Abstract
Traumatic spinal cord injuries (SCIs) lead to axonal damage at the trauma site, as well as disconnections within the central nervous system. While the exact mechanisms of the long‐term pathophysiological consequences of SCIs are not fully understood, it is known that neuronal damage and degeneration are not limited to the direct proximity of the trauma. Instead, the effects can be detected even in the cerebrum. We examined SCI‐induced chronic brain changes with a case‐control design using 32 patients and 70 control subjects. Whole‐brain white matter (WM) tracts were assessed with diffusion tensor imaging (DTI). In addition, we analysed associations between DTI metrics and several clinical SCI variables. Whole‐brain analyses were executed by tract‐based spatial statistics (TBSS), with an additional complementary atlas‐based analysis (ABA). We observed widespread, statistically significant (P ≤ 0.01) changes similar to neural degeneration in SCI patients, both in the corticospinal tract (CST) and beyond. In addition, associations between DTI metrics and time since injury were found with TBSS and ABA, implying possible long‐term post‐injury neural regeneration. Using the ABA approach, we observed a correlation between SCI severity and DTI metrics, indicating a decrease in WM integrity along with patient sensory or motor scores. Our results suggest a widespread neurodegenerative effect of SCI within the cerebrum that is not limited to the motor pathways. Furthermore, DTI‐measured WM integrity of chronic SCI patients seemed to improve as time elapsed since injury. Hum Brain Mapp 38:3637–3647, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, spinal cord injuries, white matter, humans, cerebrum
INTRODUCTION
In a traumatic spinal cord injury (SCI), an external force causes immediate damage and death of the neural cells at the injury site, resulting in a secondary neuropathological process that further aggravates neuronal damage. Secondary degeneration of white matter (WM) tracts has been shown to spread in both anterograde and retrograde directions from the injury site over several years after the injury [Beirowski et al., 2005; Buss et al., 2004]. Degeneration includes a slow and progressive demyelination, which eventually leads to gliosis [Buss et al., 2004].
Secondary degeneration of the corticospinal tract (CST) in humans after SCI has been shown histologically to reach cerebral regions. Furthermore, histological evidence supports atrophy of the CST neurons [Yamamoto et al., 1989]. In line with these findings, atrophy‐related changes have been detected on volumetric MRI scans in the CST and the sensorimotor cortex in SCI patients [Freund et al., 2011; Wrigley et al., 2009].
After an injury to the central nervous system, complex neuroplastic mechanisms are initiated, similar to those associated with learning processes that occur during normal development of the brain [Keyvani and Schallert, 2002; Zatorre et al., 2013]. Neuroplasticity involves several underlying mechanisms, including changes in myelin structure, axon diameter and packing density changes, axonal sprouting, rerouting, and elimination [Zatorre et al., 2013]. These processes can potentially alter the functional and structural fabric of the brain's neural network to compensate for at least part of the possible damage to the brain [Nudo et al., 2001; Zatorre et al., 2013]. Functional MRI has provided evidence of cortical reorganization that compensates for sensorimotor loss after SCI [Freund et al., 2011; Henderson et al., 2011; Jurkiewicz et al., 2007]. The changes in activation observed by functional MRI after SCI can be explained by both functional and structural alterations in nervous tissue. After experimental SCI structural alterations, the remodelling of synaptic structures and axonal sprouting and the formation of new connections have been demonstrated together with reorganisation in both the cortex and subcortical regions [Florence et al., 1998; Kim et al., 2006; Ramu et al., 2008].
Diffusion tensor imaging (DTI), which measures the diffusion of water molecules in tissues, provides quantitative information on tissue microstructures. In nervous tissue, the orientation of fibre bundles, axonal diameter, density, and myelination affect diffusion metrics [Beaulieu, 2002; Sen and Basser, 2005]. Spinal DTI has been shown to have potential for quantifying the extent of clinical disability following SCI and radiological SCI severity [Chang et al., 2010; Koskinen et al., 2013]. Additionally, DTI could detect diffusion changes at a distance from the macroscopic spinal lesions seen on conventional MRI, suggesting secondary degeneration of WM tracts in the spinal cord [Chang et al., 2010; Cohen‐Adad et al., 2011; Koskinen et al., 2013; Petersen et al., 2012]. Degeneration‐associated abnormalities in cerebral DTI values after SCI have also been demonstrated in humans, although only in a few studies that were mostly focused on the CST [Freund et al., 2012; Gustin et al., 2010; Koskinen et al., 2014; Wei et al., 2008; Wrigley et al., 2009]. Wrigley et al. [2009] found volumetric and DTI metrics changes in multiple cortical areas beyond the primary sensory and motor cortices, indicating that subcortical WM changes after SCI could also extend beyond the CST.
