Abstract
Emerging evidence has suggested that abnormalities in regional spontaneous brain activity following stroke may be detected by intrinsic low‐frequency oscillations (LFO) in resting‐state functional MRI (R‐fMRI). However, the relationship between hand function outcomes following stroke and local LFO synchronization in different frequency bands is poorly understood. In this study, we performed R‐fMRI to examine the regional homogeneity (ReHo) at three different frequency bands (slow‐5: .01–.027 Hz; slow‐4: .027–.08 Hz; and typical band: .01–.1 Hz) in 26 stroke patients with completely paralyzed hands (CPH) and 26 matched patients with partially paralyzed hands (PPH). Compared to the PPH group, decreased ReHo in the bilateral cerebellum posterior lobes and the contralesional cerebellum anterior lobe was observed in the slow‐5 band and the slow‐4 band in the CPH group, respectively. The mean ReHo values in these regions were positively correlated with the Fugl‐Meyer assessment (FMA) scores. In contrast, increased ReHo in the contralesional supplementary motor area and the contralesional superior temporal gyrus was observed in the slow‐4 band and the slow‐5 band, respectively. The mean ReHo values in these regions were negatively correlated with the FMA scores. Importantly, significant interactions were identified between the frequency bands and the subgroups of patients in the contralesional precentral gyrus and middle frontal gyrus. These findings indicate that frequency‐dependent R‐fMRI patterns may serve as potential biomarkers of the neural substrates associated with hand function outcomes following stroke.
Keywords: completely paralyzed hands, frequency‐dependent, partially paralyzed hands, regional homogeneity, resting‐state fMRI, stroke
1. INTRODUCTION
It is well known that stroke often causes the persistent motor disability and create a great burden on families and society (Stinear, 2010; Sun, Zou, & Liu, 2013a). Only approximately 30% of patients fully recover after stroke, and stroke survivors often suffer from upper limb weakness (Beebe & Lang, 2009). The level of self‐care of hemiplegic patients mainly relies on their upper limbs, particularly their hands, which play a vital role in an individual's daily life and social participation (Veerbeek, Kwakkel, van Wegen, Ket, & Heymans, 2011). To date, the pathological mechanisms underlying disabled hands in stroke patients remain unclear, and an approach that precisely evaluates the prognosis of paralyzed hands is lacking in clinical practice. Many fMRI studies have reported that functional reorganization is associated with motor recovery following stroke (Loubinoux et al., 2007; Rehme, Eickhoff, Rottschy, Fink, & Grefkes, 2012; Wang et al., 2010). Nevertheless, the current methods used to assess the recovery of paralyzed hands after stroke are in the preliminary stages. Recently, our group employed MRI techniques to reveal that the patterns of brain reorganization differed in both structure and function between patients with partially paralyzed hands (PPH) and patients with completely paralyzed hands (CPH) after subcortical stroke (Yin et al., 2012, 2013b, 2014). Specifically, using functional connectivity (FC), we discovered that CPH patients had decreased FC between the ipsilesional primary motor cortex (M1) and the contralesional postcentral gyrus, superior parietal lobule, and ipsilesional inferior parietal lobule (Yin et al., 2012). By performing voxel‐based morphometric analyses of DTI data, the results demonstrated that the fractional anisotropy (FA) in the CPH group was significantly reduced in the ipsilesional medial frontal gyrus, precentral gyrus, superior temporal gyrus (STG), supplementary motor area (SMA), and contralesional postcentral gyrus (Yin, Yan, et al., 2013b). Moreover, the betweenness centrality in motor‐related regions, such as the ipsilesional dorsolateral premotor cortex, middle frontal cortex, and superior parietal lobule, displayed striking differences between the CPH and PPH groups using graph theoretical approaches (Yin et al., 2014). In addition, the abovementioned brain changes between the subgroups were all correlated with the Fugl‐Meyer Assessment of Hand and Wrist (FMA‐HW) scores across all stroke patients, indicating that brain reorganization is closely related to hand‐function outcomes after subcortical stroke. In addition, a previous longitudinal study suggested that the changes in the pattern of motor‐related brain activation were related to motor recovery rather than the passage time after stroke (Ward, Brown, Thompson, & Frackowiak, 2003). Therefore, a subgroup classification of patients with different hand functions may be helpful in stroke studies to successfully interpret the different motor recovery patterns after subcortical stroke.
Task‐dependent fMRI and task‐free resting‐state fMRI (R‐fMRI) are currently the two main ways of obtaining information regarding whole‐brain activity. In contrast to conventional task‐driven fMRI, R‐fMRI is a rapidly evolving, promising technology for clinical studies because this approach does not require patients to perform complicated tasks to probe the intrinsic low‐frequency oscillations (LFO) and connectivity networks of the brain (Damoiseaux et al., 2006; Fox & Raichle, 2007; Schölvinck, Maier, Frank, Duyn, & Leopold, 2010). Regional homogeneity (ReHo) is an analytical method with high test–retest reliability (Zuo & Xing, 2014) in the R‐fMRI domain and is calculated using Kendall's coefficient of concordance (Gibbons & Kendall, 1990). This method measures the similarity between the time series of a given voxel and that of its nearest neighbors in a voxel‐wise manner (Zang, Jiang, Lu, He, & Tian, 2004). This method is suitable and convenient for exploring the regional synchronization of brain activity at rest because it is largely parametric free and does not require a priori knowledge of the structure or function of the brain (Zuo et al., 2013). In addition, this metric is highly robust against temporospatial noise and outliers (Zuo et al., 2013). ReHo abnormalities within regions of the brain reflect alterations in the synchronization of temporal neural activities (Zhong et al., 2011), which suggests that there is unbalanced local functionality or noncompensatory reactions in the whole‐brain network (Dai et al., 2012). This approach has been used to identify specific pathophysiological functional signatures and to reveal the neural mechanisms underlying dysfunctions in various brain disorders (Huang et al., 2016; Wang et al., 2014; Wu et al., 2011).
To date, most R‐fMRI studies have detected LFO activities at a typical frequency band of .01–.1 Hz that are thought to reflect spontaneous neuronal activities (Biswal, Yetkin, Haughton, & Hyde, 1995). However, the pattern of intrinsic brain activity has recently been shown to be sensitive to specific frequency bands, and the LFO amplitudes in different frequency bands are thought to reflect meaningful differences among brain regions (Zuo et al., 2010). Specifically, by decomposing the R‐fMRI LFOs into five distinct frequency bands [slow‐6 (0–.01 Hz), slow‐5 (.01–.027 Hz), slow‐4 (.027–.073 Hz), slow‐3 (.073–.198 Hz), and slow‐2 (.198–.25 Hz)], it has been shown that the oscillation amplitudes of the slow‐4 and slow‐5 bands occur in the gray matter, while the signals of the slow‐6, slow‐3 and slow‐2 bands mainly reflect low‐frequency drift, white matter signals, and high‐frequency physiological noises, respectively (Li et al., 2014; Zuo et al., 2010). In addition, frequency‐dependent changes in the LFOs have been reported across a wide range of diseases including mild cognitive impairment (Han et al., 2011), Parkinson's disease (Hou, Wu, Hallett, Chan, & Wu, 2014; Song et al., 2015) and schizophrenia (Yu et al., 2014). Recently, Zhu et al. used the ReHo method to show that relative to healthy controls (HCs), abnormalities in LFOs in the parietal lobules in stroke patients were frequency‐dependent, and the authors suggested that R‐fMRI studies investigating stroke should consider frequency effects when measuring intrinsic brain activity (Zhu et al., 2015). Another cross‐sectional study using independent component analysis demonstrated that the oscillations in the slow‐5 band in the default mode network (DMN), primary visual, and sensorimotor networks were reduced in the stroke population, and this reduction in the slow‐5 oscillation amplitude in the posterior component of the DMN is likely associated with lower phonemic verbal fluency scores (La et al., 2016). However, these two studies did not explore whether the altered LFOs among stroke patients with different hand outcomes were associated with specific frequency bands.
