Abstract
Trauma to the spinal cord rarely results in complete division of the cord with surviving nerves sometimes remaining silent or failing to function normally. The term motor or sensory discomplete has been used to describe this important but unclassified subgroup of complete SCI. Importantly, silent motor or sensory pathways may contribute to aversive symptoms (spasticity, pain) or improved treatment success. To demonstrate more objectively the presence of subclinical preserved somatosensory pathways in clinically complete SCI, a cross‐sectional study using functional MRI (fMRI) was undertaken. The presence of brain activation following innocuous brushing of an insensate region below‐injury (great toe) was analyzed in 23 people (19 males (83%), mean ± SD age 43 ± 13 years) with clinically complete (AIS A) SCI with (n = 13) and without (n = 10) below‐level neuropathic pain and 21 people without SCI or pain (15 males (71%); mean ± SD age 41 ± 14 years). Location appropriate, significant fMRI brain activation was detected in 48% (n = 11/23) of subjects with clinically complete SCI from below‐injury stimulation. No association was found between the presence of subclinical sensory pathways transmitting innocuous mechanical stimuli (dorsal column medical lemniscal) and below‐level neuropathic pain (χ 2 = 0.034, P = 0.9). The high prevalence of sensory discomplete injuries (∼50% complete SCI) strengthens the case to explore inclusion of this category into the international SCI taxonomy (ISNCSCI). This would ensure more widespread inclusion of discomplete SCI in ongoing pain and motor recovery research. Neurophysiological tests such as fMRI may play a role in this process. Hum Brain Mapp 39:588–598, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: classification, magnetic resonance imaging, neuralgia, somatosensory cortex, spinal cord injury
INTRODUCTION
The International Standard for Neurological Classification of Spinal Cord Injury (ISNCSCI) distinguishes between two types of functional impairment: complete and incomplete based on sparing of the sacral segments (S4–5) [Kirshblum et al., 2011]. Categorizing people into these two groups relies on a standardized examination and assists in predicting recovery [Waters et al., 1991]. Clinical examination however has limited sensitivity to detect partially preserved motor and sensory spinal cord pathways [Ellaway et al., 2007; Nicotra and Ellaway, 2006] and therefore poses problems for more detailed research assessing spinal cord impairment especially with complete injuries [Kirshblum et al., 2014].
Post‐mortem human and animal studies demonstrate that the spine is rarely completely severed following blunt trauma [McKay et al., 2004]. Indeed, central neural tissue connectivity has been shown in postmortem histological studies in clinically complete injuries in over 50% of cases [Bunge et al., 1993; Kakulas, 1999]. This has led to the term discomplete spinal cord injury (SCI) where no clinical evidence of fiber tract function is detectable however residual fibers possessing the potential to influence perception and function remain [motor: Dimitrijevic, 1987; Sherwood et al., 1992; sensory: Awad et al., 2015; Finnerup et al., 2004; Sabbah et al., 2002; Wasner et al., 2008].
Preserved spinal cord pathways may contribute to positive or negative health outcomes. Partially preserved motor pathways may assist with efforts to improve motor function or be associated with spasticity [Sherwood et al., 1992]. In addition, the presence of partially preserved sensory pathways may affect both ascending and descending modulatory systems and contribute to the development of pain [Cruz‐Almeida et al., 2012; Finnerup et al., 2004; Wasner et al., 2008; Widerström‐Noga et al., 2015] or provide input to avoid pressure area breakdown [Awad et al., 2015]. The presence of residual spinothalamic tract function has been proposed as an important determinant of below‐level neuropathic (central) pain following SCI [Wasner et al., 2008; Widerström‐Noga et al., 2015].
Various techniques have been used to supplement clinical examination to assess the integrity of sensory spinal pathways. Somatosensory evoked potentials (SSEPs) have been used [Finnerup et al., 2004; Sherwood et al., 1992]; however, their sensitivity to detect partially preserved spinal cord function appears extremely low (no SSEPs in 15 complete SCI subjects of which 8 had discomplete injuries) [Finnerup et al., 2004]. While psychophysical techniques such as Quantitative Sensory Testing (QST) have confirmed sensory continuity in up to 50% of people with complete injuries [Finnerup et al., 2004], the ability to demonstrate brain activation linked to stimulation would more objectively establish the presence of preserved pathways.
In a previous case series (AIS A: n = 8, AIS B: n = 1) [Sabbah et al., 2002] and case report (AIS A) [Awad et al., 2015], functional magnetic resonance imaging (fMRI) has been used to demonstrate partially preserved sensory spinal cord pathways in SCI. Both of these pilot studies raise the possibility that fMRI may be a useful technique to assess spinal cord pathway preservation. However, larger controlled studies are required to provide further objective evidence of partially preserved somatosensory preservation. While a consensus approach to identify preserved pathways would need to be determined [Krishna et al., 2014], the inclusion of discomplete SCI subgroups (motor and sensory) in the international SCI taxonomy (ISNCSCI) [Kirshblum et al., 2011] has potential benefits. It would facilitate research aiming to (1) improve the selection of people with acute SCI more likely to benefit from enhanced reparative rehabilitation [Gerasimenko et al., 2015; Donati et al., 2016]; (2) protect against injury [Awad et al., 2015]; and (3) explain how SCI injury type influences the development of pain [Cruz‐Almeida et al., 2012; Finnerup et al., 2004, 2014; Wasner et al., 2008; Widerström‐Noga et al., 2015].