Fractional anisotropy (FA) and mean diffusivity (MD) are currently the most commonly used DTI metrics [Guleria et al., 2008; Hulkower et al., 2013; Wei et al., 2008], and both reflect WM integrity in various pathological conditions [Alexander et al., 2007]. FA is mostly lower and MD higher in pathological regions compared to healthy tissue. Additionally, radial diffusivity (RD), and axial diffusivity (AD) can be examined. RD is thought to be largely affected by the integrity of the myelin sheath, while changes in AD reflect the degree of axonal degeneration [Alexander et al., 2007; Song et al., 2002]. With pathological tissue, RD is usually higher in myelin degradation, while AD can be lower in axonal disruption.
In this study, we applied tract‐based spatial statistics (TBSS) [Smith et al., 2006], which is a whole‐brain group comparison analysis method, adapting a unique approach on whole‐brain analysis by its registration and “skeletonisation” phases. Previously, only one study [Wei et al., 2008] addressed cerebral WM changes after SCI with TBSS. Contrary to the Wei group's region of interest findings, they found no structural between‐group differences in their TBSS analyses.
The purpose of this study was to investigate the effects of SCI on the entire cerebral WM, detectable by DTI. We also investigated the association between cerebral DTI values and clinical SCI parameters, including injury severity, and time since injury (TSI), and applied an atlas‐based analysis (ABA) method to supplement the TBSS results. We hypothesized that (i) SCI‐induced WM changes would be detectible beyond the CST with TBSS and (ii) that the motor and sensory functions of chronic SCI patients would be related to brain WM integrity.
MATERIALS AND METHODS
Subjects
All consecutive patients with a chronic traumatic cervical spine injury (n = 88) who were admitted to either the ward or an outpatient clinic at Tampere University Hospital between 1989 and 2010 (the annual incidence rate of SCI in Finland mirrored the area of responsibility of Tampere University Hospital, leading to ∼8.3 new tetraplegia patients per year [Ahoniemi et al., 2008], of which not all are sent to Tampere University Hospital) were contacted in 2011 to participate in the study. The inclusion criteria were as follows: (i) over 18 years of age, (ii) resident of the hospital district, (iii) clinically significant neurological findings due to a traumatic cervical SCI after 24 h of monitoring in the hospital, and (iv) TSI was greater than 1 year. The exclusion criteria were as follows: (i) known neurological illness other than SCI (including traumatic brain injury), (ii) respiratory arrest, (iii) contraindication for MRI, and (iv) refusal to participate in the study. In addition to the exclusion criteria, two subjects were dismissed due to severe microangiopathy on brain MRI. The final SCI population sample consisted of 32 patients.
The control subject sample comprised two separate groups of DTI study controls, both imaged at Tampere University Hospital using the same scanner and imaging protocol. We enrolled a total of 70 control subjects, of which 40 were orthopedically injured patients evaluated in the ED of Tampere University Hospital. This group of control subjects was categorised in an age‐ and gender‐stratified manner, with five men and five women in the following age groups: (i) 18–30 years, (ii) 31–40 years, (iii) 41–50 years, and (iv) 51–60 years. The remaining 30 control subjects were healthy voluntary hospital staff members. Conventional MRI findings of the control subjects were interpreted as normal by a neuroradiologist (A.B.). All subjects included in this study provided written informed consent according to the Declaration of Helsinki.
Clinical Data
All patients with SCI were examined at an outpatient clinic at Tampere University Hospital. The collection of clinical data was performed by a neurologist (E.K.). The aetiology of the SCI was classified using the International SCI Core Data Set [DeVivo et al., 2006]. The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) were used to evaluate and classify the neurological consequence of SCI [Waring et al., 2010]. Ten of the SCI patients had complete injury [American Spinal Injury Association Impairment Scale (AIS), grade A]. See Table 1 for demographic information on the control and patient groups.
Table 1.
Subject demographics
| Group comparisons | Controls (n = 70) | Patients (n = 32) | AIS A (n = 10) | P (con v. pat) | P (con v. AIS A) |
|---|---|---|---|---|---|
| Age (yrs, mean ± SD) | 39.5 ± 11.8 | 56.5 ± 14.2 | 51.3 ± 15.7 | <0.001 | 0.008 |
| Gender (male/female) | 29/41 | 25/7 | 7/3 | <0.001 | 0.089 |
| TSI (yrs, mean ± SD) | 13.8 ± 12.3 | 23.5 ± 13.1 | |||
| ASIA impairment scale | |||||
| AIS A | 10 | 10 | |||
| AIS B | 1 | ||||
| AIS C | 4 | ||||
| AIS D | 16 | ||||
| AIS E | 1 | ||||
| Injury etiology | |||||
| Fall | 13 | 3 | |||
| Transport | 11 | 5 | |||
| Sports | 6 | 2 | |||
| Assault | 1 | ||||
| Other | 1 |
The gender distribution between the controls and the patients was tested with a chi‐squared test and the age distribution with a Mann‐Whitney U test.
