Abstract
This study used resting state functional magnetic resonance imaging (rsfMRI) to investigate whole brain networks in patients with persistent postural perceptual dizziness (PPPD). We compared rsfMRI data from 38 patients with PPPD and 38 healthy controls using whole brain and region of interest analyses. We examined correlations among connectivity and clinical variables and tested the ability of a machine learning algorithm to classify subjects using rsfMRI results. Patients with PPPD showed: (a) increased connectivity of subcallosal cortex with left superior lateral occipital cortex and left middle frontal gyrus, (b) decreased connectivity of left hippocampus with bilateral central opercular cortices, left posterior opercular cortex, right insular cortex and cerebellum, and (c) decreased connectivity between right nucleus accumbens and anterior left temporal fusiform cortex. After controlling for anxiety and depression as covariates, patients with PPPD still showed decreased connectivity between left hippocampus and right inferior frontal gyrus, bilateral temporal lobes, bilateral insular cortices, bilateral central opercular cortex, left parietal opercular cortex, bilateral occipital lobes and cerebellum (bilateral lobules VI and V, and left I–IV). Dizziness handicap, anxiety, and depression correlated with connectivity in clinically meaningful brain regions. The machine learning algorithm correctly classified patients and controls with a sensitivity of 78.4%, specificity of 76.9%, and area under the curve = 0.88 using 11 connectivity parameters. Patients with PPPD showed reduced connectivity among the areas involved in multisensory vestibular processing and spatial cognition, but increased connectivity in networks linking visual and emotional processing. Connectivity patterns may become an imaging biomarker of PPPD.
Keywords: functional connectivity, persistent postural perceptual dizziness, resting state functional imaging, support vector machine, vertigo
1. INTRODUCTION
Persistent postural perceptual dizziness (PPPD) was recently defined by the World Health Organization (World Health Organization) and the Bárány Society (Staab et al., 2017) based on 30 years of research on phobic postural vertigo (PPV; Brandt, 1996), space‐motion discomfort (Jacob et al., 1993), visual vertigo (Bronstein, 1995), chronic subjective dizziness (CSD; Staab & Ruckenstein, 2007) and related entities (Longridge, Mallinson, & Denton, 2002; Marks, 1981; Page & Gresty, 1985). PPPD is characterized by persistent non‐vertiginous dizziness or unsteadiness lasting three months or more. Symptoms are present most days with some fluctuation. Affected individuals usually feel worse when upright or exposed to moving or complex visual stimuli, or during active or passive head motion. Neuro‐otologic, general medical and psychiatric conditions that cause vertigo, unsteadiness, or dizziness such as peripheral and central vestibular disorders, vestibular migraine, panic attacks and generalized anxiety, mild traumatic brain injuries, and autonomic or cardiac conditions may trigger PPPD (Staab et al., 2017). Clinical epidemiologic studies of PPV (Brandt, 1996) and CSD (Staab & Ruckenstein, 2007) indicate that PPPD is one of the most common causes of chronic vestibular symptoms, and ranks among the top three diagnoses in tertiary neuro‐otology centers (Dieterich & Staab, 2017; Dieterich, Staab, & Brandt, 2016).
Although the pathophysiology of PPPD remains to be elucidated, hypotheses based on physiological investigations of its four main predecessors suggest that it may arise in part from a persistent shift in multi‐sensory processing of space‐motion information to favor visual over vestibular or somatosensory inputs for determining spatial orientation (i.e., visual dependence) and stiffening of postural control for controlling locomotion (Dieterich & Staab, 2017; Dieterich et al., 2016). Anxiety‐related factors seem to play two important roles in promoting these functional changes. First, the anxiety‐related personality trait of neuroticism appears to confer risk for developing CSD (Chiarella et al., 2016) and PPPD (Yan et al., 2017), whereas resilient personality traits may reduce risk (Tschan et al., 2011). Second, high anxiety in the context of acute vestibular syndromes predicted persistent dizziness at 3 (Heinrichs, Edler, Eskens, Mielczarek, & Moschner, 2007), 6 (Cousins et al., 2017), and 12 (Godemann et al., 2005) months of prospective follow‐up to a significantly greater degree than the extent of structural vestibular deficits (Tschan et al., 2011). Thus, an anxious predisposition and the acute interaction of elevated anxiety with structural/metabolic conditions appear to promote a persistent shift to visual dependence and stiffening of stance and gait that are hypothesized to underlie critical aspects of the chronic dizziness and sensitivity to postural and perceptual challenges that characterize PPPD (Staab et al., 2017).
Four recent neuroimaging investigations support this hypothesis. A task‐based functional magnetic resonance imaging (fMRI) study using sound‐evoked vestibular stimulation in 26 normal right‐handed Italian individuals (Indovina, Riccelli, Staab, Lacquaniti, & Passamonti, 2014) found that neuroticism correlated positively with activity in the pons, cerebellar fastigium bilaterally, and left para‐striate cortex (V2–visual association area), and negatively with activity in the left supra‐marginal gyrus (a portion of the non‐dominant vestibular cortex). Introversion correlated positively with activity in the right amygdala and negatively with connectivity between the right amygdala and right inferior frontal gyrus. Furthermore, neuroticism correlated positively with increased connectivity between the right amygdala and both left pons and left cerebellar fastigium and between the left inferior frontal gyrus and both left supra‐marginal gyrus and left para‐striate cortex. A second task‐based fMRI study in 24 normal right‐handed Italian individuals (Riccelli et al., 2017) using visual motion stimulation from a virtual reality rollercoaster ride found that neuroticism correlated positively with activity in the left (non‐dominant) parieto‐insular vestibular cortex and with connectivity between that area and the right amygdala when comparing travel on vertical versus horizontal sections of the ride. A resting state fMRI study (Van Ombergen et al., 2017) in 10 right‐handed Belgian patients with visually‐induced dizziness (as known as visual vertigo, a key symptom of PPPD) compared to 10 healthy right‐handed controls found that patients had decreased functional connectivity in the right central opercular cortex (superior temporal gyrus, a component of the dominant vestibular cortex) and increased functional connectivity in the occipital pole in whole brain analyses. Seed‐based analyses centered on vestibular and visual regions identified increased functional connectivity between the thalamus and lateral occipital cortex bilaterally and cerebellar regions (left central cerebellar lobules I and VI, bilateral Crus I and II) and between the visual association cortex and both middle frontal gyrus and precuneus. Seed‐based analyses also found decreased functional connectivity between the visual association cortex and the left parahippocampal gyrus and between the thalamus and most of the right putamen in patients versus controls. Finally, a task‐based fMRI study (Indovina et al., 2015) used sound‐evoked vestibular stimulation to investigate brain activity and connectivity in 18 right‐handed patients with CSD and 18 healthy right‐handed controls matched for anxiety, depression, neuroticism and introversion. Compared to controls, patients showed localized hypofunction in the right posterior insula and adjacent superior temporal gyrus (both portions of the dominant vestibular cortex), left anterior insula extending to the left frontal opercular cortex, left inferior frontal gyrus, left anterior cingulate cortex (all regions that modulate instinctive stimulus‐response and emotional behaviors), and left hippocampus. Patients with CSD also showed reduced connectivity between the left anterior insula/inferior frontal gyrus and right middle occipital cortex, left anterior cingulate cortex and right superior temporal gyrus, and left hippocampus and right superior temporal gyrus. In sum, the two imaging studies in normal individuals were consistent with preceding psychological investigations of CSD (Chiarella et al., 2016; Staab, Rohe, Eggers, & Shepard, 2014) and PPPD (Yan et al., 2017) and suggested that anxiety‐related personality traits increase the sensitivity of vestibular, visual, and anxiety regions of the brain to vestibular and visual motion stimuli and increase their connectivity with one another in a manner that may enhance responses to visual over vestibular inputs. The imaging studies of patients with visually induced dizziness and CSD were consistent with previous psychophysiological studies of patients with persistent dizziness (Cousins et al., 2017) and more strongly indicated that a shift to reliance on visual versus vestibular information occurs in patients with PPPD. In addition, these investigations identified reduced connectivity between frontal brain regions that modulate instinctive stimulus‐response behaviors and posterior regions that process space‐motion information.