Recently, we used the ReHo method at a frequency band of .01–.08 Hz and detected the alterations in ReHo among stroke subgroups in certain motor‐related regions, which were correlated with the FMA‐HW scores (Yin et al., 2013a). However, the number of subgroup patients in the abovementioned study was small (12 CPH vs. 12 PPH stroke patients). In this study, we increased the total number of subgroup patients (26 CPH vs. 26 PPH patients) and extended the ReHo approach to examine the local synchronization of LFOs at different frequency bands (slow‐5 [.01–.027 Hz], slow‐4 [.027–.073 Hz] and the typical band [.01–.1 Hz]), and the relationship between ReHo changes and FMA‐HW scores. Specifically, we first examined whether frequency‐specific changes in ReHo between the total stroke group (PPH + CPH) and HCs were affected by different hand function outcomes in stroke patients. Second, we detected whether ReHo differences in cortical regions among stroke patients with different outcomes in hand function were frequency‐dependent. Third, we explored the correlations between ReHo values at the specific frequency bands and the FMA‐HW scores of hand function across all the stroke patients. Based on the typical frequency band studies among stroke subgroups (Yin, Luo, et al., 2013a; Yin et al., 2012, 2014) and a subfrequency study between stroke patients and HCs (Zhu et al., 2015), we hypothesized that spontaneous brain activity following stroke would be influenced by both the specific frequency of LFO and the severity of stroke. We predicted that the results in the typical band would be consistent with those of our previous studies among stroke subgroups, which might be found in the primary sensorimotor cortex, SMA, and several high‐order motor regions, such as the frontal gyrus and the parietal lobule. In addition, the distribution of ReHo differences in those regions would be frequency‐specific. Moreover, we also expected to find some new regions in the subfrequency bands compared with the results of previous studies in the typical band. Importantly, frequency‐specific ReHo in certain brain regions detected to be correlated with the FMA‐HW scores could provide more help for clinical evaluations of the hand outcomes of motor function in stroke patients.
2. METHODS
2.1. Subjects
Fifty‐two left‐only subcortical stroke patients in the chronic stage and 52 well‐matched (the demographics of the 52 healthy subjects are shown in Supporting Information Table S1) HCs were recruited in this study. The inclusion criteria were as follows: (1) first onset left‐subcortical stroke; (2) age between 30 and 75 years; (3) illness duration of stroke >3 months; (4) pure motor deficit and Mini‐Mental State Examination score (MMSE) >27 (Sun et al., 2013b); and (5) right handedness before stroke. The exclusion criteria for this study included the following: (1) any contraindication to MRI; (2) neurological disorders other than stroke, such as psychiatric disease; (3) unstable conditions, such as severe atrial fibrillation; (4) severe aphasia that hindered basic communication and tests; and (5) excessive head motion during fMRI scanning. The global cognition of each participant was evaluated by an experienced therapist using the Mini‐Mental State Examination on the day of admission. To assess the motor performance of the patients, the FMA scale was administered in each stage of stroke survivors. The FMA scale evaluates the ability to isolate movements at each joint and the influence of unwanted synergies during movement execution, thereby representing a reduction in impairment rather than a compensatory pattern; thus, the impairment changes reflect substantial recovery at the neurobiological and behavioral levels. Only the hand and wrist domains of the FMA motor component, which includes five wrist items and seven hand items with a maximal score of 24, were selected as two of our primary outcome measures. The statistical analysis of the demographical variables was performed using the Statistical Package for Social Sciences (SPSS) version 17 for Windows.
According to the Paralyzed Hand Function Assessment as described in our previous works (Yin et al., 2012, 2014; Yin, Luo, et al., 2013a; Yin, Yan, et al., 2013b), the stroke patients were divided into two subgroups: the partially paralyzed hand (PPH = 26) group and the completely paralyzed hand (CPH = 26) group. This scale involves five functional actions of hand in daily life (see Supporting Information Figure 1). All those who could not complete any action were regarded as CPH while those who could complete at least one of the five actions were regarded as PPH.
The Institutional Review Boards of East China Normal University approved this work. The study procedures were conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants or their legal guardian as appropriate.
2.2. MRI data acquisition
Images were acquired on a 3‐T MRI scanner (Siemens, Erlangen, Germany) at Shanghai Key Laboratory of Magnetic Resonance. Structural images were acquired using a magnetization‐prepared rapid gradient echo sequence: TR/TE/TI = 1900/3.42/900 ms, FOV = 240 × 240 mm2, flip angle = 9°, matrix = 256 × 256, 192 sagittal slices, slice thickness = 1 mm, gap = .5 mm. T2‐weighted images were collected to identify the lesions using a turbo‐spin‐echo sequence: 30 axial slices, slice thickness = 5 mm, no gap, TR/TE = 6,000/93 ms, FOV = 220 × 220 mm2, flip angle = 120°, and matrix = 320 × 320. Resting‐state fMRI data were obtained in an interleaved acquisition mode using an echo‐planar imaging sequence: 30 axial slices, slice thickness = 4 mm, gap = 0.8 mm, TR/TE = 2,000/30 ms, flip angle = 90°, FOV = 220 × 220 mm2, matrix = 64 × 64, 240 volumes, and acquisition time lasted for 8 min 6 s (the first 6 s was dummy scanning). During fMRI scans, subjects were instructed to keep motionless with their eyes closed but remaining awake.
2.3. fMRI data preprocessing
Standard image preprocessing analyses were performed using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and Data Processing Assistant for Resting‐State fMRI (DPARSF, http://resting-fmri.sourceforge.net). The first 10 volumes of each participant were discarded to allow for magnetization equilibrium and environment adaptation. The remaining 230 images were corrected for the time delay between slices based on the middle slice in time and for rigid‐body head movement by coregistering the images to the first image. Head motion was defined as a greater than 2.0 mm translation or greater than 2.0° of rotation at any direction. Any head motions greater than these criteria were removed from the analysis. None of the participants were excluded due to excessive motion. In addition, no significant differences were observed in the frame‐wise displacement (Jenkinson, Bannister, Brady, & Smith, 2002) between the groups (t = −1.70, p = .10). The linear trend, mean white matter and CSF signals, and 24 head motion covariates (translations, rotations, their derivatives, and quadratic terms) were regressed from each voxel's time course. Previous studies have demonstrated that global signal regression could introduce various anticorrelations and contaminate the final results in the function connectivity preprocessing (He & Liu, 2012; Weissenbacher et al., 2009). Therefore, global signal regression was not performed in our preprocessing analysis. Subsequently, using the lesion mask (Andersen, Rapcsak, & Beeson, 2010) and unified segmentation algorithm (Ashburner & Friston, 2005), the functional images were normalized to the Montreal Neurological Institute space. The data were then resampled every 3 mm using the parameters estimated during the unified segmentation.
2.4. Lesion analysis
Lesion volume of each patient was determined by two experienced neuroradiologist who manually outlined the profiles on T2‐weighted images slice by slice using the software MRIcron (http://www.mricro.com). Also, maps of the lesion overlap for each stroke subgroup and whole stroke patients are shown in Figure 1.
Figure 1.
