Abstract
Prior work has established that that an acute ethanol challenge that mimics high intensity alcohol consumption increased IL-6 and suppressed of IL-1β and TNFα mRNA in intoxication, with the opposite pattern seen in withdrawal. These experiments Sprague-Dawley rats to further extend these results across time course (from 45 min to 6 hours after ethanol), sex, and central versus peripheral expression. Furthermore, these data show that cannulation surgery may selectively modify the central neuroimmune response to ethanol. These findings highlight a unique plasticity of IL-6 that is specific to central structures and responsive to alterations by environmental factors.
Keywords: ethanol, cytokine, IL-6, surgery, hippocampus, amygdala
1. Introduction.
Alcoholism is a world-wide issue that impacts social, medical, and financial spheres. This disease is progressive and complicated, involving many signaling networks in the central and peripheral nervous system as well as other organs. An emergent connection between these systems and the development of alcohol use disorders is the immune system, the main effectors of which are cytokines. It has been shown that alcohol can activate cytokine signaling across the brain and periphery (Szabo and Saha, 2015, Crews et al., 2015), and that these alterations contribute to the pathology and etiology of alcoholism (de Timary et al., 2017, Crews et al., 2017, Montesinos et al., 2016, Cui et al., 2014).
The immune response to alcohol is a dynamic process that evolves as frequency of alcohol exposures increases. Prolonged alcohol exposure is associated with a shift toward a pro-inflammatory state in both peripheral structures (Laso et al., 2007) and the brain (He and Crews, 2008, Crews et al., 2017), yet paradoxically is associated with an increased susceptibility to infectious diseases and impaired recovery from wounds and trauma (Szabo and Mandrekar, 2009, Szabo and Saha, 2015). For instance, it has been shown that acute versus chronic ethanol results in opposing effects on the immune response to a challenge such as an injection of lipopolysaccharide (LPS). Specifically, acute alcohol dampened the human monocyte response to LPS, whereas chronic alcohol sensitized, host defense against this challenge (Mandrekar et al., 2009). As exposure to ethanol is repeated and becomes chronic, the immune response shows cytokine-specific plasticity in its adaptations. Our previous work has shown that a history of chronic ethanol consumption using an intermittent access procedure resulted in a blunted Interleukin-6 (IL-6) mRNA response to an acute ethanol challenge which was observed in the paraventricular nucleus of the hypothalamus, but not other brain regions or in other cytokines that were assessed (Doremus-Fitzwater et al., 2014). In contrast, cues (context + odor) associated with ethanol exposure produced the opposite effect, leading to a sensitized IL-6 gene expression response to a subthreshold dose of ethanol in the presence of ethanol-paired cues (Gano et al., 2017b). These adaptations can vary based on a number of factors and understanding their progression may help tease apart the mechanisms that drive the progression of alcohol-related health problems or alcoholism itself.
Because the immune system is in bidirectional communication with neurotransmitter and endocrine systems (Franco et al., 2007, Miller et al., 2013), cytokine signaling is also subject to modification by environmental factors such as injury (for a recent review, see Morganti-Kossmann et al., 2019), stress (Marsland et al., 2017, for reviews, see Maier and Watkins, 1998, Doremus-Fitzwater et al., 2018), and subject characteristics such as sex. For instance, while fewer women are impacted by alcohol use disorders, those that are may suffer an increased rate of brain damage and medical consequences (Erol and Karpyak, 2015, Agartz et al., 2003, Schweinsburg et al., 2003, Hommer et al., 2001) as compared to men. In rodent models, it has been shown that there are sex differences in the neuroimmune response to chronic ethanol, though it should be noted that most of these studies have focused on cytokine expression after ethanol clearance (i.e., during acute withdrawal or beyond). Several rodent studies have found CNS gene and protein expression changes during withdrawal that may indicate greater damage in females (Alfonso-Loeches et al., 2013, Wilhelm Clare et al., 2015, Hashimoto and Wiren, 2008). Wilhelm and colleagues have shown that females expressed greater levels of genes associated with inflammatory signaling (IL-6 and p38 MAPK) and astrocytic-related activity (connective growth factor - CTGF - and sphingosine kinase 1 - Sphk1) in the medial prefrontal cortex during peak withdrawal following 72 hours of continuous ethanol inhalation (Wilhelm Clare et al., 2015). Another chronic ethanol intoxication study found that female mice in peak withdrawal showed greater inducible nitric oxide synthase (iNOS) expression and glial fibrillary acidic protein (GFAP) protein levels in the brain than males. Moreover, females showed higher central caspase-3 expression as well as lower levels of neuronal nuclei (NeuN) and microtubule associated protein (MAP)-2 protein levels, indicating greater neuronal injury (Alfonso-Loeches et al., 2013). Much less is known about whether these sexual dimorphisms in inflammatory signaling are present after just a single ethanol administration, the manner in which they adapt as the exposure becomes chronic, and whether changes observed in withdrawal may differ from those seen at other phases of intoxication.
Another variable that may impact the cytokine response to ethanol is the presence of any other immune disturbances. It has been shown that traumatic brain injury (TBI) can cause changes in the cytokine response to ethanol. Various studies have demonstrated these effects to be specific to injury, timing and dose of ethanol administration, and the timing of sampling. For instance, acute alcohol exposure prevented the resolution of IL-1β, IL-6, and tumor necrosis factor alpha (TNFα) responses to mild TBI 24 h following the injury (Teng and Molina, 2014). Yet other work shows that pre-treatment with an acute dose of ethanol (5 g/kg intragastric) attenuated the inflammatory response to TBI, as evidenced by suppressed IL-6 and monocyte chemoattractant protein (MCP)-1 levels during acute intoxication (Goodman et al., 2013). Another study demonstrated that pre-treatment with ethanol (low and moderate doses resulting in ethanol concentrations of 100–220 mg/dL) attenuated the IL-1β and TNFα response to a moderate cortical impact injury (Gottesfeld et al., 2002). More recent work from our lab has utilized large-molecule microdialysis to assess evoked cytokine and chemokine responses in the hippocampus, demonstrating long-lasting effects of adolescent ethanol exposure on the trajectory of inflammatory responses to an acute ethanol challenge (Gano et al., 2019). Although it would not be prudent to equate cannulation with TBI due to external forces, these findings prompt some consideration of the inherent change in immunological state that occurs due to the use of invasive approaches. Specifically, tissue damage and the resultant neuroimmune response may alter the kinetics of neuroimmune changes evoked by later ethanol exposure. Thus, understanding damage-ethanol interactions may have important implications for pre-clinical studies utilizing invasive neural manipulations such as stereotaxic surgery for cannulation, microinjection, or ablation.
In our previous work, acute ethanol challenge in adult male rats evinced a highly reproducible neuroimmune response that was cytokine, region, and timing particular. Specifically, following an acute supra-binge ethanol challenge resulting in blood ethanol concentrations (BECs) of 200 mg/dL and higher, brain gene expression levels of IL-6 were elevated and IL-1β and TNFα were suppressed, a pattern that reversed after ethanol clearance (i.e., during acute withdrawal). In contrast, liver and spleen levels of these cytokines escalated across time as ethanol was cleared and were all significantly elevated in withdrawal (Doremus-Fitzwater et al., 2014). Nevertheless, a limitation of existing studies is that very little is known about the rapid timecourse of cytokine gene induction after acute ethanol and its temporal coherence between the brain and peripheral organs, which formed the primary goal of Experiment 1. Though some of these findings in males have since been replicated in other studies (Gano et al., 2016) and across strains (Gano et al., 2017a), few studies have examined the effects of acute ethanol on female cytokine responses. Thus, the goal of Experiment 2 was to determine whether rapid changes in neuroimmune gene expression produced during acute ethanol intoxication would differ between males and females. Finally, in Experiment 3 cannulation surgery was performed to assess the influence of invasive neuroscience techniques (e.g. cannulation for viral infusion, microdialysis, microinjection, etc.) on neuroimmune gene expression changes produced by acute ethanol challenge.
