Abstract
Electroconvulsive therapy (ECT) is an effective and rapid treatment for major depressive disorder (MDD). However, the neurobiological underpinnings of ECT are still largely unknown. Recent studies have identified dysregulated brain networks in MDD. Therefore, we hypothesized that ECT may improve MDD symptoms through reorganizing these networks. To test this hypothesis, we used resting‐state functional connectivity to investigate changes to the intra‐ and internetwork architecture of five reproducible resting‐state networks: the default mode network (DMN), dorsal attention network (DAN), executive control network (CON), salience network (SAL), and sensory‐motor network. Twenty‐three MDD patients were assessed before and after ECT, along with 25 sex‐, age‐, and education‐matched healthy controls. At the network level, enhanced intranetwork connectivities were found in the CON in MDD patients after ECT. Furthermore, enhanced internetwork connectivities between the DMN and SAL, and between the CON and DMN, DAN, and SAL were also identified. At the nodal level, the posterior cingulate cortex had increased connections with the left posterior cerebellum, right posterior intraparietal sulcus (rpIPS), and right anterior prefrontal cortex. The rpIPS had increased connections with the medial PFC (mPFC) and left anterior cingulate cortex. The left lateral parietal had increased connections with the dorsal mPFC (dmPFC), left anterior prefrontal cortex, and right anterior cingulate cortex. The dmPFC had increased connection with the left anterolateral prefrontal cortex. Our findings indicate that enhanced interactions in intra‐ and internetworks may contribute to the ECT response in MDD patients. These findings provide novel and important insights into the neurobiological mechanisms underlying ECT.
Keywords: electroconvulsive therapy, internetwork, intranetwork, major depressive disorder, resting‐state functional connectivity
1. INTRODUCTION
Major depressive disorder (MDD) is characterized by the core symptoms of low mood, lack of interest, anhedonia, and reduced energy. It is one of the most prevalent and serious psychiatric disorders, often leading to an increased risk of suicide (Belzung, Willner, & Philippot, 2015; Jia et al., 2010). Electroconvulsive therapy (ECT) is an effective and widely used treatment for MDD, especially for treatment‐resistant depression because of the rapid response to the disease (Kellner et al., 2012). However, the neurobiological underpinnings of ECT are still largely unknown. Recent studies have shown that MDD exhibits not only affective deficits, but also cognitive impairments and somatic symptoms, which are driven by different functional networks (Disner, Beevers, Haigh, & Beck, 2011; Drevets, 2001; Veer et al., 2010; Zhang et al., 2016). Increasingly, MDD is being recognized as a disorder of dysregulated interconnected brain networks, rather than a disruption in one single brain area (Wang, Hermens, Hickie, & Lagopoulos, 2012b; Wu et al., 2017, 2016).
Resting‐state functional magnetic resonance imaging (fMRI), which enables the identification of spontaneous neural activity related to self‐initiated behavior, can provide a task‐free approach to detect the intrinsic functional modules in the brain (Eickhoff & Grefkes, 2011; Fox & Raichle, 2007; Wang et al., 2017c, 2015b, 2016b). Using seed‐based analysis and independent components analysis (Calhoun, Adali, Pearlson, & Pekar, 2001; Damoiseaux et al., 2006), five resting‐state networks, the default mode network (DMN), dorsal attention network (DAN), executive control network (CON), salience network (SAL), and sensory‐motor network (SMN) have been reproducibly identified in many previous studies (Mantini, Perrucci, Del Gratta, Romani, & Corbetta, 2007; Yeo et al., 2011). The DMN primarily participates in self‐referential functioning and autobiographical memory (Buckner, Andrews‐Hanna, & Schacter, 2008; Raichle et al., 2001; Zhang et al., 2014). In contrast to the DMN, the DAN is typically involved in top‐down attention processing, and the ECN in the management of exogenous cognitive functions, and externally guided cognition (Corbetta & Shulman, 2002; Seeley et al., 2007; Sridharan, Levitin, & Menon, 2008; Wang et al., 2016a). The SAL and SMN are separately implicated in allocating attentional resource to salient events, sensory‐motor processing, and emotional experience (Lindquist, Wager, Kober, Bliss‐Moreau, & Barrett, 2012; Menon & Uddin, 2010; Menon, 2011).
The alteration of these five brain networks in MDD has been documented in previous studies. Greicius et al. (2007) first reported abnormal functional connectivities of the DMN in MDD patients. Subsequently, a large number of studies have also found abnormal activity in the DMN (Broyd et al., 2009; Zhu et al., 2012). This is mainly related to negative rumination, and can be normalized by antidepressant treatment (Dichter, Gibbs, & Smoski, 2015; Li et al., 2013). Sheline, Price, Yan, and Mintun (2010) found abnormal functional activity not only in the DMN, but also in the CON and the affective network in MDD patients, and that the three networks are mediated by a dorsal nexus area of the medial prefrontal cortex. Studies testing MDD patients after ECT have shown decreased global functional connectivities in the CON subcomponent of the dorsolateral prefrontal cortex (dlPFC), and reversed functional connectivity between the posterior DMN and dlPFC (Abbott et al., 2013; Perrin et al., 2012). Abnormal activity in the SAL network has been widely reported in depression, and the functional activities in SAL are of high relevance to the treatment response (Anand et al., 2005; Dutta, McKie, & Deakin, 2014; Wu et al., 2011). For the DAN network, a recent meta‐analysis revealed that disrupted connectivity between the frontoparietal network and DAN may reflect biases toward ruminative thoughts at the cost of attending to the external world (Kaiser, Andrews‐Hanna, Wager, & Pizzagalli, 2015). In addition to the aforementioned networks, structural and functional changes in the SMN have also been observed (Guo et al., 2011; Schmaal et al., 2017; Wu et al., 2016; Zhang et al., 2016). Our recent study demonstrated that the functional connectivity density in the SMN is closely related to the response to ECT in MDD patients, which may be an early predictor of treatment response (Wang et al., 2017b). Although previous studies have identified structural and functional changes in these five large‐scale networks in MDD patients, how these networks reorganize their interaction after ECT remains largely unknown.
