ABSTRACT
Background: A western high fat, high carbohydrate diet has been shown to be associated with decreased gut bacterial diversity and reductions in beneficial bacteria. This gut bacteria dysbiosis could develop in early life and contribute to chronic disease risk such as obesity, type 2 diabetes and non-alcoholic fatty liver disease.
Objective: To determine how dietary macronutrients are associated with the relative abundance of gut bacteria in healthy adolescents.
Methods: Fifty-two obese participants (12–19 years) from two studies, many who were primarily of Hispanic background, provided fecal samples for 16S rRNA gene sequencing. Dietary macronutrients were assessed using 24-hour diet recalls and body composition was assessed using DEXA. General regression models assuming a negative binomial distribution were used to examine the associations between gut bacteria and dietary fiber, saturated fat, unsaturated fats, protein, added sugar, total sugar and free fructose after adjusting for age, gender, race/ethnicity, body fat percentage, study and caloric intake.
Results: The genera Eubacterium (Benjamini-Hochberg (BH) corrected p-value = 0.10) and Streptococcus (BH corrected p-value = 0.04) were inversely associated with dietary fructose intake. There were no other significant associations between abundances of gut microbes and other dietary macronutrients, including fiber, fat, protein, total sugar or added sugar.
Conclusions: High dietary fructose was associated with lower abundance of the beneficial microbes Eubacterium and Streptococcus, which are involved with carbohydrate metabolism.
KEYWORDS: Fructose, macronutrients, nutrition, gut microbiota, adolescents, Eubacterium, Streptococcus
Introduction
The human gut is home to thousands of bacterial species known as microbiota, which have been shown to contribute to host immunity, nutrient metabolism, growth, and energy harvesting.1–4 In healthy adults, the gut is dominated by the phyla Bacteroidetes, Firmicutes and Proteobacteria.5 While there is still much to be understood about the gut microbiota, previous studies show that in healthy adolescents the composition of the gut is primarily made of the genera Bacteroides (Bacteriodetes), Faecalibacterium (Firmicutes), Alistipes (Bacteroidetes) and Bifidobacterium (Actinobacteria).6,7 Along with age, dietary factors impact the gut microbiota, 8 suggesting that different microbes are needed to handle metabolism of dietary macronutrients.9–11 It has been shown that human microbial communities can be divided into two prominent clusters (known as enterotypes).12 Examples include the Bacteroides enterotype, which is associated with high consumption of animal protein and saturated fat consumption, and the Prevotella enterotype, which is associated with a diet high in carbohydrates and simple sugars.13
Studies in animal models have demonstrated that the composition of gut microbiota is related to macronutrient intake.14–16 One study found that kittens who consumed a high protein low carbohydrate diet had a lower abundance of Bifidobacterium compared to those consuming a moderate protein and carbohydrate diet, which is a genus of bacteria has been linked to decreased intestinal health.14 In a murine model, a high fat diet resulted in a microbiota that had a low percentage of Bacteroidetes and higher percentages of Firmicutes and Proteobacteria.15 Lastly, a recent study by our group found that rats with ad libitum access to a sugar solution had a higher abundance of pathogenic bacteria, including the phylum Proteobacteria, compared to rats given a water control.16 Generally among mammals, not only has it been shown that carnivores and herbivores have distinct gut microbial communities but there is also a distinction between carnivores and omnivores, suggesting that the digestion of complex plant-based carbohydrates helps to shape the composition of the gut microbiota.8 Despite this, few studies have examined the associations between specific dietary macronutrients and the composition of the gut microbiota in adolescents.
Differences in the gut microbial composition by diet can be due to the specific gut microbes that are needed to metabolize certain nutrients. In humans, gut microbiota influences health as inefficient metabolism has been linked to metabolic diseases. Several studies support the link between gut microbiota and chronic diseases, including obesity17–20 and type 2 diabetes.21–23 Additionally, poor diet habits such as the high consumption of soft drinks has been shown to contribute to obesity during adolescence.24,25 Therefore, it is important to examine the association of macronutrient intake and the composition of gut microbiota during adolescence. Thus, the aim of this study was to determine the associations between dietary macronutrient intake and the compositional abundance of gut microbiota in adolescents. Based on previous studies, 26 we hypothesized that in our cohort, a high abundance of the Prevotella-dominant enterotype would be associated with a high consumption of carbohydrates while a Bacteroides-dominant enterotype would be associated with a high consumption of protein and fat.
