ABSTRACT
Vitamin B12 is a critical nutrient for humans as well as microbes. Due to saturable uptake, high dose oral B12 supplements are largely unabsorbed and reach the distal gut where they are available to interact with the microbiota. The aim of this study was to determine if oral B12 supplementation in mice alters 1) the concentration of B12 and related corrinoids in the distal gut, 2) the fecal microbiome, 3) short chain fatty acids (SCFA), and 4) susceptibility to experimental colitis. C57BL/6 mice (up to 24 animals/group) were supplemented with oral 3.94 µg/ml cyanocobalamin (B12), a dose selected to approximate a single 5 mg supplement for a human. Active vitamin B12 (cobalamin), and four B12-analogues ([ADE]CN-Cba, [2Me-ADE]CN-Cba, [2MeS-ADE]CN-Cba, CN-Cbi) were analyzed in cecal and fecal contents using liquid chromatography/mass spectrometry (LC/MS), in parallel with evaluation of fecal microbiota, cecal SCFA, and susceptibility to dextran sodium sulfate (DSS) colitis. At baseline, active B12 was a minor constituent of overall cecal (0.86%) and fecal (0.44%) corrinoid. Oral B12 supplementation increased active B12 at distal sites by >130-fold (cecal B12 increased from 0.08 to 10.60 ng/mg, fecal B12 increased from 0.06 to 7.81 ng/ml) and reduced microbe-derived fecal corrinoid analogues ([ADE]CN-Cba, [2Me-ADE]CN-Cba, [2MeS-ADE]CN-Cba). Oral B12 had no effect on cecal SCFA. Microbial diversity was unaffected by this intervention, however a selective decrease in Bacteroides was observed with B12 treatment. Lastly, no difference in markers of DSS-induced colitis were detected with B12 treatment.
KEYWORDS: Inflammatory bowel disease, colitis, vitamin B12, microbiota
Introduction
Vitamin B12 is synthesized exclusively by microbes.1 In the human gut, such synthesis occurs distal to the site of absorption, therefore this nutrient must be obtained from exogenous sources. The quantity of vitamin B12 required by adults, 2 µg/day, is lower than any other micronutrient.2 Commercially available supplements contain up to 5 mg/tablet, greatly exceeding the daily requirement. Unlike other water-soluble vitamins, which are largely absorbed and enter the circulation, receptor-mediated B12 absorption in the ileum becomes saturated around 2 µg/meal1 and only a small fraction of high dose oral supplements are absorbed by non-specific pathways. Following supplement ingestion, non-absorbed vitamin B12 reaches the large intestine where it is available to interact with the colonic microbiota. Microbes require vitamin B12 or related corrinoids for their own functions. It is estimated that 20% of gut bacteria are able to synthesize vitamin B12, yet >80% of gut bacteria require this nutrient for their own metabolic purposes.3,4 Further, microbes produce various B12 analogues by altering the lower ligand base (Figure 1(a)), and only a small fraction of the corrinoid in human feces is active B12.5 Auxotrophic bacteria rely on B12 transporters for acquisition, even encoding multiple transporters with varied preference for different B12 analogues to gain competitive advantage.3 B12 destined for utilization by the host is chaperoned throughout the digestive tract and then during circulation by glycoproteins (haptocorrin, intrinsic factor, transcobalamin II), possibly as a means of preserving them from microbial acquisition.6 The importance of B12 for microbial physiology is illustrated by early B12 assays which derive B12 concentration from the rate of bacterial growth.7 Collectively, these observations led us to hypothesize that high dose oral B12 could fundamentally alter the corrinoid economy and microbial composition in the distal gut.
Figure 1.
