Abstract
Noninvasive brain stimulation can modify phantom sounds for longer periods by modulating neural activity and putatively inducing regional neuroplastic changes. However, treatment response is limited and there are no good demographic or clinical predictors for treatment outcome. We used state‐of‐the‐art voxel‐based morphometry (VBM) to investigate whether transcranial magnetic stimulation‐induced neuroplasticity determines therapeutic outcome. Sixty subjects chronically experiencing phantom sounds (i.e., tinnitus) received repetitive transcranial magnetic stimulation (rTMS) of left dorsolateral prefrontal and temporal cortex according to a protocol that has been shown to yield a significantly higher number of treatment responders than sham stimulation and previous stimulation protocols. Structural magnetic resonance imaging was performed before and after rTMS. In VBM whole‐brain analyses (P < 0.05, FWE corrected), we assessed longitudinal gray matter changes as well as structural connectivity between the ensuing regions. We observed longitudinal mesoscopic gray matter changes of left dorsolateral prefontal (DLPFC), left operculo‐insular, and right inferior temporal cortex (ITC) in responders (N = 22) but not nonresponders (N = 38), as indicated by a group × time interaction and post‐hoc tests. These results were neither influenced by age, sex, hearing loss nor by tinnitus laterality, duration, and severity at baseline. Furthermore, we found robust DLPFC–insula and insula–ITC connectivity in responders, while only relatively weak DLPFC–insula connectivity and no insula–ITC connectivity could be demonstrated in nonresponders. Our results reinforce the implication of nonauditory brain regions in phantom sounds and suggest the dependence of therapeutic response on their neuroplastic capabilities. The latter in turn may depend on (differences in) their individual structural connectivity. Hum Brain Mapp 39:554–562, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: magnetic resonance imaging, phantom sounds, tinnitus, transcranial magnetic stimulation, voxel‐based morphometry
INTRODUCTION
Tinnitus, the perception of sound in the absence of a corresponding external acoustic stimulus is an excellent paradigm to gain insight into the neural basis of phantom sensations [De Ridder et al., 2011, 2014; Elgoyhen et al., 2015]. While auditory hallucinations mainly occur in psychotic disorders and typically involve hearing voices, tinnitus as an auditory phantom phenomenon is usually of an unformed acoustic nature and described as ringing, hissing, or buzzing [Langguth et al., 2013]. Neural changes underlying these nonpsychotic phantom perceptions involve structure, function, and connectivity of not only auditory but also nonauditory brain regions [Elgoyhen et al., 2015]. Particularly frontostriatal gating may contribute to tinnitus pathophysiology [Leaver et al., 2011; Rauschecker et al., 2015].
According to its prevalence ranging from 10–15%, tinnitus can be considered a common disorder, which severely impairs quality of life of about 1–2% of the general population [Langguth et al., 2013]. No effective specific drug treatments are available and other treatment options are limited [Baguley et al., 2013]. Besides hearing aids in cases of concomitant hearing loss, evidence is most robust for sound therapy and cognitive behavioral therapy (CBT) [Baguley et al., 2013; Langguth et al., 2013]. In addition, the use of noninvasive brain stimulation techniques has gained momentum. A recent meta‐analysis on the effect of repetitive transcranial magnetic stimulation (rTMS) on tinnitus in randomized, placebo‐controlled trials indicated medium to large effect sizes, although not all subjects respond to this treatment [Soleimani et al., 2016]. These results underscore the potential of rTMS in modulating auditory phantom phenomena but leave open why some subjects show treatment response and others do not.
Unfortunately, there exist no good demographic or clinical predictors of treatment outcome [Lehner et al., 2012]. It must therefore be assumed that success depends on neurobiological properties that are not reflected in available demographic or clinical variables. Given known morphologic and connectional abnormalities of the tinnitus brain, it is to be expected that brain stimulation‐induced changes in tinnitus are linked to neuroplastic changes that affect brain structure and connectivity. Evidence for such causal relationship is however missing.
Here, we tested the hypothesis that rTMS‐induced brain plasticity determines therapeutic response in tinnitus. To this end, we obtained high‐resolution structural magnetic resonance images of tinnitus patients before and after rTMS following a protocol that has been shown to yield significantly more treatment responders than sham‐stimulation and previous stimulation protocols [Langguth et al., 2014]. In addition to auditory networks (i.e., the temporal cortex), this procotol targets the dorsolateral prefrontal cortex (DLPFC) according to electrophysiological evidence that tinnitus might occur as the result of a dysfunction in the top–down inhibitory processes that originate in the prefrontal lobe [Kleinjung et al., 2008; Norena et al., 1999]. Using whole‐brain voxel‐based morphometry (VBM), we assessed whether a reduction of tinnitus distress after rTMS is explained by structural gray matter changes and if treatment response can be predicted by brain morphology before the intervention. In addition, we investigated whether anticipated neuroplastic effects and associated treatment response could be predicated on differential structural connectivity.
