Abstract
Cognitive control is one of the most important skills in day‐to‐day social and intellectual functioning but we are yet to understand the neural basis of the group of behaviors required to carry this out. Here, we probed changes over time in the brain network associated with cognitive control (the dorsolateral prefrontal cortex, the dorsal posterior parietal cortex, and the dorsal anterior cingulate cortex) using both behavioral assays and functional brain imaging during a selective working memory task in 69 healthy participants within the age range 18–38 years (mean: 25, SD: ±6), assessed twice, 2 years apart. We aimed to explore the relationship of changing network activation and connectivity with behavioral tasks associated with cognitive control in this otherwise neurodevelopmentally stable group. We found that increased connectivity between frontoparietal cognitive control network regions during the working memory task was associated with improved memory and executive functions over the 2‐year period and that this association was not impacted by age, gender, or baseline performance. These results provide evidence that changes in the functional organization of the cognitive control brain network occur despite the absence of neurodevelopment, aging or targeted cognitive training effects, and could modulate cognitive performance in early to mid‐adulthood. Understanding how and why this change is occurring could provide insights into the mechanisms through which cognitive control ability is cultivated over time. This could aid in the development of interventions in cases where cognitive control is impaired.
Keywords: brain networks, cognitive control, connectivity, fMRI, neurodevelopment, working memory
1. INTRODUCTION
Cognitive control describes the ability to effectively and adaptively identify, manipulate and process relevant information in order to direct behavior towards one's internal goals (Badre, 2011). Evidence from functional magnetic resonance imaging (fMRI) has shown that coordinated exertion from a brain network comprising primarily the dorsolateral prefrontal cortex (DLPFC), the dorsal/posterior parietal cortex (DPC) and the dorsal anterior cingulate cortex (dACC) is responsible for a range of cognitive control behaviors such as working memory, executive function, attention, impulsivity, and information processing (Cole & Schneider, 2007; Niendam et al., 2012; Ochsner, Silvers, & Buhle, 2012). However, the relationship of this network, often referred to as the cognitive control network (CCN; alternately, the central executive network or executive control network [ECN]), to cognitive control ability is yet to be well defined except during neurodevelopment where whole brain and behavioral changes are occurring rapidly and in relation to age (Luna, Marek, Larsen, Tervo‐Clemmens, & Chahal, 2015 [review]). How changes in the functional recruitment and organization of this brain network underlie execution of these cognitive control abilities independent of age is not yet understood. Here, we focus on a group where age is no longer the primary driving factor in behavioral development in order to investigate if these brain‐behavior relationships are still changing over time.
Improvements in cognitive control abilities including working memory are thought to reach adult levels between 16 and 20 years of age (Clark et al., 2006; Luciana, Conklin, Hooper, & Yarger, 2005; Luna, Garver, Urban, Lazar, & Sweeney, 2004; Luna, Padmanabhan, & O'Hearn, 2010) and these parallel the stabilization of widespread change in activation and connectivity, as well as grey matter volume with only white matter growth continuing well into the twenties (Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; Gogtay et al., 2004; Rubia, 2013; Velanova, Wheeler, & Luna, 2008;). Despite this, there is reason to believe organization and functional brain changes could still be occurring well beyond 18 years of age driven by modification of environmental demands (Enriquez‐Geppert, Huster, & Herrmann, 2013). The early and mid‐adult years corresponds with the time of great change, in which individuals start gaining responsibility, gaining tertiary levels of education and entering and developing careers, and are also subject to range of complex social changes in their lives, all of which involve important cognitive skills such as decision making, social cognition and executive functions. Importantly it is also the period in which psychiatric illnesses most commonly emerge (Kessler et al., 2007). This link is particularly important in the CCN as cognitive control dysfunction is common across many of these illnesses despite varying affective symptoms. Discerning how this functional network operates and varies in healthy controls in this age group is the first step in understanding if this network is vulnerable to both negative and positive brain changes during this time and their behavioral correlates.
There have been a limited number of longitudinal studies which are able to establish a direct link between age or behavior and changes in the brain in this age group. A previous study by our group found that in healthy individuals aged 8–38 changes in grey matter volume were occurring and were correlated with cognitive control behavior even after controlling for the effects of age (Breukelaar et al., 2016). In terms of functional brain measures, very little change in activation or task performance has been observed beyond adolescence, but where changes did occur neural activation has been reported to be a better predictor of improvement in cognitive performance than age, suggesting others factors may be able to drive variability beyond adolescence (Koolschijn, Schel, de Rooij, Rombouts, & Crone, 2011; Ordaz, Foran, Velanova, & Luna, 2013). During this period, cognitive training has also been shown to impact both cognitive performance as well as the brain changes associated with them (Buschkuehl, Jaeggi, & Jonides, 2012). These studies demonstrate that brain and cognitive performance measures in this group are still susceptible to experience‐driven brain plasticity even though from a neurodevelopment perspective they have reached stability.
The brain is increasingly conceptualized as an interconnected network of brain regions (Fox et al., 2005) and a breakdown in this interconnectivity has been linked to functional deficits and observed across a number of mental disorders (Just, Keller, Malave, Kana, & Varma, 2012; Lynall et al., 2010; Tomasi & Volkow, 2012; Yue et al., 2013). In order to provide a comprehensive picture of how fMRI could provide a useful measure of the relationship between brain and behavior, functional connectivity between CCN areas was investigated in addition to activation. Previous functional connectivity analyses during both resting state and cognitive tasks, mostly using a graph theory approach, indicate that, with age, functional connectivity between regions that are further apart increases while short range connectivity decreases (Dosenbach et al., 2010; Edin, Macoveanu, Olesen, Tegner, & Klingberg, 2007; Fair et al., 2009; Hwang, Velanova, & Luna, 2010;). In addition, studies have shown increased frontoparietal connectivity is associated with disease recovery and cognitive training (Sharp, Turkheimer, Bose, Scott, & Wise, 2010; Voss et al., 2012). However, very little work has been done investigating how these changes in network connectivity are associated with behavioral performance in healthy individuals once age is not a factor driving brain or behavioral changes.
Here, we investigate how neural activation and connectivity patterns during a working memory task relate to changes in cognitive performance. Working memory performance has been shown to have a high transfer rate to performance on a selection of other tasks (Buschkuehl et al., 2012; Klingberg, 2010). Using this modality as our primary fMRI task was done in the hope to engage the brain circuitry that is central to a broad range of cognitive control task. In this study, we investigate connectivity and activation across key CCN regions of interests (ROIs), both at baseline and longitudinally, in a group of 69 healthy participants between 18 and 38 years of age who had two MRI scans with a 2 year interval between them. We hypothesized that changes in both activation and connectivity during this period would be correlated with change in cognitive performance but not necessarily with age.
