Abstract
Musical expertise is visible both in the morphology and functionality of the brain. Recent research indicates that functional integration between multi‐sensory, somato‐motor, default‐mode (DMN), and salience (SN) networks of the brain differentiates musicians from non‐musicians during resting state. Here, we aimed at determining whether brain networks differentially exchange information in musicians as opposed to non‐musicians during naturalistic music listening. Whole‐brain graph‐theory analyses were performed on participants' fMRI responses. Group‐level differences revealed that musicians' primary hubs comprised cerebral and cerebellar sensorimotor regions whereas non‐musicians' dominant hubs encompassed DMN‐related regions. Community structure analyses of the key hubs revealed greater integration of motor and somatosensory homunculi representing the upper limbs and torso in musicians. Furthermore, musicians who started training at an earlier age exhibited greater centrality in the auditory cortex, and areas related to top‐down processes, attention, emotion, somatosensory processing, and non‐verbal processing of speech. We here reveal how brain networks organize themselves in a naturalistic music listening situation wherein musicians automatically engage neural networks that are action‐based while non‐musicians use those that are perception‐based to process an incoming auditory stream. Hum Brain Mapp 38:2955–2970, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: music, fMRI, musical training, functional connectivity, graph theory
INTRODUCTION
The ubiquitous existence of music production and listening renders it an important element of human existence [Huron, 2001]. However, as learning to play music at high levels is a daily process that takes place over many years, musical abilities and the underlying neural substrates for music perception and action differ significantly between musicians and non‐musicians. Hence, the study of musicians' brain function is a human model for studying brain plasticity and learning [Münte et al., 2002]. In particular, musical training influences perception and action networks in the brain involved in listening to and producing music. Perception and execution of actions are strongly coupled in the human brain as a result of learning a sensorimotor task, which facilitates not only predicting the action of others but also interacting with them [Novembre and Keller, 2014]. In music, a tight coupling occurs between the perception and production of hierarchically organized sequential information [Molnar‐Szakacs and Overy, 2006]. Because musical activities, such as ensemble playing or singing, involve imitation and synchronization, they may engage brain regions largely overlapping with the human mirror neuron system [Wan et al., 2010].
It has been established that adults' musical training causes structural [Gaser and Schlaug, 2003; Schlaug et al., 1995; Schneider et al., 2002] and functional changes in the adult [Reybrouck and Brattico, 2015] and developing brain [Baer et al., 2013; Bailey et al., 2014; Watanabe et al., 2007] leading to enhanced sensitivity in the processing, representation, and discrimination of sounds and music [Brattico et al., 2016; Kraus and Chandrasekaran, 2010; Mikutta et al., 2014; Musacchia et al., 2008; Pantev et al., 2015; Strait et al., 2009; Tervaniemi et al., 2001; Wong et al., 2007] depending on instrument [Pantev et al., 2001], style of music [Vuust et al., 2012], and starting age and years of musical training [Amunts et al., 1997; Bengtsson et al., 2005; Brattico et al., 2009; Imfeld et al., 2009; Steele et al., 2013; Vaquero et al., 2016]. Furthermore, the effect of musical training transfers to non‐auditory functions, such as attention, verbal intelligence, academic performance, and overall cognitive development [Hansen et al., 2013; Miendlarzewska and Trost, 2014].
However, the central question of how the functional connectivity in the brain differs as a function of musical expertise during real music listening has still not been answered. Functional connectivity allows us to investigate brain states, or even categorize subjects, for example, distinguishing between patients and controls, and is a data‐driven approach [Friston, 2011]. This study is the first to investigate the effect of musical expertise on whole‐brain networks and identify key hubs in an fMRI naturalistic music listening paradigm [Abrams et al., 2013; Alluri et al., 2012, 2013; Burunat et al., 2014, 2015; Toiviainen et al., 2014], wherein the fMRI acquisition takes place while the participant is continuously listening to music thereby emulating real‐life listening experiences.
Connectivity studies in music neuroscience have provided new insights about the relationship between integrative brain function and musical training. By means of fMRI, an increase in auditory‐motor connectivity as a function of musical training was previously observed [Chen et al., 2008]. Similarly, the authors of a study on auditory‐motor interactions during beat‐based rhythm processing found that the coupling between cortical motor (bilateral supplementary motor area; SMA) and auditory areas (superior temporal gyrus; STG) was facilitated by musical training [Grahn and Rowe, 2009]. In a recent study [Wilkins et al., 2014], the authors used network theory to identify key hubs during naturalistic listening to music which was rated as “liked” or “disliked” or “favorite” by the participant and how the community structure of an auditory seed varied in those three conditions. They found that the precuneus emerged as a key hub with its community structure closely resembling the DMN. However, they did not examine the effect of musical training. Similarly, eigenvector centrality mapping, a measure of global network connectivity, was used to identify networks associated with music‐evoked joy and fear in non‐musicians during continuous presentation [Koelsch and Skouras, 2014]. However, studies that have thus far investigated the effect of musical expertise on functional connectivity during continuous music listening have been scarce. The authors of the study that focused on limbic‐region seeds connectivity [Alluri et al., 2015], reported that during music listening musicians' deep perceptual and motoric knowledge of music increases the coupling between areas that process musical emotions with areas that process motor commands and pleasure. In a second study [Burunat et al., 2015], the authors observed several foci of increased functional voxel‐mirrored connectivity between the two cerebral hemispheres in keyboardists compared to string players during continuous music listening. They also observed a more general increased functional symmetry in the musician than in the control group, primarily in somatomotor and cerebellar regions. The different symmetry profiles may thus result from adaptation to intensive musical training rather than from early predisposition. However, neither of the studies has established “baseline” networks that emerge while listening to music and how musical expertise modulates them.
Two recent connectivity studies have focused on effects of music training in a special case of brain state, namely during rest, by measuring resting‐state fMRI (rsMRI). The first study limited the functional connectivity analysis to a total of five seed‐regions representing the right primary motor, left primary auditory, primary somatosensory, primary visual, and V2 areas [Luo et al., 2012]. They found a significant increase in functional connectivity for all the seed regions in musicians. Effective pair‐wise connectivity between these areas revealed that musicians display high coupling of the motor region with the remaining perceptual systems and conclude that the auditory cortex plays an important role in modulating the observed functional plasticity. Non‐musicians on the other hand did not display any such consistent pattern. The limited choice of brain regions in this study, however, portrays only an incomplete picture of connectivity differences between musicians and non‐musicians.
In a subsequent study, the same research group investigated the effect of musical expertise on functional connectivity patterns by extending the analysis to the whole‐brain [Luo et al., 2014]. They found greater integration of the anterior cingulate cortex (ACC) and the frontoinsular cortex (FIC) in musicians than non‐musicians. These structures represent the main nodes of the salience network (SN), which is described as a bridge between the DMN‐related state and the central executive network (CEN)‐related state by assessing salience or importance of incoming sensory information [Goulden et al., 2014; Sridharan et al., 2008]. Furthermore, the authors summarize studies that describe how a special set of neurons, the von Economo, are found only in the SN, and that the structural and functional connections between these regions is strengthened by age, which thereby result in flexible and adaptive behavior [Goulden et al., 2014].
In this context, the authors of [Luo et al., 2014] contend that musical training expedites the strengthening of these connections, thereby causing transfer effects leading to “enhanced higher‐level cognitive processes in musicians.” This result is interesting in light of longitudinal studies that have indeed shown that musical training enables children to perform better at several cognitive tasks than their untrained counterparts [Kraus and Chandrasekaran, 2010]. A very recent resting‐state study performed with high‐density EEG revealed using graph‐theoretical analysis that the right primary auditory cortex displayed significant differences in functional connectivity of low frequency oscillations (theta and alpha1 bands) between musicians and non‐musicians [Klein et al., 2015]. In addition, they found increased functional connectivity in music perception and production related regions including the sensorimotor and prefrontal cortices, which was further found to be positively associated with total number of training hours and musical aptitude. Evidence exists suggesting similarities in connectomes of rs‐low frequency oscillations of EEG (delta, theta, and alpha) and rsMRI [Deligianni et al., 2014]. In this light, the enhanced connectivity observed in musicians by Luo et al. [2014] and Klein et al. [2015] encompass dissimilar regions. Moreover, resting‐state fMRI or EEG data is typically acquired while the participant does not perform any task and is instructed to stay awake. This engenders too much unaccounted variance of brain function in terms of mind‐wandering and may not reflect the entire picture concerning the connectivity differences due to musical expertise. As it has been discussed already that musical training leads to structural differences and enhanced encoding of sounds and music, resting‐state connectivity may be more biased to highlighting these differences between the groups. Hence, it makes a better case to study musical‐expertise modulated functional differences in the presence of music as a stimulus which then allows for tracking the neural networks that are supposedly more affected by long‐term daily instrumental practice.