METHODS
Subjects
We recruited 60 subjects with chronic subjective tinnitus who were all TMS‐naïve. All patients provided written informed consent to participate in the study. The study protocol was approved by the local ethics committee. Patients were treated in the context of a clinical trial [Langguth et al., 2014] or rTMS was done as compassionate use treatment between years 2006 and 2010. Magnetic resonance imaging (MRI) was performed immediately before the first and after the last of all 10 rTMS sessions that subjects underwent on 10 consecutive working days. No subject received concomitant psychotropic medication. Treatment response was defined according to accepted standards on the basis of previously calculated minimal clinically important difference (MCID) [Adamchic et al., 2012]. More specifically, classification as “responder” presupposed a reduction by at least 5 points on the Tinnitus Questionnaire, a validated and commonly used instrument for assessment of tinnitus severity [Adamchic et al., 2012; Goebel and Hiller, 1994]. As expected, there were no baseline differences in age, sex, hearing loss, tinnitus laterality, tinnitus duration, or tinnitus severity between responders and nonresponders (Table 1).
Table 1.
Patients' characteristics
| Nonresponders | Responders | P value | |
|---|---|---|---|
| Subjects [N] | 38 | 22 | N/A |
| Age [years] | 52.2 ± 9.2 | 52.1 ± 12.4 | 0.955 |
| Sex [male/female] | 33/5 | 15/7 | 0.082 |
| Hearing loss [dB]a | 20.0 ± 12.3 | 19.4 ± 11.3 | 0.865 |
| Tinnitus laterality [L/R/B]b | 15/12/11 | 13/5/3 | 0.230 |
| Tinnitus duration [months]c | 74.2 ± 79.4 | 98.4 ± 117.0 | 0.362 |
| Tinnitus severity [TQ] | 47.6 ± 20.1 | 44.4 ± 16.0 | 0.527 |
Data available for 35/20 non‐/responders.
Data available for 38/21 non‐/responders.
Data available for 36/20 non‐/responders.
Values are reported as mean ± standard deviation. P values were determined by a two‐sample t test for age, hearing loss, tinnitus duration, and tinnitus severity, and a χ 2 test of independence for sex and tinnitus laterality.
B, bilateral; L, left; N/A, not applicable; R, right; TQ, Tinnitus Questionnaire [Goebel and Hiller, 1994].
Repetitive Transcranial Magnetic Stimulation
Initially, the resting motor threshold (RMT) was determined for the right M. abductor digiti minimi and defined as the lowest intensity at which at least four of eight consecutive magnetically evoked potentials were ≥50 µV in amplitude while the investigated muscle was at rest. In each of the subsequent 10 sessions, patients received rTMS of the left DLPFC (40 trains with 50 stimuli; 25 s intertrain interval; 20 Hz; 110% RMT), immediately followed by low‐frequency rTMS (2000 Stimuli; 1 Hz; 110% RMT) of the left temporal cortex. Coil positioning over the temporal cortex was based on 10–20 EEG coordinates [Langguth et al., 2014, 2006] and over the left DLPFC on a standard algorithm by moving the coil 6 cm from the RMT hot spot of the right M. abductor digiti minimi in anterior direction [Langguth et al., 2014].
Structural Magnetic Resonance Imaging
Structural MRI scans were acquired on a 1.5 T scanner (MAGNETOM Sonata, Siemens Medical Solutions, Erlangen, Germany) equipped with a standard 8‐channel birdcage head coil. T1‐weighted images were obtained using a 3D magnetization‐prepared rapid acquisition with gradient echo sequence (repetition time 1880 ms, echo time 3.42 ms, flip angle 15°, matrix size 256 × 256, 176 sagittal slices, voxel size 1 × 1 × 1 mm3).
Image Preprocessing
All image preprocessing steps followed the default longitudinal preprocessing approach (cf. Supplement) implemented in VBM8 (http://dbm.neuro.uni-jena.de/vbm/), integrated as a toolbox into SPM8 software (Wellcome Trust Centre for Neuroimaging, UK). Default preprocessing involved intra‐subject realignment, bias correction, segmentation, and normalization. The resulting gray matter images were smoothed with an isotropic Gaussian kernel of 8 mm full‐width at half‐maximum.
Statistical Analyses
To investigate brain structural changes associated with treatment response to rTMS, ensuing unmodulated gray matter images were entered into a repeated measures ANOVA with the between‐subjects factor group (non‐/responders) and the within‐subjects factor time (pre‐/post‐rTMS) in SPM8. Whole‐brain results were evaluated using threshold free cluster enhancement (TFCE) inference at a family‐wise error (FWE) corrected threshold of P < 0.05 [Smith and Nichols 2009]. Such combination of unmodulated images and TFCE has been proposed the optimal setting for advanced VBM due to an ideal balance between (high) sensitivity and (low) false‐positive rates [Radua et al., 2014].
To test if treatment response to rTMS is predicted by brain morphology before the intervention, pre‐rTMS images were correlated with changes in tinnitus severity (i.e., differences in Tinnitus Questionnaire scores) in whole‐brain analyses. Also here, the TFCE approach was used for statistical inference at P < 0.05, FWE corrected.