In the present study, we aimed to increase our understanding of the neurobiological basis of ECT in MDD by investigating the altered intra‐ and internetwork functional connectivities in five brain networks: the DMN, DAN, CON, SAL, and SMN. We hypothesized that ECT may reconfigure the intranetwork connectivities of the CON to enhance internetwork connectivities between the DMN, DAN, SAL and SMN, which may contribute to the response to ECT in MDD patients.
2. MATERIALS AND METHODS
2.1. Participants
Twenty‐three MDD patients participating in ECT treatment were recruited at Anhui Mental Health Center. MDD was diagnosed using the Diagnostic and Statistical Manual of Mental Disorders‐IV (DSM‐IV) criteria which is the Chinese translation of the English DSM‐IV (Jiang, 2012). Patients with treatment‐resistant MDD or with acute suicidal tendencies were entered for ECT. Patients with substance dependence, pregnancy, life threatening somatic disease, neurological disorders, other comorbid mental disorders, previous ECT treatment, as well as MRI‐contraindications were excluded. A total of 23 participants (11 males and 12 females, age range 18–55 years, mean age = 38.74, standard deviation = 11.02; mean education level = 8.83 years, standard deviation = 3.89) were enrolled in the present study. All patients continued to take anti‐depressive medication during the course of receiving ECT. The severity of depression was assessed using the 17‐item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). The scale was administered 12–24 h before the first ECT session and 24–72 h after the last ECT session. The antidepressive ECT response was evaluated using a paired two‐sided t test on the HRSD scores, and the threshold for significance was set at p < .05. Additionally, 25 sex, age, and education matched healthy controls (HC) (12 males and 13 females, range 26–51 years, mean = 39.52 years, standard deviation = 8.07; mean education level = 8.84 years, standard deviation = 3.05) were included. All participants were right‐handed and provided written informed consent. The study was conducted in accordance with the latest revision of the Declaration of Helsinki and had full ethical approval from the local ethics committees of Anhui Medical University.
2.2. ECT procedures
A modified bi‐frontal ECT protocol using a Thymatron System IV Integrated ECT System (Somatics, Lake Bluff, IL, USA) in Anhui Mental Health Center was used in the present study. The first three ECT sessions were administered on consecutive days, and the subsequent ECT sessions were conducted every other day with a break over the weekends until patients reached symptom remission. The criterion for remission was defined as the HRSD score of patient was not more than 7. The mean of the total duration of the ECT sessions was 14.6 ± 5.8 days. During ECT, all the MDD patients were anesthetized using propofol. A previous study has revealed that patients were anesthetized using propofol affecting resting state brain activity. Given that patients were scanned 24–72 h after the last ECT and propofol half‐life is between 1 and 8 h (dependent on infusion duration), this should not have a confounding effect on the data (Guldenmund et al., 2013). In addition, succinylcholine and atropine were used to relax the musculature and suppress the secretion of glands. Electroencephalography was used to monitor seizure activity. The initial percent energy dial was set based on the age of each participant (e.g., 50% for a 50‐year‐old patient). The stimulation strength was evenly adjusted with an increment of 5% of the maximum charge (approximately 1,000 millicoulombs) in our treatment strategy. If no seizure activity was detected with the initial stimulation setting, the percent energy was increased until seizure was visually observed (Wang et al., 2017a, 2017b; Wei et al., 2014). The final energy used was 42.1 ± 11.7% of the maximum charge.
2.3. MRI data acquisition
All MRI data were acquired on a clinical 3.0T whole‐body MRI system (Signa HDxt, GE Healthcare, Buckinghamshire, UK) located at the First Affiliated Hospital of Anhui Medical University. Patients were scanned 12–24 h before the first ECT session and 24–72 h after the last ECT session. In addition, the healthy controls were scanned once to determine pre‐treatment neural alterations in the patients. Participants were instructed to relax and to keep their eyes closed, to remain awake and not to think of anything during the MRI acquisition. The resting‐state functional images were acquired using a standard echo planar imaging (EPI) sequence. The main parameters were: repetition time/echo time ratio = 2,000/22.5 ms, 240 volumes, flip angle = 30°, 33 slices, thickness/gap = 4.0/0.6 mm, voxel size = 3.4 × 3.4 × 4.6 mm3, matrix size = 64 × 64, field of view = 220 × 220 mm2. Total acquisition of resting‐state MRI was 8 min. We also acquired a T1‐weighted anatomical image with 188 slices for each patient to further identify and discard gross radiological alterations. (TR = 8.676 ms; TE = 3.184 ms; inversion time = 800 ms; flip angle = 8°; field of view = 256 × 256 mm; slice thickness = 1 mm; voxel size = 1 × 1 × 1 mm).
