Abstract
Objectives
The innate alarm system (IAS) models the neurocircuitry involved in threat processing in posttraumatic stress disorder (PTSD). Here, we investigate a primary subcortical structure of the IAS model, the superior colliculus (SC), where the SC is thought to contribute to the mechanisms underlying threat‐detection in PTSD. Critically, the functional connectivity between the SC and other nodes of the IAS remains unexplored.
Experimental design
We conducted a resting‐state fMRI study to investigate the functional architecture of the IAS, focusing on connectivity of the SC in PTSD (n = 67), its dissociative subtype (n = 41), and healthy controls (n = 50) using region‐of‐interest seed‐based analysis.
Principal observations
We observed group‐specific resting state functional connectivity between the SC for both PTSD and its dissociative subtype, indicative of dedicated IAS collicular pathways in each group of patients. When comparing PTSD to its dissociative subtype, we observed increased resting state functional connectivity between the left SC and the right dorsolateral prefrontal cortex (DLPFC) in PTSD. The DLPFC is involved in modulation of emotional processes associated with active defensive responses characterising PTSD. Moreover, when comparing PTSD to its dissociative subtype, increased resting state functional connectivity was observed between the right SC and the right temporoparietal junction in the dissociative subtype. The temporoparietal junction is involved in depersonalization responses associated with passive defensive responses typical of the dissociative subtype.
Conclusions
Our findings suggest that unique resting state functional connectivity of the SC parallels the unique symptom profile and defensive responses observed in PTSD and its dissociative subtype. Hum Brain Mapp 39:563–574, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: post‐traumatic stress disorder, dissociative subtype, functional magnetic resonance imaging, resting state, superior colliculus
INTRODUCTION
In post‐traumatic stress disorder (PTSD), threat‐detection mechanisms operating at conscious and subconscious levels are responsible for eliciting trauma‐related defensive responses. From an evolutionary perspective, subconscious threat‐detection mechanisms allow ultra‐fast defensive responses to threat, thereby promoting survival. The innate alarm system (IAS) [Lanius et al., 2017; Liddell et al., 2005] model has been successful in identifying many components of the neurocircuitry underpinning this subconscious threat‐detection mechanism and its associated defensive responses. Nonetheless, the neurocircuitry underpinning the IAS is yet to be fully explored. Specifically, the role of critical midbrain structures such as the superior colliculus (SC) remains to be further elucidated in PTSD.
The SC is a powerful subcortical structure processing multisensory integration and sensorimotor transformations [King, 2004; May, 2006; Stein and Meredith, 1993]. Critically, extensive animal studies [Carello and Krauzlis, 2004; Comoli et al., 2012; Merker, 2013], more recently replicated in healthy humans [Gitelman et al., 2002; Krebs et al., 2010b; Steuwe et al., 2015; Vuilleumier, 2015], indicate that the SC is involved in a series of cognitive and motor processes relevant to threat‐detection mechanisms. Its involvement is also associated with a cluster of symptoms observed clinically in both active (fight/flight) and passive (emotional detachment with accompanying symptoms of depersonalization/derealisation) defensive responses [Harricharan et al., 2016; Kozlowska et al., 2015; Schauer and Elbert, 2010].
The SC plays a critical role in target selection [Gitelman et al., 2002; Krauzlis et al., 2004], a process central to threat detection. Both animal and human studies implicate the SC in tasks involving visual detection and recognition of threatening stimuli, such as snakes, face, and whole‐body emotional expressions [Almeida et al., 2015; Celeghin et al., 2015; Maior et al., 2012; Van den Stock et al., 2011], and in eye‐contact processing in healthy individuals [Senju and Johnson, 2009] and patients with PTSD [Steuwe et al., 2014]. Moreover, both animal and healthy human studies demonstrate that the SC processes target selection or visual detection and recognition of faces and whole‐body emotional expressions independently from cortical structures [Carello and Krauzlis, 2004; Celeghin et al., 2015; Merker, 2007; Van den Stock et al., 2011]. These findings are in line with the proposed central role of the SC in the fast subconscious threat‐detection pathway modeled in the IAS, itself hypothesized to operate at the subcortical level only, without initial engagement of cortical structures [Lanius et al., 2017].
The SC may also play a critical role in motor processes associated specifically with threat‐related defensive responses. Here, human studies point to the role of the SC in cognitive processes and in associated oculomotor tasks, including the allocation of attentional resources described recently in healthy humans [Krebs et al., 2010a, 2010b]. Together, these studies suggest the SC plays a central role in underpinning hypervigilance behaviours associated with heightened threat sensitivity in PTSD [Steuwe et al., 2014; Thome et al., 2014]. Critically, several studies [Vuilleumier, 2015] revealed recently that affective and motivational mechanisms may modulate collicular allocation of attentional resources and oculomotor behavior to emotionally salient information with either negative (threatening) or positive (rewarding) stimuli, a process most likely at play during hypervigilant behaviors. Moreover, as noted above, animal studies have demonstrated robustly that the SC computes motor outputs in response to detected sensory inputs, which are characterized by approaching or avoidance defensive movements [Comoli et al., 2012]. These findings suggest a strong linkage between hypervigilant behaviour and fast fight‐or‐flight active defensive responses operant at the SC [Kozlowska et al., 2015]. Notably, a previous study [Olive et al., 2015] points further to the role of the SC in passive defensive responses, characterized by detachment of emotion and anomalous bodily experience, including feelings of distortion of the body size, mass or shape, and out‐of‐body experiences [Lanius et al., 2006, 2012].
Taken together, both animal and human studies suggest that the SC may serve as a main processing hub underlying threat‐detection by igniting associated active and passive defensive responses in PTSD. This hypothesis is of particular relevance to understanding the role of the IAS in PTSD and its dissociative subtype. Yet, to date, there are no studies examining the collicular network in PTSD.
Accordingly, we sought to explore resting state functional connectivity in the SC among three groups: PTSD, its dissociative subtype (PTSD + DS), and healthy controls. We hypothesized the involvement of the SC during resting state, suggestive of defensive posturing at rest in PTSD [Harricharan et al., 2016; Lanius et al., 2017]. We expected further that functional connectivity with the SC and brain regions involved in hypervigilance and emotional anticipation, including the dorsolateral prefrontal cortex (DLPFC) [Aupperle et al., 2012; Herz et al., 2016], would emerge in the PTSD patient group, a population exhibiting predominantly active defensive responses. Moreover, we expected to observe functional connectivity between the SC and brain regions involved in depersonalization, with an emphasis on abnormal bodily self‐consciousness, that is, the temporoparietal junction (TPJ) [Blanke et al., 2005], in the PTSD + DS, where these individuals display predominantly passive defensive responses associated with depersonalization and derealization symptomatology [Kozlowska et al., 2015; Schauer and Elbert, 2010]. Overall, we hypothesized that together these connectivity patterns would identify the SC as the threat‐detection hub of the IAS, serving to rapidly ignite both active (hyperarousal) and passive (depersonalization/derealization) defensive mechanisms.
