Abstract
Background
The multi-site Prescription Opioid Addiction Treatment Study (POATS), conducted by the National Drug Abuse Treatment Clinical Trials Network, was the largest clinical trial yet conducted with patients dependent upon prescription opioids (N = 653). In addition to main trial results, the study yielded numerous secondary analyses, and included a 3.5-year follow-up study, the first of its kind with this population. This paper reviews key findings from POATS and its follow-up study.
Methods
The paper summarizes the POATS design, main outcomes, predictors of outcome, subgroup analyses, the predictive power of early treatment response, and the long-term follow-up study.
Results
POATS examined combinations of buprenorphine-naloxone of varying duration and counseling of varying intensity. The primary outcome analysis showed no overall benefit to adding drug counseling to buprenorphine-naloxone and weekly medical management. Only 7% of patients achieved a successful outcome (abstinence or near-abstinence from opioids) during a 4-week taper and 8-week follow-up; by comparison, 49% of patients achieved success while subsequently stabilized on buprenorphine-naloxone. Long-term follow-up results were more encouraging, with higher abstinence rates than in the main trial. Patients receiving opioid agonist treatment at the time of follow-up were more likely to have better outcomes, though a sizeable number of patients succeeded without agonist treatment. Some patients initiated risky use patterns, including heroin use and drug injection. A limitation of the long-term follow-up study was the low follow-up rate.
Conclusions
POATS was the first large-scale study of the treatment of prescription opioid dependence; its findings can influence both treatment guidelines and future studies.
Keywords: Prescription opioids, Opioid use disorder, Treatment, Outcome, Addiction, Follow-up
1. Introduction
Non-medical prescription opioid use has emerged as a major public health challenge over the past two decades. In 2014, 4.3 million Americans used prescription opioids for non-medical reasons, making prescription opioids the second most used illicit drug (Center for Behavioral Health Statistics and Quality, 2015). On average, more than 1000 patients per day visited an emergency department because of non-medical use of prescription opioids in 2011 (Crane, 2015). Moreover, overdose deaths from prescription opioids climbed steadily throughout the end of the last decade. Although they declined slightly in 2012, they increased again by 9% in 2014, comprising the majority of all opioid-related deaths (Rudd et al., 2016). Treatment of prescription opioid use disorders has become extremely common; in 2013, 746,000 patients received treatment for prescription opioid use disorders in inpatient locations or mental health centers (Substance Abuse and Mental Health Services Administration, 2014), and 24% of patients who were started on pharmacotherapy for opioid use disorders primarily used prescription opioids.
Since its approval in 2002, buprenorphine-naloxone (bup-nx) has become a mainstay of pharmacotherapy for opioid use disorders. However, because the approval of bup-nx derived from large-scale clinical trials conducted predominantly in heroin users (Fudala et al., 2003; Ling et al., 1998), it was unclear the degree to which its use in those dependent upon prescription opioids would yield similar outcomes. Indeed, evidence suggests that prescription opioid users differ in important ways from those who use heroin. Prescription opioid users, on average, use less total opioid per day, have fewer co-occurring substance use disorders, have better family and social functioning, shorter treatment histories, are less likely to administer by injection, and experience fewer legal consequences (Moore et al., 2007; Rosenblum et al., 2007). Overall, prescription opioid users have better treatment outcomes than those who use heroin (Nielsen et al., 2013; Potter et al., 2013). It has even been suggested that the favorable characteristics of prescription opioid users may mean that these patients might not require the same treatments as do heroin users, with some researchers questioning the necessity of long-term agonist therapy for this population (Sigmon, 2006). Empirical evidence can best validate our treatments for prescription opioid use disorder.
One factor that may play an important role in the successful treatment of prescription opioid use disorders is drug counseling. One study of heroin users in methadone maintenance treatment programs showed that adding drug counseling increases opioid-negative urine-screens (McLellan et al., 1993). However, other studies have not found meaningful differences when drug counseling is added (Senay et al., 1973; Gruber et al., 2008; Schwartz et al., 2011). It is unclear, however, the degree to which we can generalize from studies of heroin users receiving methadone maintenance treatment to prescription opioid users receiving office-based bup-nx. Prior to the study we will describe below, only one study, with a modest population size, had examined the role of counseling in patients receiving office-based bup-nx, finding no benefit from more intensive counseling over standard medical management (Fiellin et al., 2006). However, no study had looked specifically at the role of counseling in prescription opioid users.
Given the lack of guidance on pharmacologic and psychosocial treatments for prescription opioid dependence, the National Drug Abuse Treatment Clinical Trials Network sponsored the Prescription Opioid Addiction Treatment Study (POATS) to address these issues (Weiss et al., 2011). The Clinical Trials Network, under the auspices of the National Institute on Drug Abuse, is a partnership between addiction researchers, community treatment program directors, and the National Institute on Drug Abuse itself to design and conduct multi-site clinical trials in community substance use disorder treatment programs and general medical settings.