Results
As shown in Table 1, participants included 16 overweight and 36 obese adolescents with a mean age of 17.3 years. We aimed to determine the association between macronutrients intake and gut microbiota in these participants. When examining the gut microbiota, a total of 6,009,400 sequence reads were mapped to Operational Taxonomic Units (OTUs) (Supplemental Table 1). Most of the reads mapping to the phyla Bacteroidetes, Firmicutes and Actinobacteria (Supplemental Figure 1–2 & Supplemental Table 2) and at the genus level the taxa with the highest number of the reads were mapped to the genera Bacteroides, Prevotella, Ruminococcus and Blautia. (Supplemental Figure 1–2 & Supplemental Table 3). Most of the cohort exhibited a Bacteroides enterotype (71% of cohort) while others had a Prevotella enterotype (29% of cohort) (Supplemental Figure 3).
Table 1.
Demographic characteristics of participants included in this study.
| General Characteristics (N = 52) |
Mean (SD*) |
|---|---|
| Age (years) | 17.3 (2.4) |
| Sex (female/male) | 23/29 |
| BMI (kg/m2) | 32.7 (5.35) |
| BMI Category (%) | |
| Overweight | 30.8 |
| Obese | 69.2 |
| Hispanic (%) | 82.7 |
Figure 1.
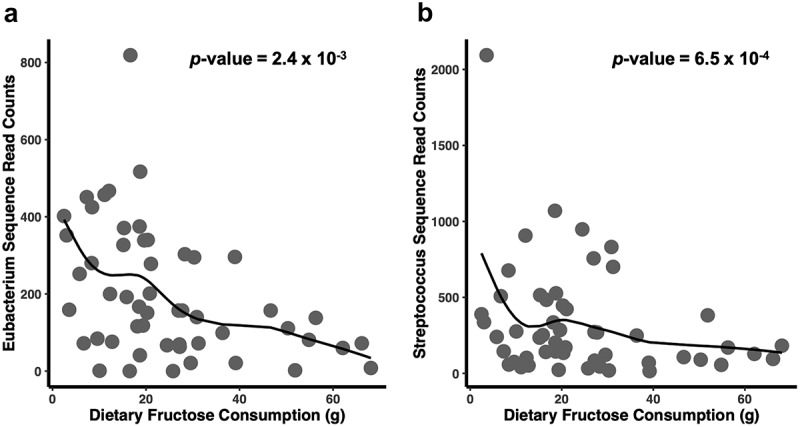
The genera Eubacterium and Streptococcus are negatively associated with fructose intake. Fructose intake was measured using self-reported 24-hour dietary recalls and abundance of genera was computed using the number of 16S rRNA sequence reads that mapped to the genera. P-values were calculated using negative binomial regression models.
Table 2.
Average dietary intake of participants included in this study.
