ABSTRACT
Despite the well-known biochemistry of the major pathways involved in central carbon and amino acid metabolism, there are still gaps regarding their regulation or regulatory interactions. Recent research demonstrated the physiological significance of the mitochondrial redox machinery, particularly thioredoxin o1 (TRXo1), for proper regulation of the tricarboxylic acid cycle, components of the mitochondrial electron transport chain and photorespiration. These findings imply that TRXo1 regulation contributes to the metabolic acclimation toward changes in the prevailing environmental conditions. Here, we analyzed if TRXo1 is involved in the light induction of photosynthesis. Our results show that the trxo1 mutant activates CO2 assimilation rates to a significantly lower extend than wild type in response to short-term light/dark changes. Metabolite analysis suggests that activation of glycine-to-serine conversion catalyzed through glycine decarboxylase in conjunction with serine hydroxymethyltransferase in trxo1 is slowed down at onset of illumination. We propose that redox regulation via TRXo1 is necessary to allow the rapid induction of mitochondrial steps of the photorespiratory cycle and, in turn, to facilitate light-induction of photosynthesis.
KEYWORDS: Photorespiration, mitochondria, redox regulation, thioredoxin, light acclimation
Considerable attention has been paid to resolve the biochemistry of the major pathways involved in central carbon and amino acid metabolism, including the Calvin-Benson (CB) cycle,1,2 the tricarboxylic acid (TCA) cycle,3-6 and photorespiration7-9 in plants. Moreover, the physiological significance of these metabolic branches for optimal plant growth has been demonstrated and the enzyme-encoding genes have been well characterized with respect to transcriptional regulation and effector-mediated regulation.10,11,12,6,13 However, there are still open questions regarding potential regulatory mechanisms of enzyme activities, particularly via posttranslational modifications, and the interaction of different pathways to orchestrate plant metabolism.
To regulate metabolic fluxes, especially in response to light/dark transitions, thiol‐disulfide redox changes play the most important role to regulate enzyme activities at the posttranslational level.14 Disulfide bond formation between conserved cysteine residues is, among others, catalyzed by ubiquitous thioredoxins (TRX). Hence, TRX are involved in either the (de)activation of enzymes or contribute to correct folding of proteins.15,16 To date, TRX-mediated enzyme regulation is best studied in chloroplasts. Within this compartment, a multitude of TRX proteins regulate the activity of parts of the photosynthetic electron transport chain and of the CB cycle, whereas the latter becomes activated after onset of illumination through TRX-mediated reduction of disulfide bonds in several participating enzymes.17,18,16,12 Hence, TRX regulation is key for light induction of photosynthetic CO2 assimilation. Moreover, redox-control is also important to regulate the activities of different malate dehydrogenase (MDH) isoforms in various subcellular compartments. For example, NADP-dependent MDH activity in chloroplasts was shown to increase around 100-fold within less than a minute after onset of illumination through redox activation and thus accounts for a major regulatory component to adjust stromal ATP/NADPH ratios and the flux through the photosynthetic C4 cycle.19–21 However, redox regulation of MDH is not restricted to the chloroplast itself but also contributes to the entire cellular malate metabolism via the well-known malate valves to exchange redox equivalents between the different subcellular compartments.22,23 In addition to chloroplasts, plant mitochondria also possess a TRX regulation system. Whereas the TRXo1 protein was found to exclusively localize to mitochondria,24 TRXh2 localization is shared between mitochondria, the endoplasmic reticulum and the cytoplasm.25-27 Recently, both proteins were shown to contribute to the redox regulation of mitochondrial metabolism. Daloso and colleagues28 provided compelling evidence that either TRXo1 or TRXh2 are involved in the regulation of TCA cycle enzyme activities and, thus, are able to modulate the carbon flux through the entire cycle in heterotrophic and photosynthesizing tissue. Moreover, it was demonstrated that lack of TRXo1 affects the in vivo activation state of the alternative oxidase (AOX), constituting for a nonphosphorylated pathway to allow more flexibility to the energy supply via the mitochondrial electron transport chain.29 Finally, TRXo1 and TRXh2 also impact on photorespiration, since both contribute to the redox regulation of the four protein (P, T, H, and L), multienzyme system glycine decarboxylase (GDC), where its regulation was anticipated to mainly occur at the GDC L-protein (mtLPD).30,31 Given that mtLPD is shared between GDC, pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase, and the branched-chain 2-oxoacid dehydrogenase complex,32,33;34 it is likely that other mitochondrial pathways such as the TCA cycle and the degradation of branched chain amino acids might be affected via this mechanism, too.
