Abstract
Creativity is imperative to the progression of human civilization, prosperity, and well‐being. Past creative researches tends to emphasize the default mode network (DMN) or the frontoparietal network (FPN) somewhat exclusively. However, little is known about how these networks interact to contribute to creativity and whether common or distinct brain networks are responsible for visual and verbal creativity. Here, we use functional connectivity analysis of resting‐state functional magnetic resonance imaging data to investigate visual and verbal creativity‐related regions and networks in 282 healthy subjects. We found that functional connectivity within the bilateral superior parietal cortex of the FPN was negatively associated with visual and verbal creativity. The strength of connectivity between the DMN and FPN was positively related to both creative domains. Visual creativity was negatively correlated with functional connectivity within the precuneus of the pDMN and right middle frontal gyrus of the FPN, and verbal creativity was negatively correlated with functional connectivity within the medial prefrontal cortex of the aDMN. Critically, the FPN mediated the relationship between the aDMN and verbal creativity, and it also mediated the relationship between the pDMN and visual creativity. Taken together, decreased within‐network connectivity of the FPN and DMN may allow for flexible between‐network coupling in the highly creative brain. These findings provide indirect evidence for the cooperative role of the default and executive control networks in creativity, extending past research by revealing common and distinct brain systems underlying verbal and visual creative cognition. Hum Brain Mapp 38:2094–2111, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: visual creativity, verbal creativity, default mode network, frontoparietal network, functional connectivity
INTRODUCTION
Creativity is imperative to human survival, the progression of human civilization, prosperity, and well‐being and is considered the ability to generate output that is both original and appropriate [Runco and Jaeger, 2012; Sternberg and Lubart, 1996]. Accordingly, the creative person is characterized by the ability to produce novel and useful ideas and to discern which ideas are appropriate, worthwhile, and meaningful [Runco, 2003]. Creative thought may pertain to goal‐directed, self‐generated cognition, which may involve spontaneous cognition and the top‐down control of spontaneous thought [Beaty et al., 2014a, 2015, 2016]. In this context, creative thought may depend on the cooperation of control processing supported by the frontoparietal control network (FPN) and spontaneous processing supported by the default mode network (DMN). However, the neurocognitive mechanisms of creative thought remain elusive largely due to the different measurement methods and tasks used in studies [Arden et al., 2010; Chávez‐Eakle et al., 2007; Dietrich and Kanso, 2010]. Past research regarding the neural mechanisms of creative cognition has focused on specific brain regions. Few studies have investigated the neural mechanisms of creativity from the perspective of functional connectivity within and across large‐scale networks. Creativity is widely measured with divergent thinking (DT), which is a central aspect of creativity [Torrance, 1968]. DT requires individuals to generate several possible solutions to a given problem [Guilford, 1967], and it has strong predictive value for creative achievement [Kim, 2008] DT is widely measured with the Torrance Tests of Creative Thinking (TTCT) in different domains. In this study, we investigate how verbal creativity, as measured by the verbal TTCT, and visual creativity, as measured by the figural TTCT, correlates with intranetwork and internetwork connectivity derived from resting‐state brain imaging.
The DMN and the FPN are two of the most widely studied networks. The DMN comprises the medial frontal, precuneus, and temporoparietal junction [Raichle et al., 2001]. The DMN is involved in cognitive processes that are internally focused, including various mental states conducive to creative thought, such as mind wandering [Andrews‐Hanna, 2012; Christoff et al., 2009; Mason et al., 2007], perspective‐taking [Buckner and Carroll, 2007], and imagining one's personal future or recollecting one's past [Burgess, 2008; Christoff et al., 2009; Mason et al., 2007]. Recent findings suggest that the DMN plays a critical role in spontaneous cognition (e.g., imaginative thought), and spontaneous thought involving establishing new connections among existing ideas in a quasi‐random or random way is imperative to the generation of novel ideas [Jung et al., 2013; Mok, 2012]. Structural neuroimaging of DT has shown that both cortical thickness and volume of the precuneus, right posterior cingulate are significantly correlated with individual verbal creativity [Chen et al., 2015; Jauk et al., 2015; Jung et al., 2010]. Moreover, evidence from functional neuroimaging studies implicates the DMN in verbal creative cognition, including DT [Jauk et al., 2015; Niendam et al., 2012] creative story generation [Howard‐Jones et al., 2005] and insight problem solving [Kounios et al., 2008; Subramaniam et al., 2009]. A recent resting‐state functional magnetic resonance imaging (rs‐fMRI) study showed that higher creativity was associated with increased resting‐state functional connectivity (RSFC) between the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC), which are key nodes of the DMN [Takeuchi et al., 2012]. Furthermore, Wei et al. reported that verbal creativity was positively correlated with the strength of RSFC between the mPFC and the middle temporal gyrus and that the strength of RSFC increased after creativity training [Wei et al., 2014].
Although the DMN and spontaneous cognitive processes appear to be important for creative cognition, previous research also points to an important role of the FPN in creative cognition. The FPN comprises the dorsolateral PFC, middle frontal gyrus (MFG), and posterior parietal lobule [Vincent et al., 2008]. The control network is associated with a diverse range of cognitive processes, such as selective retrieval of ideas from memory, integrating information to solve complex problems, inhibition of inappropriate information, working memory, and task‐set switching [Niendam et al., 2012], all of which are essential for creative information processing [Dietrich, 2004]. Supporting evidence from structural magnetic resonance imaging studies on DT have revealed that significantly increased cortical thickness and/or volume in regions of the FPN corresponding to the right dorsolateral prefrontal cortex (DLPFC) and the bilateral inferior frontal gyrus (IFG) are associated with higher verbal creativity [Takeuchi et al., 2012; Zhu et al., 2013]. Regions of the FPN have been recruited during a variety of creative thought processes, including DT [Goel and Vartanian, 2005; Gonen‐Yaacovi et al., 2013], visual art design [Aziz‐Zadeh et al., 2013; Huang et al., 2013; Kowatari et al., 2009], poetry composition [Liu et al., 2015], and music improvisation [Beaty et al., 2015; Bengtsson et al., 2014; de Manzano and Ullén, 2012]. Recent research on visual creativity has also shown that the left MFG and the superior parietal lobule are recruited during visual creativity [Aziz‐Zadeh et al., 2013; Gansler et al., 2011], and other work has shown that highly creative individuals exhibit greater activation in the ventral anterior cingulate cortex during verbal DT [Mayseless et al., 2015]. Furthermore, in a recent meta‐analysis of DT using Activation Likelihood Estimation, Wu et al. reported robust activation of the posterior parietal cortex [including the superior parietal cortex (SPL)] and the lateral prefrontal cortex across several functional neuroimaging studies [Wu et al., 2015]. Together, these results suggest that brain regions linked to cognitive control play a central role in creative cognition by inhibiting unoriginal ideas and selecting useful and original ideas [Beaty et al., 2014a, 2015, 2016; Jung et al., 2013].
Past studies have tended to emphasize the DMN or the FPN somewhat exclusively. Emerging evidence, however, has reported coactivation of the DMN and FPN control networks within the context of cognitive tasks requiring the evaluation of internal information [Beaty et al., 2014a, 2015, 2016]. Creative cognition involves goal‐directed and self‐generated thought processes. Thus, creative thought needs both the DMN and the FPN [Beaty et al., 2016]. Interaction between cognitive control and the DMN was earlier proposed by Jung et al. [2013]. The brain structure can predict functional connectivity [Segall et al., 2012]. Highly creative individuals have shown greater connectivity between the DMN and the left IFG [Beaty et al., 2014a,b]—a region associated with cognitive control—and increased connectivity between the ACC within the control network and the occipital‐temporal area within the DMN [Mayseless et al., 2015]. Creative generation may be supported by the DMN [Andrews‐Hanna, 2012; Beaty et al., 2014a, 2015, 2016; Fox et al., 2015; Stawarczyk and D'Argembeau, 2015]. Moreover, creative evaluation may depend on cooperation between control processing supported by the FPN and spontaneous processing supported by the DMN, and the FPN supports creative thought by persistently generating ideas around the current goal and inhibiting unoriginal ideas [Beaty et al., 2014a, 2014b; De Dreu et al., 2012].
Furthermore, the theory of blind variation and selective retention (BVSR) considers blind variation—a spontaneous process that involves establishing new connections among existing ideas in a quasi‐random or random way—as dependent on activation the default mode network (DMN), while selective retention—a cognitive control process that involves evaluating these novel combinations—tends to recruit the FPN [Jung et al., 2013]. Similarly, within the context of two‐stage models, the generation of novel ideas may be supported by the DMN, while the evaluation of ideas may be supported by the control network. Moreover, cognitive control mechanisms may be responsible for monitoring and directing spontaneous activity stemming from the DMN [Beaty et al., 2014a], and the FPN may integrate information from the DMN. Accordingly, we speculate that the cooperation of the DMN and the FPN may play a key role in creative cognition, and the FPN may mediate the relationship between the DMN and creative cognitive ability.
METHODS
Participants
This study is a long‐term program that is a part of an ongoing project investigating the associations among brain imaging, mental health, and creativity. In total, 364 right‐handed college students (145 men, mean age = 19.97) from Southwest University participated in this study. Forty‐eight participants were excluded due to a potential to suffer from depression, because their Beck Depression Inventory scores were higher 13. Three subjects were excluded because of behavioural data [intelligence (IQ) and creativity] beyond three standard deviations, and 33 participants were excluded due to issues with the imaging data: 6 subjects had missing rfMRI images, and 27 met the exclusion criteria of head movement during rest‐fMRI scanning (i.e., >2 mm translation in any axis and >2° angular rotation in any axis). Consequently, a total of 282 participants (132 males; mean age = 19.98; SD = 1.25) were included in the analyses. This project was approved by the Institutional Review Board of the Southwest University Brain Imaging Center. All participants without a history of psychiatric diseases or neurological disorders provided informed consent and received payment for their participation.