This article will discuss the design and findings of the POATS trial, including a 3.5-year follow-up study, and will comment on some of its implications and current perspectives. POATS was intended a priori to answer the following questions: (1) Does adding opioid drug counseling to buprenorphine-naloxone plus medical management improve opioid use outcomes? (2) How many patients dependent upon prescription opioids can achieve successful opioid use outcomes with a brief taper of bup-nx, as opposed to bup-nx stabilization? (3) Which patient characteristics predict successful outcomes? (4) Which patient characteristics predict successful response to counseling? Questions later raised included: (1) Can initial response to treatment predict outcomes at the end of treatment? and (2) What are the long-term (up to 42 months) outcomes of study participants?
2. Methods
2.1. POATS design considerations
Designing POATS presented several challenges. The investigators had to weigh the relative importance of studying a new population (those dependent upon prescription opioids rather than heroin) vs. choosing a population that was generalizable to treatment-seeking patients (many of whom have experimented with heroin). To resolve this, study investigators included those who had used heroin unless they (1) had ever injected heroin, (2) had ever met criteria for opioid dependence based on heroin use alone, or (3) had used heroin on >4 days in the month before study entry (Weiss et al., 2010).
The other design issue relevant to this population was related to chronic pain, which is common in these patients (Barry et al., 2009; Potter et al., 2008; Rosenblum et al., 2003). The investigators chose to include those with chronic non-cancer pain if they had not experienced a major pain event in the previous 6 months. Moreover, for those being prescribed opioids for pain, the prescriber had to agree that it was safe and medically appropriate for the patient to stop opioid use.
2.2. Overall study design
POATS employed a two-phase adaptive treatment research design, which is intended to approximate clinical practice by beginning with a non-intensive treatment approach and utilizing a more intensive treatment strategy for patients who fail to respond to the initial treatment. In this study, Phase 1 consisted of a 4-week bup-nx taper, with participants randomized to receive either standard medical management (SMM) alone or SMM plus individual opioid drug counseling (ODC). Patients who were successful in this first phase, i.e., they were abstinent or nearly abstinent from opioids during both the taper and an 8-week follow-up period, were deemed to have successfully finished the study. Those who returned to opioid use during Phase 1 were offered the second phase, consisting of 12 weeks of bup-nx stabilization followed by a 4-week taper and 8 weeks of follow-up; again, participants were randomized to receive either SMM alone or SMM + ODC. The statistical analysis included the intention-to-treat population (i.e., all randomized participants) to compare outcomes between the two counseling conditions; generalized estimating equation models were employed to account for the potential correlation of outcomes among participants at each of the 10 study sites. To be considered to have had an abstinent week, a participant had to self-report no opioid use and have an opioid-negative urine test; a missing urine sample was considered positive for opioids. Planned secondary analyses included examination of the role of pain and heroin use; predictors of outcome; and predictors of response to counseling. The study was powered for the main outcome, not the secondary analyses. A long-term follow-up study (see below for details) was proposed and approved during the main trial.
2.3. Study population
The POATS population consisted of 653 participants age >18, at 10U.S. sites. Participants were 60% male, 91% Caucasian, half never-married, and 63% employed full-time; mean age was 33. Participants were near-daily users of opioid analgesics, but had relatively little other substance use; cannabis was the most frequently used non-opioid, with an average of 5 days a month. Interestingly, two-thirds of the population had never sought opioid use disorder treatment before. Twenty-three per cent of patients had a lifetime history of heroin use, and 42% reported current chronic pain at study entry, defined by self-report as pain (excluding pain from withdrawal) beyond everyday kinds of pain, for >3 months.
2.4. Assessments and outcome measures
The Composite International Diagnostic Interview (World Health Organization, January 1997) was used to diagnose substance use disorders, major depressive disorder, and posttraumatic stress disorder. Substance use was assessed at weekly visits, using a calendar technique (Sobell and Sobell, 1992), and supplemented by a urine drug screen. Pain was assessed using the Brief Pain Inventory-Short Form (Keller et al., 2004) at baseline and monthly thereafter. Withdrawal was measured with the Clinical Opioid Withdrawal Scale (Tompkins et al., 2009).
The main outcome measure was the percentage of participants who were “successful” in the second phase, comparing those who received SMM alone versus those who received SMM + ODC. Success in Phase 2 was defined as urine-confirmed abstinence in ≥3 of the final 4 weeks of bup-nx stabilization (weeks 9–12), including week 12. “Success” in Phase 1 was defined as completing all 12 weeks of Phase 1, opioid use on <4 days in a month, no 2 consecutive opioid-positive urine screens, and no additional formal substance use disorder treatment (mutual-help groups such as Narcotics Anonymous were allowed).
2.5. Treatments
All participants were inducted as outpatients onto sublingual bup-nx and were stabilized on allowable doses ranging from 8 to 32 mg per day. In Phase 1, the medication was tapered in weeks 3 and 4; in Phase 2, the taper occurred in weeks 13–16. All participants also received manual-based SMM (Fiellin et al., 1999). This consisted of an initial 45–60 min visit and subsequent weekly 15–20 min with a buprenorphine-certified physician, who reviewed the impact of medication, side effects, and adherence; recommended abstinence, and encouraged attendance at mutual-help groups such as Narcotics Anonymous. Half of the participants also received individual manual-based ODC (Pantalon et al., 1999), delivered by a trained substance use disorder or mental health professional; ODC sessions, 45–60 min long, were delivered at the same treatment sites and, when possible, linked to medical management visits. ODC covered standard relapse prevention topics: recommendation for abstinence, encouragement to attend mutual-help groups, and methods to deal with interpersonal relationships and risky situations.
