Abstract
Perinatal maternal depressive symptoms influence brain development of offspring. Such effects are particularly notable in the amygdala, a key structure involved in emotional processes. This study investigated whether the functional organization of the amygdala varies as a function of pre‐ and postnatal maternal depressive symptoms. The amygdala functional network was assessed using resting‐state functional magnetic resonance imaging (rs‐fMRI) in 128 children at age of 4.4 to 4.8 years. Maternal depressive symptoms were obtained at 26 weeks of gestation, 3 months, 1, 2, 3, and 4.5 years after delivery. Linear regression was used to examine associations between maternal depressive symptoms and the amygdala functional network. Prenatal maternal depressive symptoms were significantly associated with the functional connectivity between the amygdala and the cortico‐striatal circuitry, especially the orbitofrontal cortex (OFC), insula, subgenual anterior cingulate (ACC), temporal pole, and striatum. Interestingly, greater pre‐ than post‐natal depressive symptoms were associated with lower functional connectivity of the left amygdala with the bilateral subgenual ACC and left caudate and with lower functional connectivity of the right amygdala with the left OFC, insula, and temporal pole. These findings were only observed in girls but not in boys. Early exposure to maternal depressive symptoms influenced the functional organization of the cortico‐striato‐amygdala circuitry, which is intrinsic to emotional perception and regulation in girls. This suggests its roles in the transgenerational transmission of vulnerability for socio‐emotional problems and depression. Moreover, this study underscored the importance of gender‐dependent developmental pathways in defining the neural circuitry that underlies the risk for depression.
Keywords: amygdala functional network, maternal depressive symptoms, resting‐state functional magnetic resonance imaging, sexual dimorphism, transgenerational transmission
1. INTRODUCTION
Early exposure to maternal depression has detrimental effects on emotional (Field, 1992; Goodman & Gotlib, 1999), behavioral (Brennan et al., 2000), and cognitive development (Sohr‐Preston & Scaramella, 2006), and increases a risk for depression in offspring (Goodman et al., 2011; Pearson et al., 2013). Twin children of depressed mothers showed a high risk for depression, underscoring the importance of “shared” environmental influences on offspring (Silberg, Maes, & Eaves, 2010). Likewise, perinatal maternal depressive symptoms also impact brain development in offspring and highlight the profound influence of the early environment on brain development (Tau & Peterson, 2010).
Different pathways may contribute to the influence of pre‐ and post‐natal maternal depressive symptoms on offspring. Prenatal maternal depressive symptoms can cause a number of physiological changes that may influence intra‐uterine environment and alter fetal development. On the other hand, postnatal maternal depressive symptoms influence the offspring most likely through forms of parenting (Fleming, Ruble, Flett, & Shaul, 1988; Rifkin‐Graboi et al., 2015a; Wen et al., 2017) that enhance fearfulness, social withdrawal (Bruder‐Costello et al., 2007; Degnan, Almas, & Fox, 2010; Moffitt, Caspi, Newman, & Silva, 1996; Murray, Halligan, Goodyer, & Herbert, 2010) and predict an increased risk for later psychopathology in offspring (Biederman et al., 2001). Hence, we expect that pre‐ and post‐natal maternal depressive symptoms may independently influence brain development of offspring. Moreover, children who experience the fluctuation of maternal depressive symptoms pre‐ and post‐natally show decreased motor and mental development during the first few years of life (Sandman, Davis, & Glynn, 2012), suggesting that incongruence between prenatal and postnatal environments may confer a disadvantage for critical survival functions during early development. We may expect that the fluctuation between pre‐ and post‐natal maternal depressive symptoms would influence early brain development in the same manner as shown in motor and mental development in early life.
In this study, we aimed to examine the abovementioned two hypotheses using resting‐state magnetic resonance imaging (rs‐fMRI) technique in 4.5‐year‐old children. We focused on the amygdala that has featured prominently in developmental studies examining the influence of maternal mental health. Prenatal maternal depressive symptoms associate with the amygdala microstructure of neonates (Rifkin‐Graboi et al., 2013) and with amygdala functional connectivity with the prefrontal and limbic cortex as well as with the sensory cortex in infants (Posner et al., 2016; Qiu et al., 2015). Moreover, 10‐year‐old children of mothers with postnatal depression show larger bilateral amygdala volumes compared with those of mothers without depression (Lupien et al., 2011). Children with a maternal history of major depressive disorder display a reduced functional connectivity between the amygdala and inferior limbic regions (Luking et al., 2011), and show a failure to integrate emotional experience and memory. In addition, only female adolescents with early life stress show a positive association between amygdala‐ventromedial prefrontal cortex (vmPFC) functional connectivity and depressive symptoms (Burghy et al., 2012). Similarly, a recent study suggests that influences of maternal depressive symptoms are gender‐specific in a certain developmental time window (Wen et al., 2017). Greater prenatal maternal depressive symptoms were associated with larger right amygdala volume in girls, but not in boys (Wen et al., 2017). Increased postnatal maternal depressive symptoms were associated with greater water diffusivity in right amygdala only in girls (Wen et al., 2017). These findings demonstrate the vulnerability of the amygdala's emotional perception and regulation networks to maternal depression and such vulnerability may be specific to gender. Hence, this study examined the two hypotheses in girls and boys separately to elucidate potential gender‐dependent amygdala functional connectivity in response to maternal depressive symptoms in early life. We expect that independent influences of pre‐ and post‐natal maternal depressive symptoms on the amygdala functional network would predominantly occur in the prefrontal and limbic cortex as well as sensory regions (Lebel et al., 2016; Sandman, Buss, Head, & Davis, 2015). These brain regions, together with the amygdala, are in the emotion perception and regulation circuitry with implications for major depressive disorder (MDD) (Price & Drevets, 2010). As the sample in this study was drawn from a general population, the severity of maternal depressive symptoms lay largely within the normal range. Hence, the results of this study provide, to our knowledge, the first analysis linking maternal depressive symptoms and the amygdala functional organization in early childhood from a general population.