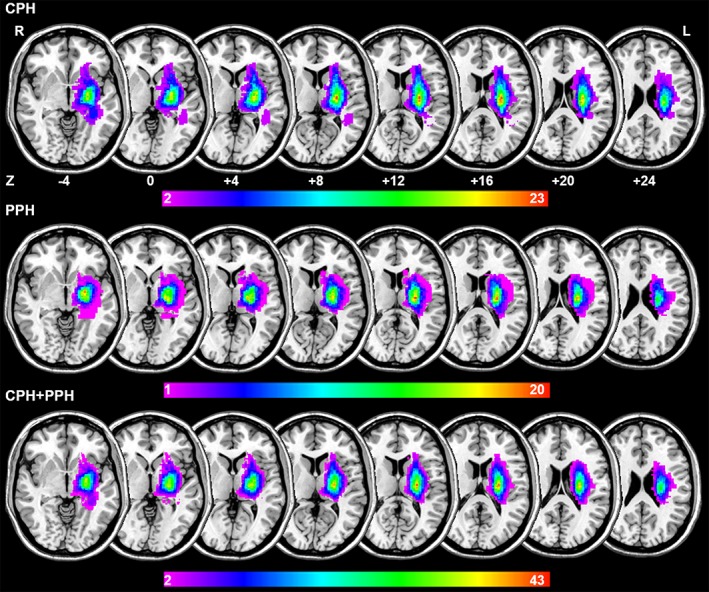
Lesion overlapped across the 26 PPH patients (the first raw), 26 CPH patients (the second raw) and total patients (the third raw). Color bar indicates the number of subjects having lesions in each voxel [Color figure can be viewed at http://wileyonlinelibrary.com]
2.5. ReHo measure and statistical analysis
The ReHo analysis was performed for normalized images of each subject using the Resting‐State fMRI Data Analysis Toolkit (REST, http://restfmri.net). First, to examine the frequency effects on brain activity changes in patients, we used REST to conduct temporal band‐pass filtering in the typical band (.01–.1 Hz), slow‐5 (.01–.027 Hz) and slow‐4 (.027–.073 Hz) bands (Hou et al., 2014; Yu et al., 2014), respectively. Next, the ReHo value was calculated across the whole brain with Kendall's coefficient of concordance in a voxel‐wise way at three frequency bands to assess the similarity of the time series at a given voxel with the time series of its 26 nearest neighbors (the sensitivity of 26 voxels was better than 19 and 7 voxels; Zang et al., 2004). The Kendall's coefficient of concordance value ranging from 0 to 1 was calculated voxel‐by‐voxel across the whole brain to produce an individual ReHo map for each subject. Finally, to reduce the global effects of variability across participants, the ReHo of each voxel was divided by the global mean ReHo value across the whole brain for each subject (Zang et al., 2004; Zhong et al., 2011), and the ReHo maps for all subjects were smoothed using a Gaussian filter of 6‐mm full width at half maximum to reduce noise and residual differences.
To determine the main effects of the group, frequency band, and the interaction effect between the frequency band and group on ReHo, we performed a repeated‐measures analysis of variance (anova) with SPM8 software. The ReHo results of each subject were modeled using a flexible factorial design with group (CPH and PPH) as a between‐subject factor and frequency bands (slow‐4 and slow‐5) as a repeated measure. The threshold produced an F‐map of the “frequency by group” interaction was set to a significance level of p < .05 (combined voxel height threshold of p < .005 and an extent threshold of k > 24 voxels, AlphaSim corrected) according a Monte Carlo simulation (1,000 simulations, FWHM = 10 mm) within the gray matter mask using REST software. For those findings showing significant main effects of group, post hoc two‐sample t‐tests were further performed in which age, gender, and mean frame‐wise displacement were entered as covariates. The t‐maps of the post hoc t‐tests and group main effects were corrected for multiple comparisons at a significance level of p < .05 (combined voxel height threshold of p < .005 and an extent threshold of k > 74 voxels, AlphaSim corrected). Subsequently, we also performed an anova analysis to compare the patient group (PPH + CPH) and the HCs, PPH and the HCs, and CPH and the HCs. Notably, this study primarily focused on whether the ReHo changes between CPH and PPH depended on the different subfrequency bands.
2.6. Correlation analysis
To determine the relationship between the identified brain regions with subgroup differences and FMA‐HW scores, we extracted the ReHo index of each stroke patient in the identified clusters with significant differences between CPH and PPH. Subsequently, a Spearman correlation (nonparametric) analysis was performed between the ReHo index and FMA‐HW for all stroke patients, and p value < .05 (FDR corrected) was considered to be statistically significant.
3. RESULTS
3.1. Participant characteristics
In all patients, the FMA‐HW scores were significantly lower in patients with CPH than in patients with PPH (two‐sample t‐test, p < 10−9). There were no significant differences in age (two‐sample t‐test, p = 0.89), gender (chi‐square test, p = .16), disease duration (two‐sample t‐test, p = .87), lesion volume (two‐sample t‐test, p = .13), MMSE (two‐sample t‐test, p = .75), and mean frame‐wise displacement (two‐sample t‐test, p = .10) between the CPH and PPH patients, as shown in Table 1.
Table 1.
Demographics and clinical details of the subjects
| PPH (n = 26) mean ± SD | CPH (n = 26) mean ± SD | CPH vs. PPH p value | |
|---|---|---|---|
| Age (years)a | 56 ± 9.92 | 56 ± 10.22 | .89 |
| Sex (male: female)b | 25:1 | 22:4 | .16 |
| Hand dominance | R | R | – |
| Duration of illness (months)a | 16 ± 15.58 | 16 ± 17.36 | .87 |
| Lesion volume (ml)a | 5.53 ± 4.89 | 7.77 ± 5.51 | .13 |
| MMSEa | 29 ± 1.36 | 29 ± 1.21 | .75 |
| FMA‐HWa | 12 ± 7.32 | 1.38 ± 1.20 | <10−9 |
| FDa | .11 ± .05 | .15 ± .10 | .10 |
Independent t‐test.
Chi‐square test.
MMSE = Mini‐Mental State Examination; FD = frame‐wise Displacement; FMA‐HW = Fugl‐Meyer Assessment of hand and wrist section.
3.2. Full band
Compared with the PPH group, in the typical band, the CPH group presented decreased ReHo in the bilateral cerebellum posterior lobes (CPL) and increased ReHo in the contralesional SMA (Figure 2a and Table 2).
Figure 2.
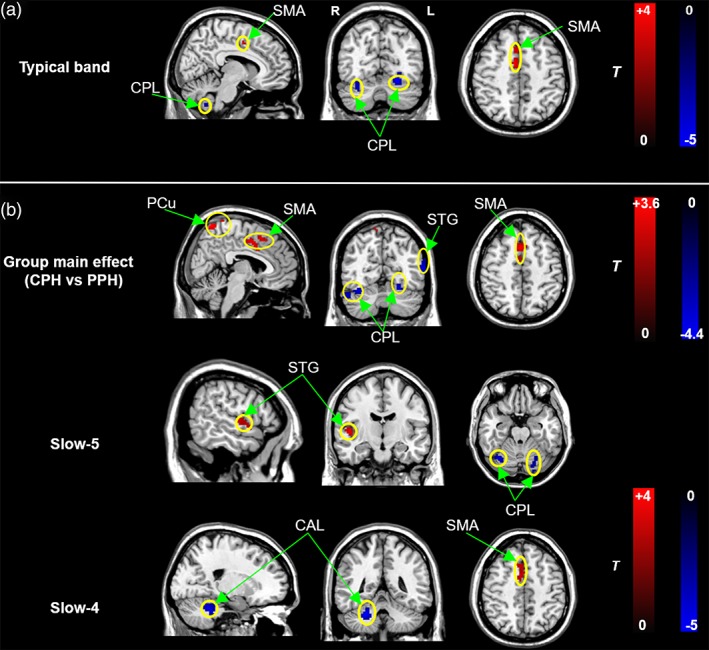
Subgroup differences in ReHo in the full band (a) and specific frequency bands (b). Cold and hot colors indicate regions showing lower and higher ReHo in the CPH vs. PPH groups, respectively. Threshold of ReHo: p < .05 (corrected). SMA = supplementary motor area; PCu = precuneus; STG = superior temporal gyrus; CPL = cerebellum posterior lobe; CAL: cerebellum anterior lobe; L = left; R = right [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Regions showing significantly different ReHo in the CPH group compared with PPH group
| Regions | BA | MNI coordinates (x, y, z) | Cluster (voxel number) | T value |
|---|---|---|---|---|
| CPH < PPH | ||||
| TB | ||||
| Cerebellum posterior lobe (CL) | – | (33, −66, −21) | 181 | 4.08 |
| Cerebellum posterior lobe (IL) | – | (−24, −69, −18) | 83 | 4.83 |
| Slow‐5 | ||||
| Cerebellum posterior lobe (CL) | – | (33, −66, −21) | 118 | 4.32 |
| Cerebellum posterior lobe (IL) | – | (−21, −72, −15) | 98 | 5.17 |
| Slow‐4 | ||||
| Cerebellum anterior lobe (CL) | – | (24, −45, −33) | 132 | 4.81 |
| CPH > PPH | ||||
| TB | ||||
| Supplementary motor area (CL) | 6 | (6, 9, 33) | 85 | 3.74 |
| Slow‐5 | ||||
| Superior temporal gyrus (CL) | 22 | (63, 0, 3) | 94 | 4.18 |
| Slow‐4 | ||||
| Supplementary motor area (CL) | 6/8 | (3, 12, 51) | 87 | 4.09 |
CPH = completely paralyzed hand; PPH = partially paralyzed hand; IL = ipsilesional; CL = contralesional.