Assessment of Creativity
The TTCT [Ye et al., 1988] were designed as a measure of creativity (i.e., DT ability). In this study, the verbal TTCT (TTCT‐V) was used to assess individual verbal creativity abilities [Carson et al., 1994; Kim, 2006]. The TTCT‐V comprises seven tasks: generating questions, causes, and consequences; improving products (a toy elephant); alternate uses (cardboard boxes); manipulating objects; and imagining the consequences of a scenario. Scoring consisted of three components: flexibility (the number of different categories of responses, which reflects the ability to shift between conceptual fields); fluency (the number of meaningful and relevant responses, which is associated with the ability to generate and consider other possibilities); and originality (the degree of originality of the responses, which is associated with thinking “outside the box”). The total TTCT‐V score is the sum of these three components. Three trained postgraduates who were blind to the goal of this research took part in the scoring. The inter‐rater reliability for the scoring of the TTCT‐V was 0.90.
The figural TTCT (TTCT‐FTTCT‐F) consisted of three type of tasks used to measure individual visual creativity abilities. The first task asked participants to make an object or picture using 10 incomplete figures. The second task asked participants to draw as many objects or pictures on three pages of parallel lines. Like the TTCT‐V, the TTCT‐FTTCT‐F provides a total score which that consists of three components: flexibility (the number of different categories of responses, which reflects the ability to shift between conceptual fields); fluency (the number of relevant and meaningful responses, which is associated with the ability to generate a number of pictures or objects); and originality (the number of infrequent ideas, which reflects the ability to produce unique or uncommon responses) [Kim et al., 2006]. The total TTCT‐FTTCT‐F score reflects the sum of these three components. Scoring was performed by three trained raters who were all blind to this study. The inter‐rater reliability for scoring of the TTCT‐FTTCT‐F was 0.85.
The separate total creativity score was also used because it was correlated with each component score (each correlation coefficient > 0.88), and the scores of the dimensions were highly correlated with each other (each correlation coefficient > 0.78).
Assessment of General IQ
To examine intellectual ability, participants performed the Combined Raven's Test (CRT), which is widely used for IQ testing and has a high degree of reliability and validity [Wang, 2007]. The CRT, which includes 72 items and is based on Raven's standard progressive matrix [Raven, 1960] and Raven's coloured progressive matrix [Raven, 1958] was revised by the Psychology Department of East China Normal University in 1994. The total index score of this test, which is equal to the number of correct answers given by participants in 40 min, is used as a psychometric index of individual IQ.
Image Acquisition
All participants were scanned in a 3T Trio scanner (Siemens Medical, Erlangen, Germany) from Southwest University, China. Resting‐state functional images were obtained using a gradient echo planar imaging sequence: repetition time = 2000 ms; echo time = 30 ms; slices = 32; thickness = 3.0 mm; resolution matrix = 64 × 64; flip angle = 90°; field of view = 220 × 220 mm2; voxel size = 1 × 1 × 1 mm; slice gap = 1 mm, voxel size = 3.4 × 3.4 × 4 mm3. Each section contained 242 volumes. During the functional image acquisition, all subjects were instructed to close their eyes, not think about anything in particular, and remain awake.
Preprocessing of Imaging Data
The processing of resting‐state functional MRI data was performed using the Data Processing Assistant for Resting‐State fMRI (DPARSF, http://resting-fmri.sourceforge.net/) [Yan and Zang, 2010] based on SPM8. First, the first 10 volumes from each subject's functional imaging data were discarded to account for steady‐state magnetization. The remaining 232 volumes were included in the subsequent analysis. Second, slice timing correction was used to correct slice order effects, and head motion correction was used to correct head movement artefacts. Twenty‐seven subjects who exhibited a head motion of 2 mm maximum displacement and 2° rotation throughout the course of scans were excluded. Third, each participant's functional image was spatially normalized to the standard MNI template with a resampled voxel size of 3 × 3 × 3 mm. The data were then smoothed with an isotropic 8 mm full‐width at half maximum Gaussian kernel. To reduce the residual effects of motion artefacts, the mean framewise displacement (FD) derived with Jenkinson's relative root mean square algorithm was regressed out in group statistical analysis as a regressor of no interest.
STATISTICAL ANALYSIS
Behavioral Data Analysis
We analysed behavioural data with SPSS 19.0 statistical software (SPSS, Chicago, IL). Pearson's correlation coefficient was used to analyse the relationship between the three sub‐dimensional TTCT scores (originality, flexibility, and fluency) and the total TTCT scores (TTCT‐FTTCT‐F, TTCT‐V). In addition, we tested sex differences on the TTCT‐FTTCT‐F, TTCT‐V, and CRT using two‐tailed t‐tests. The results were considered statistically significant at P < 0.05 for all analyses.
Independent Component Analysis (ICA)
Emerging neuroimaging studies have focused on exploring the RSFC, which detects the synchronization of inter‐regional spontaneous neuronal signals [Biswal et al., 1995] and has been shown useful for examining brain regions or networks that are related to different cognitive tasks, such as working memory [Zou et al., 2013], attention [Mennes et al., 2010, 2011]. ICA is a multivariate and data‐driven method used to identify independent spatial‐temporal patterns of coherent activity without prior knowledge about locations or activity waveforms [Beckmann et al., 2005; McKeown et al., 1997], and it is widely used to examine functional network connectivity (FNC), particularly with resting state fMRI data [Esposito et al., 2005]. ICA can provide greater sensitivity to detect subtle differences between individuals [Koch et al., 2010], and it takes into consideration the relationships between all voxels. Using this method, previous studies have consistently revealed several networks in the human brain, for example, the visual network, auditory network, sensorimotor network, anterior DMN (aDMN), posterior DMN (pDMN), and FP control network [Allen et al., 2011; Jafri et al., 2008; Liao et al., 2010; Smith et al., 2009]. Recent studies have shown that creative cognition does not depend on a single brain region or cognitive process [Dietrich and Kanso, 2010; Jung et al., 2013; Wu et al., 2015]. Thus, specific neural networks and their dynamic interplay may be crucial in creative cognition.
We identified resting state networks (RSNs) using spatial ICA as performed in the GIFT toolbox (http://mialab.mrn.org/software/gift/) [Calhoun et al., 2001]. We chose a relatively low model order ICA (number of components, C = 20), as previous fMRI studies have demonstrated that 20 independent components (ICs) yield refined components corresponding to known functional and anatomical segmentations [Abou‐Elseoud et al., 2010; Ray et al., 2013; Smith et al., 2009], thus providing a reliable and integrated representation of large‐scale networks [Calhoun et al., 2001; Zuo et al., 2010]. Each data set was reduced by methods of principal component analysis in two steps. After we performed a single ICA analysis on each subject, decomposition was estimated on concatenated datasets using the Infomax algorithm [Bell and Sejnowski, 1995]. The Infomax algorithm was repeated 100 times in Icasso with different initial values and different bootstrapped data sets, and the components were clustered to estimate the reliability of the decomposition [Himberg and Hyvarinen, 2003]. The quality of conservative clusters was quantified using the index IQ, which reflects the difference between extra‐cluster and intracluster similarity and ranges from 0 to 1. Then, single‐subject spatial maps and time courses were back‐reconstructed using the results from the data reduction step and the aggregated components [Calhoun et al., 2001; Jafri et al., 2008]. The mean spatial map of each group was transformed to Z‐scores for display.
RSN Detection
We used the DMN and FPN from previous fMRI studies [Allen et al., 2011; Biswal et al., 2010; Di and Biswal, 2015; Smith et al., 2009] as network spatial templates to classify the components. We selected RSNs corresponding to the cerebral components with the largest spatial associations with the network templates [Mantini et al., 2009; van de Ven et al., 2008, 2004]. We visually inspected all components to verify the automated component selection [Zuo et al., 2010]. This led to the identification of 4 RSNs: left and right FPN, anterior DMN (aDMN), and posterior DMN (pDMN). The ICs corresponding to four RSNs were extracted from all participants. We then entered the spatial maps of each RSN for all participants into a one‐sample t‐test in SPM8. The statistical threshold was set at P < 0.05, corrected for multiple comparisons using family wise error, forming a binary mask for further analysis (see Table 3, Fig. 1).
Table 3.
Peak foci for the group‐level lPFN, rPFN, aDMN and, pDMN defined by ICA
| Regions | Coordinate (X Y Z) MNI | No. Voxels |
|---|---|---|
| aDMN (IC 13) | ||
| mPFC | −3 54 18 | 2262 |
| PCC | −3 −51 30 | 125 |
| L angular Gyrus | −45 −69 36 | 49 |
| R angular Gyrus | 51 −60 33 | 27 |
| pDMN (IC 5) | ||
| Precuneus | −3 −72 33 | 2464 |
| Inferior Parietal Lobule | −39 −60 48 | 173 |
| lPFN (IC 6) | ||
| Inferior Frontal Gyrus | −48 −18 33 | 3404 |
| Inferior Parietal Lobule | −36 −66 39 | 2606 |
| Inferior Temporal Gyrus | −60 −45 −6 | 433 |
| Angular Gyrus | 39 −66 48 | 276 |
| MFG | 51 24 33 | 32 |
| rPFN (IC 1) | ||
| Inferior Temporal Gyrus | 63 −24 −21 | 240 |
| MFG | 39 18 51 | 2735 |
| Inferior Parietal Lobule | 45 −54 51 | 1475 |
| Middle cingulate Gyrus | 6 −36 39 | 189 |
| Angule | −42 −63 48 | 286 |
rFPN, right frontoperital network; lFPN, lift frontoperital network; aDMN, anterior default network; pDMN, posterior default network.