2. Methods.
2.1. Subjects.
Adult male (all Experiments) and female (Experiment 2) Sprague Dawley rats (280–320 g) were ordered from Harlan (Frederick, MD) and allowed 2 weeks to acclimate to the colony (22±1°C with 12:12 light–dark cycle, lights on 0700) prior to experimental manipulation. Rats were pair-housed in standard Plexiglas cages with ad libitum access to food and water. Prior to experimentation, all rats were handled (3–5 min) for 2 days. In Experiment 3, rats were separated into individual housing immediately after surgery (the non-surgical controls were likewise separated into individual housing at that time) and remained individually housed until tissue was collected. These procedures were approved by the Institutional Animal Care and Use Committee at Binghamton University and rats were treated in accordance with Public Health Service (PHS) policy.
2.2. Ethanol administrations.
All drug solutions were mixed fresh daily the morning of intubations or injections. In Experiment 1, 95% ethanol was mixed with sterile physiological saline (0.9%, Teknova, Hollister, CA) for intraperitoneal (i.p.) administration (20% v/v), with the same saline used for all vehicle injections. In Experiments 2 and 3, 95% ethanol was mixed with tap water (30% v/v) for intragastric intubation, with vehicle animals receiving equivolumetric tap water intubations.
2.3. Tissue collection.
Trunk blood, brains, and spleens were harvested using rapid, unanesthetized decapitation. Trunk blood was collected in EDTA-coated Vacutainers (BD Vacutainers, VWR cat. no. VT6450, Radnor, PA), and plasma was separated via refrigerated centrifugation and stored at −20°C until time of use. Brains were extracted, immediately submerged in methylbutane on dry ice for 10 seconds in order to flash-freeze, and then stored at −80 °C until subsequent dissection. Brain dissection took place on a freezing cryostat with temperature maintained at −15 to −20 °C. Structures of interest were located using an atlas (Watson and Paxinos, 2005) and excised using biopsy punches. These samples were placed into 2.0 mL Eppendorf tubes for later extraction and returned to −80°C, thereby avoiding a complete thaw of the tissue prior to RNA extraction. In Experiment 1, punches were separated by hemisphere, with one side allocated to RNA analysis, and other side examined for protein. In Experiment 2, tissue was pooled from both hemispheres for each structure. In Experiment 3, tissue was separated into hemispheres and analyzed separately for effects on the cannulated side vs. those on the uncannulated side. As no significant effects of side of cannulation emerged here, data shown were pooled across side.
2.4. Blood ethanol concentrations (BECs).
Ethanol concentrations were determined in 5-μl aliquots using an Analox AM-1 alcohol analyzer (Analox Instruments, Lunenburg, MA). The machine was calibrated every 15 samples using the appropriate (50, 100, or 200) mg% industry standard, with output recorded in milligram per deciliter (mg/dL). The floor of assay sensitivity using the Analox is 12–15 mg/dL, as evidenced by background measurements obtained from rats never exposed to ethanol. As such, measurements at or below this threshold were interpreted as zero values.
2.5. Plasma corticosterone (CORT).
Concentrations of CORT were determined using a commercially available EIA kit (Cat No: ADI-901–097; Enzo Life Sciences, Farmingdale, NY) according to manufacturer’s instructions, with the exception that samples were heat-inactivated to denature endogenous corticosteroid binding globulin (CBG) by immersion in 75°C water for 60 min (Buck, Hueston, Bishop, & Deak, 2011). The CORT assay had a sensitivity of 27.0 pg/mL, an inter-assay variability of 28.1%, and an intra-assay variability of 2.9%.
2.6. RT-PCR.
Gene expression in the brain and in the spleen was analyzed using real-time RT-PCR as described elsewhere (Gano et al., 2017a). Briefly, tissue was homogenized using 500 μL Trizol RNA reagent and 5 mm stainless steel beads in 2.0 mL Eppendorf tubes using a Tissue Lyser II (Qiagen). Total RNA was extracted using a RNeasy mini kit (Qiagen #74106), and cDNA synthesis including a DNAse treatment step was performed using a QuantiTect Reverse Transcription Kit (Cat. No. 205313, Qiagen). cDNA amplification was performed using the CFX384 Real-Time PCR Detection System (Bio-Rad, #185–5485). All data were analyzed using the 2−ΔΔC(T) method (Livak and Schmittgen, 2001) and are shown as % of the control group for the particular experiment. All gene expression data were adjusted to a housekeeper gene (Glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) after a separate analysis ensured the stability of GAPDH across experimental groups. All primer information is reported in Table 1.
Table 1.
Primers, accession numbers, and sequences used in real-time RT-PCR for all gene expression studies.
| Primer | Accession # | Oligo | Sequence |
|---|---|---|---|
| Gapdh1 | NM_017008 | Forward | 5’-ATGACTCTACCCACGGCAAG-3’ |
| Reverse | 5’-AGCATCACCCCATTTGATGT-3’ | ||
| IκBα2 | NM_080899 | Forward | 5’-CTGTTGAAGTGTGGGGCTGA-3’ |
| Reverse | 5’-AGGGCAACTCATCTTCCGTG-3’ | ||
| IL-13 | NM_031512 | Forward | 5’-AGGACCCAAGCACCTTCTTT-3’ |
| Reverse | 5’-AGACAGCACGAGGCATTTTT-3’ | ||
| IL-64 | NM_012589 | Forward | 5’-TAGTCCTTCCTACCCCAACTTCC-3’ |
| Reverse | 5’-TTGGTCCTTAGCCACTCCTTC-3’ | ||
| TNFα5 | NM_012675 | Forward | 5’-GGGGCCACCACGCTCTTCTG-3’ |
| Reverse | 5’-CGACGTGGGCTACGGGTTG-3’ |
Glyceraldehyde 3-phosphate dehydrogenase;
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha;
Interleukin-1 beta (β);
Interleukin-6;
Tumor necrosis factor alpha (α).
2.7. IL-6 protein.
In Experiment 1, tissue for protein measurement was homogenized in a Tris buffer (20 mM Tris, 2 mM EDTA, 1 % Triton X, 1 protease inhibitor tablet/10 mL total buffer) for 5 seconds and then assayed using a commercially available IL-6 protein ELISA (R&D Systems, #R6000B) in accordance with manufacturer’s instructions. Plasma protein was assayed directly. Total protein quantification was performed using the Bradford method (Bradford, 1976) for the hippocampus, and a BCA (Fisher Scientific, # 23225) for the spleen, amygdala, and plasma. Data are presented adjusted to total protein (pg IL-6 per 100 μg total protein). The IL-6 assay had a sensitivity of 31.2 pg/mL, an inter-assay variability of 26.0 %, and an intra-assay variability of 2.8%.
2.8. Statistics.
Data were analyzed using ANOVAs particular to the study design as noted in each results section. All post hoc testing was performed using Fisher’s LSD test. The criterion for significance was set at an α level of 0.05 for all tests. Power analyses were performed for all significant effects and are reported as partial eta squared (ηp2).
2.9. Experiment 1 specific methods.
Our prior work has indicated that while changes seen during withdrawal from an acute ethanol challenge are largely similar to those seen in withdrawal from chronic intermittent ethanol, a unique cytokine signature occurs at the time of peak blood ethanol concentrations (~3 h after challenge). Having previously examined cytokine patterns at the peak of intoxication (3 h after injection) and withdrawal (~15–18 h after injection), the goal of the present study was to examine a more detailed time course of intoxication-related cytokine changes in the brain and periphery. Adult male Sprague Dawley rats (N = 40; n = 8 per group) were distributed across a one factor design with 5 levels (VEH vs. 45 min vs. 90 min vs. 180 min vs 360 min). Rats were given an acute 4 g/kg intraperitoneal (i.p.) injection of ethanol (or sterile physiological saline for the VEH group). Trunk blood, brain tissue, and spleens were collected 45, 90, 180, or 360 min after injection for assessment of plasma corticosterone, blood ethanol concentrations, and brain/spleen gene expression and protein analysis. To minimize the number of animals used, rats assigned to the VEH group were distributed across timepoints and then collapsed across this variable after a preliminary analysis was performed to ensure that no effects of time emerged.