The focus of this study was on sensory discomplete SCI. While somatosensory preservation in complete SCI has previously been demonstrated using psychophysical testing [Cruz‐Almeida et al., 2012; Finnerup et al., 2004; Wasner et al., 2008; Widerström‐Noga et al., 2015], the role of neurophysiological testing remains to be determined. To further address this issue we used fMRI to measure primary somatosensory cortical (S1) activity in response to innocuous stimulation of the great toe in people with complete thoracic SCI (with and without below‐level neuropathic pain) and controls without SCI or neuropathic pain.
MATERIALS AND METHODS
Experimental Design
A cross‐sectional study was undertaken of adults (18–65years) with complete spinal cord injury with and without below level neuropathic pain recruited sequentially through advertising and an established volunteer database. Age‐ and sex‐matched controls without spinal cord injury or neuropathic pain were recruited in a similar manner.
Subjects
Twenty‐three subjects with established (>12 months) clinically complete thoracic SCI (19 males (83%), mean ± SD age 43 ± 13 years) and 21 age‐ and gender‐matched controls without pain or SCI (15 males (71%); mean ± SD age 41 ± 14 years) were recruited for the study (Table 1). For each subject with SCI, the extent of spinal cord damage was recorded according to the ISNCSCI [Kirshblum et al., 2014, 2011]. In particular, the most caudal level of the spinal cord with normal sensory and motor function on both sides of the body (Neurological Level of Injury, NLI) was determined and if the injury was complete or incomplete. An injury was termed complete if there was an absence of sensory and motor function in the lowest sacral segments (S4–5). In addition, the sensory zone of partial preservation (ZPP) was mapped and the presence of hypersensitivity to light touch and pin prick recorded. Each subject was examined by the same clinician (PW).
Table 1.
Spinal cord injury subject characteristics
| Subject | Sex | Age | Years since injury | AIS | NLI | Sensory discomplete | Motor level | Sensory level | Sensory ZPP | ZPP hypersensitivity | Av pain prior to MRI | Pain at MRI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | ||||||||||
| SCI patients with below‐level neuropathic pain | |||||||||||||||
| 1 | M | 41 | 5 | A | T7 | N | T1 | T1 | T7 | T8 | T12 | T12 | Y | 4.2 | 6.1 |
| 2 | M | 54 | 27 | A | T2 | Y | T1 | T1 | T5 | T2 | T12 | L2 | Y | 2.8 | 1.6 |
| 3 | M | 30 | 4 | A | T5 | N | T1 | T1 | T6 | T5 | L1 | L1 | Y | 0.9 | 1.2 |
| 4 | M | 35 | 8 | A | T4 | N | T1 | T1 | T4 | T4 | T6 | T5 | Y | 4.6 | 3.9 |
| 5 | M | 58 | 32 | A | T4 | N | T1 | T1 | T4 | T4 | T5 | T6 | N | 3.4 | 5.6 |
| 6 | M | 41 | 14 | A | T3 | Y | T1 | T1 | T4 | T3 | T5 | T6 | N | 3.5 | 5.2 |
| 7 | M | 26 | 2 | A | T1 | Y | T1 | T1 | T1 | T1 | T5 | T4 | N | 2.1 | 1.7 |
| 8 | M | 51 | 3 | A | T9 | N | T1 | L2 | T9 | T9 | S2 | S2 | Y | 3.2 | 0.2 |
| 9 | F | 55 | 37 | A | T3 | N | T1 | T1 | T3 | T4 | T6 | T5 | N | 2.3 | 3.7 |
| 10 | M | 72 | 5 | A | T4 | Y | T1 | T1 | T4 | T5 | T7 | T7 | N | 5.7 | 5.8 |
| 11 | F | 63 | 5 | A | T10 | Y | T1 | T1 | T11 | T10 | T12 | T12 | N | 6.9 | 7.3 |
| 12 | M | 54 | 37 | A | T3 | N | T1 | T1 | T3 | T4 | T6 | T5 | N | 2.3 | 4.4 |
| 13 | M | 24 | 4 | A | T5 | Y | T1 | T1 | T5 | T5 | T12 | T11 | N | 6 | 1.8 |
| Mean | 46 | 14 | Mean | 4 | 4 | ||||||||||
| (±SD) | 15 | 14 | (±SEM) | 0.5 | 0.6 | ||||||||||
| SCI patients without neuropathic pain | |||||||||||||||
| 14 | M | 43 | 21 | A | T8 | Y | T1 | T1 | T8 | T9 | T10 | T10 | N | 0 | 0 |
| 15 | M | 26 | 7 | A | T10 | N | T1 | T1 | T10 | T10 | L1 | T12 | N | 0 | 0 |
| 16 | M | 44 | 27 | A | T5 | Y | T1 | T1 | T6 | T5 | L3 | S2 | N | 0 | 0 |
| 17 | M | 53 | 9 | A | T3 | N | T1 | T1 | T3 | T3 | T4 | T4 | N | 0 | 0 |
| 18 | M | 37 | 16 | A | T3 | N | T1 | T1 | T3 | T3 | T4 | T4 | N | 0 | 0 |
| 19 | M | 39 | 7 | A | T3 | N | T1 | T1 | T3 | T3 | T5 | T7 | N | 0 | 0 |
| 20 | F | 22 | 3 | A | T5 | Y | T1 | T1 | T5 | T5 | T8 | T11 | ‐* | 0 | 0 |
| 21 | M | 37 | 11 | A | T6 | Y | T1 | T1 | T6 | T9 | T11 | T12 | N | 0 | 0 |
| 22 | M | 54 | 17 | A | T6 | N | T1 | T1 | T6 | T6 | T11 | T12 | N | 0 | 0 |
| 23 | F | 32 | 3 | A | T5 | Y | T1 | T1 | T5 | T5 | T12 | L3 | N | 0 | 0 |
| Mean | 39 | 12 | |||||||||||||
| (±SD) | 10 | 8 | |||||||||||||
ZPP hypersensitivity, hypersensitivity to light touch or pin prick within the zone of partial preservation (ZPP); Av pain prior to MRI, average neuropathic pain intensity (10 cm visual analog scale, VAS) during week prior to MRI; pain at MRI, neuropathic pain intensity (10 cm VAS) during MRI; *, missing data.
