Abstract
While anterior temporal lobe (ATL) resection is an effective treatment for temporal lobe epilepsy, surgery on the dominant hemisphere is associated with variable decline in confrontation naming. Accurate prediction of naming impairment is critical to inform clinical decision making, and while there has been some degree of success using task‐based functional MRI (fMRI) paradigms, there remains a growing interest in the predictive utility of resting‐state connectivity as it allows for relatively shorter scans with low task demands. Our objective was to assess the relationship between measures of preoperative resting‐state connectivity and postoperative naming change in patients following left ATL resection. We compared the resting language network connectivity of each patient to a normative healthy control template using a novel measure called “matrix similarity,” and found that patients with more abnormal global language‐network connectivity—particularly of regions spared from surgery—showed greater postoperative naming decline than those with normative patterns of connectivity. When we interrogated the degree centrality of to‐be‐resected regions in a more targeted approach of the pathological temporal lobe, we found that greater functional integration of those regions with the rest of the language network at rest was related to greater decline in naming following surgery. Finally, we found that matrix similarity was a better predictor of postoperative outcome than degree within to‐be‐resected regions, network clustering, modularity, and language task fMRI laterality. We provide some of the first evidence that using this novel measure, a relatively short preoperative resting scan can be exploited to inform naming ability following ATL resection.
Keywords: graph theory, postoperative outcome, resting‐state connectivity, temporal lobe epilepsy
1. INTRODUCTION
While anterior temporal lobectomy has proven to be effective in the treatment of refractory temporal lobe epilepsy (TLE) (Josephson et al., 2013; Wiebe, Blume, Girvin, & Eliasziw, 2001), removal of the anterior temporal lobe (ATL) in the dominant hemisphere is associated with variable postoperative language deficits (Ives‐Deliperi & Butler, 2012; Sherman et al., 2011) due to this region's involvement in semantic integration (Binder et al., 2011), as well as its close proximity and connectivity to critical language areas (Saur et al., 2008). The task of identifying patients who are at risk for significant language morbidity postoperatively remains challenging, and is crucial to inform the surgical decision‐making process.
Several studies have related measures of activation (Bonelli et al., 2011), laterality (Rosazza et al., 2013), and regional connectivity (Barnett, Marty‐Dugas, & McAndrews, 2014) during task‐based fMRI to preoperative language performance. However, research on the capacity of such measures to inform postoperative morbidity is relatively scarce. Various indices of language lateralization have been used to inform surgical candidacy for patients with TLE, with the expectation that patients with functional language lateralized to the epileptic hemisphere are at greater risk for language disruption after surgery on that hemisphere (Szaflarski et al., 2002; Wada & Rasmussen, 1960). Functional lateralization has traditionally been established with the intracarotid amobarbital procedure (Wada test) or using dichotic listening tests, and more recently, task‐based functional MRI (fMRI) has been accepted as a viable alternative wherein patients engage with language tasks in the scanner, and the proportion of activated voxels in each hemisphere comprises the index of language laterality (Bahn et al., 1997; Binder et al., 1996; Szaflarski et al., 2017). Research on the ability of task‐based fMRI to inform postoperative morbidity has generally focused on particular brain regions, showing that stronger activation (Bonelli et al., 2012) and lateralization to left frontal regions (Bonelli et al., 2012; Rosazza et al., 2013; Sabsevitz et al., 2003) or left temporal regions (Sabsevitz et al., 2003) is associated with greater naming decline following left temporal lobe excisions.
Task‐based fMRI is less invasive, less uncomfortable, and carries less cerebrovascular risks than the Wada procedure (Abou‐Khalil & Schlagger, 2003) and can provide a more direct measure of brain lateralization than dichotic listening tests. Nonetheless, it places high demands on compliance with scanning procedures and ability to engage in language tasks during scanning, which is particularly troublesome for individuals with lower language abilities, and individuals who communicate in languages that do not have immediately available or adapted tests. Moreover, the resulting maps of task‐active language regions are highly dependent on arbitrary thresholds of significance, which leaves open the possibility of reaching quite different conclusions about lateralization among professional raters (Benjamin et al., 2017). In light of such limitations, clinical researchers have begun to explore resting‐state connectivity as an alternative to task‐based measures (Fox & Greicius, 2010; Kamran et al., 2014), as the only requirement is a 5–10 min scan where the patient is told to stay awake, lay still, and think about nothing in particular. The low frequency fluctuations extracted from such scans are thought to index the brain's intrinsic connectivity, and multiple networks—many of which map on to task activation patterns—can be evaluated (Beckmann, DeLuca, Devlin, & Smith, 2005; Smith et al., 2009).
Of particular interest, resting‐state fMRI is reliably able to identify the language network in healthy controls using seed‐based measures of connectivity (Tomasi & Volkow, 2012) and independent component analysis (Tie et al., 2014), and in TLE, patterns of resting connectivity correlate with task‐based laterality indices (Doucet, Pustina, et al., 2015; Smitha, Arun, Rajesh, Thomas, & Kesavadas, 2017). While this technique is being increasingly acknowledged as a potentially useful clinical tool (Barnett, Audrain, & McAndrews, 2017; Tracy & Doucet, 2015), little has been done to assess the utility of resting state in the context of informing potential postoperative language morbidity for individual patients, and the two studies that have endeavored to do so have yielded mixed results. Doucet, Rider, et al. (2015) used graph theory measures from resting‐state data collected preoperatively to inform various measures of cognitive outcome in a group of 16 patients with TLE. Of relevance here, they investigated subregions of the left inferior frontal gyrus (IFG) due to this region's involvement in language production, as well as bilateral hippocampi due to focal seizure activity originating from these regions, and the precuneus as it is a major brain network hub. They found that a complex model successfully explained 73% of variance in language outcome (a combination of confrontation naming and semantic and phonemic fluency measures) in people with left TLE. The predictors in this model included distance (an inverse measure of how well integrated a region is with the rest of the brain) within the left pars orbitalis, pars triangularis, pars opercularis, and the right hippocampus, as well as participation (which reflects the presence of numerous intermodular connections for a given brain region) within the left pars orbitalis of the IFG. While this finding demonstrates the promise of resting fMRI in informing postoperative language change, some issues are apparent in the calculation of their measures. They used the absolute values of negative correlations within the connectivity matrices in their calculation of distance, which likely distorts this measure as negative correlation weights—which often signify competition rather than cooperation—are treated as positive weights (Fornito, Zalesky, & Breakspear, 2013). Furthermore, utilizing 5 predictors to fit 16 observations likely over‐fits the data, as evidenced by fact that some of their models predicted 98–99% of postoperative change in cognition. A subsequent study by this group used a different strategy for examining resting‐state connectivity, identifying language networks by seeding from multiple regions of peak activity found during verb generation and assessing deviation of functional connectivity in patients relative to controls (Osipowicz, Sperling, Sharan, & Tracy, 2016). In this case, however, the measure of deviation was not associated with postoperative change in semantic fluency. Thus, it remains unclear how to explore resting‐state networks in a manner that may be maximally sensitive to language change and thus of greatest utility in preoperative planning.
These resting‐state studies thus far have tended to focus on relatively focal and primarily left‐lateralized language areas to serve as predictors of postoperative decline in naming or fluency, in keeping with literature from studies of task activation and neuropsychology that underscore the importance of the left hemisphere to language functioning. However, the language network that has typically been extracted from resting‐state fMRI is quite bilateral (Doucet, He, Sperling, Sharan, & Tracy, 2017; Tomasi & Volkow, 2012). In their large multisite study, Tomasi and Volkow (2012) characterized the resting‐state language network using a seed‐to‐voxel approach from Broca's and Wernike's areas, and found that both seeds demonstrated robust connectivity to language regions that were much more bilateral than typically reported in studies of task activation. Doucet et al. (2017) also reported that resting‐state language networks were more bilateral in both patients with left TLE and controls in comparison to sets of coactivated regions recruited by task activation which were much more left‐lateralized. They suggested that bilateral activity captured with resting‐state reflects “prepotent” language regions of which specific areas are recruited depending on task demands. Furthermore, it has since been proposed that the concept of language “dominance,” at least as assessed using fMRI, may actually be inappropriate when considering regional effects rather than at the level of the entire hemisphere (Tailby, Abbott, & Jackson, 2017). Even though lesions or inactivation procedures generally confirm strong laterality for language in the majority of TLE and other patients, it may be important to interrogate the more bilateral networks revealed by fMRI to characterize changes in functional capacity following epilepsy surgery.
Thus, in this study, we aimed to harness the broader and more bilateral pattern of language network connectivity that resting state engenders to find predictors of postoperative language decline in TLE, especially given higher rates of bilateral language representation in this population (Berl et al., 2005; Brázdil, Zákopčan, Kuba, Fanfrdlová, & Rektor, 2003; Weber et al., 2006). We sought to craft a novel measure that was free of arbitrary thresholding issues that impact the clinical utility of task activation, and that characterized both positive and negative connectivity in the service of evaluating functional subsystems operating within broader language networks. To do this, we derived the language networks of patients with left TLE using expansive bilateral frontal‐temporal regions of interest (ROIs) and compared each patient's language network to that of a normative language network template derived from healthy controls. We used the measure of how similar each patient's language network was to the normative one (here termed “matrix similarity”) as a predictor of postoperative language decline. In order to situate this novel index with existing network analysis measures, we explored how matrix similarity was related to established graph theoretical measures of network modularity, network clustering, network community organization, and degree centrality of the to‐be‐resected regions. We then compared matrix similarity to task‐based activation laterality with respect to naming change. We further examined how these measures were related to preoperative language, to characterize the relative merits of each predictor. Finally, to determine which of the described measures was most predictive of postoperative decline, we examined the variance structure of all significant single predictors within a global model of language change.
