | (4, 172) | ||
| UPDRS‐III score | |||||
| Mean | NA | 28.9 | 24.10 | NA | .061 |
| (Min, Max) | NA | (4, 72) | (7, 63) | ||
Note. Data is provided as mean ± SD or mean (Min, Max).
Entire PD group vs. controls.
PD with PET group vs. total PD group.
3.1. Cognitive profile in PD patients and healthy controls
An overview of neuropsychological results for PD patients and controls is provided in Table 2. Although the global cognitive function (MMSE) did not reveal any difference between the two groups, PD patients had significantly poorer scores on nearly all individual neuropsychological tests (Table 2). The highest percentages of PD patients with impairment (i.e., >2 SDs below control mean) on the individual neuropsychological tests were for TMT‐B (20.2%) and TMT‐A (19.6%). The lowest percentages with impairment were present with the VFT (7.1%) and BNT (10.1%). The numbers and percentage of PD patients with impairment according to each cognitive domain were: executive function, 41 (24.4%); attention, 38 (22.6%); memory, 38 (22.6%); visuospatial, 31 (18.5%); language, 23 (13.7%). The information of the neuropsychological tests in the 53 PD patients with PET imaging scores was listed in Supporting Information Table S1.
Table 2.
Cognitive profile of the PD patients and healthy controls
| Control (n = 100) | PD (n = 168) | p a | p b | |||
|---|---|---|---|---|---|---|
| Mean ± SD | No. with impairment (percentage) | Mean ± SD | No. with impairment (percentage) | |||
| MMSE | 27.82 ± 1.81 | 0(0%) | 27.35 ± 2.87 | 0(0%) | .690 | / |
| Executive function | 3(3%) | 41(24.4%) | <.001 | |||
| CWT‐C | 77.94 ± 20.91 | 7(7%) | 92.57 ± 58.36 | 27(16.1%) | .078 | .031 |
| TMT‐B | 141.80 ± 43.36 | 4(4%) | 187.52 ± 87.01 | 34(20.2%) | <.001 | <.001 |
| Attention | 4(4%) | 38(22.6%) | <.001 | |||
| TMT‐A | 57.42 ± 20.23 | 3(3%) | 80.10 ± 46.87 | 33(19.6%) | <.001 | <.001 |
| SDMT | 39.52 ± 11.46 | 1(1%) | 29.15 ± 13.16 | 27(16.1%) | <.001 | <.001 |
| Memory | 1(1%) | 38(22.6%) | <.001 | |||
| AVLT | 1(1%) | 31(18.5%) | <.001 | |||
| – Short delayed recall | 6.38 ± 1.64 | / | 4.42 ± 2.59 | / | <.001 | / |
| – Long delayed recall | 5.93 ± 1.41 | / | 4.24 ± 2.50 | / | <.001 | / |
| – Cued recall | 5.77 ± 2.01 | / | 4.14 ± 2.57 | / | <.001 | / |
| – Recognition | 21.49 ± 2.03 | / | 19.91 ± 3.56 | / | <.001 | / |
| CFT‐delay recall | 16.44 ± 5.52 | 0 | 14.36 ± 7.07 | 22(13.1%) | .010 | <.001 |
| Visuospatial function | 3(3%) | 31(18.5%) | <.001 | |||
| CFT | 3(3%) | 21(12.5%) | .008 | |||
| – Time of copy | 188.67 ± 99.99 | / | 191.25 ± 87.47 | / | .422 | / |
| – Score of copy | 33.51 ± 3.25 | / | 30.99 ± 7.05 | / | .044 | / |
| CDT | 25.81 ± 6.31 | 12(12%) | 20.44 ± 6.95 | 23(13.7%) | <.001 | .691 |
| Language | 0 | 23(13.7%) | <.001 | |||
| VFT | 1(1%) | 12(7.1%) | .024 | |||
| – Animal category | 17.96 ± 4.29 | / | 15.09 ± 4.22 | / | <.001 | / |
| – City category | 16.78 ± 4.58 | / | 15.33 ± 6.59 | / | .006 | / |
| – Alternating form | 15.48 ± 3.58 | / | 14.20 ± 5.01 | / | .028 | / |
| BNT | 24.82 ± 3.22 | 2(2%) | 22.70 ± 4.12 | 17(10.1%) | <.001 | .012 |
Note. MMSE = Mini Mental State Examination; CWT‐C = Stroop Color‐Word Test C; TMT = Trail Making Test; SDMT = Symbol Digit Modalities Test; AVLT = Auditory Verbal Learning Test; CFT = the Rey–Osterrieth Complex Figure Test; VFT = Verbal Fluency Test; BNT = Boston Naming Test; CDT = Clock Drawing Test. Data represented as mean ± SD.
p Values for the comparison of cognitive test scores between PD and control groups.
p Values for the comparison between the PD and control groups of the frequency (i.e., percentage in each group) of significant cognitive impairment.
3.2. Clinical characteristics of cognitive impairments in PD patients
According to MDS Task Force Level II criteria, 54 cases (32.1%) of 168 PD patients had cognitive impairment, with 39 cases (23.2%) classified as PD‐MCI and 15 cases (8.9%) as PD‐D. In the group of PD‐MCI, there were two cases (5.1%) with single‐domain impairment (executive and attention domains, respectively) and 37 cases (94.9%) with multiple‐domain impairment.