Therefore, we hypothesized that the pathophysiology of PPPD involves functional changes in brain pathways through the multisensory vestibular cortex, visual cortex (primary and association areas), and frontal regions that together regulate instinctive and conscious control of locomotion and spatial cognition. However, the previous neuroimaging work must be extended to larger groups of patients from additional countries and cultures who completely fulfill the new diagnostic criteria for PPPD in order to create a more detailed and generalizable model of the pathophysiologic mechanisms underlying this common condition. In this investigation, we chose resting state fMRI to compare functional networks of the brain in an unconstrained (i.e., not task dependent) state in 38 right‐handed Korean patients with PPPD versus 38 age and gender matched right‐handed healthy controls.
The aims of this study were: (a) to define the whole‐brain functional connectivity related to PPPD using a well‐established data‐driven method, (b) to evaluate the functional connectivity centered on the primary vestibular and visual areas, and hippocampus bilaterally, (c) to determine whether these changes in functional connectivity correlate with the severity of dizziness handicap, anxiety and depression in patients with PPPD, and (d) to evaluate how well whole‐brain functional connectivity results may serve as a biomarker for PPPD by distinguishing patients with PPPD from normal controls using machine learning algorithms.
2. METHODS
2.1. Subjects
Thirty‐eight patients with PPPD (26 women; mean age ± SD = 48.6 ± 12 years) were enrolled at the Dizziness Clinic of Seoul National University Bundang Hospital between January 2015 and August 2016. The diagnosis of PPPD was made according to draft definitions established by the World Health Organization (World Health Organization) and the Classification Committee of the Bárány Society (Staab et al., 2017). The diagnostic criteria for PPPD included (a) dizziness, unsteadiness, or non‐spinning vertigo that are present on most days for 3 months or more, (b) symptoms are present without specific provocation, but are exacerbated by upright posture, exposure to moving or complex visual stimuli, and active or passive motion, (c) the disorder follows acute or episodic vestibular or balance‐related problems, other neurologic or medical illnesses, or psychological distress (d) symptoms cause significant distress or functional impairment, and (e) symptoms are not better accounted for by another disease or disorder. Patients were excluded when they had (a) active neuro‐otologic disorders, (b) organic brain diseases that may affect the resting state brain activities, (c) previous history of head trauma, or (d) medication that may affect cerebral function, such as antidepressants or anxiolytics.
Thirty‐eight age‐ and gender‐matched healthy volunteers (26 women; mean age ± SD = 47.5 ± 13 years) served as controls. Especially, the control subjects should have (a) no history of neurological or neurotological disorders, and (b) no medication that may affect the resting state brain activities.
All subjects were right‐handed according to the Edinburgh Handedness Inventory (Oldfield, 1971) and had completed Korean versions of the Dizziness Handicap Inventory (DHI) (Han et al., 2004), Beck Anxiety Inventory (BAI; Kwon, 1997), and Beck Depression Inventory (BDI) (Lee et al., 1995). The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B‐1609/362‐108).
2.2. Imaging acquisition
All subjects had MRIs using a 3T Philips Achieva MRI scanner (Philips Healthcare, Inc., Best Netherlands) at Seoul National University Bundang Hospital. Subjects were asked to rest with their eyes closed and lie still during the scanning. High resolution three‐dimensional anatomic T1‐weighted images of the brain were acquired with 0.5‐mm isotropic voxels using a fast field‐echo planar imaging sequence (TR = 8.1 ms, TE = 4.6 ms, flip angle = 8°, slice thickness = 1 mm, 175 slices). Functional images of the whole brain were acquired using a fast field‐echo planar imaging sequence (TR = 3,000 ms, TE = 30 ms, matrix = 64 × 64, field of view = 224 mm, flip angle = 90°, slice thickness = 3.5 mm/0 gap, numbers of slice = 42, number of volumes = 120, total scan time = 369 s).
2.3. Preprocessing
The images were preprocessed using Statistical Parametric Mapping 12 software (SPM12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12) and the CONN toolbox (version 16a (http://www.nitrc.org/projects/conn) working on MATLAB R2015a (MathWorks, Inc., Natick, MA). The preprocessing steps were applied with default parameters in CONN as follows: The fMRI data were preprocessed with the default parameters in CONN as follows: all normalization procedures adopted non‐linear registration methods. (a) realignment, (b) slice timing correction, (c) segmentation and normalization (non‐linear transformation to Montreal Neurological Institute space), (d) scrubbing using the artifact detection tools with the threshold for global‐signal above 5 (z‐value) and for subject‐motion above 0.9 mm, and (e) smoothing using a 6 mm full‐width half‐maximum Gaussian kernel. After the preprocessing steps, a band‐pass filter (0.008–0.09 Hz) was applied to the time series and white matter and cerebrospinal fluid time series were regressed out. There was no difference in head motion during the scan between the groups (p = .897). Age and gray matter volume were treated as nuisance covariates. Total gray matter volume was calculated using MATLAB get_totals script implemented in SPM (http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m).
2.4. Functional connectivity analysis of the whole brain
The whole‐brain functional connectivity analysis was carried out using the CONN toolbox. The parcellation scheme of the Harvard‐Oxford Atlas (Caviness, Meyer, Makris, & Kennedy, 1996) and spatially unbiased infratentorial template (SUIT, http://www.diedrichsenlab.org/imaging/suit.htm) determined 105 supratentorial and 28 infratentorial regions of interests (ROIs; Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009). We conducted the whole‐brain functional connectivity analysis (ROI‐to‐ROI) as described previously (Whitfield‐Gabrieli & Nieto‐Castanon, 2012). The averaged time‐series for each ROI from the preprocessed images were extracted. Then, as a measure of functional connectivity, a Pearson's correlation coefficient for each pair of the 133 time‐series was calculated and converted to z scores using Fisher's r‐to‐z transformation. We performed whole‐brain functional connectivity analyses in two ways: (a) we compared whole‐brain functional connectivity between patients with PPPD and control subjects and (b) we performed correlation analyses between functional connectivity and clinical variables (DHI, BAI, and BDI scores) in patients with PPPD. In order to correct multiple comparison problems, we applied the statistical threshold at seed‐level false discovery rate‐corrected two‐sided (FDR) p values at p < .05 for group comparison and correlation analysis of functional connectivity (Ludbrook, 2013). The graphic presentation of group and correlation analyses was prepared using BrainNet Viewer (https://www.nitrc.org/projects/bnv; Xia, Wang, & He, 2013).
2.5. Functional connectivity analysis centered on specific vestibular and visual brain regions
We also performed a seed to voxel approach using a priori defined seeds related to key vestibular and visual areas of the brain. Since the symptoms of PPPD are typically worse with upright posture, head or body motion and exposure to complex or motion‐rich environments (Staab et al., 2017), we selected the multimodality vestibular cortex and primary visual cortex bilaterally. For the vestibular cortex, we chose the parietal opercular cortex 2 (OP2) because a recent meta‐analysis suggested it as the primary vestibular cortical region in humans (zu Eulenburg, Caspers, Roski, & Eickhoff, 2012). We also added the hippocampus because it plays a critical role in the processing of spatial information and was identified as a region of altered activity and connectivity in the fMRI study of patients with CSD (Indovina et al., 2015). Four ROIs were extracted from the Juelich histological atlas available on the FMRIB Software Library (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) and were defined by creating binary masks with a probability threshold of 20%: the bilateral OP2 (Eickhoff, Grefkes, Zilles, & Fink, 2007), the bilateral primary visual cortices (V1). The other two ROIs, bilateral hippocampi, were extracted from the Harvard‐Oxford Atlas: bilateral hippocampi. In order to correct multiple comparisons, the cluster‐defining threshold was set at p < .001 voxel level (Friston, Worsley, Frackowiak, Mazziotta, & Evans, 1994). Then, cluster‐wise correction for multiple comparisons was applied using family‐wise error (FWE) with corrected p < .05 and cluster extent threshold of 200. Finally, the number of seeds was corrected using Bonferroni method. The graphic presentation of the analyses was prepared using MRIcron (http://people.cas.sc.edu/rorden/mricron/index.html).
2.6. Group differences in functional connectivity after controlling for effects of psychiatric comorbidities
In cross‐sectional and longitudinal studies of PPV and CSD, approximately 60% of patients had comorbid anxiety (moderate on average) and about 40% had comorbid depression (mild on average; Dieterich et al., 2016; Staab, 2012). Anxiety and mood symptoms often co‐existed, but 25% of patients had no psychiatric morbidity. Therefore, we sought to identify alterations in functional connectivity attributable to PPPD itself by repeating our between group analyses of whole brain and a priori vestibular and visual regions of interest with BAI and BDI scores added as covariates to our general linear models.
