Abstract
Currently, a combination of marijuana cannabinoids including delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) is used as a drug to treat muscle spasticity in patients with Multiple Sclerosis (MS). Because these cannabinoids can also suppress inflammation, it is unclear whether such patients benefit from suppression of neuroinflammation and if so, what is the mechanism through which cannabinoids act. In the currently study, we used a murine model of MS, experimental autoimmune encephalomyelitis (EAE), to study the role of gut microbiota in the attenuation of clinical signs of paralysis and inflammation caused by cannabinoids. THC+CBD treatment attenuated EAE and caused significant decrease in inflammatory cytokines such as IL-17 and IFN-γ while promoting the induction of anti-inflammatory cytokines such as IL-10 and TGF-β. Use of 16S rRNA sequencing on bacterial DNA extracted from the gut revealed that EAE mice showed high abundance of mucin degrading bacterial species, such as Akkermansia muciniphila (A.muc), which was significantly reduced after THC+CBD treatment. Fecal Material Transfer (FMT) experiments confirmed that THC+CBD-mediated changes in the microbiome play a critical role in attenuating EAE. In silico computational metabolomics revealed that LPS biosynthesis, a key component in gram-negative bacteria such as A.muc, was found to be elevated in EAE mice which was confirmed by demonstrating higher levels of LPS in the brain, while treatment with THC+CBD reversed this trend. EAE mice treated with THC+CBD also had significantly higher levels of short chain fatty acids such as butyric, isovaleric, and valeric acids compared to naïve or disease controls. Collectively, our data suggest that cannabinoids may attenuate EAE and suppress neuroinflammation by preventing microbial dysbiosis seen during EAE and promoting healthy gut microbiota.
Keywords: THC, CBD, EAE, Multiple sclerosis, Gut microbiome, Akkermansia muciniphila, SCFAs, LPS
1.1. Introduction
Multiple sclerosis (MS) is a chronic neuroinflammatory and demyelinating autoimmune disorder of the central nervous system (CNS) mainly affecting young adults worldwide and it is more common in women than men (Rogers and MacDonald, 2015). The pathogenesis of this disease is characterized by neuroinflammation leading to demyelination and consequently paralysis. Although the complete etiology and the pathogenesis of MS remains unclear, there is evidence that increased migration of myelin-reactive CD4+ Th1 and Th17 cells across the blood-brain barrier (BBB) causing inflammation in the CNS which leads to axonal demyelination of neurons, axonal injury, oligodendrocyte loss, neuronal damage, and glial plaques (Wu and Chen, 2016). Experimental autoimmune encephalomyelitis (EAE), an animal model of MS, is often used to study disease development and is characterized by neurological dysfunction and demyelination closely mimicking conditions in the human MS patient population (Baxter, 2007; Bjelobaba et al., 2018; Glatigny and Bettelli, 2018). Most current treatments of MS, such as interferon (IFN)-β, fingolimod, and glatiramer acetate act by regulating the immune response (Fragoso et al., 2019; Scheu et al., 2019; Wu and Chen, 2016), however these drugs are only partially effective in many cases (20–30% successful in MS patients) and can have negative side-effects and increased toxicity (Freedman, 2014; Hocevar et al., 2019). Because current treatments present many challenges to the patient population, new treatments are needed to improve the outcomes of patients who are affected by MS.
Cannabis is a product of Cannabis sativa, an annual herbaceous plant, and for several centuries this plant product has been used as an alternative medicine in many countries (Jensen et al., 2015). Cannabis sativa produces over 421 chemical compounds, including about 80 terpenophenolic compounds named phytocannabinoids, which include both THC and CBD (Andre et al., 2016). Research has shown cannabinoids are effective treatment options against neurodegenerative diseases, including MS, because these plant products can reduce disease-associated effects such as oligodendrocyte death and axonal damage (Kubajewska and Constantinescu, 2010; Nagarkatti et al., 2009). In addition, THC+CBD combination therapy has been shown to effectively reduce MS-associated tremors and spasticity, leading to clinical use (Izquierdo, 2017; Keating, 2017; Maccarrone et al., 2017; Mallada Frechin, 2018). Cannabinoids have also been shown to exert potent anti-inflammatory activities, particularly through regulating immune cells involved in the innate and adaptive immune response (Elliott et al., 2018; Nagarkatti et al., 2009; Rao et al., 2015; Sido et al., 2016; Yang et al., 2016). Combination of THC+CBD has also been shown to ameliorate EAE by suppressing neuroinflammation (manuscript under consideration, (Moreno-Martet et al., 2015), (Feliu et al., 2015) ). More recent reports are exploring the role cannabinoids have in regulating the gut microbiome, a major player in overall human host health and disease (Cluny et al., 2015; Cluny et al., 2012; Mehrpouya-Bahrami et al., 2017; Mestre et al., 2018).
The microbiome, consisting of a large population of commensal microbes, plays such an important role in the health of the host it has been referred to as the “forgotten organ” (O’Hara and Shanahan, 2006). Studies of MS patients and the EAE mouse model of MS have shown that the gut microbiome plays a significant role in disease both progression and severity (Chitrala et al., 2017; Chu et al., 2018; Gandy et al., 2019). For example, it was found that germ-free mice developed a less severe EAE disease with a reduction in pro-inflammatory IFN-γ and IL-17A T cell responses accompanied with increase production of anti-inflammatory regulatory T cell (Tregs) (Lee et al., 2011). A more recent study has shown that unaltered commensal bacteria can trigger a spontaneous form of EAE after exposure to myelin oligodendrocytes glycoprotein (MOG) (Berer et al., 2011). Interestingly, studies also found that the microbiome influences the development of the BBB, as germ-free mice had disrupted formation of this important barrier protecting the host CNS (Braniste et al., 2014). Among the factors related to the microbiome which influence the host immune system response, short chain fatty acids (SCFAs) are some of the most significant functioning metabolites synthesized by the gut microbiome. SCFAs were found to be critical modulators in the gastrointestinal tract capable of regulating the pro-inflammatory Th1/Th17 and anti-inflammatory Treg responses during autoimmune neuroinflammation (Haghikia et al., 2015).
In the current study, we investigated the use of combination cannabinoid treatment (THC+CBD at 1:1 ratio) during EAE to determine how this treatment affects the gut microbiome and whether these alterations influenced THC+CBD-mediated protective effects. The results showed THC+CBD treatment of EAE results in reduction in disease severity and alterations in the pro-inflammatory immune response. 16S rRNA sequencing of colon contents revealed THC+CBD treatment altered the gut microbiome during EAE, particularly by reducing the abundance of Akkermansia muciniphila (A.muc) which was increased during the disease state. In addition to the alterations in the microbial phylogenetic profile, THC+CBD treatment during EAE resulted in changes in the gut metabolome, specifically leading to increases in SCFAs such as anti-inflammatory butyrate. Lastly, fecal transfer experiments revealed that the protective effects of THC+CBD during EAE was at least partly due to the alterations of the microbiome during treatment with these combined cannabinoids.
1.2. Material and Methods
1.2.1. Mice
6–8-week-old female C57BL/6 mice were purchased from the Jackson laboratories (Bar Harbor, ME). Mice were housed in the Animal Resource Facility (ARF) at the University of South Carolina, School of Medicine. All animal experiments were performed in accordance with National Institutes of Health (NIH) guidelines under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina.