2. METHODS AND MATERIALS
2.1. Participants
Children participating in the prospective birth cohort study, Growing Up in Singapore Towards healthy Outcomes (GUSTO), were invited to this neuroimaging study at 4.5 years of age. Detailed GUSTO recruitment criteria are stated elsewhere (Soh et al., 2014). In general, the GUSTO cohort recruited pregnant Singapore citizens or Permanent Residents of Chinese, Malay or Indian ethnic backgrounds from two major birthing hospitals in Singapore at the first antenatal visit. Their children were then followed up after birth. The GUSTO study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB) and the SingHealth Centralized Institutional Review Board (CIRB). Written consent was obtained from mothers.
Maternal education level and maternal ethnicity were extracted from survey questionnaires conducted as part of a scheduled appointment during the 26th week of pregnancy. Birth outcomes (i.e., gestational age, birth weight, and gender) and pregnancy measures were obtained from the hospital record.
This study only included children with maternal report on prenatal and postnatal maternal depression scales and with gestational age ≥ 34 weeks, birth weight ≥ 2 kg and a 5‐minute Appearance, Pulse, Grimace, Activity, Respiration (APGAR) score ≥ 8 to avoid their potential impacts on brain development.
2.2. Maternal depression scales
In GUSTO, the Edinburgh Postnatal Depression Scale (EPDS) (Cox, Holden, & Sagovsky, 1987) questionnaire was administered to mothers at 26 weeks of pregnancy and 3 months after delivery to quantify depressive symptomatology. The EPDS is a widely used 10‐item self‐report scale designed as a screening instrument for postnatal depression and is valid for use in prenatal and early postnatal depression. Each item of the EPDS is scored on a four‐point scale (0–3), and while items 3 and 5–10 are reverse scored.
The Beck's Depression Inventory‐II (BDI‐II) was administered to mothers at 12 months, 24 months, 36 months and 54 months after delivery. The BDI‐II is a widely used 21‐item questionnaire that assesses the existence and severity of symptoms of depression and predicts the severity of clinical depressive symptoms (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). Each item of the BDI‐II is scored on a four‐point scale (0–3). Higher total scores indicate more severe depressive symptoms.
Prorated imputation was performed when 8 or 9 questions were answered on the EPDS and 19 or 20 questions were answered for the BDI‐II. To incorporate the EPDS and BDI scores, each EPDS/BDI score at each time point was first standardized. The standardization analysis was not restricted in the sample of this imaging study, rather it was applied to the whole GUSTO sample (n = 1,162). Latent growth curve analysis suggested that maternal depressive symptoms in most of the GUSTO subjects (∼92%) were stable over the course of the first 54 months after delivery (Supporting Information Figure S1). Because of the stability of postnatal maternal depressive symptoms over time, this study quantified postnatal maternal depressive symptoms using the average value over the course of the first 54 months to represent the severity of postnatal maternal depressive symptoms. The fluctuation of maternal depressive symptoms was calculated by subtracting the postnatal score from the prenatal score. Its value greater than 0 indicated a higher prenatal maternal depressive score than its corresponding postnatal score. The severity of maternal depressive symptoms was calculated by averaging the pre‐ and postnatal scores.
2.3. MRI acquisition
Children underwent the structural and functional brain scans at 4.5 years of age (–2 months to +4 months). The MRI scans were acquired using a 3T Siemens Skyra scanner with a 32‐channel head coil at KK Women's and Children's hospital. The image protocols were: (i) high‐resolution isotropic T1‐weighted Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE; 192 slices, 1 mm thickness, in‐plane resolution 1 mm, sagittal acquisition, field of view 192 × 192 mm, matrix = 192 × 192, repetition time = 2,000 ms, echo time = 2.08 ms, inversion time = 877 ms, flip angle = 9°); (ii) isotropic axial resting‐state functional MRI (rs‐fMRI) protocol (single‐shot echo‐planar imaging; 48 slices with 3 mm slice thickness, no inter‐slice gaps, matrix = 64 × 64, field of view= 192 × 192 mm, repetition time = 2,660 ms, echo time = 27 ms, flip angle = 90°, scanning time of the first run = 5.27 min (120 volumes), scanning time of the second run = 3.19 min (70 volumes)). The children watched their favorite movie during the scanning except for the rs‐fMRI session. They were asked to close their eyes during the rs‐fMRI scanning session.
To ensure image quality, children underwent the MRI home and on‐site training programs prior the MRI visit (see details in the Supporting Information). Additionally, the image quality was verified immediately after the acquisition through visual inspection while the children were still in the scanner. If the motion artifact in MPRAGE was large, a repeated MPRAGE scan was conducted. The image was removed from the study if no acceptable image was acquired after three repetitions. After the data acquisition, the image quality was further checked and 230 children with good T1 and rs‐fMRI imaging quality and their rs‐fMRI scan longer than 5 min were included for the following MRI processing. The image data were further excluded if they do not pass below image processing as described in depth.
2.4. Structural MRI analysis
FreeSurfer was used to label each voxel in the T1‐weighted image as gray matter (GM), or white matter (WM), or cerebrospinal fluid (CSF), or subcortical structures (e.g., hippocampus, amygdala, thalamus, caudate, putamen, globus pallidus) (Fischl et al., 2002). FreeSurfer employed a Markov random field (MRF) model that requests for a prior probability obtained from a training dataset with T1‐weighted images and their manual structural labels. In this study, we reconstructed the prior probability for the MRF model based on the manual segmentation of 30 Asian children and embedded it in FreeSurfer (replacing RB_all_2008‐03‐26.gca under freesurfer/average). FreeSurfer was then performed to each T1‐weighted image in this study. Post‐processing quality check was conducted following by the instruction on https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingData. The segmentation accuracy was assessed using a volume overlap ratio (VOR) between the automated and manual segmentation (Fischl et al., 2002). The VOR values for the amygdala is 0.90 ± 0.05, suggesting the high accuracy of the automated segmentation when compared with the manual labeling. Finally, nonlinear image normalization was achieved by co‐aligning individual T1‐weighted MRI images to the atlas space via large deformation diffeomorphic metric mapping (LDDMM) (Du, Younes, & Qiu, 2011; Tan & Qiu, 2016).