Neuropathic pain
Thirteen (11 males (85%); mean ± SD age 46 ± 15 years) of the 23 SCI subjects (57%) experienced persistent neuropathic pain (Fig. 1). All 13 SCI subjects had below‐level neuropathic pain as defined by the International Spinal Cord Injury Pain Classification [Bryce et al., 2012]. These subjects experienced constant shooting, electric or burning pain in the region of sensory loss beginning at least three segments below the NLI. To assess the intensity of their pain, each subject completed a pain diary in which they indicated, with a vertical pencil stroke on a 10 cm horizontal line, the intensity of their pain (0 cm = no pain to 10 cm = maximum imaginable pain) three times a day for the week prior to the scanning session. These pain diary values were averaged to provide an indication of each subject's chronic pain rating. Each subject also rated their ongoing pain intensity during the MRI scanning session (Table 1). The remaining 10 SCI subjects (8 males (80%); mean ± SD age 39 ± 10 years) did not report persistent neuropathic pain. Informed written consent was obtained for all procedures and the study was approved by the local institutional Human Research Ethics Committees.
Figure 1.
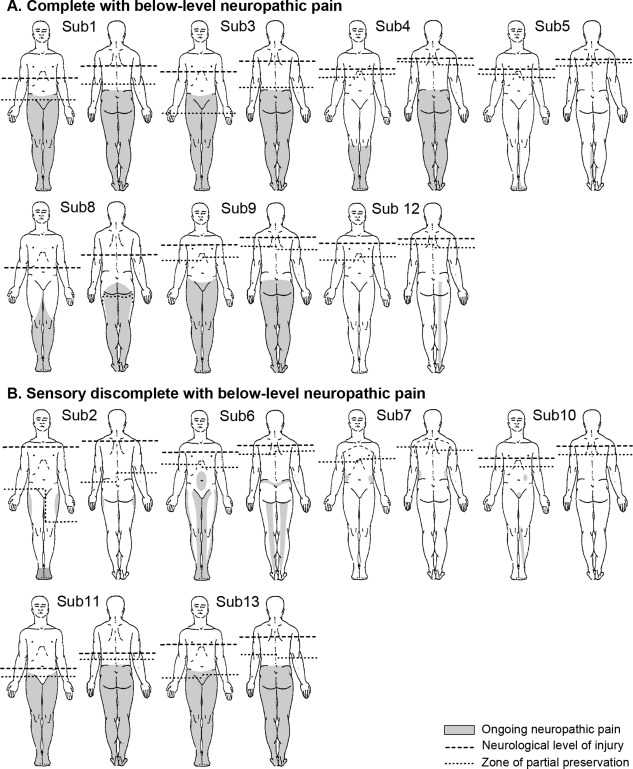
Diagrams showing neurological level of injury (NLI), zone of partial preservation (ZPP), and pain distribution in subjects with below‐level neuropathic pain. Individual illustrations of the NLI, ZPP, and distribution of ongoing pain in SCI subjects with below‐level neuropathic pain are depicted. Subjects are grouped into those identified as complete or sensory discomplete SCI based on fMRI brain activation (see results). The light grey shading indicates each SCI subject's ongoing pain distribution. The dashed line indicates the NLI and the dotted line, the most caudal segment of the sensory ZPP. Subject numbers correspond to those in Table 1.
Functional Magnetic Resonance Imaging (fMRI)
fMRI scans were acquired using a Philips Achieva 3T MRI machine. Each subject lay in a supine position with their arms by their sides. Head movement was restricted by using tight fitting headphones and foam padding placed between the subject's head and the MRI head coil. A series of 130 gradient echo echo‐planar fMRI image volumes using blood oxygen level dependent contrast were collected. Each image volume contained 43 axial slices covering the entire brain (voxel = 1.95 × 1.95 × 3.00 mm thick, repetition time = 3000 ms; echo time = 40 ms).
During each fMRI series, the pad of the right big toe was brushed using a plastic tool in a downward sweeping motion (1–2 cm length) at ∼1 stroke/s. In one SCI participant (subject 16), the left big toe was brushed due to amputation of the right big toe. The stimulation paradigm was performed for a period of 10 fMRI volumes (30 s) followed by a baseline period of 10 fMRI volumes (30 s). This was repeated a further 5 times for a total of 6 stimulation and 7 baseline periods. The plastic tool delivered a vertical force between 700 and 900 mN measured by a custom designed load sensor (FN3280, S‐Beam load cell 0–1 N, FGP Sensors, France) across an approximate area of 8 mm2. Control subjects did not report the stimuli as painful. Stimulation was undertaken by the same researcher (SG).