1.2.2. Reagents
The following reagents were used during the experiments and purchased as follows: THC and CBD were from Cayman Chemical (Ann Arbor, MI); red blood cell (RBC) lysis buffer and β-mercaptoethanol were from Sigma-Aldrich (St. Louis, MO); percoll was purchased from GE Healthcare Life Sciences (Pittsburgh, PA); RPMI 1640, l-glutamine, HEPES, phosphate-buffered saline, and fetal bovine serum (FBS) were from VWR (West Chester, PA); myelin oligodendrocyte glycoprotein (MOG35–55) peptide and H-MEVGWYRSPFSRVVHLYRNGK-OH were from PolyPeptide Laboratories (San Diego, CA); Mycobacterium tuberculosis (strain H37Ra) and complete Freund’s adjuvant were purchased from Difco (Detroit, MI); pertussis toxin was purchased from List Biological Laboratories (Campbell, CA); Neural Tissue Dissociation Kit (P) was purchased from Miltenyi Biotech Inc. (Auburn, CA); QIAamp DNA Stool mini kit was purchased from Qiagen (Germantown, MD); Illumina MiSeq reagents were purchased from Illumina, Inc. (San Diego, CA) except The Agencourt AMPure XP system beads were purchased from Beckman Coulter Life Science (Indianapolis, IN); Lipopolysaccharide (LPS) ELISA Kit (Sandwich ELISA) was purchased from LifeSpan Biosciences, Inc. (Seattle, WA. USA). In addition, the following mouse-specific ELISA kits were purchased from BioLegend (San Diego, CA): IL-10, IL-17A, IFN-γ, and TGF-β.
1.2.3. EAE induction and treatment with THC+CBD
To investigate the effect of the THC+CBD combination, EAE was induced as described previously (Rouse et al., 2013; Singh et al., 2007). Briefly, mice were given a subcutaneous injection with 100 μl of 150 μg MOG35–55 peptide emulsified in complete Freund’s adjuvant containing 8 mg/ml killed Mycobacterium tuberculosis (strain H37Ra), followed by an intraperitoneal injection (i.p.) of 200 ng of pertussis toxin on day 0. These injections were repeated with 400 ng pertussis toxin on day 2. For treatment, EAE mice were randomized and treated with an i.p. injection of 10 mg/kg each of THC+CBD (1:1 ratio) or vehicle (2% dimethylsulfoxide, DMSO; 20% ethanol diluted in PBS) on day 10 after the induction of EAE, and this treatment regimen continued once daily until the end of the experiment. Animals were monitored, and clinical scores were observed and evaluated daily.
1.2.4. EAE clinical scoring
The measured parameters for clinical EAE scores were recorded as follow: 0 = normal; 1 = partial loss of tail tonicity/inability to curl the distal end of the tail; 2 = tail atony/ moderately clumsy/impaired righting; 3 = hind limbs weakness/partial paralysis; 4 = complete hind limp paralysis/fore limb weakness; 5 = tetraplegia/moribund (O’Neill et al., 1992). The mean score was calculated for each group every day. Proper care of paralytic animals unable to access food and water was ensured by providing supplementary DietGel Boost and Hydrogel from Daily H2O (Portland, ME) on a daily basis. Death was not used as an index for clinical scores, and any mice that were moribund were immediately euthanized with an overdose anesthetic isoflurane (%5), a method approved by the American Association for Laboratory Animal Science and university IACUC.
1.2.5. Histopathological analysis
For histopathological evaluation, brain tissues from experimental mice were isolated at day 15 after EAE induction during the peak of disease. Prior to brain isolation, euthanized mice were perfused with 10 mL heparinized PBS to flush out vascular circulating cells. Isolated brain tissues obtained after perfusion were immersed in 4% paraformaldehyde (PFA) overnight prior to embedding in paraffin. Microtome sections (7 μm) were obtained, and tissue sections were stained with Hematoxylin and Eosin (H&E) to evaluate damage and cellular infiltration into the CNS.
1.2.6. Analysis of cytokine production
Pro-inflammatory (IFNγ and IL17A) and anti-inflammatory (IL-10 and transforming growth factor-β, TGF-β) cytokines were evaluated in the serum and ex vivo splenocyte supernatants from experimental groups using ELISA kits as previously described (Rouse et al., 2013). For serum, blood was collected on day 15 and serum was separated and stored at −80°C until analysis. For evaluation of the peripheral response in the spleen during EAE, spleens from experimental mice were excised at the end of the experiment and processed into a cell single suspension. Isolated splenocytes were seeded (1×106 cells/ml) cultured for 24 hours at 37°C and 5% CO2 overnight in complete RPMI 1640 media supplemented with 10% fetal bovine serum, 10 mM l-glutamine, 10 mM HEPES, 50 μM β-mercaptoethanol, and 100 μg/ml penicillin/streptomycin. After 24 hours, cell culture supernatants were cultured and stored at −80°C until further analysis
1.2.7. Flow cytometry analysis for myeloid-derived suppressor cells (MDSCs)
To determine peripheral MDSC induction, spleens were excised prior to perfusion in experimental mice. Whole splenocyte tissues were homogenized into a single cell suspension and subjected to red blood cell (RBS) lysis. Cells suspensions from experimental groups were tagged with fluorescently-labeled monoclonal antibodies (mAbs) purchased from Biolegend. Specifically, PE-labeled Gr-1 Abs and AlexaFluro700-labeled CD11b Abs were used to identify MDSC populations in the spleen. Antibody-tagged samples were run in a BD FACs Celesta flow cytometer and analyzed using FlowJo software from BD Biosciences (San Jose, CA).
1.2.8. Bacterial phylogenetic and metabolomic profiling by 16S rRNA analysis
16S rRNA sequencing using the Illumina MiSeq platform and analysis with Nephele were performed as previously described (Alrafas et al., 2019). Briefly, cecal flushes were collected from Naïve, EAE-VEH and EAE-(THC+CBD) mice under antiseptic conditions, and collected samples were kept frozen at - 80 °C until DNA extraction. Isolation of DNA from stool was performed using the QIAamp DNA Stool Mini Kit from Qiagen as per the instructions from the manufacturer. Cecal content (200 mg) was collected per experimental mouse. Sequencing was performed on the Illumina MiSeq platform to generate reads, and all further downstream analysis was performed using the Nephele platform from the National Institute of Allergy and Infectious Diseases (NIAID) Office of Cyber Infrastructure and Computational Biology (OCICB) in Bethesda, MD (Weber et al., 2018). To analyze phylogenetic and possible metabolomic alterations within samples, Nephele-based analysis using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PiCRUSt) option was used which requires a closed reference against the Greengenes database (Greengene_99) at taxa levels 2 and 3 (Phylum and Class) for KEGG annotations. In order to determine significantly altered bacteria and processes within the samples, linear discrimination analysis of effect size (LefSE) was performed on operational taxonomic unit (OTU) tables generated from Nephele (Segata et al., 2011). To validate A.muc (F 5′GACTAGAGTAATGGAGGGGGAA 3′ R 5′GTATCTAATCCCTTTCGCTCCC 3′) expression, PCR was performed using Qiagen miScript cDNA synthesis kit and Biorad SSO advanced SYBR Green PCR reagents for analysis on a CFX96 qPCR system. Fold change of A.muc was determined using the delta-delta-CT method expressed relatively to a Eubacteria (Forward: 5′ACTCCTACGGGAGGCAGCAGT; 3′ Reverse: 5′ATTACCGCGGCTGCTGGC 3′) control.
