Abstract
Openness/Intellect (i.e., openness to experience) is the Big Five personality factor most consistently associated with individual differences in creativity. Recent psychometric evidence has demonstrated that this factor consists of two distinct aspects—Intellect and Openness. Whereas Intellect reflects perceived intelligence and intellectual engagement, Openness reflects engagement with fantasy, perception, and aesthetics. We investigated the extent to which Openness and Intellect are associated with variations in brain structure as measured by cortical thickness, area, and volume (N = 185). Our results demonstrated that Openness was correlated inversely with cortical thickness and volume in left middle frontal gyrus (BA 6), middle temporal gyrus (MTG, BA 21), and superior temporal gyrus (BA 41), and exclusively with cortical thickness in left inferior parietal lobule (BA 40), right inferior frontal gyrus (IFG, BA 45), and MTG (BA 37). When age and sex were statistically controlled for, the inverse correlations between Openness and cortical thickness remained statistically significant for all regions except left MTG, whereas the correlations involving cortical volume remained statistically significant only for left middle frontal gyrus. There was no statistically significant correlation between Openness and cortical area, and no statistically significant correlation between Intellect and cortical thickness, area, or volume. Our results demonstrate that individual differences in Openness are correlated with variation in brain structure—particularly as indexed by cortical thickness. Given the involvement of the above regions in processes related to memory and cognitive control, we discuss the implications of our findings for the possible contribution of personality to creative cognition.
Keywords: cortical thinning, creativity, Openness/Intellect, personality neuroscience
1. INTRODUCTION
Historically, the scientific study of creativity has been concerned with elucidating the contributions of personality to creative thought and behavior (Barron & Harrington, 1981). Robust evidence in support of this link has emerged based on the Big Five personality factor of Openness/Intellect (i.e., openness to experience)—defined as “the breadth, depth, originality, and complexity of an individual's experiential life” (John, Naumann, & Soto, 2008, p. 120). Openness/Intellect has been shown to be correlated consistently and positively with creativity across tasks, measures, and ages (Batey & Furnham, 2006; Carson, Peterson, & Higgins, 2003; Feist & Barron, 2003; King, Walker, & Broyles, 1996; McCrae, 1987; Silvia, Kaufman, & Pretz, 2009a). Indeed, regardless of whether one is focusing on creative self‐beliefs, creative performance (e.g., divergent thinking), or creative achievement, Openness/Intellect has been consistently shown to be the best predictor of creativity compared to the other Big Five traits of extraversion, conscientiousness, neuroticism and agreeableness (Karwowski & Lebuda, 2016; Silvia, Nusbaum, Berg, Martin, & O'connor, 2009b).
Feist (1998, 1999, 2010) has proposed an influential model to explain the relationship between creativity and personality traits associated with creativity, such as Openness/Intellect. Specifically, he has argued that personality influences creativity by lowering the behavioral thresholds that make creativity more likely. According to this functional and causal model, genetic and epigenetic factors influence brain characteristics (e.g., structure and function), which in turn influence the four clusters of personality traits most consistently associated with creativity—namely, cognitive, social, motivational‐affective, and clinical—which ultimately influence creative thoughts and behaviors. As such, within Feist's model, personality is perceived to mediate the link between brain characteristics and creativity (Eysenck, 1993, 1995).
1.1. Openness/Intellect and creativity: Insights from brain structure and function
There has been much interest in assessing the neural bases of individual differences in personality. However, the available evidence has painted a rather variable picture regarding the neural bases of Openness/Intellect (for detailed review, see Vartanian, 2018). Here we will review the neural findings, broken down by structural versus functional approaches.
1.1.1. Openness/Intellect and brain structure
In cases where investigators focused on variations in cortical brain volume, Bjørnebekk et al. (2013) found no correlation with Openness/Intellect, DeYoung et al. (2010) reported a positive correlation in the inferior parietal lobule, and Kapogiannis, Sutin, Davatzikos, Costa, and Resnick (2013) reported a positive correlation in the right frontopolar cortex and left thalamus but a negative correlation in the right medial orbitofrontal cortex (OFC), bilateral fusiform gyrus, left insular cortex, right superior frontal gyrus, left supplemental motor area, left postcentral gyrus, right precuneus, and left inferior parietal cortex (see also Jauk, Neubauer, Dunst, Fink, & Benedek, 2015). DeYoung et al. linked the involvement of the parietal lobule to its role in working memory, control of attention and intelligence (Gray, Chabris, & Braver, 2003). Similarly, Kapogiannis et al. noted that the regions exhibiting positive correlation with Openness/Intellect are associated with cognitive control, whereas those exhibiting a negative correlation are associated with inhibitory or cautionary responses to aversive and/or fearful stimuli, suggesting that those tendencies might be attenuated in people with higher Openness/Intellect scores. Taking a different analytic approach, Li et al. (2015) found that Openness/Intellect mediated the association between the regional volume in the right posterior middle temporal gyrus (MTG) and trait creativity, which they attributed to the involvement of MTG in semantic processing. This is a plausible idea given that individual differences in creativity have been shown to be related to structural differences in semantic memory (Kenett, Anaki, & Faust, 2014; Mednick, 1962).
Switching to white matter integrity, Jung, Grazioplene, Caprihan, Chavez, and Haier (2010a) assessed fractional anisotropy (FA) and found that Openness/Intellect was related inversely to FA within the right inferior frontal white matter (i.e., regions overlapping the uncinate fasciculus and anterior thalamic radiation). In turn, Xu & Potenza (2012), measuring FA as well as mean diffusivity (MD), found no correlation with FA but a negative correlation between Openness/Intellect with white matter MD in superior longitudinal fasciculus and corona radiata (tracts that connect the prefrontal cortex [PFC], the parietal cortex, and subcortical structures), as well as in the anterior cingulum, forceps minor, and corpus callosum. In addition, Openness/Intellect was correlated negatively with white matter MD adjacent to the dorsolateral PFC (DLPFC) in both hemispheres, including the middle and inferior frontal gyrus. In conjunction, Jung et al.'s and Xu et al.'s results suggest that Openness/Intellect is correlated both positively and negatively with white matter integrity in regions that extend beyond the PFC, including connective tissues.
1.1.2. Openness/Intellect and brain function
Sutin, Beason‐Held, Resnick, and Costa (2009) investigated the relationship between resting‐state positron‐emission tomography (PET) and Openness/Intellect scores, and found that Openness/Intellect was correlated positively with PFC activity in females but with anterior cingulate activity in males, and with OFC activity in both sexes. Also focusing on resting‐state data but collected with fMRI, Sampaio, Soares, Coutinho, Sousa, and Gonçalves (2014) found that greater Openness/Intellect was associated with increased activity in right inferior parietal cortex but with decreased activity in bilateral superior parietal cortex and in the left precuneus. Using resting‐state fMRI data to assess patterns of functional connectivity and network integrity in relation to individual differences in Openness/Intellect, Adelstein et al. (2011) examined the contribution of each Big Five personality factor to resting‐state functional connectivity involving two “hubs” that are known to exhibit connectivity with numerous regions in the brain—the anterior cingulate cortex (ACC) and the precuneus. The researchers found that Openness/Intellect scores predicted resting‐state functional connectivity with midline hubs of the default‐mode network (DMN) known to underlie internally‐oriented cognition, as well as with the DLPFC, a region associated with intelligence, executive functions, and the Intellect aspect of the Openness/Intellect factor.