2.5. Rs‐fMRI analysis
Precaution of head motion in rs‐fMRI was taken. The rs‐fMRI data that had large motion or checkerboard image appearance through more than 10 volumes in a row were discarded for any further analysis. Then, the rest of the rs‐fMRI scans were preprocessed using FSL (Smith et al., 2004), including slice time correction, motion correction, and skull stripping. For each subject, the head motion was corrected in two steps. First, rs‐fMRI volumes were aligned to the first volume and the mean volume was then created via averaging across all the rs‐fMRI volumes. Second, all rs‐fMRI volumes were aligned to the mean volume. This study further excluded rs‐fMRI data if one or multiple volumes had framewise displacement greater than 0.5 mm. The mean and standard deviation values of the maximal framewise displacement among the subjects included in this study were 0.169 mm and 0.130 mm and the range was from 0.03 mm to 0.49 mm. Within subjects, the mean functional volume was aligned to the corresponding T1‐weighted anatomical image via rigid body alignment. Subsequently, intensity normalization was applied.
Six motion parameters, whole brain, WM and CSF signals were partialled out from rs‐fMRI signals. Here, whole brain, WM, and CSF signals were averaged over the voxels in the eroded whole brain, WM, and CSF masks that were obtained from the above FreeSurfer analysis. Even though it is well known that the global signal regression may generate anti‐correlated functional networks, it was considered as an appropriate preprocessing step particularly for studying pediatric, clinical and elderly populations to eliminate artifactual variance due to micro‐motion (Van Dijk et al., 2010; Yan et al., 2013). Hence, the global signal regression approach was employed in this study. Band‐pass filtering (0.01–0.08 Hz) was then applied. The functional data were finally transformed to the atlas space via LDDMM obtained based on the T1‐weighted MRI.
Functional connectivity of the amygdala was computed via Pearson's correlation of the fMRI time courses between the amygdala and the rest of the brain, where the fMRI time course of the amygdala was averaged across voxels within the amygdala mask obtained from the T1‐weighted MRI data. The Pearson's correlation was then transformed to z‐scores using Fisher's transformation for below statistical analysis. To identify left and right amygdala functional networks, the z‐maps from individual subjects were first transformed to the atlas via large deformation diffeomorphic metric mapping (Du et al., 2011; Tan & Qiu, 2016). One‐sample t‐test was then performed on the z‐maps in the atlas space using SPM to identify brain regions significantly connected to left or right amygdala. The amygdala functional networks were identified when p‐value was less than 0.001 at individual voxels and cluster size with its corrected cluster p < .05 was greater than 28 voxels that was determined via Monte Carlo simulation using the new version of 3dFWHMx and 3dClustSim in AFNI (Forman et al., 1995). The amygdala functional networks were shown in Supporting Information Figures S2 and S3.
2.6. Statistical analysis
Multiple regression analyses were used to examine independent effects of prenatal and postnatal maternal depressive symptoms and their fluctuation on the amygdala functional networks.
2.6.1. Covariates
Maternal ethnicity and maternal education were thought to represent socio‐economic status, which have been suggested to impact brain development (Barch et al., 2016; Sripada, Swain, Evans, Welsh, & Liberzon, 2014). Hence, these two variables were included as covariates in all regression analyses. Maternal ethnicity was represented by two dummy variables in which one variable had value of 1 for Indian and the other variable had value of 1 for Malay before they were entered into the regression model to ensure their suitability for regression. Maternal education was coded as a variable ranging from 1 to 5, where 1 to 5 respectively represent the primary, secondary, general certification of education/institute of technical education, university, and above university. Additionally, the child's age at MRI and rs‐fMRI data length were also included as covariates in all the following regression analyses. The rs‐fMRI data had 120 volumes (only with the first rs‐fMRI scan) or 192 volumes (with both the first and second rs‐fMRI scans). In sum, maternal ethnicity and education, child's age at MRI and rs‐fMRI data length were entered as covariates in all regression models below.
2.6.2. Independent effects
The independent effects of prenatal or postnatal maternal depressive symptoms on bilateral amygdala functional networks were examined using regression models on individual voxels on the aforementioned z‐maps. Prenatal and postnatal maternal depressive symptoms were considered as main factors while adjusting child's age at MRI, rs‐fMRI length, maternal ethnicity, and maternal education. The aforementioned analyses were conducted for girls and boys separately.
2.6.3. Fluctuation effects
The influence of fluctuation in maternal depressive symptoms between the pre‐ and post‐natal periods on the amygdala functional networks were examined using regression where the fluctuation was considered as a main factor and age at MRI, rs‐fMRI length, maternal ethnicity, maternal education, as well as the severity of maternal depressive symptoms were entered as covariates. The aforementioned analyses were conducted for girls and boys separately.
2.6.4. Correction for multiple comparisons
As mentioned above, the image masks of the amygdala functional networks were determined at the group level. The above regression analysis was only performed on the voxels within the masks of the amygdala functional networks. Statistical results from the regression analyses on independent and fluctuation effects were considered significant when p‐value was less than 0.001 at individual voxels and cluster size was greater than 28 voxels that was determined via Monte Carlo simulation using the new version of 3dFWHMx and 3dClustSim with option of ACF in AFNI (Forman et al., 1995) at a corrected cluster p < .05/12(=0.0042). The 12 tests included the tests for effects of pre‐ and post‐natal maternal depressive symptoms and their fluctuation on the left and right amygdala networks in boys and girls. The same linear regression models were employed to further examine the association direction of maternal depressive symptoms with the amygdala functional connectivity averaged within each cluster.
3. RESULTS
3.1. Demographics
Among 230 subjects with both T1 and rs‐fMRI data, 177 passed through visual inspection for obvious head motion of rs‐fMRI, such as large motion and checkerboard image appearance through more than 10 volumes in a row. Additional 24 rs‐fMRI datasets had maximal framewise displacement greater than 0.5 mm, 9 did not meet the study inclusion criteria, and 16 mothers of children did not complete maternal questionnaires. Hence, this study included 128 subjects (57 boys and 71 girls) with the complete data. Table 1 lists the demographic information of the 128 subjects used in this study, which was largely comparable with the demographic information of the subjects with poor MRI quality (Supporting Information Table S1).
Table 1.
