Abstract
Objective
Posttraumatic stress disorder (PTSD) is associated with decreased top–down emotion modulation from medial prefrontal cortex (mPFC) regions, a pathophysiology accompanied by hyperarousal and hyperactivation of the amygdala. By contrast, PTSD patients with the dissociative subtype (PTSD + DS) often exhibit increased mPFC top–down modulation and decreased amygdala activation associated with emotional detachment and hypoarousal. Crucially, PTSD and PTSD + DS display distinct functional connectivity within the PFC, amygdala complexes, and the periaqueductal gray (PAG), a region related to defensive responses/emotional coping. However, differences in directed connectivity between these regions have not been established in PTSD, PTSD + DS, or controls. Methods: To examine directed (effective) connectivity among these nodes, as well as group differences, we conducted resting‐state stochastic dynamic causal modeling (sDCM) pairwise analyses of coupling between the ventromedial (vm)PFC, the bilateral basolateral and centromedial (CMA) amygdala complexes, and the PAG, in 155 participants (PTSD [n = 62]; PTSD + DS [n = 41]; age‐matched healthy trauma‐unexposed controls [n = 52]). Results: PTSD was characterized by a pattern of predominant bottom–up connectivity from the amygdala to the vmPFC and from the PAG to the vmPFC and amygdala. Conversely, PTSD + DS exhibited predominant top–down connectivity between all node pairs (from the vmPFC to the amygdala and PAG, and from the amygdala to the PAG). Interestingly, the PTSD + DS group displayed the strongest intrinsic inhibitory connections within the vmPFC. Conclusions: These results suggest the contrasting symptom profiles of PTSD and its dissociative subtype (hyper‐ vs. hypo‐emotionality, respectively) may be driven by complementary changes in directed connectivity corresponding to bottom–up defensive fear processing versus enhanced top–down regulation. Hum Brain Mapp 38:5551–5561, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: posttraumatic stress disorder, dynamic causal modeling, amygdala, prefrontal cortex, periaqueductal gray, fMRI, connectivity
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric illness, characterized by symptoms of vivid re‐experiencing of traumatic events, avoidance, alterations in cognitions and mood, as well as hyperarousal [American Psychiatric Association, 2013]. Dissociation involves detachment from immediate somatic or environmental experience, and often occurs during trauma, modulating its immediate psychophysiological impact [Spiegel, 2012]. Recently, a dissociative subtype of PTSD (PTSD + DS) has been recognized, characterizing individuals experiencing significant emotional detachment and hypoemotionality, including symptoms of depersonalization and derealization (albeit PTSD + DS can oscillate between symptoms of hyper‐ and hypo‐emotionality) [American Psychiatric Association, 2013]. Typically, individuals with PTSD + DS have a history of more severe early‐life trauma [Stein et al., 2013], higher PTSD severity scores [Wolf et al., 2012], and single‐nucleotide polymorphisms associated with dissociation [Wolf et al., 2014]. Neurobiologically, among PTSD as compared to PTSD + DS, differential patterns of neural activation have been documented within the amygdala and prefrontal cortex (PFC) [Felmingham et al., 2008; Hopper et al., 2007; Lanius et al., 2010; Mickleborough et al., 2011]. Specifically, PTSD is characterized by emotion undermodulation, associated with decreased regulatory activation from the medial (mPFC), hyperactivation of the amygdala, and hyper‐arousal/emotionality [Hayes et al., 2012; Hopper et al., 2007; Lanius et al., 2010; Sadeh et al., 2014; Stevens et al., 2013; Yehuda et al., 2015]. By contrast, PTSD + DS is characterized neurobiologically by emotion overmodulation and is associated with increased regulatory activation of the mPFC, resulting in hypoactivation of the amygdala during symptom provocation with concomitant emotional detachment and autonomic blunting [Hopper et al., 2007; Lanius et al., 2010; Mickleborough et al., 2011; Yehuda et al., 2015], a pattern supported by trans‐diagnostic evidence from other dissociative disorders and healthy individuals (for review see [Brand, 2012]).
In the absence of external stimuli, differential patterns of resting‐state functional connectivity between the amygdala and PFC [Nicholson et al., 2015], as well as of the periaqueductal gray (PAG) [Harricharan et al., 2016; Thome et al., 2016], a midbrain region involved in defense (fight‐or‐flight) and emotional coping responses [Bandler et al., 2000; Linnman et al., 2012], are also apparent in PTSD, PTSD + DS, and healthy controls. Specifically, PTSD + DS is associated with increased amygdala subregion resting‐state functional connectivity with PFC emotion regulation regions, which may parallel increased top–down inhibition in this group [Nicholson et al., 2015]. Moreover, as compared to controls, PTSD patients display widespread PAG connectivity with regions involved in threat responses and emotional reactivity, suggesting exacerbated defensive reactions at rest likely reflective of instinctual hypervigilant tendencies in preparation for threat [Harricharan et al., 2016; Thome et al., 2016]. Critically, in healthy individuals, as a threat approaches and is perceived as more imminent, defense processing shifts from higher‐order vmPFC fear regulation sites toward more primitive automatic emotion/defensive regions, such as the PAG and amygdala [Mobbs et al., 2010a, 2010b].
A deeper understanding of the directed connectivity among the vmPFC, amygdala, and PAG is required given their aforementioned functioning in fear/defense circuits [Mobbs et al., 2010a, 2010b] in PTSD. Here, the basolateral (BLA) and centromedial (CMA) amygdala complexes are thought to mediate cortical integration of fear and the execution of behavioral fear responses, respectively [Duvarci and Pare, 2014; LeDoux, 2007], and display differential patterns of connectivity in PTSD, PTSD + DS, and controls [Brown et al., 2014; Nicholson et al., 2015]. Notably, related PAG signaling drives learned and innate fear responses in the amygdala [Johansen et al., 2010; Kim et al., 2013], where the PAG modulates BLA synaptic plasticity [Kim et al., 2013]. Crucially, the vmPFC, amygdala complexes, and the PAG have rich structural and functional connections [Bandler et al., 2000; Etkin et al., 2015; LeDoux, 2007; Linnman et al., 2012]; however, the directionality of these complex connections has yet to be elucidated in PTSD, PTSD + DS, and healthy controls.