In turn, motivated by the idea that individual differences in Openness/Intellect might be related to dopamine—given dopamine's role in regulating adaptive behaviors and the orientation of attention toward salient and/or rewarding stimuli (DeYoung, 2010; DeYoung, Peterson, & Higgins, 2005)—Passamonti et al. (2015) investigated the relationship between Openness/Intellect and dopaminergic circuits in three studies. Their first study involved the collection of task‐independent resting‐state fMRI, whereas the next two studies involved tasks based on the presentation of pleasant odors and pictures of food. Across all three studies, Openness/Intellect was associated positively with the functional connectivity between the right substantia nigra/ventral tegmental area—a major source of dopaminergic inputs in the brain—and the ipsilateral DLPFC—a key working memory region for encoding, maintaining, and updating information relevant for adaptive behaviors. Consistent with Feist's (1998, 1999) hypothesis that personality influences creativity by lowering the behavioral thresholds that make creativity more likely, Passamonti et al. (2015) reasoned that “increased dopaminergic inputs within the DLPFC reduce the threshold for information processing in open people and make them highly ‘permeable’ and receptive to relevant information” (p. 307).
In summary, a diverse set of findings has emerged regarding the correlation between various measures of brain structure and function in relation to Openness/Intellect scores. Nevertheless, across studies, there is evidence to suggest that variations in Openness/Intellect are associated with structural and functional variation in regions of the brain that underlie both internally directed cognition and memory such as the DMN and the semantic system, as well as regions that underlie cognitive control (e.g., DLPFC). This pattern is consistent with the historically influential idea that creative thinking is underpinned by both bottom–up processes that underlie the generation of ideas as well as top–down goal‐oriented processes using which the generated ideas are selected for goal completion as a function of task demands (Campbell, 1960; Eysenck, 1993; Simonton, 1999; see also Jung, 2013).
1.2. From Openness/Intellect to Openness and Intellect
We argue that the variability observed in the abovementioned studies might be due to the conceptualization of Openness/Intellect as a unitary construct. Specifically, DeYoung, Quilty, and Peterson (2007) demonstrated that psychometrically, Openness/Intellect has two distinct aspects: Intellect and Openness. Whereas Intellect reflects perceived intelligence and intellectual engagement, Openness reflects engagement with fantasy, perception, and aesthetics. Indeed, intelligence and working memory were shown to be correlated more strongly with Intellect than Openness (DeYoung et al., 2005). In addition, Kaufman et al. (2016) provided evidence regarding the predictive validity of this distinction by demonstrating that whereas Openness predicts creative achievement in the arts, Intellect predicts creative achievement in the sciences (see also Kaufman et al., 2010).
The psychometric distinction between Openness and Intellect suggests that they might have dissociable biological substrates. DeYoung, Shamosh, Green, Braver, and Gray (2009) demonstrated that Intellect, but not Openness, was correlated positively with accuracy‐related brain activity in left lateral anterior PFC and medial frontal cortex during a working memory task. Based on resting‐state data, Beaty et al. (2016b) measured the relationship between Openness and Intellect with global network efficiency within the DMN in two studies. The results of Study 1 demonstrated that Intellect was a significant predictor of DMN network efficiency. In turn, Study 2 demonstrated that when the focus is shifted from the aspect level to the facet level, all but one of the six facets of Openness (Openness to Fantasy, Aesthetics, Feelings, Actions, Ideas, and Values) predict DMN network efficiency. These results support the utility of exploring individual differences in intrinsic brain function in relation to Openness/Intellect at finer gradations of analysis than the factor level.
Despite the apparent benefits of distinguishing between Openness and Intellect, their specific and dissociable associations with brain structure and/or function have not received sufficient attention. Here we describe a structural MRI study aimed at examining the dissociable correlations of Openness and Intellect with measures of brain structure. Specifically, we sought to explore brain regions that would exhibit structural relationships with Openness and/or Intellect. This approach was motivated by the idea that those regions that are functionally involved with the cognitive tendencies and thinking styles associated with Openness and/or Intellect would also exhibit structural differences in association with those tendencies and styles, similar to what has been observed elsewhere in relation to hippocampal contributions to navigational ability (Maguire et al., 2000; Maguire, Woollett, & Spiers, 2006). Toward this aim, we conducted a whole‐brain morphometric investigation to compute correlations between Openness and Intellect on the one hand, and cortical thickness, area, and volume on the other hand (Fischl & Dale, 2000). We opted to focus on cortical thickness, area, and volume to offer a more comprehensive picture of the relationship between Openness, Intellect, and brain structure. We hypothesized that Intellect would be correlated with variation in brain structure in fronto‐parietal regions of the brain associated with intelligence (Jung & Haier, 2007), whereas Openness would be correlated with variations in brain structure in regions of the brain associated with cognitive flexibility (Chrysikou & Thompson‐Schill, 2011), conceptual expansion (Abraham et al., 2012), and creativity (for meta‐analyses, see Boccia, Piccardi, Palermo, Nori, & Palmiero, 2015; Gonen‐Yaacovi et al., 2013; Wu et al., 2015). Chief among those, we were specifically interested in structures within networks that underlie internally generated cognition, including the DMN (Beaty, Benedek, Silvia, & Schacter, 2016a; Zabelina and Andrews‐Hanna, 2016) and temporal‐lobe regions that underlie semantic and episodic memory (Abraham 2014; Roberts & Addis, 2017). We reasoned that if a brain region were to exhibit correlation with Intellect or Openness involving cortical thickness, area, or volume, then this would represent evidence regarding its role as a possible structural contributor to aspects of personality related to creativity.
2. METHOD
2.1. Participants
Two hundred and sixty Science, Technology, Engineering and Mathematics, (STEM) participants either working in the field or studying within STEM fields were recruited for our study. Seventy‐one participants were not included in the analysis due to missing personality data and four participants were excluded due to incidental MRI findings (e.g., cavum septum pellucidum), leaving a final sample of 185 participants for analysis. The participants ranged from 16 to 32 years of age (M = 22.06 ± 3.6 years), and were well matched by sex (91 males and 94 females). They were recruited by postings in various departments and classrooms around the University of New Mexico, local high schools, and various professional STEM‐related businesses. The study protocol was approved by the institutional review board of the University of New Mexico. The study protocol also received retrospective support from the Human Research Ethics Committee of Defence Research and Development Canada. All participants signed an informed consent form prior to participation in the experimental protocol.
Prior to entry into the study, participants were screened by a questionnaire and met no criteria for neurological or psychological disorders that would impact experimental hypotheses (e.g., learning disorders, traumatic brain injury, and major depressive disorder). Participants were also screened for conditions that would prohibit undergoing an MRI scan (e.g., metal implant, orthodontic braces, and severe claustrophobia).