To this end, we examined differences in whole‐brain functional connectivity between musicians and non‐musicians while they listened to three 8 min‐long instrumental pieces representing different styles (tango nuevo, modern classical, and progressive rock). We adopted a graph theoretical framework to identify the main hubs characterized by node degree, followed by community structure analysis for these nodes to identify the dominant networks that emerge during continuous listening to music in both groups. We hypothesized that we would observe connectivity differences between musicians and non‐musicians in networks engaged in musical perception and action. As a result, we found that musicians' key hubs encompassed cerebral sensorimotor regions whereas non‐musicians' focal hubs included parietal and left‐hemispheric auditory regions. Furthermore, we were able to show enhanced connectivity of the motor and sensory homunculus representing the upper limbs and torso in the musicians whereas no regions with enhanced connectivity were observed for the non‐musicians.
MATERIALS AND METHODS
Participants
Thirty‐six healthy participants with no history of neurological or psychological disorders participated in the fMRI experiment. The participant pool was equally divided between musically trained (n = 18) and untrained participants (n = 18). Both groups were comparable with respect to gender, age distribution, cognitive measures (Processing Speed and Working Memory Index Scores from the WAIS‐WMS III), and socioeconomic status (according to Hollingshead's Four‐Factor Index). See Table 1 for demographic data. The musicians' group was homogeneous in terms of the duration of their musical training and amount of years of active instrument playing.
Table 1.
Demographic information of the participants
| Groups | MUS | NMUS |
|---|---|---|
| N | 18 | 18 |
| Age | 28.2 ± 7.8 | 29.2 ± 10.7 |
| Gender | 9F | 10F |
| Hand | 18R | 17R |
| Soc‐eco status | 43.6 | 35.4 |
| WAIS‐III PSI | 116.3 | 115.7 |
| Active listening (h/week) | 7.5 ± 5.8 | 5.3 ± 4.8 |
| Passive listening (h/week) | 10.6 ± 7.5 | 7.1 ± 3.9 |
| Total listening (h/week) | 18.2 ± 11.2 | 12.4 ± 6.7 |
| Instrument starting age | 8.6 ± 5.5 | — |
| Instrument playing (years) | 21.2 ± 7.8 | — |
| Instrument practicing (h/week) | 16.6 ± 11 | — |
| Musical training (years) | 16 ± 5.7 | — |
| Style | 12 class | 5 jazz | 1 p/r | — |
Abbreviations: MUS: musicians, NMUS: non‐musicians, class: classical, p/r: pop‐rock; soc‐eco: socioeconomic, PSI: Processing Speed Index.
Stimuli
Three musical pieces were used in the experiment: (a) Stream of Consciousness by Dream Theater; (b) Adios Nonino by Astor Piazzolla; and (c) Rite of Spring (comprising the first three episodes from Part I: Introduction, Augurs of Spring, and Ritual of Abduction) by Igor Stravinsky. These are a progressive rock/metal piece, an Argentinian New Tango, and an iconic 20th century classical work, respectively, thus covering distinct musical genres and styles. All three selected pieces are instrumental and have a duration of about 8 min. These pieces of music were chosen based on the following criteria: to have an appropriate duration for the experimental setting used; to belong to different genres in order allow generalization of the obtained findings; to contain a high amount of acoustic variation and that the amount be comparable between the three pieces (the mean normalized standard deviation across all 25 extracted musical features [Alluri et al., 2012] differed less than 10% between the stimuli); to have a comparable musical structure (starting with a session of solo instrument and then introducing the larger ensemble after few minutes); and lastly to not contain lyrics to avoid the confounding effects of semantics. The order of presentation of the musical pieces in the experiment was counter‐balanced across participants and the volume level of the music was adjusted individually prior to the start of the experiment. Furthermore, the participants were instructed to fix their gaze on the screen while being scanned. In addition, at the end of each piece, the participants responded to questions by the experimenter via the intercom, expressing orally their ratings of liking, arousal, and familiarity on a discrete five‐point scale.
fMRI Data Acquisition and Preprocessing
Participants' brain responses were acquired while they listened to each of the musical stimuli in a counterbalanced order. Participants' only task was to attentively listen to the music delivered via high‐quality MR‐compatible insert earphones while keeping their eyes open. Foam was used to attenuate the gradient noise. The sound level of the stimuli was individually adjusted so that they were audible above the scanner noise but the volume stayed within safety limits (below 80 dB). The study protocol proceeded on acceptance by the ethics committee of the Coordinating Board of the Helsinki and Uusimaa Hospital District. The data collection was part of a broader project (Tunteet) involving additional tests and neuroimaging and neurophysiological measures [Alluri et al., 2015; Bogert et al., 2016; Burunat et al., 2015, 2016; Carlson et al., 2015; Haumann et al., 2016; Kliuchko et al., 2015, 2016].
Scanning was performed using a 3T MAGNETOM Skyra whole‐body scanner (Siemens Healthcare, Erlangen, Germany) and a standard 32‐channel head‐neck coil, at the Advanced Magnetic Imaging (AMI) Centre (Aalto University, Espoo, Finland). Using a single‐shot gradient echo planar imaging (EPI) sequence thirty‐three oblique slices (field of view = 192 × 192 mm; 64 × 64 matrix; slice thickness = 4 mm, interslice skip = 0 mm; echo time = 32 ms; flip angle = 75°; voxel size: 2 × 2 × 2 mm3) were acquired every 2 sec, providing whole‐brain coverage per participant. T1‐weighted structural images (176 slices; field of view = 256 × 256 mm; matrix = 256 × 256; slice thickness = 1 mm; interslice skip = 0 mm; pulse sequence = MPRAGE) were also collected for individual coregistration. Functional MRI scans were preprocessed on a Matlab platform using SPM8 (Statistical Parametric Mapping), VBM5 for SPM (Voxel Based Morphometry; Wellcome Department of Imaging Neuroscience, London, UK), and customized scripts developed by the present authors. For each participant, low‐resolution images were realigned on six dimensions using rigid body transformations (translation and rotation corrections did not exceed 2 mm and 2°, respectively), segmented into grey matter, white matter, and cerebrospinal fluid, and registered to the corresponding segmented high‐resolution T1‐weighted structural images. These were in turn normalized to the MNI (Montreal Neurological Institute) segmented standard a priori tissue templates using a 12‐parameter affine transformation. Functional images were then blurred to best accommodate anatomical and functional variations across participants as well as to enhance the signal‐to‐noise by means of spatial smoothing using an 8 mm full‐width‐at‐half‐maximum Gaussian filter. Movement‐related variance components in fMRI time series resulting from residual motion artifacts, assessed by the six parameters of the rigid body transformation in the realignment stage were regressed out from each voxel time series. Following this, spline interpolation was used to detrend the fMRI data. Next, temporal filtering was performed by Gaussian smoothing (kernel width = 4 sec), as it provides a good compromise between efficiency and bias [Friston et al., 2000]. Because functional connectivity analysis has been found to be susceptible to head movement [Van Dijk et al., 2012], we performed two‐tailed t‐tests on the standard deviation of each of the six movement parameters between musicians and non‐musicians. No significant differences were found, indicating that there was no difference in the amount of head movement between the groups. Nevertheless, the motion parameters were regressed out from the analysis to further minimize any influence of slight movements on the brain connectivity patterns.
Connectivity Analyses
For functional connectivity analysis, the fMRI data obtained with each stimulus were spatially resampled to a 4 × 4 × 4 mm3 voxel size, resulting in 28,542 voxels within the scanning volume. Networks were generated by calculating, for each participant, the Pearson correlation between the time series of all possible combinations of voxel pairs. This yielded a cross‐correlation matrix for each participant representing the strength of association between each voxel pair.
Subsequently, each correlation matrix was thresholded to generate a binary adjacency matrix that indicates whether any two voxels are connected. These binary connections are subsequently referred to as edges. To this end, fixed average degree thresholding [Simpson et al., 2013; Telesford et al., 2011] was used. This thresholding method fixes the proportion of the number of edges to the number of elements in the network matrix. The threshold is commonly expressed as S = log(N)/log(K), where N is the total number of nodes and K is the number of edges. In biological networks, edge density has been observed to be approximately S = 2.5 [Laurienti et al., 2011]. In the present study, we used thresholds of S = 2.5 and S = 4.0. Similar ranges of thresholds have commonly been used in other studies applying graph theory to fMRI data [Power et al., 2011, 2013]. Overall, the results were similar between the different threshold values. The most significant differences between the two participant groups were however observed with S = 4.0, indicating that the differences are most prominent at the strongest end of functional connectivity. In the present article, only these results will be reported.