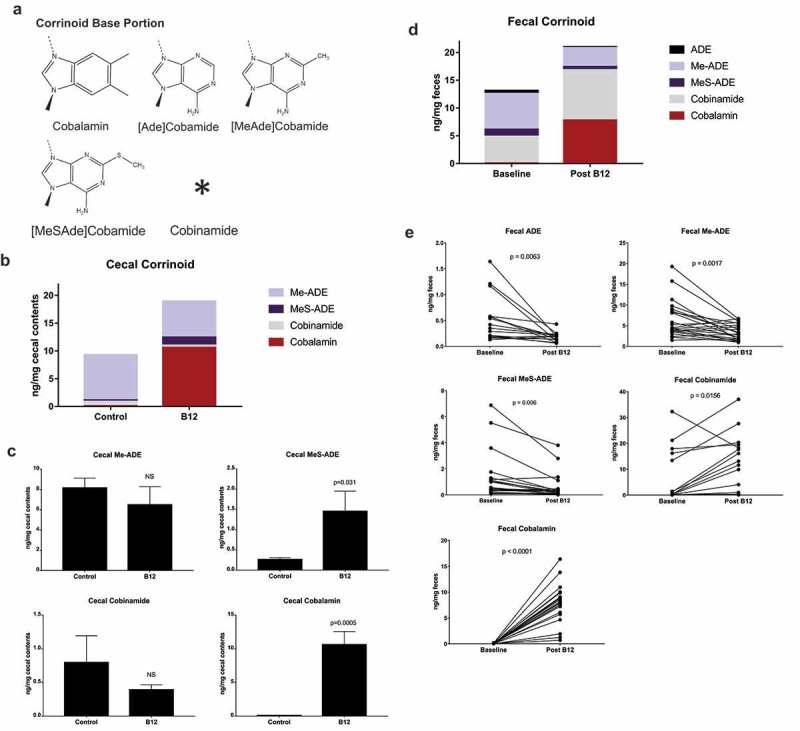
B12 supplement alters the corrinoid profile in the cecum and feces. (a) Base moieties of the most abundant corrinoids. Cobinamide lacks the base as well as ribose and phosphate moieties present in other corrinoids. (b) Cecal corrinoid profile overall and (c) by individual corrinoid in control (n = 5) and B12 treated (n = 4) mice by unpaired two-tailed Student’s t-test. (d) Fecal corrinoid profile overall and (e) by individual corrinoid at baseline and after 16-day B12 supplementation (ADE n = 16/group, others n = 22/group, paired two-tailed t-test).
Two known B12-dependent enzymes have been described in humans, but at least 15 B12-dependent enzymes exist among gut microbes.8 B12 has additional roles in microbial gene regulation, functioning in B12-dependent riboswitches (regulatory RNA elements),9 and as a cofactor for gene regulatory proteins.10 L-Methylmalonyl-CoA mutase is a B12-dependent enzyme shared by humans and microbes (though the direction of product-substrate is reversed). Anaerobic microbes use this enzyme to generate CO2, a critical electron acceptor in the anaerobic lumen.11 Propionate, a short-chain fatty acid (SCFA) generated as a byproduct of this reaction, is important in host physiology and immunity.4,12 In one study, the addition of B12 to bacterial growth media increased propionate production by three bacterial species.13 This knowledge prompted the hypothesis that oral B12 might alter microbial SCFA production and thereby, resistance to colitis in the distal gut.
Here we report results from murine experiments that evaluated the effect of oral B12 supplementation on luminal B12 and related corrinoids in the distal gut. In addition, we assessed the effect of B12 supplement on the fecal microbiome, cecal SCFA, and resistance to experimental colitis.
Results
As a starting point to profile B12 metabolites in mice, we measured the influence of oral B12 supplementation (3.94 µg/ml) on corrinoids in the distal gut. Cobalamin, and four other corrinoid analogues were selected for analysis based on their abundance in human feces5 and the availability of standards for LC/MS. These included cobalamin, [ADE]CN-Cba, [2Me-ADE]CN-Cba, [2MeS-ADE]CN-Cba, and CN-Cbi, which differ from cobalamin in the lower ligand base moiety (Figure 1(a)). Cobalamin was found to be a minor constituent representing just 0.86% of cecal and 0.44% of fecal corrinoid at baseline (Figure 1(b–d)). Supplemented animals had 132-fold higher cobalamin concentration in cecal contents (Figure 1(c). 0.08 vs 10.60 ng/mg, p = 0.0005) and 136-fold higher concentration in fecal contents (Figure 1(e). 0.06 vs 7.81 ng/mg, p = 0.0001) with Bonferroni-corrected α of 0.0125 and 0.01 respectively. Likewise, MeS-Ade was 5.6-fold higher in cecal contents of supplemented animals (Figure 1(b,c). 0.26 vs 1.45 ng/mg) but this was non-significant after Bonferroni correction. In contrast, there was a robust decrease in cobamide analogues (Figure 1(d,e)) in feces of supplemented mice except for cobinamide which was increased. To control for change over time we compared corrinoids in non-supplemented control animals at days 1 vs 16, and found a marginal decrease in fecal cobalamin, which was non-significant after Bonferroni correction (α = 0.01; Fig S1).