To reduce unintended increases in somatosensory cortical activity from awareness, subjects were prevented from seeing cutaneous stimulation during the fMRI by asking them to close their eyes and carefully orienting the head coil mirror away from the brushing site. Subjects were also asked if they were aware of when stimulation was being applied and whether this altered their pain. A 3D T1‐weighted image set was also collected (voxel size: 0.90 × 0.90 × 0.90 mm).
fMRI Image Processing and Statistical Analysis
All fMRI images were processed using SPM8 software [Friston et al., 1994]. The images were motion corrected, global signal drifts removed using the detrending method described by Macey et al. [2004], spatially normalized to the Montreal Neurological Institute (MNI) template and spatially smoothed using a 6 mm full‐width‐at‐half‐maximum Gaussian filter. Significant increases in fMRI signal intensity were determined using a repeated box car model convolved with hemodynamic delay function. Each subject's T1‐weighted anatomical image set was spatially normalized to the MNI template and segmented into grey matter, white matter, and cerebrospinal fluid images. The grey matter image was co‐registered to an individual's fMRI image set so that both the fMRI and T1‐anatomical images were in the same three‐dimensional space. A second‐level group analysis was performed to determine the significant signal intensity increases during the big toe brushing periods in the control group (random effects, whole‐brain analysis, corrected for multiple comparisons (false discovery rate (FDR), P < 0.05). Furthermore significant signal intensity increases during big toe brushing periods for every SCI subject were determined (P < 0.05, corrected for multiple comparisons (FDR), whole‐brain analysis). In the SCI subjects in which no significant signal intensity increase was detected within the S1, the threshold was set to P < 0.05 (uncorrected).
Furthermore, differences in signal intensity change between the discomplete and the complete SCI group as well as the healthy and discomplete SCI group were determined using a second‐level, two‐sample t‐test (random effects, whole‐brain analysis, P < 0.05, uncorrected). With regards to this analysis, the contrast image of subject 16 was flipped. To obtain neuroanatomical labels for significant differences in signal intensity increases in somatosensory brain regions during big toe brushing, masks from the AAL software [Tzourio‐Mazoyer et al., 2002] were used, for example, S1, the secondary somatosensory cortex (S2), the thalamus, and the cerebellum. Finally, a chi‐square test (P < 0.05) was used for categorical data to detect if the existence of a sensory discomplete SCI was associated with the presence of neuropathic pain among people with clinically complete SCI.
Changes in S1 activation with toe brushing
To determine whether toe representation within S1 significantly changed in people with discomplete SCI compared to control subjects, we compared the Euclidean distance (ED) between an anatomical marker and the maximally activated voxel in the contralateral (left) S1. The point at which the central sulcus meets the longitudinal fissure at the dorsal aspect of the brain was used as a standardized anatomical marker. A two‐sample t‐test was used to determine significant differences in ED between discomplete SCI and control subjects (P < 0.05). This method has been described in detail previously [Wrigley et al., 2009].
All data were assessed by Kolmogorov–Smirnov Test for normality. Data identified as not normally distributed were analyzed using nonparametric tests.
RESULTS
Location of S1 Representation for Toe Brushing
Individual and mean subject characteristics are shown in Table 1. In the control group, big toe brushing was associated with a significant increase in signal intensity in the contralateral representation area of the toe within S1 (mean coronal coordinate: −39, sagittal coordinate: −11, axial coordinate: 68) (P < 0.05, FDR corrected, Fig. 2). The mean activations were located within the somatosensory representation area of the big toe of the human S1 [Kaas, 2012; Nakamura et al., 1998]. Furthermore, S1 toe activity was seen in 19 control subjects using a threshold of P < 0.05 FDR corrected over the entire brain. In the remaining two controls the threshold had to be lowered to P < 0.005 uncorrected to see S1 toe activity. Big toe brushing was also associated with a significant increase in signal intensity in further somatosensory brain areas such as the ipsilateral cerebellar cortex, the contralateral thalamus, and S2 (P < 0.05, FDR corrected, Fig. 2).
Figure 2.
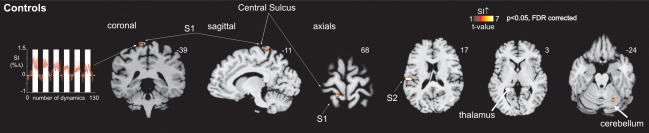
Activation of the brain's somatosensory system during brushing the big toe in controls. The mean S1 activation during brushing the big toe in 21 control subjects is illustrated according to the color scale. The mean (±SEM) percentage change in signal intensity in S1 is also shown. The vertical white bars indicate each brushing period. Note that brushing activates further somatosensory areas of the brain such as the ipsilateral cerebellar cortex, contralateral thalamus, and S2. The slice location is indicated by the Montreal Neurological Institute co‐ordinate at the top right of each image. FDR, false discovery rate.
In 12 (52%) of the 23 SCI subjects, brushing did not result in any significantly activated voxels in the contralateral representation area of the toe within S1. In the remaining 11 (48%) of the SCI subjects, significant activation in the contralateral representation area of the toe within S1 (compared to baseline) was demonstrated during toe brushing (n = 7: P < 0.05, FDR corrected; n = 4: P < 0.05, uncorrected, Fig. 3). These signal intensity increases were located in similar locations in all 11 SCI subjects (Fig. 3), consistent with the mean significant signal intensity increases of the 21 control subjects during big toe brushing (Fig. 2). Furthermore, S1 toe signal change was less in discomplete people compared to healthy controls (Fig. 4, P = 0.05, uncorrected). The ED of the maximally activated voxel were not significantly different between discomplete SCI and control subjects (P = 0.64; ED (SE) control subjects: 16.1 (1.0); discomplete SCI subjects: 15.2 (1.3)). Thus the location of toe activation within S1 did not differ between discomplete SCI and control subjects.
Figure 3.