3. Results
3.1. Primary outcome results
In Phase 1, i.e., the 4-week taper, only 43 of the 653 study participants (7%) were successful, In contrast, 49% of the 360 participants who entered phase 2 of the trial achieved successful outcomes while stabilized on bup-nx. At week 24 (8 weeks after completing the second taper), the success rate had dropped precipitously to 9%. Notably, the primary analysis showed no difference in successful outcome rates between those who did and those who did not receive ODC (Weiss et al., 2011).
3.2. Secondary analyses
3.2.1. The role of pain
Among the 42% of POATS participants who reported current chronic pain, spine (48%) and lower extremity (32%) pain were most common; the mean pain severity score was 4.4 on a 10-point scale, indicating moderate overall levels of pain. Eighty-three percent of patients with chronic pain reported that the primary reason for their first use of opioids was to relieve pain. Interestingly, when these 83% of pain patients were asked about their primary motivation for current opioid use, 57% reported now using opioids primarily to avoid withdrawal; only 23% reported using opioids at present to relieve pain. This finding demonstrates that the reason for initial use of a drug may differ from the reason for maintenance of use once one has developed a substance use disorder (Weiss et al., 2014b). When we examined the relationship between chronic pain and POATS treatment outcome, we found no significant difference in successful outcomes among those with and without chronic pain (Weiss et al., 2011).
3.2.2. Predictors of outcome
We examined a variety of sociodemographic and clinical characteristics, to see which patient traits predicted successful outcomes in Phase 2 in the POATS population (Dreifuss et al., 2013). Many of the findings were unsurprising; older patients had better outcomes, as did those who had never used heroin or had initially used opioids for pain rather than to get high. Using opioid analgesics via a route of administration for which it was not intended (e.g., snorting, crushing, chewing) was a particularly poor prognostic sign. Those who were seeking treatment for the first time fared better than those who had previously engaged in either formal opioid use disorder treatment or mutual-help group attendance. An interesting and somewhat unexpected finding was that those who had major depressive disorder had nearly twice the odds of achieving a successful outcome. Potential explanations for this finding include the potential antidepressant effects of buprenorphine in some patients (Bodkin et al., 1995; Nyhuis et al., 2008) and the increased motivation to change one’s life that a depressed patient may have. The finding regarding the good prognostic significance of major depressive disorder was corroborated in an analysis of patients with all psychiatric disorders, in which we found that the overall population of patients with psychiatric illness had better opioid use outcomes than patients without psychiatric illness (Griffin et al., 2014).
3.2.3. Do subgroups of prescription opioid dependent patients benefit from counseling?
The fact that drug counseling did not improve outcome for the study population as a whole does not mean that certain patients or subgroups of patients might not benefit from counseling. Studies of alcohol dependence, for example, have shown that patients with certain genetic subtypes are more likely to respond to specific medications (Anton et al., 2008; Johnson et al., 2011; Kranzler et al., 2014). We therefore examined the possibility that certain subgroups of POATS participants might benefit from counseling (Weiss et al., 2014a). We hypothesized that two factors might influence treatment response: severity of the disorder and session attendance. For many health disorders, more severely ill patients require more intensive treatment. Moreover, we posited that participants who attended counseling more often would benefit more from counseling than those who attended infrequently.