In addition, we performed structural connectivity analyses of (longitudinal) morphologic changes associated with response to rTMS (i.e., of regions under the interaction term of the ANOVA) to investigate (i) whether spatially distributed neuroplastic effects occur in (structurally) connected regions and (ii) whether treatment response could hence be predicated on differential structural connectivity. To this end, pre‐rTMS images were subtracted from post‐rTMS images for each participant, respectively. From the ensuing difference images, we then extracted gray matter eigenvariates of the candidate regions. Correlations of these gray matter data were analyzed using SPSS Statistics software (Version 22.0.0.0, IBM Corp., 2013, USA). Correlations of local gray matter are considered indicative of axonal connectivity between covarying regions because connectivity should exert mutually trophic effects on connected neurons and regions [Bullmore and Bassett, 2011]. This approach has been applied in the context of both neuroplasticity of the healthy brain and disease‐related alterations of brain networks [Gerber et al., 2014; Labus et al., 2014; Rüsch et al., 2007].
Anatomical Labeling
For anatomical labeling, we capitalized on cytoarchitectonic maps of the human brain provided by the SPM Anatomy Toolbox [Eickhoff et al., 2006b, 2007, 2005] Clusters were thus assigned to the most probable histologically defined area at the respective location. This probabilistic histology‐based labeling is reported in the imaging results table.
RESULTS
We found a significant group (non‐/responders) × time (pre‐/post rTMS) interaction indicating specific neuroplastic changes associated with response to rTMS in a cluster comprising the left DLPFC and ventrolateral prefrontal cortex (VLPFC; ipsilateral stimulation site), in the left operculo‐insular cortex including the claustrum, and in the right inferior temporal cortex (ITC) (Fig. 1, Table 2). Post‐hoc tests revealed that the interaction was driven by a gray matter decrease of all regions after rTMS (compared with pre‐rTMS gray matter) in responders (but not nonresponders) (Fig. 2). More specifically, within‐group analyses showed a robust gray matter decrease after rTMS in the responder group (P < 0.05, FWE corrected) but no gray matter changes (in either direction) in the nonresponder group in the corresponding regions (even) at an uncorrected threshold of P < 0.001.
Figure 1.
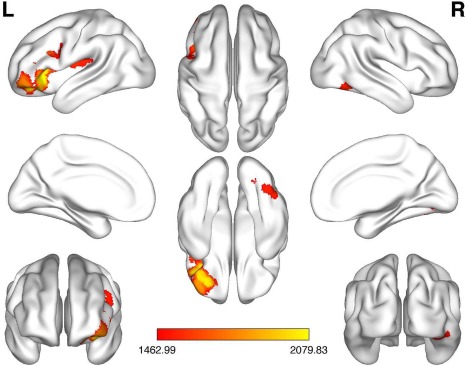
Group (non‐/responders) × time (pre‐/post rTMS) interaction. Significant specific neuroplastic changes associated with response to rTMS in a cluster comprising the left dorsolateral/ventrolateral prefrontal cortex, in the left operculo‐insular cortex including the claustrum, and in the right inferior temporal cortex (P < 0.05, FWE corrected). The color bar indicates TFCE values.
FWE, familywise error; L, left; R, right; TFCE, threshold‐free cluster enhancement. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Brain stimulation‐induced neuroplastic changes associated with therapeutic response in phantom sounds
| Brain region | Cluster size | MNI coordinates | |||||
|---|---|---|---|---|---|---|---|
| Macroanatomic | Cytoarchitectonic | in voxels | x | y | z | P value | |
| L | DLPFC/VLPFC | 3610 | −34 | 27 | −5 | 0.007 | |
| L | Operculo‐insular cortex/Claustrum | Areae OP2/3, TE 1.1 | 1149 | −30 | −40 | 6 | 0.029 |
| R | Inferior temporal cortex | FG1 | 498 | 46 | −60 | −15 | 0.036 |
Significant group (non‐/responders) × time (pre‐/post‐stimulation) interactions in whole‐brain voxel‐based morphometric (VBM) analyses (P < 0.05, FWE corrected). Post‐hoc tests revealed that the interaction was driven by a longitudinal gray matter (GM) decrease in all three regions specifically in responders. Coordinates represent peaks within a cluster. For detailed information on cytoarchitectonics, see publications by Caspers (FG1), Eickhoff (OP2/3), Lorenz (FG1), Morosan (TE 1.1), and colleagues [Caspers et al., 2013; Eickhoff et al., 2006a, 2006c; Lorenz et al., 2017; Morosan et al., 2001].
DLPFC, dorsolateral prefrontal cortex; FWE, familywise error; L, left; MNI, Montreal Neurological Institute; R, right; TFCE, threshold‐free cluster enhancement; VLPFC, ventrolateral prefrontal cortex.
Figure 2.
