Abstract
This article explores neural and self‐report responses to gender congruence in product‐voice combinations in commercials. An fMRI study was carried out in which participants (n = 30) were presented with gender‐targeted pictures of characteristic male or female products accompanied by either gender congruent or incongruent voices. The findings show that attitudes are more positive toward commercials with gender congruent than with gender incongruent product‐voice combinations. fMRI analyses revealed that primary visual brain areas, namely calcarine and cuneus, responded stronger to congruent than incongruent combinations suggesting that participants enhanced their endogenous attention toward congruent commercials. Incongruent combinations, by contrast, elicited stronger activation in areas related to the perception of conflicts in information processing and error monitoring, such as the supramarginal, inferior parietal gyri and superior, and middle temporal gyri. Interestingly, increased activation in the posterior cingulate cortex (an area related to value encoding) predicted more positive attitudes toward congruent commercials. Together, these results advance our understanding of the neural correlates of processing congruent and incongruent audiovisual stimuli. These findings may advice advertising professionals in designing successful campaigns of everyday products, namely by making use of congruent instead of incongruent product‐voice combinations.
Keywords: commercials, congruency, endogenous attention, fMRI, gender‐targeted products, voice gender
1. INTRODUCTION
Advertising plays an important role in the business world as it influences attention, attitude, and recall of commercials (Tu, Kao, & Tu, 2013), as well as consumer attitude toward product purchase and intention of purchase (Connell, Brucks, & Nielsen, 2014). If designed properly, commercials can shape consumer decisions and direct consumption toward specific products. Previous studies evaluated the impact of several media features (such as color, packaging, or commercial content, Pilelienė & Grigaliūnaitė, 2017; Van der Laan, De Ridder, Viergever, & Smeets, 2012; Martínez‐Fiestas, del Jesus, Sánchez‐Fernández, & Montoro‐Rios, 2015) on media effects (such as attitude, intention, and choice). Audio visual (AV) commercials usually combine visual (video or image) and auditory components (a voice providing information). Traditionally, the visual and auditory elements of these commercials are gender congruent as products targeting females (FP) are combined with a female voice (FV) and products targeting males (MP) are combined with a male voice (MV). For example, commercials of men's swim wears, typically male‐targeted products, are most often accompanied by MVs, whereas, hair removal creams, typically female‐targeted products, are generally presented by females.
Communication effectiveness research has focused on the attitudes toward commercials including male/FVs, gender‐targeted products and, above all, congruent/incongruent gender product‐voice combinations. Yet, the studies reveal inconsistent results with regard to the impact of those media features on consumer attitudes toward commercials (Rodero, Larrea, & Vázquez, 2013). The two determinants that may explain the lack of unanimity in previous research on gender‐targeted products and voices are both related to the use of self‐report tools (surveys, questionnaires). First, these tools can be subject to social desirability and subjectivity biases: they may show biased responses to sensitive gender‐related issues which people are uncomfortable to reveal opinions about (Lewinski, Fransen, & Tan, 2014). Second, self‐report tools cannot measure processes of which the person itself is not aware, that is, unconscious and automatic cognitive processes related to endogenous attention, value encoding or conflict monitoring in response to advertising with product‐voice combinations.
As neuroscientific techniques enable the measurement of these unconscious processes in a nonbiased manner, they may be a useful complement to self‐report tools in the study of product‐voice combinations. Neuroscientific techniques are able to identify psychological responses of consumers based on objective physiological data and track consumer response in real time (i.e., measurements are possible during the processing of the stimulus of interest, something which is not possible with self‐reports as taking self‐reports during a task can raise conscious processes and biases). Applying neurological tools to the study of consumers' emotional and cognitive responses to marketing stimuli has indeed sparked growing interest in recent years and resulted in the birth of a new field commonly referred to as “consumer neuroscience” or “neuromarketing.” This interdisciplinary field studies the neural background of consumption decisions and consumer behavior (Reimann, Schilke, Weber, Neuhaus, & Zaichkowsky, 2011). Recent studies have employed neuroscientific techniques to evaluate processes underlying consumer decision‐making in marketing areas such as e‐commerce, political advertising, environmental communication, and packaging research (Casado‐Aranda, Martínez‐Fiestas, & Sánchez‐Fernández, 2018; Dimoka, 2010). One of the main benefits of neuroscientific techniques in communication research is that it facilitates exploring the neural origin of media effects provoked by specific media features. In other words, it sheds light on the underlying mechanisms of media effects (Falk, Berkman, & Lieberman, 2012; Weber, Mangus, & Huskey, 2015).
Following this mainstream, the current study applies a neuroscientific tool aiming to: (i) elucidate the neural background of the effects of congruent and incongruent gender product‐voice combinations in advertising; and (ii) investigate in which brain areas activation in response to gender congruent AV commercials covaries with traditional self‐reported attitudes toward the commercials. This study employed functional Magnetic Resonance Imaging (fMRI), a technique that offers an indirect measure of brain activation (Solnais, Andreu‐Perez, Sánchez‐Fernández, & Andréu‐Abela, 2013).
2. THEORETICAL BACKGROUND
2.1. Gender‐targeted products
Many consumer products are imbued with gender association (e.g., Grohman, 2009). This starts already at a young age with, for example, advertisements associating color pink with toys for girls while dark blue is used for toys for boys. Moreover, examples of gender association in advertising are also often showcased through props (Prieler, 2016). Gender identity is therefore generated and attained in a large manner through consumption. As a consequence of purchasing male‐targeted products, males are thought to see themselves as part of a group that behaves according to masculine norms, while females are part of a different group with consumption following a feminine identity (Gal & Wilkie, 2010). Commercials are designed for each target group so that individuals can identify themselves with particular products. For example, personal relevance of the product to a certain target audience (e.g., male or female) can be increased by making references to characteristics typical of the specific target audience (Sherry et al., 2004).
The findings of a recent fMRI study by Wang et al. (2016) reveal that safe sex video commercials targeted specifically at the participant's race and sexual orientation are associated with greater activity in the brain regions related to processing self‐relevance (such as the medial prefrontal cortex and precuneus) and regions related to learning, memory, and language processing (i.e., hippocampus, bilateral inferior, and middle temporal gyrus [MTG]). Similarly, fMRI studies analyzing the underlying processing of own‐group versus other group‐related stimuli found that evaluating personality traits in own‐age versus other‐age individuals (Ebner et al., 2013), or comparing relevant personal qualities to those of an acquaintance (Modinos, Ormel, & Aleman, 2009), increase the activity in the brain areas related to self‐relevance processing (the medial Prefrontal Cortex). In addition, studies comparing own‐ and other‐race faces indicate that categorizing own‐race faces induces greater activations in the right (R) medial frontal cortex and in the bilateral ventral occipito‐temporal cortex (Proverbio & De Gabriele, 2017).
2.2. Voice gender
The gender of the voice pronouncing the information about the product is an element of great interest in communication research. Several authors have noted that changing the gender of voice‐over announcer campaigns may alter the perceived credibility of the commercial's messenger and message (Potter, Jamison‐Koenig, Lynch, & Sites, 2016), the attitude toward the commercial (Potter & Choi, 2006) and the intention to purchase (Chebat, El Hedhli, GéLinas‐Chebat, & Boivin, 2007).
Studies comparing the effects of male versus FVs in advertising reveal ambiguous results. Some conclude that MVs convey a higher level of credibility, trustfulness, authority, expertise power and, consequently, are thought to be more persuasive (Klofstad, 2016). Phonetics and social psychology studies explain these findings through biological differences in terms of pitch and factors like noise or perceived sensuality (Pennock‐Speck & del Saz Rubio, 2009). However, Martín‐Santana, Muela‐Molina, Reinares‐Lara, and Rodríguez‐Guerra (2015) find no evidence for effectiveness differences related to the gender of the voice. Certain authors suggest that the higher level of confidence associated with the MV is based more on traditional stereotypes (Rodero et al., 2013; Whipple & McManamon, 2002).
Neuroimaging studies show evidence of involvement of the inferior frontal gyrus and the cerebellum in voice gender perception (Casado‐Aranda, Sánchez‐Fernández, & Montoro‐Ríos, 2017; Joassin, Maurage, & Campanella, 2011). Analyses of emotional speech processing or perception of opposite/same‐gender voices could, in fact, betray an interaction between voice gender and the listener's gender (Junger et al., 2014) as listening to voices of the opposite sex induces stronger activation of the fronto‐temporal neural network (Junger et al., 2013) and listening to voices of both sexes induces an increase of activation in the posterior cingulate (Chun, Park, Park, & Kim, 2012). The parahippocampal gyrus, in turn, is activated in both males and females while hearing laughter of the same sex (Chun et al., 2012). Thus, although the general mechanisms underlying voice perception and gender differences are becoming increasingly clear, no advertising research to date has focused on brain responses to male and FVs.