Imaging
A head MRI was done with a 3 Tesla MRI scanner (Siemens Trio, Siemens AG Medical Solutions, Erlangen, Germany). A 12‐channel head coil and a 4‐channel neck coil were used simultaneously for the SCI patients, but only the head MRI data were used in this study. The MRI protocol included sagittal T1‐weighted 3D inversion recovery prepared gradient echo, axial T2 turbo spin echo, conventional axial and high‐resolution sagittal FLAIR, axial T2*, axial susceptibility‐weighted imaging, and diffusion‐weighted imaging series.
The brain DTI data were collected by a single‐shot, spin echo‐based and diffusion‐weighted echo planar imaging sequence. The parameters for the DTI sequence were TR 5144 ms, TE 92 ms, field of view 230 mm, matrix 128 × 128, 3 averages, slice/gap 3.0/0.9 mm, voxel dimensions of 1.8 × 1.8 × 3.0 mm, and b‐factors 0 and 1000 s/mm2 with 20 diffusion gradient orientations. Diffusion tensors were calculated from the gradient data and further derived into DTI scalars used in the analyses (FA, MD, RD, and AD).
Statistical Analysis
Whole‐brain voxel‐wise statistical analysis for the DTI data was carried out using TBSS [Smith et al., 2006], a part of FSL, version 5.0.6 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) [Smith et al., 2004]. After pre‐processing the data, a mean FA image was derived and thinned to create a mean FA skeleton. The threshold for FA values for the creation of the skeleton was chosen as ≥0.25. Each subject's aligned data were then projected onto this skeleton for each DTI parameter, and the resulting skeletonised data were fed into voxel‐wise cross‐subject statistics.
Statistical group comparison analysis was performed using FSL with TFCE [Smith and Nichols, 2009]. Nonparametric two‐sample permutation test using general linear model (GLM) design was used for statistics [Winkler et al., 2014]. Inference was obtained through 50,000 permutations, testing the resulting clusters for significance at P ≤ 0.01 (one‐sided), corrected for multiple comparisons across space. The type I error caused by multiple analyses on the same dataset was potentially minimized by adopting a more conservative significance level (P ≤ 0.01). Statistical regression was performed in a similar manner, using a GLM to check the DTI data for partial regression with clinical parameters. Effects of age and gender were controlled by adding them as covariates to the design matrices in all analyses.
Two group analyses were performed: (i) a comparison between the whole SCI group (n = 32) and the healthy control subjects (n = 70) and (ii) a comparison between patients with complete SCI (AIS grade A, n = 10) and healthy control subjects (n = 70). The clinical variables used in the partial correlation analyses were (i) the ISNCSCI‐derived total motor score (TMS), (ii) motor subscore for upper extremities (UEMS), (iii) motor subscore for lower extremities, (iv) total sensory score (TSS), and (v) the TSI = time between injury and MRI. Due to the findings in stepwise linear regression analyses performed on the significant clusters in the group comparison, we decided to control TSI analysis with UEMS and motor and sensory subscores with TSI, respectively.
To further specify our findings, we utilised the JHU‐ICBM‐DTI‐81 WM labels atlas in an ABA approach, which, in theory, should complement the TBSS results [Faria et al., 2010]. Additionally, it can help to further localize the findings. Regional DTI metric values were derived for each atlas tract by taking the arithmetic mean of the skeleton voxels inside the corresponding atlas volume. The mean values were then fed to JASP (JASP Team, 2016, Version 0.8) for statistical analysis. The group comparison was carried out using analysis of covariance (ANCOVA), controlling for age and gender, and correlation analyses by linear regression. To distinguish between the anatomical regions in general and the volume defined by the JHU atlas, the abbreviated atlas volumes will be referred to with a subscript JHU (e.g., CSTJHU).
RESULTS
Both group comparisons [i.e., patient group (n = 32) vs. control subjects (n = 70) and full injury patients (n = 10) vs. control subjects (n = 70)] resulted in statistically significant differences at a significance level of P ≤ 0.01. The ABA group comparison results were mostly in concordance with the TBSS results. Most linear regression analyses did not reach statistical significance in TBSS; TSI was the only variable that produced a statistically significant correlation with DTI metrics. The ABA, however, revealed individual correlations that did not reach significance in the TBSS analysis. The complete list of results from the ABA are displayed in Table 2. Detailed information on the ABA results are provided as Supporting Information.
Table 2.
