Abstract
To psychoradiologically investigate the topological organization of single‐subject gray matter networks in patients with PTSD. Eighty‐nine adult PTSD patients and 88 trauma‐exposed controls (TEC) underwent a structural T1 magnetic resonance imaging scan. The single‐subject brain structural networks were constructed based on gray matter similarity of 90 brain regions. The area under the curve (AUC) of each network metric was calculated and both global and nodal network properties were measured in graph theory analysis. We used nonparametric permutation tests to identify group differences in topological metrics. Relationships between brain network measures and clinical symptom severity were analyzed in the PTSD group. Compared with TEC, brain networks of PTSD patients were characterized by decreased clustering coefficient (C p) (p = .04) and local efficiency (E loc) (p = .04). Locally, patients with PTSD exhibited altered nodal centrality involving medial superior frontal (mSFG), inferior orbital frontal (iOFG), superior parietal (SPG), middle frontal (MFG), angular, and para‐hippocampal gyri (p < .05, corrected). A negative correlation between the segregation (C p) of gray matter and functional networks was found in PTSD patients but not the TEC group. Analyses of topological brain gray matter networks indicate a more randomly organized brain network in PTSD. The reduced segregation in gray matter networks and its negative relation with increased segregation in the functional network indicate an inverse relation between gray matter and functional changes. The present psychoradiological findings may reflect a compensatory increase in functional network segregation following a loss of segregation in gray matter networks.
Keywords: gray matter network, PTSD, psychoradiology, single‐subject, structural MRI, topological organization
1. INTRODUCTION
Posttraumatic stress disorder (PTSD) is a psychiatric condition that can develop following exposure to extremely traumatic life events. Its lifetime prevalence is 6.8% of the adult population (Kessler et al., 2005), and it occurs in 24% of individuals after severe stressors such as occur in military veterans and earthquake survivors (Dai et al., 2016). With chronic and fluctuating symptoms including re‐experiencing the trauma, avoidance of trauma‐related stimuli, negative cognitions and mood, and hyperarousal (APA, 2013), PTSD is believed to impact multiple neural structures and functions of the human brain network (Yehuda et al., 2015).
Investigators have documented multiple regional brain abnormalities in PTSD during fear and stress processing in task‐based fMRI studies, particularly in default mode (DMN), central executive (CEN), and salience brain networks (SN) (Menon, 2011; Rauch, Shin, & Phelps, 2006). With long‐term exposure to stress, these networks might be damaged by high levels of glucocorticoids and related stress‐induced neurochemical changes (Sapolsky, Uno, Rebert, & Finch, 1990), leading to reductions in gray matter volume and cortical thickness as previously reported (Kuhn & Gallinat, 2013; Li et al., 2014). Dystrophic changes in gray matter have been associated with cortical dysfunction in PTSD (Yehuda et al., 2015). However, most previous gray matter morphological studies focused on individual brain regions, an approach which can fail to capture the complex connectivity alterations of gray matter networks that support higher cognitive and affective processes (Alexander‐Bloch, Giedd, & Bullmore, 2013).
The development of psychoradiology (https://radiopaedia.org/articles/psychoradiology), allow the noninvasive examination of brain in patients with psychiatric disorders. Amongst recently technical advances, graph theory analysis has become a powerful method to examine the complex connectivity of brain structure. Human brain networks have been shown to have a small‐world topological organization with a high level of local clustering and short path lengths. Such network organization provides an economic trade‐off between minimizing costs and allowing high global and local efficiency in information processing (Bullmore & Sporns, 2012; Watts & Strogatz, 1998).
One previous study reported decreased small world organization of gray matter networks in a group of 40 military veterans with PTSD (Mueller et al., 2015). However, that study investigated network properties in a group‐level analysis, limiting detection of individual patient network differences and their relationship with illness severity. Moreover, most patients in that study were being treated with psychotropic medications that can affect brain networks.
We investigated graph properties of single‐subject gray matter networks in PTSD using a method proposed by Tijms et al. to statistically describe gray matter networks in individual subjects using T1‐weighted MRI scans (Tijms, Series, Willshaw, & Lawrie, 2012). This approach has been successfully used to study Alzheimer's disease (Tijms, Moller, et al., 2013a; Tijms et al., 2016; Tijms et al., 2014; Tijms, Wink, et al., 2013b) and individuals at risk for schizophrenia (Tijms et al., 2015). We have previously used the method in pediatric PTSD and found increased small world network characteristics, the opposite finding from the Mueller et al. (2015) study in adult PTSD. This divergence may be due to age effects or network extraction methods. Given previous evidence of more randomly organized gray matter networks in many adult psychiatric disorders and a loss of small world organization of gray matter network in adult PTSD as noted above (Mueller et al., 2015), we predicted that gray matter networks in individual PTSD patients would be shifted toward a randomized configuration. We also hypothesized that altered nodal degree and efficiency would be found in PTSD patients based on previous findings (Suo et al., 2017), and that there would be a correlation between those network alterations and clinical severity of PTSD.
2. MATERIALS AND METHODS
2.1. Participants
Individuals who survived a massive earthquake (8.0 magnitude on the Richter scale) in Sichuan Province of western China in 2008 were recruited in Hanwang and nearby areas of Beichuan County located 80–115 km from the earthquake epicenter respectively from a group of 4,200 individuals who completed the PTSD Checklist (PCL) (Weathers, Litz, Herman, Huska, & Keane, 1994) 8–15 months after the earthquake(Jin et al., 2014). From that sample we selected participants who met the following criteria: (i) physically experienced the earthquake and personally witnessed death, serious injury or the collapse of buildings, (ii) no previous lifetime psychopharmacological or psychological interventions, and (iii) no known PTSD prior to the earthquake. The presence or absence of PTSD diagnosis and other psychiatric comorbidities were determined using the Structured Clinical Interview for DSM‐IV (SCID; First, Gibbon, Spitzer, Benjamin, & Williams, 1997). The Clinician Administered PTSD Scale (CAPS) was used to evaluate PTSD symptom severity in all study participants. PTSD patients were required to have a CAPS score of 50 or greater to ensure that patients had significant PTSD symptoms and met diagnostic criteria for PTSD at the time of scans. Individuals with PCL scores less than 30 and without a SCID diagnosis of PTSD were recruited as non‐PTSD controls who had experienced trauma but did not have significant symptoms of PTSD.