The PD‐MCI, PD‐D, and PD with normal cognition (PD‐NC) groups were similar regarding education, although there were significant differences for age, PD duration, and UPDRS‐III scores between the three groups. With respect to cognitive domains, both PD‐MCI and PD‐D patients showed impairment following a similar pattern: prominent in attention and executive dysfunction and less prominent in language (Table 3).
Table 3.
Comparisons of demographic profiles in the three cognitive groups of Parkinson's disease patients
| Variable | PD‐NC | PD‐MCI | PD‐D | Post hoc significance |
|---|---|---|---|---|
| (n = 114) | (n = 39) | (n = 15) | ||
| Age (years) | 59.69 ± 8.62 | 63.77 ± 5.82 | 69.27 ± 5.23 | [D < N c][D < M a] |
| Education (years) | 11.70 ± 3.91 | 11.72 ± 3.44 | 12.39 ± 3.09 | |
| PD duration (months) | 21(10,52) | 43(21,117) | 119(26,151) | |
| UPDRS‐III | 22.67 ± 12.85 | 32.19 ± 16.19 | 44.43 ± 24.16 | |
| MMSE | 28.26 ± 1.73 | 26.79 ± 2.53 | 21.87 ± 4.16 | [D < N c][D < M b][M < N b] |
| Executive function | ||||
| Z‐scores | 0.13(−0.47,0.57) | −1.76(−2.96,‐0.94) | −3.2(−6.43,‐2.54) | [D < N c][M < N c] |
| Impairment (%) | 66.67% | 100.00% | ||
| Attention | ||||
| Z‐scores | −0.24(−0.66,0.27) | −1.57(−2.5,−0.83) | −3.98(−5.69,−2.85) | [D < N c][D < M a][M < N c] |
| Impairment (%) | 58.97% | 100.00% | ||
| Memory | ||||
| Z‐scores | −0.33(−0.94,0.34) | −1.25(−2.09,−0.63) | −2.56(−2.93,−2.05) | [D < N c][D < M a][M < N c] |
| Impairment (%) | 58.97% | 100.00% | ||
| Visuospatial function | ||||
| Z‐scores | −0.10(−0.47,0.14) | −1.13(−1.8,−0.28) | −2.87(−3.56,−1.65) | [D < N c][D < M b][M < N c] |
| Impairment (%) | 46.15% | 86.7% | ||
| Language | ||||
| Z‐scores | −0.18(−0.82,0.36) | −0.9(−1.72,−0.49) | −1.97(−2.51,−1.19) | [D < N c][M < N c] |
| Impairment (%) | 30.77% | 73.3% | ||
Note. N = PD‐NC; M = PD‐MCI; D = PD‐D. Data are showed in mean ± SD or median (25%, 75%). p Value represents the significance level of the analysis of variance performed for each test across the three groups.
p < .05.
p < .01.
p < .001.
3.3. The neuroanatomical regions correlating with cognitive dysfunction in PD
Global metabolic values were comparable among the three subgroups of PD patients (ANOVA: F 2,50 = 0.884, p = .419) and did not correlate with UPDRS motor scores and any test scores of five cognitive domains in the combined group (regression analysis: absolute value r ≤ .258, p ≥ .072). We found positive correlations between the Z‐score of each cognitive domain and 18F‐FDG metabolism in the following areas: executive function with cuneus, superior temporal gyrus, and inferior parietal lobule; attention score correlated positively with the areas in precuneus, superior parietal lobule, and inferior parietal lobule metabolism; memory with superior temporal gyrus, precuneus, and posterior cingulate; and visuospatial function with precuneus, inferior parietal lobule, and superior temporal gyrus (Table 4 and Figure 1). Negative correlations between cognitive domain scores and FDG uptake were found mainly in putamen, frontal lobe, and temporal lobe (Table 5 and Figure 1). We also highlighted the clusters that survived FDR‐corrected p < .05 in both tables. Uptake in these related regions was generally symmetrical. The scatterplots and associated correlations between cognitive domain Z‐score and metabolism for each of the identified regions are provided in Figure 2. No significant correlation between the Z‐score of language domain and 18F‐FDG metabolism was observed.
Table 4.
Brain regions in the 53 PD patients exhibiting positive correlations between the Z‐score of cognitive domains and regional brain metabolism
| Domain | Regions | BA | MNI coordinates | Z max | Voxel number | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Executive function | Rt cuneusa | 7 | 10 | −70 | 34 | 5.31 | 76,192 |
| Rt superior temporal gyrusa | 39 | 54 | −62 | 24 | 4.93 | ||
| Lt inferior parietal lobulea | 40 | −48 | −48 | 26 | 4.92 | ||
| Attention | Rt Precuneusa | 7 | 6 | −70 | 34 | 4.72 | 20,864 |
| Rt superior parietal lobulea | 7 | 42 | −66 | 48 | 4.15 | ||
| Lt inferior parietal lobulea | 40 | −44 | −50 | 46 | 4.09 | 2,920 | |
| Lt superior parietal lobulea | 7 | −38 | −70 | 44 | 3.63 | ||
| Memory | Lt superior temporal gyrus | 39 | −48 | −58 | 32 | 3.94 | 2,440 |
| Lt Precuneus | 19 | −38 | −72 | 40 | 3.93 | ||
| Lt posterior cingulate | 30 | −14 | −66 | 12 | 3.89 | 2,464 | |
| Lt Precuneus | 31 | −10 | −76 | 28 | 3.53 | ||
| Visuospatial function | Rt inferior parietal lobulea | 40 | 44 | −66 | 44 | 4.71 | 6,776 |
| Rt inferior parietal lobulea | 40 | 58 | −52 | 42 | 3.69 | ||
| Rt superior temporal gyrus | 39 | 54 | −62 | 26 | 3.63 | ||
| Lt Precuneusa | 7 | −4 | −58 | 34 | 4.21 | 6,952 | |
| Rt Precuneusa | 7 | 6 | −58 | 40 | 4.12 | ||
Note. Lt = left; Rt = right; BA = Brodmann area; MNI = Montreal Neurological Institute. Statistical threshold: p < .001.