3.3. Frequency‐dependent effects
A two‐way repeated‐measure anova of the ReHo values showed a significant main effect of group and a significant main effect of frequency. The brain regions showing the significant main effect of group included the contralesional precuneus (PCu), SMA, cingulate gyrus, ipsilesional STG, and bilateral CPL (Figure 2b). Post hoc two‐sample t‐tests were applied to reveal the differences between the subgroups at each frequency band. Interestingly, several clear differences also existed between the two bands. Compared to the PPH group, decreased ReHo in the bilateral CPL and increased ReHo in the contralesional STG were revealed in the slow‐5 band in the CPH group, while decreased ReHo in the contralesional cerebellum anterior lobe (CAL) and increased ReHo in the contralesional SMA were observed in the slow‐4 band (Figure 2b and Table 2).
The analysis of the main effect of frequency band showed the ReHo in the slow‐4 band was higher in the pons, midbrain, CAL, basal ganglia, temporal lobe, SMA, and premotor cortex than in the slow‐5 band. In contrast, the slow‐4 band showed lower ReHo in the middle temporal gyrus, middle occipital gyrus, medial frontal gyrus (MedFG), inferior frontal gyrus, PCu and CPL than the slow‐5 band did (Supporting Information Figure 2).
The two‐way repeated‐measures anova revealed a significant “frequency by group” interaction effect. The affected brain regions included the contralesional MFG and precentral gyrus (PreCG) (Figure 3).
Figure 3.
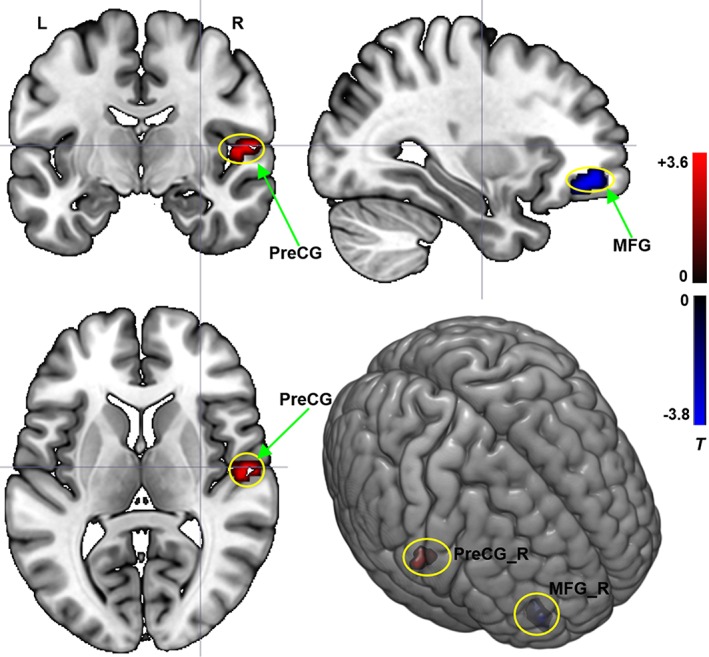
Interaction effect between the frequency band (slow‐4 and slow‐5) and the group (CPH and PPH) on ReHo. Repeated‐measures anova (p < .05, corrected). T value bar is shown on the right. L = left; R = right; MFG = middle frontal gyrus; PreCG = precentral gyrus [Color figure can be viewed at http://wileyonlinelibrary.com]
In addition, compared with the HCs, significantly increased ReHo in the bilateral superior frontal gyrus was revealed in the typical band, slow‐4 band, and slow‐5 band in patients. Higher ReHo in the bilateral superior frontal gyrus was observed in typical band, and higher ReHo in the contralesional superior frontal gyrus was only observed in both the slow‐5 and slow‐4 bands in CPH patients than in the HCs; these regions highly overlapped with the regions detected in the comparison of the total patient group and the HC group. Moreover, increased ReHo in the ipsilesional superior frontal gyrus was found in the typical band in PPH patients compared with that of the HCs, while increased ReHo in the bilateral parietal cortex and the ipsilesional superior frontal gyrus was found in both the slow‐5 and slow‐4 bands (Figure 4).
Figure 4.
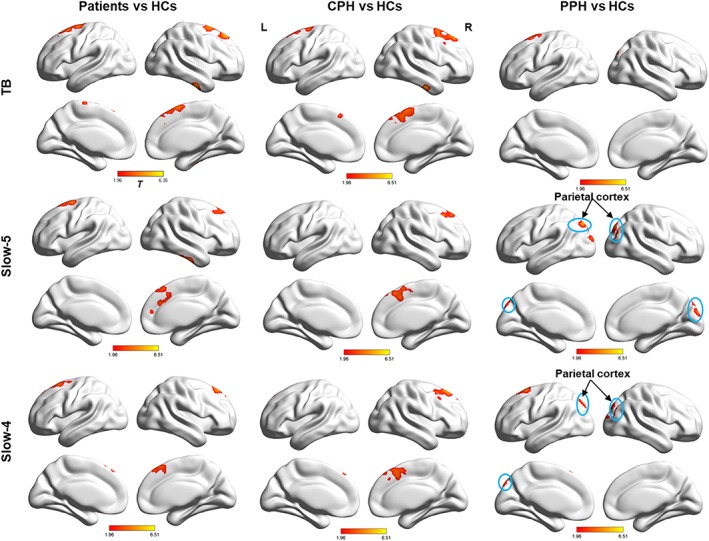
Changes in ReHo between the patient group and the HC group in three different FBs. Hot colors indicate regions showing higher ReHo in the patient group than in the HC group. Threshold of ReHo: p < .05 (corrected). L = left; R = right [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Correlations between ReHo and FMA scores
In the typical band, significant (p < .05, FDR corrected) positive correlations were found between the mean ReHo index in the ipsilesional CPL as well as the contralesional CPL and the FMA‐HW scores, and the r values were .501 (p < .001) and .444 (p = .001), respectively. A significant negative correlation was found between the mean ReHo index in the contralesional SMA and the FMA‐HW scores, and the r value was −.527 (p < .001). In the slow‐5 band, a significant positive correlation between the mean ReHo index in the contralesional CPL and the FMA‐HW scores and a significant negative correlation between the mean ReHo index in the contralesional STG and the FMA‐HW scores were observed; the r values were .520 (p < .001) and − .430 (p = .001), respectively. For the slow‐4 band, the mean ReHo index in the contralesional CAL was positively correlated with the FMA‐HW scores, and that in the contralesional SMA was negatively correlated with the FMA‐HW scores; the r values were .453 (p = .01) and − .531 (p < .001), respectively (Figure 5).
Figure 5.