2.2. Psychometric measures
All participants were administered the Big Five Aspects Scale (BFAS), an instrument that further refines the big five personality traits model by distinguishing between two aspects for each trait (DeYoung et al., 2007). Ten items are used to assess each of the ten aspects. Participants rated their agreement with how well each statement described them using a five‐point scale ranging from strongly disagree to strongly agree. Scores for each aspect were computed by taking the mean of the corresponding ten items. Although all participants completed the entire BFAS inventory, here we focus on the two aspects of Openness/Intellect exclusively: Openness and Intellect.
2.3. Image acquisition and processing
Structural imaging was obtained with a 3 T Siemens scanner using a 32‐channel head coil to obtain a T1 5 echo sagittal MPRAGE sequence [TE = 1.64 ms; 3.5 ms; 5.36 ms; 7.22 ms; 9.08 ms; TR = 2,530 ms; voxel size = 1.0 × 1.0 × 1.0 mm3; FOV = 256 mm; slices = 192; acquisition time = 6:03]. For all scans, each T1 was reviewed for image quality. Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer‐v5.3.0 image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The methodology for FreeSurfer is described in full in several papers (Dale, Fischl, & Sereno, 1999; Desikan et al., 2006; Fischl & Dale, 2000; Fischl, Sereno, & Dale, 1999a; Fischl, Sereno, Tootell, & Dale, 1999b; Fischl, Liu, & Dale, 2001; Fischl et al., 2002, 2004a, 2004b; Segonne, Pacheco, & Fischl, 2007). Thickness measurements were obtained by reconstructing representations of the GM/WM boundary and the cortical surface and then calculating the distance between those surfaces at each point across the cortical mantle (Dale et al., 1999). Procedures for the measurement of cortical thickness, area, and volume have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). The results of the automatic segmentations were reviewed and any errors were manually corrected.
2.4. Statistical analysis
To investigate the correlation between measurements of cortical thickness, area and volume and Openness and Intellect scores, we performed a surface‐based group analysis using tools within FreeSurfer. First, the participants' surface was smoothed using a full‐width/half‐maximum Gaussian kernel of 10 mm via the “qcache” command. This smoothing was done so that all participants could be displayed on a common template, which is an average brain as described in http://surfer.nmr.mgh.harvard.edu/. To perform and visualize a group analysis, we used the Query, Design, Estimate, Contrast (QDEC) interface of FreeSurfer. QDEC is a single‐binary application included in the FreeSurfer distribution that is used to perform group averaging and inference on the cortical morphometric data produced by the FreeSurfer processing stream (http://surfer.nmr.mgh.harvard.edu/fswiki/Qdec). The design matrix consisted of two discrete groups (male and female), Openness and Intellect raw scores and age as covariates and the slope used was different offset/intercept, different slope (DODS). Correction for multiple comparisons was done by a cluster‐wise procedure using the Monte Carlo Null‐Z simulation method adapted for cortical surface analysis and incorporated into the QDEC processing stream. For these analyses, a total of 10,000 iterations of simulation were performed for each comparison, using a threshold of p = .05. This is the probability of forming a maximum cluster of that size or larger during the simulation under the null hypothesis. This procedure replaces FDR and FWE procedures commonly used in structural or functional paradigms to correct for multiple corrections and presents the likelihood that the cluster of vertices would have arisen by chance.
3. RESULTS
As expected (DeYoung et al., 2007), psychometrically, there was a weak positive correlation between Openness and Intellect, r(182) = .152, p = .041.
To begin with, we focused on the relationship between Openness, Intellect, and cortical thickness. We found six discrete clusters that exhibited a statistically significant negative correlation between Openness and cortical thickness, indicating decreased cortical thickness in relation to higher Openness scores. The regions in the left hemisphere where cortical thickness was correlated negatively with Openness consisted of left MTG (BA 21), superior temporal gyrus (STG, BA 41), inferior parietal lobule (BA 40), and middle frontal gyrus (BA 6) (Figure 1 and Table 1). The regions in the right hemisphere where cortical thickness was correlated negatively with Openness consisted of inferior frontal gyrus (IFG, BA 45) and MTG (BA 37) (Figure 1 and Table 1). There was no statistically significant correlation between Intellect and cortical thickness. Next, we shifted our focus to cortical area, and found no statistically significant correlation between it and Openness or Intellect scores. Shifting our focus to cortical volume last, we found statistically significant inverse correlations between Openness and cortical volume in left MTG (BA 21), STG (BA 41), and middle frontal gyrus (BA 6). There was no statistically significant correlation between Intellect and cortical volume.
Figure 1.
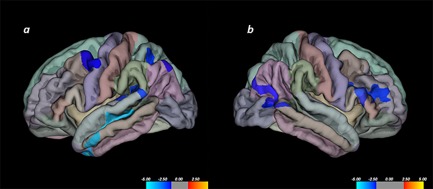
Regions exhibiting inverse correlation between Openness and cortical thickness. Notes. (a) Left hemisphere exhibiting significant correlations in middle temporal gyrus (BA 21), superior temporal gyrus (BA 41), inferior parietal lobule (BA 40), and middle frontal gyrus (BA 6). (b) Right hemisphere exhibiting significant correlations in inferior frontal gyrus (BA 45) and middle temporal gyrus (BA 37)
Table 1.
Regions exhibiting inverse correlation between Openness and cortical thickness
| Region | BA | Coordinates | Size (mm2) |
|---|---|---|---|
| Middle temporal gyrusa | 21 | −53.1, −24.0, −4.0 | 1,676 |
| Superior temporal gyrusa | 41 | −32.4, −31.9, 17.1 | 924 |
| Inferior parietal lobule | 40 | −39.7, −49.7, 35.0 | 821 |
| Middle frontal gyrusa | 6 | −41.4, 3.5, 46.8 | 820 |
| Inferior frontal gyrus | 45 | 48.4, 25.9, 5.2 | 1,275 |
| Middle temporal gyrus | 37 | 44.0, −67.7, 7.0 | 1,165 |
Note. Abbreviation: BA = Brodmann area; regions are designated in Talairach coordinates.
Also exhibited an inverse correlation between Openness and cortical volume.
To test the robustness of our analyses, we computed the correlations between Openness, Intellect and brain structure measures (thickness, area, and volume), controlling statistically for variations in age and sex (in the form of partial correlations). Our results demonstrated that the above six inverse correlations between Openness and cortical thickness remained statistically significant for all regions except left MTG. In contrast, the correlations involving cortical volume remained statistically significant only for left middle frontal gyrus.