Atlas − based analysis results (P ≤ 0.01)
| SCI v controls* | AIS A v controls* | TSI correlation** | UEMS** | TMS** | TSS** | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WM tract (ICBM DTI−81) | FA | MD | RD | AD | FA | MD | RD | AD | FA | MD | RD | AD | FA | MD | RD | FA | FA | MD |
| Anterior corona radiata L | ↓ | ↑ | ↑ | + | − | |||||||||||||
| Anterior corona radiata R | ↓ | ↑ | ↑ | |||||||||||||||
| ALIC L | ↓ | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | − | ||||||||||
| ALIC R | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ||||||||||||
| Body of CC | ↑ | ↑ | ||||||||||||||||
| Cingulum (cingulate gyrus) L | ↑ | ↑ | − | − | ||||||||||||||
| Cingulum (cingulate gyrus) R | ↑ | ↑ | ↑ | + | ||||||||||||||
| CST R | ↓ | |||||||||||||||||
| External capsule R | ↑ | ↑ | ||||||||||||||||
| Fornix (column and body of fornix) | ↑ | − | − | − | ||||||||||||||
| Fornix (cres)/Stria terminalis L | ↓ | ↑ | + | − | ||||||||||||||
| Genu of CC | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ||||||||||||
| Inferior cerebellar peduncle L | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ||||||||||||
| Inferior cerebellar peduncle R | ↓ | ↑ | ↓ | ↓ | ↓ | |||||||||||||
| Medial lemniscus L | ↓ | |||||||||||||||||
| Medial lemniscus R | ↓ | |||||||||||||||||
| Middle cerebellar peduncle | + | |||||||||||||||||
| Posterior corona radiata L | − | − | − | |||||||||||||||
| Posterior corona radiata R | ↓ | ↑ | ↑ | ↓ | ↑ | − | − | |||||||||||
| Posterior limb of internal capsule L | ↑ | ↑ | ↑ | |||||||||||||||
| Posterior thalamic radiation La | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | − | |||||||||||
| Retrolenticular part of internal capsule L | ↓ | − | − | |||||||||||||||
| Retrolenticular part of internal capsule R | + | + | ||||||||||||||||
| Sagittal stratum Lb | ↓ | ↑ | ↑ | ↓ | ↑ | + | − | − | ||||||||||
| Sagittal stratum Rb | − | |||||||||||||||||
| Splenium of CC | + | − | − | |||||||||||||||
| Superior cerebellar peduncle L | + | |||||||||||||||||
| Superior cerebellar peduncle R | + | + | + | |||||||||||||||
| Superior corona radiata L | ↑ | ↑ | − | − | − | |||||||||||||
| Superior corona radiata R | − | |||||||||||||||||
| Superior fronto−occipital fasciculus L | ↓ | ↑ | ↑ | |||||||||||||||
| Superior fronto−occipital fasciculus R | ↓ | ↑ | ↑ | |||||||||||||||
| Superior longitudinal fasciculus L | ↑ | − | − | − | − | |||||||||||||
| Tapetum L | + | − | ||||||||||||||||
| Tapetum R | ↑ | ↑ | ||||||||||||||||
| Uncinate fasciculus L | ↓ | ↑ | ||||||||||||||||
*Up arrows (↑) indicate higher values, and down arrows (↓) indicate lower values in the patient group compared with the control subjects.
**Plus signs (+) indicate positive correlation, and minus signs (−) indicate negative correlation.
Includes optic radiation.
Includes inferior longitudinal fasciculus and inferior fronto − occipital fasciculus.
Group Comparison
The TBSS group comparison between all SCI patients (n = 32) and control subjects (n = 70) yielded statistically significant (P ≤ 0.01) differences in FA, MD, RD, and AD values. The FA values were found to be lower and the MD, RD, and AD values higher in the patients when compared with the control subjects.
Instead of being mainly focused on the CST as expected, the changes related to SCI were more widespread. The coverage of statistically significant voxels in FA was 30.3% of the skeleton's total volume, 32.9% in MD, 38.7% in RD, and 13.0% in AD, respectively. The areas with significantly decreased FA and increased MD can be seen in Figure 1, and the actual differences in DTI values are displayed as bar graphs in Figure 2. The significant areas with increased RD and AD are presented as Supporting Information. Due to the widespread nature of the findings, we also created images with the P‐value threshold set to 0.002 to emphasize the areas with the most change in DTI values. The areas of FA, MD, and RD with P ≤ 0.002 can be seen in the Supporting Information.
Figure 1.
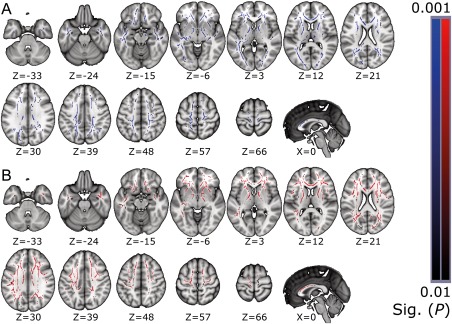
Statistically significant clusters of lower FA acquired in the TBSS group comparison analysis shown in the two upper rows (A), with the significant clusters of higher MD shown in the two lower rows (B). Results are limited to P‐values ≤ 0.01. The results are overlaid on the MNI152 standard‐space template, with corresponding MNI coordinates below the slices. Neurological convention, left = left. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
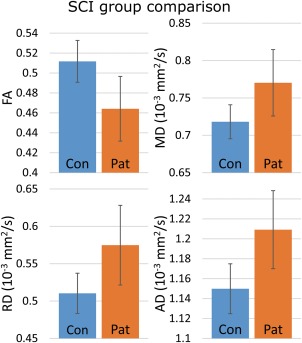
The differences in absolute DTI values taken from the statistically significant clusters in the group comparison for each DTI metric (P ≤ 0.01; FA, MD, RD, and AD). The bar graph values represent mean values of the significant voxels, with error bars showing the ± SD for the volume's mean. [Color figure can be viewed at http://wileyonlinelibrary.com]
The significant findings were extensive, with clusters spread nearly throughout the whole cerebrum. Areas of WM affected by SCI in the group analysis included projection, commissural, and association fibres. From the projection fibres, the majority of the CST and the thalamocortical projections were affected. Between‐group differences in the area of the CST extended from the cerebral peduncle (CP), through the posterior limb of internal capsule (PLIC), up to the subcortical WM underneath the primary motor and sensory cortices. The genu and the anterior part of the body of the corpus callosum (CC) were the most affected of the commissural fibres. The association fibres were widely affected, with most prominent findings in the inferior and superior longitudinal fasciculi, inferior fronto‐occipital fasciculus, and uncinate fasciculus. The anterior cingulum was also affected. The findings in MD are notably similar to FA, although with slightly wider coverage.