2.10. Experiment 2 specific methods.
The results of Experiment 1 indicated that the 3 h time point during intoxication was a time at which the cytokine signature of intoxication was most apparent. Experiment 2 focused on this time point in order to assess sex differences in this response in adult male and female rats. Adult male and female Sprague Dawley rats (N = 32; n = 8 per group) were distributed in a 2 (Sex: Male vs. Female) × 2 (Drug: VEH vs. EtOH) between-subjects design. Rats were given a 5 g/kg intragastric (i.g.) intubation of either ethanol or equivolumetric vehicle. Since adult females rats display faster ethanol metabolism than males (Varlinskaya and Spear, 2004), the dose was increased from the previous study to ensure that BECs achieved an effective level for neuroimmune gene induction. Given the increase in dose, the change in route of administration from i.p. (Exp 1) to i.g. (Exp 2,3) was determined to more closely mimic the typical route of ethanol absorption in humans. Trunk blood and brains were collected 3 h after the intubation for assessment of plasma corticosterone, blood ethanol concentrations, and brain gene expression analysis.
2.11. Experiment 3 specific methods.
Experiments 1 and 2 replicated findings previously published from our group, specifically, the unique pattern of cytokine change that emerges during intoxication consisting of an elevation of IL-6 and a suppression of IL-1β and TNFα in central limbic structures. This pattern was found to be durable across dose, route of administration, and sex, and to be largely independent of changes observed in the periphery (spleen, plasma). The goal of the final experiment was to examine whether cannulation surgery such as that needed for in vivo cytokine sampling would alter rapid changes in neuroimmune gene expression observed during acute ethanol intoxication. Adult male Sprague Dawley rats (N = 50; n = 6–8 per group) were distributed in a 2 (Surgery: Intact vs. Cannulated) × 3 (Group: VEH vs. 3 h vs. 12 h) design. Cannulated animals underwent a unilateral hippocampal cannulation. Briefly, rats were anesthetized with isoflurane (1–4% in oxygen) and were administered analgesia (0.05 mg/kg Buprenorphine, Reckitt Benckiser Healthcare Ltd, Hull, England). Guide cannulae (EICOM, CA) were implanted dorsal to the hippocampus (from Bregma: AP −5.28, ML +4.84, DV −3.30) and secured using 2–3 skull screws next to the cannula and dental acrylic (Butler Schein, Ohio). The guide cannulae were located above the hippocampus, as in our other work (Gano et al., 2019) without entering the structure itself. Cannulated rats were allowed 7 days to recover from surgery before ethanol administration; the Intact surgery control group was similarly handled for weights but otherwise remained in the home cage at this time. During ethanol testing, both groups were administered either 5 g/kg i.g. ethanol or given a vehicle intubation. Trunk blood and brain tissue were collected either 3 or 12 h after intubation for assessment of plasma corticosterone, blood ethanol concentrations, and brain gene expression analysis. The VEH group brains were collected at both 3 (n = 4) and 12 h (n = 4) after intubation and, after ensuring the time points did not create differences in any measures, were collapsed into a single control group (n = 8).
3. Results.
3.1. Experiment 1.
3.1.1. Blood ethanol concentrations and plasma corticosterone.
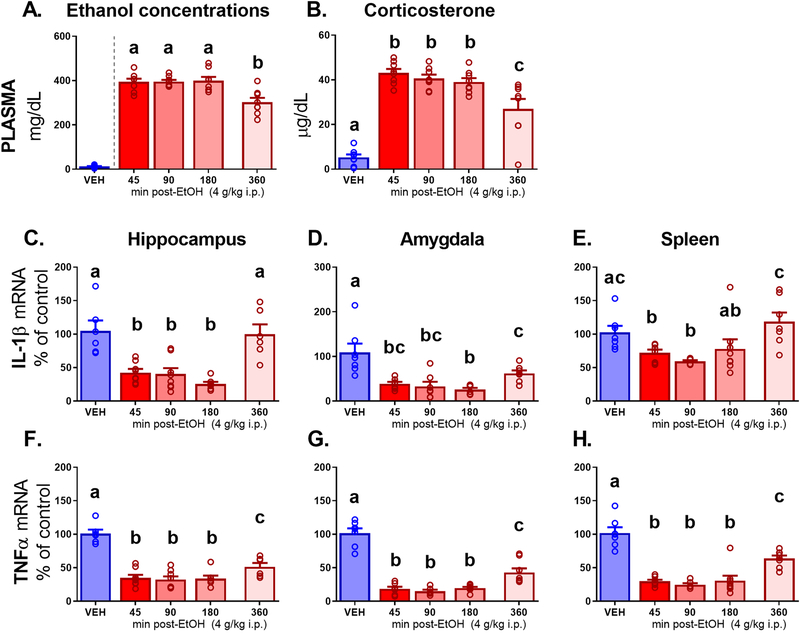
After ensuring that VEH group ethanol concentrations were below the level of detection, BECs (Figure 1A) were assessed in rats that received an ethanol injection (one-way between-subjects ANOVA, 45 min vs. 90 min vs. 180 min vs. 360 min, F3, 28 = 8.97, p < 0.001, ηp2 =0.49). Peak levels were maintained across the first three groups (394.33 ± 14.64 in 45 min group, 395.10 ± 8.39 in 90 min group, and 399.15 ± 17.57 in the 180 min group), with a significant (p < 0.001 for all) decline observed in the 360 min group (302.26 ± 19.81). A similar pattern was observed in plasma corticosterone. Concentrations of CORT (Figure 1B) were assessed using a one-way between-subjects ANOVA (VEH vs. 45 min vs. 90 min vs. 180 min vs. 360 min). CORT was elevated (F4, 34 = 66.28, p < 0.0001, ηp2 = 0.89) in all ethanol-injected animals as compared to vehicle (p < 0.0001 for all post-hocs). At the final 360 min time point, CORT levels began to decline (difference from all previous time points, p < 0.01), but did not reach VEH-like levels.
Figure 1.
Experiment 1 plasma measures and brain gene expression in adult males acquired 45, 90, 180, or 360 min after an intraperitoneal (i.p.) injection of 4 g/kg ethanol (EtOH) or vehicle (VEH). Plasma measures shown include (A) ethanol concentrations and (B) corticosterone. Gene expression outcomes in the hippocampus, amygdala, and spleen are shown for (C, D, E) Interleukin-1β - IL-1β; and (F, G, H) Tumor necrosis factor alpha - TNFα. Letters indicate differences divulged by post hoc testing of a significant main effect of Group (groups that share a letter in common did not differ statistically, whereas differences between groups are indicated by letters that do not match).
3.1.2. Brain and peripheral cytokines.
IL-1β and TNFα mRNA.
All cytokine data were analyzed using a between-subjects one-way ANOVA (VEH vs. 45 min vs. 90 min vs. 180 min vs. 360 min). The same pattern of suppression due to ethanol was observed for TNFα and IL-1β across peripheral and central structures. In the hippocampus, IL-1β (Figure 1C) expression was suppressed (F4, 30 = 13.43, p = 0.0001, ηp2 = 0.64) in the 45, 90, and 180 min groups as compared to VEH (all p < 0.0001). At 360 min, IL-1β levels returned to baseline-like levels similar to VEH and were significantly higher than in the previous time points (all p < 0.0001). In the amygdala, IL-1β (Figure 1D) was suppressed (F4, 31 = 10.17, p < 0.0001, ηp2 = 0.57) at 45, 90, 180 and 360 min as compared to VEH (all p < 0.0001). The 45, 90 and 180 min groups did not differ from each other. In the 360 min group, levels were different from the VEH and 180 min groups (p < 0.01 and 0.05, respectively) but did not differ from the 45 or 90 min time point group. In the spleen, IL-1β (Figure 1E) was suppressed (F4, 31 = 4.95, p < 0.01, ηp2 = 0.40) at 45 and 90 min as compared to VEH (p < 0.05 and 0.01 respectively). At 180 min, levels were similar to both the previous time points and VEH, and at 360 min IL-1β was expressed at levels higher than all other time points (p < 0.01) and did not differ from VEH.
TNFα in the hippocampus (Figure 1F) was suppressed (F4, 30 = 29.68, p = 0.0001, ηp2 = 0.80) by ethanol at 45, 90, and 180 min (p < 0.0001). At the 360 min time point, TNFα showed a shift toward return to baseline that was observed to be higher than the previous time points (all p < 0.05), but still lower than VEH (p < 0.0001). In the amygdala, TNFα (Figure 1G) was suppressed (F4, 30 = 54.94, p < 0.0001, ηp2 = 0.88) in all time point groups as compared to VEH controls (all p < 0.0001). Similar to the hippocampus, the last 360 min time point group displayed a partial return to baseline, in that expression was elevated compared to the 45, 90 and 180 time point groups (all p < 0.01) but was still lower than VEH levels (p < 0.0001). In the spleen, TNFα (Figure 1H) was suppressed (F4, 32 = 32.68, p < 0.0001, ηp2 = 0.80) in all groups as compared to VEH (all p < 0.0001). The last time point group displayed a partial return to baseline levels as expression was elevated compared to 45, 90, and 180 min (all p < 0.001).