Figure 1.
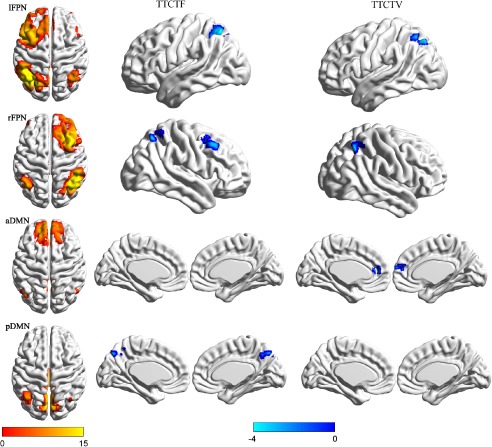
Regions in which the functional connectivity strengths within each RSN (lFPN, rFPN, aDMN, pDMN) were significantly related to creativity. Higher visual creativity was negatively correlated with decreased functional connectivity in the precuneus of the pDMN, the right middle frontal and superior parietal regions of the right FPN, and the left superior parietal region of the left FPN. Higher verbal creativity was correlated with decreased functional connectivity in the medial frontal region of the aDMN, the right superior parietal region of the right FPN, and the left superior parietal region of the left FPN. Significant clusters are shown at a statistical threshold of P < 0.05 (corrected by the AlphaSim program in REST with a combined threshold of P < 0.001 for each voxel). [Color figure can be viewed at http://wileyonlinelibrary.com]
Second‐Level Analysis of the RSNS
To identify regions where functional connectivity was significantly correlated with individual verbal creativity in DMN and PFN, we performed multiple linear regression in SPM. The verbal and visual creativity scores were considered variables of interest, while gender, age, and general IQ were used as covariates of no interest. The binary mask was used as an explicit mask in the analysis. A multiple comparison correction was performed within an ICA‐derived network using the AlphaSim program in the REST software [Yan and Zang, 2010], with individual voxel P = 0.001 and cluster‐level P < 0.05.
We examined the regions that were significantly related to individual visual creativity in the same way. To further investigate whether the exclusive regions of verbal or visual creativity were affected by the correction standard, we also examined regions at a less conservative level of P < 0.05, 200 voxels, uncorrected (see Fig. A1 in the Appendix).
IQ, as a cognitive component, may facilitate creative thought [Beaty et al., 2014b; Nusbaum and Silvia, 2011]. To determine whether the effect is specific to “creativity” or to “cognition” writ large, multiple regression was used to investigate the correlation between regions of RSN and individual IQ, controlling for possible confounding variables (creativity, gender, FD, and age).
Association between Regions–Networks Connectivity and Creativity Scores
To test whether the association between ROIs and functional networks was related to visual creativity scores, we calculated the association between ROIs that were significantly correlated with TTCT‐F and the functional network. First, the representative mean time series was computed by averaging the time series of voxels in this ROI and these networks. Then, we calculated the Pearson correlation coefficient between each ROI and every other network, averaging the time course for each subject. The correlation coefficients were standardized using Fisher's r‐to‐z transformation, allowing further correlation analysis. Then, to examine whether the correlation between ROIs and FNC was related to visual creativity, we correlated the TTCT‐F scores with associations between ROIs and FNC, regressing out age, IQ, gender, and mean FD. We also examined whether correlation between ROI and the functional network was related to verbal creativity. Correction for multiple comparisons was performed using the false discovery rate (FDR) for the final results.
FNC in Relation to Creativity Scores and General IQ
We conducted FNC analysis using the Pearson correlation between each and every other summary time courses. This allowed us to assess whether the effects of specific network hubs and functional networks on creativity were consistent with the FNC analysis. The results consisted of an FNC matrix with dimensions of 4 times 4 times 282 (subjects). The correlation coefficients were standardized using Fisher's r‐to‐z transformation, allowing further correlation analysis. We then correlated creativity scores (TTCT‐F scores and TTCT‐V scores) with FNC separately. Multiple comparisons were performed using the FDR for the final results.
To determine whether the effect is specific to “creativity” or to “cognition” writ large, we further correlated the general IQ scores with FNC in the same way. Multiple comparisons were performed using the FDR for the final results.
Mediation Analysis
Mediation analysis was then employed to explore whether the FPN mediated the relationship between the DMN and both verbal and visual creativity, using an INDIRECT macro implemented in SPSS [Preacher and Hayes, 2008] This macro utilized bootstrapped sampling (10,000 bootstrapped samples) and bias‐corrected 95% bootstrap confidence intervals (CI). Note that if the CIs do not include zero, there is a significant indirect effect of the independent variable on the dependent variable through the mediators [Preacher and Hayes, 2008].
We began by examining whether the FPN mediated the relationship between the DMN and visual creativity. The proposed mediators included the mean z values within the bilateral FPN, which were correlated with the verbal creativity scores. Moreover, the mean z values of the precuneus were added as an independent variable, and the figural creativity scores were added as a dependent variable in the model; IQ, age, and gender were modelled as covariates of no interest.
Next, we examined whether the FPN mediated the relationship between the DMN and verbal creativity. The proposed mediators again included the mean z values within the bilateral FPN, which were correlated with verbal creativity scores. Moreover, the mean z values of the mPFC were added as an independent variable, and the verbal creativity scores were added as a dependent variable in the model; IQ, age, and gender were modelled as covariates of no interest.
RESULTS
Behavioral Results
Table 1 shows the mean and SD for age and the CRT, TTCT‐FTTCT‐F, and TTCFV scores of the females and males in our sample. For the TTCT‐FTTCT‐F, the correlations among flexibility, originality, fluency, and the total TTCT score were high (r > 0.93, P > 0.001). For the TTCT‐FTTCT‐F, the correlations among flexibility, originality, fluency, and the total TTCT score were similarly high (r > 0.88, P > 0.001). No statistically significant difference was found between females and males on the TTCT‐FTTCT‐F (P = 0.289) or the CRT (P = 0.564). However, a significant difference between females and males was found on the TTCT‐V (P = 0.039, see Table 1).
Table 1.
Descriptive statistics of behavioral measures (n = 282)
| Males | Females | |||
|---|---|---|---|---|
| Means | SD | means | SD | |
| Age | 20.23 | 1.30 | 19.75 | 1.17 |
| CRT | 66.02 | 3.40 | 66.25 | 3.50 |
| TTCTF | 64.87 | 17.06 | 67.18 | 19.18 |
| TTCTV | 126.14 | 39.47 | 135.78 | 38.58 |
Note: n, number; SD, standard deviation; TTCTF, figural torrance tests of creative thinking; TTCTV, verbal torrance tests of creative thinking; CRT, combined Raven's test.
Visual Creativity Correlations with Functional Networks
Multiple regression was used to investigate the correlation between regions of RSNs and individual visual creativity, controlling for possible confounding variables (IQ, gender, and age). The regression analysis showed that the TTCT‐F score was significantly and negatively associated with functional connectivity in the precuneus of the posterior DMN, the left superior parietal region of the left FPN, and the right superior parietal and MFG of the right FPN (see Table 2, Fig. 1). To eliminate the residual effects of head motion in this analysis, we added FD as a nuisance covariate. After controlling for sex, age, FD, and IQ, the regression analysis showed that the statistical values and coordinates of the peak voxel did not change.
Table 2.
Regions in which functional connectivity strengths within each RSN were significantly related to creativity
| Regions | Coordinate (X Y Z) MNI | Voxels | Z Value | |
|---|---|---|---|---|
| rFPN | ||||
| TTCTF | SPL | 39 −57 66 | 49 | −4.02 |
| MFC | 48 15 48 | 9 | −3.52 | |
| TTCTV | SPL | 33 −66 57 | 37 | −4.21 |
| lFPN | ||||
| TTCTF | SPL | −27 −66 54 | 16 | −3.79 |
| MFC | −36 18 36 | 29 | −4.07 | |
| TTCTV | SPL | −24 −72 51 | 26 | −3.75 |
| pDMN | ||||
| TTCTF | precuneus | 9 −75 45 | 17 | −3.62 |
| TTCTV | ____ | |||
| aDMN | ||||
| TTCTF | ____ | |||
| TTCTV | mPFC | −3 45 15 | 9 | −3.66 |
MFC indicates middle frontal cortex; SPL, Superior parietal; mPFC, medial prefrontal cortex; rFPN, right frontoperital network; lFPN, lift frontoperital network; aDMN, anterior default network; pDMN, posterior default network.
Correlation analysis revealed that the figural creativity scores were significantly and negatively correlated with mean Z values of the right MFG (r = −0.212, P < 0.001), left SPL (r = −0.256, P < 0.001), right SPL (r = −0.245, P < 0.001), and precuneus (r = −0.194, P = 0.002). Verbal creativity scores were significantly and negatively correlated with mean Z values of the left SPL (r = −0.181, P = 0.002) and the right SPL (r = 0.202, P =0.001) but not significantly correlated with the mean Z values of the right MFC (r = −0.055, P = 0.356) or the precuneus (r = −0.009, P = 0.879). Thus, the right MFG and precuneus may be distinct neural correlates for visual creativity.