| Macronutrient intake | Absolute Mean (SD) | Percent Caloric Intake Mean (SD) |
|---|---|---|
| Total caloric intake (kcal) | 1854.3 (649.8) | - |
| Total carbohydrates (g) | 239.7 (87.9) | 51.6 (9.2) |
| Total sugar (g) | 100.0 (49.7) | 21.9 (9.9) |
| Added sugar (g) | 57.2 (42.6) | 12.2 (8.5) |
| Fructose (g) | 24.8 (16.7) | 5.6 (3.9) |
| Glucose (g) | 22.9 (14.7) | 5.1 (3.1) |
| Fiber (g) | 16.1 (6.7) | 3.7 (1.5) |
| Total fat (g) | 68.8 (32.6) | 31.8 (7.4) |
| Saturated fat (g) | 23.2 (12.0) | 11.1 (3.6) |
| Unsaturated fat (g) | 39.7 (21.4) | 18.9 (5.5) |
| Monounsaturated fat (g) | 23.3 (10.7) | 11.2 (3.2) |
| Polyunsaturated fat (g) | 16.4 (13.8) | 7.7 (3.7) |
| Total protein (g) | 74.3 (27.3) | 16.5 (4.5) |
There were only two significant relationships between macronutrients and individual microbes when fitting the negative binomial distribution regression models, but these were only observed with dietary fructose (Supplemental Table 3 & 4). At the taxonomic rank of genus, there was an inverse association between dietary fructose intake and the genera Eubacterium (p-value = 2.4x10−3, Benjamini-Hochberg (BH) corrected p-value = 0.10, effect size = −0.03) and Streptococcus (p-value = 6.5x10−4, BH corrected p-value = 0.04, effect size = −0.03) (Figure 1). At the OTU level, we found that fructose consumption was negatively associated with an OTU with sequence reads mapping to Eubacterium eligens (p-value = 2.4x10−3, BH corrected p-value = 0.10, effect size = −0.03) and an OTU with reads mapping to Streptococcus thermophilus (p-value = 4.3x10−5, BH corrected p-value = 0.003, effect size = −0.33). These relationships remained significant after controlling for total caloric intake, body fat percent, sex, race/ethnicity, study and age. The results were also consistent when the assessment of fructose intake is the percent of fructose intake relative to caloric intake instead of absolute fructose intake (Eubacterium: p-value = 5.5x10−3, Benjamini-Hochberg (BH) corrected p-value = 0.18, effect size = −0.12; Eubacterium eligens: p-value = 6.5x10−3, Benjamini-Hochberg (BH) corrected p-value = 0.15, effect size = −0.12; Streptococcus: p-value = 1.6x10−3, Benjamini-Hochberg (BH) corrected p-value = 0.07, effect size = −0.11; Streptococcus thermophilus: p-value = 1.6x10−4, Benjamini-Hochberg (BH) corrected p-value = 0.01, effect size = −0.15). Other dietary macronutrients were examined, including intake of fiber, protein, saturated and unsaturated fats, total carbohydrates and total sugar were not associated with the overall composition and diversity of the gut microbiota (Supplemental Table 5). As expected, there was a negative association with protein intake and enterotype (measured as Prevotella: Bacteroides ratio) (p-value = 6.8x10−3, effect size = −0.02). However, carbohydrate or fat intake was not associated with enterotype (Supplemental Table 6).
Discussion
The aim of this study was to identify associations between individual dietary macronutrients and components of the gut microbiome of adolescents. Results suggest that dietary fructose intake is negatively associated with the abundance of the bacterial species Eubacterium eligens. It is known that E. eligens, along with other members of the phylum Firmicutes, have fewer polysaccharide-degrading enzymes than those members of the phylum Bacteroidetes.27 One study showed that the related species Eubacterium rectale, which is involved in butyrate production, is decreased in mice that were fed a high sugar diet.27 Results shown here also suggest that dietary fructose consumption is negatively associated with microbes belonging to the genus Streptococcus, including the species Streptococcus thermophilus. Streptococcus thermophilus has been shown to ferment lactose and sucrose and can also metabolize the monosaccharide fructose.28 However, the link between fructose and the overall genus of Streptococcus is still largely unexplored. Remarkably, while the specific species Streptococcus thermophilus is known to be non-pathogenic, the microbes belonging to the genus Streptococcus was associated with the development of multiple metabolic disorders.29 Results from the current study suggest that high levels of fructose could also be related to low abundances of the beneficial bacteria Streptococcus thermophilus.
