Abstract
The neurobiology of sexual orientation is frequently discussed in terms of cerebral sex dimorphism (defining both functional and structural sex differences). Yet, the information about possible cerebral differences between sex‐matched homo and heterosexual persons is limited, particularly among women. In this multimodal MRI study, we addressed these issues by investigating possible cerebral differences between homo and heterosexual persons, and by asking whether there is any sex difference in this aspect. Measurements of cortical thickness (Cth), subcortical volumes, and functional and structural resting‐state connections among 40 heterosexual males (HeM) and 40 heterosexual females (HeF) were compared with those of 30 homosexual males (HoM) and 30 homosexual females (HoF). Congruent with previous reports, sex differences were detected in heterosexual controls with regard to fractional anisotropy (FA), Cth, and several subcortical volumes. Homosexual groups did not display any sex differences in FA values. Furthermore, their functional connectivity was significantly less pronounced in the mesial prefrontal and precuneus regions. In these two particular regions, HoM also displayed thicker cerebral cortex than other groups, whereas HoF did not differ from HeF. In addition, in HoM the parietal Cth showed “sex‐reversed” values, not observed in HoF. Homosexual orientation seems associated with a less pronounced sexual differentiation of white matter tracts and a less pronounced functional connectivity of the self‐referential networks compared to heterosexual orientation. Analyses of Cth suggest that male and female homosexuality are not simple analogues of each other and that differences from heterosexual controls are more pronounced in HoM.
Keywords: brain, cerebral sex dimorphism, sex, sexual orientation
1. INTRODUCTION
One of the more controversial questions concerning the neurobiology of human behavior relates to the mechanisms of sexual orientation. Sexual orientation refers to sexual attraction toward persons of the opposite sex or the same sex and consists basically of two main facets: (a) interpretation of perceptual cues sensitive to sex and (b) motivational approach behaviors toward persons of the preferred sex. The primary difference between homosexual and heterosexual persons is that, for the former, the lust response is released by a stimulus emanating from a person who has the same sexual phenotype as their own. Based on rather convincing data from animal experiments showing changes in the preference of mating partner when inducing lesions in specific sexually dimorphic hypothalamic nuclei (Paredes & Baum, 1995), the neurobiology of sexual orientation has often been discussed in terms of sex dimorphism. Although dimorphism strictly refers to morphology, the expression sex dimorphism is employed to denote both structural and functional cerebral differences between males and females. Furthermore, findings congruent with same sex are usually labeled as “sex‐typical,” whereas those aligned with the opposite sex are denoted as “sex‐reversed.” To facilitate comprehension, these expressions will also be used throughout the article.
Several early postmortem studies suggested that structural/histological differences exist between homosexual male (HoM) and heterosexual male (HeM). For example, the suprachiasmatic nucleus of the hypothalamus in HoM has been found to contain twice as many cells of that of HeM (Swaab, Gooren, & Hofman, 1995). LeVay found that the size of the third interstitial nucleus of the anterior hypothalamus was smaller in HoM than HeM, a finding that was later criticized (LeVay, 1991), while Allen and Gorski (1991) observed that HoM have a larger cross‐sectional area of the anterior commissure than HeM. In accordance with these studies, which all targeted cerebral midline structures, in vivo investigations using brain imaging found that the isthmal area of the corpus callosum was larger in HoM than HeM (Witelson et al., 2008), and that HoM and homosexual females (HoF) had a sex‐reversed pattern of hemispheric volume asymmetry (Savic & Lindstrom, 2008). Furthermore, in a previous series of PET studies using putative pheromones, we noticed that activation of the anterior hypothalamus in HoM was reciprocal to that of HeM, and similar to the activation pattern of HeF (Savic, Berglund, & Lindstrom, 2005; Savic & Lindstrom, 2008). Zhou et al. subsequently also found that smelling a putative male pheromone enhanced the visual perception of male figures among HoM and HeF, but not among HeM, indicating that hypothalamic activation in HoM and HeF had downstream effects on visual perception, potentially impacting sexual partner selection (Berglund, Lindstrom, Dhejne‐Helmy, & Savic, 2008; Berglund, Lindstrom, & Savic, 2006; Zhou et al., 2014). More recently, Hu et al. (2013) used MRI to measure homogeneity in resting‐state brain activity (presumably reflecting local functional connections), and found that HoM had greater homogeneity in the mid frontal lobe and lesser homogeneity in the middle and inferior occipital lobe compared to HeM.
While congruently suggesting a less pronounced or even sex‐reversed differentiation of cerebral midline structures, most previous studies were limited to a specific cerebral area (exceptions are Hu et al., 2013, Abe et al., 2014) and a single metric; moreover, several of the studies only included male controls. Consequently, important facets of the possible neurobiology of sexual orientation remain unrevealed. One is whether possible differences between homosexual and heterosexual persons are widespread, as suggested by studies of neuropsychological test performance (Rahman, Abrahams, & Wilson, 2003) or, confined to the cerebral midline, primarily involving cerebral networks processing sexual behavior. Another issue of interest is whether possible structural and functional differences between homosexual persons and controls are coordinated, indicating a common denominator. Of further relevance is to investigate whether structural and functional underpinnings of male and female homosexuality, if existing, are fundamentally different. Although there has been a tendency to consider gay and lesbian individuals in the same category, emerging scientific data suggest that considerable differences might exist between these groups. For example, the percentage of nonheterosexual females who are attracted to both sexes is reported to be higher than that of nonheterosexual males (Hamer, Hu, Magnuson, Hu, & Pattatucci, 1993; Hu et al., 1995; Vrangalova & Savin‐Williams, 2012). Sexual orientation appears to be more fluid (i.e., with a higher degree of movement between categories) among females than males (Diamond, 2000; Diamond, Dickenson, & Blair, 2017). Also, it has been reported that a significantly higher proportion of gay brothers than heterosexual controls share Xq28 alleles (Hu et al., 1995), not found among lesbian sisters; these data need further validation. Furthermore, while the elevation of prenatal androgen levels has been found to be associated with increased homosexuality and bisexuality among females (Hines, 2010, 2011), it does not appear to have a role in male sexual orientation (Fisher et al., 2015).
In this study, which is part of a larger effort to elucidate the biology of sexual orientation and sexual identity, we set out to examine whether the sexual differentiation of cerebral structural volumes, structural and functional connections differs between homosexual and heterosexual persons. A further question was whether such differences are widespread, and if they are multimodal and possible to capture through several different types of MRI measurements. Given the previous data on performance on neuropsychological tests (Rahman, Wilson, & Abrahams, 2004a, 2004b; Rahman, Sharp, McVeigh, & Ho, 2017), we also wondered whether the possible differences would be found primarily between HoM and HeM and to a lesser degree between HoF and HeF. We, thus, investigated group differences in sex, sexual orientation and their interaction, and including measurements of
cortical thickness [usually greater in females (Bramen et al., 2012; Luders et al., 2006; Savic & Arver, 2014)];
the volumes of subcortical structures previously reported to be sexually dimorphic (Filipek, Richelme, Kennedy, & Caviness, 1994; Lentini, Kasahara, Arver, & Savic, 2013; Raz et al., 2004; Savic & Arver, 2011, 2014);
white matter connections, indexed by FA values [usually greater in males (Hahn et al., 2015; Rametti et al., 2011a)]; and
resting‐state functional connectivity within the default mode network (DMN). According to anecdotal reports, the resting‐state connectivity in the precuneus and the posterior cingulate cortex of the DMN is greater in females than males (Biswal et al., 2010; Zhang & Li, 2012).