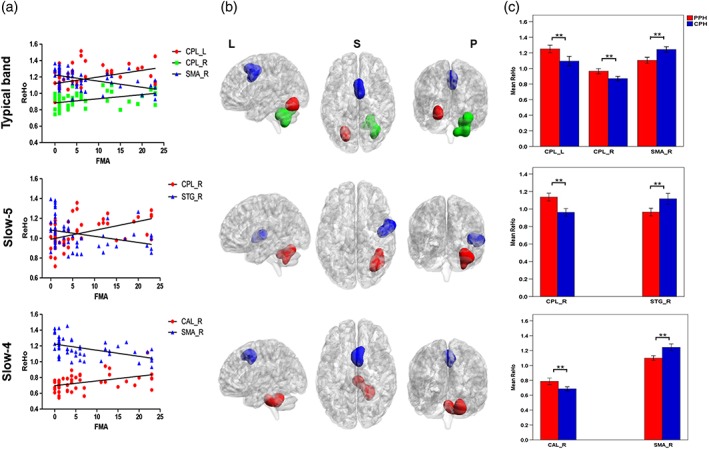
(a) Shows significant correlations between the mean ReHo with between‐subgroup differences and the FMA scores, (b) shows the corresponding brain regions, and (c) displays the between‐subgroup differences in the three different frequency bands. SMA_R = right supplementary motor area; STG_R = right superior temporal gyrus; CPL_R = right cerebellum posterior lobe; CPL_L = left cerebellum posterior lobe; CAL_R = right cerebellum anterior lobe; L = left; S = superior; P = posterior. ** Represents p value < .01 [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
To the best of our knowledge, this was the first study to investigate the changes in the frequency‐dependent effects on ReHo among left‐subcortical stroke patients with different outcomes in hand function. Our results demonstrated that, in the typical band, the CPH patients exhibited deceased ReHo in the bilateral CPL and increased ReHo in the contralesional SMA compared to the PPH patients. For the subfrequency bands, deceased ReHo in the bilateral CPL and increased ReHo in the contralesional STG were observed in the slow‐5 band in the CPH group, while decreased ReHo in the contralesional CAL and increased ReHo in the contralesional SMA were found in the slow‐4 band. These findings supported our hypothesis that spontaneous brain activity following stroke is influenced by both the severity of stroke and the specific frequency of LFOs. As we expected, the ReHo differences of the CPL and SMA between stroke subgroups in the typical band were consistent with the findings of our previous studies, and they presented a frequency‐specific distribution in which the ReHo change in the CPL was only found in the slow‐5 band, and the ReHo change in the SMA was only observed in the slow‐4 band. In addition, we also found that the ReHo in the contralesional STG increased in the slow‐5 band, and the ReHo in the contralesional CAL decreased in the slow‐4 band, which were not found in the previous studies with the typical band. Furthermore, the mean ReHo values in these regions showed a significant difference between the two subgroups and were correlated with the FMA‐HW scores across all patients, reflecting that the frequency‐specific ReHo changes may be associated with hand function outcomes of stroke patients. More importantly, we found significant interactions between the frequency band and group in the contralesional PreCG and the MFG, implying that the different intrinsic activities between the groups in the two brain regions could be modulated by the frequency band differences. Altogether, the frequency‐dependent ReHo changes in these regions not only provided new insight into the relationships between functional reorganization and outcomes in hand function after subcortical stroke, but also provided complementary evidence of the possible neural mechanism underlying hand dysfunction in stroke patients.
4.1. Full band analysis of ReHo
This study showed that, relative to the PPH group, there were reduced ReHo in the bilateral CPL and enhanced ReHo in the contralesional SMA in the typical band in the CPH group. In addition, the ReHo values in the bilateral CPL were positively correlated with FMA‐HW scores, and that in the contralesional SMA was negatively correlated with FMA‐HW scores. These findings were consistent with previous studies related to subgroups of stroke patients (Yin et al., 2012; Yin, Luo, et al., 2013a; Yin, Yan, et al., 2013b). Decreased FC with the contralesional M1 was displayed in the CPL of CPH patients compared with that in the PPH patients (Yin et al., 2012), and the CPH patients demonstrated reduced FA values in the SMA that correlated with the FMA scores across all patients (Yin, Yan, et al., 2013b). Corroborating evidence was also documented in several studies between patients and HCs. Patients with motor conversion disorder showed abnormal activity in the SMA during motor preparation compared to that of normal volunteers (Voon, Brezing, Gallea, & Hallett, 2011). In an interhemispheric FC study, stroke patients displayed decreased interhemispheric interaction in the CPL compared with that of HCs, and there was a significant positive correlation between the interhemispheric FC in the CPL and the FMA scores of the stroke patients (Tang et al., 2016). Therefore, the findings of abnormal activation in the SMA and CPL in the typical band indicated that the impairment in the cerebellar–cerebral information circuit may contribute to reduced hand function (O'Reilly, Beckmann, Tomassini, Ramnani, & Johansen‐Berg, 2010), and the poor hand function outcomes in CPH patients may be associated with dysfunctional motor control (Amengual et al., 2014; Brugger, Galovic, Weder, & Kägi, 2015).
It is worth noting that although our findings replicated those of the previous ReHo study, such as those related to the SMA, a difference was still presented in that the CPL was not revealed in the Yin's study (Yin, Luo, et al., 2013a). There are several possible reasons for the inconsistent results. First, the frequency band used in the former study was .01–.08 Hz, which was different from the typical band (.01–.1 Hz) used in this study. Second, the lesion, which was reported to influence the final statistical results (Andersen et al., 2010), was not considered in the normalization of the fMRI data during preprocessing for patients in the prior study and the covariates (age, gender, and head motion) were removed from the comparison between the subgroups in the current study but not in the previous study. Third, the sample size of each subgroup in the former study was smaller (n = 12) than that of this study (n = 26), and the results may not be robust. Therefore, the current findings in the typical band may be a supplement for the results of our prior study related to subgroups of patients after stroke.
4.2. Differences in ReHo between the two subgroups
We showed that the differences in ReHo observed between the subgroups were frequency‐dependent. Specifically, in the slow‐5 band, compared to the PPH group, we found decreased ReHo in the bilateral CPL and increased ReHo in the contralesional STG in the CPH group, while decreased ReHo in the contralesional CAL and increased ReHo in the contralesional SMA were observed in the slow‐4 band. In addition, the ReHo values in the contralesional STG and contralesional SMA were negatively correlated with the FMA‐HW scores, and the ReHo values in the contralesional CPL and contralesional CAL were positively correlated with the FMA‐HW scores. The CAL has been found to be closely related to sensorimotor function (O'Reilly et al., 2010; Schmahmann, MacMore, & Vangel, 2009). In addition, a previous study reported that activation of the contralesional CAL 20 days after stroke was positively correlated with the motor performance involving finger‐tapping (Loubinoux et al., 2007). Several FC studies have suggested that the activity in sensorimotor areas is significantly correlated with the activity in the CAL (Krienen & Buckner, 2009; O'Reilly et al., 2010). In contrast to M1, the STG cannot directly control movement execution, but this region is a motor‐related area. Several previous animal studies and lesion studies found that the STG was involved in the parallel organization of the cortico‐striato‐thalamic circuits that support both actual and potential movement (i.e., motor preparation and motor planning) (Alexander, DeLong, & Strick, 1986; Yin, Luo, et al., 2013a). A task‐based fMRI study revealed that compared with imagery‐related activities, the bilateral STG generated increased activation during movement‐related activities (Hanakawa, Dimyan, & Hallett, 2008). Thus, the decreased ReHo of the CAL may represent functional impairment, and the increased ReHo in the STG is likely a reflection of compensation. Our findings suggest that the ReHo changes in these regions induced by stroke may be associated with the functional reorganization of specific frequency bands in CPH and PPH patients.