2. METHODS
2.1. Participants and study design
A total of 138 healthy participants between the ages of 18 and 38 (mean age at first visit 25 ± 6 and 54% male) participated in a longitudinal fMRI study along with a well‐established cognitive battery (Mathersul et al., 2009; Williams et al., 2009) as part of a National Health & Medical Research Council funded project at the Brain Dynamics Centre, Westmead Institute for Medical Research and University of Sydney (Sydney, Australia). Participants were recruited using community advertisements such as flyers in public spaces, libraries, and other community spaces throughout the Westmead region as well as on broader community and university websites. Out of this full baseline sample (Breukelaar et al., 2016), 101 of these same participants returned for a follow‐up a median of 1.84 (range: 0.73–4.21) years after their initial scan where they completed the same protocol. Three participants were excluded due to the presence of mental illness at follow‐up and/or an incidental finding of structural abnormality in their brain. Of these, 69 participants had complete cognitive behavioral data, responded to in‐scanner task instructions correctly and had no motion artifacts in their structural scan or excessive motion outliers in their functional scan (see below). The 69 participants who were included in this study did not differ significantly from the full baseline sample on age, gender, Depression and Anxiety Stress Scale (DASS) measures, education level, or cognitive scores.
Participants were healthy volunteers with no reported history of neurological or psychiatric problems, and without any physical impediment that would impact ability to complete the study. Psychological and physical health were assessed using the Somatic and Psychological Health Report (SPHERE‐12) (Hickie et al., 2001) and the DASS (Lovibond & Lovibond, 1995). Participants had a median of 16 (11–18) years of education and were 89% right handed. DASS measures for the cohort were in the normal range (>90% of the sample with normal DASS scores at both visits) and not significantly different between the two visits (Visit 1: depression = 3.84 ± 5.09, anxiety = 2.61 ± 3.11 and stress = 5.98 ± 4.68; at follow‐ups, depression = 4.351 ± 5.46, anxiety = 2.37 ± 2.99, and stress = 6.08 ± 5.59).
Human subject participation was approved by the local Institutional Review Board; the University of Sydney Human Ethics Committee and the Western Sydney Local Health District Human Research Ethics Committee. After the study, procedures were fully explained in accordance with the ethical guidelines of the institutional review board, participants provided written consent.
2.2. Cognitive test battery
A validated computerized test battery, “WebNeuro” (Silverstein et al., 2007) consisting of 12 tests covering five cognitive domains was used to assess cognitive capacities in participants. The 12 WebNeuro tests take 30–40 min to complete. The reproducibility of WebNeuro computerized platforms has been established (Silverstein et al., 2007). These cognitive tests (in touchscreen format) have been shown to have excellent validity in terms of relationships with conventional paper‐and‐pencil measures of similar constructs of cognitive performance (Paul et al., 2005; Silverstein et al., 2007). The tests also have sound test retest reliability (Williams et al., 2005) and sound cross‐site reliability (Paul et al., 2007). WebNeuro age and sex norms across 9 decades have been established for a norm sample of 1,000 participants (Mathersul et al., 2009; Williams et al., 2009). Test instructions were delivered on‐screen immediately prior to test practice and test materials. An examiner provided assistance where necessary, in addition to an interactive, automated protocol used to ensure test comprehension. Response time and error measures for choice reaction time, switching of attention (information processing), forward digit span, memory recognition (memory) and go/no‐go (impulsivity) tasks as well as a continuous performance task (attention) and maze (executive function) are normalized for age and sex and converted to z‐scores relative to the normative mean of 0, with positive z‐scores reflecting better than average performance, and negative values reflecting poorer than average performance. Summary scores are then computed as the average of individual test scores for each test and labeled according to the domain they represent indicated in brackets above (Table SI, Supporting Information).
2.3. fMRI experimental design and image acquisition
We probed the cognitive control functional brain network using an event‐related 1‐back working memory fMRI task (Korgaonkar, Grieve, Etkin, Koslow, & Williams, 2013). During the task, a series of 120 letters (B, C, D, or G) in either yellow (target or nontarget trials) or white (distractor) were presented sequentially for 200 ms each with an interstimulus interval of 2,300 ms. Participants were required to press a button when the same yellow letter appeared twice in a row (1‐back target) ignoring any white letters that appeared in between (see Figure 1). Total task time was 5 min and 8 s. Reaction time and number of errors were used to establish whether or not participants had performed the task sufficiently. Participants had an average accuracy rate of 90% on the task and there was no significant difference in accuracy or response times between time points.
Figure 1.
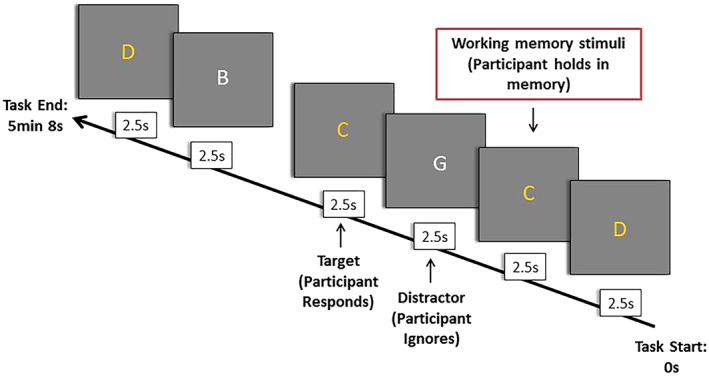
N‐back working memory task. An n‐back task was used inside the MRI scanner to elicit brain activity involved in working memory (Korgaonkar et al., 2013). A series of 120 letters (B, C, D, G) are presented for 200 ms in either yellow or white, with a 2,300 ms interval between then. Participants are required to press a button when the same yellow letter appears twice in a row (1‐back), ignoring any white letters that appear in between. A total of 60 letters are the nontarget stimuli or working memory stimulus, not to be responded to (do not appear twice in a row, in yellow) but held in the mind till the next letter appears; 20 letters are target or sustained attention stimuli, which the participant responds to (appear twice in a row, also in yellow); and 40 letters are distractor stimuli, to be ignored (in white). One fMRI volume per stimulus is acquired. Response time and errors are used to measure task performance [Color figure can be viewed at http://wileyonlinelibrary.com]
MRI was performed using an 8‐channel phased‐array head coil on a 3T GE Signa Twinspeed HDxT MR Scanner (GE Healthcare, Milwaukee, WI) at the Department of Radiology, Westmead Hospital. Functional MRI data were acquired using an echo‐planar imaging sequence with the following parameters: repetition time (TR) = 2,500 ms, echo time (TE) = 27.5 ms, matrix = 64 × 64, field of view (FOV) = 24 cm, flip angle = 90°, 120 volumes with a total scan time of 5 min and 8 s. Three dummy scans were also acquired at the start of every acquisition to allow magnetization to stabilize to steady state. T1‐weighted images were acquired in the sagittal plane using a 3D SPGR sequence (FOV = 256 mm, TR = 8.3 ms; TE = 3.2 ms; flip angle = 11°; TI = 500 ms; NEX = 1, and ASSET = 1.5; frequency direction: S/I) and used for normalization of the functional data. A total of 180 contiguous 1 mm slices were acquired with a 256 × 256 matrix, with an in‐plane resolution of 1 × 1 mm2 resulting in isotropic voxels in a total scan time of 7 min and 12 s.