Stochastic dynamic causal modeling (sDCM) [Friston et al., 2003; Li et al., 2011] is a procedure for estimating directed or effective connectivity from resting‐state fMRI data, which allows for the comparison of different functional architectures [Penny et al., 2004]. Importantly, DCM estimates directed connections at the level of neuronal coupling—as opposed to (undirected) functional connectivity based on hemodynamic fluctuations. Furthermore, DCM estimates regional variations in hemodynamic parameters, mitigating the uncertainty attending measures of functional connectivity [Friston, 2009].
The purpose of the current study was to uncover foundational markers of effective resting‐state connectivity between the vmPFC, amygdala subregions, and the PAG, among PTSD, PTSD + DS, and healthy controls using separate sDCM analyses for each node pair. Our motivation for this approach was to focus on the hierarchical coupling between pairs of nodes, while allowing for any top–down or bottom–up effective connectivity to be mediated directly or indirectly via nodes not included in the DCM. Hence, our aim was to inform future, more complex/elaborate models of fear and emotion circuitry related to PTSD. Within the PTSD group, we predicted predominant ascending or bottom–up connectivity from the PAG to the amygdala and vmPFC—and from the amygdala to the vmPFC. Ascending connections are responsible for conveying fear inputs and driving defensive responses and thus may mediate chronic hyperarousal in this group. By contrast, we predicted predominantly descending or top–down connectivity from the vmPFC to the amygdala and PAG—and from the amygdala to PAG—among PTSD + DS, a pattern corresponding to increased top–down modulation of limbic and defense regions [Lanius et al., 2010].
METHODS
Participants
Our sample consisted of 155 participants (PTSD [n = 62]; PTSD + DS [n = 41]; age‐matched healthy trauma‐unexposed controls [n = 52]; Table 1). Most PTSD patients (90%) experienced childhood trauma as assessed by the Childhood Trauma Questionnaire (CTQ‐ see below). Exclusion criteria for patients included: alcohol or substance abuse/dependence not in sustained full remission, and diagnosis of bipolar disorder or schizophrenia. Exclusion criteria for the control group included lifetime Axis‐I or Axis‐II disorders (see Supporting Information).
Table 1.
Demographic and clinical information
| Measure | PTSD | PTSD + DS | Healthy controls | |||
|---|---|---|---|---|---|---|
| N | 62 | 41 | 52 | |||
| Gender | 35 female | 33 female | 36 female | |||
| n | SD | n | SD | n | SD | |
| Age | 37.8 | 11.6 | 40.72 | 13.37 | 34.96 | 11.52 |
| CAPS‐IV Total* | 67.9 | 13.4 | 81.6 | 12.7 | 0.93 | 3.4 |
| CAPS‐5 Total | 31.63 | 10.1 | 39.0 | 8.2 | — | — |
| CTQ‐Total* | 56.87 | 24.68 | 68 | 19.10 | 32.28 | 8.99 |
| BDI* | 23.1 | 7.6 | 33.30 | 10.30 | 1.62 | 2.48 |
| MDI‐Total* | 54.10 | 15.29 | 77.20 | 22.00 | 34.36 | 3.86 |
| MDI‐Dep/Dereal* | 7.63 | 2.73 | 12.85 | 4.60 | 5.11 | 0.91 |
| n | % | n | % | n | % | |
| MDD | 11 | 17.7 | 23 | 56.1 | — | — |
| Panic Disorder/Agoraphobia | 10 | 16.1 | 9 | 21.9 | — | — |
| Social Phobia | 2 | 3.2 | 6 | 14.6 | — | — |
| OCD | 3 | 4.8 | 0 | 0 | — | — |
| GAD | 1 | 1.6 | 0 | 0 | — | — |
Proportion of participants evaluated with CAPS‐IV (PTSD n = 53, PTSD + DS n = 30, Controls n = 52), and CAPS‐5 (PTSD n = 9, PTSD + DS n = 11, Controls n = 0).
Abbreviations: PTSD= posttraumatic stress disorder, PTSD + DS= dissociative subtype posttraumatic stress disorder patients, CAPS = Clinician Administered PTSD Scale, CTQ = Childhood Trauma Questionnaire (none or minimal childhood trauma = 25–36, moderate = 56–68, extreme trauma > 72), BDI = Beck's Depression Inventory, MDI = Multiscale Dissociation Inventory, Dep/Dereal = Depersonalization and derealization average, MDD = major depressive disorder, OCD = obsessive compulsive disorder, GAD = generalized anxiety disorder, SD = standard deviation, * indicates the clinical variables on which all groups differed significantly from one another.
All participants were evaluated using the Clinician Administered PTSD Scale (CAPS; versions IV and 5) [Blake et al., 1995] and the DSM‐IV Structured Clinical Interview (SCID) [First et al., 2002]. Dissociative subtype patients were identified by scoring ≥ 2 for both frequency and intensity on either depersonalization or derealization CAPS symptoms as per standard methods [Harricharan et al., 2016; Nicholson et al., 2015]. A battery of questionnaires was also administered (CTQ, Beck's Depression Inventory [BDI], and Multiscale Dissociation Inventory [MDI]; see Table 1 and Supporting Information for group comparisons on clinical variables). Participants took part in a 6‐minute eyes‐closed resting‐state scan following standard methods [Harricharan et al., 2016; Nicholson et al., 2015].
Image Acquisition
We utilized a 3 Tesla MRI Scanner (Trio, Siemens Medical Solutions, Germany) with a 32‐channel head coil for brain imaging. During the resting‐state scan, 120‐volumes were collected (see Supporting Information for details).
fMRI Preprocessing
Standard preprocessing of the functional images was performed with SPM12 and consisted of spatial re‐alignment, reslicing, coregistration, segmentation, and normalization to MNI standard template (see Supporting Information). We smoothed the data with a 4mm kernel FWHM (see [Harricharan et al., 2016]) and bandpass filtered (0.012–0.1 Hz). We used ART software to calculate additional regressors for motion outliers and movement, which were included in each participant's first‐level GLM (see Supporting Information).
