Abstract
Quantifying white matter (WM) tract disruption in people with multiple sclerosis (PwMS) provides a novel means for investigating the relationship between defective network connectivity and clinical markers. PwMS exhibit perturbations in personality, where decreased Conscientiousness is particularly prominent. This trait deficit influences disease trajectory and functional outcomes such as work capacity. We aimed to identify patterns of WM tract disruption related to decreased Conscientiousness in PwMS. Personality assessment and brain MRI were obtained in 133 PwMS and 49 age‐ and sex‐matched healthy controls (HC). Lesion maps were applied to determine the severity of WM tract disruption between pairs of gray matter regions. Next, the Network‐Based‐Statistics tool was applied to identify structural networks whose disruption negatively correlates with Conscientiousness. Finally, to determine whether these networks explain unique variance above conventional MRI measures and cognition, regression models were applied controlling for age, sex, brain volume, T2‐lesion volume, and cognition. Relative to HCs, PwMS exhibited lower Conscientiousness and slowed cognitive processing speed (p = .025, p = .006). Lower Conscientiousness in PwMS was significantly associated with WM tract disruption between frontal, frontal‐parietal, and frontal‐cingulate pathways in the left (p = .02) and right (p = .01) hemisphere. The mean disruption of these pathways explained unique additive variance in Conscientiousness, after accounting for conventional MRI markers of pathology and cognition (ΔR 2 = .049, p = .029). Damage to WM tracts between frontal, frontal‐parietal, and frontal‐cingulate cortical regions is significantly correlated with reduced Conscientiousness in PwMS. Tract disruption within these networks explains decreased Conscientiousness observed in PwMS as compared with HCs.
Keywords: lesion, neuropsychology, pathology, personality, structural connectivity, white matter
1. INTRODUCTION
Newer methods for measuring in vivo white matter (WM) tract disruption allow for novel investigation of the relationship between networks of structurally connected gray matter (GM) regions and neuropsychological phenomena (Kuceyeski et al., 2015). Although such methods were previously applied, little is known about the association with personality traits, factors that like cognitive reserve (Modica et al., 2015; Sumowski et al., 2014), may buffer the effects of structural brain injury in neurological disease (Benedict et al., 2013).
Of the Five‐Factor Model personality traits, Conscientiousness shows the most robust effects in discriminating healthy samples from neurological disease and predicting behavioral outcomes such as decline in ADLs (Roy, Ficarro, et al., 2016) and economic burden (Pocnet, Rossier, Antonietti, & von Gunten, 2013; Suchy, Williams, Kraybill, Franchow, & Butner, 2010). Conscientiousness, or the proclivity to be well organized and deliberate, is associated with increased longevity in aging adults (Bogg & Roberts, 2013) and more favorable outcomes in diseases such as diabetes (Jokela et al., 2014), Alzheimer's disease (Roy, Ficarro, et al., 2016), and multiple sclerosis (MS) (Roy, Schwartz, et al., 2016). In MS, particularly, deficits of this trait most robustly distinguishes patients from healthy controls (HCs) (Benedict et al., 2001), predicts work disability (Strober, et al., 2012) and decreased quality of life (Strober, 2016), correlates with cortical volume (Benedict, et al., 2008) and cognitive impairment (Roy, Drake, Eizaguirre, et al., 2018), moderates the relationship between atrophy and neuropsychiatric symptoms (Benedict et al., 2013), and it is the trait most likely to decline longitudinally (Roy, Drake, Fuchs, et al., in press). Therefore, trait Conscientiousness holds considerable promise for efforts to identify risk factors for poorer disease course.
Previous studies of healthy adults have examined the neuroanatomical relationship between regional GM structural characteristics and Conscientiousness (DeYoung et al., 2010; Privado, Román, Saénz‐Urturi, Burgaleta, & Colom, 2017; Riccelli, Toschi, Nigro, Terracciano, & Passamonti, 2017). In these studies, regional indices of voxel‐based morphology and surface‐based cortical thickness were associated with differences in Conscientiousness, as measured by the NEO Five Factor Inventory (NEOFFI; Costa & McCrae, 1992). The NEOFFI is one of the most widely used tools for assessing personality in healthy adults as well as in disease (Schwartz, Chapman, Duberstein, Weinstock‐Guttman, & Benedict, 2011) . These studies reveal correlation between Conscientiousness and GM volumes in frontal and parietal cortical regions. If there is a positive relationship between GM structure of these regions and trait Conscientiousness, then disease‐related damage to these regions or the connected WM tracts ought to produce defects in trait Conscientiousness of the affected individuals.
MS is an appropriate disease for investigating neuroanatomical associations with personality because PwMS exhibit markedly reduced Conscientiousness (Benedict et al., 2001) and the location of pathology, such as cerebral lesions, varies greatly from person to person. Like recent investigations of structural connectivity in MS, tools are available to measure where lesions disconnect WM tracts in a spatially defined and localized manner (Kuceyeski et al., 2013). This localized disruption leads to disconnection and impaired signaling between GM regions. Thus, in the context of PwMS, these measures of WM tract disruption can be applied to better understand the relationship between networks of GM regions and complex emergent phenomenon such as Conscientiousness.
We therefore aimed to investigate the impact of WM tract disruption in PwMS on levels of trait Conscientiousness. Because previous studies reported positive correlations between Conscientiousness and cortical thickness/volume of frontal and parietal GM regions (DeYoung et al., 2010; Riccelli et al., 2017), we hypothesized that increased lesion‐associated WM tract disruption between these regions and other related GM regions would be associated with lower Conscientiousness in PwMS.
