Abstract
Altered striatocortical functional connectivity has been suggested to be a trait marker of schizophrenia spectrum disorders, including schizotypal personality. In the present study, we examined the association between schizotypal personality traits and striatocortical functional connectivity in a sample of healthy adults. The German version of the Schizotypal Personality Questionnaire was obtained from N = 111 participants recruited from the general public. Resting‐state functional magnetic resonance imaging scans were acquired at 3T. Six striatal seed regions in each hemisphere were defined and striatocortical resting‐state functional connectivity (rsFC) as well as its lateralization indices was calculated. Regression analysis showed that schizotypy scores, especially from the positive dimension, were positively correlated with rsFC between ventral striatum and frontal cortex and negatively associated with rsFC between dorsal striatum and posterior cingulate. No significant associations were found between negative dimension schizotypy and striatocortical rsFC. We also found positive correlations between schizotypy total scores and lateralization index of right dorsal caudate and right rostral putamen. In conclusion, the present study extends previous evidence of altered striatocortical rsFC in the schizophrenia spectrum. The observed associations resemble in part the alterations observed in psychotic patients and their relatives, providing support for dimensionality from schizotypal personality to the clinical disorder. Hum Brain Mapp 39:288–299, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: schizotypy, functional magnetic resonance imaging, resting state, connectivity, striatum, lateralization
INTRODUCTION
This study investigates the association of schizotypal personality traits with striatocortical functional connectivity (FC) and its hemispheric asymmetry in healthy adults. Schizotypy refers to a constellation of traits that can be structured into positive, disorganized and negative dimensions [Raine, 2006], akin to the symptom structure of schizophrenia [e.g., Liddle, 1987]. At their most extreme expression, high schizotypy scores result in a diagnosis of schizotypal personality disorder [Raine, 1991]. Interindividual variation across the entire spectrum from low‐to‐high schizotypy questionnaire scores is associated with alterations in brain structure, brain function and cognition, with the pattern of alterations in relation to higher schizotypy bearing some resemblance with those observed in full‐blown schizophrenia [Ettinger et al., 2014; Nelson et al., 2013].
A particular focus in structural and functional neuroimaging as well as neuropathological studies in schizophrenia has been on the striatum [Shepherd, 2013; Simpson et al., 2010]. The striatum is involved in a variety of motor, affective and cognitive functions as part of several cortico‐striato‐thalamo‐cortical loops [Alexander et al., 1990] and receives prominent innervations from midbrain dopaminergic neurons. Dysfunction in striatal dopamine turnover is thought to be involved in the pathophysiology of schizophrenia [Howes and Kapur, 2009; Simpson et al., 2010], with a meta‐analysis confirming increased presynaptic dopamine synthesis in the striatum in schizophrenia patients [Howes et al., 2012]. Dopaminergic dysfunction in the striatum is also thought to occur in schizotypy [Mohr and Ettinger, 2014].
Resting‐state functional magnetic resonance imaging (rs‐fMRI) has been established as a powerful method to examine FC of distributed brain networks in healthy and diseased populations [Fornito and Bullmore, 2010]. A recent study provided detailed evidence of striatocortical functional organization emerging from six seeds in caudate and putamen in the healthy brain [Di Martino et al., 2008]. FC approaches to rs‐fMRI data have also been applied to schizophrenia spectrum populations [e.g., Giraldo‐Chica and Woodward, 2017]. Advantages of resting‐state FC (rsFC) studies include the absence of a task on which index groups may differ, resulting in differential levels of performance. Instead, rsFC data reflect brain function “at rest,” that is, without specific task‐induced cognitive demands, thereby allowing the investigation of the neural correlates of naturally occurring thought and its association with external variables such as schizotypy.
A recent study found reduced FC between dorsal caudate and prefrontal cortex as well as increased FC between ventral caudate and orbitofrontal cortex, insula and dorsolateral prefrontal cortex in first‐episode psychosis, indicating a dorsal‐to‐ventral gradient of striatocortical hypoconnectivity to hyperconnectivity [Fornito et al., 2013]. A similar pattern was found in relatives, suggesting sensitivity of this pattern to genetic influences [Fornito et al., 2013].
Striatocortical FC has also been investigated in individuals in the at‐risk mental state (ARMS). ARMS patients displayed reduced FC between dorsal caudate and prefrontal cortex and thalamus and between dorsal putamen and thalamus and lenticular nucleus [Dandash et al., 2014]. Increased FC, however, was observed between ventral putamen and frontotemporal cortex.
In relation to schizotypy, we previously found that participants with high social anhedonia (i.e., negative schizotypy) showed hyperconnectivity between ventral striatum and anterior cingulate cortex (ACC) and insula, and between dorsal striatum and motor cortex, compared to controls [Wang et al., 2016]. However, given that our previous study focused on people with high levels of negative schizotypy, we could not characterize FC in relation to overall schizotypy or the positive schizotypy and disorganization dimensions. This is important, since previous studies have found associations between positive symptoms and striatocortical FC in both patients and at‐risk individuals [Dandash et al., 2014; Fornito et al., 2013]. The first aim of the current study, therefore, was to fill this gap by applying a comprehensive schizotypy inventory, the Schizotypal Personality Questionnaire (SPQ) [Raine, 1991], which allows an assessment of the three key dimensions of schizotypy, viz., the cognitive‐perceptual (positive), interpersonal (negative), and disorganization dimensions.
A further, important finding from neuroimaging studies concerns the hemispheric asymmetry of striatal structure and function. For example, volumes of caudate, nucleus accumbens and putamen have been found to be larger in the left hemisphere than the right [Ahsan et al., 2007; Ettinger et al., 2012a]. There is also evidence from fMRI of lateralized striatal rsFC, with Mueller et al. [2015] reporting that schizophrenia patients show weaker left hemispheric but stronger right hemispheric specialization of the caudate.
Evidence of altered asymmetry in schizotypy comes from a diffusion tensor imaging (DTI) study showing greater right > left asymmetry in the uncinate fasciculus of people with high total schizotypy scores compared to controls [DeRosse et al., 2015]. Additionally, in a functional near‐infrared spectroscopy study of a verbal fluency task, a high total schizotypy group showed a right hemispheric preference of prefrontal activation compared to a low schizotypy group [Hori et al., 2008]. However, hemispheric asymmetry in FC has not yet been investigated in relation to schizotypy using rs‐fMRI.
