Abstract
Previous research suggests a role of the dorsomedial prefrontal cortex (dmPFC) in metacognitive representation of social information, while the right posterior superior temporal sulcus (pSTS) has been linked to social perception. This study targeted these functional roles in the context of spontaneous mentalizing. An animated shapes task was presented to 46 subjects during functional magnetic resonance imaging. Stimuli consisted of video clips depicting animated shapes whose movement patterns prompt spontaneous mentalizing or simple intention attribution. Based on their differential response during spontaneous mentalizing, both regions were characterized with respect to their task‐dependent connectivity profiles and their associations with autistic traits. Functional network analyses revealed highly localized coupling of the right pSTS with visual areas in the lateral occipital cortex, while the dmPFC showed extensive coupling with instances of large‐scale control networks and temporal areas including the right pSTS. Autistic traits were related to mentalizing‐specific activation of the dmPFC and to the strength of connectivity between the dmPFC and posterior temporal regions. These results are in good agreement with the hypothesized roles of the dmPFC and right pSTS for metacognitive representation and perception‐based processing of social information, respectively, and further inform their implication in social behavior linked to autism. Hum Brain Mapp 38:3791–3803, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI, posterior superior temporal sulcus, dorsomedial prefrontal cortex, spontaneous mentalizing, autistic traits
INTRODUCTION
The involvement of the dorsomedial prefrontal cortex (dmPFC) and posterior superior temporal sulcus (pSTS) in social cognition has long been established. The dmPFC has been linked to higher‐order other‐referential processing, such as the inference of time‐invariant traits, intentions, and beliefs of a social partner [Denny et al., 2012; Ferrari et al., 2016; Wagner et al., 2016]. The pSTS, in contrast, is reliably implicated in the perception of animacy and agency [Gao et al., 2012], biological motion [Grossman and Blake, 2002], faces [Haxby et al., 2000], and voices [Belin et al., 2000]. More posteriorly, the temporoparietal junction (TPJ) has been related to the representation of other people's temporary mental state, such as false beliefs [Saxe and Kanwisher, 2003]. These findings imply that posterior temporal areas are in charge of processing the building blocks of our proximate social environment [Deen et al., 2015; Hein and Knight, 2008; Lahnakoski et al., 2012], while the dmPFC is responsible for flexible and context‐independent metacognitive representations of the social world [Bzdok et al., 2013; Spunt and Adolphs, 2015].
While the characterization of the dmPFC and pSTS has to a large extent been based on their task‐related functional activation [Schurz et al., 2014], it has also been informed by their coactivation profiles and resting‐state functional connectivity patterns [Eickhoff et al., 2016; Habas et al., 2011]. For instance, a substantial portion of the social brain is part of the so‐called default mode network (DMN) [Gusnard et al., 2001; Schilbach et al., 2012], with the dmPFC being proposed as highly interconnected key node of DMN subnetworks related to social processing [Amft et al., 2015; Andrews‐Hanna et al., 2010]. Numerous studies have demonstrated robust functional connectivity of the dmPFC with other instances of the social brain, such as the temporal poles, precuneus, or TPJ [Amft et al., 2015; Andrews‐Hanna et al., 2010; Bzdok et al., 2013; Sallet et al., 2013]. Interestingly, these resting‐state connectivity profiles of the dmPFC commonly do not include the pSTS. Instead, pSTS functional connectivity has been shown to be particularly pronounced in temporal, insular, and higher‐order motor regions [Deen et al., 2015; Habas et al., 2011]. In contrast to these resting‐state investigations, less is known about both regions' brain‐wide connectivity profiles during the execution of a social task and how these connectivity profiles are modulated by social demands. This question is important as differences in task‐specific connectivity should delineate differential cognitive contributions and are therefore highly informative about the functional roles of both regions for social cognition. In addition, it would be interesting to know whether the task‐specific functional responses and connectivity profiles of the dmPFC and pSTS are related to individual differences in social abilities and traits.
To target these research questions, we selected an established animated shapes task [Abell et al., 2000] for a functional magnetic resonance imaging (fMRI) study in a well‐powered sample of healthy adults. The animated shapes task probes the tendency to ascribe mental states to clearly inanimate objects, such as cartoon characters or animated shapes [Heider and Simmel, 1944]. These highly automatic processes are referred to as spontaneous mentalizing and are essential for successful social interactions [Apperly and Butterfill, 2009; Mar and Macrae, 2007]. Spontaneous mentalizing has been studied in various ways, such as the presentation of animated shapes [Castelli et al., 2000], task‐irrelevant trait descriptions [Ma et al., 2011], or uninstructed viewing of social scenes in photographs or movies [Powers et al., 2016; Wagner et al., 2016, 2011]. Imaging evidence suggests that spontaneous mentalizing recruits the core areas of the so‐called social brain [Adolphs, 2009], with varying degrees depending on the task at hand [Van Overwalle and Vandekerckhove, 2013]. Several reports have demonstrated robust responses of the social brain to animated shapes, including pSTS and dmPFC [Castelli et al., 2002, 2000; Mar, 2011; Moessnang et al., 2016], with particularly stronger involvement of temporal and inferior frontal areas when compared to other mentalizing tasks [Schurz et al., 2014].
Besides its significance for daily social functioning and its reliance on social brain regions including dmPFC and pSTS, spontaneous mentalizing has also been shown to be sensitive for individual differences in social abilities. For instance, the level of dmPFC activation during spontaneous mentalizing could be related to differences in autism‐related traits [Spunt et al., 2015; Wagner et al., 2011], real‐life social expertise [Powers et al., 2016], and the proneness to adopt the intentional stance [Kestemont et al., 2013; Moran et al., 2014]. Another line of research suggests that spontaneous mentalizing is particularly impaired in patients with autism spectrum disorders (ASD) [Abell et al., 2000; Senju et al., 2009; White et al., 2011]. These observations were paralleled by findings of blunted social brain responses in ASD subjects during an animated shapes task [Castelli et al., 2002]. More generally, altered activation of the dmPFC and pSTS in social tasks has repeatedly been reported in ASD [Castelli et al., 2002; von dem Hagen et al., 2014; Zilbovicius et al., 2006], which additionally motivates research on both brain regions and their relation to social behavior.
Based on the evidence reviewed above, we used the animated shapes task to study the impact of spontaneous mentalizing on the connectivity profiles of the dmPFC and pSTS and their relation to autistic‐like traits. So far, connectivity of the dmPFC and pSTS during active mentalizing has been described within preselected regions of interest [Hillebrandt et al., 2013, 2014; Shultz et al., 2015], which precludes any conclusion on brain‐wide connectivity patterns, including task‐specific coupling with other instances of the social brain. This study aims at addressing this question by following a whole‐brain connectivity approach.