We tested two plausible hypotheses with opposite predictions for the relationship between matrix similarity and postoperative naming decline. The first was that a normative language network would be more robust than an abnormal network, and thus would be resilient to network disruption secondary to surgery. This would be expected particularly if regions that remained intact following surgery predicted resilience when they were appropriately connected. The second hypothesis was that a more abnormal language network may have reorganized away from the epileptogenic hemisphere in an adaptive way, which might be optimal for preserving performance after surgery. In this case, we would expect that normative connectivity of the language network would be related to greater language decline postoperatively. In either case, we expected that matrix similarity would capture individual differences in network modularity, community clustering, and community organization, and as such it would be a better predictor of postoperative naming change than any of these measures alone. Further, we hypothesized that if to‐be‐resected regions had a high number of connections (measured via the graph theory metric of degree), then removal of these regions would result in greater naming decline as these regions would no longer be able to contribute to network processing. For individuals with a low number of connections, we postulated that removal would have less of an impact on naming as these regions were not widely participating in network information transfer at baseline. Finally, based on previous research (Bonelli et al., 2012; Rosazza et al., 2013; Sabsevitz et al., 2003), we anticipated that strong left lateralized task activation presurgically would predict naming decline, though we were particularly interested in how this measure would compare with those based on resting‐state network connectivity.
2. METHODS
2.1. Participants
Twenty adults with medically intractable TLE were recruited for this study from the Epilepsy Clinic at Toronto Western Hospital (12 females, Mean age = 37.6; age range: 23–53). Continuous recording of scalp electroencephalography and video monitoring during an inpatient evaluation in our epilepsy monitoring unit were used to determine seizure focus in the left temporal lobe. All patients underwent a standard left ATL resection (Mansouri et al., 2014), wherein approximately 1 cm was removed from the superior temporal gyrus, 3 cm were removed from the middle temporal gyrus, and 4–5 cm from the inferior temporal gyrus extending caudal from the temporal pole. The amygdala and hippocampus were also completely removed. Nineteen healthy control subjects were recruited (8 females, mean age = 34.4; age range: 22–59) with no history of neurological or psychiatric disorders. All controls gave prospective written informed consent. Patients either gave prospective written informed consent or, for an older cohort, permission for retrospective analysis of clinical data was obtained from the University Health Network Ethics Board.
2.2. Neuropsychological testing
All patients underwent an extensive neuropsychological battery of tests preoperatively and at a postoperative session approximately 12 months after surgery. For the current study, we examined language outcome using total number of correct words, without phonemic cueing, on the Boston Naming Test (BNT), as naming performance is one of the main cognitive functions at risk for decline following ATL surgery (Sherman et al., 2011). Change scores were calculated by taking the difference in performance from preoperative to postoperative testing. Preoperative BNT scores were also retained as a predictor of postoperative change, as better baseline performance has been associated with a higher risk for decline (Busch et al., 2016; Sabsevitz et al., 2003).
2.3. MRI data acquisition
A high‐resolution 3D anatomical scan was collected on a 3T Signa MR system (GE Medical Systems, Milwaukee, WI) for each subject (T1‐weighted sequence, FOV 220 mm, 146 slices, 256 × 256 matrix, resulting in voxel size of 0.86 × 0.86 × 1.0). Task and resting‐state fMRI (T2*‐weighted) scans were acquired with an echo‐planar pulse imaging sequence (FOV 240 mm, 28–32 slices depending on head size, TR = 2000 ms, TE = 25 ms, 64 × 64 matrix, 3.75 × 3.75 × 5 mm voxels, for 180 volumes). During resting state scans, subjects were instructed to lie still, clear their thoughts, and “not to think about anything in particular,” with their eyes closed.
2.4. fMRI language tasks
Four language tasks were used to characterize functional activation laterality; all required covert word generation to visual cues (Supporting Information Figure S1). In the first (verb generation), participants were presented with a noun (e.g., ball) and were instructed to produce as many verbs as they could that were associated with that noun (e.g., kick, toss, throw, and catch). In each block of verb generation, participant viewed five nouns for 5 s each. In the second task (sentence completion), participants were instructed to complete a sentence (e.g., The boy wore red ______) with words that logically made sense (e.g., socks, shorts). Each block had five trials for 5 s each. In the third task (category fluency), participants were visually presented with a category (e.g., flowers) and asked to generate as many examples of that category as they could (e.g., rose, daisy, tulip). Each block had two categories of 12.5 s each. In the fourth task (naming to description), participants were presented with a sentence (e.g., furniture that you sleep on) and were instructed to generate words that matched the description (e.g., bed, couch). Each block had five trials for 5 s each. Prior to each task block, was a fixation block which was cued by “Relax” and involved the presentation of a string of symbols (e.g., ^&*%#$@) and participants were told to fixate on the middle of the strings. Each fixation block had five trials at 4 s each. An instruction screen was provided at the beginning of each block for 1 s to ensure participants understood the task requirements. Seventeen patients performed all four of the language tasks during one functional run. For these subjects, the four tasks were interleaved and presented for three blocks each. Three subjects performed only the verb generation task and category fluency task. None of the healthy controls performed these language tasks.
2.5. fMRI preprocessing
Preprocessing was performed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Anatomical and functional images were reoriented so that the origin aligned with the anterior commissure. Then, the functional images were coregistered to the anatomical image and motion corrected using the realign and unwarp function. Anatomical images for each subject were segmented into gray matter, white matter, and cerebral spinal fluid and normalized into standard Montreal Neurological Institution (MNI) space using a linear transformation. Functional images were then normalized to standard space using the parameters from the anatomical transformation and smoothed with an 8‐mm full‐width half‐max Gaussian kernel. We used the Artifact detection toolbox (Whitfield‐Gabrieli & Nieto‐Castanon, 2012) to identify fluctuations in global signal greater than three SDs, translational motion greater than 1 mm, and rotational motion greater than 0.05 radians. The resulting outliers were saved as regressors and used as covariates of no interest along with motion regressors. Finally, for the resting state data only, we used the CONN toolbox (http://www.nitrc.org/projects/conn; Whitfield‐Gabrieli & Nieto‐Castanon, 2012) to temporally filter the data to exclude low (<0.008 Hz) and high (>0.09 Hz) frequency fluctuations and used aCompCor (Behzadi, Restom, Liau, & Liu, 2007) to exclude measures of physiological noise by regressing out the top five components of a principal component analysis, run on the unsmoothed data, from white matter and cerebrospinal fluid masks.
2.6. Matrix similarity analysis
To construct our language network for resting‐state analyses, we selected all of the ROIs from the Brainnetome Atlas (Fan et al., 2016) that were characterized as involved in the language processing domain using the meta data labels of the BrainMap Database (http://www.brainmap.org/taxonomy)), along with their contralateral homologs. In the Brainnetome Atlas, ROIs were labeled as language regions if a given region activated at p < .05, corrected during tasks in the language domain (forward inference) or if a task in the language domain was most likely administered given that there was activation in the region (reverse inference) using Bayes' rule (Fan et al., 2016). For our language nodes, we included regions that were labeled as involved in speech, semantics, syntax, and phonology, and excluded regions that were exclusively involved orthography, as we wanted our ROIs to represent a broad array of language processes that could be involved in lexical access in our activation tasks. In addition, we included the left anterior and posterior hippocampal ROIs and their homologs (also acquired from the Brainnetome Atlas) given epileptogenicity of this region in our patient population. We obtained a total of 33 ROIs in each hemisphere (66 total) spanning frontal and temporal regions (Figure 1a).
Figure 1.
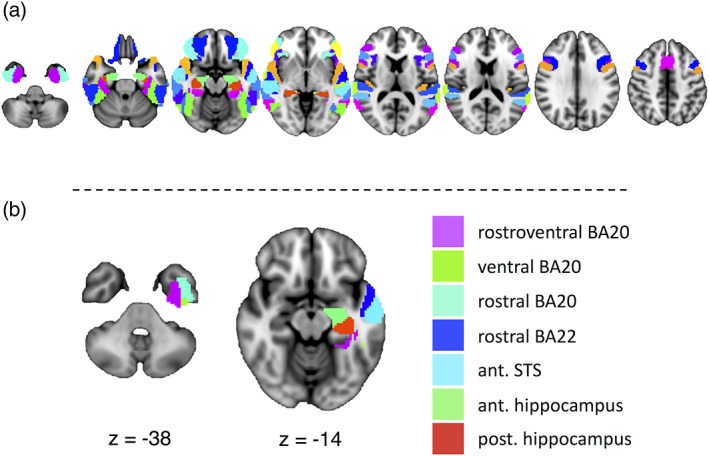
(a) The 33 selected regions of interest from the Brainnetome Atlas involved in language functioning, and their homologs. (b) To‐be‐resected regions of interest from which we extracted degree centrality across a range of thresholds to correlate with postoperative naming change. The regions are displayed on the MNI152 brain in radiological convention on axial slices. Ant., anterior; BA, Brodmann area; post., posterior; STS, superior temporal sulcus
Using the resting‐state fMRI data, we extracted the mean corrected time course across all the voxels in each ROI, which we correlated with every other ROI in a pairwise fashion and converted to z‐scores using a Fisher's z‐transformation, resulting in a 66 × 66 language connectivity matrix for each subject. The connectivity matrices of the 19 healthy control subjects were averaged together to create a normative template, representative of typical language network connectivity in the healthy brain (Figure 2). Then, we investigated how similar each patient's language network connectivity pattern was to the normative template using a Pearson's correlation between the vectorized normative matrix and each of the vectorized patient matrices. The resulting correlation coefficients were transformed using a Fisher's z‐transformation which served as our measure of matrix similarity. We then used linear regression to predict preoperative BNT performance, as well as preoperative to postoperative change in BNT performance using matrix similarity as a predictor.
Figure 2.