The present study, therefore, aimed to examine the association of schizotypal personality traits with (1) striatocortical FC and (2) its lateralization. We adopted a recently described striatocortical FC method [Di Martino et al., 2008] and investigated associations of FC with both total schizotypy score and schizotypal dimensions in order to provide a comprehensive and fine‐grained analysis of the different features of schizotypy. We tested a large sample of adults who were carefully screened regarding current psychopathology, in order to avoid influences on FC that may confound true effects of schizotypy. Given the findings from patients with schizophrenia, their first‐degree relatives and at‐risk individuals, we hypothesized that we would find associations between the total score of schizotypal traits and striatal–cortical FC compatible with those previous studies. In particular, we expected that these associations would be found in prefrontal cortex. Moreover, we hypothesized that higher levels of schizotypal trait would be associated with reduced left and increased right lateralization of the FC.
METHOD
Participants
One hundred and seventeen participants were recruited into the study. Recruitment and assessment of participants was carried out at the University of Munich, Germany. Participants were carefully screened on two occasions, first on the telephone and then in the laboratory, to ensure they did not fulfill any of the following exclusion criteria:
(a) Any current DSM‐IV Axis I disorders (according to the German version of the Mini‐International Neuropsychiatric Interview); (b) any past diagnoses of psychotic disorders; (c) a past or current diagnosis of ADHD; (d) any diagnoses of psychotic disorders or ADHD amongst first‐degree relatives; (e) a history or current evidence of neurological disorders; (f) any current physical condition; (g) any current consumption of over‐the‐counter or prescription medication (except for contraceptives); (h) any visual impairments (other than the use of corrective lenses or glasses); and (i) not right handed.
Demographic and Psychometric Measures
Information was obtained on age, gender, handedness (Edinburgh Handedness Inventory) [Oldfield, 1971], and verbal IQ (Mehrfachwahl‐Wortschatz‐Intelligenztest, MWT‐B) [Lehrl et al., 1995]. The MWT‐B is a widely used German measure of verbal ability and requires the identification of a word among 4 nonword distractors in each of 37 trials. Higher scores indicate better verbal ability. Internal reliability (Cronbach's alpha) of the MWT‐B in this study α = 0.67.
Schizotypy was measured using the German translation of the SPQ full version [Klein et al., 1997]. The SPQ is a 74‐item self‐report scale based on DSM‐III‐R criteria for SPD [Raine, 1991]. It is scored using a two‐point response format (yes = 1, no = 0) and includes nine subscales: Ideas of Reference (IR, 9 items), Social Anxiety (SA, 8 items), Magical Thinking (MT, 7 items), Unusual Perceptual Experiences (UPE, 9 items), Eccentric Behavior (EB, 7 items), No Close Friends (NCF, 9 items), Odd Speech (OS, 9 items), Constricted Affect (CA, 8 items), and Suspiciousness (S, 8 items). MT, UPE, IR, and S scores are summed to construct the cognitive‐perceptual (positive schizotypy) dimension. NCF, CA, and SA scores are summed to yield the interpersonal (negative schizotypy) factor. EB and OS scores are summed to make up the disorganization dimension. Internal reliability of the SPQ in this study was good (α = 0.88).
Resting‐State fMRI Data Acquisition
The fMRI data have not previously been published. Resting‐state fMRI data depicting the blood oxygen level dependent (BOLD) signal were collected using a MAGNETOM Verio scanner (Siemens, Erlangen, Germany) at 3 Tesla field strength. A T2*‐weighted echo planar pulse sequence was applied (TE = 30 ms, TR = 3,000 ms, flip angle = 90°, spatial resolution 3 × 3 × 4 mm isotropic voxels, transverse orientation, 36 slices fully covering the cerebral cortex and the cerebellum, and acquisition time = 6 min). Participants were instructed to keep their eyes closed during the scan. For anatomical reference a high‐resolution isotropic Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence was acquired (TE = 7.6 ms, TR = 14 ms, flip angle = 20°, spatial resolution 0.8 × 0.8 × 0.8 mm isotropic voxels, FOV = 256 mm × 256 mm, and acquisition time = 10 min).
fMRI Data Preprocessing
Preprocessing was performed using the Data Processing Assistant for Resting‐State fMRI (DPARSF) software [Yan and Zang, 2010] implemented on the Matlab 2012a. The first 10 volumes were removed, leaving 110 volumes for each participant. The time delay of image acquisition and head motion was corrected during the slice timing and realignment. The structural images were segmented into gray matter and white matter images using the Segmentation toolbox [Ashburner and Friston, 2005]. Then, the DARTEL toolbox [Ashburner, 2007] was used to create a study specific template for the accurate normalization. Then, resting‐state functional images were coregistered to the structural images and normalized into Montreal Neurological Institute (MNI) space, resliced to 3 mm cubic voxels and smoothed using a 4 mm full width at half maximum (FWHM) Gaussian kernel. Temporal band‐pass filtering (0.01 < f < 0.10 Hz) was performed. Nuisance covariates, including head motion parameters calculated using the Friston 24 model [Friston et al., 1996], global signal, white matter, and cerebrospinal fluid (CSF) signal were regressed out. Time points with Power's frame‐wise displacement (FD) > 0.5 [Power et al., 2012] as well as one volume before and two volumes after were regressed out during the nuisance covariate regression. Furthermore, in order to exclude the potential effect of the head motion, the individuals’ mean FD values were taken as covariates in the subsequent analysis.
Functional Connectivity Analysis
To examine the FC between seeds of the striatum and whole brain voxels, we first defined six seeds of the striatum in each hemisphere with a radius of 4 mm as described previously [Di Martino et al., 2008; Gabbay et al., 2013]. These included the ventral striatum inferior/nucleus accumbens (VSi; ±9,9,–8), ventral striatum superior (VSs; ±10,15,0), dorsal caudate (DC; ±13,15,9), dorsal caudal putamen (DCP; ±28,1,3), dorsal rostral putamen (DRP; ±25,8,6), and ventral rostral putamen (VRP; ±20,12,–3) [see Di Martino et al., 2008]. The mean time series of each seed was calculated and voxel‐wise FC analyses were conducted between brain activity of each seed region and voxels across the whole brain using the toolkit for Resting‐State Functional MRI analysis (REST) [Song et al., 2011]. The participants’ correlation r‐maps of each seed were then transformed to Fisher z‐maps for further analysis.
Asymmetry of Functional Connectivity Analysis
In order to examine the laterality of the FC of striatal seeds, we took participants’ FC maps of each seed region and calculated the total voxel numbers strongly correlated with the seed region of the ipsilateral and contralateral hemispheres. This resulted in measures of each seed region for its FC of both within‐ and cross‐hemisphere. Then, based on the algorithm adopted by Mueller et al. [2015], we calculated the Asymmetry Index (AI) to indicate the lateralization of the FC of each seed using the following equation: AI = N i/H i – N c/H c, where N i and N c are the numbers of voxels strongly correlated (threshold at r > 0.25) with the seed in the ipsilateral and contralateral hemispheres, respectively, and H i and H c are the total number of voxels in the ipsilateral and contralateral hemispheres, respectively. For example, for the AI of the left VSi, the equation would be: AI (left VSi) = number of voxels strongly correlated with left VSi in left hemisphere/total voxel number in left hemisphere – number of voxels strongly correlated with left VSi in right hemisphere/total voxel number in right hemisphere.