2. METHODS
2.1. Subjects
The participants were 30 HoM (age 31.4 ± 6.1, education 15.6 ± 2.6 years), 40 HeM (age 29.5 ± 6.2, education 16.0 ± 2.7 years), 40 HeF (age 29.3 ± 5.5, education 16.8 ± 2.8 years), and 30 HoF (age 27.9 ± 6.1, education 16.0 ± 2.9 years). They were recruited through friends and social connections and through local campus advertisements. All of them were right handed (Oldfield, 1971), healthy, and HIV negative. HeM and HeF scored 0–1 and HoM scored 5–6 on the Kinsey et al., 1948 heterosexual–homosexual rating scale (0 = maximally heterosexual, 6 = maximally homosexual), (Berglund et al., 2006; Kinsey, 1948). In addition to scoring themselves on the Kinsey scale, the subjects also participated in interviews addressing three dimensions of their sexual orientation (fantasy, romantic attraction, and sexual behavior), divided into consecutive 5‐year historical time periods, from age 16 to the present (Berglund et al., 2006). Only subjects reporting stable sexual orientation were subsequently included. All decisions about the sexual orientation of subjects were made without consulting the MR data.
Subjects were excluded if they had a previous history of psychosis, personality disorder, sexual dysfunction, gender dysphoria, hypogonadism, HIV infection, paraphilia, major or bipolar depression, alcohol or substance abuse, chronic fatigue, chronic pain, systemic disease, or sexual offences. No daily medication was allowed during the two months prior to the study.
This study was approved by the Ethics Committee at Karolinska Institutet, and written informed consent was received from each participant.
2.2. Magnetic resonance imaging
2.2.1. Data acquisition
Magnetic resonance imaging data were acquired on a 3 T MRI medical scanner (Discovery 3T GE‐MR750, General Electric, Milwaukee, Wisconsin) equipped with a 32‐channel and/or 8‐channel phased array receiving coil. 3D T1‐weighted spoiled gradient (SPGR) images were acquired with 1 mm3 isotropic voxel size (TE = 3.1 ms, TR = 7.9 ms, TI = 450 ms, FoV = 24 cm, 176 axial slices, flip angle of 12°). Other MR sequences included resting‐state functional MRI (closed eyes, 10 min) performed with a gradient echo pulse sequence using a voxel size of 3 × 3 mm (TE = 30 ms, TR = 2500 ms, FoV = 28.8 cm, 44 interleaved axial slices, 3 mm thickness, flip angle of 90°). The acquisition of resting state fMRI lasted for eight minutes, during which the subjects were instructed to keep their eyes closed, let their mind wander, not fall asleep, and not to try to solve problems. An assistant was present to make sure that the subjects were not sleeping. In addition, multislice DTI was performed using an echo planar imaging sequence with 2 × 2 mm in‐plane resolution (FoV = 23 cm, 60 interleaved axial slices, thickness = 2.9 mm, TE = 83.7 ms, TR = 8000 ms, 60 diffusion gradient directions [b = 1000], flip angle of 90°). Finally, clinical sagittal FLAIR imaging was utilized (TE/TR = 117/8000, TI = 2255, ETL = 140, ARC acceler. R = 2 × 2 (slice, phase), FoV: 27 cm, 224 × 224, slice thickness, 1.2 mm).
The female controls were tested between day 10 and 14 of their menstrual cycles (all had regular 4 weeks cycles).
2.2.2. Calculations of cortical thickness
The MR volumes were processed using FreeSurfer software version 5.3 as described in our previous studies (Savic, 2015; Savic & Arver, 2014). Briefly, a calculation of surface‐based anatomical measures was produced by reconstructing models of white matter (WM), and gray matter (GM) surfaces from MR volumes. The reconstruction of the MRI images was inspected visually after the Talairach transformation, after the skull striping, and after the surfaces had been built and the volumes labeled. Necessary corrections were made after each inspection, including correcting erroneous skull striping by adjusting watershed parameters or manually editing out the skull tissue, and adding control points to normalize intensity for erroneous WM surface reconstruction.
Possible group differences were evaluated for each vertex using a 10 mm filter, using Monte Carlo correction with 5000 permutations (p < .05). Age was employed as the nuisance variable (different slope and different offset approaches in qdec statistics), although there were no significant age differences between the groups.
For further information about the MRI protocol and data analysis, please see a review by Fischl (2012) and our previous publications (Savic, 2015; Savic & Arver, 2011, 2014).
FreeSurfer pipeline was also used for subcortical segmentations. The subcortical structures of interest were the amygdala, hippocampus, caudate, and putamen, because these are the structures most consistently reported to be sexually dimorphic (Lentini et al., 2013; Raz et al., 2004; Savic & Arver, 2014). Ratios between the respective VOIs and the total intracranial volume (ICV), retrieved from the FreeSurfer program, were entered into the statistical analyses. After ensuring that the data were normally distributed, group comparisons of relative structural volumes (VOI/ICV) were performed through a 2 × 2 MANOVA, with sex and sexual orientation as the independent factors, and the structural volumes as the dependent factor. If significant, this calculation was followed by group comparisons for each type of structure (one‐way ANOVAs, p < .0055, after Bonferroni correction for the nine comparisons). Group (HoM, HoF, HeM, HeF) was here used as the fixed parameter. The eight relative structural volumes (structure/ICV) and the ICV constituted the nine dependent parameters. When a group difference was detected for a certain structural volume, we tested which specific groups differed from each other using Scheffe's post‐hoc test (p < .05).
The analyses were carried out with PASW Statistics 23 (SPSS Inc., Chicago, IL).
2.2.3. DTI analysis
Diffusion tensor imaging (DTI) was used to examine the integrity of white matter tracts. DTI techniques utilize the magnetic resonance signal to visualize water movement within axons, which can help characterize axonal microstructure. Information about the diffusion of water is considered anisotropic because it is an index for axonal connectivity (Basser & Jones, 2002). FA yields values between 0 (isotropic diffusion: diffusion that is equal in all directions) and 1 (maximum anisotropic diffusion: the hypothetical case of diffusion along one axis). Data were analyzed in a standardized way using FMRIB's Diffusion Toolbox implemented in FSL v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/) and a group‐wise mean FA white matter skeleton.
Diffusion images were first corrected for (motion) artifacts and eddy current distortions using DTIPrep (Oguz et al., 2014). They were then realigned by means of DTIfit, which is part of FMRIB's Diffusion Toolbox. Non‐brain tissue was removed with BET (part of FSL). A tensor model was fitted to the diffusion data, defining the eigenvalues of the tensor for each voxel and calculating individual FA maps. All subjects' FA maps were registered to the FMRIB58_FA template, and then transformed to MNI152 space. The normalized individual FA maps were averaged to create a group‐wise mean FA white matter skeleton. A threshold of 0.2 was applied to reduce partial volume effects. Finally, the individual aligned FA images were projected onto the mean FA skeleton for subsequent voxel‐wise statistical analyses with Tract‐Based‐Spatial Statistics (TBSS) and by using the Randomise Tool (part of FSL).