2. METHODS
2.1. Subjects
We studied 133 people with MS (PwMS) who completed MRI and NEOFFI as part of a case‐control cardiovascular, environmental, and genetics study (Kappus et al., 2015; Zivadinov et al., 2016). Three subjects with MS were not included in this study due to poor image quality. Patients were diagnosed with MS as defined by the 2010 revised McDonald criteria (Polman et al., 2011). Of the 133 subjects included in the study, 107 (80.5%) were receiving disease‐modifying therapy. Disease course and disease duration were determined by clinical assessment. A cohort of 49 age‐ and sex‐matched HCs was also included in this study in order to characterize the MRI and neuropsychological status of the PwMS under investigation.
Inclusion criteria for PwMS in the study were diagnosis of relapsing‐remitting, progressive or primary‐progressive MS diagnosis, and neurologic/physical examination within 30 days from the standardized MRI. Exclusion criteria were substance dependence/abuse, psychiatric disorders, or history of any neurological disease other than MS. The study protocol was approved by the University and Buffalo Institutional Ethics Review Board. All subjects’ written informed consent were obtained before participation.
2.2. MR image acquisition
MRI data were collected from a 3T GE scanner. Imaging included a 3D T1‐weighted (IR‐FSPGR, repetition time [TR] 5.9 ms, echo time [TE] 2.8 ms, inversion time [TI] 900 ms, flip angle [FA] 10°, field‐of‐view [FOV] 25.6 × 19.2 cm2 [256 × 256 matrix with phase FOV 0.75], 1 × 1 mm2, 180 slices of 1 mm) and a 2D T2‐FLAIR scan (TR 8,500 ms, TE 120 ms, TI 2,100 ms, FA 90°, echo train length 24, FOV 25.6 × 19.2 cm (256 × 256 matrix with phase FOV 0.75), 1 × 1 mm2, 48 slices of 3 mm without gap).
2.3. MR image processing
N4 bias field correction was applied to all T1w and T2‐FLAIR images. Global volumetric measures were acquired via brain extraction and tissue segmentation techniques. Images were pre‐processed using a lesion filling tool to minimize the impact of T1 hypointensities (Gelineau‐Morel et al., 2012). The SIENAX cross‐sectional software tool was then applied (version 2.6; Smith et al., 2002). Whole brain volumes (WBV) were normalized for head size using the head size scaling factor estimated by SIENAX. Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/; Dale, Fischl, & Sereno, 1999). All free‐surfer derived data were visually inspected for quality. Manual corrections, such as control points, were applied when needed.
T2‐FLAIR WM lesion masks were obtained using a semi‐automated edge detection contouring/thresholding technique, described previously (Zivadinov et al., 2012). In order to account for WM areas with a greater severity of neuropathology, lesion masks were also obtained using T1w images. High resolution T1 weighted images were registered into Montreal Neurological Institute (MNI) space non‐linearly using ANTs (Avants et al., 2011). The same transforms were then applied to the corresponding WM lesion masks using nearest neighbor interpolation.
The transformed WM lesion masks were processed with the Network Modification (NeMo) tool (Kuceyeski, et al., 2013). This tool infers changes to the structural connectivity network resulting from the WM lesion mask by referencing a within software database of 73 healthy control tractograms. The proportion of WM tract disruption for each GM region is calculated as the proportion of tractogram streamlines connected to that region, according to the tractogram database, which pass through the given lesion mask and are therefore disrupted. The identification of tract streamlines which go through a lesion is binary, such that a streamline which goes through a single lesion is not treated differently from those which pass through multiple lesions. Percent tract disruption was evaluated for each of the GM structures included in the 86 region FreeSurfer brain parcellation. See Figure 1 for additional explanation. The percent tract disruption was calculated for each of the tractograms in the NeMo software database of 73 controls, and then the mean over these scores was taken to be the final region‐specific value. Tract disruption was also calculated in a pairwise manner, indicating the proportion of disrupted tract streamlines between each pair of structurally connected GM regions. This was done for all possible pairs of GM regions, resulting in the generation of subject‐specific 86 × 86 matrices which account for the proportion of WM tract disruption between each pair of connected GM regions.
Figure 1.
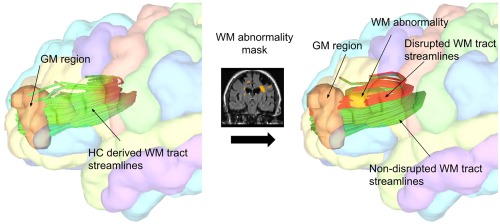
The Network Modification tool: A WM abnormality mask is registered to the Montreal Neurological Institute standard space. Then, an in‐software database of HC tractograms are referenced in order to determine the proportion of streamlines in a WM tract bundle, connected to a GM region, which are disrupted by WM abnormalities. WM tract disruption was evaluated for all GM structures included in the 86 region FreeSurfer brain parcellation. Tract disruption was also calculated in a pairwise manner, indicating the proportion of disrupted tracts between each pair of structurally connected GM regions [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. Psychometric assessment
All participants agreed to complete tests of personality according to the NEO Five Factor Inventory (NEOFFI; Costa & McCrae, 1992). The NEOFFI is a 60‐item questionnaire derived from the original NEO Personality inventory that assesses the following traits: Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness. Subjects are asked to rate the degree to which they agree with each statement as it relates to their own beliefs, behavior patterns or attributes, on a 5‐point Likert scale. The subscale raw scores were converted to sex‐corrected T scores (mean [SD] = 50 [10]) in accordance with published manual guidelines (Costa & McCrae, 1992). Herein we focused our analysis on Conscientiousness as it is the single trait most markedly affected in MS.