Verbal Creativity Correlations with Functional Networks
Multiple regression was used to investigate regions where functional connectivity was significantly related to individual verbal creativity, controlling for possible confounding variables (IQ, gender, and age). The regression analysis showed that verbal creativity was significantly and negatively associated with functional connectivity in the anterior DMN, left FPN, and right FPN. The hubs included the left SPL in the left FPN, the right SPL in the right FPN, and the medial frontal cortex in the anterior DMN (see Table 2, Fig. 1). To assess the residual effects of head motion, we added FD as a nuisance covariate. After controlling for sex, age, FD, and IQ, the regression analysis showed that the statistical values and coordinates of the peak voxel did not change.
Correlation analysis revealed that verbal creativity scores were significantly and negatively correlated with mean Z values of the left superior parietal (r = −0.225, P < 0.001) and right superior parietal (r = −0.244, P < 0.001) regions of the FPN and negatively correlated with the medial frontal region (r = −0.214, P = 0.001) of the aDMN. Visual creativity scores were also negatively correlated with mean Z values within the left superior parietal (r = −0.119, P = 0.047) and right superior parietal (r = −0.189, P = 0.001) regions within the left and right FPNs, respectively, but not significantly correlated with the mean Z values within the medial frontal region of the aDMN (r = −0.090, P = 0.133). This analysis therefore identified common (left and right superior parietal regions in the FPN) and distinct (precuneus in the pDMN, medial frontal region in the aDMN) brain regions for visual and verbal creativity.
To further examine this result, a correlation coefficient difference test was conducted. We found that the mean Z values of the left and right superior parietal regions and the visual creativity scores were not significantly different from the figural creativity scores (t < 1.645). However, the association between the mean Z values of the precuneus, MFG, and visual creativity was significantly greater than with verbal creativity (t = 2.49 > 1.645; t = 2.38 > 1.645), and the association between the mean Z values of the medial frontal and verbal creativity was significantly greater than with visual creativity (t = 1.73 > 1.645).
To test the stability and reliability of the results, we repeated the analysis using half of the subjects, selected randomly. After controlling for age, gender, IQ, and FD, the regression analysis revealed that the coordinates and statistical values of the peak voxel in the precuneus of the posterior DMN, the left inferior parietal region of left FPN, and the right inferior parietal and MFG of the right FPN correlated with visual creativity changes as x, y, z = −3 −78 42 (214 voxels); −24, −60, 57 (56 voxels); 42, −54, 63 (27 voxels, a threshold of P < 0.005, uncorrected, and a minimum cluster size of 15 contiguous voxels). The coordinates and statistical values of the peak voxel in the left SPL in the left FPN, the right SPL in the right FPN, and the medial frontal cortex in the anterior DMN correlated with visual creativity changes as x, y, z = −27 −78 48 (40 voxels); 33 −66 57 (25 voxels); −3 42 15 (16 voxels). The findings using data from only half of the subjects were similar to those using data from all participants, which may indicate the stability of the results.
General IQ Correlations with Functional Networks
We further explored the correlation between RSN regions and individual IQ, controlling for possible confounding variables (creativity, gender, FD, and age). No significant correlation between RSN regions and individual IQ scores was found.
Association between Regions‐Networks Connectivity and Creativity Scores
We then assessed the effects of association between ROIs and functional networks on both visual and verbal creativity. The visual creativity results revealed significant and positive associations between figural creativity scores and the right superior parietal and left FPN, the left superior parietal region and the pDMN, the right MFG and the aDMN, and the precuneus and the left FPN [q(FDR) = 0.05]. The verbal creativity results revealed positive and marginally significant associations between verbal creativity scores and the right superior parietal and left FPN (P = 0.061) and the medial frontal and right FPN (P = 0.062; see Table 4, Fig. 2).
Table 4.
Association Between ROI and networks and TTCT Scores, and Between FNC and the TTCT Scores
| TTCTF Brain region ∼ network pair | r | P | TTCTV Brain region ∼ network pair | r | P |
|---|---|---|---|---|---|
| rMFC ∼ pDMN | 0.044 | 0.465 | mPFC ∼ pDMN | 0.091 | 0.131 |
| rMFC ∼ lFPN | 0.098 | 0.102 | mPFC ∼ lFPN | 0.061 | 0.312 |
| rMFC ∼ aDMN | 0.154 | 0.010 | mPFC ∼ rFPN | 0.112 | 0.062 |
| rIPL ∼ pDMN | 0.123 | 0.040 | rIPL ∼ pDMN | 0.065 | 0.277 |
| rIPL ∼ aDMN | −0.009 | 0.883 | rIPL ∼ aDMN | 0.058 | 0.337 |
| precuneus ∼ rFPN | −0.054 | 0.369 | – | ||
| precuneus ∼ lFPN | 0.166 | 0.018 | – | ||
| precuneus ∼ aDMN | 0.018 | 0.765 | – | ||
| lIPL ∼ rFPN | −0.021 | 0.726 | lIPL ∼ rFPN | −0.047 | 0.434 |
| lIPL ∼ pDMN | 0.128 | 0.033 | lIPL ∼ pDMN | 0.080 | 0.183 |
| lIPL ∼ aDMN | 0.020 | 0.738 | lIPL ∼ aDMN | 0.044 | 0.463 |
| Network ∼ network pair | Network ∼ network pair | ||||
| rFPN ∼ pDMN | −0.045 | 0.456 | rFPN ∼ pDMN | −0.015 | 0.806 |
| rFPN ∼ pDMN | 0.137 | 0.022 | rFPN ∼ pDMN | 0.111 | 0.063 |
| rFPN ∼ aDMN | 0.021 | 0.726 | rFPN ∼ aDMN | 0.129 | 0.032 |
| lFPN ∼ pDMN | 0.194* | 0.001 | lFPN ∼ pDMN | 0.066 | 0.270 |
| lPFN ∼ aDMN | 0.065 | 0.280 | lPFN ∼ aDMN | −0.006 | 0.915 |
| aDMN ∼ pDMN | 0.022 | 0.713 | aDMN ∼ pDMN | 0.006 | 0.918 |
*P < 0.01 (q[FDR] = 0.05); MFC indicates middle frontal cortex; r, right; l, left; IPL, inferior parietal; mPFC, medial prefrontal cortex; rFPN, right frontoperital network; lFPN, left frontoperital network; aDMN, anterior default network; pDMN, posterior default network.
Figure 2.
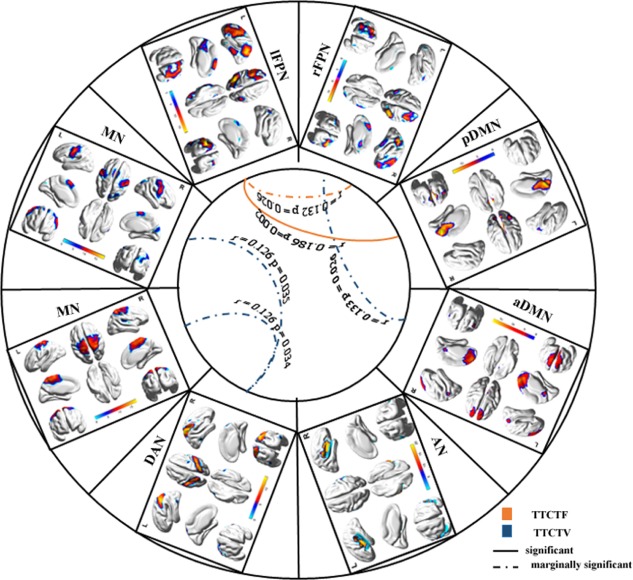
Association between FNC and the creativity scores. Regarding visual creativity, significant and positive associations were found between figural creativity scores and functional connectivity between the left FPN and pDMN (P = 0.002, q[FDR] = 0.05), and marginally significant associations and functional connectivity were found between the right and left FPN (P = 0.026). Regarding verbal creativity, positive and marginally significant associations between verbal creativity scores and functional connectivity between the right FPN and aDMN and between the DAN and MN was found. [Color figure can be viewed at http://wileyonlinelibrary.com]
FNC in Relation to Creativity Scores and General IQ
We examined the effects of the DMN and FPN on visual and verbal creativity. The visual creativity results showed significant and positive associations between figural creativity scores and functional connectivity between the right and left FPN (P < 0.05) and between the left FPN and the pDMN (P = 0.002, q[FDR] = 0.05). The verbal creativity results showed positive and marginally significant associations between verbal creativity scores and functional connectivity between the right FPN and the aDMN (see Table 4).
Considering that many other ICs were extracted by ICA (see Fig. 2), we explored whether and how some other components out of the DMN and the FPN are related to verbal and visual creativity. Multiple regression was used to investigate the correlation between the regions of all the RSNs and visual creativity, controlling for possible confounding variables (IQ, gender, and age). A multiple comparison correction was performed within an ICA‐derived network using the AlphaSim program in the REST software [Yan and Zang, 2010], with individual voxel P = 0.001 and cluster‐level P < 0.05.
The regression analysis showed that the TTCT‐F score was significantly and negatively associated with functional connectivity in the inferior parietal region of the DAN. There were no significant associations in the other networks. We examined the regions that were significantly related to individual verbal creativity in the same way. No significant associations between verbal creativity scores and functional connectivity of these RSNs was found. To eliminate the residual effects of head motion in this analysis, we added FD as a nuisance covariate. After controlling for sex, age, FD, and IQ, the regression analysis showed that the statistical values and coordinates of the peak voxel did not change.
Next, we examined the effects of FNC on visual and verbal creativity. The visual creativity results showed no significant associations between figural creativity scores and functional connectivity among these components. The verbal creativity results showed positive and marginally significant associations between verbal creativity scores and functional connectivity between the DAN and MN (see Fig. 2).
The effects of FNC on general IQ showed no significant associations between IQ scores and FNC among the FPN and DMN.