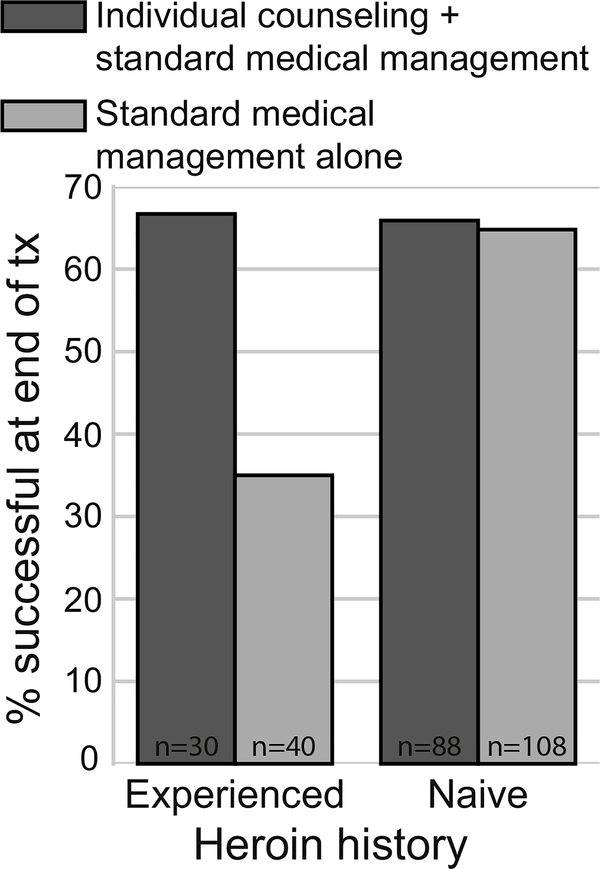
Severity was measured in three ways: (1) the Addiction Severity Index (McLellan et al., 1992) drug composite score, (2) presence of chronic pain, and (3) lifetime history of heroin use at study entry. Adequate attendance was defined a priori as attending ≥60% of all offered visits. We found that among these variables, only a history of heroin use was associated with poorer outcomes. Patients with more severe problems, by any measure, did not have better outcomes when randomized to receive counseling. Similarly, among participants with adequate attendance, counseling did not improve outcome. However, the interaction of heroin use and attendance was significant; among patients with adequate attendance, those who had used heroin had significantly better outcomes (odds ratio 3.7, CI 1.1–11.8, p = 0.03) when assigned to SMM + ODC than when assigned to SMM alone (see Fig. 1). No such outcome difference occurred in participants without a heroin use history. Thus, patients who had used heroin were able to achieve outcomes comparable to non-heroin users if they were offered counseling and attended regularly. The findings from this analysis are limited by the fact that there was no attention control, so it is possible that the treatment-adherent heroin users assigned to SMM + ODC benefited from the extra attention, not necessarily from the content of the counseling sessions. Moreover, the better outcomes among this population may have been a result of their high motivation, although that explanation is somewhat mitigated by the fact that similarly motivated study participants without a history of heroin use did not have better outcomes when assigned to SMM + ODC.
Fig. 1.
The impact of lifetime heroin use and treatment condition on the rate of successful opioid use outcomes on buprenorphine-naloxone in treatment-adherent patients (N = 266).
3.2.4. The importance of early response to buprenorphine-naloxone treatment
Early response to treatment can be an important predictor of later outcome, but this is not always the case; for example, a delayed response is common in antidepressant treatment (Trivedi et al., 2006). POATS represented the first opportunity to examine the prognostic importance of early response to buprenorphine in patients with prescription opioid dependence (McDermott et al., 2015).
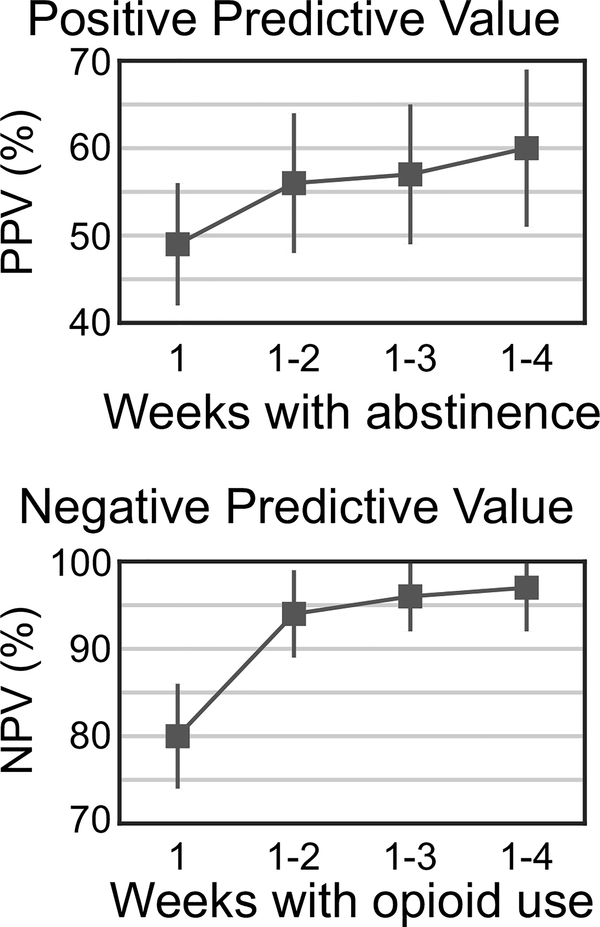
We therefore examined the positive and negative predictive values of early outcomes (see Fig. 2). We found that abstaining from opioids in week 1 did not predict the likelihood of abstinence in weeks 9–12 at all: only 49% of those who abstained in week 1 also did so in weeks 9–12. Abstaining in both weeks 1 and 2 improved the positive predictive value somewhat, to 56%. Continuing to abstain in weeks 3 and 4 only marginally improved positive predictive value.
Fig. 2.
Prognostic significance of opioid abstinence/use in first 4 weeks of buprenorphine-naloxone treatment in predicting abstinence/use in weeks 9–12 (N = 360). The positive predictive value (top) and negative predictive value (bottom) are computed based on abstinence or use, respectively, over the first n weeks of the study, where n varies from 1 to 4. Error bars are 95% confidence intervals.