As an addition to the TBSS analyses, we utilised the JHU‐ICBM‐DTI‐81 WM atlas to extract parts of the skeleton and ran statistical tests on the areas' mean values. We compared the patient group with the control subjects via ANCOVA. Statistically significant (P ≤ 0.01) lower FA values were found in several locations, along with higher MD and RD values. Several atlas locations included changes in more than one DTI metric: changes in both FA and MD were found in the anterior corona radiata (CRJHU), the anterior limb of internal capsule (ALICJHU), the genu of the CCJHU, the right posterior CRJHU, the left posterior thalamic radiation, the left sagittal stratum, and in the superior fronto‐occipital fasciculus. According to our analysis, AD correlated positively in the left ALICJHU, the body of the CCJHU, and in the fornix, whereas negative correlation was found in the right CSTJHU and in the inferior CPJHU. The complete list of locations is shown in Table 2.
Complete SCI
Patients with AIS grade A (n = 10) were compared with the healthy control subjects (n = 70) with TBSS. Age and gender were used as covariates in the GLM setup. The analysis resulted in statistically significant (P ≤ 0.01) differences in FA, MD, and RD: patients had lower FA and higher MD and RD values compared with the control subjects. AD did not reach statistical significance at the 0.01 level. The results were reminiscent of the group comparison (whole SCI group and control subjects), but considerably more spatially restricted. Significant clusters covered 9.2% of the skeleton volume in FA and only 2.8% in MD, while the coverage was 10.4% in RD.
Differences in FA were somewhat asymmetric, occurring predominantly on the left side of the cerebrum. Statistically significant areas of FA and MD can be seen in Figure 3, with the absolute DTI values of the significant clusters displayed in Figure 4. The affected areas in FA were mostly in the projection fibres, including the PLIC, the anterior limb of the internal capsule, the posterior thalamic radiation, and the subcortical CST. While higher MD values were found mostly in the genu of the CC, a small, significant cluster was located near the subcortical CST. The significant clusters of RD can be seen in the Supporting Information.
Figure 3.
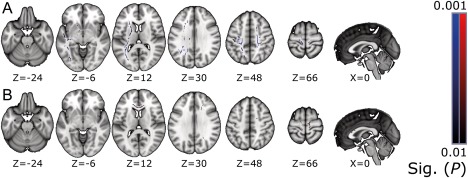
Areas with statistically significant (P ≤ 0.01) differences between patients with full injury and control subjects. Areas with lower FA values are shown above (A), and areas with higher MD are below (B). The results are overlaid on the MNI152 standard‐space template, with corresponding MNI coordinates below the slices. Neurological convention, left = left. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
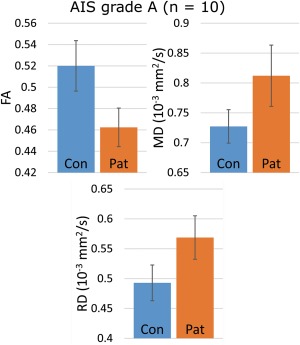
The differences in absolute DTI values for the full injury group, taken from the statistically significant clusters for each significant DTI metric (P ≤ 0.01; FA, MD, and RD). The values represent mean values of the significant voxels, with error bars showing the ± SD for the volume mean. Note the apparent increase in difference compared to the whole‐group comparison. [Color figure can be viewed at http://wileyonlinelibrary.com]
Comparing the complete (AIS A) SCI patient group (n = 10) with the control subjects (n = 70) in an ABA, we found significantly lower FA and AD values and higher MD and RD values. Again, there were some locations with findings in multiple DTI metrics: FA was found lower and MD higher in the ALICJHU, the genu of CCJHU, and in the left posterior thalamic radiation. See Table 2 for listing of the ABA results.
Partial Correlations with Injury Parameters
None of the sensory or motor score variables correlated significantly (P ≤ 0.01) with the DTI metrics in our TBSS analyses. Of all the tested clinical parameters, only the TSI produced a statistically significant partial correlation with the DTI metrics in the patient group. The TSI correlation analysis was conducted using age, gender, and UEMS as covariates.