Exclusion criteria were: (i) history of depression, bipolar disorder or psychotic disorder, history of major psychological trauma before or after the earthquake and its aftermath, and alcohol or drug abuse, (ii) contraindication to MR imaging, (iii) significant systemic or neurologic disorders, (iv) younger than 18 years, (v) left handedness, (vi) brain lesions identified at the MR imaging examination, and (vii) history of brain injury.
First‐episode patients with PTSD (n = 89) and a demographically matched group of 88 trauma‐exposed individuals who did not develop PTSD participated in MRI studies. The two groups had similar demographic characteristics, lifestyles, and earthquake experiences (Table 1). Selecting healthy individuals who also experienced the earthquake as a comparison group was done to control for stress exposure effects independent from the later development of PTSD, and to have comparisons with a more clinical relevance with regard to differential diagnosis. This study was approved by the Sichuan University research ethics committee. Each participant provided written informed consent.
Table 1.
Demographic data and clinical characteristicsa
| PTSD (n = 89) | Non‐PTSD (n = 88) | p value | |
|---|---|---|---|
| Age (years)b | 42.48 ± 10.42 | 43.50 ± 10.06 | .51c |
| Gender (M/F) | 28/61 | 25/63 | .78d |
| Handedness (R/L) | 89/0 | 88/0 | NA |
| Years of educationb | 7.08 ± 3.20 | 6.88 ± 3.30 | .68c |
| Time since trauma (months)b | 11.04 ± 2.33 | 11.60 ± 2.40 | .12c |
| Gray matter volume (cm3)e | 1,092.15 ± 193.17 | 1,127.94 ± 142.49 | .16c |
| Sparsity of networksf | 86.17 ± 1.17 | 85.99 ± 1.34 | .35c |
| PCL score | 46.29 ± 14.82 | 28.09 ± 7.22 | NA |
| CAPS score | 54.77 ± 17.97 | 22.83 ± 11.31 | NA |
PTSD = posttraumatic stress disorder; PCL = PTSD checklist; CAPS = Clinician‐administered PTSD scale.
Data are presented as means±standard deviations. No significant differences were identified between the pediatric PTSD and the trauma controls in age, gender, years of education, time since trauma, and gray matter volume.
Age, years of education, and time since trauma were determined at the time of MRI scanning.
p value was obtained by two‐tailed two‐sample t test.
p value was obtained by two‐tailed Pearson χ 2 test.
Gray matter volume was obtained from whole brain after segmentation.
Sparsity of individual networks before normalization.
2.2. Data acquisition and preprocessing
Participants underwent T1 structural imaging with a 3.0‐T MR imaging system (Excite; GE Healthcare, Milwaukee, WI) using an eight‐channel phased‐array head coil. The head was stabilized with cushions and ear plugs. Images were acquired using a spoiled gradient recalled sequence with repetition time (TR) = 8.5 ms, echo time (TE) = 3.4 ms, flip angle (FA) = 12°, 156 axial slices with slice thickness = 1 mm, field of view (FOV) = 24 × 24 cm2, and data matrix = 256 × 256.
Structural images were preprocessed using Statistical Parametric Mapping (SPM) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Individual structural images were first segmented into gray matter, white matter, CSF, bone, soft tissue, and air/background using the default tissue probability maps as priors (Ashburner & Friston, 2005). We visually confirmed all automatic segmentations. Then, DARTEL tools in SPM were used to create a template to determine nonlinear deformations for warping gray and white matter images into Montreal Neurological Institute (MNI) coordinate space. In this process, gray matter data were resampled to 2 mm3 voxels and spatially smoothed (Gaussian kernel with a full width at half maximum of 6 mm).
2.3. Extraction of brain networks
Single‐subject gray matter networks were obtained based on intracortical similarity using a completely automated, data‐driven method that has been previously described (Tijms et al., 2012). Briefly, the method starts with defining the network's nodes as 3 × 3 × 3 voxel cubes (27 voxels) in standard space. These cubes keep the 3D structure of the cortex intact, thereby including spatial information from the MRI scan in addition to gray matter values in voxels. By keeping spatial information intact, the cubes contain a quantity reflecting the local thickness and folding structure of the cortex. Then the structural similarity between every pair of cubes was quantified by correlating the 27 voxels in each cube. It is this step that allows brain networks to be computed on individual patients.
Because neocortex is a curved structure, rotating a cube over cortex can change its similarity to a seed cube. Therefore, cubes were rotated to identify their maximum correlation value with seed cubes over the different rotations. After this step, a single‐subject network for each participant was established. Next, unweighted and undirected graphs were constructed by binarizing the similarity matrices using a threshold for significant similarity (p < .05) determined using a FDR correction for each network. Because the sparsity threshold of individual networks might induce spurious changes that could drive the higher order integration/segregation measures in patient‐control studies (van den Heuvel, et al., 2017; Van Wijk, Stam, & Daffertshofer, 2010), we calculated the overall sparsity for each individual and then compared these values across groups (Table 1). Groups did not differ in brain network sparsity.