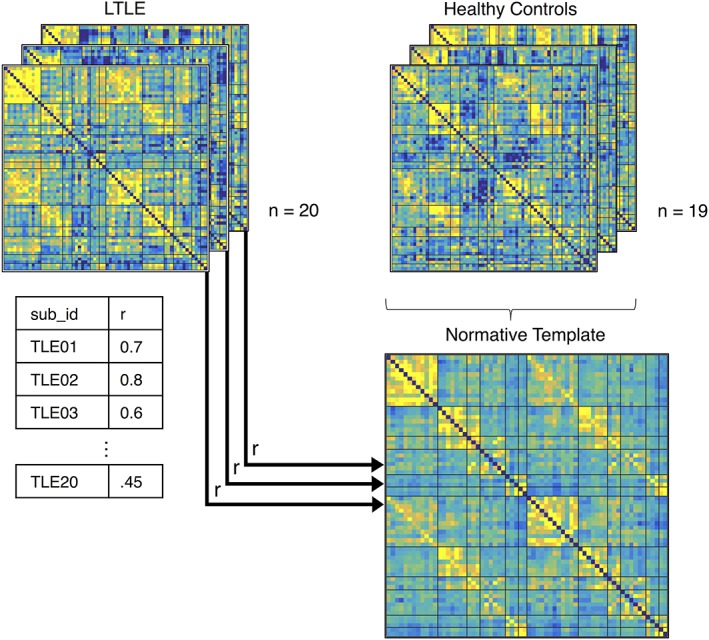
Matrix similarity derivation. Matrices representing each participant's resting language network connectivity were calculated. A normative template was made by averaging the matrices from 19 healthy controls. Each patient's connectivity matrix was correlated with the average normative template to quantify how similar patient networks were to “normal” language network connectivity. The resulting z‐transformed Pearson correlations were used to predict postoperative naming change
To characterize further what regional patterns were related to postoperative language change, we calculated the ROI‐level similarity between each patient and the normative template. In this analysis, we examined how similar an ROI's pattern of connectivity to every other ROI—its connectivity fingerprint—was to the normative template. We did this by correlating each ROI's connectivity fingerprint with the corresponding ROI connectivity fingerprint of the normative template for each subject (i.e., we correlated each row of every patient matrix with the corresponding row of the normative template matrix). We then Fisher z‐transformed each correlation and evaluated the relationship between each region's connectivity fingerprint similarity and postoperative naming change using a Pearson's correlation, and thresholded these regions at p < .05, corrected for False Discovery Rate (FDR).
2.7. Graph theoretical analysis of the language network
We additionally wanted to quantify the representative characteristics of the healthy language network, and to investigate how matrix similarity compared with existing measures of network organization in terms of its descriptive and predictive ability. Visual inspection of the normative template generally revealed a pattern of community organization within anatomical lobes of the brain characterized by greater within‐lobe connectivity, moderate connectivity to contralateral homologs, and relatively weaker across‐lobe connectivity. We therefore chose to characterize the community structure of the normative language network using measures of clustering and modularity, which we calculated using a combination of Brain Connectivity Toolbox functions (https://sites.google.com/site/bctnet; Rubinov & Sporns, 2010) and in‐house scripts.
The clustering coefficient describes how often a given node's neighbors are also connected with each other (Rubinov & Sporns, 2010). Using the brain connectivity toolbox, we calculated the weighted clustering coefficient for each node, which is the proportion of a given node's neighbors that are connected to each other, renormalized by the average intensity of neighborhood clusters at that node (Onnela, Saramäki, Kertész, & Kaski, 2005). The average of the weighted clustering coefficients across the network is thought to be indicative of specialized processing and can range from 0 (there is no clustering or specialized processing) to 1 (there is maximal clustering).
Modularity (Q) describes how well a network can be divided into communities that have high within community connectivity and low between community connectivity and can range from −1 (highest between community connectivity, with lowest within community connectivity) to 1 (highest within community connectivity, with lowest between) (Rubinov & Sporns, 2010). Community detection for the modularity measure was performed using the Louvain method (Blondel, Guillaume, Lambiotte, & Lefebvre, 2008) which iteratively performs a greedy optimization of modularity by randomly selecting nodes and merging them into the community that maximally increases modularity, until no more gains in modularity are possible. These resulting communities are then treated as nodes in a network and the Louvain method of community detection is performed on these resulting communities. As this detection is influenced by the random node that the algorithm begins with, we ran the algorithm 1,000 times and created consensus co‐classification matrices (Dwyer et al., 2014; Lancichinetti & Fortunato, 2012) for each patient and the healthy template. The consensus co‐classification matrices represent how often pairwise selections of nodes (node i and node j) were assigned to the same community across the 1,000 iterations. Finally, the Louvain community detection algorithm was applied to these consensus matrices for each patient and the template to derive the final community arrangements. The modularity measure, Q, was calculated for each subject on their final community arrangement.
Given that TLE patients may have a modular language network even though the nodes within the communities of that network differ from the normative template, we additionally investigated the adjusted Rand index—a measure of similarity between two community assignments, adjusted for the chance grouping of nodes (Rand, 1971)—to compare the community assignment of the normative template to community assignment of each patient's language network in a measure of community similarity. We calculated the adjusted Rand index for each subject using their final community arrangements derived from their consensus co‐classification matrices. The Rand index is scaled between 0 (match is at chance level) and 1 (perfect match). We investigated the degree to which matrix similarity captured these descriptors by computing these measures (clustering, modularity, adjusted Rand index) for each TLE patient's language network and comparing them to matrix similarity scores using Pearson's correlations and corrected for the number of comparisons using FDR correction. In instances of significant relationship between graph theoretical measures and matrix similarity, we additionally investigated if these descriptors were related to BNT change.
2.8. Graph theoretical analysis of to‐be‐resected regions
Using the CONN toolbox, individual subject matrices were thresholded and binarized so that only the top positive connections survived at 5, 10, 15, 20, 25, 30, 35, 40, and 45 percentile connection density thresholds, in order to reduce bias in selecting any one arbitrary threshold, as is typical for this type of analysis (Rubinov & Sporns, 2010). These proportional thresholds, in contrast to absolute thresholds, ensure that each participant's matrices have similar numbers of edges. Degree centrality—the number of connections a particular region has to the rest of the network—was extracted for each ROI that was to‐be‐resected (in full or in part) in the left ATL with a standard ATL resection at our centre (Mansouri et al., 2014). This metric was chosen as a relatively straightforward measure of regional functional integration. These regions included rostral Brodmann area 22 along the superior temporal gyrus, the anterior superior temporal sulcus of the middle temporal gyrus, three regions from Brodmann area 20 (intermediate ventral area 20, rostral area 20, and rostroventral area 20) of the inferior temporal gyrus, and the rostral and caudal hippocampus (Figure 1b). We summed together the degree from each of the to‐be‐resected ROIs, subtracting out the connections that link to‐be‐resected ROIs with other to‐be‐resected ROIs. These summed degree measures served as our index of ATL integration to the rest of the network. The summed degree was correlated with preoperative BNT scores and postoperative BNT change across the range of thresholds.
2.9. Task activation and laterality calculation
For each patient, we modeled first level activation using a general linear model with a block design and computed subject level t‐contrasts for all language tasks versus all fixations in SPM8. To evaluate language laterality, we used the laterality index (LI) toolbox (Wilke & Lidzba, 2007), which extracts voxel values that exceed a particular threshold over a range thresholds and computes a LI for each threshold according to the formula (Leftactivation – Rightactivation)/(Leftactivation + Rightactivation). We used a bootstrapping method and calculated the weighted mean LI across a range of thresholds using 10,000 bootstrap resamples. The weighted mean LI is calculated by averaging the resulting LIs at each threshold, weighted by the height of the threshold, such that more conservative thresholds have a greater influence. Thus, this procedure does not rely on arbitrary thresholding, but also allows voxels with stronger activation more influence than those with lower activation. The resulting LI values range from −1 to 1, with values closer to −1 indicating right lateralized activation and values closer to 1 indicating left lateralized activation. This method has been used in the past by our group (Barnett et al., 2014) and others (Bonelli et al., 2012) and the details of these calculations have been described elsewhere (Wilke & Lidzba, 2007).
We calculated laterality using three different masks, given reports of regional differences in LI (Tailby et al., 2017). The first was a relatively inclusive mask that included the frontal, temporal, and parietal lobes, which was made out of masks included with the LI toolbox and defined using the AAL atlas (Tzourio‐Mazoyer et al., 2002). Second, we calculated LI within the IFG exclusively using the AAL atlas as described in Rosazza et al. (2013), with the exception that we mirrored the left mask onto the right hemisphere to ensure equal number of voxels in each hemisphere for the LI calculation. Finally, we calculated LIs in the temporal lobes exclusively using a whole temporal lobe mask that is provided with the LI toolbox. This mask was selected because it was visually quite similar and as inclusive as the mask used by Sabsevitz et al. (2003) who successfully predicted language outcome with LI from their temporal lobe mask. The occipital lobe and all voxels within 5 mm of the midline were excluded from the calculations, as activation here has been shown to be a major source of discrepancy between fMRI LI and Wada laterality (Arora et al., 2009). The LIs from each of the three masks were separately used as predictors of postoperative language change using linear regression. Given previous research, we used a one‐tailed approach, hypothesizing that greater left lateralization would be related to greater naming decline.
2.10. Combined model prediction
Using the independent variables that were significantly related to language change as predictors (with a cut‐off of p < .05), we performed a multiple linear regression on postoperative BNT change to assess the unique influence of each variable on naming outcome.
3. RESULTS
3.1. Participant demographics
There were no significant differences in age, t(37) = 0.91, p = .37, sex distribution, χ 2 = 0.64, p = .43, or handedness, Fisher Exact probability test, p = 1 between the patient group and the control group. There were, however, significantly higher levels of education in the healthy control group, t(37) = 2.57, p = .01. Demographics are displayed in Table 1 (also see Supporting Information for more detail).
Table 1.
Patient demographic data
| Controls | LTLE | |
|---|---|---|
| N | 19 | 20 |
| Age, years (SD) | 34.4 (10.7) | 37.6 (11.2) |
| Education, y (SD) | 18 (3.1) | 15.6 (2.6) |
| Sex, M/F | 11/8 | 8/12 |
| Handedness, L/R/BI | 2/17/0 | 1/18/1 |
| Language dominance, L/R/BI | – | 13/2/5 |
| Disease duration, y (SD) | – | 19.2 (14.8) |
| Onset of seizures, y (SD) | – | 19 (15.1) |
| Presence of MTS, yes/no | – | 11/9 |
BI = bilateral; F = female; L = left; LTLE = left temporal lobe epilepsy; M = male; MTS = mesial temporal sclerosis; R = right; SD = standard deviation; y = years. Language dominance was determined using LI scores from the combined frontal, temporal, and parietal mask, wherein LIs > 0.2 indicated left‐dominance, LIs < −0.2 indicated right‐dominance, and LIs between −0.2 and 0.2 denoted bilateral language. Characterization of MTS was based on radiology (3T MRI protocol).