2.7. Functional connectivity analysis of the hippocampus along the anterior–posterior axis
Based on the initial results of our study, we also created four ROIs representing the head (anterior) and tail (posterior) portions of the hippocampus. Seeds were derived from the Juelich Histological Atlas and had a minimum of 60% likelihood of being the hippocampus. We excluded the voxels that had any probability of being in the amygdala to prevent any possibility that the anterior hippocampus connectivity may partially reflect the amygdala connectivity. The hippocampus was divided along the A–P axis into three sections as was performed in the previous studies (Chen & Etkin, 2013).
2.8. Identification of whole‐brain functional connectivity as a potential biomarker of PPPD
To determine if alterations in whole‐brain functional connectivity could be a potential biomarker of PPPD, effectively distinguishing patients with PPPD from normal controls, we developed a classification method to separate the two groups using machine learning algorithms. Specifically, we constructed functional connectivity matrices from selected brain regions that showed significant differences between the groups. We arranged these regions into arrays and applied a principal component analysis to reduce the length of the arrays, thus avoiding excessive dimensionality. Then, we employed a support vector machine (a discriminative classifier) to develop a separation criterion using the well‐established leave‐one‐out cross‐validation method (Chen et al., 2011; Zeng et al., 2012). In this approach, one subject at a time was set aside and all remaining subjects were used as a training dataset. The left‐out subject was then used as test data. This entire process was repeated until each subject had served as a test subject, and the results of all leave out training runs (76 processes in this case) were combined to create the final separation criterion. To quantify the success of the separation criterion, we calculated the sensitivity, specificity, and accuracy of the resulting classification and the area under a receiver operating characteristic (ROC).
2.9. Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows v18.0 (SPSS Institute Inc., Chicago, IL). Significance levels for all analyses were set at p < .05. Group comparisons of clinical variables were performed using two‐sample t test for parametric continuous variables and chi‐square tests for categorical variables.
3. RESULTS
3.1. Clinical features
The demographic and clinical characteristics of the participants are summarized in Table 1. As expected, patients with PPPD had higher mean scores on the DHI, BAI, and BDI than healthy controls. Scores for the PPPD group reflected moderate dizziness handicap, moderate anxiety symptoms, and mild depressive symptoms. Preceding peripheral vestibular disorders were confirmed in 9 patients (24%), vestibular neuritis in four and benign paroxysmal positional vertigo in five. Four patients had migraine and one patient reported a history of anxiety disorder. The duration of symptoms ranged from 3 to 360 months (median = 48.2). All of these demographic and clinical variables are consistent with the typical presentation of patients with PPPD in tertiary neuro‐otology centers around the world (Dieterich & Staab, 2017; Dieterich et al., 2016).
Table 1.
Profiles of the patients with PPPD and healthy controls
| PPPD (n = 38) | Control (n = 38) | Difference | |
|---|---|---|---|
| Gender (man/woman), | 12/26 | 12/26 | NSa |
| Age (years) | 48.6 ± 12 | 47.5 ± 13 | NSb |
| Duration (months) | 45.6 ± 69.9 | – | – |
| DHI | 37.2 ± 20.3 | 0 | p < 0.05b |
| BAI | 18.1 ± 8.4 | 3.6 ± 2.9 | p < 0.05b |
| BDI | 14.7 ± 7.2 | 1.5 ± 1.7 | p < 0.05b |
BAI: Beck Anxiety Inventory, BDI: Beck Depression Inventory, DHI: Dizziness Handicap Inventory, PPPD: persistent postural perceptual dizziness.
Pearson chi‐square test.
t Test, NS: not significant.
3.2. Altered functional connectivity of the whole brain in patients with PPPD
Compared to normal controls, patients with PPPD showed increased functional connectivity between the subcallosal cortex and both the left superior lateral occipital cortex and left middle frontal gyrus. Patients with PPPD showed decreased functional connectivity between the left hippocampus and bilateral central opercular cortices, left parietal opercular cortex, right insular cortex, bilateral cerebellar lobules VI, right cerebellar lobule V and left Crus I. Connectivity was also decreased between the right nucleus accumbens and the anterior portion of the left temporal fusiform cortex (Figure 1a, Supporting Information Table S1).
Figure 1.
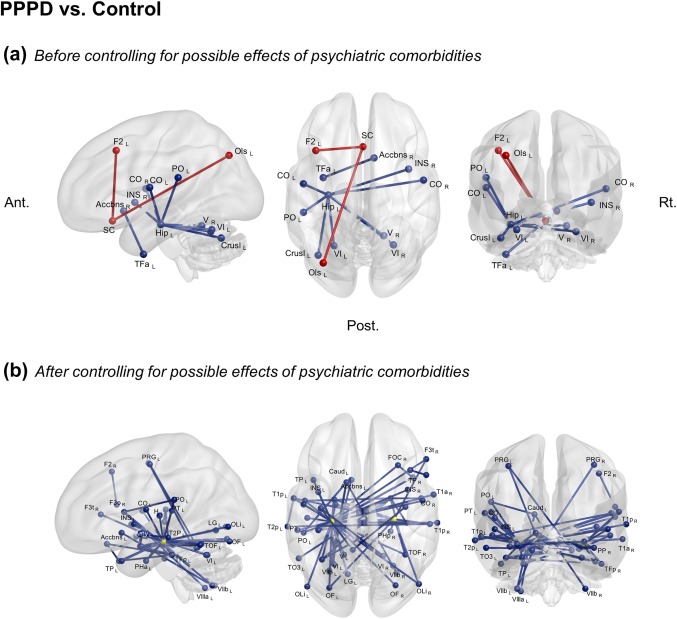
ROI‐to‐ROI analyses of the resting‐state fMRI in the whole brain. The connectivity with a significant difference between the patients with PPPD and healthy controls. Red lines show increased functional connectivity in PPPD patients compared to healthy controls while blue lines illustrate decreased functional connectivity in patients with PPPD. a and b showed the results before or after excluding possible effects of psychiatric comorbidities. Two yellow circle seeds represent bilateral hippocampus. Seed‐level two‐sided false discovery rate (FDR)‐corrected p < .05. Accbns: nucleus accumbens, Ant.: anterior side, Amy: amygdala, Caud: caudate CO: central opercular cortex, Crus I: cerebellar Crus I, F2: Middle frontal gyrus, Hip: Hippocampus, INS: insular cortex, F3o: inferior frontal gyrus, pars opercularis, F3t:inferior frontal gyrus, pars triangularis, FOC: frontal orbital cortex, H: Heschl's gyrus, Hip: hippocampus, INS: insular cortex, LG: lingual gyrus, OF: occipital fusiform gyrus, OLi: lateral occipital cortex, inferior, Ols: lateral occipital cortex superior division, PO: parietal opercular cortex, Post.: posterior side, PPPD: persistent postural perceptual dizziness, Rt.: right side, SC: subcallosal cortex, T1a:superior temporal gyrus, anterior, T1p: superior temporal gyrus, posterior, T2p: middle temporal gyrus, posterior, TFa: temporal fusiform cortex, anterior division, TFp: temporal fusiform cortex, posterior, TO3: inferior temporal gyrus, temporo‐occipital, TOF: temporal occipital fusiform cortex, TP: temporal pole, I–IV: cerebellar lobules I‐IV, V: cerebellar lobule V, VI: cerebellar lobule VI, VIIb: cerebellar lobule VIIb, VIIIa: cerebellar lobule VIIIa
3.3. Functional connectivity analysis centered on specific vestibular and visual brain regions
Patients with PPPD differed significantly in functional connectivity from healthy controls in several a priori vestibular, visual, and hippocampal regions of interest. Specifically, they showed increased connectivity between the left OP2 and the left lateral occipital cortex. They also had increased connectivity between left V1 and the left temporal pole. Patients with PPPD had decreased connectivity between the left hippocampus and the left cerebellar lobules VI and Crus I (Figure 2a, Supporting Information Table S3).
Figure 2.