METHODS
Participants
One‐hundred and fifty‐eight age‐matched subjects were included in the study: 67 patients with a primary diagnosis of PTSD without the dissociative subtype (PTSD), 41 patients with a primary diagnosis of PTSD with the dissociative subtype (PTSD + DS), and 50 healthy control individuals. Of these, 86.5% of PTSD patients (PTSD‐DS and PTSD + DS) met criteria for interpersonal childhood trauma according to responses on the Childhood Trauma Questionnaire (CTQ) [Bernstein and Fink, 1998; DiLillo et al., 2006]. Participants were recruited from 2009 to 2016 via referrals from family physicians, mental health professionals, psychology/psychiatry clinics, community programs for traumatic‐stress survivors, and posters/advertisements within the London, Ontario community.
A primary PTSD diagnosis was determined using the CAPS‐4 (n = 133) or CAPS‐5 (n = 25; Clinician‐Administered PTSD Scale; CAPS‐4 cut‐off score ≥50) [Blake et al., 1995]. As per standard methods, PTSD patients with the dissociative subtype were further required to score at least “2” on both the frequency and intensity scales assessing depersonalization and derealization symptoms [Nicholson et al., 2015; Steuwe et al., 2014]. For each participant, comorbid Axis‐I disorders were assessed using the SCID (Structured Clinical Interview for DSM‐IV Axis I disorders) [First et al., 2002]. A battery of questionnaires was also administered, including the Beck Depression Inventory (BDI) [Beck et al., 1997], the CTQ [Bernstein and Fink, 1998], and the Multiscale Dissociative Inventory (MDI) [Briere et al., 2005]. State reliving and depersonalization/derealization symptoms experienced during the resting state scan were assessed using the Response to Script Driven Imagery Scale (RSDI), adapted to resting state [Hopper et al., 2007].
Exclusion criteria for all participants included the presence of metal implants that violate 3.0T scanner safety regulations, a previous head injury associated with loss of consciousness, current or past history of neurological disorders, significant untreated medical illness, and pervasive developmental mental disorders. PTSD patients were excluded further if they met criteria for current or past history of bipolar or psychotic disorders, or if patients had alcohol/substance dependency or abuse that had not sustained full remission for at least 6 months prior to study entry. Control participants were excluded if lifetime criteria were met for any Axis‐I psychiatric disorder.
All scanning took place at Robarts Research Institute's Center for Functional and Metabolic Mapping or at Lawson Health Research Institute in London, Ontario, Canada. The study was approved by the Research Ethics Board of Western University of Canada. All participants provided written informed consent to partake in the study.
Data Acquisition
Whole‐brain fMRI (functional magnetic resonance imaging) data was obtained using a 3.0T scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany) with a 32‐channel phased array head coil where the participant's head was supported with foam padding. BOLD (blood‐oxygen level dependent) fMRI data was collected using a manufacturer's standard gradient‐echo planar imaging (EPI) pulse sequence (single‐shot, blipped‐EPI) with an interleaved slice acquisition order with the following parameters: Time Resolution = 3,000 ms; Echo‐Time = 20 ms; voxel size = 2 × 2 × 2 mm3; Field of View = 192 × 192 × 128 mm3 (94 × 94 matrix, 64 contiguous slices); Flip Angle = 90°. High‐resolution T1‐weighted anatomical images were also obtained (MP‐RAGE: 192 slices, voxel size = 1 × 1 × 1 mm3). Resting state data was obtained for six minutes according to standard methods [Bluhm et al., 2009; Fransson, 2005].
Resting‐State fMRI Data Preprocessing
Image pre‐processing and statistical analyses were performed using statistical parametric mapping software (SPM12, Wellcome Trust Center for Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/spm) within MATLAB R2016b (Mathworks, MA). The functional images for each subject were realigned to the first functional image to correct for motion in the scanner and resliced. The mean functional image was created and then coregistered to the T1‐weighted structural image for each subject to spatially realign functional images to the subject's anatomical space. The coregistered images were segmented into gray matter, white matter, cerebrum spinal fluid, bone, soft tissue and air. The forward deformation fields were generated and used to spatially normalize the functional images to MNI space. In keeping with a previous SC functional neuroimaging study [Olive et al., 2015], the images were then smoothed with a three‐dimensional isotropic Gaussian kernel of 6mm FWHM (full‐width at half maximum). The smoothed functional images were further motion corrected with ART software [Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA], which generates outlier motion regressors that were used as a covariate of no interest during within‐subject (first‐level) analysis. The smoothed functional images were subsequently de‐noised with the Compcor method [Behzadi et al., 2007] and bandpass‐filtered to reduce the signal‐to‐noise ratio using 0.012 and 0.1 Hz as the high‐pass and low‐pass frequency cut‐offs, respectively [CONN toolbox, http://www.nitrc.org/projects/conn].
Seed‐Based Regions of Interest
Using the MRICROn toolbox developed by Chris Rorden [https://www.nitrc.org/projects/mricron], seed regions‐of‐interest (ROI) masks were generated separately for the left and right SC of each participant. This procedure followed the anatomical description provided in Martin [2012].
Statistical Analysis
Demographic and psychological measures
Quantile‐Quantile plots demonstrated that participants’ ages across all three groups were not normally distributed. Accordingly, a Kruskall‐Wallis test was performed to assess age differences across participant groups, and to ensure that groups were age‐matched. Critically, Levene's test of homogeneity of variances demonstrated that the principle of homogeneity of variances was violated in all tested measures. As such, a one‐way between‐groups analysis of variance (ANOVA), followed by post‐hoc Games‐Howell testing, was employed to assess between‐group differences for the following psychological measures: total CAPS‐IV scores, averaged MDI scores for trait depersonalization and derealization, state reliving and depersonalization and derealisation RSDI scores, BDI, and CTQ scores.