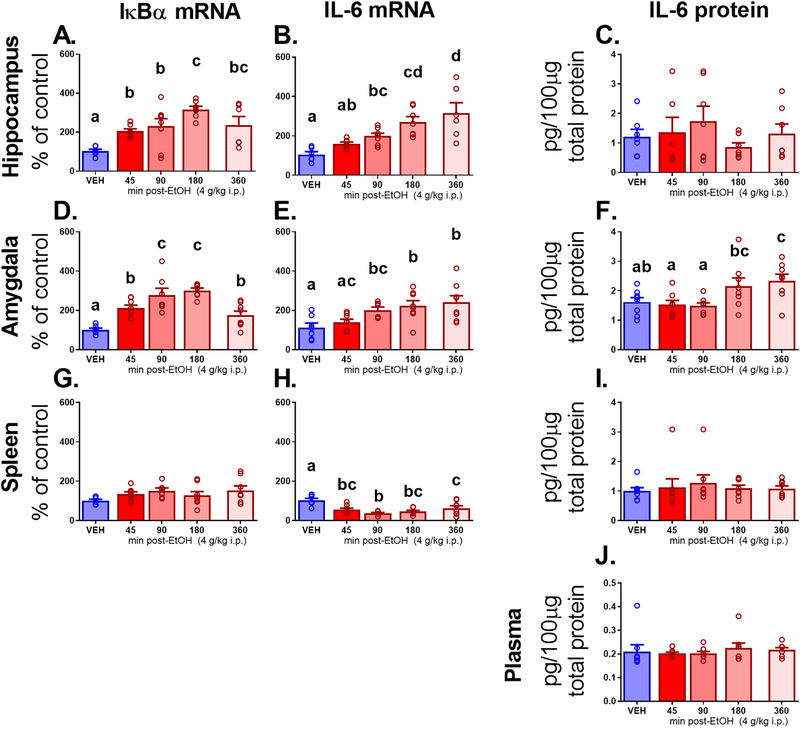
IL-6 and IκBα.
Unlike TNFα and IL-1β, IL-6 and IκBα differed in central versus peripheral expression. In the hippocampus, IκBα mRNA (Figure 2A) showed an increase in expression across time after ethanol (F4, 29 = 6.92, p < 0.0001, ηp2 = 0.49), with all groups elevated above VEH (all p < 0.05), and a peak seen in the 180 min group. Signs of decreasing expression emerged in the 360 min group. Likewise, in the amygdala, IκBα gene expression (Figure 2D) showed a similar pattern. Levels were elevated progressively more across all ethanol groups (F4, 32 = 14.78, p < 0.0001, ηp2 = 0.65), with all groups higher than VEH (p < 0.05), until the 360 min group, which showed a return to levels that were higher than VEH, but equivalent to the 45 min group. The spleen, however, showed no IκBα gene expression effects (Figure 2G).
Figure 2.
Experiment 1 brain, peripheral, and plasma measures pertaining to Interleukin-6 (IL-6) and a gene expression marker of Nf-κB activity, Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα). Gene expression is shown in the hippocampus, amygdala, and spleen for (A, D, G) IκBα and (B, E, H) IL-6, respectively. Additionally, IL-6 protein expression shown adjusted to total protein is also shown in these structures for IL-6 (C, F, I) as well as in (J) plasma. Letters indicate differences divulged by post hoc testing of a significant main effect of Group (groups that share a letter in common did not differ statistically, whereas differences between groups are indicated by letters that do not match).
IL-6 mRNA effects were again similar in the central structures. In the hippocampus, IL-6 (Figure 2B) was progressively more elevated in all ethanol-injected groups (F4, 29 = 9.44, p < 0.0001, ηp2 = 0.57), with 90, 180, and 360 min showing significant elevation compared to VEH (p < 0. 05, 0.001, 0.0001, respectively), and peak observed at 360 min. In the amygdala, IL-6 (Figure 2E) was also elevated at later time points after ethanol (F4, 32 = 5.02, p < 0.01, ηp2 = 0.38), with steadily elevated levels observed at 90, 180, and 360 min as compared to VEH (all p < 0.01). Conversely, in the spleen, IL-6 expression (Figure 2H) was suppressed (F4, 32 = 8.08, p < 0.001, ηp2 = 0.50) in all groups that received ethanol as compared to VEH (all p < 0.01).
IL-6 protein expression was also assessed in these structures as well as in plasma. There were no effects on IL-6 protein in the hippocampus (Figure 2C), spleen (Figure 2I), or plasma (Figure 2J). However, there was a main effect of Group on IL-6 expression in the amygdala (F4, 35 = 4.85, p < 0.01, ηp2 = 36; Figure 2F). There was progressive elevation of IL-6 across time after ethanol, with elevation observed at 180 min as compared to 45 and 90 min (p < 0.01 and < 0.05, respectively), and peak observed at 360 min, elevated beyond levels observed in VEH (p < 0.05), 45 min, and 90 min (both p < 0.01). Correlation analysis indicated that gene expression was not related to protein in any structure, and neither did plasma IL-6 protein correlate with IL-6 protein presence in the hippocampus, amygdala, or spleen.
3.2. Experiment 2.
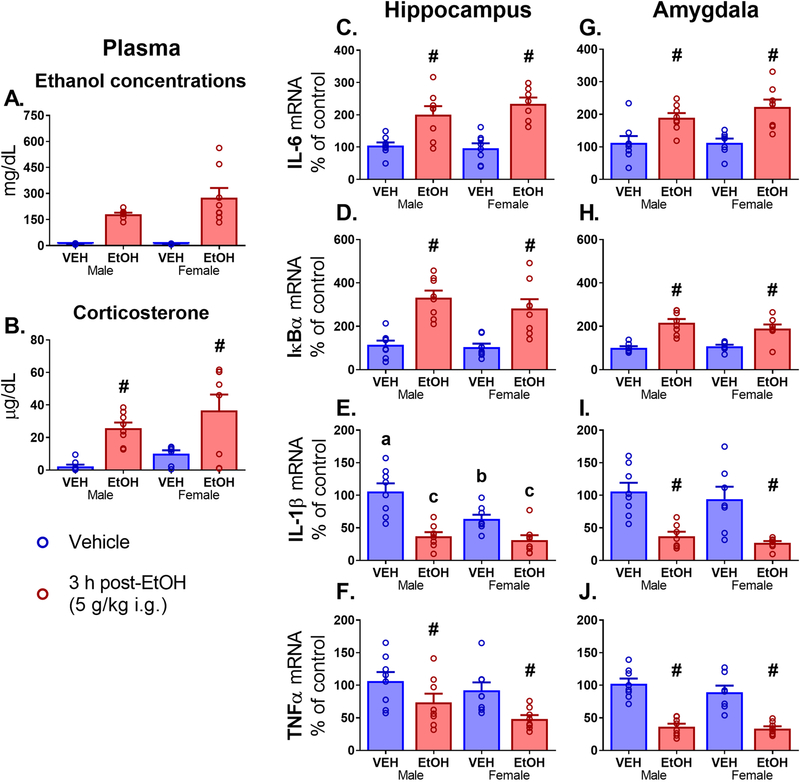
3.2.1. Plasma corticosterone and blood ethanol concentrations.
After verifying that the two VEH groups had no detectable ethanol, BEC differences between Male and Female EtOH groups were assessed using a t-test (Fig. 3A). There were no differences found in Male and Female BECs. Plasma CORT levels (Fig. 3B) showed a main effect of Drug (F1, 28 = 22.27, p < 0.0001, ηp2 = 0.44), with EtOH-intubated animals showing elevated CORT levels (p < 0.0001) as well as a trend for a main effect of Sex (p = 0.087). It should be noted that the substantially reduced BECs observed in Experiment 2 as compared to Experiment 1 reflect the route of ethanol administration.
Figure 3.