Dynamic Causal Modeling
VOI extraction
The six nodes of interest comprised the vmPFC, BLA, and CMA amygdala complexes, and the PAG. Amygdala complexes were delineated using anatomical masks via SPM Anatomy Toolbox. We defined 6 mm spheres based on coordinates from the literature for the vmPFC and PAG [Thome et al., 2016], where this vmPFC regions was found to display increased functional connectivity in PTSD to areas involved in emotional reactivity and motor readiness. This sphere size was chosen based on previous PAG, amygdala, and PFC connectivity manuscripts (see [Thome et al., 2016]). All nodes of interest have been shown to be structurally and functionally connected [Bandler et al., 2000; Etkin et al., 2015; LeDoux, 2007; Linnman et al., 2012], are highly implicated in fear, emotion and defense processing [Mobbs et al., 2009, 2010a, 2010b], and display altered connectivity among PTSD, PTSD + DS, and controls [Harricharan et al., 2016; Nicholson et al., 2015]. We generated a first‐level GLM to model each participant's resting‐state data, adjusting for signal from white matter and cerebrospinal fluid, and correcting for motion by including ART regressors as covariates‐of‐no‐interest. We extracted the principal eigenvariate from each node (volume) of interest from the first‐level GLM of each participant to summarize regional activity at each node.
Bayesian model selection
Regional activities of patients and controls were modeled using stochastic DCM in SPM12 [Bastos‐Leite et al., 2014; Friston et al., 2003; Li et al., 2011], where stochastic DCM is a conventional method for estimating directed resting state connectivity in patient groups, albeit a more conservative method as compared to deterministic spectral DCM [Razi et al., 2015]. We first defined three models of directed hierarchical connectivity (bidirectional, bottom–up, top–down) between each of the amygdala complexes, the PAG and the vmPFC, and between the PAG and vmPFC (Fig. 1). Following the construction and inversion of the three models (for each pair of regions), we performed random‐effects Bayesian model selection [Bastos‐Leite et al., 2014; Stephan et al., 2009]. The superior model was identified in terms of its exceedance probability (xp), which denotes the probability a given model is more likely to have generated the observed data than any other model considered. For clarity, we will focus on models with exceedance probabilities of greater than 0.8 (see Supporting Information). In other words, models we can be 80% sure were more likely than any other model to have generated the data.
Figure 1.
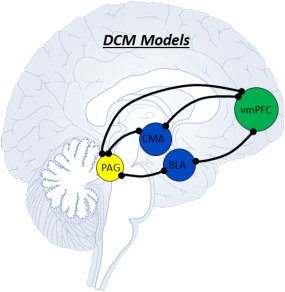
Each black line represents a separate pairwise stochastic DCM analysis, and corresponds to connections between nodes for which we modeled bi‐directional, top–down, and bottom–up directed connectivity. We subsequently identified superior models using Bayesian model selection. [Color figure can be viewed at http://wileyonlinelibrary.com]
Notably, we compared models of hierarchical reciprocal connectivity using separate DCMs for each pair of nodes. Our motivation for this approach was to focus on the hierarchical coupling between pairs of nodes, while allowing for any top–down or bottom–up effective connectivity to be mediated directly or indirectly via nodes not included in the DCM. The aim was to assess direct (monosynaptic) and vicarious (polysynaptic) extrinsic or between region connectivity contributing to hierarchical coupling between regions of interest. This provides an inclusive measure of directed coupling that speaks to our hypothesis about bottom–up fear/defense driving inputs and top–down emotional regulation. This use of Bayesian model comparison was restricted to comparing different models within each group.
Bayesian model averaging and correlations with psychopathology
To supplement the Bayesian model comparison above, we performed quantitative analyses of the underlying parameter estimates using the Bayesian Model Average of each connectivity parameter over the three models for each pair of nodes, within each group. Estimates of connection strengths were used as summary statistics for: (a) classical inference delineating group differences in the strength of connections and (b) correlations with PTSD psychopathology (CAPS IV‐total and depersonalization/derealization MDI average scores) using Pearson bivariate correlations. Notably, whereas directed (extrinsic) connections between nodes can be positive or negative (i.e., excitatory or inhibitory), inhibitory intrinsic self‐connections are inhibitory. We conducted a MANOVA to first observe any significant relationships between BMA parameters denoting node connectivity and group, with each BMA parameter treated as a dependent variable. On significance, this would justify examining separate univariate ANOVAS for each BMA parameter with three levels of group (PTSD, PTSD + DS, and controls). However, if inhomogeneity of variance was detected via Levene's test, we conducted Welch's ANOVA and Games‐Howell post hoc analyses. To control for depression symptoms that may be driving differences in directed connectivity, we also computed separately correlations between estimates of connection strengths between nodes and BDI scores.
RESULTS
Bayesian Model Selection
PTSD patient group
Clear model superiority was identified for node pairs, denoted by high exceedance probabilities (>0.80) for one particular model (see Fig. 2a,c and Table 2). Specifically, PTSD patients displayed bilateral BLA top–down coupling to the PAG, in contrast to bottom–up connectivity from the PAG to the bilateral CMA. Whereas the bilateral BLA complexes showed bottom–up coupling to the vmPFC, the vmPFC displayed top–down connectivity to the right CMA. Finally, the PAG evidenced bottom–up connectivity to the vmPFC.
Figure 2.