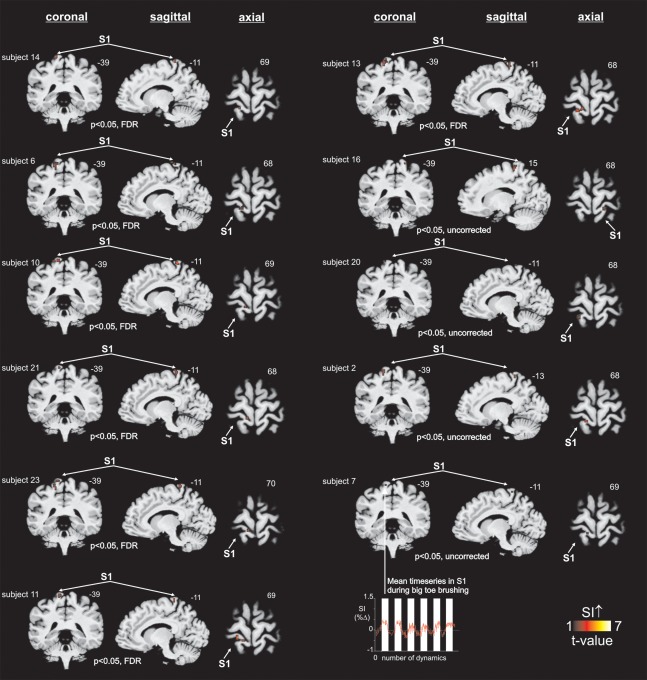
Sensory continuity between the big toe and S1 in people with complete thoracic spinal cord injury. S1 activation during brushing of the big toe in 11 discomplete SCI subjects (diagnosed clinically as having a complete injury). The slice location is indicated by the Montreal Neurological Institute co‐ordinate at the top right of each image. Subject numbers match information in Table 1. The mean (±SEM) percentage change in S1 signal intensity in discomplete SCI subjects (n = 11) is also shown. FDR, false discovery rate.
Figure 4.
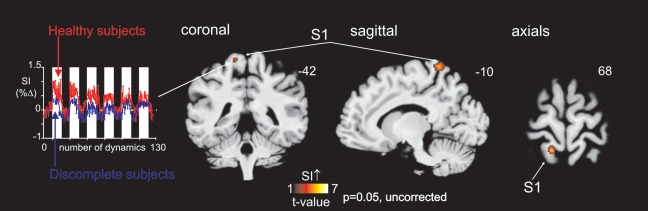
S1 activation during toe brushing in healthy subjects compared to discomplete subjects. Note that healthy subjects show more signal increase than discomplete subjects within S1 during toe brushing (P = 0.05 uncorrected). The slice location is indicated by the Montreal Neurological Institute co‐ordinate at the top right of each image. The mean (±SEM) percentage change in S1 signal intensity in discomplete SCI subjects (blue) compared to healthy controls (red) is also shown.
Based on these findings, 11/23 (48%) of the subjects with complete SCI (AIS A) demonstrate intact sensory pathways between the great toe and brain. These 11 subjects with AIS A SCI and intact sensory pathways to the toe on fMRI were classified as having a sensory discomplete SCI.
Relationship between Completeness and Brain Activation Pattern
When the fMRI activation volumes for toe brushing were compared between the groups defined as having discomplete (AIS A with S1 activation on toe brushing, n = 11) and complete (AIS A without S1 activation on toe brushing, n = 12) SCI injuries (Fig. 5), significant increase in evoked signal was noted in the discomplete SCI group in the contralateral representation area of the toe within S1 (P < 0.001, uncorrected) and other areas consistent with sensory preservation including contralateral S2 (P < 0.05, uncorrected), contralateral thalamus (P < 0.005, uncorrected) and the ipsilateral cerebellum (P < 0.001, uncorrected). Brain activation within contralateral S2, contralateral thalamus and ipsilateral cerebellum overlapped with the activation of somatosensory brain areas in controls subjects (Fig. 2).
Figure 5.
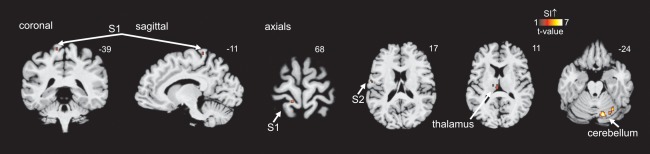
Brain activation during toe brushing in discomplete subjects. Significant S1 increases during brushing the big toe in 11 discomplete SCI subjects compared to 12 complete SCI subjects. Note that brushing activates the ipsilateral cerebellar cortex, contralateral thalamus, and S2 in discomplete SCI subjects compared to complete SCI subjects. The slice location is indicated by the Montreal Neurological Institute co‐ordinate at the top right of each image.
Relationship between Completeness and Below‐Level Neuropathic Pain
When subjects classified as having discomplete SCI were examined, 6/11 (55%) had below‐level neuropathic pain and 5/11 (45%) did not. Similarly, of the subjects classified as having complete SCI (AIS A with no S1 activation on toe brushing), 7/12 (58%) had below‐level neuropathic pain and 5/12 (42%) did not. When discomplete and complete SCI were examined there was no association between neuropathic pain and the existence of a sensory discomplete SCI (χ 2 = 0.034, P = 0.9). In all SCI subjects, brushing was not perceived nor did it trigger and/or increase pain below, at and/or above the injury.
Hypersensitivity in the ZPP
When the presence of a sensory discomplete SCI was examined there was no association between a sensory discomplete SCI and the presence of hypersensitivity in the ZPP (χ 2 = 2.33, P = 0.13). There was, however, a weak association between the presence of below‐level neuropathic pain and hypersensitivity in the ZPP (χ 2 = 4.480, P = 0.054 Fisher's Exact Test), where 38% (n = 5/13) of subjects reporting below‐level neuropathic pain had hypersensitivity while no subject (n = 0/9, 1 missing) without neuropathic pain reported hypersensitivity.