1.2.9. Quantification of SCFAs
Quantification of SCFAs was performed as previously described (Alrafas et al., 2019; Chitrala et al., 2017). Briefly, 100mg of cecal contents was weighted and suspended in dIH2O to a final concentration of 250 mg/mL. Samples were vortexed until completely homogenized, then acidified with 1:4 volumes of 25% metaphosphoric acid. Acidified samples were vortexed and placed on ice for 30 min. Cold acidified samples were centrifuged at 12,000xg for 15 minutes at 4°C. Supernatants were filtered over ultra-free MC columns (0.22 um GVDurapore, UFC30GV0S, ThermoFisher Scientific) and centrifuge at 12,000xg for 4 minutes at 4°C. Samples were stored until later downstream quantification at −80°C. To quantify SCFAs and determine their concentrations within experimental samples, standards were prepared as shown in Table 1. Ethylbutyric acid (0.10 mM) was added as an internal standard (IS) for all samples and standards tubes. The acidified samples were thawed and 100µL was transferred to a new microfuge tube, along with 100µL of the standards. For the IS-blank, 100µL ddH2O was transferred to a new microcentrifuge tube. Then, 60µL of 0.1mM IS was added to each sample, standard, and IS-blank for a final volume of 160 µL. Two sets of glass GC vials (Supelco 29056-U) were labeled for each sample, standard, and IS-blank. Approximately 400µL of methyl tert-butyl ether (MTBE) was added to one set of glass vials using a 2mL glass serological pipette. Samples, standards, and IS-blank from the tubes that had final volume of 160µL (100µL ddH2O+60µL of 0.1mM IS) were added into the glass vials containing MTBE. Samples were vortexed ~5–10 seconds, then spun down at 1.300 rpm for 5 minutes at room temperature. Lastly, 100µL of the top organic layer from the samples was placed into the other set of glass vials for analysis. Samples were stored at −20°C until analyzed with the gas chromatography flame-ionized detection (GC-FID) instrument (Zhao et al., 2006).
Table 1:
Source and concentration of SCFAs standards
| Acid | Vendor (Cat. No.) | Concentration(mM) | µL |
|---|---|---|---|
| Water(ddH2O) | - | - | 422.3 |
| Acetic | Acros Organics (64–19-7) | 400 | 11.5 |
| Propionic | Sigma (402907) | 400 | 14.9 |
| n-Butyric | Sigma (B103500) | 400 | 18.3 |
| Isovaleric | Sigma (129542) | 200 | 11.0 |
| Valeric | Sigma (240370) | 200 | 10.9 |
| Isobutyric | Sigma (58360) | 100 | 4.6 |
| Caproic | MP Biomedicals (101242) | 50 | 3.0 |
| n-Heptanoic | Sigma (75190) | 50 | 3.5 |
1.2.10. Lipopolysaccharide (LPS) Detection in brain lysates
Brain tissues were collected from naïve, EAE-VEH and EAE-(THC+CBD), rinsed with 1X PBS, and minced into a single cell suspension. Cells were collected and pelleted by centrifugation, and the supernatant was removed. Cells were washed 3 times with 1X cold-PBS and suspended in 1X PBS to be lysed by freezing the cells at −20°C and allowing to thaw at room temperature 3 times. Cells were centrifuged at 1500×g for 10 minutes at 4°C to remove cellular debris. The collected cell supernatants were analyzed for detection of LPS by using a LPS ELISA Kit (Sandwich ELISA) for LifeSpan Biosciences, Inc. (Seattle, WA) according to the manufacturer’s instructions.
1.2.11. Fecal microbial transplantation (FMT)
In order to perform FMT experiments, the EAE was induced as described above. Cecal flushes from Naïve, EAE-VEH, and EAE-(THC+CBD), were harvested aseptically at the peak of the disease (day 15) using an anaerobic chamber and suspended in sterile PBS containing 30% glycerol for storage at −80C until used. To prepare mice for FMTs, 6-week-old C57Bl/6J mice we administered antibiotics (streptomycin 1g/L and ampicillin 1g/L, referred to as “Abx”) by oral gavage (100μL) daily for 4 weeks. Abx-treated mice were kept in autoclaved cages with autoclaved water and chow. To ensure depletion of the microbiome, PCR validation (DNA collected from stool) using a primer for Eubacteria (Forward: 5′ACTCCTACGGGAGGCAGCAGT; 3′ Reverse: 5′ATTACCGCGGCTGCTGGC 3′) and bacterial culture plates were performed. EAE was induced in mice treated with Abx for 4 weeks and after symptoms started, mice were randomized into 4 groups: FMT EAE+Control (PBS only), FMT EAE+Naive, FMT EAE+VEH, FMT EAE+(THC+CBD). We performed daily FMTs (50mg) by oral gavage starting at day 10 until the end of the experiment (day 18).
1.2.12. Statistics
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Inc, La Jolla, CA). The data shown in this study represent at least three independent experiments unless otherwise stated. Average values ± standard error mean (SEM) are shown for experiments to determine to significance. The statistical differences between two experimental groups was calculated using ANOVA and Student’s t test. For comparisons of three or more groups, one-way ANOVA and Tukey’s multiple comparisons posthoc test were performed. EAE clinical scores over a period of time were evaluated using groups of at least five mice and significance was determined using two-way ANOVA and Tukey’s multiple comparisons test in order to determine significance during each individual time point (e.g. days). A p value <0.05 was considered significant for all experiments.
1.3. Results
1.3.1. Combination THC+CBD treatment reduces EAE disease and promotes anti-inflammatory response
Combination of THC+CBD is currently being used to treat muscle spasticity in patients with MS ((Markova et al., 2019)). Recently, we demonstrated that THC+CBD combination can attenuate EAE by suppressing neuroinflammation (manuscript under review). In order to determine the mechanisms through which THC+CBD attenuate EAE, we undertook studies as described in Fig 1A using the following groups: EAE mice treated with vehicle (EAE-VEH) and EAE mice treated with cannabinoid combination (EAE-(THC+CBD)). It should be noted that initially, we tested several controls including mice that received the adjuvants but no MOG, such as CFA+PTX+VEH, CFA+PTX+THC+CBD, and found that these groups did not develop any signs of EAE (data not shown). However, when we studied clinical EAE scores in EAE-(THC+CBD) group when compared to EAE-VEH disease controls, we found the cannabinoid treated mice had reduced disease severity (Figure 1B), consistent with our earlier studies. Histological results from the brains taken at day 15 of the experimental model revealed that when compared to naïve wild-type (WT) mice, EAE-VEH mice had high number of infiltrating cells and signs of tissue damage, however, EAE-(THC+CBD) group showed significant decrease in neuroinflammation and damage (Figure 1C). Serum samples also showed that EAE-(THC+CBD) mice had a decrease in the pro-inflammatory cytokines levels for IL-17A (Figure 1D) but not IFN-γ (Figure 1E), when compared to the disease controls. However, EAE-(THC+CBD) serum had increases in the levels of anti-inflammatory cytokines, TGF-β (Figure 1F) and IL-10 (Figure 1G) compared to EAE-VEH groups. In the periphery, spleen cells showed that pro-inflammatory cytokines IL-17A (Figure 1H) and IFN-γ (Figure 1I) to be reduced with THC+CBD treatment, and this correlated with an increase in anti-inflammatory myeloid-derived suppressor cells (MDSCs) when compared to disease controls. Collectively, these data showed that THC+CBD treatment was able to reduce EAE disease severity and promote and anti-inflammatory response.
Figure 1. Combination THC+CBD treatment attenuates EAE disease severity and promotes anti-inflammatory immune response.