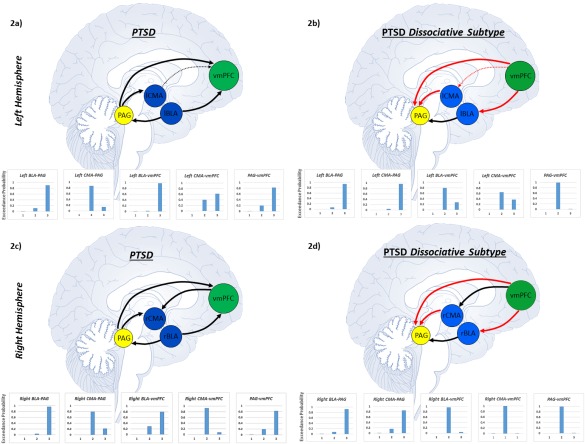
Arrows correspond to the superior model delineating directional connectivity between nodes (brain regions) as identified via Bayesian model selection in each PTSD group. Each line represents a separate pairwise stochastic DCM analysis. Red arrows indicate unique directed connectivity in the dissociative subtype of PTSD as compared to PTSD patients without the subtype. Arrows that appear smaller and dashed represent directed connectivity approaching model superiority. Individual graphs display the exceedance probabilities for each model of directed connectivity between node pairs. Here, superior models were identified using the exceedance probability as criterion, which denotes the probability that a given model was more likely to have generated the observed data than any other model considered. Model 1 refers to bi‐directional connectivity between nodes, Model 2 refers to connectivity from node 2 to node 1, and Model 3 refers to connectivity from node 1 to node 2. Node 1 and node 2 are denoted by the order in which they appear in the title of each graph. (a) Figure displaying directed connectivity within PTSD for the left BLA and CMA complexes as well as for the PAG and vmPFC. (b) Directed connectivity within the dissociative subtype of PTSD for the left BLA and CMA complexes as well as for the PAG and vmPFC. (c) Directed connectivity within PTSD for the right BLA and CMA complexes as well as for the PAG and vmPFC. (d) Directed connectivity within the dissociative subtype of PTSD for the right BLA and CMA complexes as well as for the PAG and vmPFC. Abbreviations: BLA = basolateral amygdala, CMA = centromedial amygdala, PAG = periaqueductal gray, vmPFC = ventromedial prefrontal cortex, PTSD = posttraumatic stress disorder. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Bayesian model selection [Color table can be viewed at http://wileyonlinelibrary.com]
| Group | Nodes 1 and 2 | Exceedance probability | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| PTSD | Left BLA‐PAG | 0.0001 | 0.1117 | 0.9 |
| Right BLA‐PAG | 0 | 0.0375 | 0.9625 | |
| Left CMA‐PAG | 0.0002 | 0.87 | 0.1382 | |
| Right CMA‐PAG | 0 | 0.8 | 0.2029 | |
| Left BLA‐vmPFC | 0.0045 | 0.0197 | 0.9758 | |
| Right BLA‐vmPFC | 0.0002 | 0.2088 | 0.80 | |
| Left CMA‐vmPFC | 0.0035 | 0.4054 | 0.62 | |
| Right CMA‐vmPFC | 0 | 0.9263 | 0.0737 | |
| PAG‐vmPFC | 0.004 | 0.1853 | 0.82 | |
| PTSD+DS | Left BLA‐ PAG | 0 | 0.0659 | 0.9341 |
| Right BLA‐PAG | 0.0008 | 0.08 | 0.92 | |
| Left CMA‐PAG | 0 | 0.0391 | 0.9609 | |
| Right CMA‐PAG | 0.0043 | 0.159 | 0.85 | |
| Left BLA‐vmPFC | 0 | 0.80 | 0.208 | |
| Right BLA‐vmPFC | 0 | 0.9622 | 0.0378 | |
| Left CMA‐vmPFC | 0 | 0.65 | 0.3757 | |
| Right CMA‐vmPFC | 0.0001 | 0.9991 | 0.0008 | |
| PAG‐vmPFC | 0.0001 | 0.9894 | 0.0105 | |
| Healthy controls | Left BLA‐PAG | 0.0001 | 0.6914 | 0.3085 |
| Right BLA‐PAG | 0.0026 | 0.3769 | 0.6205 | |
| Left CMA‐PAG | 0.0034 | 0.9882 | 0.0084 | |
| Right CMA‐PAG | 0.0733 | 0.8095 | 0.1172 | |
| Left BLA‐vmPFC | 0.0001 | 0.0924 | 0.9075 | |
| Right BLA‐vmPFC | 0.0025 | 0.1796 | 0.8179 | |
| Left CMA‐vmPFC | 0.0495 | 0.7607 | 0.1898 | |
| Right CMA‐vmPFC | 0.009 | 0.099 | 0.892 | |
| PAG‐vmPFC | 0.0089 | 0.1024 | 0.8887 | |
Model 1 refers to bi‐directional connectivity between nodes, Model 2 refers to connectivity from node 2 to node 1, and Model 3 refers to connectivity from node 1 to node 2. Exceedance probabilities in bold indicate superior models (>0.80). Abbreviations: PTSD = posttraumatic stress disorder, PTSD + DS = dissociative subtype posttraumatic stress disorder patients, CMA = centromedial amygdala, BLA = basolateral amygdala, PAG = periaqueductal gray.
PTSD dissociative subtype group (PTSD + DS)
We found clear top–down model superiority between all pairs of nodes in the PTSD + DS group (see Fig. 2b,d and Table 2). Specifically, all amygdala complexes (bilateral BLA and CMA) exerted top–down influences on the PAG. Similarly, the vmPFC evidenced top–down connectivity to the bilateral BLA, right CMA, and PAG.
Controls
The controls exhibited unique directed connectivity profiles as compared to both PTSD groups (see Fig. 3a,b and Table 2). Specifically, the bilateral CMA evidenced bottom–up connectivity from the PAG to the amygdala. Moreover, the bilateral BLA and right CMA evidenced bottom–up connectivity from the amygdala to the vmPFC. Finally, the PAG also exerted bottom–up influences on the vmPFC.
Figure 3.
