Abstract
Motor functions are supported through functional integration across the extended motor system network. Individuals following stroke often show deficits on motor performance requiring coordination of multiple brain networks; however, the assessment of connectivity patterns after stroke was still unclear. This study aimed to investigate the changes in intra‐ and inter‐network functional connectivity (FC) of multiple networks following stroke and further correlate FC with motor performance. Thirty‐three left subcortical chronic stroke patients and 34 healthy controls underwent resting‐state functional magnetic resonance imaging. Eleven resting‐state networks were identified via independent component analysis (ICA). Compared with healthy controls, the stroke group showed abnormal FC within the motor network (MN), visual network (VN), dorsal attention network (DAN), and executive control network (ECN). Additionally, the FC values of the ipsilesional inferior parietal lobule (IPL) within the ECN were negatively correlated with the Fugl‐Meyer Assessment (FMA) scores (hand + wrist). With respect to inter‐network interactions, the ipsilesional frontoparietal network (FPN) decreased FC with the MN and DAN; the contralesional FPN decreased FC with the ECN, but it increased FC with the default mode network (DMN); and the posterior DMN decreased FC with the VN. In sum, this study demonstrated the coexistence of intra‐ and inter‐network alterations associated with motor‐visual attention and high‐order cognitive control function in chronic stroke, which might provide insights into brain network plasticity following stroke.
Keywords: fMRI, independent component analysis, motor deficits, resting‐state networks, stroke
1. INTRODUCTION
Stroke often results in persistent disability, particularly due to motor impairments (Stinear, 2010). Patients typically show hemiplegia, and more than 50% of patients are left with residual motor deficits, especially deficits affecting an upper limb (Duncan, Goldstein, Matchar, Divine, & Feussner, 1992). However, exactly how stroke lesions lead to persistent motor impairments remains difficult to understand, and this limits the clinical assessment and restricts the ability to select homogenous groups for targeted clinical treatments.
Abnormalities of brain function following stroke have previously been demonstrated using a range of neuroimaging techniques (Calautti & Baron, 2003; Elsner, Kugler, Pohl, & Mehrholz, 2013; Finnigan, Walsh, Rose, & Chalk, 2007). Previous task‐based functional magnetic resonance imaging (fMRI) studies of stroke and motor impairments have often focused on differences in brain activity associated with the performance of motor demanding tasks (Cramer et al., 1997; Pineiro, Pendlebury, Johansen‐Berg, & Matthews, 2002; Sun et al., 2013). As patients are often impaired on this type of task, the neural changes have generally been interpreted as representing an adaptive or compensatory response to the effects of brain injury, namely, motor function reorganization. Although primary sensorimotor areas are the most frequently reported regions involved in the reorganization of motor function, higher order cognitive control regions, such as frontal and parietal regions, are often affected after a subcortical motor pathway stroke. For example, fMRI studies showed overactivations in the prefrontal and posterior parietal regions with the movement of a paretic hand, and these overactivations decreased in parallel with motor recovery (Marshall et al., 2000; Ward, Brown, Thompson, & Frackowiak, 2003). However, many stroke survivors with severe motor deficits cannot complete even the simple tasks, and the results may be confounded due to differences in movement performance.
Resting‐state fMRI has the advantage that it does not require the performance of any specific tasks during functional neuroimaging acquisition. Therefore, a growing number of studies are employing this technique to map the spatio‐temporal covariance structure of networks of spontaneous brain activity to investigate the reorganization of motor function following stroke (Golestani, Tymchuk, Demchuk, Goodyear, & Group, 2013; Wang et al., 2010; Yin et al., 2012). Many resting‐state studies have accordingly shown the altered functional connectivity of both sensorimotor and higher order cognitive control regions (Liu, Tian, Qin, Li, & Yu, 2016; Park et al., 2011; Zhao et al., 2016). In addition, many previous studies that described tasks and seed‐based FC engaged more than basic sensorimotor processes, such as the abnormal activation or FC in temporal and occipital regions (Park et al., 2011; Ward et al., 2003; Zhao et al., 2016). However, the link between sensorimotor regions and these nonsensorimotor regions in motor deficits after stroke remains unclear. In particular, it is impossible to determine which affected nonsensorimotor regions contribute to which network based on conventional task and seed‐based FC research.
Motor dysfunction in stroke survivors may be viewed as a system‐level disruption of brain networks based on previous network studies in patients with stroke (Wang et al., 2014a, 2010; Yin et al., 2014). The interaction between brain regions within a network (i.e., their functional connectivity), and the interactions between networks are both important for efficient motor function. It now appears that a detailed understanding of these networks may be particularly important for understanding motor reorganization following stroke. Furthermore, in combination with behavioral analyses, it is possible to determine which connections in a widely distributed brain network are most relevant for clinical outcome and neurorehabilitation.