Mediation Analysis
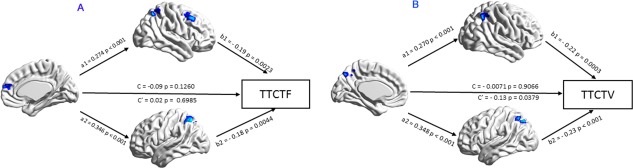
We examined whether the FPN mediated the relationship between the DMN and visual creativity. The results showed that the direct effect (c′ = −0.11) was not significant (P = 0.08), but we found two significant indirect effects (see Fig. 3). The first indirect effect of the precuneus on the figural creativity scores through the right FPN (superior parietal) was the product of a1 = 0.27 and b1 = −0.19, a1*b1 = −0.05 [CI: −0.10, −0.02]. The next indirect effect of the precuneus on the figural creativity scores through the right superior parietal (−0.06) was also significant [CI: −0.11, −0.01], and the total indirect effect (−0.11) was statistically significant [CI: −0.17, −0.06]. The FPN mediated the relationship between pDMN and visual creativity.
Figure 3.
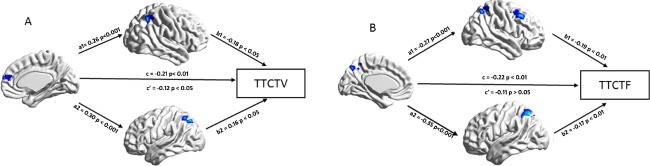
Mediation analysis: (A) The FPN mediated the relationship between the aDMN and verbal creativity. B: The FPN mediated the relationship between the pDMN and visual creativity. [Color figure can be viewed at http://wileyonlinelibrary.com]
Next, we examined whether the FPN mediated the relationship between the DMN and verbal creativity. The results showed that the direct effect (c′ = 0.12) was significant (P = 0.045), and we again found two significant indirect effects (see Fig. 3). The first indirect effect of the mPFC on verbal creativity scores through the right superior parietal region was the product of a1 = 0.26 and b1 = −0.18, a1*b1 = −0.046 [CI: −0.09, −0.02]. The next indirect effect (−0.046) of the mPFC on verbal creativity scores through the left PFN (superior parietal and MFG) was significant [−0.9, −0.02], and the total indirect effect (−0.09) was statistically significant [−0.15 −0.05]. The FPN mediated the relationship between aDMN and verbal creativity. To further investigate whether visual and verbal creativity recruit specialized brain networks, we ran two additional mediation models (see Fig. A2 in the Appendix).
DISCUSSION
The present study explored the relation between creative capacity and RSFC within the default and FP control networks using ICA. Our results showed that decreased functional connectivity within common regions of the FPN was associated with both visual creativity and verbal creativity, and the strength of connectivity between the FPN and the DMN was positively related to both creative domains. Furthermore, the FPN mediated the relation between the DMN and creative ability. In addition, there were specialized hubs and interactive systems for verbal and visual creativity. Higher visual creativity was related to decreased functional connectivity in the precuneus and MFC of the pDMN, while higher verbal creativity was related to decreased functional connectivity in the mPFC of the aDMN. Mediation analysis revealed that the FPN mediated the relationship between the aDMN and verbal creativity. It also mediated the relationship between the pDMN and visual creativity. Our results extend previous research by revealing how the DMN and FPN cooperate to contribute to creative cognitive ability during the resting state.
We found that decreased functional connectivity in common regions of the FPN (bilateral SPL) was related to both visual and verbal creativity. Previous studies have suggested that the SPL plays an important role in multimodal information processing as well as a wide range of other functions, including attention to action, monitoring in working memory, response selection, and suppression of irrelevant information [Booth et al., 2002; d'Esposito et al., 1998; Niendam et al., 2012]—cognitive processes that are central to both verbal and visual creativity [Dietrich, 2004]. Notably, the SPL has been implicated in studies of visual creativity. For example, in a study comparing brain activity during creative versus uncreative visual creativity, Aziz‐Zadeh et al. [2013] reported greater activation of the SPL. The SPL has also shown increased involvement during a creative drawing task [Ellamil et al., 2012]. Studies of verbal creativity have also implicated the SPL, including DT [Gansler et al., 2011] and “brainstorming” during creative writing [Shah et al., 2013].
The findings provide direct evidence for the cooperative role of the default mode and control networks in creative cognition. The DMN is related to spontaneous and self‐generated thought, such as mental simulation, mind‐wandering, social cognition, and autobiographical retrieval [Andrews‐Hanna, 2012; Christoff et al., 2009; Hassabis and Maguire, 2007; Schacter et al., 2012]. Such “self‐generated thought” has previously been associated with creative cognition [e.g., mind‐wandering, Baird et al., 2012]. Self‐generated thought involves the spontaneous integration of previously unassociated information [Baird et al., 2012; Perkins et al., 2015], which could lead to more available mental elements for creative generation. Carson and coworkers [2003] required subjects to disregard inconsequential information using latent inhibition tests and found that highly creative individuals were less likely to screen out irrelevant information and focus on the task. Further evidence suggests that creative individuals show an increased propensity to mind‐wander during cognitive tasks [Perkins et al., 2015].
Notably, mind‐wandering has been characterized as the antithesis of executive control processing [Kane et al., 2007]. However, recent evidence has demonstrated a critical role of cognitive control factors in creative thought, such as fluid IQ [Beaty et al., 2014b; Benedek et al., 2014], working memory capacity [De Dreu et al., 2012; Lee and Therriault, 2013], verbal fluency [Silvia et al., 2013], and attentional flexibility [Zabelina and Robinson, 2010]. Such executive functions are thought to support creative cognition by providing the cognitive control needed to inhibit salient but irrelevant information and to manage complex search processes [Beaty et al., 2015]. In the absence of such control, DT can be compromised by an inability to effectively overcome prepotent response tendencies [Gilhooly et al., 2007]. Accordingly, highly creative individuals may be characterized by both enhanced idea generation and evaluation abilities.
Cooperation of the default and control networks has been shown to be important for goal‐directed, self‐generated thought, including autobiographical future planning and even mind‐wandering [Fox et al., 2015; Spreng et al., 2015]. Such processes appear to involve the top‐down modulation of self‐generated information. Considered within the context of this study, the DMN may be associated with the process of idea generation, in light of its role in self‐generated cognition, while the control network may be associated with evaluating the efficacy of ideas and modifying them to meet the constraints of task‐specific goals. Thus, DMN and FPN coupling may reflect the ability to exert top‐down control over the process of idea generation. Increased connectivity between FPN and DMN may correspond to a greater ability of creative individuals to evaluate and revise self‐generated ideas by inhibiting salient task‐irrelevant information and selecting goal‐congruent ideas among a wide range of competing alternatives [Beaty et al., 2015].
Our results are consistent with several recent studies on the role of brain networks in creative cognition. One such study found that highly creative individuals exhibited greater cooperation between regions associated with the control network and the DMN [Beaty et al., 2014a,b]. Other work examining functional connectivity during DT reported enhanced connectivity between the ACC within the control network and the occipital‐temporal area within the DMN [Mayseless et al., 2015]. In a similar vein, Pinho et al. [2016] asked professional pianists to improvise following one of two different experiment conditions during fMRI: expressing certain emotional content or using specific piano keys (i.e., “pitch‐sets”). In the pitch‐set condition, the DLPFC showed increased coupling with the bilateral dorsal promotor and the supplementary motor area. In the emotional condition, in contrast, the DLPFC showed increased coupling with several regions associated with the DMN, including the mPFC [Pinho et al., 2016]. Such work provides further support for the cooperative role of the default and control networks across a range of creative tasks and domains.
Our findings are also consistent with the “two stage” model as well as the BVSR theory of creativity. Regarding the “two stage” theory, a recent study on creative drawing showed differential contributions of default and executive control networks during different stages of the drawing process [Ellamil et al., 2012]. Creative generation was related to greater recruitment of regions within the DMN, and creative evaluation was related to greater recruitment of regions within the executive control network. Regarding the BVSR theory, blind variation appears to involve spontaneous idea generation processes that may occur in the DMN, whereas selective retention may recruit cognitive control processes in the control network [Jung et al., 2013]. In a similar vein, Beaty et al. suggested that the DMN contributes to the spontaneous generation of candidate ideas, while the cognitive control network monitors, directs, and evaluates ideas stemming from the DMN [Beaty et al., 2015]. Our results extend such work by revealing how the default and control networks cooperate to support creative thought.
In addition, our results showed specialized hubs and interactive mechanisms for verbal and visual creativity. In terms of cognitive processes, the generation of novel mental images may involve the retrieval of existing representations from memory and the establishment of new connections by transforming and synthesizing these existing memory units. The precuneus, as a core hub of the pDMN, may support highly integrated and complex behavioral functions, including the processing of visual‐spatial information [Selemon and Goldman‐Rakic, 1988], retrieval from episodic memory [Shallice et al., 1994], and mental imagery [Burgess, 2008; Hassabis and Maguire, 2007]—processes that are essential to visual creativity. Moreover, the right MFC is important for visual thinking [Prabhakaran et al., 1997], object representation [Takahama et al., 2010], and mental rotation [Gauthier et al., 2002], which may account for its involvement in visual creativity. This notion is supported by Huang et al. [2013], who examined brain mechanisms underlying visual creativity using fMRI and reported decreased activity in the right middle MFC [cf., Hassabis and Maguire, 2007].