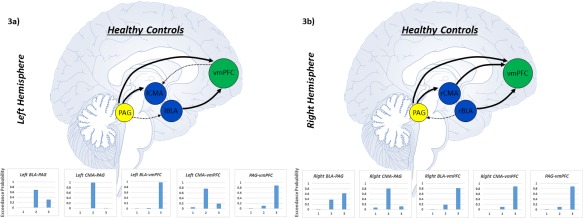
Arrows corresponds to the superior model delineating the direction of connectivity between nodes as identified via Bayesian model selection in healthy trauma‐unexposed controls. Each line represents a separate pairwise stochastic DCM analysis in controls. Arrows that appear smaller and dashed represent directed connectivity approaching model superiority. Graphs represent the exceedance probability for each model of directed connectivity between node pairs. Exceedance probabilities were used as criterion to identify superior models. Model 1 refers to bi‐directional connectivity between nodes, Model 2 refers to connectivity from node 2 to node 1, and Model 3 refers to connectivity from node 1 to node 2. Node 1 and node 2 are denoted by the order in which they appear in the title of each graph. (a) Figure displaying directed connectivity within healthy controls for the left BLA and CMA complexes as well as for the PAG and vmPFC. (b) Directed connectivity within healthy controls for the right BLA and CMA complexes as well as for the PAG and vmPFC. Abbreviations: BLA = basolateral amygdala, CMA = centromedial amygdala, PAG = periaqueductal gray, vmPFC = ventromedial prefrontal cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
Bayesian Model Averaging
We detected significant multivariate effects via a MANOVA when examining the relationship between BMA parameters and group (Pillai's Trace = 0.699, F(70, 234) = 1.797, P < 0.001; see Supporting Information Table S2 for descriptive statistics). When examining univariate effects, we found significant main effects only for intrinsic inhibitory connections spanning the left CMA (when averaging over the left CMA‐PAG models), left BLA (left BLA‐vmPFC models), right CMA (right CMA‐vmPFC models), and PAG (PAG‐vmPFC models; see Table 3). Here, the PTSD + DS group repeatedly demonstrated the strongest intrinsic inhibitory self‐connection of the vmPFC (within bilateral CMA‐vmPFC, right BLA‐vmPFC, and PAG‐vmPFC models).
Table 3.
Bayesian model averaging statistical analyses
| Node pair in Bayesian model averaging | Intrinsic inhibitory connection | Levene's homogeneity test | ANOVA or Welch's ANOVA | Post hoc comparison | Tukey's HSD or games Howell |
|---|---|---|---|---|---|
| Left CMA & PAG | Left CMA | F(2, 153) = 9.729, P < 0.001* | F(2, 83.056) = 4.902, P < 0.010 | PTSD & PTSD+DS | ns |
| PTSD & Control | ns | ||||
| PTSD+DS > Control | P = 0.034 | ||||
| Left BLA & vmPFC | Left BLA | F(2, 153) = 3.440, P < 0.001* | F(2, 90.601) = 3.675, P < 0.030 | PTSD & PTSD+DS | ns |
| PTSD & Control | ns | ||||
| PTSD+DS < Control | P = 0.031 | ||||
| Left CMA & vmPFC | vmPFC | F(2, 153) = 9.129, P < 0.001* | F(2, 85.146) = 3.835, P < 0.025 | PTSD & PTSD+DS | ns |
| PTSD > Control | ns | ||||
| PTSD+DS > Control | P = 0.020 | ||||
| Right BLA & vmPFC | vmPFC | F(2, 153) = 5.874, P < 0.005* | F(2, 87.498) = 3.880, P < 0.025 | PTSD & PTSD+DS | ns |
| PTSD & Control | ns | ||||
| PTSD+DS > Control | P = 0.023 | ||||
| Right CMA & vmPFC | Right CMA | F(2, 153) = 0.315, ns | F(2, 153) = 7.372, P < 0.001 | PTSD > PTSD+DS | P = 0.018 |
| PTSD & Control | ns | ||||
| PTSD+DS < Control | P = 0.001 | ||||
| vmPFC | F(2, 153) = 13.168, P < 0.001* | F(2, 81.156) = 12.635, P < 0.001 | PTSD < PTSD+DS | ns | |
| PTSD > Control | P = 0.005 | ||||
| PTSD+DS > Control | P < 0.001 | ||||
| PAG & vmPFC | PAG | F(2, 153) = 0.473, ns | F(2, 153) = 7.103, P < 0.001 | PTSD > PTSD+DS | P = 0.019 |
| PTSD & Control | ns | ||||
| PTSD+DS < Control | P < 0.001 | ||||
| vmPFC | F(2, 153) = 5.587, P < 0.005* | F(2, 86.350) = 6.004, P < 0.004 | PTSD & PTSD+DS | ns | |
| PTSD & Control | ns | ||||
| PTSD+DS > Control | P = 0.003 |
Univariate analyses following significant multivariate effects. If a significant Levene's test was detected (*), Welch's ANOVA and Games Howell post hoc tests were conducted to account for unequal variances. The “post hoc comparison” column denotes the direction of group differences via “< or >” if a significant post hoc analysis was detected. Abbreviations: PTSD = posttraumatic stress disorder, PTSD + DS = dissociative subtype posttraumatic stress disorder patients, CMA = centromedial amygdala, BLA = basolateral amygdala, PAG = periaqueductal gray, vmPFC = ventromedial prefrontal cortex, HSD = Tukey's honestly significant difference.
Correlations with PTSD Psychopathology
After correcting for multiple comparisons for our a priori hypothesized clinical variables, we found a significant negative correlation between CAPS total scores and the strength of connectivity between the left BLA to the vmPFC (r = –0.259, P = 0.015). Analysis further revealed a positive correlation between depersonalization/derealization average scores and the strength of connectivity from the right CMA to the PAG (r = 0.363, P < 0.001). These correlations lend construct validity to the effective connectivity estimates, given that the psychopathology scores were completely independent of the DCM estimates. Control analyses examining BDI scores were found to be non‐significant.
DISCUSSION
This is the first study to report unique patterns of directed connectivity within fear/defense and emotion regulation circuitry among PTSD, PTSD + DS, and healthy controls. Our results suggest PTSD is characterized by predominately bottom–up connections from the PAG to the amygdala and vmPFC, and from the amygdala to the vmPFC. By contrast, PTSD + DS is characterized by predominately top–down connections from the vmPFC to the amygdala and PAG, and from the amygdala to PAG.
