Abstract
Adolescents’ exposure to community violence is a significant public health issue in urban settings and has been associated with poorer cognitive performance and increased risk for psychiatric illnesses, including PTSD. However, no study to date has investigated the neural correlates of community violence exposure in adolescents. Sixty‐five healthy adolescents (age = 14–18 years; 36 females, 29 males) from moderate‐ to high‐crime neighborhoods in Los Angeles reported their violence exposure, parents’ education level, and free/reduced school lunch status (socio‐economic status, SES), and underwent structural neuroimaging and intelligence testing. Violence exposure negatively correlated with measures of SES, IQ, and gray matter volume. Above and beyond the effect of SES, violence exposure negatively correlated with IQ and with gray matter volume in the left inferior frontal gyrus and anterior cingulate cortex, regions involved in high‐level cognitive functions and autonomic modulation, and previously shown to be reduced in PTSD and combat‐exposed military populations. The current results provide first evidence that frontal brain regions involved in cognition and affect appear to be selectively affected by exposure to community violence, even in healthy nondelinquent adolescents who are not the direct victims or perpetrators of violence.
Keywords: adolescent development, brain, post‐traumatic stress disorder, stress, voxel‐based morphometry
1. INTRODUCTION
Community violence exposure is a significant public health issue for urban adolescent populations (Stein, Jaycox, Kataoka, Rhodes, & Vestal, 2003) and is linked to poorer cognitive (Delaney‐Black et al., 2002; Sharkey, 2010) and psychological outcomes (Fowler, Tompsett, Braciszewski, Jacques‐Tiura, & Baltes, 2009). Such criminal activity is vastly disproportionate across communities. In certain urban communities, such as those in South Los Angeles, children and adolescents are exposed to the highest rates of violent crimes per capita, with rates of up to 188 violent crimes per 10,000 people per year, compared with the citywide average of 29, and some areas that experience almost none (Los Angeles Times, 2017).
The detrimental effects of stress on physical and mental health have long been recognized (Lupien, McEwen, Gunnar, & Heim, 2009; McEwen, 2000; Selye, 1936). It has been shown that chronic stress leads to a continuous release of glucocorticoids, which is associated with reduced neurogenesis and increased dendritic atrophy (Lupien et al., 2009). Volumetric reductions in regions particularly sensitive to the effects of glucocorticoids, such as the hippocampus (Sapolsky, Krey, & McEwen, 1985) and prefrontal cortex (Arnsten, 2009), have been related to chronic stress exposure (McEwen & Morrison, 2013; Sapolsky, Uno, Rebert, & Finch, 1990). This study focuses on community violence exposure as a form of chronic stress (for a review, see Tolan, 2016). This is supported by a wide body of literature demonstrating the negative effects of community violence at the cognitive (Delaney‐Black et al., 2002; Sharkey, 2010), psychological (Fowler et al., 2009; Margolin & Gordis, 2000), and endocrinological levels (Aiyer, Heinze, Miller, Stoddard, & Zimmerman, 2014; Kliewer, 2006; Wilson, Kliewer, Teasley, Plybon, & Sica, 2002). Importantly, the strength of an individual's stress response is determined in part by the ability to predict upcoming events and to exert control over the situation (de Kloet, Joëls, & Holsboer, 2005). As such, community violence exposure is likely to elicit a strong stress response due to its unpredictability and the low level of control an individual can exert over the situation.
Community violence exposure is likely higher in youths from low socioeconomic status (SES) backgrounds due to criminal and gang activity in the neighborhoods in which these youths live, though some youths will have witnessed more than others. Adolescents spend more time outside of their homes and away from their parents/caregivers than do children, making it likely that the community social environment may especially impact them. Adolescence is also a critical period in development, during which time individuals are particularly sensitive to social stimuli (Albert, Chein, & Steinberg, 2013) and may be particularly susceptible to the effects of social‐relational stress (Lupien et al., 2009). Adolescents, when compared with children or adults, show increased autonomic responses to social stress (Hollenstein, McNeely, Eastabrook, Mackey, & Flynn, 2012) and demonstrate increased and protracted release of stress‐related hormones (Romeo et al., 2014; Stroud et al., 2009). In addition, brain regions known to be the most sensitive to stress in adulthood, including the hippocampus, prefrontal cortex, and amygdala, all continue to mature during adolescence (Giedd & Rapoport, 2010; Gogtay et al., 2004). Given this constellation of factors, it is very possible that violence exposure presents an additional liability for brain development in adolescence, beyond that known to be associated with SES (Noble et al., 2015).
Previous neuroimaging studies of pediatric stress exposure have demonstrated that adverse experiences during childhood and adolescence correlate with smaller hippocampal and medial prefrontal gray matter volumes (Andersen et al., 2008; Dannlowski et al., 2012; Van Harmelen et al., 2010), and may increase the risk for psychiatric illnesses in adulthood (Kaufman, Plotsky, Nemeroff, & Charney, 2000). The majority of neuroimaging studies on the effects of stress in pediatric and adult populations have used clinical samples, specifically post‐traumatic stress disorder (PTSD). In a recent review of neuroimaging studies of child abuse (Hart & Rubia, 2012), 27 of the 29 structural magnetic resonance imaging (MRI) studies reviewed included participants with psychopathology, while the remaining two did not report psychiatric diagnoses. As these studies mainly used clinical samples, the results may be due to stress, the psychiatric condition, or an interplay between the two. In addition, the majority of pediatric stress exposure studies have investigated the effects of abuse and neglect experienced within the home during childhood and adolescence. In this study, we assessed healthy adolescents only, without any current or previous psychiatric diagnosis and without a history of abuse or neglect. We consider the current sample to represent an important extension of previous studies of stress exposure in human adolescents, as the effects observed should not be confounded by interactions with psychiatric disorders or factors related to abuse experienced within the home.
In this research, we tested whether community violence exposure correlates with cognitive ability and gray matter volume in a healthy nondelinquent adolescent population (participants recruited were in full‐time education, passing all courses, and not the subject of disciplinary action; they reported no use of drugs or alcohol). We hypothesized that community violence exposure would correlate with lower IQ and reduced prefrontal and hippocampal gray matter in regions similar to those observed in childhood maltreatment and PTSD, even after controlling for SES (parental education levels and eligibility for free or reduced‐price school lunch). Structural MRI, demographic, and cognitive ability data were collected. Whole‐brain analysis and a region of interest approach were used to investigate the neural hypothesis.