The TSI correlated positively with FA and negatively with MD, RD, and AD at the 0.01 P‐value level. Statistically significant clusters in positive correlation between FA and the TSI covered 5.0% of the skeleton volume, while coverages were 26.3% for MD, 31.5% for RD, and 2.4% for AD. Partial correlation maps for FA and MD can be seen in Figure 5. Correlation maps for RD and AD are provided as Supporting Information. Partial correlation coefficients for the significant clusters of FA, MD, RD, and AD were 0.378, −0.331, −0.328, and −0.366, respectively.
Figure 5.
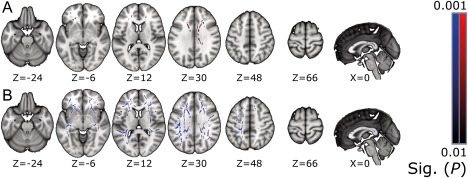
Partial correlations maps (P ≤ 0.01) for TSI obtained from TBSS analysis. FA correlated positively with TSI (above, A), and MD correlated negatively (below, B). The results are overlaid on the MNI152 standard‐space template, with corresponding MNI coordinates below the slices. Neurological convention, left = left. [Color figure can be viewed at http://wileyonlinelibrary.com]
A positive correlation between the TSI and FA was spatially concentrated to the commissural fibres: virtually the whole volume of the CC was affected. No correlations were found in the caudal parts of the projection fibres. For MD, the areas with correlation were similar to the FA findings, but with additional correlation findings widely in the association fibres and subcortically in the projection fibres.
In addition to TSI, we found a correlation between DTI metrics and the UEMS motor subscore, TMS, and TSS in ABA. TSI correlated positively with FA and negatively with MD, RD, and AD in our analysis. UEMS correlated positively with FA and negatively with MD and RD. TSS correlated positively with FA and negatively with MD, and TMS correlated positively with FA. See Table 2 for a complete list of the ABA results.
DISCUSSION
Group Comparison
The direction of the differences found in FA and MD were in agreement with previous studies, but the spatial extent of the findings was significantly larger than has been previously reported [Guleria et al., 2008; Koskinen et al., 2014; Wrigley et al., 2009]. The results suggest degenerative‐type changes in the majority of the cerebral WM, with the bulk of lower FA and higher MD extending beyond the CST. These changes could signify large‐scale post‐SCI secondary anterograde (Wallerian) and retrograde cerebral degeneration [Beirowski et al., 2005; Buss et al., 2004; Guleria et al., 2008].
Previous studies focusing on separate pathological conditions have hypothesized that areas of WM containing increased RD and AD values in addition to lower FA are associated with axonal degeneration [Della Nave et al., 2011; Metwalli et al., 2010; Roosendaal et al., 2009; Song et al., 2002]. In light of these previous studies, the rise in RD and AD found in our TBSS results may be associated with post‐SCI axonal degeneration. However, the increase of AD beyond the spinal cord and brain stem, which include mainly coherent WM tracts, could also be a consequence of crossing WM fibres, causing fictitious change in directional diffusivities [Wheeler‐Kingshott and Cercignani, 2009].
The CSTJHU and the inferior CPJHU had significantly lower AD in contrast to the otherwise found higher AD in our ABA. This is, however, convergent with previous DTI studies of chronic SCI which have reported a reduction of AD associated with axonal degeneration in areas like CP and CST [Freund et al., 2012; Wrigley et al., 2009], as well as in the spinal cord remote from the site of injury [Cohen‐Adad et al., 2011]. The inconsistency in AD results may be linked to post‐SCI neuroplasticity [Nudo et al., 2001; Schallert et al., 2000] and secondary degeneration [Beirowski et al., 2005; Buss et al., 2004; Guleria et al., 2008] associated with different regions of the cerebrum. Unfortunately, this type of speculation based only on AD cannot fully be confirmed with our current results and requires further research.
AIS Grade A
The absolute differences between the groups in the AIS A analysis were slightly larger for FA and MD compared with the full‐group comparison, but the lower sample size may distort the results. However, low power, along with the strict P‐value limit, may imply an even larger effect size of the findings compared with the group comparison. Future studies with a larger sample of AIS grade A patients could provide interesting results.
The ABA results of AIS A seem logical due to findings in areas of the brain often associated with secondary degeneration [Beirowski et al., 2005; Buss et al., 2004]. Unfortunately, no definite conclusions can be made with the current sample size.
Correlations
Contrary to previous studies with chronic SCI patients [Freund et al., 2012; Koskinen et al., 2014; Wrigley et al., 2009], we found a correlation between DTI scalars and TSI with both TBSS and ABA. However, owing to the limited sample of patients, the regression analyses could only reliably detect large effect sizes. While TSI correlations with DTI metrics have not been previously detected in the cerebrum [Freund et al., 2012; Koskinen et al., 2014; Wrigley et al., 2009], these studies have concentrated on the CST instead of the whole brain volume. It is worth noting that Guleria et al. [2008] observed increased FA and decreased MD values in the rostral part of the CST in SCI patients compared with control subjects, and this trend seemed to increase with TSI during the first 12 months after injury. According to experimental studies [Ramu et al., 2008], these types of changes have been suggested to reflect post‐SCI subcortical regeneration.