Past research suggests that verbal creativity involves the retrieval of existing concepts from semantic memory and the establishment of new connections among these existing concepts. The mPFC, a core region of the aDMN, supports the integration of linguistic information [Liu et al., 2015] and is involved in a wide range of functions, including inhibitory control, response selection, spontaneous counterfactual thinking, and conflict processing [Botvinick et al., 1999; Crottaz‐Herbette and Menon, 2006; Kray et al., 2006; van Veen and Carter, 2005]—processes that are central to verbal creativity. Furthermore, previous research suggests that verbal creativity tasks frequently induce activation of the mPFC, such as verbal insight problems solving [Jung‐Beeman et al., 2004] and creative story generation [Howard‐Jones et al., 2005]. Recent work has also reported differential coupling of the mPFC with other brain regions associated with individual differences in creativity measured by the verbal TTCT [Wei et al., 2014] and the S‐A creativity test [Takeuchi et al., 2012]. Taken together, such findings are consistent with these results and point to a central role of the default and control networks in creative cognition.
Finally, our results point to interesting patterns of within‐ and between‐network connectivity in the creative brain. Specifically, we found that verbal and visual creativity were negatively correlated with functional connectivity within the DMN and FPN. On the other hand, creative ability was associated with increased between‐network connectivity of the DMN and FPN. We suspect that decreased within‐network connectivity of the FPN and DMN may allow for flexible between‐network connectivity, allowing the FPN and DMN to more easily couple with each other (see more empirical evidence in the Supporting Information material). This finding may provide key insights into how brain networks that typically work in opposition come to cooperate to support complex cognitive processes. Future research should further explore the extent to which other creative thought processes involve similar patterns of within‐ and between‐network connectivity.
However, because our conclusions stem from resting‐state data, the causal relation among the DMN, FPN, and creative thinking ability should be further explored in future research. Subsequent studies should employ event‐related designs to elucidate the complex network dynamics underlying creative cognition.
CONCLUSION
The present study demonstrated common and distinct brain network contributions to visual and verbal creativity. Higher visual and verbal creativity was related to decreased functional connectivity in the bilateral SPL of the FPN. We also provided direct evidence for the notion that creative cognition benefits from the interaction of the default mode and control networks. Our analysis revealed that the FPN mediated the relationship between the DMN and both verbal and visual creative ability, but specialized hubs and interactive systems were also observed for each domain. Higher visual creativity was related to decreased functional connectivity within the precuneus cortex and the MFC of the pDMN, whereas higher verbal creativity was related to decreased connectivity within the mPFC of the aDMN. Furthermore, the control network mediated the relationship between the aDMN and verbal creativity, and it also mediated the relationship between the pDMN and visual creativity. Together, these results extend prior research by revealing how the default and control networks cooperate to support creative thought.
COMPETING INTERESTS
The authors declare that they have no competing interests.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to all study participants for their contributions.
APPENDIX A.
COMMON AND DISTINCT BRAIN NETWORKS UNDERLYING VERBAL AND VISUAL CREATIVITY
Mediation Analysis
To further investigate whether visual and verbal creativity recruit specialized brain networks, we reran another two mediation models with age, gender, and IQ as covariates. Proposed mediators again included the mean z values within bilateral FPN, which were correlated with verbal creativity scores. The figural creativity scores were added as dependent variable. However, the mean z values of the mPFC was added as an independent variable in the model, Next, Proposed mediators again included the mean z values within the bilateral FPN, which were correlated with verbal creativity scores. The verbal creativity scores were added as dependent variable in the model. However, the mean z values of the precuneus was added as an independent variable, and IQ, age, and gender were modeled as covariates of no interest.
Figure AI.
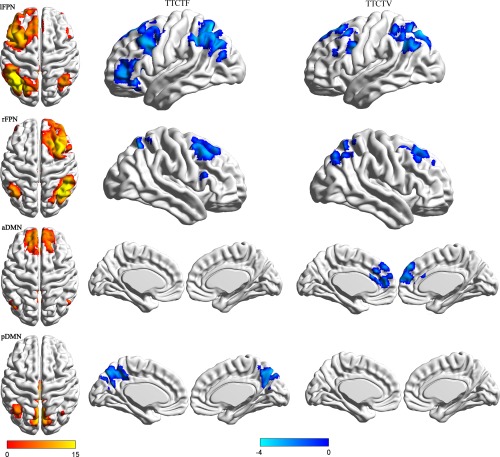
Regions in which functional connectivity strengths within each RSN (lFPN, rFPN, aDMN, pDMN) were significantly related to creativity. Higher visual creativity was negatively correlated with decreased functional connectivity in the precuneus of the pDMN, right middle frontal and inferior parietal of the right FPN, and left inferior parietal, superior frontal gyrus (SFG), IFG, and DLPFC of the left FPN. Higher verbal creativity was correlated with decreased functional connectivity in the medial frontal of the aDMN, right inferior parietal, and DLPFC of the right FPN, and left inferior parietal and superior frontal gyrus of the left FPN. We examined regions at a less conservative level of P < 0.05, 200 voxels, uncorrected. [Color figure can be viewed at http://wileyonlinelibrary.com]
Because results showed the independent variable was not significantly correlated with the dependent variable in the two models above, there was reason to suspect suppression effects in the models. According to model suppression theories [MacKinnon et al., 2000; McFatter 1979], one reason for the nonsignificant effects of the aDMN on figural creativity and the pDMN on verbal creativity may be the suppressing effect of the FPN (see Fig. A2).
Figure A2.
Mediation analysis: (A) and (B) show the suppressing effects of the FPN in the model, which may account for the nonsignificant correlation of the aDMN with visual and the nonsignificant correlation of the pDMN with verbal creativity scores TTCTF. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table AI.
Regions in which functional connectivity strengths within each RSN were significantly related to creativity
| Regions | Coordinate (X Y Z) MNI | Voxels | Z Value | |
|---|---|---|---|---|
| rFPN | ||||
| Figure | MFC | 48 15 48 | 422 | −3.68 |
| IPL | 39 −57 66 | 211 | −3.72 | |
| Verbal | DLPFC | 45 48 27 | 307 | −3.09 |
| IPL | 33 −66 57 | 313 | −4.21 | |
| lFPN | ||||
| Figure | DLPFC | −39 18 36 | 354 | −3.95 |
| IPL | −27 −66 54 | 823 | −4.10 | |
| IFG | −36 27 29 | 326 | −3.83 | |
| SFG | −6 27 48 | 254 | −3.24 | |
| Verbal | IPL | −24 −72 51 | 558 | −3.75 |
| SFG | −3 24 48 | 491 | −3.36 | |
| pDMN | ||||
| Figure | precuneus | −3 −72 45 | 551 | −3.31 |
| Verbal | ____ | |||
| aDMN | ||||
| Figure | ____ | |||
| Verbal | mPFC | −3 45 15 | 338 | −3.66 |
MFC indicates middle frontal cortex; IPL, inferior parietal; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; mPFC, medial prefrontal cortex; rFPN, right frontoperital network; lFPN, left frontoperital network; aDMN, anterior default network; pDMN, posterior default network; P < 0.05, 200 voxels, uncorrected.
APPENDIX B.
CONNECTIVITY ANALYSIS
Preprocessing of Connectivity Analysis
The processing of resting‐state functional MRI data was performed using the DPARSF (http://resting-fmri.sourceforge.net/) [Yan and Zang, 2010] based on SPM8. First, the first 10 volumes from each subject's functional imaging data were discarded to account for steady‐state magnetization. The remaining 232 volumes were included in the subsequent analysis. Second, slice timing correction was used to correct slice order effects, and head motion correction was used to correct head movement artifacts, respectively. Twenty‐seven subjects, who exhibited head motion of 2 mm maximum displacement and 2° rotation throughout the course of scans, were discarded. Third, each participant's functional image was spatially normalized to the standard MNI template with a resampled voxel size of 3 × 3 × 3 m3. The data was then smoothed with an isotropic 8 mm full‐width at half maximum Gaussian kernel. Then, the linear trend and a band‐pass filter (0.01–0.08 HZ) was performed to reduce low‐frequency drift and high‐frequency noise [Biswal et al., 1995]. Finally, the nuisance signals (cerebrospinal fluid, white matter, head‐motion profiles, and global signal) were regressed out to remove the impact of those physiological artifacts.
CONNECTIVITY ANALYSIS
To further explore the relationship between intra‐ and inter‐network connectivity, we conducted connectivity analysis using the seed regions selected based on a study by [Dosenbach et al., 2010]. Fifty‐five spherical (3 mm radius) regions of interest (ROI) represented the DMN and the FPN (see Table AII). The representing mean time series was computed by averaging the time series of voxels in this ROI. We conducted intranetwork and internetwork connectivity using the Pearson correlation between each pair of ROIs for each subject. The correlation coefficients were standardized using Fisher's r‐to‐ transformation, increasing the normality of the distribution and allowing further correlation analysis. For each of the two RSNs, the intranetwork strength was calculated as the mean connection strength of all ROIs in the same network. The internetwork connectivity was calculated as the mean connection strength between each ROI of a network and all of ROIs of the other network. And we explored the relationship between the intranetwork and internetwork connectivity using the Pearson correlation. The result showed that reduced intraconnectivity in the DMN and FPN was significantly correlated with increased interconnectivity of the DMN and FPN(r = −0.449, P = 0.000; r = −0.284, P = 0.000), which provide more evidence to support the idea that the reduced intranetwork connectivity within the DMN and FPN may allow for more flexible internetwork connectivity in the highly creative brain.