2.3. Congruency between gender‐targeted products and gender of the Presenter's voice
2.3.1. Behavioral studies
As noted earlier, traditionally, gender‐targeted products are most commonly presented by a gender congruent voice. However, results of studies on the effects of gender congruence in commercial effectiveness are ambiguous]. Some of these studies found more positive product/commercial evaluations, higher purchase intention, credibility, and commercial effectiveness when the gender of a product matches the gender of the presenter (the so‐called “match‐up hypothesis,” Debevec & Iyer, 1986; Pedelty & Kuecker, 2014). In other words, consumers of both genders evaluate commercials as more positive when the gender‐targeted product is combined with a gender congruent voice. An explanation proposed for this finding is that the gender congruent voice is linked to a conception of higher expertise and a strong personal identification with the presenter (Strach, Zuber, Fowler, Ridout, & Searles, 2015).
Other studies, on the other hand, do not find that gender congruence increases positive evaluations toward the commercials or that it does only under specific conditions. Whipple and McManamon (2002) Whipple and McManamon (2002) indicate that though there is a significant increase of effect of gender congruent presenters among female targeted products, this is not the case for products targeting males. Lien, Chou, and Chang (2012) investigated the match‐up effect between a spokesperson's attractiveness type, a product's image and the effect of the spokesperson's sex. Their results indicate that for female‐gendered products, the advertising effect is more positive with a female spokesperson than with a male spokesperson, regardless of the image of the product (sexy or cute). However, ads with male spokespersons selling cute‐image products can create more favorable reactions that those with male spokespersons selling sexy‐image products. Rodero et al. (2013) suggest that congruence only increases media effects when there is a strong association between the product and a pre‐existing stereotype. For example, since male stereotypes linked to a tie are stronger than those to a lawn mower, then the MV will be more effective in commercials for a tie than for a lawn mower. More recently, Hendriks, van Meurs, and van der Meij (2015) assessed whether the use of foreign accents in radio commercials is more effective for congruent (e.g., a German accent and sausage) than incongruent products (e.g., a German accent and wine) combinations. Their results show that despite foreign‐accented commercials resulted in more negative evaluations than nonaccented commercials, incongruent accent‐product combinations did not differ from congruent accent‐product combinations with regard to the attitude toward the commercial.
2.3.2. Neural studies
To date no research has examined brain responses to male and female targeted products and to male and FVs in a commercial context, let alone their congruent and incongruent combinations. Research in other fields (such as the more basal level AV integration) indicate that passive viewing and listening to AV stimuli (i.e., faces and voices, faces and music, or letters and speeches) consistently elicits activation in the superior temporal gyrus (STG) (Callan et al., 2003; Erickson, Heeg, Rauschecker, & Turkeltaub, 2014; Hölig, Föcker, Best, Röder, & Büchel, 2017; Kokinous, Kotz, Tavano, & Schröger, 2015; Van Atteveldt, Formisano, Blomert, & Goebel, 2007; Venezia et al., 2017). A few studies assessed whether different brain areas were activated when participants were exposed to congruent compared to incongruent AV stimulation. Erickson et al. (2014), in a recent activation likelihood estimation meta‐analysis, concluded that specific brain activation patterns are only found when comparing AV congruent versus incongruent (or vice versa) contrasts. The findings reveal, in particular that brain areas involved in the processing of congruent (vs. incongruent) AV syllables (e.g., either written or heard) are detected in the more proximal visual areas of the occipital cortex (e.g., the calcarine sulcus, the cuneus, and the lateral occipital cortex). Congruent versus incongruent AV language/signal processing also elicits an increase of activity in visual areas such as the calcarine sulcus and the ventral occipitotemporal cortex (Blau, van Atteveldt, Formisano, Goebel, & Blomert, 2008; Heim, Friederici, Schiller, Rüschemeyer, & Amunts, 2009).
Other studies suggest that areas involved in error processing and the perception of conflict in information processing, such as the left inferior parietal (Durston, 2003; Gau & Noppeney, 2016; Ojanen et al., 2005) and the supramarginal gyrus (Lange, Christian, & Schnitzler, 2013), are activated when participants are exposed to discrepant audio‐visual stimuli. Furthermore, Szycik, Jansma, and Münte (2009) find stronger signal changes for incongruent (vs. congruent) stimuli in the posterior part of STG. Together with the STG, the MTG is also more strongly activated when subject to incongruent versus congruent AV speech (Erickson et al., 2014). Furthermore, these findings are bolstered by the conclusions of a number of specialists (Komeilipoor, Cesari, & Daffertshofer, 2017; Pekkola et al., 2006; Szycik et al., 2009).
3. RESEARCH HYPOTHESES
Although the studies above assess congruence in completely different fields, their findings serve to formulate the following hypotheses which are tested in the current study. Hypothesis 1: We hypothesize stronger activation in areas related to visual encoding (occipital areas, calcarine, or cuneus gyri) when processing gender congruent (MV × MP + FV × FP) as opposed to incongruent (MV × FP + FV × MP) AV commercial combinations. Hypothesis 2: we hypothesize that the areas involved in error monitoring and conflicting AV processing (STG, MTG, supramarginal, and left inferior parietal lobe) are more strongly activated in response to commercials with incongruent (MV × FP + FV × MP) compared to congruent (MV × MP + FV × FP) product‐voice combinations. Furthermore, given the importance from the neuromarketing perspective of understanding the role of specific brain areas in predicting self‐report responses such as attitudes toward commercials, this study also investigates which brain regions activated during viewing commercials with gender congruent (vs. incongruent) product‐voice combinations covary with individual differences in self‐reported attitudes toward congruent‐incongruent commercials. In line with previous research, we expect these attitudes to covary in the areas most commonly involved in value encoding such as the orbitofrontal cortex, the posterior cingulate cortex (PCC) and the striatum (Bartra, McGuire, & Kable, 2013).
4. MATERIALS AND METHODS
4.1. Participants
Thirty heterosexual right‐handed subjects—15 females and 15 males, average age 30.0 years (SD: 10.4)—were recruited via social networks and the institutional website of the University of Granada between April and June 2017. Through a screening questionnaire that participants filled at the beginning of the fMRI session, participants were screened subjects to make sure the MRI exclusion criteria were not met. This questionnaire assessed whether subjects had a history of head injury, psychiatric illness, claustrophobia, pregnancy, and metal implants in the body. Further, prior to the MRI scan, informed consent was obtained from each participant. The study protocol was approved by the Vice‐rector for Research and Transfer of University of Granada (through Ethics Committee of Human Research, REF: 828) and the Research Centre of the Mind, Brain and Behavior. For approval, those institutions followed the protocol of the World Medical Association Declaration of Helsinki (2013) as well as that suggested by The Neuromarketing Science and Business Association. The latter constitutes an institution that has drawn up a code of ethics for consumer neuroscience studies and calls on researchers: (i) to be honest in their analyses and findings; (ii) not to take advantage of the incomprehension of participants of neuroscience measurements; and (iii) to offer transparency throughout the duration of experiments. The high level of requirement of those protocols assures a drastic protection of the subject in the current research.
4.2. Procedure
The study was carried out during one session. Participants arrived at the laboratory 1 hr prior to the fMRI task and after instruction and verification that all study procedures were understood, they completed the informed consent questionnaire. Participants then underwent a series of fMRI scans including two localizer scans, a structural scan, and functional scans. Over the course of the functional scans, participants performed a fMRI task in which they were asked to pay attention to the stimuli. After leaving the scanner, participants viewed a set of product‐voice combinations. After completion of the session, participants were thanked and reimbursed.
4.3. Stimuli experimental design
The study followed a 2 × 2 design with two within‐subject independent variables (Gender‐targeted product and Gender Voice) of two levels each: Male Product (MP)/Female Product (FP), and MV/FV. An independent sample (n = 70), rated a total of 60 products on gender stereotype (7‐likert scale, where 1 = very masculine and 7 = very feminine). For this study, only products that scored as extreme gender‐targeted were selected. This resulted in a total of 12 products (M mp= 2.11 vs. M fp = 6.48; t [69] = −21.92, p < .001) of which six were typically male and six were typically female. Male‐targeted products were an electric shaver, men's swimwear, men's deodorant, shaving cream, a tie, and men's shoes. Female‐targeted products, in turn, were a bikini, sanitary napkins, lipstick, nail polish, high‐heels, and hair removal cream. In the same independent sample (n = 70), the manipulation checks of the experimental stimuli were done: (i) differences in voice clarity between male and FVs was tested (t [69] = −1.10, p = .28), (ii) it was tested whether congruent versus incongruent product‐voice combinations were different in comprehension (t [69] = 1.44, p = .15), and (iii) it was tested whether congruent product‐voice combinations were perceived as more congruent than incongruent product‐voice combinations (t [69] = 12.51, p < .001).
All spoken messages were adapted from existing commercials. Each message consisted of 8 to 12 words pronounced by one neutral female and one neutral MV. The messages were recorded digitally with a high‐quality microphone (type C 2000 B by AKG) at a sampling rate of 44,100 Hz and a 16 bit quantization in a sound‐proofed room using Audacity software (http://audacity.sourceforge.net). An average fundamental frequency for each speaker was set (male, 124 Hz; female, 205 Hz) and the stimuli were shifted in fundamental frequency by the amount of the F0‐difference, that is, 81 Hz so as to assure objective voice comparisons. The recordings were then equalized by the PSOLA re‐synthesis function of the PRAAT speech editing software. Intensities were normalized using the Cool edit speech software. Furthermore, all auditory stimuli were filtered for ambient noise and standardized for the average root mean square power and set at a sound pressure level sensation of 70 dB on an average to assure comfort, intelligibility, and audibility given the background noise of the scanner. The presenters were professionals with similar tessitura and voices regulated by the same type of intonation, inflections, emphasis, and pauses.