2.4. MRI processing and first‐level analysis
The fMRI data were preprocessed and analyzed using the SPM8 software package (http://www.fil.ion.ucl.uk/spm) running on MATLAB R2012a (MathWorks, Natick, MA). The details of the preprocessing methodology have been described previously (Korgaonkar et al., 2013). In brief, all functional images were first corrected for rigid motion by realignment and unwarping. To account for any physiological noise, average signal using a mask from CSF and white matter was removed from the motion‐corrected fMRI time series and then smoothing performed using an 8‐mm Gaussian kernel. For normalization to stereotactic MNI space, the T1‐weighted SPGR images were normalized to standard space using the FMRIB nonlinear registration tool and the fMRI EPI data were coregistered to the T1 data using FMRIB linear registration tool. Normalization warps from these two steps were stored for use in functional to standard space transformations of the contrast maps. A general linear model framework was used to model the BOLD signal for first level analyses for each participant and included three regressors; one for each experimental condition (target, non‐target, and distractor trials) convolved using canonical hemodynamic response function. We defined contrasts for nontargets (working memory stimuli) versus baseline, our primary condition of interest to assess working memory function, as well as sustained attention (targets) versus baseline and distractor versus baseline which were not of interest in this particular study.
Before analyses, we screened for gross motion artifacts in our dataset using the Artifact Detection Tools (ART, http://www.nitrc.org/projects/artifact_detect/). The motion parameters (three translation and three rotational) estimated during the realignment preprocessing stage were used to identify any problematic fMRI volumes in each participant's entire scan at both Visit 1 (T1) and Visit 2 (T2). A volume frame was defined as an outlier (artifact) if the head displacement in x, y, or z direction was greater than .5 mm from the previous frame, or if the global mean intensity in the volume was greater than 3 SDs from the mean image intensity for the entire scan (mean number of outliers across our cohort at T1: 3.35 ± 4.60, T2: 3.26 ± 5.08, max at T1: 28, T2: 29) (Chai, Ofen, Gabrieli, & Whitfield‐Gabrieli, 2014). Participant data (n = 5) with more than 25% outlier volumes were rejected from further analysis. Paired‐sample t‐tests results showed that participant movement was not significantly different at the two time points (p = .862).
2.5. Whole‐brain activation analysis
We first performed a one sample second level voxel‐wise t test of fMRI contrast images for the whole group to determine CCN activation during working memory using the nontargets versus baseline contrast (p < .05, family wise error [FWE] corrected). For the same contrast, contrast estimates for seven 10 mm radii spheres defining ROI for the CCN were extracted subject‐by‐subject. ROI coordinates were chosen based on a peak activations observed in a meta‐analysis by Niendam et al., 2012, of 193 functional neuroimaging studies of 2,832 healthy individuals aged 18–60 performing tasks across a range of executive function domains. These regions included the bilateral DLPFC (Left (L):‐40, 26, 28 and Right (R): 40, 30, 28), bilateral inferior and superior dorsal parietal cortices (DPC; L inferior:‐38, −52, 40 and R inferior: 38, −50. 42 (iDPC); L superior:‐28, −60, 44 and R superior: 28, −60, 44 (sDPC) and the dACC (−2, 16, 40). Extracted data were used for all further statistical analyses in SPSS.
2.6. Functional connectivity analysis
Connectivity within the CCN was investigated using a generalized psychophysiological interaction (gPPI) model run in the CONN functional connectivity toolbox (https://www.nitrc.org/projects/conn). A PPI model explores the physiological response (HRF convolved BOLD signal) in one region of the brain in terms of the context‐dependent response of another region; this effectively provides a measure of task‐modulated connectivity between two or more regions (Friston et al., 1997; O'Reilly, Woolrich, Behrens, Smith, & Johansen‐Berg, 2012). A standard PPI creates a psychological regressor as the product of the condition onset times and a weighting/contrast vector creating a psychological term that is only inclusive of the task conditions as a difference vector. However, generalized context‐dependent PPI (gPPI) model, used here, generates a regressor of each condition's onset times, in this case for each targets, nontargets and distractor time courses, which are individually convolved with the HRF, which has been shown to generate a more accurate model of the interaction between the conditions and neural activity (McLaren, Ries, Xu, & Johnson, 2012). These psychological regressors for each condition were then multiplied by the physiological regressor which is derived from the raw seed region's neural activity to create the interaction term. This interaction term was then modelled against the time course for the six remaining CCN ROIs to test for significant connectivity effects. First‐level contrasts were generated for the psychophysiological interaction at nontargets versus the psychophysiological interaction during rest. The seed region for our gPPI analysis was chosen based on the activation analysis which showed a strong relationship between executive function performance and information processing and strength of left iDPC activation (see Section 3). Fisher‐transformed correlation coefficients for each participant at both visits were extracted for the left inferior dorsal parietal cortex (L‐iDPC) connectivity to the remaining six CCN ROIs for 1‐back > rest and used for further analyses described below.
2.7. Statistical analysis
Analysis of age and gender relationships with baseline and longitudinal cognition, activation and connectivity were investigated using a GLM to test for sensitivity of cognition and activation differences to these covariates. Paired‐sample t tests were used to examine whether cognitive performance, brain activation, and network connectivity changed over the 2‐year period.
Correlations between baseline activation, connectivity and cognitive scores were also investigated. Change in brain measure and cognitive z‐scores were calculated by subtracting relevant measure at Time 1 (T1) from Time 2 (T2) and dividing by the exact number of years between scans adjusted to 2 years (Breukelaar et al., 2016). The relationship between these measures of change was investigated using Partial correlations, controlling for age. A secondary analysis was done on all significant longitudinal findings to see if T1 cognitive performance/connectivity could predict longitudinal change. In cases where this was found to be significant, we included relevant T1 performance/connectivity measure as an additional covariate in a sensitivity analysis measuring the impact of baseline performance on degree of longitudinal change. In addition, a mediation analyses was performed to test if change in connectivity mediated the difference between Time 1 and Time 2 performance.
For all analyses, Benjamini–Hochberg FDR corrections for multiple comparisons was applied at an adjusted significant level of p < .05 (Benjamini & Hochberg, 1995). For activation analysis, a total of five behavioral measures were correlated with activation in seven CCN ROIs resulting in a total of 35 tests. This resulted in an FDR corrected p value (or q‐value) of .004. For connectivity analyses, there were six possible connections throughout the network and these were correlated with five behavioral tests resulting in a total of 30 tests. This resulted in an FDR corrected p value of .007.
3. RESULTS
3.1. fMRI activation during the working memory task
Whole‐brain functional activation during the working memory task showed recruitment of our predefined CCN areas as seen in previous studies (FWE corrected p < .05; Figure 2 and Table 1).