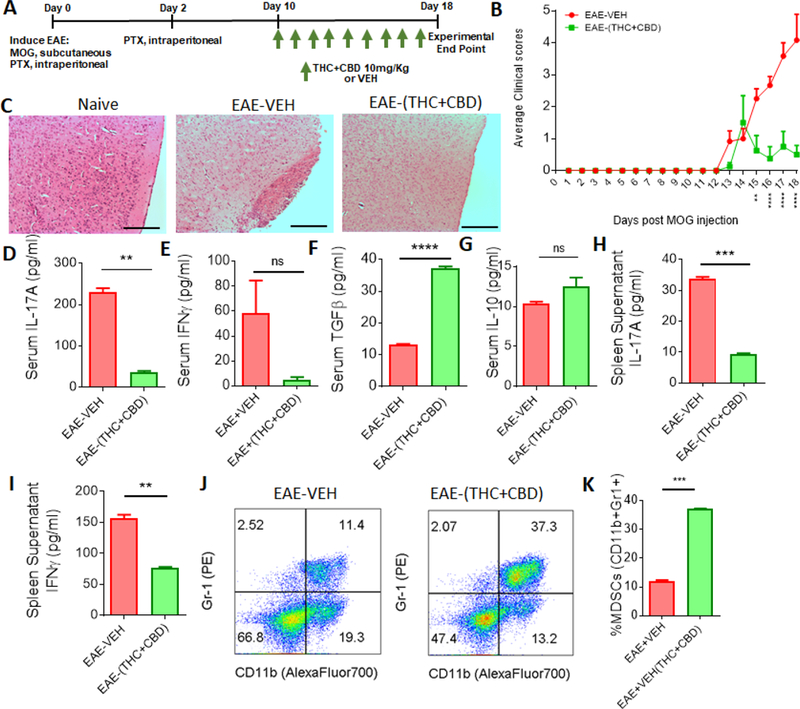
(A) Experimental design of EAE using combined treatment of THC+CBD (10mg/kg). (B) Clinical scores, outlined in the Materials and Methods section, of EAE mice treated with VEH (n = 10) or THC+CBD (n = 10). Significance was determined using two-way ANOVA and Tukey’s multiple comparisons test to evaluate significance at each day. Significant p values (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001) are indicated below respective days after EAE induction. (C) Representative images of brain histopathology stained with H&E (10×) at day 15 from experimental groups: Naïve, EAE+VEH, and EAE+THC; scale bars, 100 µM. (D-G) Serum was collected at the peak of the disease (day 15) to determine levels of IL-17A (D), IFN-γ (E), TGF-β (F), and IL-10 (G). (H-I) Supernatants from cultured splenocytes were collected after 24hrs to analyze IL-17A (H) and IFN-γ (I) by ELISA. (J-K) Representative flow cytometry plots(J) to determine MDSC (Gr-1+CD11b+) percentages (K). Bar graph data are expressed as the mean ± SEM and statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 when compared between EAE-VEH and EAE-(THC+CBD) unless otherwise stated.
1.3.2. Combination THC+CBD treatment alters the gut microbiome during EAE induction
In order to test the potential role of gut microbiota in THC+CBD-mediated attenuation of EAE and inflammation, 16S rRNA gene sequencing was performed on cecal flushes from the following groups: Naïve, EAE-VEH, and EAE-(THC+CBD). The Illumina MiSeq platform with primers targeting the V3 and V4 variable regions was used. Sequences were translated by using the 16S QIIME Paired-End pipeline implemented in Nephele platform (release 1.6 which uses QIIME 1.9.1) (DeSantis et al., 2006). OTUs were picked with QIIME’s unclust-based (Edgar, 2010), open-reference OTU picking protocol (Caporaso et al., 2010), and the taxonomic assignment was done against Greengenes-99 reference sequence set at 99% similarity, as previously described (Rideout et al., 2014). Alpha diversity, as assessed using Chao1 rarefraction measure, showed that EAE-induced mice had slightly lower abundances of bacteria in the gut (Figure 2A). Beta diversity, depicted in a 3D principle coordinate analysis (PCOA) plot, showed that the three experimental groups clustered together well within their own groups, suggesting there was divergence in the gut microbiome population (Figure 2B). At the phylum level (Figure 2C), there were notable differences in the taxa. EAE-induced mice had significantly lower abundances of Bacteriodetes when compared to Naïve mice (Figure 2D), whereas EAE mice treated with THC+CBD had higher levels of Firmicutes when compared to the disease controls (Figure 2E). Both EAE groups had a significant drop in Tenericutes when compared to the controls (Figure 2F), but had increased abundance of Verrucomicrobia, although EAE-(THC+CBD) had reduced levels compared to EAE-VEH (Figure 2G). Interestingly, Proteobacteria was significantly higher in EAE-VEH mice compared to controls, but the abundance of this phylum was reduced to normal levels after treatment with THC+CBD (Figure 2H). Significant alterations in the gut microbiome were observed throughout the various taxa levels from class to genus among the experimental groups with an interesting consistent trend showing EAE-associated increase in Verrucomicrobia sub-levels was decreased after THC+CBD treatment (Supplementary Figures 1–4).
Figure 2. THC+CBD alters the gut microbiome during EAE disease.
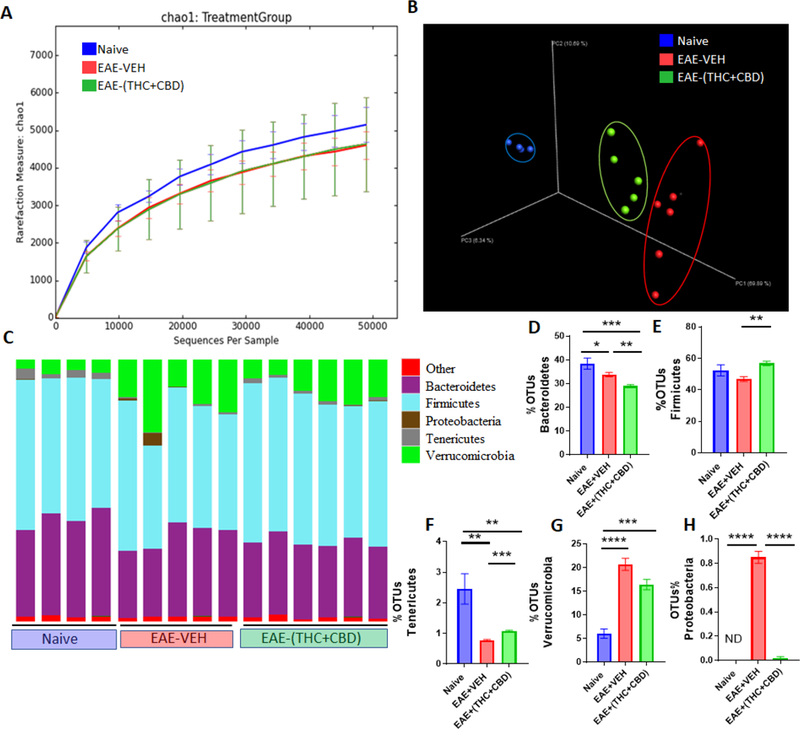
(A-B)16S rRNA sequencing from the cecal flushes was performed on Naive (n=4), EAE-VEH (n=5), and EAE-(THC+CBD) (n=6) experimental mice and data are representative of one experiment. Sequenced reads were analyzed using Nephele to generate chao1 alpha diversity (A) and beta diversity PCOA (B) plots. (C) Depicted stacked bar graphs of percent OTUs at the phylum level. (D-H) Individual bar graphs are depicted for the following phylum level: Bacteriodetes (D), Firmicutes (E), Tenericutes (F), Verrucomicrobia (G), and Proteobacteria (H). Bar graph data are expressed as the mean ± SEM and statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Significance was determined using one-way ANOVA and Tukey’s multiple comparisons test.
In order to ascertain the most significantly altered bacteria between naïve and disease controls and identify any potential microbial biomarkers of disease, LefSE was performed on the OTU output data (Figure 3A). When examining microbial disruption between the Naïve and EAE-VEH groups, the bacterial species Akkermansia muciniphilia (A.muc) within the Verrucomicrobiae class had the highest LDA score in EAE-induced mice (Figure 3B). When taking into account the levels in treated groups, EAE-(THC+CBD) mice had significantly reduced levels of this EAE-associated bacteria (Figure 3C). The sequencing data was validated using primers specific for A.muc, reinforcing the observation that THC+CBD treatment reduced the levels of this bacteria that were significantly higher in EAE mice when compared to naïve controls (Figure 3D).