In light of the multitude of targets of the mitochondrial TRX system, it is likely to assume that TRX are involved in the acclimation of metabolic fluxes toward changes in the prevailing environment. Indeed, Fonseca-Pereira and colleagues,35 showed participation of the mitochondrial TRX system under drought. Additionally, absence of TRXo1-affected carbon metabolism in response to changes in the light intensity.29 Here we analyzed whether or not TRXo1 regulation in mitochondria is somehow involved in the light induction of metabolism, particularly photosynthesis, given that impairment of mitochondrial performance was reported to negatively affect chloroplastidial functions.36,37
Photosynthesis measurements on trxo1 mutant-plants grown under standard conditions did not show major changes.29,31 However, the trxo1 mutant is characterized by lower photosynthetic rates (A) and an increased CO2 compensation point under conditions that require an elevated photorespiratory flux.31 Interestingly, trxo1 mutant plants show also decreased A, if measured in alternating light/dark cycles (Figure 1a). As shown before, A of trxo1 is comparable to the wild type if determined at a light intensity similar to the light applied during plant growth (150–200 µmol m−2 s−1) without previous dark adaption. However, if the measurements were performed after the light was switched off for 15 min and plants were reilluminated at 200 following 500 µmol m−2s−1, a significant decrease in A was seen. The difference was even more pronounced when measured after another two phases of dark incubation, and if measurements were carried out with stepwise increasing light intensities from 50 to 1000 µmol m−2 s−1 (Figure 1a). Despite the changes in photosynthesis, very minor effects on dark respiration (Rd) were observed during our experiment (Figure 1a). Given that photorespiration and photosynthesis form an overlapping network, and both rates show positive correlation,39,40 we assumed absence of proper redox regulation of photorespiration at the GDC/serine hydroxymethyltransferase (SHMT) step might impair the flux through photorespiration and in turn photosynthesis. Indeed, the quantification of both metabolites involved in the GDC/SHMT reaction cycle, glycine and serine, respectively, revealed that lack of TRXo1 affects glycine-to-serine conversion. As expected, no changes were found in the dark (inactive photorespiration). However, after onset of illumination on dark-incubated plants for 2 and 5 min (active photorespiration), trxo1 leaves accumulated significantly increased glycine contents compared to wild type, whereas the serine levels showed the opposite behavior, that is, they were lower in trxo1 at both time points (significant after 5 min). Interestingly, elevated glycine accumulation and the decrease in serine disappeared 30 min after light was switched on (Figure 1b). Such unaltered levels in both amino acids are in agreement with our previous metabolite analysis of trxo1 at later stages in the light phase.31
Figure 1.
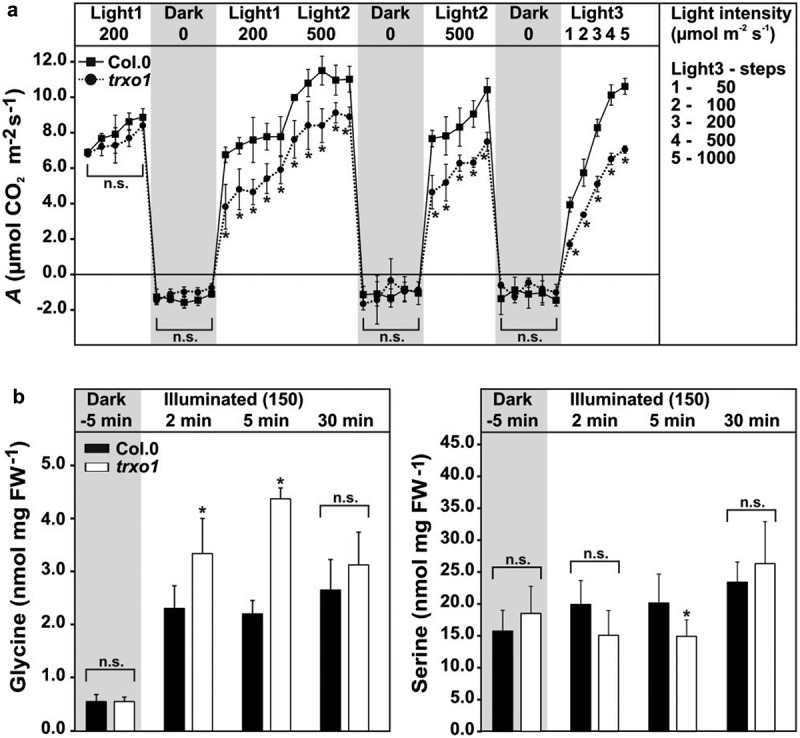
Light acclimation of photosynthesis and absolute glycine and serine contents in leaves of the wild type and the trxo1 mutant. Depicted are (a) net CO2 uptake (A) and dark respiration (Rd) rates of wild-type and trxo1 plants grown in normal air (390 ppm CO2) to growth stage 5.138 with a 12/12 h day/night cycle (20/18°C) and a light intensity of 150 µmol m−2 s−1. Fully expanded leaves were incubated into the measuring chamber of a Licor-6400. Then, A and Rd were determined for at least 15 min in each condition during alternating light/dark cycles as indicated. Absolute glycine and serine contents (b) were determined by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) as described previously.31 Plants were grown under the same conditions as indicated above and leaf-material harvested in the end of the dark phase (5 min prior onset of illumination) and 2, 5, and 30 min after light was switched on (150 µmol m−2 s−1). Shown are mean values ± SD from three independent biological replicates. Asterisks indicate significant alterations of the trxo1 mutant compared to the wild type according to Student’s t test (*p < .05, n.s. – not significant).