4. DISCUSSION
We examined the correlation between two aspects of Openness/Intellect and cortical thickness, area and volume. Previous studies that have investigated the relationship between composite Openness/Intellect measures and cortical thickness have reported mixed findings. Wright, Feczko, Dickerson, and Williams (2007) reported a negative correlation between Openness/Intellect and cortical thickness in right inferior parietal cortex in a sample of healthy elderly participants. Bjørnebekk et al. (2013) reported no statistically significant association between Openness/Intellect and cortical thickness. Riccelli, Toschi, Nigro, Terracciano, and Passamonti (2017) examined the relationship between the Big Five factors and multiple surface‐based morphometry indices including not only cortical thickness but also cortical volume, surface area, and cortical folding. Openness/Intellect was correlated negatively with cortical thickness in rostral and superior prefrontal regions. In contrast, it was correlated positively with surface area and folding in parietal and temporal areas, and in OFC. This pattern of results—consisting of reduced cortical thickness coupled with increased surface area and folding—was interpreted by the authors as an indication of greater cortical maturation in the service of enlarging the range of sensory experiences in people higher in Openness/Intellect. When the focus has been on the relationship between composite Openness/Intellect measures and cortical brain volume instead, the results have implicated the involvement of prefrontal and parietal lobe regions involved in intelligence and executive functions, as well as temporal lobe regions involved in semantic processing (Bjørnebekk et al., 2013; DeYoung et al., 2010; Jauk et al., 2015; Kapogiannis et al., 2013; Li et al., 2015).
This study was motivated in part by the hypothesis that Openness and Intellect might reveal different patterns of correlation with variations in brain structure. Indeed, our results revealed a negative correlation between Openness and cortical thickness in six regions: left middle frontal gyrus (BA 6), MTG (BA 21), STG (BA 41) and inferior parietal lobule (BA 40), as well as right IFG (BA 45), and right MTG (BA 37) (Figure 1 and Table 1). With the exception of the correlation involving left MTG, all remaining correlations remained statistically significant after controlling for variations in age and sex. When we shifted our focus to cortical volume, our results demonstrated that Openness was correlated inversely with cortical volume in left middle frontal gyrus, MTG, and STG, although only the former correlation remained statistically significant after controlling for variations in age and sex.
The association of Openness with reduced cortical thickness is consistent with the findings of Wright et al. (2007) and Riccelli et al. (2017), although the specific regions where cortical thickness reductions were observed varied across studies. One reason for this discrepancy might be that whereas Wright et al. (2007) and Riccelli et al. (2017) used composite Openness/Intellect scores, here we examined the relationship with cortical thickness separately for each Aspect. Similarly, in terms of cortical volume, Kapogiannis et al. (2013) reported negative correlations between composite Openness/Intellect scores and volume in the right medial OFC, bilateral fusiform gyrus, left insular cortex, right superior frontal gyrus, left supplemental motor area, left post‐central gyrus, right precuneus, and left inferior parietal cortex (see also Jauk et al., 2015). As noted earlier, given the association of these regions with inhibitory or cautionary responses, Kapogiannis et al. suggested that the negative correlations might reflect attenuated inhibitory tendencies in people with higher Openness/Intellect scores.
Nevertheless, it is important to ask what this reduction in cortical thickness and volume means in relation to Openness. Reduced cortical thickness has been associated with a host of clinical disorders such as Alzheimer's Disease (Dickerson et al., 2009; Lerch et al., 2008; Singh et al., 2006; Westlye et al., 2009), schizophrenia (Douaud et al., 2007; Kuperberg et al., 2003; Nesvag et al., 2008), attention‐deficit hyperactivity disorder (Makris et al., 2007; Shaw et al., 2006), and neuropsychiatric systemic lupus erythematosus (Jung et al., 2010b). Reduced cortical thickness has also been associated with normal ageing (Fjell et al., 2009a, 2009b; Salat et al., 2004; Westlye et al., 2010). Surveying this literature, Westlye, Grydeland, Walhovd, and Fjell (2011) noted that adult cortical thinning is typically interpreted as an indication of degenerative processes such as shrinkage of large neurons (Terry, DeTeresa, & Hansen, 1987), loss of myelinated axonal fibers (Nairn, Bedi, Mayhew, & Campbell, 1989), deafferentation (Bertoni‐Freddari et al., 2002), and reduction in synaptic density that in turn causes a shrinkage of the cortical ribbon (Morrison & Hof, 1997). In other words, reduced cortical thickness has normally been associated with decreased cognitive function.
The reason why Openness was associated with reduced cortical thickness and volume here could be gleaned by a closer examination of the distinguishing features of this facet within the Big Five model of personality. Recall that Openness reflects engagement with fantasy, perception, and aesthetics. Items within the BFAS that tap Openness include “Get deeply immersed in music,” “See beauty in things that others might not notice,” “Seldom get lost in thought” (reverse scored), and “Seldom daydream” (reverse scored) (DeYoung et al., 2007). These items suggest that higher scores on Openness reflect greater immersion in sensory, cognitive, and emotional information that might otherwise have been filtered out of consciousness (Riccelli et al., 2017). Martindale (1999) argued that this reduced inhibitory ability—what he termed cognitive disinhibition—is a hallmark of creativity, and is the reason why measures of creative ability exhibit positive correlations with personality measures that tap cognitive disinhibition—including Openness/Intellect as well as psychoticism (see Eysenck, 1993, 1995). This idea is consistent with the conclusions drawn from a recent review of structural studies of creativity (and traits related to creativity such as Openness/Intellect) involving structural MRI, diffusion tensor imaging, proton magnetic resonance spectroscopy, and patient/lesion studies (Jung, Mead, Carrasco, & Flores, 2013). What the authors of that review noted was that unlike studies focusing on intelligence where greater ability is typically associated with increased cortical thickness and/or volume (e.g., Draganski et al., 2004; Haier, Jung, Yeo, Head, & Alkire, 2005), creativity has been found to be associated with decreases as well as increases in cortical thickness and/or volume across a broad network of brain regions, with decreases observed in the lingual gyrus, cuneus, angular gyrus, inferior parietal gyrus, fusiform gyrus, the orbitofrontal cortex, and the splenium of the corpus callosum (Gansler et al., 2011). What Jung et al. (2013) concluded was that the brains of more creative individuals are more disinhibited in their organization, measured in terms of lower cortical volume (Jung et al., 2010c), lower white matter fidelity (Jung et al., 2010a), and anterior cingulate biochemistry that tends to gate frontal information flow (at or below a verbal IQ of 116) (Jung et al., 2009). According to this view, lower cortical thickness and volume associated with Openness is not a marker of decreased cognitive function, but rather a function of cognitive disinhibition characteristic of flexible cognition.
Jung et al.'s (2013) interpretation is also consistent with Grazioplene, Chavez, Rustichini, and DeYoung (2016)'s recent observation of a negative correlation between Openness and FA, interpreted as possibly “reflecting a more ‘diffuse’ connectivity pattern, which may contribute to the divergent and associative cognitive style linked to Openness and positive schizotypy. It is possible that a more diffuse connectivity pattern in the frontal lobes underpins adaptive and beneficial behaviors linked to Openness (e.g., creativity, innovation, and curiosity) when paired with higher intelligence and a supportive developmental environment” (p. 1141). Grazioplene et al.'s interpretation also emphasizes the need to consider the benefits of specific types of neural variation within a larger systems model that takes into account the interactions between vulnerabilities and protective factors for bringing about creativity (Carson, 2017).