A primary strength of this study is the use of detailed dietary questionnaires in conjunction with characterization of the gut microbiota in overweight and obese adolescents. Our cohort was made of over 80% Hispanic participants. Our results show that this population has a different microbial profile than previously published studies examining the gut microbiomes of adolescents. While previous studies that are conducted in mostly healthy Caucasian pre-adolescents and adolescents show that the gut is dominated by the genera Bacteroides, Faecalibacterium and Bifidobacterium, our cohort is dominated by the genera Bacteroides, Prevotella and Ruminoccus6,7 with relatively low levels of Bifidobacterium (an average of 4.6% of composition versus an average of 9.0% in previous studies). A study examining the gut microbiome of Mexican children aged 6–12 lacked Bifidobacterium30 implying that the difference in gut microbial composition of the adolescents could be impacted by cultural, genetic and environmental differences in the cohorts. Our study examines overweight and obese Hispanic adolescents essentially adding to the literature by characterizing their gut microbiome and showing the impact of diet on the gut microbiome in this diverse cohort. This is important because diverse populations have diverse dietary patterns, health outcome sand eating behavior that can impact or is impacted by the gut microbiome. However, a limitation of exclusively including overweight/obese participants is that we do not have a sufficient sized healthy cohort to compare our results. Also, while there may have been under or over reporting of dietary fructose, this study utilized 24-hour diet recalls with the multi-pass method that has been shown to increase the accuracy of dietary reports.31 There is also difficulty in assessing dietary fructose levels because the true amount of fructose in a soft drinks is unknown and previous lab analysis by our group suggests we might be underestimating dietary fructose in products.32 It must be noted that in our study, while there was a negative association between Eubacterium and Streptococcus abundance and fructose intake, there was a subset of participants who consumed lower dietary fructose but also had a low Eubacterium and Streptococcus abundance. Also, this study is limited by its relatively small sample size. As such, larger studies are needed to confirm the observed relationships dietary fructose intake with Streptococcus and Eubacterium. Repeated measures of fecal samples and longitudinal studies are also necessary because these could help to lower variances in any associations found. Additionally, future animal studies could be used to determine how strongly the association between Eubacterium and Streptococcus and fructose exists in a controlled environment system. Increased levels of these microbes are associated with the increase of short chain fatty acids33 and degradation of dietary fiber,9 however it is unknown whether depletion of these two microbes affects metabolic outcomes. As a follow-up, it would be of great interest to determine if the production of short chain fatty acids is reduced by the increase consumption of fructose.
In the United States, there is an increase of adolescent obesity that has paralleled the increase of dietary fructose intake in the form of fruit juice and sugar-sweetened beverages sweetened with high-fructose corn syrup (HFCS).25 HFCS is a liquid sweetener that is made of a combination of fructose and glucose, usually 55% fructose and 45% glucose34 however, our group has conducted laboratory measures showing that there is higher than expected fructose in HFCS sweetened beverages and in fruit juice.33,35 Soft-drinks are the primary source of HFCS in our diets but it is also in present in breakfast cereals, jams, and canned drinks.36 The impact of HFCS has been well documented in numerous studies. In one study by Bocarsly et al., rats that were given access to HFCS not only gained significantly more weight than their counterparts who were given equal access to sucrose, but also had higher triglyceride levels and more abdominal fat.37 Bocarsly et al. also showed that although fructose and glucose are present in similar proportions in the blood stream, the two sugars had different effects on weight gain. This difference could be because fructose from HFCS is metabolized at an earlier point than that of sucrose which could result in unregulated creation of carbon molecules that are transformed into fatty acids.38 It is possible that gut microbiota is involved with this transformation.
While there has been several studies showing an association between gut microbes and dietary fructose in rodents, 39–41 to our knowledge, this is the first report of negative associations between dietary fructose and non-pathogenic microbes in a cohort of adolescents with a high percentage of overweight/obese participants. These associations appeared to be specific to dietary fructose, as the composition of the gut microbiota was not associated with dietary protein, fats, or added sugars and total sugars. In summary, in our cohort there was not a relationship between the two enterotypes and individual macronutrient intake. However, results from this study show that independent of sex, race/ethnicity, body fat percentage and total caloric intake, increased dietary fructose intake was associated with lower levels of gut bacterial taxa that have been shown to be involved in carbohydrate metabolism, including the genera Eubacterium and Streptococcus.
