Abstract
Classical trigeminal neuralgia (TN) is a specific type of neuropathic orofacial pain of which the plasticity of brain structure and connectivity have remained largely unknown. A total of 62 TN patients were included and referred to MRI scans. Voxel‐based morphometry was used to analyze the change of gray matter volume. Resting‐state functional imaging was used to analyze the connectivity between brain regions. The results showed gray matter volume reduction in components of the prefrontal cortex, precentral gyrus, cerebellar tonsil, thalamus, hypothalamus, and nucleus accumbens among right TN patient and in the inferior frontal gyrus, precentral gyrus, cerebellum, thalamus, ventral striatum, and putamen among left TN patients. The connections between the right superior frontal gyrus and right middle frontal gyrus were lower in right TN patients. The connection between the left precentral gyrus and the left superior frontal gyrus was lower while the connection between bilateral thalamus was higher in left TN patients. The changes of volume in bilateral thalamus of right TN patients and left ventral striatum of left TN patients, and the connectivity between bilateral thalamus of left TN patients were moderately correlated with pain duration. These findings suggest that brain regions such as the thalamus may not only be involved in processing of pain stimuli but also be important for the development of TN. The left hemisphere may be dominant in processing and modulation of TN pain signal. Chronification of TN induces volume changes in brain regions which are associated with emotional or cognitive modulation of pain. Hum Brain Mapp 39:609–621, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: trigeminal neuralgia, voxel‐based morphometry, functional connectivity, neuronal plasticity, neuropathic pain
INTRODUCTION
Trigeminal neuralgia (TN) is one of the most common facial pains. It is most frequently described as a “stabbing”‐ or “electric‐shock”‐like pain that is localized to the sensory supply area of trigeminal nerve. Neurovascular compression of the trigeminal nerve at root entry zone is the most common cause of idiopathic TN [Love and Coakham, 2001; Maarbjerg et al., 2015]. TN that develops without apparent cause other than neurovascular compression is defined as classical TN [Headache Classification Committee of the International Headache Society (IHS), 2013], which is distinct from trigeminal neuropathy that has a similar clinical presentation. Trigeminal neuropathy is defined as a facial pain in the distribution of trigeminal nerve cause by another disorder (such as herpes zoster infection and multiple sclerosis) or trauma and is indicative of neural damage. It is more often a constant orofacial pain and is different from classical TN that presents with recurring and paroxysmal attacks. TN is often associated with sensory neurological deficits [Nurmikko and Eldridge, 2001] and is usually treated by anticonvulsants, microvascular decompression, or minimally invasive percutaneous lesioning of the trigeminal nerve such as radiofrequency rhizotomy [Obermann, 2010; Yang et al., 2010]. However, the pathophysiology of classical TN is still debated. Postoperative histopathological studies have shown axonal atrophy and demyelination in patients with TN [Hilton et al., 1994; Love and Coakham, 2001].
Over the past decade, a number of functional and structural imaging studies have dealt with the topic of pain processing in human subjects and identified a variety of brain regions and networks encompassed in pain, including the thalamus, basal ganglia, somatosensory (SMC), prefrontal (PFC), anterior cingulate (ACC), and the insular cortices [Bushnell et al., 2013; Legrain et al., 2011]. The thalamus and SMC encode information about sensory features, such as the location and duration of pain. The thalamus, ACC, insula, and PFC, which have been considered components of the limbic system, are responsible for encoding the emotional and motivational aspects of pain [Bushnell et al., 2013; Lamm et al., 2011]. Neuroimaging has also been used to investigate the changes in brain structure and function associated with TN [Becerra et al., 2006; Blatow et al., 2009; DeSouza et al., 2014; Gustin et al., 2011; Gustin et al., 2012; Henderson et al., 2013; Moisset et al., 2011; Obermann et al., 2013; Scrivani et al., 2010]. Although functional and structural changes in the thalamus, SMC, PFC, and basal ganglia were frequently reported, these observed results were somehow heterogeneous. Noticeably, most of these studies enrolled patients with painful trigeminal neuropathy instead of classical TN, which is a specific type of chronic neuropathic orofacial pain with distinct pathophysiology and alteration in brain activity [Gustin et al., 2011; Lin, 2014; Youssef et al., 2014]. Furthermore, a majority of these studies evaluated brain responses to experimentally induced pain, which were more likely to reveal the CNS pathways that are involved in the pain processing of acute TN. The plasticity of brain and the alterations of connectivity between pain‐associated brain regions in chronic classical TN patients have remained largely unknown. In addition, the lateralization of processing pain signals from TN has seldom been discussed.
This study uses structural and resting‐state functional magnetic resonance imaging (rs‐fMRI) to achieve three specific aims. The first aim is to analyze regional gray matter (GM) volume changes, and also evaluate the change of connectivity between each brain regions with GM volume change in patients with classical TN. This can help to identify specific brain areas and networks that may be associated with the development and persistence of this specific type of chronic neuropathic orofacial pain. The second aim is to see the different brain structural and functional changes between TN patients presenting with right‐ versus left‐sided symptoms. This may enhance our understanding of the lateralization of pain processing pathways. Because brain structural and functional change may be dynamic and progressively pain‐driven over time, the third aim of this study is to estimate the relation between the duration of TN with the amplitude of structural and functional change, which may illustrate the brain plasticity due to pain chronicity.
MATERIALS AND METHODS
Subjects
Sixty‐two patients with TN were prospectively enrolled into this study. Thirty‐six patients suffered from right TN and 26 suffered from left side TN. Patients presenting with bilateral TN were excluded. All patients were diagnosed with classical TN according to the criteria of the International Headache Society for TN, and underwent MRI. Patients on analgesic medication were asked to discontinue their medication one day before their scheduled scanning session. In addition, 19 healthy subjects without history of neurological disease or any pathological findings in conventional MRI were enrolled. The study was approved by the Institutional Review Board of our institution and all patients gave their written informed consent prior to their participation in the study.