Figure 3. LefSE analysis identifies A.Muc as a potential biomarker of EAE disease, which is reduced after THC+CBD treatment.
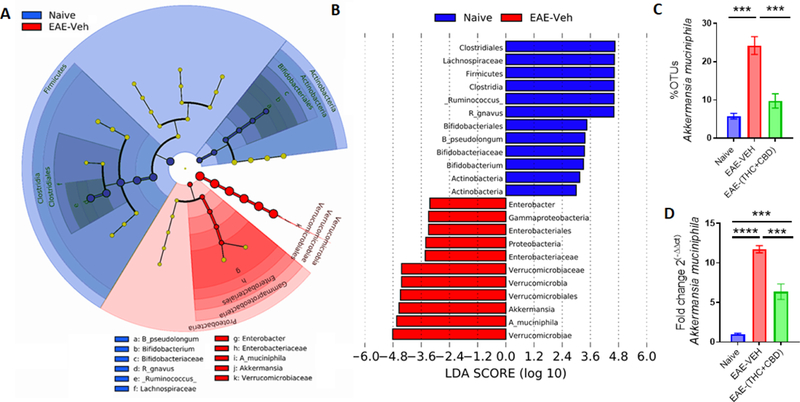
(A-B)16S rRNA sequencing from the cecal flushes was performed on Naive (n=4), EAE-VEH (n=5), and EAE-(THC+CBD) (n=6) experimental mice and OTU data was subjected to LefSE analysis to generate cladogram (A) and LDA scores (B) between Naïve and EAE-VEH groups. For LefSe data, the alpha factorial Kruskal-Wallis test among classes was set to 0.05, and the threshold on the logarithmic LDA score for discriminative features was set at3. (C) Bar graph depicting the percent OTUs of A.Muc generated from 16S rRNA sequencing in Naïve, EAE-VEH, and EAE-(THC+CBD) groups. (D) Bar graph depicting PCR validation and fold change of A.Muc in Naïve (n=5), EAE-VEH (n=5), and EAE-(THC+CBD) (n=5) groups. Bar graph data are expressed as the mean ± SEM and statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Significance was determined using one-way ANOVA and Tukey’s multiple comparisons test.
1.3.3. Treatment with THC+CBD during EAE alters gut bacterial metabolite production
In addition to the phylogenetic data from 16s rRNA sequencing, in silico computational metabolomics was performed using the PICRUSt method. LefSE analysis of the output data showed that when comparing disease controls (EAE-VEH) to EAE-(THC+CBD) treated groups, several KEGG pathways had high Linear discriminant analysis (LDA) scores in the disease state (Figure 4A). For example, LPS biosynthesis, a key component in gram-negative bacteria such as A.muc was found to be elevated in disease controls compared to naïve mice, but this was greatly reduced after treatment with THC+CBD (Figure 4B). In order to corroborate this in silico data, brain lysates from experimental groups were analyzed for detection of LPS levels. When comparing all the experimental groups, lysates from the brains of EAE-VEH mice had significantly higher levels of LPS compared to controls, however, EAE-(THC+CBD) mice reduced this level significantly (Figure 4C). Production of known SCFAs was also evaluated from cecal flushes. Compared to Naïve controls, EAE-induced mice (untreated or treated) had higher levels of acetic (Figure 4D) and propionic acids (Figure 4E), though only EAE-(THC+CBD) mice had increased isobutyric acid (Figure 4F). In addition, EAE-(THC+CBD) samples had significantly higher levels of butyric (Figure 4G), isovaleric (Figure 4H), and valeric (Figure 4I) acids compared to naïve or disease controls. Collectively, these data showed that EAE- (THC+CBD) mice had global increases in SCFA production, in addition to altering other bacterial metabolomic pathways such as LPS production.
Figure 4. THC+CBD alters the gut microbiome metabolome during EAE disease.
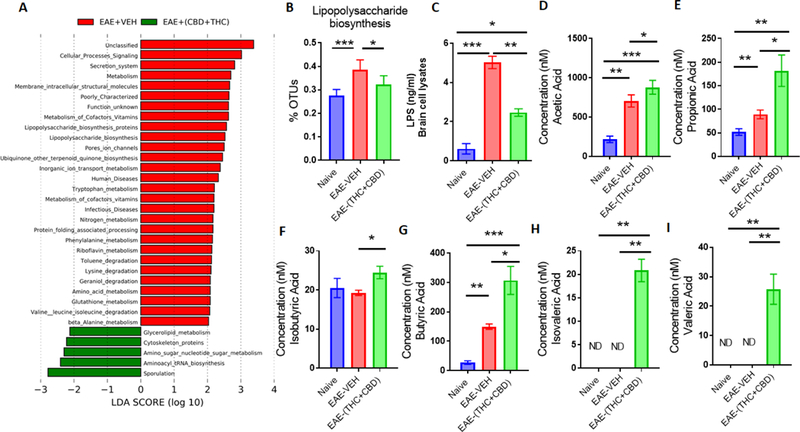
(A) LDA scores between EAE-VEH and EAE-(THC+CBD) groups using PICRUSt-generated Level 3 (L3) KEGG pathways of 16S rRNA sequencing data from Naive (n=4), EAE-VEH (n=5), and EAE-(THC+CBD) (n=6) experimental mice. (B) Individual bar graph depicting percent OTUs attributed to LPS biosynthesis from Naive (n=4), EAE-VEH (n=5), and EAE-(THC+CBD) (n=6) experimental mice after PICRUSt analysis. (C) Concentration of LPS levels from cultured brain lysates detected by ELISA for Naive (n=5), EAE-VEH (n=5), and EAE-(THC+CBD) (n=5) samples. (D-I) Concentrations of SCFAs from cecal flushes of Naive (n=5), EAE-VEH (n=5), and EAE-(THC+CBD) (n=5) samples. Bar graph data are expressed as the mean ± SEM and statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Significance was determined using one-way ANOVA and Tukey’s multiple comparisons test.
1.3.4. FMT of THC+CDB-altered microbiome attenuates EAE disease severity
In order to determine if the alterations in the microbiome by THC+CBD treatment was responsible for any protective effects against EAE induction, FMT experiments were performed as described in Figure 5A. To deplete the microbiota, mice were treated with Abx for 4 weeks, and confirmation of this depletion was perfomed using PCR (Figure 5B) and bacterial culturing (Figure 5C). For FMTs, EAE-induced mice were fed fecal material from controls (FMT EAE Control), from Naïve (FMT EAE-Naïve), disease controls (FMT EAE-VEH), or disease mice treated with THC+CBD (FMT EAE-(THC+CBD)). When assessed for clinical scores after EAE induction, all mice treated with Abx had reduced disease severity compared to normal EAE experiments (Figure 5D), more than likely due to the dependency of EAE induction on the microbiota. However, FMT EAE-(THC+CBD) mice had significantly reduced scores compared to the other groups. These data confirmed that alterations in the microbiome by combination THC+CBD may play an important role in protection against EAE-induction.
Figure 5. FMT of THC+CBD altered microbiome attenuates EAE severity.