Collectively, the results presented here suggest that TRXo1-mediated redox regulation is essential for short-term acclimation of mitochondrial metabolism, mainly activation of the photorespiratory GDC/SHMT reaction cycle after onset of illumination. Hence, the mitochondrial TRX system is a pivotal feature for rapid light induction of photosynthesis (Figure 1a). Adaptation to fluctuations in light intensities might also involve the TRXo1 protein as previously also suggested by Florez-Sarasa et al.29 However, on the longer time scale, mitochondria are able to adjust their metabolism to alterations in light intensities including adjustment in the transcriptional and translational regulation of photorespiration as reported previously41 and also in the absence of TRXo1. Currently, we assume that other TRX proteins, presumably TRXh2,30 compensate for the loss of TRXo1 to prevent from severe damage to mitochondrial metabolism. To fully elucidate potential redundancy within the mitochondrial thiol redox system future work is needed, including the production of multiple mutants and comprehensive analysis under different environmental conditions.
Acknowledgments
We wish to thank Danilo M. Daloso (University of Fortaleza) and Wagner L. Araújo (University of Viscosa) for a fruitful collaboration on the topic of redox regulation of mitochondrial metabolism and Alisdair R. Fernie (Max Planck Institute for Molecular Plant Physiology, Golm) for the trxo1 mutant and his long-term support. This work was financially supported by the University of Rostock. The LC-MS/MS equipment at the University of Rostock used during this study was financed through the HBFG program (GZ: INST 264/125-663 1 FUGG).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Raines CA. The Calvin cycle revisited. Photosynth Res. 2003;75(1):1–4. doi: 10.1023/A:1022421515027. [DOI] [PubMed] [Google Scholar]
- 2.Bassham BA, Benson AA, Calvin M. The path of carbon in photosynthesis. J Biol Chem. 1950;185:781–787. [PubMed] [Google Scholar]
- 3.Zhang S, Bryant DA. The tricarboxylic acid cycle in cyanobacteria. Science. 2011;334(6062):1551–1553. doi: 10.1126/science.1210858. [DOI] [PubMed] [Google Scholar]
- 4.Beevers H. Respiratory metabolism in plants. New York, NY: Harper and Row; 1961. [Google Scholar]
- 5.Sweetlove LJ, Beard KFM, Nunes-Nesi A, Fernie AR, Ratcliffe RG. Not just a cycle: flux modes in the plant TCA cycle. Trends Plant Sci. 2010;15(8):462–470. doi: 10.1016/j.tplants.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Nunes-Nesi A, Araújo WL, Obata T, Fernie AR. Regulation of the mitochondrial tricarboxylic acid cycle. Curr Opin Plant Biol. 2013;16(3):335–343. doi: 10.1016/j.pbi.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Ogren WL, Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nature New Biol. 1971;230:159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- 8.Bauwe H, Hagemann M, Fernie AR. Photorespiration: players, partners and origin. Trends Plant Sci. 2010;15:330–336. doi: 10.1016/j.tplants.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Bauwe H, Hagemann M, Kern R, Timm S. Photorespiration has a dual origin and manifold links to central metabolism. Curr Opin Plant Biol. 2012;15:269–275. doi: 10.1016/j.pbi.