Experiment 2 plasma measures and brain gene expression in adult males and females 3 hours after an intragastric (i.g.) intubation of vehicle (VEH) or 5 g/kg ethanol (EtOH). Plasma measures shown include (A) ethanol concentrations and (B) corticosterone. Gene expression outcomes in the hippocampus and amygdala are shown for (C, G) Interleukin-6 - IL-6 gene expression; (D, H) Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha - IκBα; (E, I) Interleukin-1β - IL-1β; and (F, J) Tumor necrosis factor alpha - TNFα. Significant effects of Drug are indicated with a pound sign (#) above the EtOH groups indicating significant difference form the VEH groups, and letters indicate differences divulged by post hoc testing of a significant interaction (groups that share a letter in common did not differ statistically, whereas differences between groups are indicated by letters that do not match).
3.2.2. Brain cytokine gene expression
Hippocampus.
All cytokine data were analyzed using a between-subjects 2 (Sex: Male vs. Female) × 2 (Drug: VEH vs EtOH) ANOVA. There was a main effect of Drug in the hippocampus for IL-6 (Fig. 3C), IκBα (Fig. 3D), and TNFα (Fig. 3F) expression (F1, 27 = 39.48, p < 0.0001, ηp2 = 0.59; F1, 28 = 43.53, p < 0.0001, ηp2 = 0.61; F1, 28 = 10.43, p < 0.0001, ηp2 = 0.27, respectively). EtOH-intubated animals showed elevated IL-6 and IκBα levels and suppressed TNFα levels as compared to their VEH control (p < 0.0001, 0.0001, 0.01, respectively). A Drug × Sex interaction was found for IL-1β (Fig. 3E) expression (F1, 28 = 4.30, p < 0.05, ηp2 = 0.13), and Fishers LSD post hoc analysis revealed both Male and Female EtOH-intubated animals showed lower levels of IL-1β as compared to both VEH groups (p < 0.05 for all comparisons). Moreover, VEH-intubated Females showed lower levels of IL-1β than VEH-intubated Males (p < 0.01).
Amygdala.
In the amygdala, there was a main effect of Drug for IL-6, IκBα, IL-1β and TNFα (Fig. 3G–J) expression (F1, 27 = 25.64, p < 0.0001, ηp2 = 0.49; F1, 27 = 47.43, p < 0.0001, ηp2 = 0.64; F1, 26 = 32.60, p < 0.0001, ηp2 = 0.56; F1, 27 = 76.88, p < 0.0001, ηp2 = 0.74, respectively). EtOH-intubated animals showed elevated levels of IL-6 and IκBα and lowered levels of IL-1β and TNFα, as compared to VEH (all p < 0.0001). There were no main effects or interactions involving the Sex variable.
3.3. Experiment 3.
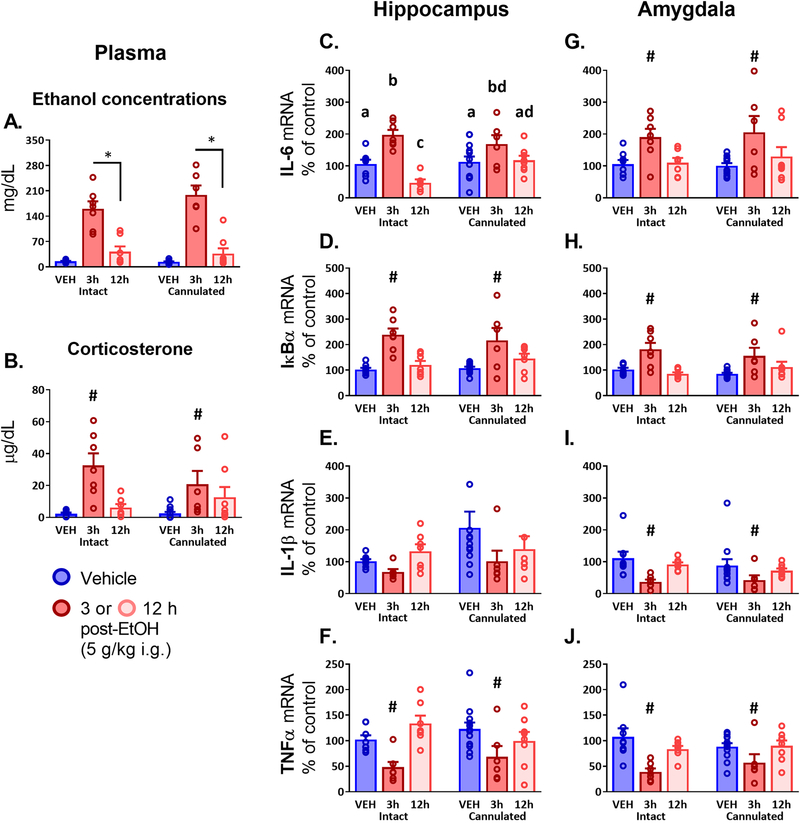
3.3.1. Plasma corticosterone and blood ethanol concentrations.
After ensuring that VEH groups did not have detectable ethanol levels, BECs (Figure 4A) were compared using a between-subjects 2 (Surgery: Intact vs. Cannulated) × 2 (Group: 3 h vs. 12 h) ANOVA. There was a main effect of Group (F1, 24 = 54.56, p < 0.0001, ηp2 =0.69), with higher ethanol concentrations observed in the 3 h group as compared to 12 h. Plasma corticosterone (Figure 4B) was analyzed using a between-subjects 2 (Surgery: Intact vs. Cannulated) × 3 (Group: VEH vs. 3 h vs. 12 h) ANOVA (after a t-test confirmed that the VEH groups could be collapsed across time points into a single VEH group; this analysis was also performed for all other measures and was not significant in any case). There was a main effect of Group (F2, 43 = 13.98, p < 0.0001,ηp2 = 0.39), with levels in the 3 h group observed to be higher than those of both VEH and 12 h groups (both p < 0.0001).
Figure 4.
Experiment 3 plasma measures and brain gene expression in adult males 3 or 12 hours after an intragastric (i.g.) intubation of vehicle (VEH) or 5 g/kg ethanol (EtOH), assayed in groups that have either been given a unilateral hippocampal cannulation (Cannulated) or remained surgically unmanipulated (Intact). Plasma measures shown include (A) ethanol concentrations and (B) corticosterone. Gene expression outcomes in the hippocampus and amygdala are shown for (C, G) Interleukin-6 - IL-6 gene expression; (D, H) Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha - IκBα; (E, I) Interleukin-1β - IL-1β; and (F, J) Tumor necrosis factor alpha - TNFα. In panel (A) displaying ethanol concentrations, an asterisk (*) is used to indicate a significant difference between the ethanol-intubated animals only, excluding the VEH group. Group-specific differences emerging in post hoc testing for a significant effect of Drug are indicated with a pound sign (#), indicating that the 3 h EtOH group differed significantly from both VEH and the 12 h group. Letters indicate differences divulged by post hoc testing of a significant interaction (groups that share a letter in common did not differ statistically, whereas differences between groups are indicated by letters that do not match).
3.3.2. Brain cytokine gene expression.
All cytokine data were analyzed using between-subjects 2 (Surgery: Intact vs. Cannulated) × 3 (Group: VEH vs. 3 h vs. 12 h) ANOVAs. In the hippocampus, IL-6 (Figure 4C) expression exhibited an interaction effect of Surgery and Group (F2, 41 = 3.63, p < 0.05, ηp2 = 0.15). In the Intact group, rats showed an elevation of IL-6 in the 3 h group compared to VEH and 12 h (p < 0.0001 for both), and a suppression in the 12 h compared to VEH (p < 0.05). In the Cannulated group, peak IL-6 levels in the 3 h group were higher than its VEH comparator (p < 0.05), but the 12 h group did not differ from 3 h or VEH. The peak levels between Intact and Cannulated rats at 3 h did not differ. The 12 h groups were different, with higher levels seen in the Cannulated animals compared to Intact, indicating a failure to resolve the IL-6 response to ethanol in the cannulated hippocampus. Amygdala IL-6 (Figure 4G) displayed a main effect of Group (F2, 42 = 8.43, p < 0.001, ηp2 =0.29), with higher levels of IL-6 observed in the 3 h group than VEH and 12 h (p < 0.001 and p < 0.01, respectively).