Previous studies have suggested a connection between DTI scalars and neuroplasticity (axonal regeneration, glial processes, or synaptogenesis), detectible as an increase in FA and/or decrease in MD and RD [Keller and Just, 2016; Sagi et al., 2012; Steele et al., 2013]. Due to the restrictions in our study setting, it is difficult to draw a solid causal conclusion on the findings. Hypothetically, positive correlation with FA and negative with MD over time suggest axonal regeneration or other plastic mechanisms. However, it is uncertain whether post‐SCI neuroplasticity could affect the brain over a decade post‐injury, and whether the observed correlation is actually associated with the post‐injury neurophysiology.
The directions of the correlations between UEMS, TMS, and TSS and DTI scalars in the ABA are in agreement with our previous study [Koskinen et al., 2014], where FA was found to correlate positively and MD negatively with UEMS, TMS, and TSS, suggesting that the clinical state of the patient tends to be better with higher FA and lower MD values. These findings imply that the severity and extent of SCI have an effect on the cerebral WM microstructure, detectable with DTI in the chronic phase.
Study Design and Limitations
In our study, we decided to combine two separate control groups to form a single larger pool of controls in favour of statistical power. After some consideration, we deemed the groups sufficiently homogeneous, and comparable from a pathological point of view, for them to be combined. Had we opted to create a pool of age‐ and gender‐matched controls, the raw statistical power of our group analysis would have been lower than in our current setup. Regarding the effect of ageing on DTI scalars, previous studies have suggested that the slight quadratic trend of the relationship between age and DTI metrics can be modelled as linear with sufficient precision [Kodiweera et al., 2016; Westlye et al., 2010]. It should thus be possible to reliably control for the effect of age in a GLM. In addition, controlling for gender in the analyses is straightforward, as it is a binary categorical variable. Nevertheless, our study would have benefited from a large pool of age‐ and gender‐matched subjects.
While our patient data were screened carefully to exclude any previous neurological diseases, including traumatic brain injury, the possibility of a concomitant mild brain injury with SCI cannot fully be ruled out [Wei et al., 2008]. However, it has been generally postulated that the traumatic brain injury mechanism affects the largest axon bundles, of which the CC is a perfect example [Hulkower et al., 2013], while our results were concentrated in areas less severely affected by brain trauma. Additionally, the different types of medication and rehabilitation methods possibly adapted to various patients in our sample could influence the magnitude of neuroplasticity [Schallert et al., 2000] and cause slight bias when not controlled for. In general, numerous physical and mental factors can affect DTI results, and while these cannot all be controlled for, several known confounding factors were used as exclusion criteria for our subject pool.
In terms of clinical relevance and rehabilitation, the acute phase is the most crucial stage of SCI, and while studies on neuroplasticity mostly focus on the acute to sub‐acute phase, our study focused on the chronic phase of SCI. Another concern is the large deviation of the mean TSI of our patient population, which could distort our results.
The fundamental microstructural mechanisms of neurodegeneration and plasticity are diverse, and the changes perceived by a single imaging modality are the combined effects of these mechanisms [Zatorre et al., 2013]. DTI alone is not sufficient to fully classify the mechanisms affecting WM post‐SCI, and multimodal imaging would be preferred. In addition, glial processes are likely to be present in post‐SCI cerebral WM [Keyvani and Schallert, 2002], and the presence of gliosis may cause increased FA values [Budde et al., 2011]. A known limitation of DTI is the crossing fibre problem, which may lead to anomalous DTI scalars in voxels containing neural tracts of different orientations [Wheeler‐Kingshott and Cercignani, 2009]. While crossing fibres may be considered as a fixed attribute of DTI, a possible solution could be acquiring the data with a high angular resolution diffusion imaging (HARDI) suited imaging sequence [Berman et al., 2013; Frank, 2001; Tuch, 2004; Tuch et al., 2002]. However, no direct replacement for DTI scalars, mainly FA, as the measure for axonal integrity currently exist for HARDI and finding such a measure may prove time‐consuming.
Future Prospects
Based on the results of our group comparison, future studies of the effects of SCI on the human brain WM should be extended outside the CST. Examining brain WM outside the sensorimotor tracts may also give a more thorough outlook on the link between neural structure and clinical function in chronic SCI. Nevertheless, additional evidence, especially longitudinal studies, is needed in the future to evaluate the presumptive change in DTI metrics with time after an acute injury and the association of that change with clinical recovery. This knowledge could facilitate the prognostication of recovery and our ability to understand and monitor the changes induced by treatment and rehabilitative interventions beyond clinical disability scales and conventional MRI. However, it should be noted that group‐level research results are not directly applicable to individual clinical cases.