Amygdala Connections with the PAG
Amygdala complexes evidenced unique patterns of directed connectivity with the PAG among all three groups. The PTSD group was characterized by bottom–up connections from the PAG to the bilateral CMA and with top–down connections from the bilateral BLA to the PAG. By contrast, PTSD + DS was best characterized by top–down connections from both the bilateral BLA and bilateral CMA to the PAG. This pattern of inverse directional connectivity suggests exacerbated fear and defense‐related driving inputs from the PAG to the amygdala in PTSD patients, which may lead to hyperarousal/hypervigilance [Lanius et al., 2010]. By contrast, top–down connectivity in the PTSD + DS group may be related to overmodulation of PAG defense/fear processing (shut down of fight‐or‐flight responses) and associated emotional detachment [Hopper et al., 2007; Lanius et al., 2010]. The control group showed a pattern of connectivity more similar to the PTSD group; however, exceedance probabilities for the BLA‐PAG connection were considerably lower. Here, we hypothesize that this amygdala‐PAG fear/defense circuit may not be as active at rest in controls, corresponding to the lower exceedance probabilities observed in our control group Bayesian model selection analysis.
From a neurophysiological perspective, the BLA mediates cortical integration of fear and emotions and is regulated by feedforward inhibition from the mPFC, with outputs to the PFC and PAG [Duvarci and Pare, 2014; LeDoux, 2007]. The CMA is more involved in execution/expression of fear responses, with GABAergic outputs to the PAG (Duvarci & Pare, 2014; LeDoux, 2007). The CMA complex provides the majority of projections to the brainstem and PAG [Duvarci and Pare, 2014], which may explain why we only observed an inverse pattern of directed connectivity between the CMA and PAG in PTSD and PTSD + DS. Critically, the PAG is involved in coordinating instinctual defensive reactions (i.e., fight or flight responses, tonic immobility and feigning death), emotional coping, and responding to threatening stimuli [Bandler et al., 2000; Linnman et al., 2012]. PAG signaling drives learned and innate fear responses in the amygdala [Johansen et al., 2010; Kim et al., 2013], where the PAG can modulate BLA synapses [Kim et al., 2013]. Hence, whereas bottom–up connections from the PAG to the amygdala in PTSD may indicate central inputs signaling chronic fear responses, top–down connections from the amygdala to the PAG in PTSD + DS may indicate overmodulation of defensive reactions and emotion‐related responses. In support of this, pharmacological inactivation of the PAG attenuates fear‐evoked responses in the amygdala, indicating that the PAG may relay instructive fear signals to the amygdala [Johansen et al., 2010]. Accordingly, it is probable that top–down connectivity from the BLA and CMA to the PAG in PTSD + DS represents an inhibitory pathway involved in shutting down active defensive responses related to hyperarousal flight‐or‐flight, thus enabling passive defensive responses, including depersonalization and derealization states and associated emotional detachment in PTSD + DS. This in line with defense cascade models of PTSD [Harricharan et al., 2016; Kozlowska et al., 2015; McKinnon et al., 2016], where fight or flight sympathetic nervous system activation is associated with increased processing in the amygdala via the dorsolateral PAG, resulting in the downstream activation of skeletal muscles via premotor centers in the pons and medulla. By contrast, dissociative responses (i.e., compromised consciousness, depersonalization, derealization and tonic collapsed immobility) are thought to be associated with opioid‐mediated analgesia via the ventrolateral PAG [Kozlowska et al., 2015; McKinnon et al., 2016].
Amygdala Connections with the vmPFC
Supporting unique biomarkers of PTSD, PTSD + DS, and controls, we observed differences in the pattern of amygdala and vmPFC effective connectivity between these groups. Specifically, PTSD was characterized by bottom–up connections from bilateral BLA to the vmPFC. Only the right CMA evidenced top–down connections from the vmPFC to the CMA. By contrast, PTSD + DS was characterized by top–down connections from the vmPFC to bilateral BLA and right CMA. The control group displayed similar BLA connectivity to the vmPFC as the PTSD group, albeit the right CMA showed bottom–up connectivity to the vmPFC. Furthermore, healthy controls have previously been found to display weaker functional connectivity patterns between these regions at rest (Nicholson et al., 2015). In keeping with previous emotion modulation models of PTSD [Lanius et al., 2010; Nicholson et al., 2015], our findings support the notion that the vmPFC is most dominant over the amygdala in PTSD + DS. Here, the vmPFC may exert top–down inhibition on amygdala emotional processing leading to emotional detachment, including depersonalization/derealization. On balance, we found that PTSD + DS demonstrated the strongest intrinsic inhibitory self‐connections of the vmPFC, as compared to the PTSD and controls.
Broadly, the vmPFC is involved heavily in implicit regulation of fear and emotions [Etkin et al., 2015]. Given this role, the amygdala may serve as an emotion processing region resulting from the integration of top–down emotion regulation from the vmPFC (cognitive circuit) and bottom–up defense/fear generation from the PAG/midbrain (defensive survival circuit) [Åhs et al., 2015; Etkin et al., 2015; Ledoux, 2016; Panksepp, 2003; Panksepp et al., 2011]. Interestingly, recent studies have shown more emotional dysregulation and numbing, as well as self‐blame, detachment, and an inability to feel positive emotions among PTSD + DS patients [Bennett et al., 2015; Hansen et al., 2017]. Our results suggest that emotional processing in the amygdala may be blunted as a result of increased top–down connectivity from the vmPFC in PTSD + DS. This conclusion is in line with our previous work demonstrating increased resting‐state functional connectivity of the PFC with the BLA and CMA among PTSD + DS as compared to PTSD, which was correlated to dissociative symptoms [Nicholson et al., 2015]. It is interesting to note from a treatment perspective that down‐regulating amygdala activation during emotional processing via real‐time fMRI neurofeedback has been shown to increase PFC‐amygdala connectivity in PTSD patients [Nicholson et al., 2017].
PAG and vmPFC Connections
Whereas in PTSD the PAG displayed bottom–up directional connectivity to the vmPFC, PTSD + DS was characterized by top–down directional connectivity. The control group also displayed bottom–up directional connectivity from the PAG to the vmPFC. In the PTSD group, we predicted the direction of information flow would go from the PAG to the vmPFC, corresponding to increased fear/defense processing inputs from the PAG related to chronic hyperarousal. Furthermore, in PTSD + DS, we predicted top–down connectivity from the vmPFC to the PAG; here, the vmPFC may over‐regulate limbic reactivity corresponding to hypoarousal in PTSD + DS.