We performed a Sex (male, female) by Sexual orientation (heterosexual, homosexual) ANOVA including the four groups, using randomise and permutation‐based non‐parametric testing (5000 permutations), and applying the Threshold‐Free Cluster Enhancement option, with a cluster threshold of p FWE = .05 and a minimal cluster size of 100 voxels. In the event of significant effects, possible group differences were evaluated with separate one‐way ANOVAs. Cluster locations were identified using the JHU White Matter Tractography atlas and JHU ICBM‐DTI‐81 white matter labels (Hua et al., 2009; Mori et al., 2008).
2.2.4. Resting‐state functional MRI
The analysis of resting‐state functional connectivity, using model‐free independent component analysis (MELODIC), focused on possible group differences in relation to the DMN. The analyses were carried out in FSL v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/), as described in a previous study from our group (Feusner et al., 2016).
Spatial preprocessing of the functional images was performed using SPM8 (Welcome Department of Cognitive Neurology) according to the standardized procedure and by incorporating fieldmap correction. Movement correction was conducted with 18 movement regressors (SPM8 software), and also through the use of ICA‐AROMA, which automatically identifies and subsequently removes data‐driven derived components that represent motion‐related artifacts (Pruim et al., 2015). The spatial parameters were then applied to the slice‐timed and realigned functional volumes that were finally resampled to 2 × 2 × 2 mm voxels and smoothed with a 6‐mm full‐width at half‐maximum kernel.
The data were, after initial preprocessing, analyzed in FSL software v5.0 (FMRIB Software Library, Oxford, http://fsl.fmrib.ox.ac.uk/), using a high‐pass filter at 100s before running individual independent component analyses (ICA), (Beckmann & Smith, 2004), (Multivariate Exploratory Linear Decomposition into Independent Components, Version 3.14), with automatic determination of dimensionality. The resulting component maps were then manually classified into components of interest and nuisance components in accordance with the criteria proposed in Kelly et al. (2010). The nuisance components were subsequently regressed out of the original data set using fsl_regfilt. In light of our a priori hypothesis, only the DMN component was further examined in the present group comparison study.
Group concat‐ICA was performed on the entire cleaned dataset, resulting in 22 components. These components were used to run dual regression analysis, and the resulting general linear model (GLM) parameter estimate images were fed into FSL's Randomise tool for nonparametric permutation inference in order to test the separate hypotheses about differences in connectivity within the DMN among groups. As we did not have an a priori hypothesis for the other components, they were not used in the further analysis for the present publication. The design included using mean DVARS (Root Mean Square intensity difference of volume N to volume N + 1; Power, Barnes, Snyder, Schlaggar, & Petersen, 2012), which are an index of the effects of motion, as a nuisance covariate. The results are reported at a threshold of FWE corrected p < .05, (using mask as described by Feusner and Savic) (Feusner et al., 2016).
3. RESULTS
3.1. Demographical data
The groups differed with respect to the Kinsey scale (p < .001), but not in respect to age or education (Table 1).
Table 1.
Demographic data
| HeM | HeF | HoM | HoF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 40 | N = 40 | N = 30 | N = 30 | ||||||||
| Unit | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F(df) | p value | |
| Age | year | 29.5 | 6.2 | 29.3 | 5.5 | 31.4 | 6.1 | 27.9 | 6.1 | 1.7 (3,176) | .168 |
| Education | year | 16.0 | 2.7 | 16.8 | 2.8 | 15.6 | 2.6 | 16.0 | 2.9 | 1.2 (3,176) | .332 |
| Kinsey Score | 0.3 | 0.5 | 0.4 | 0.5 | 5.6 | 0.5 | 5.5 | 0.5 | 1076.8 (3,176) | .000 | |
Note. HeM = heterosexual men; HoM = homosexual men; HeW = heterosexual women; HoW = homosexual women.
F values from group comparisons (one way ANOVA).
3.2. Cortical thickness
We first compared the four groups with respect to cortical thickness (Cth). Congruent with previous reports, the parietal lobe cortex (including the postcentral gyrus and the right and left superior parietal lobes, left > right) was thicker in HeF than HeM, whereas Cth in the left superior/middle temporal gyrus was thicker in HeM than HeF (Figure 1 and Table 2). In HoM the Cth in the left superior temporal gyrus was similar to HeM and greater than in HeF. HoM, however, also showed deviations from this “sex‐congruent pattern.” First of all, like HeF, they displayed a significantly thicker left occipito‐parietal cortex than HeM (Figure 1 and Table 2). Second, their cuneus cortex was thinner than in HeM. In addition, HoM differed from all other groups by having greater Cth in the superior frontal and mPFC and in the precuneus. In contrast, no significant differences were detected between HoF and HeF (Figure 1 and Table 2). Just like HeF, HoF had thicker parietal cortex than HeM, however, not in relation to HoM. There were no differences between HeF and HoF, (Figure 1 and Table 2). Thus, with respect to Cth we detected group differences with respect to sex, and sexual orientation (with less pronounced dimorphism in the parietal and left superior temporal cortices driven by data from HoM), and also an effect of sex by sexual orientation (with singular features in HoM).
Figure 1.
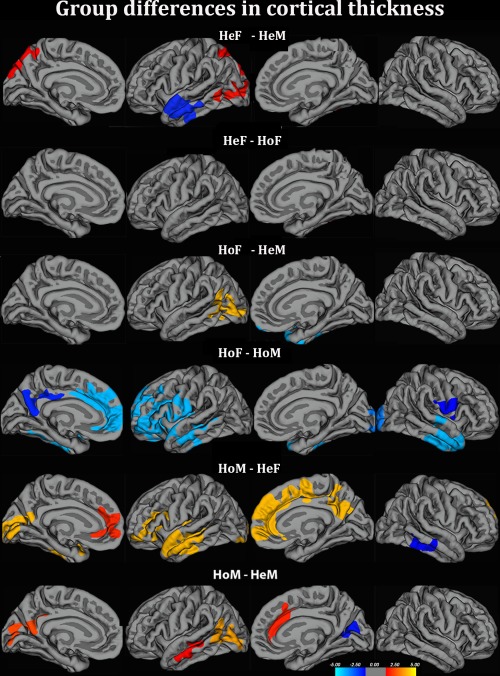
Group differences in cortical thickness. HoM = homosexual men; HeF = heterosexual women; HoM = homosexual men; HeM = heterosexual men. The contrasts were calculated at p < .05, FWE corrected for multiple comparisons (Monte Carlo permutation). The projection of cerebral hemispheres (MR images of the FreeSurfer atlas) is standardized. Scale is logarithmic and sHoFs −log 10(P), with cool colors indicating negative contrast, and warm colors positive contrast. The figure illustrates two major findings: (a) Whereas no differences were detected between HoF and HeF, the HoM‐HeM contrast displayed clusters suggesting both “sex reversed” (left parietal cortex) and singular (mPFC‐ and precuneus‐clusters) features. (b) Cth values in the left superior parietal cortex in the two homosexual groups were in‐between those of HeF and HeM
Table 2.