DISCUSSION
This study demonstrates clear, location appropriate, significant fMRI brain activation from innocuous stimulation of clinically insensate regions below clinically complete spinal cord injuries, confirming the presence of preserved subclinical spinal cord sensory connections from these regions. This indicates the presence of a SCI that may be termed discomplete. No association was found between the presence of an innocuous sensory discomplete injury and below‐level neuropathic pain.
Ability of fMRI to Detect Subclinical Sensory Spinal Cord Pathways
When individual subjects with complete SCI were examined, approximately half (48%, n = 11/23) demonstrated significant activation of the contralateral S1 toe region after stimulation of the clinically insensate foot. These subjects were classified as having sensory discomplete SCI. The frequency of detection in this study is consistent with previous estimates made on post‐mortem anatomical and clinical sensory assessment studies (50%) [Bunge et al., 1993; Finnerup et al., 2004; Kakulas, 1999]. This study, however, provides more objective evidence of preserved sensory pathways capable of conducting afferent information centrally. If multimodal sensory assessment (innocuous and noxious mechanical and thermal testing) was performed the frequency of sensory discomplete SCI may have been higher.
Characteristics of Cortical Activation
The S1 activation location in subjects with discomplete SCI and no SCI were the same when differences in ED were compared (P = 0.64). Evidence for the concordance of S1 activation with that expected for the great toe is strengthened when each individual cortical map is examined and clear activation voxels can be seen in the area of interest (Figs. 2 and 3). The stable nature of toe S1 cortical location contrasts with the changes in topographical organization seen in cortical locations of body regions near or above an area of SCI deafferentation [Jutzeler et al., 2015b; Wrigley et al., 2009]. While the influence of neuropathic pain on the degree and direction of changes in S1 cortical topography following SCI remain controversial [Jutzeler et al., 2015a, 2015b; Wrigley et al., 2009], cortical regions serving the more rostral regions with normal sensation may be more prone to reorganization as they become involved in postinjury neuroadaptation to restore sensory communication [Henderson et al., 2011].
As expected, the increase in evoked signal within S1 during brushing was less in subjects with discomplete SCI than in subjects without SCI (P = 0.05). This reduction in cortical activation would be consistent with the absence of stimulus perception in any of the SCI subjects.
Brain Activation Stimulation Pattern
The differences in brain activation detected between the two SCI groups (sensory discomplete and complete, Fig. 5) are consistent with the known neuroanatomy of ascending innocuous sensory afferent input. The observed differences in contralateral thalamus and S1 is consistent with loss of sensory afferent information transmitted through the dorsal column medial lemniscal (DCML) or spinothalamic tract (STT) [Mtui et al., 2016]. In the same way, differences in contralateral S2 activation which is involved in tactile discrimination is consistent with reductions in S1 activation [Mtui et al., 2016]. Ipsilateral cerebellar differences would also be expected via loss of the ascending unconscious proprioceptive input received from the toe projecting via the dorsal nucleus (Clarkes column, T1‐L2) and the dorsal spinocerebellar tract [Mtui et al., 2016]. The spinocerebellar tract carries information from a range of primary afferents including muscles and joints but also receives collateral input from cutaneous sensory neurons [Mtui et al., 2016].
Association Between Discomplete Injury and Below‐Level Neuropathic Pain
The proportions of subjects with below‐level neuropathic pain in complete (58%) and discomplete (55%) SCI were the same (χ 2 = 0.034, P = 0.9). In this study population, therefore, the presence of a partially preserved sensory pathway, transmitting innocuous mechanical sensation had no association with the presence of below‐level neuropathic pain.
The presence of hypersensitivity (allodynia or hyperalgesia) in the sensory ZPP did not differentiate people with sensory discomplete or complete SCI. Hypersensitivity was, however, associated with the presence of below‐level neuropathic pain as noted in previous cross sectional studies [Finnerup et al., 2003, 2007]. In a more recent prospective study, early below‐level evoked pain was a predictor for the development of below‐level neuropathic pain [Finnerup et al., 2014).
In previous studies using QST [Cruz‐Almeida et al., 2012; Finnerup et al., 2004; Wasner et al., 2008], patients reporting conscious perception of stimuli localized to an area of sensory loss were classified as having a sensory discomplete SCI. In two of these studies [Cruz‐Almeida et al., 2012; Finnerup et al., 2004], no association was found between partially preserved STT function and the presence of neuropathic pain. Wasner et al. [2008], however, did note a marked difference proposing a crucial role for partially preserved STT in the maintenance of below‐level neuropathic SCI pain. In Wasner et al. [2008] topical solutions were used to sensitize peripheral receptors (capsaicin, menthol, and histamine) to maximize primary afferent activation and enhance perception.
Greater reductions in DCML‐mediated function (at injury level) have also been associated with the presence of SCI neuropathic pain [Cruz‐Almeida et al., 2012] suggesting extensive damage affecting both innocuous and noxious pathways, with the maintenance of STT function may be necessary for SCI neuropathic pain to the develop or continue [Cruz‐Almeida et al., 2012]. This broad hypothesis is also supported by a magnetic spectroscopy (MRS) study correlating somatosensory changes occurring following SCI and brain neurotransmitter levels [Widerström‐Noga et al., 2015].
The stimulus delivered in this study, while repetitive (1 Hz), was not reported as painful in any of the control subjects. The balance between nociceptive and non‐nociceptive ascending input has been suggested as a possible cause for neuropathic pain [Cruz‐Almeida et al., 2012; Finnerup et al., 2004; Widerström‐Noga et al., 2015]. In addition, damage to the posterior cord is also likely to influence descending inhibitory circuits [Cruz‐Almeida et al., 2012]. However, this balance was not examined in this study. Further neurophysiological studies using both innocuous and noxious stimuli with chemical peripheral sensitization would assist in determining this.