In addition, the decreased ReHo in the bilateral CPL in the slow‐5 band and increased ReHo in the contralesional SMA in the slow‐4 band replicated the findings observed in the typical band, which were consistent with those of recent studies investigating the changes in LFOs in stroke patients (Tang et al., 2016; Yin et al., 2012; Yin, Yan, et al., 2013b; Zhu et al., 2015). This outcome further demonstrated that the bilateral CPL and contralesional SMA may play pivotal roles in functional reorganization in stroke patients with different hand function outcomes, and these two regions could serve as rehabilitative target regions for improving hand function recovery in chronic stroke patients. However, the increased ReHo in the contralesional STG in the slow‐5 band and the decreased ReHo in the contralesional CAL in the slow‐4 in the CPH group, which correlated with the FMA‐HW scores across all patients, were not found in the typical band. Although the origins, relationships, and specific physiological functions of the different frequency bands are not fully understood, the abnormalities in the low‐frequency fluctuation amplitudes detected in clinical populations have been associated with the choice of the slow‐5 and slow‐4 bands (Di Martino et al., 2008; Han et al., 2012). Hence, the ReHo changes between CPH and PPH in the subfrequency bands may imply an abnormal pattern of intrinsic brain activity in the contralesional STG and CAL in stroke patients with differential paralyzed hands that is more sensitive to the slow‐5 and slow‐4 bands than typical band, respectively. These findings indicated that a subfrequency band study may be able to identify the specific frequency bands through the abnormal brain regions occurred between the subgroups of patients, and more prominent regions related to hand dysfunctions were found in the subfrequency bands than in the full frequency band. Moreover, a recent study did not find ReHo changes in the regions discussed above in a comparison of mixed patients and HCs in either the slow‐4 or slow‐5 bands (Zhu et al., 2015). Another study (Yin, Luo, et al., 2013a) investigating mixed frequency bands did not uncover altered ReHo in the cerebellum or the SMA but found a decrease in the ipsilesional STG in the CPH group relative to the PPH group. Thus, these findings further demonstrated that the pattern of brain functional reorganization in stroke patients with differential paralyzed hands was not similar in different subfrequency bands. Therefore, considering the frequency effect and the characteristics of the patients will be necessary in future stroke studies.
4.3. Differences in ReHo between the two sub‐frequency bands
There was a significant difference in stroke‐related activity between the two bands. The frequency effect analysis showed that higher ReHo was observed in the pons, midbrain, CAL, basal ganglia, temporal lobe, SMA, and premotor cortex in the slow‐4 band than in the slow‐5 band, but lower ReHo in the middle temporal gyrus, middle occipital gyrus, MedFG, inferior frontal gyrus, PCu, and CPL was found in the slow‐4 band. In general, these findings were compatible with those of previous studies that LFOs in the slow‐5 band showed higher power distributed in widespread cortical regions, whereas greater LFO in the slow‐4 band was mainly located in the subcortical regions (Hou et al., 2014; Yu et al., 2014). Specifically, the identified regions (slow‐4 > slow‐5) were primarily associated with preliminary motor execution and were distributed in the corticospinal tracts involved in the movement circuit (Lee, Han, Kim, Kwon, & Kim, 2005), while the regions (slow‐5 > slow‐4) were mainly related to the higher‐order motor functions (e.g., motor control and preparation) (Inman et al., 2012). This outcome may demonstrate that, in the motor network, the oscillations in different frequencies respond to different levels of motor behavior by the corresponding brain regions. In addition, it has been suggested that the oscillations of the brain cover a wide range of frequencies, and each of these oscillatory bands is generated by different mechanisms and has different physiological functions (Buzsáki & Draguhn, 2004; Han et al., 2011). These findings suggest that a properly chosen frequency band can be helpful for exploring stroke‐related neural changes.
4.4. Frequency‐specific changes in ReHo between the two subgroups
The most remarkable finding in this study was that the ReHo changes in the contralesional PreCG and MFG were modulated by the frequency bands. Recently, Hou et al. (2014) revealed that the amplitude of the LFOs decreased in the striatum and increased in the midbrain of Parkinson's disease patients relative to that in the controls, and these changes were greater in the slow‐4 band than in the slow‐5 band. Yu et al. (2014) observed a significant interaction between the frequency band and group in the inferior occipital gyrus, PCu, and thalamus in schizophrenia patients. These findings indicated that the pattern of intrinsic brain activity is sensitive to the specific frequency bands. Moreover, cross‐sectional studies have suggested that the contralesional M1, specifically the PreCG, might functionally inhibit ipsilesional M1 in subcortical stroke patients (Murase, Duque, Mazzocchio, & Cohen, 2004), and this pattern of activation was also shown in a longitudinal study (Loubinoux et al., 2003). Our recent ReHo study and FC analysis in the .01–.08 HZ band found decreased ReHo in the ipsilesional PreCG in the CPH group relative to the HC group (Yin, Luo, et al., 2013a), and altered connectivity was observed in the bilateral PreCG between the PPH and CPH groups (Yin et al., 2012). In addition, several studies (Park et al., 2011; Yin et al., 2014; Yin, Luo, et al., 2013a) have demonstrated that the organization of the connectivity in the MFG in patients with subcortical stroke was altered, which reflected the role of the frontal lobe in higher‐order planning of movement. As mentioned above, the results of the present study suggest that the two motor‐related regions are dysfunctional in stroke patients with different levels of paralysis in their contralesional hands and are dependent on the stroke disease and frequency bands, which enhanced our understanding of frequency‐specific pathology in patients with stroke. The underlying neural mechanisms of these interactions in the temporal and frequency domains are interesting topics that require further investigation.
In addition, the findings of two recent ReHo studies in stroke patients were inconsistent; Zhu et al. (2015) only found increased ReHo in the left SPL/PCu in the .01–.1 Hz range in the patient group relative to the HC group, whereas Yin et al., (2013a) revealed not only increased ReHo in the .01–.08 HZ band in widespread motor‐related regions, such as the precentral gyrus, postcentral gyrus, premotor cortex, middle frontal gyrus, and inferior parietal lobule but also decreased ReHo in nonmotor regions, such as the STG and occipital lobe, in stroke patients relative to the HCs. However, these inconsistent findings cannot be simply explained by the frequency band effect because there were differences in the inclusion criteria between Zhu's study and Yin's study. Therefore, this study may be helpful in clarifying these dissimilar ReHo findings by showing that the local synchronization between CPH and PPH patients was strongly modulated by the frequency band. Future studies should further explore this possibility. Moreover, Zhu's study had several limitations, including a small sample size (15 patients). The lesions in their patients were widely distributed, involving both the left and right hemispheres, and extended to the cortex (parietal cortex and frontal cortex). This distribution may have influenced the robustness of their results. Therefore, this study also detected the differences in ReHo at three frequency bands between HCs and patients. Interestingly, we did not find increased ReHo in the parietal cortex in the patient group, which was a primary outcome reported in Zhu's study (Zhu et al., 2015), in the comparisons between the HC group and the patient group (CPH + PPH, 52 patients) in the three frequency bands. However, after dividing the patients into the two subgroups (CPH and PPH) and comparing them with the HC group, consistent findings in the parietal cortex were observed between the PPH group and the HC group in both the slow‐5 and slow‐4 bands, but not in the typical band, and this result was not found in the comparisons of the CPH group and the HC group in the three frequency bands (Figure 4). Thus, we speculated that the patients with stroke in Zhu's study may have been mostly similar to the PPH group. These findings were consistent with those of our previous studies (Yin et al., 2012, 2014; Yin, Luo, et al., 2013a), which demonstrated that the stroke disorder in patients with different levels of paralysis in their contralesional hands may induce distinct functional organizations. Therefore, we recommend that future stroke‐related studies consider the outcomes of hand function in the inclusion criteria of patients because this adjustment can ensure that the final conclusion is more robust and persuasive.