Figure 2.
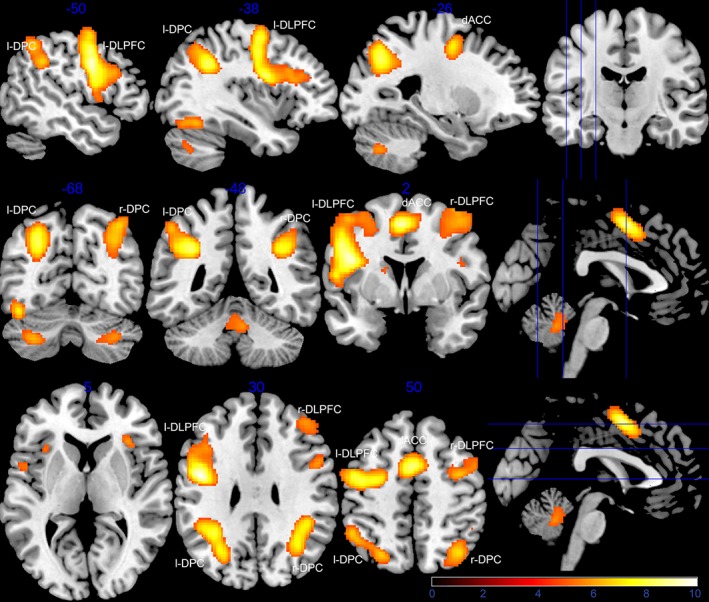
Activation of the CCN during working memory functional MRI task. Significant (p < .05, FWE corrected) clusters from the whole‐brain activation analysis during the working memory contrast (nontargets vs. rest) across all 69 subjects at Visit 1 is shown in three slices (top: sagittal, middle: coronal, bottom: axial) [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Brain region clusters with significant activation during the working memory task (non‐targets vs. rest)
| Brain region | Side | BA | ROIa | MNIb | Voxels | z‐scores | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Inferior frontal gyrus | L | 9 | DLPFC | −44 | −2 | 26 | 3,939 | Inf |
| Inferior parietal lobule | R | 40 | infDPC | 34 | −56 | 36 | 1,890 | 7.74 |
| Medial frontal gyrus | L | 6 | dACC | −4 | 2 | 52 | 1,034 | 7.71 |
| Supramarginal gyrus | L | 40 | DPC | −36 | −50 | 34 | 2,149 | 7.43 |
| Fusiform | L | NA | −42 | −66 | −16 | 415 | 6.57 | |
| Middle frontal gyrus | R | 6 | 38 | −2 | 52 | 742 | 5.98 | |
| Posterior cerebellum | L | NA | −30 | −64 | −38 | 226 | 5.9 | |
| Middle frontal gyrus | R | 10 | DLPFC | 36 | 40 | 26 | 393 | 5.71 |
| Posterior cerebellum | R | NA | 34 | −66 | −38 | 182 | 5.61 | |
| Insula | R | NA | 34 | 26 | 2 | 116 | 5.57 | |
| Anterior cerebellum | R | NA | 4 | −50 | −28 | 241 | 5.49 | |
| Inferior frontal gyrus | R | 9 | 52 | 8 | 30 | 220 | 5.48 | |
| Fusiform | R | 37 | 48 | −54 | −20 | 59 | 5.26 | |
| Insula | L | 13 | −34 | 20 | 2 | 28 | 5.09 | |
| Caudate | L | NA | −16 | 0 | 16 | 27 | 4.99 | |
| Middle frontal gyrus | L | 10 | −32 | 56 | 16 | 2 | 4.59 | |
Abbreviations: BA = Broadmann area; CCN = cognitive control network; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; DPC = dorsal/posterior parietal cortex; FWE = family wise error; MNI = Montreal Neurological Institute; L = Left; R = Right; ROI = regions of interest.
Cluster includes CCN regions of interest as identified in previous studies.
MNI coordinates are of peak P values at p < .05 FWE corrected.
3.2. Stability of cognitive performance, brain activation, and network connectivity over time
There were no significant differences in performance during the in‐scanner working memory fMRI task. Mean cognitive scores at both time points were centered on zero (Impulsivity Norm: −0.02; Attention Norm: −0.27, Information Processing: −0.03; Memory Norm: 0.49; Executive Function: 0.19) reflecting that this group accurately reflected average cognitive ability. Only cognitive measures of executive function performance significantly increased over the 2‐year time period (182% increase, p = .002). There were no significant changes in activation measures over the 2‐year time period. Similarly, there were no significant changes in connectivity measures from the left iDPC to the rest of the CCN over the 2‐year period.
3.3. Associations of baseline activation and connectivity with cognitive behavioral measures
At baseline, there were no significant age or gender relationships with cognition, activation, or connectivity within the CCN.
There were significant relationships between cognitive performance and activation of the CCN (Table 2). Lower activation in the CCN was correlated with better performance across three different cognitive domains: dACC with memory domain (p = .001); left iDPC with executive function (p = .002); and left sDPC with information processing performance (p = .003).
Table 2.
Cross‐sectional and longitudinal correlations of activations and connectivity in the CCN with cognitive performance while controlling for age
| Cross sectional | Longitudinal | |||||
|---|---|---|---|---|---|---|
| Activation | Connectivity—LinfDPCa | |||||
| ROI | Corr | p | Corr | p | Task | |
| dACC | −.392 | .001 | Memory | .391 | .000 | Executive function |
| Left DLPFC | NS | NS | NS | .419 | .000 | Executive function |
| .384 | .001 | Memory | ||||
| Right DLPFC | NS | NS | NS | .429 | .000 | Executive function |
| aLeft iDPC | −.352 | .003 | Executive function | NS | NS | NS |
| Right iDPC | NS | NS | NS | NS | NS | NS |
| Left sDPC | −.365 | .002 | Information processing | NS | NS | NS |
| Right sDPC | NS | NS | NS | NS | NS | NS |
Abbreviation: CCN = cognitive control network; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; DPC = dorsal/posterior parietal cortex; NS = not significant; ROI = regions of interest.
Note. NB: No significant results for cross‐sectional connectivity analyses or longitudinal activation analyses were observed and are not shown here.
Seed for gPPI.
There were no significant correlations between cognitive performance and connectivity at baseline.
3.4. Change in activation and connectivity with change in cognitive behavioral measures
There were no longitudinal associations between change in activation and change in cognitive performance that were significant after correcting for multiple comparisons.
Increases in connectivity between the left iDPC and the left and right DLPFC (p < .001) as well as the dACC (p < .001) were significantly correlated with improvements in the executive function domain. Increases in connectivity between the left iDPC and the left DLPFC were also significantly correlated with improvements in the memory domain (p = .001) (Figure 3).
Figure 3.
