Abstract
The extent to which one can use cognitive resources to keep information in working memory is known to rely on (1) active maintenance of target representations and (2) downregulation of interference from irrelevant representations. Neurobiologically, the global capacity of working memory is thought to depend on the prefrontal and parietal cortices; however, the neural mechanisms involved in controlling interference specifically in working memory capacity tasks remain understudied. In this study, 22 healthy participants completed a modified complex working memory capacity task (Reading Span) with trials of varying levels of interference control demands while undergoing functional MRI. Neural activity associated with interference control demands was examined separately during encoding and recall phases of the task. Results suggested a widespread network of regions in the prefrontal, parietal, and occipital cortices, and the cingulate and cerebellum associated with encoding, and parietal and occipital regions associated with recall. Results align with prior findings emphasizing the importance of frontoparietal circuits for working memory performance, including the role of the inferior frontal gyrus, cingulate, occipital cortex, and cerebellum in regulation of interference demands.
Keywords: cognitive control, fMRI, interference, working memory
1. INTRODUCTION
Working memory involves a diverse set of functions responsible for managing “online” cognitive activities, including temporary storage and processing of goal‐directed cognition (Conway et al., 2005). Working memory ability is critical for a host of cognitive activities necessary in daily life, as it facilitates maintenance of attention on specific goals and overriding automatic responses (Faraco et al., 2011). The multicomponent account of working memory suggests that working memory is supported by both modality‐specific (e.g., phonological) storage processes and executive attention processes that perform operations and/or manipulations of stored representations irrespective of modality (Baddeley, 2003; Engle, Tuholski, Laughlin, & Conway, 1999). Individual differences in working memory capacity (WMC), or the limit of information that can be held active for manipulation and use in the working memory store at a given time, are thought to be a function of both executive attention and short‐term storage ability (Daneman and Carpenter, 1980; Kane and Engle, 2002). Together, the availability of storage space for maintaining representations and the efficiency of processing operations on those representations are thought to determine individual differences in WMC performance.
1.1. Complex span tasks as working memory capacity assessments
Working memory span tasks, which require temporary storage of stimuli under conditions of secondary processing demands, are considered to be a “gold standard” paradigm for WMC assessment (Conway et al., 2005; Cowan et al., 2005). In a typical span task, participants are asked to remember stimuli (e.g., words, numbers), while simultaneously solving unrelated problems (e.g., sentence reading, math operations). The primary behavioral outcome in the task is commonly the number or percentage of items recalled. Behavioral outcomes from these tasks have demonstrated strong psychometric properties across diverse samples (Conway et al., 2005).
Numerous studies have documented associations between span task performance and complex, higher order cognitive functions (e.g., language, problem solving, and learning (Kane and Engle, 2002)). Moreover, span task performance is also associated with affective functions that require cognitive capacity. For example, individuals with higher span task performance are better able to downregulate negative cognitions (Brewin and Smart, 2005), and have greater emotion regulation functioning (Schmeichel, Volokhov, & Demaree, 2008), both of which are considered important for maintaining psychological health. Conversely, disorders characterized by dysregulation of affectively‐relevant cognitions (e.g., anxiety), have demonstrated lower span task performance (Amir and Bomyea, 2011). Thus, WMC tasks provide valuable cognitive assessments both because of their correspondence to the functions of working memory in daily life (e.g., ability to maintain information in the face of potential distractions) and because they account for substantial variability in higher order cognitive functioning outcomes that may be important for academic/vocational, social, and psychological well‐being (Chein, Moore, & Conway, 2011).
1.2. Executive attention in WMC tasks: The importance of interference control
Although span tasks provide a metric of capacity, it is executive attention ‐ more so than domain‐specific storage—that likely underlies the strong association between WMC performance and higher order cognitive abilities (Unsworth, 2010). The executive attention component of working memory is utilized for processing operations or functions on current representations, keeping specific representations active in working memory, and simultaneously keeping unneeded representations out of working memory by downregulating competing but irrelevant representations during retrieval and maintenance (i.e., controlling potential sources of interference (Unsworth and Engle, 2007)). The extent to which one can prevent alternate representations from entering working memory and remove them once they emerge is one of the most critical aspects of successfully maintaining desired representations, making interference control a key determinant of WMC performance (Unsworth, 2010).
In the case of complex span tasks, interference control over representations from trial to trial is a fundamental requisite for performance, because span score outcome (i.e., correct number recalled) relies on downregulation of representations on previous trials in addition to storage and processing capacity on the current trial (Lustig, May, & Hasher, 2001). Thus, new encoding and retrieval depends on the ability to control “internal” memory interference from former learning (i.e., proactive interference control), while also controlling “external” current interference that may be incurred by new information presented in the processing task. For example, in one study, Bunting (2006) manipulated interference control demands across trials within a complex span task by varying similarity of the to‐be‐remembered storage items. Interference is thought to accumulate based on the extent of similarity of stimuli encountered (Lustig, May, & Hasher, 2001), so in this study, interference demands were manipulated across trials during a complex span task by varying the category of the to‐be‐remembered storage items. High interference trials involved continual presentation of stimuli in the same category (e.g., all numbers), while interference release trials alternated category (e.g., numbers to letters). Results revealed that interference demands (based on performance outcomes) increased as a function of the number of stimuli presented within the same category type both within a given trial and across trials, and the degree of interference influenced recall performance accuracy. Moreover, only high interference control trial performance was associated with a measure of general fluid intelligence, suggesting that interference control demands across trials contribute significantly to both span task performance and to the relationship between WMC performance and other cognitive abilities.
