Abstract
Deficits in inhibitory control and visual processing are common in youths with attention‐deficit/hyperactivity disorder (ADHD), but little is known about endophenotypes for unaffected siblings of youths with ADHD. This study aimed to investigate the potential endophenotypes of brain activation and performance in inhibitory control and visual processing among ADHD probands, their unaffected siblings, and neurotypical youths. We assessed 27 ADHD probands, 27 unaffected siblings, and 27 age‐, gender‐, and IQ‐matched neurotypical youths using the counting Stroop functional magnetic resonance imaging and two tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB): rapid visual information processing (RVP) for inhibitory control and spatial span (SSP) for visual processing. ADHD probands showed greater activation than their unaffected siblings and neurotypical youths in the right inferior frontal gyrus (IFG) and anterior cingulate cortex. Increased activation in the right IFG was positively correlated with the mean latency of the RVP in ADHD probands. Moreover, ADHD probands and their unaffected siblings showed less activation in the left superior parietal lobule (SPL) than neurotypical youths. Increased activation in the left SPL was positively correlated with the spatial length of the SSP in neurotypical youths. Our findings suggest that less activation in the left SPL might be considered as a candidate imaging endophenotype for visual processing in ADHD.
Keywords: CANTAB, counting Stroop fMRI, inhibitory control, visual processing
1. INTRODUCTION
Attention‐deficit/hyperactivity disorder (ADHD) is known to be highly heritable (Friedman & Rapoport, 2015) with adverse social outcomes (Faraone et al., 2005). Individuals with ADHD may have life‐long deficits in executive functions (Gau & Huang, 2014; Gau & Shang, 2010) and visual memory (Shang & Gau, 2011). Due to its clinical and genetic heterogeneity, despite extensive research in searching genetic etiologies for ADHD, our knowledge about the genetic basis of ADHD is insufficient (Akutagava‐Martins et al., 2013; Bonvicini, Faraone, & Scassellati, 2016). Among the approaches proposed to search for the genetic etiologies (Hawi et al., 2017), endophenotype has been adopted by several studies to help improving phenotyping and to obtain a more genetically homogenous group (Kendler & Neale, 2010).
The endophenotypic marker has been proposed to be more proximal to the risk genes and potential pathophysiological deficits of ADHD than behavioral phenotypes (Doyle et al., 2005). Endophenotypes or intermediate phenotypes for ADHD are broadly defined as heritable and quantifiable neurobiological traits not only that are correlated with ADHD in the probands but also that correlation reflects shared genes (Kendler & Neale, 2010). The focus of our study lies on the following key criteria to define endophenotypes (Doyle et al., 2005; Gottesman & Gould, 2003). First, it is associated with youths with ADHD. Second, the unaffected siblings would exhibit the trait to some extents or demonstrate more severe deficits than the general population (Doyle et al., 2005; Durston et al., 2004). Third, the endophenotype can be measured by instruments with good psychometric properties (Gottesman & Gould, 2003), like the Cambridge Neuropsychological Test Automated Battery (CANTAB) and the counting Stroop test used in this study.
Unaffected siblings usually have similar genetic backgrounds and early‐life environments with probands, with a higher risk of developing the same disorder than the general populations (Doyle et al., 2005). Our previous work based on the unaffected sibling design has provided evidence to support that executive functions (including inhibitory control) (Gau and Shang, 2010), visual memory (Shang & Gau, 2011), and sustained attention (Gau & Huang, 2014) are the potential endophenotypes for ADHD. Among these neuropsychological deficits, impaired inhibitory control is the most prominent cognitive deficit in ADHD (Gau and Shang, 2010; Paloyelis, Mehta, Kuntsi, & Asherson, 2007). Poor performance on visual memory is also noted in children with ADHD (Shang & Gau, 2011). Previous studies solely examined either executive functions (Gau & Huang, 2014; Gau & Shang, 2010; Ni et al., 2013) or visual processing (Shang & Gau, 2011). In this study, we examined both inhibitory control and visual processing for potential endophenotypes of ADHD by using the combination of the neuropsychological tests (CANTAB) and the counting Stroop task in the fMRI assessments.
Previous studies have suggested that intermediate imaging traits linking clinical diagnosis and familial etiology might help accelerate the discovery of risk genes (Paloyelis et al., 2007). Using the GO/NOGO task, previous studies reported that unaffected siblings showed reduced activation in the frontal lobe and anterior cingulate cortex (ACC) compared to healthy controls, suggesting that brain activation of unaffected siblings was similar to that of ADHD probands during inhibitory control (Durston et al., 2006). Using the stop‐signal task, ADHD probands and their unaffected siblings showed decreased activity in the frontostriatal and frontoparietal networks compared to healthy controls (van Rooij et al., 2015). A meta‐analysis of imaging studies of ADHD (Hart et al., 2013) revealed that dysfunction in the inferior frontal gyrus (IFG) and ACC reduced the ability to optimally recruit subsidiary brain regions to perform cognitive tasks in ADHD (Fassbender & Schweitzer, 2006). Notably, the right IFG and ACC are thought to be involved in inhibitory control. Our previous work also showed increased activation in the right IFG and ACC during inhibitory control in youths with ADHD as compared to neurotypical youths during a counting Stroop task (Fan, Gau, & Chou, 2014; Fan, Chou, & Gau, 2017). Moreover, parietal activation is involved in attention and visual processing (Fassbender & Schweitzer, 2006). Less activation within the parietal regions was found during visual processing in children with ADHD (Silk et al., 2008). Moreover, several studies showed robust activity in the left SPL/precuneus (BA 7) during a counting Stroop task, suggesting that ADHD was associated with impaired visual processing (Bush et al., 1998; Fan et al., 2017, 2014). Previous studies have demonstrated that changes in brain activity related to cognitive control are sensitive to a familial risk for ADHD (Durston et al., 2006; van Rooij et al., 2015). Recent studies suggest that inhibitory control and visual memory may serve as endophenotypes for ADHD (Gau & Huang, 2014; Gau & Shang, 2010; Shang & Gau, 2011). The present study aimed to examine whether changes in brain activations during inhibitory control and visual processing in ADHD probands could be observed in their unaffected siblings.