Similarity based gray matter networks defined in this way have different sizes. As network properties can vary with network size (Van Wijk et al., 2010), it is critical to normalize gray matter networks to have the same number of nodes across participants. To achieve this, we followed the methodology proposed by Batalle et al. (2013) to normalize single subject gray matter networks using the 90‐node Automated Anatomical Labeling (AAL) parcellation template. To perform the normalization, each cube was assigned to a ROI (brain region) of the AAL atlas based on the ROI to which most of the voxels of the cube belonged (Batalle et al., 2013). Each pair of ROIs in the AAL atlas was considered to be connected with a weight corresponding to the ratio of actual significant cube‐to‐cube correlations by the total possible connections between all the cubes belonging to the two ROIs included in the previous binary network. The weight obtained is bounded between 0 and 1. Self‐connections were excluded. This procedure resulted in a 90 × 90 weighted normalized network for each subject. The network measures were then calculated from the weighted normalized networks. Note that in the present study, we use the term “connection” to refer to brain network edges that have statistically similar gray matter morphology of two cubes, which can exist in the absence of direct axonal connectivity or anatomic proximity.
2.4. Network properties
GRETNA (http://www.nitrc.org/projects/gretna/) software was used to calculate network properties (Wang et al., 2015). A wide range of sparsity (S) thresholds was applied to each correlation matrix. The upper and lower limit of S values used was determined ensuring that the thresholded networks were estimable for the small‐worldness scalar and that the small‐world index was larger than 1.0. With these limits, our threshold range was 0.10 < S < 0.34 with an interval of 0.01. The area under the curve (AUC) reflecting measures across the sparsity parameter S was calculated for each network metric, providing a summarizing scalar for the topological characterization of brain networks that avoided the use of an arbitrary single threshold. This approach has been shown to be sensitive for detecting topological alterations of brain networks (Achard & Bullmore, 2007; He et al., 2009; Zhang et al., 2011).
Both global and nodal network properties were calculated at each sparsity threshold. Global metrics included small‐world parameters (Watts & Strogatz, 1998): clustering coefficient C p, characteristic path length L p, normalized clustering coefficient γ, normalized characteristic path length λ, and small worldness σ. Network efficiency parameters (Achard & Bullmore, 2007) included local efficiency E loc and global efficiency E glob. Nodal degree and efficiency were examined in each ALL region.
2.5. Statistical analysis
Between‐group differences of network characteristics were analyzed using nonparametric permutation tests. We adopted the false discovery rate (FDR) to correct for multiple comparisons when comparing nodal centralities across groups (Genovese, Lazar, & Nichols, 2002) After significant between‐group differences were identified, partial correlations using age, gender and whole brain gray matter volume as covariates were performed to evaluate relationships between network metrics and CAPS scores both in the combined sample and each group separately.
Region pairs with between‐group differences of nodal characteristics were identified using the network‐based statistics (NBS) toolbox (http://www.nitrc.org/projects/nbs/). First, we chose nodes that exhibited between group differences of both nodal degree and efficiency, and then created a connection matrix among those nodes for each participant. The size of the subnetwork fed into NBS was 17 × 17. Second, connections were tested for significance (p < .05 FWE‐corrected network‐level). The threshold t‐value was set as 4.5 and the number of permutations was 5,000. Details of this approach are described elsewhere (Zalesky, Fornito, & Bullmore, 2010).
3. RESULTS
3.1. Alterations of global brain network properties
The topology of brain networks was altered in patients with PTSD. The graphs from PTSD patients were characterized by decreased clustering coefficient (C p) (0.0702 ± 0.0005 vs. 0.0691 ± 0.0004, p = .04) and local efficiency (E loc) (0.0800 ± 0.0004 vs. 0.0791 ± 0.0003, p = .04). There were no significant differences between groups in characteristic path length (L p) (1.1410 ± 0.0053 vs. 1.1437 ± 0.0046, p = .35), global efficiency (E glob) (0.0511 ± 0.0003 vs. 0.0510 ± 0.0002, p = .35), normalized clustering coefficient (γ) (0.2548 ± 0.0009 vs. 0.2562 ± 0.0006, p = .12), normalized characteristic path length (λ) (0.2392 ± 0.0001 vs. 0.2392 ± 0.0001, p = .31), or small worldness (σ) (0.2556 ± 0.0008 vs. 0.2571 ± 0.0006, p = .08) (Figure 1).
Figure 1.
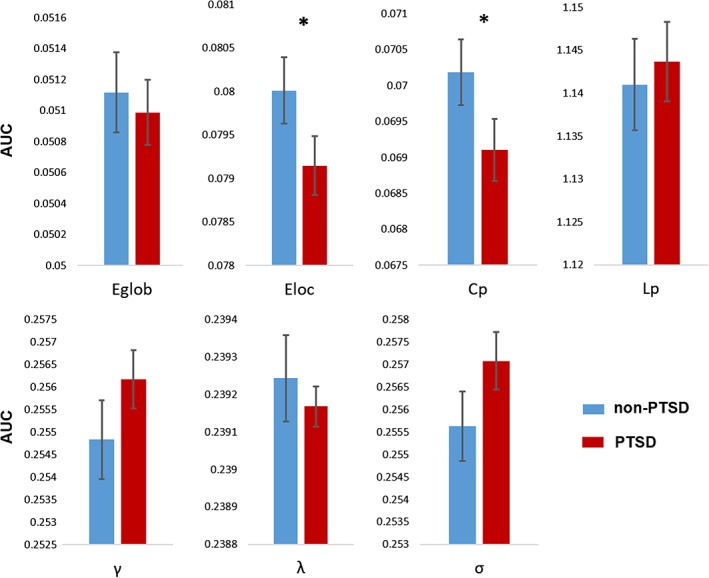
Group differences in global topological properties between PTSD patients and trauma‐exposed non‐PTSD controls. Abbreviations: C p, clustering coefficient; L p, characteristic path length; γ, normalized clustering coefficient; λ, normalized characteristic path length; σ, small worldness. An asterisk designates network metrics with significant group differences [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Alterations of nodal brain network properties
Brain regions exhibiting significant between‐group differences both in nodal degree and nodal efficiency were identified (FDR corrected p < .05). Compared with control subjects, patients with PTSD exhibited decreased nodal degree and nodal efficiency in right inferior orbital, right medial superior frontal, left superior and inferior occipital, and right middle temporal gyri, left parahippocampus, and bilateral caudate nucleus. Nodal degree and nodal efficiency were increased in right precuneus, right middle frontal, left middle cingulum, right superior and inferior occipital, left superior parietal, and right angular gyri in PTSD (Table 2; Figure 2).