First‐level analysis
The individual bilateral SC ROI masks, created in MRICROn, generated time‐course‐of‐activation tables in WFU Pickatlas (http://fmri.wfubmc.edu/software/PickAtlas) that were associated with seed activity for all subjects based on whole‐brain resting state data. In‐house software developed by coauthor Dr. Jean Théberge read these tables and extracted a subject‐specific mean‐signal‐intensity time course, and output it in a format suitable for within‐subject multiple regression model along with ART movement regressors in SPM 12. Functional connectivity was then assessed using a voxel‐wise approach by calculating both positive and negative correlations between ROIs and other voxels of the brain.
Second‐level analysis
A mixed 3 × 2 ANOVA was conducted for the second‐level analyses. Whereas the between‐group factor GROUP consisted of three levels: PTSD, PTSD + DS, and healthy controls, the within‐group factor REGION consisted of two levels: left SC (lSC) and right SC (rSC). To determine significant gray matter clusters, a family wise error (FWE) whole‐brain corrected (P < 0.05) threshold was set for both the interaction and post‐hoc analyses. Post‐hoc t‐tests were used to assess connectivity patterns between and within each group and region. Results were explored at the whole‐brain activation level at P = 0.05 FWE‐corrected and through a ROI approach. Using the MRICROn toolbox developed by Chris Rorden (https://www.nitrc.org/projects/mricron), customized ROI masks were generated as 10 mm spheres for the right TPJ and the right DLPFC from coordinates reported in the literature [Chechlacz et al., 2012; Cieslik et al., 2013].
Correlational analysis
Using SPM12 multiple regression analyses, CAPS‐IV total scores, averaged MDI depersonalization and derealization scores, state reliving and depersonalization/derealization (during the resting state scan) RSDI scores were assessed as predictors of collicular connectivity among individuals in the PTSD and PTSD + DS groups.
RESULTS
Strikingly, initial analyses contrasting the PTSD and PTSD + DS patient groups revealed a preferential connectivity of the SC with frontal areas involved in emotion regulation (i.e., the DLPFC) in PTSD as compared with PTSD + DS. By contrast, as compared with PTSD, the PTSD + DS exhibited preferential SC connectivity with a region associated with depersonalization responses and somatic processing (i.e., the TPJ).
Between‐Group Analysis of Clinical Variables Scores at Behavioral Level
A one‐way between‐group ANOVA performed on total CAPS scores, followed by a post‐hoc Games‐Howell test (given heterogeneity of variance), yielded significant differences between all three groups. Here, the PTSD + DS group exhibited higher scores than the PTSD (P < 0.001) and the control (P < 0.001) groups. The PTSD group exhibited lower scores than the PTSD + DS group and higher scores than the control group only (P < 0.001).
A one‐way between‐group ANOVA performed on averaged RSDI items assessing state reliving symptoms during the resting state scan also violated the assumption of homogeneity of variance. Follow‐up post‐hoc Games‐Howell testing revealed a significant difference between the control and PTSD groups. Specifically, both the PTSD (P < 0.001) and PTSD + DS (P < 0.001) groups exhibited higher scores as compared to the control group. The same procedure was repeated with averaged RSDI assessing state depersonalization and derealization symptoms during the resting state scan. A significant difference between all three groups was observed. Here, the PTSD + DS group exhibited higher depersonalization and derealization RSDI scores as compared to the PTSD (P = 0.01) and control (P < 0.001) groups. Both RSDI results were obtained with partial sample analysis (control n = 49, PTSD n = 63, PTSD + DS n = 29). Similarly, the PTSD + DS group exhibited higher MDI depersonalization and derealization scores as compared to the PTSD (P < 0.001) and the control (P < 0.001) groups.
A one‐way between‐group ANOVA performed on CTQ scores violated the assumption of homogeneity of variance between the three groups. Follow‐up post‐hoc Games‐Howell testing yielded higher scores in the PTSD + DS group as compared to the PTSD group (P = 0.026) and the control group (P < 0.001). Similar patterns were observed for BDI scores. Please see Table 1 for details.
Table 1.
Description of groups
| (a) Demographic and clinical information | |||
|---|---|---|---|
| Measure | PTSD (n = 67) | PTSD + DS (n = 41) | Control (n = 50) |
| M ± SD | M ± SD | M ± SD | |
| Age | 37.59 ± 11.78 | 41.12 ± 13.34 | 35.2 ± 11.59 |
| Sex | 35 F/32 M | 33 F/8 M | 26 F/24 M |
| CAPS‐4 | (n = 53) 68.28 ± 13.40 | (n = 30) 81.6 ± 12.89 | (n = 50) 0.6 ± 2.7 |
| CAPS‐5 | (n = 14) 35.86 ± 8.6 | (n = 11) 39 ± 8.64 | – |
| CTQ | 55.63 ± 23.64 | 68 ± 18.57 | 32.1 ± 9.11 |
| BDI | 23.38 ± 7.78 | 33.66 ± 13.24 | 1.06 ± 2 |
| MDI DepDer | 7.8 ± 2.75 | 12.71 ± 4.48 | 5.2 ± 0.54 |
| RSDI DepDera | (n = 63) 3.5 ± 1.4 | (n = 29) 4.83 ± 2.02 | (n = 49) 2.66 ± 0.48 |
| RSDI Relivinga | (n = 63) 3 ± 1.31 | (n = 29) 3.34 ± 1.47 | (n = 49) 2 ± 0.28 |
| (b)Clinical variables statistics | ||||
|---|---|---|---|---|
| Variable | Levene's test | ANOVA | Games‐Howell | |
| M ± SE | ||||
| CAPS‐4 | F(2,130) = 50.109; P < 0.001 | NA | PTSD and PTSD + DS | Mean dif = 13.32 ± 2.98; P < 0.001 |
| PTSD and Control | Mean dif = 67.68 ± 1.9; P < 0.001 | |||
| PTSD + DS and Control | Mean dif = 81 ± 2.4; P < 0.001 | |||
| CAPS‐5 | – | – | PTSD and PTSD + DS | Mean dif = 3.14 ± 3.47; P = 0.829 |
| CTQ | F(2,147) = 25.643; P < 0.001 | NA | PTSD and PTSD + DS | Mean dif = 12.37 ± 4.2; P = 0.011 |
| PTSD and Control | Mean dif = 23.52 ± 3.27; P < 0.001 | |||
| PTSD + DS and Control | Mean dif = 35.89 ± 3.22; P < 0.001 | |||
| BDI | F(2,145) = 25.637; P < 0.001 | NA | PTSD and PTSD + DS | Mean dif = 9.2 ± 2.3; P = 0.001 |
| PTSD and Control | Mean dif = 22.32 ± 1.04; P < 0.001 | |||
| PTSD + DS and Control | Mean dif = 31.6 ± 2.11; P < 0.001 | |||
| MDI DepDer | F(2,149) = 36.63; P < 0.001 | NA | PTSD and PTSD + DS | Mean dif = 4.89 ± 0.79; P < 0.001 |
| PTSD and Control | Mean dif = 2.6 ± 0.35; P < 0.001 | |||
| PTSD + DS and Control | Mean dif = 7.5 ± 0.72; P < 0.001 | |||
| RSDI DepDera | F(2,138) = 24.327; P < 0.001 | NA | PTSD and PTSD + DS | Mean dif = 1.3 ± 0.4; P = 0.01 |
| PTSD and Control | Mean dif = 0.88 ± 0.18; P < 0.001 | |||
| PTSD + DS and Control | Mean dif = 2.2 ± 0.38; P < 0.001 | |||
| RSDI Relivinga | F(2,138) = 36.962; P < 0.001 | NA | PTSD and PTSD + DS | Mean dif = 0.4 ± 0.32; P = 0.438 |
| PTSD and Control | Mean dif = 0.91 ± 0.17; P < 0.001 | |||
| PTSD + DS and Control | Mean dif = 1.3 ± 0.27; P < 0.001 | |||
Abbreviations: CAPS, clinician administered PTSD scale; CTQ, childhood trauma questionnaire; BDI, beck depression inventory; MDI, multiscale dissociation inventory; DEP, depersonalization; DER, derealization; RSDI, the responses to script‐driven imagery scale; n, number of participants corresponding to a group; PTSD, nondissociative PTSD group; PTSD + DS, dissociative subtype PTSD group; Control, age‐matched control group; M, mean; SD, standard deviation; SE, standard error; NA, not applicable; CAPS‐5 assessed through independent 2‐sample T‐test.