Given the widespread microbial requirement for exogenous corrinoid, we hypothesized that B12 supplementation would select for microbes whose growth was otherwise limited by this nutrient. Stool samples were collected for 16S rRNA gene sequencing at baseline (T1) and after 16 days (T2) of treatment. Including samples from DSS colitis experiments, described below, 54 stool and cecal samples were collected for microbiome analysis, of which 52 were successfully profiled (two animals given DSS had inadequate cecal contents to generate adequate 16S rRNA gene amplicons). In total 3,242,801 sequences were obtained (average 60,051 per sample). Goods coverage exceeded 99.8% for all samples analyzed demonstrating adequate sequencing. Beta diversity analysis at phylum level (Figure 2(a)) showed similar overall composition before and after B12 supplementation, while a significant difference was seen at the genus level (Figure 2(b), p = 0.048). Additionally, this analysis yielded one conspicuous finding: the closely related genera Bacteroides (2.50% OTU baseline vs 0.54% OTU post, p = 0.027) was significantly reduced after B12 supplementation (Figure 2(c)). Furthermore, while alpha diversity in these samples demonstrated no difference by treatment, principal coordinates analysis (PCoA) revealed that the control vs. B12 groups post-supplementation differed significantly (Fig S2).
Figure 2.
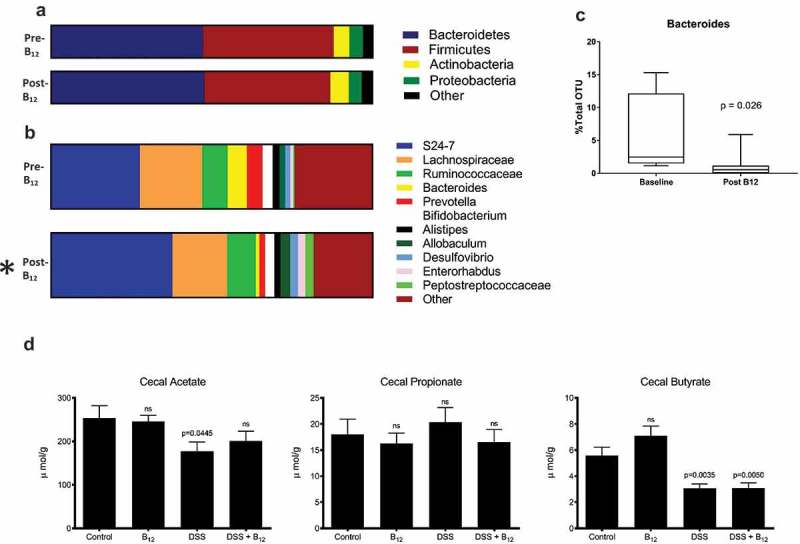
Influence of oral B12 supplement on the fecal microbiome. Beta diversity analysis of (a) phylum and (b) genus level (n = 12 mice/time point) at baseline and following 16-day B12 supplementation. *p < 0.05. (c) Significant reduction in the relative abundance of Bacteroides following B12 supplementation (paired two-tailed t-test). (d) Cecal short-chain fatty acids in animals administered H2O (Control) and experimental colitis (DSS) with and without B12. (Control n = 15, B12 n = 15, DSS n = 20, DSS/B12 n = 18; paired two-tailed t-test (c) and ANOVA with Tukey’s post-test (d).
Culture experiments have shown increased propionate synthesis by some bacteria with the addition of B12 to culture media.13 Therefore, we tested the hypothesis that B12 would alter cecal SCFA concentrations. Cecal contents rather than stool was used for SCFA analysis because a large portion of SCFA produced in the gut is absorbed in the colon before luminal contents are expelled as fecal pellets. This analysis did not identify any effect of B12 on cecal acetate, propionate, or butyrate levels (Figure 2(d)).