MRI Instrumentation and Procedures
All data were collected with a three Tesla Siemens Verio MRI system (Siemens Medical System, Erlangen, Germany) using a 32‐channel head coil. 3D MP‐RAGE anatomical images were obtained using a gradient echo sequence (TR = 1900 ms; TE = 2.98 ms; FOV = 230 mm; matrix = 220 × 256; slice number: 160, spatial resolution of 0.9 × 0.9 × 0.9 mm). Functional images were obtained using a gradient EPI sequence that is sensitive to blood‐oxygen‐level‐dependent contrast (TR = 2500 ms, TE = 27 ms, FOV = 220 mm, matrix = 64 × 64 × 36, slice thickness = 4 mm. Each scan consisted of 240 image volumes). All subjects were instructed to stay awake and relax with their eyes closed during scan.
Voxel‐Based Morphometry Analysis
The VBM8 toolbox (Gaser, C., http://dbm.neuro.uni-jena.de/vbm/) was used to analyze all anatomical images. Preprocessing steps included bias‐field correction and segmentation into GM, white matter, and cerebrospinal fluid. Segmented images were registered to standard Montreal Neurological Institute (MNI) space. The GM images were then modulated by correcting non‐linear normalization wrap with non‐linear‐only modulation, while correcting for the different brain sizes (http://www.neuro.uni-jena.de/vbm/segmentation/modulation/). Finally, the normalized and modulated images were smoothed with an 8 mm Gaussian kernel. The final images can be regarded as relative gray matter volume with correction for the total intracranial volume (http://www.neuro.uni-jena.de/vbm/segmentation/modulation/). Based on these final processed images, voxel‐wised two‐sample T tests were conducted to compare left‐sided TN versus normal controls and right‐sided TN versus normal controls. Age and gender were included into these tests as covariates. Results from each pair were corrected for multiple comparisons. Family‐wise error with an initial voxel wise threshold of P < 0.05 was used.
Functional Images Processing and Analysis
Functional images were motion corrected, and co‐registered to the individual anatomical image using the AFNI package (http://afni.nimh.nih.gov/afni/). Six motion parameters, derivatives of each motion parameter, forward derivatives of each motion parameter and squared forward derivatives of each motion parameter were regressed out from all time‐series. The anatomical images were also partitioned into gray matter, white matter (WM), and CSF. Each subject's deformation map obtained from the anatomical image was applied to the functional images for normalization into the Montreal Neurological Institute (MNI) space with the voxel size of isotropic 3 mm. Five principal components from both CSF and WM were regressed out. A band pass filter ranging from 0.01 to 0.1 HZ was performed on all time‐series. Finally, the functional images were spatially smoothed by a 6 mm full‐width‐at‐half‐maximum kernel.
Based on the VBM analysis results, we generated spherical ROIs with an 6 mm radius on the peak coordinates of all abnormal regions for the left TN group and right TN group respectively (Fig. 4). The average time series were extracted from each ROI. The Pearson's correlation between the averaged time series from each ROI and the rest of brain were calculated. For each subject, a correlation map was obtained, then Fisher‐Z transformed. First of all, one sample t test is performed separately on both groups to determine the functional connectivity profile of this seed. Then to compare each patient group (left and right) to normal controls, a general linear model was built, with the functional connectivity strength as the dependent variable, the diagnosis as the predictor and the age and gender as covariates. The linear regression model was performed separately to address the differences between the left TN group and right TN group to normal controls. FSL's randomize function was used to conduct the permutation test based on the above models. The significant results were thresholded at FWE corrected P < 0.05.
Figure 4.
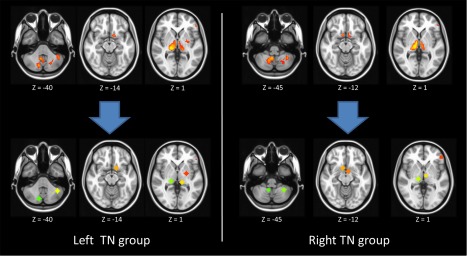
The pain related ROIs have been defined based on the VBM results. Two sets of ROIs were made for left TN and right TN separately. [Color figure can be viewed at http://wileyonlinelibrary.com]
Statistical Analysis
Statistical analysis tested GM volume differences between TN patients and healthy controls. Continuous variables, including age, duration of pain, and intensity of pain were expressed as mean ± standard deviation. The age of each group was compared by performing the analysis of variance (ANOVA). The difference of gender between groups was compared using the Pearson χ 2 test. To control for age‐ and gender‐related GM changes, age and gender were implemented into our statistical model as covariates. The relationship between duration of pain and GM volume of the significant clusters and functional connectivity were also explored. The correlations were estimated by Pearson's correlation coefficients. A P value of <0.05 was deemed as a significant correlation.
RESULTS
Demographics and clinical characteristics are summarized in Table 1. No difference in age (ANOVA, P = 0.193) or gender (χ 2, P = 0.197) was found in comparing right TN patient, left TN patient and healthy control groups. The duration of TN was 69.6 ± 75.4 months for right TN group and 63.2 ± 59.0 months for left TN group. The majority of pain distribution was in V2 and V3 divisions of the trigeminal nerve. The visual analogue scale (VAS) for pain of all patients ranged from 7 to 10, which has high subjective pain intensity.
Table 1.
Demographics and patient characteristics
| Number of patients | Healthy subjects (n = 19) | Right TN (n = 36) | Left TN (n = 26) | P value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 55.6 ± 8.2 | 58.0 ± 7.7 | 59.0 ± 6.6 | 0.193 |
| Gender (women/men) | 15/4 | 20/16 | 18/8 | 0.197 |
| Duration of TN, months | n.a. | 69.6 ± 75.4 | 63.2 ± 59.0 | |
| Pain location V1/V2/V3 (%) | n.a. | 11.1/72.2/80.6 | 15.4/73.1/84.6 | |
| Pain intensity (VAS) | n.a. | 9.3 ± 0.7 | 9.4 ± 0.9 |
VAS, visual analog scale; TN, trigeminal neuralgia.