Two approaches are commonly used to construct functional brain networks. One is a seed‐based FC analysis approach that measures the similarity between the time series of a given seed and that of other brain regions in an ROI‐wise (region of interest, ROI) way (Rosazza, Minati, Ghielmetti, Mandelli, & Bruzzone, 2012; van de Ven, Formisano, Prvulovic, Roeder, & Linden, 2004). However, this method needs to identify the ROIs based on prior knowledge. In contrast, independent component analysis (ICA) is a data‐driven approach to delineating spatially independent patterns of coherent signals (Roosendaal et al., 2010; Skidmore et al., 2012; Smith et al., 2009; Wang et al., 2014b). Compared with seed‐based FC analysis, the distinct advantage of ICA is that it allows a direct and fairly straightforward measure of interactions within and between multiple brain networks. It has been demonstrated to be efficient for investigating the intra‐ and inter‐network connectivity patterns in patients with Alzheimer's disease, Parkinson's disease, and so on (Song et al., 2013; Tessitore et al., 2012). However, relative to a priori seed‐based FC studies, ICA studies of both intra‐ and inter‐network connectivity changes of motor deficits following stroke have rarely been investigated. Wang et al. (2014a) first reported functional connectivity alterations in and between multiple networks by using ICA, but their enrolled subjects were a group of subcortical stroke patients with well‐recovered motor function. The interactions of multiple networks in stroke patients who are suffering severe motor impairments are unknown. More importantly, the clarification of the impairment and compensation patterns of multiple networks in stroke motor dysfunction would provide valuable information for more targeted treatments.
This study is an exploratory analysis that tests the following hypotheses. First, subcortical stroke with pure motor deficits is associated with abnormalities of FC in multiple brain networks, including the sensorimotor network and motor cognitive networks that support motor function. Beyond these intra‐network changes, inter‐network FC alterations are also associated with motor dysfunction as a result of stroke. Last, changes in FC are correlated with impairments in motor function, which we test by investigating whether intra‐ and inter‐network FC changes correlate with abnormalities in upper limb motor performance. Taken together, our results, for the first time, reveal abnormalities in intra‐ and inter‐network FC that describe motor deficits after motor stroke and demonstrate how sustained changes in network function relate to the adaption of motor behavior.
2. METHODS
2.1. Subjects
A total of 35 first‐episode left subcortical stroke patients were recruited from Huashan Hospital, which is affiliated with Fudan University. In addition, 34 age‐, gender‐, and handedness matched control subjects were selected from the local community. All patients underwent clinical assessments, including the Mini‐Mental State Examination (MMSE) and the Fugl‐Meyer Assessment (FMA). All patients fulfilled the following inclusion criteria: (a) first‐ever left subcortical stroke; (b) at least 3 months had passed since the stroke; (c) normal cognitive ability with a score of ≥27 on the MMSE; (d) pure motor deficits; and (e) right handedness. Global cognition of all participants was evaluated by an experienced therapist using the Mini‐Mental State Examination (MMSE) on the admission day. To assess the motor performance of patients, a battery of motor‐specific scales, which have been validated for each stage in stroke survivors, was administered. The FMA scale evaluates the ability to isolate movements at each joint and the influence of unwanted synergies during movement execution, which represents a reduction in impairment rather than in compensatory pattern. Thus, the changes in impairment reflect substantial recovery at neurobiological and behavioral levels. Only the hand and wrist domain in FMA (FMA‐HW), which includes 5 wrist items and 7 hand items (the maximal score is 24), was selected as one of our primary outcome measures.
Exclusion criteria for all subjects were as follows: (a) a contraindication to MRI (pacemaker, metallic foreign bodies, and severe claustrophobia); (b) severe quadriplegia; (c) a history of neurological and psychiatric disorders; and (d) a history of hand dysfunction.
Two patients were excluded due to excessive motion during the MRI scanning. The final subjects included 33 patients (29 males and 4 females, age ± SD: 59.88 ± 8.60 (years)) and 34 control subjects (26 males and 8 females, age ± SD: 59.76 ± 10.05). We manually outlined the profiles on T2‐weighted images slice by slice using the software MRIcron (http://www.mricro.com). Additionally, a map of the lesions overlapped across all stroke patients is shown in Figure 1. In addition to descriptive purposes, the lesion masks were used to evaluate the lesion volume of the stroke patients. Moreover, the individual masks were used to exclude intralesion voxels from ICA. There were no significant differences between the two groups in age (t = 0.05, p = .96) or gender (t = 1.48, p = .22). The stroke volume was not correlated with the FMA‐HW scores across all patients (Spearman correlation: r = −.083, p = .647). The clinical characteristics and demographic data are summarized in Supporting Information, Table I.
Figure 1.
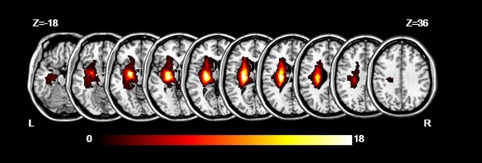
The map of lesions overlapped across 33 stroke patients. The color bar indicates the number of subjects with lesions in each voxel. Z axis from Z = −18 to Z = 36 in MNI coordinates, with an incremental interval of 6. L = left; R = right [Color figure can be viewed at http://wileyonlinelibrary.com]
This study was approved by the Institutional Ethics Committee of East China Normal University, and all participants signed informed consent forms before undergoing the MRI examination.
2.2. MRI data acquisition
All data were acquired with a Siemens Trio 3.0 Tesla MRI scanner (Siemens, Erlangen, Germany) at the Shanghai Key Laboratory of Magnetic Resonance, East China Normal University. We used a 12‐channel phased‐array head coil with foam padding and headphones to restrict head motion and scanner noise. Resting‐state functional images were acquired using an echo‐planar imaging (EPI) sequence (30 transverse slices, slice thickness/gap = 4 mm/0.8 mm, matrix = 64 × 64, repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, field of view = 220 mm × 220 mm, number of volumes = 240). T1‐weighted anatomical images in a sagittal orientation were obtained using magnetization prepared by rapid gradient echo sequence (MPRAGE) (192 slices covered the whole brain, slice thickness/gap = 1 mm/0.5 mm, repetition time = 1,900 ms, echo time = 3.42 ms, field of view = 240 mm × 240 mm, matrix = 256 × 256). To identify the location of the lesion, T2‐weighted images were collected using a turbo‐spin‐echo sequence: (30 axial slices, thickness = 5 mm, no gap, repetition time = 6,000 ms, echo time = 93 ms, field of view = 220 mm × 220 mm, matrix = 320 × 320). During the resting‐state functional MRI collection, the participants were instructed to close their eyes but to remain awake while trying not to think about anything specific. All images were visually inspected to ensure that they did not contain MRI artifacts or excessive movement before analysis.