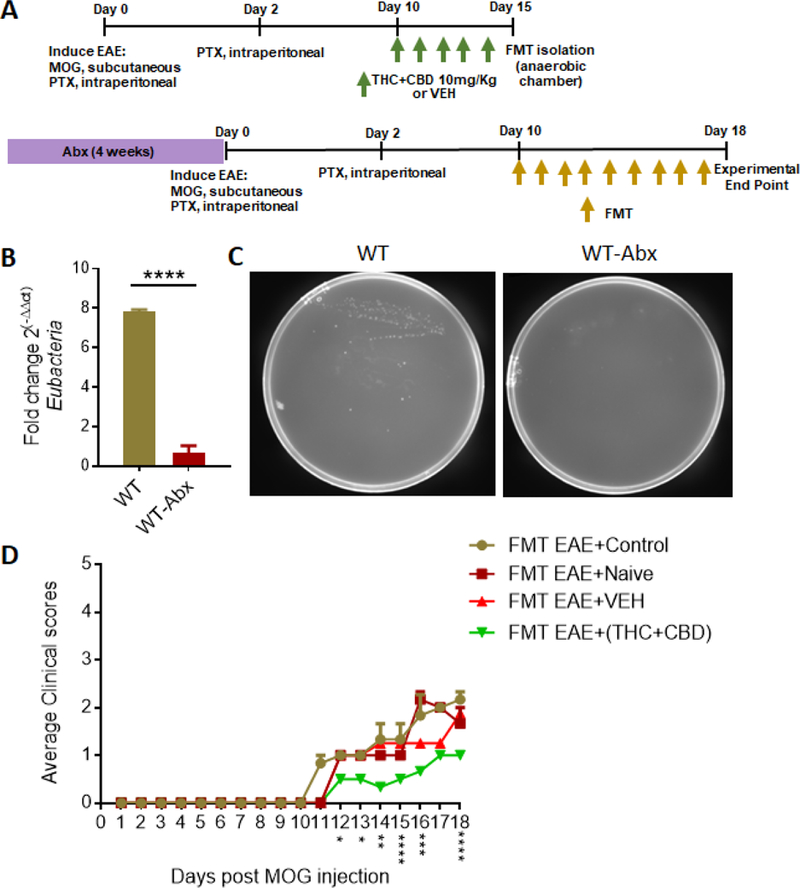
(A) Experimental design of FMT EAE experiments where 4-week Abx-treated mice received FMTs (50mg) from either Control PBS (n=3), Naïve (n=3), EAE-VEH (n=5), or EAE-(THC+CBD) (n=5) mice. (B) PCR validation confirming Abx mice had depleted microbiome. Depicted is the fold change of Eubacteria normalized to 18S compared between WT(n=5) and WT+Abx (n=5). Bar graph data are expressed as the mean ± SEM and statistical significance is indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 (C) Representative feces cultured plates from WT (n=4) and WT+Abx (n=4) mice to confirm depletion of microbiome. (D) Clinical scores, outlined in the Materials and Methods section, of FMT EAE mice treated with Control PBS (n=3), Naïve (n=3), EAE-VEH (n=5), or EAE-(THC+CBD) (n=5) fecal material. Significance was determined using two-way ANOVA and Tukey’s multiple comparisons test to evaluate significance at each day. Significant p values (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001) are indicated below respective days after EAE induction.
1.4. Discussion
MS is a neurological autoimmune disease caused by a combination of genetic and environmental exposures which has increased in incidence in the past several decades suggesting changes in environmental risk factors (Granieri et al., 2000), and gut microbiota might be one of these environmental factors (Jangi et al., 2016). Our study demonstrated treatment of EAE mice with a mixture of cannabinoids (THC and CBD) resulted in alleviation of the clinical symptoms and this was associate with dysbiosis in the gut. Fecal transfer experiments confirmed that THC+CBD-induced alterations in the gut microbiota played a significant role in attenuating EAE. These data further advance the findings from our lab and that from others that THC+CBD can attenuate EAE and neuroinflammation [manuscript under review], (Moreno-Martet et al., 2015), (Feliu et al., 2015). Moreover, we have also previously shown that CBD can attenuate EAE-induced disease (Elliott et al., 2018). The efficacy of cannabinoids against MS was observed over 20 years ago when it was reported that a MS patient who smoked marijuana had decreased incident of MS-associated ataxia and spasticity (Meinck et al., 1989), and since then several reports have shown cannabinoids are effective at lessening MS disease burden (Corey-Bloom et al., 2012; Zajicek et al., 2012). Recently, an oromucosal pharmaceutical cannabinoid spray consisting of THC+CBD (1:1 ratio) from marijuana (Sativex) has become available to reduce spasticity and pain attributed to MS (Vermersch, 2011). However, whether such a combination also suppress neuroinflammation in MS patients and if so, what are the mechanism involved, remains to be further elucidated. It should be noted that initially, we performed preliminary studies and found that THC alone (10mg/kg) or CBD alone (10mg/kg) were not effective in attenuating clinical signs of EAE but when we used a combination of THC+CBD (10mg/kg each), we noted significant efficacy against EAE. Moreover, the dose of THC used in our study is well within the range used in humans. Based on body surface area normalization guidelines from FDA (Nair and Jacob, 2016), 10 mg/kg THC dose in mice converts to 30 mg/m2. In humans, THC (Marinol) used as an antiemetic is recommended at a dose of 90 mg/m2/day by FDA, which is 3 times higher than what we used in mice. Similarly, CBD (Epidiolex) that was recently approved by FDA to treat epilepsy in children was recommended at the highest dose of 20 mg/kg. Thus, CBD used in this study (10 mg/kg) in mice converts to 30 mg/m2 while the CBD in humans converts to 740 mg/m2, thereby making the dose of CBD used in the current study, markedly less than that currently approved for human use.
Several mechanisms have been attributed to the beneficial effects of cannabinoids such as THC and CBD, to include those that affect the immune system. In the current report, treatment with THC+CBD resulted in reduction of pro-inflammatory cytokine production and increase in anti-inflammatory cytokines production. In early reports, Kozela et al found that CBD suppressed microglial activity and prevented T cell proliferation associated with inducible EAE (Kozela et al., 2011). The same research group later reported that THC and CBD suppressed Th17 cells and proinflammatory cytokines such as TNFα and IFNγ during EAE (Kozela et al., 2013). In addition to suppressing the pro-inflammatory phenotype, our lab has also shown that cannabinoids are able to increase abundance and activity of anti-inflammatory immune cell phenotypes, such as Tregs and MDSCs, in various inflammatory models of disease (Elliott et al., 2018; Hegde et al., 2011; Hegde et al., 2015; Jackson et al., 2014; Rao et al., 2015; Sido et al., 2016). Therapeutic agents that can reduce pro-inflammatory Th1/Th17 subsets and increase anti-inflammatory Th2/Treg/MDSC subsets can potentially help patients suffering from MS, reinforcing the case that these cannabinoids are a good source of alternative therapy for the patient population. Despite such studies that have helped understand the immunological mechanisms through which cannabinoids suppress inflammation including that seen in MS, there are no reports evaluating the role of microbiota in cannabinoid-mediated attenuation of EAE or MS.
There is evidence to suggest that MS is characterized by alterations in amount, composition, and metabolomic profile of the microbiome (Zoledziewska, 2019). Of particularly note in the current study was during EAE induction and treatment, significant changes were observed in A. Muc which is reported to be involved in exacerbation of both human MS patients and animal models of MS, like EAE (Cekanaviciute et al., 2018; Zoledziewska, 2019). For example, it was found that increased A. Muc in MS patients was capable of increasing the severity of EAE when transplanted into mice (Cekanaviciute et al., 2017). In a study done by Jangi et al., researchers found that the genus Akkermansia increased in patients with MS (Jangi et al., 2016). In another study, researchers found that untreated MS subjects showed increase in Akkermansia as well (Cantarel et al., 2015). The current report suggests that cannabinoid treatment was able to regulate A. Muc, which is supported by previous studies demonstrating that blocking cannabinoid receptors leads to significant increase in A. Muc in a model of obesity (Mehrpouya-Bahrami et al., 2017).