Gray Matter Volume Changes
According to the two‐sample test results, GM volume decrease in the right TN subjects was found in (1) PFC, such as the superior and inferior frontal gyrus, (2) precentral gyrus, (3) cerebellar tonsil, and (4) subcortical regions, such as the thalamus, hypothalamus, and nucleus accumbens (Fig. 1 and Table 2). For patients with left TN, GM volume decrease was found in (1) PFC, such as inferior frontal gyrus, (2) precentral gyrus, (3) cerebellum, such as tonsil, pyramis, and culmen, and (4) subcortical regions, such as the thalamus, ventral striatum, and putamen (Fig. 2 and Table 2). We further calculated the absolute gray matter volume in milliliter (ml) by multiplying the relative gray matter volume by the size of image voxel (1.5 × 1.5 × 1.5 mm) and then divided by 1000.
Figure 1.
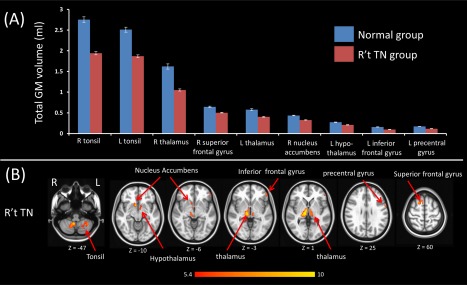
The VBM comparison of right side TN vs controls. (A) Bar plot demonstrates the grey matter volume of each cluster, which are significantly different between right TN and controls and (B) illustrates the anatomic locations of the GM volume differences. There are significant volume reductions among patients with right side TN in the superior, inferior frontal, and precentral gyrus; cerebellar tonsil, thalamus, hypothalamus, and nucleus accumbens. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Regions of reduced gray matter volume in patients with trigeminal neuralgia compared to healthy controls
| Anatomical region | Cluster size | MNI coordinates (MM) | Side | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Patient with right trigeminal neuralgia | |||||
| Cerebellar tonsil | 1,466 | 8 | −51 | −46 | R |
| Cerebellar tonsil | 1,353 | −34 | −51 | −45 | L |
| Thalamus, ventral posterior nucleus | 1,143 | 14 | −21 | 0 | R |
| Superior frontal gyrus | 411 | 20 | 13 | 60 | R |
| Thalamus, medial dorsal nucleus | 341 | −6 | −10 | 3 | L |
| Nucleus accumbens | 182 | 9 | 10 | −12 | R |
| Hypothalamus | 119 | −6 | 0 | −10 | L |
| Inferior frontal gyrus | 108 | −50 | 40 | −3 | L |
| Precentral gyrus | 106 | −58 | 6 | 27 | L |
| Patient with left trigeminal neuralgia | |||||
| Cerebellum, pyramis | 1,024 | 10 | −74 | −38 | R |
| Thalamus, pulvinar | 1,018 | 18 | −26 | 2 | R |
| Cerebellar tonsil | 457 | −27 | −60 | −45 | L |
| Cerebellum culmen | 398 | −40 | −52 | −39 | L |
| Thalamus, pulvinar | 336 | −14 | −28 | 2 | L |
| Ventral striatum | 297 | −12 | 9 | −14 | L |
| Precentral gyrus | 231 | −60 | 3 | 27 | L |
| Putamen | 202 | −24 | −6 | 0 | L |
| Inferior frontal gyrus | 171 | −51 | 34 | 6 | L |
Figure 2.
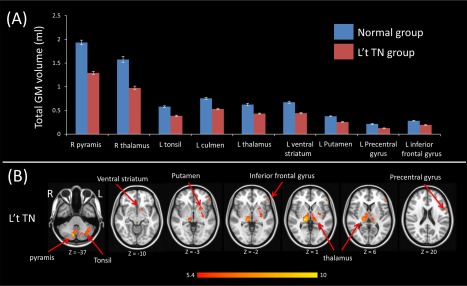
The VBM comparison of left side TN vs controls. (A) Bar plot demonstrates grey matter volume of each cluster, which are significantly different between left TN and controls. (B) Illustrative anatomic locations of the GM volume differences. There are significant volume reductions among patients with left side TN in the cerebellar tonsil, pyramis, culmen; inferior frontal gyrus, precentral gyrus, thalamus, ventral striatum, and putamen. [Color figure can be viewed at http://wileyonlinelibrary.com]
Functional Connectivity Changes
To estimate the functional connectivity changes, we utilized the results of VBM to derive several separate ROIs for left and right TN (Fig. 4). Based on those ROIs, we performed the seed‐based correction to estimate the functional connectivity between ROIs and the whole brain. The results were summarized in Table 3. As compared to the healthy controls, patients with right TN showed significantly lower spatial extension of the motor network and lower connections of the right superior frontal gyrus with the right middle frontal gyrus (FWE corrected P < 0.05; Fig. 5 and Table 3). Patients with left TN showed lower spatial extension of the salient network and lower connection between the left precentral gyrus with the left superior frontal gyrus (FWE corrected P < 0.05; Fig. 6 and Table 3). On the contrary, there was significantly higher connection between the pulvinar of bilateral thalamus (FEW corrected P < 0.05; Fig. 7 and Table 3).
Table 3.
Significant differences in network connectivity between patients with trigeminal neuralgia compared and healthy controls
| Connectivity change | Seed region | Connection | Cluster size | MNI coordinates (MM) | T value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Patient with right trigeminal neuralgia | |||||||
| Lower connectivity | Right superior frontal gyrus | Right middle frontal gyrus | 62 | 42 | 32 | 51 | 6.85 |
| Patient with left trigeminal neuralgia | |||||||
| Higher connectivity | Right thalamus, pulvinar | Left thalamus, pulvinar | 61 | −21 | −24 | 9 | 4.79 |
| Lower connectivity | Left precentral gyrus | Left superior frontal gyrus | 118 | −6 | −6 | 69 | 5.08 |
Figure 5.