MATERIALS AND METHODS
Experimental Procedure
Participants
A sample of 46 healthy, right‐handed subjects (mean age: 24.7 ± 5.3 years, 21 females) participated in the study. The same data set was used in a prior investigation on specificity and test–retest reliability of task activation [Moessnang et al., 2016]. None of the subjects reported any history of neurological or psychiatric disorder, significant general medical problems including liver, cardiac, or renal dysfunctions, a history of head trauma, current intake of psychoactive substances, or pregnancy. The majority of subjects (98%) completed Gymnasium or Fachhochschule (comparable to high school) and more than 90% of subjects were enrolled in university (or had already completed a university degree) at the time of testing, suggesting an overall high level of cognitive functioning of our sample. All individuals provided written informed consent for the study protocol which was approved by the institutional review board of the Medical Faculty Mannheim.
Paradigm
We used an animated shapes task based on Frith‐Happé animations [Abell et al., 2000] which allows for a reliable assessment of mentalizing‐specific responses of the social brain, including dmPFC and right pSTS [Moessnang et al., 2016]. Stimuli consisted of animated video clips featuring a big and a small triangle moving about the screen. In the Theory of Mind (ToM) condition, the triangles appear to engage in complex intentional interactions requiring mind reading (e.g., deception). In the goal‐directed (GD) control condition, the triangles interact purposefully without apparent mentalizing efforts (e.g., imitating each other), thereby conveying the perception of agency. In the random (R) control condition, the triangles move randomly without interacting with each other. Clips were integrated in a block‐designed fMRI paradigm with a pseudorandomized order of the three task conditions. For behavioral control, each video clip was followed by a multiple‐choice question (Fig. 1, MCQ‐cat), where subjects were asked to categorize the depicted interaction according to the perceived social significance (i.e., ToM, GD or R) [White et al., 2011]. In case of ToM animations, subjects were additionally asked to rate the perceived emotional state of each triangle (i.e., positive, neutral, or negative emotional valence; Fig. 1, MCQ‐feeling). These ratings were used to ensure successful comprehension of the intended cover story of each ToM animation and are reported elsewhere [Moessnang et al., 2016]. The fMRI scan was preceded by a training session involving three established training animations.
Figure 1.
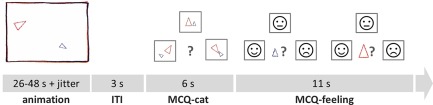
Animations were presented at the beginning of each trial. Example video clips can be retrieved from https://sites.google.com/site/utafrith/research. Subjects were subsequently asked to categorize the animation to one of the three conditions (R, GD, and ToM), represented by simplified icons. Following ToM videos, subjects were additionally asked to rate the emotional state of each triangle (“How did the small/big triangle feel at the end of the animation?”). Responses were given with the right thumb, using the left, upper, and right key of an MRI compatible button box (Current Designs, PA, USA). As soon as responses were given during MCQ ratings (MCQ‐cat, MCQ‐feeling), the chosen icon was framed in red for the duration of one additional second, followed by a blank screen for the remainder of the respective MCQ phase. No feedback on response accuracy was given. A jitter with variable duration (M = 996 ms, SD = 418 ms) was included before video presentation. [Color figure can be viewed at http://wileyonlinelibrary.com]
MRI data acquisition
MRI was performed on a 3 T Siemens Trio Scanner (Siemens, Erlangen, Germany) equipped with a 12‐channel head coil. Functional images were acquired using an echo‐planar imaging (EPI) sequence (TE: 30 ms, TR: 2 s, α: 80°, matrix: 64 × 64, FOV: 192 × 192 mm, in‐plane resolution: 3 × 3 mm, slice thickness: 4 mm, gap: 1 mm, 28 axial slices, 331 volumes).
Definition of Regions of Interest
Regions of interest (ROIs) were derived from a meta‐analysis on nonstory‐based (i.e., nonverbal) Theory of Mind studies [Mar, 2011], which show a good regional fit with brain responses to our animated shapes task and have been used for reliability assessment in a previous study [Moessnang et al., 2016; see Supporting Information for more details). Although both left and right pSTS are implicated in social cognition, we selected the right pSTS based on meta‐analytical evidence of a higher consistency of right compared to left temporal activation during mentalizing [Schurz et al., 2014]. In addition to masks of the dmPFC (290 voxels) and right pSTS (227 voxels), we used an extended set of ROIs to specifically assess connectivity patterns of the dmPFC and right pSTS within key regions of the social brain (left pSTS [189 voxels], precuneus [201 voxels], inferior frontal gyrus [left: 84 voxels, right 104 voxels], anterior middle temporal gyrus [left: 75 voxels, right: 81 voxels], and temporal poles [left: 254, right: 391 voxels]). Note that masks of the temporal poles were extracted from the Anatomical Automatic Labeling Atlas [Tzourio‐Mazoyer et al., 2002] due to insufficient coverage by Mar clusters.
DATA ANALYSIS
Image Preprocessing
Image preprocessing followed standard processing routines in SPM8 (http://www.fil.ion.ucl.ac.uk/spm), including realignment to the first image, slice time correction, spatial normalization based on the Montreal Neurological Institute (MNI) template, resampling to 3 mm isotropic voxels, and smoothing with an 8 mm full‐width at half‐maximum Gaussian Kernel.
Overall subject motion was low (mean framewise displacement: M = 0.09 mm, SD = 0.04 mm, Max = 0.31 mm) [Jenkinson et al., 2002]. Translation did not exceed the conventional threshold of 3 mm. One dataset was identified with sporadic rotation peaks >3°, which however did not occur during video presentations. Supplementary analyses showed that the exclusion of this dataset did not change the reported results and that motion was not associated with the investigated phenotypes (Supporting Information).
Analysis of Mentalizing‐Specific Connectivity Profiles of the dmPFC and Right pSTS
A psychophysiological interaction (PPI) analysis [Friston et al., 1997; O'Reilly et al., 2012] was performed to model mentalizing‐related connectivity patterns of the dmPFC and right pSTS. As a first step, we defined a first‐level statistical model to estimate mentalizing‐specific responses of the dmPFC and right pSTS. To this end, the different video conditions were modeled as box‐car functions, convolved with the canonical hemodynamic response function, and entered as regressors into individual general linear models (GLMs), with realignment parameters included as covariates of no interest. A high‐pass filter with a cutoff of 256 s and an autoregressive model of the first order were applied during model estimation. Mentalizing‐specific functional activation was defined as the differential response to ToM compared to GD animations (i.e., ToM > GD). This contrast allows for a reliable differentiation of brain responses related to mentalizing from those related to subordinate processes of agency perception [Moessnang et al., 2016].