2.3. Preprocessing of fMRI data
Preprocessing of the imaging data was performed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and Data Processing Assistant for Resting‐State fMRI (DPARSF, http://resting-fmri.sourceforge.net). The following steps were adopted: (a) the first 10 volumes were discarded to allow the signal to reach equilibrium and the participants adapt to the environment, leaving 230 images for further process; (b) the time delay between slices and rigid‐body head movement during scans were corrected. Excessive motion was defined as more than 2.0 mm of translation or greater than a 2.0° rotation in any direction and two patients were excluded, thus 33 patients and 34 control subjects were included for further analysis; (c) structural images of each subject were then co‐registered to the mean functional image, and segmented into gray matter, white matter, and cerebrospinal fluid; (4) the functional images were normalized to the Montreal Neurological Institute space following motion correction using a lesion mask (Andersen, Rapcsak, & Beeson, 2010) and unified segmentation with both a linear affine transform and a nonlinear deformation (Ashburner & Friston, 2005) and, and they were then resampled to a 3‐mm isotropic voxel; (5) the normalized images were then smoothed using a 6 mm full‐width half‐maximum Gaussian kernel.
2.4. Identification of resting‐state networks
We conducted ICA via MICA (http://www.nitrc.org/projects/cogicat/), which implements Subject Order‐Independent Group ICA (SOI‐GICA). The traditional temporal concatenated ICA adopts three steps of principle component analysis (PCA) for data reduction, which could result in inconsistent and variable components when different subject orders are used. The SOI‐GICA fixes the problem by implementing the algorithm multiple times with randomized initial values and different subject orders. Then, the multiple results were integrated to form the final output. The toolbox supports a GICA approach that first concatenates the individual data across time and subsequently computes the subject‐specific components and time courses. The toolbox performed the analysis in three stages: (a) data reduction, (b) application of the ICA algorithm, and (c) back‐reconstruction for each individual subject. We performed the GICA 100 times, and the data were decomposed into 30 components; 11 components were identified as RSNs, which were described previously via visual inspection and spatial correlation coefficients between the selected components and the templates reported (Smith et al., 2009). For each given template, a spatial cross‐correlation coefficient between each thresholded component volume and the template was calculated using Pearson correlation. The per‐component correlation coefficients obtained this way were sorted in decreasing order, and the three component candidates with the highest correlation coefficient scores were presented to the researcher together with the corresponding volume slices. Single subject time courses and spatial maps of components were then computed through back‐reconstruction and converted into z scores, which reflect the degree to which the time series of a given voxel correlates with the mean time series of the component to which it belongs (Wang et al., 2014a).
2.5. Intra‐network functional connectivity analysis
Each component of all subjects, including both patients and healthy controls, was entered into a one‐sample t test using FDR correction (p < .005) and a cluster size of >100 to create a sample‐specific component map and a network mask. A two‐sample t test was then conducted to compare the intra‐network FC difference between the two groups for each component within the corresponding network mask (Wang et al., 2014b; Zhang et al., 2015) in a voxel‐wise manner with stroke volume as a covariate (AlphaSim corrected significance level of p < .05 was obtained by clusters with a minimum size of 70 voxels and individual voxel height threshold of p < .01 according to a Monte Carlo simulation (1000 simulations, FWHM = 12 mm) within the network mask using dpabi software (Yan, et al., 2016).
2.6. Inter‐network functional connectivity analysis
To examine the pattern of FC changes between functional networks, the temporal correlation among different RSNs was computed. First, the time‐course of each RSN was extracted from the ICA procedure. Then, the time courses of each pair of the 11 RSNs were used to calculate the temporal correlation, namely the functional network connectivity (FNC), and normalized with Fisher r‐to‐z transformation. A two‐sample t test was used to compare group differences in the FNCs (p < .05, uncorrected).
2.7. Correlation analysis
To determine the relationship between FCs with significant between‐group differences and clinical assessments, we performed a nonparametric Spearman correlation analysis between the FCs and FMA‐HW scores in stroke patients. Specifically, for the intra‐network FC, those brain regions based on the location of voxels showing significant differences in FC between the two groups were extracted as masks. Then by averaging the z scores of the FC value of each voxel within the mask, the FC value for each brain region was obtained and correlated to the corresponding FMA‐HW scores of the stroke patients. For the inter‐network FC, correlation analysis was performed between the FNCs with significant between‐group differences and FMA‐HW scores across all patients. A value of p < .05 was considered statistically significant.
