Abstract
Increasing evidence shows that thinner retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL), assessed on optical coherence tomography (OCT), are reflecting global brain atrophy. Yet, little is known on the relation of these layers with specific brain regions. Using voxel‐based analysis, we aimed to unravel specific brain regions associated with these retinal layers. We included 2,235 persons (mean age: 67.3 years, 55% women) from the Rotterdam Study (2007–2012) who had gradable retinal OCT images and brain magnetic resonance imaging (MRI) scans, including diffusion tensor (DT) imaging. Thicknesses of peripapillary RNFL and perimacular GCL were measured using an automated segmentation algorithm. Voxel‐based morphometry protocols were applied to process DT‐MRI data. We investigated the association between retinal layer thickness with voxel‐wise gray matter density and white matter microstructure by performing linear regression models. We found that thinner RNFL and GCL were associated with lower gray matter density in the visual cortex, and with lower fractional anisotropy and higher mean diffusivity in white matter tracts that are part of the optic radiation. Furthermore, thinner GCL was associated with lower gray matter density of the thalamus. Thinner RNFL and GCL are associated with gray and white matter changes in the visual pathway suggesting that retinal thinning on OCT may be specifically associated with changes in the visual pathway rather than with changes in the global brain. These findings may serve as a basis for understanding visual symptoms in elderly patients, patients with Alzheimer's disease, or patients with posterior cortical atrophy.
Keywords: brain, magnetic resonance imaging, population‐based study, retina, visual pathways, voxel‐based morphometry
1. INTRODUCTION
In search of identifying novel biomarkers for Alzheimer's disease, markers of retinal neurodegeneration are increasingly being recognized as potential candidates (Frost, Martins, & Kanagasingam, 2010; Javaid, Brenton, Guo, & Cordeiro, 2016; Lim et al., 2016). Markers of retinal neurodegeneration such as thinner retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL), assessed on optical coherence tomography (OCT), have repeatedly been observed in Alzheimer's disease patients indicating that atrophy in the brain and the retina may occur concomitantly (Coppola et al., 2015; Thomson, Yeo, Waddell, Cameron, & Pal, 2015). Indeed, studies have shown that thinner RNFL and GCL were associated with global cerebral gray matter and white matter atrophy, even in nondemented individuals (Casaletto et al., 2017; Mutlu et al., 2017; Ong et al., 2015). It has been suggested that the retina and the brain suffer from a shared underlying pathology such as vascular disease or the accumulation of misfolded proteins, leading to concomitant atrophy. Yet, another possibility is that retinal thinning may be directly linked to changes in specific brain areas, most likely those that are anatomically connected and involved in visual processing. For instance, damage to the primary visual cortex may trigger a cascade of events leading to changes in the retina, or vice versa.
Previous studies have exclusively focused on global brain changes, but it remains unclear whether retinal thinning in nondiseased individuals reflects changes in specific brain regions. While researchers hypothesize that changes in the retina or specific brain regions may manifest itself in concurrent changes in these structures, and neuroanatomical studies suggest so, this putative link has not been convincingly established yet in a nonclinical setting without overt brain pathology or retinal pathology. Such a link needs to be studied in a large sample of relatively healthy persons in which potential confounders should be taken into account. Voxel‐based analysis (VBA) enables studying the relation between retinal layers and brain tissue on the smallest regional level, the voxel level. Moreover, this method provides the opportunity to study associations hypothesis‐free, and thereby allows to identify whether specific brain regions are related to retinal layer thickness. Investigating the link between retinal layer thickness and brain voxels may provide new insights into mechanisms underlying neurodegenerative brain diseases. Within a large population of nondemented aging people, we conducted a VBA of diffusion tensor magnetic resonance imaging (DT‐MRI) data to identify whether RNFL and GCL are associated with local gray matter and white matter density. Given that the retina is connected to the brain via the optic nerve, we hypothesize that thinner retinal layers are related to changes in the visual pathway of the brain. Also, given that the GCL is composed of cell bodies, and the RNFL of axons, it is possible that the GCL may reflect more the condition of the cerebral gray matter, whereas the RNFL may reflect more the condition of the cerebral white matter.
2. MATERIALS AND METHODS
2.1. Study setting and population
This study was part of the Rotterdam Study: a prospective population‐based cohort study that investigates causes and consequences of chronic diseases in residents of the Ommoord district in the city Rotterdam, the Netherlands, aged 45 years or older (Ikram et al., 2017). The original cohort started in 1990 (n = 7,983), and was extended in 2000 (n = 3,011) and 2006 (n = 3,932). Follow‐up examinations take place every three to four years. Participants were interviewed at home and examined at the research center. In 2007, spectral‐domain OCT scanning was added to the protocol, and thus was performed in the fifth follow‐up of the first cohort (RS‐I‐5), the third follow‐up of the second cohort (RS‐II‐3), and at the baseline visit in about half of the third cohort (RS‐III‐1), see Figure 1. As the introduction of OCT in the Rotterdam Study, a total of 5,065 persons were eligible for OCT scanning, but 664 persons did not undergo OCT scanning due to logistic reasons (e.g., lack of personnel, device maintenance or replacement) or personal reasons (e.g., sickness or unable to attend). A further 803 persons were excluded because OCT scans were ungradable due to motion artifacts, segmentation failures, missing data, or poor signal strength. We also excluded persons with dementia (n = 43), persons that did not undergo dementia screening (n = 21), and persons with a history of clinical stroke (n = 137). Dementia and stroke diagnosis was established during a consensus panel led by a neurologist according to Diagnostic and Statistical Manual of Mental Disorders‐III‐Revised and World Health Organization criteria, respectively. Continuous monitoring of the cohort for incident dementia and stroke took place through electronic linkage of the study database with medical records from general practitioners and the regional institute for outpatient mental health care. Available information on clinical neuroimaging was used when required for diagnosis of subtype. For dementia diagnosis, information on cognitive tests obtained during center visits was also evaluated.
Figure 1.
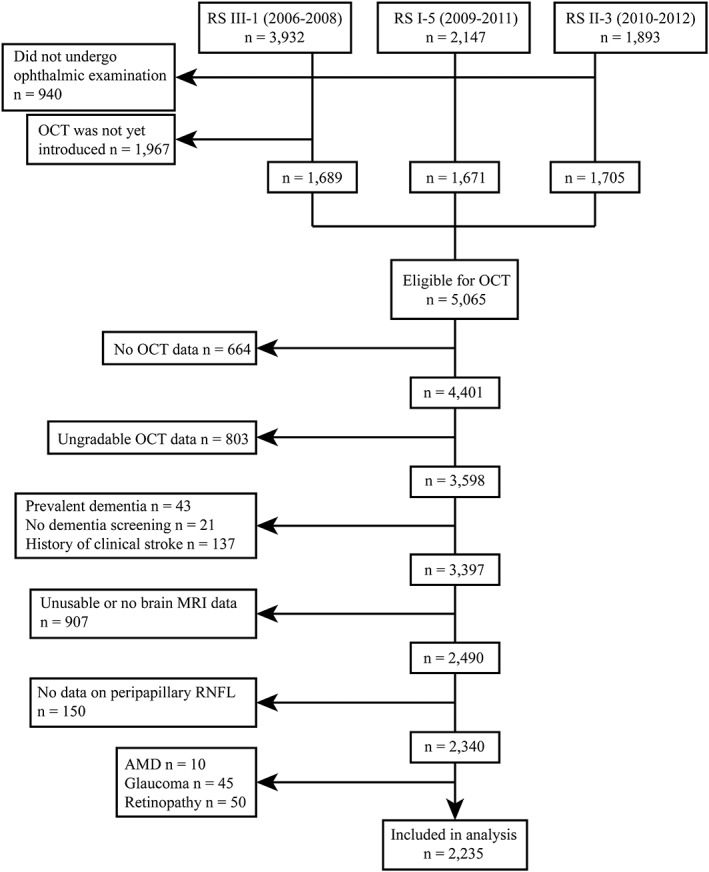
Flow diagram of the study population
From the remaining 3,397 persons, 907 persons had no brain MRI scans (n = 799) or the scans were unusable (n = 108) for the following reasons: nonrespondent or not visited the research center, refused or physical/mentally unable to attend, had MRI contra‐indications, could not complete MRI scan, or poorly segmented scans. Subsequently, we only included persons if they had data on GCL thickness measured at the macular region, and RNFL thickness measured at the peripapillary region on the same eye (n = 2,340). Finally, we excluded persons with age‐related macular degeneration (n = 10), glaucoma (n = 45), and hypertensive or diabetic retinopathy (n = 50), that could affect retinal thickness measurement. This resulted in 2,235 persons from which primarily the right eye (94%) was chosen for further analysis. Table 1 shows the characteristics of the study population. Mean age at time of OCT scanning was 67.3 years (SD: 9.7), and 55% of the participants were women.