3.2. Matrix similarity analysis
Matrix similarity was significantly lower in people with TLE compared to the healthy control group, t(37) = 6.3, p < .001, suggesting that the TLE group had abnormal patterns of preoperative network connectivity (note, the control similarity was calculated by taking the matrix similarity between a given healthy control's network to a template made from all other controls; see Supporting Information Figure S2). We did not find a significant relationship between matrix similarity and preoperative BNT scores (R 2 = 0.01, F[1,18] = 0.24, p = .63). However, matrix similarity showed a significant, positive relationship with preoperative to postoperative change on the BNT (R 2 = 0.4, F[1,18] = 11.8, p = .003). Patients who showed a pattern of language network connectivity that was more similar to that of the normative template showed the least decline in naming performance (Figure 3a). To better understand which regions contributed to this effect, we interrogated the relationship between ROI fingerprint similarity and naming change. We found that patients who had a similar pattern of connectivity to the template in bilateral supplementary motor area, Broca's area (left opercular part of the IFG), bilateral middle temporal, right posterior middle temporal, and right inferior temporal regions showed less naming decline following surgery, p < .05 FDR corrected for the 66 regions (Figure 3c). That is, demonstrating a more normative pattern of connectivity from each of the aforementioned ROIs to the rest of the language network was associated with better language outcome postoperatively. No regions showed a substantial negative relationship between ROI similarity and naming change, indicating there were no regions where normal connectivity patterns predicted worse outcome. Plotted in Figure 3d, we have presented an example of the patient with the most similar and the patient with the most dissimilar language network connectivity to the template. The individual with the most similar pattern to the template showed almost no change in naming performance, increasing by two points (a 4% increase) on the BNT, while the individual with the most dissimilar pattern to the template showed a loss of 19 points (a 39% decrease) following surgery.
Figure 3.
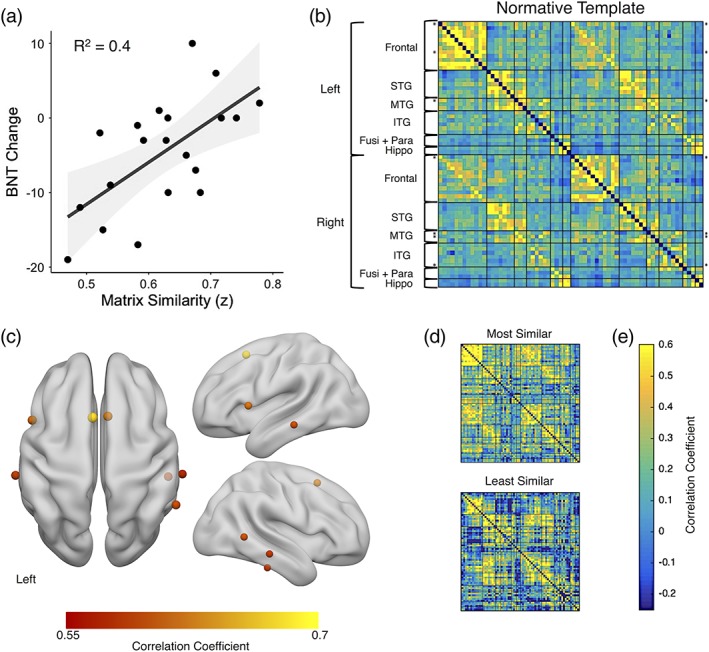
(a) Correlation between matrix similarity and postoperative BNT change. Higher matrix similarity scores represent patient language networks that were more similar to normative template, p < .01. Negative BNT change scores represent a decline in naming postoperatively. (b) the healthy patterns of connectivity. The rows with asterisks (*) are the ROIs for which fingerprint similarity is significantly related to BNT change (r > .45, p < .05, FDR corrected). (c) the centers of gravity for the top regions where ROI fingerprint similarity is related to BNT change (the rows with asterisks in b) depicted on a rendered brain. (d) Two example TLE connectivity matrices are provided for comparison: The patient with the highest matrix similarity score and the lowest. (e) The color bar represents correlation coefficient. BNT, Boston naming test; Fusi + Para, fusiform and parahippocampal gyrus; hippo, hippocampus; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; STG, superior temporal gyrus
3.3. Graph theoretical analysis of the language network
We next investigated how other measures of network similarity fared with respect to descriptive and predictive ability. There was a positive relationship between network clustering and matrix similarity (t[18] = 2.27, r = .47, p = .04), wherein greater clustering of the language network was associated with greater matrix similarity. However, while greater clustering was associated with better naming outcome, this relationship was not significant (R 2 = 0.12, F[1,18] = 2.28, p = .07). Network modularity was not related to matrix similarity (t[18] = 0.57, r = .13, p = .29), indicating that there was no relationship between how modular an individual's language network was and how similar their network was to the normative template. This means that a patient's network can be modular and yet dissimilar from the template, suggesting that the communities formed in their network are likely distorted relative to the template communities. When looking at how modules grouped together, we found a strong, positive relationship between adjusted Rand index and matrix similarity (t[18] = 6.08, r = .82, p < .001), wherein patients who had more similar community assignment to healthy controls also had greater matrix similarity (Figure 4b). Adjusted Rand index was also positively correlated with BNT change, wherein patients with more similar community structure to that of the normative template performed better on the naming task after surgery, though this relationship did not reach significance (R 2 = 0.12, F[1,18] = 2.37, p = .07). When we investigated the topology of the community organization, we saw that the healthy template was characterized by a frontal community, a superior and mid‐temporal community, an inferior temporal community, and a medial temporal community (Figure 4a). We again characterized the highest and lowest matrix similarity networks and saw that the patient with the lowest matrix similarity had relatively discordant community assignment with the template, showing a loss of frontal community specificity, especially in the right hemisphere (Figure 4c). The patient with the most similar matrix showed a preserved frontal community, although both patients highlighted here did not show a clear medial temporal community, which is perhaps not surprising given that is the seizure focus.
Figure 4.
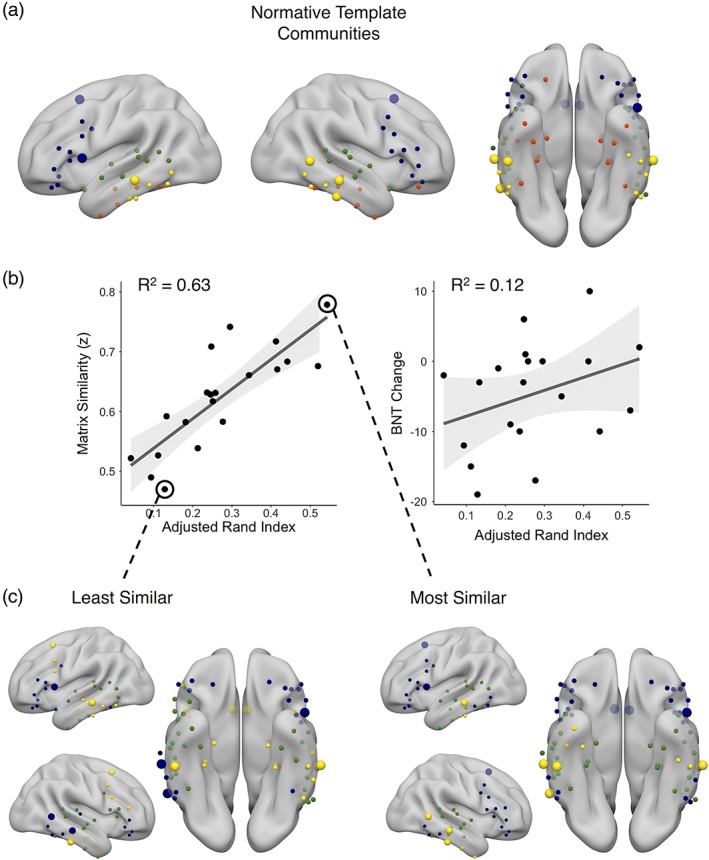
(a) The nodes of the language network color coded by the community to which they were assigned using iterative Louvain community detection on the normative template network, projected onto an inflated standard brain. Blue: frontal module; green: superior‐middle temporal module; yellow: middle‐inferior temporal module; red: medial temporal module. (b) Scatterplots showing the relationship between adjusted Rand index and matrix similarity (left), p < .001 and the relationship between adjusted Rand index and change in naming following surgery on the Boston naming test (right), p = .07. (c) The community organization of the patient with the least similar language network matrix similarity (left) and the community organization of the most similar language network matrix similarity (right). The larger spheres represent the nodes where connectivity pattern similarity is related to naming change following surgery and are highlighted in Figure 3
3.4. Graph theoretical analysis of to‐be‐resected regions
We found a positive relationship between preoperative BNT scores and degree centrality in to‐be‐resected ROIs across a range of thresholds from 5 to 40 percentile (Figure 5a), wherein greater degree in the dominant ATL was associated with better naming scores preoperatively. Thus, greater integration of the dominant ATL with the rest of the language network supports better confrontation naming before surgery.
Figure 5.