3. RESULTS
3.1. Resting‐state networks
Out of 30 components, 11 components were identified, which were in line with the previous studies (Wang et al., 2014b; Zhao et al., 2017; Zuo et al., 2010) (Figure 2). The sensorimotor network (SMN) mainly includes the bilateral precentral gyrus (PreCG) and postcentral gyrus (PostCG). The motor network (MN) mainly includes the bilateral PreCG and supplementary motor areas (SMA). The medial visual network (MVN) mostly encompasses the occipital pole. The lateral visual network (LVN) is composed of the bilateral part of the occipital lobe. The auditory network (AN) primarily includes the bilateral superior temporal gyrus. The dorsal attention network (DAN) includes the interparietal sulcus and the junction of the precentral superior frontal sulcus bilaterally. The anterior default mode network (aDMN) mainly involves the medial prefrontal (mPFC) and anterior cingulate cortex (ACC), and the posterior DMN (pDMN) primarily includes the posterior cingulate cortex (PCC)/precuneus (Pcu), as well as the bilateral lateral parietal cortex. The left and right frontoparietal network (L/RFPN) involves the dorsolateral PFC and posterior parietal cortex. In addition, the executive control network (ECN) mainly involves the bilateral dorsolateral PFC and the lateral parietal cortex.
Figure 2.
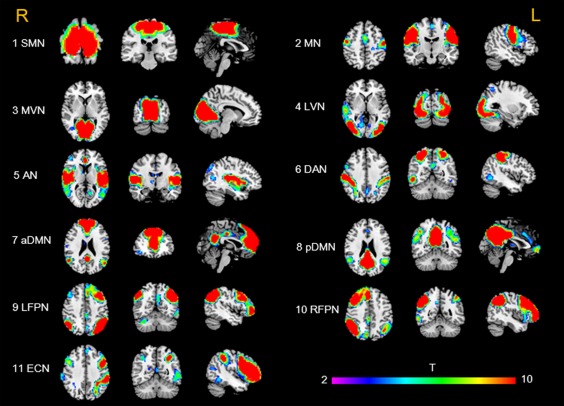
Cortical representations of resting‐state networks identified via independent component analysis. The color bar indicates the T values ranging from 2 to 10. SMN = sensory motor network; MN = motor network; MVN = medial visual network; LVN = lateral visual network; AN = auditory network; DAN = dorsal attention network; aDMN = anterior default mode network; pDMN = posterior default mode network; LFPN = left frontoparietal network; RFPN = right frontoparietal network; ECN = executive control network [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Altered FC within RSNs
Compared with healthy controls, patients showed significant FC differences in multiple RSNs (Figure 3 and Table 1). For the MN, FC decreased in the contralesional precentral (M1) and postcentral gyrus (S1) (Figure 3a). For the MVN, FC decreased in the bilateral cuneus/calcarine (Figure 3b) but increased in the ipsilesional fusiform/lingual gyrus (Figure 3c); FC decreased in a similar region (ipsilesional fusiform/lingual gyrus) of the LVN (Figure 3d). For the ECN, FC increased in the ipsilesional IPL (Figure 3e). For the DAN, FC decreased in the ipsilesional IPL (Figure 3f).
Figure 3.
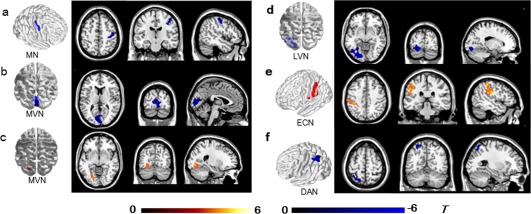
Brain regions with significant differences in intra‐network functional connectivity (FC) between stroke patients and controls (a–f). Warm and cold colors indicate regions with higher or lower intranetwork FC in stroke patients compared with healthy controls (p < .05, AlphaSim corrected). The colored bars indicate the T values of the two‐sample t test. MN, motor network; MVN, medial visual network; LVN, lateral visual network; DAN, dorsal attention network; ECN, executive control network [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Brain regions with significant differences in intra‐network functional connectivity (FC) between stroke patients and healthy controls
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Regions | Side | Brodmann area | X | Y | Z | Peak T value | Cluster size |
| MN | |||||||
| Precentral gyrus | R | BA4 | 48 | −9 | 51 | −4.94 | 76 |
| Postcentral gyrus | R | BA3 | 50 | −14 | 51 | −4.37 | 43 |
| MVN | |||||||
| Cuneus/calcarine | L/R | BA18/17 | 3 | −96 | 15 | −5.08 | 215 |
| Fusiform/lingual gyrus | L | BA18/19 | −30 | −63 | −3 | 5.04 | 78 |
| LVN | |||||||
| Fusiform/lingual gyrus | L | BA19/18 | −18 | −84 | −12 | −5.73 | 191 |
| DAN | |||||||
| Inferior parietal lobule | L | BA40 | −57 | −27 | 42 | −4.54 | 95 |
| ECN | |||||||
| Inferior parietal lobule | L | BA40 | −36 | −45 | 57 | 5.08 | 98 |
Note. Abbreviations: DAN = dorsal attention network; ECN = executive control network; LVN = lateral visual network; MVN = medial visual network; RFPN = right frontoparietal network; SMN = sensory motor network.
3.3. Altered inter‐network FC
In stroke patients, decreased FNC was detected between the MN and LFPN, between the DAN and LFPN, between the MVN and pDMN, between the LVN and pDMN, and between the RFPN and ECN. In contrast, increased FNC was detected between the RFPN and aDMN, and between the RFPN and pDMN (p < .05, uncorrected). No FNCs survived when the p values were corrected for multiple comparisons (FDR, p < .05). Between‐group differences in inter‐network FC are shown in Figure 4 and Table 2.
Figure 4.