Contrary to our expectation, we did not find a correlation between Intellect and cortical thickness, area, or volume in regions of the brain associated with intelligence, particularly in the fronto‐parietal system (Jung & Haier, 2007). As is typically the case, it is difficult to make inferences about null findings. We can likely rule out low statistical power as the reason for this observation, given our relatively large sample size (N = 185). However, because ours was the first study to probe this specific association, the reliability of our findings will have to be determined by the results of future efforts to assess this correlation.
Notably, the regions where cortical thickness and/or volume were correlated negatively with Openness—including left middle frontal gyrus (BA 6), MTG (BA 21), STG (BA 41), and inferior parietal lobule (BA 40), and right IFG (BA 45) and MTG (BA 37)—have been shown to be activated reliably by cognitive flexibility (Chrysikou & Thompson‐Schill, 2011), conceptual expansion (Abraham et al., 2012), and/or creativity tasks (for meta‐analyses, see Boccia et al., 2015; Gonen‐Yaacovi et al., 2013; Wu et al., 2015). Importantly, research has demonstrated that creative individuals exhibit structural differences in the organization of their semantic memory (Kenett et al., 2014; Mednick, 1962), as well as superior ability in accessing its contents (Benedek & Neubauer, 2013). The left temporal lobe, specifically MTG, has a well‐established role in episodic and semantic memory (Binder, Desai, Graves, & Conant, 2009; Gabrieli, 1998; Martin & Chao, 2001). The correlation of brain activation in left temporal lobe regions with Openness could reflect individual differences captured by Openness involving the retrieval of episodic and semantic content from memory in the service of flexible cognition. In turn, right IFG is involved in cognitive control and executive functions, including inhibition (Aron, Robbins, & Poldrack, 2004, 2014). As argued elsewhere, there is reason to believe that this region plays a key role in the reduction of the cognitive constraints placed on concepts in the service of idea generation (Goel &Vartanian, 2005; Vartanian & Goel, 2005; see also Vartanian, 2011). Specifically, in the context of tasks where participants must break cognitive or perceptual task sets for generating correct solutions, right IFG has been shown to be activated more when participants generate successful solutions. In this sense, IFG could be related to individual differences captured by Openness involving one's ability to reduce the constraints placed on cognition (Benedek, Jauk, Sommer, Arendasy, & Neubauer, 2014).
Insofar as Openness is related to the ability to think creatively, the involvement of left MTG and right IFG is also consistent with empirical data demonstrating that creativity appears to arise as a function of the interplay between regions of the brain that underlie the processing of semantic and episodic knowledge and those that underlie cognitive control (Abraham, 2014; Beaty et al., 2016a, 2014; Beaty, Benedek, Kaufman, & Silvia, 2015; Chen et al., 2018; Zabelina & Andrews‐Hanna, 2016; see also Chrysikou, Weber, & Thompson‐Schill, 2014). Our findings provide evidence to suggest that the contribution of Openness to creativity might be mediated by structural variations in memory and cognitive control regions, consistent with the emergent idea that multiple systems contribute to the generation of new ideas in the brain.
In addition to left MTG and right IFG, another region that also exhibited an inverse correlation between Openness and cortical thickness was left inferior parietal lobule. This structure has functions that are related conceptually to Openness. For example, it forms part of several frontoparietal control systems that regulate top–down control of cognition (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Seeley et al., 2007; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). The negative correlation between cortical thickness and Openness in this region could mean a possible reduction of the control processes that regulate cognition. However, this region is also associated with the DMN (Igelström & Graziano, 2017), and its involvement could reflect its role in features of Openness that are driven by internally generated cognition (e.g., daydreaming and fantasy) rather than reductions in cognitive control per se. Importantly, these possibilities need not be mutually exclusive, and more research is needed to determine the precise functional contributions of the highlighted regions to individual differences in Openness.
5. CONCLUSION
This study was motivated by the idea that our understanding of the neural underpinnings of Openness/Intellect would benefit from focusing on the aspects of Openness and Intellect separately. Indeed, whereas Openness exhibited negative correlations with cortical thickness and volume in several regions, Intellect was unrelated to brain structure. Importantly, regions implicated in Openness are known to contribute functionally to memory and cognitive control processes, and their involvement here could reflect the relevance of those processes to individual differences in Openness.
ACKNOWLEDGMENTS
The authors report no conflict of interest. This work was supported by a grant from the John Templeton Foundation to Rex E. Jung, and by a Department of National Defence fund under the direction of Oshin Vartanian (04KI04).
Vartanian O, Wertz CJ, Flores RA, et al. Structural correlates of Openness and Intellect: Implications for the contribution of personality to creativity. Hum Brain Mapp. 2018;39:2987–2996. 10.1002/hbm.24054
Funding information John Templeton Foundation; Department of National Defence, Grant/Award Number: 04KI04
REFERENCES
- Abraham, A. (2014). Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Frontiers in Human Neuroscience, 8, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham, A. , Pieritz, K. , Thybusch, K. , Rutter, B. , Kröger, S. , Schweckendiek, J. , … Hermann, C. (2012). Creativity and the brain: Uncovering the neural signature of conceptual expansion. Neuropsychologia, 50(8), 1906–1917. [DOI] [PubMed] [Google Scholar]
- Adelstein, J. S. , Shehzad, Z. , Mennes, M. , Deyoung, C. G. , Zuo, X.‐N. , Kelly, C. , … Milham, M. P. (2011). Personality is reflected in the brain's intrinsic functional architecture. PLoS One, 6(11), e27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2004). Inhibition and the right inferior cortex: One decade on. Trends in Cognitive Sciences, 8(4), 170–177. [DOI] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2014). Inhibition and the right inferior cortex: One decade on. Trends in Cognitive Sciences, 18(4), 177–185. [DOI] [PubMed] [Google Scholar]
- Barron, F. , & Harrington, D. M. (1981). Creativity, intelligence, and personality. Annual Review of Psychology, 32(1), 439–476. [Google Scholar]