Table 2.
Nodal network properties exhibiting significant alterations in patients with PTSD versus control participants
| Nodal degree | Nodal efficiency | |||||
|---|---|---|---|---|---|---|
| Brain regions | Non‐PTSD (mean ± SE) | PTSD (mean ± SE) | p value | Non‐PTSD (mean ± SE) | PTSD (mean ± SE) | p value |
| Middle frontal gyrus L | 3.0523 ± 0.0463 | 3.1757 ± 0.0315 | .0014 | 0.0628 ± 0.0003 | 0.0644 ± 0.0002 | .0025 |
| Middle frontal gyrus R | 3.4181 ± 0.0442 | 3.6725 ± 0.0356 | .0002 | 0.0657 ± 0.0002 | 0.0668 ± 0.0002 | .0004 |
| Inferior orbital frontal gyrus R | 2.2750 ± 0.0466 | 2.0982 ± 0.0400 | .0014 | 0.0564 ± 0.0005 | 0.0549 ± 0.0003 | .0026 |
| Medial superior frontal gyrus R | 0.2920 ± 0.0645 | 0.1138 ± 0.0082 | .0002 | 0.0294 ± 0.0013 | 0.0193 ± 0.0010 | .0002 |
| Middle cingulum cortex L | 0.1172 ± 0.0153 | 0.2437 ± 0.0330 | .0002 | 0.0194 ± 0.0016 | 0.0264 ± 0.0017 | .0018 |
| ParaHippocampus L | 3.2193 ± 0.0995 | 2.7384 ± 0.0874 | .0002 | 0.0658 ± 0.0005 | 0.0631 ± 0.0004 | .0002 |
| Superior occipital gyrus L | 0.5403 ± 0.0395 | 0.4311 ± 0.0246 | .0094 | 0.0486 ± 0.0005 | 0.0455 ± 0.0007 | .0006 |
| Superior occipital gyrus R | 0.4393 ± 0.0276 | 0.8359 ± 0.0403 | .0002 | 0.0451 ± 0.0007 | 0.0499 ± 0.0004 | .0002 |
| Inferior occipital gyrus L | 0.9270 ± 0.0825 | 0.6048 ± 0.0548 | .0004 | 0.0524 ± 0.0006 | 0.0498 ± 0.0005 | .0004 |
| Inferior occipital gyrus R | 0.8888 ± 0.0479 | 1.3068 ± 0.0560 | .0002 | 0.0507 ± 0.0004 | 0.0532 ± 0.0004 | .0002 |
| Superior parietal gyrus L | 2.3177 ± 0.0452 | 2.4886 ± 0.0509 | .0056 | 0.0611 ± 0.0003 | 0.0624 ± 0.0003 | .0018 |
| Angular gyrus R | 2.9672 ± 0.0687 | 3.2364 ± 0.0735 | .0040 | 0.0639 ± 0.0004 | 0.0653 ± 0.0004 | .0082 |
| Precuneus R | 1.5879 ± 0.0522 | 1.8706 ± 0.0613 | .0006 | 0.0568 ± 0.0004 | 0.0581 ± 0.0004 | .0058 |
| Caudate nucleus L | 5.3673 ± 0.0628 | 5.1354 ± 0.0552 | .0042 | 0.0764 ± 0.0004 | 0.0748 ± 0.0004 | .0036 |
| Caudate nucleus R | 4.8419 ± 0.0580 | 4.4666 ± 0.0665 | .0004 | 0.0726 ± 0.0005 | 0.0704 ± 0.0005 | .0008 |
| Superior temporal pole R | 1.0870 ± 0.0444 | 1.3304 ± 0.0452 | .0002 | 0.0520 ± 0.0004 | 0.0539 ± 0.0004 | .0002 |
| Middle temporal gyrus R | 2.7298 ± 0.0518 | 2.4163 ± 0.0521 | .0002 | 0.0619 ± 0.0003 | 0.0604 ± 0.0003 | .0004 |
Both nodal degree and nodal efficiency of the regions above were significantly changed in PTSD group.
The Benjamini–Hochberg false discovery rate correction was applied to correct for multiple group comparisons of brain regions.
The p value thresholds for nodal degree and nodal efficiency were .0134 and .0118, respectively. All p values were obtained by using a permutation test.
All the brain regions are from automated anatomical labeling (AAL).
R = right; L = left.
Figure 2.
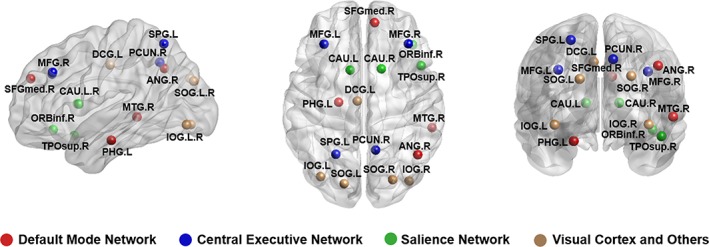
Regions with significant alterations both in nodal degree and nodal efficiency in PTSD patients in comparison with trauma‐exposed non‐PTSD controls. Associations of these nodes with specific brain networks are shown in different color, default mode network (DMN; in red), central executive network (CEN; in blue), and salience network (SN; in green), and nodes not associated with these networks (in yellow) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. PTSD‐related alterations in network connectivity
Using NBS to identify network alterations of brain regions showing between‐group differences of nodal degree and nodal efficiency, we identified significantly altered networks composing 15 brain regions and 21 anatomic connections in PTSD group (corrected for multiple comparisons using FDR). The networks involved brain regions in frontal, occipital, and parietal lobes (Figure 3). Detailed information is provided in supplementary materials (Supporting Information Tables S1 and S2).