Demographics
| Measure |
Overall Sample (N = 128) |
Male sample (N = 57) |
Female sample (N = 71) |
|---|---|---|---|
| Child characteristics | |||
| Gestational Age (week), mean (SD) | 38.61 (1.27) | 38.26 (1.39) | 38.89 (1.10) |
| Birth weight (gram), mean (SD) | 3,096.73 (418.98) | 3,180.88 (451.01) | 3,029.17 (381.28) |
| APGAR Score, mean (SD) | 8.99 (0.08) | 9.00 (0.00) | 8.99 (0.12) |
| Age (year), mean (SD) | 4.58 (0.08) | 4.58 (0.09) | 4.59 (0.08) |
| Mother characteristics | |||
| 26 week EPDS raw score, mean (SD) | 7.80 (4.57) | 7.03 (4.44) | 8.41 (4.62) |
| 3 month EPDS raw score, mean (SD) | 6.71 (5.11) | 6.20 (4.78) | 7.1 (5.38) |
| 12 month BDI raw score, mean (SD) | 7.34 (8.58) | 6.49 (7.34) | 8.06 (9.52) |
| 24 month BDI raw score, mean (SD) | 7.70 (7.93) | 6.93 (5.92) | 8.40 (9.44) |
| 36 month BDI raw score, mean (SD) | 7.89(8.20) | 6.00 (5.55) | 9.39 (9.61) |
| 54 month BDI raw score, mean (SD) | 6.65 (8.87) | 4.91 (6.04) | 8.21 (10.64) |
| Prenatal Maternal Depression standardized score, mean (SD) | 0.07 (1.01) | −0.10 (0.98) | 0.21 (1.02) |
| Average Postnatal Maternal Depression standardized score, mean (SD) | 0.10 (0.98) | −0.01 (0.77) | 0.19 (1.12) |
| Maternal ethnicity, % | |||
| Chinese | 42.2 | 36.8 | 46.5 |
| Malay | 39.1 | 49.1 | 31.0 |
| Indian | 18.8 | 14.0 | 22.5 |
| Maternal Education, % | |||
| Primary School | 7.8 | 7.7 | 7.0 |
| Secondary School | 32.0 | 11.5 | 35.2 |
| Pre‐university, Diploma or Technical course | 37.5 | 38.5 | 35.2 |
| University Undergraduate level | 20.3 | 23.1 | 18.3 |
| Above University Undergraduate level | 2.3 | 19.2 | 4.2 |
Among the subjects in this study, pre‐ and post‐natal maternal depressive symptoms were significantly correlated (r 128 = .537, p < .001). Prenatal maternal depressive symptoms significantly differed among the three ethnic groups (F 125,2 = 3.706, p = .027). However, there was no ethnic group difference in postnatal maternal depressive symptoms (F 125,2 = 0.709, p = .494). Maternal education, considered as indicator of family socio‐economic status, was not correlated with prenatal maternal depressive symptoms (r = –.009, p = .922), but was negatively correlated with postnatal maternal depressive symptoms (r = –.200, p = .024). Neither pre‐ nor post‐natal maternal depressive symptoms differed between boys and girls (p > .09). The female sample differed from the male sample on gestational age (t 126 = −2.872, p = .005) and birth weight (t 126 = 2.062, p = .041) when assessed using independent sample t‐tests. There were no gender differences in terms of maternal ethnicity as assessed by a Chi‐square test (p = .101) or differences in maternal education as assessed by Fisher's exact test (p = .543).
3.2. Boys
Neither pre‐, nor post‐natal maternal depressive symptoms, nor their fluctuation were associated with the left and right amygdala functional networks in boys.
Since late preterms may be a high risk population with brain alterations, we repeated our analysis by removing late preterms. In our study, 8 (6 boys and 2 girls) among 128 children had gestational age between 34 and 36 weeks. The repeated analysis showed that neither pre‐, nor post‐natal maternal depressive symptoms, nor their fluctuation were associated with the left and right amygdala functional networks in boys.
3.3. Girls
Our study revealed significant associations between prenatal maternal depressive symptoms and left and right amygdala functional connectivity with the right insula, and putamen, bilateral subgenual anterior cingulate cortex (ACC), and caudate in girls (Table 2). Greater prenatal maternal depressive symptoms in girls were associated with lower functional connectivity of the left amygdala with the right insula and putamen (Figure 1a), as well as with the bilateral subgenual anterior cingulate cortex (ACC) and caudate (Figure 1a). Likewise, greater prenatal maternal depressive symptoms were associated with lower functional connectivity of the right amygdala with the left orbitofrontal cortex (OFC) and temporal pole in girls (Figure 2a). These findings were obtained after partialing out the variance associated with maternal education and ethnicity, age at the MRI visit day, rs‐fMRI data length, and postnatal maternal depressive symptoms. When additionally controlling for the left amygdala volume, these findings remained significant (p < .001). Without controlling for postnatal maternal depressive symptoms, the aforementioned effects due to prenatal maternal depressive symptoms remained significant on the functional connectivity of the left amygdala with the right insula and putamen (p < .001), the bilateral subgenual ACC and caudate (p = .003), and on the functional connectivity of the right amygdala with the left OFC and temporal pole (p = .007).
Table 2.
Statistical results of association between positive left and right amygdala network and maternal depressive symptoms
| Sample | Effects | Anatomy | Cluster Size (voxels) |
|---|---|---|---|
| Left Amygdala Functional Network | |||
| Girls | Prenatal | R Insula, R Putamen | 85 |
| L/R subgenual ACC, L/R Caudate | 92 | ||
| Fluctuation | L/R subgenual ACC, L Caudate | 64 | |
| Right Amygdala Functional Network | |||
| Girls | Prenatal | L OFC, L Temporal Pole | 127 |
| Fluctuation | L OFC, L Insula, L Temporal Pole | 228 | |
Abbreviations: L – left; R – right; ACC – anterior cingulate cortex; OFC – orbitofrontal cortex.
Figure 1.