2. METHODS AND MATERIALS
2.1. Participants
Sixty‐five right‐handed adolescents (55% female, 45% male; 45% Latino, 52% Asian American, 3% African American) were recruited from public high schools in low‐SES Los Angeles neighborhoods (mean age = 15.8 years, SD = 1.1, ranging between 14 and 18). These neighborhoods have high levels of immigrants and all participants had at least one immigrant parent. Enrollment criteria included no history of neurological or psychiatric disorders, physical or emotional abuse or neglect, use of psychotropic medication, drugs or alcohol, or presence of a medical condition that would preclude scanning. Criteria also included full‐time enrollment in school, passing all classes, and not under any disciplinary action. All participants and their parents gave written informed consent in accordance with the requirements of the Institutional Review Board of the University of Southern California (USC).
Prior to the study, adolescent participants filled eligibility questionnaires, and then they and their parents separately underwent structured private interviews to determine in more detail the status of the participant's home situation, emotional wellbeing, social relations at home and at school, drug and alcohol use, and future life plans. By their own confidential report, all reported stable, safe homes and relationships, and positive plans for the future. None of the participants reported any history of drug or alcohol use, none had perpetrated violence or other criminal acts, and none had been physically assaulted. All participants were therefore included in the analysis.
The neural and violence exposure data for the current study were collected as the first wave of a longitudinal project investigating psychosocial and neurobiological aspects of social emotional development in adolescence. Sample size was determined to allow sufficient power to detect neurobiological effects. A sample size of 62 or higher would allow us to detect medium to large effect sizes (r ≥ .3), with a voxel‐wise threshold of p < .005, as can be expected based on a comparison of non‐PTSD trauma‐exposed individuals and healthy controls from a meta‐analysis of PTSD neuroimaging studies (Karl et al., 2006).
2.2. Community violence exposure
Cumulative lifetime community violence exposure was assessed via a 13‐item modified version of the Survey of Children's Exposure to Community Violence – self‐report version (Margolin et al., 2009). Given that no externally verifiable measure for individual community violence exposure exists, self‐report arguably provides the best practical index, and is commonly used in studies of community violence exposure (McDonald & Richmond, 2008). Exposure to violence was measured by providing participants with a list of 13 events that involved either victimization (e.g., Item 9. Has anyone ever threatened to beat you up?) or witnessing and/or hearing about violence (e.g., Item 13. Have you ever witnessed or heard about someone being shot?), as previous work has demonstrated that both being a victim and being a witness must be considered to capture the full impact of violence exposure (Rosenthal, 2000). For each item, participants received a score from 1 (“Never”) to 4 (“More than twice”), giving a potential final score of between 13 and 52. Participants scored an average 24.8 (SD = 8.9, ranging between 13 and 47) on the violence exposure questionnaire. After completing the questionnaire, each participant then underwent a private interview to confirm their answers and to determine whether the reported violence exposure occurred in the community (neighborhood or school), in the home, or in the media (e.g. on television). Incidents that happened in the home and involved family members would disqualify the participant (no incidents in the home were reported). Incidents witnessed in the media were not counted toward the violence exposure score.
2.3. Socioeconomic status (SES)
All subjects were recruited from public schools in low‐SES neighborhoods; however, to get insight into further variation within this sample, we also collected information on (1) whether participants received free or reduced‐price school meals and (2) highest parental education level. The school meals program provides government‐subsidized meals for students from financially low‐resourced families (annual family income ≤130% of federal poverty line for free meals, ≤185% of federal poverty line for reduced‐price meals; https://www.fns.usda.gov/school-meals/applying-free-and-reduced-price-school-meals). Forty‐six participants reported receiving free meals, 5 reported receiving reduced‐price meals, and 14 reported paying full price for meals. (As only 5 participants received reduced‐price lunch, free and reduced‐price categories were collapsed.) Following work by Noble et al. (2015), parental education level was recoded as years of formal education (Supporting Information, Table S1). Average reported parental education was 12.3 years, SD = 3.9, ranging between 8 and 18 years. Parental education data were missing from four Latino participants. Their parental years of education were imputed as the mean of Latino participants in their neighborhood (10 years of schooling).
2.4. Cognitive ability
Participants completed the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence – Second Edition (WASI‐II; Wechsler, 2013). Owing to funding and design considerations for the bigger longitudinal project, this was collected at a follow‐up measurement, ∼2 years following collection of the neural and violence exposure data. Participants had an average Full Scale IQ (FSIQ) score of 105.3 (SD = 11.3, ranging between 79 and 131). WASI data for 5 participants were not available, giving a final sample size of 60.
2.5. MRI data acquisition
Structural images were collected on a Siemens 3 T MAGNETON TIM Trio scanner (Erlangen, Germany) with a 12‐channel matrix coil at the Dana and David Dornsife Neuroimaging Center, University of Southern California. The images were obtained using a three‐dimensional T1‐weighted magnetization prepared gradient‐echo sequence (MPRAGE) (repetition time = 2,530 ms, echo time = 3.09 ms, TI = 800 ms, acquisition matrix = 256 × 256 × 176, flip angle = 10°; 1 × 1 × 1 mm3 voxel size).
2.6. MRI data preprocessing
Prior to processing, raw images were visually inspected for motion artifacts, quality and gross anatomical abnormalities. In addition, following processing, segmented and normalized data were visually inspected. No issues with artifacts, data quality, anatomical abnormalities, or segmentation and normalization were identified. Structural data were processed with voxel‐based morphometry (VBM8; http://dbm.neuro.uni-jena.de/vbm.html) and statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) using default parameters running on MATLAB 9.1 (Mathworks, Sherborn, MA). VBM is a neuroimaging analytic technique that allows whole‐brain investigation of focal differences in brain anatomy based on statistical parameter mapping of structural images (Ashburner & Friston, 2000; Mechelli, Price, Friston, & Ashburner, 2005). Images were normalized to Montreal Neurological Institute (MNI) space and segmented into gray matter, white matter, and cerebrospinal fluid based on voxel signal intensity and a priori expectation of tissue type based on anatomical location, using default parameters. Modulation was applied to preserve the volume of a particular tissue within a voxel by multiplying voxel values in the segmented images by the Jacobian determinants derived from the spatial normalization step. Images were smoothed with a full‐width at half‐maximum kernel of 8 mm.