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Fridlyand LE, Scheibe R. Regulation of the Calvin cycle for CO2 fixation as an example for general control mechanisms in metabolic cycles. Biosyst. 1999;2:79–93. doi: 10.1016/S0303-2647(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 11.Timm S, Florian A, Wittmiß M, Jahnke K, Hagemann M, Fernie AR, Bauwe H. Serine acts as metabolic signal for the transcriptional control of photorespiration-related genes in Arabidopsis thaliana. Plant Physiol. 2013;162::379–389. doi: 10.1104/pp.113.215970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelet L, Zaffagnini M, Morisse S, Sparla F, Pérez-Pérez ME, Francia F, Danon A, Marchand CH, Fermani S, Trost P, et al. Redox regulation of the Calvin–benson cycle: something old, something new. Front Plant Sci. 2013;4:470. doi: 10.3389/fpls.2013.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leegood RC, PJ L, Adcock MD, Häusler RE. The regulation and control of photorespiration. J Exp Bot. 1995;46:1397–1414. doi: 10.1093/jxb/46.special_issue.1397. [DOI] [Google Scholar]
- 14.Mock HP, Dietz KJ. Redox proteomics for the assessment of redox‐related posttranslational regulation in plants. Biochimica Et Biophysica Acta, Proteins and Proteomics. 2016;1864:967–973. doi: 10.1016/j.bbapap.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer Y, Buchannan BB, Vignols F, Recihheld JP. Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu Rev Genet. 2009;43:335–367. doi: 10.1146/annurev-genet-102108-134201. [DOI] [PubMed] [Google Scholar]
- 17.Hashida SN, Kawai-Yamada M. Inter-organelle NAD metabolism underpinning light responsive NADP dynamics in plants. Front Plant Sci. 2019;10:960. doi: 10.3389/fpls.2019.00960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geigenberger P, Thormählen I, Daloso DM, Fernie AR. The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci. 2017;22:249–262. doi: 10.1016/j.tplants.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Ashton AR, Hatch MD. Regulation of C4 photosynthesis: regulation of activation and inactivation of NADP-malate dehydrogenase by NADP and NADPH. Arch Biochem Biophys. 1983;227:416–425. doi: 10.1016/0003-9861(83)90471-x. [DOI] [PubMed] [Google Scholar]
- 20.Miginiac-Maslow M, Lancelin JM. Intrasteric inhibition in redox signalling: light activation of NADP-malate dehydrogenase. Photosynth Res. 2002;72:1–12. doi: 10.1023/A:1016099228450. [DOI] [PubMed] [Google Scholar]
- 21.Scheibe R. NADP-malate dehydrogenase in C3 plants: regulation and role of a light-activated enzyme. Physiol Plant. 1987;71:393–400. doi: 10.1111/j.1399-3054.1987.tb04362.x. [DOI] [Google Scholar]
- 22.Selinski J, Scheibe R. Malate valves: old shuttles with new perspectives. Plant Biol. 2018;21:21–30. doi: 10.1111/plb.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheibe R. Malate valves to balance cellular energy supply. Physiol Plant. 2004;120: 21–26. [DOI] [PubMed] [Google Scholar]
- 24.Laloi C, Rayapuram N, Chartier Y, Grienenberger JM, Bonnard G, Meyer Y. Identification and characterization of a mitochondrial thioredoxin system in plants. Proc Natl Acad Sci U S A. 2001;98:14144–14149. doi: 10.1073/pnas.241340898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus F, Chamberlain SH, Chu C, Masiarz FR, Shin S, Yee BC, Buchanan BB. Plant thioredoxin h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys. 1991;287:195–198. doi: 10.1016/0003-9861(91)90406-9. [DOI] [PubMed] [Google Scholar]
- 26.Bodenstein-Lang J, Buch A, Follmann H. Animal and plant mitochondria contain specific thioredoxins. FEBS Lett. 1989;258:22–26. doi: 10.1016/0014-5793(89)81606-0. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan BB. The path to thioredoxin and redox regulation beyond chloroplasts. Annu Rev Plant Biol. 2017;58:1–24. [DOI] [PubMed] [Google Scholar]