Previous studies showed the effect of local overconnectivity would positively stabilize and reinforce local physical connections while is coupled with long‐range underconnectivity [Belmonte et al., 2004; Cerliani et al., 2015; Courchesne and Pierce, 2005]. Accordingly, at the network level, the effect of connectivity within networks negatively affect the development of efficient connections between networks [Cerliani et al., 2015; Rudie et al., 2012; Shih et al., 2011]. In this study, the increased connectivity between DMN and FPN may mean increasing synchrony in the activity of the two networks and more effective information transfer between the two networks.
Table AII.
This set of ROIs are from Dosenbach et al. [2010], Science
| Regions | MNI coordinates (x y z) | Subnetwork | Regions | MNI coordinates (x y z) | Subnetwork |
|---|---|---|---|---|---|
| vmPFC | 6 64 3 | Default | precuneus | 11 −68 42 | Default |
| mPFC | 0 54 32 | Default | IPS | −36 −69 40 | Default |
| aPFC | −25 51 27 | Default | Occipital | −9 −72 41 | Default |
| vmPFC | 9 51 16 | Default | Occipital | 45 −72 29 | Default |
| vmPFC | −6 50 −1 | Default | Occipital | −2 −75 32 | Default |
| vmPFC | −11 45 17 | Default | Occipital | −42 −76 26 | Default |
| vmPFC | 8 42 −5 | Default | aPFC | 29 57 18 | FPN |
| ACC | 9 39 20 | Default | aPFC | −29 57 10 | FPN |
| vlPFC | 46 39 −15 | Default | vent aPFC | 42 48 −3 | FPN |
| SFG | 23 33 47 | Default | Vent aPFC | −43 47 2 | FPN |
| SFG | −16 29 54 | Default | vlPFC | 39 42 16 | FPN |
| ITG | 52 −15 −13 | Default | dlPFC | 40 36 29 | FPN |
| ITG | −59 −25 −15 | Default | ACC | −1 28 40 | FPN |
| Post cingulate | 1 −26 31 | Default | dlPFC | 46 28 31 | FPN |
| Fusiform | 28 −37 −15 | Default | vPFC | −52 28 17 | FPN |
| Precuneus | −3 −38 45 | Default | dlPFC | −44 27 33 | FPN |
| post cingulate | −8 −41 3 | Default | dFC | 40 17 40 | FPN |
| ITG | −61 −41 −2 | Default | dFC | 44 8 34 | FPN |
| Occipital | −28 −42 −11 | Default | dFC | −42 7 36 | FPN |
| Post cingulate | −5 −43 25 | Default | IPL | −41 −40 42 | FPN |
| Precuneus | 9 −43 25 | Default | IPL | 54 −44 43 | FPN |
| Precuneus | 5 −50 33 | Default | post parietal | −35 −46 48 | FPN |
| Post cingulate | −5 −52 17 | Default | IPL | −48 −47 49 | FPN |
| Post cingulate | 10 −55 17 | Default | IPL | −53 −50 39 | FPN |
| precuneus | −6 −56 29 | Default | IPL | 44 −52 47 | FPN |
| Post cingulate | −11 −58 17 | Default | IPS | −32 −58 46 | FPN |
| AG | 51 −59 34 | Default | IPS | 32 −59 41 | FPN |
| AG | −48 −63 35 | Default |
vmPFC, ventromedial prefrontal cortex; mPFC, medial prefrontal cortex; aPFC, anterior prefrontal cortex; ACC, Anterior cingulate cortex; vlPFC, ventromedial prefrontal cortex; SFG, superior frontal gyrus; ITG, inferior temporal gyrus; AG, Angular gyrus; IPS, Intraparietal sulcus; dlPFC, dorsolateral prefrontal cortex; dFC, dorsal frontal cortex; IPL, inferior parietal lobule.
Wenfeng Zhu and Qunlin Chen contributed equally to this article.
Contributor Information
Guikang Cao, Email: cgk@swu.edu.cn.
Jiang Qiu, Email: qiuj318@swu.edu.cn.
REFERENCES
- Abou‐Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V (2010): The effect of model order selection in group PICA. Hum Brain Mapp 31:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R (2011): A baseline for the multivariate comparison of resting‐state networks. Front Syst Neurosci 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR (2012): The brain's default network and its adaptive role in internal mentation. Neuroscientist 18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden R, Chavez RS, Grazioplene R, Jung RE (2010): Neuroimaging creativity: A psychometric view. Behav Brain Res 214:143–156. [DOI] [PubMed] [Google Scholar]
- Aziz‐Zadeh L, Liew SL, Dandekar F (2013): Exploring the neural correlates of visual creativity. Soc Cogn Affect Neurosci 8:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Mrazek MD, Kam JW, Franklin MS, Schooler JW (2012): Inspired by distraction mind wandering facilitates creative incubation. Psychol Sci 10:1117–1122. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Wilkins RW, Jauk E, Fink A, Silvia PJ, Hodges DA, Koschutnig K, Neubauer AC (2014a): Creativity and the default network: a functional connectivity analysis of the creative brain at rest. Neuropsychologia 64:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Silvia PJ, Nusbaum EC, Jauk E, Benedek M (2014b): The roles of associative and executive processes in creative cognition. Mem Cognit 42:1186–1197. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Kaufman SB, Silvia PJ (2015): Default and executive network coupling supports creative idea production. Sci Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, Schacter DL (2016): Creative cognition and brain network dynamics. Trends Cogn Sci 20:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B: Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159. [DOI] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Sommer M, Arendasy M, Neubauer AC (2014): Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence 46:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Csíkszentmihályi M, Ullén F (2014): Cortical regions involved in the generation of musical structures during improvisation in pianists. J Cogn Neurosci 5:830–842. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar mri. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S (2010): Toward discovery science of human brain function. Proc Natl Acad Sci 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2002): Functional anatomy of intra‐and cross‐modal lexical tasks. Neuroimage 16:7–22. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402:179–181. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC (2007): Self‐projection and the brain. Trends Cogn Sci 11:49–57. [DOI] [PubMed] [Google Scholar]
- Burgess N (2008): Spatial cognition and the brain. Ann N Y Acad Sci 1124:77–97. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson G, Pekar J (2001): Spatial and temporal independent component analysis of functional MRI data containing a pair of task‐related waveforms. Hum Brain Mapp 13:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DK, Bittner MT, Cameron BR, Brown DM, Meyer SS (1994): Creative thinking as a predictor of school‐aged children's stress responses and coping abilities. Creat Res J 7:145–158. [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C (2015): Increased functional connectivity between subcortical and cortical resting‐state networks in autism spectrum disorder. JAMA Psychiatry 72:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez‐Eakle RA, Graff‐Guerrero A, García‐Reyna JC, Vaugier V, Cruz‐Fuentes C (2007): Cerebral blood flow associated with creative performance: a comparative study. Neuroimage 38:519–528. [DOI] [PubMed] [Google Scholar]
- Chen QL, Xu T, Yang WJ, Li YD, Sun JZ, Wang KC, Beaty RE, Zhang QL, Zuo XN, Qiu J (2015): Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia 75:441–449. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Scis 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K (2005): Why the frontal cortex in autism might be talking only to itself: local over‐connectivity but long‐distance disconnection. Current opinion in Neurobiology 15:225–230. [DOI] [PubMed] [Google Scholar]
- Crottaz‐Herbette S, Menon V (2006): Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. J Cogn Neurosci 18:766–780. [DOI] [PubMed] [Google Scholar]
- d'Esposito M, Aguirre G, Zarahn E, Ballard D, Shin R, Lease J (1998): Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res 7:1–13. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Nijstad BA, Baas M, Wolsink I, Roskes M (2012): Working memory benefits creative insight, musical improvisation, and original ideation through maintained task‐focused attention. Pers Soc Psychol Bull 38:656–669. [DOI] [PubMed] [Google Scholar]
- de Manzano Ö, Ullén F (2012): Goal‐independent mechanisms for free response generation: Creative and pseudo‐random performance share neural substrates. Neuroimage 59:772–780. [DOI] [PubMed] [Google Scholar]
- Di X, Biswal BB (2015): Dynamic brain functional connectivity modulated by resting‐state networks. Brain Struct Funct 220:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A (2004): The cognitive neuroscience of creativity. Psychon Bull Rev 11:1011–1026. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R (2010): A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychol Bull 136:822. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov‐Schlaggar CN (2010): Prediction of individual brain maturity using fMRI. Science 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K (2012): Evaluative and generative modes of thought during the creative process. Neuroimage 59:1783–1794. [DOI] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, Tedeschi G, Goebel R, Seifritz E, Di Salle F (2005): Independent component analysis of fMRI group studies by self‐organizing clustering. Neuroimage 25:193–205. [DOI] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews‐Hanna JR, Christoff K (2015): The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. Neuroimage 111:611–621. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Moore DW, Susmaras TM, Jerram MW, Sousa J, Heilman KM (2011): Cortical morphology of visual creativity. Neuropsychologia 49:2527–2532. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Hayward WG, Tarr MJ, Anderson AW, Skudlarski P, Gore JC (2002): BOLD activity during mental rotation and viewpoint‐dependent object recognition. Neuron 34:161–171. [DOI] [PubMed] [Google Scholar]
- Gilhooly K, Fioratou E, Anthony S, Wynn V (2007): Divergent thinking: Strategies and executive involvement in generating novel uses for familiar objects. Br J Psychol 98:611–625. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O (2005): Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set‐shift problems. Cereb Cortex 15:1170–1177. [DOI] [PubMed] [Google Scholar]
- Gonen‐Yaacovi G, de Souza LC, Levy R, Urbanski M, Josse G, Volle E (2013): Rostral and caudal prefrontal contribution to creativity: A meta‐analysis of functional imaging data. Front Hum Neurosci 7:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford JP (1967): The nature of human intelligence McGraw‐Hill: New York. pp 249–256.