1.3. Neuroimaging of complex span tasks
Work on the neurobiological mechanisms of complex span tasks documents associations with activation bilaterally in the dorsolateral prefrontal cortex (PFC), anterior cingulate cortex (ACC), and inferior frontal and parietal cortices (Bunge, Klingberg, Jacobsen, & Gabrieli, 2000; Osaka et al., 2004), with greater levels of activation observed during dual task conditions (i.e., conditions requiring both storage and processing of a secondary task, versus one or the other). Studies examining the effect of span set size (i.e., number of to‐be‐remembered stimuli on the trial) also suggest that greater cognitive exertion, as indexed by larger set size, involves the ACC and insular cortex (Engstrom, Karlsson, Landtblom, & Craig, 2015). Moreover, when contrasting individuals with high versus low WMC performance, greater recruitment of the ACC and connectivity between ACC and multiple areas in the PFC has been observed in those with high WMC, and greater activation in the ACC is related to behavioral performance during a span task within this group (Kondo et al., 2004; Osaka et al., 2004).
Though WMC performance appears to rely heavily on the regulation of interference control across trials, to date, it remains unclear whether interference control demands in such tasks recruit neural substrates that are distinct from general task demands. The few published neuroimaging studies of span tasks have primarily compared contrasts across single versus dual task conditions, which cannot differentiate a specific effect of interference control mechanisms, or have examined the impact of load as a metric of difficulty without separately considering interference demands (Engstrom et al., 2015). One exception is Chein et al. (2011), who evaluated the potential effect of modality similarity between storage and processing phases (e.g., verbal storage with verbal processing versus nonverbal storage with verbal processing) on performance and neural activity. In these data, PFC and ACC regions were activated regardless of modality similarity in the storage and processing phase. These data do not directly address the question of which neural regions are involved in controlling interference that is generated by prior trial learning, which appears to be a significant contributor to the utility of these tasks for predicting higher order cognition.
Isolation of key neural regions involved in the interference control component of span task performance may be informative regarding neural circuitry in conditions associated with cognitive deficits (e.g., psychiatric conditions like anxiety, or affective regulation more broadly), and may also inform understanding of neural circuits modulated by cognitive training with these types of tasks (Bomyea, Stein, & Lang, 2015). Other tasks requiring interference control for memory performance show involvement of a number of key cortical areas that could be similarly hypothesized to regulate interference in span tasks. For example, Burgess et al. (2011) identified areas of the lateral and ventromedial PFC and parietal lobe as key interference control regions. Activity in these regions accounted for variance in the relationship between span task performance and higher order cognition, supporting the hypothesis that interference control brain regions are a critical component of effective WMC. In a number of reviews, regions identified as key for cognitive control over memory representation selection and resolution of interference across other kinds of tasks (e.g., recent probes task, n‐back) have most commonly included the inferior frontal cortex and ventrolateral PFC, as well as the precuneus, parietal cortex, and frontopolar cortex (Badre & Wagner, 2007; Nee, Jonides & Berman, 2007). Inferior frontal gyrus (IFG) activation in particular appears to be sensitive to load increase across trials, but does not appear to reflect load difficulty or the amount of time spent on the task (Nee, Jonides & Berman, 2007). Together, these data informed a model whereby the IFG is responsible for selecting among competing representations by activating the necessary representation and inhibiting or selecting from competing representations during goal‐directed cognitive activity (Nee, Jonides & Berman, 2007)
This study sought to further examine the neurobiological substrates of interference control demands in WMC using a complex span task. We used a modified Reading Span task (Rspan) to extend the small body of existing literature on neural activity during complex span tasks in a number of ways. First, we sought primarily to examine neural substrates that are specifically associated with differential interference control demands, accounting for memory demand difficulty as indexed by set size (memory load). To date, the body of literature examining neural correlates of span tasks has focused on delineating neural correlates of single (memory storage or secondary task processing) versus dual task performance or other task manipulations (e.g., span size). Because interference control of previously learned representations has been proposed as a key element of both task performance and the predictive utility of WMC tasks, identifying the brain regions most directly influenced by interference control demands is a critical next step for work in this area. Based on literature employing different cognitive paradigms, we hypothesized that greater interference control demands would be associated with activation in the inferior frontal cortex (Derrfuss, Brass, Neumann, & von Cramon, 2005), and regions of the dorsolateral PFC, ACC, and parietal cortex. Second, the design of this task also allowed us to separately evaluate the effects of mounting interference control demands during encoding and retrieval, which has been an understudied differentiation in prior fMRI studies of WMC tasks (Chein et al., 2011).
2. METHODS
2.1. Participants
Twenty‐two right‐handed healthy participants participated in a modified Rspan task while undergoing fMRI (see Table 1 for sample characteristics). All participants provided written informed consent and the project was approved by the UCSD Human Research Protection Program. Prior to participation in the fMRI session, participants completed a screening interview to confirm that they had no lifetime history of Axis I DSM‐IV disorders. Participants were compensated with $125 for participation in the study.
Table 1.