Figure 3.
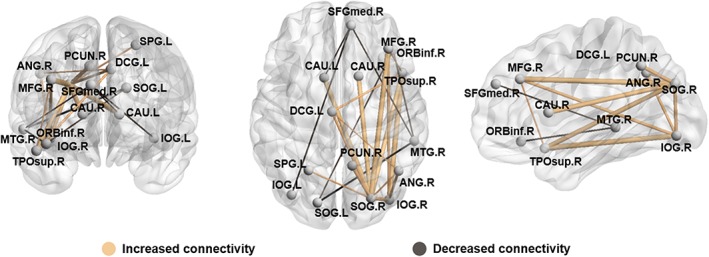
PTSD‐related alterations in network connectivity significantly increased connectivity is presented in yellow and decreased connectivity in gray. MFG, middle frontal gyrus; ORBinf, inferior frontal gyrus; orbital part; SFGmed , superior frontal gyrus; medial ; DCG , median cingulate and paracingulate gyri; SOG , superior occipital gyrus; IOG , inferior occipital gyrus; SPG , superior parietal gyrus; ANG , angular gyrus; PCUN , precuneus; CAU , caudate nucleus; TPOsup , temporal pole; superior temporal gyrus; MTG , middle temporal gyrus. L, left; R , right. The results are presented using BrainNet software (http://www.nitrc.org/projects/bnv) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Correlation of network alterations with PTSD symptom severity
Nodal degrees of left and right middle frontal gyri were significantly correlated with CAPS scores (r = .24, p = .03; r = .23, p = .04, respectively) in the PTSD group, using age, gender, and whole brain gray matter volume as covariates. We did not detect significant correlations between CAPS scores and network parameters in either participant group, or when correlations were examined in the combined group (Figure 4).
Figure 4.
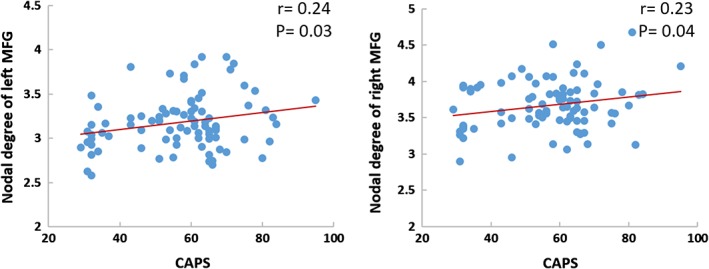
Scatterplots of the relationship between nodal topological measures and CAPS scores treating age, gender and whole brain gray matter volume as covariates in partial correlation analyses [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. Relation between structural and functional network parameters
We have previously published a functional network analysis of PTSD in this sample (Lei et al., 2015), and conducted secondary analyses to investigate the relationship between structural and functional network properties from the same patients, imaging acquisition system and network analysis software. This analysis is described in detail in Supporting Information materials. Both global and nodal network parameters including C p, L p, E loc, E glob, sigma, nodal degree, and nodal efficiency were used to examine the relation between structural and functional network characteristics. In PTSD patients, a negative correlation between C p of gray matter and functional networks was observed (r = −.25, p = .03), which was significantly different from the correlation seen in controls (r = .20, p = .10; F = 7.55, p = .007, corrected for multiple comparisons). No other significant group differences were found in the relationship of functional and anatomic modalities (Figure 5).
Figure 5.
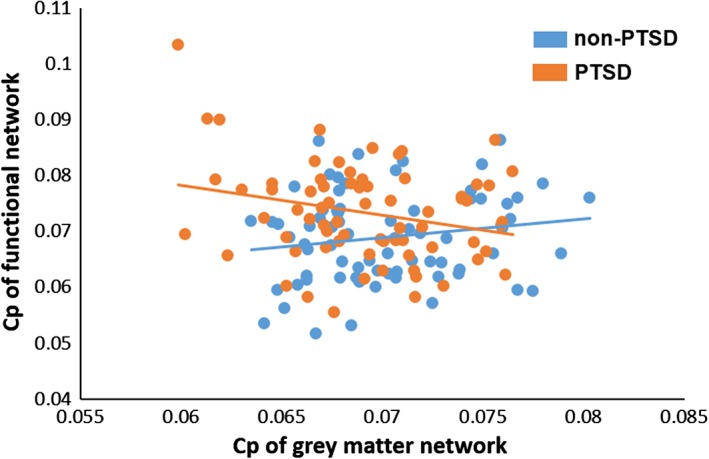
Relationships between structural and functional brain network characteristics [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
The present psychoradiological study demonstrated significant alterations in topological properties of single‐subject gray matter networks in a relatively large population of patients with untreated PTSD relative to similarly stressed individuals who did not develop PTSD. This was achieved combining graph theoretical analysis with a method of describing patterns of intracortical similarities in individual participants using structural MRI data. We found that PTSD patients showed a more randomly organized brain network reflected in decreased C p and E loc relative to non‐PTSD trauma‐exposed controls. Further, the C p in the gray matter network was negatively correlated to C p of the functional network in PTSD group, which was significantly different from the association seen in the trauma‐exposed controls.
The human brain is typically organized as a “small world” network with two main organizational principles: segregation, reflected by clustering coefficient (C p) or local efficiency (E loc), and integration, reflected by shorter path length (L p), and global efficiency (E glob) (Bullmore & Sporns, 2012). Our findings of decreased clustering coefficient and local efficiency in PTSD indicate that the gray matter networks of PTSD adults are shifted toward to a more random organization, which is a form of global network disruption previously reported in other neuropsychiatric disorders including Alzheimer's disease (Tijms, Moller, et al., 2013a), schizophrenia (Bassett et al., 2008), and major depressive disorder (Singh et al., 2013). The reduced segregation of brain networks reflected by lower C p and E loc might contribute to impairments in cognitive function and emotion processing in patients with PTSD, as suggested by correlations between MRI measures and psychiatric ratings in PTSD patients in the present study.