Published work implicates commensal Bacteroides species in the pathogenesis of murine colitis,14,15 in contrast, lower levels of Bacteroides are associated with human inflammatory bowel disease (IBD).16–18 Given our finding that oral B12 supplementation decreased the proportion of Bacteroides in feces, we sought to determine the effect of oral B12 in murine colitis. The DSS model of experimental colitis was chosen because prior studies using this model demonstrated a role for SCFA-mediated signaling.19 As expected, induction of experimental colitis with the addition of 2.25% DSS in drinking water resulted in lower weight, shorter colon length, and increased gut permeability as reflected by the appearance of serum fluorescence following gavage of FITC-dextran (Figure 3(a–c)). However, B12 supplementation, which was sustained during DSS administration, did not significantly influence these endpoints (Figure 3(a–c)). Similarly, there was no significant difference in IL-1β, TNF-α, IL-6, IL-10, IL-12p70, IFN-γ, or murine KC in colonic mucosal scrapings with oral B12 supplementation in DSS colitis (Figure 3(d)). Given the potential for host B12 status to influence response to colitis,20 we included a control group that received intraperitoneal B12 injection (parenteral B12) – an intervention that did not alter fecal corrinoids (Fig S3).
Figure 3.
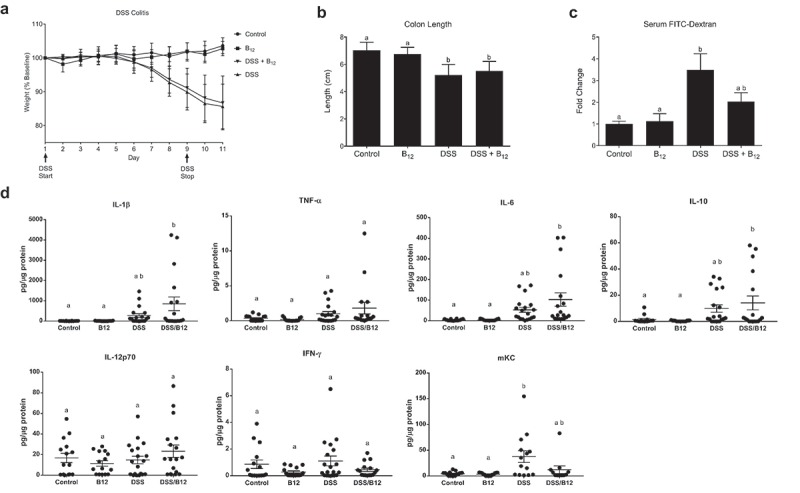
B12 supplement in DSS colitis. (a) Vitamin B12 had no effect on weight loss in DSS colitis (sum of three replicate experiments, Control (n = 15), B12 (n = 15), DSS (n = 23), DSS/B12 (n = 24) by ANOVA and Dunnett’s multiple comparisons (Control vs DSS or DSS/B12: p < 0.0001, but DSS vs DSS/B12: p = ns) or measures of disease including (b) colon length, Control (n = 15), B12 (n = 15), DSS (n = 20), DSS/B12 (n = 19) by ANOVA and Tukey’s multiple comparisons, or (c) enteral administered FITC-dextran detected in circulation of Control (n = 14), B12 (n = 15), DSS (n = 20), DSS/B12 (n = 19) by ANOVA and Tukey’s multiple comparisons. (d) Colon tissue cytokines were not significantly different comparing DSS vs DSS/B12 by ANOVA and Tukey’s multiple comparisons. Unique letter represents p < 0.05.
Discussion
It is predicted that 80% of gut microbes require B12 or similar corrinoids, though a minority harbor the B12 biosynthetic pathway.8 Therefore, most gut microbes rely on acquisition of exogenous corrinoid or precursor molecules from their environment and successful competition confers a survival advantage.3 Therefore, we hypothesized that oral supplementation would deliver excess B12 to the distal gut, disrupting the corrinoid economy and thereby alter the microbiome, SCFA, and resistance to colitis.
To our knowledge, this is the first study to profile corrinoids in the distal gut of mice since the LC/MS method was originally described by Allan and Stabler.5 Importantly, it demonstrates that active B12 is a minor constituent of the fecal corrinoid pool in mice (0.44%, 59 ng/g stool) which is similar to published results from human feces (1.4%, 19 ng/g stool).5 We show that oral B12 supplementation in mice, at a level corresponding to human consumption of a 5 mg daily supplement, increased B12 in the distal gut >130 fold. B12 supplementation decreased the absolute concentration of several non-B12 corrinoids. Cobinamide was increased in fecal contents which indicates microbial modification of cobalamin salvaging the corrin ring. Given the ability of microbes to sense and acquire B12,3,9 this likely reflects negative regulation of microbial corrinoid synthesis in the setting of environmental excess.