Group differences in cortical thickness
| HoM − HeF | HoM − HeM | HoF − HoM | HeF − HeM | HoF − HeM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Max, log10 (p) |
Sizecm2 |
Coordinates | Max, log10 (p) |
Sizecm2 |
Coordinates | Max, log10 (p) |
Sizecm2 |
Coordinates | Max, log10 (p) | cm2 | Coordinates | Max, log10 (p) |
Sizecm2 |
Coordinates |
| L superior and middle temporal cortex | 5.1 | 41.1 | −60 −33 −8b | 2.5 | 18.2 | −7 −51 53a, c | ‐ | ||||||||
| L superior frontal cortex | 3.9 | 12.5 | −14 40 16 | ||||||||||||
| L pericalcarine | 3.3 | 26.6 | −8 −83 4 | ||||||||||||
| L precuneus | 2.9 | 10.4 | −13 −57 18 | 3.6 | 14.3 | −6 −58 18a | −4.2 | 12.7 | −6 −66 36 | 2.3 | 20.1 | −29 −84 2a | |||
| L lateral occipital and parietal cortex | 4.6 | 14.0 | −42 −81 2 | ||||||||||||
| L pars triangularis + prefrontal cortex | −5.3 | 178.4 | −41 30 −2d | ||||||||||||
| L rostral middle frontal | 5.1 | 32.2 | −34 33 8 | ||||||||||||
| and rostral anterior cingulate | |||||||||||||||
| L superior temporal cortex | −2.8 | 11.3 | −48 −25 −7 | −2.8 | 15.3 | −50 −1 −20 | |||||||||
| R precuneus | 5.3 | 66.7 | 5 −57 26 | ||||||||||||
| R cuneus | −3.4 | 11.6 | 4 −73 16 | ||||||||||||
| R rostral anterior cingulate | 3.5 | 12.5 | 11 35 12 | ||||||||||||
| R inferior temporal cortex | −5.5 | 10.6 | 53 −28 −14 | ||||||||||||
| R insular, pre–post central | −6.0 | 13.2 | 56 3 6 | ||||||||||||
| R superior frontal cortex | −5.5 | 35 | 8 51 15 | ||||||||||||
| R lateral occipital cortex | −2.9 | 17.1 | 32 −78 7 | ||||||||||||
Note. R = right; L = left.
Negative values denote reverse contrast. The “Region” column describes the coverage of the cluster.
Clusters are calculated at p < .05 corrected, using a 10 mm filter.
Includes left inferior and superior parietal lobes.
Includes L superior temporal gyrus and supramarginal cortex.
Includes part of left occipital cortex.
Large cluster including superior frontal and anterior cingulate cortex, left superior temporal gyrus comprises left parietal cortex; observe that there we no differences between HoF and HeF.
3.3. Subcortical structural volumes
The data from subcortical analyses are presented in Table 3. There was a significant main effect of sex (Wilks' lambda = 0.72, F(10,126) = 4.89, p < .001); Difference between males and females were found in regard to the relative caudate volumes and the relative hippocampus volumes, with larger values among HeF and HoF than among HeM and HoM (Table 3). Notably there was no significant effect of sexual orientation (Wilks' lambda = 0.92, F(10,126) = 0.89, p = .342), and no overall interaction between sex and sexual orientation (Wilks' lambda = 0.89, F(10,126) = 1.02, p = .301. Thus, in contrast to Cth, the regional patterns of structural volumes were similar in homo‐ and hetero‐sexual populations.
Table 3.
Group comparisons of subcortical values
| Structural volumes | HeM | HeF | HoM | HoF | ||
|---|---|---|---|---|---|---|
| (cm3) | N = 40 | N = 40 | N = 30 | N = 30 | F(df) | p values |
| L caudate volume | 4.3 ± 0.6 | 4.0 ± 0.5 | 4.2 ± 0.6 | 3.9 ± 0.5 | 3.5 (3,136) | .018 |
| R caudate volume | 4.3 ± 0.6 | 4.0 ± 0.5 | 4.2 ± 0.6 | 3.9 ± 0.4 | 5.3 (3,136) | .002 |
| L putamen volume | 5.5 ± 0.7 | 4.9 ± 0.6 | 5.3 ± 0.5 | 5.1 ± 0.7 | 3.1 (3,136) | .030 |
| R putamen volume | 5.4 ± 0.6 | 4.8 ± 0.6 | 5.2 ± 0.6 | 4.9 ± 0.7 | 2.2 (3,136) | .094 |
| L hippocampus volume | 4.4 ± 0.5 | 4.0 ± 0.3 | 4.2 ± 0.5 | 4.2 ± 0.4 | 10.2 (3,136) | .000 |
| R hippocampus volume | 4.5 ± 0.5 | 4.2 ± 0.3 | 4.3 ± 0.6 | 4.3 ± 0.5 | 11.1 (3,136) | .000 |
| L amygdala | 2.0 ± 0.3 | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.8 ± 0.2 | 0.4 (3,136) | .751 |
| R amygdala | 2.1 ± 0.3 | 1.9 ± 0.2 | 2.1 ± 0.4 | 1.9 ± 0.3 | 0.1 (3,136) | .941 |
| ICV volume | 1632 ± 131 | 1426 ± 117 | 1618 ± 123 | 1420 ± 131 | 30.4 (3,136) | .000 |
Note. ICV = total intracranial volume.
p‐ and F values indicate groups differences for the separate structural volumes (one‐way ANOVA, p < .0054 after Bonferroni correction for nine comparisons. The input data for subcortical volumes in the statistical analyses were based on ratios with ICV to correct for effects of individual brain size. Scheffe's post hoc tests, p < .05, were used to define which groups differed from each other. The ICV was significantly greater in HeM and HoM than in HeW and HoW, without any differences between the male and female groups. The left hippocampus volume was larger in HeW and HoF than in HoM, and in HeM. The right hippocampus volume was larger in HeW and HoF than in HoM, and in HeM. The right and left caudate volumes were larger in HeF and HoF than in HeM and HoM.
3.3.1. Diffusion tensor imaging—FA, voxel‐wise whole brain analysis
Effects of sex and sexual orientation
A 2 (Sex) by 2 (Sexual Orientation) ANOVA including all four groups revealed no significant interaction, nor main effect of sexual orientation. There was a significant (p FWE < .05) main effect of sex, with males having higher FA values than females in a number of bilateral white matter tracts (Figure 2 and Supporting Information, Table S1a).
Figure 2.
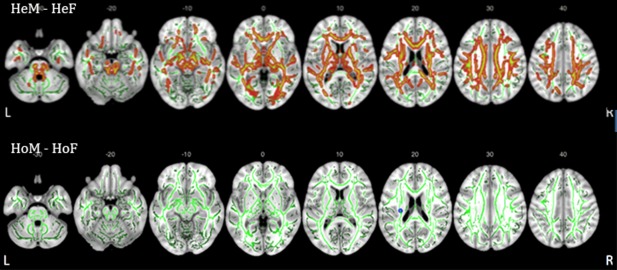
Group difference in FA values. The differences are illustrated in red–yellow and blue and superimposed on the group skeleton (green). These clusters were calculated at p < .05, FWE corrected for multiple comparisons. HeM‐HeF (upper row, indicated in red–yellow) and HoM‐HoF (lower raw, indicated in blue, showing only a small cluster). z coordinates of the MNI atlas are indicated. R = right side; L = left side
At variance from the heterosexual groups, the comparison between HoM and HoF revealed only a small cluster located in the left CST where HoM had significantly (p FWE < .05) higher FA (Figure 2, lower raw). Thus, while there were highly significant sex differences between heterosexual men and women, the homosexual groups barely differed from each other.
3.3.2. Resting‐state functional connectivity
In contrast to the results from DTI analysis, the resting state functional connectivity analysis revealed a significant effect of sexual orientation, but no main effect of sex. At p FWE < .01, significant clusters were detected in the mPFC/ACC and in the precuneus, indicating a less pronounced connectivity in HoF and HoM than in HeF and HeM, respectively. When lowering the threshold to p FWE < .05, a cluster appeared in the precuneus when comparing HoF and HoM, revealing a less pronounced connection in HoM. No other significant group differences were detected within the DMN (Figure 3).