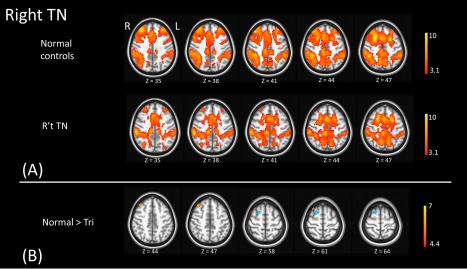
The panel A illustrates the functional connectivity map of right superior frontal gyrus (the seed) in normal controls and right TN patients. The seed region (blue) is shown in the panel B. The connection between right superior gyrus (the seed) and right middle frontal gyrus (warm color) is significantly higher in normal controls than in right TN patients. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
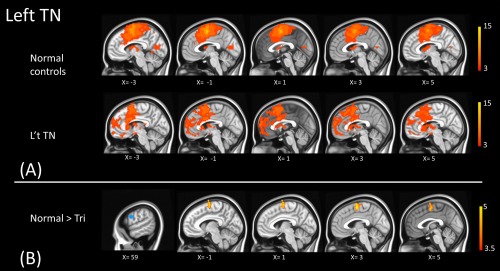
The panel A illustrates the functional connectivity map of the left precentral gyrus (the seed) in normal controls and left TN patients. The seed region (blue) is shown at the first column of the panel B. The significant group differences are shown in the panel B (warm color). The connection between left precentral gyrus (the seed) and left superior frontal gyrus is higher in normal controls than in left TN patients. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 7.
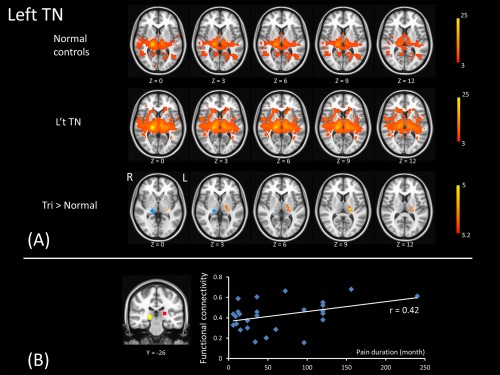
The upper two rows of panel A illustrate the functional connectivity map of the right pulvinar (the seed, shown in the lower row) in normal controls and left TN patients. For each subject, the functional connectivity map of this seed region was measured. The voxel‐wise two‐sample t test results reveal a higher connection of bilateral pulvinar in the patient group than in normal controls (the bottom row of panel A). The panel B illustrates the connectivity between bilateral pulvinar in left TN and pain duration is positively correlated. [Color figure can be viewed at http://wileyonlinelibrary.com]
Brain Structure and Connectivity That are Correlated With Pain Duration
Within the left TN group, moderate correlation between GM volume in the left ventral striatum and pain duration (r = 0.346, P = 0.084) was observed (Fig. 3). Within the right TN group, the GM volume of the ventral posterior nucleus right thalamus and the medial dorsal nucleus of left thalamus showed moderate correlation with pain duration (r = 0.362, P = 0.039 and r = 0.373, P = 0.032, respectively). Both failed to pass multiple comparisons (Fig. 3). Three outliers with pain duration exceeded the value of 1.5× interquartile range above third quartile or outside 3 SD were excluded.
Figure 3.
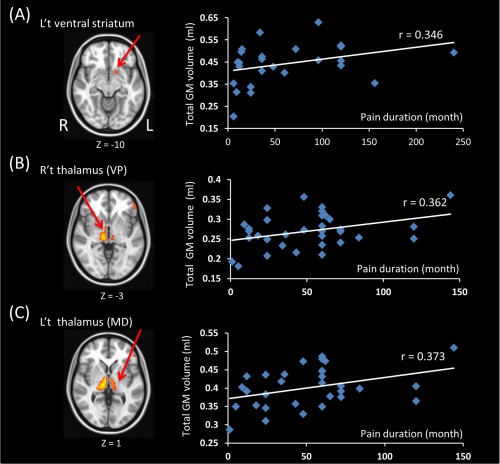
The correlation between total GM volume in each cluster derived from VBM results and pain duration. (A) In left TN patients, the volume of left ventral striatum has a moderate correlation with pain duration but do not reach statistically significance. (B,C) In right TN patients, 3 outliers which have very long pain duration were removed. The GM volume in the ventral posterior nucleus of right thalamus and pain duration are positively correlated. The medial dorsal nucleus of left thalamus and pain duration are also positively correlated. [Color figure can be viewed at http://wileyonlinelibrary.com]
The connectivity between pulvinar of bilateral thalamus observed in patients with left TN showed significant correlation with pain duration (r = 0.42, P = 0.032) (Fig. 7). Other networks that are significantly different between left TN and normal subjects did not have any trend to be correlated with pain duration.
DISCUSSION
Over the past decade, many human neuroimaging studies have led to the characterization of a network of brain regions forming a “pain matrix,” including the thalamus, basal ganglia, SMC, insular cortex, ACC, and PFC. These studies have shown that acute noxious stimuli activate brain regions which are thought to code location and intensity of pain, such as SMC, and emotional and motivational aspects of pain such as the insula, ACC, and PFC [Apkarian et al., 2005; Bushnell et al., 2013; Duerden and Albanese, 2013; Legrain et al., 2011]. On the contrary, reports from studies of chronic pain showed more heterogeneous changes in structure and function of these brain regions. The volume of GM involved in pain processing was decreased in most studies but increased volume of various brain regions was also frequently reported [Bushnell et al., 2013; Cauda et al., 2014; Smallwood et al., 2013; Schmidt‐Wilcke, 2015]. Understanding the mechanism that leads to pain chronification is important for treatments aimed at reversing cortical reorganization [Gustin et al., 2012; Hashmi et al., 2013]. In this study, GM volume reduction in chronic TN patients were found in brain regions that are known to be associated with the pain perception and modulation like the PFC, precentral gyrus, cerebellum, and the subcortical structures such as the thalamus, hypothalalmus, putamen, and nucleus accumbens. Up to now, only few neuroimaging studies have focused on the structural and functional changes of brain in chronic TN and the reported results were heterogeneous. Gustine et al. [2011] first reported that in patients of chronic orofacial pain, there was GM volume reduction in the SMC, anterior insula, putamen, nucleus accumbens, and thalamus, whereas GM volume was increased in the posterior insula. Only 8 out of 21 patients in that study were classical TN and the GM changes were not found in this subgroup alone, probably due to lack of statistical power. Obermann et al. [2013] reported similar results of GM volume reduction in TN patients in the SMC, orbitofrontal cortices, thalamus, insula, ACC, cerebellum, and dorsolateral PFC. DeSouza et al. [2013] observed chronic TN patient had increased GM volume in the sensory thalamus, amygdala, periaqueductal GM, putamen, caudate, and nucleus accumbens. As studies of other chronic pain show, not all brain regions of the pain matrix showed significant change in studies of TN, generally owing to differences in sample size, image acquisition, postprocessing, and other methodological factors [Obermann et al., 2013]. These studies, and our results, suggest that GM volume change in patients with TN is similar to other causes of chronic pain. The change of GM volume in patients with chronic classical TN may be related to the perception and processing of pain but not specific for this disorder.