Starting from the adjacency matrices, we calculated the node degree and global efficiency for each participant. Node degree refers to the number of edges connected to each node i, defined by
where is the connection status between nodes i and j. As the focus of the article is on hub‐related‐ and community structure‐related differences between musicians and non‐musicians regardless of musical style, we chose to concatenate the data obtained with the individual stimuli and evaluate the node degree with the added advantage of a boost in statistical power of the results. Subsequent t‐test results between the two groups revealed regions in the brain that possessed significantly different node degrees. To assess the extent to which the important hubs correspond to functional networks observed in resting state, the Z‐map obtained from the t‐test result were correlated using Pearson Correlation with the resting state network maps reported in [Damoiseaux et al., 2006].
Correlation Analyses between Functional Connectivity Measure and Starting Age of Musical Training
We further investigated the plausible relationship between the starting age of musical training and node degree in musicians. Given the non‐normality of the distribution of starting ages, Spearman's rank correlation coefficient was chosen as the suitable non‐parametric measure of statistical dependence, which is in addition less sensitive to outliers. Moreover, the potential relationship between starting age and node degree may be monotonic, and not necessarily a linear one.
Community Structure Analysis
In addition to the degree, we performed community structure analysis to identify groups of nodes that are more connected to each other than to nodes in other groups. To this end, we used the QCut algorithm [Ruan and Zhang, 2008], which uses a combination of spectral graph partitioning and local search to optimize the modularity measure:
where is the proportion of edges that connect nodes in module u with nodes in module v [Newman and Girvan, 2004]. As QCut is a stochastic algorithm, ten runs were performed for each participant, and the partition yielding the highest modularity value was retained. Subsequently, scaled inclusivity [Steen et al., 2011] was used to determine, for selected voxels, the consistency of community structure across participants. For the selected seed voxels, the main focus was on the difference in scaled inclusivity between the two participant groups. The significance of this difference was estimated using a permutation test (with replacement) on group labels with 100,000 simulations. To assess the extent to which the community structure of the important hubs corresponds to functional networks observed in resting state, the group‐wise inclusivity maps were correlated with RSN maps obtained by [Damoiseaux et al., 2006].
RESULTS
Node Degrees
First, t‐tests on the behavioral ratings revealed no significant differences between the groups for the mean arousal ratings for two of the three stimuli with the exception of the Stravinsky stimulus which received significantly higher arousal ratings from the musicians as compared with the non‐musicians (t(16) = 2.55, P < 0.05). Conversely, the non‐musicians rated the Dream Theater stimulus higher on the Liking scale (t(17) = 2.29, P < 0.05) and vice‐versa for the Stravinsky (t(17) = 3.17, P < 0.01) with no significant differences observed between the groups for the Piazzolla. The only group differences observed for the familiarity ratings were of the Piazzolla stimulus, which was found to be more familiar to the musicians (t(17) = 2.7, P < 0.05). However, no significant differences were observed between the groups for the concatenated stimulus for the arousal, liking, and familiarity ratings. The ratings for concatenated stimulus were obtained by averaging the ratings for individual stimuli.
Figure 1 and Table 2 show the regions that possessed significantly higher node degree for musicians in red and for non‐musicians in blue.
Figure 1.
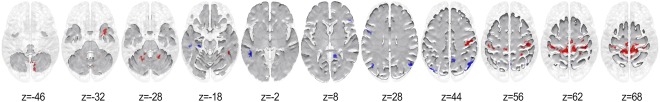
Thresholded map showing significantly larger node degrees in musicians and non‐musicians in red and blue, respectively (P < 0.01, two‐tailed; cluster size = 50 voxels, FWE = 0.05). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Results of the group level t‐test performed on the node degrees maps
| Left hemisphere | k | BA | x | y | z | Right hemisphere | k | BA | x | y | z |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MUS > NMUS | |||||||||||
| Postcentral and precentral gyrus | 140 | 4 | −40 | −28 | 62 | Paracentral lobule, precuneus, postcentral gyrus | 412 | 4 | −6 | −38 | 72 |
| Postcentral and precentral gyrus, Supplementary motor area, precuneus, paracentral lobule | 952 | 3 | 46 | −22 | 52 | Lobules VIIb, VIII, IX of cerbellum | 190 | 10 | −76 | −46 | |
| Lobules VI, IV‐V of cerbellum | 74 | −14 | −50 | −24 | Lobules VI, IV‐V of cerbellum | 73 | 22 | −52 | −26 | ||
| Temporal pole, parahippocampal gyrus | 65 | 36 | 36 | 4 | −32 | ||||||
| Postcentral gyrus, supramarginal and angular gyri, superior parietal gyrus | 55 | 2 | 28 | −44 | 58 | ||||||
| Fusiform and inferior temporal gyrus | 51 | 20 | 40 | −38 | −24 | ||||||
| NMUS > MUS | |||||||||||
| Middle occipital gyrus, angular gyrus, supramarginal and angular gyrus | 227 | 19 | −34 | −76 | −36 | Angular gyrus, middle occipital gyrus, supramarginal and angular gyrus | 470 | 39 | 50 | −54 | 36 |
| Middle temporal gyrus | 147 | 21 | −48 | −48 | 2 | Precuneus | 170 | 7 | 10 | −54 | 40 |
| Precentral gyrus, pars opercularis | 63 | 44 | −48 | 4 | 28 | Precuneus, calcarine fissure | 85 | 29 | 8 | −48 | 12 |
| Hippocampus and parahippocampal gyrus | 51 | 20 | −26 | −26 | −14 | Pars opercularis and triangularis | 54 | 45 | 60 | 22 | 12 |
The significant voxels were obtained at a threshold of P < 0.01 (cluster corrected at FWE P < 0.05). The clusters were obtained using the 18‐connectivity scheme used in SPM. Anatomical labels correspond to the Automated Anatomical Labeling (AAL) [Tzourio‐Mazoyer et al., 2002]. The table reports within‐cluster region size (k; i.e., number of voxels) and the MNI coordinates represent the location of the maximum within each cluster. Abbreviations: MUS: musicians, NMUS: non‐musicians.
As can be seen, the hubs for the musicians for the concatenated stimulus lie in the premotor (BA 6), primary motor (BA 4), and somatosensory (BA 3) cortices extending to the anterior regions of the right dorsal precuneus. In addition, musicians showed significantly higher node degrees in cerebellar regions with focal points lying in lobules VI, VIII, and smaller clusters encompassing lobules IV‐V, VIIB, and IX. Furthermore, significantly higher node degree was observed for the musicians in the right temporal pole extending dorsally to the parahippocampal gyrus (BA 36), and in the vicinity of the inferior temporal gyrus and fusiform gyrus (BA 20).
Conversely, non‐musicians displayed higher node degree in several regions of the parietal lobe with focal points in the middle precuneal sulcus (BA 7), and the bilateral angular gyrus (BA 39) further extending into the middle occipital gyrus (BA 19). In addition, a relatively large region in the left middle temporal gyrus (MTG; BA 21) was found to possess greater node degree for them. Also, the left pars opercularis (BA 44) and right pars triangularis (BA 45) displayed significantly higher node degree for non‐musicians. Furthermore, non‐musicians exhibited significantly higher node degree at the juncture encompassing the right‐hemispheric isthmus of the cingulate gyrus, calcarine fissure and inferior precuneus (BA 29), and also in the left hippocampus and parahippocampal gyrus.
The correlation of the Z‐map with the RSN revealed that the musicians had overall higher node degree in regions belonging to the visual, sensorimotor, and cerebellar RSNs whereas the non‐musicians displayed higher node degree in the DMN, Salience, and CEN RSNs (Fig. 2).
Figure 2.
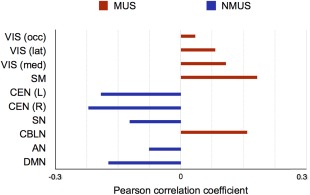
Results of correlation between the Z‐map of node degree differences and RSNs. Positive correlation signifies greater node degree for the musicians in the regions pertaining to the respective RSN and negative correlation represents the same for non‐musicians. Legend: VIS (occ) – occipital visual, VIS (lat) – lateral visual network, VIS (med) – medial visual network, SM – sensorimotor network, CEN (L) – left central executive network, CEN ® – right central executive network, SN – salience network, CBLN – cerebellar network, AN – auditory network, DMN – default mode network. MUS – musicians, NMUS – non‐musicians. [Color figure can be viewed at http://wileyonlinelibrary.com]
Correlation between Starting Age of Musical Training and Node
Since the correlation between starting age and years of training is low (Spearman's rho = −0.28, P = 0.27), the potential confound effect of overall years of training was discarded. Cluster size thresholding was used for multiple comparisons correction, with threshold size estimates obtained from a Monte Carlo simulation, whereby randomized starting ages were correlated voxelwise with the node degrees in the musician group and subsequently thresholded at P < 0.001 (one‐tailed). A critical cluster size of 19 voxels was obtained from a distribution of 5,000 cluster sizes (FWE = 0.05). Results of Spearman's correlation between these regions and starting age of training can be seen in Figure 3. A significant negative correlation (P < 0.001) was observed mainly in primary and surrounding auditory areas including Heschl's gyrus (HG) and STG, but also the left medial superior frontal gyrus (SFG), the right temporal pole (in the MTG), the anterior division of the cingulate gyrus, the right insula, and the right Broca's area (inferior frontal gyrus, IFG; see Table 3 for a complete list of regions). No significant positive correlation survived cluster correction. This indicates that the early starters tended to have higher node degree in those regions than the late starters.