Patterns of IκBα were the same in the hippocampus (Figure 4D) and amygdala (Figure 4H), with main effects of Group (F2, 41 = 19.30, p < 0.0001, ηp2 = 0.48, F2, 42 = 11.72, p < 0.0001, ηp2 =0.36, respectively) showing that at 3 h, IκBα was elevated above VEH and 12 h groups (all p < 0.001). There were no effects on IL-1β in the hippocampus (Figure 4E), though increased variability was observed in Cannulated animals as compared to Intact controls. In the amygdala, there was a main effect of Group on IL-1β (Figure 4I) expression (F2, 42=6.82, p < 0.05, ηp2 = 0.25) with suppression seen in the 3 h group compared to both VEH and 12 h (p < 0.001 and p < 0.05, respectively). TNFα expression patterns in the hippocampus (Figure 4F) and amygdala (Figure 4J) were the same, with main effects of Group (F2, 42 = 8.96, p < 0.001, ηp2 = 0.30, F2, 42 = 10.60, p < 0.001, ηp2 =0.34, respectively) revealing suppression in the 3 h group as compared to VEH controls (p < 0.001 for both) and 12 h rats (p < 0.001 and 0.01, respectively).
4. Discussion
There is accumulating evidence that the most immediate effects of ethanol on neuroimmune status involve the induction of key cytokines IL-1β, TNF-α, and IL-6 (Doremus-Fitzwater et al., 2014; Gano et al., 2016; 2017a, 2019; Lippai et al., 2013). This has been observed in the chronic ethanol literature, and work from our laboratory and others have corroborated these results using acute administration models. The use of an acute model of alcohol administration has allowed us to examine the naïve response of an organism to alcohol, particularly the native role of these cytokines across a single cycle of ethanol intoxication and withdrawal. In particular, initial doses of ethanol yielding BECs in the range of High Intensity Drinking, a particularly dangerous pattern of alcohol consumption, seem especially capable of inducing “waves” of neuroimmune gene expression across the intoxication-withdrawal cycle. Indeed, we have previously demonstrated that across the rise and fall of blood ethanol concentrations, individual cytokines follow unique patterns of gene expression, though these studies utilized relatively crude time courses with only 1–2 time points being examined. Specifically, the rise of IL-6 that is in conjunction with the rise of BECs and CORT heralds the subsequent increase of IL-1β and TNFα as BECs are cleared. We conducted the studies discussed here to further probe the unique and divergent expression patterns of these cytokines and examine other factors that may differentially influence their expression, including biological sex and prior surgical history.
In Experiment 1, we examined a detailed time course of cytokine gene expression in the brain and spleen following an acute dose of ethanol with more temporal resolution than in prior work. These results largely recapitulated findings from our previous experiments (Doremus-Fitzwater et al., 2014), showing IL-1β and TNFα mRNA to be suppressed at the time of intoxication in both central (hippocampus, amygdala) and peripheral (spleen) structures. However, similar to Experiment 2, IL-6 again showed a unique response signature in both protein and mRNA analysis. Whereas in the brain, IL-6 expression was increased in intoxication, IL-6 was suppressed in the spleen at the same time points. Again, similar findings were found previously in the hippocampus and spleen 3 hours after an alcohol challenge (Doremus-Fitzwater et al., 2014). IL-6 changes were paralleled by IκBα, a marker of Nf-κB activation, which was upregulated in the brain but remained unaffected in the spleen. Plasma IL-6 protein was also unaltered at these time points. Together, these data indicate that the IL-6 response to ethanol is particular to central structures and is likely independent of either splenic cytokine response or the IL-6 response of circulating immune cells. This adds to previous data from our lab showing that the effects of both acute and chronic ethanol administration on cytokine signaling are not only target-specific but region-specific as well (Doremus-Fitzwater et al., 2014), while adding greater temporal clarity to the early timecourse of gene alterations after acute ethanol.
In Experiment 2, we replicated the pattern of cytokine response found in our previous work in adult males and, for the first time, extended these findings to adult females in Sprague Dawley rats. Surprisingly, the patterns of cytokine response did not differ across sex. This is of particular interest as sex effects in both the immune response and the response to ethanol have been well established. In the chronic alcohol literature, there is overwhelming evidence that women are particularly vulnerable to the toxic effects of long-term alcohol use. While the female population consumes less alcohol, drinks less frequently and has lower risk for development of alcohol use disorders than men, women that are heavy drinkers are more vulnerable to health complications associated with chronic alcohol use (Erol and Karpyak, 2015). The susceptibility to the deleterious effects of long-term alcohol use is linked to higher levels of inflammation and cellular damage in females, as well as a unique signature of cytokines relative to males (Pascual et al., 2017, Da Pozzo et al., 2018). In this present study, we found that the pattern of cytokine gene expression that follows the first binge in a naïve rat does not differ as a function of sex. This indicates that the differences seen after chronic ethanol exposure are more likely a result of divergent patterns of inflammatory pathway adaptation in the two sexes rather than an inherent sex difference in the neuroimmune response to ethanol. However, it should be noted that Experiment 2 focused exclusively on a single time point (3 hr) after ethanol exposure, and we cannot rule out the possibility that sex differences in the kinetics of ethanol-induced neuroimmune changes might differ between males and females. Indeed, adult females have been shown to metabolize ethanol substantially faster than males (Varlinskaya and Spear, 2004), which is likely to impact the time course of both acute intoxication and acute withdrawal effects.
It is also important to note that while the cytokines we examined are amongst the few that are detectable in the central nervous system, they are just a sampling of the more global inflammatory response to ethanol. Sex differences to acute ethanol may have been revealed if we had examined other inflammatory-related genes implicated in chronic ethanol studies, such as genes more directly involved in neurotoxicity or glial cell activation. Various studies have indicated that chronic ethanol administration may result in greater levels of neurodegeneration in females as well as enhanced gene expression related to apoptotic/cell death signaling and astrocytic function (Hashimoto and Wiren, 2008, Alfonso-Loeches et al., 2013, Wilhelm Clare et al., 2015) Moreover, we may have found sex differences in cytokine expression if we had also investigated peripherally; it has been shown that women develop alcohol-related medical issues, such as alcoholic liver disease (Becker et al., 1996) more rapidly and following lower levels of consumption than in men. This differences in susceptibility to alcohol-related health issues may be due, in part, to pharmacokinetic differences in how females and males respond to alcohol. Females tend to have a lower first pass metabolism due to lower gastric alcohol dehydrogenase activity (Kosobud and Crabbe, 1986). Moreover, higher fat content and lower body water content result in less distribution of alcohol in women than in men. All these factors are thought to contribute to women achieving higher alcohol content than men during acute intoxication for equivalent doses of alcohol and help explain why women are particularly sensitive to the effects of alcohol (Frezza, 1990). Of note are the sexual dimorphisms found in peripheral cytokine activity following chronic ethanol. Studies have shown that females exhibit greater liver injury following chronic ethanol, along with greater TNFα gene expression and Nf-κB activity in the liver (Iimuro et al., 1997, Kono et al., 2000, Fulham and Mandrekar, 2016). Interestingly, while one study has found greater adipose tissue IL-6 expression in males following chronic ethanol administration (Fulham and Mandrekar, 2016), others have found that females show higher levels of IL-6 and IL-6 receptor (IL-6R) α and STAT3, part of the signal transduction pathway for IL-6R, in the liver after 2 weeks of ethanol exposure (Gallucci Randle et al., 2006). Though there are studies that have examined the effects of chronic ethanol on spleen immune function in males and females, these have largely focused on prenatal ethanol exposure and have not exposed significant sex differences in splenic function (Bodnar et al., 2016, Weinberg and Jerrells, 1991). As shown in Experiment 1, central and peripheral cytokine expression differed in males, so additional work will be necessary to examine whether acute alcohol exposure induces different cytokine expression in peripheral organs across sex.