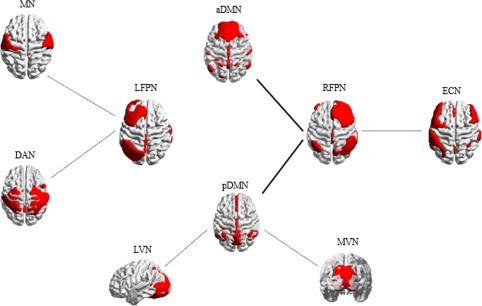
Inter‐network connectivity differences between stroke patients and healthy controls. The dashed lines indicate decreased inter‐network functional connectivity in stroke patients. The solid lines denote increased connectivity in stroke patients (p < .05, uncorrected). MN = motor network; MVN = medial visual network; LVN = lateral visual network; DAN = dorsal attention network; ECN = executive control network; aDMN = anterior default mode network; pDMN = posterior default mode network; RFPN = right frontoparietal network; LFPN = left frontoparietal network [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
FNCs showing the significant differences between patient group and healthy groups (HCs)
| Network | HCs (mean ± std) | Patient group | p value | t value |
|---|---|---|---|---|
| MN‐LFPN | −0.06 ± 0.21 | −0.18 ± 0.21 | .02 | −2.28 |
| LVN‐pDMN | 0.35 ± 0.30 | 0.18 ± 0.26 | .01 | −2.42 |
| MVN‐pDMN | 0.38 ± 0.27 | 0.24 ± 0.25 | .03 | −2.19 |
| DAN‐LFPN | 0.03 ± 0.24 | −0.12 ± 0.21 | .01 | −2.60 |
| aDMN‐RFPN | 0.17 ± 0.23 | 0.31 ± 0.24 | .02 | 2.37 |
| RFPN‐pDMN | 0.25 ± 0.22 | 0.43 ± 0.23 | .002 | 3.21 |
| RFPN‐ECN | 0.39 ± 0.21 | 0.20 ± 035 | .01 | −2.62 |
Note. Abbreviations: aDMN = anterior default mode network; DAN = dorsal attention network; ECN = executive control network; FNC = functional network connectivity; LFPN = left frontoparietal network; LVN = lateral visual network; MN = motor network; MVN = medial visual network; pDMN = posterior default mode network; RFPN = right frontoparietal network.
3.4. Correlation between FC and FMA‐HW scores
In the stroke group, a significant negative correlation between the FC of the IPL within ECN and FMA‐HW scores was revealed (uncorrected, r = −.382, p = .028; Figure 5). There were no significant correlations between FNCs with significant between‐group differences and FMA‐HW scores across all patients.
Figure 5.
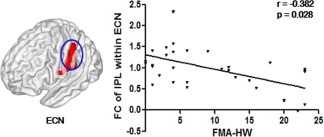
Correlation results between altered FC within the ECN and the FMA‐HW scores of the stroke patients. The vertical axis indicates the mean z‐score of FC, and the horizontal axis indicates the FMA (hand + wrist) scores [Color figure can be viewed at http://wileyonlinelibrary.com]
Additionally, we also used a predetermined network atlas (https://brainnexus.com/resting-state-fmri-templates/) to reanalyze the intranetwork FC and the correlation between FC and FMA‐HW scores in a voxel‐based way. The results were consistent with our main findings except for the bilateral precentral (M1) gyrus within SMN (Supporting Information, Figures 1 and 2).
4. DISCUSSION
To our knowledge, this is the first exploratory study to investigate intra‐ and inter‐network FC and their correlations with hand motor deficits in patients with subcortical stroke. Using an ICA approach, 5 of the 11 well‐known RSNs were found to be abnormal and involved visual, attention, motor and motor executive control. Moreover, the FC values of the ipsilesional IPL within the ECN showed a negative correlation with FMA‐HW scores across all patients with chronic stroke. On the other hand, chain alterations of the inter‐network functional coupling in motor‐visual attention and high‐order cognitive control networks were found in stroke patients. These findings support our hypothesis and are particularly important for understanding the pathophysiology of stroke because motor reorganization is a common mechanism of cortical injury following subcortical stroke, which disrupts the related networks that support motor behavior.
Our connectivity analysis revealed dysfunction after subcortical lesions in the motor network. The most consistent finding in previous resting‐state studies is that patients with motor impairment after stroke show decreased interhemispheric connectivity of the ipsilesional M1 with the contralesional SMC (Park et al., 2011; Zheng et al., 2016). Compared to our previous M1‐based resting‐state FC study (Yin et al., 2012), although both studies enrolled stroke patients with a similar left motor pathway dysfunction, the FC changes in the pattern of the MN are somewhat different, which is possibly due to the adoption of different FC analysis methods. The ROI‐based FC analyses focus on FC of other brain regions with the seed of M1, while the data‐driven ICA approach focuses on the entire complexity of the network.
With the exception of the MN, alteration of FC also appeared in another sensory network (i.e., the visual network). The visual cortex receives top–down modulation from frontal and parietal areas in relation to visual attention (Bressler, Tang, Sylvester, Shulman, & Corbetta, 2008), and transcranial magnetic stimulation (TMS) showed that the disruption of top‐down signals from frontal and parietal regions to the visual cortex during spatial attention can cause behavioral deficits in healthy subjects (Ruff et al., 2008, 2006). In previous motor‐task fMRI and FC studies, the abnormal activity of visual occipital regions has been generally regarded as a noneffective compensatory reorganization in motor deficits after stroke or has been ignored (Park et al., 2011; Ward et al., 2003). Our previous study using effective connectivity analysis reported a higher influence from the ipsilesional M1 to the ipsilesional occipital lobe in the stroke group with more severe paralysis and regarded it as compensation for the impaired connectivity to the ipsilesional somatosensory cortex (Zhao et al., 2016). We also found reduced FC of the ipsilesional inferior parietal lobule within the DAN, further suggesting that the disruption of the attention network contributed to the impairment of the motor function. Here, FNC changes between the MN, VN and DAN may provide strong evidence that the motor‐visual attention function was disrupted in stroke patients. Besides, the motor‐visual attention network also decreased the interaction with higher order cognitive motor control networks, as the internetwork FNC analysis showed that stroke patients exhibited weak inter‐network FC between the MN, and DAN and LFPN, and between the VN and pDMN. Together, our results support the view that sensory information from proprioception and vision plays an important role in guiding and adjusting movement by providing bottom–up and top–down control (Fogassi & Luppino, 2005), which deepens our understanding of the importance of eye–hand coordination for upper extremity motor recovery and may enhance the rehabilitation of subjects after stroke.