Figure 3.
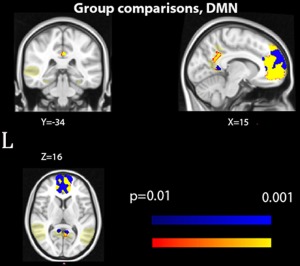
Group difference, in rs‐fMRI, default mode network (DMN) significant group differences in resting‐state functional connections within the default mode network (which was also used as a mask and is indicated in pale yellow). Differences between HeF and HoF are indicated in red–yellow; the corresponding cluster for the HeM‐HoM contrast is indicated in blue. Clusters calculated at p < .01, FWE corrected. MNI coordinates for the two clusters are indicated. The Y‐coordinate for the lower precuneus cluster is −50. See also Table 4
4. DISCUSSION
Multimodal MRI was used to investigate whether sexual orientation could be linked to sexual dimorphism of the brain. We replicated the previously reported findings (Chou, Cheng, Chen, Lin, & Chu, 2011; Huster, Westerhausen, Kreuder, Schweiger, & Wittling, 2009; Inano, Takao, Hayashi, Abe, & Ohtomo, 2011; Menzler et al., 2011; Savic et al., 2017; Takao, Hayashi, & Ohtomo, 2013; van Hemmen et al., 2017) regarding the differences between the male and female heterosexual controls with respect to FA values, regional Cth (Luders et al., 2006; Manzouri, Kosidou, & Savic, 2015), ICV and in the relative caudate and hippocampal volumes (Lentini et al., 2013; Raz et al., 2004).
As opposed to controls, no sex differences were detected in regard to FA values among the homosexual groups, with both HoM and HoF displaying values in‐between those of their sex‐matched controls. The two homosexual groups also shared a functional connectivity pattern that differed from those of both control groups, in that the precuneus and the pregenual anterior cingulate cortex connections within the DMN were significantly less pronounced. The two homosexual groups displayed less pronounced sex dimorphic features also in regard to Cth (left parietal and right superior temporal cortex), though mainly due to values in HoM. In addition, HoM differed from all the other groups by having a thicker mPFC and precuneus cortex. In contrast to HoM, Cth of HoF was congruent with HeF's.
Together, these data suggest a less pronounced sex differentiation among the homosexual groups, particularly along the cerebral midline. Furthermore, and again along the midline, there was a significant sex by sexual orientation effect in that HoM differed from HeM, whereas HoF showed similar values as HoF.
Table 4.
Group differences in resting‐state connectivity, DMN
| HeF–HoF | HeM–HoM | HeF–HoM | HeM–HoF | |||||
|---|---|---|---|---|---|---|---|---|
| Region | Cluster size, cm3 | MNI coordinates | Cluster size, cm3 | MNI coordinates | Cluster size, cm3 | MNI coordinates | Cluster size, cm3 | MNI coordinates |
| ACC | 17.2 | −1 50 17 | 9.9 | 15 51 3 | 1.0 | −15 56 17 | 0.5 | −15 56 17 |
| Precuneus | 3.9 | −6 −50 3.0 | 0.6 | 11 −48 6 | ||||
Note. ACC = anterior cingulate cortex; HeF = heterosexual women; HeM = heterosexual men; HoF= homosexual women; HoM = homosexual men.
Clusters calculated at p FWE < .01.
None of the studied subjects had a known sex‐chromosome aberration, nor did we identify any key environmental factors that differed between HoM and HoF; According to our data on educational background, perceived stress, socioeconomic status, and life traumas, which had been specially collected for another study, these factors were similar among our HoM and HoF groups. The present observations will, therefore, primarily be discussed in relation to sex hormones. Changes in testosterone and estrogen levels have indeed shown to coincide with changes in Cth/grey matter density and FA values, both in longitudinal studies of endogenous estrogen/testosterone, (Herting, Gautam, Spielberg, Dahl, & Sowell, 2015; Peper, van den Heuvel, Mandl, Hulshoff Pol, & van Honk, 2011), and studies of responses to cross sex hormone treatment in transsexual populations (Burke et al., 2017; Rametti et al., 2012; Zubiaurre‐Elorza, Junque, Gomez‐Gil, & Guillamon, 2014).
FA values (believed to reflect axonal packing and caliber as well as the degree of myelinization) are, according to several publications (Gong et al., 2009; Lazar, Miles, Babb, & Donaldson, 2014), influenced by testosterone, and typically higher in males (Rametti et al., 2011b). A lack of difference in FA values between HoM and HoF could thus signify weaker sexual differentiation, possibly indicating a locally increased androgenization in HoF compared to HeF and/or reduced androgenization in HoM compared to HeM. Testosterone affects also Cth (leading to thinning of the parietal and thickening of the superior temporal lobe cortex, and decreased frontal lobe grey matter volume in boys, (Bramen et al., 2012; Nguyen et al., 2013; Witte, Savli, Holik, Kasper, & Lanzenberger, 2010), and a thickening of the cuneus, lingual and pericalcarine cortex in trans men (Zubiaurre‐Elorza et al., 2014), and the detected differences in Cth between HoM and HeM, could reflect a regional hypoandrogenization in HoM. Altered androgenization in regard to both FA and Cth could be result of altered fetal testosterone exposure (Hines, 2010), altered circulating testosterone later in life (Bramen et al., 2012; Nguyen, 2017; Nguyen et al., 2013; Nopoulos, Flaum, O'leary, & Andreasen, 2000; Savic & Arver, 2014), or changes in androgen receptor binding properties, which may occur, for example, as an effect of different CAG repeats (Paus et al., 2010; Raznahan et al., 2010).
Cth is also moderated by circulating estrogen. Estrogen has been found to contribute to thickening of the inferior parietal cortex and thinning of the temporal cortex in females, which is just opposite to testoterone's effects in boys, with thinning of the parietal and thickening of the superior temporal cortex (Bramen et al., 2012). Estrogen is also associated with thinning of the frontal cortex, and decreasing in grey matter volume in boys and reduced pruning of gray matter in girls (Herting et al., 2014; Peper et al., 2009; Witte et al., 2010). Considering that the frontal lobe cortex in our HoM was thicker rather than thinner than in controls, it seems unlikely that it was related to estrogen. Estrogen levels were normal in HoM, whereas their testosterone levels were at the lower end of the normal range [total testosterone, (mean ≠ SD) was 9.5 ≠ 0.7 nmol/L, active testosterone was 5.4 ≠ 0.5nmol/L].
From a functional perspective, it is interesting that all of the principal observations in this study relate to circuits of the DMN. Of particular interest are findings in the precuneus cortex because this region is tightly connected with networks processing visual and pheromonal stimuli and sexual arousal (Berglund et al., 2006; Cavanna & Trimble, 2006, 2008; Witelson et al., 2008; Zhang & Li, 2012). It is plausible to hypothesize that the precuneus, together with the mPFC, could be involved in processing sexual orientation. For homosexual individuals, it may thus be that the downstream information from the precuneus may lead to a different interpretation and/or perception of sexual stimuli, thus allowing the body of a same‐sex individual to be perceived as sexually attractive. Whether such a mechanism could be a cause or a consequence of having homosexual orientation is not known. Such information would require longitudinal studies that start when the sexual orientation becomes explicit or studies of individuals who for some reason change their sexual preference in adulthood.