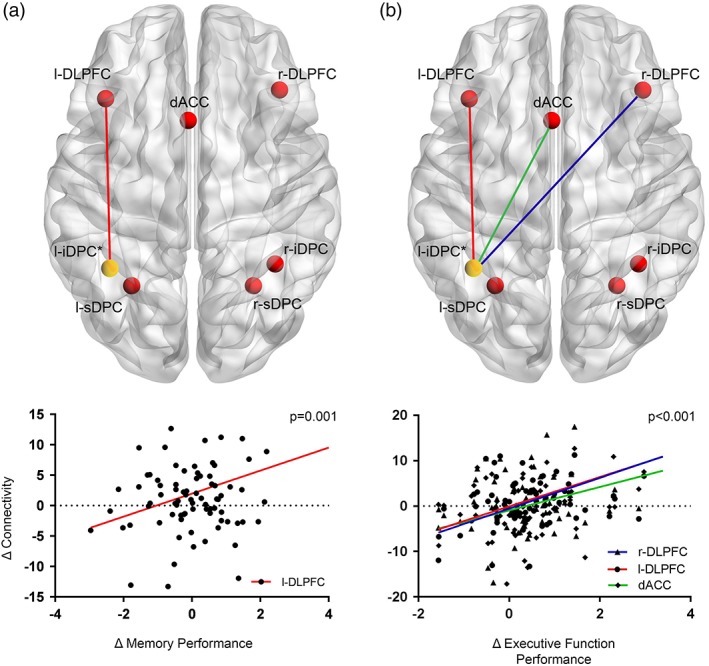
Change in connectivity during a working memory task with change in cognitive performance. (a) Increases in connectivity between the l‐iDPC and the l‐DLPFC are significantly correlated with improvements in the memory domain (p = .001). (b) Increases in connectivity between the left iDPC and bilateral DLPFC and dACC are all significantly correlated with improvements in executive function performance at a significance level of p < .001 [Color figure can be viewed at http://wileyonlinelibrary.com]
To explore these relationships further, a mediation analysis was done to investigate if changing connectivity mediated the improvements between T1 and T2 executive function and memory performance, as well as vice versa but no significant mediation effects were identified.
3.5. Relationship between baseline function and degree of change
We found Time 1 executive function performance to be significantly associated with change in executive function (corr. = −0.584, p < .001) and Time 1 memory performance to be significantly associated with change in memory performance (corr = −0.502, p < .001). In addition, Time 1 left inferior DPC to left and right DLPFC and iDPC to dACC connectivity were significantly correlated with their respective change measure (iDPC‐left DLPFC: corr. = − .604, p < .001; iDPC‐Right DLPFC corr. = −549, p < .001; iDPC‐dACC: corr. = −380, p = .001).
Based on these findings, we tested if the longitudinal associations reported above (Section 3.4) remained significant controlling for baseline performance, that is, with T1 executive function and T1 memory performance included as covariates in the correlation between connectivity change and performance change. The positive correlation between connectivity for the left iDPC and left and right DLPFC and executive function performance were maintained at a corr. = 0.368, p = .002 and corr. = 347, p = .004 significance level, respectively. The positive correlation between the left iDPC with left DLPFC and change in memory was also maintained with the inclusion of baseline memory at corr. =0.374, p = .001. The correlation with dACC connectivity was also maintained although with a drop in significance (corr. = 0.275, p = .024) below the FDR correction. Similarly, correlation of connectivity between the left iDPC and left DLFPC and memory also remained significant (p = .002) controlling for baseline memory performance.
In addition, we also tested if the relationship between changes in connectivity and behavioral performance remained significant controlling for baseline connectivity. As above, all findings remained significant except for the relationship between change in left iDPC‐left DLPFC connectivity and executive performance, which dropped to p = .014 (from a p < .001 significance rate without baseline connectivity included) below the FDR correction.
4. DISCUSSION
This study found that changes in the functional organization of the CCN while performing a working memory task directly relate to changes in cognitive control performance in a healthy population aged between 18 and 38 years. In this group, there was no detectible relationship between activation, connectivity or behavior with age or gender permitting us to instead capture novel relationships of change in cognitive control neural circuitry with cognitive abilities occurring over time. We found less activation in the dACC was associated with better memory performance while less activation in the DPC was associated with better executive function and information processing function. We then found that increased connectivity between the DPC and bilateral DLPFC as well as with the dACC was moderately correlated with improvements in the executive function domain. In addition, increased left‐sided parietal to DLPFC connectivity was associated with increased memory performance. These findings suggest that less neural recruitment of specific areas of the cognitive control brain network may relate to better performance cross sectionally. However, it additionally provides evidence, using longitudinal data, that increased connectivity between long‐range frontoparietal regions is correlated with improvements in cognitive control behaviors. These findings suggest that the CCN is changing over time along with behavioral performance, potentially implicating experience‐driven plasticity as having a role in cognitive development even in healthy individuals in early to mid‐adulthood.
In this study, we observed the level of activation in specific nodes of the CCN was correlated with cognitive performance in specific domains. Prior studies have also found that variation in dorsal/posterior parietal and prefrontal cortex activation are correlated to individual differences in working memory/cognitive control performance among adults (Klingberg, 2010; McNab & Klingberg, 2007; Todd & Marois, 2004; Vogel & Machizawa, 2004). We found that lower dACC activation was significantly correlated with greater memory performance, while lower left inferior DPC was significantly correlated with greater executive function performance and lower left superior DPC was significantly correlated with greater information processing performance. While given that greater activation in cognitive control areas has been previously associated with better cognitive performance throughout development, in healthy adults the association between activation especially through the parietal cortex has been found to be more complex (Finn, Sheridan, Kam, Hinshaw, & D'Esposito, 2010; Klingberg, Forssberg, & Westerberg, 2002). Several studies of inter individual differences in working memory and other cognitive control behavioral tasks have found that decreased parietal activation is associated with better working memory performance and additional studies of cognitive training effects have found a decrease in activation throughout the CCN is associated with better performance (Buschkuehl et al., 2012). While results in this field have been inconsistent and associations of performance with both directions of activity have been reported, one theory for the observed decrease in activation is the “neural efficiency theory” (Haier et al., 1988). This theory hypothesizes that when participants are doing well on a task lower neuronal recruitment is required. Previous studies have identified that this is particularly true for the level of activity observed in the posterior parietal cortex, which is thought to be particularly important in allocating storage space and limiting storage capacity during working memory tasks (McNab & Klingberg, 2007; Todd & Marois, 2004). The fMRI task used in this study was designed to have a low level of difficulty, and it is plausible that those with the highest working memory capacity, reflected in higher memory, executive function, and information processing behavioral task performances, would have had to expend the lowest level of neural energy to meet task demands (Vogel & Machizawa, 2004). Interestingly, this pattern mirrors that found in a previous study of grey matter volume in this group, where decreases in grey matter volume in left DLPFC, bilateral DPFC and dACC were associated with better performance in executive function and information processing tasks possibly associated with refinement of highly efficient brain areas (Breukelaar et al., 2016).