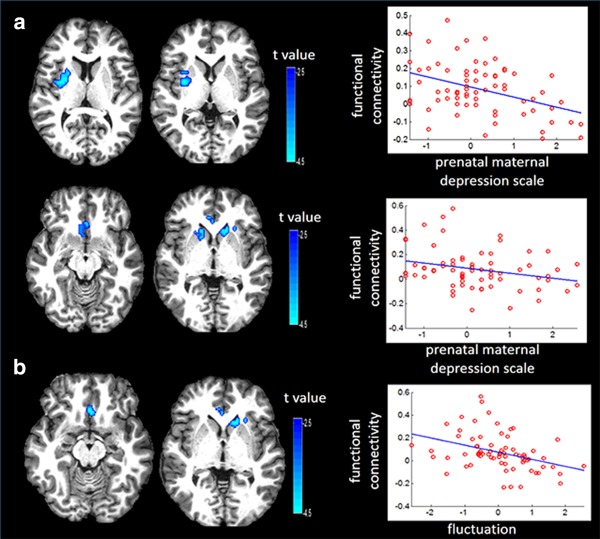
Influences of maternal depressive symptoms on the left amygdala functional network in girls. Panel (a) illustrates influence of prenatal maternal depressive symptoms on the left amygdala functional network. Panel (b) illustrates influence of fluctuation between prenatal and postnatal maternal depressive symptoms on the left amygdala functional network. The third column shows scatter plots of maternal depressive symptoms with the functional connectivity between the left amygdala and the brain regions corresponding to those on the same row
Figure 2.
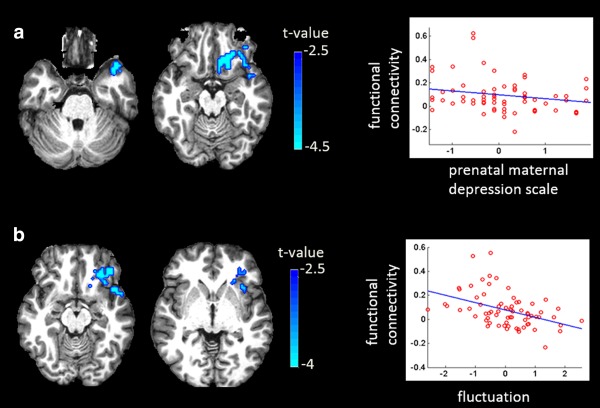
Influences of maternal depressive symptoms on the right amygdala functional network in girls. Panel (a) illustrates influence of prenatal maternal depressive symptoms on the right amygdala functional network. Panel (b) illustrates influence of fluctuation between prenatal and postnatal maternal depressive symptoms on the right amygdala functional network. The third column shows scatter plots of maternal depressive symptoms with the functional connectivity between the right amygdala and the brain regions corresponding to those on the same row
Moreover, greater pre‐ than post‐natal depressive symptoms were strongly associated with lower functional connectivity of the left amygdala with bilateral subgenual ACC and left caudate (Figure 1b), and with lower functional connectivity of the right amygdala with the left OFC, insula, and temporal pole (Figure 2b). This finding was the same with and without adjusting for the severity of maternal depressive symptoms.
We repeated our analysis by removing the 2 girls whose gestational age was between 34 and 36 weeks and showed the same results for girls as seen in Figures 1 and 2.
Nevertheless, statistical analyses did not reveal any interaction of gender with prenatal or postnatal maternal depressive symptoms on amygdala functional connectivity.
4. DISCUSSION
This study examined the relationship of pre‐ and post‐natal maternal depressive symptoms and their fluctuation with the amygdala functional networks in young children. Our findings suggested that maternal depressive symptoms are associated with significant variation in the functional connectivity of the amygdala with the cortico‐striatal circuitry, especially with the OFC, insula, subgenual ACC, temporal pole, and striatum. These maternal influences were most prominent in girls, which is consistent with an extensive meta‐analysis showing that the association between maternal depressive symptoms and internalizing problems is prominent in girls in community samples (Goodman et al., 2011). Similarly, Burghy and colleagues (2012) found that the association between early life stress, defined largely by maternal emotional well‐being, and amygdala‐prefrontal connectivity was unique to females. Finally, higher maternal cortisol levels in early gestation were associated with larger right amygdala volume in childhood, but only in girls (Buss et al., 2012). Taken together with the current findings, these studies underscore the importance of gender‐dependent developmental pathways in defining the neural circuitry that underlies the risk for depression.
Our study found that maternal depressive symptoms altered the functional connectivity in the cortico‐amygdala‐striatal circuitry in young children, suggesting the importance of this circuitry in transgenerational transmission of vulnerability for depression. The amygdala‐striatal connections are essential for the formation of signals to the prefrontal cortex. These connections are responsible for motivational responses appropriate for emotional state (Liu, Hairston, Schrier, & Fan, 2011; Ramasubbu et al., 2014). The cortico‐amygdala‐striatal circuitry is suggested to play an important role in amygdala‐mediated encoding of emotional salience across various stimuli and stages of processing (Cho, Ernst, & Fudge, 2013; Phelps & LeDoux, 2005; Salzman & Fusi, 2010) and in gauging inner emotional states through attention to inner physiology (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004), motivation (Liljeholm & O'Doherty, 2012) and guiding social responses, such as reappraisal (Etkin, Egner, & Kalisch, 2011) and complex behavioral tasks (Morrison, Saez, Lau, & Salzman, 2011). These emotional processes have been implicated as major components in MDD. Children with preschool‐onset MDD show reduced connectivity between the subgenual ACC and prefrontal cortex (Gaffrey et al., 2010). Similarly, children at a high familial risk for MDD show decreased connectivity between the caudate and cingulate (Frost Bellgowan et al., 2015). Children with a maternal history of MDD or bipolar disorder also show reduction in the functional connectivity of the amygdala with the prefrontal cortex (Luking et al., 2011). As early as in infancy, 6‐month old infants born to mothers with greater prenatal maternal depressive symptoms show alterations of the amygdala functional connectivity with the prefrontal cortex (Qiu et al., 2015). Moreover, mothers with depressive symptomatology show reduced activities of the OFC, insula, and striatal regions (Laurent & Ablow, 2013) to joy‐distress of their own infants, suggesting the neural basis of parenting difficulties in depressed mothers. This parenting‐related neuronal circuitry of depressed mothers significantly overlaps with that shown in children with greater maternal depressive symptoms. These findings may suggest that the cortico‐amygdala‐striatal circuitry could be a neural circuitry for intrinsic emotional regulation and be relevant to transgenerational transmission of vulnerability for depression.