The changes of GM volume in chronic pain patients may reflect the cortical plasticity arising from a long‐term, repeated nociceptive input, pain modulation, and compensatory motor response to avoid triggering pain [Peck et al., 2008; Rodriguez‐Raecke et al., 2009]. However, it is not well understood if the GM volume changes in patients with TN are a consequence of pain or a predisposing factor that make patients more susceptible to development of TN [Obermann et al., 2013]. Although it is widely accepted that a neurovascular contact in the cisternal segment of the trigeminal nerve is the primary cause of classical TN, neurovascular contact alone does not necessarily cause the disease [Headache Classification Committee of the International Headache Society (IHS), 2013; Hamlyn and King, 1992]. Previous studies reported highly varying prevalence of neurovascular contact in the general population. Maarbjerg et al. [2015] reported that only severe neurovascular contact with displacement or atrophy of the trigeminal nerve is associated with classic TN while simple neurovascular contact is common among asymptomatic patients or normal population. In addition to trigeminal root compression, pathology in the dorsal horns and trigeminal ganglion is also important for developing TN [Devor et al., 2002]. Activation of the second order wide dynamic range neurons in lamina V of the dorsal horns and the trigeminal ganglion due to hypersensitivity of tactile A‐beta fibers could promote the perception of pain in response to non‐noxious touch stimuli via convergent information from both tactile (A‐beta) and nociceptive (A‐delta and C) fibers [Devor et al., 2002; Obermann et al., 2013]. Interestingly, in this study, the GM volume in the ventral posterior nucleus of right thalamus and medial dorsal nucleus of left thalamus are smaller in chronic right TN patients but progressively enlarges with pain duration. The thalamus plays a critical role in chronic pain processing. Reduced thalamic volume and biochemical changes indicating neural loss and reduced inhibitory neurotransmitter content have been observed in patients of neuropathic pain [Apkarian et al., 2004; Gustin et al., 2011; Henderson et al., 2013]. This can result in more frequent bursting activity of thalamic neurons and in development of thalamocortical dysrhythmia [Gerke et al., 2003; Schmidt‐Wilcke, 2015]. Henderson et al. reported significant negative correlations between the connectivity of somatosensory thalamus with multiple cortical regions and thalamic inhibitory neurotransmitter (GABA) content in patients of trigeminal neuropathy [Henderson et al., 2013]. They concluded that initial loss of neurons in the thalamus results in a loss of excitatory inputs to the thalamic reticular nucleus, which in turn leads to reduced GABAergic content of the thalamus. This alters thalamic firing patterns and thalamocortical rhythm, and may result in the constant perception of pain. Based on our data and in the light of previous research, we assume that a relatively smaller volume of thalamus in patients of TN may represent an impaired ability of the thalamus in procession and modulation of neuropathic pain signals. This makes patients more susceptible to the development of TN. Along with the duration of the disease, the gradually increased volume of the thalamus might be due to its role in motor and psychological responses of chronic pain or in response to pain medications, which usually are antiepileptic agents modulating sodium channels and GABA receptors. Longitudinal investigations on TN patients with an analysis of substructure of thalamus and thalamus‐associated networks would add further evidence in this observation.
For the functional connectivity analysis, we utilized ROIs derived from VBM results as seed regions and performed seed‐based correlation with the while brain. Results of two‐sample t tests showed differences in the salient network for right TN patients and motor network for left TN patients (Figs. 5 and 6). The salient network, including the anterior insula and midcingulate cortex, is responsible for integrating information of an impending stimulation into perceptual decision‐making in the context of pain [Wiech et al., 2010]. The noxious stimuli can trigger the salience network that ensures prioritized processing through connections with the cognitive and emotional control networks [Wiech and Tracey, 2013]. Sensory‐motor integration at a reflex such as a motor withdrawal reflex in response to noxious stimuli is well understood [Borsook, 2007]. Interactions between pain and motor systems have long been integrated in several therapeutic approaches such as physical therapy or exercise or providing pain relief to enhance motor activity in conditions such as the back pain or postoperative pain.
Results of the functional connectivity analysis have shown that lower connection between components of ipsilateral PFC in patients with right TN. The PFC has been shown to be activated by pain stimuli and also has clear implications in pain perception and modulation [Schmidt‐Wilcke, 2015]. The PFC is one of the key regions in affective, cognitive, and emotional aspects of pain, may be able to modulate cortico‐subcortical and cortico‐cortical pathways [Lorenz et al., 2003] with inhibitory control of nociceptive transmission system [Fierro et al., 2010]. On the other hand, the dorsolateral PFC is a target of repetitive transcranial magnetic stimulation (rTMS) for pain control [Brighina et al., 2011; Mariano et al., 2015]. Results from left TN patients showed lower connectivity between the left precentral gyrus and ipsilateral superior frontal gyrus. The primary motor cortex (M1) is the key driver of motor output and may contribute to motor response or movement dysfunction in pain [Chang et al., 2015]. An fMRI study also showed an increased activation of M1 with movement of the affected hand in the complex regional pain syndrome [Maihöfner et al., 2007]. Studies of patients with fibromyalgia showed increased connectivity between M1 and supplementary motor area [Cifre et al., 2012], and the reduction of GABAergic and glutamatergic M1 function is associated with fatigue [Mhalla et al., 2010]. Schwenkreis et al. [2010] reported a close correlation between M1 disinhibition and chronic neuropathic pain. Youssef et al. [2014] reported the blood flow increased in motor‐related regions in patients of non‐neuropathic pain but not neuropathic pain. M1 is also a target for chronic pain therapy with rTMS; and stimulation of M1 can induce long‐lasting, potentially therapeutic brain plasticity [Klein et al., 2015]. In a study of capsaicin‐induced pain, dorsolateral PFC rTMS was able to revert the effect of pain on motor cortex [Fierro et al., 2010]. Along with decreased GM volume in the precentral gyrus detected by VBM, we assumed that structural changes in the M1 region may result in disinhibition of M1 activity and consequently alter cortical connectivity with M1. This may be associated with brain plasticity and behavior responses in chronic pain. As M1 is a potential target for pain control and cortical disinhibition may be a specific feature of chronic neuropathic pain [Schwenkreis et al., 2010], further investigations are necessary to understand how alterations of M1 connectivity will effect pain related psychological and behavioral responses and how these alterations can be modulated by transcranial magnetic stimulation.