In contrast, opioid use in just the first week of bup-nx treatment had a negative predictive value of 80%, and use in weeks 1 and 2 was associated with a negative predictive value of 94%, meaning that the likelihood of opioid abstinence in weeks 9–12 among participants who used opioids in weeks 1 and 2 was only 6%. This analysis revealed the importance of early response to bup-nx treatment of prescription opioid dependence; in contrast to antidepressant treatment, patients are unlikely to respond later when they have not started off well. Although increasing the intensity of behavioral treatment was not possible in POATS, doing so in clinical practice (or, alternatively, switching to methadone or naltrexone) could help patients who struggle early in bup-nx treatment.
3.2.5. The POATS long-term follow-up study
POATS presented an important opportunity to examine long-term outcomes in this population in a naturalistic, exploratory study (Potter et al., 2015; Weiss et al., 2015). Primary areas of interest in the follow-up study included opioid use outcomes, treatment participation, the course of chronic pain, and the initiation of risky new behaviors, specifically heroin use and drug injection.
The follow-up study consisted of three interviews, which took place 18, 30, and 42 months following initial randomization. Interviews lasted approximately 45–60 min and were all conducted over the telephone by research staff at McLean Hospital, Belmont, Massachusetts, where the study lead investigators were located. A total of 375 POATS participants agreed to enter the follow-up study. This number was limited to some degree by the fact that the follow-up study was not approved until near the end of the main trial; participants who had participated in and completed the study during its early phases were least likely to participate in the follow-up study, in large part because of the difficulty in locating them. Otherwise, the only differences between those who entered and those who did not enter the follow-up study were that follow-up participants were more likely to be female (44% vs. 35%, p < 0.05) and to have a non-cocaine stimulant use disorder (3.5% vs. 0%, p < 0.01), A total of 275 participants completed a month-18 interview; month 30 and 42 interviews were completed by 312 and 306 participants, respectively; retention was over 90%.
Follow-up results were generally encouraging. At month 42,32% of participants had abstained from opioids in the previous month and were not receiving agonist treatment; 29% had abstained from illicit opioids while receiving agonist therapy; 31% were using opioids and not receiving agonist therapy, and 8% were receiving agonist therapy and using illicit opioids. Given that all participants had entered POATS using either daily or nearly every day, this represented marked improvement. There was a clear decline in the rate of opioid dependence (excluding a diagnosis of opioid dependence on agonist therapy), from 16% to 12% to 8% at months 18, 30, and 42 respectively; by definition, all participants had a diagnosis of opioid dependence at study initiation. There was no compensatory increase in the use of other substances when prescription opioid use declined. Indeed, the use of cannabis, the most frequently used drug at baseline, declined from an average of 5 days in the month prior to study entry to 3 days per month at months 18, 30, and 42. A limitation of the long-term follow-up study was the relatively low follow-up rate; only 52% of main-trial participants entered the follow-up study. It is possible, then, that those participants who were doing well may have been more likely to enter the follow-up study. The inclusion of more non-cocaine stimulant users in the follow-up study than in the general sample (even though it was only 3.5% of the follow-up sample) may have influenced the rate of risk behaviors.
Approximately two-thirds of the patients in the follow-up study participated in some form of treatment during the follow up period. Buprenorphine maintenance was most common, with approximately one-third of participants receiving that treatment during each of the follow-up assessments; a similar number of participants attended mutual-help groups. Patients receiving agonist treatment were significantly more likely to be abstinent from illicit opioids. Indeed, 80% of participants receiving opioid agonist treatment at both months 18 and 42 had abstained from illicit opioids in the previous month, compared to abstinence rates of 37% and 50% among those not receiving agonist treatment at months 18 and 42, respectively. Notably, these abstinence rates for those receiving and for those not receiving agonist treatment were substantially higher than those achieved during the main trial.
Participants who had successful outcomes either in the taper phase (Phase 1) or the bup-nx stabilization phase (Phase 2) of the main trial were both more likely to have better outcomes at month 18 than those who did not achieve successful outcomes in the main trial. However, by month 42, those with and those without successful outcomes in the main trial had similar long-term outcomes. Indeed, the only predictor from the main trial of month-42 treatment outcome was the use of heroin before study entry: study participants who had ever used heroin before entering the trial had more than 3 times greater odds of having a diagnosis of opioid dependence at month 42 then those who had never used heroin.
Unfortunately, the follow-up study also revealed some worrisome trends, specifically the initiation of heroin use among participants who had never used it before study entry, and the initiation of drug injection. Ten percent of the participants in the follow-up study used heroin for the first time after study entry, all by month 30. Moreover, 10% of the follow-up sample reported intravenous injection of heroin at least 5 times in the previous year; any lifetime injection of heroin was an exclusion criterion for the POATS study. Thus, although the study outcomes were generally encouraging, some participants switched from prescription opioid use to riskier behaviors—a pattern that has become an increasing problem in recent years (Jones, 2013; Jones et al., 2015).