2.7. MRI data analysis
Statistical analysis was conducted on preprocessed gray matter images using SPM8.
2.8. Whole‐brain analysis
A whole‐brain voxel‐wise multiple regression with community violence exposure was computed. Whole‐brain analysis allows for unbiased and unconstrained characterization of anatomy, rather than restricting analysis to a priori hypothesized regions of interest (Friston, Rotshtein, Geng, Sterzer, & Henson, 2006). Parental education level, eligibility for free/reduced price lunch, gender, ethnicity, age (in days), and total gray matter volume were included as covariates of no interest. Parental education level and eligibility for free/reduced price lunch were included to control for known effects of SES on brain development (Noble et al., 2015). Total gray matter volume was used as a covariate of no interest to allow detection of regional specific effects that are independent from global differences in gray matter volume (Mechelli et al., 2005; Peelle, Cusack, & Henson, 2012). Ethnicity was included to control for potential morphological differences between the ethnic groups (Bai et al., 2012; Chee, Zheng, Goh, Park, & Sutton, 2011; Isamah et al., 2010). Gender was included to control for differences in mean gray matter volume and developmental trajectories between males and females (Lenroot et al., 2007). An absolute gray matter probability threshold of 0.4 was applied. The resulting maps were thresholded at p < .005 at the voxel level and cluster‐extent thresholded at expected voxels per cluster according to random field theory (k = 205) in combination with correction for nonisotropic smoothness (implemented in VBM 8; Hayasaka & Nichols, 2004) to control for type‐I error at α = .05.
2.9. Region of interest (ROI) analysis
Separate anatomical masks for the bilateral hippocampus and amygdala were anatomically defined using the anatomical automatic labeling (AAL; Tzourio‐Mazoyer et al., 2002) template. The Region‐of‐Interest Extraction Toolbox (REX; Whitfield‐Gabrieli, 2009) was used to extract gray matter volumes from the identified clusters.
3. RESULTS
3.1. Socioeconomic status (SES)
Parental education level was negatively associated with community violence exposure, and positively associated with FSIQ (Table 1). Compared to participants who paid full price for lunch, participants who received free/reduced lunch on average had more community violence exposure (t 44 = 2.38, p = .02, 95% CI [.68, 8.10], Hedges' g = 0.50; Levene's test indicated unequal variances, F 1,63 = 7.36, p = .009, so degrees of freedom were adjusted from 63 to 44) and lower FSIQs (t 58 = −2.98, p = .004, 95% CI [−16.41, −3.21], Hedges' g = 0.91).
Table 1.
Correlation coefficients with 95% confidence intervals for the relationships between community violence exposure, IQ, and SES
| Variable | 1 | 2 | |
|---|---|---|---|
| 1. | Community violence | – | |
| 2. | Parental education (years) |
−.438** [−.62, −.22] |
|
| 3. | FSIQ |
−.338** [−.55, −.09] |
.458** [.23, .64] |
Note. Bivariate correlations are presented. FSIQ data were missing for 5 participants. Correlations for variable 3 are based on 60 subjects, while correlations between variables 1 and 2 are based on 65 subjects.
**p < .01.
3.2. Cognitive ability
FSIQ score was negatively associated with community violence exposure (Table 1), and this relationship remained significant when controlling for SES, age, and gender (one‐tailed, p = .04; Figure 1).
Figure 1.
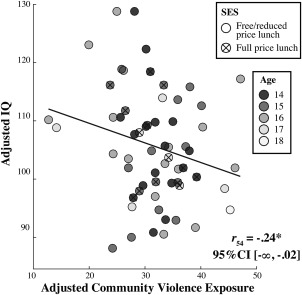
Association between community violence exposure and FSIQ. A scatter plot with the best‐fitting regression line illustrates the negative association between FSIQ and community violence exposure, controlling for SES (parental education level and eligibility for free/reduced school lunch), age, and gender. FSIQ and community violence exposure values are adjusted for parental education level, eligibility for free/reduced school lunch, age, and gender. The correlation coefficient is reported, along with the lower bound one‐tailed 95% confidence interval in brackets. For illustration purposes, participants are labeled by school lunch status and age in years. *p < .05, one‐tailed
3.3. Neuroimaging
Controlling for SES (parental education and free/reduced lunch), gender, age, ethnic group, and whole‐brain gray matter volume, whole‐brain regression analysis yielded a significant negative association between community violence exposure and gray matter volume in the left anterior cingulate cortex (ACC) and the left inferior frontal gyrus (IFG; see Figure 2a and Table 2). No region showed a significant positive association between gray matter volume and community violence exposure. For visualization purposes, Figure 2b shows the average gray matter volume of the clusters plotted against community violence exposure. Supporting Information, Figure S1 presents scatterplots for each region separately.
Figure 2.
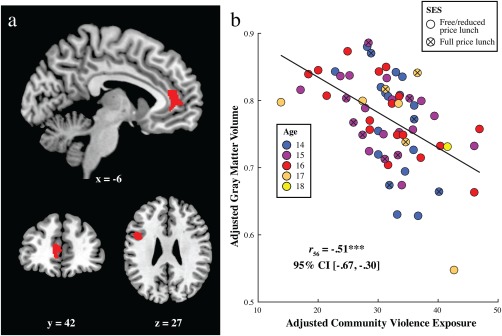
Association between community violence exposure and gray matter volume. (a) The brain image displays negative correlations between community violence exposure and gray matter volume, controlling for SES (parental education level and free/reduced lunch status), total gray matter volume, age, gender, and ethnicity, in the left anterior cingulate cortex (ACC, Montreal Neurological Institute coordinates: x = −10.5, y = 45, z = 7.5; k = 471) and left inferior frontal gyrus (IFG; Montreal Neurological Institute coordinates: x = −49.5, y = 10.5, z = 27; k = 325). Clusters that exceeded a voxel level threshold of p < .005 and a cluster size of k > 205 (expected voxels per cluster and nonisotropic smoothness corrected) are displayed. (b) A scatter plot with the best‐fitting regression line reillustrates the association between community violence exposure and average gray matter volume across the clusters depicted in (a). Gray matter and community violence exposure values are adjusted for parental education level, eligibility for free/reduced school lunch, total gray matter, age, gender, and ethnicity. The correlation coefficient is reported, along with the 95% confidence interval in brackets. For illustration purposes, participants are labeled by school lunch status and age in years. ***p < .001
Table 2.