Impaired inhibitory control and visual memory were also found in unaffected siblings of patients with ADHD (Gau & Huang, 2014; Gau & Shang, 2010; Shang & Gau, 2011). Previous studies have shown that patients with ADHD demonstrated poorer performance of inhibitory control in the rapid visual information processing (RVP) test (Gau & Huang, 2014; Gau & Shang, 2010; Ni et al., 2013), and visual processing in the spatial span (SSP) test (Gau and Shang, 2010; Ni et al., 2013), as compared to neurotypical children. The counting Stroop task in the scanner can be used to evaluate the neural substrate of inhibitory control (Bush et al., 1998; Chou, Chia, Shang, & Gau, 2015; Fan et al., 2017, 2014) and visual processing (Chou et al., 2015; Fan et al., 2017, 2014). Furthermore, the contrast of incongruent versus congruent stimuli was associated with inhibitory control, and the contrast of the larger versus the fewer number of words was associated with visual processing in the counting Stroop (Chou et al., 2015; Fan et al., 2017, 2014). Hence, combining the CANTAB and the counting Stroop fMRI offers a better understanding of these two processes.
In this study, we used the CANTAB and the counting Stroop fMRI to investigate the neural correlates of inhibitory control and visual processing in ADHD probands, their unaffected siblings, and neurotypical youths. According to the a priori hypothesis, we expected that the involvement of the right IFG and ACC might be related to the inhibitory control and that the parietal regions might be related to the visual processing. We also anticipated the probands with ADHD as well as their unaffected siblings would have some activation changes in these two brain regions.
2. MATERIALS AND METHODS
2.1. Participants and procedures
We assessed 27 youths (mean age ± standard deviation (SD), 12.1 ± 2.0 years old, ages 9–15, 3 females) with a clinical diagnosis of ADHD according to the DSM‐IV diagnostic criteria, 27 unaffected siblings (mean age ± SD, 13.0 ± 2.6 years old, ages 7–17, 8 females), and 27 neurotypical youths (mean age ± SD, 11.8 ± 1.7 years old, ages 9–16, 6 females) according to the distribution of age, gender, handedness, and IQ of the ADHD group. ADHD probands were recruited from the Department of Psychiatry, National Taiwan University Hospital (NTUH), Taipei, Taiwan. The ADHD probands and their parents were interviewed to confirm their diagnosis of ADHD and to exclude any other psychiatric disorders by the corresponding author (SSG) using the Chinese Kiddie epidemiologic version of the Schedule for Affective Disorders and Schizophrenia (K‐SADS‐E) interview. The neurotypical group was recruited from similar school districts as the ADHD group with the help of principals and school teachers rather than by advertisement outside schools. Similar to the ADHD group, they and their parents were interviewed using the Chinese K‐SADS‐E to ensure that they did not meet the DSM‐IV diagnosis of ADHD or any other psychiatric disorder.
All participants were native Mandarin‐Chinese speakers, had standard scores of the full‐scale IQ >80 as assessed by the Weschler Intelligence Scale for Children‐3rd Edition (WISC‐III) (Wechsler, 1991), and had normal hearing and normal or corrected‐to‐normal vision. Participants with a clinical diagnosis of any other psychiatric disorders were excluded from the study. The study was approved by the Research Ethics Committee at the NTUH, and all the participants and their parents provided written informed consent before study implementation (IRB ID: 201204071RIC; http://ClinicalTrials.gov number, NCT01682915). No participants with ADHD took any medication before the assessments.
2.2. Neuropsychological measures
Rapid visual information processing (RVP) and spatial span (SSP) were chosen from the CANTAB to assess inhibitory control (Gau & Huang, 2014) and visual processing (Gau and Shang, 2010), respectively.
In the RVP test, participants were asked to respond to the specific sequence of digits, when a white box was presented in the center of the screen with digits (ranging from 2 to 9) appearing one at a time (100 digits/min) in a pseudo‐random order. Participants were instructed to respond to three specific number sequences of digits (i.e., 2–4‐6, 3–5‐7, 4–6‐8) by pressing the touchpad. Four measures were recorded: (a) A′: a signal detection measure of sensitivity to the target, regardless of response tendency (Sahgal, 1987); (b) mean latency: mean time taken to respond correctly; (c) probability of false alarms (if the participant responding inappropriately): total false alarms divided by the sum of total false alarms and total correct rejections; and (d) probability of hits (hit, the participant responding correctly): total hits divided by the sum of total hits and total misses.
In the SSP test, participants were asked to remember the order of visual stimuli, when nine white boxes were presented in fixed locations on the screen. In the beginning, some of the boxes changed color in a variable sequence. The participants were instructed to point the boxes with changing color in the same order. The test began with 2‐box problems and proceeded up to 9‐box problems. Two measures were recorded: (a) span length: the most extended sequence being successfully recalled and (b) total usage errors: the number of times the subject selected a box, not in the sequence being recalled.