5. LIMITATIONS
Although we revealed the frequency‐specific alterations in spontaneous neuronal activity between patients with different hand function outcomes and that these changes were correlated with the clinical assessments, this study had several limitations. First, this study primarily focused on the ReHo differences between the CPH group and the PPH group in two frequency bands (slow‐4 and slow‐5 bands). Future studies involving stroke patients could include more frequency bands, such as the slow‐1, slow‐2, and slow‐3 bands. Second, the stroke patients in this study were only left‐subcortical stroke patients in the chronic stage. A study of stroke patients with different lesions (e.g., only right and bilateral lesions) needs to be conducted, and further cross‐sectional studies should include acute and subacute patients. Finally, the nature of these resting‐state signal fluctuations remains unclear. Future studies should investigate the biological and functional significance of the LFOs.
6. CONCLUSIONS
In summary, this study uncovered the changes in ReHo in three frequency bands between patients with different levels of hand paralysis. Importantly, we found that the altered ReHo in several regions, including the cerebellum, SMA and STG, were frequency‐dependent and significantly correlated with the FMA‐HW scores across all patients. Moreover, the interaction between the group and frequency suggested that the ReHo differences between the patient subgroups in the contralesional PreCG and MFG were modulated by different subfrequency bands. This finding indicated that the frequency bands are closely related to the functional reorganization in CPH and PPH. Altogether, our findings suggest that the use of specific frequency bands could be helpful in detecting neural changes in stroke patients with different outcomes in hand function, which should be considered in future investigations.
Supporting information
Figure 1 Illustration of Paralyzed Hand Function Assessment: ① the affected hand stabilizes a piece of paper on the table, with the unaffected hand controlling a shear to cut the paper; ② the affected hand holds a wallet, with the unaffected hand taking a coin from the wallet; ③ the affected hand holds an unfolded umbrella in the air for at least 10 seconds; ④ the affected hand controls a nail scissor to trim nails of the unaffected hand; and ⑤ the affected hand buttons the cuff of the unaffected side. The hand in dark denotes the affected hand.
Figure 2. Main effects of frequency band. The color bar indicates regions showing higher ReHo in slow‐5 versus slow‐4 (p <0.05, FDR‐corrected). L: left; R: right.
Table I. Demographics and clinical details of the subjects
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China and China's National Strategic Basic Research Program.
Zhao Z, Tang C, Yin D, et al. Frequency‐specific alterations of regional homogeneity in subcortical stroke patients with different outcomes in hand function. Hum Brain Mapp. 2018;39:4373–4384. 10.1002/hbm.24277
Funding information 12th Five‐Year Plan Supporting Project of Ministry of Science and Technology of the People's Republic of China, Grant/Award Number: 2013BAI10B03; China National Key R&D Program, Grant/Award Number: 2017YFC1308502; China National Natural Science Young Foundation, Grant/Award Number: 81401859; China National Natural Science Foundation, Grant/Award Number: 81471734; National Natural Science Foundation of China, Grant/Award Number: 31600869/81471651
Contributor Information
Dongrong Xu, Email: xu.dongrong@columbia.edu.
Mingxia Fan, Email: mxfan@phy.ecnu.edu.cn.
REFERENCES
- Alexander, G. E. , DeLong, M. R. , & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Amengual, J. L. , Münte, T. F. , Marco‐Pallarés, J. , Rojo, N. , Grau‐Sánchez, J. , Rubio, F. , … Rodríguez‐Fornells, A. (2014). Overactivation of the supplementary motor area in chronic stroke patients. Journal of Neurophysiology, 112, 2251–2263. [DOI] [PubMed] [Google Scholar]
- Andersen, S. M. , Rapcsak, S. Z. , & Beeson, P. M. (2010). Cost function masking during normalization of brains with focal lesions: Still a necessity? NeuroImage, 53, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J. , & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Beebe, J. A. , & Lang, C. E. (2009). Active range of motion predicts upper extremity function 3 months after stroke. Stroke, 40, 1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. , Yetkin, F. Z. , Haughton, V. M. , & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic Resonance in Medicine, 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Brugger, F. , Galovic, M. , Weder, B. J. , & Kägi, G. (2015). Supplementary motor complex and disturbed motor control–a retrospective clinical and lesion analysis of patients after anterior cerebral artery stroke. Frontiers in Neurology, 6, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki, G. , & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304, 1926–1929. [DOI] [PubMed] [Google Scholar]
- Dai, X.‐J. , Gong, H.‐H. , Wang, Y.‐X. , Zhou, F.‐Q. , Min, Y.‐J. , Zhao, F. , … Xiao, X.‐Z. (2012). Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: A resting‐state fMRI study. Sleep Medicine, 13, 720–727. [DOI] [PubMed] [Google Scholar]
- Damoiseaux, J. , Rombouts, S. , Barkhof, F. , Scheltens, P. , Stam, C. , Smith, S. M. , & Beckmann, C. (2006). Consistent resting‐state networks across healthy subjects. Proceedings of the National Academy of Sciences, 103, 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, A. , Ghaffari, M. , Curchack, J. , Reiss, P. , Hyde, C. , Vannucci, M. , … Castellanos, F. X. (2008). Decomposing intra‐subject variability in children with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 64, 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Gibbons, J.D. , & Kendall, M. (1990) Rank correlation methods. Edward Arnold; New York, NY: Oxford University Press, London. [Google Scholar]
- Han, Y. , Lui, S. , Kuang, W. , Lang, Q. , Zou, L. , & Jia, J. (2012). Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One, 7, e28664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , Wang, J. , Zhao, Z. , Min, B. , Lu, J. , Li, K. , … Jia, J. (2011). Frequency‐dependent changes in the amplitude of low‐frequency fluctuations in amnestic mild cognitive impairment: A resting‐state fMRI study. NeuroImage, 55, 287–295. [DOI] [PubMed] [Google Scholar]
- Hanakawa, T. , Dimyan, M. A. , & Hallett, M. (2008). Motor planning, imagery, and execution in the distributed motor network: A time‐course study with functional MRI. Cerebral Cortex, 18, 2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, H. , & Liu, T. T. (2012). A geometric view of global signal confounds in resting‐state functional MRI. NeuroImage, 59, 2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Y. , Wu, X. , Hallett, M. , Chan, P. , & Wu, T. (2014). Frequency‐dependent neural activity in Parkinson's disease. Human Brain Mapping, 35, 5815–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T. , Zhao, Z. , Yan, C. , Lu, J. , Li, X. , Tang, C. , … Luo, Y. (2016). Altered spontaneous activity in patients with persistent somatoform pain disorder revealed by regional homogeneity. PLoS One, 11, e0151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman, C. S. , James, G. A. , Hamann, S. , Rajendra, J. K. , Pagnoni, G. , & Butler, A. J. (2012). Altered resting‐state effective connectivity of fronto‐parietal motor control systems on the primary motor network following stroke. NeuroImage, 59, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Krienen, F. M. , & Buckner, R. L. (2009). Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex, 19, 2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La, C. , Mossahebi, P. , Nair, V. A. , Young, B. M. , Stamm, J. , Birn, R. , … Prabhakaran, V. (2016). Differing patterns of altered Slow‐5 oscillations in healthy aging and ischemic stroke. Frontiers in Human Neuroscience, 10, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. S. , Han, M.‐K. , Kim, S. H. , Kwon, O.‐K. , & Kim, J. H. (2005). Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: Topographical correlation with clinical symptoms. NeuroImage, 26, 771–776. [DOI] [PubMed] [Google Scholar]