4.4. The fMRI task
During the fMRI task subjects passively viewed the products and listened to the voices. All 12 products (six male and six female‐targeted) were combined with a male and FV presenting persuasive messages. This, therefore, resulted in 24 unique blocks: six blocks for the MP × MV condition (i.e., six different male products accompanied by the MV), six blocks for the FP × FV condition (i.e., six different female products accompanied by the FV), six blocks for the MP × FV condition (i.e., the same male products but accompanied by the FV) and six blocks for the FP × MV condition (i.e., the same female products but accompanied by the MV). There were, therefore, 12 blocks of product‐voice congruent conditions (MP × MV and FP × FV) and 12 blocks of incongruent conditions (MP × FV and FP × MV). The order of presentation of the blocks was random.
Each block, 27 s in length, consisted of five different images of the same gender‐targeted product. Each image, displayed for 5.4 s, was accompanied by a voice message pronounced by either a male or FV. Blocks were separated by 15 s inter trial baseline periods (“+”) (see Figure 1). Participants were required after every four blocks to answer the following question: “Taking into account the presenter of the last product, what is your attitude toward the commercial?” Stimuli were presented via an MRI compatible sound system with electrostatic headphones with E‐Prime Version 2 Professional software. The total duration of the task was 23.8 min. All images of the gender‐imaged products and all the messages can be consulted in the Supporting Information Appendix A.
Figure 1.
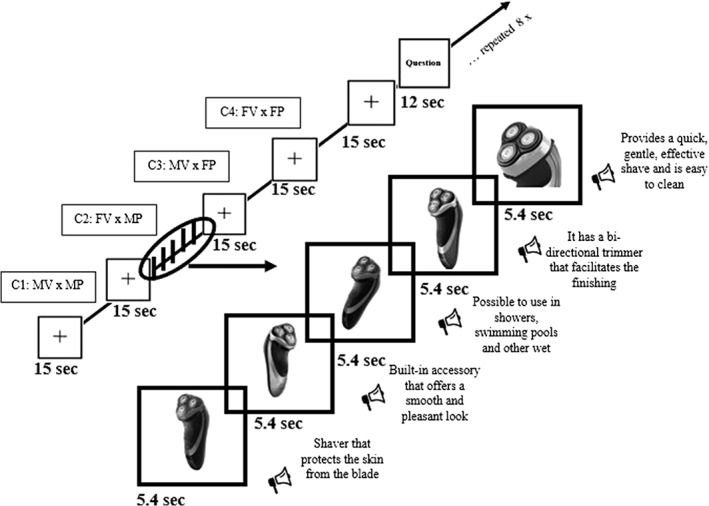
The left side is the fMRI task structure. The order corresponds to the first group of four blocks. The conditions (MV × MP, FV × MP, MV × FP, and FV × FP) are presented in random order in the subsequent seven repetitions. To the right is an example of a condition with five pictures of a male‐targeted product (MP) presented by a female voice (MV)
4.5. Self‐report measures
Directly after the MRI scan, the participants carried out a behavioral task where they evaluated one commercial of each of the four conditions (MP × MV; MP × FV; FP × MV; FP × FV) viewed in the fMRI task. After each message, the subject responded by means of a semantic differential scale using the following five pairs of adjectives: (a) sad/happy, (b) boring/exciting, (c) noninformative/informative, (d) irrelevant/relevant, and (e) dislike/like. A composite score of attitude toward the commercial in line with earlier self‐report studies (e.g., Venkatraman et al., 2015) was calculated from the average of the five questions. The results of the internal consistency analysis (Cronbach's alpha) of the attitude toward the four messages was acceptable in all cases (α = .782 for MP × MV; α = .95 for MP × FV; α = .93 for FP × MV; and α = .94 for FP × FV).
4.6. Statistical analysis of self‐reports
Statistical analyses were carried out with the IBM Statistical Package of Social Science (IBM SPSS Version 20). Paired‐Sample t tests were carried out to determine whether participants showed significantly higher attitudes toward commercials presenting congruent or incongruent AV combinations.
4.7. Image acquisition and preprocessing
MRI scanning was implemented with a Siemens Trio 3 T scanner equipped with a 32‐channel head coil. The structural image T1 was acquired by a 3D MP‐RAGE sequence with a sagittal orientation and a 1 × 1 × 1 mm3 voxel size (TR = 2,300 ms, TE = 2.96 ms). Functional scans were acquired with a (T2*‐weighted) echo‐planar imaging sequence (TR = 3,000 ms, TE = 35 ms, Flip Angle 90° and a plane reduction of 3 × 3 × 3 mm corresponding to the slice thickness, slice order: descending). The distance factor was 25% so as to attain a total of 36 slices, a slice matrix of 64 × 64 mm, and a Field of View of 192 mm with an axial orientation. A total of 477 functional scans were acquired.
Data were preprocessed and analyzed using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12) run with MATLAB R2012a (The MathworksInc, Natick, MA). Default settings were used unless stated otherwise. To allow stabilization of the BOLD signal, the first five volumes (15 s with a “cross” on the screen) of each run were discarded prior to analysis. Corrections were then applied by means of interpolation as to the differences in the time of slice acquisition with the initial slice serving as the reference. Functional images were realigned to the first image of the time series. Functional and structural images were coregistered and normalized (retaining 3 × 3 × 3 mm voxels) to the Montreal Neurological Institute (MNI) template. Finally, functional images were smoothed with the Gaussian kernel (FWHM = 6 mm). The mean functional images were visually inspected for artifacts. Furthermore, the realignment parameters of all subjects were examined. The Volume Artifact tool from ArtRepair (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) then served to detect and repair anomalously noisy volumes. Volumes that moved more than .5 mm/TR were repaired. Based on this, two participants (one female one male) were excluded from the analysis because too many volumes (>30%) required repair.
4.8. The fMRI analyses
Statistical maps were generated for each participant by fitting a boxcar function to the time series, convolved with the canonical hemodynamic response function. Data were high pass filtered with a cutoff of at 128 s. The following conditions were modeled as regressors: (1) six onsets referred to the MP × MV blocks, each 27 s in length, (2) six onsets referred to the MP × FV blocks, each 27 in length, (3) six onsets referred to the FP × MV blocks, each 27 s in length, (4) six onsets referred to the FP × FV blocks, each 27 s in length, (5) six onsets of the inquiries about positive or negative attitudes toward the commercial, each 12 s in length; and (6) the rest periods (fixation points of 15 s in length) were treated as the baseline on the General Linear Model implemented in SPM12. Six rigid body motion correction parameters (the parameters from the realignment) were also included as nuisance covariates.
On the first level, two contrasts were calculated: (i) congruent (MP × MV + FP × FV) minus incongruent (MP × FV + FP × MV) onsets, applying a T‐contrast to the first and fourth regressors of the model [1 –1 –1 1]; and (ii) incongruent (MP × MV + FP × FV) minus congruent (MP × MV + FP × FV) onsets, applying a T‐contrast to the second and third regressors of the model [−1 1 1 –1].
To determine which brain regions showed differential activation for congruent and incongruent periods, the contrast images of congruent minus incongruent periods (and vice versa) were entered into one‐sample t test analyses in the second level random‐effects phase. To identify the brain regions where AV congruency‐related activation varies with individual differences in attitudes toward congruent (vs. incongruent) combinations, the contrast image of congruent (MP × MV + FP × FV) minus incongruent (MP × FV + FP × MV) periods were entered into a one‐sample t test with as covariate the subtraction of the average scores of attitudes toward congruent combinations and the average scores of attitudes toward incongruent combinations. This procedure constitutes a common practice in neuroscience studies applied to Social Sciences and marketing in particular (Gearhardt, Yokum, Stice, Harris, & Brownell, 2014; Langleben et al., 2009).
The cp_cluster_Pthresh (https://goo.gl/kjVydz) routine served to set the cluster extent threshold to a meaningful value. This function is implemented in MATLAB and it can be employed to find the cluster‐size threshold (number of voxels) for a given (whole brain corrected) p value after the SPM estimation of the second level. Particularly, this tool offers a nonarbitrary uncorrected threshold and cluster extent equal to p < .05 corrected for multiple comparisons (FWE) across the whole brain. In the congruent versus incongruent analyses (and vice versa), the threshold resulted in p < .001 uncorrected with a cluster (k) 36. Following previous research in the Social Science arena, (Gearhardt et al., 2014), we used a more liberal threshold in the exploratory analysis regarding the relation between neural responses and attitudes toward commercials, since we look into the most important areas involved in value (e.g., orbitofrontal cortex, striatum, or PCC). In this case, the study resorted to a threshold of p < .001 uncorrected with a cluster extent of 20 voxels.