Figure 3.
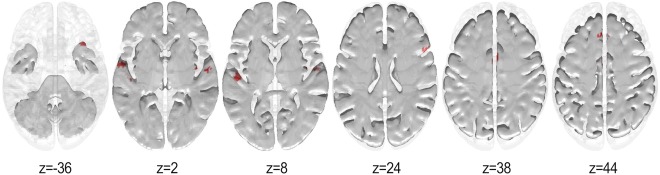
Correlation results (P < 0.05, cluster size = 19 voxels, FWE < 0.05) between starting age and node degree for the musicians. Red represents negative correlation. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Correlation results between starting age of training and node degree in musicians
| k | P‐value | x | y | z | BA | |
|---|---|---|---|---|---|---|
| Cluster 1 | ||||||
| Superior temporal gyrus (L) | 68 | 0.001 | −62 | −6 | −2 | 48 |
| Rolandic operculum (L) | 4 | 0.001 | −62 | −2 | 4 | 48 |
| Cluster 2 | ||||||
| Heschl's gyrus (L) | 32 | 0.001 | −48 | −12 | 6 | 48 |
| Superior temporal gyrus (L) | 27 | 0.001 | −52 | −16 | 4 | 48 |
| Cluster 3 | ||||||
| Superior frontal gyrus, medial (L) | 24 | 0.0008 | −6 | 30 | 46 | 8 |
| Superior frontal gyrus, medial (R) | 9 | 0.001 | 4 | 32 | 48 | 8 |
| Cluster 4 | ||||||
| Temporal pole, middle temporal gyrus (R) | 58 | 0.001 | 40 | 20 | −36 | 38 |
| Cluster 5 | ||||||
| Median cingulate and paracingulate gyrus (L) | 31 | 0.001 | −2 | 8 | 38 | 24 |
| Median cingulate and paracingulate gyrus (R) | 19 | 0.001 | 2 | 10 | 38 | 24 |
| Cluster 6 | ||||||
| Insula (R) | 22 | 0.0009 | 40 | −6 | 4 | 48 |
| Heschl's gyrus (R) | 14 | 0.001 | 48 | −8 | 8 | – |
| Superior temporal gyrus (R) | 10 | 0.001 | 52 | −10 | 2 | 48 |
| Cluster 7 | ||||||
| Inferior frontal gyrus, opercular part (R) | 12 | 0.001 | 56 | 16 | 26 | 44 |
| Inferior frontal gyrus, triangular part (R) | 8 | 0.0009 | 56 | 16 | 24 | 44 |
Brain areas showing significant negative correlation (P < 0.001, cluster corrected at FWE P < 0.05) between the starting age of musical training and the node degrees (musicians). Clusters were obtained via the 18‐connectivity scheme used in SPM. Anatomical labels correspond to the Automated Anatomical Labeling (AAL) [Tzourio‐Mazoyer et al., 2002]. The table reports within‐cluster region size (k; i.e., number of voxels), lowest P‐value per region within the cluster, and its respective MNI coordinates. Small regions within the cluster (k < 4 voxels) were discarded from the resulting table. Abbreviations: L = left, R = right.
Community Structure
To investigate differences in connectivity structure between the two participant groups, community structure analyses were performed using a set of seed voxels. These were chosen based on the differences in the node degree difference z‐score maps between the groups. The voxel with the highest node degree difference for the concatenated stimulus (Fig. 1) was chosen provided it was also found to exhibit significant node degree difference for all of the individual stimuli. For each participant and seed, the binary inclusivity [Steen et al., 2011]1 values were calculated for the voxels contained in a sphere with 4‐mm radius centered at the seed voxel, and then averaged. For each group, the inclusivity maps for each seed of interest (SOI) were obtained separately for each stimulus and then averaged. Group difference maps for each seed were determined by a subtraction operation. The significance of the observed differences was estimated via permutation tests.
Seeds with high node degree for musicians
The seed selection procedure resulted in two common seeds across all stimuli that possessed significantly higher node degree for musicians. Both reside in the motor regions (x = −6, y = −38, z = 72, Z = 3.43 in the left paracentral lobule, BA 4; and x = −10, y = −40, z = 64, Z = 3.62 in the left precuneus) among which the later seed possessed the highest node degree difference for the concatenated stimulus.
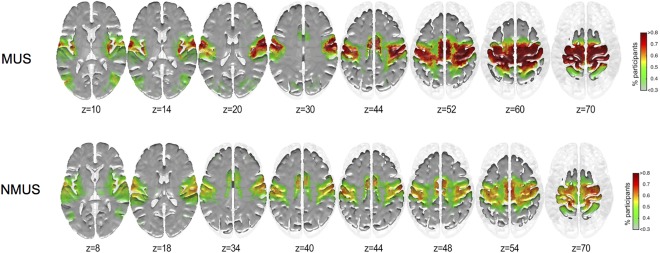
The areas showing higher inclusivity in each group for seed #1 (left Paracentral lobule) and #2 (left Precuneus) are shown in Figures 4 and 5 respectively.
Figure 4.
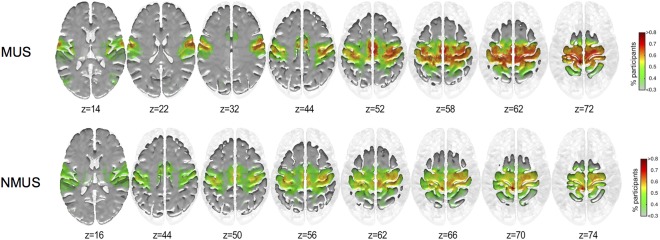
Inclusivity maps of seed #1 for musicians and non‐musicians. x = −6, y = −38, z = 72, left paracentral lobule. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
Inclusivity maps of seed #2 for musicians and non‐musicians. x = −10, y = −40, z = 64, Z = 3.62, left precuneus. [Color figure can be viewed at http://wileyonlinelibrary.com]
As can be seen from Figures 4 and 5, a larger proportion of musicians display greater inclusivity particularly in the motor cortex (BA 4). No significant differences were observed between the groups for the inclusivity maps for seed #1. However, group differences were observed for seed #2 (Fig. 6).
Figure 6.
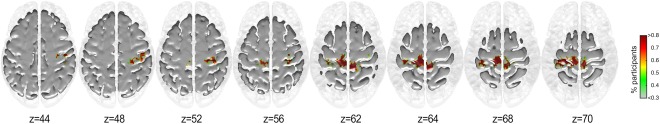
Group differences of the inclusivity maps of seed #2 (MUS > NMUS). [Color figure can be viewed at http://wileyonlinelibrary.com]
As can be seen, the musicians exhibited more consistent coupling of seed #2 with the primary motor cortex (BA 4) particularly in the left paracentral lobule extending anteriorly to the SMA and the right paracentral lobule extending posteriorly to the precuneus. These regions primarily encompass the lower and upper limbs, and upper torso representations of the motor homunculus of both hemispheres further encompassing the right‐hemispheric homuncular representation of the fingers. Furthermore, greater consistency in connectivity for the musicians was observed between seed #2 and the right primary somatosensory cortex (BA 3) in the vicinity of the sensory homunculus representing the hand and fingers.
Seeds with high node degree for non‐musicians
Overall, the non‐musicians did not display significantly greater node degree than musicians at any one voxel across all the stimuli. As a result, we chose only one voxel that displayed significantly higher node degree difference for the concatenated stimulus and for the majority of the individual stimuli. This SOI was located in the right precuneus (x = 10, y = −54, z = 40, Z = −3.46). The areas showing higher inclusivity in each group for seed #3 (right precuneus) are shown in Figure 7.
Figure 7.
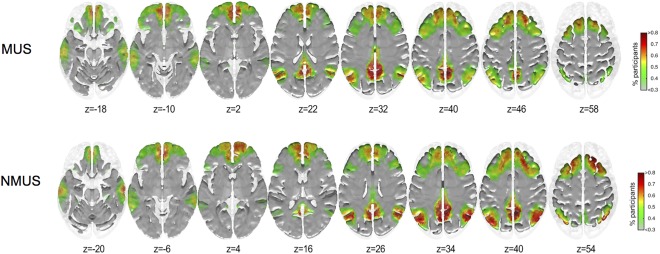
Inclusivity maps of seed #3 for musicians and non‐musicians. x = 10, y = −54, z = 40, Z = −3.46, right precuneus. [Color figure can be viewed at http://wileyonlinelibrary.com]
As can be seen in Figure 7, non‐musicians displayed more consistent and focalized inclusivity maps than the musicians. The inclusivity maps show high inclusivity in frontoparietal executive and DMN resting‐state networks.