In the second step, the peak voxel (maximum t value) for mentalizing‐specific activation was identified within each search mask (i.e., ROI mask of the dmPFC and right pSTS) for each subject (see Supporting Information, Table S3 and Fig. S2 for detailed information on peak distribution within masks). We subsequently extracted the first eigenvariate from a 6 mm sphere around the identified peak voxel, yielding individually defined volumes of interest (VOI) within the larger mask. The extracted VOI time series were corrected for nuisance effects (i.e., adjustment for effects of interest). After time‐series extraction, PPI regressors were generated as element‐by‐element product of the task conditions of interest (psychological regressors: GD, ToM) and the sphere time course (physiological regressor). Separate GLMs were calculated for dmPFC and right pSTS, where the resulting interaction terms (PPIToM and PPIGD) and the respective sphere time course were included in the first‐level model. Individual contrast images (PPIToM > PPIGD) were subjected to one‐sample t tests for group‐level inference, with age and sex included as covariates of no interest. Significance of group‐level results was defined at a level of P FWE < 0.05, family‐wise error (FWE) corrected for multiple comparisons across the whole brain. In addition, we specifically analyzed their condition‐dependent coupling with other key regions of the social brain using small volume correction (SVC; P FWE < 0.05, FWE corrected for multiple comparisons in the combined search mask).
Analysis of Associations with Autism Traits
Autism traits were assessed using the German short version of the Autism‐Spectrum Quotient Inventory (AQ‐K) [Baron‐Cohen et al., 2001; Freitag et al., 2007] which quantifies traits and social abilities on the subscales “social interaction and spontaneity” (11 items), “imagination” (12 items), and “communication and reciprocity” (10 items). Higher scores reflect higher degrees of autism traits. A total score is calculated as the sum of individual items, with a clinical threshold of 17 and a maximum of 33. AQ‐K data were available for a subset of 36 individuals (18 males) and total AQ‐K scores were included as covariate of interest in voxel‐wise one‐sample t tests of the high‐level contrast, separately for the activation (ToM > GD) and connectivity (PPIToM > PPIGD) phenotypes. Sex and age were included as covariates. In the first step, we tested whether autism traits were associated with functional responses in the dmPFC or right pSTS per se, which was evaluated in a combined dmPFC‐right pSTS mask using SVC (P FWE < 0.05). In the second step, we tested whether the functional connectivity with specific key regions of the social brain, as identified in the previous PPI analysis, was modulated by autism traits using SVC (P FWE < 0.05, FWE corrected for multiple comparisons in the combined search mask).
Owing to a strong sex effect in autism, with higher prevalence and higher autism trait scores in males [Baron‐Cohen et al., 2001], we performed supplementary analyses to assess the effect of sex on autism traits and associated brain responses (see Supporting Information).
RESULTS
Functional Connectivity of the dmPFC and Right pSTS During Spontaneous Mentali‐Zing
Whole‐brain analysis
During ToM compared to GD animations, the dmPFC (as defined by individual VOIs within the dmPFC ROI) showed increased coupling with multiple areas (all P FWE < 0.05, whole brain), including instances of large‐scale control systems [Power et al., 2011] such as the frontoparietal network (intraparietal sulcus, dorsal frontal cortex near the frontal eye fields) and cingulo‐opercular network (dorsal anterior cingulate/medial superior frontal cortex, anterior TPJ [overlapping with the pSTS ROI; see Supporting Information, Fig. S3]; Table 1). Strongest connectivity effects were observed in a region of the lateral occipital cortex of both hemispheres which showed highest correspondence to visual area LO2 [Malikovic et al., 2015] implicated in visual scene processing [Larsson and Heeger, 2006]. In addition, significant coupling of the dmPFC was found with a cluster in the depth of the right pSTS (Fig. 2A and Supporting Information, Fig. S3). In contrast, the whole‐brain mentalizing‐specific connectivity profile of the right pSTS (as defined by individual VOIs within the right pSTS ROI) was limited to visual area LO2 in the lateral occipital complex of both hemispheres (P FWE < 0.05, whole brain; Table 1 and Fig. 2B).
Table 1.
Whole‐brain and region of interest connectivity results during spontaneous mentalizing compared to agency perception (PPIToM > PPIGD)
| Region | x | y | z | t | P FWE | |
|---|---|---|---|---|---|---|
| Mentalizing‐specific connectivity of the dmPFC | ||||||
| Exploratory whole‐brain analysis (FWE significance threshold: T > 5.25) | ||||||
| Middle occipitotemporal gyrus [hOc4la (LO2), Area PGp (IPL)] | 51 | −73 | 13 | 6.99 | <0.001 | |
| Precuneus [Area 7A (SPL)] | −9 | −64 | 49 | 6.54 | <0.001 | |
| Precuneus [Area 5L (SPL)] | 6 | −61 | 64 | 6.14 | 0.004 | |
| Middle occipital gyrus [hOc4la (LO2), Area PGp (IPL)] | −42 | −79 | 16 | 5.98 | 0.006 | |
| Cerebellum [Lobule VIIa crus 1] | 39 | −61 | −32 | 5.76 | 0.012 | |
| Cerebellum [Lobule VIIa crus 1] | −12 | −85 | −29 | 5.75 | 0.012 | |
| Superior temporal gyrus | 51 | −40 | 22 | 5.67 | 0.015 | |
| Middle temporal gyrus | 60 | −40 | 10 | 5.66 | 0.016 | |
| Cerebellum [Lobule VIIa crus 1] | −15 | −73 | −29 | 5.58 | 0.020 | |
| Angular gyrus [Area hlP3 (IPS)] | 36 | −55 | 43 | 5.57 | 0.020 | |
| Middle frontal gyrus | 27 | 8 | 55 | 5.57 | 0.020 | |
| Cerebellum [Lobule VIIa crus 2] | −39 | −67 | −50 | 5.50 | 0.025 | |
| Precuneus [Area 5M (SPL)] | 9 | −49 | 52 | 5.39 | 0.034 | |
| Inferior parietal lobule [Area hlP3 (IPS)] | −33 | −55 | 49 | 5.38 | 0.035 | |
| Fusiform gyrus | 39 | −7 | −32 | 5.35 | 0.038 | |
| Superior medial frontal gyrus | −6 | 20 | 43 | 5.34 | 0.038 | |
| Middle temporal gyrus | 51 | −37 | −5 | 5.33 | 0.040 | |
| Cuneus (BA 17, V1) | 18 | −94 | 13 | 5.30 | 0.043 | |
| Angular gyrus | 45 | −52 | 31 | 5.25 | 0.050 | |
| Region of interest analysis within social brain mask (FWE significance threshold: T > 4.16) | ||||||
| ROI label: right pSTS | 51 | −43 | 25 | 5.43 | 0.001 | |
| ROI label: precuneus | −3 | −61 | 49 | 5.08 | 0.004 | |
| ROI label: left pSTS | −57 | −49 | 16 | 4.93 | 0.006 | |
| ROI label: right TP | 54 | 8 | −23 | 4.35 | 0.030 | |
| Mentalizing‐specific connectivity of the right pSTS | ||||||
| Exploratory whole‐brain analysis (FWE significance threshold: T > 5.25) | ||||||
| Middle occipital gyrus [hOc4la (LO2)] | −48 | −73 | 10 | 6.09 | 0.005 | |
| Middle temporal gyrus [hOc4la (LO2)] | 51 | −76 | 10 | 6.01 | 0.006 | |
| Region of interest analysis within social brain mask (FWE significance threshold: T > 4.16) | ||||||
| ROI label: right TP | 48 | 14 | −36 | 4.33 | 0.032 | |
| ROI label: precuneus | −3 | −58 | 46 | 4.31 | 0.034 | |
Regions were classified according to the Automated Anatomical Labeling Atlas [Tzourio‐Mazoyer et al., 2002]. If applicable, functional labels were added in square brackets based on Anatomical Probability Maps (Anatomy toolbox) [Eickhoff et al., 2006]. x‐, y‐, and z‐coordinates (MNI) and statistical information refer to peak voxels in the identified clusters. P values are adjusted for family‐wise error correction for multiple comparisons across the whole brain or across the combined mask of predefined social brain regions, respectively. Age and sex were included as covariates in the analysis. BA, Brodmann area; dmPFC, dorsomedial prefrontal cortex; pSTS, posterior superior temporal sulcus; TP, temporal pole.