The study aim was, in fact, to assess the effect of congruency and not to assess the main effects of product (male vs. female) and voice (male vs. female). However, for completeness and to facilitate future meta‐analyses, we report the analyses and results of the main effects in the Supporting Information Appendix B.
5. RESULTS
5.1. Self‐report results
A Paired‐samples t test indicated that attitudes to commercials with congruent combinations yielded significantly more positive scores (MP × MV + FP × FV) than incongruent (FP × MV + MP × FV) combinations (t [27] = 2.90, p = .007, Cohen's r = .55) (see Figure 2).
Figure 2.
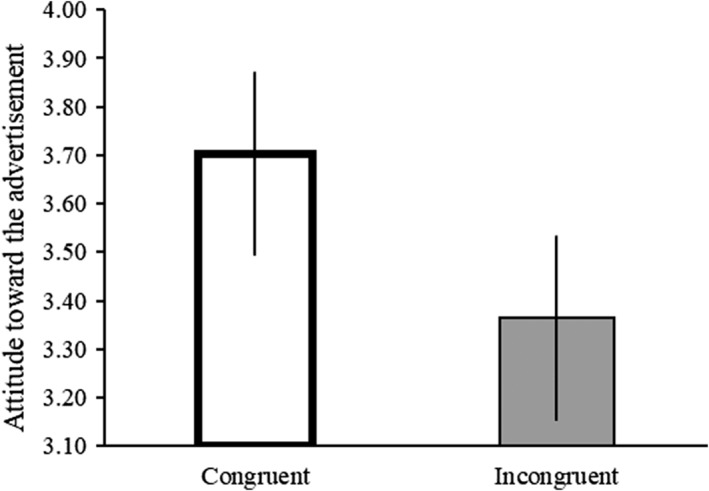
Results of the behavioral analysis. y axis: Attitude toward the commercial (at); x axis: Congruent (MP × MV + FP × FV) and incongruent (FP × MV + MP × FV) voice combinations. Attitudes toward commercials with gender congruent product‐voice combinations are more positive when compared to incongruent combinations (t [27] = 2.90, p = .007). Error bars indicate standard deviation
A two‐way RM ANOVA with attitudes toward advertisements as dependent variable, congruent and incongruent combinations as independent variables and the gender as a between subject factor did not reveal a significant interaction effect between the level of congruency of the advertising and the gender (F [1,27] = .05, p = .825). Thus, both males and females showed higher scores toward congruent AV advertisements (M males = 3.77, SD males = .23; M females = 3.63, SD females = .23) when compared to the incongruent counterparts (M males = 3.40, SD males = .28; M females = 3.32, SD females = 0.28).
5.2. Functional imaging results
5.2.1. Congruent and incongruent combinations
Clusters in the left calcarine gyrus and left cuneus areas were more strongly activated (p < .001 uncorrected, k = 36) when exposed to commercials with gender congruent (MP × MV + FP × FV) as opposed to incongruent (MP × FV + FP × MV) combinations (see Figure 3A, Table 1).
Figure 3.
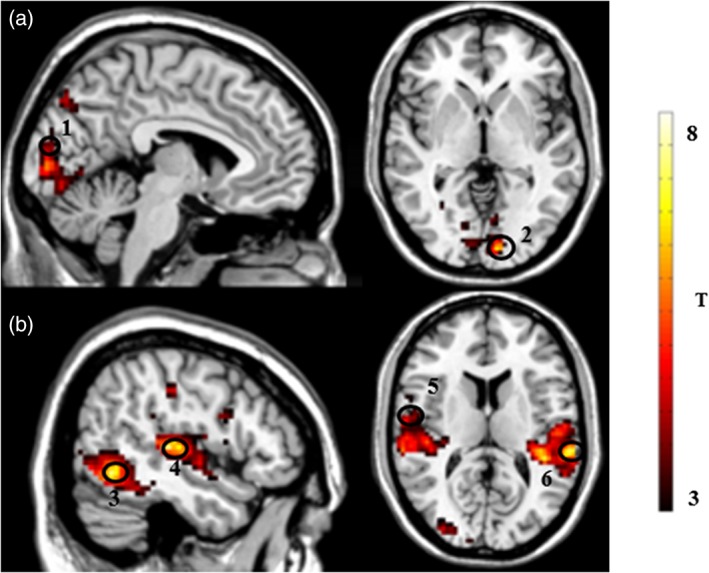
Illustration of the brain regions activated during (a) the congruent (MP × MV + FP × FV) > incongruent (MP × FV + FP × MV) contrast: (1) calcarine gyrus and (2) cuneus gyrus; (b) incongruent (MP × FV + FP × MV) > congruent (MP × MV + FP × FV) contrast: (3) inferior and middle occipital gyri (4) bilateral superior temporal gyri, (5) middle temporal gyrus, and (6) supramarginal gyrus and inferior parietal lobe. T‐map thresholded at p < .001 uncorrected for multiple comparisons (3 < T < 8), superimposed on the mean anatomical image of all subjects (MNI‐space) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Brain regions differentially activated in response to congruent (FP × MV + MP × FV) versus incongruent (MP × MV + FP × FV) commercials
| Brain region | Peak MNI‐coordinates (mm) | ||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Z value | T | K | Effect Sizea | |
| Congruent minus incongruentb | |||||||
| L calcarine gyrus | –9 | −91 | 2 | 5.25 | 7.02 | 158 | .99 |
| L cuneus | −9 | −94 | 14 | 4.83 | 6.17 | .91 | |
| −15 | −88 | 20 | 5.09 | 6.69 | .96 | ||
| Incongruent minus congruentb | |||||||
| L middle temporal gyrus | −57 | −32 | 5 | 5.76 | 8.19 | 578 | 1.09 |
| L superior temporal gyrus | −51 | −22 | 5 | 5.56 | 7.72 | 1.05 | |
| R superior temporal gyrus | 57 | −22 | 5 | 5.68 | 8.01 | 477 | 1.07 |
| R inferior occipital gyrus | 48 | −58 | −13 | 5.59 | 7.79 | 395 | 1.06 |
| R middle occipital gyrus | 27 | −88 | 8 | 4.18 | 5.02 | .79 | |
| R supramarginal gyrus | 60 | −22 | 41 | 4.34 | 5.28 | 36 | .82 |
| R inferior parietal lobe | 51 | −22 | 38 | 3.81 | 4.44 | .72 | |
Effect Size = Z/√N.
Peak coordinates of clusters significant at p < .001, k ≥ 36 voxels are reported.
Clusters in several brain regions such as the middle temporal (MTG), bilateral superior temporal (STG), inferior and middle occipital gyri, supramarginal gyrus, and inferior parietal lobes were more strongly activated (p < .001 uncorrected, k = 36, see Table 1, Figure 3B) in response to commercials with gender incongruent (FP x MV + MP x FV) than congruent (MP × MV + FP × FV) combinations.
Differences between males and females in brain responses to congruent and incongruent conditions were also tested. A two‐sample t test analysis revealed no significant differences between males and females when the congruent condition (MP × MV + FP × FV) was compared to the incongruent condition (MP × FV + FP × MV), or vice versa.
5.2.2. Relation between neural responses and attitudes toward commercials
Activation in the superior temporal gyri, R Heschl's gyri and R PCC during viewing of congruent minus incongruent combinations covaried significantly (positive) with the difference in attitudes score toward congruent minus incongruent commercials (r STG = .57; r Heschl = .61; r PCC = .56). Thus, participants with more positive attitudes to congruent (as opposed to incongruent commercials) reveal significantly stronger activation in these areas while viewing gender congruent compared to incongruent commercials (See Figure 4, Table 2).
Figure 4.
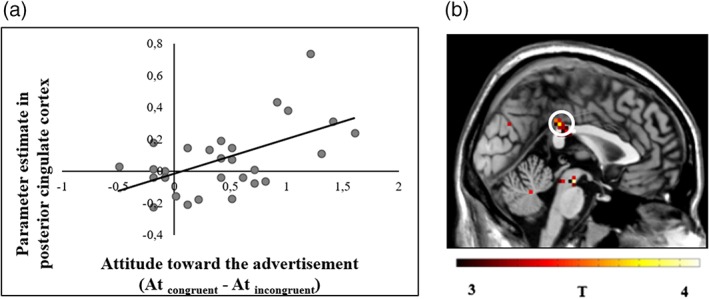
Activation in the left posterior cingulate cortex during commercials with gender congruent (minus incongruent) combinations of voice and product covaries with attitude toward congruent minus incongruent commercials. (a) Plot showing the correlation between parameter estimate of congruent vs. incongruent in the PCC cluster and the attitude toward congruent (vs. incongruent) commercials. (b) Brain regions in which activation during viewing commercials with gender congruent minus incongruent combinations covaries with differences in attitudes toward congruent minus incongruent commercials. Circle indicates right PCC cluster. T‐map thresholded at p < .001 uncorrected for multiple comparisons (3 < T < 4 for visualization), superimposed on the mean anatomical image of all subjects (MNI‐space) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Brain regions in which activation covaries with differences in attitudes toward congruent (MP × MV + FP × FV) minus incongruent (MP × FV + FP × MV) commercials
| Brain region | Peak MNI‐coordinates (mm) | ||||||
|---|---|---|---|---|---|---|---|
| x | y | z | Z value | T | K | Effect Sizea | |
| Congruent minus incongruentb | |||||||
| L superior temporal gyrus | −51 | −34 | 14 | 4.44 | 5.51 | 57 | .84 |
| L Heschl's gyri | −36 | −22 | 17 | 4.38 | 5.40 | .83 | |
| R posterior cingulate cortex | 6 | −31 | 23 | 4.04 | 4.82 | 22 | .76 |
| R posterior cingulate cortex | 6 | −40 | 23 | 3.39 | 3.85 | .64 | |
Effect Size = Z/√N.