Cognitive testing was performed by research assistants under the supervision of a board‐certified neuropsychologist (RHBB) blinded to other findings. Visuospatial memory was assessed with the Brief Visuospatial Memory Test Revised (BVMT‐R; Benedict, 1997). For our analysis, we applied the subscale relating to delayed visuospatial memory recall. Cognitive processing speed was assessed with The Symbol Digit Modalities Test (SDMT; Smith, 1982). The SDMT is sensitive in PwMS, has excellent test‐retest reliability, construct validity, and robust associations with brain MRI metrics (Benedict, Deluca, Phillips, Larocca, & Hudson, 2017). Participants were presented with a series of nine symbols, each paired with a single digit in a key at the top of an 8 × 11‐inch sheet and were asked to respond orally with the digit associated with each symbol as rapidly as possible for 90 s. The number of correct responses was tabulated for each test. For final analysis, raw scores provided by the SDMT and BVMT‐R were converted to z‐scores, correcting for age, sex, and education.
2.5. Statistical analysis
In order to characterize our sample of PwMS, group comparisons were carried out relative to a sample of age‐ and sex‐matched HCs. Fisher's exact test was applied to compare sex of MS and HCs. Independent sample t‐tests were applied to compare both groups in terms of age, WBV, Conscientiousness, and cognitive processing speed. One sample t tests were applied to determine which pairs of GM regions exhibited statistically significant proportions of WM tract disruption. Additionally, ANCOVA controlling for age and sex was applied in order to determine whether different disease course phenotypes (relapse‐remitting vs. secondary‐progressive) are associated with different levels of trait Conscientiousness.
The Network‐Based‐Statistics (NBS) tool (Zalesky, Fornito, & Bullmore, 2010) was applied to associate NEOFFI trait Conscientiousness differences with variations in structural network disruption, as indicated by the 86 × 86 structural connectivity matrices described in the methods above. This tool is a method for controlling family‐wise error while retaining power by accounting for network structure—and is similar to more conventional cluster statistics. First, NBS computes a test statistic representing the strength of association between Conscientiousness and the structural connectivity of each pair of gray matter regions in the 86 × 86 structural connectivity matrix. Then networks of structurally connected suprathreshold pairs are identified and a p‐value is assigned to the entire identified component by comparing its size with the null distribution of maximal component size based on 5,000 permutations. Like conventional cluster statistics, NBS identifies significant sets of edges (networks) rather than individual edges themselves. We first applied NBS to identify patterns of structural network disruption which are associated with abnormal Conscientiousness. For the analyses, structural connectivity matrices derived from T2‐FLAIR lesion masks were associated with trait Conscientiousness variation, controlling for age, sex, and WBV. Component size was determined according to the sum of individual edge scores with a test‐statistic threshold of 3.3. Results were considered significant at an α‐level of 0.05.
To further confirm that these results provided unique information above and beyond what would otherwise already be predicted from conventional MRI measures and cognition, post‐hoc hierarchical regressions, including these measures, were applied. For these analyses, mean proportional tract disruption was calculated for all the region‐pairs included in the Conscientiousness‐associated networks, identified by the previous NBS analysis. This was done for T2‐FLAIR lesion based tract disruption as well as T1w lesion based tract disruption. Additionally, the mean volume for the cortical regions included in these networks was calculated. Each region's volume was corrected for surface area, before calculation of the mean. Prior to final analysis, all data were inspected for normality using the Shapiro‐Wilk test. The following variables were cubed‐root transformed in order to maintain data normality: T2LV, mean T2‐FLAIR lesion‐based tract disruption between region‐pairs included in Conscientiousness‐associated networks, and mean T1 lesion‐based tract disruption between region‐pairs included in Conscientiousness‐associated networks.
The first model predicting trait Conscientiousness contained age, sex, WBV, T2 lesion volume, cognitive processing speed, and visuospatial memory as predictors. Then, retaining the predictor variables from model 1, the following variables were added to the second model: mean pairwise T2‐FLAIR lesion based tract disruption for the Conscientiousness‐associated networks, mean pairwise T1w lesion based tract disruption for these networks, and mean volume of cortical regions included in these networks. Significance of ΔR2 was determined according to the F change statistic from model 1 to model 2.
3. RESULTS
3.1. Study participant characteristics
The PwMS participating in our study had a mean (SD) age and disease duration of 53.91 (11.15) and 20.86 (10.87) years respectively. Their median EDSS (IQR) score was 3.0 (2.0–6.0). The PwMS did not significantly differ from HCs by age or sex. Refer to Table 1 for additional participant demographic and clinical information.
Table 1.