Figure 2.
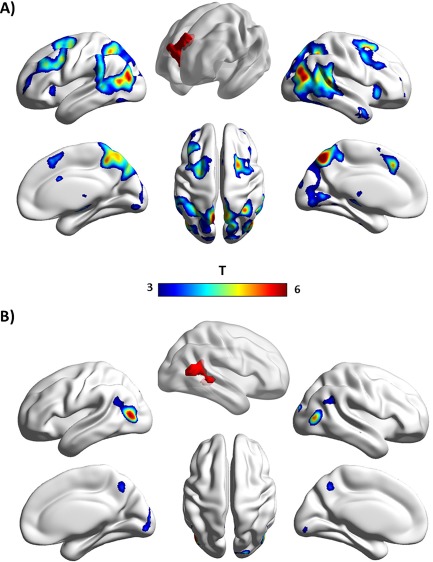
Whole‐brain connectivity profiles of the (A) dmPFC and (B) right pSTS during spontaneous mentalizing compared to agency perception (PPIToM > PPIGD). For illustrative purposes, a height threshold of t = 3 (P uncorr = 0.002) was used. Both ROIs, which were used for seed region definition, are illustrated in red. Data are displayed with BrainNet Viewer (http://www.nitrc.org/projects/bnv/). [Color figure can be viewed at http://wileyonlinelibrary.com]
Region of interest analysis within the social brain
When evaluating mentalizing‐specific coupling of the dmPFC (as defined by individual VOIs within the dmPFC ROI) with key regions of the social brain, significant connectivity was observed within both pSTS, the precuneus, and the right temporal pole (P FWE < 0.05, SVC for the combined search mask, Table 1). In contrast, effects of the right pSTS (as defined by individual VOIs within the right pSTS ROI) were restricted to the right temporal pole and the precuneus (P FWE < 0.05, SVC for the predefined social brain mask; Table 1). Figure 3 allows for a visual assessment of differences between both regions' mentalizing‐specific connectivity with social brain regions.
Figure 3.

Illustration of differences in mentalizing‐specific coupling of the dmPFC (blue) and right pSTS (red) with key regions of the social brain. For each region, the maximum t value (voxel level) is plotted for the mentalizing‐specific contrast (PPIToM > PPIGD), which reflects the difference in connectivity strength during ToM compared to GD animations. The dashed line represents the minimum t value required to pass the significance threshold (P FWE < 0.05, SVC for the combined mask of social brain regions). TP, temporal pole; aMTG, anterior middle temporal gyrus; pSTS, posterior superior temporal sulcus; dmPFC, dorsomedial prefrontal cortex; IFG, inferior frontal gyrus; L, left; R, right. [Color figure can be viewed at http://wileyonlinelibrary.com]
Association of Mentalizing‐Specific Phenotypes of the dmPFC and Right pSTS With Autism Traits
Activation phenotype
Within the combined mask, consisting of the ROIs of the right pSTS and dmPFC, a cluster within the dmPFC was negatively associated with the total AQ‐K score (MNI x = 12, y = 59, z = 28; t = 3.81, P FWE = 0.044, SVC for the combined mask; Fig. 4). No association was observed with differential activation in the right pSTS. Follow‐up analyses suggested no relevant differential effect of AQ‐K subscales (see Supporting Information).
Figure 4.
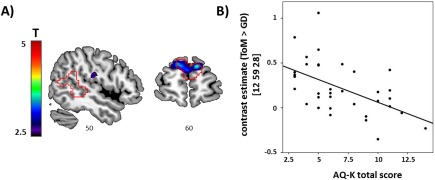
(A) Sections depicting voxel‐wise associations of mentalizing‐specific activation (i.e., ToM > GD) with autism traits. ROI outlines of dmPFC and right pSTS are overlaid in red. (B) Scatter plot illustrating the association between the individual's total AQ‐K score and contrast estimate of the peak voxel at x = 12, y = 59, z = 28 MNI) within the dmPFC. [Color figure can be viewed at http://wileyonlinelibrary.com]
Connectivity phenotype
A significant association with total AQ‐K scores was observed for the mentalizing‐specific connectivity of the dmPFC, but not pSTS. More precisely, higher AQ‐K scores were related to increased connectivity of the dmPFC (as defined by individual VOIs within the dmPFC ROI) with a cluster in the posterior‐ventral part of the pSTS, located on the middle temporal gyrus (BA 39; MNI x = 57, y = −61, z = 10; t = 4.38, P FWE = 0.020, SVC for the combined search mask; Fig. 5). Again, we did not observe a differential effect of AQ subscales (see Supporting Information).
Figure 5.
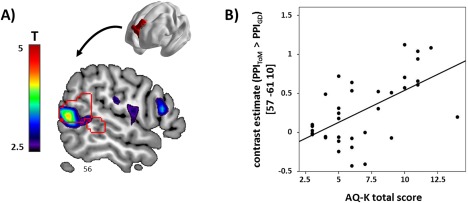
(A) Sections depicting voxel‐wise associations of mentalizing‐specific connectivity of the dmPFC (i.e., PPIToM > PPIGD) with autism traits. ROI outlines of dmPFC and right pSTS are overlaid in red. (B) Scatter plot illustrating the association between total AQ‐K scores and contrast estimates (PPIToM > PPIGD, seed region in the dmPFC) of the peak voxel at x = 57, y = −61, z = 10 (MNI) within the right pSTS ROI. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
The dmPFC and right pSTS have long been recognized as key nodes of the social brain, but their functional roles for social cognition are incompletely understood. Using an animated shapes task that triggers spontaneous mentalizing, we observed substantial differences between the dmPFC and right pSTS with respect to their brain‐wide connectivity profiles and their associations with autistic‐like traits. These findings not only corroborate the hypothesized differential relevance for perception‐based vs metacognition‐based processing of social information in these regions but also lend support for their implication in clinical conditions with social relevance, such as ASD.