Table 1.
Characteristics of the study population
| Characteristic | Descriptive |
|---|---|
| N | 2,235 |
| Age, years | 67.3 (9.7) |
| Female sex | 1,235 (55) |
| Systolic blood pressure, mmHg | 144.2 (21.7) |
| Diastolic blood pressure, mmHg | 84.7 (10.8) |
| Blood pressure lowering medication | 881 (39) |
| Body mass index, kg/m2 | 27.3 (3.9) |
| Diabetes mellitus | 231 (10) |
| Total cholesterol, mmol/L | 5.5 (1.1) |
| High‐density lipoprotein cholesterol, mmol/L | 1.5 (0.4) |
| Smoking status | |
| Never smoker | 715 (32) |
| Past smoker | 1,171 (52) |
| Current smoker | 349 (16) |
| Education | |
| Lower education | 632 (28) |
| Intermediate education | 1,088 (49) |
| Higher education | 515 (23) |
| White matter lesion volume, mLa | 6.8 (9.2) |
| Lacunar infarct | 180 (8) |
| Cerebral microbleed | 377 (17) |
| Right eyes | 94 |
| Axial length, mm | 23.5 (1.1) |
| Retinal nerve fiber layer optic nerve, μm | 96.1 (14.3) |
| Ganglion cell layer macula, μm | 33.9 (5.2) |
Note. Values are presented as mean (standard deviation) or as numbers (percentage).
White matter lesion volumes are further natural log‐transformed due their skewed distribution.
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC and by the Ministry of Health, Welfare and Sport of the Netherlands, implementing the Wet Bevolkingsonderzoek: ERGO (Population Studies Act: Rotterdam Study). All participants provided written informed consent to participate in the study and to obtain information from their treating physicians.
2.2. Spectral‐domain OCT
From September 2007 to June 2011 participants underwent a standard ophthalmic examination after pharmacological mydriasis, including fundus photography and OCT scanning of the macula and optic nerve. Initially, participants’ eyes were scanned with the Topcon 3D OCT‐1000 (n = 1,805; Topcon optical Co., Tokyo, Japan). From August 2011 onwards, due to an update during the study, this device was replaced with the Topcon 3D OCT‐2000 (n = 430). The macula and optic nerve head were scanned in the horizontal direction in an area of 6 × 6 × 1.68 mm with 512 × 512 × 480 voxels for OCT‐1000 and in an area of 6 × 6 × 2.30 mm with 512 × 512 × 885 voxels for OCT‐2000, enabling us to detect structures with a resolution of 5 μm (Figure 2). We measured the RNFL in the peripapillary region, and the GCL in the perimacular region as these layers are the thickest in those regions. Thickness of the peripapillary RNFL was measured automatically by Topcon's built‐in segmentation algorithm. This was done in 12 peripapillary segments of 30° each, and average RNFL thickness was derived from the calculation circle. For the macula, volumes were segmented using Iowa Reference Algorithms 3.6, which has been validated for most widely available commercial OCT scanners (available from https://www.iibi.uiowa.edu/content/shared-software-download; Terry et al., 2016). Thickness of the GCL was measured in nine regions of the Early Treatment Diabetic Retinopathy Study Grid. The average retinal layer thickness was calculated and used in further statistical analyses. Indices of quality control were used to preserve good quality images (high signal, low noise) and to exclude scans with segmentation errors. Scans included in our study had a segmentability index of more than 30%, an undefined region of less than 20%, and a quality factor of more than 30, as explained previously (Keane et al., 2016; Lee et al., 2016; Mutlu et al., 2017; Patel et al., 2016).
Figure 2.
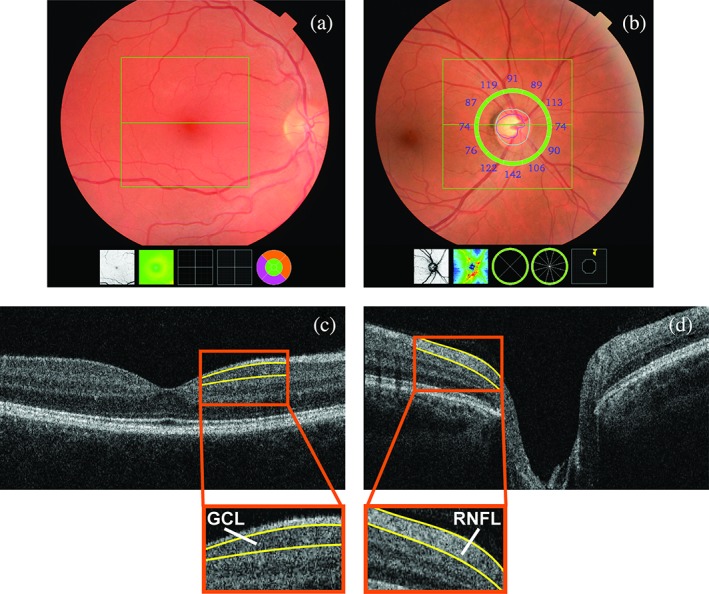
Fundus photograph centered on the macula (A) and optic nerve (B) with corresponding cross‐sectional images of the fundus (C and D, respectively). GCL indicates ganglion cell layer; RNFL indicates retinal nerve fiber layer [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. Brain image acquisition and processing
Brain MRI scanning was performed on a 1.5T MRI scanner (GE Signa Excite; GE Healthcare, Waukesha, WI; Ikram et al., 2015).
The scan protocol consisted of four high‐resolution axial sequences: a 3D T1‐weighted spoiled‐gradient echo (slice thickness 1.6 mm, number of slices 96, voxel size 0.49 × 0.49 × 0.80 mm, zero‐padded to 0.8, repetition time 13.8, echo time 2.8, flip angle 20), a proton‐density weighted, a fluid‐attenuated inversion recovery, a 3D T2‐weighted gradient‐recalled echo, and a DT‐MRI sequence. For DT‐MRI, we performed a single‐shot, diffusion‐weighted, spin‐echo, echo‐planar imaging sequence with maximum b‐value of 1,000 s/mm2 in 25 noncollinear directions (slice thickness 3.5 mm, number of slices 39, voxel size 3.3 × 2.2 × 3.5 mm, repetition time 8,000, echo time 74.6, flip angle 90–180; Ikram et al., 2015).
Using a k‐nearest‐neighbor classifier algorithm trained on manually segmented data acquired on the same scanner, we classified supratentorial voxels on T1 images into gray matter, white matter, white matter lesion volume, and cerebrospinal fluid (Vrooman et al., 2007). After quality control, persons with insufficient registration quality were excluded. Of the 2,235 persons with successfully segmented tissues, 39 did not have DT‐MRI sequences.