Demographic and clinical characteristics of study participants
| MS (n = 133) | HC (n = 49) | p (t test and fishers) | |
|---|---|---|---|
| Age in years (mean ± SD) | 53.91 ± 11.15 | 50.50 ± 15.14 | 0.099 |
| Female/male; % Female | 94/39; 70.7 | 37/12; 75.5 | 0.580 |
| Disease duration in Years (mean ± SD) | 20.86 ± 10.87 | – | – |
| Relapse remitting MS; %; | 83; 62.4 | – | – |
| Primary progressive MS; % | 7; 5.3 | – | – |
| Secondary progressive MS; % | 43; 32.3 | – | – |
| EDSS (median; IQR) | 3.0; 2.0–6.0 | – | – |
| White; % white | 127; 95.5 | 43; 87.8 | – |
| Hispanic/Latino; % | 1; 0.8 | 0; 0 | – |
| Black/African‐American; % | 2; 1.5 | 4; 8.2 | – |
| Asian; % | 1; 0.8 | 1; 2.0 | – |
Abbreviations: MS, multiple sclerosis; HC, healthy control; IQR: inter‐quartile range; EDSS, expanded disability status scale.
3.2. Reduced brain volume, trait conscientiousness, and cognitive processing speed in PwMS
In comparison with HCs, the PwMS exhibited significantly reduced trait Conscientiousness (p = .025) and cognitive processing speed (p = .006). Additionally, the PwMS had significantly lower brain volume (p < .001). There was no significant difference between relapsing remitting and secondary progressive PwMS in regards to levels of trait Conscientiousness. Refer to Table 2 for additional information.
Table 2.
MRI and neuropsychological characteristics of study participants
| MS (n = 133) | HC (n = 49) | p | |
|---|---|---|---|
| WBV (ml; mean ± SD) | 1,444.9 ± 89.0 | 1,523.6 ± 99.7 | <.001 |
| T2LV (ml; mean ± SD) | 13.4 ± 15.1 | 0.7 ± 1.4 | <.001 |
| Conscientiousness (mean ± SD) | 46.3 ± 13.5 | 51.2 ± 10.7 | .025 |
| C‐Net T2‐FLAIR disruption (%, mean ± SD) | 34.7 ± 25.4 | – | – |
| Cognitive processing speed (mean ± SD) | 49.6 ± 13.9 | 56.0 ± 13.4 | .006 |
p values relate to significance of independent‐sample t test comparisons.
MS, multiple sclerosis; HC, healthy control; WBV, whole brain volume; T2LV, T2 lesion volume; C‐Net T2‐FLAIR disruption, mean T2‐FLAIR lesion‐based tract disruption between region‐pairs included in Conscientiousness‐associated networks.
3.3. Network alterations associated with reduced conscientiousness
NBS analysis showed that lower Conscientiousness was characterized by a focal pattern of structural connectivity disruption, predominantly involving frontal, frontal‐parietal, and frontal‐cingulate pathways and connections to the basal ganglia. This pattern was observed in both the left (p = .02) and right (p = .01) hemisphere (Figure 2). This network consisted of 19 pairs of GM regions. In comparison, one sample t tests identified 450 pairs of GM regions with statistically significant proportions of WM tract disruption in this cohort, overall.
Figure 2.
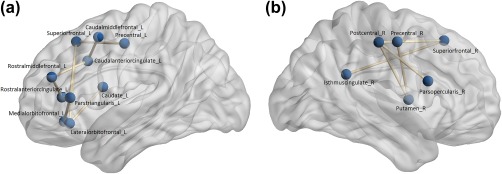
Conscientiousness‐associated networks. Lesion‐based WM tract disruption between the regions shown in the left (a) and right (b) hemisphere is significantly associated with reduced Conscientiousness in PwMS [Color figure can be viewed at http://wileyonlinelibrary.com]
Mean (SD) tract disruption in the Conscientiousness‐associated structural network was 34.7% (25.4). See Table 3 for details about region‐pairs included in each network as well as the strength of the relationship between each region pair and trait Conscientiousness.
Table 3.
Strength of association between region‐pair connectivity and Conscientiousness in PwMS (df = 131)
| Network | Region‐pair | t value | p |
|---|---|---|---|
| 1 | L. Caudal Anterior Cingulate‐L. Caudal Middle Frontal | 3.50 | <.001 |
| L. Lateral Orbitofrontal‐L. Superior Frontal | 3.41 | <.001 | |
| L. Caudal Anterior Cingulate‐L. Rostral Middle Frontal | 3.39 | <.001 | |
| L. Parstriangularis‐L. Superior Frontal | 3.38 | <.001 | |
| L. Superior Frontal‐L. Superior Parietal | 3.38 | <.001 | |
| L. Caudal Middle Frontal‐L. Posterior Cingulate | 3.37 | <.001 | |
| L. Rostral Anterior Cingulate‐L. Rostral Middle Frontal | 3.34 | <.001 | |
| L. Parstriangularis‐L. Rostral Anterior Cingulate | 3.31 | <.001 | |
| 2 | R. Cingulate Isthmus‐R. Superior Frontal | 3.96 | <.001 |
| R. Precentral‐R. Putamen | 3.60 | <.001 | |
| R. Postcentral‐R. Putamen | 3.48 | <.001 | |
| R. Postcentral R. Superior Frontal | 3.45 | <.001 | |
| R. Caudal Anterior Cingulate‐R. Insula | 3.43 | <.001 | |
| R. Caudal Middle Frontal‐R. Posterior Cingulate | 3.41 | <.001 | |
| R. Posterior Cingulate‐R. Precentral | 3.39 | <.001 | |
| R. Postcentral‐R. Posterior Cingulate | 3.37 | <.001 | |
| R. Caudal Anterior Cingulate‐R. Precentral | 3.36 | <.001 | |
| R. Parsopercularis‐R. Postcentral | 3.34 | <.001 | |
| R. Posterior Cingulate‐R. Pallidum | 3.33 | <.001 |
3.4. Post hoc regression analysis
In regression models predicting levels of Conscientiousness in PwMS while controlling for conventional MRI measures and cognitive function, the addition of network‐based measures resulted in a statistically significant increase in explained variance. This unique explained variance was above and beyond what was otherwise explained by age, sex, T2 lesion volume, WBV, cognitive processing speed, and visuospatial memory (ΔR 2 = .049, p = .029). The network based measures consisted of mean T2‐FLAIR lesion‐based tract disruption in the Conscientiousness‐associated networks (β = −0.466, p = .032), mean T1w lesion‐based tract disruption of these networks (β = −0.012, p = .934), and mean cortical volume of regions included in these networks (β = −0.202, p = .043). See Table 4 for additional details.