Critically, the vmPFC has direct connections with the PAG [Bandler et al., 2000; Linnman et al., 2012], where as threat comes closer or is perceived as more imminent, processing shifts from higher‐order vmPFC and orbital frontal regions toward more primitive emotion/defense regions, such as the PAG and amygdala [Mobbs et al., 2010a, 2010b]. Mobbs et al. [2009] suggest that whereas higher‐order forebrain areas (vmPFC) are involved in the regulation of fear, imminent danger results in automatic and “hard‐wired” defensive reactions mediated by the PAG. Hence, the PTSD group may perceive threats as more chronically imminent, thus displaying bottom–up driving inputs form the PAG to the vmPFC [Panksepp, 2003; Panksepp et al., 2011]. Inversely, the PTSD + DS group may display over‐regulation from the vmPFC on defensive fear processing within the PAG. Notably, we found that PTSD + DS demonstrated the strongest intrinsic inhibitory self‐connections of the vmPFC. In controls, by contrast, top–down emotion regulation from the vmPFC on the PAG may not be needed during resting state, given that this group does not possess pathological activation of fear and defense circuits due to trauma exposure. Critically, resting‐state functional connectivity between the PAG and prefrontal regions was found to be significantly weaker in controls as compared to both PTSD and PTSD+DS patient groups (Harricharan et al., 2016). Accordingly, we hypothesize that whereas bottom–up directional connectivity in the control group may be related to normal interoceptive/limbic ascending sensory signal transfer [Harricharan et al., 2016; Nicholson et al., 2015], in the PTSD group this may instead relate to pathological bottom–up limbic activation related to hyperarousal [Hopper et al., 2007; Stevens et al., 2013].
Associations between sDCM Findings and Psychopathology
Among patients, we found a significant negative correlation between CAPS total scores and strength of connectivity from the left BLA to the vmPFC. Notably, higher CAPS scores are reported by PTSD + DS patients [Wolf et al., 2012]. This negative correlation between CAPS and connectivity from the left BLA to the vmPFC may be related to enhanced prefrontal top–down modulation among PTSD + DS. Indeed, the vmPFC may adapt, through necessity, to shut down and contain activations that repeatedly threaten to overwhelm the functioning of basic physiological systems. In support of increased top–down connectivity among PTSD + DS, we also found a significant positive correlation between depersonalization/derealization average scores and the strength of connectivity from the right CMA to the PAG.
LIMITATIONS AND FUTURE DIRECTIONS
The current approach needs to be applied longitudinally, in larger samples. It would also be of interest to conduct a separate examination in complex PTSD, and with a resting‐state protocol that obtains more functional volumes. The majority of our sample was also female. Hence, sex differences will need to be examined in future studies. In addition to implementing, the new parametric empirical Bayes framework, examining node connectivity during trauma provocation and elucidating inhibitory/excitatory connections, future studies should also examine PAG subregions separately, as well as other areas of the PFC, insula and cingulate cortex. Interestingly, we found that the PTSD + DS group exhibited significantly higher levels of childhood trauma severity as assessed by the CTQ. This was expected as more severe childhood trauma exposure has been identified as a risk factor for developing the dissociative subtype of PTSD [Wolf et al., 2012]. Future studies are needed to assess the role of childhood trauma exposure and severity on directional connectivity in PTSD neural architecture. Furthermore, it should be noted that somewhat different results of effective connectivity within neural architectures have been reported when using stochastic versus spectral DCM model inversions [Razi et al., 2015]. Lastly, the results of this study can only be generalized to the specified regions of interest in each Bayesian model selection analysis; hence, additional DCM analyses of more elaborate circuits will need to be conducted.
CONCLUSION
Here, we describe the first study to examine cortical and subcortical biomarkers of directed connectivity in PTSD and its dissociative subtype, as well as in healthy controls. We found that PTSD patients were characterized predominately by bottom–up connections from the PAG to the vmPFC and amygdala, and from the amygdala to vmPFC. By contrast, PTSD + DS was characterized predominately by top–down connections from the vmPFC to amygdala and PAG, and from the amygdala to PAG. These results suggest that the contrasting symptom profiles of PTSD and its dissociative subtype (hyper‐ vs. hypo‐ emotionality, respectively) may be related to their opposing patterns of directional connectivity.
Supporting information
Supporting Information
David Spiegel shared senior author.
The authors declare no conflicts of interest.
REFERENCES
- Åhs F, Kragel PA, Zielinski DJ, Brady R, LaBar KS (2015): Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. Neuroimage 122:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders. American Journal of Psychiatry, 5th ed Washington DC, Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J (2000): Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53:95–104. [DOI] [PubMed] [Google Scholar]
- Bastos‐Leite AJ, Ridgway GR, Silveira C, Norton A, Reis S, Friston KJ (2014): Dysconnectivity within the default mode in first‐episode schizophrenia: A stochastic dynamic causal modeling study with functional magnetic resonance imaging. Schizophr Bull 41:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC, Modrowski CA, Kerig PK, Chaplo SD (2015): Investigating the dissociative subtype of posttraumatic stress disorder in a sample of traumatized detained youth. Psychol Trauma 7:465–472. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995): The development of a clinician‐administered PTSD scale. J Trauma Stress 8:75–90. [DOI] [PubMed] [Google Scholar]
- Brand BL, Lanius R, Vermetten E, Loewenstein RJ, Spiegel D (2012): Where are we going? An update on assessment, treatment, and neurobiological research in dissociative disorders as we move toward the DSM‐5. J Trauma Dissociation 13:9–31. [DOI] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Beall SK, Van Voorhees E, Marx CE, Calhoun PS, Fairbank JA, Green KT, Tupler LA, Weiner RD, Beckham JC, Brancu M, Hoerle JM, Pender M, Kudler H, Swinkels CM, Nieuwsma JA, Runnals JJ, Youssef NA, McDonald SD, Davison R, Yoash‐Gantz R, Taber KH, Hurley R, McCarthy G, Morey RA (2014): Altered resting‐state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D (2014): Amygdala microcircuits controlling learned fear. Neuron 82:966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ (2015): The neural bases of emotion regulation. Nat Rev Neurosci 16:693–700. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Kemp a. H, Williams L, Falconer E, Olivieri G, Peduto a, Bryant R (2008): Dissociative responses to conscious and non‐conscious fear impact underlying brain function in post‐traumatic stress disorder. Psychol Med 38:1771–1780. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Patient Edition (SCID‐I/P, 11/2002 revision) for DSMIV. New York, NY: Biometrics Research, New York State Psychiatric Institute.