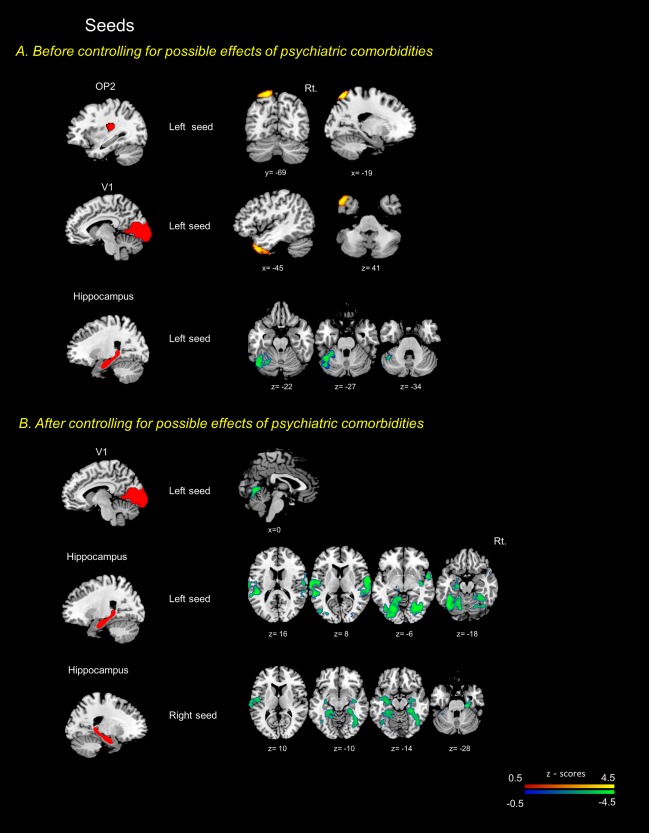
Seed to voxel analyses centered on specific brain regions associated dizziness. Seed placements are represented in the left side. The red‐yellow colored regions indicate increased functional connectivity in PPPD patients compared to healthy controls. In contrast, the blue‐green colored regions denote decreased functional connectivity in PPPD. a and b showed the results before or after excluding possible effects of psychiatric comorbidities. Cluster sized family‐wise error (FWE) corrected p < .05/number of seeds using Bonferroni correction with a cluster extent threshold of 200. The T1 template of the Montreal Neurological Institute (MNI) was used to render results. The x, y, and z values indicate the MNI coordinates of represented sections. Color bar is presented with z scores, respectively. OP2: parietal opercular cortex 2, PPPD: persistent postural perceptual dizziness, Rt.: right side
3.4. Group differences in functional connectivity after controlling for effects of psychiatric comorbidities
When the possible effects of anxiety and depression were controlled as covariates, whole brain analyses showed significantly decreased functional connectivity centered on the hippocampus bilaterally, but especially on the left side, in patients with PPPD versus controls. In patients, connectivity was decreased between left hippocampus and right inferior frontal gyrus, bilateral temporal lobes, bilateral insular cortices, bilateral central opercular cortices, left parietal opercular cortex, bilateral occipital lobes and cerebellum (bilateral lobules VI and V, and left I–IV). Connectivity was decreased between right hippocampus and right inferior frontal gyrus, bilateral temporal lobes, bilateral parahippocampal gyri, bilateral occipital lobes, and left amygdala. Connectivity also was decreased between bilateral temporal lobes and both right amygdala and right frontal lobe (inferior frontal gyrus, and orbital frontal cortex) as well as within the temporal lobes. Additionally, connectivity was decreased between the cerebellum (bilateral lobules VIIb, left lobules VIIIb, left lobules VIIIa and I–IV and right lobule V) and bilateral precentral gyri, left caudate, and left nucleus accumbens (Figure 1b, Supporting Information Table S2). After controlling for psychiatric state, no increased connectivity was identified.
From seed to voxel analyses centered on OP2 and V1 regions, the only significant finding was decreased connectivity between the bilateral V1 and lingual gyri in patients with PPPD versus controls. No differences were found in the connectivity centered on the OP2. Additional analyses centered on the hippocampus showed decreased connectivity in patients versus controls between left hippocampus and bilateral central opercular cortices, bilateral occipital fusiform gyrus, right temporal occipital fusiform cortex and left amygdala. Right hippocampus disclosed decreased connectivity with bilateral parahippocampal gyri, bilateral temporal occipital fusiform, right lateral occipital cortex, left hippocampus and left central opercular cortex (Figure 2b, Supporting Information Table S4). No increase in connectivity was identified.
3.5. Functional connectivity of the hippocampus according to the anterior–posterior axis
Without controlling for psychiatric comorbidities, the left anterior hippocampus showed decreased connectivity with left parietal opercular cortex, right insular cortex, bilateral lingual gyri, cerebellar lobules VI and Crus I in patients with PPPD compared to controls. No differences were found in the connectivity centered on the bilateral posterior hippocampi
After controlling for psychiatric comorbidities, patients with PPPD compared to controls showed decreased connectivity between left anterior hippocampus and bilateral lingual gyri and bilateral cerebellar lobules VI. They also had decreased connectivity between left posterior hippocampus and bilateral central opercular cortices and right middle temporal gyrus. The right posterior hippocampus had decreased connectivity with right middle temporal gyrus, right supramarginal gyrus, left superior temporal gyrus and left postcentral gyrus (Figure 3, Supporting Information Table S5). Again, no increase in connectivity was found after controlling for psychiatric variables.
Figure 3.
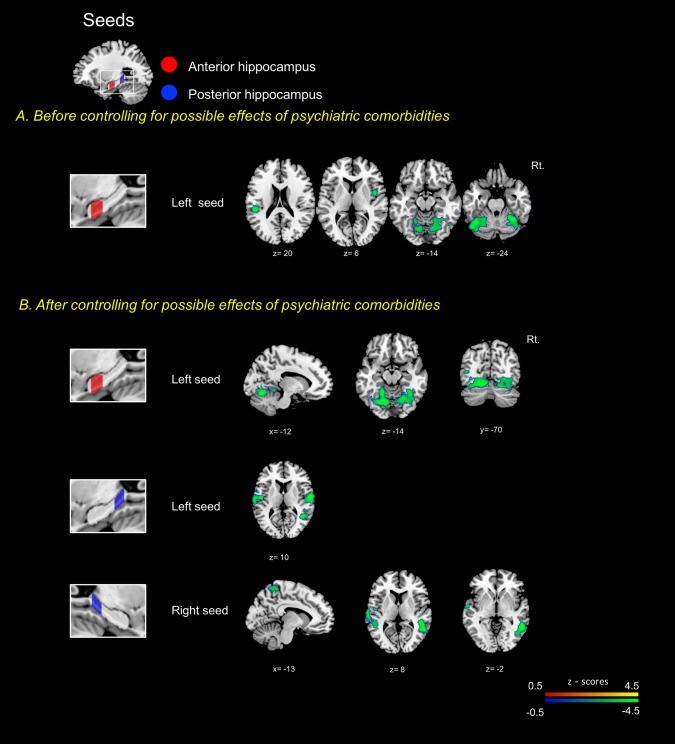
Seed to voxel analyses centered on the hippocampus along the anterior‐posterior axis. Seed placements are represented on the left side. The red‐yellow colored regions indicate increased functional connectivity in PPPD patients compared to healthy controls. In contrast, the blue‐green colored regions denote decreased functional connectivity in PPPD. a and b showed the results before or after excluding possible effects of psychiatric comorbidities. Cluster sized family‐wise error (FWE) corrected p < .05/number of seeds using Bonferroni correction with a cluster extent threshold of 200. The T1 template of the Montreal Neurological Institute (MNI) was used to render results. The x, y, and z values indicate the MNI coordinates of represented sections. Color bar is presented with z scores, respectively. PPPD: persistent postural perceptual dizziness
3.6. Correlation of the altered connectivity with the severity of dizziness handicap, anxiety, and depression
In patients with PPPD, positive correlations were found between severity of dizziness handicap (DHI scores) and (a) connectivity among bilateral supramarginal gyri and left posterior lobes of the cerebellum (Crus II, VIIb, VIIIa, and VIIIb), (b) connectivity centered on right inferior temporal gyrus, and (c) connectivity centered on the cerebellar lobule X. Negative correlations were found with the connectivity of left parietal opercular cortex, left hippocampus and left amygdala (Figure 4, Supporting Information Table S6).
Figure 4.