- Hassabis D, Maguire EA (2007): Deconstructing episodic memory with construction. Trends Cogn Sci 11:299–306. [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A (2003): Icasso: software for investigating the reliability of ICA estimates by clustering and visualization. IEEE pp 259–268.
- Howard‐Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G (2005): Semantic divergence and creative story generation: An fMRI investigation. Cogn Brain Res 25:240–250. [DOI] [PubMed] [Google Scholar]
- Huang P, Qiu L, Shen L, Zhang Y, Song Z, Qi Z, Gong Q, Xie P (2013): Evidence for a left‐over‐right inhibitory mechanism during figural creative thinking in healthy nonartists. Hum Brain Mapp 34:2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD (2008): A method for functional network connectivity among spatially independent resting‐state components in schizophrenia. Neuroimage 39:1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauk E, Neubauer AC, Dunst B, Fink A, Benedek M (2015): Gray matter correlates of creative potential: A latent variable voxel‐based morphometry study. Neuroimage 111:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung‐Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel‐Liu S, Greenblatt R, Reber PJ, Kounios J (2004): Neural activity when people solve verbal problems with insight. PLoS Biol 2:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Jeremy Bockholt H, Flores RA, Smith SM, Chavez RS, Haier RJ (2010): Neuroanatomy of creativity. Hum Brain Mapp 31:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA (2013): The structure of creative cognition in the human brain. Front Hum Neurosci 7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin‐Germeys I, Kwapil TR (2007): For whom the mind wanders, and when an experience‐sampling study of working memory and executive control in daily life. Psychol Sci 18:614–621. [DOI] [PubMed] [Google Scholar]
- Kim KH (2006): Can we trust creativity tests? A review of the Torrance Tests of Creative Thinking (TTCT). Creat Res J 18:3–14. [Google Scholar]
- Kim KH (2008): Meta‐analyses of the relationship of creative achievement to both IQ and divergent thinking test scores. J Creat Behav 42:106–130. [Google Scholar]
- Kim KH, Cramond B, Bandalos DL (2006): The latent structure and measurement invariance of scores on the Torrance Tests of Creative Thinking–Figural. Edu Psychol Measurement 66:459–477. [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T (2010): Effects of aging on default mode network activity in resting state fMRI: Does the method of analysis matter? Neuroimage 51:280–287. [DOI] [PubMed] [Google Scholar]
- Kounios J, Fleck JI, Green DL, Payne L, Stevenson JL, Bowden EM, Jung‐Beeman M (2008): The origins of insight in resting‐state brain activity. Neuropsychologia 46:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M (2009): Neural networks involved in artistic creativity. Hum Brain Mapp 30:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray LJ, Galinsky AD, Wong EM (2006): Thinking within the box: The relational processing style elicited by counterfactual mind‐sets. J Pers Soc Psychol 91:33. [DOI] [PubMed] [Google Scholar]
- Lee CS, Therriault DJ (2013): The cognitive underpinnings of creative thought: A latent variable analysis exploring the roles of intelligence and working memory in three creative thinking processes. Intelligence 41:306–320. [Google Scholar]
- Liao W, Mantini D, Zhang Z, Pan Z, Ding J, Gong Q, Yang Y, Chen H (2010): Evaluating the effective connectivity of resting state networks using conditional Granger causality. Biol Cybern 102:57–69. [DOI] [PubMed] [Google Scholar]
- Liu S, Erkkinen MG, Healey ML, Xu Y, Swett KE, Chow HM, Braun AR (2015): Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Hum Brain Mapp 36:3351–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM (2000): Equivalence of the mediation, confounding and suppression effect. Prevention Science 1:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Caulo M, Ferretti A, Romani GL, Tartaro A (2009): Noxious Somatosensory Stimulation Affects the Default Mode of Brain Function: Evidence from Functional MR Imaging. Radiology 253:797–804. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayseless N, Eran A, Shamay‐Tsoory SG (2015): Generating original ideas: The neural underpinning of originality. Neuroimage 116:232–239. [DOI] [PubMed] [Google Scholar]
- McFatter RM (1979): The use of structural equation models in interpreting regression equations including suppressor and enhancer variables. Applied Psychological Measurement 3:123–135. [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung T‐P, Kindermann SS, Bell AJ, Sejnowski TJ ( 1997): Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6:160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP (2010): Inter‐individual differences in resting‐state functional connectivity predict task‐induced BOLD activity. Neuroimage 50:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP (2011): Linking inter‐individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 54:2950–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok L (2012): Short‐term retrospective versus prospective memory processing as emergent properties of the mind and brain: Human fMRI evidence. Neuroscience 226:236–252. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012): Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum EC, Silvia PJ (2011): Are intelligence and creativity really so different? ⋆: Fluid intelligence, executive processes, and strategy use in divergent thinking. Intelligence 39:36–45. [Google Scholar]
- Perkins AM, Arnone D, Smallwood J, Mobbs D (2015): Thinking too much: Self‐generated thought as the engine of neuroticism. Trends Cogn Sci 19:492–498. [DOI] [PubMed] [Google Scholar]
- Pinho AL, Ullén F, Castelo‐Branco M, Fransson P, de Manzano Ö (2016): Addressing a paradox: Dual strategies for creative performance in introspective and extrospective networks. Cereb Cortex 26:3052–3063. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD (1997): Neural substrates of fluid reasoning: An fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cogn Psychol 33:43–63. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF (2008): Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 40:879–891. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC (1958) Guide to Using the Coloured Progressive Matrices London: HK Lewis. p 40.
- Raven JC (1960) Guide to the standard progressive matrices: sets A, B, C, D and E. London: HK Lewis.
- Ray KL, McKay DR, Fox PM, Riedel MC, Uecker AM, Beckmann CF, Smith SM, Fox PT, Laird A (2013): ICA model order selection of task co‐activation networks. Front Neurosci 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Shehzad Z, Hernandez LM, Colich NL, Bookheimer SY, Iacoboni M, Dapretto M (2012): Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cerebral Cortex 22:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runco, M. (2003) Idea evaluation, divergent thinking, and creativity. Crit Creat Process 69‐94.
- Runco MA, Jaeger GJ (2012): The standard definition of creativity. Creat Res J 24:92–96. [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: Remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall JM, Allen EA, Jung RE, Erhardt EB, Arja SK, Kiehl K, Calhoun VD (2012): Correspondence between structure and function in the human brain at rest. Front Neuroinform 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman‐Rakic PS (1988): Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8:4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah C, Erhard K, Ortheil HJ, Kaza E, Kessler C, Lotze M (2013): Neural correlates of creative writing: An fMRI study. Hum Brain Mapp 34:1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith C, Grasby P, Frackowiak R, Dolan R (1994): Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368:633–635. [DOI] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller RA (2011): Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry 70:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Beaty RE, Nusbaum EC (2013): Verbal fluency and creativity: General and specific contributions of broad retrieval ability (Gr) factors to divergent thinking. Intelligence 41:328–340. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Gerlach KD, Turner GR, Schacter DL (2015): Autobiographical planning and the brain: Activation and its modulation by qualitative features. J Cogn Neurosci 11:2147–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, D'Argembeau A (2015): Neural correlates of personal goal processing during episodic future thinking and mind‐wandering: An ALE meta‐analysis. Hum Brain Mapp 36:2928–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg RJ, Lubart TI (1996): Investing in creativity. Am Psychol 51:677. [Google Scholar]
- Subramaniam K, Kounios J, Parrish TB, Jung‐Beeman M (2009): A brain mechanism for facilitation of insight by positive affect. J Cogn Neurosci 21:415–432. [DOI] [PubMed] [Google Scholar]
- Takahama S, Miyauchi S, Saiki J (2010): Neural basis for dynamic updating of object representation in visual working memory. Neuroimage 49:3394–3403. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R (2012): The association between resting functional connectivity and creativity. Cereb Cortex 22:2921–2929. [DOI] [PubMed] [Google Scholar]
- Torrance EP (1968): Torrance tests of creative thinking. Personnel Press, Incorporated.
- van de Ven V, Bledowski C, Prvulovic D, Goebel R, Formisano E, Di Salle F, Linden DE, Esposito F (2008): Visual target modulation of functional connectivity networks revealed by self‐organizing group ICA. Hum Brain Mapp 29:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE (2004): Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp 22:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2005): Separating semantic conflict and response conflict in the Stroop task: A functional MRI study. Neuroimage 27:497–504. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D (2007): A report on the third revision of combined raven's test (CRT‐C3) for children in China. Chin J Clin Psychol 15:559. [Google Scholar]
- Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J (2014): Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex 51:92–102. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, Zhang Q, Zhang M, Qiu J (2015): A meta‐analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Hum Brain Mapp 36:2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C‐G, Zang Y‐F (2010): DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4. [DOI] [PMC free article] [PubMed]
- Ye R, Hong D, Torrance EP (1988): Cross cultural comparion of creative thinking between Chinese and American students using Torrance Test. Chin J Appl Psychol 3:22–29. [Google Scholar]
- Zabelina DL, Robinson MD (2010): Creativity as flexible cognitive control. Psychol Aesthet Creat Arts 4:136. [Google Scholar]
- Zhu F, Zhang Q, Qiu J (2013): Relating inter‐individual differences in verbal creative thinking to cerebral structures: an optimal voxel‐based morphometry study. PLoS One 8:e79272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, Gao JH, Stein EA, Zang YF, Yang Y (2013): Intrinsic resting‐state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp 34:3204–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP (2010): Reliable intrinsic connectivity networks: test–retest evaluation using ICA and dual regression approach. Neuroimage 49:2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