As the community structure for seeds #1 & #2 encompassed sensorimotor cortical regions, we correlated the difference inclusivity maps (MUS minus NMUS) with the sensorimotor RSN. The correlations for the seeds #1 and #2 were r = 0.329, P < 0.05, and r = 0.314, P < 0.05, respectively, indicating that both seeds displayed a significantly stronger connectivity to the sensorimotor RSN in musicians than in non‐musicians. For seed #3, no significant correlations were observed between the difference inclusivity maps (NMUS minus MUS) and RSNs.
DISCUSSION
Comparing functional connectivity between musicians and non‐musicians during continuous music listening, using full‐brain connectivity analyses, we found higher connectivity in action‐related networks in musicians. Musicians' primary hubs comprised cerebral and cerebellar sensorimotor regions whereas non‐musicians' dominant hubs encompassed DMN‐related regions. Community structure analyses of the key hubs revealed greater consistency in coupling between the motor and somatosensory homunculi representing the upper limbs and torso in musicians while listening to music. These results are interesting in light of previous studies that support the notion that musical expertise strengthens the brain mechanisms for linking action and perception. Clark [2013] discusses models of cognition in the brain in a predictive coding framework [Friston, 2005; Vuust et al., 2009], a general theory of brain function. Predictive coding describes the brain as a predictive encoder that processes sensory information in a way such that it encodes change of incoming sensory stream and attempts to minimize prediction error. The prediction model however can either be perception‐based or action‐based. Perception‐based models attempt to match incoming sensory information based on modification of top down predictions, whereas action‐based or action‐oriented predictive processing (AOPP) models try to minimize the prediction error by actively engaging the motor system internally to generate the motor commands needed to fulfill the predictions [Gebauer et al., 2015; Hawkins and Blakeslee, 2004].
Importantly, the ability to predict changes in a sensory stream of information in the AOPP framework is highly dependent on one's own action repertoire [Konig et al., 2013]. It is therefore crucial to understand the role of expertise in relation to how brain networks integrate and segregate information from sensory modalities, the body, and memories. Expert dancers were found to react to dance movements by internal motor simulation more so for their own dance form, versus non‐experts who showed no such differences to any dance form [Calvo‐Merino et al., 2005]. Several such studies support the notion that observing an action from one's repertoire indeed engages the regions involved in AOPP [Calvo‐Merino et al., 2006; Cross et al., 2006]. Similarly, athletes while passively listening to sounds familiar to their sport engaged neural areas involved in action planning [Woods et al., 2014] thereby further lending support to our current results that perceiving auditory information indeed recruits the regions required to produce them.
While it has been established that both passive and affective listening of musical pieces leads to increase in activity in the motor cortex in the musicians' brains [Brattico et al., 2016; Novembre and Keller, 2014], there are barely any studies that investigate the link between somatosensory cortex activity and music listening. As hypothesized, musicians exhibited higher node degrees in motor‐related cerebral regions (BA 3,4,6) and primary somatosensory cortex, in addition to cerebellar regions (lobules, VI, VIII and small clusters in IV‐V, VIIb, and IX), the inferior temporal gyrus (BA 20), and the temporal pole extending medially to the parahippocampal gyrus (BA 36). The results of the present study, however, are consistent with existing evidence on the interdependency of the motor and somatosensory processes in context of mirror neurons [Keysers et al., 2010; van Ede and Maris, 2013] and also as a result of musical training [Kuchenbuch et al., 2014; Schulz et al., 2003].
In their extensive cerebellar meta‐analysis [Stoodley and Schmahmann, 2009, 2010], the authors provide strong evidence supporting the sensorimotor role of anterior parts (IV‐V), medial lobule VI and VIII of the cerebellum, which we found to possess higher node degree for musicians than non‐musicians. To add to this, a previous study reported that tactile stimulation of the hand activated lobule V ipsilaterally in addition to a lobule IX, a prominent hub in the cerebellar RSN (CBLN) [Bushara et al., 2001]. However, in a recent rsMRI study, the authors provide evidence of lobule IX's possible inclusion in the DMN [Habas et al., 2009]. Lobule VIIb has been associated with executive functioning [Stoodley and Schmahmann, 2009]. In light of these studies, it appears that that music listening automatically engages both cerebral and cerebellar sensorimotor regions in the brain in musicians.
The significantly higher node degree observed for the musicians in the temporal pole encompassing the parahippocampal gyrus extending to the ectorhinal cortex (BA 36) is interesting in light of the review on the functionality of the temporal pole [Olson et al., 2007]. They advocate the key role of the temporal pole in binding complex perceptual inputs to visceral emotional responses. Furthermore, Brodmann area 36, which is part of the perirhinal cortex, has been implicated to play a mnemonic role [Insausti et al., 1998; Svoboda et al., 2006] and that it is key in communication between extensive areas of sensory cortex and the hippocampus [Mishkin et al., 1997]. This allows us to posit that during continuous music listening, musically complex stimuli recruit regions known to process working memory [Burunat et al., 2014], that is, the temporal pole, albeit the right hemispheric counterpart and surrounding regions in the vicinity of the parahippocampal gyrus and as a result evoke more visceral emotional responses in musicians. Furthermore, in a recent study examining limbic seed‐based (3 seeds: amygdala, hippocampus, and nucleus accumbens) connectivity differences between musicians and non‐musicians during continuous music listening [Alluri et al., 2015], the authors evidenced greater connectivity of the amygdalae and left NAc with the left temporal pole. This further lends support to the possible notion that listening to the current music, which is characterized by rhythmically and tonally complex information causes greater emotional responses in musical experts than in non‐musicians. Additionally, they reported greater connectivity of the amygdala with the ITG in the vicinity of the fusiform gyrus in musicians. To add to this, regional activation of similar brain structures as those found here in musicians was previously found to correlate negatively with the clarity of musical pulse in musicians [Alluri et al., 2012] and with processing temporal unpredictability [Engel and Keller, 2011]. These further highlight the functioning of the musical brain as one involved in deriving emotional responses as a result of assessing ongoing musical structure, and hence trying to minimize the prediction error by actively engaging the motor system internally. Despite previous studies evidencing greater integration of the SN/DMN during resting state, music listening engages other brain networks differently between the groups. However, further research that investigates resting‐state networks before, during, and post music listening is called for to clarify these differences.
Finally, the effect of the starting age of musical training revealed that the earlier the onset in musical training, the higher the node degrees in auditory areas (HG and STG), as well as areas involved in top‐down cognitive processes and emotion such as the dorsomedial prefrontal cortex (dmPFC), the right temporal pole (in the MTG), the cingulate gyrus (anterior division), the rolandic operculum, the right insula, and right Broca's area (IFG). The dmPFC (medial SFG) is an area known to be implicated in executive mechanisms and decision‐related processes executive [Narayanan and Laubach, 2006; Talati and Hirsch, 2005], as well as in social behavior [Finger et al., 2006], and self‐referential mental activity [Wolf et al., 2010]. The TP, as mentioned above, is thought to reflect underlying emotional processing [Jimura et al., 2009; Olson et al., 2007]. The rolandic operculum is integrated in the somatosensory homuncular representation of oral structures [McCarthy et al., 1993] and seems to respond to mechanical stimulation of the mouth and tongue [Hari et al., 1993; Nakamura et al., 1998]. The rolandic operculum has been linked to the perception of pleasant tunes, as it may enable premotor representations for vocal sound production during the perception of pleasant auditory information [Koelsch et al., 2006]. Additionally, rolandic opercular areas were also implicated in the processing of various musical features during continuous music listening [Alluri et al., 2012].
The ACC and the insula were also areas reported to exhibit increasing node degree for decreasing onset ages of musical training. These areas are important anchors of the SN and thus these areas are sensitive to behaviorally salient events and ready to initiate appropriate remedial responses [Menon and Uddin, 2010]. This could mean that the SN is more integrated in early onset musicians, and so starting age may be a factor for increased integration of this network. This interpretation is supported by recent findings showing that effects of training are immediately visible in ACC, insula and hippocampus [Groussard et al., 2014]. In other words, early training would seem to intensify the salience quality of or awareness toward musical stimuli among musicians.