2.3. Functional activation task
In the counting Stroop task (Fan et al., 2017, 2014), experimental stimuli were divided into congruent, incongruent, and control conditions, with 24 trials in each condition. In the “congruent” condition, the number of words was consistent with the meaning of the word such as “one,” “two,” “three,” or “four.” In the ”incongruent” condition, the number of words was inconsistent with the meaning of the word. In the “control” condition, the Chinese words did not give any clue to the number. All the words of the three conditions were matched for the number of syllables, visual complexity, and frequency. Participants were instructed to report the number of words in each set via button‐press with one, two, three, and four buttons from left to right on the keypad.
2.4. MRI image acquisition
Participants lay in the scanner with their head position secured. The head coil was positioned over the participants’ head. Participants viewed visual stimuli projected onto a screen via a mirror attached to the inside of the head coil. Images were acquired using a 3 T Siemens Tim‐Trio scanner. The functional and structural imaging parameters were the same as our prior study (Fan et al., 2014). The task was administered in a pseudorandom order for all participants for event‐related designs (Burock et al., 1998). We used the Optseq script for randomized event‐related design (http://surfer.nmr.mgh.harvard.edu/optseq, written by D. Greve, Charlestown, MA) that implemented the Burock, Buckner, Woldorff, Rosen, and Dale (1998) approach. The Optseq is a tool for automatically randomizing the order and timing of events for rapid‐presentation event‐related fMRI experiments.
2.5. Clinical and neuropsychological analysis
We used SPSS to conduct statistical analysis. The descriptive results were displayed as frequency and percentage for the categorical variables, and mean and SD for the continuous variables. We used the analyses of variance (ANOVAs) to compare their performance among the three groups, and all reported results were significant using p < .05. Post‐hoc Fisher's least significant difference (LSD) tests were applied to determine the differences between groups.
2.6. Image analysis
Data analysis was performed using SPM12 (Statistical Parametric Mapping). The preprocessing procedures were the same as our prior study (Chou et al., 2015; Fan et al., 2017, 2014). The functional images were corrected for the differences in slice‐acquisition time to the middle volume and were realigned to the first volume in the scanning session using affine transformations. No participant had more than 3 mm of movement in any plane. Co‐registered images were normalized to the MNI (Montreal Neurological Institute) average template. Statistical analyses were calculated on the smoothed data (10 mm isotropic Gaussian kernel), with a high pass filter (128 s cutoff period) to remove low‐frequency artifacts. Data from each participant were entered into a general linear model using an event‐related analysis procedure (Josephs & Henson, 1999). Stimuli were treated as individual events for analysis and modeled using a canonical hemodynamic response function (HRF). Parameter estimates from contrasts of the canonical HRF in single subject models were entered into random‐effects analysis across all participants in a whole‐brain analysis. There were three event types: congruent, incongruent, and control. To observe the neural correlates of inhibitory control, we compared the incongruent to the congruent condition. To further observe the neural correlates of visual processing, we compared the larger number of words (i.e., “three” and “four”) to the fewer number of words (i.e., “one” and “two”) in the incongruent condition and the congruent condition. For the within‐group analyses, reported areas of significant activations were p < .01 uncorrected in a whole‐brain analysis.
Two‐sample t tests between groups (ADHD probands vs unaffected siblings, ADHD probands vs neurotypical youths, and neurotypical youths vs unaffected siblings) were conducted to test for the potential endophenotypes. Reported areas of significant activations are p < .0005 uncorrected in a whole‐brain analysis. The WFU PickAtlas was integrated into the SPM software environment by using the standard toolbox method and provided a sort of ROI masks in MNI space. In addition, when a mask was applied, the SPM small volume correction was automatically implemented on the basis of the mask to limit the number of multiple statistical comparisons for more robust inference. Therefore, we used the WFU PickAtlas to define ROI masks by selecting the anatomical masks of the right IFG, ACC, and left SPL based on our a priori hypothesis. The masks were used to control for multiple comparisons at p < .05 FWE (familywise error) corrected at the voxel level (Chou et al., 2015; Fan et al., 2017, 2014).
2.7. Correlation analyses of brain activation and neuropsychological measures
For neuropsychological measures, either the RVP or SSP tasks had more than one testing score. We utilized a multiple regression analysis provided in SPM to examine the correlations between neuropsychological performance and brain activation in a whole‐brain analysis. Specifically, we entered the continuous variables of the performance of the RVP task (four testing scores) with signal intensity changes for the incongruent versus congruent contrast, and the SSP task (two testing scores) with signal intensity changes for the contrast of the larger number of words versus the fewer number of words, respectively. This allowed us to examine neuropsychological performance‐related increases or decreases in activation. Reported areas of significant activations are p < .005 uncorrected in a whole‐brain analysis. To visualize correlations, we extracted the beta values from the peak voxels of brain regions for these correlation analyses.