Demographic characteristics
| Variable | |
|---|---|
| Female (%) | 12 (54.5%) |
| Mean age (SD) | 21.86 (2.76) |
| Mean education (SD) | 15.63 (1.47) |
| Race (%) | |
| White | 9 (40.9%) |
| Black | 1 (4.5%) |
| Asian | 5 (22.7%) |
| Biracial/other | 7 (31.8%) |
2.2. Task
In the modified Rspan, participants were instructed to remember items presented while simultaneously solving a secondary processing task, in which they decided if a sentence was logically correct (Daneman and Carpenter, 1980; Unsworth, Heitz, Schrock, & Engle, 2005). The program was computer‐administered, consistent with studies of automatized span tasks (Unsworth, Redick, Heitz, Broadway, & Engle, 2009). Each trial began with a fixation cross in the center of the screen for 500 ms. Then, a sentence (e.g., “Jane walks her car in the park”) appeared on the screen (Figure 1). The participant indicated when they had finished reading the sentence, and then were shown a screen where they selected whether the sentence made sense by selecting a box on the screen using a joystick‐operated mouse cursor (left box for “yes,” right box for “no”). Half of the sentences presented were grammatically correct (i.e., made sense) and half were not. Sentences were presented until the subject made a response or after 5 s (in which case the sentence was considered a time‐out error). A to‐be‐remembered item (a letter or number) appeared on the screen for 500 ms after the participant completed the sentence problem (i.e., an ideographic timing occurred between sentence reading offset and onset of the to‐be‐remembered item). The participant continued to view sentences and items until the end of the trial, and then viewed a recognition screen listing twelve letters or numbers. Using the joystick‐operated mouse cursor, participants were asked to select the previously presented items in the correct serial order. After each item was selected, a small box adjacent to the item showed the selection order number of the item (e.g., the first item showed the number “1,” the second item showed the number “2”, etc.). Once the recognition test for the set was completed, the next trial began. During the task, the participant received feedback about their sentence accuracy at the conclusion of each trial. During the instructions, participants were informed that it was important for them to retain their sentence performance accuracy above 85% (Conway et al., 2005). The task contained 9 sets of trials, each of which tested trials of set size two, four and six to‐be‐remembered items (i.e., memory load of two, four, or six). Within each set, the number of to‐be‐remembered items was presented in a different random order to all participants, and sets were presented in a consistent order to participants.
Figure 1.
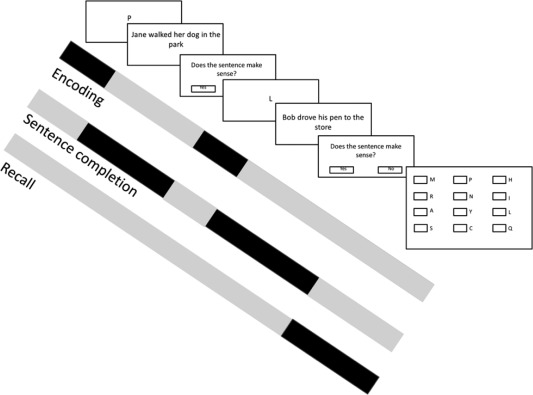
Schematic of Reading Span Task with a trial size of 2, depicting basic regressors (1–3) for each phase of the task
Trials of the task varied in the amount of proactive interference control required based on alternation of the category of the to‐be‐remembered stimuli (letters or numbers). Trials where the to‐be‐remembered stimuli were the same category as the prior trials (i.e., current trial was numbers and prior trial was numbers) were considered interference control trials. Thus, interference control trials were those in which a high degree of interference had built across trials due to item similarity (Bunting, 2006). The amount of interference control required on each trial was a product of (1) prior stimuli type (same or different category), (2) the number of memoranda stimuli previously to‐be‐remembered in prior trials in the same category, and (3) number of memoranda stimuli on the current trial. When the stimuli changed type, it was considered a release from interference and the interference from prior trials was reset. For example, if a trial contained letter stimuli and had 2 to‐be‐remembered stimuli, and the prior two trials contained a total of 10 to‐be‐remembered letter stimuli, the interference control requirement would be 12 (Figure 1). If the same 2‐item trial followed prior trials that contained a different category, then the interference control requirement would be only 2, based only on the number of stimuli on the current trial. On each trial, letters were randomly selected from F, H, J, K, L, N, P, Q, R, S, T, and Y, and numbers were digits randomly selected from the set 1 through 12, with each letter/number shown as a potential item during recall. All items within each set were letters or numbers, such that each set tested trials of size 2, 4, and 6 within a specific category type. Because no differences were anticipated in responses to specific stimuli type (letters versus numbers), type of stimuli was collapsed. Reaction time and accuracy were recorded for all responses.
2.3. Experimental procedures
During the fMRI session, participants provided consent, then completed a brief battery of MRI safety and self‐report questionnaires. Prior to completing the scan, participants completed a behavioral practice version of the task outside of the scanner. Stimulus presentation and response registration were collected using Eprime software.
2.4. fMRI scanning
Participants were scanned in a 3 T GE 750 scanner using an 8‐channel head array coil. Each scanning session included a three‐plane scout scan, a sagittally acquired spoiled gradient recalled (SPGR) sequence for acquiring T1‐weighted images (FOV 256 cm; matrix: acquired 192 × 256 matrix resampled to 256 × 256; 172 slices; thickness: 1 mm; TR = 8 ms, TE: 3 ms, flip angle: 12°, inversion time = 450 ms) and one T2*‐weighted axially acquired echo‐planar imaging (EPI) scans to measure blood oxygen level dependent (BOLD) signals (parameters: 3.75 mm × 3.75 mm × 3 mm; 64 × 64 acquisition matrix with a 1 mm gap, TR = 1500 s, TE = 32 ms, flip angle = 80°, and 30 slices (whole brain)). The length of task ranged from 544 to 894 acquisitions (M = 17.58 min). The stimuli for the Rspan task were synchronized with the scanner sequence and visible to participants using a projected screen visible through a mirror in the head coil.