No clusters survived at p < .001, k ≥ 36. Peak of clusters significant at p < .001, k ≥ 20 voxels are reported.
6. DISCUSSION AND CONCLUSIONS
No advertisement research to date has examined brain responses and behavioral reactions to male and female targeted products presented with male and FVs. This is the first study that examines the neural origin of the gender congruence effect on product and presenter combinations in advertising with both self‐report and neural tools. Generally, the findings show that congruent and incongruent product‐voice combinations are processed differentially at both neural and self‐report levels. In line with literature on the media effects of these combinations (Rodero et al., 2013), the current findings reveal more positive attitudes toward congruent compared to incongruent commercials. Interestingly, the neural findings provide some explanation for the origin of the higher attitudes conferred to congruent commercials: congruent product‐voice combinations give rise to stronger activation in primary visual areas which is tantamount to higher endogenous attention, while incongruent AV commercials elicit activation in areas linked to error and inconsistency processing.
More specifically, brain areas identified in earlier studies involved with congruent AV stimuli integration, namely the left calcarine gyrus and left cuneus, are more strongly activated in response to congruent compared to incongruent combinations. The meta‐analysis of Erickson et al. (2014) on the AV integration of speech signals concluded that the brain areas involved in the processing of congruent AV speech (e.g., the same written and heard syllable) are in the more proximal visual areas of the occipital cortex (such as the middle or superior occipital gyri), the bilateral fusiform gyrus, the cuneus, and the calcarine. Although the earlier studies on congruence in AV processing used very simple stimuli, the results of this study suggest that these areas also activate in response to congruence when stimuli are more complex, that is, gender congruence of product and voice.
Earlier studies on the neural processing of multisensory stimuli (e.g., AV) showed that activation in primary visual areas (such as the calcarine and cuneus areas) are the result of attentional shifts toward visual cues (Yamagishi, Goda, Callan, Anderson, & Kawato, 2005) and enhancements of the endogenous attention (Liu, Pestilli, & Carrasco, 2005; Pekkola et al., 2006). In the framework of this article, the found increases in primary visual activations during congruent product‐voice combinations may suggests that participants took more notice of congruent (vs. incongruent) commercials. Indeed, this reasoning is in line with the conclusions of van Ee, van Boxtel, Parker, and Alais (2009) and Fairhall and Macaluso (2009) who posited that congruent auditory or tactile information, and combined auditory‐tactile information, promote attentional control over incongruent visual stimuli. According to these authors, the enhanced capacity for attentional selection of the congruent stimulus results from a boost of its perceptual gain which is attributed to top‐down feedback from multisensory attentional processes that select the congruent feature of the input signal. In other words, audiovisual integration and spatial attention interact to contribute to a more thorough perception of a fused AV commercial, thus supporting Hypothesis 1.
In line with Hypothesis 2, areas involved in the processing of incongruent (vs. congruent) AV stimuli, namely the bilateral posterior superior (STG) and the middle temporal (MTG) gyri as well as the supramarginal and parietal lobes, are more strongly activated in response to incongruent as opposed to congruent combinations. The role of the posterior STG and MTG areas in AV integration is largely supported by specialized literature (Blau et al., 2008; Möttönen, Schürmann, & Sams, 2004; Naghavi, Eriksson, Larsson, & Nyberg, 2011). Recent studies suggest that these areas are specifically involved in processing of incongruent (vs. congruent) AV speech (Erickson et al., 2014; Ojanen et al., 2005; Szycik et al., 2009), combinations of faces and voices (Kokinous et al., 2015) and incongruent visual‐semantic information (Hein et al., 2007). Other research points to an increase in activation in those areas when comparing congruent vs. incongruent combinations of music and faces (Jeong et al., 2011), speech information (Van Atteveldt, Formisano, Goebel, & Blomert, 2004) and spoken phrases in audio and video (Calvert, Brammer, Bullmore, Campbell, Iversen, & David, 1999). The main differences between this study and that of Calvert et al. (1999) are the type of stimulation. Calvert et al. (1999) analyzed the congruency of stimuli that differ in the temporal domain (out of sync sound and video), while, as noted above, this study indicates that the congruency effect on the neural level is also found with a higher cognitive level stimulus (gender voice and gender product). Moreover, this paper suggests that incongruent combinations of AV stimuli of a higher cognitive level and complexity (e.g., gender products and voices) are processed in specific areas in the temporal lobe, notably the STS, and MTS.
The stronger activation in the supramarginal and left inferior parietal lobe during incongruent stimulation might be related to increased conflict detection, mismatch and contradiction when presented with inconsistent (vs. consistent) AV inputs (Durston, 2003). These results are bolstered by research comparing incongruent vs. congruent AV speech (Gau & Noppeney, 2016; Szycik et al., 2009) and language production (Heim et al., 2009). The superior, middle and inferior occipital gyri were also strongly activated by incongruent AV combinations. Previous research revealed that those areas may play a key role in (incongruent) multisensory stimuli. Specifically, Macaluso, George, Dolan, Spence, and Driver (2004) related those areas to spatial multisensory interactions and Meienbrock, Naumer, O. Doehrmann, Singer, and Muckli (2007) to the incongruent AV integration. Consequently, different areas in the occipital lobe are involved in congruent and incongruent AV stimuli. While the calcarine and the cuneus appear to be more affected by AV synchrony and increases in endogenous attention (e.g., congruent product‐voice combinations), more superior and inferior areas seem to be associated with the multisensory interaction of incongruent combinations (e.g., incongruent product‐voice combinations).
This article's last goal was to identify the brain regions where product‐voice congruency‐related activation varies with individual differences in attitudes toward congruent (vs. incongruent) product voice combinations. The findings reveal that participants with more positive attitudes toward congruent commercials show stronger activation during congruent (vs. incongruent) periods in several brain regions, including the right PCC. The PCC receives input via connections and integration from the ventromedial prefrontal cortex, a brain area that reflects subjective value of stimuli (Bartra et al., 2013). Specifically, the right PCC is thought to encode stimulus salience (Maddock, 1999). It also responds during selection of a favorite brand in a forced choice paradigm (Deppe, 2005) and during purchasing decisions (Knutson, Rick, Wimmer, Prelec, & Loewenstein, 2007). It has also been shown that the right ventral PCC plays a role in evaluating emotion content from visual sensory input (Vogt, Vogt, & Laureys, 2006). In our study, the stronger PCC activation during congruent commercials in participants who granted a higher rating to the congruent AV commercials may reflect a higher subjective value and reward conveyed by commercials which include congruent (vs. incongruent) combinations of voice gender and gender‐targeted products.
Theoretically, the current findings contribute to the literature challenging the notion of the importance of increasing positive perceptions toward commercials. Previous research focused on the self‐reported media effects of smartphone advertising (Martins, Costa, Oliveira, Gonçalves, & Branco, 2018) or exposing the viewer to idealized female images (Wan, Ansons, Chattopadhyay, & Leboe, 2013) and congruent product‐voice combinations (Rodero et al., 2013). This study sheds light on the subconscious origin of the more positive self‐reported attitudes conferred to very common elements in commercials (such as gender congruence in product‐voice combinations) aiming to encourage the purchase of products of daily use. It also constitutes a new step in the neuromarketing field with the application of neurological tools aiming to explore consumer processing of visual and audio information. Previous research in this direction employed fMRI to compare the neural processing of successful and unsuccessful advertising (Daugherty, Hoffman, & Kennedy, 2016), to test the effect of counterarguing in antidrug campaigns (Weber, Huskey, Mangus, Westcott‐Baker, & Turner, 2015), to explore advertising appeals and their relationship to product attractiveness (Chang, O'Boyle, Anderson, & Suttikun, 2016) or to elucidate the neural mechanisms of social influence (Mason, Dyer, & Norton, 2009). This study advances and spells out the processing of the combination of two elements, voice gender and gender product, whose subconscious mechanisms were previously not investigated in advertising research. Finally, this article contributes to the literature analyzing the neural processing of multisensorial integration. Previous studies examined the role of emotion in the neural processing of music and face combinations (Jeong et al., 2011) or the neural correlates of visual letters and speech sounds (Blau et al., 2008). The current research goes further and sheds light on the neural responses to more complex AV stimuli (product‐voice combinations) with different levels of congruency.