2.4. Voxel‐based analysis of gray matter
Voxel‐based analysis of the gray matter was performed with an optimized protocol using the FSL software (Good et al., 2001; Smith et al., 2004), and as previously described (Roshchupkin et al., 2016). Briefly, gray matter density maps derived from T1‐weighted images were nonlinearly registered to the International Consortium of Brain Mapping Montreal Neurological Institute 152 template. To preserve the gray matter volume, a spatial modulation procedure was applied, and voxel densities were multiplied by the Jacobian determinants of transformation field. Finally, images were smoothed using an isotropic Gaussian kernel of 3 mm (full width half maximum 8 mm). Brain regions were segmented using atlas‐based segmentation based on the Hammer atlas (Hammers et al., 2003). The modulation step in the voxel‐based morphometry pipeline preserves the volume of a particular tissue within a voxel. The multiplication of the voxel values in the segmented images by the Jacobian determinants derived from the spatial normalization step allows us to calculate volumes by aggregating voxels.
Given that the lateral geniculate nucleus is part of the visual pathway, we used the Oxford thalamic connectivity atlas to identify probability of anatomical connections from subthalamic regions (Behrens et al., 2003).
2.5. Voxel‐based analysis of white matter tracts
We performed a VBA of DT‐MRI data using FSL software (de Groot et al., 2013; Smith et al., 2004) for preprocessing. All fractional anisotropy (FA) and mean diffusivity (MD) maps were nonlinearly registered to the standard FA template from the FSL package with a 1 × 1 × 1 mm3 voxel resolution. Additionally, we created a Rotterdam Study specific tracts atlas. Participants’ specific tract segmentation masks were registered to Montreal Neurological Institute template in the same way as FA and MD maps and then merged to one tracts probability atlas image (de Groot et al., 2015). To map voxels from VBA, we used a 10% probability cutoff to define tracts boundaries microstructure. Moreover, because the Rotterdam Study tracts atlas did not contain segmentation tracts of the optic radiation, we used the Jülich histological atlas to assess changes in the optic radiation (Eickhoff et al., 2007). In general, FA is lower and MD is higher in older and damaged brains, which is thought to reflect worse white matter microstructure of the brain. A representative tractogram of the visual pathway has been shown previously (Kamali et al., 2014).
Diffusion tensor magnetic resonance imaging provides multiple measures of diffusion, with FA and MD most widely used. FA describes the directionality of diffusion, and a lower value typically reflects reduced microstructural organization in regions where white matter fibers are aligned. MD represents the overall magnitude of water diffusion, and a higher value generally reflects reduced microstructural organization. Both values are thought to provide complimentary information on white matter pathology (Alexander, Lee, Lazar, & Field, 2007).
2.6. Cognition
Participants underwent routine cognitive assessment during their visit to the center, including verbal fluency test (animal categories), 15‐word learning test (immediate and delayed recall, and recognition), letter–digit substitution test, Stroop test (reading and interference task, color naming task) and the Purdue pegboard test (sum score of both hands simultaneously; Hoogendam, Hofman, van der Geest, van der Lugt, & Ikram, 2014). We transformed the distribution of all tests to a normal distribution and calculated z‐scores.
2.7. Covariates
White matter lesion volume (in milliliters) was obtained supratentorially as described previously. The presence of cerebral microbleeds and lacunar infarcts was rated by one of five trained research physicians, blinded to the participants’ data, on a 3D T2‐weighted gradient‐recalled echo MRI. The presence of lacunes of presumed ischemic origin was rated on fluid‐attenuated inversion recovery, proton‐density weighted and T1‐weighted sequences (Vernooij et al., 2008). We defined lacunes as focal lesions ≥3 and <15 mm in size with the same signal intensity as cerebrospinal fluid on all sequences and a hyperintense rim on fluid‐attenuated inversion recovery. The axial length of eyes was measured using Lenstar LS900 (Haag‐Streit AG, Koeniz, Switzerland). Blood pressure was measured twice in sitting position at the right brachial artery with a random‐zero sphygmomanometer, and the average of two readings was used for analysis. Body mass index was computed as weight divided by height squared. Fasting serum total and high‐density lipoprotein cholesterol concentrations were determined by an automated enzymatic procedure. Diabetes mellitus was considered present if fasting serum glucose level was ≥7.0 mmol/L, or when persons reported use of anti‐diabetic medication. Information on smoking (non, former, or current) and blood pressure lowering medication use was obtained during the home interview by a computerized questionnaire.
2.8. Statistical analysis
As eyes were scanned with two different devices and to standardize the measurements, we calculated z‐scores for the OCT measurements of each device. We investigated the association of retinal layer thickness (per SD increase) with neuroimaging outcomes using linear regression models adjusted for age, sex, education, subcohort, axial length of the eyes, intracranial volume, natural log‐transformed white matter lesion volume, lacunar infarcts, cerebral microbleeds, and for the following cardiovascular risk factors: systolic blood pressure, diastolic blood pressure, use of blood pressure lowering medication, body mass index, total cholesterol, high‐density lipoprotein cholesterol, diabetes mellitus, and smoking. We have used a linear model for the association between each OCT measurement and every voxel of the brain measures, and thus the exact number of these models depends on the total number of voxels and differs per brain region. Missing data on covariates were handled using fivefold multiple imputations based on determinant, outcome, and included covariates. As the voxels are correlated, the actual number of independent tests was calculated using 10,000 permutations. For α = 0.05 and corrected by number of tested models, this yielded a p value threshold for statistical significance of 9.97 × 10−8 for the VBA of gray matter, 1.97 × 10−8 for the VBA of FA, and 2.16 × 10−8 for the VBA of MD. The threshold for the p values are slightly different because we decided to run permutations for each measurement separately to be stricter regarding the number of false positives. Moreover, these p values are calculated using permutations, which depends on the number of independent tests and on the property of the data, and thus might be different for FA and MD. Furthermore, we performed several additional analyses by stratifying for age (median: 69.0 years), sex, global gray matter atrophy, and after excluding intracranial volume from the models. For gray matter atrophy, we first created a new variable by dividing the total gray matter volume by intracranial volume (GM/ICV), and took the median of this variable. Persons with high GM/ICV are considered to have less atrophy than persons with low GM/ICV. We stratified for global gray matter atrophy to further investigate whether our associations are independent of global gray matter atrophy. Finally, we investigated the association of the retinal layer thickness with cognitive tests by adjusting for age, sex, subcohort, axial length of the eye, and additionally for cerebral gray matter volume.
3. RESULTS
Figures 3 and 4 show the projection of voxel‐based gray matter areas and white matter tracts on axial coupes associated with RNFL and GCL, respectively. We found that mainly thinner RNFL and to a lesser extent thinner GCL was associated with lower gray matter density of the occipital lobe, in the area of the calcarine sulcus. In addition, thinner GCL was associated with lower gray matter density of the thalamus. Furthermore, we found that both thinner RNFL and GCL were associated with lower FA and higher MD in white matter tracts that are part of the optic radiation as well as in major neighboring tracts such as the inferior longitudinal fasciculus and inferior fronto‐occipital fasciculus. These associations were more widespread for FA than MD.
Figure 3.