2.5. Image processing and analysis
The data were preprocessed and normalized to MNI coordinates using tools available in ANTsR, a statistical interface between Advanced Normalization Tools Software, R software, and Analysis of Functional NeuroImages (AFNI). fMRI preprocessing steps consisted of removal of temporal outliers (AFNI:3dDespike), field inhomogeneity correction (ANTsR:n3BiasFieldCorrection), slice time correction, and temporal whitening (ANTsR:preprocessing). Motion correction and CompCor estimation correction were also included as part of this processing pathway, and motion and CompCor correction regressors were removed as part of the preprocessing steps. Outlying acquisitions (AFNI 3dToutcount) were censored from the time series. Regressors with hemodynamic shifts (AFNI:waver) were entered into a regression (R:lm) to calculate normalized beta weights. Data were aligned to individual anatomical and MNI template (ANTsR:antsRegistration/antsApplyTransforms) for group comparisons. Five response regressors were generated for phases of the task based on the idiographic timing of presentation for each phase for each participant (Figure 2). Task‐based regressors were included to model basic features of each of the trials in the paradigm that were considered distinct from inference demands, including (1) sentence reading and verification, (2) stimuli encoding weighted by set size of a given trial to reflect memory load variance, and (3) stimuli recall weighted by set size of a given trial to reflect memory load variance. Interference‐based regressors of interest were (4) one for interference in encoding and (5) one for interference in recall, that reflected the cumulative build of interference control requirements across like trials (once memory load variance has been covaried in regressors #2 and #3). These allowed for examination of neural activity in response to increasing interference control demands during the trials.
Figure 2.
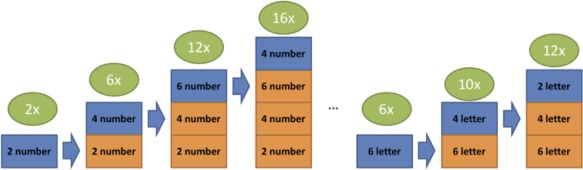
Schematic of regressors for interference control load on current trial (i.e., blue boxes) based on size of prior trials (i.e., orange boxes) [Color figure can be viewed at http://wileyonlinelibrary.com]
We tested for differential blood oxygenation level‐dependent (BOLD) signal during the task by examining voxels that showed significant activation in response to interference control requirements (regressors 4 and 5). Within the encoding and recall phases for each trial, we examined a regressor that modeled the amount of interference control required. The regressor reflected a linear “weighting” of the number of prior and current stimuli presented within the same category (i.e., the amount of interference build engendered by the current and prior trials). If the stimuli changed type, the regressor reflected a reset of interference from prior trials by returning to zero. Thus, for all trials, the regressor provided a marker of interference‐based effort, when accounting for regressors that represented general task demands (e.g., the phase of encoding or recall, the set size of the given trial). Voxel‐wise percent signal change data were entered into a t test (3dttest++) to evaluate regions of significant activation (Cox et al., 2016) to the two regressors of interest as compared to baseline. Voxel‐wise percent signal changes were also entered into a paired‐samples t test to evaluate differences in signal during encoding versus recall phases of the task. Permutations testing within AFNI's 3dttest++(ClustSim), which computes a three‐parameter spatial autocorrelation function from the model residuals using 3dFHWMx to create an optimal smoothing kernel, were used to guard against identifying false positive activations. A voxel‐wise a priori probability of .005 was found to result in a corrected cluster‐wise activation probability of .05 if a minimum of 14 contiguous voxels was considered. The average percent signal change was extracted from regions of activation that were found to survive this threshold/cluster method.
3. RESULTS
3.1. Behavioral results
Total raw accuracy (i.e., a traditional working memory capacity score) was indexed as the percent of correctly recalled items in the correct serial order (Conway et al., 2005). Total raw accuracy for participants was 79.9% (SD = 10; 86.5 total items) of the memoranda. Two methods were used to examine the behavioral effects of the increasing interference demand designed within the task. In the first, the relative percentage correct on each trial was analyzed as a linear effect of the number of prior same‐category trials (e.g., whether the trial was the first, second, third, etc. of the same category, letters or numbers). A linear mixed effects model was used to analyze the slope of trial‐level performance as a function of the number of prior same‐category trials. Results revealed that performance decreased over the course of same‐category trials, B = −.01, t = 2.16, p = .03, suggesting a linear pattern of decreased performance as the number of trials with the same stimuli type increased.1 In the second, the effect of interference on performance was also determined by calculating a simple binary comparison of the total number of correctly recalled items for trial sets that contained low interference (novel presentation of stimuli from a category) and high interference (second presentation of stimuli from a category). Results revealed lower performance on high interference trial sets as compared to low interference trial sets, t(21) = 2.20, p < .05 (Figure 3a), suggesting performance decrement based on interference demands. The effect of load size was also examined using a linear mixed effects model analyzing trial‐level performance as a function of set size. Results revealed that performance worsened as set size increased, B = −.06, t = 10.23, p < .001, (Figure 3b).
Figure 3.