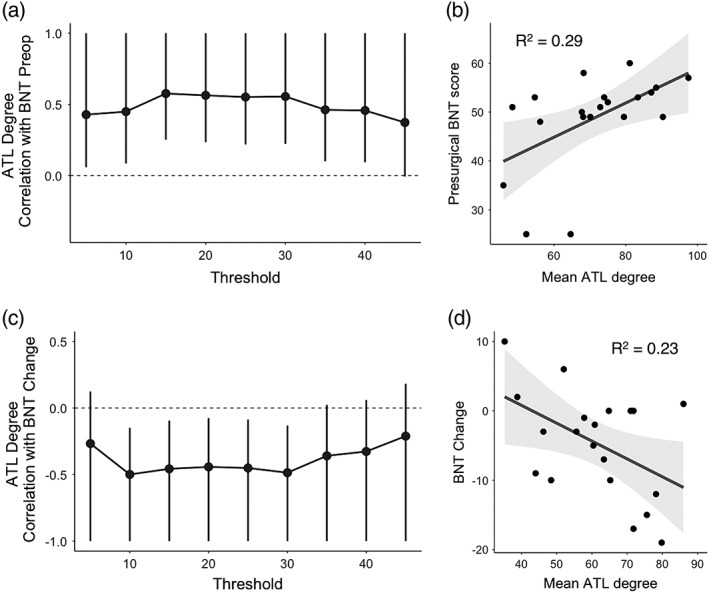
Top panels: Association between degree and preoperative naming ability. (a) Correlation between degree within left ATL regions and preoperative BNT scores across a range of thresholds. ATL degree represents summed connections of ROIs within the to‐be‐resected region to the rest of the language network (and not to each other). Whiskers represent 95% confidence intervals, and those which do not cross 0 denote a significant relationship at p < .05 uncorrected levels. (b) A representative scatterplot showing the average relationship between ATL degree across thresholds and preoperative BNT scores. Here, ATL degree was averaged across thresholds where this measure was significantly related to preoperative BNT scores (5–40 percentile as shown in a) to get a single R 2 value representing the strength of this relationship (p < .01). Bottom panels: Association between degree and postoperative naming change. (c) Correlation between degree within ATL regions and postoperative BNT change across a range of thresholds. As in (a), ATL degree at each threshold represents summed connections of ROIs within the to‐be‐resected region to the rest of the language network. Whiskers represent 95% confidence intervals, and those which do not cross 0 denote a significant relationship at p < .05 uncorrected levels. (d) A representative scatterplot showing the average relationship between ATL degree across thresholds and postoperative BNT change. ATL degree was averaged across thresholds where this measure was significantly related to preoperative BNT scores (10–30 percentile as shown in c) to get a single R 2 value representing the strength of this relationship (p < .05)
In parallel, we found a negative relationship between BNT change after surgery and degree in to‐be‐resected ROIs across a range of thresholds from 10 to 30 percentile. Figure 5c demonstrates the consistency of this finding across the different thresholds. Patients who had greater degree in these to‐be‐resected ROIs showed the greatest decline in naming following surgery, indicating that removal of dominant ATLs that are highly integrated with the rest of the language network is detrimental to language outcome. For visualization, we have plotted the mean degree centrality across the range of significant thresholds against postoperative naming change in Figure 5d. Across this range of significant thresholds, there was no difference in the mean ATL degree between the left temporal lobe epilepsy and control group at baseline (t[37] = 1.1, p = .3).
3.5. Other indices of language functioning
Preoperative BNT scores were negatively associated with postoperative BNT change (R 2 = 0.14, F[1,18] = 2.89, p = .053, one‐tailed), meaning that those who had better naming ability before surgery experienced a bigger decline post‐resection. In terms of fMRI activation, task laterality showed left lateralization in 13 out of 20 patients (LI > 0.2) using the combined frontal–temporal–parietal mask (range = −0.37–0.94), 15 out of 20 patients using the IFG mask (range = −0.58–0.98), and 12 out of 20 patients using the temporal lobe mask (range = −0.50–0.94). Laterality was not significantly related to BNT scores preoperatively (all R 2 < 0.03, p > .2), or to postoperative change using any of the masks (all R 2 < 0.02, p > .28).
3.6. Combined regression model
Since matrix similarity and degree within to‐be‐resected regions were both related to postoperative BNT change (p < .05), we used these measures in a combined regression model (for degree, we used the average degree across the range of significant thresholds). The combined model was significantly related to BNT naming change following surgery, R 2 = 0.46, F(2,17) = 7.3, p = .005. To ensure there was no collinearity among our predictors, we examined the variance inflation factor (VIF) and found a VIF of 1.17. Typically, a VIF < 10 is considered to indicate that there no evidence for consequential collinearity (Hair, Anderson, Tatham, & Black, 1995; Menard, 1995). Matrix similarity was a significant predictor, β = 0.52, t(17) = 2.72, p = .01, and explained 23% of unique variance in outcome, but mean degree in the to‐be‐resected temporal lobe regions was no longer significant, β = −0.28, t(17) = −1.47, p = .16, explaining only 7% of unique variance.
4. DISCUSSION
Our main objective in this study was to assess the relationship between measures of resting language network connectivity with postoperative change in patients following left ATL resection. Using a novel measure, matrix similarity, we found that individuals who showed more normative global patterns of resting‐state language network connectivity at baseline were less likely to show postoperative naming decline than those with abnormal patterns of connectivity. TLE patients with greater matrix similarity also had greater network clustering and greater community similarity to the normative template (which consisted of relatively circumscribed bilateral frontal, superior temporal, mid‐inferior temporal, and medial temporal communities), implicating these as important properties of the normative network, but these indices were weaker predictors of language outcome compared to matrix similarity. Normative connectivity patterns in predominantly bilateral supplementary motor area, Broca's area, bilateral middle temporal, right posterior middle, and inferior temporal cortex—regions spared (except for the most anterior portion of left middle temporal gyrus) in surgery for left TLE—were especially important regional predictors of functional reserve. Thus, healthy patterns of connectivity in regions that are not directly impacted by surgery appear to allow for stable performance following damage to other components of the network. When we interrogated the degree centrality of to‐be‐resected regions in a more targeted approach of the pathological temporal lobe, we found that high numbers of connections from to‐be‐resected regions to the rest of the language network at rest was related to better preoperative naming ability and greater decline in naming following surgery. This suggests that degree within these regions is a good indicator of language functioning, and that losing nodes that are highly integrated with the language network is detrimental to language ability after surgery. When we combined the significant predictors of naming change in one model, we found that matrix similarity was more strongly related to outcome, with degree explaining only an additional 7% of unique variance in naming change. Altogether, results from the present study indicate that of those connectivity features examined, matrix similarity is the strongest predictor of functional outcome after ATL resection.
Our findings accord with others in the literature in demonstrating that low frequency fluctuations at rest characterize network properties that subserve cognition. While task activation is certainly invaluable in terms of describing how the brain operates during a specific mental operation, and in identifying functional engagement of particular regions, it is not without limitations in clinical contexts (Fox & Greicius, 2010). Resting‐state fluctuations may provide a window to the intrinsic connections that can be harnessed for any relevant task and thus reflect the capacity of a network and not how it is being engaged during a particular occasion (Cole et al., 2016; Doucet et al., 2017). Indeed, resting state analysis has been shown to identify regions involved in task‐based activity across a wide range of cognitive tasks including those of attention, emotion, reward learning, relational reasoning, social cognition, working memory, motor, and language (Cole et al., 2016; Mennes et al., 2010; Tavor et al., 2016), and subject‐specific functional connectivity fingerprints at rest have been identified for simple motor and visual operations (Finn & Constable, 2016; Geerligs, Rubinov, Cam‐Can, & Henson, 2015). There have been criticisms that “resting state” is a poorly understood and uncontrolled set of mental operations that can vary considerably across participants (Buckner, Krienen, & Yeo, 2013; Campbell & Schacter, 2017) which implies that this may have little clinical value. Nonetheless, an emerging body of research suggests these network characteristics may depict key properties that relate to functional integrity and prediction of change at the individual patient level (Dierker et al., 2017; Doucet, Rider, et al., 2015; McCormick et al., 2014; McCormick, Quraan, Cohn, Valiante, & McAndrews, 2013; Sair, Agarwal, & Pillai, 2017; Zhou, Liu, Ng, & Wang, 2017).
4.1. Normative resting‐state language network connectivity of spared regions is related to better outcome postoperatively
Patients whose resting‐state language network was more similar to that of healthy controls had better naming outcome after surgery. This finding suggests that focal insult to the language network, at least in TLE, is less catastrophic when resting connectivity within the broader network is normal, and is perhaps able to compensate for the damage with redundant or degenerate processes—processes that can perform the same function following impairment of a subset of regions (Tononi, Sporns, & Edelman, 1999). This may be specific to the epileptic brain, although we found preoperative naming ability was not related to matrix similarity, suggesting that network alterations caused by longstanding seizures may not cause dramatic functional impairment. Nonetheless, these altered networks may be especially vulnerable given that surgical disruption leads to greater impairment. As discussed earlier, Doucet et al. (2017) demonstrated that resting connectivity of the language network in epilepsy is broad, bilateral, and seems to be reflective of a prepotent set of regions that may subserve language. In this context, those with normal connectivity have greater opportunity to rely on the integrity of network components capable of subserving language that are spared during surgery. However, deviation from this pattern of typical network connectivity may restrict the opportunity to capitalize on redundant or degenerate processes.
When we examined which ROIs were contributing most to this pattern, we found that it was largely driven by frontal and temporal regions that were not excised during surgery (with the exception of part of one middle temporal region). While the pattern of connectivity of left lateralized ROIs were important drivers of the matrix similarity effect, the only completely left lateralized region in this respect was Broca's area. Interestingly, we found that most of the left temporal ROIs that were to‐be‐resected were not actually driving the matrix similarity effect, indicating that the pattern of connectivity from most of these regions were not important in determining outcome. These results, which are influenced by the topological pattern of connections, differ from the results showing that the arbitrary number of connections (degree) preoperatively in the to‐be‐resected regions are related to naming change. Instead, the connectivity patterns of right temporal regions were generally more predictive of outcome than the pattern of connectivity of regions within the epileptogenic temporal lobe, suggesting that abnormal reorganization of connectivity within the right temporal lobe (perhaps a functional response to impairment in the left temporal lobe or in response to bilateral interictal discharges) results in a network organization that is more fragile. It is important to note that conclusions based on resting‐state topography may be quite different from those regarding reorganization in the context of task‐related activation (Berl et al., 2005; Brázdil et al., 2003; Weber et al., 2006). Indeed, it could be that there is increased activation and functional load assumed by right temporal regions that is adaptive to preserved language functioning, but that reorganized connections with other language regions cannot withstand network insult as efficiently as a normative connectome. This finding suggests that resting connectivity of right temporal regions is informative and use of resting‐state fMRI to interrogate language networks must reflect the bilateral nature of the network.