The alteration of network segregation reflected by reduced C p and E loc in the present study was in the opposite direction from that seen in our previous functional study with a highly overlapping adult sample (Lei et al., 2015). Previous studies have shown that in the healthy adult brain, volumetric covariance, and resting‐state functional connectivity are positively correlated (Di, Gohel, Thielcke, Wehrl, & Biswal, 2017). There was a similar trend for such a positive correlation in our control group between C p in gray matter networks with C p in functional networks (p < .10). In contrast, our PTSD patients demonstrated a significant inverse correlation between C p of structural and functional networks. Because disruptions of regional anatomy might lead to compensatory increases in functional connectivity, our findings of an inverse relation between C p of functional and anatomic networks might represent a compensatory response of functional networks to anatomic alteration. This pattern has similarities with one seen in a previous study that reported decreased white matter connectivity and increased functional connectivity in fronto‐limbic areas in depression (De Kwaasteniet et al., 2013).
The adult PTSD findings in the present study had similarities and differences from those of our previous study using the same MRI analysis pipeline and patient recruitment strategies with pediatric PTSD patients (Niu et al., 2018). For instance, the gray matter network organization of pediatric PTSD patients showed increased small‐worldness while in the present study we observed more randomly organized networks in adult patients. As reduction of small world characteristics was also seen in adults with PTSD in Mueller et al.'s study (Mueller et al., 2015), our findings thus suggest that PTSD affects brain gray matter networks may differ in adult and pediatric patients. Brain structural networks have been shown to exhibit significant network evolution through childhood and adolescents (Cao, Huang, Peng, Dong, & He, 2016; Khundrakpam et al., 2013), and the impact of PTSD on brain maturation trajectory may lead to different neuroanatomic network alterations in pediatric patients.
At a regional level, our findings included decreased nodal degree and nodal efficiency in the DMN involving parahippocampus, right medial superior frontal and middle temporal gyri, and in SN regions including right inferior orbital frontal gyrus and bilateral caudate also showed decreased nodal degree and nodal efficiency. We also observed increased nodal degree and nodal efficiency in the CEN involving right precuneus, and right middle frontal and left superior parietal gyri, as well as in nodes outside this network (Table 2). These findings provide partial support for the “triple network model” in PTSD (Akiki, Averill, & Abdallah, 2017; Menon, 2011; Patel, Spreng, Shin, & Girard, 2012; Sylvester et al., 2012).
However, the absence of case–control nodal differences in the hippocampus and posterior cingulate in the DMN, and the insula in the SN does not provide full support for the model (Akiki et al., 2017). Given that results of network properties from different modalities can vary (Di et al., 2017), our present results that failed to identify nodal gray matter alterations in the hippocampus, PCC, and insula may result from functional and structural dissociations in some brain regions that will need to be resolved by network analysis in multimodal imaging studies.
Among regions with nodal alterations, higher nodal degree of right and left middle frontal gyrus were both significantly correlated with CAPS scores reflecting PTSD symptom severity, consistent with our hypothesis of a relationship between network alterations and clinical severity of the disorder. Middle frontal gyrus is crucial for executive control, working memory and emotion processing that are disrupted in PTSD (Morey et al., 2009). A previous task‐based fMRI study also showed that PTSD patients demonstrated disrupted responses to trauma stimuli in bilateral middle frontal cortex(Hou et al., 2007), and it has been speculated that heightened encoding of negative expected social value in middle frontal gyrus might contribute to hypervigilance and attentional biases for threat in PTSD patients (Cisler et al., 2015).
We examined anatomic connectivity of pairs of brain regions in which nodal alterations were observed in patients with PTSD using NBS tools. These connections formed a single connected network with 15 nodes and 21 connections, involving brain regions in frontal, temporal, occipital, and parietal lobes. Of note, 19 of 21 connections are long‐distance connections linking different lobes. Because a previous resting‐state study has shown that combat‐related PTSD soldiers display long‐range hyperconnectivity in frontal, temporal, and parietal lobes (Dunkley et al., 2014), also using NBS tools, our findings emphasize the importance of abnormal long‐distance connectivity in the pathophysiology of PTSD.
The present study had certain limitations. All participants were recruited after the earthquake occurred, thus it is not clear whether findings we report reflect consequences of PTSD or prior risk factors for developing the illness. Second, using Network‐Based Statistic, we only tested the connectivity within the subnetwork of PTSD defined by regions with abnormal nodal properties. This was done to focus the NBS analysis specifically on pathological changes in the PTSD brain. Studies of the whole brain network might provide further information in future studies. Finally, we selected an earthquake‐exposed control group who did not develop PTSD to differentiate PTSD and general stress effects. Whether and how both of these groups might differ from unstressed community controls remains to be determined. Present study particularly adds to psychoradiology, an evolving specialty as opposed to neuroradiology, mainly for psychiatric and psychological brain (Lui et al., 2016; Kressel et al., 2017; Sun et al., 2018; Port, 2018).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81501452, 81621003, 81761128023, 81220108031 and 81227002), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, grant IRT16R52) of China, the Changjiang Scholar Professorship Award (Award No. T2014190) of China, and the CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education. D.L. was supported by Newton International Fellowship from the Royal Society, UK. The authors would like to thank the study participants and their families, and also, Dafnis Batalle (King's College London, UK) and Jinghui Wang (Hangzhou Normal University, China) for their generous help throughout this project.
Niu R, Lei D, Chen F, et al. Reduced local segregation of single‐subject gray matter networks in adult PTSD. Hum Brain Mapp. 2018;39:4884–4892. 10.1002/hbm.24330
Running Niu and Du Lei contributed equally to this work.