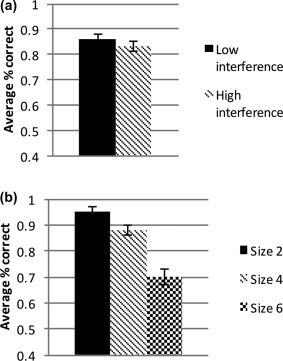
Behavioral performance on low versus high interference trials (a), and small medium and large set size trials (b)
3.2. fMRI results
3.2.1. Activation areas
We performed one‐sample t tests to evaluate activation under each of the conditions of interest, that is, interference demand load during encoding and recall. In interference demand encoding, positive cortical activations were found in a large area of the bilateral middle frontal gyrus extending to the inferior frontal gyrus, a region of the left dorsal medial PFC, middle occipital gyrus, left parietal cortex, bilateral cerebellum, bilateral superior frontal gyrus, left precentral gyrus, and left insula (Figures 4, 5, 6). Negative activation in response to greater interference demand encoding was observed in the posterior cingulate, motor cortex, medial frontal gyrus, middle cingulate gyrus, left ventromedial PFC, and superior frontal cortex (Table 2). During interference demand recall, positive activation in response to interference demands was observed in the middle to anterior cingulate cortex, right middle occipital gyrus, right paracentral lobule/motor cortex, left cuneus, left postcentral gyrus, and right precuneus (Table 3 and Figure 7). A paired‐sample t test was used to evaluate differential interference‐based activation during encoding and recall, which revealed greater activation to recall versus encoding in the posterior cingulate, supplemental motor area, and medial frontal gyrus (Table 4). We also evaluated activation to memory load during encoding and recall (i.e., set size; Table 5) and found significant activations spanning the prefrontal cortex (bilateral middle frontal, right medial frontal), temporal, and occipital lobes during encoding only (see Supporting Information, Figure 1 for a depiction of distinct and overlapping regions involved in memory load during encoding versus interference control during encoding).
Figure 4.
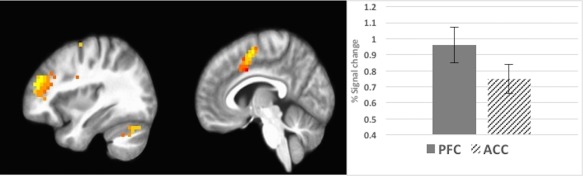
Activation during encoding in prefrontal and cerebellar regions (x = 35) and cingulate (x = 6); voxels > 14 [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
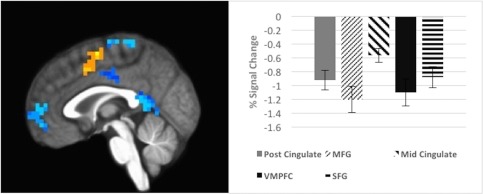
Deactivations during encoding (less activation in response to greater interference; x = 1); voxels > 14 [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
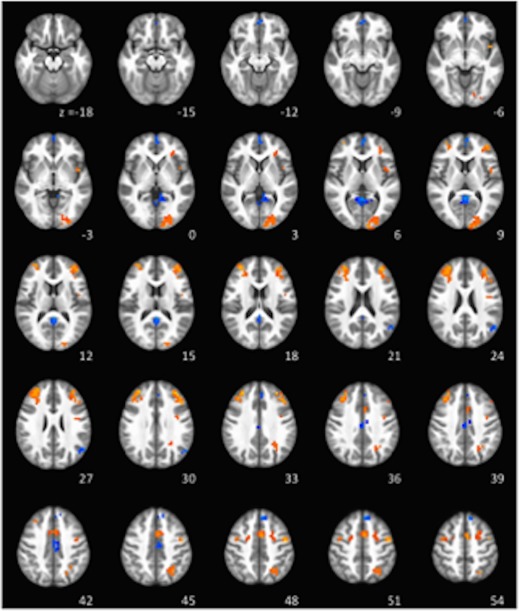
Whole brain signal change in during interference control in encoding; voxels > 14 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Activation during interference‐based encoding phase
| Voxels | x | y | z | Region | BA | t test |
|---|---|---|---|---|---|---|
| 107 | 35 | 42 | 26 | Right middle frontal gyrus | 10/45 | 3.33 |
| 106 | −12 | 4 | 56 | Left dorsal medial frontal gyrus | 6 | 3.43 |
| 104 | −39 | 40 | 22 | Left middle frontal gyrus | 10/45 | 3.33 |
| 79 | −20 | −93 | 5 | Left cuneus | 17 | 3.58 |
| 44 | −28 | −60 | 44 | Left superior parietal lobule | 7 | 3.29 |
| 41 | 36 | −67 | −29 | Right cerebellum | 3.70 | |
| 41 | 29 | 3 | 59 | Right middle frontal gyrus | 6 | 3.30 |
| 24 | −45 | −2 | 45 | Left precentral gyrus | 6 | 3.30 |
| 15 | −46 | 5 | 2 | Left insula | 13 | 3.11 |
| 14 | −34 | −61 | −34 | Left cerebellum | 3.31 | |
| 14 | −32 | −72 | −26 | Left cerebellum | 3.09 | |
| 54 | −1 | −50 | 9 | Left posterior cingulate | 29 | −3.39 |
| 37 | −2 | −31 | 74 | Left medial frontal gyrus | 6 | −3.15 |
| 26 | −3 | −14 | 42 | Middle cingulate gyrus | 24 | −3.12 |
| 24 | −1 | 59 | 1 | Left ventromedial cortex | 10 | −3.19 |
| 19 | −7 | 39 | 46 | Left superior frontal gyrus | 8 | −3.04 |
Table 3.