4.1. Are there differences between male and female homosexuality?
While functional connection in the mPFC and the precuneus region was less pronounced in both HoF and HoM, thickening of the precuneus and the mPFC/ACC was present only in HoM. Could male and female homosexuality have partially different cerebral underpinnings? One hypothesis, in light of the present findings, is that the coding of cerebral DMN circuits for sexual response to body morphology of the sex same could be stronger in HoM than in HoF; this difference may thus be reflected not only in the functional connections but also in structural anatomy along the cerebral midline and in the portion of the left parieto‐occipital cortex comprising the extrastriatal body area—a region known to mediate body perception (Hodzic, Kaas, Muckli, Stirn, & Singer, 2009). Notably, at a threshold of p FWE < .05, the weaker connection in the precuneus was more marked among HoM than HoF. This tentative explanation also accords with the general view that female sexual orientation is more fluid than male sexual orientation (Diamond, 2000, 2003, 2008).
An alternative and not mutually exclusive explanation is that behavioral differences over the course of a lifetime could account for the morphometric differences between HoF and HoM. All of our participants described having an early life (puberty) awareness of their sexual orientation and that it did not change over time. Moreover, there was no significant difference between the Kinsey scores of HoF and HoM (Table 1). These data do not suggest that there are fundamental differences in the type of behavior in question among our groups.
4.2. Methodological considerations
The group size was sufficient for the results to be regarded as reliable (Liem et al., 2015). Worth mentioning is that reduction of control group size to 30 HeM and 30 HeF did not change the results with regard to sex differences among heterosexual controls. Thus, as for the FA values, the less pronounced sex dimorphism in Cth in the parietal and superior temporal cortex among the homosexual groups is unlikely to be a simple effect of smaller group size. It should also be noted that the lack of difference in FA values between HoF and HoM cannot be attributed to brain size, as the ICV was different between HoF and HoM and the ICV of HeF and HeM was in a similar range. Although FA is a rather nonspecific measure (axial and radial diffusivity provide more specific information), we chose to limit our DTI analysis to FA because it is the most sensitive metric. The observed differences in FA could reflect differences in axonal density or the diameter, or differences in the degree of myelination.
Although there is no reason to suspect that sex hormone levels in HoF would be different from those in HeF, it would, admittedly, have been more optimal if we had collected data on these levels to verify this. However, given that we only included subjects who did not display any of the likely factors that would have predisposed them to changes in sex hormone levels, we did not foresee that collecting sex hormone data could be worthwhile. Furthermore, sex hormone levels were available via medical charts (with subject's permission) for two‐thirds of the lesbian population, and these were within the normal range.
In contrast to previous reports (Lentini et al., 2013; Savic & Arver, 2011), no sex differences were detected in regard to amygdala volumes, which can be attributed to the fact that this structure is small and not optimally segmented with the FreeSurfer software (Grimm et al., 2015; Schoemaker et al., 2016).
5. CONCLUSION
Our findings add to the body of literature showing relationships between sexual orientation and both the cortical structure and the structural and functional connections along the cerebral midline. Specifically, the present data display a reduced sexual dimorphism in FA values, and a weaker functional connection between the precuneus and pACC among the homosexual subjects. The data suggest that one possible mechanism behind homosexuality could be that the encoding regarding what sensory stimuli are perceived as attractive by the aforementioned midbrain circuits is different in homosexual as compared to heterosexual persons, which may be due to different cerebral organization, possibly related to sex hormone effects. The study also advances the previous literature by suggesting that cerebral signatures of male and female homosexuality are not just analogues of each other. Whereas HoF showed no differences from HeF in regard to Cth, HoM displayed both singular and “female” patterns, a finding that deserves further investigation.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
CONFLICT OF INTEREST
None
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Figure S1
Supporting Information
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Research Council (I.S Dnr 2007–3107); Stockholm Brain Institute (I.S.); FORTE (I.S.); and AFA (I.S.). The authors are grateful to Sarah Burke for recruiting the HoF participants, to be extensively presented in a separate publication.
Manzouri A, Savic I. Cerebral sex dimorphism and sexual orientation. Hum Brain Mapp. 2018;39:1175–1186. 10.1002/hbm.23908
Funding information Swedish Science Council, Grant/Award Number: Dnr 2007‐3107; Stockholm Brain Institute; FORTE; AFA
REFERENCES
- Abe, C. , Johansson, E. , Allzen, E. , & Savic, I. (2014). Sexual orientation related differences in cortical thickness in male individuals. PLoS One, 9, e114721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, L. S. , & Gorski, R. A. (1991). Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. The Journal of Comparative Neurology, 312, 97–104. [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , & Jones, D. K. (2002). Diffusion‐tensor MRI: Theory, experimental design and data analysis ‐ a technical review. NMRi N Biomedicine, 15, 456–467. [DOI] [PubMed] [Google Scholar]
- Beckmann, C. F. , & Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–152. [DOI] [PubMed] [Google Scholar]
- Berglund, H. , Lindstrom, P. , Dhejne‐Helmy, C. , & Savic, I. (2008). Male‐to‐female transsexuals show sex‐atypical hypothalamus activation when smelling odorous steroids. Cerebral Cortex (New York, N.Y.: 1991), 18, 1900–1908. [DOI] [PubMed] [Google Scholar]
- Berglund, H. , Lindstrom, P. , & Savic, I. (2006). Brain response to putative pheromones in lesbian women. Proceedings of the National Academy of Sciences of the United States of America, 103, 8269–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes, M. , Zuo, X. N. , Gohel, S. , Kelly, C. , Smith, S. M. , … Milham, M. P. (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America, 107, 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen, J. E. , Hranilovich, J. A. , Dahl, R. E. , Chen, J. , Rosso, C. , Forbes, E. E. , … Sowell, E. R. (2012). Sex matters during adolescence: Testosterone‐related cortical thickness maturation differs between boys and girls. PLoS One, 7, e33850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, S. M. , Manzouri, A. H. , Dhejne, C. , Bergstrom, K. , Arver, S. , Feusner, J. D. , & Savic‐Berglund, I. (2017). Testosterone effects on the brain in transgender men. Cerebral Cortex (New York, N.Y.: 1991), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129, 564–583. [DOI] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2008). Behavioral correlates of posteromedial parietal cortex hypometabolism in a family with idiopathic basal ganglia calcifications. Journal of the Neurological Sciences, 266, 190–191. [DOI] [PubMed] [Google Scholar]