Interestingly, patients with left TN showed higher connectivity between pulvinar of bilateral thalamus; and the connectivity has an increasing trend with the duration of pain. Thalamus plays a key role in processing of nociceptive stimuli by projection of nociceptive input from the thalamus to SMC for detection of the quality (such as stinging, burning, or aching), location, intensity, and duration of pain. It also delivers the nociceptive information to ACC and insula for encoding the emotional and motivational aspects of pain [Bushnell et al., 2013]. It has been shown that chronic pain disrupts thalamo‐cortical connections [Cauda et al., 2014; Jensen et al., 2012]. Pulvinar is the largest of thalamic nuclei and consists of several divisions. It is an association nucleus in the posterolateral portion of the thalamus that has reciprocal connections with large areas of the frontal, parietal, and occipital cortex [Yuan et al., 2015]. Stereotactic radiofrequency lesioning of the pulvinar (pulvinotomy) has been used to alleviate chronic neuropathic or cancer pain without the production of motor or sensory deficits [Yoshii et al., 1980; Yoshii and Fukuda, 1979]. In a magnetic resonance spectroscopy study, Wang et al. [2014] reported a lower N‐acetylaspartate to creatine ratio in the affected side of pulvinar in idiopathic trigeminal neuralgia patient suggesting the existence of thalamic neural dysfunction or loss. Lesions developing at or near the anterior pulvinar nucleus are reported to be associated with thalamic pain [Vartiainen et al., 2016]. Moreover, the anterior pulvinar is a target of spinothalamic afferents, and electrical stimulation of the anterior pulvinar can evoke thermal and painful sensation [Lenz et al., 2010, 1993]. These studies, together with our results of small volume of pulvinar from VBM and higher connections between bilateral pulvinar, we assume that a reduction or dysfunction of pulvinar nucleus will trigger (or lost inhibition) the processing of pain signal and make people more vulnerable to develop chronic TN. The internuclear connections of pulvinar and other thalamic nuclei may play a role in perception and modulation of pain.
In this study, the gray matter volume of brain regions involved in acute nociceptive stimuli, including SMC, insula, and ACC were not significantly different between patients and healthy subjects. In a longitudinal study of back pain by Hashmi et al., the brain activity for back pain in acute back pain patients is confined to regions involved in the acute pain, whereas in the chronic pain patients, brain activity is confined to emotion‐related networks. Brain activity diminished in time with patients who recovered from acute back pain; whereas in patients with persistent back pain, brain activity diminished in acute pain regions but increased in emotion‐related network [Hashmi et al., 2013]. Gustin et al. [2012] reported cortical reorganization and structural change in SMC in patients with chronic neuropathic pain but not in patients with non‐neuropathic chronic pain. They concluded that the key factor for the SMC to undergo reorganization is when there is a constant painful stimulus and S1 input, instead of subtype of pain. Patients with painful trigeminal neuropathy were categorized as a neuropathic pain group in that study. Painful trigeminal neuropathy is the damage of trigeminal nerve caused by other disorders, and characterized as episodic sharp and shooting pains combined with constant or long episodes of background aching and burning pain [Gustin et al., 2012; Headache Classification Committee of the International Headache Society (IHS), 2013]. On the contrary, a patient with classical TN, as enrolled in our study, is characterized by the episodic sharp and shooting pain without constant background stimuli. Our results are consistent with their observations that long‐standing persistent pain stimuli may induce gray matter volume changes among brain regions of acute pain perception whereas classical TN, episodic pain stimulation, may lead to changes only in brain regions that are involving in emotional or cognitive modulation.
For the functional connectivity analysis, we utilized ROIs derived from VBM results as seed regions. By doing so, we were able to estimate how functional connectivity changes of those regions along with GM volume loss. As we mentioned above, it has not been well understood if the GM volume changes in patients with TN are a consequence of pain or a predisposing factor that make patients more susceptible to the development of TN [Obermann et al., 2013]. Smaller GM volume in patients of TN, as shown in this study, may represent an impaired ability in procession and modulation (loss of inhibition or altered thalamic firing) of neuropathic pain signals[Alshelh et al., 2016; Henderson et al., 2013]. On the other hand, central sensitization or use‐dependent synaptic plasticity can occur when there is the sustained or repetitive activation of nociceptive afferent fibers [DeSouza et al., 2016; Woolf, 2010]. The GM or neurons change the gain of their response to sensory input such that they have amplified responses to noxious stimuli. This change is triggered by intracellular cascades, which lead to facilitated excitatory transmission and depressed inhibition [Woolf, 2010]. This may be the reason of altered connectivity between the regions of GM loss and other brain regions.