Microbial dysbiosis in MS/EAE hosts characterized by increased gram-negative bacteria, such as A.Muc, could be linked to increased LPS, which was observed in brain lysates from EAE mice in the current study. Elevated LPS levels suggest a shift to increased presence of gram negative bacterial endotoxin and a study has shown LPS results in degeneration of myelin and results in white matter damage (Felts et al., 2005). It is noteworthy that LPS is linked to a proinflammatory response in the host capable of activating microglia, disrupting the BBB, and causing a defect in Treg functions (Escribano et al., 2017). In the current study, to perform FMT, we used mice that were depleted of microbiota following treatment with antibiotics (ABX) for 4 weeks. The long-term treatment approach ensures that most of the microbiota is eliminated thereby making the results more conclusive on the efficacy of FMT-induced changes. We wish to point out that we have also used in the past, short-term treatment with antibiotics for 2 days followed by FMT to study EAE (Chitrala et al., 2017). The disadvantage in short-term ABX model is that the treatment may not eliminate all gut microbiota, and thus some concern remains on whether the results observed are contributed by heightened residual gut microbiota activity. On the other hand, the advantages short-term ABX model in studying EAE is that the EAE is fully developed comparable to normal mice (Chitrala et al., 2017), while in the long-term ABX model, the EAE is significantly reduced, as seen in the current study, because the normal gut microbiota may be necessary to induce full-blown EAE.
In the current study, we also noted that THC+CBD treatment resulted in widespread increases in SCFAs. SCFAs play a very important role in regulating the inflammatory response and preventing autoimmune diseases such as MS by suppressing the production of Th17 cells and promoting the production of Treg cells from CD4 naïve T cells (Bhutia and Ganapathy, 2015). In fact, a recent report showed that gut dysbiosis and lack of SCFAs were seen in Chinese MS patients (Zeng et al., 2019). The results of all these aforementioned studies, combined with the results in EAE mice treated with THC+CBD, provide an interesting therapeutic approach and target in the future treatment of MS patients, by way of altering the gut microbiome and preventing MS-associated microbial dysbiosis.
In summary, the current study demonstrates that a combination of THC+CBD, which is currently used to treat muscle spasticity can also suppress neuroinflammation and clinical signs of EAE. Mechanistically, using fecal transfer experiments, we validated that the beneficial effects of these cannabinoids may result from alterations that they cause in the gut microbiota. Our studies have translational impact inasmuch as various combinations of THC+CBD can now be clinically tested for their efficacy to treat neuroinflammation and thus long-term relief from MS. Also, further characterization of these cannabinoid-induced microbiota may lead to clinical translation of microbiome-based therapies against MS.
Supplementary Material
Research Highlights.
Combination of THC+ CBD attenuates mouse model of multiple sclerosis (EAE)
THC+CBD attenuates neuro-inflammation
THC+CBD treatment lead to alterations in the gut microbiome and metabolome
Fecal transfer of THC+CBD-altered microbiome attenuate EAE disease severity
Cannabinoids suppress EAE through regulation of microbiota
1.7. Acknowledgments
The authors would like to thank the National Institute of Allergy and Infectious Diseases (NIAID) Office of Cyber Infrastructure and Computational Biology (OCICB) in Bethesda, MD to provide the Nephele platform that was used in this study to analyze the microbiome data.
1.5 Funding
This work was supported by NIH grants P01AT003961, R01AT006888, R01AI123947, R01AI129788, R01MH094755, and P20GM103641 provided by MN and PSN. The Ministry of Higher Education and Scientific Research (MOHESR)/Iraq also provided support to ZZA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that there is no conflict of interest to report.
1.8 References:
- Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS, 2019. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J Leukoc Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre CM, Hausman JF, Guerriero G, 2016. Cannabis sativa: The Plant of the Thousand and One Molecules. Frontiers in plant science 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AG, 2007. The origin and application of experimental autoimmune encephalomyelitis. Nature reviews. Immunology 7, 904–912. [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G, 2011. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541. [DOI] [PubMed] [Google Scholar]
- Bhutia YD, Ganapathy V, 2015. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity 43, 629–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelobaba I, Begovic-Kupresanin V, Pekovic S, Lavrnja I, 2018. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. Journal of neuroscience research 96, 1021–1042. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S, 2014. The gut microbiota influences blood-brain barrier permeability in mice. Science translational medicine 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, Venkatesan A, Fraser CM, Mowry EM, 2015. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med 63, 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R, 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E, Probstel AK, Thomann A, Runia TF, Casaccia P, Katz Sand I, Crabtree E, Singh S, Morrissey J, Barba P, Gomez R, Knight R, Mazmanian S, Graves J, Cree BAC, Zamvil SS, Baranzini SE, 2018. Multiple Sclerosis-Associated Changes in the Composition and Immune Functions of Spore-Forming Bacteria. mSystems 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, Crabtree-Hartman E, Sand IK, Gacias M, Zhu Y, Casaccia P, Cree BAC, Knight R, Mazmanian SK, Baranzini SE, 2017. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proceedings of the National Academy of Sciences of the United States of America 114, 10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitrala KN, Guan H, Singh NP, Busbee B, Gandy A, Mehrpouya-Bahrami P, Ganewatta MS, Tang C, Chatterjee S, Nagarkatti P, Nagarkatti M, 2017. CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur J Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F, Shi M, Lang Y, Shen D, Jin T, Zhu J, Cui L, 2018. Gut Microbiota in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis: Current Applications and Future Perspectives. Mediators Inflamm 2018, 8168717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Keenan CM, Reimer RA, Le Foll B, Sharkey KA, 2015. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Delta9-Tetrahydrocannabinol. PloS one 10, e0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Reimer RA, Sharkey KA, 2012. Cannabinoid signalling regulates inflammation and energy balance: the importance of the brain-gut axis. Brain, behavior, and immunity 26, 691–698. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, Gouaux B, 2012. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 184, 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL, 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72, 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC, 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. [DOI] [PubMed] [Google Scholar]
- Elliott DM, Singh N, Nagarkatti M, Nagarkatti PS, 2018. Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. Frontiers in immunology 9, 1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano BM, Medina-Fernandez FJ, Aguilar-Luque M, Aguera E, Feijoo M, Garcia-Maceira FI, Lillo R, Vieyra-Reyes P, Giraldo AI, Luque E, Drucker-Colin R, Tunez I, 2017. Lipopolysaccharide Binding Protein and Oxidative Stress in a Multiple Sclerosis Model. Neurotherapeutics 14, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliu A, Moreno-Martet M, Mecha M, Carrillo-Salinas FJ, de Lago E, Fernandez-Ruiz J, Guaza C, 2015. A Sativex((R)) -like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. Br J Pharmacol 172, 3579–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts PA, Woolston AM, Fernando HB, Asquith S, Gregson NA, Mizzi OJ, Smith KJ, 2005. Inflammation and primary demyelination induced by the intraspinal injection of lipopolysaccharide. Brain 128, 1649–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso YD, Adoni T, Gomes S, Goncalves MVM, Parolin LF, Rosa G, Ruocco HH, 2019. Severe Exacerbation of Multiple Sclerosis Following Withdrawal of Fingolimod. Clinical drug investigation [DOI] [PubMed] [Google Scholar]
- Freedman MS, 2014. Treatment options for patients with multiple sclerosis who have a suboptimal response to interferon-beta therapy. Eur J Neurol 21, 377–387, e318–320. [DOI] [PubMed] [Google Scholar]