Funding information
National Natural Science Foundation of China, Grant/Award Numbers: 81501452, 81621003, 81761128023, 81220108013, 81227002; Program for Changjiang Scholars and Innovative Research Team in University, Grant/Award Number: IRT16R52; Changjiang Scholar Professorship Award, Grant/Award Number: T2014190; CMB Distinguished Professorship Award administered by the Institute of International Education, Grant/Award Number: F510000/G16916411
REFERENCES
- Achard, S. , & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3, 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki, T. J. , Averill, C. L. , & Abdallah, C. G. (2017). A network‐based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Current Psychiatry Reports, 19, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander‐Bloch, A. , Giedd, J. N. , & Bullmore, E. (2013). Imaging structural co‐variance between human brain regions. Nature Reviews. Neuroscience, 14, 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Bassett, D. S. , Bullmore, E. , Verchinski, B. A. , Mattay, V. S. , Weinberger, D. R. , & Meyer‐Lindenberg, A. (2008). Hierarchical organization of human cortical networks in health and schizophrenia. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28, 9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalle, D. , Munoz‐Moreno, E. , Figueras, F. , Bargallo, N. , Eixarch, E. , & Gratacos, E. (2013). Normalization of similarity‐based individual brain networks from gray matter MRI and its association with neurodevelopment in infants with intrauterine growth restriction. NeuroImage, 83, 901–911. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2012). The economy of brain network organization. Nature Reviews, Neuroscience, 13, 336–349. [DOI] [PubMed] [Google Scholar]
- Cao, M. , Huang, H. , Peng, Y. , Dong, Q. , & He, Y. (2016). Toward developmental Connectomics of the human brain. Frontiers in Neuroanatomy, 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J. M. , Bush, K. , Scott Steele, J. , Lenow, J. K. , Smitherman, S. , & Kilts, C. D. (2015). Brain and behavioral evidence for altered social learning mechanisms among women with assault‐related posttraumatic stress disorder. Journal of Psychiatric Research, 63, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, W. , Chen, L. , Lai, Z. , Li, Y. , Wang, J. , & Liu, A. (2016). The incidence of post‐traumatic stress disorder among survivors after earthquakes:A systematic review and meta‐analysis. BMC Psychiatry, 16, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kwaasteniet, B. , Ruhe, E. , Caan, M. , Rive, M. , Olabarriaga, S. , Groefsema, M. , … Denys, D. (2013). Relation between structural and functional connectivity in major depressive disorder. Biological Psychiatry, 74, 40–47. [DOI] [PubMed] [Google Scholar]
- Di, X. , Gohel, S. , Thielcke, A. , Wehrl, H. F. , & Biswal, B. B. (2017). Do all roads lead to Rome? A comparison of brain networks derived from inter‐subject volumetric and metabolic covariance and moment‐to‐moment hemodynamic correlations in old individuals. Brain Structure & Function, 222(8), 3833–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley, B. T. , Doesburg, S. M. , Sedge, P. A. , Grodecki, R. J. , Shek, P. N. , Pang, E. W. , & Taylor, M. J. (2014). Resting‐state hippocampal connectivity correlates with symptom severity in post‐traumatic stress disorder. NeuroImage Clinical, 5, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Gibbon, M. , Spitzer, R. L. , Benjamin, L. S. , & Williams, J. B. (1997). Structured clinical interview for DSM‐IV axis II personality disorders: SCID‐II. Arlington, VA: American Psychiatric Pub. [Google Scholar]
- Genovese, C. R. , Lazar, N. A. , & Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage, 15, 870–878. [DOI] [PubMed] [Google Scholar]
- He, Y. , Dagher, A. , Chen, Z. , Charil, A. , Zijdenbos, A. , Worsley, K. , & Evans, A. (2009). Impaired small‐world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain : A Journal of Neurology, 132, 3366–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, C. , Liu, J. , Wang, K. , Li, L. , Liang, M. , He, Z. , … Jiang, T. (2007). Brain responses to symptom provocation and trauma‐related short‐term memory recall in coal mining accident survivors with acute severe PTSD. Brain Research, 1144, 165–174. [DOI] [PubMed] [Google Scholar]
- Jin, C. , Qi, R. , Yin, Y. , Hu, X. , Duan, L. , Xu, Q. , … Li, L. (2014). Abnormalities in whole‐brain functional connectivity observed in treatment‐naive post‐traumatic stress disorder patients following an earthquake. Psychological Medicine, 44, 1927–1936. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Khundrakpam, B. S. , Reid, A. , Brauer, J. , Carbonell, F. , Lewis, J. , Ameis, S. , … Evans, A. C. (2013). Developmental changes in organization of structural brain networks. Cerebral Cortex (New York, NY: 1991), 23, 2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressel, H. Y. (2017), Setting Sail: 2017, Radiology, 282(1), 4–6. [DOI] [PubMed] [Google Scholar]
- Kuhn, S. , & Gallinat, J. (2013). Gray matter correlates of posttraumatic stress disorder: A quantitative meta‐analysis. Biological Psychiatry, 73, 70–74. [DOI] [PubMed] [Google Scholar]
- Lei, D. , Li, K. , Li, L. , Chen, F. , Huang, X. , Lui, S. , … Gong, Q. (2015). Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology, 276, 818–827. [DOI] [PubMed] [Google Scholar]
- Li, L. , Wu, M. , Liao, Y. , Ouyang, L. , Du, M. , Lei, D. , … Gong, Q. (2014). Grey matter reduction associated with posttraumatic stress disorder and traumatic stress. Neuroscience and Biobehavioral Reviews, 43, 163–172. [DOI] [PubMed] [Google Scholar]