Activation during interference‐based recall phase
| Voxels | x | Y | z | Region | BA | t test |
|---|---|---|---|---|---|---|
| 62 | −3 | −2 | 51 | Left cingulate gyrus | 24 | 3.20 |
| 31 | 18 | −96 | 10 | Right middle occipital gyrus | 18 | 3.35 |
| 30 | 7 | −30 | 72 | Right paracentral lobule/motor cortex | 6 | 3.24 |
| 22 | −8 | −75 | 10 | Left cuneus | 23/17 | 3.17 |
| 15 | −51 | −10 | 23 | Left postcentral gyrus | 43 | 3.29 |
| 14 | 9 | −41 | 49 | Right precuneus | 5 | 3.09 |
Figure 7.
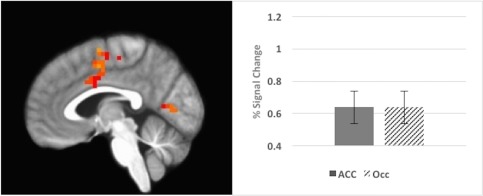
Activations during recall (x = 5); voxels > 14 [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Differential activation between interference‐based encoding and recall phases
| Voxels | x | y | z | Region | BA | t test |
|---|---|---|---|---|---|---|
| 33 | 1 | −51 | 8 | Right posterior cingulate | 29 | −3.21 |
| 21 | 3 | −37 | 72 | Right paracentral lobule | 6 | −3.30 |
| 20 | 0 | −16 | 75 | Left medial frontal gyrus | 6 | −3.37 |
Table 5.
Activation during memory load‐based encoding
| Voxels | x | y | z | Region | BA | t test |
|---|---|---|---|---|---|---|
| 655 | −14 | −64 | 39 | Left precuneus | 7 | 3.64 |
| 506 | −29 | 3 | 43 | Left middle frontal gyrus | 6 | 3.60 |
| 132 | −2 | −82 | 22 | Left cuneus | 18 | −3.36 |
| 104 | 11 | −56 | −5 | Right culmen | 19 | −3.17 |
| 74 | 25 | −4 | 54 | Right middle frontal gyrus | 6 | 3.32 |
| 60 | 35 | −1 | −41 | Right inferior temporal gyrus | 20 | −3.43 |
| 56 | 32 | −84 | −1 | Right middle occipital gyrus | 18 | 3.34 |
| 34 | 4 | −63 | −28 | Cerebellum | 3.23 | |
| 31 | −54 | −62 | 35 | Left angular gyrus | 39 | −3.28 |
| 24 | −30 | 6 | −41 | Left superior temporal gyrus | 38 | −3.30 |
| 17 | 59 | −56 | 19 | Right superior temporal gyrus | 40 | −3.09 |
| 15 | −29 | 19 | −1 | Left claustrum | 13 | 3.07 |
| 15 | −37 | 49 | 13 | Left middle frontal gyrus | 10 | 3.01 |
| 15 | 0 | 43 | 46 | Right medial frontal gyrus | 8 | −3.23 |
4. DISCUSSION
Interference control is considered to be a key process implicated in successful WMC performance. This study sought to better understand the neural substrates supporting interference control using a novel adaptation of a complex span task designed to build on earlier fMRI studies of working memory capacity in a number of ways. First, we examined the impact of interference demands during dual task performance when accounting for difficulty due to set size. Second, the design of the study enabled the differentiation of interference‐based neural regions involved in encoding and recall (c.f. a design that compares activation in both encoding and recall to either encoding or recall without differentiating between these two phases (Osaka et al., 2004)). Interference demands recruited the PFC, and cingulate, parietal, insular, and cerebellar regions during encoding. Greater interference‐based encoding demand was also associated with deactivation of regions including the posterior cingulate cortex, paracentral gyrus, and orbitofrontal areas. In contrast, memory load in encoding activated a widespread network of frontal, temporal and occipital lobes (see Supporting Information, Figure 1 for shared and distinct neural activation patterns across set size versus interference). During recall, clusters in regions including the ACC, parietal, occipital, and medial PFC were significantly activated.
Consistent with earlier work on the neurobiology of complex span tasks (e.g., Bunge et al., 2000; Kondo et al., 2004), the current data support the role of the medial and inferior PFC and insula, and the parietal, premotor, and occipital cortices as substrates for the adaptive management of interference in working memory. The overlap between the current interference‐based task activations and data from earlier studies comparing single versus dual task WMC processing suggests commonality in the systems that subserve interference control and those that manage performance more generally. Data also converge with extant findings on the neural substrates of interference control. For example, the left inferior frontal gyrus has repeatedly been shown to play a critical role in resolving proactive interference in alternative verbal working memory tasks (Bunge, Matsumoto, Desmond, Glover, & Gabrieli, 2000; Jonides and Nee, 2006; Jonides, Smith, Marshuetz, Koeppe, & Reuter‐Lorenz, 1998), and also appears to be one region involved in WMC under conditions of high interference control requirements.