Materials and methods
Participants
This study included 58 participants from two studies using identical methods in the collection of dietary recalls, clinical assessments and fecal samples to quantify gut microbiota. Briefly, 18 were obese Hispanic adolescents (12–19 years of age) from the baseline visit of a 16-week parallel, double-blind and placebo-controlled trial examining the efficacy of probiotic supplementation in changing gut microbiota (clinical trial registered at www.clinicaltrials.gov: NCT03115385).42 Participants also included 40 adolescents (17–19 years of age, 72.5% are Hispanic and 85% overweight/obese) who were recruited from the ongoing Meta-AIR (Metabolic and Asthma Incidence Research) study at the University of Southern California between 2014–2016, which aims to elucidate the impact of environmental exposures and metabolic outcomes during adolescence as previously described.23 Written parental consent and child assent for inclusion in these studies were obtained prior to any testing procedure for participants under 18 years of age. The University of Southern California Institutional Review Board approved that these studies were conducted in accordance with the Declaration of Helsinki.
Dietary recall
To assess mean daily intakes of energy, fiber, protein, fat, carbohydrate, sugars and free fructose, 24-hour diet recalls were collected and analyzed using Nutrition Data System for Research (NDSR) software (version 2014). Two 24-hour diet recalls (1 weekend and 1 weekday) were collected from 70% of the participants while the rest provided one 24-hour diet recall. There were four participants whose dietary recalls was removed from further analysis because of low total energy intake (< 600 kcal/day if female and < 800 kcal/day if male).43
Sequencing and taxonomic assignment
Fecal samples were collected using commercial collection kits developed by Second Genome (South San Francisco, CA) that contained a preservative, and the samples were stored at – 80°C immediately after receipt. If a participant was unable to provide the fecal sample in person at the study visit, then the study team provided a prepaid envelope containing the collection kit so the participant could mail their sample to the lab within 1–2 days of the study visit; all samples were received at the lab within 5 days of the visit. The relative abundance of bacterial taxa was determined using 16S rRNA amplicon sequencing conducted by Second Genome (San Francisco, CA). Briefly, nucleic acid isolation was performed with the MoBio PowerMag® Microbiome kit (Carlsbad, CA) according to manufacturer’s guidelines and optimized for high-throughput processing. All samples were quantified via the Qubit® Quant-iT dsDNA High Sensitivity Kit (Invitrogen, Life Technologies, Grand Island, NY) to ensure that they met minimum concentration and mass of DNA. To enrich the sample for bacterial 16S V4 rDNA region, DNA was amplified utilizing V4 fusion primers described by Caporaso et al.44 The complete sequences of the primers were: Forward – 5ʹ GTGCCAGCMGCCGCGGTAA 3ʹ and Reverse – 5ʹ GGACTACHVGGGTWTCTAAT 3ʹ. Samples that met the post-PCR quantification minimum and were advanced for pooling and sequencing on the Illumina Miseq v3 sequencer platform. The 16S rDNA sequence reads were quality filtered, clustered into operational taxonomic units (OTUs) with a shared 97% identity by UPARSE (de novo OTU clustering), and a representative consensus sequence per de novo OTU was aligned against the Greengenes reference database (version 13.5)45 and assigned taxonomy to determine community profiles. The UPARSE clustering algorithm comprises a chimera filtering and discards likely chimeric OTUs. All non-strain sequences that passed the quality filtering were mapped to the representative consensus sequences to generate an abundance table for de novo OTUs.
Statistical analysis
Linear regression models were used to determine the association between microbial abundance and macronutrient intake. Negative binomial linear models were conducted using the function “gamlss” with “NBI” family and default parameters in the R package “gamlss” .46 This model allows for more accurate modeling by allowing for non-linear relationships and modeling of the high amount of low abundant taxa. The “gamlss” (Generalized Additive Models for Location, Scale and Shape) package does this by accounting for the mean and variance of the outcome variable in the model. Sequence read counts of taxa, diversity measures (Shannon Diversity, Simpson Diversity, evenness and richness) and enterotype (measured as Prevotella sequence read counts/(Prevotella sequence read counts + Bacteroides sequence read counts)), were each used as the outcome variable with an offset variable for read counts as an input variable in order to normalize the read counts when necessary. Standardized outcome variables were regressed against standardized dietary intake and the coefficient of the dietary variable from this model was used as a measure of the effect size. All models considered individual dietary macronutrient intake as an input variable and adjusted for caloric intake, body fat percentage from whole body dual-energy x-ray absorptiometry (DEXA) scans, sex, study, age, and Hispanic race/ethnicity based on self-report. Additionally, we repeated these models on a subset of the cohort including only Hispanic participants. Benjamini-Hochberg (BH) corrected p-values with a false discovery rate that is less than 10% when were considered to be significant.