Statistical Analysis
First, descriptive statistics of demographic information, MWT‐B scores, SPQ scores and FD of the resting‐state images were calculated. Second, to examine correlations between SPQ total and dimension scores and cortical FC of striatal seeds, participants’ Fisher z‐maps were adopted using a linear regression model with SPQ total score (or dimension scores) as regressor in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software) implemented in Matlab 2012a (The MathWorks, Natick, MA). Age, verbal IQ, and mean FD were used as covariates. Clusters were considered significant if they reached a Cluster Defining Threshold (CDT) of P < 0.001 and cluster P FWE corrected < 0.05. In order to further reduce the possibility of false positive results, we only reported clusters with k ≥ 30 voxels. Finally, we calculated AIs for each striatal seed and conducted one‐sample t tests to confirm the lateralization of all the seeds. Then, we included significant lateralized seeds in further correlation analysis between AIs and SPQ total and dimension scores. Given that the handedness of participants might confound the results of brain asymmetry analysis, we only performed the analyses of asymmetry in a subsample (N = 97) of EHI score > 40, which indicates right handedness [Oldfield, 1971]. The threshold for significance was set at P < 0.05, Bonferroni corrected. SPSS v19.0 (IBM, Armonk, NY) was used for statistical analysis.
RESULTS
Descriptive Statistics
A sample of N = 117 participants completed the study. Five participants were excluded due to excessive head motion (translation > 2 mm, rotation > 2°) and one participant was excluded because of evidence of brain structural abnormalities. Descriptive statistics of the final sample of N = 111 are presented in Table 1. Descriptive statistics regarding individual SPQ subscale scores are shown in Supporting Information Table 1.
Table 1.
Demographic and psychometric variables
| Entire sample (N = 111) | ||||
|---|---|---|---|---|
| Min | Max | Mean/N | SD | |
| Age (years) | 18 | 50 | 26.91 | 7.9 |
| Gender (N male:female) | – | – | 55:56 | – |
| Education years | 11 | 23 | 15.8 | 2.3 |
| EHI | 5 | 100 | 66.49 | 21.96 |
| Verbal ability score (MWT‐B) | 19 | 36 | 30.36 | 2.96 |
| SPQ cognitive‐perceptual | 0 | 15 | 3.15 | 3.01 |
| SPQ interpersonal | 0 | 17 | 2.43 | 3.09 |
| SPQ disorganized | 0 | 14 | 2.24 | 2.86 |
| SPQ total score | 0 | 35 | 7.81 | 6.72 |
EHI, Edinburgh Handedness Inventory; MWT‐B, Mehrfachwahl‐Wortschatz‐Intelligenztest; SPQ, Schizotypal Personality Questionnaire.
Functional Connectivity
Functional connectivity in entire sample
The results of the FC analysis from the entire sample are shown in Supporting Information Figure 1. The observed connectivity patterns are broadly compatible with those reported by Di Martino et al. [2008] using the same striatal seeds.
Associations between functional connectivity and SPQ total and dimension scores
For each striatal seed, we conducted regression analysis to identify clusters with significant correlations between striatocortical FC and SPQ total and dimension scores (Fig. 1 and Table 2).
Figure 1.
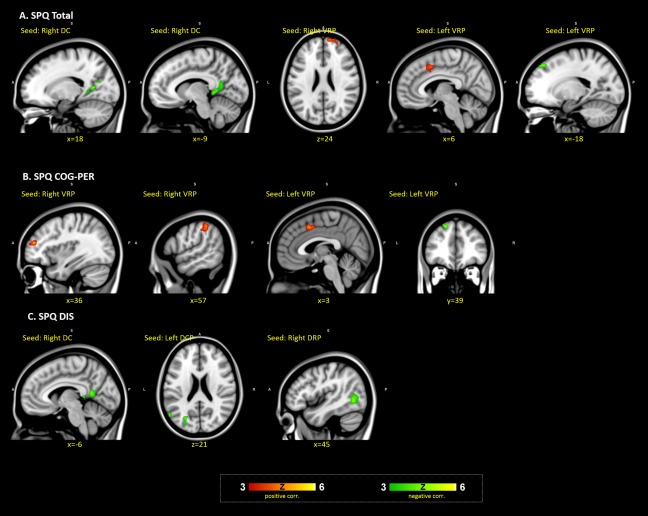
Associations of SPQ total and dimension scores with striatocortical functional connectivity. (A) The clusters with significant correlations with SPQ total scores. (B) The clusters with significant correlations with the SPQ cognitive‐perceptual factor. (C) Clusters with significant correlations with the SPQ disorganized dimension. All regression analyses included age, verbal IQ and mean FD as covariates. Thresholds for significance were set as Cluster Defining Threshold (CDT) of P < 0.001 and cluster P FWE corrected < 0.05 and cluster size k ≥ 30 voxels.
Table 2.
Associations of SPQ total and dimension scores with striatocortical functional connectivity
| Direction of association | Z | Peak coordinate (MNI) | Brain region | |||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| SPQ total | ||||||||
| ROI5 | Right DC | Negative | 45 | 4.57 | 18 | −57 | 9 | BA30, posterior cingulate |
| Right DC | Negative | 71 | 4.11 | −9 | −48 | 3 | Parahippocampal gyrus | |
| ROI11 | Right VRP | Positive | 38 | 4.31 | 12 | 63 | 24 | BA9, superior frontal gyrus |
| ROI12 | Left VRP | Positive | 35 | 4.04 | 6 | 9 | 45 | BA24, cingulate |
| Left VRP | Negative | 37 | 4.49 | −18 | 39 | 48 | BA8, superior frontal gyrus | |
| SPQ COG‐PER | ||||||||
| ROI11 | Right VRP | Positive | 49 | 4.91 | 36 | 48 | 18 | BA10, middle frontal gyrus |
| Right VRP | Positive | 54 | 4.44 | 57 | −39 | 48 | BA40, IPL | |
| ROI12 | Left VRP | Positive | 34 | 4.38 | 3 | 12 | 45 | BA32, medial frontal gyrus |
| Left VRP | Negative | 34 | 4.75 | −18 | 39 | 48 | BA8, superior frontal gyrus | |
| SPQ DIS | ||||||||
| ROI5 | Right DC | Negative | 38 | 4.42 | −6 | −54 | 12 | BA30, posterior cingulate |
| ROI8 | Left DCP | Negative | 31 | 4.12 | −24 | −72 | 21 | BA18, cuneus |
| ROI9 | Right DRP | Negative | 56 | 4.55 | 45 | −63 | −3 | BA37, middle temporal gyrus |
k, number of voxels in cluster; MNI, Montreal Neurological Institute; BA, Brodmann Area; SPQ, Schizotypal Personality Questionnaire; COG‐PER, cognitive‐perceptual dimension; INT, interpersonal dimension; DIS, disorganized dimension. Thresholds for significance were set as Cluster Defining Threshold of P < 0.001, cluster P FWE correction of P < 0.05 and cluster size k >= 30 voxels.