Survived after FDR correction, p < .05.
Figure 1.
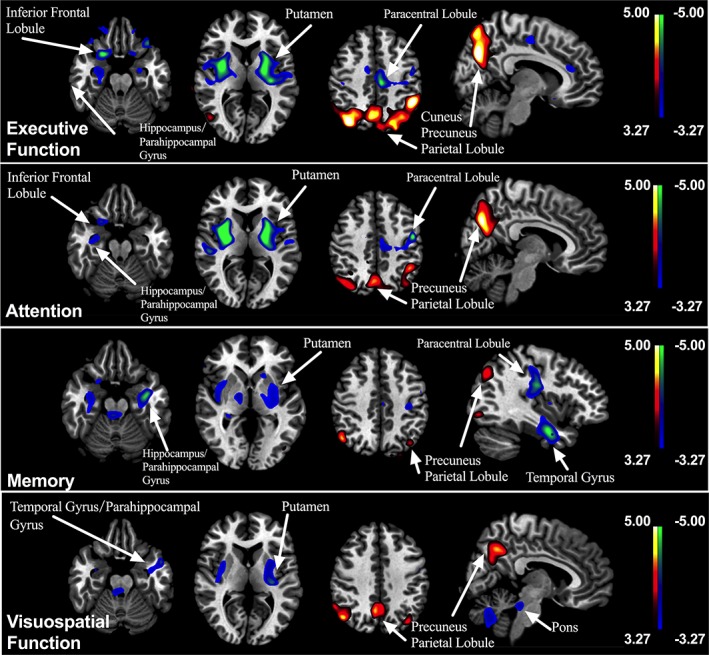
Correlations between the Z‐score of cognitive domains and 18F‐FDG uptake in PD patients. Positive correlations are displayed using a red–yellow scale and negative correlations using a blue–green scale. The overlays are depicted in neurologic orientation. The gray‐scale image is the standard T1‐weighted structural magnetic resonance image (MRI) in Montreal Neurological Institute (MNI) space. The thresholds of the color bars represent T values. Voxel threshold was set at p < .001
Table 5.
Brain regions in the 53 PD patients exhibiting negative correlations between the Z‐score of cognitive domains and regional brain metabolism
| Domain | Regions | BA | Coordinates | Z max | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Executive function | Lt Lentiform nucleusa | Putamen | −30 | −18 | 12 | 4.98 | 38,008 |
| Lt paracentral lobule | 31 | −8 | −24 | 48 | 4.55 | ||
| Rt claustruma | / | 34 | −2 | 8 | 4.71 | 17,232 | |
| Rt Lentiform nucleus | Putamen | 24 | 6 | 16 | 3.81 | ||
| Rt inferior frontal gyrus | 47 | 24 | 14 | −22 | 4.55 | 6,096 | |
| Rt Parahippocampal gyrus | Hippocampus | 26 | −10 | −24 | 3.63 | ||
| Attention | Rt claustruma | / | 32 | −6 | 12 | 5.5 | 21,984 |
| Rt insulaa | 13 | 42 | −28 | 22 | 4.15 | ||
| Rt superior temporal gyrusa | 41 | 50 | −26 | 6 | 4.13 | ||
| Lt precentral gyrusa | 6 | −50 | −6 | 40 | 4.94 | 35,000 | |
| Lt Lentiform nucleusa | Putamen | −30 | −18 | 12 | 4.87 | ||
| Memory | Lt insulaa | 13 | −46 | −16 | 22 | 4.85 | 13,336 |
| Lt precentral gyrusa | 6 | −22 | −22 | 56 | 4.38 | ||
| Lt postcentral gyrus | 3 | −36 | −24 | 46 | 3.79 | ||
| Lt fusiform gyrusa | 20 | −40 | −4 | −24 | 4.50 | 22,712 | |
| Lt Lentiform nucleus | Putamen | −30 | −6 | 10 | 4.36 | ||
| Lt Parahippocampal gyrus | 28 | −26 | −22 | −12 | 4.34 | ||
| Rt sub‐Gyrala | Hippocampus | 30 | −26 | −10 | 4.05 | 5,960 | |
| Rt fusiform gyrus | 20 | 40 | −6 | −26 | 3.67 | ||
| Rt Parahippocampal gyrus | Amygdala | 32 | −8 | −20 | 3.61 | ||
| Rt precentral gyrusa | 6 | 56 | 0 | 14 | 3.91 | 5,896 | |
| Rt insula | 13 | 40 | −4 | 6 | 3.69 | ||
| Rt claustrum | / | 36 | 8 | −2 | 3.38 | ||
| Visuospatial function | Lt Lentiform nucleusa | Putamen | −30 | −20 | 10 | 4.13 | 10,808 |
| Lt Parahippocampal gyrusa | 28 | −24 | −24 | −12 | 3.96 | ||
| Lt insulaa | 13 | −38 | −24 | 16 | 3.93 | ||
| Lt middle frontal gyrusa | 6 | −20 | −22 | 62 | 4.12 | 3,776 | |
| Lt superior frontal gyrus | 6 | −14 | −6 | 68 | 3.34 | ||
| Rt insulaa | / | 42 | −16 | −2 | 3.99 | 4,200 | |
| Rt claustruma | / | 32 | 4 | 6 | 3.44 | ||
| Rt uvula | / | 8 | −66 | −36 | 3.78 | 5,528 | |
| Lt uvulaa | −10 | −64 | −36 | 3.41 | |||
| Rt Culmena | / | 22 | −62 | −34 | 3.35 | ||
Note. Lt = left; Rt = right; BA = Brodmann area; MNI = Montreal Neurological Institute. Statistical threshold: p < .001.