RSDI scores were not available for the whole sample.
Within‐Group Results
Within‐group analyses revealed the presence of a strong collicular functional network in the PTSD + DS patient group that was not observed in the PTSD or the control group. Here, whole‐brain analysis (P fwe = 0.05) revealed that the PTSD + DS group demonstrated connectivity between the left SC and the left vermis 3 (MNI = −2 −38 −8; P fwe = 0.036) and between the right SC and the right caudate (MNI = 12 6 6; P fwe < 0.001). No suprathreshold clusters were found for the PTSD and control groups. Please see Table 2 for details.
Table 2.
Within‐group SC connectivity
| Contrast | Seed region | Target region | MNI | P fwe | Cluster size | T‐score | Z‐score |
|---|---|---|---|---|---|---|---|
| x y z | |||||||
| PTSD + DS | Right SC | Right Caudate | 12 6 18 | <.001 | 263 | 5.98 | 5.82 |
| 14 20 4 | |||||||
| PTSD + DS | Left SC | Left Vermis 3 | −2 −38 −8 | 0.036 | 32 | 4.59 | 4.51 |
| PTSD | Right SC | No suprathreshold clusters | – | – | – | – | – |
| PTSD | Left SC | No suprathreshold clusters | – | – | – | – | – |
| Control | Right SC | No suprathreshold clusters | – | – | – | – | – |
| Control | Left SC | No suprathreshold clusters | – | – | – | – | – |
Whole‐brain P fwe = 0.05 corrected for multiple comparisons, df = [1.0,310.0].
Abbreviations: PTSD, nondissociative PTSD group; PTSD + DS, dissociative subtype PTSD group; SC, superior colliculus; TPJ, temporoparietal junction; DLPFC, dorsolateral prefrontal cortex; MNI, Montreal Neurological Institute.
Between‐Group Results: Interaction and Main Effects
Analysis of the results at the whole‐brain level (P fwe = 0.05) revealed a significant interaction between the main factors of group (PTSD, PTSD + DS, Control) and of region (left SC, right SC). Main activation foci were in subcortical areas. Specifically, we observed significant activation in the left vermis (MNI = −2 −38 −8; P fwe < 0.001) and the right caudate (MNI = 6 6 2; P fwe = 0.004). Please see Table 3 for details.
Table 3.
Interaction factor
| Contrast | Seed region | Target region | MNI | P fwe | Cluster size | F‐score | Z‐score |
|---|---|---|---|---|---|---|---|
| x y z | |||||||
| Interaction | – | Left Vermis 3 | −2 −38 −8 | <0.001 | 279 | 12.08 | 6.87 |
| – | Right Caudate | 6 6 2 | 0.004 | 297 | 7.41 | 5.07 |
Whole‐brain P fwe = 0.05 corrected for multiple comparisons, df = [6.0,310.0].
Abbreviations: PTSD, nondissociative PTSD group; PTSD + DS, dissociative subtype PTSD group; SC, superior colliculus; TPJ, temporoparietal junction; DLPFC, dorsolateral prefrontal cortex; MNI, Montreal Neurological Institute.
Differential Functional Connectivity between PTSD, PTSD + DS, and Controls
A ROI analysis revealed that as compared with the PTSD + DS patient group, the PTSD group showed an increase in functional connectivity between the left SC and the contralateral right DLPFC (MNI = 30 36 30; P fwe = 0.021). By contrast, when compared with the PTSD group, the PTSD + DS group exhibited a lateralized increase in functional connectivity between the right SC and the right TPJ (TPJ‐r) (MNI = 44 −28 14; P fwe = 0.01).
When contrasted to healthy controls, neither PTSD nor PTSD + DS patient groups exhibited any suprathreshold clusters. Similarly, healthy controls, when compared with both PTSD and PTSD + DS patient groups, did not reveal any suprathreshold clusters. Please see Table 4 and Figure 1 for details.
Table 4.
PTSD, dissociative subtype PTSD, and controls: Between‐group differences in connectivity
| Contrast | Seed region | Target region | MNI | p fwe | Cluster size | Cluster size before ROI | T‐voxel | Z‐score |
|---|---|---|---|---|---|---|---|---|
| x y z | ||||||||
| PTSD + DS > PTSD | Right SC | right TPJ | 44 −28 14 | 0.01 | 19 | 55 | 3.22 | 3.19 |
| PTSD + DS > PTSD | Left SC | No suprathreshold clusters | – | – | – | – | – | – |
| PTSD > PTSD+DS | Right SC | No suprathreshold clusters | – | – | – | – | – | – |
| PTSD > PTSD + DS | Left SC | right DLPFC | 30 36 30 | 0.021 | 5 | 12 | 3.36 | 3.33 |
| PTSD + DS > Control | Right SC | No suprathreshold clusters | – | – | – | – | – | – |
| PTSD + DS > Control | Left SC | No suprathreshold clusters | – | – | – | – | – | – |
| PTSD > Control | Right SC | No suprathreshold clusters | – | – | – | – | – | – |
| PTSD > Control | Left SC | No suprathreshold clusters | – | – | – | – | – | – |
| Control > PTSD + DS | Right SC | No suprathreshold clusters | – | – | – | – | – | – |
| Control > PTSD + DS | Left SC | No suprathreshold clusters | – | – | – | – | – | – |
| Control > PTSD | Right SC | No suprathreshold clusters | – | – | – | – | – | – |
| Control > PTSD | Left SC | No suprathreshold clusters | – | – | – | – | – | – |
ROI analysis, P fwe = 0.025 corrected for multiple comparisons, df = [1.0,310.0].