Funding Statement
This work was supported by L. K. Whittier Foundation [003457]; National Institute of Environmental Health Sciences [P30ES007048]; National Institute of Environmental Health Sciences [P01ES022845]; National Institute of Environmental Health Sciences [R00 ES027853].
Acknowledgments
T.A., F.G., and M.G. designed study; T.A., J.K., F.G., and M.G. and conducted research; J.M. provided statistical input; R.J. analyzed data; and R.J. wrote the paper. R.J. had primary responsibility for final content. All authors read and approved the final manuscript.
Disclosure of interest
We disclose that no financial interest or benefit that has arisen from the direct applications of this research.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Azcarate-Peril MA, Sikes M, Bruno-Barcena JM.. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301: G401–24. doi: 10.1152/ajpgi.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boleij A, Tjalsma H. Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer. Biol Rev Camb Philos Soc. 2012;87: 701–730. doi: 10.1111/j.1469-185X.2012.00218.x. [DOI] [PubMed] [Google Scholar]
- 3.Lepage P, Leclerc MC, Joossens M, Mondot S, Blottière HM, Raes J, Ehrlich D, Doré J. A metagenomic insight into our gut’s microbiome. Gut. 2013;62: 146–158. doi: 10.1136/gutjnl-2011-301805. [DOI] [PubMed] [Google Scholar]
- 4.Vipperla K, O’Keefe SJ. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr Clin Pr. 2012;27: 624–635. doi: 10.1177/0884533612452012. [DOI] [PubMed] [Google Scholar]
- 5.Human Microbiome PC. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486: 207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol. 2011;77: 404–412. doi: 10.1111/j.1574-6941.2011.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta T-A, Raza S, Doddapaneni HV, Metcalf GA, Muzny DM, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3: 36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, et al. Evolution of mammals and their gut microbes. Science. 2008;320: 1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K, DeCoffe D, Molcan E, Gibson DL, Bocarsly ME, Powell ES, Avena NM, Hoebel BG. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4: 1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fodor A, Winglee K. Intrinsic association between diet and the gut microbiome: current evidence. Nutr Diet Suppl. 2015;69. doi: 10.2147/NDS.S62362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. . Human nutrition, the gut microbiome, and immune system: envisioning the future. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen -Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334: 105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooda S, Vester Boler BM, Kerr KR, Dowd S, Swanson K. The gut microbiome of kittens is affected by dietary protein: carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br J Nutr. 2013;109:1637–1646. doi: 10.1017/S0007114512003479 [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand F, Nguyen TL, Brinkman B, Yunta R, Cauwe B, Vandenabeele P, Liston A, Raes J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013;14: R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble EE, Hsu TM, Jones RB, Fodor AA, Goran MI, Kanoski SE. Early-life sugar consumption affects the rat microbiome independently of obesity. J Nutr. 2016;147:20–28. doi: 10.3945/jn.116.238816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado MV, Cortez-Pinto H. Diet, microbiota, obesity, and NAFLD: a dangerous quartet. Int J Mol Sci. 2016;17: 481. doi: 10.3390/ijms17040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444: 1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 19.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. [DOI] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon, JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3: 213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57: 1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 22.Devaraj S, Hemarajata P, Versalovic J. The human gut microbiome and body metabolism: implications for obesity and diabetes. Clin Chem. 2013;59: 617–628. doi: 10.1373/clinchem.2012.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurmann F, Gilliland FD, Goran MI. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ Res. 2018;161:472–478. doi: 10.1016/j.envres.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington S. The role of sugar-sweetened beverage consumption in adolescent obesity: a review of the literature. J Sch Nurs. 2008;24: 3–12. doi: 10.1177/10598405080240010201. [DOI] [PubMed] [Google Scholar]