- Chou, K. H. , Cheng, Y. , Chen, I. Y. , Lin, C. P. , & Chu, W. C. (2011). Sex‐linked white matter microstructure of the social and analytic brain. NeuroImage, 54, 725–733. [DOI] [PubMed] [Google Scholar]
- Diamond, L. M. (2000). Sexual identity, attractions, and behavior among young sexual‐minority women over a 2‐year period. Developmental Psychology, 36, 241–250. [DOI] [PubMed] [Google Scholar]
- Diamond, L. M. (2003). What does sexual orientation orient? A biobehavioral model distinguishing romantic love and sexual desire. Psychological Review, 110, 173–192. [DOI] [PubMed] [Google Scholar]
- Diamond, L. M. (2008). Female bisexuality from adolescence to adulthood: Results from a 10‐year longitudinal study. Developmental Psychology , 44, 5–14. [DOI] [PubMed] [Google Scholar]
- Diamond, L. M. , Dickenson, J. A. , & Blair, K. L. (2017). Stability of sexual attractions across different timescales: The roles of bisexuality and gender. Archives of Sexual Behavior, 46, 193–204. [DOI] [PubMed] [Google Scholar]
- Feusner, J. D. , Lidstrom, A. , Moody, T. D. , Dhejne, C. , Bookheimer, S. Y. , & Savic, I. (2016). Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek, P. A. , Richelme, C. , Kennedy, D. N. , & Caviness, V. S. Jr. (1994). The young adult human brain: An MRI‐based morphometric analysis. Cerebral Cortex (New York, N.Y.: 1991), 4, 344–360. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, A. D. , Castellini, G. , Casale, H. , Fanni, E. , Bandini, E. , Campone, B. , … Maggi, M. (2015). Hypersexuality, paraphilic behaviors, and gender dysphoria in individuals with Klinefelter's syndrome. The Journal of Sexual Medicine, 12, 2413–2424. [DOI] [PubMed] [Google Scholar]
- Gong, G. , He, Y. , Concha, L. , Lebel, C. , Gross, D. W. , Evans, A. C. , & Beaulieu, C. (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cerebral Cortex (New York, N.Y.: 1991), 19, 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, O. , Pohlack, S. , Cacciaglia, R. , Winkelmann, T. , Plichta, M. M. , Demirakca, T. , & Flor, H. (2015). Amygdalar and hippocampal volume: A comparison between manual segmentation, Freesurfer and VBM. Journal of Neuroscience Methods, 253, 254–261. [DOI] [PubMed] [Google Scholar]
- Hahn, A. , Kranz, G. S. , Kublbock, M. , Kaufmann, U. , Ganger, S. , Hummer, A. , … Lanzenberger, R. (2015). Structural connectivity networks of transgender people. Cerebral Cortex (New York, N.Y.: 1991), 25, 3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, D. H. , Hu, S. , Magnuson, V. L. , Hu, N. , & Pattatucci, A. M. (1993). A linkage between DNA markers on the X chromosome and male sexual orientation. Science (New York, N.Y.), 261, 321–327. [DOI] [PubMed] [Google Scholar]
- Herting, M. M. , Gautam, P. , Spielberg, J. M. , Dahl, R. E. , & Sowell, E. R. (2015). A longitudinal study: Changes in cortical thickness and surface area during pubertal maturation. PLoS One, 10, e0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting, M. M. , Gautam, P. , Spielberg, J. M. , Kan, E. , Dahl, R. E. , & Sowell, E. R. (2014). The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping, 35, 5633–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, M. (2010). Sex‐related variation in human behavior and the brain. Trends in Cognitive Sciences, 14, 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, M. (2011). Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Frontiers in Neuroendocrinology, 32, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic, A. , Kaas, A. , Muckli, L. , Stirn, A. , & Singer, W. (2009). Distinct cortical networks for the detection and identification of human body. NeuroImage, 45, 1264–1271. [DOI] [PubMed] [Google Scholar]
- Hu, S. , Pattatucci, A. M. , Patterson, C. , Li, L. , Fulker, D. W. , Cherny, S. S. , … Hamer, D. H. (1995). Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nature Genetics, 11, 248–256. [DOI] [PubMed] [Google Scholar]
- Hu, S. , Xu, D. , Peterson, B. S. , Wang, Q. , He, X. , Hu, J. , … Xu, Y. (2013). Association of cerebral networks in resting state with sexual preference of homosexual men: A study of regional homogeneity and functional connectivity. PLoS One, 8, e59426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, K. , Oishi, K. , Zhang, J. , Wakana, S. , Yoshioka, T. , Zhang, W. , … Mori, S. (2009). Mapping of functional areas in the human cortex based on connectivity through association fibers. Cerebral Cortex (New York, N.Y.: 1991), 19, 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster, R. J. , Westerhausen, R. , Kreuder, F. , Schweiger, E. , & Wittling, W. (2009). Hemispheric and gender related differences in the midcingulum bundle: A DTI study. Human Brain Mapping, 30, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano, S. , Takao, H. , Hayashi, N. , Abe, O. , & Ohtomo, K. (2011). Effects of age and gender on white matter integrity. American Journal of Neuroradiology, 32, 2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, R. E. , Alexopoulos, G. S. , Wang, Z. , Gunning, F. M. , Murphy, C. F. , Morimoto, S. S. , … Hoptman, M. J. (2010). Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods, 189, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey, A. C. , Pomeroy, W. B. , & Martin, C. E. (1948). Sexual behavior in the human male. Philadelphia: Indiana University Press. [Google Scholar]
- Lazar, M. , Miles, L. M. , Babb, J. S. , & Donaldson, J. B. (2014). Axonal deficits in young adults with high functioning autism and their impact on processing speed. NeuroImage. Clinical, 4, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini, E. , Kasahara, M. , Arver, S. , & Savic, I. (2013). Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cerebral Cortex (New York, N.Y.: 1991), 23, 2322–2336. [DOI] [PubMed] [Google Scholar]
- LeVay, S. (1991). A difference in hypothalamic structure between heterosexual and homosexual men. Science (New York, N.Y.), 253, 1034–1037. [DOI] [PubMed] [Google Scholar]
- Liem, F. , Merillat, S. , Bezzola, L. , Hirsiger, S. , Philipp, M. , Madhyastha, T. , & Jancke, L. (2015). Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. NeuroImage, 108, 95–109. [DOI] [PubMed] [Google Scholar]
- Luders, E. , Narr, K. L. , Thompson, P. M. , Rex, D. E. , Woods, R. P. , Deluca, H. , … Toga, A. W. (2006). Gender effects on cortical thickness and the influence of scaling. Human Brain Mapping, 27, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri, A. , Kosidou, K. , & Savic, I. (2015). Anatomical and functional findings in female‐to‐male transsexuals: Testing a New Hypothesis. Cerebral Cortex (New York, N.Y.: 1991). [DOI] [PubMed] [Google Scholar]
- Menzler, K. , Belke, M. , Wehrmann, E. , Krakow, K. , Lengler, U. , Jansen, A. , … Knake, S. (2011). Men and women are different: Diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. NeuroImage, 54, 2557–2562. [DOI] [PubMed] [Google Scholar]
- Mori, S. , Oishi, K. , Jiang, H. , Jiang, L. , Li, X. , Akhter, K. , … Mazziotta, J. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage, 40, 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. V. (2017). Developmental effects of androgens in the human brain. Journal of Neuroendocrinology. [DOI] [PubMed] [Google Scholar]