Analyses of longitudinal changes in connectivity outlined an opposite directional pattern as compared to the activation measures. We found that increased connectivity between the left parietal cortex and the dACC to be associated with an increase in general executive function performance. Whether or not this relates to the observed cross‐sectional relationships between dACC activation and memory performance or the inferior parietal cortex and executive function cannot be ascertained as no associations between change in activation and changes in connectivity were statistically observed (data not shown). It was also observed that increased connectivity between the left parietal and bilateral DLPFC was significantly correlated with improvements in the executive function domain, while left parietal to left dorsolateral prefrontal connectivity was associated with increase in memory performance. These findings suggest that interindividual differences in strength of long‐range connectivity in the CCN could relate to how participants improved on a range of cognitive control tasks. These findings were additionally maintained controlling for baseline executive function and memory performance suggesting that while baseline ability is strongly negatively correlated to degree of change in performance over time (i.e., those with lower baseline performance showed the biggest improvement), the relationship between change in connectivity and change in cognitive ability is not solely determined by baseline ability. This was not the case for the parietal‐cingulate connectivity which was affected substantially by the inclusion of a baseline covariate, suggesting low performers at Time 1 had more to gain from the strengthening of this connection (left iDPC to dACC) than high performers. While we were unable to detect what might be driving cognitive control improvements and/or their corresponding brain changes we suggest these changes are due to experience‐dependent plasticity for two main reasons. First, this entire study cohort improves in executive function suggesting there is a linear increase in function at least between these two time points. Second, the relationship between increasing frontoparietal connectivity and cognitive performance mirrors the impact seen in cognitive training, disease recovery, and neurodevelopmental findings (Damoiseaux et al., 2006; Fair et al., 2007; Jung & Haier, 2007; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008) that report stronger integration of long range connections in the CCN to be associated with improvements in function. This could indicate that frontoparietal connectivity is a central mechanism in modulating cognitive function even where the driving factor varies (age, neurodevelopment, cognitive training, and disease state) or is unknown. Understanding these relationships better has important implications for targeted nonpharmacological treatments, such as cognitive training, neurofeedback or brain stimulation, which could improve function in brain disorders, including psychiatric illness, where cognition is impaired (Enriquez‐Geppert et al., 2013; Keshavan, Vinogradov, Rumsey, Sherrill, & Wagner, 2014; Snowball et al., 2013).
However, one major limitation of the study is that we could not determine the specific factor/factors that may be driving these changes in brain and behavior and/or mediating their relationship. This means we cannot rule out that some of these could findings could be driven by state‐based differences at the two time points. We account for some of these factors by testing for differences in psychological measures (DASS scores), education and motion across time points. Also, both our behavioral tasks and previous observations of the PFC and parietal cortex activation have strong test–retest reliability (Crone et al., 2006; Williams et al., 2005) and we decided to correlate behavioral tasks done outside the MRI scanner with a different task in the scanner to limit some of these state‐based interactions. While we observed an association between an increase in frontoparietal connectivity and an increase in executive function and memory performance across two time points, it cannot be assumed this trajectory would continue over the longer term with multiple further time points. In addition, the nature of the fMRI task used to probe the cognitive control brain network could have likely introduced bias towards significant associations with predominantly memory and executive functioning and may not be generalizable across all cognitive tasks. It is likely that tasks designed for other cognitive domains could identify other interesting neural associations with these domains. The influence of “effort” on performance variability between time‐points is also a possible confound associated with task familiarity and increased ease of performance during the second visit. We attempted to account for this by a long time gap between visits and by using a task that would be easy to perform in the scanner and in which performance is high across all participants and across visits while still requiring cognitive engagement relative to rest. The strength of using such an easy task is that it is less likely to significantly advantage or disadvantage people based on age, education level or familiarity with the task, allowing us to capture age and performance independent effects. However, the ceiling effect of such an easy task limits the more subtle neural differences that can only be detected using a more difficult task (Vogan, Morgan, Powell, Smith, & Taylor, 2016). In addition, we chose to use composite scores (see Breukelaar et al., 2016 for more detail) in order to better reflect an overall ability to do a task (taking into account both accuracy and time to respond). However previous studies have indicated that in some cases response time alone can be a more sensitive measure task performance (Finn et al., 2010; Sheppard & Vernon, 2008). While some of these methodological decisions were made in order to answer the question at hand it is important to consider these issues when comparing with prior and future studies.
5. CONCLUSION
This study provides new insight into the potentially bidirectional relationship between CCN organization and cognitive behavior. Our findings indicate that gains in cognitive control performance in the healthy adult population directly relate to change in CCN activation and functional connectivity. As one of the first studies to investigate this longitudinally in the healthy young to mid‐adult population, it identifies new relationships between brain network changes and cognition that are not driven by age‐correlated neurodevelopment. This supports future work investigating the root of cognitive impairments and how differences in the functional organization of the cognitive control brain network may relate to this. A better understanding of the relationship between brain and behavior will provide the foundation to develop novel effective tools to repair and improve this relationship in cases where it is damaged, especially in psychiatric and neurological illnesses.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest in relation to the work presented in this manuscript.
Supporting information
Supplementary Figure S1. Age distribution of participants (years). Participants we aged 18–38 at visit 1 and returned approximately 2 years later (n = 69, 54% males). The age at each scan is indicated by a circle joined by a line.
Supplementary Figure S2. Activation of the cognitive control network during working memory functional MRI task at visit 2. Significant (p < .05, FWE corrected) clusters from the whole‐brain activation analysis during the working memory contrast (Nontargets vs rest) across all sixty‐nine subjects is shown in three slices (top: sagittal, middle: coronal, bottom: axial)
Supplementary Figure S3. Difference in brain activation between visit 1 and 2. Clusters represent remaining activation after minusing the visit 1 p < .05 FWE corrected T‐map from the visit 2 p < .05 FWE corrected T‐map using the imcalc function in SPM (top: sagittal, middle: coronal, bottom: axial)
Supplementary Table S1. Summary of the tasks comprising the WebNeuro test battery ‐ the constructs they assess, the individual test scores they generate and the summary score domain (Breukelaar et al., 2017).
Supplementary Table S2. Age, DASS measures, years of education and cognitive performance mean scores for baseline and longitudinal sample as well as a comparison between these groups.
Supplementary Table S3. Highest Level of Education
ACKNOWLEDGMENTS
This study was funded by the National Health and Medical Research Council (NHMRC) of Australia project grant (APP1008080) awarded to M.S.K., S.M.G. and L.M.W. S.M.G. acknowledges the support of the Parker Hughes Bequest, the Frecker Foundation and the Heart Research Institute. I.B. was supported by a University Postgraduate Award granted by the University of Sydney. M.S.K. was supported by a NHMRC Career Development Fellowship (APP1090148).