- Li, C. , Liu, C. , Yin, X. , Yang, J. , Gui, L. , Wei, L. , & Wang, J. (2014). Frequency‐dependent changes in the amplitude of low‐frequency fluctuations in subcortical ischemic vascular disease (SIVD): A resting‐state fMRI study. Behavioural Brain Research, 274, 205–210. [DOI] [PubMed] [Google Scholar]
- Loubinoux, I. , Carel, C. , Pariente, J. , Dechaumont, S. , Albucher, J.‐F. , Marque, P. , … Chollet, F. (2003). Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage, 20, 2166–2180. [DOI] [PubMed] [Google Scholar]
- Loubinoux, I. , Dechaumont‐Palacin, S. , Castel‐Lacanal, E. , De Boissezon, X. , Marque, P. , Pariente, J. , … Chollet, F. (2007). Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cerebral Cortex, 17, 2980–2987. [DOI] [PubMed] [Google Scholar]
- Murase, N. , Duque, J. , Mazzocchio, R. , & Cohen, L. G. (2004). Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology, 55, 400–409. [DOI] [PubMed] [Google Scholar]
- O'Reilly, J. X. , Beckmann, C. F. , Tomassini, V. , Ramnani, N. , & Johansen‐Berg, H. (2010). Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral Cortex, 20, 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C.‐h. , Chang, W. H. , Ohn, S. H. , Kim, S. T. , Bang, O. Y. , Pascual‐Leone, A. , & Kim, Y.‐H. (2011). Longitudinal changes of resting‐state functional connectivity during motor recovery after stroke. Stroke, 42, 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme, A. K. , Eickhoff, S. B. , Rottschy, C. , Fink, G. R. , & Grefkes, C. (2012). Activation likelihood estimation meta‐analysis of motor‐related neural activity after stroke. NeuroImage, 59, 2771–2782. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , MacMore, J. , & Vangel, M. (2009). Cerebellar stroke without motor deficit: Clinical evidence for motor and non‐motor domains within the human cerebellum. Neuroscience, 162, 852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck, M. L. , Maier, A. , Frank, Q. Y. , Duyn, J. H. , & Leopold, D. A. (2010). Neural basis of global resting‐state fMRI activity. Proceedings of the National Academy of Sciences, 107, 10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X. , Hu, X. , Zhou, S. , Xu, Y. , Zhang, Y. , Yuan, Y. , … Gao, J.‐H. (2015). Association of specific frequency bands of functional MRI signal oscillations with motor symptoms and depression in Parkinson's disease. Scientific Reports, 5, 16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear, C. (2010). Prediction of recovery of motor function after stroke. The Lancet. Neurology, 9, 1228–1232. [DOI] [PubMed] [Google Scholar]
- Sun, H. , Zou, X. , & Liu, L. (2013a). Epidemiological factors of stroke: A survey of the current status in China. Journal of stroke, 15, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L. , Yin, D. , Zhu, Y. , Fan, M. , Zang, L. , Wu, Y. , … Hu, Y. (2013b). Cortical reorganization after motor imagery training in chronic stroke patients with severe motor impairment: A longitudinal fMRI study. Neuroradiology, 55, 913–925. [DOI] [PubMed] [Google Scholar]
- Tang, C. , Zhao, Z. , Chen, C. , Zheng, X. , Sun, F. , Zhang, X. , … Jia, J. (2016). Decreased functional connectivity of homotopic brain regions in chronic stroke patients: A resting state fMRI study. PLoS One, 11, e0152875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerbeek, J. M. , Kwakkel, G. , van Wegen, E. E. , Ket, J. C. , & Heymans, M. W. (2011). Early prediction of outcome of activities of daily living after stroke a systematic review. Stroke, 42, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Voon, V. , Brezing, C. , Gallea, C. , & Hallett, M. (2011). Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Movement Disorders, 26, 2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Qin, W. , Liu, Y. , Zhang, Y. , Jiang, T. , & Yu, C. (2014). Altered resting‐state network connectivity in congenital blind. Human Brain Mapping, 35, 2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Yu, C. , Chen, H. , Qin, W. , He, Y. , Fan, F. , … Zang, Y. (2010). Dynamic functional reorganization of the motor execution network after stroke. Brain, 133, 1224–1238. [DOI] [PubMed] [Google Scholar]
- Ward, N. , Brown, M. , Thompson, A. , & Frackowiak, R. (2003). Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain, 126, 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher, A. , Kasess, C. , Gerstl, F. , Lanzenberger, R. , Moser, E. , & Windischberger, C. (2009). Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage, 47, 1408–1416. [DOI] [PubMed] [Google Scholar]
- Wu, Q. Z. , Li, D. M. , Kuang, W. H. , Zhang, T. J. , Lui, S. , Huang, X. Q. , … Gong, Q. Y. (2011). Abnormal regional spontaneous neural activity in treatment‐refractory depression revealed by resting‐state fMRI. Human Brain Mapping, 32, 1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, D. , Luo, Y. , Song, F. , Xu, D. , Peterson, B. S. , Sun, L. , … Fan, M. (2013a). Functional reorganization associated with outcome in hand function after stroke revealed by regional homogeneity. Neuroradiology, 55, 761–770. [DOI] [PubMed] [Google Scholar]
- Yin, D. , Song, F. , Xu, D. , Peterson, B. S. , Sun, L. , Men, W. , … Fan, M. (2012). Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLoS One, 7, e52727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, D. , Song, F. , Xu, D. , Sun, L. , Men, W. , Zang, L. , … Fan, M. (2014). Altered topological properties of the cortical motor‐related network in patients with subcortical stroke revealed by graph theoretical analysis. Human Brain Mapping, 35, 3343–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, D. , Yan, X. , Fan, M. , Hu, Y. , Men, W. , Sun, L. , & Song, F. (2013b). Secondary degeneration detected by combining voxel‐based morphometry and tract‐based spatial statistics in subcortical strokes with different outcomes in hand function. American Journal of Neuroradiology, 34, 1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, R. , Chien, Y. L. , Wang, H. L. S. , Liu, C. M. , Liu, C. C. , Hwang, T. J. , … Tseng, W. Y. I. (2014). Frequency‐specific alternations in the amplitude of low‐frequency fluctuations in schizophrenia. Human Brain Mapping, 35, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, Y. , Jiang, T. , Lu, Y. , He, Y. , & Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage, 22, 394–400. [DOI] [PubMed] [Google Scholar]
- Zhong, Y. , Lu, G. , Zhang, Z. , Jiao, Q. , Li, K. , & Liu, Y. (2011). Altered regional synchronization in epileptic patients with generalized tonic–clonic seizures. Epilepsy Research, 97, 83–91. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Jin, Y. , Wang, K. , Zhou, Y. , Feng, Y. , Yu, M. , & Jin, X. (2015). Frequency‐dependent changes in the regional amplitude and synchronization of resting‐state functional MRI in stroke. PLoS One, 10, e0123850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, X.‐N. , Di Martino, A. , Kelly, C. , Shehzad, Z. E. , Gee, D. G. , Klein, D. F. , … Milham, M. P. (2010). The oscillating brain: Complex and reliable. NeuroImage, 49, 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, X.‐N. , & Xing, X.‐X. (2014). Test‐retest reliabilities of resting‐state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neuroscience & Biobehavioral Reviews, 45, 100–118. [DOI] [PubMed] [Google Scholar]
- Zuo, X.‐N. , Xu, T. , Jiang, L. , Yang, Z. , Cao, X.‐Y. , He, Y. , … Milham, M. P. (2013). Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. NeuroImage, 65, 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 Illustration of Paralyzed Hand Function Assessment: ① the affected hand stabilizes a piece of paper on the table, with the unaffected hand controlling a shear to cut the paper; ② the affected hand holds a wallet, with the unaffected hand taking a coin from the wallet; ③ the affected hand holds an unfolded umbrella in the air for at least 10 seconds; ④ the affected hand controls a nail scissor to trim nails of the unaffected hand; and ⑤ the affected hand buttons the cuff of the unaffected side. The hand in dark denotes the affected hand.
Figure 2. Main effects of frequency band. The color bar indicates regions showing higher ReHo in slow‐5 versus slow‐4 (p <0.05, FDR‐corrected). L: left; R: right.
Table I. Demographics and clinical details of the subjects