These findings have remarkable managerial implications. First, the findings indicate that managers and communication professionals desiring to design successful advertising campaigns of everyday products should employ female presenters for female‐targeted products and male presenters, for male‐targeted products. In line with Strach et al. (2015), congruent product‐voice combinations not only increase positive attitudes toward commercials but attain a higher degree of subconscious attention than their incongruent counterparts. Despite the scarce unanimity in literature regarding the attitudes provoked by congruent and incongruent product‐voice combinations, and regardless of the nature of the match‐up condition, this study suggests that incongruent combinations in advertising may trigger subconscious ambivalence and dispute. This article goes further to propose, thirdly, that congruent combinations of voice and product in commercials also increase the consumer value and relevance toward the advertised product. The congruent combination elicits brain mechanisms similar to those involved with the maternal love (Noriuchi, Yoshiaki, & Senoo, 2008) or food desire (Linder et al., 2010). Therefore, carefully matching the type of product with a gender‐congruent voice in advertising may be key in creating positive expectations and obtaining long‐term competitive advantages through purchase relationships with consumers. Accordingly, all efforts made by businesses on offering high‐quality products or services, may be worthless when the combination between voice and product is not congruent in the commercial.
It must be noted that this work only measured self‐reported attitudes toward commercials and not actual purchasing behavior. Although, it is widely demonstrated that more positive attitudes toward commercials are linked to an increase in purchase intention (Raza, Bakar, & Mohamad, 2018) future neuromarketing research should link neural responses to gender congruence in commercials with actual purchasing behaviors. Second, the conclusions of this article should be received with caution due to sample size of 30 participants. Future research applying neuromarketing techniques in AV advertising with larger sample sizes is needed. Thirdly, previous self‐report research analyzing product‐voice combinations in advertising used a gender‐balanced sample aged from 21 to 23 years old (Rodero et al., 2013) or from 19 to 65 years old (Whipple & McManamon, 2002). The former study extrapolated the findings to other age groups; and the latter one did not find age differences in the results. Although the current research can provoke similar results in older individuals, more studies increasing the sample age are needed to corroborate the results.
Despite the large amount of literature analyzing the effects of gender congruence on advertising valuation, it is surprising to observe how most studies omit clarifying the question of the origin of the media effects provoked by congruent and incongruent product‐voice combinations in advertising. This is the first study that applies a neuromarketing approach to face this research gap and advances that congruent product‐voice combinations receive the greatest attitudes possibly due to the increases in attention, value and relevance they induce during advertising valuation. By contrast, incongruent combinations trigger error monitoring and inconsistency detection. Therefore, this exploratory study constitutes an advance in the understanding of the origin of attitudes and preferences induced by congruent and incongruent advertising.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
This work was supported by an Excellence Project awarded by the Junta de Andalusia [REF: P12‐SEJ‐1980]; and a FPU contract awarded by the Ministry of Education, Culture and Sports of Spain. [REF: FPU14 / 04736].
Casado‐Aranda L‐A, Van der Laan LN, Sánchez‐Fernández J. Neural correlates of gender congruence in audiovisual commercials for gender‐targeted products: An fMRI study. Hum Brain Mapp. 2018;39:4360–4372. 10.1002/hbm.24276
Funding information Excellence Project by the Junta de Andalusia, Grant/Award Number: P12‐SEJ‐1980; Ministry of Education, Culture and Sports of Spain, Grant/Award Numbers: REF: FPU14 / 04736, FPU14 / 04736; Junta de Andalusia, Grant/Award Number: P12‐SEJ‐1980
REFERENCES
- Bartra, O. , McGuire, J. T. , & Kable, J. W. (2013). The valuation system: A coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau, V. , van Atteveldt, N. , Formisano, E. , Goebel, R. , & Blomert, L. (2008). Task‐irrelevant visual letters interact with the processing of speech sounds in heteromodal and unimodal cortex. European Journal of Neuroscience, 28, 500–509. 10.1111/j.1460-9568.2008.06350.x [DOI] [PubMed] [Google Scholar]
- Callan, D. E. , Jones, J. A. , Munhall, K. , Callan, A. M. , Kroos, C. , & Vatikiotis‐Bateson, E. (2003). Neural processes underlying perceptual enhancement by visual speech gestures. Neuroreport, 14, 2213–2218. https://insights.ovid.com/pubmed?pmid=14625450 [DOI] [PubMed] [Google Scholar]
- Calvert, G. A. , Brammer, M. J. , Bullmore, E. T. , Campbell, R. , Iversen, S. D. , & David, A. S. (1999). Response amplification in sensory‐specific cortices during crossmodal binding. Neuroreport, 10, 2619–2623. https://insights.ovid.com/pubmed?pmid=14625450 [DOI] [PubMed] [Google Scholar]
- Casado‐Aranda, L.‐A. , Martínez‐Fiestas, M. , & Sánchez‐Fernández, J. (2018). Neural effects of environmental advertising: An fMRI analysis of voice age and temporal framing. Journal of Environmental Management, 206, 664–675. 10.1016/j.jenvman.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Casado‐Aranda, L.‐A. , Sánchez‐Fernández, J. , & Montoro‐Ríos, F. J. (2017). Neural correlates of voice gender and message framing in advertising: A functional MRI study. Journal of Neuroscience, Psychology, and Economics, 10(4), 121–136. 10.1037/npe0000076 [DOI] [Google Scholar]
- Chang, H. J. J. , O'Boyle, M. , Anderson, R. C. , & Suttikun, C. (2016). An fMRI study of advertising appeals and their relationship to product attractiveness and buying intentions: An fMRI study of advertising appeals. Journal of Consumer Behaviour., 15, 538–548. 10.1002/cb.1591 [DOI] [Google Scholar]
- Chebat, J.‐C. , El Hedhli, K. , GéLinas‐Chebat, C. , & Boivin, R. (2007). Voice and persuasion in a banking telemarketing context. Perceptual and Motor Skills, 104, 419–437. 10.2466/pms.104.2.419-437 [DOI] [PubMed] [Google Scholar]
- Chun, J.‐W. , Park, H.‐J. , Park, I.‐H. , & Kim, J.‐J. (2012). Common and differential brain responses in men and women to nonverbal emotional vocalizations by the same and opposite sex. Neuroscience Letters, 515, 157–161. 10.1016/j.neulet.2012.03.038 [DOI] [PubMed] [Google Scholar]
- Connell, P. M. , Brucks, M. , & Nielsen, J. H. (2014). How childhood advertising exposure can create biased product evaluations that persist into adulthood. Journal of Consumer Research, 41, 119–134. 10.1086/675218 [DOI] [Google Scholar]
- Debevec, K. , & Iyer, E. (1986). The influence of spokespersons in altering a product's gender image: Implications for advertising effectiveness. Journal of Advertising , 15, 12–20. Retrieved from http://eds.a.ebscohost.com/eds/pdfviewer/pdfviewer?vid=3&sid=13682b49-0d9d-4a72-b979-75c7f70b72e3%40sessionmgr4009&hid=4102 [Google Scholar]
- Dimoka, A. (2010). What does the brain tell us about trust and distrust? Evidence from a functional neuroimaging study. MIS Quarterly, 2(34), 373–396. Retrieved from https://www.jstor.org/stable/20721433?seq=1#page_scan_tab_contents [Google Scholar]
- Daugherty, T. , Hoffman, E. , & Kennedy, K. (2016). Research in reverse: Ad testing using an inductive consumer neuroscience approach. Journal of Business Research, 69(8), 3168–3176. 10.1016/j.jbusres.2015.12.005 [DOI] [Google Scholar]
- Deppe . (2005). Nonlinear responses within the medial prefrontal cortex reveal when specific implicit information influences economic decision making. Journal of Neuroimaging, 15, 171–182. 10.1177/1051228405275074 [DOI] [PubMed] [Google Scholar]
- Durston, S. (2003). Parametric manipulation of conflict and response competition using rapid mixed‐trial event‐related fMRI. NeuroImage, 20, 2135–2141. 10.1016/j.neuroimage.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Ebner, N. C. , Johnson, M. R. , Rieckmann, A. , Durbin, K. A. , Johnson, M. K. , & Fischer, H. (2013). Processing own‐age vs. other‐age faces: Neuro‐behavioral correlates and effects of emotion. NeuroImage, 78, 363–371. 10.1016/j.neuroimage.2013.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, L. C. , Heeg, E. , Rauschecker, J. P. , & Turkeltaub, P. E. (2014). An ALE meta‐analysis on the audiovisual integration of speech signals: ALE meta‐analysis on AV speech integration. Human Brain Mapping, 35, 5587–5605. 10.1002/hbm.22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall, S. L. , & Macaluso, E. (2009). Spatial attention can modulate audiovisual integration at multiple cortical and subcortical sites. European Journal of Neuroscience, 29(6), 1247–1257. 10.1111/j.1460-9568.2009.06688.x [DOI] [PubMed] [Google Scholar]