While conventional activation analyses reflect average responses to experimental manipulations, they provide limited information about the underlying network dynamics. The analysis of task‐specific activation and connectivity can therefore yield complementary information about a brain region's functional profile, as exemplified in this study. Previous studies have repeatedly shown that both dmPFC and right pSTS are activated during ToM conditions of the employed animated shapes task [Castelli et al., 2002; Moessnang et al., 2016; Schurz et al., 2014]. The analysis of mentalizing connectivity, however, revealed striking differences between both regions which are suggestive of their complementary roles in social cognition.
Consistent with a top–down account of the dmPFC, processing of ToM animations resulted in stronger synchronization with well‐known large‐scale networks associated with executive control [Vincent et al., 2008], task‐set maintenance [Dosenbach et al., 2006], and salience detection [Seeley et al., 2007]. Our data therefore suggest that the connectivity profile of the dmPFC adaptively changes during spontaneous mentalizing, most likely in terms of a stronger recruitment of task‐control networks with increasing mentalizing demands. Context‐dependent network reconfigurations are an important principle for adaptive task control [Braun et al., 2015; Fornito et al., 2012; Gao and Lin, 2012] and might represent a top–down control mechanism of the dmPFC on earlier processing stages [Hillebrandt et al., 2013]. Indeed, dmPFC connectivity during mentalizing was also increased to the visual area LO2 as well as a cluster located in the depth of the pSTS, suggesting a dmPFC‐driven coupling of functional systems that are involved in perception‐based, “bottom–up”‐like and attentional, “top–down”‐like information processing. We propose that this finding reflects a mechanism for functional integration which enables the dmPFC to exert top–down control for enhanced processing of social information.
In contrast, whole‐brain connectivity of the right pSTS during mentalizing was restricted to a region which most likely represents the visual area LO2 [Malikovic et al., 2015]. Region LO2 has been proposed to be crucially involved in perceptual organization based on spatial relations, such as grouping of shape information in a visual image [Larsson and Heeger, 2006]. In a recent meta‐analysis, activation of areas LO1/LO2 could be related not only to processing of shapes but also to action observation [Malikovic et al., 2015]. This suggests that social meaning in the animations is primarily conveyed by spatial configurations generated by the moving triangles, which is subsequently propagated to higher‐order areas of the social brain via the pSTS. Another novel finding is the high specificity of pSTS connectivity during mentalizing. Previous studies on pSTS connectivity used dynamic causal modeling [Friston et al., 2003] in a priori defined regions and reported significant modulation of directed information flow between the pSTS and (1) area V5 during the perception of animated shapes [Hillebrandt et al., 2014], (2) fusiform gyrus during the perception of faces and biological motion [Shultz et al., 2015], and (3) superior occipital gyrus during perspective taking [Hillebrandt et al., 2013]. Following a whole‐brain correlational approach, we extend these findings by demonstrating a highly specific coupling of the right pSTS with areas involved in the processing of the critical sensory information. This is consistent with the proposed role as “bottom–up hub” of the social brain.
When focusing on the mentalizing‐specific connectivity of the dmPFC and right pSTS with other key regions of the social brain, a similar picture emerged. Across regions, the dmPFC showed a stronger increase in mentalizing‐specific coupling compared to the right pSTS. Interestingly, however, significant effects of both ROIs converged on the precuneus and right temporal pole, which suggests enhanced functional integration between these regions [Knight, 2007]. The precuneus (and adjacent posterior cingulate cortex) has long been recognized as a core area of the DMN [Gusnard et al., 2001; Hagmann et al., 2008], with extensive functional connectivity with key regions of the social brain such as the dmPFC [Amft et al., 2015; Andrews‐Hanna et al., 2010]. Our data suggest that active mentalizing leads to an increase of this region's connectivity with task‐relevant brain regions within (i.e., dmPFC) and outside (i.e., pSTS) the DMN. The precuneus has been linked to highly integrative functions, including self‐awareness, autobiographical memory, and perspective taking [Cavanna and Trimble, 2006], and likely interacts closely with the dmPFC for the generation of context‐ and time‐independent representations of the (social) world [Amft et al., 2015]. The temporal poles have also been shown to be functionally connected to the dmPFC and pSTS during rest [Andrews‐Hanna et al., 2010; Deen et al., 2015; Eickhoff et al., 2016; Pascual et al., 2015] and have been suggested to represent a multimodal integration hub for socioaffective semantics (e.g., social concepts) [Olson et al., 2013; Pascual et al., 2015]. While less consistent with resting‐state data, our observation of a lateralized connectivity pattern in our task‐specific analysis might relate to a stronger implication of the left temporal pole in the retrieval of person‐specific knowledge (e.g., names) [Olson et al., 2013; Waldron et al., 2014]—a function which is not specifically addressed by ToM as compared to GD animations in our task.
No significant effects were observed for the aMTG and IFG. That is, while these regions jointly increased their activation during ToM compared to GD animations [Moessnang et al., 2016], this was not paralleled by an increase in connectivity with the dmPFC or right pSTS. This dissociation is interesting as it suggests that the differentiation between ToM and GD animations is not, or to a smaller extent, dependent on an exchange of information between the dmPFC/right pSTS and these regions. Some resting‐state studies reported functional connectivity of the aMTG with dmPFC [Amft et al., 2015; Bzdok et al., 2013; Sallet et al., 2013] and posterior temporal areas [Jackson et al., 2016; Xu et al., 2015]. Similarly, the IFG was shown to be part of a resting‐state network with posterior and middle temporal areas [De Luca et al., 2006], as well as of a resting‐state network seeded in the dmPFC [Bzdok et al., 2013]. Both aMTG and IFG are highly integrative regions, with the former being implicated in language and semantic cognition [Deen et al., 2015; Jackson et al., 2016], whereas the IFG is proposed to support action understanding and empathy [Schurz et al., 2014]. To date, we can only speculate about the reason why both regions did not increase their connectivity with dmPFC or right pSTS during spontaneous mentalizing. To this end, their task‐specific connectivity profiles would be highly informative and should be followed up in future analyses.
Our second aim was to explore the roles of the dmPFC and right pSTS from a social behavioral perspective using autism trait scores. These scores describe a continuum of social traits related to communication, social interaction, and imagination, with autistic patients reliably scoring in the upper range [Baron‐Cohen et al., 2001]. Our analyses in healthy adults revealed an association of autistic‐like traits with functional responses in the dmPFC, but not the pSTS. More precisely, higher autism trait scores were related to (1) lower differential activation to ToM compared to GD animations in the dmPFC and (2) stronger differential connectivity of the dmPFC during ToM animations with a cluster in the right pSTS.