Table 4.
Post hoc multivariate models predicting trait Conscientiousness in people with MS
| Predictor | β | R 2 | ΔR 2 | |
|---|---|---|---|---|
| Model 1 | Age | 0.077 | 0.084 * | – |
| Sex | 0.039 | – | – | |
| T2LV | −0.145 | – | – | |
| WBV | 0.119 | – | – | |
| Cognitive processing speed | 0.282 * | – | – | |
| Visuospatial memory | −0.076 | – | – | |
| Model 2 | Age | 0.101 | 0.133** | 0.049 * |
| Sex | 0.008 | – | – | |
| T2LV | 0.284 | – | – | |
| WBV | 0.017 | – | – | |
| Cognitive processing speed | 0.243 * | – | – | |
| Visuospatial memory | −0.082 | – | – | |
| C‐Net cortical volume | 0.202 * | – | – | |
| C‐Net T2‐FLAIR disruption | −0.466 * | – | – | |
| C‐Net T1w disruption | −0.012 |
All βs are standardized. All R 2 values are adjusted for the number of predictors in the models.
Abbreviations:
T2LV, T2 lesion volume; WBV, whole brain volume; C‐Net cortical volume, mean volume of cortical regions included in the Conscientiousness‐associated network; C‐Net T2‐FLAIR disruption, mean FLAIR lesion‐based tract disruption between region‐pairs included in Conscientiousness‐associated networks; BH C‐Net disruption, mean T1w lesion‐based tract disruption between region‐pairs included in Conscientiousness‐associated networks.
*p ≤ .05; **p ≤ .01; ***p ≤ .001.
4. DISCUSSION
The PwMS under investigation exhibited reduced trait Conscientiousness compared with HCs. This is consistent with findings in previous studies (Akbar, Honarmand, & Feinstein, 2011; Benedict et al., 2001; Bruce, Hancock, Arnett, & Lynch, 2010) and supports our assumption that MS is an appropriate disease for addressing the neural correlates of personality. Previously, studies of healthy adults indicated a relationship between Conscientiousness and frontal and parietal cortical thickness (DeYoung et al., 2010; Riccelli et al., 2017). This may therefore indicate a relationship between structural integrity of these cortical brain regions and Conscientiousness, and perturbation of the structural connections between these regions ought to produce deficits in Conscientiousness. Our results support this supposition in that we found a significant correlation between structural network disruption of these regions and reduced Conscientiousness. Specifically, structural disruption of WM tracts between frontal, frontal‐parietal, and frontal‐cingulate cortical region pairs was significantly associated with reduced Conscientiousness in PwMS. Furthermore, reduced volume of these cortical brain regions was also significantly correlated with reduced Conscientiousness. These associations remained significant, even after controlling for age, sex, cognitive dysfunction, and conventional MRI measures of brain pathology (T2 lesion volume and WBV).
The relationship between reduced Conscientiousness in PwMS and network damage of frontal, frontal‐parietal, and frontal‐cingulate connections may relate to known functions of the involved regions, such as executive control and the formation of association between action and reward (Hayden & Platt, 2010; Wagner, Maril, Bjork, & Schacter, 2001). However, because the relationship between the identified network damage and reduced Conscientiousness remained significant after controlling for cognitive dysfunction, these networks may relate to unique components of trait Conscientiousness that are independent of cognition, or at least cognitive processing speed and visuospatial memory. Future work is planned to examine the influence of higher executive function, or neuropsychological tasks known to be more frontally mediated such as the Stroop task. Future work is also planned to examine the networks of GM regions whose disconnection is associated with frontally‐mediated cognitive tasks so that these networks may be compared with those observed for Conscientiousness.
In comparison with previous studies investigating neuroanatomical associations with personality, results from this study were derived through complementary methods of analysis. Rather than relying on measures of cortical volume alone, we also employed measures relating to network disruption caused by focal WM lesions. Because these analysis were driven by lesion‐based WM tract disruption, we were unable to generate meaningful data about structural network alterations in HCs. Lesions measured in HC MRI images, when present, were quite minimal and so network connectivity in HCs showed negligible variance. Localized WM tract disruption is unique to diseases such as MS. Consequently, this disease provides a unique window into the functional impact of overt disruption to regions and their connections with one another. Thus, our results corroborate previously reported brain‐behavior relationships via an independent sample of individuals with neurological disease, rather than healthy adults. Furthermore, the use of structural disconnection suggests that not only individual structures, but also the relationships and connections between these structures, may underlie trait Conscientiousness.