While it is tempting to focus on strong positive correlations between regions as indicative of better outcome, negative, or null connections within the network are equally as important to consider in the context of the present findings, as it is the entire pattern of connectivity that correlates with outcome, not just the positive connections. This distinguishes matrix similarity from other metrics which may focus on only the strongest available positive connections such as degree. Greater connectivity between regions that are not normally connected would contribute just as much to an abnormal resting language network as null correlations where there should be positive ones. In short, it is the balance between positive and negative connections to the rest of the language network that determines how much influence a given ROI has in driving matrix similarity and also determines the overall predictive value of this metric. Similarly, this may be the reason matrix similarity better captures language outcome than clustering or adjusted Rand index. Matrix similarity was moderately positively correlated with clustering and strongly positively correlated with adjusted Rand index—suggesting that it overlaps with both of these descriptors of network structure. While matrix similarity was associated with naming outcome, clustering, and adjusted Rand index were not. General clustering of the network is valuable for describing overall network topology, but it does not take into account which regions are clustering together, whether those regions are within the same community, or whether those communities map on to normative network communities. Conversely, adjusted Rand index does take into account which regions are normatively grouped together into the same community, but it does not consider the patterns of those relationships within and between communities in the same way that matrix similarity does. One example of this is illustrated in the community assignments used to calculate adjusted Rand index in the individual who had the highest matrix similarity score (Figure 4c). While communities within the temporal lobes were less circumscribed and there was greater integration between some frontal and temporal communities compared to the normative template (Figure 4a), the ROIs that drove the matrix similarity effect (at the group level) were clustered within the same communities in this individual as they were in the normative template. This may indicate that the community membership and pattern of connectivity of some nodes or regions may be more important than others, and matrix similarity is better able to capture these covarying magnitudes of community alignment compared to the categorical assignments examined with the Rand index.
4.2. Greater degree in to‐be‐resected regions is associated with worse outcome postoperatively
In a more targeted approach, we examined the number of connections from to‐be‐resected ROIs to the rest of the language network (excluding the number of connections within the to‐be‐resected regions themselves) and found that patients who had more connections running through these regions performed better preoperatively and sustained a greater decrement in their naming ability after these regions were resected. This finding suggests that functional integration of the left ATL to the rest of the language network is important to consider in the context of surgical removal of this region, where the loss of an ATL characterized by greater integration is more detrimental to naming ability than the loss of an ATL that has fewer connections to the rest of the language network.
Previous studies have demonstrated the importance of to‐be‐resected regions to cognitive outcome. Encoding activation in the to‐be‐resected hippocampus (Bonelli et al., 2010) and greater connectivity between the to‐be‐resected hippocampus and the posterior cingulate cortex (McCormick et al., 2013) is related to greater memory decline after surgery. Our findings also correspond with other literature that has demonstrated that loss or damage to high degree nodes results in greater impairment in cognitive ability (Fagerholm, Hellyer, Scott, Leech, & Sharp, 2015; Warren et al., 2014). Further, simulations of lesions that target random nodes compared to high degree nodes, demonstrate that loss of high degree nodes is more devastating to network architecture relative to loss of random nodes (Crossley et al., 2014). Crossley et al. (2014) suggested that this is because the loss of high degree nodes is more likely to lead to reductions in global network efficiency—the ability of a network to transfer information. Thus, removal of high degree regions with surgery for TLE may lead to reduced network efficiency, and greater naming decline. Together, these studies demonstrate the importance of functional adequacy—the integrity/functional capability—of the to‐be‐resected tissue in predicting postoperative changes in cognition. In the context of our matrix similarity findings, this suggests that high left ATL integration with the rest of the language network is an important indicator of language functioning, regardless of if those connections are normative. Preoperatively, patients with higher degree in the left ATL showed greater naming ability irrespective of how normal those connections were (indeed, matrix similarity was not predictive of preoperative naming ability). However, normative connectivity of regions spared from surgery explained more variance in postoperative outcome than integration of the to‐be‐resected ATL, suggesting that while even abnormal connectomes may preserve language functioning preoperatively, they are much less resilient to network insult. This may in part be impacted by the fact that there are more connections considered in the rest of the language network than from the to‐be‐resected ATL on its own, but it also underscores the importance of functional reserve in cognitive outcome.
4.3. Preoperative language ability, LI, and postoperative naming decline
Better preoperative BNT scores were only marginally associated with postoperative decline in naming ability, though this trend is consistent with existing literature (Busch et al., 2016; Sabsevitz et al., 2003). This relationship is also in agreement with findings from this study that individuals with greater degree within the to‐be‐resected region had better naming ability before surgery and worse ability afterward. Thus, individuals with better integrated and perhaps better functioning ATLs appear to be at greater risk for postoperative language morbidity.
Surprisingly, we failed to replicate the finding that LI extracted from task‐active language regions is related to postoperative language change (Bonelli et al., 2012; Rosazza et al., 2013; Sabsevitz et al., 2003). This was true for a relatively inclusive frontal–temporal–parietal mask and for separate temporal and frontal masks. There are several potential reasons for the discrepant findings and indeed, among the three studies in the literature that have shown a relationship between LI and postsurgical language outcome, there is considerable variability in methodological choices. The tasks used to identify language activation across these studies include a semantic decision‐making task (Sabsevitz et al., 2003), a naming task (Rosazza et al., 2013), and a verbal fluency task (Bonelli et al., 2012). Our task activation map was composed of activations from naming, verb generation, category fluency, and sentence completion tasks for the majority of subjects. While we believe that the composite of these measures better reflects “true” language activation (Gaillard et al., 2004), we would not necessarily expect this measure to engender the same LI or activation patterns as any one given measure (e.g., naming tasks tend to engage more temporal language regions whereas fluency tasks better engage frontal regions). Another methodological consideration is the calculation of laterality. Sabsevitz et al. (2003) calculated LI based on voxel count at a threshold of p < .001 in left and right homologous ROIs. We, and others, would argue that calculating LI across a range of thresholds is much more robust than when using an arbitrary threshold, given that one can get substantially different results depending on the threshold chosen (Branco et al., 2006; Wilke & Lidzba, 2007). However, Bonelli et al. (2012) used the same bootstrapping method as the current study and to a more limited extent Rosazza et al. (2013) did calculate LI across a discrete range of thresholds and found that relatively greater rightward lateralization was related to better outcome. The third possible reason for the discrepant findings lies within the ROIs chosen for these analyses, though we probed several different regions for a relationship between LI and language outcome based on reported ROIs from the aforementioned studies and still found no relationship. We repeated our LI analysis using only the naming and category fluency subsets of our tasks with our IFG mask, using the same threshold as Rosazza et al. (2013) and we still did not find a relationship with naming change (see Supporting Information). Previous studies did, however, have fewer instances of bilateral or right‐lateralization in their sample of patients than reported here (and indeed, Bonelli et al. (2012) excluded these patients from their analysis). To investigate this possibility, we examined the left lateralized patients (LI > 0.2 using the combined frontal–temporal–parietal mask) independently. We found that while there was a small negative correlation between the degree of leftward lateralization and naming change, it was not significant (t[11] = 0.93, p = .19, r = −.27); note that due to a smaller sample size in this instance, power was diminished. The fact that we did not replicate this finding may be the product of any such differences, and future studies will be needed to investigate the impact of such varied methodological choices.
4.4. Limitations
The first limitation of this study, and one that affects many studies of language fMRI, is that all patients were using antiepileptic medication (AEDs) during scanning. Some AEDs have been shown to affect language performance and activation in fMRI, specifically topiramate is associated with reduced activation (Wandschneider et al., 2017), but the degree to which they affect resting state properties has not been explored. However, of our sample, only three patients were taking this drug. Second, the results pertaining to degree centrality are partially contingent upon methodological decisions such as thresholding, and choice of parcellation scheme. We presented our findings across a range of thresholds that have previously been used in the TLE literature (Bernhardt, Chen, He, Evans, & Bernasconi, 2011; Doucet et al., 2014), and took steps to ensure that our non‐graph theory measures were minimally dependent on threshold. For the calculation of LI in task fMRI we took a weighted mean across many thresholds, bootstrapped 10,000 times. Also, our measure of matrix similarity is threshold independent, as the full pattern of connections was taken into account. For the choice of parcellation scheme, we used an atlas that was based on anatomical and connectivity‐based boundaries (Fan et al., 2016) and selected language regions based on their taxonomy determined via the BrainMap database (http://www.brainmap.org/taxonomy)) which draws on over 3,600 publications and over 110,000 subjects. We are, thus, confident that our ROIs are bound by anatomy and shared connectivity providing meaningful division, while avoiding the redundancy of using each voxel as an ROI.
5. CONCLUSIONS AND FUTURE DIRECTIONS
Here, using a novel measure of matrix similarity, we provide some of the first evidence that preoperative resting‐state connectivity measures can be exploited as predictors of postoperative language decline in adults with left TLE, and that a quick 6‐min scan at rest provides enough information to inform language change after surgery. We found that the healthy resting‐state language network is quite broad, bilateral, and consists of relatively circumscribed frontal and temporal lobe communities. Notably, we found that normative connectivity of regions spared from surgery is critical for optimal language functioning postoperatively. Finally, we found that a high level of integration of the to‐be‐resected ATL with the rest of the network was indicative of better language functioning preoperatively regardless of the pattern of those connections, and was also associated with worse outcome postoperatively. These results highlight the difference between networks subserving functional adequacy versus reserve and the complexities of interactions between these processes in the context of network damage.
We have speculated that resting networks that look healthy presurgically are able to better adapt to network disruption due to surgery, and future studies should address this by examining network organization following surgery to observe how network reorganization may take place. Further, longitudinal tracking of naming ability may be explored to determine whether network plasticity and recovery of language performance can be predicted from preoperative neuroimaging measures. Finally, matrix similarity could serve as a metric for other types of prediction such as memory decline following surgery for epilepsy, or potentially serve as predictive measure for cognitive decline in children with TLE following surgery or other neurological disorders such as individuals with mild cognitive impairment. Work will need to be done to test the efficacy and limitations of this method as applied to other domains.
Supporting information
Supplemental Table S1. Specific Patient Demographics.
Supplemental Figure S1. Illustration of task presentation in during language fMRI scan. Each block was presented three times over the course of a scanning session. Verb Gen, verb generation task; Sentence, sentence completion task; Category, category fluency task; Naming, naming to description task
Supplemental Figure S2. Boxplots and individual subject scatter points for Matrix Similarity in the left temporal lobe epilepsy (LTLE) group and control group. The control similarity was calculated by taking the Matrix similarity between a given healthy control's network to a template made from all other controls
Supplemental Figure S3. Scatterplots depicting the relationship between naming change on the Boston Naming Test (BNT) from pre‐ to post‐surgery with laterality index (LI) during the category fluency task (Fluency) and the naming to description task (Naming) using voxels greater than t=2
ACKNOWLEDGMENTS
The authors would like to thank Irene Giannoylis and Dr. David Gold for their assistance with data collection and organization.