- Batey, M. , & Furnham, A. (2006). Creativity, intelligence, and personality: A critical review of the scattered literature. Genetic, Social, and General Psychology Monographs, 132(4), 355–429. [DOI] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Kaufman, S. B. , & Silvia, P. J. (2015). Default and executive network coupling supports creative idea production. Scientific Reports, 5(1), 10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Silvia, P. J. , & Schacter, D. L. (2016a). Creative cognition and brain network dynamics. Trends in Cognitive Sciences, 20(2), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Benedek, M. , Wilkins, R. W. , Jauk, E. , Fink, A. , Silvia, P. J. , … Neubauer, A. C. (2014). Creativity and the default network: A functional connectivity analysis of the creative brain at rest. Neuropsychologia, 64, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty, R. E. , Kaufman, S. B. , Benedek, M. , Jung, R. E. , Kenett, Y. N. , Jauk, E. , … Silvia, P. J. (2016b). Personality and complex brain networks: The role of openness to experience in default network efficiency. Human Brain Mapping, 37, 773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek, M. , & Neubauer, A. C. (2013). Revisiting Mednick's model on creativity‐related differences in associative hierarchies. Evidence for a common path to uncommon thought. Journal of Creative Behavior, 47(4), 273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek, M. , Jauk, E. , Sommer, M. , Arendasy, M. , & Neubauer, A. C. (2014). Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence, 46, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni‐Freddari, C. , Fattoretti, P. , Delfino, A. , Solazzi, M. , Giorgetti, B. , Ulrich, J. , & Meier‐Ruge, W. (2002). Deafferentative synaptopathology in physiological aging and Alzheimer's disease. Annals of the New York Academy of Sciences, 977, 322–326. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Desai, R. H. , Graves, W. W. , & Conant, L. L. (2009). Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cerebral Cortex (New York, N.Y. : 1991), 19(12), 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnebekk, A. , Fjell, A. M. , Walhovd, K. B. , Grydeland, H. , Torgersen, S. , & Westlye, L. T. (2013). Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. NeuroImage, 65, 194–208. [DOI] [PubMed] [Google Scholar]
- Boccia, M. , Piccardi, L. , Palermo, L. , Nori, R. , & Palmiero, M. (2015). Where do bright ideas occur in our brain? Meta‐analytic evidence from neuroimaging studies of domain‐specific creativity. Frontiers in Psychology, 6, 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, D. T. (1960). Blind variation and selective retentions in creative thought as in other knowledge processes. Psychological Review, 67(6), 380–400. [DOI] [PubMed] [Google Scholar]
- Carson, S. H. (2017). Creativity and psychopathology: A relationship of shared neurocognitive vulnerabilities In Jung R. E. & Vartanian O. (Eds.), Cambridge handbook of the neuroscience of creativity (pp. 136–158). Cambridge University Press. [Google Scholar]
- Carson, S. H. , Peterson, J. B. , & Higgins, D. M. (2003). Decreased latent inhibition is associated with increased creative achievement in high‐functioning individuals. Journal of Personality and Social Psychology, 85(3), 499–506. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Beaty, R. E. , Wei, D. , Yang, J. , Sun, J. , Liu, W. , … Qiu, J. (2018). Longitudinal alterations of frontoparietal and frontotemporal networks predict future creative cognitive ability. Cerebral Cortex (New York, N.Y. : 1991), 28(1), 103–115. [DOI] [PubMed] [Google Scholar]
- Chrysikou, E. G. , & Thompson‐Schill, S. L. (2011). Dissociable brain states linked to common and creative object use. Human Brain Mapping, 32(4), 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou, E. G. , Weber, M. , & Thompson‐Schill, S. (2014). A matched filter hypothesis for cognitive control. Neuropsychologia, 62, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, A. M. , Fischl, B. , & Sereno, M. I. (1999). Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Desikan, R. S. , Segonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. (2010). Personality neuroscience and the biology of traits. Social and Personality Psychology Compass, 4(12), 1165–1180. [Google Scholar]
- DeYoung, C. G. , Hirsh, J. B. , Shane, M. S. , Papademetris, X. , Rajeevan, N. , & Gray, J. R. (2010). Testing predictions from personality neuroscience: Brain structure and the Big Five. Psychological Science, 21(6), 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung, C. G. , Peterson, J. B. , & Higgins, D. M. (2005). Sources of openness/intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality, 73(4), 825–858. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Quilty, L. C. , & Peterson, J. B. (2007). Between facets and domains: Ten aspects of the Big Five. Journal of Personality and Social Psychology, 93(5), 880–896. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Shamosh, N. A. , Green, A. E. , Braver, T. S. , & Gray, J. R. (2009). Intellect as distinct from openness: Differences revealed by fMRI of working memory. Journal of Personality and Social Psychology, 97(5), 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, B. C. , Feczko, E. , Augustinack, J. C. , Pacheco, J. , Morris, J. C. , Fischl, B. , & Buckner, R. L. (2009). Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiology of Aging, 30(3), 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. F. , Fair, D. A. , Cohen, A. L. , Schlaggar, B. L. , & Petersen, S. E. (2008). A dual‐networks architecture of top‐down control. Trends in Cognitive Sciences, 12, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud, G. , Smith, S. , Jenkinson, M. , Behrens, T. , Johansen‐Berg, H. , Vickers, J. , … James, A. (2007). Anatomically related grey and white matter abnormalities in adolescent‐onset schizophrenia. Brain, 130(Pt 9), 2375–2386. [DOI] [PubMed] [Google Scholar]
- Draganski, B. , Gaser, C. , Busch, V. , Schuierer, G. , Bogdahn, U. , & May, A. (2004). Changes in grey matter induced by training. Nature, 427(6972), 311–312. [DOI] [PubMed] [Google Scholar]
- Eysenck, H. J. (1993). Creativity and personality: Suggestions for a theory. Psychological Inquiry, 4(3), 147–178. [Google Scholar]
- Eysenck, H. J. (1995). Genius. Cambridge, MA: Cambridge University Press. [Google Scholar]
- Feist, G. J. (1998). A meta‐analysis of the impact of personality on scientific and artistic creativity. Personality and Social Psychology Review, 2(4), 290–309. [DOI] [PubMed] [Google Scholar]
- Feist, G. J. (1999). The influence of personality on artistic and scientific creativity In Sternberg R. J. (Ed.), Handbook of creativity (pp. 273–296). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Feist, G. J. (2010). The function of personality in creativity In Kaufman J. C. & Sternberg R. J. (Eds.), The Cambridge handbook of creativity (pp. 113–130). New York: Cambridge University Press. [Google Scholar]