3. RESULTS
3.1. Clinical and neuropsychological results
There were no statistically significant differences in age, handedness, gender, and IQ profiles among the three groups (Table 1). Table 1 presents that unaffected siblings and neurotypical youths performed similarly on the forward and backward digit spans, RVP, and SSP scores. One‐way ANOVA (ADHD probands, unaffected siblings, neurotypical youths) on the scores of digit span showed that ADHD probands had significantly fewer digits recalled backward and forward than the unaffected siblings and neurotypical youths. Regarding the RVP task, one‐way ANOVA revealed significant group differences on the A′ (target sensitivity), mean latency, and the probability of false alarms. The neurotypical youth performed better than ADHD probands on A′, mean latency, and probability of false alarms (t(52) = 3.04, p < .05; t(52) = 2.80, p < .05; and t(52) = 2.45, p < .05, respectively). One‐way ANOVAs in the SSP showed significant group differences in span length and total usage errors. For span length and total usage errors, the neurotypical youths performed better than ADHD probands (t(52) = 2.59, p < .05; and t(52) = 2.17, p < .05, respectively). For total usage errors, ADHD probands had more errors than unaffected siblings (t(52) = 2.00, p < .05).
Table 1.
The age, IQ scores, performance in the RVP and SSP for the youths with ADHD, unaffected siblings, and neurotypical groups
| ADHD (n = 27) | Unaffected siblings (n = 27) | Neurotypical youths (n = 27) | Group main effect | Pairwise group comparisons | |
|---|---|---|---|---|---|
| Handedness (left/right) | 1/26 | 1/26 | 1/26 | ||
| Gender (male/female) | 24/3 | 19/8 | 21/6 | 1.411 | |
| Age in years, mean ± SD (range) | 12.1 ± 2.0 (9–15) | 13.0 ± 2.6 (7–17) | 11.8 ± 1.7 (9–16) | 2.354 | |
| Wechsler Intelligence Scale for Children, 3rd version | |||||
| Verbal IQ | 109.0 ± 12.0 | 108.4 ± 11.8 | 109.5 ± 9.1 | 0.074 | |
| Performance IQ | 104.9 ± 12.6 | 110.1 ± 14.4 | 110.2 ± 13.8 | 1.250 | |
| Full‐scale IQ | 105.2 ± 13.7 | 109.6 ± 12.1 | 110.4 ± 10.2 | 1.448 | |
| Digit span | |||||
| Forward | 7.8 ± 1.1 | 8.4 ± 0.8 | 8.7 ± 0.6 | 7.197** | U, N>A |
| Backward | 5.3 ± 1.7 | 6.3 ± 1.7 | 6.6 ± 1.3 | 5.038** | U, N>A |
| Rapid visual information processing (RVP) | |||||
| A′ (target sensitivity) | 0.84 ± 0.07 | 0.89 ± 0.05 | 0.89 ± 0.05 | 7.093** | U, N>A |
| Mean latency | 533.4 ± 184.7 | 478.2 ± 135.9 | 426.7 ± 70.6 | 4.005 * | A>N |
| Probability of false alarms | 0.06 ± 0.11 | 0.01 ± 0.01 | 0.01 ± 0.01 | 6.206** | A>U, N |
| Probability of hits | 0.51 ± 0.18 | 0.59 ± 0.20 | 0.58 ± 0.18 | 1.450 | |
| Spatial span (SSP) | |||||
| Span length | 6.52 ± 1.70 | 7.30 ± 1.94 | 7.63 ± 1.47 | 3.094 * | N>A |
| Total usage errors | 2.11 ± 2.08 | 1.11 ± 1.55 | 1.15 ± 0.99 | 3.372 * | A> U, N |
Note. Abbreviations: A, ADHD probands; ADHD, attention‐deficit/hyperactivity disorder; A′, signal detection measure of sensitivity to the target; N, neurotypical youths; SD, standard deviation; U, unaffected siblings.
* p < .05; ** p < .01.
3.2. Behavioral performance of the counting Stroop
A three groups (ADHD probands, unaffected siblings, neurotypical youths) by three conditions (incongruent, congruent, control) ANOVA was performed on reaction time and accuracy of the counting Stroop task separately (Table 2). Analysis on reaction time revealed a significant interaction between group and condition (F(4,156) = 2.96, p < .05), and the post hoc t tests showed that ADHD probands were slower in the incongruent versus congruent condition than unaffected siblings and neurotypical youths (t(52) = 2.15, p < .05; t(52) = 2.44, p < .05, respectively). There was a significant main effect of condition (F(2, 156) = 66.48, p < .001), with shorter reaction time in the control and congruent conditions compared to the incongruent condition (t(80) = −8.94, p < .001; t(80) = 9.28, p < .001, respectively). Moreover, analysis of accuracy showed a significant main effect of condition (F(2, 156) = 35.65, p < .001), with higher accuracy in the control and congruent conditions compared to the incongruent condition (t(80) = 6.60, p < .001; t(80) = −6.50, p < .001, respectively).
Table 2.