Audrain S, Barnett AJ, McAndrews MP. Language network measures at rest indicate individual differences in naming decline after anterior temporal lobe resection. Hum Brain Mapp. 2018;39:4404–4419. 10.1002/hbm.24281
Samantha Audrain and Alexander James Barnett contributed equally to this work and share first authorship.
Funding information Ontario Brain Institute, Grant/Award Number: EpLink
REFERENCES
- Abou‐Khalil, B. W. , & Schlagger, B. L. (2003). Is it time to replace the Wada test?: Reply. Neurology, 60(2), 354–355. 10.1212/WNL.60.2.354 [DOI] [PubMed] [Google Scholar]
- Arora, J. , Pugh, K. , Westerveld, M. , Spencer, S. , Spencer, D. D. , & Constable, R. T. (2009). Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia, 50(10), 2225–2241. 10.1111/j.1528-1167.2009.02136.x [DOI] [PubMed] [Google Scholar]
- Bahn, M. M. , Lin, W. , Silbergeld, D. L. , Miller, J. W. , Kuppusamy, K. , Cook, R. J. , … Cross, D., III . (1997). Localization of language cortices by functional MR imaging compared with intracarotid amobarbital hemispheric sedation. American Journal of Roentgenology, 169(2), 575–579. [DOI] [PubMed] [Google Scholar]
- Barnett, A. , Audrain, S. , & McAndrews, M. P. (2017). Applications of resting‐state functional MR imaging to epilepsy. Neuroimaging Clinics of North America, 27(4), 697–708. 10.1016/j.nic.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Barnett, A. , Marty‐Dugas, J. , & McAndrews, M. P. (2014). Advantages of sentence‐level fMRI language tasks in presurgical language mapping for temporal lobe epilepsy. Epilepsy and Behavior, 32, 114–120. 10.1016/j.yebeh.2014.01.010 [DOI] [PubMed] [Google Scholar]
- Beckmann, C. F. , DeLuca, M. , Devlin, J. T. , & Smith, S. M. (2005). Investigations into resting‐state connectivity using independent component analysis. Philosophical Transactions of the Royal Society, 360(1457), 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, C. F. , Walshaw, P. D. , Hale, K. , Gaillard, W. D. , Baxter, L. C. , Berl, M. M. , … Bookheimer, S. Y. (2017). Presurgical language fMRI: Mapping of six critical regions. Human Brain Mapping, 4255, 4239–4255. 10.1002/hbm.23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl, M. M. , Balsamo, L. M. , Xu, B. , Moore, E. N. , Weinstein, S. L. , Conry, J. A. , … Gaillard, W. D. (2005). Seizure focus affects regional language networks assessed by fMRI. Neurology, 65(10), 1604–1611. 10.1212/01.wnl.0000184502.06647.28 [DOI] [PubMed] [Google Scholar]
- Bernhardt, B. C. , Chen, Z. , He, Y. , Evans, A. C. , & Bernasconi, N. (2011). Graph‐theoretical analysis reveals disrupted small‐world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cerebral Cortex, 21(9), 2147–2157. 10.1093/cercor/bhq291 [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Gross, W. L. , Allendorfer, J. B. , Bonilha, L. , Chapin, J. , Edwards, J. C. , … Koenig, K. (2011). Mapping anterior temporal lobe language areas with fMRI: A multicenter normative study. NeuroImage, 54(2), 1465–1475. 10.1016/j.neuroimage.2010.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. , Swanson, S. , Hammeke, T. , Morris, G. , Mueller, W. , Fischer, M. , … Haughton, V. (1996). Determination of language dominance using functional MRI. Neurology, 46, 978–984. 10.1212/WNL.46.4.978 [DOI] [PubMed] [Google Scholar]
- Blondel, V. D. , Guillaume, J. L. , Lambiotte, R. , & Lefebvre, E. (2008). Fast unfolding of communities in large networks. Journal of Statistical Mechanics: Theory and Experiment, 2008(10), 1–12. 10.1088/1742-5468/2008/10/P10008 [DOI] [Google Scholar]
- Bonelli, S. B. , Powell, R. , Thompson, P. J. , Yogarajah, M. , Focke, N. K. , Stretton, J. , … Koepp, M. J. (2011). Hippocampal activation correlates with visual confrontation naming: FMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy Research, 95(3), 246–254. 10.1016/j.eplepsyres.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, S. B. , Powell, R. H. W. , Yogarajah, M. , Samson, R. S. , Symms, M. R. , Thompson, P. J. , … Duncan, J. S. (2010). Imaging memory in temporal lobe epilepsy: Predicting the effects of temporal lobe resection. Brain, 133(4), 1186–1199. 10.1093/brain/awq006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli, S. B. , Thompson, P. J. , Yogarajah, M. , Vollmar, C. , Powell, R. H. W. , Symms, M. R. , … Duncan, J. S. (2012). Imaging language networks before and after anterior temporal lobe resection: Results of a longitudinal fMRI study. Epilepsia, 53(4), 639–650. 10.1111/j.1528-1167.2012.03433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco, D. M. , Suarez, R. O. , Whalen, S. , O'Shea, J. P. , Nelson, A. P. , da Costa, J. C. , & Golby, A. J. (2006). Functional MRI of memory in the hippocampus: Laterality indices may be more meaningful if calculated from whole voxel distributions. NeuroImage, 32(2), 592–602. 10.1016/j.neuroimage.2006.04.201 [DOI] [PubMed] [Google Scholar]
- Brázdil, M. , Zákopčan, J. , Kuba, R. , Fanfrdlová, Z. , & Rektor, I. (2003). Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions. Epilepsy and Behavior, 4(4), 414–419. 10.1016/S1525-5050(03)00119-7 [DOI] [PubMed] [Google Scholar]
- Buckner, R. L. , Krienen, F. M. , & Yeo, B. T. T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience, 16(7), 832–837. 10.1038/nn.3423 [DOI] [PubMed] [Google Scholar]
- Busch, R. M. , Floden, D. P. , Prayson, B. , Chapin, J. S. , Kim, K. H. , Ferguson, L. , … Najm, I. M. (2016). Estimating risk of word‐finding problems in adults undergoing epilepsy surgery. Neurology, 87, 2363–2369. 10.1212/WNL.0000000000003378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, K. L. , & Schacter, D. L. (2017). Ageing and the resting state: Is cognition obsolete? Language, Cognition and Neuroscience, 32(6), 661–668. 10.1080/23273798.2016.1227858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W. , Ito, T. , Bassett, D. S. , & Schultz, D. H. (2016). Activity flow over resting‐state networks shapes cognitive task activations. Nature Neuroscience, 19(12), 1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley, N. A. , Mechelli, A. , Scott, J. , Carletti, F. , Fox, P. T. , Mcguire, P. , & Bullmore, E. T. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain, 137(8), 2382–2395. 10.1093/brain/awu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker, D. , Roland, J. L. , Kamran, M. , Rutlin, J. , Hacker, C. D. , Marcus, D. S. , … Leuthardt, E. C. (2017). Resting‐state functional magnetic resonance imaging in Presurgical functional mapping: Sensorimotor localization. Neuroimaging Clinics, 27(4), 621–633. 10.1016/j.nic.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. , He, X. , Sperling, M. R. , Sharan, A. , & Tracy, J. I. (2017). From rest to language task: Task activation selects and prunes from broader resting‐state network. Human Brain Mapping, 38(5), 2540–2552. 10.1002/hbm.23539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. , Pustina, D. , Skidmore, C. , Sharan, A. , Sperling, M. , & Tracy, J. (2015). Resting‐state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normals. Biophysical Chemistry, 36(1), 288–303. 10.1002/hbm.22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. , Sharan, A. , Pustina, D. , Skidmore, C. , Sperling, M. R. , & Tracy, J. I. (2014). Early and late age of seizure onset have a differential impact on brain resting‐state organization in temporal lobe epilepsy. Brain Topography, 28(1), 113–126. 10.1007/s10548-014-0366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet, G. E. , Rider, R. , Taylor, N. , Skidmore, C. , Sharan, A. , Sperling, M. , & Tracy, J. I. (2015). Presurgery resting‐state local graph‐theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia, 56(4), 517–526. 10.1111/epi.12936 [DOI] [PubMed] [Google Scholar]
- Dwyer, D. B. , Harrison, B. J. , Yucel, M. , Whittle, S. , Zalesky, A. , Pantelis, C. , … Fornito, A. (2014). Large‐scale brain network dynamics supporting adolescent cognitive control. Journal of Neuroscience, 34(42), 14096–14107. 10.1523/JNEUROSCI.1634-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm, E. D. , Hellyer, P. J. , Scott, G. , Leech, R. , & Sharp, D. J. (2015). Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain, 138(6), 1696–1709. 10.1093/brain/awv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, L. , Li, H. , Zhuo, J. , Zhang, Y. , Wang, J. , Chen, L. , … Jiang, T. (2016). The Human Brainnetome Atlas: A new brain atlas based on connectional architecture. Cerebral Cortex, 26(8), 3508–3526. 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, E. S. , & Constable, R. T. (2016). Individual variation in functional brain connectivity: Implications for personalized approaches to psychiatric disease. Dialogues in Clinical Neuroscience, 18(3), 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito, A. , Zalesky, A. , & Breakspear, M. (2013). Graph analysis of the human connectome: Promise, progress, and pitfalls. NeuroImage, 80, 426–444. 10.1016/j.neuroimage.2013.04.087 [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , & Greicius, M. (2010). Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience, 4, 19 10.3389/fnsys.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, W. D. , Balsamo, L. , Xu, B. , McKinney, C. , Papero, P. H. , Weinstein, S. , … Theodore, W. H. (2004). fMRI language task panel improves determination of language dominance. Neurology, 63(8), 1403–1408. 10.1212/01.WNL.0000141852.65175.A7 [DOI] [PubMed] [Google Scholar]
- Geerligs, L. , Rubinov, M. , Cam‐Can , & Henson, R. N. (2015). State and trait components of functional connectivity: Individual differences vary with mental state. Journal of Neuroscience, 35(41), 13949–13961. 10.1523/JNEUROSCI.1324-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair, J. F., Jr. , Anderson, R. E. , Tatham, R. L. , & Black, W. C. (1995). Multivariate data analysis (3rd ed.). New York, NY: Macmillan. [Google Scholar]