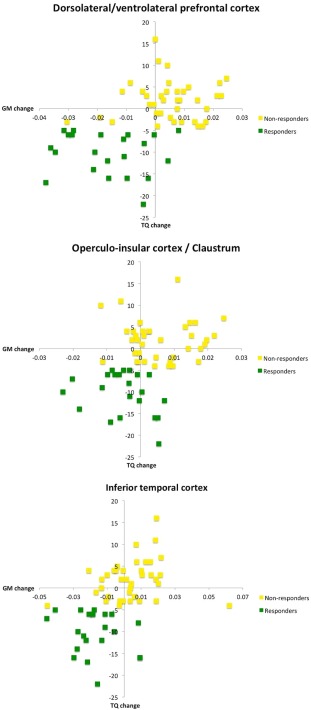
Relationship between gray matter (GM) changes and tinnitus improvement in responders and nonresponders. Responders to repetitive transcranial magnetic stimulation (rTMS) showed GM decrease in the specified regions, while nonresponders did not. Tinnitus improvement is expressed by a Tinnitus Questionnaire (TQ) score reduction. Abscissae represent changes in GM values (after minus before rTMS), ordinates depict changes in TQ values (after minus before rTMS). The supplementary figure depicts a plot of changes in TQ scores for all subjects. [Color figure can be viewed at http://wileyonlinelibrary.com]
There was no significant association of changes in tinnitus severity with gray matter before rTMS, precluding the prediction of therapeutic response on the basis of brain morphology before treatment. This was true for correlational analyses across all subjects and also within the groups of non‐/responders.
We found significant structural connectivity with medium effect size for rTMS induced changes between left DLPFC/VLPFC and operculo‐insular/claustral gray matter (Pearson's r = 0.482, P = 0.023, two‐sided) and between left operculo‐insular/claustral gray matter and right ITC (r = 0.458, P = 0.032) in responders. In contrast, there was significant connectivity between DLPFC/VLPFC and opercular‐insular/claustral gray matter (r = 0.373, P = 0.021) but not between the latter and the right ITC (r = 0.131, P = 0.434) in non‐responders. Structural connectivity between left DLPFC/VLPFC and right ITC was not significant, neither in responders (r = 0.168, P = 0.456) nor in nonresponders (r = −0.084, P = 0.615). Connectivity results are illustrated in Figure 3.
Figure 3.
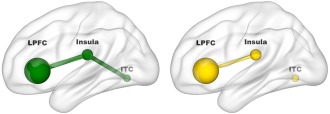
Structural connectivity between gray matter regions associated with treatment response. Correlational analyses demonstrated structural connectivity between left lateral prefrontal cortex (LPFC) and operculo‐insular/claustral region (r = 0.482, P = 0.023) and between the latter and right inferior temporal cortex (ITC; r = 0.458, P = 0.032) in responders (green). Nonresponders (yellow) showed structural connectivity also between LPFC and operculo‐insular/claustral region (r = 0.373, P = 0.021) but not between the latter and right ITC (r = 0.131, P = 0.434). [Color figure can be viewed at http://wileyonlinelibrary.com]
As there was a nonsignificant trend (P < 0.1) toward an imbalance in sex ratios between responders and nonresponders, we repeated the aforementioned interaction, correlational, and connectivity analyses using sex as a covariate. Considering sex in the statistical models did not change the results (i.e., the same (and no additional) clusters emerged at the stated significance level). To exclude that the normalization approach (nonmodulation) affected the results, we assessed the potential effect of modulation on our data. As the deformations due to nonlinear normalization are the same for all time points, the modulation only depends on the affine component that is driven by different brain sizes. We therefore reanalyzed our data including total intracranial volume (TIV) as a covariate to account for this potential effect. Also this additional analysis yielded similar results, albeit accompanied by a general slight increase in cluster sizes and extension of the left lateral prefrontal cluster into the medial prefrontal cortex. More precisely, the prefrontal cluster now also included the dorsomedial prefrontal and anterior cingulate cortex.
DISCUSSION
We provide first evidence of brain stimulation‐induced neuroplastic changes in cortical morphology underlying the reduction of phantom sounds. Notably, brain morphology before rTMS did not predict therapeutic success. It is therefore to be assumed that idiosyncratic neuroplastic properties of gray matter determine outcome. These do neither depend on age, sex, or hearing loss, nor on tinnitus laterality, duration, or severity, because responders did not differ from nonresponders in these variables. However, it seems possible that these characteristics are associated with the mechanism of tinnitus, which may differ between responders and nonresponders. In general, various factors have been implicated in the neurobiological basis of mesoscopically traceable gray matter changes, for example, changes in cell body sizes, synaptic proliferation, changes in spine density, neural and glial cell genesis, but also changes in blood flow or interstitial fluid. More specifically, however, a study on dynamic aspects of brain stimulation‐induced neuroplasticity in the healthy brain suggests that rTMS affects fast adjusting neuronal systems, such as spine and synapse turnover [May et al., 2007]. It may therefore be concluded that response to rTMS in subjects suffering from phantom sounds is associated with particular neuroplastic capabilities of dendrites and/or synapses. This view would also be supported by recently reported prediction of shifts in dendritic spine density and morphology with auditory conditioning by VBM signal [Keifer et al., 2015]. The detailed nature of the (probably) microscopic characteristics underlying rTMS response however remains unclear but is an important matter that needs to be addressed in further studies. More specifically, these studies could address whether and which mechanisms on the cellular level determine the neural potential for reorganization induced by rTMS. More detailed information on these rTMS‐related “neuroplastic capabilities” may allow for predicting individual response to rTMS in tinnitus in the future.