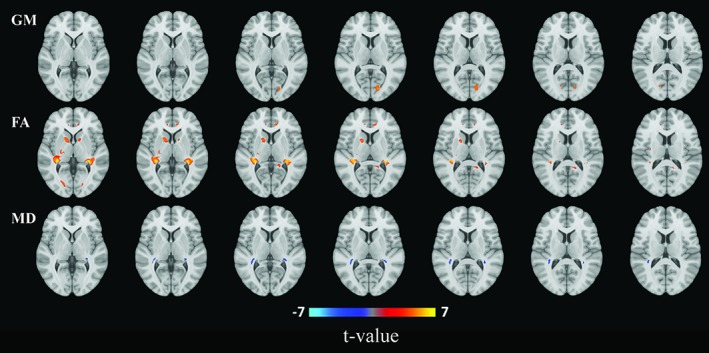
Areas of gray matter (GM) density and white matter microstructure from voxel‐based analysis that were significantly associated with retinal nerve fiber layer thickness. Colors correspond to t‐values and reflect the direction of the associations from regression models: red for a positive (increase of gray matter, fractional anisotropy (FA), or mean diffusivity (MD)), blue for a negative (decrease of GM, FA, or MD) association per standard deviation increase of retinal nerve fiber layer thickness [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
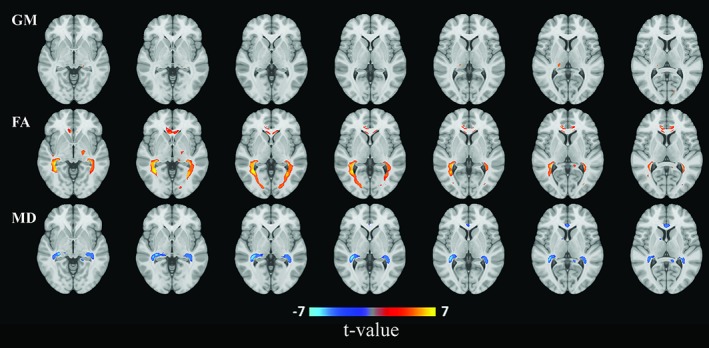
Areas of gray matter (GM) density and white matter microstructure from voxel‐based analysis that were significantly associated with ganglion cell layer thickness. Colors correspond to t‐values and reflect the direction of the associations from regression models: red for a positive (increase of gray matter, fractional anisotropy (FA), or mean diffusivity (MD)), blue for a negative (decrease of GM, FA, or MD) association per standard deviation increase of ganglion cell layer thickness [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2 shows the gray matter regions from VBA that were significantly associated with RNFL and GCL. All gray matter regions associated with thinner RNFL and GCL belong to the visual cortex, except for the lingual gyrus and thalamus. Furthermore, comparing RNFL with GCL, the number of significant voxels was higher and the lowest p values were smaller for associations with RNFL. All significant voxels showed a positive association, that is, with an increase in retinal layer thickness, the voxel‐wise gray matter density was also increasing. We used the Oxford thalamic connectivity atlas to identify probability of anatomical connections from subthalamic regions that were associated with the retinal layers. This showed that the associated thalamic structures had their connections to the posterior parietal cortex and occipital cortex with probabilities up to .84.
Table 2.
Retinal layer thickness associated with voxel‐based gray matter areas
| Retinal nerve fiber layer | Ganglion cell layer | ||||
|---|---|---|---|---|---|
| Region | Number of voxels | Lowest p value | Number of significant voxels | Lowest p value | Number of significant voxels |
| Cuneus left | 14,454 | 1.3 × 10 −10 | 302 | 1.3 × 10 −08 | 60 |
| Cuneus right | 13,755 | 4.5 × 10 −09 | 131 | 1.5 × 10−06 | 0 |
| LROOL left | 64,895 | 1.8 × 10 −10 | 186 | 2.8 × 10 −08 | 12 |
| LROOL right | 66,957 | 6.0 × 10 −08 | 8 | 6.1 × 10−06 | 0 |
| Lingual gyrus left | 18,132 | 1.4 × 10 −10 | 141 | 2.2 × 10 −08 | 24 |
| Lingual gyrus right | 18,495 | 1.4 × 10 −08 | 33 | 1.1 × 10−06 | 0 |
| Thalamus left | 10,524 | 8.1 × 10 −08 | 2 | 3.8 × 10 −08 | 22 |
| Thalamus right | 10,429 | 3.6 × 10−07 | 0 | 6.5 × 10 −09 | 158 |
Note. Abbreviation: LROOL, lateral remainder of occipital lobe. Table shows the gray matter regions from voxel‐based analysis that were significantly associated with retinal nerve fiber layer and ganglion cell layer. Numbers indicate total number of voxels within a region, the lowest p value observed in that region, and the number of voxels that had a p value lower than the p value threshold for significance: 9.97 × 10−8. Bolded values are significant voxels.
Tables 3 and 4 show for FA and MD, respectively, the white matter tract voxels which were significantly associated with RNFL and GCL. In general, comparing RNFL with GCL, the number of significant voxels was lower and the lowest p values were larger for associations with RNFL. All significant voxels for the FA showed a positive association, whereas the significant voxels for the MD showed a negative association, as expected. These tables together with the figures show us that even within specific tracts, certain parts are more strongly associated. For instance, the inferior fronto‐occipital fasciculus passes along the frontal lobe, but the figures show us that only the posterior part is associated with RNFL and GCL. Finally, we found that particularly the GCL was associated with the corpus callosum.
Table 3.
Fractional anisotropy of white matter tracts from voxel‐based analysis
| Retinal nerve fiber layer | Ganglion cell layer | ||||
|---|---|---|---|---|---|
| Tracts | Number of voxels | Lowest p value | Number of significant voxels | Lowest p value | Number of significant voxels |
| ATR left | 14,618 | 2.9 × 10 −17 | 765 | 2.4 × 10 −09 | 3 |
| ATR right | 15,241 | 2.6 × 10 −13 | 1,011 | 7.9 × 10 −09 | 1 |
| CGC left | 4,183 | 6.0 × 10 −12 | 283 | 2.8 × 10 −12 | 449 |
| CGC right | 3,903 | 3.4 × 10 −11 | 102 | 3.1 × 10 −11 | 462 |
| CGH left | 2,485 | 2.0 × 10 −11 | 56 | 1.3 × 10 −08 | 1 |
| CGH right | 2,336 | 1.8 × 10 −09 | 48 | 1.6 × 10−07 | 0 |
| CST left | 18,423 | 3.3 × 10 −22 | 303 | 1.4 × 10 −20 | 486 |
| CST right | 18,322 | 5.2 × 10 −25 | 1,481 | 8.6 × 10 −21 | 203 |
| FMA | 19,451 | 1.2 × 10 −13 | 578 | 5.1 × 10 −10 | 64 |
| FMI | 11,974 | 1.2 × 10 −11 | 244 | 4.9 × 10 −10 | 493 |
| Fornix right | 2,670 | 4.0 × 10−06 | 0 | 1.7 × 10 −08 | 1 |
| IFO left | 15,152 | 2.3 × 10 −17 | 2,326 | 1.0 × 10 −14 | 1,671 |
| IFO right | 15,706 | 2.7 × 10 −16 | 2,686 | 5.3 × 10 −19 | 2,607 |
| ILF left | 16,422 | 2.3 × 10 −17 | 2,833 | 1.0 × 10 −14 | 1,651 |
| ILF right | 17,938 | 7.5 × 10 −17 | 3,701 | 5.3 × 10 −19 | 2,837 |
| ML left | 4,846 | 4.4 × 10 −10 | 35 | 2.3 × 10 −11 | 60 |
| ML right | 4,552 | 1.2 × 10 −10 | 54 | 1.5 × 10 −09 | 3 |
| Optic radiation, lefta | 51,440 | 7.1 × 10 −19 | 2,509 | 4.4 × 10 −16 | 2,953 |
| Optic radiation, righta | 48,435 | 7.1 × 10 −20 | 3,186 | 8.3 × 10 −19 | 3,485 |
| PTR left | 13,000 | 7.5 × 10 −19 | 2,573 | 4.4 × 10 −16 | 2,044 |
| PTR right | 13,943 | 7.1 × 10 −20 | 3,373 | 5.3 × 10 −19 | 3,083 |
| SLF left | 23,378 | 1.3 × 10 −10 | 332 | 4.7 × 10−08 | 0 |
| SLF right | 24,206 | 2.5 × 10 −11 | 392 | 3.8 × 10−08 | 0 |
| STR left | 14,096 | 2.4 × 10 −09 | 32 | 3.0 × 10−07 | 0 |
| STR right | 14,096 | 4.7 × 10 −13 | 304 | 4.9 × 10−08 | 0 |
| Uncinatus left | 5,443 | 6.0 × 10 −11 | 43 | 1.1 × 10−05 | 0 |
| Corpus callosumb | 30,572 | 2.5 × 10 −11 | 819 | 5.1 × 10 −11 | 1,915 |
Note. Table shows the white matter tracts from voxel‐based analysis that were significantly associated with retinal nerve fiber layer and ganglion cell layer. Numbers indicate total number of voxels within a tract, the lowest p value observed in that tract, and the number of voxels that had a p value lower than the p value threshold for significance: 1.97 × 10−8. Bolded values are significant voxels.