COMPLIANCE WITH ETHICAL STANDARDS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
A. Breukelaar I, Williams LM, Antees C, et al. Cognitive ability is associated with changes in the functional organization of the cognitive control brain network. Hum Brain Mapp. 2018;39:5028–5038. 10.1002/hbm.24342
Funding information University of Sydney, Grant/Award Number: University Postgraduate Award; Heart Research Institute; National Health and Medical Research Council, Grant/Award Number: APP1008080 Career Development Fellowship (APP1090148)
Contributor Information
Isabella A. Breukelaar, Email: isabella.breukelaar@sydney.edu.au.
Mayuresh S. Korgaonkar, Email: m.korgaonkar@sydney.edu.au.
REFERENCES
- Badre, D. (2011). Defining an ontology of cognitive control requires attention to component interactions. Topics in Cognitive Science, 3, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Statistical Methodology), 57, 289–300. [Google Scholar]
- Breukelaar, I. A. , Antees, C. , Grieve, S. M. , Foster, S. L. , Gomes, L. , Williams, L. M. , & Korgaonkar, M. S. (2016). Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Human Brain Mapping. 38, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschkuehl, M. , Jaeggi, S. M. , & Jonides, J. (2012). Neuronal effects following working memory training. Developmental Cognitive Neuroscience, 2, S167–S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, X. J. , Ofen, N. , Gabrieli, J. D. , & Whitfield‐Gabrieli, S. (2014). Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of Cognitive Neuroscience, 26, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, C. R. , Paul, R. H. , Williams, L. M. , Arns, M. , Fallahpour, K. , Handmer, C. , & Gordon, E. (2006). Standardized assessment of cognitive functioning during development and aging using an automated touchscreen battery. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 21, 449–467. [DOI] [PubMed] [Google Scholar]
- Cole, M. W. , & Schneider, W. (2007). The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage, 37, 343–360. [DOI] [PubMed] [Google Scholar]
- Crone, E. A. , Wendelken, C. , Donohue, S. , van Leijenhorst, L. , & Bunge, S. A. (2006). Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America, 103, 9315–9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux, J. S. , Rombouts, S. A. , Barkhof, F. , Scheltens, P. , Stam, C. J. , Smith, S. M. , & Beckmann, C. F. (2006). Consistent resting‐state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103, 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Nardos, B. , Cohen, A. L. , Fair, D. A. , Power, J. D. , Church, J. A. , … Schlaggar, B. L. (2010). Prediction of individual brain maturity using fMRI. Science, 329, 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin, F. , Macoveanu, J. , Olesen, P. , Tegner, J. , & Klingberg, T. (2007). Stronger synaptic connectivity as a mechanism behind development of working memory‐related brain activity during childhood. Journal of Cognitive Neuroscience, 19, 750–760. [DOI] [PubMed] [Google Scholar]
- Enriquez‐Geppert, S. , Huster, R. J. , & Herrmann, C. S. (2013). Boosting brain functions: Improving executive functions with behavioral training, neurostimulation, and neurofeedback. International Journal of Psychophysiology, 88, 1–16. [DOI] [PubMed] [Google Scholar]
- Fair, D. A. , Cohen, A. L. , Power, J. D. , Dosenbach, N. U. , Church, J. A. , Miezin, F. M. , … Petersen, S. E. (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5, e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Dosenbach, N. U. , Church, J. A. , Cohen, A. L. , Brahmbhatt, S. , Miezin, F. M. , … Schlaggar, B. L. (2007). Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America, 104, 13507–13512.17679691 [Google Scholar]
- Finn, A. S. , Sheridan, M. A. , Kam, C. L. , Hinshaw, S. , & D'Esposito, M. (2010). Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Buechel, C. , Fink, G. R. , Morris, J. , Rolls, E. , & Dolan, R. J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6, 218–229. [DOI] [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J. N. , Lusk, L. , Hayashi, K. M. , Greenstein, D. , Vaituzis, A. C. , … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier, R. J. , Siegel, B. V. , Nuechterlein, K. H. , Hazlett, E. , Wu, J. C. , Paek, J. , … Buchsbaum, M. S. (1988). Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence, 12, 199–217. [Google Scholar]
- Hickie IB, Davenport TA, Hadzi‐Pavlovic D, Koschera A, Naismith SL, Scott EM, Wilhelm KA (2001): Development of a simple screening tool for common mental disorders in general practice. The Medical Journal of Australia, 175, S10–7. [DOI] [PubMed] [Google Scholar]
- Hwang, K. , Velanova, K. , & Luna, B. (2010). Strengthening of top‐down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 15535–15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , & Haier, R. J. (2007). The Parieto‐frontal integration theory (P‐FIT) of intelligence: Converging neuroimaging evidence. The Behavioral and Brain Sciences, 30, 135–154 discussion 154‐87. [DOI] [PubMed] [Google Scholar]
- Just, M. A. , Keller, T. A. , Malave, V. L. , Kana, R. K. , & Varma, S. (2012). Autism as a neural systems disorder: A theory of frontal‐posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36, 1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A. M. , Uddin, L. Q. , Biswal, B. B. , Castellanos, F. X. , & Milham, M. P. (2008). Competition between functional brain networks mediates behavioral variability. NeuroImage, 39, 527–537. [DOI] [PubMed] [Google Scholar]
- Keshavan, M. S. , Vinogradov, S. , Rumsey, J. , Sherrill, J. , & Wagner, A. (2014). Cognitive training in mental disorders: Update and future directions. The American Journal of Psychiatry, 171, 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Angermeyer, M. , Anthony, J. C. , R DEG , Demyttenaere, K. , Gasquet, I. , … Ustun, T. B. (2007). Lifetime prevalence and age‐of‐onset distributions of mental disorders in the World Health Organization's world mental health survey initiative. World Psychiatry: Official Journal of the World Psychiatric Association, 6, 168–176. [PMC free article] [PubMed] [Google Scholar]
- Klingberg, T. (2010). Training and plasticity of working memory. Trends in Cognitive Sciences, 14, 317–324. [DOI] [PubMed] [Google Scholar]
- Klingberg, T. , Forssberg, H. , & Westerberg, H. (2002). Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience, 14, 1–10. [DOI] [PubMed] [Google Scholar]
- Koolschijn, P. C. , Schel, M. A. , de Rooij, M. , Rombouts, S. A. , & Crone, E. A. (2011). A three‐year longitudinal functional magnetic resonance imaging study of performance monitoring and test‐retest reliability from childhood to early adulthood. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31, 4204–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar, M. S. , Grieve, S. M. , Etkin, A. , Koslow, S. H. , & Williams, L. M. (2013). Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: First wave results from the iSPOT‐D study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond, P. F. , & Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behaviour Research and Therapy, 33, 335–343. [DOI] [PubMed] [Google Scholar]
- Luciana, M. , Conklin, H. M. , Hooper, C. J. , & Yarger, R. S. (2005). The development of nonverbal working memory and executive control processes in adolescents. Child Development, 76, 697–712. [DOI] [PubMed] [Google Scholar]