We observed gray matter changes in three distinct clusters. It is reasonable, yet remarkable that one cluster was located in the left DLPFC, that is, precisely under the stimulation site, even if its larger part comprised the VLPFC. This suggests a direct neuroplastic effect of the magnetic pulses on subjacent brain tissue. The DLPFC is an inherent part of current models of tinnitus generation, which rely on structural and functional imaging in humans [De Ridder et al., 2011; Elgoyhen et al., 2015]. According to electroencephalographic studies, (abnormal) activity of particularly the left DLPFC modulates tinnitus‐related distress and could represent the neural correlate of a maladaptive coping style [Song et al., 2015; Vanneste et al., 2014]. Flawed coping strategies in turn may be based on a specific deficit of top–down executive control of attention in tinnitus patients [Heeren et al., 2014]. These correlative imaging and electrophysiological findings are supported by brain stimulation trials demonstrating that (putatively) increasing the excitability of the left DLPFC by high‐frequency rTMS can reduce tinnitus annoyance and hence pointing to a causative functional role of this region in tinnitus modulation if not generation [Faber et al., 2012; Langguth et al., 2014]. Following our high‐frequency rTMS of the left DLPFC, we observed gray matter changes also in the adjacent VLPFC. Importantly, it has been shown that high‐frequency but not low‐frequency or sham rTMS of the left VLPFC can modulate tinnitus loudness, indicating its etiologic involvement in tinnitus [Vanneste and De Ridder 2012]. Although we did not directly target the left VLPFC, the conjunction of these imaging and brain stimulation findings suggests that high‐frequency stimulation of left DLPFC induces cortical remodeling also of the adjacent VLPFC resulting in a reduction of phantom sounds.
Our analyses identified another two clusters in remote areas that could not have been directly affected by the magnetic pulses over the left DLPFC. One cluster was located ipsilateral to the stimulation site and comprised the operculo‐insular cortex and claustrum, the other the contralateral ITC. Structural connectivity between these regions, as here evidenced by the correlation of their gray matter changes, supports the notion that tinnitus is an auditory phantom sensation involving network dysfunction [Leaver et al., 2016; Schlee et al., 2008]. Despite the broad clinical use, evidence for an effect of rTMS on gray matter morphology is limited to three studies in 36 healthy volunteers, 77 subjects with tinnitus, and 27 patients suffering from major depression (as well as one negative study in 22 depressed patients) [Lan et al., 2016; Lehner et al., 2014; May et al., 2007; Nahas et al., 2000]. The positive study on brain morphological changes induced by rTMS to the left DLPFC of 27 depressive patients reported effects that did not coincide with our findings [Lan et al., 2016]. This discrepancy suggests that rTMS induces disease‐specific effects on brain plasticity. The study in tinnitus subjects (which used a different rTMS protocol than the present investigation) observed structural brain changes but was not able to relate them to treatment outcome, i.e., tinnitus reduction [Lehner et al., 2014]. The reason for this lack of a relationship may be the specific rTMS protocol that achieves only negligible clinical effects [Landgrebe et al., in press]. Our results go beyond these findings by demonstrating fast morphologic adaptions that occurred specifically in responders and should hence represent the neurobiological basis of symptom improvement. That we found robust DLPFC–insula and insula–ITC connectivity in responders, but only relatively weak DLPFC–insula and no insula–ITC connectivity in nonresponders, moreover suggests that the neuroplastic capabilities associated with rTMS response may depend on individual structural connectivity.