Based on Jülich histological atlas.
Based on Hammer atlas.
Table 4.
Mean diffusivity of white matter tracts from voxel‐based analysis
| Retinal nerve fiber layer | Ganglion cell layer | ||||
|---|---|---|---|---|---|
| Tracts | Number of voxels | Lowest p value | Number of significant voxels | Lowest p value | Number of significant voxels |
| CGH left | 2,485 | 9.6 × 10−07 | 0 | 2.2 × 10 −10 | 201 |
| CST left | 18,423 | 2.6 × 10 −09 | 91 | 1.7 × 10 −09 | 24 |
| CST right | 18,322 | 8.6 × 10 −09 | 6 | 1.2 × 10 −11 | 41 |
| FMA | 19,451 | 2.5 × 10−06 | 0 | 3.0 × 10 −10 | 125 |
| FMI | 11,974 | 1.5 × 10−05 | 0 | 3.4 × 10 −11 | 421 |
| IFO left | 15,152 | 1.6 × 10 −10 | 760 | 8.1 × 10 −11 | 711 |
| IFO right | 15,706 | 4.3 × 10 −12 | 399 | 8.6 × 10 −14 | 636 |
| ILF left | 16,422 | 1.6 × 10 −10 | 718 | 8.1 × 10 −11 | 638 |
| ILF right | 17,938 | 4.3 × 10 −12 | 686 | 8.6 × 10 −14 | 698 |
| ML right | 4,552 | 1.9 × 10−05 | 0 | 3.3 × 10 −08 | 2 |
| Optic radiation, lefta | 51,440 | 3.1 × 10 −11 | 666 | 3.1 × 10 −14 | 1,854 |
| Optic radiation, righta | 48,435 | 4.2 × 10 −12 | 287 | 3.8 × 10 −14 | 1,387 |
| PTR left | 13,000 | 3.1 × 10 −11 | 1,387 | 4.3 × 10 −15 | 1,510 |
| PTR right | 13,943 | 4.3 × 10 −12 | 661 | 5.2 × 10 −15 | 1,662 |
| SLF left | 23,378 | 1.2 × 10−06 | 0 | 6.7 × 10 −09 | 6 |
| Corpus callosumb | 30,572 | 2.0 × 10−06 | 0 | 3.4 × 10 −11 | 837 |
Note. Abbreviations: ATR, anterior thalamic radiation; CGC, cingulate gyrus part of cingulum; CGH, parahippocampal part of cingulum; CST, corticospinal tract; FMA, forceps major; FMI, forceps minor; IFO, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; ML, medial lemniscus; PTR, posterior thalamic radiation; SLF, superior longitudinal fasciculus. Table shows the white matter tracts from voxel‐based analysis that were significantly associated with retinal nerve fiber layer and ganglion cell layer. Numbers indicate total number of voxels within a tract, the lowest p value observed in that tract, and the number of voxels that had a p value lower than the p value threshold for significance: 2.16 × 10−8. Bolded values are significant voxels.
Based on Jülich histological atlas
Based on Hammer atlas.
Supporting Information Tables S1–S3 show the additional analysis that we have performed by stratifying for age and sex. While we did not observe clear differences for the gray matter density between older and younger persons, and males and females, we found that the associations with white matter microstructure density were in general more widespread (i.e., more significant voxels) for younger persons and males.
Supporting Information Tables S4–S6 show the results of the analyses that we have performed by stratifying for gray matter atrophy, and excluding intracranial volume from the models. We found that in general the associations were weaker (i.e., less significant voxels) in persons with higher degree of gray matter atrophy (low GM/ICV) compared to persons with lower degree of gray matter atrophy (high GM/ICV). However, in both strata, significant associations in areas of the visual pathway were still present. Further, more associations became significant after excluding intracranial volume from the model.
Table 5 shows the association of retinal layer thickness with cognition. This table shows that the GCL is significantly and stronger associated with various cognitive tests than RNFL.
Table 5.
Associations of retinal layer thickness with cognition, n = 1,958
| Cognitive domain | Retinal nerve fiber layer | Ganglion cell layer | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Mini mental state examination | 0.040 (−0.005; 0.085) | 0.029 (−0.015; 0.074) | 0.065 (0.021; 0.109) | 0.056 (0.012; 0.100) |
| Executive function | ||||
| Letter–digit substitution test | 0.033 (−0.006; 0.073) | 0.030 (−0.010; 0.070) | 0.052 (0.013; 0.091) | 0.049 (0.010; 0.088) |
| Stroop 1 | −0.025 (−0.069; 0.019) | −0.020 (−0.064; 0.024) | −0.040 (−0.084; 0.003) | −0.036 (−0.080; 0.007) |
| Stroop 2 | −0.024 (−0.068; 0.021) | −0.019 (−0.064; 0.026) | −0.047 (−0.091; −0.003) | −0.044 (−0.088; 0.000) |
| Stroop 3 | −0.047 (−0.087; −0.006) | −0.038 (−0.079; 0.002) | −0.039 (−0.079; 0.001) | −0.031 (−0.071; 0.008) |
| Word fluency test | −0.003 (−0.048; 0.041) | −0.009 (−0.053; 0.035) | 0.014 (−0.029; 0.058) | 0.010 (−0.034; 0.053) |
| Memory | ||||
| Word learning test immediate | 0.001 (−0.043; 0.045) | 0.002 (−0.042; 0.046) | 0.045 (0.002; 0.088) | 0.046 (0.003; 0.090) |
| Word learning test delayed | −0.022 (−0.067; 0.023) | −0.018 (−0.063; 0.027) | 0.023 (−0.021; 0.067) | 0.027 (−0.017; 0.070) |
| Word learning test recognition | −0.016 (−0.062; 0.030) | −0.016 (−0.063; 0.030) | 0.022 (−0.023; 0.068) | 0.022 (−0.024; 0.068) |
| Fine motor speed | ||||
| Purdue pegboard test | 0.035 (−0.004; 0.074) | 0.035 (−0.004; 0.074) | 0.045 (0.007; 0.083) | 0.044 (0.006; 0.083) |
Note. Values are z‐scores per SD increase in retinal layer thickness (95% confidence interval). Model 1: age, sex, subcohort, and axial length of the eye. Model 2: additionally for gray matter volume. Bolded values are p value <.05.