One important limitation of this study is the cross‐sectional nature of the analysis. Future studies may derive more robust conclusions by assessing longitudinal change in personality in PwMS and its relationship with changes in structural brain characteristics. Of note, we have recently shown for the first time that Conscientiousness declines in MS over 5 years (Roy, Drake, Fuchs et al., in press). This time frame may therefore be appropriate for future studies incorporating MRI analysis. Importantly, this study does not address the impact of different phenotypes of PwMS, such as relapsing‐remitting vs. secondary progressive, on patterns of WM tract disruption and subsequent changes in trait Conscientiousness. Future studies with larger sample sizes may be better suited for addressing this question.
It is also important to note that structural network disruption measured for our analysis was derived through methods which are independent of diffusion characteristics for each individual subject. Because diffusion imaging was not used for these analysis, more subtle alterations in pairwise structural connectivity may have been missed by our approach. Nonetheless, we addressed variations in severity of structural network damage by accounting for disruption caused by different forms of MRI lesions in our regression models, both T2‐FLAIR and T1w lesions. Notably, network disruption caused by T2‐FLAIR lesions was a significant predictor of Conscientiousness, after accounting for other predictor variables. However, disruption specifically caused by T1w lesions, a subset of T2‐FLAIR lesions considered to represent more severe pathology (Van Walderveen et al., 1998), did not account for significant additional variance in these models. Future analysis may additionally benefit from the application of microstructural measures in order to account for structural network abnormalities which are otherwise not captured by lesion‐based imaging. Nonetheless, the NeMo tool is useful for getting around problems associated with tractography in PwMS. Lesions caused by MS result in changes to diffusion‐imaging based measures, and contribute to poor prediction of WM tract streamline pathways. Thus, we believe that the NeMo tool allows us to consider the impact of lesions on structural connectivity, without the unreliability of tractography in this particular population of individuals affected by neurological disease.
5. CONCLUSION
The PwMS under investigation exhibited reduced Conscientiousness relative to HCs. This decreased Conscientiousness was significantly associated with disruption in WM tracts connecting frontal, fronto‐parietal, and fronto‐cingulate cortical regions. These findings are consistent with previous studies of healthy adults which indicated positive relationships between frontal and parietal GM structure and Conscientiousness. Our results were derived through very different methods of assessing structural brain characteristics in comparison with these previous studies. We therefore believe that the current study provides robust support for the previously observed neuro‐anatomical relationship between fronto‐parietal structure and Conscientiousness, and also underscores the importance of connectivity as well as individual cortical regions.
6. DISCLOSURES
Tom Fuchs, Amy Kuceyeski, Keith Carolus, Xian Li, Sanjeevani Choudhery, Matthew Mallory, Dejan Jakmovski, and Deepa Ramasamy have nothing to disclose.
Robert Zivadinov received personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Genzyme‐Sanofi, Claret Medical, IMS Health and Novartis for speaking and consultant fees. He received financial support for research activities from Teva Pharmaceuticals, Genzyme‐Sanofi, Novartis, Claret Medical, Intekrin and IMS Health.
Ralph H. B. Benedict has received research support from Accorda, Novartis, Genzyme, Biogen Idec, and Mallinkrodt, and is on the speakers’ bureau for EMD Serono, and consults for Biogen Idec, Genentech, Roche, Sanofi/Genzyme, Takeda, NeuroCog Trials, and Novartis. Dr. Benedict also receives royalties for Psychological Assessment Resources.
Michael G Dwyer received personal compensation from Novartis and Claret Medical for speaking and consultant fees. He received financial support for research activities from Novartis.
Fuchs TA, Dwyer MG, Kuceyeski A, et al. White matter tract network disruption explains reduced conscientiousness in multiple sclerosis. Hum Brain Mapp. 2018;39:3682–3690. 10.1002/hbm.24203
Funding information Teva Pharmaceuticals; Genzyme‐Sanofi; Novartis; Claret Medical; Intekrin; IMS Health; Accorda; Genzyme; Biogen Idec; Questcor Pharmaceuticals
REFERENCES
- Akbar, N. , Honarmand, K. , & Feinstein, A. (2011). Self‐assessment of cognition in multiple sclerosis : The role of personality and anxiety. Cognitive and Behavioral Neurology : Official Journal of the Society for Behavioral and Cognitive Neurology, 24(3), 115–121 [DOI] [PubMed] [Google Scholar]
- Avants, B. B. , Tustison, N. J. , Song, G. , Cook, P. A. , Klein, A. , & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict, R. H. B. (1997). Brief visuospatial memory test–revised: Professional manual. Lutz, FL: PAR. [Google Scholar]