- Falk, E. B. , Berkman, E. T. , & Lieberman, M. D. (2012). From neural responses to population behavior: Neural focus group predicts population‐level media effects. Psychological Science, 23, 439–445. 10.1177/0956797611434964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt, A. N. , Yokum, S. , Stice, E. , Harris, J. L. , & Brownell, K. D. (2014). Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience, 9(7), 932–938. 10.1093/scan/nst059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölig, C. , Föcker, J. , Best, A. , Röder, B. , & Büchel, C. (2017). Activation in the angular gyrus and in the pSTS is modulated by face primes during voice recognition. Human Brain Mapping, 38(5), 2553–2565. 10.1002/hbm.23540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal, D. , & Wilkie, J. (2010). Real men Don't eat quiche: Regulation of gender‐expressive choices by men. Social Psychological and Personality Science, 1, 291–301. 10.1177/1948550610365003 [DOI] [Google Scholar]
- Gau, R. , & Noppeney, U. (2016). How prior expectations shape multisensory perception. NeuroImage, 124, 876–886. 10.1016/j.neuroimage.2015.09.045 [DOI] [PubMed] [Google Scholar]
- Grohman, B. (2009). Gender dimensions of brand personality. Journal of Marketing Research, 46, 105–119. 10.1509/jmkr.46.1.105 [DOI] [Google Scholar]
- Heim, S. , Friederici, A. D. , Schiller, N. O. , Rüschemeyer, S.‐A. , & Amunts, K. (2009). The determiner congruency effect in language production investigated with functional MRI. Human Brain Mapping, 30, 928–940. 10.1002/hbm.20556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein, G. , Doehrmann, O. , Muller, N. G. , Kaiser, J. , Muckli, L. , & Naumer, M. J. (2007). Object familiarity and semantic congruency modulate responses in cortical audiovisual integration areas. Journal of Neuroscience, 27, 7881–7887. 10.1523/JNEUROSCI.1740-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks, B. , van Meurs, F. , & van der Meij, E. (2015). Does a foreign accent sell? The effect of foreign accents in radio commercials for congruent and non‐congruent products. Multilingua , 34(1), 119–130. Retrieved from https://www.degruyter.com/view/j/mult.2015.34.issue-1/multi-2013-0048/multi-2013-0048.xml [Google Scholar]
- Jeong, J.‐W. , Diwadkar, V. A. , Chugani, C. D. , Sinsoongsud, P. , Muzik, O. , Behen, M. E. , & Chugani, D. C. (2011). Congruence of happy and sad emotion in music and faces modifies cortical audiovisual activation. NeuroImage, 54, 2973–2982. 10.1016/j.neuroimage.2010.11.017 [DOI] [PubMed] [Google Scholar]
- Joassin, F. , Maurage, P. , & Campanella, S. (2011). The neural network sustaining the crossmodal processing of human gender from faces and voices: An fMRI study. NeuroImage, 54, 1654–1661. 10.1016/j.neuroimage.2010.08.073 [DOI] [PubMed] [Google Scholar]
- Junger, J. , Habel, U. , Bröhr, S. , Neulen, J. , Neuschaefer‐Rube, C. , Birkholz, P. , … Pauly, K. (2014). More than just two sexes: The neural correlates of voice gender perception in gender dysphoria. PLoS One, 9, e111672 10.1371/journal.pone.0111672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger, J. , Pauly, K. , Bröhr, S. , Birkholz, P. , Neuschaefer‐Rube, C. , Kohler, C. , & Habel, U. (2013). Sex matters: Neural correlates of voice gender perception. NeuroImage, 79, 275–287. 10.1016/j.neuroimage.2013.04.105 [DOI] [PubMed] [Google Scholar]
- Komeilipoor, N. , Cesari, P. , & Daffertshofer, A. (2017). Involvement of superior temporal areas in audiovisual and audiomotor speech integration. Neuroscience, 343, 276–283. 10.1016/j.neuroscience.2016.03.047 [DOI] [PubMed] [Google Scholar]
- Klofstad, C. A. (2016). Candidate voice pitch influences election outcomes: Voice pitch influences voters. Political Psychology, 37(5), 725–738. 10.1111/pops.12280 [DOI] [Google Scholar]
- Kokinous, J. , Kotz, S. A. , Tavano, A. , & Schröger, E. (2015). The role of emotion in dynamic audiovisual integration of faces and voices. Social Cognitive and Affective Neuroscience, 10, 713–720. 10.1093/scan/nsu105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, B. , Rick, S. , Wimmer, G. E. , Prelec, D. , & Loewenstein, G. (2007). Neural predictors of purchases. Neuron, 53, 147–156. 10.1016/j.neuron.2006.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, J. , Christian, N. , & Schnitzler, A. (2013). Audio–visual congruency alters power and coherence of oscillatory activity within and between cortical areas. NeuroImage, 79, 111–120. 10.1016/j.neuroimage.2013.04.064 [DOI] [PubMed] [Google Scholar]
- Langleben, D. D. , Loughead, J. W. , Ruparel, K. , Hakun, J. G. , Busch‐Winokur, S. , Strasser, A. , & Lerman, C. (2009). Reduced prefrontal and temporal processing and recall of high “sensation value” ads. NeuroImage, 46(1), 219–225. 10.1016/j.neuroimage.2008.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski, P. , Fransen, M. L. , & Tan, E. S. H. (2014). Predicting advertising effectiveness by facial expressions in response to amusing persuasive stimuli. Journal of Neuroscience, Psychology, and Economics, 7, 1–14. 10.1037/npe0000012 [DOI] [Google Scholar]
- Lien, N. H. , Chou, H. Y. , & Chang, C. H. (2012). Advertising effectiveness and the match‐up hypothesis: Examining spokesperson sex, attractiveness type, and product image. Journal of Current Issues & Research in Advertising, 33(2), 282–300. 10.1080/10641734.2012.700809 [DOI] [Google Scholar]
- Linder, N. S. , Uhl, G. , Fliessbach, K. , Trautner, P. , Elger, C. E. , & Weber, B. (2010). Organic labeling influences food valuation and choice. NeuroImage, 53(1), 215–220. 10.1016/j.neuroimage.2010.05.077 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Pestilli, F. , & Carrasco, M. (2005). Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron, 45(3), 469–477. 10.1016/j.neuron.2004.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso, E. , George, N. , Dolan, R. , Spence, C. , & Driver, J. (2004). Spatial and temporal factors during processing of audiovisual speech: A PET study. NeuroImage, 21(2), 725–732. 10.1016/j.neuroimage.2003.09.049 [DOI] [PubMed] [Google Scholar]
- Maddock, R. J. (1999). The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences, 22, 310–316. 10.1016/S0166-2236(98)01374-5 [DOI] [PubMed] [Google Scholar]
- Martín‐Santana, J. D. , Muela‐Molina, C. , Reinares‐Lara, E. , & Rodríguez‐Guerra, M. (2015). Effectiveness of radio spokesperson's gender, vocal pitch and accent and the use of music in radio advertising. BRQ Business Research Quarterly, 18, 143–160. 10.1016/j.brq.2014.06.001 [DOI] [Google Scholar]
- Martínez‐Fiestas, M. , del Jesus, M. I. V. , Sánchez‐Fernández, J. , & Montoro‐Rios, F. J. (2015). A psychophysiological approach for measuring response to messaging: How consumers emotionally process green advertising. Journal of Advertising Research, 55, 192–205. 10.2501/JAR-55-2-192-205 [DOI] [Google Scholar]
- Martins, J. , Costa, C. , Oliveira, T. , Gonçalves, R. , & Branco, F. (2018). How smartphone advertising influences consumers' purchase intention. Journal of Business Research. (in press) 10.1016/j.jbusres.2017.12.047 [DOI] [Google Scholar]
- Mason, M. F. , Dyer, R. , & Norton, M. I. (2009). Neural mechanisms of social influence. Organizational Behavior and Human Decision Processes, 110(2), 152–159. 10.1016/j.obhdp.2009.04.001 [DOI] [Google Scholar]
- Meienbrock, A. , Naumer, M. J. , Doehrmann, O. , Singer, W. , & Muckli, L. (2007). Retinotopic effects during spatial audio‐visual integration. Neuropsychologia, 45(3), 531–539. 10.1016/j.neuropsychologia.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Modinos, G. , Ormel, J. , & Aleman, A. (2009). Activation of anterior insula during self‐reflection. PLoS One, 4, e4618 10.1371/journal.pone.0004618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möttönen, R. , Schürmann, M. , & Sams, M. (2004). Time course of multisensory interactions during audiovisual speech perception in humans: A magnetoencephalographic study. Neuroscience Letters, 363, 112–115. 10.1016/j.neulet.2004.03.076 [DOI] [PubMed] [Google Scholar]
- Naghavi, H. R. , Eriksson, J. , Larsson, A. , & Nyberg, L. (2011). Cortical regions underlying successful encoding of semantically congruent and incongruent associations between common auditory and visual objects. Neuroscience Letters, 505, 191–195. 10.1016/j.neulet.2011.10.022 [DOI] [PubMed] [Google Scholar]