Comparison of accuracy and reaction time of the counting Stroop test in three conditions for the ADHD probands, unaffected siblings, and neurotypical youths
| ADHD (n = 27) | Unaffected siblings (n = 27) | Neurotypical youths (n = 27) | |
|---|---|---|---|
| Accuracy (%) | |||
| Congruent | 0.988 ± 0.023 | 0.999 ± 0.008 | 0.977 ± 0.040 |
| Incongruent | 0.937 ± 0.076 | 0.949 ± 0.056 | 0.937 ± 0.048 |
| Control | 0.983 ± 0.041 | 0.994 ± 0.015 | 0.983 ± 0.032 |
| Reaction time (ms) | |||
| Congruent | 875.1 ± 202.5 | 834.4 ± 214.2 | 819.9 ± 132.1 |
| Incongruent | 1003.9 ± 226.0 | 913.5 ± 243.9 | 885.1 ± 160.9 |
| Control | 898.7 ± 221.1 | 832.1 ± 213.4 | 832.2 ± 158.5 |
3.3. fMRI results
For inhibitory control, ADHD probands had greater activation in the right IFG (BA 47) and ACC than the unaffected siblings and neurotypical youths for the incongruent versus congruent condition in the whole brain analyses (Figure 1 and Table 3). In addition, ADHD probands had greater activation in the right middle frontal gyrus (BA 9) and right postcentral gyrus (BA5) than the unaffected siblings; ADHD probands showed greater activation in the left middle temporal gyrus (BA 21) than neurotypical youths; while the neurotypical youths showed greater activation in left superior temporal gyrus (BA 42) than the unaffected siblings (Table 3). Within each group in the whole brain analyses, ADHD probands showed greater activation in the right precentral gyrus (BA 4/6), right ACC (BA 24/32), left middle temporal gyrus (BA 21), and right IFG (BA 47); the unaffected siblings showed greater activation in the left superior frontal gyrus (BA 8); while neurotypical youths showed greater activation in the right precentral gyrus (BA 4/6) and superior temporal gyrus (BA 41) (Supporting Information, Table 1).
Figure 1.
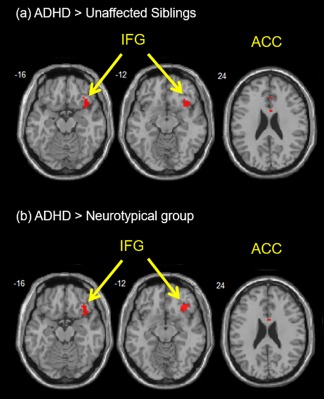
Inhibitory control: greater activation were found in the right IFG (BA 45) and anterior cingulate cortex (ACC) with ROI‐masked analysis during the contrast of “incongruent versus congruent condition” (a) for ADHD probands as compared to the unaffected siblings and (b) for ADHD probands as compared to neurotypical youths [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Brain regions for the inhibitory control and visual processing between groups in the whole‐brain analyses
| Cortical regions | H | BA | Voxels | Z test | MNI coordinates | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Inhibitory control: the incongruent versus the congruent conditions | |||||||||
| ADHD probands > unaffected siblings | |||||||||
| Middle frontal gyrus | R | 9 | 42 | 3.50 | 30 | 11 | 32 | ||
| Postcentral gyrus | R | 5 | 15 | 3.47 | 12 | −43 | 62 | ||
| Anterior cingulate cortexa | R | 24/32 | 38 | 2.69 | 6 | 8 | 23 | ||
| Inferior frontal gyrusa | R | 47 | 25 | 2.80 | 42 | 11 | −13 | ||
| ADHD probands > neurotypical youths | |||||||||
| Middle temporal gyrus | L | 21 | 56 | 3.51 | −48 | −31 | −4 | ||
| Anterior cingulate cortexa | R | 24/32 | 62 | 3.25 | 3 | 8 | 17 | ||
| Inferior frontal gyrusa | R | 47 | 19 | 2.84 | 36 | 23 | −16 | ||
| Unaffected siblings > ADHD probands | |||||||||
| None | |||||||||
| Unaffected siblings > neurotypical youths | |||||||||
| None | |||||||||
| Neurotypical youths > ADHD probands | |||||||||
| None | |||||||||
| Neurotypical youths > unaffected siblings | |||||||||
| Superior temporal gyrus | R | 42 | 37 | 3.71 | 60 | −13 | 11 | ||
| Visual processing: the larger number of words versus the fewer number of words | |||||||||
| ADHD probands > unaffected siblings | |||||||||
| None | |||||||||
| ADHD probands > neurotypical youths | |||||||||
| None | |||||||||
| Unaffected siblings > ADHD probands | |||||||||
| Postcentral gyrus | R | 2 | 72 | 4.29 | 39 | −25 | 26 | ||
| L | 2 | 24 | 3.74 | −33 | −19 | 32 | |||
| Medial frontal gyrus | L | 6 | 40 | 4.25 | −12 | −19 | 65 | ||
| R | 6 | 15 | 3.67 | 15 | −16 | 59 | |||
| Middle temporal gyrus | L | 21/22 | 12 | 3.73 | −39 | −31 | −1 | ||
| Unaffected siblings > neurotypical youths | |||||||||
| None | |||||||||
| Neurotypical youths > ADHD probands | |||||||||
| Medial frontal gyrus | R | 6 | 59 | 4.10 | 15 | −16 | 59 | ||
| Postcentral gyrus | R | 2 | 94 | 3.94 | 36 | −25 | 29 | ||
| Superior parietal lobulea | L | 7/40 | 43 | 2.41 | −30 | −49 | 65 | ||
| Neurotypical youths > unaffected siblings | |||||||||
| Precentral gyrus | R | 4/6 | 105 | 3.69 | 45 | −16 | 44 | ||
| Superior parietal lobulea | L | 7/40 | 18 | 2.50 | −33 | −49 | 62 | ||
Note. Abbreviations: BA, Brodmann's area; H, hemisphere; L, left; R, right; voxels, number of voxels in cluster at p < .0005 uncorrected.
Coordinates of activation peak(s) within a region based on a z test are given in the MNI stereotactic space (x, y, z). ROI masks (anatomical masks of the right inferior frontal gyrus and the right anterior cingulate cortex) were only used in between group analysis.
p < .05 for familywise error (FWE) corrected with the use of an anatomical mask.