Survived after FDR correction, p < .05.
Figure 2.
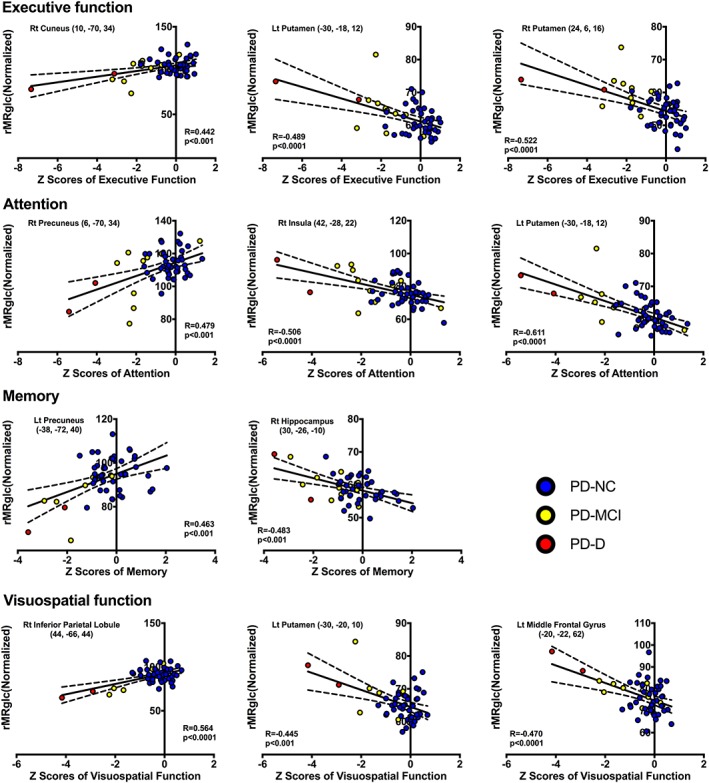
Scatter plots showing correlations between cognitive domain scores and relative metabolic values for the significantly correlated regions in SPM. Cognitive status of each patient is depicted by filled colored circle (blue, PD‐NC; yellow, PD‐MCI; red, PD‐D). PD‐NC, Parkinson's disease with normal cognition; PD‐MCI, Parkinson's disease with mild cognitive impairment; PD‐D, Parkinson's disease dementia
4. DISCUSSION
Here, we examined the profile of cognitive impairment in a relative large Chinese cohort of PD patients using the MDS Task Force Level II criteria (Emre et al., 2007; Litvan et al., 2012) and explored possible mechanisms using PET metabolic imaging. There were four main findings. First, cognitive impairment was common in the PD group (PD‐MCI, 23.2%; PD‐D, 8.9%), with multiple rather than single domains being affected in nearly all cases. Second, cognitive impairments could be detected in almost all individual neuropsychological tests within the five domains, suggesting a broad neurocognitive phenotype. Third, the different cognitive status groups (PD‐MCI and PD‐D) showed a similar impairment pattern: executive function and attention > memory and visuospatial > language function. Finally, posterior temporo‐parieto‐occipital association cortex metabolism was significantly associated with cognitive impairment across all domains except language suggesting an important role of this region in cognitive decline in PD.
Cognitive impairment is among the most common and quality of life‐impacting non‐motor symptoms in PD. A total of 23.2% of patients met the MDS Task Force level II criteria for PD‐MCI, and 8.9% fulfilled criteria for PD‐D. In contrast to the main subtype of impairment in PD‐MCI being single‐domain as reported previously (Caviness et al., 2007; Muslimovic, Post, Speelman, & Schmand, 2005; Wang et al., 2015), the majority of our PD‐MCI patients had multiple‐domain deficits (94.9%), similar to the studies using the MDS Task Force criteria (Goldman, Weis, Stebbins, Bernard, & Goetz, 2012; Wood et al., 2016). The relatively high prevalence and predominantly multiple‐domain, impairment of PD‐MCI in our study compared to other reports, might be explained by use of the very sensitive level II diagnostic criteria of the MDS Task Force (Litvan et al., 2012), which was intended to provide more reliable characterization of widespread cognitive dysfunction.