4. Discussion
The POATS trial, the largest treatment study to date focusing on prescription opioid users, had several key results. (1) Similar to heroin users, the vast majority of prescription opioid users failed to achieve success after tapering off bup-nx, whereas about half of the patients achieved successful outcomes while maintained on bup-nx. (2) Good prognostic factors included never having used heroin, initial use of opioids to treat pain rather than to get high, and presence of major depressive disorder. (3) Patients who were randomized to receive additional opioid drug counseling (ODC) in addition to standard medical management did not have improved outcomes. This was generally borne out by subgroup analyses; only one subgroup showed a benefit from ODC—participants with a history of heroin use who also regularly attended the ODC sessions. (4) Failure of bup-nx treatment was predictable from early performance; among participants who used opioids during the first two weeks of bup-nx treatment, only 6% were abstinent in weeks 9–12 of treatment; this is consistent with other research with primarily heroin-dependent populations (Stein et al., 2005). (5) Forty-two months after the trial started, 61% of participants who entered the follow-up study had abstained from opioids in the previous month. A history of heroin use was the only predictor of (poor) month-42 outcomes.
Five years have elapsed since the main outcome paper of the POATS trial was published. In that time, a research group at the University of Vermont examined how best to conduct outpatient detoxification of prescription opioid dependent patients (N = 70) with bup-nx by randomizing participants to different durations (1 week, 2 weeks, or 4 weeks) of a bup-nx taper (Sigmon et al., 2013). Abstinence rates during the taper were 29%, 29%, and 63% for those receiving 1, 2, and 4 weeks of bup-nx, respectively; when patients were transitioned to oral naltrexone and followed through week 12, those who had undergone the 4-week taper continued to have higher abstinence rates. Further, the longer tapers were associated with milder withdrawal symptoms (Dunn et al., 2015). One important reason for the better outcomes in the Vermont trial was the use of oral naltrexone for ongoing pharmacological support following the taper.
Much evidence supports the effectiveness of bup-nx for the treatment of opioid dependence (Mattick et al., 2014; Thomas et al., 2014); POATS supports this approach specifically in those dependent upon prescription opioids, both in the main trial and the follow-up study. Fiellin et al. (2014) similarly found that participants randomized to maintenance on buprenorphine over the course of a 14-week trial had far better opioid use outcomes than did those randomized to receive a taper. However, neither these trials nor, to our knowledge, any studies that include patients dependent upon heroin, satisfactorily address the question of how long people should remain on maintenance buprenorphine therapy before they might have a good chance of tapering successfully if they desired to do so. Future studies of buprenorphine tapering should therefore focus not on the efficacy of tapering vs. maintenance, but rather on how to best taper buprenorphine for patients who are switching to naltrexone or who, for whatever reason, need to or want to stop buprenorphine treatment.
Several other studies have explored the utility of counseling in addition to bup-nx treatment. Fiellin et al. (2006) randomized patients to either a longer duration (~43 min) weekly counseling session or a briefer (~23 min) weekly counseling session, both conditions on top of thrice-weekly bup-nx dispensing over 24 weeks in a primary care setting. They found that longer counseling did not improve the frequency of opioid-negative urines or retention in the study, although the study was limited by modest sample size with a substantial drop out rate. Later, this same group randomized HIV patients with opioid use disorder into either physician management every other week or weekly physician management plus a 45-min drug counseling and medication adherence session (Tetrault et al., 2012). Again, this additional intervention and frequency did not improve retention in the study, opioid-negative urines, or adherence with HIV medications.
Fiellin et al. (2013) examined similar questions regarding cognitive behavioral therapy (CBT). Opioid dependent patients in a primary care setting were randomized to brief medical management alone or brief medical management with up to 12 weeks of CBT. Again, there was no difference in opioid-negative urines or retention in the study, either during or after the CBT. However, a secondary analysis of this study showed that patients dependent upon prescription opioids who received brief medical management plus CBT were more likely than those who received brief medical management alone to have better outcomes in terms of abstinence from all drugs; patients dependent upon heroin experienced no such benefit from CBT (Moore et al., 2016). Ling et al. (2013) inquired whether adding CBT and/or contingency management (CM) improved outcomes on top of twice weekly medical management in a private practice-like setting. Over the course of 16 weeks of treatment, or at follow-up assessments up to 52 weeks after randomization, neither CBT, CM, nor the combination of CBT and CM improved opioid-negative urines, retention, or cravings.
Overall, for the most part, the above studies support the POATS result that counseling did not improve successful outcomes in opioid users treated with bup-nx. Some might see this result as arguing against the routine need for counseling in the context of bup-nx treatment in office-based practice. However, bup-nx treatment as it is actually offered in the community is not typically as comprehensive as the study’s “standard” medical management, which included educational components, encouraged 12-step meetings and/or lifestyle changes, and discussed pain (Arfken et al., 2010). It may thus have been difficult for the more intensive counseling to separate from the standard management. The intensity of medical management delivered in POATS and other such trials was greater than that delivered in many typical medical settings and may have been sufficient to produce effects equivalent to those of ODC. Thus definitive, conclusions about the utility of counseling may require more nuanced assessment of types and amounts of counseling interventions. It is important that any future research carefully controls the quality of standard medical management to reflect what is actually delivered in practice. A more comprehensive review of the role of counseling and other behavioral interventions in the context of bup-nx treatment is beyond the scope of this paper, but can be found elsewhere (Carroll and Weiss, in press).