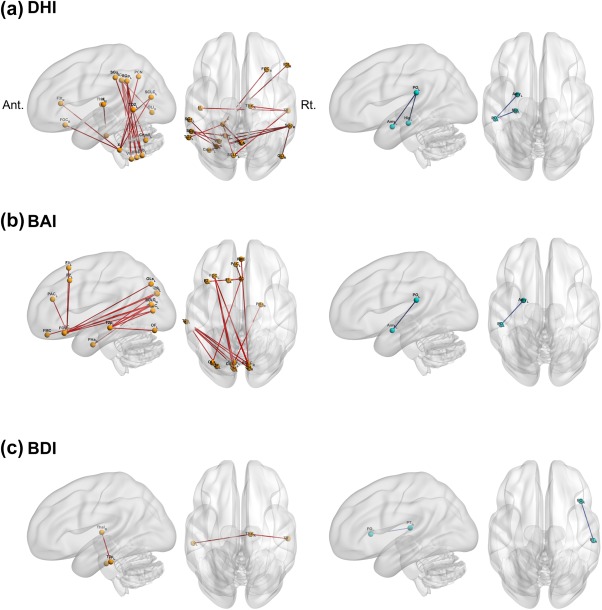
Correlation between the functional connectivity and the severity of dizziness, anxiety and depression in the whole brain. Each figure shows the functional connectivity correlated with the severity of dizziness (a), anxiety (b) and depression (c). Two‐sided seed level false discovery rate (FDR)‐corrected p < .05. Red lines: positive correlation with the severity, Blue lines: negative correlation with the severity. Ant.: anterior side, Amy: Amygdala, BAI: Beck Anxiety Inventory, BDI: Beck Depression Inventory, BDI: Beck Depression Inventory, CALC: Intracalcarine cortex, CN: Cuneal cortex, Crus I: cerebellar Crus I, DHI: Dizziness Handicap Inventory, F1: Superior frontal gyrus, F2: Middle frontal gyrus, F3t: Inferior frontal gyrus, pars triangularis, FMC: Frontal medial cortex, FO: Frontal opercular cortex, FOC: Frontal orbital cortex, H: Heschl's gyrus, Hip: Hippocampus, OF: Occipital fusiform gyrus, OLi: Lateral occipital cortex, inferior division, OLs: Lateral occipital cortex, superior division, PAC: Paracingulate gyrus, PCN: Precuneous cortex, PHa: Parahippocampal gyrus, anterior division, PO: Parietal opercular cortex, PPPD: persistent postural perceptual dizziness, PT: Planum temporale, Rt.: right side, SC: Subcallosal cortex, SCLC: Supracalcarine cortex, SGa: Supramarginal gyrus, anterior division, SGp: Supramarginal gyrus, posterior division, T2p: Middle temporal gyrus, posterior division, T3p: Inferior temporal gyrus, posterior division, Thal: Thalamus, TO2: Middle temporal gyrus, temporooccipital part, VIIb: cerebellar lobule VIIb, VIIIa: cerebellar lobule VIIIa, VIIIb: cerebellar lobule VIIIb, X: cerebellar lobule X
For anxiety, positive correlations were identified between BAI scores and (a) connectivity between bilateral calcarine cortices and subcallosal or frontal orbital cortex and (b) connectivity between bilateral calcarine cortices and middle temporal gyrus. A negative correlation was found with the connectivity between left parietal opercular cortex and left amygdala (Figure 4, Supporting Information Table S6).
For depression, a positive correlation was found between BDI scores and connectivity between right thalamus and bilateral inferior temporal gyri and a negative correlation was observed with the connectivity between right planum temporale and right frontal opercular cortex (Figure 4, Supporting Information Table S6).
3.7. Identification of whole‐brain functional connectivity as a potential biomarker of PPPD
The support vector machine developed a classification criterion based on 11 connectivity markers that differentiated patients with PPPD from normal controls with a sensitivity of 78.4%, specificity of 76.9%, accuracy of 77.6%, and areas under the ROC curve of 0.88 (Figure 5).
Figure 5.
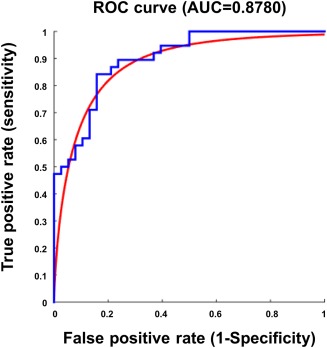
Receiver operating characteristic (ROC) curve. The ROC curve shows that use of the supporting vector machine resulted in secure differentiation of patients with persistent postural perceptual dizziness from healthy controls with an area under the curve of 0.88 [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
This is the first study on resting state functional connectivity using whole brain and seed‐based ROI analyses in patients with PPPD. The results showed altered connectivity in brain networks involved in spatial cognition and multisensory vestibular processing as well as in the networks subserving visual and emotional processing in patients compared to healthy controls.
4.1. Increased connectivity of the frontal‐occipital network
The subcallosal cortex responds to complex visual stimuli (Royet et al., 2000) and is an important component of the networks that are involved in executive and cognitive processes, responses to stress, and processing of pain (Hamani et al., 2011). The middle frontal gyrus contains the frontal eye fields and the dorsolateral prefrontal cortex, both of which are involved in high level control of saccadic eye movements. The dorsolateral prefrontal cortex also supports executive functions, such as conscious planning and prediction of forthcoming actions based on information stored in working memory (Bruce, Goldberg, Bushnell, & Stanton, 1985; Pochon, et al., 2001). The lateral occipital cortex, a part of the ventral visual stream, plays an important role in object recognition (Grill‐Spector, Kourtzi, & Kanwisher, 2001), and stores a percept assembled by motion sensitive areas such as the middle temporal and medial superior temporal cortices (Ferber, Humphrey, & Vilis, 2003). Thus, the increased connectivity of the subcallosal cortex with the middle frontal and lateral occipital cortices in patients with PPPD versus normal controls indicates that patients had a stronger connection among the brain regions involved in visual and behavioral functions, specifically ones that exert high level control over saccadic eye movements, process motion perception from visual inputs and guide executive functions such as planning, prediction of behavioral outcomes, and inhibition of reflexive responses to stress and pain. However, this result appeared to be related to patients’ psychiatric state as it was not found in analyses that controlled for anxiety and depression.
4.2. Decreased connectivity of the parieto‐insular‐hippocampal‐cerebellar network
Patients with PPPD showed significantly decreased connectivity of the left hippocampus with the cerebellar lobules VI and Crus I. The hippocampal formation is involved in spatial orientation and navigation (Hufner, Strupp, Smith, Brandt, & Jahn, 2011). Cerebellar lobules IV, VI, and Crus I are associated with judgement of spatial orientation (Lee et al., 2005). These regions are related more closely to spatial cognition than to sensorimotor processing (Stoodley, Valera, & Schmahmann, 2012). The left hippocampus and cerebellar lobule VI interact during prediction of spatio‐temporal aspects of voluntary movements, but not during reactive motor tasks or imagery (Onuki, Van Someren, De Zeeuw, & Van der Werf, 2015). These results indicate that patients with PPPD have reduced connectivity in areas of the brain that play central roles in spatial cognition.
Patients with PPPD also had decreased connectivity between the left hippocampus and the right insular cortex, bilateral central opercular cortices and left parietal opercular cortex. The hippocampus is well‐known for its roles in spatial orientation and navigation (Hufner et al., 2011), but it also may be important for high‐level compensation for peripheral or central vestibular lesions (Smith, 1997). The human vestibular cortex is centered in the OP2 region of the opercular cortices (zu Eulenburg et al., 2012) and together with adjacent areas of the insula and temporal‐parietal junction constitutes the human homologue of the parieto‐insular vestibular cortex (PIVC) that has been identified in other species (Eickhoff, Weiss, Amunts, Fink, & Zilles, 2006). Although several brain regions are known to be involved in self‐motion perception (Baier et al., 2013; Kaski et al., 2016; Nigmatullina et al., 2015), the insular and opercular cortices play an important role in multisensory integration of space‐motion information for self‐motion perception (Shinder & Newlands, 2014). These findings are consistent with reduced activity and connectivity of the PIVC, hippocampus and anterior insula observed in patients with CSD in a previous fMRI study (Indovina et al., 2015). Thus, persistent vestibular symptoms of PPPD may be related to reduced connectivity among the areas involved in spatial orientation and self‐motion perception.
Our patients with PPPD showed decreased connectivity between right nucleus accumbens and anterior portion of left temporal fusiform cortex. The nucleus accumbens, as a part of the ventral striatum, plays a key role in the cognitive processing of motivation, reward, and reinforcement learning (Day & Carelli, 2007). The function of the anterior portion of temporal fusiform gyrus is not fully understood, but it appears to be involved in visual semantic processing (Simons, Koutstaal, Prince, Wagner, & Schacter, 2003). Therefore, decreased connectivity between the nucleus accumbens and temporal fusiform gyrus may impair processing of visual reinforcement learning in patients with PPPD.