The amygdala's functional connectivity with the ACC, OFC, temporal pole, and caudate was affected by the fluctuation of maternal depressive symptoms before and after pregnancy, independent of severity of maternal depressive symptoms (r = .104, p = .241). This is in line with the previous finding showing that greater pre‐ than post‐natal maternal mood was associated with slower growth of the hippocampus (Qiu et al., 2013). These findings suggest that early development of the amygdala functional networks might be constrained by prenatal maternal depressive symptoms, but are also enhanced in response to increased postnatal maternal depressive symptoms. While seemingly counter‐intuitive, an extensive analysis of the relation between maternal depressive symptoms and the risk for later depression in offspring found independent effects and mediating pathways for the influence of pre‐ and post‐natal depressive symptoms (Pearson et al., 2013). (Barker and colleagues 2013) modeled the influence of pre‐ and post‐natal depression on cognitive development and found that prenatal depressive symptoms was associated with impairments in cognitive function, while the opposite effect was found for the association between postnatal depressive symptoms and cognitive function. Likewise, (DiPietro and colleagues 2006) reported that prenatal maternal distress associated with higher Bayley Psychomotor Development Index (PDI) scores, while the opposite effect was revealed in the relationship with postnatal depressive symptoms. These findings suggest the notion that the effects of pre‐ and post‐natal depressive symptoms on children's brain, cognition, and behaviors may be mediated via distinct mechanisms, which need to be further investigated.
Our study did not reveal any interactions between gender and maternal depression on amygdala functional conectivity. Nevertheless, our findings were apparent in 4.5‐year‐old girls, not in boys. Using the same sample, we previously also showed that pre‐ and post‐natal maternal depressive respectively impact the amygdala volume and microstructure in girls (Wen et al., 2017). In line with these findings, maternal depressive symptoms influence internalizing behaviors predominantly in girls (Goodman et al., 2011). In addition, prenatal stress leads to sexually dimorphic developmental consequences later in life (Buss, Davis, Hobel, & Sandman, 2011; David & Jones, 2006; Ellman et al., 2008; Grey, Davis, Sandman, & Glynn, 2013; Sandman, Glynn, & Davis, 2013), partly because female fetuses are more susceptible to changes in stress levels (Clifton & Murphy, 2004; Clifton, 2010). Likewise, prenatal maternal cortisol levels predict the right amygdala volume in girls, but not boys at 7 years of age (Buss et al., 2012). Hence, gender‐dependent effects on neurodevelopmental outcomes are not unique to the influence of maternal mood but they cut across various forms of maternal adversity, including maternal depression, maternal stress, maternal cortisol, and so on. Unfortunately, possible biological mechanisms for such gender‐dependent effects on neurodevelopmental outcomes are unknown.
This study, to our knowledge, was the first to investigate the influences of pre‐ and post‐natal maternal depressive symptoms on the brain functional networks using a large sample of Asian children. Despite the longitudinal design utilized in GUSTO, the rs‐fMRI was only acquired in 4.5‐year‐old children with a reasonable sample size. Several questions remain unanswered. First, the sexual dimorphic impact on the brain functional network was obvious in 4.5‐year‐old girls. However, this was not observed in neonates shortly after birth, especially for the amygdala microstructure (Rifkin‐Graboi et al., 2015b). Thus, it is unclear when specific sexual differential responses to early maternal adversity, as reflected by the amygdala anatomy and function, occur. Second, a previous study with a relatively small sample (24 subjects) suggested positive associations between prenatal maternal depressive symptoms and the amygdala‐PFC connectivity in 6‐month infants (Qiu et al., 2015), which is opposite to what was observed in 4.5‐year‐old children. Recently, Luby and colleagues (2016) compared the association of maternal support at preschool or school ages with hippocampal growth and found a significant association only for maternal support at the younger age, suggesting that “a sensitive period for an environmental influence on neurodevelopment must be framed in terms of both the relevant environmental condition and the specific developmental outcome”. Studies of environmental influences on human brain development need to consider that data must be interpreted within the context of dynamic variation in structure and function, which may be absolutely critical for understanding the developmental trajectory of the brain in relation with maternal depressive symptoms. Furthermore, this study was not be able to explore possible influences of other factors, including parenting, pregnancy and birth complications, maternal and child nutrition, and so on, on early brain development. As such, our study did not claim that maternal depressive symptoms were the only factors influencing early brain development. Finally, our study did not investigate possible prediction of brain functional organization to child's mood symptoms at a later age, which needs further investigation.
This study showed the relationship of the cortico‐striato‐thalamo‐amygdala circuitry with maternal depressive symptoms particularly in girls. Our findings underscored the importance of gender‐dependent developmental pathways in defining the neural circuitry that underlies the risk for depression and suggest an important role of the emotional perception and regulation in transgenerational transmission of vulnerability for socio‐emotional problems and depression.
CONFLICT OF INTEREST
No author has conflict of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
This study is supported by National Medical Research Council (NMRC; NMRC/TCR/004‐NUS/2008, NMRC/TCR/012‐NUHS/2014, and NMRC/CBRG/0039/2013). We thank the GUSTO study group and all clinical and home visit staff involved. The voluntary participation of all participants is greatly appreciated.
The GUSTO study group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong‐Fong Chong, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Keith M. Godfrey, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D. Holbrook, Chin‐Ying Hsu, Hazel Inskip, Jeevesh Kapur, Ivy Yee‐Man Lau, Bee Wah Lee, Yung Seng Lee, Ngee Lek, Sok Bee Lim, Yen‐Ling Low, Iliana Magiati, Lourdes Mary Daniel, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Lynette Pei‐Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu‐E Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Mya Thway Tint, Hugo P S van Bever, Rob M. van Dam, Inez Bik Yun Wong, P. C. Wong, Fabian Yap, George Seow Heong Yeo.