- 28.Daloso DM, Müller K, Obata T, Florian A, Tohge T, Bottcher A, Riondet C, Bariat L, Carrari F, Nunes-Nesi A, et al (2015) Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proceedings of the National Academy of Sciences of the United States of America, 112: 1392–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florez-Sarasa I, Obata T, Del-Saz NF, Reichheld JP, Meyer EH, Rodriguez-Conception M, Ribas-Carbo M, Fernie AR. The lack of mitochondrial thioredoxin TRXo1 affects in vivo alternative oxidase activity and carbon metabolism under different light conditions. Plant Cell Physiol. 2019. doi: 10.1093/pcp/pcz123. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca-Pereira P, Souza PVL, Hou LY, Schwab S, Geigenberger P, Nunes-Nesi A, Timm S, Fernie AR, Thormählen I, Araújo WL, et al. Thioredoxin h2 contributes to the redox regulation of mitochondrial photorespiratory metabolism. Plant Cell Environ. 2019a. doi: 10.1111/pce.13640. [DOI] [PubMed] [Google Scholar]
- 31.Reinholdt R, Schwab S, Zhang Y, Reichheld JP, Fernie AR, Hagemann M, Timm S. Redox-regulation of photorespiration through mitochondrial thioredoxin o1. Plant Physiol. 2019. doi: 10.1104/pp.19.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timm S, Wittmiß M, Gamlien S, Ewald R, Florian A, Frank M, Wirtz M, Hell R, Fernie AR, Bauwe H. Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. Plant Cell. 2015;27:1968–1984. doi: 10.1105/tpc.15.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mooney BP, Miernyk JA, Randall DD. The complex fate of alpha-ketoacids. Annu Rev Plant Biol. 2002;53:357–375. doi: 10.1146/annurev.arplant.53.100301.135251. [DOI] [PubMed] [Google Scholar]
- 34.Douce R, Bourguignon J, Neuburger M, Rébeillé F. The glycine decarboxylase system: a fascinating complex. Trends Plant Sci. 2001;6:167–176. doi: 10.1016/S1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- 35.Fonseca-Pereira P, Daloso DM, Gago J, de O SFM, Condori-Apfata JA, Florez-Sarasa I, Tohge T, Reichheld JP, Nunes-Nesi A, Fernie AR, et al. The mitochondrial thioredoxin system contributes to the metabolic responses under drought episodes in Arabidopsis. Plant Cell Physiol. 2019b;60:213–229. doi: 10.1093/pcp/pcy194. [DOI] [PubMed] [Google Scholar]
- 36.Nunes-Nesi A, Sulpice R, Gibon Y, Fernie AR. The enigmatic contribution of mitochondrial function in photosynthesis. J Exp Bot. 2008;59:1675–1684. doi: 10.1093/jxb/ern002. [DOI] [PubMed] [Google Scholar]
- 37.Padmasree K, Padmavathi L, Raghavendra AS. Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit Rev Biochem Mol Biol. 2002;37:71–119. doi: 10.1080/10409230290771465. [DOI] [PubMed] [Google Scholar]
- 38.Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Gorlach J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/tpc.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timm S, Florian A, Fernie AR, Bauwe H. The regulatory interplay between photorespiration and photosynthesis. J Exp Bot. 2016;67:2923–2929. doi: 10.1093/jxb/erw083. [DOI] [PubMed] [Google Scholar]
- 40.Obata T, Florian A, Timm S, Bauwe H, Fernie AR. On the metabolic interaction of (photo)respiration. J Exp Bot. 2016;67:3003–3014. doi: 10.1093/jxb/erw128. [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Jacoby RP, Shingaki-Wells RN, Li L, Millar AH. Differential induction of mitochondrial machinery by light intensity correlates with changes in respiratory metabolism and photorespiration in rice leaves. New Phytologist. 2013;198:103–115. doi: 10.1111/nph.12123. [DOI] [PubMed] [Google Scholar]