4. DISCUSSION
In this large population‐based study of nondemented persons, we found that thinner retinal layers, that is, RNFL and GCL, were significantly associated with lower gray matter density of the visual cortex and with worse white matter microstructure of the optic radiation and of the tracts coursing adjacent to the optic radiation.
Previous studies investigating the association of retinal thickness with brain MRI markers in nondemented individuals found that thinner RNFL and GCL were associated with cerebral gray matter and white matter atrophy (Casaletto et al., 2017; Mutlu et al., 2017; Ong et al., 2015). In addition, we have previously demonstrated that both thinner RNFL and GCL were associated with worse white matter microstructure of the brain (lower FA and higher MD) suggesting that RNFL and GCL may even reflect subtle changes in the white matter that are visually not detectable (Mutlu et al., 2017).
Extending on findings from previous studies, we have now shown that thinner RNFL and GCL are associated with the gray matter density of the visual cortex, and with the microstructural integrity of white matter tracts of the optic radiation and of tracts coursing adjacent to the optic radiation. Furthermore, we found that the GCL was associated with the gray matter density of the thalamus, close to the presumed location of the lateral geniculate nucleus, a relay center for the visual pathway (Fujita et al., 2001). Furthermore, we found that the RNFL and GCL are associated with the lingual gyrus, and the GCL with the corpus callosum. While these structures are not directly recognized as being part of the visual pathway, they are connected to the visual cortex and are involved in visual information processing (Berlucchi, 2014; Bocci et al., 2014; Kitada, Johnsrude, Kochiyama, & Lederman, 2010; Takahashi & Kawamura, 2002). Similarly, most white matter tracts that were found to be associated with RNFL and GCL are not directly recognized as being part of the optic radiation, but in fact, are shown to be neighboring tracts to the optic radiation with their projections to the visual cortex (Kamali et al., 2014). That these associations were more widespread for FA than MD may demonstrate that there are fewer crossing fibers in those tracts, that is, that these tracts are rather homogenous with a single fiber population (Alexander et al., 2007). Indeed, as the visual pathway consists of long fiber bundles, it is more likely that the associations are more widespread for FA. Associations with MD are more prominent in regions close to the thalamus were the synaptic connections are present. Our findings show that in relatively healthy persons, normal variations in structure of the retina and brain are linked, but more importantly these may provide new insights into mechanisms underlying aging. Apart from studies investigating the retinal layer thickness in relation to structural brain changes, an experimental clinical‐based study focused on functional changes of the brain. They showed that degeneration of the RNFL and GC‐inner plexiform layer complex was associated with reduced activity in the primary visual cortex rather than higher‐order visual areas. Interestingly, the associations tended to be slightly stronger for the GC‐inner plexiform layer complex than the RNFL, indicating that most sensory input comes from the macula compared to the rest of the retina. Although these are generally in line with our findings pointing toward a transneuronal degeneration, differences may be explained by methodology, such as sample size, random variation, inclusion of glaucoma patients, and functional assessment of the brain. That the GCL was significantly and stronger associated with various cognitive tests than RNFL, further support the notion that the GCL reflects macular changes, whereas the RNFL reflects more changes throughout the eye. Interpreting these findings together with the findings from the VBA and cognition data, it may be that the GCL reflects more changes of the visual pathway, whereas the RNFL is more susceptible to global retinal and brain changes.
A possible mechanism that may link the retina to the brain is that damage to the visual cortex may lead to retrograde degeneration down to the optic nerve and retinal layers. Our voxel‐based analyses between retinal layers and brain tissue exclusively showed associations along the visual pathway, including the optic radiation, and the visual cortex. Although it may be that associations in other brain regions did not survive the threshold for statistical significance, these results do indicate that the relation seems more region‐specific than widespread. Indeed, a growing body of evidence suggests that damage to the visual cortex by means of an infarction, atrophy, or lobectomy may lead to retinal neurodegeneration by causing degeneration of the axons and their accompanying myelin sheaths (Bridge, Jindahra, Barbur, & Plant, 2011; Cowey, Alexander, & Stoerig, 2011; Gabilondo et al., 2014; Herro & Lam, 2015; Jindahra, Petrie, & Plant, 2009, 2012; Park, Park, Cho, & Park, 2013). For instance, a study has shown that a decrease of 1 mL in visual cortex volume relates to a reduction of 0.6 μm in RNFL thickness after one year (Gabilondo et al., 2014). Similarly, another study demonstrated a 9 μm reduction in RNFL thickness per log year following occipital lobe damage due to stroke (Jindahra et al., 2012).
Conversely, ganglion cell apoptosis in the retina may lead to anterograde degeneration along the visual pathway, leading to thinner RNFL, and eventually resulting in atrophy of the visual cortex. Studies have shown that visual deprivation alters the functional and structural organization of the human brain. These alterations were found to occur particularly in areas of the visual pathway during sensitive periods in neurodevelopment (Noppeney, 2007). As support of this, clinical studies have shown that optic nerve axotomy, or optic neuropathy due to intraocular hypertension, glaucoma or Leber's disease, may lead to changes in the lateral geniculate nucleus and visual cortex (Barcella et al., 2010; Dai et al., 2011; Gupta, Ang, Noel de Tilly, Bidaisee, & Yucel, 2006; Ito et al., 2009; Lee et al., 2014; You, Gupta, Graham, & Klistorner, 2012; Zhang et al., 2009). It should be noted that inferences from previous studies investigating retrograde or anterograde degeneration are difficult to draw due to inclusion of animals or diseased individuals (i.e., selection bias). Moreover, those studies consisted of small sample sizes and adjustment for potential confounders was not always done. We have now investigated the retina–brain connection in a large sample of relatively healthy persons being able to take potential confounders into account. Nonetheless, findings from previous studies together with our findings indicate the existence of a direct association between thinning of retinal layers and structural changes to the visual pathway.
Yet, our findings also support another hypothesis, which is that the presence of a common underlying process may affect both the retina and the brain simultaneously. Findings from previous studies demonstrating an association between retinal layer thickness and global brain structures were suggestive of a more widespread underlying process, and would support this hypothesis (Mutlu et al., 2017; Ong et al., 2015). For instance, in Alzheimer's disease pathology, vascular processes and the accumulation of misfolded proteins, such as amyloid‐beta and tau are well‐known causes of Alzheimer's disease (Serrano‐Pozo, Frosch, Masliah, & Hyman, 2011). These processes could affect both the retina and the brain, and thus could be considered as shared factors. Although we lacked information on cerebral amyloid pathology, we tried to control for potential cardiovascular risk factors, which did not change the associations. This indicates that other processes may play a role between the association of thinner RNFL and GCL with lower gray and white matter densities. Extending on findings from our previous study that suggested a more widespread underlying process affecting both the retina and brain, we have now shown that the association of retinal layers with global brain changes may be largely driven by the association of these layers with the visual pathway. Although we found our associations to be weaker in persons with higher degree of gray matter atrophy compared to persons with lower degree of gray matter atrophy, the associations between the retinal layers and voxels of the visual pathway were still significant in both strata. These findings further support the notion that changes in the visual pathway are partly, but not completely, dependent on global changes of the gray matter. We also thought that the retinal sublayers may reflect the condition of their cerebral counterpart (e.g., GCL reflects the gray matter and RNFL reflects the white matter), but this idea is less likely to be the case as the RNFL and GCL were both associated with gray and white matter densities.
Our study is the first population‐based study that investigated and confirmed the putative link between retinal layers and specific brain structures using VBA in a large sample of relatively healthy persons.