Abbreviations: PTSD, non‐dissociative PTSD group; PTSD + DS, dissociative subtype PTSD group; SC, superior colliculus; TPJ, temporoparietal junction; DLPFC, dorsolateral prefrontal cortex; MNI, Montreal Neurological Institute.
Figure 1.
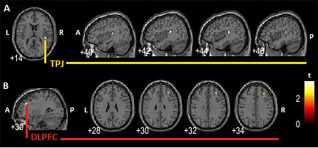
Between‐group comparison. A: PTSD + DS > PTSD, right SC increased resting state functional connectivity with ipsilateral rTPJ, a brain region implicated in depersonalization symptoms. B: PTSD > PTSD + DS, left SC increased resting state functional connectivity with contralateral right DLPFC. The right DLPFC has been involved in emotional modulation processes in the framework of PTSD. PTSD + DS: dissociative subtype PTSD group; PTSD: non‐dissociative PTSD group; SC: superior colliculus; P: posterior; A: anterior; L: left hemisphere; R: right hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
Clinical Variable Correlations to Functional Connectivity within the PTSD and PTSD + DS Groups
When evaluated as a predictor of collicular connectivity, averaged MDI depersonalization and derealization scores predicted right SC connectivity with the right TPJ (MNI = 40 −28 18; P fwe = 0.011) in the PTSD + DS group only (Table 5). Moreover, state reliving symptoms during the resting state scan predicted left SC connectivity with the right DLPFC (MNI = 26 58 −2; P fwe = 0.044) in the PTSD group. Please see Figure 2 for details.
Table 5.
Clinical variable prediction of SC connectivity by patient group
| Clinical Variable | Group | Seed region | Target region | MNI | P fwe | Cluster size | Cluster size before ROI | t‐Value | z‐Value |
|---|---|---|---|---|---|---|---|---|---|
| x y z | |||||||||
| MDI Dep/Der | PTSD + DS | Right SC | Right TPJ | 40 −28 18 | 0.011 | 13 | 19 | 3.95 | 3.58 |
| MDI Dep/Der | PTSD + DS | Left SC | No Suprathreshold clusters | – | – | – | – | – | – |
| State Reliving | PTSD | Right SC | No Suprathreshold clusters | – | – | – | – | – | – |
| State Reliving | PTSD | Left SC | Right DLPFC | 26 58 −2 | 0.044 | 4 | 8 | 3.05 | 2.85 |
ROI analysis, P fwe = 0.05 for multiple comparisons, df = [1.0,310.0].
Abbreviations: PTSD, nondissociative PTSD group; PTSD + DS, dissociative subtype PTSD group; SC, superior colliculus; TPJ, temporoparietal junction; DLPFC, dorsolateral prefrontal cortex; MNI, Montreal Neurological Institute.
Figure 2.
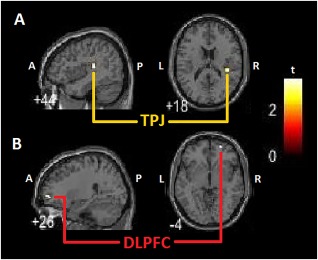
Clinical variable correlations to SC within‐group connectivity. A: PTSD + DS group MDI depersonalization and derealisation averaged scores predict right SC increased resting state functional connectivity with right TPJ. B: PTSD group in‐house questionnaire assessing reliving symptoms predicts left SC increased resting state functional connectivity with contralateral right DLPFC. PTSD + DS: dissociative subtype PTSD group; PTSD: non‐dissociative PTSD group; SC: superior colliculus; DLPFC: dorsolateral prefrontal cortex; TPJ: temporoparietal junction; MDI: multiscale dissociation inventory; P: posterior; A: anterior; L: left hemisphere; R: right hemisphere. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
Taken together, the resting state collicular functional connectivity patterns observed in this study implicate strongly the SC in PTSD and, in particular, highlight its prominent role in the functioning of the IAS. Here, we reviewed an extensive literature in animals and healthy individuals describing the multiple processes ascribed to the SC. This review led us to note the particular role of the SC in salient sensory event detection, providing a mechanistic explanation for the SC's contribution to the ultra‐fast pathway of subconscious threat detection modeled by the IAS. We suggest further that the differential collicular resting state functional networks observed in PTSD and its dissociative subtype relate to specific pathways in the IAS associated with triggering of group‐specific defensive responses. Specifically, it appears probable that the SC represents the key functional link in the IAS between threat‐detection mechanisms and the ignition of defensive responses in PTSD. The most striking finding in this respect concerns the differential functional connectivity of the SC with the frontal lobe (i.e., DLPFC), which was present in the PTSD group yet strikingly absent in its dissociative subtype where SC connectivity was observed with the TPJ. Following the framework of the IAS, frontal structures perform a central modulatory role associated with inhibition of limbic structures processing emotional responses, resulting in a characteristic under‐modulation in the PTSD group eliciting excessive hyperarousal responses. By contrast, over‐modulation observed in the dissociative subtype is thought to elicit emotional blunting associated with depersonalization processes [Lanius et al., 2017, 2010]. Taken together, our findings demonstrate that the SC is involved in the IAS frontal‐limbic pathway for modulation of emotional responses among the PTSD group only. In comparison, it appears the SC is involved in an alternative IAS pathway in the dissociative subtype group. Specifically, we propose that this pathway is related to depersonalization, with an emphasis on somatic abnormalities. Here, depersonalization responses are thought mediated by phylogenetically old areas of the brain, such as the brainstem, that are only activated secondary to previous deactivation of emotional processes [Kozlowska et al., 2015], that is, in neurobiological terms, after activity in the IAS frontal‐limbic pathway, where prefrontal dampening of limbic regions elicits emotional blunting [Kozlowska et al., 2015; Lanius et al., 2010].