2.4. Resting‐state fMRI data preprocessing
Preprocessing of the resting‐state fMRI data was carried out using SPM8 software. The first 10 volumes were discarded to allow for magnetization equilibrium. The slice timing for the remaining images was corrected, and images were realigned to the first volume to account for head motion. All participants who showed a maximum displacement of less than 3 mm and an angular motion of less than 3° were included in the subsequent analyses. All fMRI images were normalized to the Montreal Neurological Institute (MNI) template and resampled at 3 × 3 × 3 mm3. The functional images were smoothed using a Gaussian kernel of 6 mm FWHM. Subsequently, the functional images were filtered with a temporal band‐path of 0.01–0.1 Hz and six motion parameters, and white matter and cerebrospinal fluid signals were regressed out (Wang et al., 2015a, 2012a). As previous studies have shown that global mean signal regression can lead to spurious resting‐state functional correlations and false inferences, particularly at group level inference (Gotts et al., 2013; Saad et al., 2013), the global mean signal was not regressed during preprocessing.
2.5. Definition of the five networks
We defined the DMN, DAN, CON, SAL, and SMN on the basis of a previous study which identified 36 regions of interest (ROIs) derived by maximizing the topographic concordance between results obtained by seed‐based correlation mapping and by spatial independent component analysis to represent the five networks (Brier et al., 2012). Because the coordinate for the right putamen was missed in Brier and colleagues' study, the updated coordinate was adopted from study of Zhan et al. (2016). Finally, the peak MNI coordinates of the 36 voxels were used to create the ROIs with 6 mm radius to study the intra‐ and internetwork connections.
2.6. Intra‐ and internetworks connections
Intra‐ and internetwork functional connections were measured using Pearson's correlation coefficient. First, the correlation coefficient was calculated between the mean time series of each pair of the 36 ROIs for each participant. Then, the intranetwork connection was calculated by averaging the correlation coefficients of all ROI pairs in each particular network. Internetwork connection was computed by averaging the correlation coefficients of all ROI pairs belonging to different networks.
2.7. Nodal level connections
Intra‐ and internetwork connectivity analyses cannot differentiate potentially focal effects at the individual ROI pair level, thus, we evaluated the connectivity differences between all the possible pairs of ROIs. For the 36 ROIs, we calculated the correlation coefficient between any pair of the 36 ROIs, and a 36 × 36 matrix was obtained. Finally, 630 connections between the 36 ROIs were obtained. The connectivity differences for all the 630 connections between all the possible pairs of ROIs were assessed between MDD patients before and after ECT, and between HCs and MDD patients before and after ECT.
2.8. Statistical and correlation analyses
Paired t‐tests were used to determine differences in the intra‐, internetwork, and nodal level connections in MDD patients before and after ECT. Two‐sample t tests were used to identify differences in the intra‐, internetwork, and nodal level connections between HCs and MDD patients before and after ECT. For intra‐ and internetwork connections, the significance threshold was set at p < .05/15 (5 intranetworks and 10 internetwork) using a Bonferroni correction for multiple comparisons. For the ROI pair connection analyses, the significance threshold was set at p < .05/630 (630 connections between any pair of the 36 ROIs) using a Bonferroni correction for multiple comparisons.
Finally, correlation analyses between HRSD scores and intra‐, internetwork, and nodal level connections in MDD patients before ECT and after ECT were performed separately to reveal whether functional connections were associated with disease symptoms. Furthermore, correlation analyses between the changes in intra‐network, internetwork, and nodal connections of MDD patients following ECT, and the changes in HRSD scores, were performed to further explore whether the neuroimaging indices were related to changes at the symptom‐level. The significance level was set at p < .05.
3. RESULTS
3.1. Demographic and clinical characteristics
A chi‐squared test and two‐sample t test were used to determine any differences in sex, age, and education years. There were no statistically significant differences in sex (p = .99), age (p = .78), or education level (p = .99) between the MDD and HC groups (Table 1). Within the group of MDD patients, the depressive symptom load as assessed by the HRSD significantly decreased during ECT treatment (p < 10−13) (Table 1).
Table 1.
Demographic and clinical variables
| Participants | MDD | Healthy controls | P value |
|---|---|---|---|
| Number of participants | 23 | 25 | |
| Age (mean ± SD) years | 38.74 ± 11.02 | 39.52 ± 8.07 | 0.78 |
| Gender (male/female) | 11/12 | 12/13 | 0.99 |
| Education level (mean ± SD) years | 8.83 ± 3.89 | 8.84 ± 3.05 | 0.99 |
| HRSD scores (mean ± SD) | |||
| Before ECT | 22.22 ± 4.74 | ||
| After ECT | 3.83 ± 2.15 | ||
| Number of treatment (mean ± SD) | 7.26 ± 2 | ||
| Age of onset (years) | 33.90 ± 12.26 | ||
| Durations of illness (months) | 70.35 ± 83.27 | ||
| Episodes (n patients) | |||
|
First Recurrence |
8 15 |
||
| Family history (n patients) | 2 | ||
| Medication (n patients) | 23 | ||
| Medication‐free | 0 | ||
| On‐medication | 23 | ||
Note. A Pearson chi‐squared test was used for gender comparison. Two‐sample t tests were used for age, education comparisons.