- Gandy KAO, Zhang J, Nagarkatti P, Nagarkatti M, 2019. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Scientific reports 9, 6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatigny S, Bettelli E, 2018. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harbor perspectives in medicine 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granieri E, Casetta I, Govoni V, Tola MR, Marchi D, Murgia SB, Ticca A, Pugliatti M, Murgia B, Rosati G, 2000. The increasing incidence and prevalence of MS in a Sardinian province. Neurology 55, 842–848. [DOI] [PubMed] [Google Scholar]
- Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thone J, Demir S, Muller DN, Gold R, Linker RA, 2015. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 43, 817–829. [DOI] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti PS, Nagarkatti M, 2011. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PloS one 6, e18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Singh UP, Nagarkatti PS, Nagarkatti M, 2015. Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor gamma in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J Immunol 194, 5211–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar K, Ristic S, Peterlin B, 2019. Pharmacogenomics of Multiple Sclerosis: A Systematic Review. Frontiers in neurology 10, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo G, 2017. Multiple sclerosis symptoms and spasticity management: new data. Neurodegenerative disease management 7, 7–11. [DOI] [PubMed] [Google Scholar]
- Jackson AR, Nagarkatti P, Nagarkatti M, 2014. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PloS one 9, e93954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, Cook S, Tankou S, Stuart F, Melo K, Nejad P, Smith K, Topcuolu BD, Holden J, Kivisakk P, Chitnis T, De Jager PL, Quintana FJ, Gerber GK, Bry L, Weiner HL, 2016. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 7, 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Chen J, Furnish T, Wallace M, 2015. Medical Marijuana and Chronic Pain: a Review of Basic Science and Clinical Evidence. Current pain and headache reports 19, 50. [DOI] [PubMed] [Google Scholar]
- Keating GM, 2017. Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex((R))): A Review in Multiple Sclerosis-Related Spasticity. Drugs 77, 563–574. [DOI] [PubMed] [Google Scholar]
- Kozela E, Juknat A, Kaushansky N, Rimmerman N, Ben-Nun A, Vogel Z, 2013. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol 8, 1265–1276. [DOI] [PubMed] [Google Scholar]
- Kozela E, Lev N, Kaushansky N, Eilam R, Rimmerman N, Levy R, Ben-Nun A, Juknat A, Vogel Z, 2011. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol 163, 1507–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubajewska I, Constantinescu CS, 2010. Cannabinoids and experimental models of multiple sclerosis. Immunobiology 215, 647–657. [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK, 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl 1, 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Maldonado R, Casas M, Henze T, Centonze D, 2017. Cannabinoids therapeutic use: what is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert review of clinical pharmacology 10, 443–455. [DOI] [PubMed] [Google Scholar]
- Mallada Frechin J, 2018. Effect of tetrahydrocannabinol:cannabidiol oromucosal spray on activities of daily living in multiple sclerosis patients with resistant spasticity: a retrospective, observational study. Neurodegenerative disease management 8, 151–159. [DOI] [PubMed] [Google Scholar]
- Markova J, Essner U, Akmaz B, Marinelli M, Trompke C, Lentschat A, Vila C, 2019. Sativex((R)) as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci 129, 119–128. [DOI] [PubMed] [Google Scholar]
- Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT, Velazquez KT, McCellan J, Nagarkatti M, Nagarkatti P, 2017. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Scientific reports 7, 15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck HM, Schonle PW, Conrad B, 1989. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. J Neurol 236, 120–122. [DOI] [PubMed] [Google Scholar]
- Mestre L, Carrillo-Salinas FJ, Mecha M, Feliu A, Guaza C, 2018. Gut microbiota, cannabinoid system and neuroimmune interactions: New perspectives in multiple sclerosis. Biochemical pharmacology 157, 51–66. [DOI] [PubMed] [Google Scholar]
- Moreno-Martet M, Feliu A, Espejo-Porras F, Mecha M, Carrillo-Salinas FJ, Fernandez-Ruiz J, Guaza C, de Lago E, 2015. The disease-modifying effects of a Sativex-like combination of phytocannabinoids in mice with experimental autoimmune encephalomyelitis are preferentially due to Delta9-tetrahydrocannabinol acting through CB1 receptors. Mult Scler Relat Disord 4, 505–511. [DOI] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M, 2009. Cannabinoids as novel anti-inflammatory drugs. Future medicinal chemistry 1, 1333–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara AM, Shanahan F, 2006. The gut flora as a forgotten organ. EMBO Rep 7, 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill JK, Baker D, Davison AN, Maggon KK, Jaffee BD, Turk JL, 1992. Therapy of chronic relapsing experimental allergic encephalomyelitis and the role of the blood-brain barrier: elucidation by the action of Brequinar sodium. Journal of neuroimmunology 38, 53–62. [DOI] [PubMed] [Google Scholar]
- Rao R, Nagarkatti PS, Nagarkatti M, 2015. Delta(9) Tetrahydrocannabinol attenuates Staphylococcal enterotoxin B-induced inflammatory lung injury and prevents mortality in mice by modulation of miR-17–92 cluster and induction of T-regulatory cells. Br J Pharmacol 172, 1792–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout JR, He Y, Navas-Molina JA, Walters WA, Ursell LK, Gibbons SM, Chase J, McDonald D, Gonzalez A, Robbins-Pianka A, Clemente JC, Gilbert JA, Huse SM, Zhou HW, Knight R, Caporaso JG, 2014. Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. PeerJ 2, e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KA, MacDonald M, 2015. Therapeutic Yoga: Symptom Management for Multiple Sclerosis. J Altern Complement Med 21, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M, 2013. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol 169, 1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Ali S, Mann-Nuttel R, Richter L, Arolt V, Dannlowski U, Kuhlmann T, Klotz L, Alferink J, 2019. Interferon beta-Mediated Protective Functions of Microglia in Central Nervous System Autoimmunity. International journal of molecular sciences 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C, 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sido JM, Jackson AR, Nagarkatti PS, Nagarkatti M, 2016. Marijuana-derived Delta-9-tetrahydrocannabinol suppresses Th1/Th17 cell-mediated delayed-type hypersensitivity through microRNA regulation. Journal of molecular medicine 94, 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P, 2007. Resveratrol (trans-3,5,4’-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72, 1508–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermersch P, 2011. Sativex((R)) (tetrahydrocannabinol + cannabidiol), an endocannabinoid system modulator: basic features and main clinical data. Expert Rev Neurother 11, 15–19. [DOI] [PubMed] [Google Scholar]
- Weber N, Liou D, Dommer J, MacMenamin P, Quinones M, Misner I, Oler AJ, Wan J, Kim L, Coakley McCarthy M, Ezeji S, Noble K, Hurt DE, 2018. Nephele: a cloud platform for simplified, standardized and reproducible microbiome data analysis. Bioinformatics 34, 1411–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chen G, 2016. miRNAs Participate in MS Pathological Processes and Its Therapeutic Response. Mediators Inflamm 2016, 4578230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Bam M, Nagarkatti PS, Nagarkatti M, 2016. RNA-seq Analysis of delta9-Tetrahydrocannabinol-treated T Cells Reveals Altered Gene Expression Profiles That Regulate Immune Response and Cell Proliferation. The Journal of biological chemistry 291, 15460–15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG, Group MR, 2012. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry 83, 1125–1132. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Junli G, Liu X, Chen C, Sun X, Li H, Zhou Y, Cui C, Wang Y, Yang Y, Wu A, Shu Y, Hu X, Lu Z, Zheng SG, Qiu W, Lu Y, 2019. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int 129, 104468. [DOI] [PubMed] [Google Scholar]
- Zhao G, Nyman M, Jonsson JA, 2006. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr 20, 674–682. [DOI] [PubMed] [Google Scholar]
- Zoledziewska M, 2019. The gut microbiota perspective for interventions in MS. Autoimmun Rev [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