Threat‐Detection Mechanisms at Rest
We have hypothesized the importance of a collicular target selection function as a potential IAS mechanism of threat‐detection in PTSD [Lanius et al., 2017]. Threat processing in PTSD involves the detection and evaluation of real external threats in order to determine whether or not an environment can be perceived as safe and trustworthy, where an individual's own safety will be ensured and exposure to threatening stimuli will be avoided [Kozlowska et al., 2015]. Ultimately, a real external threat will be detected thus eliciting a defensive response associated with the actual individual defense response a survivor has experienced during the traumatic event [Kozlowska et al., 2015].
Both animal and human studies have already implicated the SC in tasks involving visual detection and recognition of real external threatening stimuli, such as snakes, face or whole‐body emotional expressions [Celeghin et al., 2015; Maior et al., 2012; Van den Stock et al., 2011]. Taken together, these findings highlight the importance of the SC in threat processing within the IAS. Critically, SC activation during resting state in PTSD patients suggests further that threat detection mechanisms are operational even in the absence of real external threat stimuli [Harricharan et al., 2016; Lanius et al., 2017]. Even at rest, patients with PTSD may engage in a state of defensive posturing, consistently evaluating the safety of their environment. Moreover, as we will discuss in the following sections, the collicular connectivity pattern observed in PTSD and its dissociative subtype at rest suggest that this collicular threat detection mechanism has potentially the same capacity to activate the body's defense systems ignited normally by the presence of real external threat. This suggests further that PTSD patients maintain an increased defensive posturing despite the absence of overt threat. This pattern is consistent with data demonstrating periaqueductal gray (PAG) functional connectivity at rest with areas associated with emotional reactivity and defensive responses [Harricharan et al., 2016], as well as insular cortex connectivity at rest with areas associated with emotional reactivity and anomalous bodily self‐consciousness [Nicholson et al., 2016].
Engagement of Frontal‐Collicular IAS Pathways Involved in Active Defensive Strategies in the PTSD Patient Group
When contrasting the PTSD group with the PTSD + DS group, we observed connectivity between the left SC and the contralateral right DLPFC. Interestingly, this activation pattern suggests that in the PTSD group, the SC is engaged in an IAS pathway related to frontal modulation of emotional processes. A recent study by our group highlights the role of the right DLPFC in emotional regulation in PTSD patients. Here, we identified a dynamic bidirectional modulatory flow of information with the left amygdala, such that the right DLPFC downregulates the left amygdala in a top‐down fashion, and the left amygdala modulates activity in the right DLPFC in a bottom‐up manner [Nicholson et al., 2017b]. Nevertheless, in light of evidence demonstrating the involvement of the medial prefrontral cortex (mPFC) in emotion regulation associated with PTSD [Kozlowska et al., 2015; Lanius et al., 2010], it was hypothesized that the role of the bilateral DLPFC in emotional anticipation of negative images would reflect the engagement of cognitive control networks beneficial for emotional and cognitive function [Aupperle et al., 2012]. Importantly, the DLPFC cognitive control pathway is thought to work collaboratively with affective processing (mPFC and amygdala) networks [Aupperle et al., 2012]. Finally, the right DLPFC has been involved in emotional processes in PTSD, such as conditioned generalization of danger cues to benign stimuli that resemble aspects of the trauma cue [Kaczkurkin et al., 2017].
Taken together, these studies suggest that the functional connectivity observed here between the SC and the DLPFC is related to the flow of information about threat detected at the level of the SC, which, in turn, is thought to initiate direct or indirect modulatory activity of the DLPFC upon the IAS frontal‐limbic pathway of emotion regulation in the PTSD patient group. This pattern is consistent with the proposal that PTSD patients maintain a hypervigilant state even during rest that includes preparation for active defense strategies [Kozlowska et al., 2015]. Further support for this notion stems from our results demonstrating significantly higher state reliving scores during the resting scan in the PTSD group as compared with the controls. Moreover, patterns of frontal (DLPFC)‐collicular functional connectivity were predicted by the RSDI assessing state reliving symptoms.
Engagement of IAS Pathways Involved in Passive Defensive Strategies in the PTSD + DS Patient Group
When compared with the PTSD group, the PTSD + DS group did not exhibit functional connectivity between the SC and the frontal lobe, as was observed for the PTSD group when contrasted to PTSD + DS. These results suggest that the SC is not involved in IAS pathways of emotional regulation for the PTSD + DS patient group.
When contrasting the PTSD + DS to the PTSD group, we observed increased functional connectivity between the SC and the rTPJ. The right TPJ has been shown previously to play a critical role in bodily self‐consciousness [Ionta et al., 2014; Olive et al., 2015]. The right TPJ, has been associated extensively with bodily self‐consciousness in illusion studies conducted in healthy individuals, as well as in neurological patients [Blanke et al., 2004, 2005; Heydrich et al., 2011], and has been repeatedly associated with out‐of‐body experiences [Arzy et al., 2006; Heydrich et al., 2011], the latter being the defining symptom of the dissociative subtype [Lanius et al., 2012]. Here, one study reported specifically an increase in functional connectivity between the SC and right TPJ during an illusion paradigm manipulating body‐part ownership [Olive et al., 2015], the rubber hand illusion, which recently was shown to evoke strong responses in PTSD + DS patients [Hirschmann and Lev‐Ari, 2016; Rabellino et al., 2016].
Taken together, the observed functional connectivity between the right SC and the right TPJ for the PTSD + DS patient group suggests strongly that this pattern may represent an IAS pathway processing bodily self‐consciousness. A recent study from our group revealed increased connectivity between another important node of the IAS, the PAG, and the left TPJ in PTSD+DS [Harricharan et al., 2016]. The PAG has also been shown to integrate frontal‐limbic pathways of emotional regulation in PTSD [Nicholson et al., 2017a]. This suggests that PAG and SC connectivity with the left and right aspects of the TPJ, respectively, represent complementary pathways leading to the dysfunctional bodily self‐consciousness characterizing depersonalization, which constitutes a critical component of passive defensive responses to threat [Lanius et al., 2012]. As discussed previously, we posit that this IAS bodily self‐consciousness pathway would be ignited secondary to previous activity in the IAS frontal‐limbic pathway of emotional regulation eliciting emotional blunting in PTSD + DS patients. As such, based on the empirical evidence collected thus far, we hypothesize that connectivity between the right SC and the right TPJ would be secondary to connectivity in the pathway linking the PAG to the left TPJ. Further support for this notion stems from results demonstrating significantly higher depersonalization and derealization scores during the resting scan in the PTSD + DS group when compared to both the PTSD and the control groups. Critically, here functional connectivity between the right SC and the right TPJ was predicted by averaged MDI items assessing depersonalization and derealisation symptoms.