MDD, major depressive disorder; HRSD, Hamilton Rating Scale for Depression.
3.2. Alteration of intranetwork connections
Statistical analysis identified significantly increased intra‐network connections of the CON in MDD patients after ECT compared with MDD patients before ECT, and compared with HCs (Figure 1). There were no significant differences in intra‐network connections of the CON between HCs and MDD patients before ECT (Figure 1). Additionally, we did not find significant differences in intranetwork connections in any of the other four networks, which were the DMN, DAN, SAL, and SMN, in MDD patients after ECT compared with MDD patients before ECT after correction for multiple comparisons (Figure 1).
Figure 1.
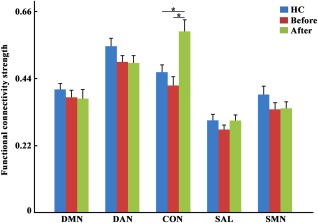
Increased intranetwork functional connectivity in the executive control network (CON) was observed in major depressive disorder (MDD) patients after electroconvulsive therapy (ECT). The increased intranetwork functional connectivity in the CON was also found between MDD patients after ECT, and healthy controls. The significance level was determined using Bonferroni correction for multiple comparisons with p < .05 [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Alteration of internetwork connections
Statistical analysis identified significantly increased internetwork connections between the DMN and SAL in MDD patients after ECT compared with MDD patients before ECT (Figure 2). The significantly enhanced internetwork connections between the CON and DMN, DAN, and SAL were also found in MDD patients after ECT compared with MDD patients before ECT (Figure 2). In addition, significantly increased internetwork connections were observed between the DMN and CON, and SAL in MDD patients after ECT compared with HCs (Figure 2). There were no significant differences in internetwork connections between HCs and MDD patients before ECT (Figure 2).
Figure 2.
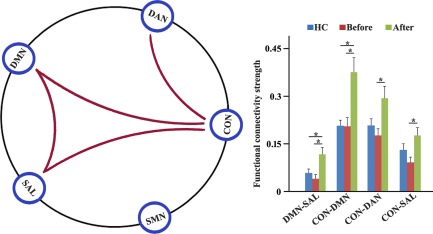
The longitudinal effect of electroconvulsive therapy (ECT) on internetwork connectivities. Five reproducible resting‐state networks: The default mode network (DMN), dorsal attention network (DAN), executive control network (CON), salience network (SAL), and sensory‐motor network (SMN) were studied. Increased inter‐network connectivities between the DMN and SAL, and between the CON and DMN, DAN and SAL were found in major depressive disorder (MDD) patients after ECT compared with MDD patients before ECT. The significantly increased internetwork connectivities between the DMN, SAL, and CON were also identified in MDD patients after ECT compared with healthy controls. The significance level was set at p < .05 using Bonferroni correction for multiple comparisons [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Nodal level interconnections
At the nodal level, enhanced connections were found in MDD patients after ECT. The posterior cingulate cortex (PCC) had increased connections with the left posterior cerebellum (lpCBL), right posterior intraparietal sulcus (rpIPS), and right anterior prefrontal cortex (raPFC) in MDD patients after ECT compared with both MDD patients before ECT, and HCs. The rpIPS had increased connections with the medial PFC (mPFC) and left anterior cingulate cortex (lACC) in MDD patients after ECT compared with MDD patients before ECT, and HCs. The left lateral parietal (lLP) had stronger connections with the dorsal mPFC (dmPFC), left aPFC, and right ACC in MDD patients after ECT than MDD patients before ECT, and HCs, except for the connectivity between the lLP and right ACC, for which no significant difference between MDD patients after ECT and HCs was found. The dmPFC had enhanced connection with laPFC in MDD patients after ECT compared with MDD patients before ECT, and HCs. In addition, altered functional connection between the rpIPS and lACC was found between all three groups. The MDD group showed significant reduction in functional connections between the rpIPS and lACC compared with both HCs and MDD patients after ECT (Figure 3).
Figure 3.
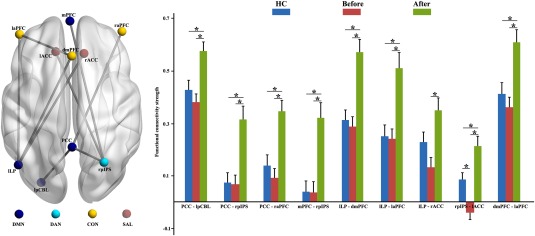
Increased resting‐state functional connectivity (RSFC) between nodal pairs in major depressive disorder (MDD) patients after electroconvulsive therapy (ECT). To account for potentially focal effects at the individual node pair level, the RSFC between each pair of nodes was calculated. Enhanced connections between the posterior cingulate cortex (PCC) and left posterior cerebellum, right posterior intraparietal sulcus (rpIPS), and right anterior prefrontal cortex (raPFC), between the rpIPS and medial PFC (mPFC), left anterior cingulate cortex (lACC), between the left lateral parietal (lLP) and dorsal mPFC (dmPFC), left aPFC, and right anterior cingulate cortex (rACC), and between the dmPFC and laPFC were found in MDD patients after ECT compared with before ECT. Similar results were also found between MDD patients after ECT and HCs except the connection between the lLP and right ACC. In addition, significantly decreased functional connection between the rpIPS and lACC was found in MDD patients compared with MDD patients after ECT, and HCs [Color figure can be viewed at http://wileyonlinelibrary.com]
3.5. Correlation analyses
We identified significant correlations between HRSD scores and intra‐network connection of the CON, internetwork connection between the CON and DMN, functional connectivity between the PCC and raPFC, and functional connectivity between the dmPFC and laPFC in MDD patients before ECT (Figure 4). In addition, significant correlations were found for MDD patients between the changed HRSD scores and changed intranetwork connections of the CON, changed internetwork connection between the CON and DMN, changed functional connectivity between the PCC and raPFC, and changed functional connectivity between the dmPFC and laPFC (Figure 4). We did not find any significant correlations between the functional connections and HRSD scores in MDD patients after ECT.