The ECN is thought to exert control over posterior sensorimotor representations and to maintain relevant data in the mind until actions are selected (Seeley et al., 2007). Higher FC was presented in the ECN in patients compared to controls, which may represent a compensatory reorganization due to the impairment of the sensorimotor and visual attention circuit. This result is consistent with previous findings that some cognitive control regions demonstrated higher activity in the acute phase of stroke and in the process of motor recovery (Marshall et al., 2000; Ward et al., 2003). Additionally, increased FC in the ipsilesional IPL within the ECN was negatively correlated with FMA‐HW scores, indicating that the higher the FC of the IPL within the ECN, the worse the hand motor function. An intriguing finding is that the ipsilesional IPL region (BA40) in the ECN showed higher FC in patients than controls; conversely, the ipsilesional IPL region (BA40) in the DAN showed lower FC in patients. We also note that the Fusiform/Lingual Gyrus (BA18/19) of the ipsilesional occipital lobe exhibited completely different FC within the MVN and LVN. These types of complex functional alterations in one brain area may suggest that a functionally defined brain cortex may exist beyond its anatomically defined cortex, which is worthy of further investigation in future studies.
Consistent with previous research, this study revealed lateralized networks of FPN via ICA, although the regions were symmetric in healthy controls. We found that inter‐network FC decreased between the ipsilesional FPN (LFPN) and the MN and DAN in stroke patients, which further suggested impairment of the dorsolateral prefrontal cortex management of cognitive load required for motor‐visual attention functions. We speculated that the increased inter‐network FC between the contralesional RFPN and DMN might be a compensatory reorganization due to the decreased inter‐network FC between the pDMN and VN in stroke patients. On the other hand, our findings showed that positive connectivity between the RFPN and ECN decreased in stroke patients, and the ECN exhibited seemingly no direct altered interaction with the DMN or motor‐visual attention networks. These serial internetwork changes appear to support that the FPN may have a closer connection to the reinforcement of the management of cognitive load required for motor performance. Therefore, the importance of the cooperation of the FPN with other related networks, which may determine the outcome of motor recovery, should be emphasized.
The DMN, a set of brain regions that typically deactivate during performance of cognitive tasks, has been considered to be involved in the integration of primary perception and high cognitive processing (Andrews‐Hanna, 2012; Broyd et al., 2009; Raichle et al., 2001). Previous studies have suggested that resting‐state DMN dysfunction is related to cognitive impairment in stroke patients (Dacosta‐Aguayo et al., 2015, 2014). In this study, not surprisingly, there were no regions that showed significant FC differences within the DMN in the stroke patients, as the enrolled subjects had good general cognitive function. FC alterations within the DMN were observed in both acute and chronic stroke patients without cognitive impairment (Ding et al., 2014). Altered intra‐ and inter‐network FC in the DMN has even appeared in well‐recovered subcortical stroke patients (Wang et al., 2014a). The inconsistent results among these studies might be related to different inclusion criteria for stroke patients with motor impairments. In this study, the relative homogeneity of the samples was carefully controlled; our patients exhibited pure motor deficits from subcortical stroke, and most lesions occurred at the ipsilesional BG regions of the left motor pathway. Although these might be the strictest inclusion criteria for stroke patients with motor impairments, our patients showed greater inter‐network FC between the RFPN and the aDMN and pDMN. We reasoned that this might reflect a comparison response to the inter‐network impairments between the pDMN and the MVN and LVN. In general, the inter‐network alterations of the DMN in this study could represent the disruption of its integrative function as a counterpoint between the primary perceptional system and the advanced cognitive system (Andrews‐Hanna, 2012; Broyd et al., 2009). More studies are necessary to clarify the role of the DMN in motor deficits after stroke. Taken together, the existence of alterations both in motor‐visual networks and in other high‐order motor cognitive networks (including the RFPN, DMN, and ECN) in chronic stroke may be further helpful for understanding the possible mechanisms underlying the beneficial effects of intervention on motor recovery.
Several limitations should be considered in this study. First, this current study had only a cross‐sectional design. It would be more helpful for us to examine the dynamic changes in multiple network functional connectivity to understand the mechanism of motor recovery, but because of our study design, we are unable to comment on this. Further studies should focus on the acute or subacute effects of stroke on multiple network function and investigate longitudinally whether these predict long‐term motor function. Second, we recruited highly specific patients who formed a homogeneous group in terms of the nature of the deficit (pure motor deficit) and the location of the lesion (subcortical lesion, involvement of a similar BG region). As a result, caution should be taken in extending the findings of this study to patients with complex motor deficits following stroke. Third, the total sample size of stroke patients is relatively small, and the further subdivision of stroke patients may exhibit a more detailed relationship between the patterns of functional connectivity and function outcomes in hand function. Finally, we did not assess cognitive functions, which may help to clarify the functional role of our findings. Future studies are needed to validate these interpretations of our results.