Significance of a Discomplete SCI
The current dichotomous classification of injury type (complete, incomplete) does not adequately represent the complexity of spinal cord damage particularly in those with complete injuries. The five‐point AIS scale allocates one category (AIS A) to complete injuries, which vary widely in motor and sensory preservation. While sacral sparing has been shown to better predict injury stability [Waters et al., 1991], it does not meet the need for more detailed research identifying those more likely to benefit from interventions or potentially at higher risk of developing persistent pain. The need for more detailed methods to quantify severity and to classify SCI type has been expressed for some time [Krishna et al., 2014]. Inclusion of discomplete SCI subgroups (motor and sensory) in the international SCI taxonomy (ISNCSCI) [Kirshblum et al., 2011] is essential to consider for its potential to improve the prediction of neurological outcome, enhance the interpretation of therapy effects and better select people with SCI for reparative trials [Krishna et al., 2014].
The differing results obtained through cross‐sectional studies examining sensory discomplete SCI highlight the importance of ongoing research in this area, in particular longitudinal studies to allow prognostic conclusions to be drawn [Finnerup et al., 2014]. Knowing whether a sensory discomplete lesion could be enhanced to improve subconscious nociceptive afferent warning of pressure injury or bladder volume [Awad et al., 2015] or could identify subgroups of patients at higher risk of developing below‐level neuropathic pain over time would be of clinical value. In the same way, the ability to identify motor discomplete SCI may help identify those at greater risk of spasticity and may also help select patients more likely to benefit from motor retraining [Donati et al., 2016] and stimulation therapy [Gerasimenko et al., 2015].
LIMITATIONS
This study examined the cortical detection of innocuous mechanical brushing to an insensate area (great toe) below injury on one side only. Stimulation of both sides and more than one site may have detected more sensory discomplete injuries but only one site was stimulated to reduce scanning time. The use of other innocuous and noxious stimuli may also have increased the number of sensory discomplete injuries identified and provided a more detailed characterization of the extent of fiber injury. The primary intention of this study was however to demonstrate the potential of fMRI to detect partially preserved sensory pathways following SCI and a single stimulus was chosen to achieve this.
A combination of brain fMRI and neurophysiological testing such as contact heat‐evoked cortical potential (CHEP) detection may provide an avenue to obtain more objective evidence of preserved pathways in SCI for both innocuous and noxious stimuli. If CHEP assessment was undertaken the additional benefit of peripheral chemical sensitization (topical capsaicin) would be important to assess given its reported benefit in QST studies [Wasner et al., 2008].
It is possible that one or more false positives may have occurred in the four SCI subjects identified using an uncorrected threshold (P < 0.05) to detect significant activation during toe brushing within S1. However, this is felt unlikely given the location of activation during toe brushing exactly matched the location detected in healthy subjects and the seven SCI subjects in which corrected FDR (P < 0.05) thresholds across the entire brain were used. In addition, strategies were employed to avoid unintentional cueing of subjects to periods of stimulation that may have inadvertently increased somatosensory cortical activity.
It is also possible one or more false positives may have occurred in the group analysis examining differences in whole brain activation between complete and discomplete SCI subjects during toe brushing as uncorrected thresholds (P < 0.05) were also used. The decision to use uncorrected thresholds was made as brain activation is likely to be extremely weak due to the somatosensory pathway loss associated with SCI. While further studies with larger sample sizes allowing corrections for multiple comparisons would be ideal, it is very difficult to recruit large numbers of this very specific group (complete SCI, thoracic level, below‐level neuropathic pain, or no neuropathic pain).
While the results of this fMRI study are very clear, applying this technique on a more routine basis would not currently be possible due to the advanced analysis required. However, with the rapid improvements in imaging analytic techniques, the potential for use of fMRI in the future remains.
CONCLUSION
These results demonstrate more objectively the presence of subclinical preserved somatosensory pathways in clinically complete SCI in a similar proportion to that reported in previous animal and human psychophysical research. An association between hypersensitivity within the ZPP and the presence of neuropathic pain was confirmed. No association was found between the presence of a sensory discomplete SCI to innocuous stimuli and below‐level neuropathic pain.
The high prevalence of sensory discomplete injuries (50% of complete injuries), strengthens the case to explore inclusion of this category into the international SCI taxonomy (ISNCSCI). This would ensure more widespread inclusion of discomplete SCI in ongoing pain and motor recovery research. For this to happen, a consensus approach on the best way to identify discomplete SCI (motor and sensory) would be needed. Neurophysiological tests such as fMRI may play a role in this process.
ACKNOWLEDGMENT
The authors declare no competing financial interests.