Brain regions showing significant relationships to violence exposure
| Region | Side | BA | Voxels | x | y | z | r |
|---|---|---|---|---|---|---|---|
| ACC | Left | 32 | 445 | −10 | 45 | 7 |
−.41*** [−.59, −.18] |
| IFG | Left | 44 | 325 | −48 | 9 | 27 |
−.47*** [−.64, −.26] |
Note. Abbreviations: ACC = anterior cingulate cortex; IFG = inferior frontal gyrus; BA = Brodmann's Area.
Correlations between community violence exposure and gray matter volume from clusters identified as significant in the whole‐brain analysis. Results are presented controlling for parental education level, eligibility for free/reduced school lunch, total gray matter volume, age, gender, and ethnicity, with 95% confidence intervals in brackets. Clusters that exceeded a voxel‐level threshold of p < .005 and a cluster size of k > 205 (expected voxels per cluster and nonisotropic smoothness corrected) are reported. Peak coordinates are given in Montreal Neurological Institute (MNI) space.
***p < .001.
ROI analyses revealed no effects in the hippocampus (r 56 = 0.12, p = .38 95% CI [−.15, .37]) or amygdala (r 56 = −.03 p = .80, 95% CI [−.29, .23]). As in the whole‐brain analysis, we controlled for SES, total gray matter volume, ethnicity, age, and gender. We also tested whether FSIQ was associated with gray matter volume and found no significant results in whole‐brain or ROI analyses.
For completeness, we repeated the analyses of violence exposure on gray matter volume controlling for FSIQ and the results were essentially unchanged. We tested for violence exposure by SES interactions on FSIQ and on gray matter volume for the clusters identified in the whole‐brain analysis, and for the amygdala and hippocampus, and found none.
4. DISCUSSION
Community violence exposure was associated with lower IQ and smaller gray matter volume in left ACC and left IFG, even after controlling for SES (parental education levels and free/reduced school lunch status, a measure of household income relative to needs). Our findings fit with literature showing that community violence exposure is associated with worse cognitive outcomes (Delaney‐Black et al., 2002; Ratner et al., 2006; Sharkey, 2010). Notably, our finding in the ACC overlaps with results from previous studies of soldiers deployed to war (Butler et al., 2017) and of a PTSD meta‐analysis (Kühn & Gallinat, 2013). Figure 3 provides the graphical overlay of the current results with results from a study of military deployment (Butler et al., 2017) and from a PTSD meta‐analysis (Kühn & Gallinat, 2013).
Figure 3.
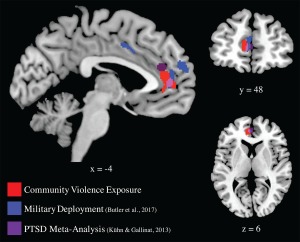
Community violence exposure, military deployment, and PTSD. Brain images display the negative correlations between community violence exposure and gray matter volume from the current study in red; results from a previous neuroimaging study showing a negative correlation between military deployment and gray matter volume (Butler et al., 2017) in blue; and results from a PTSD meta‐analysis (Kühn & Gallinat, 2013) in magenta. Note the overlapping results in anterior cingulate cortex
The ACC is part of the autonomic regulatory system and, as a centrally connected cortical “hub,” is involved in multiple processes that integrate emotion and cognition, including pain processing, arousal, modulation of memory and internal emotional responses (Bush, Luu, & Posner, 2000; Devinsky, Morrell, & Vogt, 1995), and processing social emotions (Immordino‐Yang, McColl, Damasio, & Damasio, 2009). The ACC has been previously implicated in the neurocircuitry of PTSD (Rauch, Shin, & Phelps, 2006), and smaller volumes in the ACC have been linked to depression (Drevets, Savitz, & Trimble, 2008; Frodl et al., 2008; Grieve, Korgaonkar, Koslow, Gordon, & Williams, 2013) and to PTSD (Kühn & Gallinat, 2013). The left IFG is involved in cognitive control and language production, and damage to the left IFG has been associated with cognitive and emotional impairments including reductions in emotional empathy (Shamay‐Tsoory, Aharon‐Peretz, & Perry, 2009). In addition, activation of the left IFG has been associated with cognitive control and response inhibition in children (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002).
As adolescence is a sensitive period in brain development, this age group may be particularly vulnerable to the effect of stress on the brain and cognitive functions. Though loss of gray matter volume is a normal part of brain maturation in adolescence (Gogtay et al., 2004), a longer trajectory of maturation, that is, slower loss of gray matter, has been associated with higher cognitive function (Shaw et al., 2006), while faster maturation, specifically myelination of white matter, has been associated with increased risk taking and higher rates of alcohol use disorder (Berns, Moore, & Capra, 2009; De Bellis et al., 2008). In particular, it has been hypothesized that a prolonged phase of prefrontal maturation may provide an extended period for the development of high‐level cognitive functions (Shaw et al., 2006), although there is some debate in the literature regarding this interpretation (Lu & Sowell, 2009), with some studies linking thinner cortex with higher intelligence in childhood and adolescence (e.g., Schnack et al., 2015). Our findings suggest that violence exposure during adolescence may be associated with less gray matter volume in certain frontal regions, though we cannot discern whether this finding is due to stress‐related loss, or to another process, such as accelerated maturation. Future work should investigate whether violence‐exposure‐related gray matter loss prematurely attenuates the period of adolescent neural plasticity thought to be important for the development of higher executive and cognitive functions. Although no relationship between IQ and gray matter volume was found in this study, possibly due to the cross‐sectional design, the age range of the sample or the two‐year delay between neuroimaging and IQ testing, our finding that violence exposure correlates with lower IQ would accord with this interpretation.