- Nguyen, T. V. , McCracken, J. , Ducharme, S. , Botteron, K. N. , Mahabir, M. , Johnson, W. , … Karama, S. , Brain Development Cooperative, G . (2013). Testosterone‐related cortical maturation across childhood and adolescence. Cerebral Cortex (New York, N.Y.: 1991), 23, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos, P. , Flaum, M. , O'leary, D. , & Andreasen, N. C. (2000). Sexual dimorphism in the human brain: Evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research, 98, 1–13. [DOI] [PubMed] [Google Scholar]
- Oguz, I. , Farzinfar, M. , Matsui, J. , Budin, F. , Liu, Z. , Gerig, G. , … Styner, M. (2014). DTIPrep: Quality control of diffusion‐weighted images. Frontiers in Neuroinformatics, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Paredes, R. G. , & Baum, M. J. (1995). Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. Journal of Neuroscience, 15, 6619–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus, T. , Nawaz‐Khan, I. , Leonard, G. , Perron, M. , Pike, G. B. , Pitiot, A. , … Pausova, Z. (2010). Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Hormones and Behavior, 57, 63–75. [DOI] [PubMed] [Google Scholar]
- Peper, J. S. , Brouwer, R. M. , Schnack, H. G. , van Baal, G. C. , van Leeuwen, M. , van den Berg, S. M. , … Hulshoff Pol, H. E. (2009). Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology, 34, 332–342. [DOI] [PubMed] [Google Scholar]
- Peper, J. S. , van den Heuvel, M. P. , Mandl, R. C. , Hulshoff Pol, H. E. , & van Honk, J. (2011). Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrinology, 36, 1101–1113. [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Barnes, K. A. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim, R. H. , Mennes, M. , van Rooij, D. , Llera, A. , Buitelaar, J. K. , & Beckmann, C. F. (2015). ICA‐AROMA: A robust ICA‐based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. [DOI] [PubMed] [Google Scholar]
- Rahman, Q. , Abrahams, S. , & Wilson, G. D. (2003). Sexual‐orientation‐related differences in verbal fluency. Neuropsychology, 17, 240–246. [DOI] [PubMed] [Google Scholar]
- Rahman, Q. , Sharp, J. , McVeigh, M. , & Ho, M. L. (2017). Sexual orientation‐related differences in virtual spatial navigation and spatial search strategies. Archives of Sexual Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, Q. , Wilson, G. D. , & Abrahams, S. (2004a). Biosocial factors, sexual orientation and neurocognitive functioning. Psychoneuroendocrinology, 29, 867–881. [DOI] [PubMed] [Google Scholar]
- Rahman, Q. , Wilson, G. D. , & Abrahams, S. (2004b). Developmental instability is associated with neurocognitive performance in heterosexual and homosexual men, but not in women. Behavioral Neuroscience, 118, 243–247. [DOI] [PubMed] [Google Scholar]
- Rametti, G. , Carrillo, B. , Gomez‐Gil, E. , Junque, C. , Segovia, S. , Gomez, A. , & Guillamon, A. (2011a). White matter microstructure in female to male transsexuals before cross‐sex hormonal treatment. A diffusion tensor imaging study. Journal of Psychiatric Research, 45, 199–204. [DOI] [PubMed] [Google Scholar]
- Rametti, G. , Carrillo, B. , Gomez‐Gil, E. , Junque, C. , Zubiarre‐Elorza, L. , Segovia, S. , … Guillamon, A. (2011b). The microstructure of white matter in male to female transsexuals before cross‐sex hormonal treatment. A DTI study. Journal of Psychiatric Research, 45, 949–954. [DOI] [PubMed] [Google Scholar]
- Rametti, G. , Carrillo, B. , Gomez‐Gil, E. , Junque, C. , Zubiaurre‐Elorza, L. , Segovia, S. , … Guillamon, A. (2012). Effects of androgenization on the white matter microstructure of female‐to‐male transsexuals. A diffusion tensor imaging study. Psychoneuroendocrinology, 37, 1261–1269. [DOI] [PubMed] [Google Scholar]
- Raz, N. , Gunning‐Dixon, F. , Head, D. , Rodrigue, K. M. , Williamson, A. , & Acker, J. D. (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging, 25, 377–396. [DOI] [PubMed] [Google Scholar]
- Raznahan, A. , Lee, Y. , Stidd, R. , Long, R. , Greenstein, D. , Clasen, L. , … Giedd, J. N. (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences of the United States of America, 107, 16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic, I. (2015). Structural changes of the brain in relation to occupational stress. Cerebral Cortex (New York, N.Y.: 1991), 25, 1554–1564. [DOI] [PubMed] [Google Scholar]
- Savic, I. , & Arver, S. (2011). Sex dimorphism of the brain in male‐to‐female transsexuals. Cerebral Cortex (New York, N.Y.: 1991), 21, 2525–2533. [DOI] [PubMed] [Google Scholar]
- Savic, I. , & Arver, S. (2014). Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cerebral Cortex (New York, N.Y.: 1991), 24, 3246–3257. [DOI] [PubMed] [Google Scholar]
- Savic, I. , Berglund, H. , & Lindstrom, P. (2005). Brain response to putative pheromones in homosexual men. Proceedings of the National Academy of Sciences of the United States of America, 102, 7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic, I. , Frisen, L. , Manzouri, A. , Nordenstrom, A. , & Linden Hirschberg, A. (2017). Role of testosterone and Y chromosome genes for the masculinization of the human brain. Human Brain Mapping, 38, 1801–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic, I. , & Lindstrom, P. (2008). PET and MRI show differences in cerebral asymmetry and functional connectivity between homo‐ and heterosexual subjects. Proceedings of the National Academy of Sciences of the United States of America, 105, 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker, D. , Buss, C. , Head, K. , Sandman, C. A. , Davis, E. P. , Chakravarty, M. M. , … Pruessner, J. C. (2016). Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage, 129, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab, D. F. , Gooren, L. J. , & Hofman, M. A. (1995). Brain research, gender and sexual orientation. Journal of Homosexuality, 28, 283–301. [DOI] [PubMed] [Google Scholar]
- Takao, H. , Hayashi, N. , & Ohtomo, K. (2013). White matter microstructure asymmetry: Effects of volume asymmetry on fractional anisotropy asymmetry. Neuroscience, 231, 1–12. [DOI] [PubMed] [Google Scholar]
- van Hemmen, J. , Saris, I. M. J. , Cohen‐Kettenis, P. T. , Veltman, D. J. , Pouwels, P. J. W. , & Bakker, J. (2017). Sex differences in white matter microstructure in the human brain predominantly reflect differences in sex hormone exposure. Cerebral Cortex (New York, N.Y.: 1991), 27, 2994–3001. [DOI] [PubMed] [Google Scholar]
- Vrangalova, Z. , & Savin‐Williams, R. C. (2012). Mostly heterosexual and mostly gay/lesbian: Evidence for new sexual orientation identities. Archives of Sexual Behavior, 41, 85–101. [DOI] [PubMed] [Google Scholar]
- Witelson, S. F. , Kigar, D. L. , Scamvougeras, A. , Kideckel, D. M. , Buck, B. , Stanchev, P. L. , … Black, S. (2008). Corpus callosum anatomy in right‐handed homosexual and heterosexual men. Archives of Sexual Behavior, 37, 857–863. [DOI] [PubMed] [Google Scholar]
- Witte, A. V. , Savli, M. , Holik, A. , Kasper, S. , & Lanzenberger, R. (2010). Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. NeuroImage, 49, 1205–1212. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , & Li, C. S. (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage, 59, 3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , Yang, X. , Chen, K. , Cai, P. , He, S. , & Jiang, Y. (2014). Chemosensory communication of gender through two human steroids in a sexually dimorphic manner. Current Biology, 24, 1091–1095. [DOI] [PubMed] [Google Scholar]
- Zubiaurre‐Elorza, L. , Junque, C. , Gomez‐Gil, E. , & Guillamon, A. (2014). Effects of cross‐sex hormone treatment on cortical thickness in transsexual individuals. The Journal of Sexual Medicine, 11, 1248–1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Figure S1
Supporting Information