The design of the study allowed for differentiation of neural regions activation by encoding and recall phases. Inspection of the task activations across phases suggests that activation of ACC and middle frontal regions was generally present in both encoding and recall, consistent with the use of these substrates for engagement of cognitive control resources (Burgess & Braver, 2010; Kerns et al., 2004). Encoding activated a larger and more diverse set of frontal, parietal, occipital, and cerebellar regions that were more consistent with neural regions thought to be involved in the control of interference from competing information in memory (Nee, Wager, & Jonides, 2007), which may reflect proactive maintenance and updating of representations in order to minimize interference (Burgess and Braver, 2010). Encoding also showed a specific pattern of deactivation in ventromedial and posterior cingulate regions. A subset of these regions overlaps with the default mode network, which would be anticipated to be downregulated during externally focused cognitive activity (Raichle et al., 2001). During recall, a smaller set of cingulate, parietal, and occipital regions were activated. Direct statistical comparison of these phases indicated that posterior cingulate, medial frontal, and motor regions were differentially responsive to encoding versus recall phases. Taken together, activation patterns across phases suggest that the neurobiological systems supporting regulation of interference demands initiate during presentation of to‐be‐remembered information (e.g., upregulation of fronto‐parietal systems and cerebellum), and that the later information recall relies of partially distinct regions.
There are a number of limitations in the current data that should be addressed by future work. First, the sample was modest in size and included relatively young and healthy participants. Results thus may not generalize to other individuals. Because of the specific stimuli used (letters and numbers), additional research is needed to understand the role of category in neural and behavioral data. For example, it is possible that nonverbal stimuli would result in different activation patterns. Further research is also needed to better understand how interference control demands in this type of task relate to real‐world cognitive and emotional outcomes. As part of this work, elucidating how interference control processes and corresponding neural substrates relate to psychiatric difficulties could be explored. Larger sample sizes would increase the power to examine individual differences in neural activity, behavioral responses, and symptoms.
In spite of these limitations, data highlight an activation pattern within frontoparietal and occipital regions during encoding that underlie interference control during a WMC task. The modified Rspan task appears to provide a tool for probing neural circuitry of WMC that differentiates the regions involved in interference control from set size, which may be relevant in future studies assessing populations (e.g., posttraumatic stress disorder; Bomyea, Amir, & Lang, 2012) or affective states (e.g., emotional arousal; Osaka, Yaoi, Minamoto, & Osaka, 2013) marked by deficits in this ability. In addition, empirical interest in training of cognitive functions dependent on interference control and working memory capacity in healthy and clinical samples has recently grown (Bomyea et al., 2015), with evidence that training‐related changes may occur in both structural and functional neural activity (Engvig et al., 2010). The current task may provide a sensitive assessment of neural change of interference functions, though research is needed to evaluate its utility in a treatment or training context.
DECLARATION OF INTERESTS
The authors do not have any conflicts of interest to disclose.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ROLE OF THE FUNDING SOURCE
This study was supported by a VA Merit Award grant (I01‐CX000715) to A. Simmons, a VA Career Development Award to A. Spadoni (5IK2CX000864), a VA Center of Excellence for Stress and Mental Health Pilot Award to J. Bomyea, and a National Institute of Mental Health grant to C. Taylor (ROO MH090243). This material is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs (VA) and the VA San Diego Center of Excellence for Stress and Mental Health. The sponsors were not directly involved in the design or preparation of this manuscript.
Bomyea J, Taylor CT, Spadoni A, Simmons AN. Neural mechanisms of interference control in working memory capacity. Hum Brain Mapp. 2018;39:772–782. 10.1002/hbm.23881
Funding information VA Merit Award, Grant/Award Number: I01‐CX000715; VA Career Development Award, Grant/Award Number: 5IK2CX000864; VA Center of Excellence for Stress and Mental Health Pilot Award; National Institute of Mental Health, Grant/Award Number: ROO MH090243; Office of Academic Affiliations, Department of Veterans Affairs (VA); VA San Diego Center of Excellence for Stress and Mental Health
Footnote
The possibility of a nonlinear change was examined and found to be a less optimal fit in the data using both a linear mixed effects analytic approach and a planned polynomial contrast analytic approach.
REFERENCES
- Amir, N. , & Bomyea, J. (2011). Working memory capacity in generalized social phobia. Journal of Abnormal Psychology, 120, 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley, A. (2003). Working memory: Looking back and looking forward. Nature Reviews. Neuroscience, 4, 829–839. [DOI] [PubMed] [Google Scholar]
- Badre, D. , & Wagner, A. D. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. [DOI] [PubMed] [Google Scholar]
- Bomyea, J. , Amir, N. , & Lang, A. J. (2012). The relationship between cognitive control and posttraumatic stress symptoms. Journal of Behavior Therapy and Experimental Psychiatry, 43, 844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomyea, J. , Stein, M. B. , & Lang, A. J. (2015). Interference control training for PTSD: A randomized controlled trial of a novel computer‐based intervention. Journal of Anxiety Disorders, 34, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin, C. R. , & Smart, L. (2005). Working memory capacity and suppression of intrusive thoughts. Journal of Behavior Therapy and Experimental Psychiatry, 36, 61–68. [DOI] [PubMed] [Google Scholar]