The association of higher autistic‐like traits with lower dmPFC activation during spontaneous mentalizing has been reported in previous studies involving typical adults, such as during the uninstructed viewing of social scenes [Wagner et al., 2011], during no‐task periods (i.e., rest) in an instructed mentalizing task [Spunt et al., 2015], or while listening to live as compared to recorded speech [Rice and Redcay, 2016]. At the higher end of the autism spectrum, patients with ASD have repeatedly demonstrated reduced dmPFC activation during tasks related to spontaneous mentalizing, for instance, in response to animated shapes [Castelli et al., 2002; Kana et al., 2009; Kana et al., 2015], task‐irrelevant social content in naturalistic movie clips [Kana et al., 2016], or uninstructed exposure to face and voice stimuli [Wang et al., 2007]. Our observation of a modulation of dmPFC activation by autism trait scores thus conforms to clinical and nonclinical evidence. In contrast, the lack thereof in the right pSTS needs to be compared to a more heterogeneous body of literature. In line with our result, no association between autistic‐like traits and pSTS activation during spontaneous mentalizing was observed in typical individuals [Rice and Redcay, 2016; Wagner et al., 2011]. In contrast, clinical studies involving ASD patients reported task‐related hypoactivation in the pSTS or adjacent TPJ [Castelli et al., 2002; Kana et al., 2015, 2016; Wang et al., 2007]. These reports are in line with a large body of literature suggesting alterations in posterior temporal regions in ASD [Pelphrey et al., 2011; Philip et al., 2012; Zilbovicius et al., 2006]. Interestingly, Kana et al. [2016] additionally reported a positive association of pSTS activation with trait empathizing, but only for the ASD group. Based on these findings, one possible, yet tentative interpretation in favor of our negative pSTS result is that of a qualitative difference between clinical and nonclinical groups (e.g., compensatory mechanisms in nonclinical groups or an inverted u‐shape relationship between autism traits and functional brain response; discussed for example in Lombardo et al. [2007], Nummenmaa et al. [2012], von dem Hagen et al. [2011]). It has to be noted, however, that a recent study using animated shapes did not reveal hypoactivation in the pSTS in ASD, though the animations used in this study did not include complex mentalizing [Weisberg et al., 2014]. Future work is needed to follow up on this variability of results, part of which might be due to differences in the underlying construct (e.g., spontaneous mentalizing vs social attention) or experimental methods (e.g., task design, sample characteristics).
A similar argumentation can be put forward when interpreting the effect of AQ‐K scores on connectivity. Here, stronger dmPFC connectivity with a cluster in the posterior‐ventral part of the pSTS ROI was observed in individuals with higher autistic‐like traits. This cluster is in close proximity to the group‐level activation peak reported in Moessnang et al. [2016] and to a meta‐analytically defined peak for biological motion processing [Deen et al., 2015; see also Supporting Information, Fig. S4). While previous studies in ASD subjects have similarly demonstrated altered frontal‐to‐posterior connectivity [Müller et al., 2011], the majority of findings pointed to the opposite direction, that is, reduced connectivity of the medial prefrontal cortex during spontaneous mentalizing in ASD [Kana et al., 2009, 2015, 2016]. As reasoned above, one possible explanation might be that of a qualitative difference between individuals with and without ASD. For instance, as the processing of socially relevant information might be less efficient in subjects with high autism traits [Frith, 2001], our observation of increased dmPFC‐pSTS connectivity during mentalizing might reflect a compensatory mechanism in nonclinical groups for enhanced detection and inference of social meaning [Hillebrandt et al., 2013; Nummenmaa et al., 2012]. Finally, previous studies in ASD patients have reported altered connectivity of temporal areas with lower level sensory brain regions during animated shapes tasks, such as visual area V3 [Castelli et al., 2002] and lateral fusiform gyrus [Weisberg et al., 2014]. These findings were interpreted as impaired extraction of relevant sensory information, resulting in diminished sensitivity in downstream processing areas such as the pSTS. We did not observe a modulation of pSTS connectivity with visual sensory areas by autistic‐like traits in our sample, possibly due to substantial differences in methods (e.g., connectivity assessment, seed location) and sample characteristics (e.g., nonclinical sample vs clinical samples), as discussed above.
Several limitations of our study exist. While our ROI definition based on meta‐analytically derived masks [Mar, 2011] allows for a good coverage of brain regions implicated in spontaneous theory of mind, it comes with the disadvantage of limited regional specificity on a more fine‐grained level. This needs to be taken into account when interpreting our connectivity findings. According to Deen et al. [2015], functional subdivisions adjacent to or covered by our pSTS mask (in a posterior‐to‐anterior direction) contribute to cognitive ToM, biological motion perception, and face perception (see Supporting Information, Fig. S4). The mask extends dorsally into the TPJ (e.g., overlaps with the “TPJp” coordinate defined Schurz et al. [2014]; see Supporting Information, Fig. S4), parts of which have repeatedly been included in investigations of pSTS function [Deen et al., 2015; Hein and Knight, 2008]. Similarly, functional subdivisions have been reported for the dmPFC [Eickhoff et al., 2016]. As our connectivity analyses are based on individually defined VOIs (i.e., spheres centered on the global maximum within the mask; see Supporting Information, Fig. S2), different functional subdivisions likely contributed to the group analysis results. These results, in turn, represent brain areas which show the highest consistency in connectivity across individual VOIs. For instance, even though individual pSTS VOIs presumably cover different functional subdivisions, they show a consistent connectivity pattern with area LO2, but not with the dmPFC. In contrast, individual VOIs in the dmPFC consistently connect to three areas (with potentially different functional profiles) within the right pSTS mask (see Supporting Information, Fig. S3). This asymmetry in our connectivity findings between the two ROIs is of interest as it suggests a higher functional heterogeneity within the pSTS compared to the dmPFC. A higher degree of functional heterogeneity within the pSTS might also have prevented the detection of potential associations of pSTS connectivity with AQ‐K. Adding to the functional heterogeneity, it has to be acknowledged that the identified brain responses and connectivity patterns are likely task‐specific and may not apply to other types of ToM tasks [Schurz et al., 2014].
Other limitations relate to our sample characteristics. First, our findings in healthy individuals might not generalize to individuals with ASD. Besides the possibility of a qualitative difference between subjects with and without ASD, the observed range of AQ‐K scores might capture only small, or even irrelevant, variations in autistic traits. However, subthreshold AQ scores have successfully been used in previous studies on autism traits [Ruzich et al., 2015], which suggests a sufficient degree of explained variance even in the lower scoring range. On a more general account, the AQ is a self‐report questionnaire that does not assess behavior in more demanding situations, such as real‐life social interactions, and might therefore lack sensitivity for specific types of autistic behaviors. The failure to uncover effects of AQ‐K scores on pSTS function in our study might result from this limitation. Nevertheless, good validity of the AQ‐K has been shown [Freitag et al., 2007], and the significance of spontaneous mentalizing for real‐life interactions [Apperly and Butterfill, 2009] suggests that findings related to spontaneous mentalizing can be generalized to such situations. Finally, generalizability of our findings could also be compromised by the high level of cognitive functioning in our sample, in particular in comparison to low‐functioning patient populations.