The larger of the two remote cluster comprised cytoarchitechtonic areas 2 and 3 of the parietal operculum, insular cortex, and claustrum in the left hemisphere. Opercular area 2 is the human equivalent of nonhuman primates' parieto‐insular vestibular cortex and thus closely connected to the auditory system [Eickhoff et al., 2006d]. Even more specifically, distinct activation of opercular area 3 has recently been demonstrated during provoked tinnitus‐related phantom auditory perceptions [Job et al., 2016]. Both areas of the secondary somatosensory cortex should hence be involved in processing of not only external but also of phantom auditory sensations. The insular cortex is certainly not characterized by functional specificity to the auditory modality but rather by a posterior‐to‐mid‐to‐anterior pattern of integration of interoceptive information [Craig 2009]. Moreover, it is considered an integral part of the brain's “pain matrix” and may thus represent the topofunctional link between tinnitus and pain as phantom percepts that have both been conceptualized as persisting aversive memory networks [Craig 2002; De Ridder et al., 2011]. The notion of this insular link is supported by structural and functional abnormalities of the insula in tinnitus patients and its aberrant activity in phantom limb pain, which notably also includes opercular area 3 [Elgoyhen et al., 2015; Makin et al., 2013]. The insula has furthermore been proposed to play a fundamental role in awareness [Craig 2009]. Although the area where we found improvement‐related gray matter changes is located relatively caudally in the insular cortex, one could speculate that it pertains to the network involved in the transition of interoceptive representations of phantom sounds into conscious awareness. However, the third area of the respective cluster is more likely to play a role in this context. The claustrum, a thin and sheet‐like neuronal structure, has been associated with cross‐modal processing due to its broad range of communication pathways within the brain [Calvert 2001]. Moreover, Crick and Koch conclude in their exhaustive review “that the claustrum may contain specialized mechanisms that permit information to travel widely within its anterior‐posterior and ventral‐dorsal extent to synchronize different perceptual, cognitive, and motor modalities” [Crick and Koch 2005]. Thus, the authors regard the claustrum as a “conductor coordinating a group of players in the orchestra” (i.e., various brain areas) and adumbrate its significant contribution to consciousness [Crick and Koch 2005; Stevens 2005]. In the context of auditory phantom percepts and related aberrant activity [Song et al., 2012], the claustrum may accordingly be the conductor of the tinnitus orchestra, which is composed of perceptual, emotional, cognitive, and mnestic sections represented by multiple, parallel, dynamically changing, and partially overlapping neural subnetworks [De Ridder et al., 2014]. The claustrum also connects to the posterior temporal cortex, that is, the second remote cluster where we found gray matter changes, and may thereby link the opercula with this region [Fernández‐Miranda et al., 2008]. Tinnitus‐related activation of the ITC has been demonstrated by meta‐analysis and may specifically mediate tinnitus distress [Song et al., 2012; Schecklmann et al., 2013a]. The latter seems particularly reasonable with respect to the notion of tinnitus as a persisting aversive memory because the posterior ITC interacts with the hippocampus in memory consolidation during acute stress [Henckens et al., 2009]. It may be objected that the ITC has been canonically implicated in visual processing, yet recent results strongly suggest that ITC neurons are also responsive to sole auditory stimuli [Kaposvári et al., 2011]. In summary, we also observed gray matter changes in areas that are important for interoceptive and multimodal integration of auditory percepts, putatively into consciousness.
One critical point, the direction of the gray matter changes, has not yet been addressed. It might seem puzzling that we found gray matter decreases in all regions, particularly because neuroplasticity appears to (probably misleadingly) implicate growth and proliferation. However, a recent study in mood disorders showed that electroconvulsive therapy‐induced brain plasticity involves gray matter decreases in spatially distributed regions and that also these decreases seem to be specific to the respective disease as well as responsible for the therapeutic effect [Dukart et al., 2014]. In tinnitus patients, the overall evidence for structural abnormalities specifically related to tinnitus is poor, maybe because results are quite often confounded by concomitant hearing loss and based on relatively small sample and effect sizes [Adjamian et al., 2014; Schecklmann et al., 2013b]. Our findings in contrast should not be biased by hearing loss, since there were no differences between responders and non‐responders in this regard, although we cannot exclude an inhomogeneity regarding frequencies between octave bands or the high‐frequency range (>8 kHz). However, at least an influence of the latter seems unlikely because hearing at supraclinical frequencies is correlated with gray matter in other regions than the ones observed in our study [Melcher et al., 2013]. We hence propose that the observed improvement‐related gray matter decrease does not represent gray matter loss (with a negative connotation) but rather a structural adaption involving morphologic remodeling that needs to be investigated in more detail at a microscopic level. We would go even further and put up for discussion if previously (yet inconsistently) reported gray matter alterations in tinnitus patients could mirror a (yet sometimes insufficient) adaption by cortical remodeling rather than pathology per se [Husain et al., 2011]. Such notion would be in line with recent findings that phantom pain is associated with preserved structure and function in the former limb area [Makin et al., 2013].