The right‐hemispheric homologue of Broca's area (IFG, BA 44) showed also increased node degrees for early starters. This area is known to be involved in prosody, which refers to the patterns of stress, intonation, tempo, and rhythm used in speech necessary for an appreciation of the subtleties of language (e.g., irony, stress, focus, or metaphor). Damage to this area leads aprosodia, that is, the difficulty to comprehend such subtleties and the emotional content of speech [Kandel et al., 2000] Broca's right homologue has been also discussed in fMRI studies of harmonic and melodic violation [Janata et al., 2002; Koelsch, 2006; Koelsch et al., 2002; Maess et al., 2001; Tillmann et al., 2003] evidencing that the opercular part of the IFG (BA 44) activates in response to music‐syntactic processing more predominantly in the right than in the left hemisphere. In addition, this area was part of a working memory retrieval network active in response to repetition of musical phrases [Burunat et al., 2014]. Furthermore, Broca's area and its right homologue role has been implicated in the human mirror neuron system as one that mediates sensory‐motor transformations related to imitation and hence might be key in music/speech therapy methods [Molnar‐Szakacs et al., 2006; Molnar‐Szakacs and Overy, 2006].
The regions with high node degree for early starters comprised a multimodal system, including not only the auditory cortex but also areas related to top‐down processes, attention, emotion, somatosensory processing, and non‐verbal processing of speech. Moreover, the impact of the starting age of training on the functional structure (node degree) of the brain is overtly manifested in the free listening brain responses of musicians. Because the node degree in some areas seems to be driven by early musical experience, we support the idea of a sensitive early period of high susceptibility to practice‐dependent plasticity [Trainor, 2005], which would represent an advantageous adaptation supporting musicians' multimodal skills.
Conversely, non‐musicians displayed higher node degrees in parietal and frontal regions that are known to have high membership in DMN and CEN networks, particularly the bilateral angular gyrus (BA 39), the precuneus, and in the vicinity of the posterior cingulate gyrus (BA 29). Using the same continuous listening paradigm, the angular gyrus and precuneus have been found previously to be anticorrelated to timbral features (Activity, Fullness) of ongoing musical stimulus, thereby indicative of attentive listening/attending to the musical stimulus [Alluri et al., 2012]. A similar correlation pattern with timbral features was observed in these regions using the same participants as in a current study (Alluri et al., in preparation). Furthermore, the community structure analysis of the seed belonging to the angular gyrus (Fig. 7) revealed that the DMN‐ and CEN‐related regions are indeed working in sync during passively listening to music. We can thus infer that non‐musicians display a trend in terms of greater consistency in connectivity of DMN‐ and CEN‐related nodes than the musicians. In a relevant study, the authors report that the overall structure of the DMN does not change while music listening [Kay et al., 2012]. Personal communication with the first author revealed that a major proportion of the participants in their study were not musically trained. These results are interesting in light of the recent study [Zhang et al., 2014] wherein non‐musicians were scanned before and after they were subjected to motor learning tasks that required them to tap their fingers in a particular order which can be thought of as being analogous to practicing a melody on the piano (albeit without auditory feedback). They found significant decrease in the resting‐state network strength of the DMN and increase in connectivity strength between the sensorimotor network and the left dorsal precuneus, and between the visual network and the right fusiform gyrus. A subsequent study by the same research group focusing solely on the changes in DMN for the same data added to the existing finding that motor training indeed alters the interaction of regions within the DMN [Ge et al., 2014]. Furthermore, learning new skills such as creative writing or music has been associated with neuroplasticity of the angular gyrus [Seghier, 2013]. In light of these studies and our results, we posit that musical training may indeed cause changes in the connectivity of the regions belonging to the DMN and form new connections that are eventually enhanced as a result of longitudinal practice.
Unexpectedly, the left hippocampal formation, and dorsolateral prefrontal regions encompassing the Broca's areas (BA 44) and its right hemispheric homologue (BA 45) were found to possess higher node degree for non‐musicians than musicians. In an early MEG study by [Maess et al., 2001] on musical syntax processing in non‐musicians, they found the same regions of the Broca's area (BA 44) and its right‐hemispheric homologue as regions involved in processing incoming harmonic sequences and incongruities thereof. In addition, the left MTG (BA 21) exhibited higher node degrees for non‐musicians. This result is quite interesting in light of the study [Schneider, 2005] wherein the authors found that the left HG plays a primary role in processing fundamental pitch in contrast to spectral pitch, which in turn is the primary listening strategy of non‐musicians as opposed to musicians who are more tuned to listening to the latter. Conversely, the musicians would have differing music listening strategies especially based on their primary instrument [Schneider et al., 2005] and hence would not exhibit a consistent auditory hub (across all musicians) that would indeed possess significantly higher node degree than non‐musicians. This result taken together with Broca's area and its right hemispheric homologue might indicate that non‐musicians may be processing music similar to language in terms of sequential melodic and harmonic progressions. Musicians on the other hand might have used a more action‐oriented approach, possibly one of minimizing prediction errors by internal motor simulation.
Subsequent community structure analysis revealed interesting differences for both groups. The seeds of interest that possessed significantly higher node degree for musicians belonged to the left paracentral lobule and the adjoining region of the dorsal precuneus and for the non‐musicians to the middle precuneal sulcus. The community structure of the sensorimotor seeds (seeds #1 & #2) included regions possessing high membership in the SM RSN. However, differences were observed for seed #2 wherein the musicians displayed higher consistency in the connectivity with the primary motor and somatosensory cortices specifically in the regions of the motor homunculus representing the limbs, and upper torso with an extension in the right hemisphere representing the fingers. Additionally, greater consistency in connectivity was observed for the musicians in the vicinity of the sensory homunculus representing the hand and fingers. As hypothesized, we found the motor and somatosensory cortices to be highly integrated in the musicians. These results are also in line with a study where the left premotor regions were activated more in musicians during passive listening than in non‐musicians [Bangert and Schlaug, 2006]. Moreover, this result further drives home the point that the musicians use internally generated motor action as a mechanism to reduce prediction error in the incoming auditory stream as described by the AOPP model. Conversely, the precuneus turned out to be a pivotal hub for the non‐musicians and its community structure included particularly DMN and CEN RSN regions although no differences were observed in the precuneus community structures (Fig. 6). This result permits us to postulate that non‐experts, in our case non‐musicians, indeed rely more than musicians on a perception‐based approach instead of an action‐oriented one due to their very limited action repertoire of reproducing the incoming auditory stream.
CONCLUSION
For the first time, we show here using fMRI and whole brain connectivity analysis that musicians and non‐musicians automatically use different neural networks during naturalistic condition of continuous music listening wherein musicians' brains process the auditory stimuli via an action‐based approach whereas the non‐musicians' approach is perception‐based. We identified music‐expertise‐modulated hubs in the brain that surface during continuous music listening. Key hubs that emerge during passive listening to music in the musicians lie in cerebral sensorimotor regions whereas the non‐musicians' dominant hubs lie in parietal and left‐hemispheric temporal regions. Musicians display superior integration of motor and somatosensory regions during music listening. Particularly, for the first time we show enhanced connectivity with the motor and sensory homunculus representing the upper limbs and torso during passive listening to music. In addition, we demonstrate that musicians who start training at an early age exhibit greater centrality in the auditory cortex as well as areas related to top‐down processes, attention, emotion, somatosensory processing, and non‐verbal processing of speech. Investigating modifications in brain networks due to musical training in the context of neuropsychiatry might pave way in designing better music therapy interventions and hence calls for more detailed studies. As differences in musical feature processing have been observed in the presence of lyrics, a natural extension would be to examine how these differences would manifest as connectivity patterns while listening to music with lyrics. Furthermore, investigating instrument‐specific listening strategies in musicians in addition to comparing it with rsMRI would further help reveal subtleties in reorganization of brain networks.
Footnote
We also calculated the scaled inclusivity values (Steen et al., 2011), but since they were highly similar to the binary inclusivity values, we used the latter for simplicity.