In this study, 58% patients suffered from right TN, which is slightly more than left TN. This is in line with previous studies [Bangash, 2011; Mueller et al., 2011] and suggests that TN behaves quite differently to other painful disorders that generally assumed to be more common on left side of body [Merskey and Watson, 1979]. Hypothesis for pain lateralization is that right and left hemispheres differ in their abilities to integrate and discriminate sensory inputs [Merskey and Watson, 1979]. However, this hypothesis was not supported by studies attended to confirm these findings, or by numerous neuroimaging studies of pain. Thus the theory of pain lateralization is not sustained [Campbell et al., 1985; Obermann, 2012]. In this study, we analyzed the GM volume and connectivity changes for patients with right and left TN separately. In general, although there is no significant GM volume difference between left and right TN patients, it seems that regions of GM volume loss were more limited in ipsilateral hemisphere among patients with left TN, while it was distributed in bilateral hemisphere among patients with right TN (Table 2). Moreover, the functional connectivity between pulvinar or bilateral thalamus was higher in left‐sided TN patients (Table 3). Based on these results, it is possible that left hemisphere is dominant in processing and modulation of TN pain signal and the left precentral gyrus and the left thalamus may play a key role in interhemispheric signal transduction. Further investigation with more solid scientific evidence is needed to support these assumptions.
We recognize some limitations in this study. The pain characteristics, such as pain intensity and pain distribution (V1, V2, or V3 of trigeminal nerve), were mixed in one patient group. Furthermore, TN is known to exhibit substantial variability in physical and cognitive symptoms. This heterogeneity might be translated into structural and functional brain phenomenology and thus limits the precision and specificity of the interpretation of generalization. We noted that this is a common issue in studies of TN but it is important for a better understanding of the specific orientation and lateralization of brain structure and function in terms of chronic pain. Studies with large characterized samples and many common features of TN symptomatology are necessary. Second, this is a cross‐sectional study without a longitudinal imaging or clinical data collected. Although common features have been presented in the literatures as well as in this study, the plasticity of brain should be different in each individual and it is important in studying brain modulation in the chronic pain population.
In conclusion, the TN is due to neurovascular compression of the trigeminal nerve and can result in structural and functional changes of brain. The left hemisphere may be dominant in processing and modulation of TN pain signal, but this needs further investigations. Brain regions, especially the thalamus, are not only involved in processing of pain stimuli but also important for the development of classical TN, and may help us to estimate the risk of developing classical TN in patients with vascular compression of the trigeminal nerve. Our findings also demonstrate that chronification of this specific type of neuropathic pain induces changes in GM volume of brain regions involved in the emotional or cognitive modulation, and is different from that of painful trigeminal neuropathy and other non‐neuropathic pain. These results have important clinical implications regarding the etiology of TN and the cortical plasticity which can be the target of treatments aiming at reversing cortical reorganization.
ACKNOWLEDGMENTS
The authors thank Hsueh‐Lin Wang for assisting with this study.
REFERENCES
- Alshelh Z, Di Pietro F, Youssef AM, Reeves JM, Macey PM, Vickers ER, Peck CC, Murray GM, Henderson LA (2016): Chronic neuropathic pain: It's about the rhythm. J Neurosci 36:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian a. V, Bushnell MC, Treede R‐D, Zubieta J‐K (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Apkarian a. V, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR (2004): Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24:10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangash TH (2011): Trigeminal neuralgia: Frequency of occurrence in different nerve branches. Anesthesiol Pain Med 1:70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D (2006): Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci 26:10646–10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Nennig E, Sarpaczki E, Reinhardt J, Schlieter M, Herweh C, Rasche D, Tronnier VM, Sartor K, Stippich C (2009): Altered somatosensory processing in trigeminal neuralgia. Hum Brain Mapp 30:3495–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D (2007): Pain and motor system plasticity. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, Panetta M, Giglia G, Fierro B (2011): Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain 12:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low L. a (2013): Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, Lahuerta J, Bowsher D (1985): Pain laterality in relation to site of pain and diagnosis. Pain 23:61–66. [DOI] [PubMed] [Google Scholar]
- Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DME (2014): Gray matter alterations in chronic pain: A network‐oriented meta‐analytic approach. NeuroImage Clin 4:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, O'Connell NE, Burns E, Chipchase LS, Liston MB, Schabrun SM (2015): Organisation and function of the primary motor cortex in chronic pain: Protocol for a systematic review and meta‐analysis. BMJ Open 5:e008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez‐Roldan A, Martinez‐Jauand M, Birbaumer N, Chialvo DR, Montoya P (2012): Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med 74:55–62. [DOI] [PubMed] [Google Scholar]
- DeSouza DD, Hodaie M, Davis KD (2014): Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain 155:37–44. [DOI] [PubMed] [Google Scholar]
- DeSouza DD, Hodaie M, Davis KD (2016): Structural magnetic resonance imaging can identify trigeminal system abnormalities in classical trigeminal neuralgia. Front Neuroanat 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouza DD, Moayedi M, Chen DQ, Davis KD, Hodaie M (2013): Sensorimotor and pain modulation brain abnormalities in trigeminal neuralgia: A paroxysmal, sensory‐triggered neuropathic pain. PLoS One 8:e66340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Amir R, Rappaport ZH (2002): Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin J Pain 18:4–13. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Albanese MC (2013): Localization of pain‐related brain activation: A meta‐analysis of neuroimaging data. Hum Brain Mapp 34:109–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F (2010): Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin‐induced pain: Modulatory effects on motor cortex excitability. Exp Brain Res 203:31–38. [DOI] [PubMed] [Google Scholar]
- Gerke MB, Duggan AW, Xu L, Siddall PJ (2003): Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience 117:715–722. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA (2012): Pain and plasticity: Is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci 32:14874–14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA (2011): Different pain, different brain: Thalamic anatomy in neuropathic and non‐neuropathic chronic pain syndromes. J Neurosci 31:5956–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn PJ, King TT (1992): Neurovascular compression in trigeminal neuralgia: A clinical and anatomical study. J Neurosurg 76:948–954. [DOI] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV (2013): Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136:2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS) (2013): The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33:629–808. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Peck CC, Petersen ET, Rae CD, Youssef AM, Reeves JM, Wilcox SL, Akhter R, Murray GM, Gustin SM (2013): Chronic pain: Lost inhibition? J Neurosci 33:7574–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D. a, Love S, Gradidge T, Coakham HB, Hinton DR, Jannetta PJ (1994): Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery 35:299–303. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SCR, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J (2012): Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Treister R, Raij T, Pascual‐Leone A, Park L, Nurmikko T, Lenz F, Lefaucheur J‐P, Lang M, Hallett M, Fox M, Cudkowicz M, Costello A, Carr D, Ayache S, Oaklander A (2015): Transcranial magnetic stimulation of the brain: Guidelines for pain treatment research. Pain [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T (2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A (2011): The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol 93:111–124. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Casey KL, Jones EG, Willis WD (2010): The Human Pain System: Experimental and Clinical Perspectives.
- Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH (1993): Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 70:200–212. [DOI] [PubMed] [Google Scholar]
- Lin C (2014): Brain signature of chronic orofacial pain: A systematic review and meta‐analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One 9:e94300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL (2003): Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 126:1079–1091. [DOI] [PubMed] [Google Scholar]
- Love S, Coakham HB (2001): Trigeminal neuralgia: Pathology and pathogenesis. Brain 124:2347–2360. [DOI] [PubMed] [Google Scholar]
- Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L (2015): Significance of neurovascular contact in classical trigeminal neuralgia. Brain 138:1–9. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J (2007): The motor system shows adaptive changes in complex regional pain syndrome. Brain 130:2671–2687. [DOI] [PubMed] [Google Scholar]
- Mariano TY, Van't Wout M, Garnaat SL, Rasmussen SA, Greenberg BD (2015): Transcranial direct current stimulation (tDCS) targeting left dorsolateral prefrontal cortex modulates task‐induced acute pain in healthy volunteers. Pain Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, Watson GD (1979): The lateralisation of pain. Pain 7:271–280. [DOI] [PubMed] [Google Scholar]
- Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D (2010): Alteration of cortical excitability in patients with fibromyalgia. Pain 149:495–500. [DOI] [PubMed] [Google Scholar]
- Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, Calvino B, Bouhassira D (2011): Functional brain imaging of trigeminal neuralgia. Eur J Pain 15:124–131. [DOI] [PubMed] [Google Scholar]
- Mueller D, Obermann M, Yoon M‐S, Poitz F, Hansen N, Slomke M.‐a, Dommes P, Gizewski E, Diener H‐C, Katsarava Z (2011): Prevalence of trigeminal neuralgia and persistent idiopathic facial pain: A population‐based study. Cephalalgia 31:1542–1548. [DOI] [PubMed] [Google Scholar]
- Nurmikko TJ, Eldridge PR (2001): Trigeminal neuralgia–pathophysiology, diagnosis and current treatment. Br J Anaesth 87:117–132. [DOI] [PubMed] [Google Scholar]
- Obermann M (2010): Treatment options in trigeminal neuralgia. Ther Adv Neurol Disord 3:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M (2012): Pain lateralization in trigeminal neuralgia. Anesthesiol Pain Med 2:46–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M, Rodriguez‐Raecke R, Naegel S, Holle D, Mueller D, Yoon M‐S, Theysohn N, Blex S, Diener H‐C, Katsarava Z (2013): Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 74:352–358. [DOI] [PubMed] [Google Scholar]
- Peck CC, Murray GM, Gerzina TM (2008): How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust Dent J 53:201–207. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Raecke R, Niemeier A, Ihle K, Ruether W, May A (2009): Brain gray matter decrease in chronic pain is the consequence and not the cause of pain.(osteoarthritis). J Neurosci 29:13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Wilcke T (2015): Neuroimaging of chronic pain. Best Pract Res Clin Rheumatol 29:29–41. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Scherens A, Rönnau A, Höffken O, Tegenthoff M, Maier C (2010): Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivani S, Wallin D, Moulton E. a, Cole S, Wasan AD, Lockerman L, Bajwa Z, Upadhyay J, Becerra L, Borsook D (2010): A fMRI evaluation of lamotrigine for the treatment of trigeminal neuropathic pain: Pilot study. Pain Med 11:920–941. [DOI] [PubMed] [Google Scholar]
- Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt‐Wilcke T, Farrell MJ, Eickhoff SB, Robin DA (2013): Structural brain anomalies and chronic pain: A quantitative meta‐analysis of gray matter volume. J Pain 14:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen N, Perchet C, Magnin M, Creac'h C, Convers P, Nighoghossian N, Mauguière F, Peyron R, Garcia‐Larrea L (2016): Thalamic pain: Anatomical and physiological indices of prediction. Brain 139:708–722. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li D, Bao F, Ma S, Guo C, Jin C, Zhang M (2014): Thalamic metabolic alterations with cognitive dysfunction in idiopathic trigeminal neuralgia: A multivoxel spectroscopy study. Neuroradiology 56:685–693. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin C, Brodersen KH, Bingel U, Ploner M, Tracey I (2010): Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Tracey I (2013): Pain, decisions, and actions: A motivational perspective. Front Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2010): Neuronal plasticity: Increasing the gain in pain. Science (80‐) 288:1765–1768. [DOI] [PubMed] [Google Scholar]
- Yang J, Lin M, Lee M, Weng H‐H, Liao H‐H (2010): Guided by computerized tomography with three‐dimensional image reconstruction. Chang Gung Med J 33:679–683. [PubMed] [Google Scholar]
- Yoshii N, Fukuda S (1979): Effects of unilateral and bilateral invasion of thalamic pulvinar for pain relief. Tohoku J Exp Med 127:81–84. [DOI] [PubMed] [Google Scholar]
- Yoshii N, Mizokami T, Ushikubo T, Kuramitsu T, Fukuda S (1980): Long‐term follow‐up study after pulvinotomy for intractable pain. Appl Neurophysiol 43:128–132. [DOI] [PubMed] [Google Scholar]
- Youssef AM, Gustin SM, Nash PG, Reeves JM, Petersen ET, Peck CC, Murray GM, Henderson L. a (2014): Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder. Pain 155:467–475. [DOI] [PubMed] [Google Scholar]
- Yuan R, Di X, Taylor PA, Gohel S, Tsai Y‐H, Biswal BB (2015): Functional topography of the thalamocortical system in human. Brain Struct Funct [DOI] [PMC free article] [PubMed] [Google Scholar]