Figure 4.
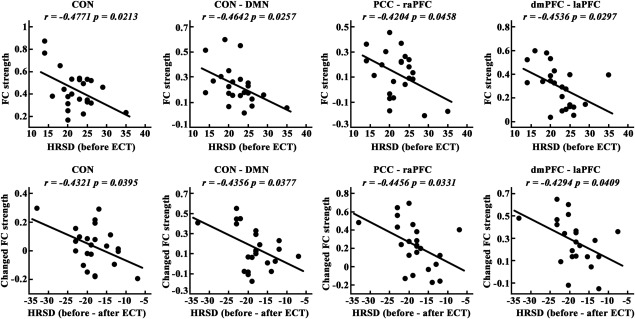
Significant correlations between Hamilton Rating Scale for Depression (HRSD) scores and intra‐network connection of the executive control network (CON), inter‐network connection between the CON and default node network (DMN), and nodal connections between the posterior cingulate cortex (PCC) and right anterior prefrontal cortex (raPFC) and between the dorsal mPFC (dmPFC) and left anterolateral prefrontal cortex (laPFC) in major depressive disorder (MDD) patients before electroconvulsive therapy (ECT). Furthermore, significant correlations between the changes of HRSD scores and changes of intra‐network connection of the CON, inter‐network connection between the CON and DMN, and nodal connections between the PCC and raPFC and between the dmPFC and laPFC were also identified in MDD patients before and after ECT
4. DISCUSSION
By assessing functional interactions within and between the five networks and nodal pairs, we aimed to reveal whether and how the interactions were modulated by ECT in MDD patients. Our findings showed that ECT can increase intra‐network connectivity of the CON and internetwork interactions between the DMN and SAL, and between the CON and DMN, DAN, and SAL. Furthermore, we also found that ECT enhanced interactions between nodal pairs. The PCC had increased connections with the lpCBL, rpIPS, and raPFC. The rpIPS had increased connections with the mPFC and lACC. The lLP had increased connections with the dmPFC, left aPFC, and right ACC. The dmPFC had increased connection with the laPFC. These findings indicate that ECT can reorganize the functional architecture of the brain in MDD patients for effective therapy.
4.1. Intranetwork connectivity
In this study, we identified increased intranetwork connectivity of the CON at the module level. At the nodal level, we further showed the increased functional connection between the CON's subareas of the dmPFC and laPFC. Our findings are supported by previous studies in MDD patients, which identified abnormal functional activity within the executive control network (Elderkin‐Thompson, Mintz, Haroon, Lavretsky, & Kumar, 2007; Sheline et al., 2006). In addition, a recent study in MDD revealed that the dmPFC is a hot‐wired hub integrating and modulating the different functional subsystems in depression (Sheline et al., 2010). The key role of the dmPFC in functional integration is supported by functionally heterogenous subareas and distinct functional connectivity profiles to the limbic system and DMN (Eickhoff, Laird, Fox, Bzdok, & Hensel, 2016). Thus, the CON is an important interface for affective and cognitive information processing in MDD. Furthermore, the laPFC, which is a key neural substrate of an emotion processing and regulation circuit, has been reported to be involved in mood regulation and cognitive reappraisal (Fuster, 2001; McRae et al., 2010; Phan et al., 2005; Phillips, Drevets, Rauch, & Lane, 2003). Thus, the increased intranetwork connectivity of the CON and nodal connectivity between the dmPFC and laPFC suggests that ECT may enhance the integration of emotional and cognitive information processing to improve emotional processing ability via cognitive reappraisal in MDD patients. However, our current study only identified significantly increased intranetwork connectivities in MDD patients after ECT compared with MDD patients and HC participants. We did not find significant differences in intranetwork connectivity between HC subjects and MDD patients. A recent study identified reduced intranetwork connectivity in the DAN between HC subjects and MDD patients using functional connectivity analyses (Sacchet et al., 2016). This discrepancy may result from the heterogeneity of MDD patients, the duration of illness, the small number of participants in our study, differing definitions for the five networks, and different methods to calculate the intra‐network connections.