Our analysis did not reveal major alterations of the fecal microbiome with B12 supplementation. Use of samples from replicate experiments reduced the chance of type I error in this analysis, the study likely was limited in its power to detect changes in the microbiome. The primary finding was the selective decrease in Bacteroides, a highly prevalent constituent of the mammalian gut microbiome.21 Bacteroides require exogenous B12,13,22 so the depletion of this genus with supplementation is paradoxical. Bacteroides derive competitive advantage from redundant B12 transporters that enable them to acquire environmental corrinoid. This leads us to speculate that Bacteroides may lose this advantage in an environment replete with B12. Conflicting data exist regarding the role of Bacteroides in colitis. Bacteroides in general16,23 and enterotoxigenic B. fragilis specifically24 are associated with IBD in humans and contribute to pathogenesis of colitis in animal models.15,25,26 To the contrary, monocolonization of germ free mice with B. fragilis is protective in DSS colitis,27 likely through immunomodulatory influences of its capsular polysaccharide-A28 and modulating secreted outer membrane vesicles.29 Given this selective decrease of Bacteroides, we subjected B12 supplemented mice to DSS colitis to evaluate their susceptibility to disease. Despite some trends toward B12-mediated protection of barrier function, no significant influence of B12 supplementation was discernable during DSS administration (Figure 3(a–d)-D). These findings agree with recent data regarding the role of cyanocobalamin in murine colitis. Zhu et al. show that cyanocobalamin supplementation during DSS administration had a negligible effect on colitis outcomes, including weight loss and colon length reduction.30 Furthermore, cyanocobalamin supplementation during DSS did not have a significant effect on microbial alpha diversity,30 similar to our findings. This is in contrast with work in other organ systems, which has shown that cyanocobalamin exhibits anti-inflammatory effects again acute and chronic inflammation in mice.31
The finding that cecal SCFA concentrations are unaffected by B12 supplementation (Figure 2(d)) contrasts with results of bacterial culture experiments in which addition of B12 increased propionate production.13 This may reflect resilience of the metabolic network in the distal gut lumen.11 Alternately, an influence of B12 on cecal SCFA may have been obfuscated in the background of B12 provided by the standard diet that all animals received. The decision to use standard B12-containing chow and the selection of the B12 dose for these studies was intended to reflect typical human exposures. One strength of this work is the number of mice included and replication of experiments. For example, we report that DSS reduces butyrate in cecal contents (Figure 2(d), n = 20/group). This finding corroborates results from other studies that employed just five animals/group.32,33
In summary, we report that oral B12 supplement, as cyanocobalamin, was effective in delivering B12 to the distal gut of C57BL/6 mice as well as modulating B12 analogues and selectively depleting Bacteroides, but did not appreciably influence cecal SCFA levels or protection in DSS colitis.
Methods
Mice and B12 treatment
All experimental protocols were approved by the University of Colorado Institutional Animal Care and Use Committee. Male and female C57BL/6 mice were bred and maintained in a specific-pathogen-free facility and used at age 8–16 weeks. Animals were evenly distributed amongst treatment groups by age and sex. Mice were fed a standard diet (Teklad Global Soy Protein-Free Extruded Rodent Diet) containing .08 mg/kg vitamin B12 as cyanocobalamin. Mice were supplemented with B12 in the form of cyanocobalamin (Sigma-Aldrich, St. Louis, MO, USA) in drinking water at a concentration of 3.94 µg/mL, which provided 1.025 µg B12/g, or on average, 30.46 µg B12 per mouse, based on water disappearance. This is the human equivalent of a single 5,000 µg oral supplement based on published data on C57BL/6 water consumption using standard body surface area based conversion between human and mouse.34,35 Mice were supplemented with B12 throughout the duration of the experiment with baseline and post supplement samples collected at day 1 and day 16. Water bottles containing B12 were replaced every other day. As a control in colitis experiments, parenteral B12 (via intraperitoneal injection) at a dose of 2.65 µg/mouse, every 3 days, was provided to the control group to account for potential systemic influences of B12 on colitis.20 This dose was selected to provide an optimistic 2.9% absorption/day.36