4.3. Functional connectivity centered on regions of interest related to vestibular and visual processing
Our patients with PPPD also showed increased connectivity between the left lateral occipital cortex and left OP2 area, which may be related to psychiatric state as it became no longer significant after controlling for anxiety and depression. In functional imaging studies in normal individuals, the visual cortex was inhibited during vestibular stimulation and vestibular cortex was inhibited during visual stimulation (Brandt, Bartenstein, Janek, & Dieterich, 1998). This reciprocal inhibition in visual‐vestibular interaction is believed to be important for perception of self‐motion. In a previous fMRI study of normal subjects (Indovina et al., 2014), the personality trait of neuroticism (a potential predisposing factor for anxiety and depressive disorders) was associated with increased connectivity of the inferior frontal gyrus with both visual and vestibular cortical regions. Thus, psychiatric factors may affect the reciprocal inhibition of visual‐vestibular interactions in healthy individuals and patients with PPPD.
We also found increased connectivity between left V1 and left temporal pole. The temporal pole is involved in various cognitive functions, including memory and emotional tagging of perceptual processes (Blaizot et al., 2010). As was the case with connectivity between visual and vestibular cortices, this result was not sustained after controlling for anxiety and depression, indicating that psychiatric state may affect the strength of association between regions that add emotional valence to sensory inputs and areas related to visual processing in patients with PPPD.
4.4. Group differences in functional connectivity after controlling for effects of psychiatric comorbidities
When we accounted for the effects of state anxiety and depression by including BAI and BDI scores in our general linear models of resting state connectivity, the findings of increased connectivity between the subcallosal cortex and the left superior lateral occipital cortex and left middle frontal gyrus and between the left OP2 and left lateral occipital cortex in patients with PPPD were no longer statistically significant. Rather, statistical significance remained only for decreased connectivity among the key vestibular, visual, and frontal regulatory regions centered mostly on the hippocampus bilaterally and amygdala on the right. This is consistent with the fMRI results of a previous study (Indovina et al., 2015) that found widespread decreases in connectivity among many of these same areas in patients with CSD in response to sound evoked vestibular stimuli. In that study, the authors carefully controlled for the effect of psychiatric variables by matching patients and healthy control subjects on personality traits and state anxiety and depression. Another study (Van Ombergen et al., 2017) reported increased connectivity in visual cortical areas and decreased connectivity in vestibular cortical areas using a resting state fMRI in patients with visually‐induced dizziness, but did not account for the psychiatric state of their subjects. Taken together, our results and the findings of these two previous studies indicate that alterations in brain functioning attributable to PPPD itself are decreases in connectivity among the vestibular and visual cortices, the frontal regulatory regions and the hippocampus. Increased activity and connectivity in the visual cortex may be more attributable to the effects of co‐existing anxiety or depression.
4.5. Correlation of the altered connectivity with the severity of dizziness, anxiety and depression
In our patients with PPPD, the total DHI score showed a positive correlation with the connectivity between bilateral supramarginal gyri and left cerebellum. The supramarginal gyrus is a part of the somatosensory association cortex that is involved in interpretation of tactile sensory data and perception of space and limb location (Ben‐Shabat, Matyas, Pell, Brodtmann, & Carey, 2015). It is also involved in identifying posture and gesture of other people, and is thus a part of the mirror neuron system (Reed & Caselli, 1994). In contrast, the DHI score showed a negative correlation with the connectivity between left hippocampus and left parietal opercular cortex. Thus, the severity of dizziness handicap in patients with PPPD may be associated with increased reliance not only on visual inputs but also on somatosensory rather than vestibular cues. Somatosensory dependence was identified in a previous investigation of space‐motion discomfort (Jacob, Redfern, & Furman, 2009). Dizziness handicap also may be related to impaired recognition of self‐motion and spatial working memory.
The score of BAI was positively correlated with the connectivity between the visual and frontal cortices. This is consistent with the increased connectivity between these areas identified in our patients with PPPD in the analyses that did not control for psychiatric state. It is well established that the anxiety‐related personality trait of neuroticism is a risk factor for PPPD (Chiarella et al., 2016; Staab et al., 2014; Yan et al., 2017). In an fMRI study of normal individuals, neuroticism correlated with increased activity in the visual cortex and with increased connectivity between the visual and frontal cortices, suggesting that neuroticism and state anxiety may mediate the shift to visual dependence in patients with PPPD.
In our patients, the BDI score was positively correlated with the connectivity between the thalamus and inferior temporal gyrus. Increased activation of the thalamus has been reported in patients with major depression (Hamilton et al., 2012). The inferior temporal cortex, as the final destination of the ventral visual stream, plays an important role for visual recognition of objects and memory (Kravitz, Saleem, Baker, Ungerleider, & Mishkin, 2013). Future studies will have to investigate the role of depression on visual function in patients with PPPD.
4.6. The role of hippocampus in PPPD
Based on our findings of altered connectivity centered on the hippocampus in patients with PPPD, we performed additional analyses to understand the details of those results. The hippocampus may be divided into anterior and posterior subdivisions that have a distinct functional connectivity. The anterior portion modulates stress‐induced activity of the hypothalamic‐pituitary‐adrenal axis and exerts negative‐feedback control over the axis (Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009). In contrast, the posterior portion is a crucial component of networks that mediate the cognitive processes of spatial memory and navigation as well as retrieval of episodic and semantic memories (Kjelstrup et al., 2008).
Patients with PPPD showed decreased connectivity of the left anterior hippocampus with left parietal opercular cortex, right insular cortex and bilateral cerebellar lobules VI and Crus I, which reinforced the results of our whole brain analyses before controlling for anxiety and depression. However, after controlling for psychiatric state, decreased connectivity of the left anterior hippocampus became much less pronounced whereas decreased connectivity remained statistically significant between the bilateral posterior portions of hippocampi and bilateral central opercular cortices, bilateral temporal gyri and left postcentral gyrus. Previous studies showed that the two hippocampi process complementary information with the right hippocampus mainly activated by allocentric (i.e., world‐centered) or place‐learning spatial tasks and the left hippocampus mostly activated by egocentric (i.e., body‐centered) or sequential stimulus response tasks (Igloi, Doeller, Berthoz, Rondi‐Reig, & Burgess, 2010). Our results of decreased connectivity of the hippocampi are consistent with the findings of decreased activity and connectivity identified in the fMRI study of CSD (Indovina et al., 2015), and indicate that patients with PPPD may have impaired spatial cognition.
4.7. Identification of whole‐brain functional connectivity as a potential biomarker of PPPD
In previously published works, alterations in functional connectivity successfully differentiated patients with neurologic and psychiatric disorders such as major depression (Zeng et al., 2012) and Alzheimer's disease/amnestic mild cognitive impairment (Chen et al., 2011) from healthy controls. In this study, we were able to use a machine algorithm validated by the leave‐one‐out method to discriminate patients with PPPD from controls with a reasonable precision using only 11 connections that showed significant between group differences in the whole brain analyses. If validated in future experiments, these connectivity patterns may be used as functional neuroimaging biomarkers of PPPD.
4.8. Limitations
Most fMRI studies of the brain utilize atlases derived from anatomical or cyto‐architectonic boundaries to specify ROIs (Bressler & Menon, 2010). However, the suitability of these atlases has yet to be established for the studies on resting state functional connectivity of the brain. Recent quantitative cyto‐architecture mapping techniques offer a tighter link with the functional architecture of the brain, but only a small set of cortical regions are covered well. Thus, the validity of our functional connectivity analyses depended on our ability to identify ROIs accurately. Where standard atlases were limited in this regard, we used previous vestibular imaging work to guide our methods. Several aspects should be considered when interpreting the classification results as well. The accuracy of the final model was higher than the chance level but still modest. In addition, the results may have been biased since the selection of features for machine learning were based on the results of group comparison. This study did not adopt a dizzy control group. Thus, it remains to be determined that the findings observed in our patients are specific for PPPD.