Experiment 3 examined the influence of cannulation surgery on cytokine responses to ethanol. Brain surgery is a common neuroscience procedure utilized across many types of animal studies in order to perform microdialysis or microinjections and is typically considered “minimally invasive.” However, as the surgery itself involves disrupting the CNS, as well as placement of a permanent, in-dwelling foreign object, we assessed whether surgical history would affect the cytokine response to subsequent ethanol challenge in brain sites proximal to the cannula (the hippocampus) as well as one that was more distal (amygdala). We administered ethanol to the animals 7 days after surgery, a time point commonly used for testing following recovery from surgery (Barney et al., 2018, Gano et al., 2019). The findings from this Experiment recapitulated those seen in Experiment 1 and demonstrated that in distal areas such as the amygdala, cytokine gene expression was undisturbed by cannulation. However, around the site of the cannula, minor cytokine-specific perturbations were observed. The typical IL-1β suppression due to ethanol was mild and not statistically significant, though this is likely due to highly variable levels observed in the cannulated vehicle animals. Notably, the IL-6 response to ethanol was blunted in cannulated animals, with a slightly lower peak response at 3 h and a failure to fully resolve the response at 12 h. This is similar to literature showing that ethanol prior to TBI can cause a failure to resolve the IL-6 response, though in this case the order events was reversed (Teng and Molina, 2014). It is intriguing that IL-6 was the most affected by the cannulation surgery. While it has previously been shown that IL-6 signaling is prominent in the tissue injury response and is likely strongly induced during reactive astrogliosis (Raivich et al., 1999, Penkowa et al., 2003), cytokines such as IL-1β and TNFα are also involved in these glial sequelae. Other aspects of additional work will be necessary to establish why the cannulation surgery selectively altered the IL-6 response to ethanol, yet not that of other major CNS cytokines, and whether this is a function of time point (both during ethanol intoxication as well as timing after surgery) or perhaps of cell types recruited to the site of injury.
These studies examined the cytokine response to a single ethanol administration across a variety of factors including, time, sex, and prior brain damage using exposure procedures that elicit supra-binge BECs akin to those observed with high intensity alcohol consumption. Moreover, we investigated whether the pattern of central cytokine expression would correspond to peripheral expression following acute ethanol. The cytokine response to ethanol administration was not affected by sex. Cannulation surgery had little impact on the cytokine response to ethanol, the most notable exception being a blunting of IL-6 expression in structures proximal to the cannulated structure, but not in more distal adjacent areas. Our time course of ethanol administration revealed that, again, IL-6 exhibited unique characteristics. While other cytokines exhibited complementary central vs. peripheral expression, IL-6 and Nf-κB showed a disassociation in expression in the amygdala and hippocampus as compared to the spleen. These studies highlight a feature of the cytokine response to ethanol that is also observed generally across the literature; that the expression of these factors is region-specific and sensitive to timing, yet that the general patterns observed in withdrawal versus intoxication are conserved across a variety of characteristics. For instance, other research has reliably shown that withdrawal from ethanol induces the activation of IL-1β and TNFα in areas such as the cerebellum, plasma, and whole brain (Coleman et al., 2018; Lippai et al., 2013) and in acute withdrawal models in species other than rat (Walter et al., 2017).
A strength of uniting these experiments into a multi-sided exploration of the effects of acute ethanol on a limited cadre of cytokines is the ability to observe similar patterns of change across varied parameters. In the first experiment, ethanol was delivered intraperitoneally at the dose of 4 g/kg, resulting in peak BECs of ~400 mg/dL. In contrast, in the later experiments, an intragastric route of administration was used with a higher dose of 5 g/kg, resulting in BECs of 150–300 mg/dL. However, regardless of this difference, the same patterns of cytokine change were observed in all studies at the 3 h time point and were of comparable magnitude. CORT was also assessed at this time point as one possible index of ethanol sensitivity, and unlike cytokine changes, was indeed more closely positively correlated to BECs. While glucocorticoids are immunosuppressive and CORT has been shown to affect cytokine production (for a review, see Spencer et al., 2011), we did not see evidence here for the direct influence of CORT on ethanol-induced cytokine alterations. It is nevertheless possible that CORT may exert some less prominent influence over these cytokine responses, or perhaps that these nuances would emerge in a more thorough time course or with a secondary challenge. For instance, it has been shown that while CORT does not play a primary role in mediating the effects of acute ethanol on the cytokine response to an immune challenge (polyinosine-polycytidylic acid; poly I:C), it can still modulate some cytokine and sex specific effects, i.e. the mediation of the IL-6 response to poly I:C in females after an ethanol challenge (Glover et al., 2011).
An important consideration in studies measuring gene expression is the extent to which such effects might be translated to bioactive protein. IL-6 mRNA expression did not correspond to IL-6 protein concentrations measured in the hippocampus or spleen, yet paradoxically was concordant in the amygdala. It is possible that this difference may emerge from post-transcriptional modifications enacted by other factors such as miRNA, though this is beyond the scope of the current set of studies. Such discrepancies are not uncommon for cytokines, which have short half-lives in both plasma and tissue. While these data provide further clarity on the patterns of cytokine change evoked by an acute ethanol challenge by providing a sex comparison and increased temporal resolution, they further highlight the need to be able to assess cytokine changes with higher temporal resolution and the ability to measure bioactive protein levels. In work recently emergent from our lab (Gano et al., 2019), we have begun to adapt novel technology using microdialysis and high-sensitivity multiplexing in order to assess cytokine protein levels in the brain. Studies such as Experiment 3 are necessary to properly contextualize these types of approaches by assessing the intrinsic tissue damage components inherent to invasive neuroscience techniques.
Though these cytokine responses have now been confirmed across multiple studies and have gained temporal and spatial resolution, the source and mechanism of their activity remains to be fully elucidated. All cells types in the CNS are capable of producing cytokines via multiple pathways, though in this work we have largely focused on the Nf-κB pathway via its marker, IκBα, and its connection to the expression of IL-6 during intoxication. Astrocytes are the primary source of IL-6 in the CNS, though other important sources also include microglia and neurons (as reviewed in Gruol, 2015). Further experiments are underway in our laboratory to assess the relative contributes of these cell types to these cytokine signatures under various conditions.
5. Conclusion
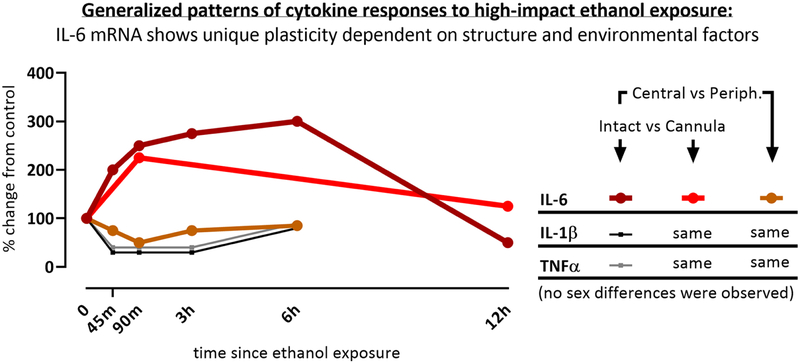
These findings add an important comparison to the wealth of chronic ethanol literature that exists, as they characterize the start of this journey by exploring the inherent neuroimmune responses following just a single ethanol exposure. Notably, these findings suggest that the sexual dimorphism of cytokine responses that exist in the immune system of chronic alcohol users may not occur after the first alcohol experience but may develop over time. IL-6 was also revealed to show a unique pattern of expression that was region specific. Figure 5 summarizes these findings and highlights the unique role of IL-6 that has emerged in these experiments. Overall, these experiments illustrate the plasticity of the neuroimmune response to ethanol administration that is particularly prominent for IL-6 expression.
Figure 5.
This figure depicts an idealized pattern of cytokine gene expression responses to high-impact ethanol exposure, summarized across the experiments in this manuscript and highlighting the unique plasticity of IL-6.
Highlights.
IL-6 showed opposite effects during ethanol intoxication relative to IL-1β and TNFα.
Central cytokine response to acute ethanol was independent of peripheral cytokines.
No sex differences in the brain cytokine response to acute ethanol were observed.
Cannulation mildly reduced the local cytokine response to acute ethanol challenge.