- Friston KJ (2009): Modalities, modes, and models in functional neuroimaging. Science 326:399–403. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Hansen M, Ross J, Armour C (2017): Evidence of the dissociative PTSD subtype: A systematic literature review of latent class and profile analytic studies of PTSD. J Affect Disord 213:59–69. [DOI] [PubMed] [Google Scholar]
- Harricharan S, Rabellino D, Frewen P, Densmore M, Theberge J, McKinnon M, Schore A, Lanius R (2016): fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav 6:e00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM (2012): Quantitative meta‐analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA (2007): Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script‐driven trauma imagery. J Trauma Stress 20:713–725. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, Blair HT (2010): Neural substrates for expectation‐modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci 13:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Horovitz O, Pellman BA, Tan LM, Li Q, Richter‐Levin G, Kim JJ (2013): Dorsal periaqueductal gray‐amygdala pathway conveys both innate and learned fear responses in rats. Proc Natl Acad Sci USA 110:14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska K, Walker P, McLean L, Carrive P (2015): Fear and the defense cascade. Harv Rev Psychiatry 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Christian S, Bremner JD, Spiegel D (2010): Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 167:640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2007): The amygdala. Curr Biol 17:868–874. [DOI] [PubMed] [Google Scholar]
- Ledoux JE (2016): Using neuroscience to help understand fear and anxiety: A two‐system framework. Am J Psychiatry 173:1083–1093. [DOI] [PubMed] [Google Scholar]
- Li B, Daunizeau J, Stephan KE, Penny W, Hu D, Friston K (2011): Generalised filtering and stochastic DCM for fMRI. Neuroimage 58:442–457. [DOI] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D (2012): Neuroimaging of the periaqueductal gray: State of the field. Neuroimage 60:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Boyd JE, Frewen PA, Lanius UF, Jetly R, Richardson JD, Lanius RA (2016): A review of the relation between dissociation, memory, executive functioning and social cognition in military members and civilians with neuropsychiatric conditions. Neuropsychologia 90:210–234. [DOI] [PubMed] [Google Scholar]
- Mickleborough MJS, Daniels JK, Coupland NJ, Kao R, Williamson PC, Lanius UF, Hegadoren K, Schore A, Densmore M, Stevens T, Lanius RA (2011): Effects of trauma‐related cues on pain processing in posttraumatic stress disorder: An fMRI investigation. J Psychiatry Neurosci 36:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD (2009): From threat to fear: The neural organization of defensive fear systems in humans. J Neurosci 29:12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD (2010a): When fear is near: Threat imminence elicits prefrontal ‐ periaqueductal grey shifts in humans. Science 317:1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T (2010b): Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA 107:20582–20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Densmore M, Frewen PA, Théberge J, Neufeld RWJ, McKinnon MC Lanius (2015): The dissociative subtype of posttraumatic stress disorder: Unique resting‐state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40:2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Schmahl C, Théberge J, Neufeld RWJ, McKinnon MC, Reiss J, Jetly R, Lanius RA (2017): The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real‐time fMRI neurofeedback. Hum Brain Mapp 38:541–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J (2003): At the interface of the affective, behavioral, and cognitive neurosciences: Decoding the emotional feelings of the brain. Brain Cogn 52:4–14. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Fuchs T, Iacobucci P (2011): The basic neuroscience of emotional experiences in mammals: The case of subcortical FEAR circuitry and implications for clinical anxiety. Appl Anim Behav Sci 129:1–17. [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ (2004): Comparing dynamic causal models. Neuroimage 22:1157–1172. [DOI] [PubMed] [Google Scholar]
- Razi A, Kahan J, Rees G, Friston KJ (2015): Construct validation of a DCM for resting state fMRI. Neuroimage 106:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Spielberg JM, Warren SL, Miller GA, Heller W (2014): Aberrant neural connectivity during emotional processing associated with posttraumatic stress. Clin Psychol Sci 2:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D (2012): Divided consciousness: Dissociation in DSM‐5. Depress Anxiety 29:667–670. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Koenen KC, Friedman MJ, Hill E, McLaughlin KA, Petukhova M, Ruscio AM, Shahly V, Spiegel D, Borges G, Bunting B, Caldas‐De‐Almeida JM, De Girolamo G, Demyttenaere K, Florescu S, Haro JM, Karam EG, Kovess‐Masfety V, Lee S, Matschinger H, Mladenova M, Posada‐Villa J, Tachimori H, Viana MC, Kessler RC (2013): Dissociation in posttraumatic stress disorder: Evidence from the world mental health surveys. Biol Psychiatry 73:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ (2009): Bayesian model selection for group studies. Neuroimage 46:1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ (2013): Disrupted amygdala‐prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, Densmore M, Frewen PA, Mckinnon MC, Th J, Nicholson AA, Koenig J, Thayer JF, Lanius RA (2017): Desynchronization of autonomic response and central autonomic network connectivity in posttraumatic stress disorder. Hum Brain Mapp 38:27–40. [DOI] [PMC free article] [PubMed]
- Wolf EJ, Miller MW, Reardon AF, Ryabchenko KA, Castillo D, Freund R (2012): A latent class analysis of dissociation and posttraumatic stress disorder. Arch Gen Psychiatry 69:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Rasmusson AM, Mitchell KS, Logue MW, Baldwin CT, Miller MW (2014): A genome‐wide association study of clinical symptoms of dissociation in a trauma‐exposed sample. Depress Anxiety 31:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, Hobfoll SE, Koenen KC, Neylan TC, Hyman SE (2015): Post‐traumatic stress disorder. Nat Rev Dis Prim 15057. doi: 10.1038/nrdp.2015.57 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