In conclusion, we demonstrated that brain plasticity in chronic stroke patients with motor deficits is not restricted to the sensorimotor network, but rather spreads to other neural networks. The intra‐ and inter‐network FC alterations involved both the primary perceptional system and higher order cognitive control networks and demonstrated the brain pathway reorganization of motor behavior after subcortical stroke. Moreover, the different FC alteration patterns in the ipsilesional IPL may reflect the coexistence of impairment of the DAN and compensation by the ECN for hand motor deficits. The findings of this study can provide complementary evidence to further understand the neural substrates of improving motor function after stroke.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
Zhao Z, Wu J, Fan M, et al. Altered intra‐ and inter‐network functional coupling of resting‐state networks associated with motor dysfunction in stroke. Hum Brain Mapp. 2018;39:3388–3397. 10.1002/hbm.24183
Funding information National Natural Science Foundation of China, Grant/Award number: 81471651/31600869; 12th Five‐Year Plan Supporting Project of Ministry of Science and Technology of the People's Republic of China, Grant/Award number: 2013BAI10B03; China National Natural Science Young Foundation,Grant/Award number: 81401859; China National Key R&D Program, Grant/Award number: 2017YFC1308502
REFERENCES
- Andersen, S. M. , Rapcsak, S. Z. , & Beeson, P. M. (2010). Cost function masking during normalization of brains with focal lesions: Still a necessity? NeuroImage, 53(1), 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. (2012). The brain's default network and its adaptive role in internal mentation. Neuroscientist, 18(3), 251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Bressler, S. L. , Tang, W. , Sylvester, C. M. , Shulman, G. L. , & Corbetta, M. (2008). Top‐down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. Journal of Neuroscience, 28(40), 10056–10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd, S. J. , Demanuele, C. , Debener, S. , Helps, S. K. , James, C. J. , & Sonuga‐Barke, E. J. (2009). Default‐mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews, 33(3), 279–296. [DOI] [PubMed] [Google Scholar]
- Calautti, C. , & Baron, J. C. (2003). Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke, 34(6), 1553–1566. [DOI] [PubMed] [Google Scholar]
- Cramer, S. C. , Nelles, G. , Benson, R. R. , Kaplan, J. D. , Parker, R. A. , Kwong, K. K. , … Rosen, B. R. (1997). A functional MRI study of subjects recovered from hemiparetic stroke. Stroke, 28(12), 2518–2527. [DOI] [PubMed] [Google Scholar]
- Dacosta‐Aguayo, R. , Grana, M. , Iturria‐Medina, Y. , Fernandez‐Andujar, M. , Lopez‐Cancio, E. , Caceres, C. , … Mataro, M. (2015). Impairment of functional integration of the default mode network correlates with cognitive outcome at three months after stroke. Human Brain Mapping, 36(2), 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacosta‐Aguayo, R. , Grana, M. , Savio, A. , Fernandez‐Andujar, M. , Millan, M. , Lopez‐Cancio, E. , … Mataro, M. (2014). Prognostic value of changes in resting‐state functional connectivity patterns in cognitive recovery after stroke: A 3T fMRI pilot study. Human Brain Mapping, 35(8), 3819–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X. , Li, C. Y. , Wang, Q. S. , Du, F. Z. , Ke, Z. W. , Peng, F. , … Chen, L. (2014). Patterns in default‐mode network connectivity for determining outcomes in cognitive function in acute stroke patients. Neuroscience, 277, 637–646. [DOI] [PubMed] [Google Scholar]
- Duncan, P. W. , Goldstein, L. B. , Matchar, D. , Divine, G. W. , & Feussner, J. (1992). Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke, 23(8), 1084–1089. [DOI] [PubMed] [Google Scholar]
- Elsner, B. , Kugler, J. , Pohl, M. , & Mehrholz, J. (2013). Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database of Systematic Reviews, Cd009645. [DOI] [PubMed] [Google Scholar]
- Finnigan, S. P. , Walsh, M. , Rose, S. E. , & Chalk, J. B. (2007). Quantitative EEG indices of sub‐acute ischaemic stroke correlate with clinical outcomes. Clinical Neurophysiology, 118(11), 2525–2532. [DOI] [PubMed] [Google Scholar]
- Fogassi, L. , & Luppino, G. (2005). Motor functions of the parietal lobe. Current Opinion in Neurobiology, 15(6), 626–631. [DOI] [PubMed] [Google Scholar]
- Golestani, A. M. , Tymchuk, S. , Demchuk, A. , Goodyear, B. G. , & Group, V.‐S. (2013). Longitudinal evaluation of resting‐state FMRI after acute stroke with hemiparesis. Neurorehabilitation and Neural Repair, 27(2), 153–163. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Tian, T. , Qin, W. , Li, K. , & Yu, C. (2016). Contrasting evolutionary patterns of functional connectivity in sensorimotor and cognitive regions after stroke. Frontiers in Behavioral Neuroscience, 10, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, R. S. , Perera, G. M. , Lazar, R. M. , Krakauer, J. W. , Constantine, R. C. , & DeLaPaz, R. L. (2000). Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke, 31(3), 656–661. [DOI] [PubMed] [Google Scholar]
- Park, C. H. , Chang, W. H. , Ohn, S. H. , Kim, S. T. , Bang, O. Y. , Pascual‐Leone, A. , & Kim, Y. H. (2011). Longitudinal changes of resting‐state functional connectivity during motor recovery after stroke. Stroke, 42(5), 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro, R. , Pendlebury, S. , Johansen‐Berg, H. , & Matthews, P. M. (2002). Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke, 33(1), 103–109. [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal, S. D. , Schoonheim, M. M. , Hulst, H. E. , Sanz‐Arigita, E. J. , Smith, S. M. , Geurts, J. J. , & Barkhof, F. (2010). Resting state networks change in clinically isolated syndrome. Brain, 133(6), 1612–1621. [DOI] [PubMed] [Google Scholar]