Funding:
this work was supported by the National Institute of Health Grant p50AA017823 to T. Deak, and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- Agartz I, Shoaf S, Rawlings RR, Momenan R & Hommer DW 2003. CSF monoamine metabolites and MRI brain volumes in alcohol dependence. Psychiatry Research: Neuroimaging, 122, 21–35. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M & Guerri C 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology, 311, 27–34. [DOI] [PubMed] [Google Scholar]
- Barney TM, Vore AS, Gano A, Mondello JE & Deak T 2018. Assessment of Interleukin-6 Signaling Effects on Behavioral Changes Associated with Acute Alcohol Intoxication in adult male rats. Alcohol, 18, 30214–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, Schnohr P & Jensen G 1996. Prediction of risk of liver disease by alcohol intake, sex, and age: A prospective population study. Hepatology, 23, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Bodnar TS, Hill LA & Weinberg J 2016. Evidence for an immune signature of prenatal alcohol exposure in female rats. Brain, Behavior, and Immunity, 58, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Zou J, Qin L, & Crews FT 2018. HMGB1/IL-1β complexes regulate neuroimmune responses in alcoholism. Brain, Behavior, and Immunity, 72, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ & Coleman LG 2017. The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N & Vetreno RP 2015. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Research: Current Reviews, 37, 331. [PMC free article] [PubMed] [Google Scholar]
- Cui C, Shurtleff D & Harris R 2014. Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol, 118, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Pozzo E, Giacomelli C, Cavallini C & Martini C 2018. Cytokine secretion responsiveness of lymphomonocytes following cortisol cell exposure: Sex differences. PloS one, 13, e0200924–e0200924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Timary P, Stärkel P, Delzenne NM & Leclercq S 2017. A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology, 122, 148–160. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner KA, Richey L, Jones ME & Deak T 2014. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcoholism, clinical and experimental research, 38, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Paniccia JE, Gano A, Vore AS & Deak T 2018. Differential effects of acute versus chronic stress on ethanol sensitivity: Evidence for interactions on both behavioral and neuroimmune outcomes. Brain, Behavior, and Immunity, 70, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A & Karpyak VM 2015. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence, 156, 1–13. [DOI] [PubMed] [Google Scholar]
- Franco R, Pacheco R, Lluis C, Ahern GP & O’connell PJ 2007. The emergence of neurotransmitters as immune modulators. Trends in Immunology, 28, 400–407. [DOI] [PubMed] [Google Scholar]
- Frezza M 1990. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. The New England Journal of Medicine, 332, 95–9. [DOI] [PubMed] [Google Scholar]
- Fulham MA & Mandrekar P 2016. Sexual Dimorphism in Alcohol Induced Adipose Inflammation Relates to Liver Injury. PLoS ONE, 11, e0164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci Randle M, Sloan Dusti K, O’dell Sijy J & Reinke Lester A 2006. Differential Expression of Liver Interleukin-6 Receptor-α in Female Versus Male Ethanol-Consuming Rats. Alcoholism: Clinical and Experimental Research, 28, 365–373. [DOI] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL & Deak T 2016. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain research, 1646, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL & Deak T 2017a. A cross-sectional comparison of ethanol-related cytokine expression in the hippocampus of young and aged Fischer 344 rats. Neurobiology of aging, 54, 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Pautassi RM, Doremus-Fitzwater TL & Deak T 2017b. Conditioned effects of ethanol on the immune system. Experimental biology and medicine, 242, 718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Vore AS, Sammakia M & Deak T 2019. Assessment of extracellular cytokines in the hippocampus of the awake behaving rat using large-molecule microdialysis combined with multiplex arrays after acute and chronic ethanol exposure. Alcoholism: Clinical and Experimental Research, 43, 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M, Cheng B, Deng X & Pruett S 2011. The role of glucocorticoids in the immediate vs. delayed effects of acute ethanol exposure on cytokine production in a binge drinking model. International immunopharmacology, 11, 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MD, Makley AT, Campion EM, Friend L.a.W., Lentsch AB & Pritts TA 2013. Preinjury alcohol exposure attenuates the neuroinflammatory response to traumatic brain injury. The Journal of surgical research, 184, 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld Z, Moore AN & Dash PK 2002. Acute Ethanol Intake Attenuates Inflammatory Cytokines after Brain Injury in Rats: A Possible Role for Corticosterone. Journal of Neurotrauma, 19, 317–326. [DOI] [PubMed] [Google Scholar]
- Gruol DL 2015. IL-6 regulation of synaptic function in the CNS. Neuropharmacology, 96, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG & Wiren KM 2008. Neurotoxic Consequences of Chronic Alcohol Withdrawal: Expression Profiling Reveals Importance of Gender Over Withdrawal Severity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 33, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J & Crews FT 2008. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology, 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Momenan R, Kaiser E & Rawlings RR 2001. Evidence for a Gender-Related Effect of Alcoholism on Brain Volumes. American Journal of Psychiatry, 158, 198–204. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Frankenberg MV, Arteel GE, Bradford BU, Wall CA & Thurman RG 1997. Female rats exhibit greater susceptibility to early alcohol-induced liver injury than males. American Journal of Physiology-Gastrointestinal and Liver Physiology, 272, G1186–G1194. [DOI] [PubMed] [Google Scholar]
- Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT & Thurman RG 2000. Gender differences in early alcohol-induced liver injury: role of CD14, NF-κB, and TNF-α. American Journal of Physiology-Gastrointestinal and Liver Physiology, 278, G652–G661. [DOI] [PubMed] [Google Scholar]
- Kosobud A & Crabbe JC 1986. Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. Journal of Pharmacology and Experimental Therapeutics, 238, 170. [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M & Orfao A 2007. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcoholism, Clinical And Experimental Research, 31, 846–854. [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA & Szabo G 2013. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leuoc Biol, 94, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD 2001. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Maier SF & Watkins LR 1998. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review, 105, 83–107. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K & Szabo G 2009. The Opposite Effects of Acute and Chronic Alcohol on Lipopolysaccharide-Induced Inflammation Are Linked to IRAK-M in Human Monocytes. Journal of immunology (Baltimore, Md. : 1950), 183, 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K & John-Henderson NA 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL & Felger JC 2013. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depression and anxiety, 30, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S & Guerri C 2016. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcoholism, Clinical And Experimental Research, 40, 2260–2270. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Semple BD, Hellewell SC, Bye N & Ziebell JM 2019. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathologica, 137, 731–755. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, García-García F, Laso FJ, Guerri C, Pascual M, Torres J-L, Costa-Alba P, García-García F & Laso F-J 2017. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addiction Biology, 22, 1829–1841. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Giralt M, Lago N, Camats J, Carrasco J, Hernández J, Molinero A, Campbell IL & Hidalgo J 2003. Astrocyte-targeted expression of IL-6 protects the CNSagainst a focal brain injury. Experimental Neurology, 181, 130–148. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss C, Werner A, Jones L & Kreutzberg G 1999. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Research Reviews, 30, 77–105. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL & Grant I 2003. Effects of Alcoholism and Gender on Brain Metabolism. American Journal of Psychiatry, 160, 1180–1183. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA & Dhabhar FS 2011. Role of Endogenous Glucocorticoids in Immune System Function: Regulation and Counterregulation. Comprehensive Physiology, 381–423. [Google Scholar]
- Szabo G & Mandrekar P 2009. A recent perspective on alcohol, immunity and host defense. Alcoholism, clinical and experimental research, 33, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G & Saha B 2015. Alcohol’s Effect on Host Defense. Alcohol Research: Current Reviews, 37, 159–170. [PMC free article] [PubMed] [Google Scholar]
- Teng SX & Molina PE 2014. Acute Alcohol Intoxication Prolongs Neuroinflammation without Exacerbating Neurobehavioral Dysfunction following Mild Traumatic Brain Injury. Journal of Neurotrauma, 31, 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI & Spear LP 2004. Acute Ethanol Withdrawal (Hangover) and Social Behavior in Adolescent and Adult Male and Female Sprague-Dawley Rats. Alcoholism: Clinical and Experimental Research, 28, 40–50. [DOI] [PubMed] [Google Scholar]
- Walters TJ & Crews FT 2017. Microglial depletion alters the brain neuroimmune respones to acute binge ethanol withdrawal. J Neuroinflammation, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C & Paxinos G 2005. The Rat Brain in Stereotaxic Coordinates, San Diego, Elsevier Academic Press. [Google Scholar]
- Weinberg J & Jerrells TR 1991. Suppression of Immune Responsiveness: Sex Differences in Prenatal Ethanol Effects. Alcoholism: Clinical and Experimental Research, 15, 525–531. [DOI] [PubMed] [Google Scholar]
- Wilhelm Clare J, Hashimoto Joel G, Roberts Melissa L, Bloom Shelley H, Andrew Melissa R & Wiren Kristine M 2015. Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathology, 26, 433–451. [DOI] [PMC free article] [PubMed] [Google Scholar]