- Ives‐Deliperi, V. L. , & Butler, J. T. (2012). Naming outcomes of anterior temporal lobectomy in epilepsy patients: A systematic review of the literature. Epilepsy & Behavior, 24(2), 194–198. 10.1016/j.yebeh.2012.04.115 [DOI] [PubMed] [Google Scholar]
- Josephson, C. B. , Dykeman, J. , Fiest, K. M. , Liu, X. , Sadler, R. M. , Jette, N. , & Wiebe, S. (2013). Systematic review and meta‐analysis of standard vs selective temporal lobe epilepsy surgery. Neurology, 80(18), 1669–1676. 10.1212/WNL.0b013e3182904f82 [DOI] [PubMed] [Google Scholar]
- Kamran, M. , Hacker, C. , Allen, M. , Mitchell, T. , Leuthardt, E. , Snyder, A. , & Shimony, J. (2014). Resting state BOLD fMRI for pre‐surgical planning. Neuroimaging Clinics of North America, 24(4), 655–669. 10.1016/j.nic.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancichinetti, A. , & Fortunato, S. (2012). Consensus clustering in complex networks. Scientific Reports, 2, 1–8. 10.1038/srep00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri, A. , Fallah, A. , McAndrews, M. P. , Cohn, M. , Mayor, D. , Andrade, D. , … Valiante, T. A. (2014). Neurocognitive and seizure outcomes of selective amygdalohippocampectomy versus anterior temporal lobectomy for mesial temporal lobe epilepsy. Epilepsy Research and Treatment, 2014, 1–8. 10.1155/2014/306382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, C. , Protzner, A. B. , Barnett, A. J. , Cohn, M. , Valiante, T. A. , & McAndrews, M. P. (2014). Linking DMN connectivity to episodic memory capacity: What can we learn from patients with medial temporal lobe damage? NeuroImage: Clinical, 5, 188–196. 10.1016/j.nicl.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick, C. , Quraan, M. , Cohn, M. , Valiante, T. A. , & McAndrews, M. P. (2013). Default mode network connectivity indicates episodic memory capacity in mesial temporal lobe epilepsy. Epilepsia, 54(5), 809–818. 10.1111/epi.12098 [DOI] [PubMed] [Google Scholar]
- Menard, S. (1995). Applied logistic regression analysis: Sage University series on quantitative applications in the social sciences. Thousand Oaks, CA: Sage. [Google Scholar]
- Mennes, M. , Kelly, C. , Zuo, X. , Di Martino, A. , Biswal, B. , Castellanos, X. , & Milham, M. P. (2010). Inter‐individual differences in resting state functional connectivity predict task‐induced BOLD activity. NeuroImage, 50(4), 1690–1701. 10.1016/j.neuroimage.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnela, J. P. , Saramäki, J. , Kertész, J. , & Kaski, K. (2005). Intensity and coherence of motifs in weighted complex networks. Physical Review E, 71(6), 65103 10.1103/PhysRevE.71.065103 [DOI] [PubMed] [Google Scholar]
- Osipowicz, K. , Sperling, M. R. , Sharan, A. D. , & Tracy, J. I. (2016). Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. Journal of Neurosurgery, 124(4), 929–937. 10.3171/2014.9.JNS131422 [DOI] [PubMed] [Google Scholar]
- Rand, W. M. (1971). Objective criteria for the evaluation of clustering methods. Journal of the American Statistical Association, 66(336), 846–850. [Google Scholar]
- Rosazza, C. , Ghielmetti, F. , Minati, L. , Vitali, P. , Giovagnoli, A. R. , Deleo, F. , … Villani, F. (2013). Preoperative language lateralization in temporal lobe epilepsy (TLE) predicts peri‐ictal, pre‐ and post‐operative language performance: An fMRI study. NeuroImage: Clinical, 3, 73–83. 10.1016/j.nicl.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Sabsevitz, D. S. , Swanson, S. J. , Hammeke, T. a. , Spanaki, M. V. , Possing, E. T. , Morris, G. L. , … Binder, J. R. (2003). Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology, 60(11), 1788–1792. 10.1212/01.WNL.0000068022.05644.01 [DOI] [PubMed] [Google Scholar]
- Sair, H. I. , Agarwal, S. , & Pillai, J. J. (2017). Application of resting state functional MR imaging to presurgical mapping: Language mapping. Neuroimaging Clinics, 27(4), 635–644. 10.1016/j.nic.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Saur, D. , Kreher, B. W. , Schnell, S. , Kümmerer, D. , Kellmeyer, P. , Vry, M.‐S. , … Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America, 105(46), 18035–18040. 10.1073/pnas.0805234105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, E. M. S. , Wiebe, S. , Fay‐Mcclymont, T. B. , Tellez‐Zenteno, J. , Metcalfe, A. , Hernandez‐Ronquillo, L. , … Jetté, N. (2011). Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia, 52(5), 857–869. 10.1111/j.1528-1167.2011.03022.x [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitha, X. K. A. , Arun, X. K. M. , Rajesh, X. P. G. , Thomas, X. B. , & Kesavadas, X. C. (2017). Resting‐state seed‐based analysis: An alternative to task‐based language fMRI and its laterality index. American Journal of Neuroradiology, 38, 1187–1192. 10.3174/ajnr.A5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Binder, J. R. , Possing, E. T. , McKiernan, K. A. , Ward, B. D. , & Hammeke, T. A. (2002). Language lateralization in left‐handed and ambidextrous people: fMRI data. Neurology, 59(2), 238–244. 10.1212/WNL.59.2.238 [DOI] [PubMed] [Google Scholar]
- Szaflarski, J. P. , Gloss, D. , Binder, J. R. , Gaillard, W. D. , Golby, A. J. , Holland, S. K. , … Theodore, W. H. (2017). Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology, 88(4), 395–402. 10.1212/WNL.0000000000003532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby, C. , Abbott, D. F. , & Jackson, G. D. (2017). The diminishing dominance of the dominant hemisphere: Language fMRI in focal epilepsy. NeuroImage: Clinical, 14, 141–150. 10.1016/j.nicl.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor, I. , Jones, O. P. , Mars, R. B. , Smith, S. M. , Behrens, T. E. J. , Jbabdi, S. , & Parker Jones, O. (2016). Task‐free MRI predicts individual differences in brain activity during task performance. Science, 352(6282), 216–220. 10.1126/science.aad8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie, Y. , Rigolo, L. , Norton, I. H. , Huang, R. Y. , Wu, W. , Orringer, D. , … Golby, A. J. (2014). Defining language networks from resting‐state fMRI for surgical planning: A feasibility study. Human Brain Mapping, 35(3), 1018–1030. 10.1002/hbm.22231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi, D. , & Volkow, N. D. (2012). Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry, 17(8), 841–854. 10.1038/mp.2011.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi, G. , Sporns, O. , & Edelman, G. M. (1999). Measures of degeneracy and redundancy in biological networks. Proceedings of the National Academy of Sciences of the United States of America, 96(6), 3257–3262. 10.1073/pnas.96.6.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy, J. I. , & Doucet, G. (2015). Resting‐state functional connectivity in epilepsy: Growing relevance for clinical decision making. Current Opinion in Neurology, 28(2), 158–165. 10.1097/WCO.0000000000000178 [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Wada, J. , & Rasmussen, T. (1960). Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. Journal of Neurosurgery, 106, 1117–1133. 10.3171/jns.2007.106.6.1117 [DOI] [PubMed] [Google Scholar]
- Wandschneider, B. , Burdett, J. , Townsend, L. , Hill, A. , Thompson, P. J. , Duncan, J. S. , & Koepp, M. J. (2017). Effect of topiramate and zonisamide on fMRI cognitive networks. Neurology, 88(12), 1165–1171. 10.1212/WNL.0000000000003736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, D. E. , Power, J. D. , Bruss, J. , Denburg, N. L. , Waldron, E. J. , Sun, H. , … Tranel, D. (2014). Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14247–14252. 10.1073/pnas.1322173111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, B. , Wellmer, J. , Reuber, M. , Mormann, F. , Weis, S. , Urbach, H. , … Fernández, G. (2006). Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain, 129(2), 346–351. 10.1093/brain/awh694 [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wiebe, S. , Blume, W. , Girvin, J. , & Eliasziw, M. (2001). A randomized, controlled trial of surgery for temporal lobe epilepsy. The New England Journal of Medicine, 345(5), 311–318. 10.1056/NEJM200108023450501 [DOI] [PubMed] [Google Scholar]
- Wilke, M. , & Lidzba, K. (2007). LI‐tool: A new toolbox to assess lateralization in functional MR‐data. Journal of Neuroscience Methods, 163(1), 128–136. 10.1016/j.jneumeth.2007.01.026 [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Liu, S. , Ng, K. K. , & Wang, J. (2017). Applications of resting‐state functional connectivity to neurodegenerative disease. Neuroimaging Clinics of North America, 27(4), 663–683. 10.1016/j.nic.2017.06.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Specific Patient Demographics.
Supplemental Figure S1. Illustration of task presentation in during language fMRI scan. Each block was presented three times over the course of a scanning session. Verb Gen, verb generation task; Sentence, sentence completion task; Category, category fluency task; Naming, naming to description task
Supplemental Figure S2. Boxplots and individual subject scatter points for Matrix Similarity in the left temporal lobe epilepsy (LTLE) group and control group. The control similarity was calculated by taking the Matrix similarity between a given healthy control's network to a template made from all other controls
Supplemental Figure S3. Scatterplots depicting the relationship between naming change on the Boston Naming Test (BNT) from pre‐ to post‐surgery with laterality index (LI) during the category fluency task (Fluency) and the naming to description task (Naming) using voxels greater than t=2