FC between right DC and bilateral posterior cingulate (extend to parahippocampal gyrus) and FC between left VRP and right superior frontal gyrus were significantly negatively correlated with SPQ total score, indicating weaker connectivity with higher overall schizotypy scores. At the same time, FC between right VRP and superior frontal gyrus as well as FC between left VRP and cingulate were significantly positively correlated with SPQ total score.
Regarding the cognitive‐perceptual dimension, there were significant positive correlations with FC between right VRP and right middle frontal gyrus and inferior parietal lobe and with FC between left VRP and right medial frontal gyrus. These correlations indicate stronger FC with higher levels of cognitive‐perceptual schizotypy.
Finally, there were negative associations between the disorganized dimension and FC of right DC with posterior cingulate, FC of left DCP with left cuneus, and FC of right DRP with middle temporal gyrus indicating lesser connectivity with higher disorganization dimension scores.
No significant associations were observed between the interpersonal (negative schizotypy) dimension and FC.
Asymmetry
Asymmetry of functional connectivity
We calculated the AI for each striatal seed in a subsample of subjects with EHI > 40. Since the AI was calculated using the connectivity with ipsilateral hemisphere minus connectivity with contralateral hemisphere, higher AI of a seed in the left hemisphere means the FC of the seed is stronger in the left than the right hemisphere (more strongly related voxels in left hemisphere than right). Conversely, higher AI of a seed in the right hemisphere means that the FC of the seed is stronger in the right than left hemisphere.
We found that the AIs of left VSs, left DC, left VRP, right DCP, right DRP, and right VRP were significantly different from 0 after Bonferroni correction based on one‐sample t tests (Fig. 2 and Table 3). Among these seeds, FC of left VSs, left DC, and left VRP were left lateralized and the FC of right DCP, right DRP, and right VRP were right lateralized. The other seeds did not show significant lateralization of FC.
Figure 2.
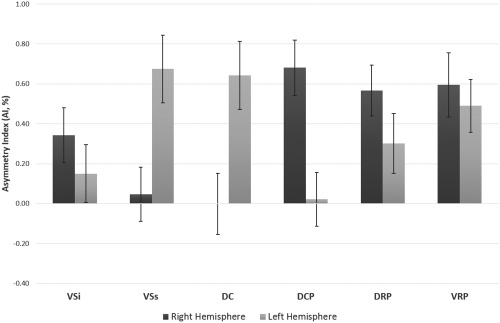
Asymmetry Index of functional connectivity of all seed regions. Asymmetry Index (AI) of functional connectivity of each seed was calculated using the following equation: AI = N i/H i – N c/H c, where N i and N c are the numbers of voxels strongly correlated (threshold at r > 0.25) with the seed in the ipsilateral and contralateral hemispheres, respectively, and H i and H c are the total number of voxels in the ipsilateral and contralateral hemispheres, respectively. VSi, ventral striatum inferior/nucleus accumbens; VSs, ventral striatum superior; DC, dorsal caudate; DCP, dorsal caudal putamen; DRP, dorsal rostral putamen; VRP, ventral rostral putamen. The error bars indicate the standard errors.
Table 3.
AI of functional connectivity of seed regions
| AI (%) | |||||
|---|---|---|---|---|---|
| Min | Max | Mean | SD | One‐sample t, P | |
| Right VSi (9,9,–8) | −4.45 | 2.61 | 0.34 | 1.36 | 2.49, 0.014 |
| Left VSi (–9,9,–8) | −4.07 | 3.59 | 0.15 | 1.43 | 1.04, 0.303 |
| Right VSs (10,15,0) | −4.06 | 2.61 | 0.05 | 1.33 | 0.35, 0.729 |
| Left VSs (–10,15,0) | −3.88 | 7.84 | 0.67 | 1.68 | 3.97, <0.001 |
| Right DC (13,15,9) | −4.67 | 4.55 | 0.00 | 1.51 | −0.01, 0.994 |
| Left DC (–13,15,9) | −4.19 | 5.88 | 0.64 | 1.68 | 3.77, <0.001 |
| Right DCP (28,1,3) | −3.70 | 6.00 | 0.68 | 1.37 | 4.91, <0.001 |
| Left DCP (–28,1,3) | −2.72 | 3.76 | 0.02 | 1.33 | 0.16, 0.872 |
| Right DRP (25,8,6) | −2.41 | 4.39 | 0.57 | 1.26 | 4.45, <0.001 |
| Left DRP (–25,8,6) | −3.29 | 5.97 | 0.30 | 1.48 | 2.00, 0.048 |
| Right VRP (20,12,–3) | −2.75 | 6.66 | 0.59 | 1.59 | 3.69, <0.001 |
| Left VRP (–20,12,–3) | −2.61 | 3.67 | 0.49 | 1.30 | 3.71, <0.001 |
The analyses were conducted in a subsample (n = 97) of subjects with EHI > 40. Numbers in parentheses indicate x, y, and z coordinates in MNI (Montreal Neurological Institute) space. AI, Asymmetry Index; VSi, ventral striatum inferior/nucleus accumbens; VSs, ventral striatum superior; DC, dorsal caudate; DCP, dorsal caudal putamen; DRP, dorsal rostral putamen; VRP, ventral rostral putamen.
Associations between asymmetry of functional connectivity and schizotypy
Correlation analyses were conducted to examine associations between the AIs of significant lateralized striatal seeds (left VSs, left DC, left VRP, right DCP, right DRP, and right VRP) and SPQ total and dimension scores. In terms of SPQ total scores, significant positive correlations were found between AI of the right DCP (r = 0.21, P = 0.039) and the right VRP (r = 0.34, P = 0.001). There were also positive correlations of the cognitive‐perceptual dimension score with AI in the right DCP (r = 0.21, P = 0.043), and of the interpersonal dimension score with AI in the right VRP (r = 0.28, P = 0.005) and the left VSs (r = 0.23, P = 0.024), and of the disorganized dimension score with AI in the right VRP (r = 0.28, P = 0.006). After Bonferroni correction, only the positive correlation of SPQ total score with AI of the right VRP survived. This significant correlation indicates that higher SPQ total scores were associated with stronger right‐lateralized striatocortical FC.