In conclusion, in this study of patients with PPPD versus healthy controls, we identified decreased connectivity in key regions of the brain involved in vestibular and visual information processing, spatial cognition, and executive functions centered primarily on the hippocampus bilaterally and including the vestibular cortex, occipital lobes, inferior frontal gyrus, insular cortex, and cerebellum. These results were consistent with a previous fMRI report of sound‐evoked vestibular stimulation in patients with CSD that showed decreased activity and connectivity in many of these same areas. Taken together, these results suggest that patients with PPPD may have widely diminished cortical integration of multi‐sensory space‐motion information and deficits in spatial cognition. We were able to separate these core features of PPPD from additional alterations, namely increased connectivity between frontal lobe regions involved in emotional regulation and occipital areas processing visual association, that were linked to state anxiety and depression in our multivariate general linear models. Additional studies are needed to validate and extend these results and link them to physiological data and specific symptoms of PPPD.
4.9. Supplementary data
This manuscript contains supplementary Tables from S1 to S7. Tables S1 to S6 show the detailed results of connectivity shown in the corresponding figures respectively. Table S7 shows the regions of interest included for analyses in this study according to the prior probability template of Harvard‐Oxford atlas and SUIT.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
We sincerely thank the technicians of Seoul National University Bundang Hospital for help in subjects’ management and image scanning. This study was supported by the supported by grant no 14–2017‐008 from the SNUBH Research Fund.
Lee J‐O, Lee E‐S, Kim J‐S, et al. Altered brain function in persistent postural perceptual dizziness: A study on resting state functional connectivity. Hum Brain Mapp. 2018;39:3340–3353. 10.1002/hbm.24080
Funding information The SNUBH Research Fund, Grant/Award Number: 14‐2017‐008
Contributor Information
Ji‐Soo Kim, Email: jisookim@snu.ac.kr.
Jae‐Hyoung Kim, Email: jaehkim@snu.ac.kr.
REFERENCES
- Baier, B. , Zu Eulenburg, P. , Best, C. , Geber, C. , Muller‐Forell, W. , Birklein, F. , & Dieterich, M. (2013). Posterior insular cortex – A site of vestibular‐somatosensory interaction? Brain and Behavior, 3(5), 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Shabat, E. , Matyas, T. A. , Pell, G. S. , Brodtmann, A. , & Carey, L. M. (2015). The right supramarginal gyrus is important for proprioception in healthy and stroke‐affected participants: A functional MRI study. Frontiers in Neurology, 6, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaizot, X. , Mansilla, F. , Insausti, A. M. , Constans, J. M. , Salinas‐Alaman, A. , Pro‐Sistiaga, P. , … Insausti, R. (2010). The human parahippocampal region: I. Temporal pole cytoarchitectonic and MRI correlation. Cerebral Cortex, 20(9), 2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, T. (1996). Phobic postural vertigo. Neurology, 46(6), 1515–1519. [DOI] [PubMed] [Google Scholar]
- Brandt, T. , Bartenstein, P. , Janek, A. , & Dieterich, M. (1998). Reciprocal inhibitory visual‐vestibular interaction. Visual motion stimulation deactivates the parieto‐insular vestibular cortex. Brain, 121(Pt 9), 1749–1758. [DOI] [PubMed] [Google Scholar]
- Bressler, S. L. , & Menon, V. (2010). Large‐scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. [DOI] [PubMed] [Google Scholar]
- Bronstein, A. M. (1995). Visual vertigo syndrome: Clinical and posturography findings. Journal of Neurology, Neurosurgery, and Psychiatry, 59(5), 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, C. J. , Goldberg, M. E. , Bushnell, M. C. , & Stanton, G. B. (1985). Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. Journal of Neurophysiology, 54(3), 714–734. [DOI] [PubMed] [Google Scholar]
- Caviness, V. S., Jr. , Meyer, J. , Makris, N. , & Kennedy, D. N. (1996). MRI‐based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience, 8(6), 566–587. [DOI] [PubMed] [Google Scholar]
- Chen, A. C. , & Etkin, A. (2013). Hippocampal network connectivity and activation differentiates post‐traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology, 38(10), 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Ward, B. D. , Xie, C. , Li, W. , Wu, Z. , Jones, J. L. , … Li, S. J. (2011). Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large‐scale network analysis based on resting‐state functional MR imaging. Radiology, 259(1), 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarella, G. , Petrolo, C. , Riccelli, R. , Giofre, L. , Olivadese, G. , Gioacchini, F. M. , … Passamonti, L. (2016). Chronic subjective dizziness: Analysis of underlying personality factors. Journal of Vestibular Research, 26(4), 403–408. [DOI] [PubMed] [Google Scholar]
- Cousins, S. , Kaski, D. , Cutfield, N. , Arshad, Q. , Ahmad, H. , Gresty, M. A. , … Bronstein, A. M. (2017). Predictors of clinical recovery from vestibular neuritis: A prospective study. Annals of Clinical and Translational Neurology, 4(5), 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day, J. J. , & Carelli, R. M. (2007). The nucleus accumbens and Pavlovian reward learning. The Neuroscientist, 13(2), 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic, K. , Duchesne, A. , Andrews, J. , Engert, V. , & Pruessner, J. C. (2009). The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage, 47(3), 864–871. [DOI] [PubMed] [Google Scholar]
- Diedrichsen, J. , Balsters, J. H. , Flavell, J. , Cussans, E. , & Ramnani, N. (2009). A probabilistic MR atlas of the human cerebellum. NeuroImage, 46(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Dieterich, M. , & Staab, J. P. (2017). Functional dizziness: From phobic postural vertigo and chronic subjective dizziness to persistent postural‐perceptual dizziness. Current Opinion in Neurology, 30(1), 107–113. [DOI] [PubMed] [Google Scholar]
- Dieterich, M. , Staab, J. P. , & Brandt, T. (2016). Functional (psychogenic) dizziness. Handbook of Clinical Neurology, 139, 447–468. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Grefkes, C. , Zilles, K. , & Fink, G. R. (2007). The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cerebral Cortex, 17(8), 1800–1811. [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Weiss, P. H. , Amunts, K. , Fink, G. R. , & Zilles, K. (2006). Identifying human parieto‐insular vestibular cortex using fMRI and cytoarchitectonic mapping. Human Brain Mapping, 27(7), 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber, S. , Humphrey, G. K. , & Vilis, T. (2003). The lateral occipital complex subserves the perceptual persistence of motion‐defined groupings. Cerebral Cortex, 13(7), 716–721. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Worsley, K. J. , Frackowiak, R. S. , Mazziotta, J. C. , & Evans, A. C. (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping, 1(3), 210–220. [DOI] [PubMed] [Google Scholar]
- Godemann, F. , Siefert, K. , Hantschke‐Bruggemann, M. , Neu, P. , Seidl, R. , & Strohle, A. (2005). What accounts for vertigo one year after neuritis vestibularis – Anxiety or a dysfunctional vestibular organ? Journal of Psychiatric Research, 39(5), 529–534. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector, K. , Kourtzi, Z. , & Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41(10–11), 1409–1422. [DOI] [PubMed] [Google Scholar]
- Hamani, C. , Mayberg, H. , Stone, S. , Laxton, A. , Haber, S. , & Lozano, A. M. (2011). The subcallosal cingulate gyrus in the context of major depression. Biological Psychiatry, 69(4), 301–308. [DOI] [PubMed] [Google Scholar]
- Hamilton, J. P. , Etkin, A. , Furman, D. J. , Lemus, M. G. , Johnson, R. F. , & Gotlib, I. H. (2012). Functional neuroimaging of major depressive disorder: A meta‐analysis and new integration of base line activation and neural response data. American Journal of Psychiatry, 169(7), 693–703. [DOI] [PubMed] [Google Scholar]
- Han, G. C. , Lee, E. J. , Lee, J. H. , Park, S. N. , Lee, H. Y. , Jeon, E. J. , … Kim, K. S. (2004). The study of standardization for a Korean adaptation of self‐report measures of dizziness. Journal of the Korean Balance Society, 3, 307–325. [Google Scholar]
- Heinrichs, N. , Edler, C. , Eskens, S. , Mielczarek, M. M. , & Moschner, C. (2007). Predicting continued dizziness after an acute peripheral vestibular disorder. Psychosomatic Medicine, 69(7), 700. [DOI] [PubMed] [Google Scholar]