In this study, PD patients had significantly poorer scores than controls on almost all individual neuropsychological tests administered, suggesting a more widespread nature of cognitive impairment in PD (Weintraub et al., 2015). Our data suggests that PD, PD‐MCI, and PD‐D patients all have a similar impairment pattern which is most prominent in executive function and attention, followed by deficits in memory and visuospatial abilities, and least impairments in language (Wang et al., 2015). However, the neuropathophysiologic mechanisms underlying this cognitive impairment remains unclear and PET imaging can provide a powerful approach for exploring the mechanisms involved or at the very least highlight regions of interest. Huang et al. reported a PD‐related cognitive pattern characterized by reduced metabolism in frontal and parietal association areas and increased metabolism in cerebellum and dentate nuclei (Huang et al., 2007). Our previous work suggested that the hypometabolism was predominantly located in the frontal and temporal cortices in Chinese PD‐MCI patients compared with PD‐NC patients (Tang et al., 2016). In PD‐D patients, the hypometabolism in the posterior cortical areas was greater than in PD‐MCI patients, suggesting an association between posterior cortical hypometabolism and more severe cognitive impairment (Tang et al., 2016). However, the association of impaired cerebral metabolism with individual cognitive domain function has not previously been examined in detail. In this study, the Z‐scores of executive function, attention, memory, and visuospatial function domains correlated positively with metabolism in posterior temporo‐parieto‐occipital cortex, with some topographical overlap (precuneus, inferior parietal lobule, and superior temporal gyrus). Thus the lower the Z‐scores, indicating greater cognitive impairment, the lower the metabolism in posterior temporo‐parieto‐occipital cortex.
The reduced posterior temporo‐parieto‐occipital cortex metabolism in the cognitively impaired PD patients likely reflects changes in regional metabolism and neurotransmitter function. The posterior key node of the so‐called default mode network (DMN) expresses hyperactivity of interlinked regions during wakeful rest (Buckner, Andrews‐Hanna, & Schacter, 2008; Raichle, 2015), and it plays a crucial role in cognitive processing both in normal aging and neurodegenerative disorders (Agosta et al., 2012; Sambataro et al., 2010). In PD, the DMN has been shown to be abnormally modulated (Boord, Madhyastha, Askren, & Grabowski, 2017) and the metabolic decrease in one or more nodes of this network might impede neural resource reallocation for normal task performance (Binder et al., 1999). In addition, the posterior cortical metabolism changes we observed might also reflect cholinergic deficiency. The posterior cortex receives cholinergic projections from the nucleus basalis of Meynert, and cholinergic denervation can be detected in this region in PD (Hilker et al., 2005; Perry et al., 1983). In support of this notion, a PET study using the tracer N‐[11C]methyl‐4‐piperidyl acetate (MP4A) showed a reduction of cholinergic function in the posterior temporo‐parieto‐occipital regions in PD patients (Klein et al., 2010). On the basis of these observations of impaired cholinergic function, in combination with our present observations of impaired metabolic function in the same territories, we speculate that cholinergic denervation in posterior association areas may play an important role in the cognitive impairments of PD.
We also found that paracentral gyrus and putamen metabolism was negatively correlated with the Z‐scores of the cognitive domains. The lower of the Z‐scores (indicating more severe cognitive deficit), the higher of the metabolism in the paracentral gyrus and putamen. In previous studies, hypermetabolism in putamen contributed to a PD‐related pattern (Eidelberg et al., 1994; Ma, Tang, Spetsieris, Dhawan, & Eidelberg, 2007; Wu et al., 2013), correlating with motor deficiency. In our previous study, we reported a significant correlation between subdomains of cognitive impairment and motor dysfunction, suggesting shared neurochemical pathways (Wang et al., 2017). In a previous study, PD‐D patients exhibit greater dopamine depletion than non‐demented patients (Emre, 2003). Therefore, it is still necessary to explore unique roles played by dopaminergic deficiency in the cognitive decline using specific radioligands. Work is underway to examine some patients included in this study who were also scanned with dopamine transporter PET imaging.
We found no correlation of 18F‐FDG uptake with language function, a somewhat unexpected result. This may be related to higher thresholds used to report the clusters of interest. At a lower voxel‐level threshold, however, we did observe positive and negative correlations in several cortical regions (p < .01; Supporting Information Table S2), indicating the need for a large sample size to detect relevant brain regions that correlate more significantly with language function in these patients. Common understanding places the function of language predominantly in the perisylvian language areas within the dominant hemisphere. However, recent studies suggest that a much broader brain network might control language. A functional MRI study in healthy Chinese volunteers showed that maximal responses were located in the right superior temporal gyrus for picture naming, and the left middle frontal gyrus and the left superior frontal gyrus for verbal fluency‐character (Ci et al., 2016). In PD patients, structural gray matter changes in temporal, frontal, and cerebellar areas correlate with semantic fluency rather than phonemic fluency scores (Pereira et al., 2009). In contrast, striatal volume is associated with phonemic verbal fluency (Ellfolk et al., 2014). However, in this study we could not confirm a clear relationship between language function and cerebral 18F‐FDG metabolisms in PD. These negative findings may be due to the Boston naming and semantic fluency tests assessing only a limited aspect of the complex and subtle functions of language (Poeppel, 2014). Therefore, they may not be sensitive enough to reveal any true, albeit subtle, metabolic reduction in a localized node (Klein et al., 2010). In addition, relative preservation of the language domain is a common finding in studies of cognitive function in PD (Goldman et al., 2012; Wood et al., 2016).