DISCUSSION
Summary of Key Findings
In this study, we examined the association between rsFC and its asymmetry with levels of schizotypal personality traits in a large sample of healthy adults. A first main finding is that the observed striatocortical rsFC largely replicates the pattern reported previously [Di Martino et al., 2008]. Our study is, to our knowledge, the largest analysis of striatocortical FC in rs‐fMRI using this method to date. The study by Di Martino et al. [2008] found differential FC patterns for subsections of the striatum. Their findings suggested that the dorsal part of the caudate, which is closely connected to the frontal lobe, is involved in cognitive processes whereas the ventral part, connected to limbic regions, is associated with affective processing. In the current study, we found similar patterns, as the seeds of DC were associated with dorsolateral prefrontal cortex (DLPFC) whereas VSi seeds were related to orbitofrontal cortex and anterior cingulate. In terms of the subsections of the putamen, although both of caudal and rostral putamen showed FC with motor regions, connectivity of the rostral putamen seeds (DRP/VRP) with frontal regions was also observed, indicating a role in executive control.
A second finding from our study was that scores in total as well as cognitive‐perceptual (positive) schizotypy were associated with widespread alterations in striatocortical FC. Most of the observed positive associations were found in ventral striatum, indicating greater FC of ventral striatum with increasing schizotypy scores. Additionally, negative associations were also observed between the SPQ total score/disorganized dimension and the FC of dorsal caudate with posterior cingulate. There were no associations between negative schizotypy (SPQ interpersonal factor) and striatocortical FC.
Finally, analysis of asymmetry indicated that higher total schizotypy scores were associated with stronger lateralized FC in the right hemisphere.
Association Between Schizotypy and Resting‐State Functional Connectivity
This study is, to our knowledge, the first to investigate striatocortical FC during resting state in relation to multidimensional schizotypy. The study thus extends previous structural and functional MRI studies in attempting to delineate the neural correlates of this important schizophrenia spectrum trait. Evidence from previous neuroimaging studies has pointed to structural and functional brain alterations in relation to higher schizotypy scores [Ettinger et al., 2015]. Specifically, structural magnetic resonance imaging studies have shown that higher schizotypy is associated with reduced gray matter in frontotemporal areas [DeRosse et al., 2015; Ettinger et al., 2012b; Wang et al., 2015; Wiebels et al., 2016], while there is also evidence of increased gray matter in posterior cingulate and precuneus [Modinos et al., 2010; Nenadic et al., 2015]. Functional magnetic resonance imaging studies have pointed to alterations in task‐related BOLD signal during performance of cognitive or motor tasks [Ettinger et al., 2015].
Importantly, there is previous evidence of altered striatal information processing in schizotypy. For example, behavioral studies have shown performance impairments on tasks known to be supported by frontostriatal networks [Ettinger et al., 2005; O'Driscoll et al., 1998]. Studies using fMRI have shown more directly that schizotypy is associated with alterations in BOLD signal in striatum during task performance [Aichert et al., 2012; Chan et al., 2016; Ettinger et al., 2013; Li et al., 2015; Yan et al., 2016].
Given the role of dopamine in the striatum and its hypothesized dysfunction in schizophrenia [Howes and Kapur, 2009; Simpson et al., 2010], these findings may implicate a role of dopaminergic dysfunction in schizotypy [Mohr and Ettinger, 2014]. The most direct, available evidence of an association between schizotypy and dopamine comes from positron emission tomography (PET) and pharmacological studies. For example, an 18F‐fallypride PET study has demonstrated a correlation of overall schizotypy with amphetamine‐induced dopamine release in the striatum, particularly the associative subdivision [Woodward et al., 2011]. Additionally, an 11C‐raclopride PET study observed that individuals with high levels of negative schizotypy showed greater stress‐induced dopamine release than controls or individuals with positive schizotypy [Soliman et al., 2011]. Finally, challenge studies using dopamine D2/D3 receptor blockers have shown differential cognitive responses in individuals with high schizotypy compared to medium‐schizotypy controls [Koychev et al., 2012; Schmechtig et al., 2013].
Here, regression analyses showed that SPQ total score was positively related to FC between ventral rostral putamen and dorsolateral prefrontal cortex, as well as anterior cingulate, indicating that individuals with higher SPQ total scores have stronger FC of ventral striatum and prefrontal cortex. In a previous study, Fornito et al. [2013] found hyperconnectivity of ventral striatum with prefrontal cortex in both patients with schizophrenia and their symptom‐free, unmedicated relatives. Interestingly, this pattern was also found in our previous study in individuals with high social anhedonia [Wang et al., 2016], suggesting this may represent a schizophrenia spectrum trait marker.
DLPFC has been considered as part of a dorsal system involved in the effortful regulation of affective states and related behaviors [Phillips and Seidman, 2008]. Dysconnections of DLPFC and striatum have been previously observed and even proposed as a potential risk biomarker for psychosis [Dandash et al., 2017]. We previously observed, in two independent studies from our groups, less gray matter volume in prefrontal cortex in relation to higher positive schizotypy [Ettinger et al., 2012a, 2012b] and in high SPQ scorers compared to low SPQ scorers [Wang et al., 2015]. The importance of the structural and functional changes of prefrontal cortex, especially its potential role in the identification of the high‐risk individuals, needs to be tested further in the future.
In addition to SPQ total score, we also examined associations between FC and SPQ dimensions. The most consistent correlate of FC was the positive schizotypy dimension, the SPQ cognitive‐perceptual factor. FC of right ventral rostral putamen with middle frontal gyrus (BA10) and FC of left ventral rostral putamen with superior frontal gyrus/DLPFC (BA8) were associated with the positive dimension. As mentioned above, recent studies suggested that the function of the putamen is not limited to motor functions but also related to cognitive processing. The rostral part of the putamen has connections with DLPFC and ACC and has been suggested to be involved in cognitive function [Postuma and Dagher, 2006]. Functional MRI has also shown that weaker FC between putamen and dorsal ACC is associated with poorer working memory in schizophrenia patients [Tu et al., 2012]. Dandash et al. [2014] adopted a similar method and examined striatocortical FC in ultra‐high‐risk (UHR) individuals, observing that positive symptom scores on the Positive and Negative Syndrome Scale (PANSS) were positively correlated with FC between ventral rostral putamen and inferior frontal gyrus. In our study, FC of ventral rostral putamen and prefrontal executive control regions (BA8/10) was found to be related to SPQ positive schizotypy. Overall, this pattern indicates potentially consistent associations between positive features and FC between rostral putamen and prefrontal cortex. The role of this pattern in the cognitive deficits of schizophrenia spectrum disorders needs to be investigated further.