- Luna, B. , Garver, K. E. , Urban, T. A. , Lazar, N. A. , & Sweeney, J. A. (2004). Maturation of cognitive processes from late childhood to adulthood. Child Development, 75, 1357–1372. [DOI] [PubMed] [Google Scholar]
- Luna, B. , Marek, S. , Larsen, B. , Tervo‐Clemmens, B. , & Chahal, R. (2015). An integrative model of the maturation of cognitive control. Annual Review of Neuroscience, 38, 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna, B. , Padmanabhan, A. , & O'Hearn, K. (2010). What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition, 72, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall, M. E. , Bassett, D. S. , Kerwin, R. , McKenna, P. J. , Kitzbichler, M. , Muller, U. , & Bullmore, E. (2010). Functional connectivity and brain networks in schizophrenia. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul, D. , Palmer, D. M. , Gur, R. C. , Gur, R. E. , Cooper, N. , Gordon, E. , & Williams, L. M. (2009). Explicit identification and implicit recognition of facial emotions: II. Core domains and relationships with general cognition. Journal of Clinical and Experimental Neuropsychology, 31, 278–291. [DOI] [PubMed] [Google Scholar]
- McLaren, D. G. , Ries, M. L. , Xu, G. , & Johnson, S. C. (2012). A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab, F. , & Klingberg, T. (2007). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience, 11, 103. [DOI] [PubMed] [Google Scholar]
- Niendam, T. A. , Laird, A. R. , Ray, K. L. , Dean, Y. M. , Glahn, D. C. , & Carter, C. S. (2012). Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience, 12, 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz, S. J. , Foran, W. , Velanova, K. , & Luna, B. (2013). Longitudinal growth curves of brain function underlying inhibitory control through adolescence. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33, 18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly, J. X. , Woolrich, M. W. , Behrens, T. E. , Smith, S. M. , & Johansen‐Berg, H. (2012). Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Gunstad J, Cooper N, Williams LM, Clark CR, Cohen RA, Lawrence JJ, Gordon E (2007): Cross‐cultural assessment of neuropsychological performance and electrical brain function measures: additional validation of an international brain database. The International journal of neuroscience 117:549–68. [DOI] [PubMed] [Google Scholar]
- Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E (2005): Preliminary validity of “integneuro”: a new computerized battery of neurocognitive tests. The International journal of neuroscience 115:1549–67. [DOI] [PubMed] [Google Scholar]
- Rubia, K. (2013). Functional brain imaging across development. European Child & Adolescent Psychiatry, 22, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, D. J. , Turkheimer, F. E. , Bose, S. K. , Scott, S. K. , & Wise, R. J. S. (2010). Increased frontoparietal integration after stroke and cognitive recovery. Annals of Neurology, 68, 753–756. [DOI] [PubMed] [Google Scholar]
- Sheppard, L. D. , & Vernon, P. A. (2008). Intelligence and speed of information‐processing: A review of 50 years of research. Personality and Individual Differences, 44, 535–551. [Google Scholar]
- Silverstein, S. M. , Berten, S. , Olson, P. , Paul, R. , Willams, L. M. , Cooper, N. , & Gordon, E. (2007). Development and validation of a world‐wide‐web‐based neurocognitive assessment battery: WebNeuro. Behavior Research Methods, 39, 940–949. [DOI] [PubMed] [Google Scholar]
- Snowball, A. , Tachtsidis, I. , Popescu, T. , Thompson, J. , Delazer, M. , Zamarian, L. , … Cohen Kadosh, R. (2013). Long‐term enhancement of brain function and cognition using cognitive training and brain stimulation. Current Biology: CB, 23, 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M. C. , Kiehl, K. A. , Pearlson, G. D. , & Calhoun, V. D. (2007). Functional neural networks underlying response inhibition in adolescents and adults. Behavioural Brain Research, 181, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M. C. , Skudlarski, P. , Pearlson, G. D. , & Calhoun, V. D. (2009). Age‐related cognitive gains are mediated by the effects of white matter development on brain network integration. NeuroImage, 48, 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, J. J. , & Marois, R. (2004). Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature, 428, 751–754. [DOI] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Abnormal functional connectivity in children with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 71, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova, K. , Wheeler, M. E. , & Luna, B. (2008). Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex, 18, 2505–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan, V. M. , Morgan, B. R. , Powell, T. L. , Smith, M. L. , & Taylor, M. J. (2016). The neurodevelopmental differences of increasing verbal working memory demand in children and adults. Developmental Cognitive Neuroscience, 17, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, E. K. , & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. [DOI] [PubMed] [Google Scholar]
- Voss, M. W. , Prakash, R. S. , Erickson, K. I. , Boot, W. R. , Basak, C. , Neider, M. B. , … Kramer, A. F. (2012). Effects of training strategies implemented in a complex videogame on functional connectivity of attentional networks. NeuroImage, 59, 138–148. [DOI] [PubMed] [Google Scholar]
- Williams, L. M. , Mathersul, D. , Palmer, D. M. , Gur, R. C. , Gur, R. E. , & Gordon, E. (2009). Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. Journal of Clinical and Experimental Neuropsychology, 31, 257–277. [DOI] [PubMed] [Google Scholar]
- Williams, L. M. , Simms, E. , Clark, C. R. , Paul, R. H. , Rowe, D. , & Gordon, E. (2005). The test‐retest reliability of a standardized neurocognitive and neurophysiological test battery: “Neuromarker”. The International Journal of Neuroscience, 115, 1605–1630. [DOI] [PubMed] [Google Scholar]
- Yue, Y. , Yuan, Y. , Hou, Z. , Jiang, W. , Bai, F. , & Zhang, Z. (2013). Abnormal functional connectivity of amygdala in late‐onset depression was associated with cognitive deficits. PLoS One, 8, e75058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Age distribution of participants (years). Participants we aged 18–38 at visit 1 and returned approximately 2 years later (n = 69, 54% males). The age at each scan is indicated by a circle joined by a line.
Supplementary Figure S2. Activation of the cognitive control network during working memory functional MRI task at visit 2. Significant (p < .05, FWE corrected) clusters from the whole‐brain activation analysis during the working memory contrast (Nontargets vs rest) across all sixty‐nine subjects is shown in three slices (top: sagittal, middle: coronal, bottom: axial)
Supplementary Figure S3. Difference in brain activation between visit 1 and 2. Clusters represent remaining activation after minusing the visit 1 p < .05 FWE corrected T‐map from the visit 2 p < .05 FWE corrected T‐map using the imcalc function in SPM (top: sagittal, middle: coronal, bottom: axial)
Supplementary Table S1. Summary of the tasks comprising the WebNeuro test battery ‐ the constructs they assess, the individual test scores they generate and the summary score domain (Breukelaar et al., 2017).
Supplementary Table S2. Age, DASS measures, years of education and cognitive performance mean scores for baseline and longitudinal sample as well as a comparison between these groups.
Supplementary Table S3. Highest Level of Education