The results of POATS suggest support for an individualized approach to behavioral interventions in the context of bup-nx treatment of prescription opioid use disorders. The fact that some patients benefited from counseling (e.g., heroin users who attended sessions regularly) suggests that some patients would do well with just medical management and others should receive additional counseling. Moreover, because POATS also found that opioid use during the first two weeks of bup-nx treatment portended a poor prognosis (McDermott et al., 2015), patients who do not stabilize within two weeks could be offered more intensive, individualized care.
Finally, there have been changes in the treatment of opioid use disorders during the past several years. Notably, extended-release injectable naltrexone has emerged as another effective tool for the pharmacologic treatment of opioid use disorders (Krupitsky et al., 2011; Lee et al., 2016). Moreover, the treatment delivery system has gradually shifted toward increasing emphasis of treatment of opioid use disorders in primary care settings (LaBelle et al., 2016). With this combination of new challenges and new tools, the POATS trial should be seen as just one important step in our understanding of how to address the prescription opioid epidemic.
Acknowledgments
Author disclosures
Role of funding source
This work was supported by National Institute on Drug Abuse (Rockville, MD) grants 2UG1DA015831 (RDW) and K24 DA022288 (RDW). NIDA had no further role in the writing of the report, or in the decisions to submit the paper for publication.
Footnotes
Conflict of interest
Dr. Weiss has served as a consultant to Indivior, U.S. WorldMeds, and GW Pharmaceuticals. Dr. Rao declares that he has no conflicts of interest.
References
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D, 2008. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch. Gen. Psychiatry 65, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfken CL, Johanson CE, di Menza S, Schuster CR, 2010. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J. Subst. Abuse Treat 39, 96–104. [DOI] [PubMed] [Google Scholar]
- Barry DT, Beitel M, Joshi D, Schottenfeld RS, 2009. Pain and substance-related pain-reduction behaviors among opioid dependent individuals seeking methadone maintenance treatment. Am. J. Addict 18, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO, 1995. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol 15, 49–57. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Weiss RD, 2017. The role of behavioral interventions in buprenorphine maintenance treatment: a review. Am. J. Psychiatry (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, 2015. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health, NSDUH Series H-50, HHS Publication No. SMA; 15–4927. [Google Scholar]
- Crane EH, 2015. The CBHSQ Report: Emergency Department Visits Involving Narcotic Pain Relievers, Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality, Rockville, MD. [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, Selzer J, Hatch-Maillette M, Sonne SC, Weiss RD, 2013. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: results from a multisit study. Drug Alcohol Depend. 131, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Saulsgiver KA, Miller ME, Nuzzo PA, Sigmon SC, 2015. Characterizing opioid withdrawal during double-blind buprenorphine detoxification. Drug Alcohol Depend. 151, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Schottenfeld RS, Gordon L, O’Connor PG, 1999. Standard Medication Management for Bup/Nx Treatment of Opioid Analgesic Dependence for Use in NIDA CTN-0030 Unpublished Manual. Yale University. [Google Scholar]
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, Schottenfeld RS, 2006. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N. Engl. J. Med. 355, 365–374. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Barry DT, Sullivan LE, Cutter CJ, Moore BA, O’Connor PG, Schottenfeld RS, 2013. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am. J. Med 126, 74.e11–74.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O’Connor PG, 2014. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern. Med 174, 1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D, Buprenorphine/Naloxone Collaborative Study, Group, 2003. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N. Engl. J. Med 349, 949–958. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Dodd DR, Potter JS, Rice LS, Dickinson W, Sparenborg S, Weiss RD, 2014. Baseline characteristics and treatment outcomes in prescription opioid dependent patients with and without co-occurring psychiatric disorder. Am.J. Drug Alcohol Abuse 40, 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber VA, Delucchi KL, Kielstein A, Batki SL, 2008. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend 94, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, DiClemente CC, Li MD, 2011. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am. J. Psychiatry 168, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, Bohm MK, 2015. Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. Morb. Mortal. Wkly. Rep. (MMWR) 64, 719–725. [PMC free article] [PubMed] [Google Scholar]
- Jones CM, 2013. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers—United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 132, 95–100. [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS, 2004. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain 20, 309–318. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM, 2014. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am. J. Psychiatry 171, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL, 2011. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet 377, 1506–1513. [DOI] [PubMed] [Google Scholar]
- LaBelle CT, Han SC, Bergeron A, Samet JH, 2016. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts Collaborative Care Model in community health centers. J. Subst. Abuse Treat 60, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA Jr., Wilson D, McDonald R, Rotrosen J, Gourevitch MN, Gordon M, Fishman M, Chen DT, Bonnie RJ, Cornish JW, Murphy SM, O’Brien CP, 2016. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N. Engl. J. Med 374, 1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr., Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, RennerJr JA, Santos E, Casadonte P, Fye C, Stine S, Wang RI, Segal D, 1998. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction 93, 475–486. [DOI] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J, 2013. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction 108, 1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev 2, CD002207. [DOI] [PubMed] [Google Scholar]