In summary, this study contributed novel information about the functional roles of the dmPFC and right pSTS for spontaneous mentalizing. We used an animated shapes task which triggers spontaneous mentalizing in an automatic fashion even in the absence of explicit social information (e.g., facial expressions). Potentially confounding effects of cognitive task demands and sensory information processing were therefore kept minimal. Our results are in line with the large body of literature that suggests complementary roles for the dmPFC and the right pSTS as top–down and bottom–up hubs of the social brain. While this view was most strongly reflected by their brain‐wide connectivity patterns, it was also compatible with connectivity patterns within the social brain. In addition, we observed an association of dmPFC activation and connectivity with self‐reported autism traits. The correlational nature of our findings, however, warrants further research allowing for causal inferences to be made, ranging from data analysis techniques such as dynamic causal modeling [Hillebrandt et al., 2013], experimental manipulations such as transcranial magnetic stimulation [Grossman et al., 2005], or longitudinal designs which take a developmental perspective on brain–behavior relationships.
CONFLICT OF INTEREST
AM‐L has received consultant fees from AstraZeneca, Elsevier, F. Hoffmann‐La Roche, Gerson Lehrman Group, Lundbeck, Outcome Europe Sárl, Outcome Sciences, Roche Pharma, Servier International, and Thieme Verlag; and has received lecture fees including travel expenses from Abbott, AstraZeneca, Aula Médica Congresos, BASF, Boehringer Ingelheim, Groupo Ferrer International, Janssen‐Cilag, Lilly Deutschland, LVR Klinikum Düsseldorf, Otsuka Pharmaceuticals, and Servier Deutschland.
TB served in an advisory or consultancy role for Actelion, Hexal Pharma, Lilly, Medice, Novartis, Oxford outcomes, PCM scientific, Shire, and Viforpharma. He received conference support or speaker's fee by Medice, Novartis, and Shire. He is/has been involved in clinical trials conducted by Shire & Viforpharma. He received royalities from Hogrefe, Kohlhammer, CIP Medien, and Oxford University Press. This work is unrelated to the above grants and relationships.
LP received conference support or speaker's fee by Shire, Lilly, Novartis, and Medice. She receives royalities from Hogrefe and Schattauer.
The other authors report no biomedical financial interests or other potential conflicts of interest.
Supporting information
Supporting Information 1
ACKNOWLEDGMENTS
This work was supported by the European Community's Seventh Framework Programme under the grant agreements No. 115300 (Project EU‐AIMS), No. 602805 (Project EU‐AGGRESSOTYPE), No. 602450 (Project EU‐IMAGEMEND), and the German Federal Ministry of Education and Research (grant No. 01ZX1314GM (Project IntegraMent); grant No. 01GQ1102 to H.T.). We thank Luanna Dixson for valuable input on the manuscript and Dagmar Gass for research assistance.
Institution at which work was performed: Central Institute of Mental Health.
REFERENCES
- Abell F, Happé F, Frith U (2000): Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cogn Dev 15:1–16. [Google Scholar]
- Adolphs R (2009): The social brain: Neural basis of social knowledge. Annu Rev Psychol 60:693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB (2015): Definition and characterization of an extended social‐affective default network. Brain Struct Funct 220:1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperly IA, Butterfill SA (2009): Do humans have two systems to track beliefs and belief‐like states? Psychol Rev 116:953–970. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E (2001): The autism‐spectrum quotient (AQ): Evidence from Asperger syndrome/high‐functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31:5–17. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B (2000): Voice‐selective areas in human auditory cortex. Nature 403:309–312. [DOI] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk‐Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H, Meyer‐Lindenberg A, Bassett DS (2015): Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci USA 112:11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB (2013): Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci 7:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U (2002): Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125:1839–1849. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C (2000): Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage 12:314–325. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM (2006): fMRI resting state networks define distinct modes of long‐distance interactions in the human brain. Neuroimage 29:1359–1367. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R (2015): Functional organization of social perception and cognition in the superior temporal sulcus. Cereb Cortex 25:4596–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN (2012): A meta‐analysis of functional neuroimaging studies of self‐ and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24:1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006): A core system for the implementation of task sets. Neuron 50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L (2016): Functional segregation of the human dorsomedial prefrontal cortex. Cereb Cortex 26:304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C, Lega C, Vernice M, Tamietto M, Mende‐Siedlecki P, Vecchi T, Todorov A, Cattaneo Z (2016): The dorsomedial prefrontal cortex plays a causal role in integrating social impressions from faces and verbal descriptions. Cereb Cortex 26:156–165. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS (2012): Competitive and cooperative dynamics of large‐scale brain functional networks supporting recollection. Proc Natl Acad Sci USA 109:12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Retz‐Junginger P, Retz W, Seitz C, Palmason H, Meyer J, Rösler M, von Gontard A (2007): Evaluation der deutschen Version des Autismus‐Spektrum‐Quotienten (AQ) ‐ die Kurzversion AQ‐k. Zeitschrift für Klinische Psychologie und Psychotherapie 36:280–289. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Frith U (2001): Mind blindness and the brain in autism. Neuron 32:969–979. [DOI] [PubMed] [Google Scholar]
- Gao T, Scholl BJ, McCarthy G (2012): Dissociating the detection of intentionality from animacy in the right posterior superior temporal sulcus. J Neurosci 32:14276–14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W (2012): Frontal parietal control network regulates the anti‐correlated default and dorsal attention networks. Hum Brain Mapp 33:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual‐Leone A (2005): Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Res 45:2847–2853. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R (2002): Brain areas active during visual perception of biological motion. Neuron 35:1167–1175. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Habas C, Guillevin R, Abanou A (2011): Functional connectivity of the superior human temporal sulcus in the brain resting state at 3T. Neuroradiology 53:129–140. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cogn Sci 4:223–233. [DOI] [PubMed] [Google Scholar]
- Heider H, Simmel M (1944): An experimental study of apparent behavior. Am J Psychol 57:243–259. [Google Scholar]
- Hein G, Knight RT (2008): Superior temporal sulcus–It's my area: Or is it? J Cogn Neurosci 20:2125–2136. [DOI] [PubMed] [Google Scholar]
- Hillebrandt H, Dumontheil I, Blakemore SJ, Roiser JP (2013): Dynamic causal modelling of effective connectivity during perspective taking in a communicative task. Neuroimage 76:116–124. [DOI] [PubMed] [Google Scholar]