This study purposefully excluded individuals with current or previous psychiatric disorders and engaged participants and their parents in detailed private interviews about their emotional wellbeing and social relationships; however, there was no measure of symptoms of psychiatric disorders at a subclinical level. As such, we do not know whether smaller gray matter volumes or higher community violence exposure correlate with increased, but subclinical, psychological symptoms in the current sample. In addition, although participants reported no current or historic psychiatric disorders, participants did not complete a full clinical screening and it is possible that some individuals had an undiagnosed clinical disorder. It is also possible that these youths’ family relationships, or some other factor, effectively buffered them. Previous studies have shown that community violence exposure is associated with increased risk for psychiatric illness (McDonald & Richmond, 2008), but also that parental warmth is protective (Chen, Miller, Kobor & Cole, 2011). Stress exposure has previously been shown to correlate with smaller PFC gray matter volumes and increased PTSD symptoms in a subclinical population (Butler et al., 2017). Future work should investigate any link to subtle psychological symptoms associated with gray matter reductions in this age group.
In this study, we were particularly interested in demonstrating that community violence correlates with smaller gray matter volume and lower IQ in healthy, nondelinquent adolescents who did not perpetrate violence. Although structural deficits in the ACC have previously been observed among youth with antisocial or aggressive behavior (Ducharme et al., 2011; Gavita, Capris, Bolno & David, 2012; Hyatt, Haney‐Caron & Stevens, 2012), adolescents engaging in antisocial behavior were excluded. It is possible the inclusion and exclusion criteria lead to a truncation of the distribution, and that some individuals with community violence exposure and smaller frontal gray matter volume may also engage in antisocial or delinquent behavior. Further work may seek to replicate or extend the current findings in delinquent populations, and to test the possibility that violence exposure may produce subtle antisocial tendencies even in healthy, high‐functioning adolescents like those in our sample.
In addition, the current sample was recruited from low‐SES neighborhoods in Los Angeles, but some individuals’ families were experiencing more severe financial difficulty than others’. We note that the portion of the sample reporting the highest levels of violence exposure was in this lower SES group, which also tended to be disproportionately Latino (reflecting the lower SES status of Latinos compared to Asians in these neighborhoods). These findings leave open the future question of whether there are differences in the impact of violence by race/ethnic background, or by socioeconomic circumstances. Previous work suggests that under conditions of economic disadvantage, environmental conditions have greater impact on IQ scores (Tucker‐Drob & Bates, 2015; Turkheimer, Harden, D'Onofrio, & Gottesman, 2009). Future research could begin to tease apart how the effects of violence exposure may disproportionately impact youth depending on their ethnic background, cultural orientation, and associated differences in demographics and access to resources.
No correlation was observed between violence exposure and hippocampal or amygdala volume at the whole brain or ROI level. Preliminary evidence suggests that abuse during childhood and early adolescence is associated with reduced hippocampal and amygdala volume while stress during middle adolescence is associated with reduced frontal cortical volume (Andersen et al., 2008; Lupien et al., 2009), although one should also note that hippocampal deficits are not consistently observed in pediatric populations (Bick & Nelson, 2016; De Bellis, Hall, Boring, Frustaci, & Moritz, 2001; Woon & Hedges, 2008). Alternatively, it is possible that due to the current sample size, we were underpowered to detect smaller effects. We note that imposing a slightly lower voxel‐level height threshold before stringently controlling for cluster extent and type‐1 error rate produces additional findings in the dorsomedial and ventromedial prefrontal cortices, and right inferior frontal gyrus in our sample (Supporting Information, Figure S2). These regions should be examined in future studies.
It has previously been shown that stress‐related changes in brain structure are reversible and can be altered by psychological and behavioral interventions (McEwen, 2007). Therefore, although we find that community violence exposure has negative effects at the neural and cognitive levels, it is unclear whether and how these effects may be reversed or buffered. Additional work on possible interventions and supports that school and community service programs could provide against the long‐term effects of violence exposure on the brain, and on cognitive and psychiatric outcomes, is required.
A potential limitation of this research is that individuals were assessed cross‐sectionally and that the reported associations between brain structure, community violence, and psychological symptoms represent interpersonal differences whose etiology has not been observed. Therefore, although we may hypothesize based on animal models that increases in stress exposure lead to decreases in gray matter volume (Lupien et al., 2009; Sapolsky et al., 1985; Selye, 1936), it is also possible that lower gray matter volume and lower cognition were present prior to community violence exposure, or even predisposed youths to exposure. For example, though no neural data were included, Danese et al. (2017) demonstrated that cognitive deficits in victimized adolescents were nonsignificant after controlling for SES, parents’ IQ, and the adolescents’ cognitive functioning in childhood. Though our neural study controlled for participants’ adolescent IQ and for SES, including parental education (which is a significant predictor of children's IQ; e.g., Neiss & Rowe, 2000), we did not assess childhood cognitive functioning or neural structure in our sample. Longitudinal data are needed to learn more about the temporal dynamics and possible reciprocal causal effects of early interpersonal differences in neural development, cognition, and context on individuals’ later vulnerability to violence exposure. In addition, the relationship between stress and brain volume has previously been shown to be mediated by alterations in stress hormones, such as cortisol (Lupien et al., 2009; Sapolsky, 1999; Sapolsky, Krey, & McEwen, 1986). However, no hormonal data were collected for the current sample. Future work that incorporates hormonal or other biological measures of long‐term stress exposure is required.
This is the first structural MRI study to provide evidence of a relationship between community violence exposure and adolescent brain morphology. Previous studies have linked community violence exposure to increases in psychological symptoms and decreases in attention and cognition. The current results provide first evidence that regions involved in cognition and affect, and that are implicated in PTSD, appear to be selectively affected by exposure to community violence, even in healthy, nondelinquent adolescents who do not perpetrate violence and who have not been direct victims. The present findings serve to underscore the need for greater understanding of how decreases in frontal gray matter volume may contribute to the functional deficits and cognitive effects observed in this and previous studies. These findings emphasize the need for greater recognition of the impact of community violence on adolescents’ brain, cognition, and affect and the urgency and importance of greater preventative measures and targeted interventions.