4.2. Internetwork connectivity
Disrupted intra‐network and internetwork connectivities in MDD have been found in many previous studies (Crowther et al., 2015; Kaiser et al., 2015; Wang et al., 2012b). In our current study, we not only identified increased intra‐network connectivity, but also found enhanced internetwork connections between the DMN and SAL, and between the CON and DMN, DAN, and SAL in MDD patients after ECT. Our findings indicate that ECT can reconfigure the architecture of the brain networks in MDD patients. The DMN is one of the most important functional modules in the human brain and plays a crucial role in retrieving autobiographical memories, envisioning the future, and conceiving the perspectives of others (Buckner et al., 2008). In MDD patients, DMN hyperactivity has been widely reported to be related to rumination and self‐associations (Disner et al., 2011; Pizzagalli, 2011). In contrast to the DMN, the DAN is involved in preparing and applying goal‐directed selection for stimuli (Corbetta & Shulman, 2002), and DAN hypoactivity in MDD is related to emotional, visceral, and autonomic dysregulation (Sheline et al., 2010). The SAL, which mainly includes the anterior cingulate cortex and anterior insula, plays an important role in segregating internal and external stimuli to guide behavior choice (Menon & Uddin, 2010). Dysregulation of this network may be related to the negative interpretation bias found in MDD (Hamilton et al., 2012). Thus, the increased internetwork connectivities between the CON and DMN, DAN, SAL suggest that the CON may provide a link with different functional networks to regulate adaptive engagement of task‐relevant and disengagement of task‐irrelevant brain regions in information detection and processing. Moreover, we found enhanced internetwork connectivity between the DMN and SAL in MDD patients responding to ECT, which indicates that ECT may regulate the SAL to disengage from a rumination state and to allocate attentional resources to goal‐guided stimuli. At the nodal level connectivity analysis, we additionally identified increased internetwork connectivities between the rpIPS and mPFC, and PCC. The rpIPS is part of the DAN, and the mPFC and PCC are part of the DMN. The enhanced internetwork connectivity between the DMN subareas of the mPFC and PCC, and DAN subarea of the rpIPS implies that ECT may promote switching between the DAN and DMN. Collectively, our results support the argument that normal and efficient behavior depend on the balance between local processing and global integration of information, and the coordinated activity of brain networks (Bullmore & Sporns, 2012; Yeo et al., 2011). Furthermore, our findings suggest that the pathology of MDD may result from the disrupted balance between different brain networks. Finally, ECT may coordinate the dynamic interaction of different networks in the effective response to ECT treatment in MDD patients.
5. CONCLUSION
We identified increased intra‐ and internetwork connectivities in MDD patients following ECT. These findings indicate that ECT can enhance the functional reorganization of disrupted brain networks in MDD. Our findings provide new insights into the underlying mechanisms of ECT, which may promote the widespread application of ECT in clinical practice.
Wang J, Wei Q, Wang L, et al. Functional reorganization of intra‐ and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum Brain Mapp. 2018;39:1403–1411. 10.1002/hbm.23928
Funding information National Natural Science Foundation of China, Grant Number(s): 31500867, 81601187, 81671354, and 81471117.
Contributor Information
Yanghua Tian, Email: ayfytyh@126.com.
Kai Wang, Email: wangkai1964@126.com.
REFERENCES
- Abbott, C. C. , Lemke, N. T. , Gopal, S. , Thoma, R. J. , Bustillo, J. , Calhoun, V. D. , & Turner, J. A. (2013). Electroconvulsive therapy response in major depressive disorder: A pilot functional network connectivity resting state FMRI investigation. Frontiers in Psychiatry, 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, A. , Li, Y. , Wang, Y. , Wu, J. , Gao, S. , Bukhari, L. , … Lowe, M. J. (2005). Antidepressant effect on connectivity of the mood‐regulating circuit: An FMRI study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 30, 1334–1344. [DOI] [PubMed] [Google Scholar]
- Belzung, C. , Willner, P. , & Philippot, P. (2015). Depression: From psychopathology to pathophysiology. Current Opinion in Neurobiology, 30, 24–30. [DOI] [PubMed] [Google Scholar]
- Brier, M. R. , Thomas, J. B. , Snyder, A. Z. , Benzinger, T. L. , Zhang, D. , Raichle, M. E. , … Ances, B. M. (2012). Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32, 8890–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd, S. J. , Demanuele, C. , Debener, S. , Helps, S. K. , James, C. J. , & Sonuga‐Barke, E. J. (2009). Default‐mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews, 33, 279–296. [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2012). The economy of brain network organization. Nature Reviews: Neuroscience, 13, 336–349. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Crowther, A. , Smoski, M. J. , Minkel, J. , Moore, T. , Gibbs, D. , Petty, C. , … Dichter, G. S. (2015). Resting‐state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40, 1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux, J. S. , Rombouts, S. A. , Barkhof, F. , Scheltens, P. , Stam, C. J. , Smith, S. M. , & Beckmann, C. F. (2006). Consistent resting‐state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103, 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter, G. S. , Gibbs, D. , & Smoski, M. J. (2015). A systematic review of relations between resting‐state functional‐MRI and treatment response in major depressive disorder. Journal of Affective Disorders, 172, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner, S. G. , Beevers, C. G. , Haigh, E. A. , & Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12, 467–477. [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. (2001). Neuroimaging and neuropathological studies of depression: Implications for the cognitive‐emotional features of mood disorders. Current Opinion in Neurobiology, 11, 240–249. [DOI] [PubMed] [Google Scholar]