Corrinoid analysis
Cobalamin and B12 analogues were measured in cecal contents and stool by stable isotope dilution liquid chromatography/mass spectrometry (LC/MS) as previously described by Allen and Stabler.5 Briefly, frozen samples were thawed and 1 mL 0.1M KPO4 buffer at pH 8.0 was added with stable isotope labeled internal standards. Samples were heated to 90°C for 45 min, then cooled on ice and centrifuged and eluted fraction added to prepared C18 reverse phase resin columns. The eluant was dried overnight before rehydration in 1 mL H2O and then passed through R-protein resin which was prewashed with 1 mL phenol, 3 mL H2O and 1 mL 0.5M glycine. Sample was eluted with 100 µl phenol, and the aqueous layer was extracted and analyzed by LC/MS as detailed previously.5
Microbiome and SCFA analysis
Bacterial profiles were determined by broad-range amplification and sequence analysis of the bacterial 16S rRNA gene V3V4 variable region following our previously described methods. Fecal pellets and cecal contents were collected directly into sterile Eppendorf tubes and flash frozen at −80°C until samples were processed for DNA isolation and sequencing. Bacterial DNA from cecal and fecal samples were isolated using the Power Fecal DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA). Paired-end sequencing was performed on the Illumina MiSeq platform using the 600-cycle v3 kit. Sequences were merged and quality filtered as previously described.37,38 Merged sequences were aligned and classified with SINA (1.3.0-r23838).39,40 Operational taxonomic units (OTUs) were produced by clustering sequences with identical taxonomic assignments. Explicet software (v2.10.5) was used for microbiome analyses, including calculation of alpha and beta diversity measures such as Goods Coverage index.41 Short chain fatty acids were analyzed by stable isotope GC/MS using an adaption of a published method.42 Briefly, cecal samples were collected directly into pre-weighed, sterile Eppendorf tubes and flash frozen at −80°C until processing. Samples were then subject to an alkylation procedure in which sample and alkylating reagent were added, vortexed for 1 min, and incubated at 60°C for 15 min. Following cooling and addition of n-hexane to allow for separation, 200 µL of the organic phase was transferred to glass inserted and analyzed by GC/MS. Results were quantified by reference to a standard curve and normalized to sample weight.
Experimental colitis
Colitis was induced using 2.25% dextran sodium sulfate (DSS), (MW 36,000–50,000, MP Biochemicals, Santa Ana, CA, USA) added to drinking water for 9 days, as previously reported.43 DSS was made fresh daily. Animals were weighed daily and on the day of euthanasia were gavaged with 3-5KD FITC dextran (Sigma Aldrich, St. Louis, MO, USA). Serum was collected at euthanasia, four hours after gavage, and fluorescence quantified. Mucosal scrapings from the colon were placed in phosphate buffered solution containing Halt Protease Inhibitor Cocktail (Fisher Scientific) and stored at −80°C. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). Tissue cytokines were quantified as previously reported44 using the Mouse Proinflammatory 7-Plex Tissue Culture Kit and according to manufacturer instructions (Meso Scale Discovery, Rockville, MD, USA).
Statistical Analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Tests include paired and unpaired two-tailed Student’s t-test and ANOVA with Tukey’s and Dunnett’s multiple comparisons as indicated in figure legends. Statistical differences reported as significant when p < 0.05 with Bonferroni correction applied when multiple comparisons were made.
Funding Statement
This work was supported by the National Institutes of Health [DK1047893, DK50189, DK095491, DK103712] and by the Veterans Administration [Merit Award BX002182].