- Hillebrandt H, Friston KJ, Blakemore SJ (2014): Effective connectivity during animacy perception–dynamic causal modelling of Human Connectome Project data. Sci Rep 4:6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RL, Hoffman P, Pobric G, Lambon Ralph MA (2016): The semantic network at work and rest: Differential connectivity of anterior temporal lobe subregions. J Neurosci 36:1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA (2009): Atypical frontal‐posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci 4:135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Maximo JO, Williams DL, Keller TA, Schipul SE, Cherkassky VL, Minshew NJ, Just MA (2015): Aberrant functioning of the theory‐of‐mind network in children and adolescents with autism. Mol Autism 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Patriquin MA, Black BS, Channell MM, Wicker B (2016): Altered medial frontal and superior temporal response to implicit processing of emotions in autism. Autism Res 9:55–66. [DOI] [PubMed] [Google Scholar]
- Kestemont J, Vandekerckhove M, Ma N, Van Hoeck N, Van Overwalle F (2013): Situation and person attributions under spontaneous and intentional instructions: An fMRI study. Soc Cogn Affect Neurosci 8:481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT (2007): Neuroscience. Neural networks debunk phrenology. Science 316:1578–1579. [DOI] [PubMed] [Google Scholar]
- Lahnakoski JM, Glerean E, Salmi J, Jaaskelainen IP, Sams M, Hari R, Nummenmaa L (2012): Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Front Hum Neurosci 6:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Heeger DJ (2006): Two retinotopic visual areas in human lateral occipital cortex. J Neurosci 26:13128–13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron‐Cohen S (2007): Self‐referential cognition and empathy in autism. PLoS One 2:e883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Vandekerckhove M, Van Overwalle F, Seurinck R, Fias W (2011): Spontaneous and intentional trait inferences recruit a common mentalizing network to a different degree: Spontaneous inferences activate only its core areas. Soc Neurosci 6:123–138. [DOI] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Kujovic M, Palomero‐Gallagher N, Eickhoff SB, Zilles K (2015): Cytoarchitecture of the human lateral occipital cortex: Mapping of two extrastriate areas hOc4la and hOc4lp. Brain Struct Funct [DOI] [PubMed] [Google Scholar]
- Mar RA (2011): The neural bases of social cognition and story comprehension. Annu Rev Psychol 62:103–134. [DOI] [PubMed] [Google Scholar]
- Mar RA, Macrae CN (2007): Triggering the intentional stance. Novartis Found Symp 278:111–120. discussion 120‐133, 216‐121. [PubMed] [Google Scholar]
- Moessnang C, Schafer A, Bilek E, Roux P, Otto K, Baumeister S, Hohmann S, Poustka L, Brandeis D, Banaschewski T, Meyer‐Lindenberg A, Tost H (2016): Specificity, reliability and sensitivity of social brain responses during spontaneous mentalizing. Soc Cogn Affect Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Jolly E, Mitchell JP (2014): Spontaneous mentalizing predicts the fundamental attribution error. J Cogn Neurosci 26:569–576. [DOI] [PubMed] [Google Scholar]
- Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK (2011): Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex 21:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Engell AD, von dem Hagen E, Henson RN, Calder AJ (2012): Autism spectrum traits predict the neural response to eye gaze in typical individuals. Neuroimage 59:3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen‐Berg H (2012): Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA (2013): Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc Cogn Affect Neurosci 8:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, Dickerson BC (2015): Large‐scale brain networks of the human left temporal pole: A functional connectivity MRI study. Cereb Cortex 25:680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC (2011): Research review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry 52:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip RC, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC (2012): A systematic review and meta‐analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev 36:901–942. [DOI] [PubMed] [Google Scholar]
- Powers JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011): Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Chavez RS, Heatherton TF (2016): Individual differences in response of dorsomedial prefrontal cortex predict daily social behavior. Soc Cogn Affect Neurosci 11:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K, Redcay E (2016): Interaction matters: A perceived social partner alters the neural processing of human speech. Neuroimage 129:480–488. [DOI] [PubMed] [Google Scholar]
- Ruzich E, Allison C, Smith P, Watson P, Auyeung B, Ring H, Baron‐Cohen S (2015): Measuring autistic traits in the general population: A systematic review of the Autism‐Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Mol Autism 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Neubert FX, Jbabdi S, O'Reilly JX, Filippini N, Thomas AG, Rushworth MF (2013): The organization of dorsal frontal cortex in humans and macaques. J Neurosci 33:12255–12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003): People thinking about thinking people. The role of the temporo‐parietal junction in “theory of mind”. Neuroimage 19:1835–1842. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB (2012): Introspective minds: Using ALE meta‐analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 7:e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J (2014): Fractionating theory of mind: A meta‐analysis of functional brain imaging studies. Neurosci Biobehav Rev 42:9–34. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S, Frith U (2009): Mindblind eyes: An absence of spontaneous theory of mind in Asperger syndrome. Science 325:883–885. [DOI] [PubMed] [Google Scholar]
- Shultz S, van den Honert RN, Engell AD, McCarthy G (2015): Stimulus‐induced reversal of information flow through a cortical network for animacy perception. Soc Cogn Affect Neurosci 10:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Adolphs R (2015): Folk explanations of behavior: A specialized use of a domain‐general mechanism. Psychol Sci 26:724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Meyer ML, Lieberman MD (2015): The default mode of human brain function primes the intentional stance. J Cogn Neurosci 27:1116–1124. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Vandekerckhove M (2013): Implicit and explicit social mentalizing: Dual processes driven by a shared neural network. Front Hum Neurosci 7:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ (2011): Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb Cortex 21:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Rowe JB, Baron‐Cohen S, Calder AJ (2014): Direct gaze elicits atypical activation of the theory‐of‐mind network in autism spectrum conditions. Cereb Cortex 24:1485–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Kelley WM, Haxby JV, Heatherton TF (2016): The dorsal medial prefrontal cortex responds preferentially to social interactions during natural viewing. J Neurosci 36:6917–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Kelley WM, Heatherton TF (2011): Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cereb Cortex 21:2788–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron EJ, Manzel K, Tranel D (2014): The left temporal pole is a heteromodal hub for retrieving proper names. Front Biosci (Schol Ed) 6:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M (2007): Reading affect in the face and voice: Neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry 64:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg J, Milleville SC, Kenworthy L, Wallace GL, Gotts SJ, Beauchamp MS, Martin A (2014): Social perception in autism spectrum disorders: Impaired category selectivity for dynamic but not static images in ventral temporal cortex. Cereb Cortex 24:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Coniston D, Rogers R, Frith U (2011): Developing the Frith‐Happe animations: A quick and objective test of Theory of Mind for adults with autism. Autism Res 4:149–154. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang J, Fan L, Li H, Zhang W, Hu Q, Jiang T (2015): Tractography‐based parcellation of the human middle temporal gyrus. Sci Rep 5:18883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N (2006): Autism, the superior temporal sulcus and social perception. Trends Neurosci 29:359–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1