For visual processing, neurotypical youths had greater activation in the left SPL (BA7/40) as compared to the unaffected siblings and ADHD probands for the larger number of words versus the fewer number of words in the whole brain analyses (Figure 2 and Table 3). In addition, the neurotypical youths showed greater activation in the right medial frontal gyrus (BA 6) and postcentral gyrus (BA 2) than ADHD probands; while the neurotypical youths showed greater activation in right precentral gyrus (BA 4/6) than the unaffected siblings (Table 3). Moreover, the unaffected siblings had greater activation in the bilateral medial frontal gyrus (BA 6), bilateral postcentral gyrus (BA 2), and left middle temporal gyrus (BA 21/22) than ADHD probands (Table 3). Within each group in the whole brain analyses, the ADHD probands showed greater activation in the left precentral gyrus (BA 4/6); the unaffected siblings showed greater activation in the left precentral gyrus (BA 4/6) and left middle occipital gyrus (BA 18/19); and the neurotypical youths showed greater activation in the left precentral gyrus (BA 4/6), bilateral middle occipital gyri (BA 18), and left SPL (BA 7) (Supporting Information, Table 1).
Figure 2.
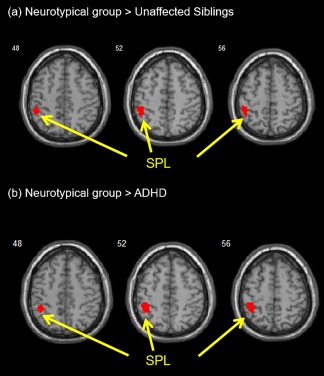
Visual processing: greater activation was found in the left SPL (BA 5/7) with ROI‐masked analysis during the contrast of “larger versus fewer numbers of words” (a) for neurotypical youths as compared to the unaffected siblings and (b) for neurotypical youths as compared to ADHD probands [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Correlations between brain activation and neuropsychological measures
We conducted Pearson's correlations between the changes in brain activation and the performance on RVP and SSP tasks. In the ADHD group, increased activation in the right IFG (for inhibitory control) was positively correlated with the mean latency of the RVP (r = .43, p < .05). In the neurotypical group, increased activation in the left SPL (for visual processing) was positively correlated with the spatial length of the SSP (r = .43, p < .05).
4. DISCUSSION
To the best of our knowledge, this study is the first study to use the counting Stroop fMRI and the CANTAB to examine the potential endophenotypes of the inhibitory control and visual processing in youths with ADHD. For inhibitory control, ADHD probands showed greater activation in the right IFG and ACC than unaffected siblings and neurotypical youths. For visual processing, neurotypical youths had greater activation in the left SPL than unaffected siblings and ADHD probands. In addition, increased activation in the right IFG was positively correlated with the mean latency of the RVP in ADHD probands, while increased activation in the left SPL was positively correlated with the spatial length of the SSP in neurotypical youths.
Our findings demonstrated that neurotypical youths had the greatest activation in the left SPL during visual processing, with no difference in the left SPL activation between ADHD probands and their unaffected siblings. Previous studies pointed out that less left parietal activation might contribute to impaired spatial memory in children with ADHD (Silk et al., 2008). The findings from this study were consistent with those of our prior study, suggesting that ADHD probands might have worse visual processing of numbers as compared to neurotypical youths during the counting Stroop task (Fan et al., 2017, 2014). Taken together, our findings provided evidence to support that less left SPL activation might be a candidate endophenotype for visual processing in ADHD.
ADHD probands showed more activation in the right IFG and ACC than their unaffected siblings and neurotypical youths during the contrast of the incongruent to the congruent condition in the counting Stroop task. In addition, increased activation in the right IFG was correlated with the mean latency of the RVP in ADHD probands. Our findings were consistent with those of previous studies related to inhibitory control, suggesting that ADHD probands might need more activation in the right IFG and ACC to compensate their deficits in inhibitory control (Fan et al., 2017, 2014; Fassbender & Schweitzer, 2006). Few studies have examined whether the deficits in the right IFG and ACC could be an index for the familial risk of ADHD. Both ADHD probands and their unaffected siblings showed reduced activation in the frontal regions as compared with the healthy controls in the GO/NOGO task (Durston et al., 2006). Previous studies also have shown hypoactivation in the frontostriatal networks of young adults with ADHD and their unaffected siblings compared to controls, whereby activation in the inferior frontal nodes in unaffected siblings was intermediate between participants with ADHD and healthy controls during the stop‐signal task (van Rooij et al., 2015). These studies on the brain activity in the right IFG and ACC have revealed inconsistent results in terms of increased or decreased activation in ADHD probands and their unaffected siblings (Durston et al., 2006; Fassbender & Schweitzer, 2006; Hart, Radua, Nakao, Mataix‐Cols, & Rubia, 2013; Silk, Vance, Rinehart, Bradshaw, & Cunnington, 2008). The discrepancy may be explained by different task designs and study populations. For the inhibition domains, the go/no‐go and stop tasks were included for motor response inhibition, while the Stroop task was included for interference inhibition with high‐load cognitive processing and conflict detection (review by Hart et al., 2013). We found that ADHD probands performed significantly worse in the RVP task than their unaffected siblings and neurotypical youths, suggesting that ADHD itself was associated with impaired inhibitory control and executive functions (Gau and Shang, 2010). Furthermore, increased activation in the right IFG during the counting Stroop task was associated with the mean latency of the RVP in ADHD probands. Previous fMRI studies have suggested that hyperactivation in a brain region can be considered as “inefficiency” because an individual with ADHD might need to use more energy than should be to perform a given task (review by Fassbender & Schweitzer, 2006). Moreover, extra activity in the ADHD probands may be viewed as compensation for under‐activity in the “appropriate” brain network (Fassbender & Schweitzer, 2006). Altogether, the right IFG might play an important role as a state marker of inhibitory control in ADHD.