For the disorganized dimension, negative correlations with the FC between dorsal striatum and posterior cingulate and middle temporal gyrus were observed, indicating that individuals with higher SPQ disorganized dimension scores have reduced FC of dorsal striatum. This pattern of reduced FC was consistent with Fornito et al. [2013]'s study in both patients with schizophrenia and first‐degree relatives. In their study, a significant association between symptom severity and FC of DC was observed. Posterior cingulate cortex (PCC) was considered as a core node of Default Mode Network (DMN) and recent studies showed that FC of PCC is involved in cognitive functions, such as attention and executive control [Sheffield and Barch, 2016].
Association Between Schizotypy and Lateralization of Resting‐State Functional Connectivity
The investigation of cerebral lateralization in schizophrenia has a long history [Berlim et al., 2003], with numerous studies suggesting abnormal lateralization in this disorder. Specifically, a reduction of lateralization in the left hemisphere has been shown not only in patients with schizophrenia, but also their first‐degree relatives [Oertel et al., 2010], supporting the notion that this abnormality may represent a trait marker for schizophrenia. Additionally, structural abnormalities of the corpus callosum have been found in patients with schizophrenia [Innocenti et al., 2003]. This structural abnormality may be expected to lead to abnormal asymmetry of FC [Ribolsi et al., 2014]. Recently, Mueller et al. [2015] examined the asymmetry of rsFC and found significantly reduced left‐hemisphere and increased right‐hemisphere lateralization of the caudate nucleus in schizophrenia patients.
In our study, we found positive correlations of SPQ total scores with asymmetry of right ventral rostral putamen. Although we did not find any reduced left‐hemispheric lateralization, the increased asymmetry of seed in the right hemisphere in relation to higher SPQ scores indicated that changes of asymmetry may be a potential marker for the identification of high schizotypy. The pattern of this altered asymmetry across different schizophrenia spectrum populations should be further examined.
Specificity of Altered Striatocortical Connectivity Across Disorders
The striatum has anatomical connections with numerous cortical and subcortical structures, thereby supporting a multitude of complex cognitive and motor functions. For example, the projections from prefrontal cortex (including orbitofrontal, ventromedial prefrontal, dorsolateral prefrontal cortex, and insula) and ACC to rostral striatum are associated with reward, motivation and cognition [Haber, 2016].
Accordingly, it should not be surprising that altered striatocortical rsFC not only is found in the schizophrenia spectrum but has also been shown in other psychiatric disorders. For example, reduced FC between nucleus accumbens and frontal regions involved in cognitive control (dorsal ACC and DLPFC) was observed in patients with substance abuse disorders [Motzkin et al., 2014]. Moreover, increased rsFC of ventral striatum and medial prefrontal cortex/ACC has been observed in patients with obsessive compulsive disorder [Harrison et al., 2009], whereas increased rsFC of striatum with prefrontal cortex has been found in medication‐free patients with major depressive disorder [Gabbay et al., 2013; Kerestes et al., 2015].
Given that schizophrenia spectrum disorders including schizotypy are frequently comorbid with anxiety and affective disorders [Anticevic et al., 2015; Buckley et al., 2009; Samsom and Wong, 2015; Zink, 2014] as well as substance abuse [Brown et al., 2012; Regier et al., 1990; Thoma and Daum, 2013], the specificity of the associations between connectivity and schizotypy observed here remains to be clarified.
Design Considerations and Limitations
A noteworthy point to consider in the discussion of our findings is that the current sample displayed rather low mean and range of SPQ scores. This incidental finding is most likely due to our recruitment and screening methods. Participants of this study were recruited via email and paper adverts specifically asking for healthy volunteers to participate in a research study. Those who contacted the experimenters were thoroughly screened for exclusion criteria of any current psychiatric or neurological disorder (incl. drug abuse/dependence) as well as any personal history of psychotic disorder. Given the associations of high schizotypy with psychotic and affective symptoms [Barrantes‐Vidal et al., 2013; Lewandowski et al., 2006; Salokangas et al., 2013] as well as substance abuse [Esterberg et al., 2009; Fridberg et al., 2011; Williams et al., 1996], our strict criteria for participation likely led to the exclusion of many individuals with high schizotypy scores.
On the one hand, this feature of our design represents a strength of the study, as it allowed us to characterize the neural correlates of variation in schizotypy in the absence of the possibly confounding effects of psychopathology or drug abuse. On the other hand, however, it should be pointed out that the interpretation of our findings is, of course, restricted to the low‐to‐moderate schizotypy range. Thus, it is unclear whether the observed correlations can be used to extrapolate to FC in high schizotypy. This remains an empirical question to be answered in future research.
Support for the importance and validity of studying correlates of schizotypy across the entire spectrum, including low scores, comes from the detection of the neural correlates of schizotypy in previous studies of low‐to‐medium scoring participants [Aichert et al., 2012]. Additionally, a recent study explicitly compared medium and low schizotypes to high schizotypes and demonstrated that low schizotypes can be differentiated from medium schizotypes, who in turn perform worse than high schizotypes, on smooth pursuit eye movements [Koychev et al., 2016], a key endophenotype and biomarker of schizophrenia [Calkins et al., 2008; Levy et al., 1993]. Thus, we feel that the observed relationships between rsFC and schizotypy scores in the low‐to‐moderate range may be indicative of associations that may emerge across the entire spectrum of schizotypy. However, this remains to be investigated with regards to the FC patterns observed here.
Conclusions
To conclude, the present study showed that schizotypal personality traits are associated with striatocortical FC during the resting state. The pattern of stronger connectivity with increasing schizotypy scores may reflect a continuum of factors underlying schizophrenia spectrum traits, from low‐to‐medium expression to the clinical condition [Fornito et al., 2013]. Associations of stronger right‐hemispheric asymmetries in relation to schizotypy similarly extend pervious work showing greater right > left asymmetry in other schizophrenia spectrum populations [DeRosse et al., 2015; Hori et al., 2008; Mueller et al., 2015].
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This study was supported by a DFG grant to U.E. R.C. was supported by a grant from the National Basic Research Programme of China, the Beijing Training Project for Leading Talents in S&T, the Beijing Municipal Science & Technology Commission Grant, and the National Science Fund China. Y.W. was supported by the National Science Fund China and China Scholarship Council. The authors thank Désirée Aichert, Nicola Wöstmann, Anna Costa, and Christine Macare for assistance in data collection and Ute Coates and Maximilian Reiser for enabling access to MRI at the University of Munich.
Contributor Information
Ulrich Ettinger, Email: ulrich.ettinger@uni-bonn.de.
Raymond C. K. Chan, Email: rckchan@psych.ac.cn.