- Dutta, A. , McKie, S. , & Deakin, J. F. (2014). Resting state networks in major depressive disorder. Psychiatry Research, 224, 139–151. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , & Grefkes, C. (2011). Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clinical Engineering & Neuroscience, 42, 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Fox, P. T. , Bzdok, D. , & Hensel, L. (2016). Functional segregation of the human dorsomedial prefrontal cortex. Cerebral Cortex, 26, 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin‐Thompson, V. , Mintz, J. , Haroon, E. , Lavretsky, H. , & Kumar, A. (2007). Executive dysfunction and memory in older patients with major and minor depression. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 22, 261–270. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Fuster, J. M. (2001). The prefrontal cortex—An update: Time is of the essence. Neuron, 30, 319–333. [DOI] [PubMed] [Google Scholar]
- Gotts, S. J. , Saad, Z. S. , Jo, H. J. , Wallace, G. L. , Cox, R. W. , & Martin, A. (2013). The perils of global signal regression for group comparisons: A case study of Autism Spectrum Disorders. Frontiers in Human Neuroscience, 7, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, M. D. , Flores, B. H. , Menon, V. , Glover, G. H. , Solvason, H. B. , Kenna, H. , … Schatzberg, A. F. (2007). Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldenmund, P. , Demertzi, A. , Boveroux, P. , Boly, M. , Vanhaudenhuyse, A. , Bruno, M. A. , … Soddu, A. (2013). Thalamus, brainstem and salience network connectivity changes during propofol‐induced sedation and unconsciousness. Brain Connectivity, 3, 273–285. [DOI] [PubMed] [Google Scholar]
- Guo, W. B. , Liu, F. , Xue, Z. M. , Yu, Y. , Ma, C. Q. , Tan, C. L. , … Zhao, J. P. (2011). Abnormal neural activities in first‐episode, treatment‐naive, short‐illness‐duration, and treatment‐response patients with major depressive disorder: A resting‐state fMRI study. Journal of Affective Disorders, 135, 326–331. [DOI] [PubMed] [Google Scholar]
- Hamilton, J. P. , Etkin, A. , Furman, D. J. , Lemus, M. G. , Johnson, R. F. , & Gotlib, I. H. (2012). Functional neuroimaging of major depressive disorder: A meta‐analysis and new integration of base line activation and neural response data. American Journal of Psychiatry, 169, 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology & Neurosurgery Psychology, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Z. , Huang, X. , Wu, Q. , Zhang, T. , Lui, S. , Zhang, J. , … Gong, Q. (2010). High‐field magnetic resonance imaging of suicidality in patients with major depressive disorder. American Journal of Psychiatry, 167, 1381–1390. [DOI] [PubMed] [Google Scholar]
- Jiang, K. (2012). Guidelines for the prevention and treatment of depressive disorder. Beijing: Peking University Medical Press. [Google Scholar]
- Kaiser, R. H. , Andrews‐Hanna, J. R. , Wager, T. D. , & Pizzagalli, D. A. (2015). Large‐Scale network dysfunction in major depressive disorder: A meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry, 72, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, C. H. , Greenberg, R. M. , Murrough, J. W. , Bryson, E. O. , Briggs, M. C. , & Pasculli, R. M. (2012). ECT in treatment‐resistant depression. The American Journal of Psychiatry, 169, 1238–1244. [DOI] [PubMed] [Google Scholar]
- Li, B. , Liu, L. , Friston, K. J. , Shen, H. , Wang, L. , Zeng, L. L. , & Hu, D. (2013). A treatment‐resistant default mode subnetwork in major depression. Biological Psychiatry, 74, 48–54. [DOI] [PubMed] [Google Scholar]
- Lindquist, K. A. , Wager, T. D. , Kober, H. , Bliss‐Moreau, E. , & Barrett, L. F. (2012). The brain basis of emotion: A meta‐analytic review. Behavioral & Brain Sciences, 35, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini, D. , Perrucci, M. G. , Del Gratta, C. , Romani, G. L. , & Corbetta, M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 104, 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae, K. , Hughes, B. , Chopra, S. , Gabrieli, J. D. , Gross, J. J. , & Ochsner, K. N. (2010). The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience, 22, 248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin, J. S. , Merz, S. , Bennett, D. M. , Currie, J. , Steele, D. J. , Reid, I. C. , & Schwarzbauer, C. (2012). Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proceedings of the National Academy of Sciences of the United States of America, 109, 5464–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, K. L. , Fitzgerald, D. A. , Nathan, P. J. , Moore, G. J. , Uhde, T. W. , & Tancer, M. E. (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57, 210–219. [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , Drevets, W. C. , Rauch, S. L. , & Lane, R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54, 504–514. [DOI] [PubMed] [Google Scholar]