- Feist, G. J. , & Barron, F. X. (2003). Predicting creativity from early to late adulthood: Intellect, potential, and personality. Journal of Research in Personality, 37(2), 62–88. [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Sereno, M. I. , & Dale, A. M. (1999a). Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. NeuroImage, 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Sereno, M. I. , Tootell, R. B. , & Dale, A. M. (1999b). High‐resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Liu, A. , & Dale, A. M. (2001). Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , van der Kouwe, A. J. , Makris, N. , Segonne, F. , Quinn, B. T. , & Dale, A. M. (2004a). Sequence‐independent segmentation of magnetic resonance images. NeuroImage, 23, S69–S84. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , van der Kouwe, A. , Destrieux, C. , Halgren, E. , Segonne, F. , Salat, D. H. , … Dale, A. M. (2004b). Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y. : 1991), 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell, A. M. , Westlye, L. T. , Amlien, I. , Espeseth, T. , Reinvang, I. , Raz, N. , … Walhovd, K. B. (2009). High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex (New York, N.Y. : 1991), 19(9), 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , Walhovd, K. B. , Fennema‐Notestine, C. , McEvoy, L. K. , Hagler, D. J. , Holland, D. , … Dale, A. M. (2009). One‐year brain atrophy evident in healthy aging. Journal of Neuroscience, 29(48), 15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli, J. D. (1998). Cognitive neuroscience of human memory. Annual Review of Psychology, 49, 87–115. [DOI] [PubMed] [Google Scholar]
- Gansler, D. A. , Moore, D. W. , Susmaras, T. M. , Jerram, M. W. , Sousa, J. , & Heilman, K. M. (2011). Cortical morphology of visual creativity. Neuropsychologia, 49(9), 2527–2532. [DOI] [PubMed] [Google Scholar]
- Goel, V. , & Vartanian, O. (2005). Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set‐shift problems. Cerebral Cortex (New York, N.Y. : 1991), 15(8), 1170–1177. [DOI] [PubMed] [Google Scholar]
- Gonen‐Yaacovi, G. , de Souza, L. C. , Levy, R. , Urbanski, M. , Josse, G. , & Volle, E. (2013). Rostral and caudal prefrontal contribution to creativity: A meta‐analysis of functional imaging data. Frontiers in Human Neuroscience, 7, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. R. , Chabris, C. F. , & Braver, T. S. (2003). Neural mechanisms of general fluid intelligence. Nature Neuroscience, 6(3), 316–322. [DOI] [PubMed] [Google Scholar]
- Grazioplene, R. G. , Chavez, R. S. , Rustichini, A. , & DeYoung, C. G. (2016). White matter correlates of psychosis‐linked traits support continuity between personality and psychopathology. Journal of Abnormal Psychology, 125(8), 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier, R. J. , Jung, R. E. , Yeo, R. A. , Head, K. , & Alkire, M. T. (2005). The neuroanatomy of general intelligence: Sex matters. NeuroImage, 25(1), 320–327. [DOI] [PubMed] [Google Scholar]
- Igelström, K. M. , & Graziano, M. S. A. (2017). The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia, 105, 70–83. [DOI] [PubMed] [Google Scholar]
- Jauk, E. , Neubauer, A. C. , Dunst, B. , Fink, A. , & Benedek, M. (2015). Gray matter correlates of creative potential: A latent‐variable voxel‐based morphometry study. NeuroImage, 111, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, O. P. , Naumann, L. P. , & Soto, C. J. (2008). Paradigm shift to the integrative Big Five trait taxonomy In John O. P., Robbins R. W., & Pervin L. A. (Eds.), Personality handbook: Theory and research (pp. 114–158). New York: Guilford. [Google Scholar]
- Jung, R. E. , Gasparovic, C. , Chavez, R. S. , Flores, R. A. , Smith, S. M. , Caprihan, A. , & Yeo, R. A. (2009). Biochemical support for the “threshold” theory of creativity: A magnetic resonance spectroscopy study. Journal of Neuroscience, 29(16), 5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , Grazioplene, R. , Caprihan, A. , Chavez, R. S. , & Haier, R. J. (2010a). White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS One, 5(3), e9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , & Haier, R. J. (2007). The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences, 30(2), 135–154. [DOI] [PubMed] [Google Scholar]
- Jung, R. E. , Mead, B. S. , Carrasco, J. , & Flores, R. A. (2013). The structure of creative cognition in the human brain. Frontiers in Human Neuroscience, 7, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , Segall, J. M. , Grazioplene, R. G. , Qualls, C. , Sibbitt, W. L. , & Roldan, C. A. (2010b). Cortical thickness and subcortical gray matter reductions in neuropsychiatric systemic lupus erythematosus. PLoS One, 5, e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , Segall, J. M. , Jeremy Bockholt, H. , Flores, R. A. , Smith, S. , Chavez, R. S. , & Haier, R. J. (2010c). Neuroanatomy of creativity. Human Brain Mapping, 31(3), 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis, D. , Sutin, A. , Davatzikos, C. , Costa, P. , & Resnick, S. (2013). The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Human Brain Mapping, 34(11), 2829–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowski, M. , & Lebuda, I. (2016). The big five, the huge two, and creative self‐beliefs: A meta‐analysis. Psychology of Aesthetics, Creativity, and the Arts, 10(2), 214–232. [Google Scholar]
- Kaufman, S. B. , DeYoung, C. G. , Gray, J. R. , Jimenez, L. , Brown, J. B. , & Mackintosh, N. (2010). Implicit learning as an ability. Cognition, 116(3), 321–340. [DOI] [PubMed] [Google Scholar]
- Kaufman, S. B. , Quilty, L. C. , Grazioplene, R. G. , Hirsh, J. B. , Gray, R. J. , Peterson, J. B. , & DeYoung, C. G. (2016). Openness to experience and intellect differentially predict creative achievement in the arts and sciences. Journal of Personality, 84(2), 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenett, Y. N. , Anaki, D. , & Faust, M. (2014). Investigating the structure of semantic networks in low and high creative persons. Frontiers in Human Neuroscience, 8, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, L. A. , Walker, L. M. , & Broyles, S. J. (1996). Creativity and the five‐factor model. Journal of Research in Personality, 30(2), 189–203. [Google Scholar]
- Kuperberg, G. R. , Broome, M. R. , McGuire, P. K. , David, A. S. , Eddy, M. , Ozawa, F. , … Fischl, B. (2003). Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry, 60(9), 878–888. [DOI] [PubMed] [Google Scholar]
- Lerch, J. P. , Pruessner, J. , Zijdenbos, A. P. , Collins, D. L. , Teipel, S. J. , Hampel, H. , & Evans, A. C. (2008). Automated cortical thickness measurements from MRI can accurately separate Alzheimer's patients from normal elderly controls. Neurobiology of Aging, 29(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Li, W. , Li, X. , Huang, L. , Kong, X. , Yang, W. , Wei, D. , … Liu, J. (2015). Brain structure links trait creativity to openness to experience. Social Cognitive and Affective Neuroscience, 10(2), 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A. , Woollett, K. , & Spiers, H. J. (2006). London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus, 16(12), 1091–1101. [DOI] [PubMed] [Google Scholar]
- Maguire, E. A. , Gadian, D. G. , Johnsrude, I. S. , Good, C. D. , Ashburner, J. , Frackowiak, R. S. J. , & Frith, C. D. (2000). Navigation‐related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America, 97(8), 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, N. , Biederman, J. , Valera, E. M. , Bush, G. , Kaiser, J. , Kennedy, D. N. , … Seidman, L. J. (2007). Cortical thinning of the attention and executive function networks in adults with attention‐deficit/hyperactivity disorder. Cerebral Cortex (New York, N.Y. : 1991), 17(6), 1364–1375. [DOI] [PubMed] [Google Scholar]