- Lui, S. , Zhou, X. , Sweeney, J. A. & Gong, Q. (2016). Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology, 281, 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Morey, R. A. , Dolcos, F. , Petty, C. M. , Cooper, D. A. , Hayes, J. P. , LaBar, K. S. , & McCarthy, G. (2009). The role of trauma‐related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatric Research, 43, 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, S. G. , Ng, P. , Neylan, T. , Mackin, S. , Wolkowitz, O. , Mellon, S. , … Weiner, M. W. (2015). Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Research, 234, 194–201. [DOI] [PubMed] [Google Scholar]
- Niu, R. , Lei, D. , Chen, F. , Chen, Y. , Suo, X. , Li, L. , … Gong, Q. (2018). Disrupted grey matter network morphology in pediatric posttraumatic stress disorder. NeuroImage Clinical, 18, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , Spreng, R. N. , Shin, L. M. , & Girard, T. A. (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: A meta‐analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 36, 2130–2142. [DOI] [PubMed] [Google Scholar]
- Port, J. (2018). Diagnosis of Attention Deficit Hyperactivity Disorder by Using MR Imaging and Radiomics: A Potential Tool for Clinicians. Radiology, 287(2), 631–632. [DOI] [PubMed] [Google Scholar]
- Rauch, S. L. , Shin, L. M. , & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research‐‐past, present, and future. Biological Psychiatry, 60, 376–382. [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. , Uno, H. , Rebert, C. S. , & Finch, C. E. (1990). Hippocampal damage associated with prolonged glucocorticoid exposure in primates. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 10, 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, M. K. , Kesler, S. R. , Hadi Hosseini, S. M. , Kelley, R. G. , Amatya, D. , Hamilton, J. P. , … Gotlib, I. H. (2013). Anomalous gray matter structural networks in major depressive disorder. Biological Psychiatry, 74, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Chen, Y. , Huang, Q. , Lui, S. , Huang,, X. , Shi, Y. , …, Gong, Q. (2018) Psychoradiologic Utility of MR Imaging for Diagnosis of Attention Deficit Hyperactivity Disorder: A Radiomics Analysis. Radiology, 287(2), 620–630. [DOI] [PubMed] [Google Scholar]
- Suo, X. , Lei, D. , Chen, F. , Wu, M. , Li, L. , Sun, L. , … Gong, Q. (2017). Anatomic insights into disrupted small‐world networks in pediatric posttraumatic stress disorder. Radiology, 282, 826–834. [DOI] [PubMed] [Google Scholar]
- Sylvester, C. M. , Corbetta, M. , Raichle, M. E. , Rodebaugh, T. L. , Schlaggar, B. L. , Sheline, Y. I. , … Lenze, E. J. (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijms, B. M. , Moller, C. , Vrenken, H. , Wink, A. M. , de Haan, W. , van der Flier, W. M. , … Barkhof, F. (2013a). Single‐subject grey matter graphs in Alzheimer's disease. PLoS One, 8, e58921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijms, B. M. , Series, P. , Willshaw, D. J. , & Lawrie, S. M. (2012). Similarity‐based extraction of individual networks from gray matter MRI scans. Cerebral Cortex (New York, NY: 1991), 22, 1530–1541. [DOI] [PubMed] [Google Scholar]
- Tijms, B. M. , Sprooten, E. , Job, D. , Johnstone, E. C. , Owens, D. G. , Willshaw, D. , … Lawrie, S. M. (2015). Grey matter networks in people at increased familial risk for schizophrenia. Schizophrenia Research, 168, 1–8. [DOI] [PubMed] [Google Scholar]
- Tijms, B. M. , Ten Kate, M. , Wink, A. M. , Visser, P. J. , Ecay, M. , Clerigue, M. , … Barkhof, F. (2016). Gray matter network disruptions and amyloid beta in cognitively normal adults. Neurobiology of Aging, 37, 154–160. [DOI] [PubMed] [Google Scholar]
- Tijms, B. M. , Wink, A. M. , de Haan, W. , van der Flier, W. M. , Stam, C. J. , Scheltens, P. , & Barkhof, F. (2013b). Alzheimer's disease: Connecting findings from graph theoretical studies of brain networks. Neurobiology of Aging, 34, 2023–2036. [DOI] [PubMed] [Google Scholar]
- Tijms, B. M. , Yeung, H. M. , Sikkes, S. A. , Moller, C. , Smits, L. L. , Stam, C. J. , … Barkhof, F. (2014). Single‐subject gray matter graph properties and their relationship with cognitive impairment in early‐ and late‐onset Alzheimer's disease. Brain Connectivity, 4, 337–346. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M.P. , de Lange, S.C. , Zalesky, A. , Seguin, C. , Yeo, B. T. T. , & Schmidt, R. (2017) Proportional thresholding in resting‐state fMRI functional connectivity networks and consequences for patient‐control connectome studies: Issues and recommendations. NeuroImage, 152, 437–449. [DOI] [PubMed] [Google Scholar]
- Van Wijk, B. C. M. , Stam, C. J. , & Daffertshofer, A. (2010) Comparing brain networks of different size and connectivity density using graph theory. PLoS One, 5: e13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wang, X. , Xia, M. , Liao, X. , Evans, A. , & He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Frontiers in Human Neuroscience, 9, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, D. J. , & Strogatz, S. H. (1998). Collective dynamics of 'small‐world' networks. Nature, 393, 440–442. [DOI] [PubMed] [Google Scholar]
- Weathers, F. , Litz, B. , Herman, D. , Huska, J. , & Keane, T. (1994). The PTSD checklist‐civilian version (PCL‐C). Boston, MA: National Center for PTSD. [Google Scholar]
- Yehuda, R. , Hoge, C. W. , McFarlane, A. C. , Vermetten, E. , Lanius, R. A. , Nievergelt, C. M. , … Hyman, S. E. (2015). Post‐traumatic stress disorder. Nature Reviews: Disease Primers, 1, 15057. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010). Network‐based statistic: Identifying differences in brain networks. NeuroImage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Wang, J. , Wu, Q. , Kuang, W. , Huang, X. , He, Y. , & Gong, Q. (2011). Disrupted brain connectivity networks in drug‐naive, first‐episode major depressive disorder. Biological Psychiatry, 70, 334–342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