- Pizzagalli, D. A. (2011). Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 36, 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad, Z. S. , Reynolds, R. C. , Jo, H. J. , Gotts, S. J. , Chen, G. , Martin, A. , & Cox, R. W. (2013). Correcting brain‐wide correlation differences in resting‐state FMRI. Brain Connectivity, 3, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet, M. D. , Ho, T. C. , Connolly, C. G. , Tymofiyeva, O. , Lewinn, K. Z. , Han, L. K. , … Yang, T. T. (2016). Large‐scale hypoconnectivity between resting‐state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41, 2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Samann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22, 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline, Y. I. , Barch, D. M. , Garcia, K. , Gersing, K. , Pieper, C. , Welsh‐Bohmer, K. , … Doraiswamy, P. M. (2006). Cognitive function in late life depression: Relationships to depression severity, cerebrovascular risk factors and processing speed. Biological Psychiatry, 60, 58–65. [DOI] [PubMed] [Google Scholar]
- Sheline, Y. I. , Price, J. L. , Yan, Z. , & Mintun, M. A. (2010). Resting‐state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107, 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan, D. , Levitin, D. J. , & Menon, V. (2008). A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer, I. M. , Beckmann, C. F. , van Tol, M. J. , Ferrarini, L. , Milles, J. , Veltman, D. J. , … Rombouts, S. A. (2010). Whole brain resting‐state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience, 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Fan, L. , Wang, Y. , Xu, W. , Jiang, T. , Fox, P. T. , … Jiang, T. (2015a). Determination of the posterior boundary of Wernicke's area based on multimodal connectivity profiles. Human Brain Mapping, 36, 1908–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Fan, L. , Zhang, Y. , Liu, Y. , Jiang, D. , Zhang, Y. , … Jiang, T. (2012a). Tractography‐based parcellation of the human left inferior parietal lobule. NeuroImage, 63, 641–652. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Tian, Y. , Wang, M. , Cao, L. , Wu, H. , Zhang, Y. , … Jiang, T. (2016a). A lateralized top‐down network for visuospatial attention and neglect. Brain Imaging & Behavior, 10, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wei, Q. , Bai, T. , Zhou, X. , Sun, H. , Becker, B. , … Kendrick, K. (2017a). Electroconvulsive therapy selectively enhanced feedforward connectivity from fusiform face area to amygdala in major depressive disorder. Social Cognitive & Affective Neuroscience 12, 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wei, Q. , Yuan, X. , Jiang, X. , Xu, J. , Zhou, X. , … Wang, K. (2017b). Local functional connectivity density is closely associated with the response of electroconvulsive therapy in major depressive disorder. Journal of Affective Disorders, 225, 658–664. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Xie, S. , Guo, X. , Becker, B. , Fox, P. T. , Eickhoff, S. B. , & Jiang, T. (2017c). Correspondent functional topography of the human left inferior parietal lobule at rest and under task revealed using resting‐state fMRI and coactivation based parcellation. Human Brain Mapping, 38, 1659–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Yang, Y. , Fan, L. , Xu, J. , Li, C. , Liu, Y. , … Jiang, T. (2015b). Convergent functional architecture of the superior parietal lobule unraveled with multimodal neuroimaging approaches. Human Brain Mapping, 36, 238–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhang, J. , Rong, M. , Wei, X. , Zheng, D. , Fox, P. T. , … Jiang, T. (2016b). Functional topography of the right inferior parietal lobule structured by anatomical connectivity profiles. Human Brain Mapping. [DOI] [PMC free article] [PubMed]
- Wang, L. , Hermens, D. F. , Hickie, I. B. , & Lagopoulos, J. (2012b). A systematic review of resting‐state functional‐MRI studies in major depression. Journal of Affective Disorders, 142, 6–12. [DOI] [PubMed] [Google Scholar]
- Wei, Q. , Tian, Y. , Yu, Y. , Zhang, F. , Hu, X. , Dong, Y. , … Wang, K. (2014). Modulation of interhemispheric functional coordination in electroconvulsive therapy for depression. Translational Psychiatry, 4, e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , Sun, H. , Wang, C. , Yu, L. , Li, Y. , Peng, H. , … Wang, J. (2017). Abnormalities in the structural covariance of emotion regulation networks in major depressive disorder. Journal of Psychiatric Research, 84, 237–242. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Sun, H. , Xu, J. , Wu, Y. , Wang, C. , Xiao, J. , … Wang, J. (2016). Changed hub and corresponding functional connectivity of subgenual anterior cingulate cortex in major depressive disorder. Frontiers in Neuroanatomy, 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q. Z. , Li, D. M. , Kuang, W. H. , Zhang, T. J. , Lui, S. , Huang, X. Q. , … Gong, Q. Y. (2011). Abnormal regional spontaneous neural activity in treatment‐refractory depression revealed by resting‐state fMRI. Human Brain Mapping, 32, 1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Buckner, R. L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, Y. , Ma, J. , Alexander‐Bloch, A. F. , Xu, K. , Cui, Y. , Feng, Q. , … Liu, Y. (2016). Longitudinal study of impaired intra‐ and inter‐network brain connectivity in subjects at high risk for Alzheimer's Disease Neuroimaging I. Journal of Alzheimer's Disease, 52, 913–927. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Li, L. , Wu, M. , Chen, Z. , Hu, X. , Chen, Y. , … Gong, Q. (2016). Brain gray matter alterations in first episodes of depression: A meta‐analysis of whole‐brain studies. Neuroscience & Biobehavioral Reviews, 60, 43–50. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Fan, L. , Zhang, Y. , Wang, J. , Zhu, M. , Zhang, Y. , … Jiang, T. (2014). Connectivity‐based parcellation of the human posteromedial cortex. Cerebral Cortex, 24, 719–727. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Wang, X. , Xiao, J. , Liao, J. , Zhong, M. , Wang, W. , & Yao, S. (2012). Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Biological Psychiatry, 71(7), 611. [DOI] [PubMed] [Google Scholar]