REFERENCES
- Awad A, Levi R, Lindgren L, Hultling C, Westling G, Nyberg L, Eriksson J (2015): Preserved somatosensory conduction in a patient with complete cervical spinal cord injury. J Rehabil Med 47:426–431. [DOI] [PubMed] [Google Scholar]
- Bryce TN, Biering‐Sorensen F, Finnerup NB, Cardenas DD, Defrin R, Lundeberg T, Norrbrink C, Richards JS, Siddall P, Stripling T, Treede RD, Waxman SG, Widerstrom‐Noga E, Yezierski RP, Dijkers M (2012): International spinal cord injury pain classification: Part I. Background and description. Spinal Cord 50:413–417. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM (1993): Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 59:75–89. [PubMed] [Google Scholar]
- Cruz‐Almeida Y, Felix ER, Martinez‐Arizala A, Widerström‐Noga EG (2012): Decreased spinothalamic and dorsal column medial lemniscus‐mediated function is associated with neuropathic pain after spinal cord injury. J Neurotrauma 29:2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic MR (1987): Neurophysiology in spinal cord injury. Paraplegia 25:205–208. [DOI] [PubMed] [Google Scholar]
- Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, Augusto PB, Tripodi S, Pires CG, Pereira GA, Brasil FL, Gallo S, Lin AA, Takigami AK, Aratanha MA, Joshi S, Bleuler H, Cheng G, Rudolph A, Nicolelis MAL (2016): Long‐term training with a brain‐machine interface‐based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep 6:30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Catley M, Davey NJ, Kuppuswamy A, Strutton P, Frankel HL, Jamous A, Savic G (2007): Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J Rehabil Res Dev 44:69–76. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Gyldensted C, Fuglsang‐Frederiksen A, Bach FW, Jensen TS (2004): Sensory perception in complete spinal cord injury. Acta Neurol Scand 109:194–199. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Johannesen IL, Fuglsang‐Frederiksen A, Bach FW, Jensen TS (2003): Sensory function in spinal cord injury patients with and without central pain. Brain 126:57–70. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Norrbrink C, Trok K, Piehl F, Johannesen IL, Sorensen JC, Jensen TS, Werhagen L (2014): Phenotypes and predictors of pain following traumatic spinal cord injury: A prospective study. J Pain 15:40–48. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sørensen L, Biering‐Sørensen F, Johannesen IL, Jensen TS (2007): Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp Neurol 207:139–149. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1994): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, Morikawa E, Haakana P, Ferguson AR, Roy RR, Edgerton VR (2015): Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma 32:1968–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ (2011): Functional reorganization of the brain in humans following spinal cord injury: Evidence for underlying changes in cortical anatomy. J Neurosci 31:7262–7268. 31:2630‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutzeler C, Curt A, Kramer J (2015a): Relationship between chronic pain and brain reorganization after deafferentation: A systematic review of functional MRI findings. NeuroImage (Amst) 9:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutzeler CR, Freund P, Huber E, Curt A, Kramer JLK (2015b): Neuropathic pain and functional reorganization in the primary sensorimotor cortex after spinal cord injury. J Pain 16:1256–1267. [DOI] [PubMed] [Google Scholar]
- Kaas JH (2012): Somatosenory system In: Mai J, Paxinos G, editors. The Human Nervous System, 3rd ed. Academic Press, Elsevier; pp 1075–1101. [Google Scholar]
- Kakulas BA (1999): The applied neuropathology of human spinal cord injury. Spinal Cord 37:79–88. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Biering‐Sorensen F, Betz R, Burns S, Donovan W, Graves DE, Johansen M, Jones L, Mulcahey MJ, Rodriguez GM, Schmidt‐Read M, Steeves JD, Tansey K, Waring W (2014): International standards for neurological classification of spinal cord injury: Cases with classification challenges. [Erratum appears in J Spinal Cord Med. 2014 Jul;37(4):481]. J Spinal Cord Med 37:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering‐Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt‐Read M, Waring W (2011): International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 34:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna V, Andrews H, Varma A, Mintzer J, Kindy MS, Guest J (2014): Spinal cord injury: How can we improve the classification and quantification of its severity and prognosis? J Neurotrauma 31:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM (2004): A method for removal of global effects from fMRI time series. NeuroImage 22:360–366. [DOI] [PubMed] [Google Scholar]
- McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM (2004): Clinical neurophysiological assessment of residual motor control in post‐spinal cord injury paralysis. Neurorehabil Neural Repair 18:144–153. [DOI] [PubMed] [Google Scholar]
- Mtui E, Gruener G, Dockery P (2016): Fitzgerald's Clinical Neuroanatomy and Neuroscience [Electronic Resource], 7th ed Philadelphia, PA: Elsevier. [Google Scholar]
- Nakamura A, Yamada T, Goto A, Kato T, Ito K, Abe Y, Kachi T, Kakigi R (1998): Somatosensory homunculus as drawn by MEG. NeuroImage 7:377–386. [DOI] [PubMed] [Google Scholar]
- Nicotra A, Ellaway PH (2006): Thermal perception thresholds: Assessing the level of human spinal cord injury. Spinal Cord 44:617–624. [DOI] [PubMed] [Google Scholar]
- Sabbah P, de SS, Leveque C, Gay S, Pfefer F, Nioche C, Sarrazin JL, Barouti H, Tadie M, Cordoliani YS (2002): Sensorimotor cortical activity in patients with complete spinal cord injury: A functional magnetic resonance imaging study. J Neurotrauma 19:53–60. [DOI] [PubMed] [Google Scholar]
- Sherwood AM, Dimitrijevic MR, McKay WB (1992): Evidence of subclinical brain influence in clinically complete spinal cord injury: Discomplete SCI. J Neurol Sci 110:90–98. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wasner G, Lee BB, Engel S, McLachlan E, Wasner G, Lee BB, Engel S, McLachlan E (2008): Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain 131:2387–2400. [DOI] [PubMed] [Google Scholar]
- Waters RL, Adkins RH, Yakura JS (1991): Definition of complete spinal cord injury. Paraplegia 29:573–581. [DOI] [PubMed] [Google Scholar]
- Widerström‐Noga E, Cruz‐Almeida Y, Felix ER, Pattany PM (2015): Somatosensory phenotype is associated with thalamic metabolites and pain intensity after spinal cord injury. Pain 156:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ (2009): Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 141:52–59. [DOI] [PubMed] [Google Scholar]