In summary, we observed rTMS‐induced neuroplastic changes underlying therapeutic response in brain areas previously implicated in attentional modulation and interoceptive integration of auditory phantom percepts. Our results reinforce the implication of nonauditory brain regions in phantom sounds and suggest the dependence of therapeutic response on their neuroplastic capabilities. The latter in turn may depend on (differences in) their individual structural connectivity.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Supporting information
Supporting Information
REFERENCES
- Adamchic I, Tass PA, Langguth B, Hauptmann C, Koller M, Schecklmann M, Zeman F, Landgrebe M (2012): Linking the tinnitus questionnaire and the subjective clinical global impression: Which differences are clinically important? Health Qual Life Outcomes 10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjamian P, Hall DA, Palmer AR, Allan TW, Langers DRM (2014): Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci Biobehav Rev 45:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley D, McFerran D, Hall D (2013): Tinnitus. Lancet 382:1600–1607. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS (2011): Brain graphs: Graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140. [DOI] [PubMed] [Google Scholar]
- Calvert GA (2001): Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb Cortex 11:1110–1123. [DOI] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K (2013): Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct 218:511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig ADB (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C (2005): What is the function of the claustrum?. Philos Trans R Soc Lond B Biol Sci 360:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Elgoyhen AB, Romo R, Langguth B (2011): Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA 108:8075–8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Weisz N, Londero A, Schlee W, Elgoyhen AB, Langguth B (2014): An integrative model of auditory phantom perception: Tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev 44:16–32. [DOI] [PubMed] [Google Scholar]
- Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, Frackowiak RS, Draganski B (2014): Electroconvulsive therapy‐induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci USA 111:1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K (2006a): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16:268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006b): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras M‐H, Evans AC, Zilles K, Amunts K (2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K (2006c): The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16:254–267. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K (2006d): Identifying human parieto‐insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp 27:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Langguth B, De Ridder D, Vanneste S (2015): Tinnitus: Perspectives from human neuroimaging. Nat Rev Neurosci 16:632–642. [DOI] [PubMed] [Google Scholar]
- Faber M, Vanneste S, Fregni F, De Ridder D (2012): Top down prefrontal affective modulation of tinnitus with multiple sessions of tDCS of dorsolateral prefrontal cortex. Brain Stimul 5:492–498. [DOI] [PubMed] [Google Scholar]
- Fernández‐Miranda JC, Rhoton AL, Kakizawa Y, Choi C, Alvarez‐Linera J (2008): The claustrum and its projection system in the human brain: A microsurgical and tractographic anatomical study. J Neurosurg 108:764–774. [DOI] [PubMed] [Google Scholar]
- Gerber P, Schlaffke L, Heba S, Greenlee MW, Schultz T, Schmidt‐Wilcke T (2014): Juggling revisited – A voxel‐based morphometry study with expert jugglers. NeuroImage 95:320–325. [DOI] [PubMed] [Google Scholar]
- Goebel G, Hiller W (1994): The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO 42:166–172. [PubMed] [Google Scholar]
- Heeren A, Maurage P, Perrot H, De Volder A, Renier L, Araneda R, Lacroix E, Decat M, Deggouj N, Philippot P (2014): Tinnitus specifically alters the top‐down executive control sub‐component of attention: Evidence from the Attention Network Task. Behav Brain Res 269:147–154. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, Hermans EJ, Pu Z, Joëls M, Fernández G (2009): Stressed memories: How acute stress affects memory formation in humans. J Neurosci 29:10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain FT, Medina RE, Davis CW, Szymko‐Bennett Y, Simonyan K, Pajor NM, Horwitz B (2011): Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Res 1369:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job A, Jacob R, Pons Y, Raynal M, Kossowski M, Gauthier J, Lombard B, Delon‐Martin C (2016): Specific activation of operculum 3 (OP3) brain region during provoked tinnitus‐related phantom auditory perceptions in humans. Brain Struct Funct 221:913–922. [DOI] [PubMed] [Google Scholar]
- Kaposvári P, Csibri P, Csete G, Tompa T, Sáry G (2011): Auditory modulation of the inferior temporal cortex neurons in rhesus monkey. Physiol Res 60 Suppl 1:S93–S99. [DOI] [PubMed] [Google Scholar]
- Keifer OP, Hurt RC, Gutman DA, Keilholz SD, Gourley SL, Ressler KJ (2015): Voxel‐based morphometry predicts shifts in dendritic spine density and morphology with auditory fear conditioning. Nat Commun 6:7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjung T, Eichhammer P, Landgrebe M, Sand P, Hajak G, Steffens T, Strutz J, Langguth B (2008): Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: A pilot study. Otolaryngol Head Neck Surg 138:497–501. [DOI] [PubMed] [Google Scholar]