- Benedict, R. H. B. , Deluca, J. , Phillips, G. , Larocca, N. , & Hudson, L. D. (2017). Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Multiple Sclerosis Journal, 1–13. 10.1177/https [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict, R. H. B. , Hussein, S. , Englert, J. , Dwyer, M. G. , Abdelrahman, N. , Cox, J. L. , … Munschauer, F. E. (2008). Cortical atrophy and personality in multiple sclerosis. Neuropsychology, 22(4), 432–441. 10.1037/0894-4105.22.4.432 [DOI] [PubMed] [Google Scholar]
- Benedict, R. H. B. , Priore, R. L. , Sc, D., Miller, C. , Munschauer, F. , & Jacobs, L. (2001). Personality disorder in multiple sclerosis correlates with cognitive impairment. Journal of Neuropsychiatry and Clinical Neuroscience, 13(1), 70–76. [DOI] [PubMed] [Google Scholar]
- Benedict, R. H. B. , Schwartz, C. E. , Duberstein, P. , Healy, B. , Hoogs, M. , Bergsland, N. , … Zivadinov, R. (2013). Influence of personality on the relationship between gray matter volume and neuropsychiatric symptoms in multiple sclerosis. Psychosomatic Medicine, 75(3), 253–261. 10.1097/PSY.0b013e31828837cc [DOI] [PubMed] [Google Scholar]
- Benedict, R. H. , Priore, R. L. , Miller, C. , Munschauer, F. , & Jacobs, L. (2001). Personality disorder in multiple sclerosis correlates with cognitive impairment. The Journal of Neuropsychiatry and Clinical Neurosciences, 13(1), 70–76. 10.1176/jnp.13.1.70 [DOI] [PubMed] [Google Scholar]
- Bogg, T. , & Roberts, B. W. (2013). Duel or diversion? Conscientiousness and executive function in the prediction of health and longevity. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 45(3), 400–401. [DOI] [PubMed] [Google Scholar]
- Bruce, J. M. , Hancock, L. M. , Arnett, P. , & Lynch, S. (2010). Treatment adherence in multiple sclerosis: Association with emotional status, personality, and cognition. Journal of Behavioral Medicine, 33(3), 219–227. 10.1007/s10865-010-9247-y [DOI] [PubMed] [Google Scholar]
- Costa, P. T. , & McCrae, R. R. (1992). Professional manual of the revised NEO personality inventory and NEO five‐factor inventory. Odessa, FL: Psychological Assessment Resources.
- Dale, A. M. , Fischl, B. , & Sereno, M. I. (1999). Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Hirsh, J. B. , Shane, M. S. , Papademetris, X. , Rajeevan, N. , & Gray, J. R. (2010). Testing predictions from personality neuroscience. Brain structure and the big five. Psychological Science, 21(6), 820–828. 10.1177/0956797610370159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau‐Morel, R. , Tomassini, V. , Jenkinson, M. , Johansen‐Berg, H. , Matthews, P. M. , & Palace, J. (2012). The effect of hypointense white matter lesions on automated gray matter segmentation in multiple sclerosis. Human Brain Mapping, 33(12), 2802–2814. 10.1002/hbm.21402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, B. Y. , & Platt, M. L. (2010). Neurons in anterior cingulate cortex multiplex information about reward and action. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 30(9), 3339–3346. 10.1523/JNEUROSCI.4874-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela, M. , Elovainio, M. , Nyberg, S. T. , Tabák, A. G. , Hintsa, T. , Batty, G. D. , & Kivimäki, M. (2014). Personality and risk of diabetes in adults: Pooled analysis of 5 cohort studies. Health Psychology, Jokela, Markus: Institute of Behavioral Sciences, University of Helsinki, Siltavuorenpenger 1A, P.O. Box 9, Helsinki, Finland, 00014, markus.jokela@helsinki.fi: American Psychological Association. 10.1037/hea0000003 [DOI] [PubMed] [Google Scholar]
- Kappus, N. , Weinstock‐Guttman, B. , Hagemeier, J. , Kennedy, C. , Melia, R. , Carl, E. , … Zivadinov, R. (2015). Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 1–7. 10.1136/jnnp-2014-310051 [DOI] [PubMed] [Google Scholar]
- Kuceyeski, A. F. , Vargas, W. , Dayan, M. , Monohan, E. , Blackwell, C. , Raj, A. , … Gauthier, S. A. (2015). Modeling the relationship among gray matter atrophy, abnormalities in connecting white matter, and cognitive performance in early multiple sclerosis. American Journal of Neuroradiology, 36(4), 702–709. 10.3174/ajnr.A4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski, A. , Maruta, J. , Relkin, N. , & Raj, A. (2013). The network modification (NeMo) tool : Elucidating the effect of white matter integrity changes on cortical and subcortical structural connectivity. Brain Connectivity, 3(5), 451 10.1089/brain.2013.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica, C. M. , Bergsland, N. , Dwyer, M. G. , Ramasamy, D. P. , Carl, E. , Zivadinov, R. , & Benedict, R. H. (2015). Cognitive reserve moderates the impact of subcortical gray matter atrophy on neuropsychological status in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England), 22(1), 36–42. 10.1177/1352458515579443 [DOI] [PubMed] [Google Scholar]
- Pocnet, C. , Rossier, J. , Antonietti, J.‐P. , & von Gunten, A. (2013). Personality features and cognitive level in patients at an early stage of Alzheimer's disease. Personality and Individual Differences, 54(2), 174–179. [Google Scholar]