Some limitations of our study merit attention. First, the cross‐sectional design of our study limits us to draw conclusions about temporality and causality. Second, persons excluded from our study had an eye or brain disease, or had unusable OCT or MRI scans, resulting in a selection of relatively healthy persons in our analysis, which may have caused underestimation of the effect sizes. Third, we lacked data on the optic chiasm and optic tract, and thus could not investigate the link between the retinal layers and those structures. Finally, we adjusted for MRI markers of small vessel disease present throughout the brain and not specifically within the visual pathway. Hence, future research may investigate whether the presence of these markers within the visual pathway may affect the retinal layer thicknesses.
Strengths of our study include the population‐based setting, large sample size, the assessment of retinal layers on OCT, the assessment of brain structures on MRI, and the extensive data on covariates.
5. CONCLUSION
In a population‐based setting of nondemented individuals, we found that thinner RNFL and GCL were significantly associated with gray and white matter changes along the visual pathway, including the optic radiation and the visual cortex. Our findings suggest that thinner RNFL and GCL may reflect areas with lesser cell densities in the visual pathway rather than reflecting lesser cell densities throughout the global brain. Yet, given the cross‐sectional design of our study and our statistical associations, inferences on causality from this study should be drawn carefully. Longitudinal research is needed to assess temporality and direction of the associations. Our findings may serve as a basis for understanding visual symptoms in elderly patients, patients with Alzheimer's disease, or patients with posterior cortical atrophy.
DISCLOSURE OF INTERESTS
Wiro Niessen is co‐founder, chief scientific officer, and shareholder of Quantib BV.
Supporting information
Table S1. Retinal layer thickness associated with voxel‐based gray matter areas.
Table S2. Fractional anisotropy of white matter tracts from voxel‐based analysis.
Table S3. Mean diffusivity of white matter tracts from voxel‐based analysis.
Table S4. Retinal layer thickness associated with voxel‐based gray matter areas.
Table S5. Fractional anisotropy of white matter tracts from voxel‐based analysis.
Table S6. Mean diffusivity of white matter tracts from voxel‐based analysis.
ACKNOWLEDGMENTS
We gratefully acknowledge the dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists of the Ommoord district to the Rotterdam Study.
Mutlu U, Ikram MK, Roshchupkin GV, et al. Thinner retinal layers are associated with changes in the visual pathway: A population‐based study. Hum Brain Mapp. 2018;39:4290–4301. 10.1002/hbm.24246
Funding information Erasmus Medisch Centrum; Erasmus Universiteit Rotterdam; Ministerie van Onderwijs, Cultuur en Wetenschap; Ministerie van Volksgezondheid, Welzijn en Sport; ZonMw; Municipality of Rotterdam; European Commission (DG XII); Ministry for Health, Welfare and Sports; Ministry of Education, Culture and Science; Research Institute for Diseases in the Elderly (RIDE); Netherlands Organization for the Health Research and Development (ZonMw); Erasmus University, Rotterdam; Erasmus Medical Center
REFERENCES
- Alexander, A. L. , Lee, J. E. , Lazar, M. , & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcella, V. , Rocca, M. A. , Bianchi‐Marzoli, S. , Milesi, J. , Melzi, L. , Falini, A. , … Filippi, M. (2010). Evidence for retrochiasmatic tissue loss in Leber's hereditary optic neuropathy. Human Brain Mapping, 31, 1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens, T. E. , Johansen‐Berg, H. , Woolrich, M. W. , Smith, S. M. , Wheeler‐Kingshott, C. A. , Boulby, P. A. , … Matthews, P. M. (2003). Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience, 6, 750–757. [DOI] [PubMed] [Google Scholar]
- Berlucchi, G. (2014). Visual interhemispheric communication and callosal connections of the occipital lobes. Cortex, 56, 1–13. [DOI] [PubMed] [Google Scholar]
- Bocci, T. , Pietrasanta, M. , Cerri, C. , Restani, L. , Caleo, M. , & Sartucci, F. (2014). Visual callosal connections: Role in visual processing in health and disease. Reviews in the Neurosciences, 25, 113–127. [DOI] [PubMed] [Google Scholar]
- Bridge, H. , Jindahra, P. , Barbur, J. , & Plant, G. T. (2011). Imaging reveals optic tract degeneration in hemianopia. Investigative Ophthalmology & Visual Science, 52, 382–388. [DOI] [PubMed] [Google Scholar]
- Casaletto, K. B. , Ward, M. E. , Baker, N. S. , Bettcher, B. M. , Gelfand, J. M. , Li, Y. , … Green, A. J. (2017). Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiology of Aging, 51, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola, G. , Di Renzo, A. , Ziccardi, L. , Martelli, F. , Fadda, A. , Manni, G. , … Parisi, V. (2015). Optical coherence tomography in Alzheimer's disease: A meta‐analysis. PLoS One, 10, e0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowey, A. , Alexander, I. , & Stoerig, P. (2011). Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain, 134, 2149–2157. [DOI] [PubMed] [Google Scholar]
- Dai, H. , Mu, K. T. , Qi, J. P. , Wang, C. Y. , Zhu, W. Z. , Xia, L. M. , … Morelli, J. N. (2011). Assessment of lateral geniculate nucleus atrophy with 3T MR imaging and correlation with clinical stage of glaucoma. AJNR American Journal of Neuroradiology, 32, 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot, M. , Ikram, M. A. , Akoudad, S. , Krestin, G. P. , Hofman, A. , van der Lugt, A. , … Vernooij, M. W. (2015). Tract‐specific white matter degeneration in aging: The Rotterdam study. Alzheimers Dement, 11, 321–330. [DOI] [PubMed] [Google Scholar]
- de Groot, M. , Vernooij, M. W. , Klein, S. , Ikram, M. A. , Vos, F. M. , Smith, S. M. , … Andersson, J. L. (2013). Improving alignment in tract‐based spatial statistics: Evaluation and optimization of image registration. NeuroImage, 76, 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Paus, T. , Caspers, S. , Grosbras, M. H. , Evans, A. C. , Zilles, K. , & Amunts, K. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage, 36, 511–521. [DOI] [PubMed] [Google Scholar]
- Frost, S. , Martins, R. N. , & Kanagasingam, Y. (2010). Ocular biomarkers for early detection of Alzheimer's disease. Journal of Alzheimer's Disease, 22, 1–16. [DOI] [PubMed] [Google Scholar]
- Fujita, N. , Tanaka, H. , Takanashi, M. , Hirabuki, N. , Abe, K. , Yoshimura, H. , & Nakamura, H. (2001). Lateral geniculate nucleus: Anatomic and functional identification by use of MR imaging. AJNR American Journal of Neuroradiology, 22, 1719–1726. [PMC free article] [PubMed] [Google Scholar]
- Gabilondo, I. , Martinez‐Lapiscina, E. H. , Martinez‐Heras, E. , Fraga‐Pumar, E. , Llufriu, S. , Ortiz, S. , … Villoslada, P. (2014). Trans‐synaptic axonal degeneration in the visual pathway in multiple sclerosis. Annals of Neurology, 75, 98–107. [DOI] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. S. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R. S. (2001). A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Gupta, N. , Ang, L. C. , Noel de Tilly, L. , Bidaisee, L. , & Yucel, Y. H. (2006). Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. The British Journal of Ophthalmology, 90, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers, A. , Allom, R. , Koepp, M. J. , Free, S. L. , Myers, R. , Lemieux, L. , … Duncan, J. S. (2003). Three‐dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping, 19, 224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herro, A. M. , & Lam, B. L. (2015). Retrograde degeneration of retinal ganglion cells in homonymous hemianopsia. Clinical Ophthalmology, 9, 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam, Y. Y. , Hofman, A. , van der Geest, J. N. , van der Lugt, A. , & Ikram, M. A. (2014). Patterns of cognitive function in aging: The Rotterdam study. European Journal of Epidemiology, 28, 133–140. [DOI] [PubMed] [Google Scholar]