The Abundant Collicular Connectivity in the PTSD + DS Patient Group
Within‐group contrasts revealed that SC resting state functional connectivity shows particular relevance for the PTSD + DS group. Specifically, the left colliculus exhibited increased connectivity with the ipsilateral left vermis, and the right colliculus demonstrated the most abundant connections with the right caudate complex.
SC connectivity with the basal ganglia has been associated previously with processes of attentional control that operate independently from cortical attentional mechanisms [Zenon and Krauzlis, 2014]. More recently, these early studies were extended to demonstrate that this subcortical attentional network constituted by the SC and the basal ganglia, and specifically the caudate complex [Kim and Hikosaka, 2015] is engaged in affective and motivational control of visual attention [Vuilleumier, 2015]. Once again, these findings suggest an intrinsic role of the SC as a threat detection mechanism in hypervigilant states characteristic of PTSD. Future research examining collicular connectivity in association with visual attention is therefore warranted in PTSD and its dissociative subtype.
LIMITATIONS AND CONCLUSION
It is important to note that the current study is cross‐sectional in nature and therefore cannot make conclusions about cause and effect. In addition, this study does not allow any conclusions regarding directionality of connectivity. Future studies employing effective connectivity analysis to determine the directionality of influences between subcortical, including the SC, and cortical structures are urgently required. Moreover, the COMPCOR denoising method, used to avoid the emergence of type I error, is an extremely conservative method for examining subcortical structures, reducing the power of this analysis. This may explain the reduced size (k < 10) of some clusters reported in this study.
On balance, this study reveals novel findings highlighting the importance of examining subcortical functional connectivity networks in PTSD and its dissociative subtype during resting state. These results are of particular relevance for deepening our understanding of the functional architecture characterizing the IAS, where a main subcortical structure, the SC emerges as a potential core hub of mechanisms of threat‐detection in PTSD, which are operational even at rest and ready to ignite specific symptoms (i.e., hyperarousal and depersonalization) and defensive responses (active and passive) associated with PTSD and PTSD + DS, respectively. Taken together, these findings represent not only an important first step in identifying neural and behavioral targets for therapeutic interventions that address both active and passive defensive strategies in trauma‐related disorders but also point to the importance of considering therapies that target deep midbrain structures rather than solely focusing on cortical interventions.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENTS
The authors would like to thank Nancy Mazza and Suzy Southwell for technical support during development of the study and preparation of the manuscript.
REFERENCES
- Almeida I, Soares SC, Castelo‐branco M (2015): The Distinct Role of the Amygdala, Superior Colliculus and Pulvinar in Processing of Central and Peripheral Snakes. PLoS One 10:e0129949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzy S, Thut G, Mohr C, Michel CM, Blanke O (2006): Neural basis of embodiment: Distinct contributions of temporoparietal junction and extrastriate body area. J Neurosci 26:8074–8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, Cissell SH, Twamley EW, Thorp SR, Norman SB, Paulus MP, Stein MB (2012): Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry 69:360–371. [DOI] [PubMed] [Google Scholar]
- Beck AT, Guth D, Steer RA, Ball R (1997): Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther 35:785–791. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L (1998) Childhood trauma questionnaire: A retrospective self‐report. San Antonio, TX: Harcourt Brace & CO. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995): The development of a Clinician‐Administered PTSD Scale. J Trauma Stress 8:75–90. [DOI] [PubMed] [Google Scholar]
- Blanke O, Landis T, Spinelli L, Seeck M (2004): Out‐of‐body experience and autoscopy of neurological origin. Brain 127:243–258. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual‐leone A, Brugger P, Seeck M, Landis T, Thut G (2005): Linking out‐of‐body experience and self processing to mental own‐body imagery at the temporoparietal junction. J Neurosci 25:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RW, Theberge J, Lanius RA (2009): Alterations in default network connectivity in posttraumatic stress disorder related to early‐life trauma. J Psychiatry Neurosci 34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Briere J, Weathers FW, Runtz M (2005): Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. J Trauma Stress 18:221–231. [DOI] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ (2004): Manipulating intent: Evidence for a causal role of the superior colliculus in target selection. Neuron 43:575–583. [DOI] [PubMed] [Google Scholar]
- Celeghin A, De Gelder B, Tamietto M (2015): From affective blindsight to emotional consciousness. Conscious Cogn 36:414–425. [DOI] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Humphreys GW (2012): Neuroanatomical Dissections of Unilateral Visual Neglect Symptoms: ALE Meta‐Analysis of Lesion‐Symptom Mapping. Front Hum Neurosci 6:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2013): Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co‐activation‐based parcellation. Cereb Cortex 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Das Neves Favaro P, Vautrelle N, Leriche M, Overton PG, Redgrave P (2012): Segregated anatomical input to sub‐regions of the rodent superior colliculus associated with approach and defense. Front Neuroanat 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilillo D, Fortier MA, Hayes SA, Trask E, Perry AR, Messman‐Moore T, Fauchier A, Nash C (2006): Retrospective assessment of childhood sexual and physical abuse: A comparison of scaled and behaviorally specific approaches. Assessment 13:297–312. [DOI] [PubMed] [Google Scholar]
- First MBS RL, Gibbon M, Williams JB (2002): Structured clinical interview for DSM IV Axis I disorders, research version, non–patient edition (SCID–I/NP). New York: Biometrics Research. [Google Scholar]
- Fransson P (2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Parrish TB, Friston KJ, Mesulam MM (2002): Functional anatomy of visual seach: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage 15:970–982. [DOI] [PubMed] [Google Scholar]
- Harricharan S, Rabellino D, Frewen PA, Densmore M, Theberge J, Mckinnon MC, Schore AN, Lanius RA (2016): fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav 6:e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz N, Reuveni I, Goldstein A, Peri T, Schreiber S, Harpaz Y, Bonne O (2016): Neural correlates of attention bias in posttraumatic stress disorder. Clin Neurophysiol 127:3268–3276. [DOI] [PubMed] [Google Scholar]