- Polman, C. H. , Reingold, S. C. , Banwell, B. , Clanet, M. , Cohen, J. A. , Filippi, M. , … Wolinsky, J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privado, J. , Román, F. J. , Saénz‐Urturi, C. , Burgaleta, M. , & Colom, R. (2017). Gray and white matter correlates of the Big Five personality traits. Neuroscience, 349, 174–184. 10.1016/j.neuroscience.2017.02.039 [DOI] [PubMed] [Google Scholar]
- Riccelli, R. , Toschi, N. , Nigro, S. , Terracciano, A. , & Passamonti, L. (2017). Surface‐based morphometry reveals the neuroanatomical basis of the five‐factor model of personality. Social Cognitive and Affective Neuroscience, 671–684. 10.1093/scan/nsw175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S. , Drake, A. , Fuchs, T. , Dwyer, M. G. , Zivadinov, R. , Chapman, B. P. , … Benedict, R. H. B. (in press). Longitudinal personality change associated with cognitive decline in multiple sclerosis. Multiple Sclerosis Journal, 135245851775372. [DOI] [PubMed] [Google Scholar]
- Roy, S. , Drake, A. S. , Eizaguirre, M. B. , Zivadinov, R. , Weinstock‐Guttman, B. , Chapman, B. P. , & Benedict, R. H. B. (2018). Trait neuroticism, extraversion, and conscientiousness in multiple sclerosis: Link to cognitive impairment? Multiple Sclerosis Journal, 24(2), 205–213. 10.1177/1352458517695467 [DOI] [PubMed] [Google Scholar]
- Roy, S. , Ficarro, S. , Duberstein, P. , Chapman, B. P. , Dubovsky, S. , Paroski, M. , … Benedict, R. H. B. (2016). Executive function and personality predict instrumental activities of daily living in Alzheimer disease. The American Journal of Geriatric Psychiatry, 24(11), 1074–1083. 10.1016/j.jagp.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S. , Schwartz, C. E. , Duberstein, P. , Dwyer, M. G. , Zivadinov, R. , Bergsland, N. , … Benedict, R. H. B. (2016). Synergistic effects of reserve and adaptive personality in multiple sclerosis. Journal of the International Neuropsychological Society, 1–8. 10.1017/S1355617716000333 [DOI] [PubMed] [Google Scholar]
- Schwartz, E. S. , Chapman, B. P. , Duberstein, P. R. , Weinstock‐Guttman, B. , & Benedict, R. H. B. (2011). The NEO‐FFI in multiple sclerosis : Internal consistency, factorial validity, and correspondence between self and informant reports. Assessment, 18(1), 39–49. 10.1177/1073191110368482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. (1982). Symbol digit modalities test (SDMT) manual (revised). Los Angeles: Western Psychological Services.
- Smith, S. M. , Zhang, Y. , Jenkinson, M. , Chen, J. , Matthews, P. M. , Federico, A. , & De Stefano, N. (2002). Accurate, robust, and automated longitudinal and cross‐sectional brain change analysis. NeuroImage, 17(1), 479–489. [DOI] [PubMed] [Google Scholar]
- Strober, L. B. (2016). Personality in multiple sclerosis (MS): Impact on health, psychological well‐being, coping, and overall quality of life. Psychology, Health & Medicine, 2, 1–10. 10.1080/13548506.2016.1164321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober, L. B. , Christodoulou, C. , Benedict, R. H. B. , Westervelt, H. J. , Melville, P. , Scherl, W. F. , … Krupp, L. B. (2012). Unemployment in multiple sclerosis: The contribution of personality and disease. Multiple Sclerosis Journal, 18(5), 647–653. 10.1177/1352458511426735 [DOI] [PubMed] [Google Scholar]
- Suchy, Y. , Williams, P. G. , Kraybill, M. L. , Franchow, E. , & Butner, J. (2010). Instrumental activities of daily living among community‐dwelling older adults: Personality associations with self‐report, performance, and awareness of functional difficulties. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 65B(5), 542–550. [DOI] [PubMed] [Google Scholar]
- Sumowski, J. F. , Rocca, M. A. , Leavitt, V. M. , Dackovic, J. , Mesaros, S. , Drulovic, J. , … Filippi, M. (2014). Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology, 82(20), 1776–1783. 10.1212/WNL.0000000000000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Walderveen, M. A. A. , Kamphorst, W. , Scheltens, P. , van Waesberghe, J. H. T. M. , Ravid, R. , Valk, J. , … Barkhof, F. (1998). Histopathologic correlate of hypointense lesions on T1‐weighted spin‐echo MRI in multiple sclerosis. Neurology, 50(5), 1282–1288. [DOI] [PubMed] [Google Scholar]
- Wagner, A. D. , Maril, A. , Bjork, R. A. , & Schacter, D. L. (2001). Prefrontal contributions to executive control : fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage, 14(6), 1337–1347. 10.1006/nimg.2001.0936 [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , & Bullmore, E. T. (2010). Network‐based statistic: Identifying differences in brain networks. NeuroImage, 53(4), 1197–1207. 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
- Zivadinov, R. , Brown, M. H. , Schirda, C. V. , Poloni, G. U. , Bergsland, N. , Magnano, C. R. , … Dwyer, M. G. (2012). Abnormal subcortical deep‐gray matter susceptibility‐weighted imaging filtered phase measurements in patients with multiple sclerosis: A case‐control study. Neuroimage, 59(1), 331–339. [DOI] [PubMed] [Google Scholar]
- Zivadinov, R. , Ramasamy, D. P. , Benedict, R. R. H. , Polak, P. , Hagemeier, J. , Magnano, C. … (2016). Cerebral microbleeds in multiple sclerosis evaluated on susceptibility‐weighted images and quantitative susceptibility maps: A case‐control study. Radiology, 281(3), 884–895. [DOI] [PubMed] [Google Scholar]