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med (Maywood). 2007;232(10):1266–1274. PubMed PMID: 17959839. doi: 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline.Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline Washington (DC): National Academies Press (US); 1998. [PubMed]
- 3.Degnan PH, Barry NA, Mok KC, Taga ME, Goodman AL. Human gut microbes use multiple transporters to distinguish vitamin B(1)(2) analogs and compete in the gut. Cell Host Microbe. 2014;15(1):47–57. PubMed PMID: 24439897; PubMed Central PMCID: PMC3923405. doi: 10.1016/j.chom.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. PubMed PMID: 24388214. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 5.Allen RH, Stabler SP. Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am J Clin Nutr. 2008;87(5):1324–1335. PubMed PMID: 18469256; PubMed Central PMCID: PMC2900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(21):2041–2042. PubMed PMID: 23697526. doi: 10.1056/NEJMc1304350. [DOI] [PubMed] [Google Scholar]
- 7.Herbert V, Colman N, Palat D, Manusselis C, Drivas G, Block E, Akerkar A, Weaver D, Frenkel E. Is there a “gold standard” for human serum vitamin B12 assay? J Lab Clin Med. 1984;104(5):829–841. PubMed PMID: 6387014. [PubMed] [Google Scholar]
- 8.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20(5):769–778. PubMed PMID: 25440056; PubMed Central PMCID: PMC4260394. doi: 10.1016/j.cmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahvi A, Barrick JE, Breaker RR. Coenzyme B12 riboswitches are widespread genetic control elements in prokaryotes. Nucleic Acids Res. 2004;32(1):143–150. PubMed PMID: 14704351; PubMed Central PMCID: PMC373277. doi: 10.1093/nar/gkh167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klug G. Beyond catalysis: vitamin B12 as a cofactor in gene regulation. Mol Microbiol. 2014;91(4):635–640. PubMed PMID: 24330414. doi: 10.1111/mmi.12490. [DOI] [PubMed] [Google Scholar]
- 11.Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011;10(4):336–347. PubMed PMID: 22018234; PubMed Central PMCID: PMC3225337. doi: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396–406 e1–e10 PubMed PMID: 23665276. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Wolin MJ. Influence of heme and vitamin B12 on growth and fermentations of Bacteroides species. J Bacteriol. 1981;145(1):466–471. PubMed PMID: 7462148; PubMed Central PMCID: PMC217295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpton T, Lyalina S, Luong J, Pham J, Deal EM, Armour C, Gaulke C, Sanjabi S, Pollard KS, Gilbert JA, et al. Development of inflammatory bowel disease is linked to a longitudinal restructuring of the gut metagenome in mice. mSystems. 2017;2(5). PubMed PMID: 28904997; PubMed Central PMCID: PMC5585689. doi: 10.1128/mSystems.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Allen PM, Stappenbeck TS. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9(5):390–403. Epub 2011/05/18. PubMed PMID: 21575910; PubMed Central PMCID: PMCPMC3241010. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Lower ZF. Level of bacteroides in the gut microbiota is associated with inflammatory bowel disease: A meta-analysis. Biomed Res Int. 2016;2016:5828959 PubMed PMID: 27999802; PubMed Central PMCID: PMC5143693. doi: 10.1155/2016/5828959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, Zhu W, Sartor RB, Boedeker EC, Harpaz N, et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):179–184. PubMed PMID: 20839241; PubMed Central PMCID: PMCPMC3834564. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. PubMed PMID: 17699621; PubMed Central PMCID: PMCPMC1959459. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. PubMed PMID: 19865172; PubMed Central PMCID: PMC3256734. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benight NM, Stoll B, Chacko S, Da Silva VR, Marini JC, Gregory JF, III, Stabler SP, Burrin DG. B-vitamin deficiency is protective against DSS-induced colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301(2):G249–59. PubMed PMID: 21596995; PubMed Central PMCID: PMC3154603. doi: 10.1152/ajpgi.00076.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wexler AG, Goodman AL. An insider‘s perspective: bacteroides as a window into the microbiome. Nat Microbiol. 2017;2:17026 PubMed PMID: 28440278; PubMed Central PMCID: PMC5679392. doi: 10.1038/nmicrobiol.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varel VH, Bryant MP. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974;28(2):251–257. PubMed PMID: 4853401; PubMed Central PMCID: PMC186696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrakas S, Mountzouris KC, Michalopoulos G, Karamanolis G, Papatheodoridis G, Tzathas C, Gazouli M. Intestinal bacteria composition and translocation of bacteria in inflammatory bowel disease. PLoS One. 2017;12(1):e0170034 PubMed PMID: 28099495; PubMed Central PMCID: PMC5242456. doi: 10.1371/journal.pone.0170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J Jr.. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6(2):171–174. PubMed PMID: 10756151; PubMed Central PMCID: PMC2640860. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yim S, Gwon SY, Hwang S, Kim NH, Jung BD, Rhee KJ. Enterotoxigenic Bacteroides fragilis causes lethal colitis in Mongolian gerbils. Anaerobe. 2013;21:64–66. PubMed PMID: 23538057. doi: 10.1016/j.anaerobe.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77(4):1708–1718. PubMed PMID: 19188353; PubMed Central PMCID: PMC2663167. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu C-C, Ching Y-H, Wang Y-C, Liu J-Y, Li Y-P, Huang Y-T, Chuang H-L. Monocolonization of germ-free mice with Bacteroides fragilis protects against dextran sulfate sodium-induced acute colitis. Biomed Res Int. 2014;2014:675786 PubMed PMID: 24971344; PubMed Central PMCID: PMC4058166. doi: 10.1155/2014/675786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. PubMed PMID: 18509436. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 29.Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu W-L, Kambal A, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352(6289):1116–1120. PubMed PMID: 27230380; PubMed Central PMCID: PMC4996125. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Xiang S, Feng X, Wang H, Tian S, Xu Y, Shi L, Yang L, Li M, Shen Y, et al. Impact of cyanocobalamin and methylcobalamin on inflammatory bowel disease and the intestinal microbiota composition. J Agric Food Chem. 2018. Epub 2018/12/24. PubMed PMID: 30572705. doi: 10.1021/acs.jafc.8b05730. [DOI] [PubMed] [Google Scholar]
- 31.Hosseinzadeh H, Moallem SA, Moshiri M, Sarnavazi MS, Etemad L. Anti-nociceptive and anti-inflammatory effects of cyanocobalamin (vitamin B12) against acute and chronic pain and inflammation in mice. Arzneimittelforschung. 2012; 62(7):324–329. Epub 2012/05/17. PubMed PMID: 22588629. doi: 10.1055/s-0032-1311635. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Wu Y, Wang J, Wu G, Long W, Xue Z, Wang L, Zhang X, Pang X, Zhao Y, et al. Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate-producing bacterium. Sci Rep. 2016;6:27572 PubMed PMID: 27264309; PubMed Central PMCID: PMC4893749. doi: 10.1038/srep27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishiro T, Kusunoki R, Otani A, Ansary MM, Tongu M, Harashima N, Yamada T, Sato S, Amano Y, Itoh K, et al. Butyric acid attenuates intestinal inflammation in murine DSS-induced colitis model via milk fat globule-EGF factor 8. Lab Invest. 2013;93(7):834–843. PubMed PMID: 23752130. doi: 10.1038/labinvest.2013.70. [DOI] [PubMed] [Google Scholar]
- 34.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32(6):435–443. PubMed PMID: 12467341; PubMed Central PMCID: PMC1397713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Services UDoHaH Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Notice by the Food and Drug Administration. National Archive Federal Register; 2005.
- 36.Rosenblum CC, Condon BF, Yamamoto GP, Oral Versus RS. Parenteral administration of Co60-Labeled Vitamin B12 to Rats. J Bio Chem. 1952;198:915. [PubMed] [Google Scholar]
- 37.Nycz BT, Dominguez SR, Friedman D, Hilden JM, Ir D, Robertson CE, Frank DN. Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patients. PLoS One. 2018;13(1):e0191232 PubMed PMID: 29329346; PubMed Central PMCID: PMCPMC5766145. doi: 10.1371/journal.pone.0191232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn KA, Schulz HM, Regner EH, Severs EL, Hendrickson JD, Mehta G, Whitney AK, Ir D, Ohri N, Robertson CE, et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018;11(2):357–368. PubMed PMID: 28812548; PubMed Central PMCID: PMCPMC5815964doi: 10.1038/mi.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruesse E, Peplies J, Glöckner FO. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28(14):1823–1829. PubMed PMID: 22556368; PubMed Central PMCID: PMCPMC3389763. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–D6. PubMed PMID: 23193283; PubMed Central PMCID: PMCPMC3531112. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson CE, Harris JK, Wagner BD, Granger D, Browne K, Tatem B, Feazel LM, Park K, Pace NR, Frank DN. Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics. 2013;29(23):3100–3101. PubMed PMID: 24021386; PubMed Central PMCID: PMCPMC3834795. doi: 10.1093/bioinformatics/btt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamarre SG, MacMillan L, Morrow GP, Randell E, Pongnopparat T, Brosnan ME, Brosnan JT. An isotope-dilution, GC-MS assay for formate and its application to human and animal metabolism. Amino Acids. 2014;46(8):1885–1891. PubMed PMID: 24748098. doi: 10.1007/s00726-014-1738-7. [DOI] [PubMed] [Google Scholar]
- 43.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless A, Scully M, Saeedi B, Golden-Mason L, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40(1):66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci U S A. 2013;110(49):19820–19825. Epub 2013 Nov 18. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