Soe NN, Wen DJ, Poh JS, et al. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum Brain Mapp. 2018;39:680–690. 10.1002/hbm.23873
Funding information National Medical Research Council (NMRC), Grant/Award Numbers: NMRC/TCR/004‐NUS/2008, NMRC/TCR/012‐NUHS/2014, and NMRC/CBRG/0039/2013
REFERENCES
- Barch, D. , Pagliaccio, D. , Belden, A. , Harms, M. P. , Gaffrey, M. , Sylvester, C. M. , … Luby, J. (2016). Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school‐age depression. The American Journal of Psychiatry, 173(6), 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, E. D. , Kirkham, N. , Ng, J. , & Jensen, S. K. G. (2013). Prenatal maternal depression symptoms and nutrition, and child cognitive function. The British Journal of Psychiatry, 203(6), 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. , Ward, C. H. , Mendelson, M. M. , Mock, J. J. , & Erbaugh, J. J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–571. [DOI] [PubMed] [Google Scholar]
- Biederman, J. , Faraone, S. V. , Hirshfeld‐Becker, D. R. , Friedman, D. , Robin, J. A. , & Rosenbaum, J. F. (2001). Patterns of psychopathology and dysfunction in high‐risk children of parents with panic disorder and major depression. The American Journal of Psychiatry, 158(1), 49–57. [DOI] [PubMed] [Google Scholar]
- Brennan, P. A. , Hammen, C. , Andersen, M. J. , Bor, W. , Najman, J. M. , & Williams, G. M. (2000). Chronicity, severity, and timing of maternal depressive symptoms: Relationships with child outcomes at age 5. Developmental Psychology, 36(6), 759–766. [DOI] [PubMed] [Google Scholar]
- Bruder‐Costello, B. , Warner, V. , Talati, A. , Nomura, Y. , Bruder, G. , & Weissman, M. (2007). Temperament among offspring at high and low risk for depression. Psychiatry Research, 153(2), 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy, C. A. , Stodola, D. E. , Ruttle, P. L. , Molloy, E. K. , Armstrong, J. M. , Oler, J. A. , … Birn, R. M. (2012). Developmental pathways to amygdala‐prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, C. , Davis, E. P. , Hobel, C. J. , & Sandman, C. A. (2011). Maternal pregnancy‐specific anxiety is associated with child executive function at 6–9 years age. Stress, 14(6), 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, C. , Davis, E. P. , Shahbaba, B. , Pruessner, J. C. , Head, K. , & Sandman, C. A. (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences, 109(20), E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, Y. T. , Ernst, M. , & Fudge, J. L. (2013). Cortico‐amygdala‐striatal circuits are organized as hierarchical subsystems through the primate amygdala. The Journal of Neuroscience, 33(35), 14017–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton, V. L. (2010). Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta, 31, (3):S33–S39. [DOI] [PubMed] [Google Scholar]
- Clifton, V. L. , & Murphy, V. E. (2004). Maternal asthma as a model for examining fetal sex‐specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta, 25, S45–S52. [DOI] [PubMed] [Google Scholar]
- Cox, J. L. , Holden, J. M. , & Sagovsky, R. (1987). Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150(6), 782–786. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Ohman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189. [DOI] [PubMed] [Google Scholar]
- David, I. W. P. , & Jones, A. (2006). Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? The Journal of Physiology, 572(1), 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan, K. A. , Almas, A. N. , & Fox, N. A. (2010). Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(4), 497–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro, J. A. , Novak Mfsx, Costigan, K. A. , Atella, L. D. , & Reusing, S. P. (2006). Maternal psychological distress during pregnancy in relation to child development at age two. Child Development, 77(3), 573–587. [DOI] [PubMed] [Google Scholar]
- Du, J. , Younes, L. , & Qiu, A. (2011). Whole brain diffeomorphic metric mapping via integration of sulcal and gyral curves, cortical surfaces, and images. Neuroimage, 56(1), 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman, L. M. , Schetter, C. D. , Hobel, C. J. , Chicz‐DeMet, A. , Glynn, L. M. , & Sandman, C. A. (2008). Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology, 50(3), 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A. , Egner, T. , & Kalisch, R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, T. (1992). Infants of depressed mothers. Development and Psychopathology, 4(1), 49–66. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fleming, A. S. , Ruble, D. N. , Flett, G. L. , & Shaul, D. L. (1988). Postpartum adjustment in first‐time mothers: Relations between mood, maternal attitudes, and mother‐infant interactions. Developmental Psychology, 24(1), 71–81. [Google Scholar]
- Forman, S. D. , Cohen, J. D. , Fitzgerald, M. , Eddy, W. F. , Mintun, M. A. , & Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magnetic Resonance in Medicine, 33(5), 636–647. [DOI] [PubMed] [Google Scholar]
- Frost Bellgowan, J. , Molfese, P. , Marx, M. , Thomason, M. , Glen, D. , Santiago, J. , … Hamilton, J. P. (2015). A neural substrate for behavioral inhibition in the risk for major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54(10), 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey, M. S. , Luby, J. L. , Repovš, G. , Belden, A. C. , Botteron, K. N. , Luking, K. R. , & Barch, D. M. (2010). Subgenual cingulate connectivity in children with a history of preschool‐depression. NeuroReport, 21(18), 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, S. H. , & Gotlib, I. H. (1999). Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review, 106(3), 458–490. [DOI] [PubMed] [Google Scholar]
- Goodman, S. H. , Rouse, M. H. , Connell, A. M. , Broth, M. R. , Hall, C. M. , & Heyward, D. (2011). Maternal depression and child psychopathology: A meta‐analytic review. Clinical Child and Family Psychology Review, 14(1), 1–27. [DOI] [PubMed] [Google Scholar]
- Grey, K. R. , Davis, E. P. , Sandman, C. A. , & Glynn, L. M. (2013). Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology, 38(7), 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent, H. K. , & Ablow, J. C. (2013). A face a mother could love: depression‐related maternal neural responses to infant emotion faces. Social Neuroscience, 8(3), 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Walton, M. , Letourneau, N. , Giesbrecht, G. F. , Kaplan, B. J. , & Dewey, D. (2016). Prepartum and postpartum maternal depressive symptoms are related to children's brain structure in preschool. Biological Psychiatry, 80(11), 859–868. [DOI] [PubMed] [Google Scholar]