- Heydrich L, Lopez C, Seeck M, Blanke O (2011): Partial and full own‐body illusions of epileptic origin in a child with right temporoparietal epilepsy. Epilepsy Behav 20:583–586. [DOI] [PubMed] [Google Scholar]
- Hirschmann S, Lev‐Ari L (2016): Learning the Rubber Hand Illusion: Implications for dissociative PTSD patients. J Trauma Stress Disor Treat 5:3. [Google Scholar]
- Hopper JW, Frewen PA, Van Der Kolk BA, Lanius RA (2007): Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script‐driven trauma imagery. J Trauma Stress 20:713–725. [DOI] [PubMed] [Google Scholar]
- Ionta S, Martuzzi R, Salomon R, Blanke O (2014): The brain network reflecting bodily self‐consciousness: A functional connectivity study. Soc Cogn Affect Neurosci 9:1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen‐Sturges T, Cooper SE, Sponheim SR, Lissek S (2017): Neural Substrates of Overgeneralized Conditioned Fear in PTSD. Am J Psychiatry 174:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O (2015): Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards. Brain 138:1776–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ (2004): The superior colliculus. Curr Biol 14:R335–R338. [DOI] [PubMed] [Google Scholar]
- Kozlowska K, Walker P, Mclean L, Carrive P (2015): Fear and the Defense Cascade: Clinical Implications and Management. Harv Rev Psychiatry 23:263–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, Carello CD (2004): Target selection and the superior colliculus: Goals, choices and hypotheses. Vision Res 44:1445–1451. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schoenfeld MA, Boehler CN, Song AW, Woldorff MG (2010a): The Saccadic Re‐Centering Bias is Associated with Activity Changes in the Human Superior Colliculus. Front Hum Neurosci 4:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Woldorff MG, Tempelmann C, Bodammer N, Noesselt T, Boehler CN, Scheich H, Hopf JM, Duzel E, Heinze HJ, Schoenfeld MA (2010b): High‐field FMRI reveals brain activation patterns underlying saccade execution in the human superior colliculus. PLoS One 5:e8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C (2006): A review of neuroimaging studies in PTSD: Heterogeneity of response to symptom provocation. J Psychiatr Res 40:709–729. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D (2010): Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 167:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Brand B, Vermetten E, Frewen PA, Spiegel D (2012): The dissociative subtype of posttraumatic stress disorder: Rationale, clinical and neurobiological evidence, and implications. Depress Anxiety 29:701–708. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Rabellino D, Boyd JE, Harricharan S, Frewen PA, Mckinnon MA (2017): The innate alarm system in PTSD: Conscious and subconscious processing of threat. Curr Opin Psychol 14:109–115. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM (2005): A direct brainstem‐amygdala‐cortical 'alarm' system for subliminal signals of fear. Neuroimage 24:235–243. [DOI] [PubMed] [Google Scholar]
- Maior RS, Hori E, Uribe CE, Saletti PG, Ono T, Nishijo H, Tomaz C (2012): A role for the superior colliculus in the modulation of threat responsiveness in primates: Toward the ontogenesis of the social brain. Rev Neurosci 23:697–706. [DOI] [PubMed] [Google Scholar]
- Martin JH (2012) Neuroanatomy: Text and atlas. New York: McGraw‐Hill Medical. [Google Scholar]
- May PJ (2006): The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151:321–378. [DOI] [PubMed] [Google Scholar]
- Merker B (2007): Consciousness without a cerebral cortex: A challenge for neuroscience and medicine. Behav Brain Sci 30:63–81. discussion 81–134. [DOI] [PubMed] [Google Scholar]
- Merker B (2013): The efference cascade, consciousness, and its self: Naturalizing the first person pivot of action control. Front Psychol 4:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M, Frewen PA, Theberge J, Neufeld RW, Mckinnon MC, Lanius RA (2015): The dissociative subtype of posttraumatic stress disorder: Unique resting‐state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40:2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Sapru I, Densmore M, Frewen PA, Neufeld RW, Theberge J, Mckinnon MC, Lanius RA (2016): Unique insula subregion resting‐state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res 250:61–72. [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Friston KJ, Zeidman P, Harricharan S, MsKinnon MC, Densmore M, Neufeld RW, Theberge J, Corrigan F, Jetly R, Spiegel D, Lanius RA (2017a): Dynamic causal modeling in PTSD and its dissociative subtype: bottom‐up versus top‐down processing within fear and emotion regulation circuitry. Hum Brain Mapp 38(11):5551–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Schmahl C, Theberge J, Neufeld RW, Mckinnon MC, Reiss J, Jetly R, Lanius RA (2017b): The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real‐time fMRI neurofeedback. Hum Brain Mapp 38:541–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive I, Tempelmann C, Berthoz A, Heinze HJ (2015): Increased functional connectivity between superior colliculus and brain regions implicated in bodily self‐consciousness during the rubber hand illusion. Hum Brain Mapp 36:717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D, Harricharan S, Frewen PA, Burin D, Mckinnon MC, Lanius RA (2016): “I can't tell whether it's my hand”: A pilot study of the neurophenomenology of body representation during the rubber hand illusion in trauma‐related disorders. Eur J Psychotraumatol 7:32918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer M, Thomas E (2010): Dissociation following traumatic stress: Etiology and treatment. Z Psychol/J Psychol 218:109–127. [Google Scholar]
- Senju A, Johnson MH (2009): The eye contact effect: Mechanisms and development. Trends Cogn Sci 13:127–134. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA (1993): The merging of the senses. Cambridge, Mass: MIT Press. [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Pannasch S, Beblo T, Reiss J, Lanius RA (2014): Effect of direct eye contact in PTSD related to interpersonal trauma: An fMRI study of activation of an innate alarm system. Soc Cogn Affect Neurosci 9:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Theberge J, Lanius RA (2015): Effect of direct eye contact in women with PTSD related to interpersonal trauma: Psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Res 232:162–167. [DOI] [PubMed] [Google Scholar]
- Thome J, Frewen P, Daniels JK, Densmore M, Lanius RA (2014): Altered connectivity within the salience network during direct eye gaze in PTSD. Borderline Personal Disord Emot Dysregul 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Stock J, Tamietto M, Sorger B, Pichon S, Grézes J, de Gelder B (2011): Cortico‐subcortical visual, somatosensory, and motor activations for perceiving dynamic whole‐body emotional expressions with and without striate cortex (V1). Proc Natl Acad Sci U S A 108:16188–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P (2015): Affective and motivational control of vision. Curr Opin Neurol 28:29–35. [DOI] [PubMed] [Google Scholar]
- Zenon A, Krauzlis R (2014): Superior colliculus as a subcortical center for visual selection. Med Sci (Paris) 30:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]