- Ikram, M. A. , Brusselle, G. G. O. , Murad, S. D. , van Duijn, C. M. , Franco, O. H. , Goedegebure, A. , … Hofman, A. (2017). The Rotterdam study: 2018 Update on objectives, design and main results. European Journal of Epidemiology, 32, 807–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram, M. A. , van der Lugt, A. , Niessen, W. J. , Koudstaal, P. J. , Krestin, G. P. , Hofman, A. , … Vernooij, M. W. (2015). The Rotterdam scan study: Design update 2016 and main findings. European Journal of Epidemiology, 30, 1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, Y. , Shimazawa, M. , Chen, Y. N. , Tsuruma, K. , Yamashima, T. , Araie, M. , & Hara, H. (2009). Morphological changes in the visual pathway induced by experimental glaucoma in Japanese monkeys. Experimental Eye Research, 89, 246–255. [DOI] [PubMed] [Google Scholar]
- Javaid, F. Z. , Brenton, J. , Guo, L. , & Cordeiro, M. F. (2016). Visual and ocular manifestations of Alzheimer's disease and their use as biomarkers for diagnosis and progression. Frontiers in Neurology, 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindahra, P. , Petrie, A. , & Plant, G. T. (2009). Retrograde trans‐synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain, 132, 628–634. [DOI] [PubMed] [Google Scholar]
- Jindahra, P. , Petrie, A. , & Plant, G. T. (2012). The time course of retrograde trans‐synaptic degeneration following occipital lobe damage in humans. Brain, 135, 534–541. [DOI] [PubMed] [Google Scholar]
- Kamali, A. , Hasan, K. M. , Adapa, P. , Razmandi, A. , Keser, Z. , Lincoln, J. , & Kramer, L. A. (2014). Distinguishing and quantification of the human visual pathways using high‐spatial‐resolution diffusion tensor tractography. Magnetic Resonance Imaging, 32, 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane, P. A. , Grossi, C. M. , Foster, P. J. , Yang, Q. , Reisman, C. A. , Chan, K. , … Consortium, U. K. B. E. V. (2016). Optical coherence tomography in the UK biobank study—Rapid automated analysis of retinal thickness for large population‐based studies. PLoS One, 11, e0164095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada, R. , Johnsrude, I. S. , Kochiyama, T. , & Lederman, S. J. (2010). Brain networks involved in haptic and visual identification of facial expressions of emotion: An fMRI study. NeuroImage, 49, 1677–1689. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. , Jeong, H. J. , Lee, J. H. , Kim, Y. J. , Kim, E. Y. , Kim, Y. Y. , … Kim, Y. B. (2014). An investigation of lateral geniculate nucleus volume in patients with primary open‐angle glaucoma using 7 tesla magnetic resonance imaging. Investigative Ophthalmology & Visual Science, 55, 3468–3476. [DOI] [PubMed] [Google Scholar]
- Lee, K. , Buitendijk, G. H. , Bogunovic, H. , Springelkamp, H. , Hofman, A. , Wahle, A. , … Abramoff, M. D. (2016). Automated Segmentability index for layer segmentation of macular SD‐OCT images. Translational Vision Science & Technology, 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J. K. , Li, Q. X. , He, Z. , Vingrys, A. J. , Wong, V. H. , Currier, N. , … Nguyen, C. T. (2016). The eye as a biomarker for Alzheimer's disease. Frontiers in Neuroscience, 10, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu, U. , Bonnemaijer, P. W. M. , Ikram, M. A. , Colijn, J. M. , Cremers, L. G. M. , Buitendijk, G. H. S. , … Ikram, M. K. (2017). Retinal neurodegeneration and brain MRI markers: The Rotterdam study. Neurobiology of Aging, 60, 183–191. [DOI] [PubMed] [Google Scholar]
- Noppeney, U. (2007). The effects of visual deprivation on functional and structural organization of the human brain. Neuroscience and Biobehavioral Reviews, 31, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Ong, Y. T. , Hilal, S. , Cheung, C. Y. , Venketasubramanian, N. , Niessen, W. J. , Vrooman, H. , … Ikram, M. K. (2015). Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neuroscience Letters, 584, 12–16. [DOI] [PubMed] [Google Scholar]
- Park, H. Y. , Park, Y. G. , Cho, A. H. , & Park, C. K. (2013). Transneuronal retrograde degeneration of the retinal ganglion cells in patients with cerebral infarction. Ophthalmology, 120, 1292–1299. [DOI] [PubMed] [Google Scholar]
- Patel, P. J. , Foster, P. J. , Grossi, C. M. , Keane, P. A. , Ko, F. , Lotery, A. , … Vision, C. (2016). Spectral‐domain optical coherence tomography imaging in 67 321 adults: Associations with macular thickness in the UK biobank study. Ophthalmology, 123, 829–840. [DOI] [PubMed] [Google Scholar]
- Roshchupkin, G. V. , Adams, H. H. , van der Lee, S. J. , Vernooij, M. W. , van Duijn, C. M. , Uitterlinden, A. G. , … Ikram, M. A. (2016). Fine‐mapping the effects of Alzheimer's disease risk loci on brain morphology. Neurobiology of Aging, 48, 204–211. [DOI] [PubMed] [Google Scholar]
- Serrano‐Pozo, A. , Frosch, M. P. , Masliah, E. , & Hyman, B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 1, a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Takahashi, N. , & Kawamura, M. (2002). Pure topographical disorientation—The anatomical basis of landmark agnosia. Cortex, 38, 717–725. [DOI] [PubMed] [Google Scholar]
- Terry, L. , Cassels, N. , Lu, K. , Acton, J. H. , Margrain, T. H. , North, R. V. , … Wood, A. (2016). Automated retinal layer segmentation using spectral domain optical coherence tomography: Evaluation of inter‐session repeatability and agreement between devices. PLoS One, 11, e0162001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, K. L. , Yeo, J. M. , Waddell, B. , Cameron, J. R. , & Pal, S. (2015). A systematic review and meta‐analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement, 1, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij, M. W. , Ikram, M. A. , Wielopolski, P. A. , Krestin, G. P. , Breteler, M. M. , & van der Lugt, A. (2008). Cerebral microbleeds: Accelerated 3D T2*‐weighted GRE MR imaging versus conventional 2D T2*‐weighted GRE MR imaging for detection. Radiology, 248, 272–277. [DOI] [PubMed] [Google Scholar]
- Vrooman, H. A. , Cocosco, C. A. , van der Lijn, F. , Stokking, R. , Ikram, M. A. , Vernooij, M. W. , … Niessen, W. J. (2007). Multi‐spectral brain tissue segmentation using automatically trained k‐nearest‐neighbor classification. NeuroImage, 37, 71–81. [DOI] [PubMed] [Google Scholar]
- You, Y. , Gupta, V. K. , Graham, S. L. , & Klistorner, A. (2012). Anterograde degeneration along the visual pathway after optic nerve injury. PLoS One, 7, e52061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Wang, H. , Lu, Q. , Qing, G. , Wang, N. , Wang, Y. , … Yan, F. (2009). Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Research, 1303, 131–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Retinal layer thickness associated with voxel‐based gray matter areas.
Table S2. Fractional anisotropy of white matter tracts from voxel‐based analysis.
Table S3. Mean diffusivity of white matter tracts from voxel‐based analysis.
Table S4. Retinal layer thickness associated with voxel‐based gray matter areas.
Table S5. Fractional anisotropy of white matter tracts from voxel‐based analysis.
Table S6. Mean diffusivity of white matter tracts from voxel‐based analysis.