REFERENCES
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, Koepp MJ, Hammers A (2007): Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. NeuroImage 38:261–270. [DOI] [PubMed] [Google Scholar]
- Aichert DS, Williams SCR, Möller HJ, Kumari V, Ettinger U (2012): Functional neural correlates of psychometric schizotypy: An fMRI study of antisaccades. Psychophysiology 49:345–356. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR (1990): Basal ganglia‐thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146. [PubMed] [Google Scholar]
- Anticevic A, Schleifer C, Youngsun TC (2015): Emotional and cognitive dysregulation in schizophrenia and depression: Understanding common and distinct behavioral and neural mechanisms. Dialogues Clin Neurosci 17:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Barrantes‐Vidal N, Gross GM, Sheinbaum T, Mitjavila M, Ballespí S, Kwapil TR (2013): Positive and negative schizotypy are associated with prodromal and schizophrenia‐spectrum symptoms. Schizophr Res 145:50–55. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Mattevi BS, Belmonte‐de‐Abreu P, Crow TJ (2003): The etiology of schizophrenia and the origin of language: Overview of a theory. Compr Psychiatry 44:7–14. [DOI] [PubMed] [Google Scholar]
- Brown RW, Maple AM, Perna MK, Sheppard AB, Cope ZA, Kostrzewa RM (2012): Schizophrenia and substance abuse comorbidity: Nicotine addiction and the neonatal quinpirole model. Dev Neurosci 34:140–151. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ (2009): Psychiatric comorbidities and schizophrenia. Schizophr Bull 35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Ones DS (2008): Eye movement dysfunction in first‐degree relatives of patients with schizophrenia: A meta‐analytic evaluation of candidate endophenotypes. Brain Cogn 68:436–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RCK, Li Z, Li K, Zeng Y, Xie W, Yan C, Cheung EFC, Jin Z (2016): Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology 30:274–280. [DOI] [PubMed] [Google Scholar]
- Dandash O, Fornito A, Lee J, Keefe RSE, Chee MWL, Adcock RA, Pantelis C, Wood SJ, Harrison BJ (2014): Altered striatal functional connectivity in subjects with an at‐risk mental state for psychosis. Schizophr Bull 40:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Pantelis C, Fornito A (2017): Dopamine, fronto‐striato‐thalamic circuits and risk for psychosis. Schizophr Res 180:48–57. [DOI] [PubMed] [Google Scholar]
- DeRosse P, Nitzburg GC, Ikuta T, Peters BD, Malhotra AK, Szeszko PR (2015): Evidence from structural and diffusion tensor imaging for frontotemporal deficits in psychometric schizotypy. Schizophr Bull 41:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP (2008): Functional connectivity of human striatum: A resting state fMRI study. Cereb Cortex 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Esterberg ML, Goulding SM, McClure‐Tone EB, Compton MT (2009): Schizotypy and nicotine, alcohol, and cannabis use in a non‐psychiatric sample. Addict Behav 34:374–379. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Flak V, Sharma T, Davis RE, Corr PJ (2005): Saccadic eye movements, schizotypy, and the role of neuroticism. Biol Psychol 68:61–78. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Schmechtig A, Toulopoulou T, Borg C, Orrells C, Owens S, Matsumoto K, Van Haren NE, Hall MH, Kumari V, McGuire PK, Murray RM, Picchioni M (2012a): Prefrontal and striatal volumes in monozygotic twins concordant and discordant for schizophrenia. Schizophr Bull 38:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Williams SCR, Meisenzahl EM, Möller H‐J, Kumari V, Koutsouleris N (2012b): Association between brain structure and psychometric schizotypy in healthy individuals. World J Biol Psychiatry 13:544–549. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Corr PJ, Mofidi A, Williams SCR, Kumari V (2013): Dopaminergic basis of the psychosis‐prone personality investigated with functional magnetic resonance imaging of procedural learning. Front Hum Neurosci 7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Meyhöfer I, Steffens M, Wagner M, Koutsouleris N (2014): Genetics, cognition, and neurobiology of schizotypal personality: A review of the overlap with schizophrenia. Front Psychiatry 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Mohr C, Gooding DC, Cohen AS, Rapp A, Haenschel C, Park S (2015): Cognition and brain function in schizotypy: A selective review. Schizophr Bull 41:S417–S426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Bullmore ET (2010): What can spontaneous fluctuations of the blood oxygenation‐level‐dependent signal tell us about psychiatric disorders? Curr Opin Psychiatry 23:239–249. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, Lennox BR, Jones PB, Suckling J, Bullmore ET (2013): Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70:1143–1151. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Vollmer JM, O'Donnell BF, Skosnik PD (2011): Cannabis users differ from non‐users on measures of personality and schizotypy. Psychiatry Res 186:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346–355. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP (2013): Striatum‐based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry 52:628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo‐Chica M, Woodward ND (2017): Review of thalamocortical resting‐state fMRI studies in schizophrenia. Schizophr Res 180:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2016): Corticostriatal circuitry. Dialogues Clin Neurosci 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano‐Mas C, Pujol J, Ortiz H, López‐Solà M, Hernández‐Ribas R, Deus J, Alonso P, Yücel M, Pantelis C, Menchon JM, Cardoner N (2009): Altered corticostriatal functional connectivity in obsessive‐compulsive disorder. Arch Gen Psychiatry 66:1189. [DOI] [PubMed] [Google Scholar]
- Hori H, Nagamine M, Soshi T, Okabe S, Kim Y, Kunugi H (2008): Schizotypal traits in healthy women predict prefrontal activation patterns during a verbal fluency task: A near‐infrared spectroscopy study. Neuropsychobiology 57:61–69. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S (2009): The dopamine hypothesis of schizophrenia: Version III—The final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi‐Dargham A, Kapur S (2012): The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J (2003): Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry 8:261–274. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Harrison BJ, Dandash O, Stephanou K, Whittle S, Pujol J, Davey CG (2015): Specific functional connectivity alterations of the dorsal striatum in young people with depression. NeuroImage Clin 7:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Andresen B, Jahn T (1997): Erfassung der schizotypen Persönlichkeit nach DSM‐III‐R: Psychometrische Eigenschaften einer autorisierten deutschsprachigen Übersetzung des “Schizotypal Personality Questionnaire” (SPQ) von Raine. Diagnostica 43:347–369. [Google Scholar]
- Koychev I, McMullen K, Lees J, Dadhiwala R, Grayson L, Perry C, Schmechtig A, Walters J, Craig KJ, Dawson GR, Dourish CT, Ettinger U, Wilkinson L, Williams S, Deakin JFW, Barkus E (2012): A validation of cognitive biomarkers for the early identification of cognitive enhancing agents in schizotypy: A three‐center double‐blind placebo‐controlled study. Eur Neuropsychopharmacol 22:469–481. [DOI] [PubMed] [Google Scholar]