DECLARATION OF CONFLICTING INTERESTS
All authors report no biomedical financial interests or potential conflicts of interest.
FUNDING
OB received funding from the International Max Planck Research School on the Life Course (LIFE). CL received funding from the Max Planck Society and the International Max Planck Research School on the Life Course (LIFE). SK has been funded by a Heisenberg grant from the German Science Foundation (DFG KU 3322/1‐1), the European Union (ERC‐2016‐StG‐Self‐Control‐677804) and the Jacobs Foundation (JRF 2016–2018). Data collection and analysis were supported by a U.S. National Science Foundation CAREER award (#11519520) to MHI‐Y, by the Brain and Creativity Institute Research Fund, by the University of Southern California Rossier School of Education, and by the USC Provost.
AUTHOR CONTRIBUTIONS
MHI‐Y and X‐FY designed and carried out the experiment. OB, MHI‐Y, X‐FY, CL, and SK analyzed the data and drafted the manuscript. All authors made final comments on the manuscript.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
The authors thank Christa Simone and Laura Garcia Cardona for their assistance with data collection, Vivian Rotenstein for her assistance with the violence exposure analysis, and Sarah Polk for her assistance with the neuroimaging analysis.
Butler O, Yang X‐F, Laube C, Kühn S, Immordino‐Yang MH. Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Hum Brain Mapp. 2018;39:2088–2097. 10.1002/hbm.23988
Funding information International Max Planck Research School on the Life Course (LIFE); Max Planck Society; German Science Foundation, Grant/Award Number: DFG KU 3322/1‐1; European Union, Grant/Award Number: ERC‐2016‐StG‐Self‐Control‐677804; Jacobs Foundation, Grant/Award Number: JRF 2016‐2018; U.S. National Science Foundation, Grant/Award Number: 11519520; Brain and Creativity Institute Research Fund; University of Southern California Rossier School of Education; USC Provost
REFERENCES
- Aiyer, S. M. , Heinze, J. E. , Miller, A. L. , Stoddard, S. A. , & Zimmerman, M. A. (2014). Exposure to violence predicting cortisol response during adolescence and early adulthood: Understanding moderating factors. Journal of Youth and Adolescence, 43(7), 1066–1079. 10.1007/s10964-014-0097-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, D. , Chein, J. , & Steinberg, L. (2013). The teenage brain: Peer influences. Current Directions in Psychological Science, 22(2), 114–120. 10.1177/0963721412471347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, S. L. , Tomada, A. , Vincow, E. S. , Valente, E. , Polcari, A. , & Teicher, M. H. , (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. TL ‐ 20. Journal of Neuropsychiatry and Clinical Neurosciences, 2 VN‐r(3), 292–301. 10.1176/appi.neuropsych.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10(6), 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2000). Voxel‐based morphometry—The methods. NeuroImage, 11(6), 805–821. 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- Bai, J. , Abdul‐Rahman, M. F. , Rifkin‐Graboi, A. , Chong, Y.‐S. , Kwek, K. , Saw, S.‐M. , … Qiu, A. (2012). Population differences in brain morphology and microstructure among Chinese, Malay, and Indian neonates. PLoS ONE, 7(10), e47816 10.1371/journal.pone.0047816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns, G. S. , Moore, S. , & Capra, C. M. (2009). Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS ONE, 4(8), 10.1371/journal.pone.0006773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick, J. , & Nelson, C. A. (2016). Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1), 177–196. 10.1038/npp.2015.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge, S. A. , Dudukovic, N. M. , Thomason, M. E. , Vaidya, C. J. , & Gabrieli, J. D. E. (2002). Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron, 33(2), 301–311. 10.1016/S0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, G. , Luu, P. , & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Butler, O. , Adolf, J. , Gleich, T. , Willmund, G. , Zimmermann, P. , Lindenberger, U. , … Kühn, S. (2017). Military deployment correlates with smaller prefrontal gray matter volume and psychological symptoms in a subclinical population. Translational Psychiatry, 7(2), e1031 10.1038/tp.2016.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee, M. W. L. , Zheng, H. , Goh, J. O. S. , Park, D. , & Sutton, B. P. (2011). Brain structure in young and Old East Asians and Westerners: Comparisons of structural volume and cortical thickness. Journal of Cognitive Neuroscience, 23(5), 1065–1079. 10.1162/jocn.2010.21513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, E. , Miller, G. E. , Kobor, M. S. , & Cole, S. W. (2011). Maternal warmth buffers the effects of low early‐life socioeconomic status on pro‐inflammatory signaling in adulthood. Molecular Psychiatry, 16(7), 729–737. 10.1038/mp.2010.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A. , Moffitt, T. E. , Arseneault, L. , Bleiberg, B. A. , Dinardo, P. B. , Gandelman, S. B. , … Caspi, A. (2017). The origins of cognitive deficits in victimized children: Implications for neuroscientists and clinicians. American Journal of Psychiatry, 174(4), 349–361. 10.1176/appi.ajp.2016.16030333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski, U. , Stuhrmann, A. , Beutelmann, V. , Zwanzger, P. , Lenzen, T. , Grotegerd, D. , … Kugel, H. (2012). Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D. , Hall, J. , Boring, A. M. , Frustaci, K. , & Moritz, G. (2001). A pilot longitudinal study of hippocampal volumes in pediatric maltreatment‐related posttraumatic stress disorder. Biological Psychiatry, 50(4), 305–309. 