- Labus JS, Dinov ID, Jiang Z, Ashe‐McNalley C, Zamanyan A, Shi Y, Hong J‐Y, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA (2014): Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 155:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M (2016): Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: An exploratory analysis. Brain Stimul 9:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Hajak G, Wolf S, Padberg F, Klupp P, Fallgatter AJ, Polak T, Höppner J, Haker R, Cordes J, Klenzner T, Schönfeldt‐Lecuona C, Kammer T, Graf E, Koller M, Kleinjung T, Lehner A, Schecklmann M, Pöppl TB, Kreuzer P, Frank E, Langguth B. 1‐Hz rTMS in the treatment of tinnitus: A sham‐controlled, randomized multicenter trial. Brain Stimul, in press. [DOI] [PubMed] [Google Scholar]
- Langguth B, Kreuzer PM, Kleinjung T, De Ridder D (2013): Tinnitus: Causes and clinical management. Lancet Neurol 12:920–930. [DOI] [PubMed] [Google Scholar]
- Langguth B, Landgrebe M, Frank E, Schecklmann M, Sand PG, Vielsmeier V, Hajak G, Kleinjung T (2014): Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World J Biol Psychiatry 15:276–285. [DOI] [PubMed] [Google Scholar]
- Langguth B, Zowe M, Landgrebe M, Sand P, Kleinjung T, Binder H, Hajak G, Eichhammer P (2006): Transcranial magnetic stimulation for the treatment of tinnitus: A new coil positioning method and first results. Brain Topogr 18:241–247. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP (2011): Dysregulation of limbic and auditory networks in tinnitus. Neuron 69:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Turesky TK, Seydell‐Greenwald A, Morgan S, Kim HJ, Rauschecker JP (2016): Intrinsic network activity in tinnitus investigated using functional MRI. Hum Brain Mapp 37:2717–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner A, Langguth B, Poeppl TB, Rupprecht R, Hajak G, Landgrebe M, Schecklmann M (2014): Structural brain changes following left temporal low‐frequency rTMS in patients with subjective tinnitus. Neural Plast 2014:132058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner A, Schecklmann M, Landgrebe M, Kreuzer PM, Poeppl TB, Frank E, Vielsmeier V, Kleinjung T, Rupprecht R, Langguth B (2012): Predictors for rTMS response in chronic tinnitus. Front Syst Neurosci 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S, Weiner KS, Caspers J, Mohlberg H, Schleicher A, Bludau S, Eickhoff SB, Grill‐Spector K, Zilles K, Amunts K (2017): Two new cytoarchitectonic areas on the human mid‐fusiform gyrus. Cereb Cortex 27:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Filippini N, Henderson Slater D, Tracey I, Johansen‐Berg H (2013): Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun 4:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Hajak G, Gänssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P (2007): Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cereb Cortex 17:205–210. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Knudson IM, Levine RA (2013): Subcallosal brain structure: Correlation with hearing threshold at supra‐clinical frequencies (>8 kHz), but not with tinnitus. Hear Res 295:79–86. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K (2001): Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage 13:684–701. [DOI] [PubMed] [Google Scholar]
- Nahas Z, DeBrux C, Chandler V, Lorberbaum JP, Speer AM, Molloy MA, Liberatos C, Risch SC, George MS (2000): Lack of significant changes on magnetic resonance scans before and after 2 weeks of daily left prefrontal repetitive transcranial magnetic stimulation for depression. J ECT 16:380–390. [DOI] [PubMed] [Google Scholar]
- Norena A, Cransac H, Chery‐Croze S (1999): Towards an objectification by classification of tinnitus. Clin Neurophysiol 110:666–675. [DOI] [PubMed] [Google Scholar]
- Radua J, Canales‐Rodríguez EJ, Pomarol‐Clotet E, Salvador R (2014): Validity of modulation and optimal settings for advanced voxel‐based morphometry. NeuroImage 86:81–90. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, May ES, Maudoux A, Ploner M (2015): Frontostriatal gating of tinnitus and chronic Pain. Trends Cogn Sci 19:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G (2007): Prefrontal‐thalamic‐cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res 93:79–89. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Landgrebe M, Poeppl TB, Kreuzer P, Männer P, Marienhagen J, Wack DS, Kleinjung T, Hajak G, Langguth B (2013a): Neural correlates of tinnitus duration and distress: A positron emission tomography study. Hum Brain Mapp 34:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Lehner A, Poeppl TB, Kreuzer PM, Rupprecht R, Rackl J, Burger J, Frank E, Hajak G, Langguth B, Landgrebe M (2013b): Auditory cortex is implicated in tinnitus distress: A voxel‐based morphometry study. Brain Struct Funct 218:1061–1070. [DOI] [PubMed] [Google Scholar]
- Schlee W, Weisz N, Bertrand O, Hartmann T, Elbert T (2008): Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS One 3:e3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Soleimani R, Jalali MM, Hasandokht T (2016): Therapeutic impact of repetitive transcranial magnetic stimulation (rTMS) on tinnitus: A systematic review and meta‐analysis. Eur Arch Otorhinolaryngol 273:1663–1675. [DOI] [PubMed] [Google Scholar]
- Song JJ, De Ridder D, Van de Heyning P, Vanneste S (2012): Mapping tinnitus‐related brain activation: An activation‐likelihood estimation metaanalysis of PET studies. J Nucl Med 53:1550–1557. [DOI] [PubMed] [Google Scholar]
- Song JJ, Vanneste S, Schlee W, Van de Heyning P, De Ridder D (2015): Onset‐related differences in neural substrates of tinnitus‐related distress: The anterior cingulate cortex in late‐onset tinnitus, and the frontal cortex in early‐onset tinnitus. Brain Struct Funct 220:571–584. [DOI] [PubMed] [Google Scholar]
- Stevens CF (2005): Consciousness: Crick and the claustrum. Nature 435:1040–1041. [DOI] [PubMed] [Google Scholar]
- Vanneste S, De Ridder D (2012): The involvement of the left ventrolateral prefrontal cortex in tinnitus: A TMS study. Exp Brain Res 221:345–350. [DOI] [PubMed] [Google Scholar]
- Vanneste S, Joos K, Langguth B, To WT, De Ridder D (2014): Neuronal correlates of maladaptive coping: An EEG‐study in tinnitus patients. PLoS One 9:e88253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