- Martin, A. , & Chao, L. L. (2001). Semantic memory and the brain. Current Opinion in Neurobiology, 11(2), 194–201. [DOI] [PubMed] [Google Scholar]
- Martindale, C. (1999). Biological bases of creativity In Sternberg R. (Ed.), Handbook of creativity, (pp. 137–152). New York: Cambridge University Press. [Google Scholar]
- McCrae, R. R. (1987). Creativity, divergent thinking, and openness to experience. Journal of Personality and Social Psychology, 52(6), 1258–1263. [Google Scholar]
- Mednick, S. A. (1962). The associative basis of the creative process. Psychological Review, 69(3), 220–232. − [DOI] [PubMed] [Google Scholar]
- Morrison, J. H. , & Hof, P. R. (1997). Life and death of neurons in the aging brain. Science (New York, N.Y.), 278(5337), 412–419. [DOI] [PubMed] [Google Scholar]
- Nairn, J. G. , Bedi, K. S. , Mayhew, T. M. , & Campbell, L. F. (1989). On the number of Purkinje cells in the human cerebellum: Unbiased estimates obtained by using the “fractionator. Journal of Comparative Neurology, 290(4), 527–532. [DOI] [PubMed] [Google Scholar]
- Nesvag, R. , Lawyer, G. , Varnas, K. , Fjell, A. M. , Walhovd, K. B. , Frigessi, A. , … Agartz, I. (2008). Regional thinning of the cerebral cortex in schizophrenia: Effects of diagnosis, age and antipsychotic medication. Schizophrenia Research, 98(1–3), 16–28. [DOI] [PubMed] [Google Scholar]
- Passamonti, L. , Terracciano, A. , Riccelli, R. , Donzuso, G. , Cerasa, A. , Vaccaro, M. , … Quattrone, A. (2015). Increased functional connectivity within mesocortical networks in open people. NeuroImage, 104, 301–309. [DOI] [PubMed] [Google Scholar]
- Riccelli, R. , Toschi, N. , Nigro, S. , Terracciano, A. , & Passamonti, L. (2017). Surface‐based morphometry reveals the neuroanatomical basis of the five‐factor model of personality. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. P. , & Addis, D. R. (2017). A common mode of processing governing divergent thinking and future imagination In Jung R. E. & Vartanian O. (Eds.), Cambridge handbook of the neuroscience of creativity (pp. 211–230). Cambridge University Press. [Google Scholar]
- Rosas, H. D. , Liu, A. K. , Hersch, S. , Glessner, M. , Ferrante, R. J. , Salat, D. H. , … Fischl, B. (2002). Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology, 58(5), 695–701. [DOI] [PubMed] [Google Scholar]
- Salat, D. H. , Buckner, R. L. , Snyder, A. Z. , Greve, D. N. , Desikan, R. S. , Busa, E. , … Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex (New York, N.Y. : 1991), 14(7), 721–730. [DOI] [PubMed] [Google Scholar]
- Sampaio, A. , Soares, J. M. , Coutinho, J. , Sousa, N. , & Gonçalves, O. F. (2014). The big five default brain: Functional evidence. Brain Structure &Amp; Function, 219(6), 1913–1922. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne, F. , Pacheco, J. , & Fischl, B. (2007). Geometrically accurate topology‐correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging, 26(4), 518–529. [DOI] [PubMed] [Google Scholar]
- Shaw, P. , Lerch, J. , Greenstein, D. , Sharp, W. , Clasen, L. , Evans, A. , … Rapoport, J. (2006). Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention‐deficit/hyperactivity disorder. Archives of General Psychiatry, 63(5), 540–549. [DOI] [PubMed] [Google Scholar]
- Silvia, P. J. , Kaufman, J. C. , & Pretz, J. E. (2009a). Is creativity domain‐specific? Latent class models of creative accomplishments and creative self‐descriptions. Psychology of Aesthetics, Creativity, and the Arts, 3, 139–148. [Google Scholar]
- Silvia, P. J. , Nusbaum, E. C. , Berg, C. , Martin, C. , & O'connor, A. (2009b). Openness to experience, plasticity, and creativity: Exploring lower‐order, higher‐order, and interactive effects. Journal of Research in Personality, 43, 1087–1090. [Google Scholar]
- Simonton, D. K. (1999). Creativity as blind variation and selective retention: Is the creative process Darwinian? Psychological Inquiry, 10, 309–328. [Google Scholar]
- Singh, V. , Chertkow, H. , Lerch, J. P. , Evans, A. C. , Dorr, A. E. , & Kabani, N. J. (2006). Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain, 129(Pt 11), 2885–2893. [DOI] [PubMed] [Google Scholar]
- Sutin, A. R. , Beason‐Held, L. L. , Resnick, S. M. , & Costa, P. T. (2009). Sex differences in resting‐state neural correlates of openness to experience among older adults. Cerebral Cortex (New York, N.Y. : 1991), 19(12), 2797–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry, R. D. , DeTeresa, R. , & Hansen, L. A. (1987). Neocortical cell counts in normal human adult aging. Annals of Neurology, 21(6), 530–539. [DOI] [PubMed] [Google Scholar]
- Vartanian, O. (2011). Brain and neuropsychology In Runco M. A. & Pritzker S. (Eds.), Encyclopedia of creativity (2nd ed., pp. 164–169). San Diego, CA: Academic Press. [Google Scholar]
- Vartanian, O. (2018). Openness to experience: Insights from personality neuroscience In Jung R. E. & Vartanian O. (Eds.), Cambridge handbook of the neuroscience of creativity (pp. 464–475). Cambridge University Press. [Google Scholar]
- Vartanian, O. , & Goel, V. (2005). Task constraints modulate activation in right ventral lateral prefrontal cortex. NeuroImage, 27(4), 927–933. [DOI] [PubMed] [Google Scholar]
- Vincent, J. L. , Kahn, I. , Snyder, A. Z. , Raichle, M. E. , & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye, L. T. , Grydeland, H. , Walhovd, K. B. , & Fjell, A. M. (2011). Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cerebral Cortex (New York, N.Y. : 1991), 21(2), 345–356. [DOI] [PubMed] [Google Scholar]
- Westlye, L. T. , Walhovd, K. B. , Dale, A. M. , Bjørnerud, A. , Due‐Tønnessen, P. , Engvig, A. , … Fjell, A. M. (2010). Differentiating maturational and aging‐related changes of the cerebral cortex by use of thickness and signal intensity. NeuroImage, 52(1), 172–185. [DOI] [PubMed] [Google Scholar]
- Westlye, L. T. , Walhovd, K. B. , Dale, A. M. , Espeseth, T. , Reinvang, I. , Raz, N. , … Fjell, A. M. (2009). Increased sensitivity to effects of normal aging and Alzheimer's disease on cortical thickness by adjustment for local variability in gray/white contrast: A multi‐sample MRI study. NeuroImage, 47(4), 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, C. I. , Feczko, E. , Dickerson, B. , & Williams, D. (2007). Neuroanatomical correlates of personality in the elderly. NeuroImage, 35(1), 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Yang, W. , Tong, D. , Sun, J. , Chen, Q. , Wei, D. , … Qiu, J. (2015). A meta‐analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapping, 36(7), 2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , & Potenza, M. N. (2012). White matter integrity and five‐factor personality measures in healthy adults. NeuroImage, 59(1), 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabelina, D. L. , & Andrews‐Hanna, J. (2016). Dynamic network interactions supporting internally‐oriented cognition. Current Opinion in Neurobiology, 40, 86–93. [DOI] [PubMed] [Google Scholar]