- Bunge, S. A. , Klingberg, T. , Jacobsen, R. B. , & Gabrieli, J. D. E. (2000). A resource model of the neural basis of executive working memory. Proceedings of the National Academy of Sciences of the United States of America, 97, 3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge, S. A. , Matsumoto, A. , Desmond, J. E. , Glover, G. H. , & Gabrieli, J. D. E. (2000). The neural basis of interference resolution: Manipulations of interference and working memory load in the Item Recognition paradigm. Journal of Cognitive Neuroscience, 108–108. [Google Scholar]
- Bunting, M. (2006). Proactive interference and item similarity in working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32, 183. [DOI] [PubMed] [Google Scholar]
- Burgess, G. C. , & Braver, T. S. (2010). Neural mechanisms of interference control in working memory: Effects of interference expectancy and fluid intelligence. PLoS One, 5, e12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein, J. M. , Moore, A. B. , & Conway, A. R. (2011). Domain‐general mechanisms of complex working memory span. NeuroImage, 54, 550–559. [DOI] [PubMed] [Google Scholar]
- Conway, A. R. , Kane, M. J. , Bunting, M. F. , Hambrick, D. Z. , Wilhelm, O. , & Engle, R. W. (2005). Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin & Review, 12, 769–786. [DOI] [PubMed] [Google Scholar]
- Cowan, N. , Elliott, E. M. , Scott Saults, J. , Morey, C. C. , Mattox, S. , Hismjatullina, A. , & Conway, A. R. (2005). On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology, 51, 42–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. , Reynolds, R. C. , & Taylor, P. A. (2016). AFNI and clustering: False positive rates redux. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman, M. , & Carpenter, P. A. (1980). Individual‐differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior, 19, 450–466. [Google Scholar]
- Derrfuss, J. , Brass, M. , Neumann, J. , & von Cramon, D. Y. (2005). Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Human Brain Mapping, 25, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, R. W. , Tuholski, S. W. , Laughlin, J. E. , & Conway, A. R. (1999). Working memory, short‐term memory, and general fluid intelligence: A latent‐variable approach. Journal of Experimental Psychology‐General, 128, 309–331. [DOI] [PubMed] [Google Scholar]
- Engstrom, M. , Karlsson, T. , Landtblom, A. M. , & Craig, A. D. (2015). Evidence of conjoint activation of the anterior insular and cingulate cortices during effortful tasks. Frontiers in Human Neuroscience, 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig, A. , Fjell, A. M. , Westlye, L. T. , Moberget, T. , Sundseth, O. , Larsen, V. A. , & Walhovd, K. B. (2010). Effects of memory training on cortical thickness in the elderly. NeuroImage, 52, 1667–1676. [DOI] [PubMed] [Google Scholar]
- Faraco, C. C. , Unsworth, N. , Langley, J. , Terry, D. , Li, K. M. , Zhang, D. G. , … Miller, L. S. (2011). Complex span tasks and hippocampal recruitment during working memory. NeuroImage, 55, 773–787. [DOI] [PubMed] [Google Scholar]
- Jonides, J. , & Nee, D. E. (2006). Brain mechanisms of proactive interference in working memory. Neuroscience, 139, 181–193. [DOI] [PubMed] [Google Scholar]
- Jonides, J. , Smith, E. E. , Marshuetz, C. , Koeppe, R. A. , & Reuter‐Lorenz, P. A. (1998). Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences of the United States of America, 95, 8410–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, M. J. , & Engle, R. W. (2002). The role of prefrontal cortex in working‐memory capacity, executive attention, and general fluid intelligence: An individual‐differences perspective. Psychonomic Bulletin & Review, 9, 637–671. [DOI] [PubMed] [Google Scholar]
- Kerns, J. G. , Cohen, J. D. , MacDonald, A. W., 3rd , Cho, R. Y. , Stenger, V. A. , & Carter, C. S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303, 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kondo, H. , Morishita, M. , Osaka, N. , Osaka, M. , Fukuyama, H. , & Shibasaki, H. (2004). Functional roles of the cingulo‐frontal network in performance on working memory. NeuroImage, 21, 2–14. [DOI] [PubMed] [Google Scholar]
- Lustig, C. , May, C. P. , & Hasher, L. (2001). Working memory span and the role of proactive interference. Journal of Experimental Psychology‐General, 130, 199–207. [DOI] [PubMed] [Google Scholar]
- Nee, D. E. , Jonides, J. , & Berman, M. G. (2007). Neural mechanisms of proactive interference‐resolution. Neuroimage, 38, 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee, D. E. , Wager, T. D. , & Jonides, J. (2007). Interference resolution: Insights from a meta‐analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience, 7, 1–17. [DOI] [PubMed] [Google Scholar]
- Osaka, M. , Yaoi, K. , Minamoto, T. , & Osaka, N. (2013). When do negative and positive emotions modulate working memory performance?. Scientific Reports, 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka, N. , Osaka, M. , Kondo, H. , Morishita, M. , Fukuyama, H. , & Shibasaki, H. (2004). The neural basis of executive function in working memory: An fMRI study based on individual differences. NeuroImage, 21, 623–631. [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod, A. M. , Snyder, A. Z. , Powers, W. J. , Gusnard, D. A. , & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel, B. J. , Volokhov, R. N. , & Demaree, H. A. (2008). Working memory capacity and the self‐regulation of emotional expression and experience. Journal of Personality and Social Psychology, 95, 1526–1540. [DOI] [PubMed] [Google Scholar]
- Unsworth, N. (2010). Interference control, working memory capacity, and cognitive abilities: A latent variable analysis. Intelligence, 38, 255–267. [Google Scholar]
- Unsworth, N. , & Engle, R. W. (2007). The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review, 114, 104–132. [DOI] [PubMed] [Google Scholar]
- Unsworth, N. , Heitz, R. R. , Schrock, J. C. , & Engle, R. W. (2005). An automated version of the operation span task. Behavior Research Methods, 37, 498–505. [DOI] [PubMed] [Google Scholar]
- Unsworth, N. , Redick, T. S. , Heitz, R. P. , Broadway, J. M. , & Engle, R. W. (2009). Complex working memory span tasks and higher‐order cognition: A latent‐variable analysis of the relationship between processing and storage. Memory, 17, 635–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