- Koychev I, Joyce DW, Barkus E, Ettinger U, Schmechtig A, Dourish CT, Dawson GR, Craig KJ, Deakin JFW (2016): Cognitive and oculomotor performance in subjects with low and high schizotypy: Implications for translational drug development studies. Transl Psychiatry 6:e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S, Triebig G, Fischer B (1995): Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91:335–345. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR (1993): Eye tracking dysfunction and schizophrenia: A critical perspective. Schizophr Bull 19:461–536. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Barrantes‐Vidal N, Nelson‐Gray RO, Clancy C, Kepley HO, Kwapil TR (2006): Anxiety and depression symptoms in psychometrically identified schizotypy. Schizophr Res 83:225–235. [DOI] [PubMed] [Google Scholar]
- Li Z, Yan C, Xie W‐Z, Li K, Zeng Y‐W, Jin Z, Cheung EFC, Chan RCK (2015): Anticipatory pleasure predicts effective connectivity in the mesolimbic system. Front Behav Neurosci 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF (1987): The symptoms of chronic schizophrenia. A re‐examination of the positive‐negative dichotomy. Br J Psychiatry 151:145–151. [DOI] [PubMed] [Google Scholar]
- Modinos G, Mechelli A, Ormel J, Groenewold NA, Aleman A, McGuire PK (2010): Schizotypy and brain structure: A voxel‐based morphometry study. Psychol Med 40:1423–1431. [DOI] [PubMed] [Google Scholar]
- Mohr C, Ettinger U (2014): An overview of the association between schizotypy and dopamine. Front Psychiatry 5:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Baskin‐Sommers A, Newman JP, Kiehl KA, Koenigs M (2014): Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp 35:4282–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wang D, Pan R, Holt DJ, Liu H (2015): Abnormalities in hemispheric specialization of caudate nucleus connectivity in schizophrenia. JAMA Psychiatry 72:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Seal ML, Pantelis C, Phillips LJ (2013): Evidence of a dimensional relationship between schizotypy and schizophrenia: A systematic review. Neurosci Biobehav Rev 37:317–327. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Lorenz C, Langbein K, Dietzek M, Smesny S, Schönfeld N, Fañanás L, Sauer H, Gaser C (2015): Brain structural correlates of schizotypy and psychosis proneness in a non‐clinical healthy volunteer sample. Schizophr Res 168:37–43. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Lenzenweger MF, Holzman PS (1998): Antisaccades and smooth pursuit eye tracking and schizotypy. Arch Gen Psychiatry 55:837–843. [DOI] [PubMed] [Google Scholar]
- Oertel V, Knöchel C, Rotarska‐Jagiela A, Schönmeyer R, Lindner M, van de Ven V, Haenschel C, Uhlhaas P, Maurer K, Linden DEJ (2010): Reduced laterality as a trait marker of schizophrenia—Evidence from structural and functional neuroimaging. J Neurosci 30:2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A (2006): Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. J Cereb Cortex 16:1508–1521. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ (2008): Emotion processing in persons at risk for schizophrenia. Schizophr Bull 34:888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A (1991): The SPQ: A scale for the assessment of schizotypal personality based on DSM‐III‐R criteria. Schizophr Bull 17:555–564. [DOI] [PubMed] [Google Scholar]
- Raine A (2006): Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol 2:291–326. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK (1990): Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 264:2511–2518. [PubMed] [Google Scholar]
- Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G (2014): Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci 8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salokangas RKR, Dingemans P, Heinimaa M, Svirskis T, Luutonen S, Hietala J, Ruhrmann S, Juckel G, Graf von Reventlow H, Linszen D, Birchwood M, Patterson P, Schultze‐Lutter F, Klosterkötter J (2013): Prediction of psychosis in clinical high‐risk patients by the Schizotypal Personality Questionnaire. Results of the EPOS project. Eur Psychiatry 28:469–475. [DOI] [PubMed] [Google Scholar]
- Samsom JN, Wong AH (2015): Schizophrenia and depression co‐morbidity: What we have learned from animal models. Front Psychiatry 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechtig A, Lees J, Grayson L, Craig KJ, Dadhiwala R, Dawson GR, Deakin JFW, Dourish CT, Koychev I, McMullen K, Migo EM, Perry C, Wilkinson L, Morris R, Williams SCR, Ettinger U (2013): Effects of risperidone, amisulpride and nicotine on eye movement control and their modulation by schizotypy. Psychopharmacology (Berl) 227:331–345. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Barch DM (2016): Cognition and resting‐state functional connectivity in schizophrenia. Neurosci Biobehav Rev 61:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG (2013): Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E (2010): A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A, O'Driscoll GA, Pruessner J, Joober R, Ditto B, Streicker E, Goldberg Y, Caro J, Rekkas PV, Dagher A (2011): Limbic response to psychosocial stress in schizotypy: A functional magnetic resonance imaging study. Schizophr Res 131:184–191. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: A Toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Daum I (2013): Comorbid substance use disorder in schizophrenia: A selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin Neurosci 67:367–383. [DOI] [PubMed] [Google Scholar]
- Tu PC, Hsieh JC, Li CT, Bai YM, Su TP (2012): Cortico‐striatal disconnection within the cingulo‐opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: A resting fMRI study. NeuroImage 59:238–247. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yan C, Yin DZ, Fan MX, Cheung EFC, Pantelis C, Chan RCK (2015): Neurobiological changes of schizotypy: Evidence from both volume‐based morphometric analysis and resting‐state functional connectivity. Schizophr Bull 41(Suppl 2):S444–S454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu WH, Li Z, Wei XH, Jiang XQ, Geng FL, Zou LQ, Lui SS, Cheung EF, Pantelis C, Chan RC (2016): Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol Med 46:125–135. [DOI] [PubMed] [Google Scholar]
- Wiebels K, Waldie KE, Roberts RP, Park HRP (2016): Identifying grey matter changes in schizotypy using partial least squares correlation. Cortex 81:137–150. [DOI] [PubMed] [Google Scholar]
- Williams JH, Wellman NA, Rawlins JNP (1996): Cannabis use correlates with schizotypy in healthy people. Addiction 91:869–877. [PubMed] [Google Scholar]
- Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, Doop M, Kessler RM, Zald DH (2011): Correlation of individual differences in schizotypal personality traits with amphetamine‐induced dopamine release in striatal and extrastriatal brain regions. Am J Psychiatry 168:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C‐G, Zang Y‐F (2010): DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang Y, Su L, Xu T, Yin DZ, Fan MX, Deng CP, Wang ZX, Lui SS, Cheung EF, Chan RC (2016): Differential mesolimbic and prefrontal alterations during reward anticipation and consummation in positive and negative schizotypy. Psychiatry Res Neuroimaging 254:127–136. [DOI] [PubMed] [Google Scholar]
- Zink M (2014): Comorbid obsessive‐compulsive symptoms in schizophrenia: Insight into pathomechanisms facilitates treatment. Adv Med 2014:317980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