- Liljeholm, M. , & O'doherty, J. P. (2012). Contributions of the striatum to learning, motivation, and performance: an associative account. Trends in Cognitive Sciences, 16(9), 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Hairston, J. , Schrier, M. , & Fan, J. (2011). Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby, J. L. , Belden, A. , Harms, M. P. , Tillman, R. , & Barch, D. M. (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences of the United States of America U S A, 113(20), 5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking, K. R. , Repovs, G. , Belden, A. C. , Gaffrey, M. S. , Botteron, K. N. , Luby, J. L. , & Barch, D. M. (2011). Functional connectivity of the Amygdala in early‐childhood‐onset depression. Journal of the American Academy of Child & Adolescent Psychiatry, 50(10), 1027–1041.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien, S. J. , Parent, S. , Evans, A. C. , Tremblay, R. E. , Zelazo, P. D. , Corbo, V. , … Séguin, J. R. (2011). Larger amygdala but no change in hippocampal volume in 10‐year‐old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America, 108(34), 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt, T. E. , Caspi, A. , Newman, D. L. , & Silva, P. A. (1996). Behavioral observations at age 3 years predict adult psychiatric disorders: Longitudinal evidence from a birth cohort. Archives of General Psychiatry, 53(11), 1033–1039. [DOI] [PubMed] [Google Scholar]
- Morrison, S. E. , Saez, A. , Lau, B. , & Salzman, C. D. (2011). Different time courses for learning‐related changes in amygdala and orbitofrontal cortex. Neuron, 71(6), 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, L. , Halligan, S. L. , Goodyer, I. , & Herbert, J. (2010). Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: A preliminary study. Journal of Affective Disorders, 122(3), 218–223. [DOI] [PubMed] [Google Scholar]
- Pearson, R. M. , Evans, J. , Kounali, D. , Lewis, G. , Heron, J. , Ramchandani, P. G. , … Stein, A. (2013). Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry, 70(12), 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, E. A. , & LeDoux, J. E. (2005). Contributions of the Amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Posner, J. , Cha, J. , Roy, A. K. , Peterson, B. S. , Bansal, R. , Gustafsson, H. C. , … Monk, C. (2016). Alterations in amygdala‐prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry, 6, e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, J. L. , & Drevets, W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, A. , Anh, T. T. , Li, Y. , Chen, H. , Rifkin‐Graboi, A. , Broekman, B. F. P. , … Meaney, M. J. (2015). Prenatal maternal depression alters amygdala functional connectivity in 6‐month‐old infants. Translational Psychiatry, 5(2), e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, A. , Rifkin‐Graboi, A. , Chen, H. , Chong, Y. S. , Kwek, K. , Gluckman, P. D. , … Meaney, M. J. (2013). Maternal anxiety and infants’ hippocampal development: timing matters. Translational Psychiatry, 3, e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu, R. , Konduru, N. , Cortese, F. , Bray, S. , Gaxiola‐Valdez, I. , & Goodyear, B. (2014). Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Frontiers in Psychiatry, 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin‐Graboi, A. , Bai, J. , Chen, H. , Hameed, WBr , Sim, L. W. , Tint, M. T. , … Qui, A. (2013). Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biological Psychiatry, 74(11), 837. [DOI] [PubMed] [Google Scholar]
- Rifkin‐Graboi, A. , Kong, L. , Sim, L. W. , Sanmugam, S. , Broekman, B. F. , Chen, H. , … Qiu, A. (2015a). Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl Psychiatry, 5, e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin‐Graboi, A. , Meaney, M. J. , Chen, H. , Bai, J. , Hameed, WBr. , Tint, M. T. , … Fortier, M. V. (2015b). Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. Journal of the American Academy of Child and Adolescent Psychiatry, 54(4), 313–321. e2. [DOI] [PubMed] [Google Scholar]
- Salzman, C. D. , & Fusi, S. (2010). Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience, 33, (1):173–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman, C. A. , Buss, C. , Head, K. , & Davis, E. P. (2015). Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry, 77(4), 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman, C. A. , Davis, E. P. , & Glynn, L. M. (2012). Prescient human fetuses thrive. Psychological Science, 23(1), 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman, C. A. , Glynn, L. M. , & Davis, E. P. (2013). Is there a viability‐vulnerability tradeoff? Sex differences in fetal programming. Journal of Psychosomatic Research, 75(4), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg, J. L. , Maes, H. , & Eaves, L. J. (2010). Genetic and environmental influences on the transmission of parental depression to children's depression and conduct disturbance: an extended Children of Twins study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(6), 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23(Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Soh, S.‐E. , Tint, M. T. , Gluckman, P. D. , Godfrey, K. M. , Rifkin‐Graboi, A. , Chan, Y. H. , … Saw, S. M. (2014). Cohort profile: Growing up in Singapore towards healthy outcomes (GUSTO) birth cohort study. International Journal of Epidemiology 43(5), 1401–1409. [DOI] [PubMed] [Google Scholar]
- Sohr‐Preston, S. L. , & Scaramella, L. V. (2006). Implications of timing of maternal depressive symptoms for early cognitive and language development. Clinical Child and Family Psychology Review, 9(1), 65–83. [DOI] [PubMed] [Google Scholar]
- Sripada, R. K. , Swain, J. E. , Evans, G. W. , Welsh, R. C. , & Liberzon, I. (2014). Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology, 39(9), 2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, M. , & Qiu, A. (2016). Large deformation multiresolution diffeomorphic metric mapping for multiresolution cortical surfaces: A coarse‐to‐fine approach. IEEE Transactions on Image Processing, 25(9), 4061–4074. [DOI] [PubMed] [Google Scholar]
- Tau, G. Z. , & Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology, 35(1), 147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk, K. R. A. , Hedden, T. , Venkataraman, A. , Evans, K. C. , Lazar, S. W. , & Buckner, R. L. (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology, 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, D. J. , Soe, N. N. , Wee, S. L. , Sanmugam, S. , Kwek, K. , Chong, Y.-S. , … Qiu, A. (2017). Infant frontal EEG asymmetry in relation with postnatal maternal depression and parenting behavior. Translational Psychiatry, 7, c1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.‐G. , Cheung, B. , Kelly, C. , Colcombe, S. , Craddock, R. C. , Di Martino, A. , … Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