We want to emphasize that correlations detected between cognitive measures and regional glucose metabolism in this study are independent of global metabolic values in individual patients. This can be attributed to three experimental and methodological factors: (1) global metabolic values were not significantly different between patients with PD‐NC, PD‐MCI, and PD‐D (ANOVA: F 2,50 = 0.884, p = .419); (2) global metabolic values did not correlate with any clinical measures including UPDRS motor ratings and all cognitive tests used as dependent variables in the regression analysis (regression analysis: absolute value r ≤ .258, p ≥ .072); and (3) any residual variation in global metabolic values was removed from differences in regional metabolic values by ANCOVA. Hence, the series of brain–behavioral relationships described in this article are not influenced by inter‐subject variability in global metabolic values.
5. LIMITATIONS
There are several limitations in our study. First, inclusion of participants in the healthy control group was based mainly on self‐report of medical history rather than medical records, so it's possible that some may have been included inappropriately. However, it is pertinent that none of the controls demonstrated cognitive impairment (e.g., MCI) on detailed assessment. Second, FDG PET scans were acquired in the static mode over 10 min which was selected to be consistent with the imaging protocol used in our previous publications (Ge et al., 2015; Wu et al., 2013). It has been known that the concentration data provided by this static imaging method is linearly related to brain glucose metabolism (Carson, 1991). Third, the activated cerebral regions identified in our study are based on the PET metabolism–cognition correlations, and so are limited in elucidating the exact neural networks and specific neurotransmitters associated with cognitive impairment in PD. Future PET studies with specific radioligands, along with autopsy and animal studies may help to provide more specific information in this regard. Fourth, although all the cognitive tests were performed in patients taking their usual daily medications, some unwanted variance in cognitive results might also exist due to the ON/OFF fluctuations.
6. CONCLUSION
In conclusion, our study has highlighted the widespread nature of cognitive impairment in PD. All of the cognitive domains examined except language function correlated positively with metabolism in posterior temporo‐parieto‐occipital cortex, suggesting a larger cortical involvement than the classic striato‐frontal hypothesis. We propose that these findings indicate that the posterior cortical areas, encompassing multiple neurotransmitters and neural circuits, participate in the pathogenesis of cognitive decline in PD. The detailed neurobiological mechanisms remain to be clarified in future studies.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
All authors’ roles in the project and preparation of the manuscript are listed as follows:
Research project: A. Conception by JW, LC, and CTZ; B. Organization by JW, LC, LW, FTL, and JJG; C. Execution by LW, FTL, JJG, JZ, YLT, WBY, and HY.
Statistical Analysis: A. Design by JW, LFT, and WL; B. Execution by WL, FTL, JJG, JZ,, YLT, and TA; C. Review and Critique by JW, HY, LC, and TA.
Manuscript: A. Writing of the first draft by WL, FTL, JJG, and JW; B. Review and Critique by JW, LC, TA, and CTZ.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Authors are grateful to Dr. Qi‐hao Guo, Dr. Qian‐Hua Zhao, and Dr. Xiao‐Niu Liang for the suggestions in data analysis, to Dr. Zhen‐Yang Liu, Dr. Ke Yang, Dr. Kui Chen, and Dr. Lu‐Lu Bu for the help in data collection, and to Dr. James B. Koprich for editing the manuscript.
Wu L, Liu F‐T, Ge J‐J, et al. Clinical characteristics of cognitive impairment in patients with Parkinson's disease and its related pattern in 18F‐FDG PET imaging. Hum Brain Mapp. 2018;39:4652–4662. 10.1002/hbm.24311
Lei Wu and Feng‐tao Liu contributed equally to this study.
Funding information National Key R&D Program of China from Ministry of Science and Technology of China, Grant/Award Numbers: 2016YFC1306500, 2016YFC1306504; National Nature Science Foundation of China, Grant/Award Numbers: 81701250, 81771372, 81671239, 81571232, 81371413, 81361120393; Scientific Research Project from Huashan Hospital affiliated to Fudan University, Grant/Award Number: 2016QD01; Project from Science and Technology Commission of Shanghai Municipality, Grant/Award Number: 15ZR1435800; the development funding for Shanghai Talents, Grant/Award Number: 201448; Shanghai Sailing Program, Grant/Award Number: 18YF1403100
Contributor Information
Ling Chen, Email: chenl2@mail.sysu.edu.cn.
Jian Wang, Email: wangjian336@hotmail.com.