REFERENCES
- Abrams DA, Ryali S, Chen T, Chordia P, Khouzam A, Levitin DJ, Menon V (2013): Inter‐subject synchronization of brain responses during natural music listening. Eur J Neurosci 37:1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alluri V, Toiviainen P, Jääskeläinen IP, Glerean E, Sams M, Brattico E (2012): Large‐scale brain networks emerge from dynamic processing of musical timbre, key and rhythm. NeuroImage 59:3677–3689. [DOI] [PubMed] [Google Scholar]
- Alluri V, Toiviainen P, Lund TE, Wallentin M, Vuust P, Nandi AK, Ristaniemi T, Brattico E (2013): From Vivaldi to Beatles and back: Predicting lateralized brain responses to music. NeuroImage 83:627–636. [DOI] [PubMed] [Google Scholar]
- Alluri V, Brattico E, Toiviainen P, Burunat I, Bogert B, Numminen J, Kliuchko M (2015): Musical expertise modulates functional connectivity of limbic regions during continuous music listening. Psychomusicology 25:443–454. [Google Scholar]
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K (1997): Motor cortex and hand motor skills: Structural compliance in the human brain. Hum Brain Mapp 5:206–215. [DOI] [PubMed] [Google Scholar]
- Baer LH, Thibodeau JL, Gralnick TM, Li KZ, Penhune VB (2013): The role of musical training in emergent and event‐based timing. Front Hum Neurosci 7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Zatorre R, Penhune V (2014): Early musical training is linked to gray matter structure Performance., in the ventral premotor cortex and auditory–motor rhythm synchronization. J Cogn Neurosci 26:755–767. [DOI] [PubMed] [Google Scholar]
- Bangert M, Schlaug G (2006): Specialization of the specialized in features of external human brain morphology. Eur J Neurosci 24:1832–1834. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. [DOI] [PubMed] [Google Scholar]
- Bogert B, Numminen‐Kontti T, Gold B, Sams M, Numminen J, Burunat I, Lampinen J, Brattico E (2016): Hidden sources of joy, fear, and sadness: Explicit versus implicit neural processing of musical emotions. Neuropsychologia 89:393–402. [DOI] [PubMed] [Google Scholar]
- Brattico E, Pallesen KJ, Varyagina O, Bailey C, Anourova I, Järvenpää M, Eerola T, Tervaniemi M (2009): Neural discrimination of nonprototypical chords in music experts and laymen: An MEG study. J Cogn Neurosci 21:2230–2244. [DOI] [PubMed] [Google Scholar]
- Brattico E, Bogert B, Alluri V, Tervaniemi M, Eerola T, Jacobsen T (2016): It's sad but i like it: The neural dissociation between musical emotions and liking in experts and laypersons. Front Hum Neurosci 9: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burunat I, Alluri V, Toiviainen P, Numminen J, Brattico E (2014): Dynamics of brain activity underlying working memory for music in a naturalistic condition. Cortex 57:254–269. [DOI] [PubMed] [Google Scholar]
- Burunat I, Brattico E, Puoliväli T, Ristaniemi T, Sams M, Toiviainen P (2015): Action in perception: Prominent visuo‐motor functional symmetry in musicians during music listening. PLoS One 10:e0138238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burunat I, Toiviainen P, Alluri V, Bogert B, Ristaniemi T, Sams M, et al. (2016): The reliability of continuous brain responses during naturalistic listening to music. Neuroimage 1–7. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Wheat JM, Khan A, Mock BJ, Turski PA, Sorenson J, Brooks BR (2001): Multiple tactile maps in the human cerebellum. Neuroreport 12:2483–2486. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Glaser DE, Grzes J, Passingham RE, Haggard P (2005): Action observation and acquired motor skills: An fMRI study with expert dancers. Cereb Cortex 15:1243–1249. [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino B, Grzes J, Glaser DE, Passingham RE, Haggard P (2006): Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16:1905–1910. [DOI] [PubMed] [Google Scholar]
- Carlson E, Saarikallio S, Toiviainen P, Bogert B, Kliuchko M, Brattico E (2015): Maladaptive and adaptive emotion regulation through music: A behavioral and neuroimaging study of males and females. Front Hum Neurosci 9:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, Zatorre RJ (2008): Moving on time: Brain network for auditory‐motor synchronization is modulated by rhythm complexity and musical training. J Cogn Neurosci 20:226–239. [DOI] [PubMed] [Google Scholar]
- Clark A (2013): Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci 36:181–204. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, de C, Grafton ST (2006): Building a motor simulation de novo: Observation of dance by dancers. NeuroImage 31:1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligianni F, Centeno M, Carmichael DW, Clayden JD (2014): Relating resting‐state fMRI and EEG whole‐brain connectomes across frequency bands. Front Neurosci 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Keller P (2011): The perception of musical spontaneity in improvised and imitated jazz performances. Front Psychol 2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Kamel N, Mitchell DGV, Blair JR (2006): Caught in the act: The impact of audience on the neural response to morally and socially inappropriate behavior. NeuroImage 33:414–421. [DOI] [PubMed] [Google Scholar]
- Friston K (2005): A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360:815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2011): Functional and effective connectivity: A review. Brain Connect 1:13. [DOI] [PubMed]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J (2000): To smooth or not to smooth? Bias and efficiency in fMRI time‐series analysis. NeuroImage 12:196–208. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G (2003): Brain structures differ between musicians and non‐musicians. J Neurosci 23:9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Zhang H, Yao L, Long Z (2014): Motor imagery learning induced changes in functional connectivity of the default mode network. IEEE Trans Neural Syst Rehabil Eng 23:138–148. [DOI] [PubMed] [Google Scholar]
- Gebauer L, Kringelbach ML, Vuust P (2015): Predictive coding links perception, action, and learning to emotions in music. Comment on “The quartet theory of human emotions: An integrative and neurofunctional model” by S. Koelsch et al. Phys Life Rev 1:21–23. [DOI] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, Mcnulty JP, Mullins PG (2014): NeuroImage The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage 99:180–190. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Rowe JB (2009): Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. J Neurosci 29:7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussard M, Viader F, Landeau B, Desgranges B, Eustache F, Platel H (2014): The effects of musical practice on structural plasticity: The dynamics of grey matter changes. Brain Cogn 90C:174–180. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009): Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Wallentin M, Vuust P (2013): Working memory and musical competence of musicians and non‐musicians. Psychol Music 41:779–793. [Google Scholar]
- Hari R, Karhu J, Hämäläinen M, Knuutila J, Salonen O, Sams M, Vilkman V (1993): Functional organization of the human first and second somatosensory cortices: A neuromagnetic study. Eur J Neurosci 5:724–734. [DOI] [PubMed] [Google Scholar]
- Haumann NT, Parkkonen L, Kliuchko M, Vuust P, Brattico E (2016): Comparing the performance of popular MEG/EEG artifact correction methods in an evoked‐response study. Comput Intell Neurosci 2016:7489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J, Blakeslee S (2004): On Intelligence. New York: Holt. [Google Scholar]
- Huron D (2001): Is music an evolutionary adaption? Ann N Y Acad Sci 930:43–61. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L (2009): White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. NeuroImage 46:600–607. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A (1998): MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am J Neuroradiol 19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Janata P, Birk JL, Van Horn JD, Leman M, Tillmann B, Bharucha JJ (2002): The cortical topography of tonal structures underlying Western music. Science (New York, N.Y.) 298:2167–2170. [DOI] [PubMed] [Google Scholar]
- Jimura K, Konishi S, Miyashita Y (2009): Temporal pole activity during perception of sad faces, but not happy faces, correlates with neuroticism trait. Neurosci Lett 453:45–48. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM (2000): Principles of Neural Science. New York: McGraw‐Hill, Health Professions Division. [Google Scholar]
- Kay BP, Meng X, Difrancesco MW, Holland SK, Szaflarski JP (2012): Moderating effects of music on resting state networks. Brain Res 1447:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V (2010): Somatosensation in social perception. Nat Rev Neurosci 11:417–428. [DOI] [PubMed] [Google Scholar]
- Klein C, Liem F, Hänggi J, Elmer S, Jäncke L (2015): The “silent” imprint of musical training the “silent” imprint of musical training. Hum Brain Mapp 37:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliuchko M, Heinonen‐Guzejev M, Monacis L, Gold BP, Heikkila KV, Spinosa V, Tervaniemi M, Brattico E (2015): The association of noise sensitivity with music listening, training, and aptitude. Noise Health 17:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliuchko M, Heinonen‐Guzejev M, Vuust P, Tervaniemi M, Brattico E (2016): A window into the brain mechanisms associated with noise sensitivity. Sci Rep 6:39236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S (2006): Significance of Broca's area and ventral premotor cortex for music‐syntactic processing. Cortex 42:518–520. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Skouras S (2014): Functional centrality of amygdala, striatum and hypothalamus in a “small‐world” network underlying joy: An fMRI study with music. Hum Brain Mapp 35:3485–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Gunter TC, V. Cramon DY, Zysset S, Lohmann G, Friederici AD (2002): Bach speaks: A cortical “language‐network” serves the processing of music. NeuroImage 17:956–966. [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Cramon DYV, Müller K, Friederici AD (2006): Investigating emotion with music: An fMRI study. Hum Brain Mapp 27:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig P, Wilming N, Kaspar K, Nagel SK, Onat S (2013): Predictions Computational, in the light of your own action repertoire as a general principle. Behav Brain Sci 36:219–220. [DOI] [PubMed] [Google Scholar]