Several features of this study constitute its strengths. First, our work is the first study to investigate the neural correlates of the inhibitory control and visual processing using the counting Stroop fMRI in unaffected siblings of ADHD probands. Second, we used standardized neuropsychological tests (CANTAB) to conduct comprehensive clinical assessments of inhibition control and visual processing. Third, we combined the neuropsychological and functional neuroimaging assessments to investigate the inhibitory control and visual processing among ADHD probands, their unaffected siblings, and neurotypical youths.
This study is limited by a lack of genetic data of the unaffected siblings to distinguish genetic influences from shared environmental influences. Moreover, the small sample size of this study may not have enough power to demonstrate the superiority of endophenotype in visual processing. Therefore, it warrants further investigations with larger samples and different experimental designs to validate our findings.
In conclusion, we used the CANTAB and the counting Stroop task in the scanner to identify the potential endophenotype of the neural mechanism for ADHD. ADHD probands showed greater activation in the right IFG and ACC as compared with their unaffected siblings and neurotypical youths, implying that the brain activation of the two regions may involve in a compensatory mechanism to help the ADHD probands dealing with inhibitory control. Furthermore, we found that both ADHD probands and their unaffected siblings have similar activation in the left SPL during visual processing indicated that familial or genetic factors might contribute to the shared deficits of visual processing in ADHD probands and their unaffected siblings. Such assumption was supported by our further analysis showing that greater activation in the left SPL was correlated with the spatial length of the SSP in neurotypical youths, which implied that ADHD probands and their unaffected siblings might share the same poor visual processing, regardless of ADHD diagnostic status. Taken together, the brain activation of the left SPL may be a candidate endophenotype for visual processing in ADHD; whereas activation in the right IFG and ACC is a state marker for inhibitory control in ADHD. Our findings may expand the understanding of the neural circuitry of inhibitory control and visual processing in ADHD probands and their unaffected siblings.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplementary Table
ACKNOWLEDGMENT
This work was supported by National Science Council (NSC101‐2321‐B‐002–079, NSC‐101–2627‐B‐002‐001, MOST103–2314‐B‐002–021‐MY3), National Health Research Institute (NHRI‐EX98‐9407PC, NHRI‐EX100‐10008PI) in part by the Department of Medical Imaging, and 3T MRI Lab in National Taiwan University Hospital.
Fan L‐Y, Shang C‐Y, Tseng W‐YI, Gau SS‐F, Chou T‐L. Visual processing as a potential endophenotype in youths with attention‐deficit/hyperactivity disorder: A sibling study design using the counting Stroop functional MRI. Hum Brain Mapp. 2018;39:3827–3835. 10.1002/hbm.24214
Funding information National Science Council, Grant/Award Numbers: NSC101‐2321‐B‐002‐079, NSC‐101‐2627‐B‐002‐001, MOST103‐2314‐B‐002‐021‐MY3; National Health Research Institute, Grant/Award Numbers: NHRI‐EX98‐9407PC, NHRI‐EX100‐10008PI; Department of Medical Imaging; 3T MRI Lab in National Taiwan University Hospital
Contributor Information
Susan Shur‐Fen Gau, Email: gaushufe@ntu.edu.tw.
Tai‐Li Chou, Email: tlchou25@ntu.edu.tw.
REFERENCES
- Akutagava‐Martins, G. C. , Salatino‐Oliveira, A. , Kieling, C. C. , Rohde, L. A. , & Hutz, M. H. (2013). Genetics of attention‐deficit/hyperactivity disorder: Current findings and future directions. Expert Review of Neurotherapeutics, 13(4), 435–445. [DOI] [PubMed] [Google Scholar]
- Bonvicini, C. , Faraone, S. V. , & Scassellati, C. (2016). Attention‐deficit hyperactivity disorder in adults: A systematic review and meta‐analysis of genetic, pharmacogenetic and biochemical studies. Molecular Psychiatry, 21(7), 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burock, M. A. , Buckner, R. L. , Woldorff, M. G. , Rosen, B. R. , & Dale, A. M. (1998). Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport, 9(16), 3735–3739. [DOI] [PubMed] [Google Scholar]
- Bush, G. , Whalen, P. J. , Rosen, B. R. , Jenike, M. A. , McInerney, S. C. , & Rauch, S. L. (1998). The Counting Stroop: An interference task specialized for functional neuroimaging—Validation study with functional MRI. Human Brain Mapping, 6(4), 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, T. L. , Chia, S. , Shang, C. Y. , & Gau, S. S. (2015). Differential therapeutic effects of 12‐week treatment of atomoxetine and methylphenidate on drug‐naive children with attention deficit/hyperactivity disorder: A counting Stroop functional MRI study. European Neuropsychopharmacology, 25(12), 2300–2310. [DOI] [PubMed] [Google Scholar]
- Doyle, A. E. , Willcutt, E. G. , Seidman, L. J. , Biederman, J. , Chouinard, V. A. , Silva, J. , & Faraone, S. V. (2005). Attention‐deficit/hyperactivity disorder endophenotypes. Biological Psychiatry, 57(11), 1324–1335. [DOI] [PubMed] [Google Scholar]