REFERENCES
- Aarsland, D. , Andersen, K. , Larsen, J. P. , Lolk, A. , & Kragh‐Sorensen, P. (2003). Prevalence and characteristics of dementia in Parkinson disease: An 8‐year prospective study. Archives of Neurology, 60, 387–392. [DOI] [PubMed] [Google Scholar]
- Aarsland, D. , Bronnick, K. , Williams‐Gray, C. , Weintraub, D. , Marder, K. , Kulisevsky, J. , … Emre, M. (2010). Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology, 75, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland, D. , Zaccai, J. , & Brayne, C. (2005). A systematic review of prevalence studies of dementia in Parkinson's disease. Movement Disorders, 20, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Agosta, F. , Pievani, M. , Geroldi, C. , Copetti, M. , Frisoni, G. B. , & Filippi, M. (2012). Resting state fMRI in Alzheimer's disease: Beyond the default mode network. Neurobiology of Aging, 33, 1564–1578. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Frost, J. A. , Hammeke, T. A. , Bellgowan, P. S. , Rao, S. M. , & Cox, R. W. (1999). Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience, 11, 80–95. [DOI] [PubMed] [Google Scholar]
- Boord, P. , Madhyastha, T. M. , Askren, M. K. , & Grabowski, T. J. (2017). Executive attention networks show altered relationship with default mode network in PD. NeuroImage: Clinical, 13, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Caffarra, P. , Vezzadini, G. , Dieci, F. , Zonato, F. , & Venneri, A. (2002). Rey‐Osterrieth complex figure: Normative values in an Italian population sample. Neurological Sciences, 22, 443–447. [DOI] [PubMed] [Google Scholar]
- Carson, R. E. (1991). Precision and accuracy considerations of physiological quantitation in PET. Journal of Cerebral Blood Flow and Metabolism, 11, A45–A50. [DOI] [PubMed] [Google Scholar]
- Caviness, J. N. , Driver‐Dunckley, E. , Connor, D. J. , Sabbagh, M. N. , Hentz, J. G. , Noble, B. , … Adler, C. H. (2007). Defining mild cognitive impairment in Parkinson's disease. Movement Disorders, 22, 1272–1277. [DOI] [PubMed] [Google Scholar]
- Ci, H. , van Graan, A. , Gonzalvez, G. , Thompson, P. , Hill, A. , & Duncan, J. S. (2016). Mandarin functional MRI language paradigms. Brain and Behavior: A Cognitive Neuroscience Perspective, 6, e00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg, D. , Moeller, J. R. , Dhawan, V. , Spetsieris, P. , Takikawa, S. , Ishikawa, T. , … Fahn, S. (1994). The metabolic topography of parkinsonism. Journal of Cerebral Blood Flow and Metabolism, 14, 783–801. [DOI] [PubMed] [Google Scholar]
- Ellfolk, U. , Joutsa, J. , Rinne, J. O. , Parkkola, R. , Jokinen, P. , & Karrasch, M. (2014). Striatal volume is related to phonemic verbal fluency but not to semantic or alternating verbal fluency in early Parkinson's disease. Journal of Neural Transmission (Vienna), 121, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre, M. (2003). Dementia associated with Parkinson's disease. Lancet Neurology, 2, 229–237. [DOI] [PubMed] [Google Scholar]
- Emre, M. , Aarsland, D. , Brown, R. , Burn, D. J. , Duyckaerts, C. , Mizuno, Y. , … Dubois, B. (2007). Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders, 22, 1689–1707 quiz 1837. [DOI] [PubMed] [Google Scholar]
- Fahn S., Marsonden C., Calne D., & Goldstein M. (Eds.). (1987). Recent developments in Parkinson's disease (Vol. 2, pp. 153–163). Florham Park, NJ: Mac‐Millan Heathcare Information. [Google Scholar]
- Foltynie, T. , Brayne, C. E. , Robbins, T. W. , & Barker, R. A. (2004). The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain, 127, 550–560. [DOI] [PubMed] [Google Scholar]
- Ge, J. , Wu, P. , Peng, S. , Yu, H. , Zhang, H. , Guan, Y. , … Wang, J. (2015). Assessing cerebral glucose metabolism in patients with idiopathic rapid eye movement sleep behavior disorder. Journal of Cerebral Blood Flow and Metabolism, 35, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, J. G. , Weis, H. , Stebbins, G. , Bernard, B. , & Goetz, C. G. (2012). Clinical differences among mild cognitive impairment subtypes in Parkinson's disease. Movement Disorders, 27, 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Q. , Zhao, Q. , Chen, M. , Ding, D. , & Hong, Z. (2009). A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Disease & Associated Disorders, 23, 253–259. [DOI] [PubMed] [Google Scholar]
- Hilker, R. , Thomas, A. V. , Klein, J. C. , Weisenbach, S. , Kalbe, E. , Burghaus, L. , … Heiss, W. D. (2005). Dementia in Parkinson disease: Functional imaging of cholinergic and dopaminergic pathways. Neurology, 65, 1716–1722. [DOI] [PubMed] [Google Scholar]
- Huang, C. , Mattis, P. , Tang, C. , Perrine, K. , Carbon, M. , & Eidelberg, D. (2007). Metabolic brain networks associated with cognitive function in Parkinson's disease. NeuroImage, 34, 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, A. J. , Daniel, S. E. , Kilford, L. , & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry, 55, 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, J. C. , Eggers, C. , Kalbe, E. , Weisenbach, S. , Hohmann, C. , Vollmar, S. , … Hilker, R. (2010). Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology, 74, 885–892. [DOI] [PubMed] [Google Scholar]