10.1016/S0006-3223(01)01105-2 [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D. , Van Voorhees, E. , Hooper, S. R. , Gibler, N. , Nelson, L. , Hege, S. G. , … MacFall, J. (2008). Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research, 32(3), 395–404. 10.1111/j.1530-0277.2007.00603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet, E. R. , Joëls, M. , & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews. Neuroscience, 6(6), 463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- Delaney‐Black, V. , Covington, C. , Ondersma, S. J. , Nordstrom‐Klee, B. , Templin, T. , Ager, J. , … Sokol, R. J. (2002). Violence exposure, trauma, and IQ and/or reading deficits among urban children. Archives of Pediatrics &Amp; Adolescent Medicine, 156(3), 280–285. 10.1097/00004703-200208000-00040 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Morrell, M. J. , & Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain, 118(1), 279–306. 10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Drevets, W. C. , Savitz, J. , & Trimble, M. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13(8), 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme, S. , Hudziak, J. J. , Botteron, K. N. , Ganjavi, H. , Lepage, C. , Collins, D. L. , … Brain, D. C. G. (2011). Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biological Psychiatry, 70(3), 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, P. J. , Tompsett, C. J. , Braciszewski, J. M. , Jacques‐Tiura, A. J. , & Baltes, B. B. (2009). Community violence: A meta‐analysis on the effect of exposure and mental health outcomes of children and adolescents. Development and Psychopathology, 21(1), 227–259. 10.1017/S0954579409000145 [DOI] [PubMed] [Google Scholar]
- Friston, K. J. , Rotshtein, P. , Geng, J. J. , Sterzer, P. , & Henson, R. N. (2006). A critique of functional localisers. NeuroImage, 30(4), 1077–1087. 10.1016/j.neuroimage.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Frodl, T. , Koutsouleris, N. , Bottlender, R. , Born, C. , Jäger, M. , Mörgenthaler, M. , … Meisenzahl, E. M. (2008). Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular Psychiatry, 13(12), 1093–1101. 10.1038/mp.2008.62 [DOI] [PubMed] [Google Scholar]
- Gavita, O. A. , Capris, D. , Bolno, J. , & David, D. (2012). Anterior cingulate cortex findings in child disruptive behavior disorders: A meta‐analysis. Aggression and Violent Behavior, 17(6), 507–513. [Google Scholar]
- Giedd, J. N. , & Rapoport, J. L. (2010). Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron, 67(5), 728–734. 10.1016/j.neuron.2010.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J. N. , Lusk, L. , Hayashi, K. M. , Greenstein, D. , Vaituzis, C. A. , … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve, S. M. , Korgaonkar, M. S. , Koslow, S. H. , Gordon, E. , & Williams, L. M. (2013). Widespread reductions in gray matter volume in depression. NeuroImage: Clinical, 3, 332–339. 10.1016/j.nicl.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, H. , & Rubia, K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 52 (March), 10.3389/fnhum.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka, S. , & Nichols, T. E. (2004). Combining voxel intensity and cluster extent with permutation test framework. NeuroImage, 23(1), 54–63. 10.1016/j.neuroimage.2004.04.035 [DOI] [PubMed] [Google Scholar]
- Hollenstein, T. , McNeely, A. , Eastabrook, J. , Mackey, A. , & Flynn, J. (2012). Sympathetic and parasympathetic responses to social stress across adolescence. Developmental Psychobiology, 54(2), 207–214. 10.1002/dev.20582 [DOI] [PubMed] [Google Scholar]
- Hyatt, C. J. , Haney‐Caron, E. , & Stevens, M. C. (2012). Cortical thickness and folding deficits in conduct‐disordered adolescents. Biological Psychiatry, 72(3), 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino‐Yang, M. H. , McColl, A. , Damasio, H. , & Damasio, A. R. (2009). Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences of the United States of America, 106(19), 8021–8026. 10.1073/pnas.0810363106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isamah, N. , Faison, W. , Payne, M. E. , MacFall, J. , Steffens, D. C. , Beyer, J. L. , … Taylor, W. D. (2010). Variability in frontotemporal brain structure: The importance of recruitment of African Americans in neuroscience research. PLoS ONE, 5(10), e13642 10.1371/journal.pone.0013642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl, A. , Schaefer, M. , Malta, L. S. , Dörfel, D. , Rohleder, N. , & Werner, A. (2006). A meta‐analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews, 10.1016/j.neubiorev.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Kaufman, J. , Plotsky, P. M. , Nemeroff, C. B. , & Charney, D. S. (2000). Effects of early adverse experiences on brain structure and function: Clinical implications. Biological Psychiatry, 48(8), 778–790. 10.1016/S0006-3223(00)00998-7 [DOI] [PubMed] [Google Scholar]
- Kliewer, W. (2006). Violence exposure and cortisol responses in urban youth. International Journal of Behavioral Medicine, 13(2), 109–120. 10.1207/s15327558ijbm1302_2 [DOI] [PubMed] [Google Scholar]
- Kühn, S. , & Gallinat, J. (2013). Gray matter correlates of posttraumatic stress disorder: A quantitative meta‐analysis. Biological Psychiatry, 73(1), 70–74. 10.1016/j.biopsych.2012.06.029 [DOI] [PubMed] [Google Scholar]
- Lenroot, R. K. , Gogtay, N. , Greenstein, D. K. , Wells, E. M. , Wallace, G. L. , Clasen, L. S. , … Giedd, J. N. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36(4), 1065–1073. 10.1016/j.neuroimage.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los Angeles Times . (2017). Mapping L.A.: Violent Crime.