- Rosazza, C. , Minati, L. , Ghielmetti, F. , Mandelli, M. L. , & Bruzzone, M. G. (2012). Functional connectivity during resting‐state functional MR imaging: Study of the correspondence between independent component analysis and region‐of‐interest‐based methods. American Journal of Neuroradiology, 33(1), 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, C. C. , Bestmann, S. , Blankenburg, F. , Bjoertomt, O. , Josephs, O. , Weiskopf, N. , … Driver, J. (2008). Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS‐fMRI. Cerebral Cortex, 18(4), 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, C. C. , Blankenburg, F. , Bjoertomt, O. , Bestmann, S. , Freeman, E. , Haynes, J. D. , … Driver, J. (2006). Concurrent TMS‐fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Current Biology, 16(15), 1479–1488. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore, E. R. , Becker, J. T. , Whyte, E. M. , Huber, L. M. , Waterstram, L. F. , Ward, A. A. , … Holm, M. B. (2012). Cognitive impairments and depressive symptoms did not impede upper extremity recovery in a clinical repetitive task practice program after stroke. A pilot study. American Journal of Physical Medicine & Rehabilitation/Association of Academic Physiatrists, 91(4), 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. , Qin, W. , Liu, Y. , Duan, Y. , Liu, J. , He, X. , … Yu, C. (2013). Aberrant functional organization within and between resting‐state networks in AD. PLoS One, 8(5), e63727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear, C. (2010). Prediction of recovery of motor function after stroke. Lancet. Neurology, 9(12), 1228–1232. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Yin, D. , Zhu, Y. , Fan, M. , Zang, L. , Wu, Y. , … Hu, Y. (2013). Cortical reorganization after motor imagery training in chronic stroke patients with severe motor impairment: A longitudinal fMRI study. Neuroradiology, 55(7), 913–925. [DOI] [PubMed] [Google Scholar]
- Tessitore, A. , Amboni, M. , Esposito, F. , Russo, A. , Picillo, M. , Marcuccio, L. , … Barone, P. (2012). Resting‐state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism and Related Disorders, 18(6), 781–787. [DOI] [PubMed] [Google Scholar]
- van de Ven, V. G. , Formisano, E. , Prvulovic, D. , Roeder, C. H. , & Linden, D. E. (2004). Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Human Brain Mapping, 22(3), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Qin, W. , Liu, Y. , Zhang, Y. , Jiang, T. , & Yu, C. (2014b). Altered resting‐state network connectivity in congenital blind. Human Brain Mapping, 35(6), 2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Qin, W. , Zhang, J. , Tian, T. , Li, Y. , Meng, L. , … Yu, C. (2014a). Altered functional organization within and between resting‐state networks in chronic subcortical infarction. Journal of Cerebral Blood Flow & Metabolism, 34(4), 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Yu, C. , Chen, H. , Qin, W. , He, Y. , Fan, F. , … Zang, Y. (2010). Dynamic functional reorganization of the motor execution network after stroke. Brain, 133(4), 1224–1238. [DOI] [PubMed] [Google Scholar]
- Ward, N. S. , Brown, M. M. , Thompson, A. J. , & Frackowiak, R. S. (2003). Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain, 126(11), 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.‐G. , Wang, X.‐D. , Zuo, X.‐N. , & Zang, Y.‐F. (2016). DPABI: Data processing & analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Yin, D. , Song, F. , Xu, D. , Peterson, B. S. , Sun, L. , Men, W. , … Fan, M. (2012). Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS One, 7(12), e52727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, D. , Song, F. , Xu, D. , Sun, L. , Men, W. , Zang, L. , … Fan, M. (2014). Altered topological properties of the cortical motor‐related network in patients with subcortical stroke revealed by graph theoretical analysis. Human Brain Mapping, 35(7), 3343–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Liu, F. , Chen, H. , Li, M. , Duan, X. , Xie, B. , & Chen, H. (2015). Intranetwork and internetwork functional connectivity alterations in post‐traumatic stress disorder. Journal of Affective Disorders, 187, 114–121. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. , Huang, T. , Tang, C. , Ni, K. , Pan, X. , Yan, C. , … Luo, Y. (2017). Altered resting‐state intra‐and inter‐network functional connectivity in patients with persistent somatoform pain disorder. PLoS One, 12(4), e0176494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Z. , Wang, X. , Fan, M. , Yin, D. , Sun, L. , Jia, J. , … Gong, J. (2016). Altered effective connectivity of the primary motor cortex in stroke: A resting‐state fMRI study with granger causality analysis. PLoS One, 11(11), e0166210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Sun, L. , Yin, D. , Jia, J. , Zhao, Z. , Jiang, Y. , … Fan, M. (2016). The plasticity of intrinsic functional connectivity patterns associated with rehabilitation intervention in chronic stroke patients. Neuroradiology, 58(4), 417–427. [DOI] [PubMed] [Google Scholar]
- Zuo, X.‐N. , Kelly, C. , Adelstein, J. S. , Klein, D. F. , Castellanos, F. X. , & Milham, M. P. (2010). Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. NeuroImage, 49(3), 2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