- Noriuchi, M. , Kikuchi, Y. , & Senoo, A. (2008). The functional neuroanatomy of maternal love: Mother's response to Infant's attachment behaviors. Stress, Depression, and Circuitry, 63(4), 415–423. 10.1016/j.biopsych.2007.05.018 [DOI] [PubMed] [Google Scholar]
- Ojanen, V. , Möttönen, R. , Pekkola, J. , Jääskeläinen, I. P. , Joensuu, R. , Autti, T. , & Sams, M. (2005). Processing of audiovisual speech in Broca's area. NeuroImage, 25, 333–338. 10.1016/j.neuroimage.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Pedelty, M. , & Kuecker, M. (2014). Seen to be heard? Gender, voice, and body in television advertisements. Communication and Critical/Cultural Studies, 11(3), 250–269. 10.1080/14791420.2014.926015 [DOI] [Google Scholar]
- Pekkola, J. , Laasonen, M. , Ojanen, V. , Autti, T. , Jääskeläinen, I. P. , Kujala, T. , & Sams, M. (2006). Perception of matching and conflicting audiovisual speech in dyslexic and fluent readers: An fMRI study at 3 T. NeuroImage, 29, 797–807. 10.1016/j.neuroimage.2005.09.069 [DOI] [PubMed] [Google Scholar]
- Pekkola, J. , Ojanen, V. , Autti, T. , Jääskeläinen, I. P. , Möttönen, R. , & Sams, M. (2006). Attention to visual speech gestures enhances hemodynamic activity in the left planum temporale. Human Brain Mapping, 27(6), 471–477. 10.1002/hbm.20190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock‐Speck, B. , & del Saz Rubio, M. M. (2009). Voice‐overs in standardized English and Spanish television commercials. Atlantis, 31, 111–127. Retrieved from http://eds.a.ebscohost.com/eds/pdfviewer/pdfviewer?vid=6&sid=2526acf9-8c50-4472-9d99-d70484c155ba%40sessionmgr4009&hid=4102 [Google Scholar]
- Pilelienė, L. , & Grigaliūnaitė, V. (2017). Relationship between Spokesperson's gender and advertising color temperature in a framework of advertising effectiveness. Scientific Annals of Economics and Business, 64, 1–13. Retrieved from http://saeb.feaa.uaic.ro/index.php/saeb/article/view/235 [Google Scholar]
- Potter, R. F. , & Choi, J. (2006). The effects of auditory structural complexity on attitudes, attention, arousal, and memory. Media Psychology, 8(4), 395–419. 10.1207/s1532785xmep0804_4 [DOI] [Google Scholar]
- Potter, R. F. , Jamison‐Koenig, E. J. , Lynch, T. , & Sites, J. (2016). Effect of vocal‐pitch difference on automatic attention to voice changes in audio messages. Communication Research, 0093650215623835, 1–18. 10.1177/0093650215623835 [DOI] [Google Scholar]
- Prieler, M. (2016). Gender stereotypes in Spanish‐and English‐language television advertisements in the United States. Mass Communication and Society, 19(3), 275–300. 10.1080/15205436.2015.1111386 [DOI] [Google Scholar]
- Proverbio, A. M. , & De Gabriele, V. (2017). The other‐race effect does not apply to infant faces: An ERP attentional study. Neuropsychologia, 42, 1–9. 10.1016/j.neuropsychologia.2017.03.028 [DOI] [PubMed] [Google Scholar]
- Raza, S. H. , Bakar, H. A. , & Mohamad, B. (2018). Relationships between the advertising appeal and behavioral intention: The mediating role of the attitude towards advertising appeal and moderating role of cultural norm. Journal of Business and Retail Management Research, 12(2), 185–193. [Google Scholar]
- Reimann, M. , Schilke, O. , Weber, B. , Neuhaus, C. , & Zaichkowsky, J. (2011). Functional magnetic resonance imaging in consumer research: A review and application. Psychology and Marketing, 28(6), 608–637. 10.1002/mar.20403 [DOI] [Google Scholar]
- Rodero, E. , Larrea, O. , & Vázquez, M. (2013). Male and female voices in commercials: Analysis of effectiveness, adequacy for the product, attention and recall. Sex Roles, 68, 349–362. 10.1007/s11199-012-0247-y [DOI] [Google Scholar]
- Sherry, J. F. , Kozinets, R. V. , Duhachek, A. , DeBerry‐Spence, B. , Nuttavuthisit, K. , & Storm, D. (2004). Gendered behavior in a male preserve: Role playing at ESPN zone Chicago. Journal of Consumer Psychology, 14, 151–158. [DOI] [Google Scholar]
- Szycik, G. R. , Jansma, H. , & Münte, T. F. (2009). Audiovisual integration during speech comprehension: An fMRI study comparing ROI‐based and whole brain analyses. Human Brain Mapping, 30, 1990–1999. 10.1002/hbm.20640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnais, C. , Andreu‐Perez, J. , Sánchez‐Fernández, J. , & Andréu‐Abela, J. (2013). The contribution of neuroscience to consumer research: A conceptual framework and empirical review. Journal of Economic Psychology, 36, 68–81. 10.1016/j.joep.2013.02.011 [DOI] [Google Scholar]
- Strach, P. , Zuber, K. , Fowler, E. F. , Ridout, T. N. , & Searles, K. (2015). In a different voice? Explaining the use of men and women as voice‐over announcers in political advertising. Political Communication, 32, 183–205. 10.1080/10584609.2014.914614 [DOI] [Google Scholar]
- Tu, J.‐C. , Kao, T.‐F. , & Tu, Y.‐C. (2013). Influences of framing effect and green message on advertising effect. Social Behavior and Personality: An International Journal, 41, 1083–1098. 10.2224/sbp.2013.41.7.1083 [DOI] [Google Scholar]
- Van Atteveldt, N. , Formisano, E. , Blomert, L. , & Goebel, R. (2007). The effect of temporal asynchrony on the multisensory integration of letters and speech sounds. Cerebral Cortex, 17, 962–974. 10.1093/cercor/bhl007 [DOI] [PubMed] [Google Scholar]
- Van Atteveldt, N. , Formisano, E. , Goebel, R. , & Blomert, L. (2004). Integration of letters and speech sounds in the human brain. Neuron, 43, 271–282. 10.1016/j.neuron.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Van der Laan, L. N. , De Ridder, D. T. D. , Viergever, M. A. , & Smeets, P. A. M. (2012). Appearance matters: Neural correlates of food choice and packaging aesthetics. PLoS One, 7(7), e41738 10.1371/journal.pone.0041738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ee, R. , van Boxtel, J. J. A. , Parker, A. L. , & Alais, D. (2009). Multisensory congruency as a mechanism for attentional control over perceptual selection. Journal of Neuroscience, 29(37), 11641–11649. 10.1523/JNEUROSCI.0873-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezia, J. H. , Vaden, K. I. J. , Rong, F. , Maddox, D. , Saberi, K. , & Hickok, G. (2017). Auditory, visual and audiovisual speech processing streams in superior temporal sulcus. Frontiers in Human Neuroscience, 11, 1–17. 10.3389/fnhum.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman, V. , Dimoka, A. , Pavlou, P. A. , Vo, K. , Hampton, W. , Bollinger, B. , & Winer, R. S. (2015). Predicting advertising success beyond traditional measures: New insights from neurophysiological methods and market response modeling. Journal of Marketing Research, 52, 436–452. 10.1509/jmr.13.0593 [DOI] [Google Scholar]
- Vogt, B. A. , Vogt, L. , & Laureys, S. (2006). Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage, 29, 452–466. 10.1016/j.neuroimage.2005.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi, N. , Goda, N. , Callan, D. E. , Anderson, S. J. , & Kawato, M. (2005). Attentional shifts towards an expected visual target alter the level of alpha‐band oscillatory activity in the human calcarine cortex. Cognitive Brain Research, 25(3), 799–809. 10.1016/j.cogbrainres.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Wan, F. , Ansons, T. L. , Chattopadhyay, A. , & Leboe, J. P. (2013). Defensive reactions to slim female images in advertising: The moderating role of mode of exposure. Organizational Behavior and Human Decision Processes, 120(1), 37–46. 10.1016/j.obhdp.2012.07.008 [DOI] [Google Scholar]
- Wang, A.‐L. , Lowen, S. B. , Shi, Z. , Bissey, B. , Metzger, D. S. , & Langleben, D. D. (2016). Targeting modulates audiences' brain and behavioral responses to safe sex video ads. Social Cognitive and Affective Neuroscience, 11, 1650–1657. 10.1093/scan/nsw070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, R. , Huskey, R. , Mangus, J. M. , Westcott‐Baker, A. , & Turner, B. O. (2015). Neural predictors of message effectiveness during Counterarguing in antidrug campaigns. Communication Monographs, 82(1), 4–30. 10.1080/03637751.2014.971414 [DOI] [Google Scholar]
- Weber, R. , Mangus, J. M. , & Huskey, R. (2015). Brain imaging in communication research: A practical guide to understanding and evaluating fMRI studies. Communication Methods and Measures, 9, 5–29. 10.1080/19312458.2014.999754 [DOI] [Google Scholar]
- Whipple, T. W. , & McManamon, M. K. (2002). Implications of using male and female voices in commercials: An exploratory study. Journal of Advertising, 31, 79–91. 10.1080/00913367.2002.10673668 [DOI] [Google Scholar]
- World Medical Association (2013). Principios Éticos para las investigaciones médicas en seres humanos. 64° Asamblea General, Retrieved from http://www.wma.net/es/20activities/10ethics/10helsinki/index.html.pdf?print-media-type&footer-right=%5Bpage%5D/%5BtoPage
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