- McDermott KA, Griffin ML, Connery HS, Hilario EY, Fiellin DA, Fitzmaurice GM, Weiss RD, 2015. Initial response as a predictor of 12-week buprenorphine-naloxone treatment response in a prescription opioid-dependent population. J. Clin. Psychiatry 76, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat 9,199–213. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP, 1993. The effects of psychosocial services in substance abuse treatment. JAMA 269, 1953–1959. [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS, 2007. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J. Gen. Intern. Med 22, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Cutter CJ, Buono FD, Barry DT, Fiellin LE, O’Connor PG, Schottenfeld RS, 2016. Cognitive Behavioral Therapy improves treatment outcomes for prescription opioid users in primary care buprenorphine treatment. J. Subst. Abuse Treat 71, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W, 2013. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. J. Addict. Med. 7, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhuis PW, Gastpar M, Scherbaum N, 2008. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J. Clin. Psychopharmacol 28, 593–595. [DOI] [PubMed] [Google Scholar]
- Pantalon MV, Fiellin DA, Schottenfeld RS, Gordon L, O’Connor PG, 1999. Manual for Enhanced Medical Management of Opioid Dependence with Buprenorphine Unpublished Manuscript. Yale University. [Google Scholar]
- Potter JS, Prather K, Weiss RD, 2008. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am. J. Addict 17, 121–125. [DOI] [PubMed] [Google Scholar]
- Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, Martin JA, Ang A, Baker R, Saxon AJ, Ling W, 2013. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START).J. Stud. Alcohol Drugs 74, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Dreifuss JA, Marino EN, Provost SE, Dodd DR, Rice LS, Fitzmaurice GM, Griffin ML, Weiss RD, 2015. The multi-site prescription opioid addiction treatment study: 18-month outcomes. J. Subst. Abuse Treat 48, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK, 2003. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 289, 2370–2378. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD, 2007. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 90, 64–71. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM, 2016. Increases in drug and opioid overdose deaths-United States, 2000–2014. MMWR. Morb. Mortal. Wkly. Rep 64, 1378–1382. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH, 2011. Interim methadone treatment compared to standard methadone treatment: 4-month findings. J. Subst. Abuse Treat 41, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senay EC,Jaffe JH, diMenza S, Renault PF, 1973. A 48-week study of methadone, methadyl acetate and minimal services In: Fisher S, Freeman A (Eds.), Opiate Dependence: Origins and Treatment. Halstead Press, New York, NY, pp. 185–201. [Google Scholar]
- Sigmon SC, Dunn KE, Saulsgiver K, Patrick ME, Badger GJ, Heil SH, Brooklyn JR, Higgins ST, 2013. A randomized, double-blind evaluation of buprenorphine taper duration in primary prescription opioid abusers. JAMA Psychiatry 70, 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, 2006. Characterizing the emerging population of prescription opioid abusers. Am. J. Addict. 15, 208–212. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline followback: a technique for assessing self-reported alcohol consumption In: Litten RZ, Allen JP (Eds.), Measuring Alcohol Consumption Psychosocial and Biochemical Methods. Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Stein MD, Cioe P, Friedmann PD, 2005. Buprenorphine retention in primary care. J. Gen. Intern. Med 20, 1038–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse, Mental Health Services Administration, 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14–4863 Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Tetrault JM, Moore BA, Barry DT, O’Connor PG, Schottenfeld R, Fiellin DA, Fiellin LE, 2012. Brief versus extended counseling along with buprenorphine/naloxone for HIV-infected opioid dependent patients. J. Subst. Abuse Treat 43, 433–439. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME, 2014. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr. Serv 65, 158–170. [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC, 2009. Concurrent validation of the clinical opiate withdrawal scale (COWS) and single-item indices against the clinical institute narcotic assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 105, 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Copersino ML, Prather K, Jacobs P, Provost S, Chim D, Selzer J, Ling W, 2010. Conducting clinical research with prescription opioid dependence: defining the population. Am. J. Addict 19, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W, 2011. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch. Gen. Psychiatry 68, 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Potter JS, Dodd DR, Dreifuss JA, Connery HS, Carroll KM, 2014a. Who benefits from additional drug counseling among prescription opioid-dependent patients receiving buprenorphine-naloxone and standard medical management? Drug Alcohol Depend. 140, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Griffin ML, McHugh RK, Haller D, Jacobs P, Gardin J, Fischer 2nd, Rosen D, 2014b. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J. Subst. Abuse Treat. 47, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Griffin ML, Provost SE, Fitzmaurice GM, McDermott KA, Srisarajivakul EN, Dodd DR, Dreifuss JA, McHugh RK, Carroll KM, 2015. Long-term outcomes from the Nation a Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug Alcohol Depend. 150, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, January 1997. Composite International Diagnostic Interview (CIDI): Core Version 2.1, Geneva, Switzerland. [Google Scholar]