- Hufner, K. , Strupp, M. , Smith, P. , Brandt, T. , & Jahn, K. (2011). Spatial separation of visual and vestibular processing in the human hippocampal formation. Annals of the New York Academy of Sciences, 1233(1), 177–186. [DOI] [PubMed] [Google Scholar]
- Igloi, K. , Doeller, C. F. , Berthoz, A. , Rondi‐Reig, L. , & Burgess, N. (2010). Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina, I. , Riccelli, R. , Chiarella, G. , Petrolo, C. , Augimeri, A. , Giofre, L. , … Passamonti, L. (2015). Role of the insula and vestibular system in patients with chronic subjective dizziness: An fMRI study using sound‐evoked vestibular stimulation. Frontiers in Behavioral Neuroscience, 9, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina, I. , Riccelli, R. , Staab, J. P. , Lacquaniti, F. , & Passamonti, L. (2014). Personality traits modulate subcortical and cortical vestibular and anxiety responses to sound‐evoked otolithic receptor stimulation. Journal of Psychosomatic Research, 77(5), 391–400. [DOI] [PubMed] [Google Scholar]
- Jacob, R. G. , Redfern, M. S. , & Furman, J. M. (2009). Space and motion discomfort and abnormal balance control in patients with anxiety disorders. Journal of Neurology, Neurosurgery, and Psychiatry, 80(1), 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, R. G. , Woody, S. R. , Clark, D. B. , Lilienfeld, S. O. , Hirsch, B. E. , Kucera, G. D. , … Durrant, J. D. (1993). Discomfort with space and motion: A possible marker of vestibular dysfunction assessed by the situational characteristics questionnaire. Journal of Psychopathology and Behavioral Assessment, 15(4), 299–324. [Google Scholar]
- Kaski, D. , Quadir, S. , Nigmatullina, Y. , Malhotra, P. A. , Bronstein, A. M. , & Seemungal, B. M. (2016). Temporoparietal encoding of space and time during vestibular‐guided orientation. Brain, 139(2), 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup, K. B. , Solstad, T. , Brun, V. H. , Hafting, T. , Leutgeb, S. , Witter, M. P. , … Moser, M. B. (2008). Finite scale of spatial representation in the hippocampus. Science, 321(5885), 140–143. [DOI] [PubMed] [Google Scholar]
- Kravitz, D. J. , Saleem, K. S. , Baker, C. I. , Ungerleider, L. G. , & Mishkin, M. (2013). The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends in Cognitive Science, 17(1), 26–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. K. , Lee, Y. H. , Park, S. H. , Sohn, C. H. , Jung, Y. J. , & Hong, S. K. (1995). Standardization study of Beck Depression Inventory (I): Korean version (K‐BDI): Reliability land factor analysis. Korean Journal of Psychopathology, 4, 77–95. [Google Scholar]
- Lee, T. M. , Liu, H. L. , Hung, K. N. , Pu, J. , Ng, Y. B. , Mak, A. K. , … Chan, C. C. (2005). The cerebellum's involvement in the judgment of spatial orientation: A functional magnetic resonance imaging study. Neuropsychologia, 43(13), 1870–1877. [DOI] [PubMed] [Google Scholar]
- Longridge, N. S. , Mallinson, A. I. , & Denton, A. (2002). Visual vestibular mismatch in patients treated with intratympanic gentamicin for Meniere's disease. The Journal of Otolaryngology, 31(01), 5–8. [DOI] [PubMed] [Google Scholar]
- Ludbrook, J. (2013). Should we use one‐sided or two‐sided P values in tests of significance? Clinical and Experimental Pharmacology & Physiology, 40(6), 357–361. [DOI] [PubMed] [Google Scholar]
- Marks, I. (1981). Space “phobia”: A pseudo‐agoraphobic syndrome. Journal of Neurology, Neurosurgery, and Psychiatry, 44(5), 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigmatullina, Y. , Arshad, Q. , Wu, K. , Seemungal, B. M. , Bronstein, A. M. , & Soto, D. (2015). How imagery changes self‐motion perception. Neuroscience, 291, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Onuki, Y. , Van Someren, E. J. , De Zeeuw, C. I. , & Van der Werf, Y. D. (2015). Hippocampal‐cerebellar interaction during spatio‐temporal prediction. Cereb. Cerebral Cortex, 25(2), 313–321. [DOI] [PubMed] [Google Scholar]
- Page, N. G. , & Gresty, M. A. (1985). Motorist's vestibular disorientation syndrome. Journal of Neurology, Neurosurgery, and Psychiatry, 48, 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon, J. B. , Levy, R. , Poline, J. B. , Crozier, S. , Lehericy, S. , Pillon, B. , … Dubois, B. (2001). The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: An fMRI study. Cerebral Cortex, 11(3), 260–266. [DOI] [PubMed] [Google Scholar]
- Reed, C. L. , & Caselli, R. J. (1994). The nature of tactile agnosia: A case study. Neuropsychologia, 32(5), 527–539. [DOI] [PubMed] [Google Scholar]
- Riccelli, R. , Indovina, I. , Staab, J. P. , Nigro, S. , Augimeri, A. , Lacquaniti, F. , & Passamonti, L. (2017). Neuroticism modulates brain visuo‐vestibular and anxiety systems during a virtual rollercoaster task. Human Brain Mapping, 38(2), 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet, J. P. , Zald, D. , Versace, R. , Costes, N. , Lavenne, F. , Koenig, O. , & Gervais, R. (2000). Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: A positron emission tomography study. Journal of Neuroscience, 20, 7752–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinder, M. E. , & Newlands, S. D. (2014). Sensory convergence in the parieto‐insular vestibular cortex. Journal of Neurophysiology, 111(12), 2445–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, J. S. , Koutstaal, W. , Prince, S. , Wagner, A. D. , & Schacter, D. L. (2003). Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage, 19(3), 613–626. [DOI] [PubMed] [Google Scholar]
- Kwon, S. M. (1997). Assessment of psychopathology in anxiety disorder. Korean Journal of Psychopathology, 6, 37–51. [Google Scholar]
- Smith, P. F. (1997). Vestibular‐hippocampal interactions. Hippocampus, 7(5), 465–471. [DOI] [PubMed] [Google Scholar]
- Staab, J. P. (2012). Chronic subjective dizziness. Continuum (Minneapolis, MN), 18(5 Neuro‐otology), 1118–1141. [DOI] [PubMed] [Google Scholar]
- Staab, J. P. , Eckhardt‐Henn, A. , Horii, A. , Jacob, R. , Strupp, M. , Brandt, T. , & Bronstein, A. (2017). Diagnostic Criteria for Persistent Postural‐Perceptual Dizziness(PPPD): Consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. Journal of Vestibular Research, 27(4), 191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab, J. P. , Rohe, D. E. , Eggers, S. D. , & Shepard, N. T. (2014). Anxious, introverted personality traits in patients with chronic subjective dizziness. Journal of Psychosomatic Research, 76(1), 80–83. [DOI] [PubMed] [Google Scholar]
- Staab, J. P. , & Ruckenstein, M. J. (2007). Expanding the differential diagnosis of chronic dizziness. Archives of Otolaryngology‐Head & Neck Surgery, 133(2), 170–176. [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , Valera, E. M. , & Schmahmann, J. D. (2012). Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage, 59(2), 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschan, R. , Best, C. , Beutel, M. E. , Knebel, A. , Wiltink, J. , Dieterich, M. , & Eckhardt‐Henn, A. (2011). Patients' psychological well‐being and resilient coping protect from secondary somatoform vertigo and dizziness (SVD) 1 year after vestibular disease. Journal of Neurology, 258(1), 104–112. [DOI] [PubMed] [Google Scholar]
- Van Ombergen, A. , Heine, L. , Jillings, S. , Roberts, R. E. , Jeurissen, B. , Van Rompaey, V. , … Wuyts, F. L. (2017). Altered functional brain connectivity in patients with visually induced dizziness. Neuroimage: Clinical, 14, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- World Health Organization, I.‐b.d. http://apps.who.int/classifications/icd11/browse/l-m/en-/http%3a%2f%2fid.who.int%2ficd%2fentity%2f2005792829.
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One, 8(7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Z. , Cui, L. , Yu, T. , Liang, H. , Wang, Y. , & Chen, C. (2017). Analysis of the characteristics of persistent postural‐perceptual dizziness: A clinical‐based study in China. International Journal of Audiology, 56(1), 33–37. [DOI] [PubMed] [Google Scholar]
- Zeng, L. L. , Shen, H. , Liu, L. , Wang, L. , Li, B. , Fang, P. , … Hu, D. (2012). Identifying major depression using whole‐brain functional connectivity: A multivariate pattern analysis. Brain, 135(5), 1498–1507. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg, P. , Caspers, S. , Roski, C. , & Eickhoff, S. B. (2012). Meta‐analytical definition and functional connectivity of the human vestibular cortex. Neuroimage, 60(1), 162–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