- Durston, S. , Mulder, M. , Casey, B. J. , Ziermans, T. , & van Engeland, H. (2006). Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention‐deficit hyperactivity disorder. Biological Psychiatry, 60(10), 1062–1070. [DOI] [PubMed] [Google Scholar]
- Durston, S. , Pol, H. E. H. , Schnack, H. G. , Buitelaar, J. K. , Steenhuis, M. P. , Minderaa, R. B. , … Engeland, H. V. (2004). Magnetic resonance imaging of boys with attention‐deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry, 43(3), 332–340. [DOI] [PubMed] [Google Scholar]
- Fan, L. Y. , Chou, T. L. , & Gau, S. S. (2017). Neural correlates of atomoxetine improving inhibitory control and visual processing in Drug‐naive adults with attention‐deficit/hyperactivity disorder. Human Brain Mapping, 38(10), 4850–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, L. Y. , Gau, S. S. , & Chou, T. L. (2014). Neural correlates of inhibitory control and visual processing in youths with attention deficit hyperactivity disorder: A counting Stroop functional MRI study. Psychological Medicine, 44(12), 2661–2671. [DOI] [PubMed] [Google Scholar]
- Faraone, S. V. , Perlis, R. H. , Doyle, A. E. , Smoller, J. W. , Goralnick, J. J. , Holmgren, M. A. , & Sklar, P. (2005). Molecular genetics of attention‐deficit/hyperactivity disorder. Biological Psychiatry, 57(11), 1313–1323. [DOI] [PubMed] [Google Scholar]
- Fassbender, C. , & Schweitzer, J. B. (2006). Is there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clinical Psychology Review, 26(4), 445–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, L. A. , & Rapoport, J. L. (2015). Brain development in ADHD. Current Opinion in Neurobiology, 30, 106–111. [DOI] [PubMed] [Google Scholar]
- Gau, S. S. F. , & Huang, W. L. (2014). Rapid visual information processing as a cognitive endophenotype for attention deficit hyperactivity disorder. Psychological Medicine, 44(02), 435–446. [DOI] [PubMed] [Google Scholar]
- Gau, S. S. F. , & Shang, C. Y. (2010). Executive functions as endophenotypes in ADHD: Evidence from the Cambridge Neuropsychological Test Battery (CANTAB). Journal of Child Psychology and Psychiatry, 51(7), 838–849. [DOI] [PubMed] [Google Scholar]
- Gottesman, I. I. , & Gould, T. D. (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636–645. [DOI] [PubMed] [Google Scholar]
- Hart, H. , Radua, J. , Nakao, T. , Mataix‐Cols, D. , & Rubia, K. (2013). Meta‐analysis of functional magnetic resonance imaging studies of inhibition and attention in attention‐deficit/hyperactivity disorder: Exploring task‐specific, stimulant medication, and age effects. JAMA Psychiatry, 70(2), 185–198. [DOI] [PubMed] [Google Scholar]
- Hawi, Z. , Cummins, T. D. , Tong, J. , Arcos‐Burgos, M. , Zhao, Q. , Matthews, N. , … Bellgrove, M. A. (2017). Rare DNA variants in the brain‐derived neurotrophic factor gene increase risk for attention‐deficit hyperactivity disorder: A next‐generation sequencing study. Molecular Psychiatry, 22(4), 580–584. [DOI] [PubMed] [Google Scholar]
- Josephs, O. , & Henson, R. N. (1999). Event‐related functional magnetic resonance imaging: Modelling, inference and optimization. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 354(1387), 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler, K. S. , & Neale, M. C. (2010). Endophenotype: A conceptual analysis. Molecular Psychiatry, 15(8), 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, H. C. , Shang, C. Y. , Gau, S. S. , Lin, Y. J. , Huang, H. C. , & Yang, L. K. (2013). A head‐to‐head randomized clinical trial of methylphenidate and atomoxetine treatment for executive function in adults with attention‐deficit hyperactivity disorder. International Journal of Neuropsychopharmacology, 16(09), 1959–1973. [DOI] [PubMed] [Google Scholar]
- Paloyelis, Y. , Mehta, M. A. , Kuntsi, J. , & Asherson, P. (2007). Functional MRI in ADHD: A systematic literature review. Expert Review of Neurotherapeutics, 7(10), 1337–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal, A. (1987). Some limitations of indices derived from signal detection theory: Evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology, 91(4), 517–520. [DOI] [PubMed] [Google Scholar]
- Shang, C. Y. , & Gau, S. S. F. (2011). Visual memory as a potential cognitive endophenotype of attention deficit hyperactivity disorder. Psychological Medicine, 41(12), 2603–2612. [DOI] [PubMed] [Google Scholar]
- Silk, T. J. , Vance, A. , Rinehart, N. , Bradshaw, J. L. , & Cunnington, R. (2008). Dysfunction in the fronto‐parietal network in attention deficit hyperactivity disorder (ADHD): An fMRI study. Brain Imaging and Behavior, 2(2), 123–131. [Google Scholar]
- van Rooij, D. , Hoekstra, P. J. , Mennes, M. , von Rhein, D. , Thissen, A. J. A. M. , Hestenfeld, D. , … Hartman, C. A. (2015). Neural activation patterns during response inhibition distinguish adolescents with ADHD, their unaffected siblings, and healthy controls. American Journal of Psychiatry, 172(7), 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1991). Wechsler Intelligence Scale for Children ‐ Third Edition (WISC‐III) manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplementary Table