- Kraus N, Chandrasekaran B (2010): Music training for the development of auditory skills. Nat Rev Neurosci 11:599–605. [DOI] [PubMed] [Google Scholar]
- Kuchenbuch A, Paraskevopoulos E, Herholz SC, Pantev C (2014): Audio‐tactile integration and the influence of musical training. PLoS One 9:e85743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurienti PJ, Joyce KE, Telesford QK, Burdette JH, Hayasaka S (2011): Universal fractal scaling of self‐organized networks. Physica A 390:3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Guo Z, Lai Y, Liao W, Liu Q, Kendrick KM, Yao DZ, Li H (2012): Musical training induces functional plasticity in perceptual and motor networks: Insights from resting‐state FMRI. PLoS One 7:e36568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, C. , Tu, S. , Peng, Y. , Gao, S. , Li, J. , Dong, L. , Li G, Lai Y, Li H, Yao, D. (2014). Long‐term effects of musical training and functional plasticity in salience system. Neural Plast 2014:180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maess B, Koelsch S, Gunter TC, Friederici AD (2001): Musical syntax is processed in Broca's area: An MEG study. Nat Neurosci 4:540–545. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Allison T, Spencer DD (1993): Localization of the face area of human sensorimotor cortex by intracranial recording of somatosensory evoked potentials. J Neurosurg 79:874–884. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miendlarzewska EA, Trost WJ (2014): How musical training affects cognitive development: Rhythm, reward and other modulating variables. Front Neurosci 7: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Suzuki WA, Gadian DG, Vargha‐Khadem F (1997): Hierarchical organization of cognitive memory. Philos Trans R Soc Lond B Biol Sci 352:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar‐Szakacs I, Overy K (2006): Music and mirror neurons: From motion to “e”motion. Soc Cogn Affect Neurosci 1:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar‐Szakacs I, Kaplan J, Greenfield PM, Iacoboni M (2006): Observing complex action sequences: The role of the fronto‐parietal mirror neuron system. NeuroImage 33:923–935. [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmüller E, Jäncke L (2002): The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3:473–478. [DOI] [PubMed] [Google Scholar]
- Musacchia G, Strait D, Kraus N (2008): Relationships between behavior, brainstem and cortical encoding of seen and heard speech in musicians and non‐musicians. Hear Res 241:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura a, Yamada T, Goto a, Kato T, Ito K, Abe Y, Kachi T, Kakigi R (1998): Somatosensory homunculus as drawn by MEG. NeuroImage 7:377–386. [DOI] [PubMed] [Google Scholar]
- Narayanan N, Laubach M (2006): Top‐down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M, Girvan M (2004): Finding and evaluating community structure in networks. Phys Rev 69:026113. [DOI] [PubMed] [Google Scholar]
- Novembre G, Keller PE (2014): A conceptual review on action‐perception coupling in the musiciansâ€TM brain: What is it good for? Front Hum Neurosci 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y (2007): The Enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130:1718–1731. [DOI] [PubMed] [Google Scholar]
- Pantev C, Roberts CALE, Schulz M, Engelien A, Ross B (2001): Timbre‐speci ® c enhancement of auditory cortical representations in musicians. Neuroreport 12:169–174. [DOI] [PubMed] [Google Scholar]
- Pantev C, Paraskevopoulos E, Kuchenbuch A, Lu Y, Herholz SC (2015): Musical expertise is related to neuroplastic changes of multisensory nature within the auditory cortex. Eur J Neurosci 41:709–717. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlagger BL, Petersen SE (2011): Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov‐Schlaggar CN, Petersen SE (2013): Evidence for hubs in human functional brain networks. Neuron 79:798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reybrouck M, Brattico E (2015): Neuroplasticity beyond sounds: Neural adaptations following long‐term musical aesthetic experiences. Brain Sci 5:69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Zhang W (2008): Identifying network communities with a high resolution. Phys Rev E 77:19. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y (1995): Increased corpus callosum size in musicians. Neuropsychologia 33:1047–1055. [DOI] [PubMed] [Google Scholar]
- Schneider P (2005): Structural, functional, and perceptual differences in Heschl's gyrus and musical instrument preference. Ann N Y Acad Sci 1060:387–394. [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A (2002): Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5:688–694. [DOI] [PubMed] [Google Scholar]
- Schneider P, Sluming V, Roberts N, Scherg M, Goebel R, Specht HJ, Dosch HG, Bleeck S, Stippich C, Rupp A (2005): Structural and functional asymmetry of lateral Heschl ' s gyrus reflects pitch perception preference. Nat Neurosci 8:1241–1247. [DOI] [PubMed] [Google Scholar]
- Schulz M, Ross B, Pantev C (2003): Evidence for training‐induced crossmodal reorganization of cortical functions in trumpet players. Neuroreport 14:157–161. [DOI] [PubMed] [Google Scholar]
- Seghier ML (2013): The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SL, Bowman FD, Laurienti PJ (2013): Analyzing complex functional brain networks: Fusing statistics and network science to understand the brain. Stat Surv 7:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Networks 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CJ, Bailey JA, Zatorre RJ, Penhune VB (2013): Early musical training and white‐matter plasticity in the corpus callosum: Evidence for a sensitive period. J Neurosci 33:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M, Hayasaka S, Joyce K, Laurienti P (2011): Assessing the consistency of community structure in complex networks. Phys Rev E 84:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD (2009): Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. NeuroImage 44:489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD (2010): Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait DL, Kraus N, Skoe E, Ashley R (2009): Musical experience and neural efficiency: Effects of training on subcortical processing of vocal expressions of emotion. Eur J Neurosci 29:661–668. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B (2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati A, Hirsch J (2005): Functional specialization within the medial frontal gyrus for perceptual go/no‐go decisions based on “what,” “when,” and “where” related information: An fMRI study. J Cogn Neurosci 17:981–993. [DOI] [PubMed] [Google Scholar]
- Telesford QK, Simpson SL, Burdette JH (2011): The brain as a complex system: Using network science as a tool for understanding the brain. 1:295–308. [DOI] [PMC free article] [PubMed]
- Tervaniemi M, Rytkönen M, Schröger E, Ilmoniemi RJ, NR (2001): Superior formation of cortical memory traces for melodic patterns in musicians. Learn Mem 8:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann B, Janata P, Bharucha JJ (2003): Activation of the inferior frontal cortex in musical priming. Ann N Y Acad Sci 999:209–211. [DOI] [PubMed] [Google Scholar]
- Toiviainen P, Alluri V, Brattico E, Wallentin M, Vuust P (2014): Capturing the musical brain with Lasso: Dynamic decoding of musical features from fMRI data. NeuroImage 88:170–180. [DOI] [PubMed] [Google Scholar]
- Trainor LJ (2005): Are there critical periods for musical development? Dev Psychobiol 46:262–278. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Maris E (2013): Somatosensory demands modulate muscular Beta oscillations, independent of motor demands. J Neurosci 33:10849–10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero L, Hartmann K, Ripollés P, Rojo N, Sierpowska J, François C, Càmara E, van Vugt FT, Mohammadi B, Samii A, Münte TF, Rodríguez‐Fornells A, Altenmüller E (2016): Structural neuroplasticity in expert pianists depends on the age of musical training onset. NeuroImage 126:106–119. [DOI] [PubMed] [Google Scholar]
- Vuust P, Ostergaard L, Pallesen KJ, Bailey C, Roepstorff A (2009): Predictive coding of music ‐ Brain responses to rhythmic incongruity. Cortex 45:80–92. [DOI] [PubMed] [Google Scholar]
- Vuust P, Brattico E, Seppänen M, Näätänen R, Tervaniemi M (2012): The sound of music: Differentiating musicians using a fast, musical multi‐feature mismatch negativity paradigm. Neuropsychologia 50:1432–1443. [DOI] [PubMed] [Google Scholar]
- Wan CY, Demaine K, Zipse L, Norton A, Schlaug G (2010): From music making to speaking: Engaging the mirror neuron system in autism. Brain Res Bull 82:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Savion‐Lemieux T, Penhune VB (2007): The effect of early musical training on adult motor performance: Evidence for a sensitive period in motor learning. Exp Brain Res 176:332–340. [DOI] [PubMed] [Google Scholar]
- Wilkins RW, Hodges DA, Laurienti PJ, Steen M, Burdette JH (2014): Network science and the effects of music preference on functional brain connectivity: From Beethoven to Eminem. Sci Rep 4:6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf I, Dziobek I, Heekeren HR (2010): Neural correlates of social cognition in naturalistic settings: A model‐free analysis approach. NeuroImage 49:894–904. [DOI] [PubMed] [Google Scholar]
- Wong PC, Skoe E, Russo NM, Dees T, Kraus N (2007): Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci 10:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods EA, Hernandez AE, Wagner VE, Beilock SL (2014): Expert athletes activate somatosensory passively, motor planning regions of the brain when listening to familiar sports sounds. Brain Cogn 87:122–133. [DOI] [PubMed] [Google Scholar]
- Zhang H, Long Z, Ge R, Xu L, Jin Z, Yao L, Liu Y (2014): Motor imagery learning modulates functional connectivity of multiple brain systems in resting state. PLoS One 9:e85489. [DOI] [PMC free article] [PubMed] [Google Scholar]