- Litvan, I. , Aarsland, D. , Adler, C. H. , Goldman, J. G. , Kulisevsky, J. , Mollenhauer, B. , … Weintraub, D. (2011). MDS task force on mild cognitive impairment in Parkinson's disease: Critical review of PD‐MCI. Movement Disorders, 26, 1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan, I. , Goldman, J. G. , Troster, A. I. , Schmand, B. A. , Weintraub, D. , Petersen, R. C. , … Emre, M. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society task force guidelines. Movement Disorders, 27, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Ge, J. , Wu, J. , Wu, P. , Ma, Y. , Zuo, C. , & Wang, J. (2018). Clinical, Dopaminergic, and Metabolic Correlations in Parkinson Disease: A Dual‐Tracer PET Study. Clinical Nuclear Medicine, doi: 10.1111/j.1399-3062.2011.00699. [DOI] [PubMed] [Google Scholar]
- Lucas, J. A. , Ivnik, R. J. , Smith, G. E. , Ferman, T. J. , Willis, F. B. , Petersen, R. C. , & Graff‐Radford, N. R. (2005). Mayo's older African Americans normative studies: Norms for Boston naming test, controlled oral word association, category fluency, animal naming, token test, WRAT‐3 reading, trail making test, Stroop test, and judgment of line orientation. The Clinical Neuropsychologist, 19, 243–269. [DOI] [PubMed] [Google Scholar]
- Ma, Y. , Tang, C. , Spetsieris, P. G. , Dhawan, V. , & Eidelberg, D. (2007). Abnormal metabolic network activity in Parkinson's disease: Test‐retest reproducibility. Journal of Cerebral Blood Flow and Metabolism, 27, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic, D. , Post, B. , Speelman, J. D. , & Schmand, B. (2005). Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology, 65, 1239–1245. [DOI] [PubMed] [Google Scholar]
- Pereira, J. B. , Junque, C. , Marti, M. J. , Ramirez‐Ruiz, B. , Bartres‐Faz, D. , & Tolosa, E. (2009). Structural brain correlates of verbal fluency in Parkinson's disease. Neuroreport, 20, 741–744. [DOI] [PubMed] [Google Scholar]
- Perry, R. H. , Tomlinson, B. E. , Candy, J. M. , Blessed, G. , Foster, J. F. , Bloxham, C. A. , & Perry, E. R. (1983). Cortical cholinergic deficit in mentally impaired parkinsonian patients. Lancet, 2, 789–790. [DOI] [PubMed] [Google Scholar]
- Poeppel, D. (2014). The neuroanatomic and neurophysiological infrastructure for speech and language. Current Opinion in Neurobiology, 28, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. (2015). The brain's default mode network. Annual Review of Neuroscience, 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Sambataro, F. , Murty, V. P. , Callicott, J. H. , Tan, H. Y. , Das, S. , Weinberger, D. R. , & Mattay, V. S. (2010). Age‐related alterations in default mode network: Impact on working memory performance. Neurobiology of Aging, 31, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, L. K. , Fitzgerald, H. E. , Adams, K. M. , Nigg, J. T. , Martel, M. M. , Puttler, L. I. , … Zucker, R. A. (2006). Normative symbol digit modalities test performance in a community‐based sample. Archives of Clinical Neuropsychology, 21, 23–28. [DOI] [PubMed] [Google Scholar]
- Steinberg, B. A. , Bieliauskas, L. A. , Smith, G. E. , & Ivnik, R. J. (2005). Mayo's older Americans normative studies: Age‐ and IQ‐adjusted norms for the trail‐making test, the Stroop test, and MAE controlled oral word association test. Clinical Neuropsychology, 19, 329–377. [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Ge, J. , Liu, F. , Wu, P. , Guo, S. , Liu, Z. , … Wang, J. (2016). Cerebral metabolic differences associated with cognitive impairment in Parkinson's disease. PLoS One, 11, e0152716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. Q. , Tang, B. S. , Yan, X. X. , Chen, Z. H. , Xu, Q. , Liu, Z. H. , … Guo, J. F. (2015). A neurophysiological profile in Parkinson's disease with mild cognitive impairment and dementia in China. Journal of Clinical Neuroscience, 22, 981–985. [DOI] [PubMed] [Google Scholar]
- Wang, Y. X. , Zhao, J. , Li, D. K. , Peng, F. , Wang, Y. , Yang, K. , … Wang, J. (2017). Associations between cognitive impairment and motor dysfunction in Parkinson's disease. Brain and Behavior: A Cognitive Neuroscience Perspective, 7, e00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub, D. , Simuni, T. , Caspell‐Garcia, C. , Coffey, C. , Lasch, S. , Siderowf, A. , … Parkinson's Progression Markers Initiative . (2015). Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson's disease. Movement Disorders, 30, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, K. L. , Myall, D. J. , Livingston, L. , Melzer, T. R. , Pitcher, T. L. , MacAskill, M. R. , … Dalrymple‐Alford, J. C. (2016). Different PD‐MCI criteria and risk of dementia in Parkinson's disease: 4‐year longitudinal study. NPJ Parkinson's Disease, 2, 15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, P. , Wang, J. , Peng, S. , Ma, Y. , Zhang, H. , Guan, Y. , & Zuo, C. (2013). Metabolic brain network in the Chinese patients with Parkinson's disease based on 18F‐FDG PET imaging. Parkinsonism & Related Disorders, 19, 622–627. [DOI] [PubMed] [Google Scholar]
- Yu, R. L. , Wu, R. M. , Tai, C. H. , Lin, C. H. , Cheng, T. W. , & Hua, M. S. (2012). Neuropsychological profile in patients with early stage of Parkinson's disease in Taiwan. Parkinsonism & Related Disorders, 18, 1067–1072. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Guo, Q. , Li, F. , Zhou, Y. , Wang, B. , & Hong, Z. (2013). The shape trail test: Application of a new variant of the trail making test. PLoS One, 8, e57333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, C. , Ma, Y. , Sun, B. , Peng, S. , Zhang, H. , Eidelberg, D. , & Guan, Y. (2013). Metabolic imaging of bilateral anterior capsulotomy in refractory obsessive compulsive disorder: An FDG PET study. Journal of Cerebral Blood Flow and Metabolism, 33, 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