- 25.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117: 673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- 26.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen -Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334: 105–108. http://science.sciencemag.org/content/334/6052/105.full. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, et al.Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106(14):5859–5864. doi: 10.1073/pnas.0901529106 http://www.pnas.org/content/106/14/5859.full.pdf [DOI] [PMC free article] [PubMed]
- 28.Hutkins RW, Morris HA. Carbohydrate metabolism by streptococcus thermophilus: a review. J Food Prot. 1987;50: 876–884. doi: 10.4315/0362-028X-50.10.876. [DOI] [PubMed] [Google Scholar]
- 29.Zeng H, Ishaq SL, Zhao F-Q, Wright ADG. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice.J Nutr Biochem. 2016;35:30–36. doi: 10.1016/j.jnutbio.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 30.López-Contreras BE, Morán-Ramos S, Villarruel-Vázquez R, Macías-Kauffer L, Villamil-Ramírez H, León-Mimila P, Vega-Badillo J, Sánchez-Muñoz F, Llanos-Moreno LE, Canizalez-Román A, et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr Obes. 2018;13: 381–388. doi: 10.1111/ijpo.12262. [DOI] [PubMed] [Google Scholar]
- 31.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104: 595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity. 2011;19: 868–874. doi: 10.1038/oby.2010.255. [DOI] [PubMed] [Google Scholar]
- 33.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10: 323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White JS. Straight talk about high-fructose corn syrup: what it is and what it ain’t. Am J Clin Nutr. 2008;88: 1716S–1721S. doi: 10.3945/ajcn.2008.25825B. [DOI] [PubMed] [Google Scholar]
- 35.Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition. 2014;30: 928–935. doi: 10.1016/j.nut.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58: 724S–732S. http://www.ncbi.nlm.nih.gov/pubmed/8213603. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- 37.Bocarsly ME, Powell ES, Avena NM, Hoebel BG. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav. 2010;97: 101–106. doi: 10.1016/j.pbb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallfrisch J. Metabolic effects of dietary fructose. FASEB J. 1990;4: 2652–2660. http://www.ncbi.nlm.nih.gov/pubmed/2189777. doi: 10.1096/fasebj.4.9.2189777. [DOI] [PubMed] [Google Scholar]
- 39.Kumar Jena P, Singh S, Prajapati B, Nareshkumar G, Mehta T, Seshadri S. Impact of targeted specific antibiotic delivery for gut microbiota modulation on high-fructose-fed rats. Appl Biochem Biotechnol. 2014;172: 3810–3826. doi: 10.1007/s12010-014-0772-y. [DOI] [PubMed] [Google Scholar]
- 40.Volynets V, Louis S, Pretz D, Lang L, Ostaff MJ, Wehkamp J, Bischoff SC. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a western-style diet or drinking water supplemented with fructose. J Nutr. 2017;147: 770–780. doi: 10.3945/jn.116.242859. [DOI] [PubMed] [Google Scholar]
- 41.Mastrocola R, Ferrocino I, Liberto E, Chiazza F, Cento AS, Collotta D, Querio G, Nigro D, Bitonto V, Cutrin JC, et al. Fructose liquid and solid formulations differently affect gut integrity, microbiota composition and related liver toxicity: a comparative in vivo study. J Nutr Biochem. 2018;55: 185–199. doi: 10.1016/J.JNUTBIO.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Jones RB, Alderete TL, Martin AA, Geary BA, Hwang DH, Palmer SL, Goran MI. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese hispanic adolescents: a 16-week, randomized, placebo-controlled trial. Pediatr Obes. 2018;13: 705–714. doi: 10.1111/ijpo.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willett W. Nutritional Epidemiology. Oxford, UK: Oxford University Press; 2012. doi: 10.1093/acprof:oso/9780199754038.001.0001. [DOI] [Google Scholar]
- 44.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Env Microbiol. 2006;72: 5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stasinopoulos M, Rigby B. Package ‘gamlss’. R Packag. version. 2017:141 [cited 2017 Apr 4]. https://cran.r-project.org/web/packages/gamlss/gamlss.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, et al.Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106(14):5859–5864. doi: 10.1073/pnas.0901529106 http://www.pnas.org/content/106/14/5859.full.pdf [DOI] [PMC free article] [PubMed]