- Lu, L. H. , & Sowell, E. R. (2009). Morphological development of the brain: What has imaging told us? In Rumsey J. M. & Ernst M. (Eds.), Neuroimaging in developmental clinical neuroscience (pp. 5–21). New York: Cambridge University Press; 10.1017/CBO9780511757402.004. [DOI] [Google Scholar]
- Lupien, S. J. , McEwen, B. S. , Gunnar, M. R. , & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Margolin, G. , & Gordis, E. B. (2000). The effects of family and community violence on children. Annual Review of Psychology, 51(1), 445–479. 10.1146/annurev.psych.51.1.445 [DOI] [PubMed] [Google Scholar]
- Margolin, G. , Vickerman, K. A. , Ramos, M. C. , Serrano, S. D. , Gordis, E. B. , Iturralde, E. , … Spies, L. A. (2009). Youth exposed to violence: Stability, co‐occurrence, and context. Clinical Child and Family Psychology Review, 12(1), 39–54. 10.1007/s10567-009-0040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, C. C. , & Richmond, T. R. (2008). The relationship between community violence exposure and mental health symptoms in urban adolescents. Journal of Psychiatric and Mental Health Nursing, 15(10), 833–849. 10.1111/j.1365-2850.2008.01321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. (2000). Effects of adverse experiences for brain structure and function. Biological Psychiatry, 48(8), 721–731. 10.1016/S0006-3223(00)00964-1 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. , & Morrison, J. (2013). The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 10.1016/j.neuron.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli, A. , Price, C. J. , Friston, K. J. , & Ashburner, J. (2005). Voxel‐based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews, 1(2), 105–113. 10.2174/1573405054038726 [DOI] [Google Scholar]
- Neiss, M. , & Rowe, D. C. (2000). Parental education and child's verbal IQ in adoptive and biological families in the National Longitudinal Study of adolescent health. Behavior Genetics, 30(6), 487–496. 10.1023/A:1010254918997 [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Brito, N. H. , Bartsch, H. , Kan, E. , Kuperman, J. M. , … Sowell, E. R. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle, J. E. , Cusack, R. , & Henson, R. N. A. (2012). Adjusting for global effects in voxel‐based morphometry: Gray matter decline in normal aging. NeuroImage, 60(2), 1503–1516. 10.1016/j.neuroimage.2011.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner, H. H. , Chiodo, L. , Covington, C. , Sokol, R. J. , Ager, J. , & Delaney‐Black, V. (2006). Violence exposure, IQ, academic performance, and children's perception of safety: Evidence of protective effects. Merrill‐Palmer Quarterly, 52(2), 264–287. 10.1353/mpq.2006.0017 [DOI] [Google Scholar]
- Rauch, S. L. , Shin, L. M. , & Phelps, E. A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research‐past, present, and future. Biological Psychiatry, 10.1016/j.biopsych.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Romeo, R. D. , Minhas, S. , Svirsky, S. E. , Hall, B. S. , Savenkova, M. , & Karatsoreos, I. N. (2014). Pubertal shifts in adrenal responsiveness to stress and adrenocorticotropic hormone in male rats. Psychoneuroendocrinology, 42, 146–152. 10.1016/j.psyneuen.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, B. S. (2000). Exposure to community violence in adolescence: Trauma symptoms. Adolescence, 35(138), 271–284. [PubMed] [Google Scholar]
- Sapolsky, R. M. (1999). Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Experimental Gerontology, 34(6), 721–732. 10.1016/S0531-5565(99)00047-9 [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. , Krey, L. C. , & McEwen, B. S. (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. Journal of Neuroscience, 5(5), 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky, R. M. , Krey, L. C. , & McEwen, B. S. (1986). The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews, 7(3), 284–301. 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. , Uno, H. , Rebert, C. S. , & Finch, C. E. (1990). Hippocampal damage associated with prolonged glucocorticoid exposure in primates. Journal of Neuroscience, 10(9), 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack, H. G. , van Haren, N. E. M. , Brouwer, R. M. , Evans, A. , Durston, S. , Boomsma, D. I. , … Hulshoff Pol, H. E. (2015). Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cerebral Cortex, 25(6), 1608–1617. 10.1093/cercor/bht357 [DOI] [PubMed] [Google Scholar]
- Selye, H. (1936). A syndrome produced by diverse nocuous agents. Nature, 138, (32 10.1038/138032a0 [DOI] [PubMed] [Google Scholar]
- Shamay‐Tsoory, S. G. , Aharon‐Peretz, J. , & Perry, D. (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–627. 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Sharkey, P. T. (2010). The acute effect of local homicides on children's cognitive performance. Proceedings of the National Academy of Sciences, 107(26), 11733–11738. 10.1073/pnas.1000690107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Greenstein, D. , Lerch, J. , Clasen, L. S. , Lenroot, R. , Gogtay, N. , … Giedd, J. N. (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440(7084), 676–679. 10.1038/nature04513 [DOI] [PubMed] [Google Scholar]
- Stein, B. D. , Jaycox, L. H. , Kataoka, S. , Rhodes, H. J. , & Vestal, K. D. (2003). Prevalence of child and adolescent exposure to community violence. Clinical Child and Family Psychology Review, 6(4), 247–264. 10.1023/B:CCFP.0000006292.61072.d2 [DOI] [PubMed] [Google Scholar]
- Stroud, L. R. , Foster, E. , Papandonatos, G. D. , Handwerger, K. , Granger, D. A. , Kivlighan, K. T. , & Niaura, R. (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21(1), 47–68. 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolan, P. H. (2016). Community violence exposure and developmental psychopathology In Developmental psychopathology, risk, resilience, and intervention (pp. 43–85). Hoboken, NJ, USA: John Wiley & Sons, Inc; 10.1002/9781119125556.devpsy402. [DOI] [Google Scholar]
- Tucker‐Drob, E. M. , & Bates, T. C. (2015). Large cross‐national differences in gene × socioeconomic status interaction on intelligence. Psychological Science, 1–12. 10.1177/0956797615612727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer, E. , Harden, K. P. , D'onofrio, B. , & Gottesman, I. I. (2009). The Scarr–Rowe interaction between measured socioeconomic status and the heritability of cognitive ability In McCartney K. & Weinberg R. A. (Eds.), Experience and development: A festschrift in honor of Sandra Wood Scarr. Taylor and Francis; 10.4324/9780203838013. [DOI] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Van Harmelen, A. L. , Van Tol, M. J. , Van Der Wee, N. J. A. , Veltman, D. J. , Aleman, A. , Spinhoven, P. , … Elzinga, B. M. (2010). Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry, 68(9), 832–838. 10.1016/j.biopsych.2010.06.011 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2013). WASI ‐II: Wechsler abbreviated scale of intelligence ‐ second edition. Journal of Psychoeducational Assessment, 31(3), 337–341. 10.1177/0734282912467756 [DOI] [Google Scholar]
- Whitfield‐Gabrieli, S. (2009). Region of interest extraction (REX) toolbox. Boston, MA.
- Wilson, D. K. , Kliewer, W. , Teasley, N. , Plybon, L. , & Sica, D. A. (2002). Violence exposure, catecholamine excretion, and blood pressure nondipping status in African American male versus female adolescents. Psychosomatic Medicine, 64(6), 906–915. 10.1097/01.PSY.0000024234.11538.D3 [DOI] [PubMed] [Google Scholar]
- Woon, F. L. , & Hedges, D. W. (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment‐related posttraumatic stress disorder: A meta‐analysis. Hippocampus, 10.1002/hipo.20437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