CONCLUSION
Patients with chronic SCI were found to show changes detectable with DTI in their cerebral WM, extending widely beyond the motor cortex and sensorimotor tracts, and those changes were associated with TSI and clinical parameters indicating the severity of injury. Primarily, the changes manifested as reduction of FA and increase of MD. Furthermore, DTI‐measured WM integrity of chronic SCI patients altered as time elapsed since injury.
In the future, additional evidence, especially longitudinal studies from the acute stage of SCI, is required to evaluate the presumptive change in DTI values with time after injury and the association of that change with clinical recovery. Optimistically, DTI metrics could serve as adjunct biomarkers in the evaluation of an individual patient's susceptibility to different types of rehabilitative interventions.
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
REFERENCES
- Ahoniemi E, Alaranta H, Hokkinen E‐M, Valtonen K, Kautiainen H (2008): Incidence of traumatic spinal cord injuries in Finland over a 30‐year period. Spinal Cord 46:781–784. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2041910&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system ‐ a technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP (2005): The progressive nature of Wallerian degeneration in wild‐type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Lanza MR, Blaskey L, Edgar JC, Roberts TPL (2013): High angular resolution diffusion imaging probabilistic tractography of the auditory radiation. Am J Neuroradiol 34:1573–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, Turtzo LC, Frank JA (2011): The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain 134:2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, Noth J, Schmitt AB (2004): Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127:34–44. [DOI] [PubMed] [Google Scholar]
- Chang Y, Jung T‐D, Yoo DS, Hyun JK (2010): Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma 27:2033–2040. [DOI] [PubMed] [Google Scholar]
- Cohen‐Adad J, El Mendili M‐M, Lehericy S, Pradat P‐F, Blancho S, Rossignol S, Benali H (2011): Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 55:1024–1033. [DOI] [PubMed] [Google Scholar]
- Della Nave R, Ginestroni A, Diciotti S, Salvatore E, Soricelli A, Mascalchi M (2011): Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich's ataxia. Neuroradiology 53:367–372. [DOI] [PubMed] [Google Scholar]
- DeVivo M, Biering‐Sørensen F, Charlifue S, Noonan V, Post M, Stripling T, Wing P (2006): International Spinal Cord Injury Core Data Set. Spinal Cord 44:535–540. http://www.ncbi.nlm.nih.gov/pubmed/16955073. [DOI] [PubMed] [Google Scholar]
- Faria AV, Zhang J, Oishi K, Li X, Jiang H, Akhter K, Hermoye L, Lee SK, Hoon A, Stashinko E, Miller MI, van Zijl PCM, Mori S (2010): Atlas‐based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. Neuroimage 52:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas Jon H, Kaas JH (1998): Large‐scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science (80‐) 282:1117–1121. http://www.ncbi.nlm.nih.gov/pubmed/9804549. [DOI] [PubMed] [Google Scholar]
- Frank LR (2001): Anisotropy in high angular resolution diffusion‐weighted MRI. Magn Reson Med 45:935–939. http://www.ncbi.nlm.nih.gov/pubmed/11378869%5Cnhttp://onlinelibrary.wiley.com/store/10.1002/mrm.1125/asset/1125_ftp.pdf?v=1&t=hh7uma40&s=e80eb97a04934cad9917e3ef0a6b725024ce9fe2. [DOI] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ (2011): Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134:1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wheeler‐Kingshott C, Nagy Z, Gorgoraptis N, Weiskopf N, Friston K, Thompson AJ, Hutton C (2012): Axonal integrity predicts cortical reorganisation following cervical injury. J Neurol Neurosurg Psychiatry 83:629–637. http://www.ncbi.nlm.nih.gov/pubmed/22492214/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M, Rathore R, Narayana PA (2008): Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J Neurosci Res 86:2271–2280. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA (2010): Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex 20:1409–1419. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ (2011): Functional Reorganization of the Brain in Humans Following Spinal Cord Injury: Evidence for Underlying Changes in Cortical Anatomy. J Neurosci 31:2630–2637. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML (2013): A decade of DTI in traumatic brain injury: 10 years and 100 articles later. Am J Neuroradiol 34:2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC (2007): Sensorimotor cortical plasticity during recovery following spinal cord injury: A longitudinal fMRI study. Neurorehabil Neural Repair 21:527–538. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA (2016): Structural and functional neuroplasticity in human learning of spatial routes. Neuroimage 125:256–266. [DOI] [PubMed] [Google Scholar]
- Keyvani K, Schallert T (2002): Plasticity‐associated molecular and structural events in the injured brain. J Neuropathol Exp Neurol 61:831–840. http://www.ncbi.nlm.nih.gov/pubmed/12387449. [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai H‐N, McAtee M, Vicini S, Bregman BS (2006): Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198:401–415. [DOI] [PubMed] [Google Scholar]
- Kodiweera C, Alexander AL, Harezlak J, McAllister TW, Wu YC (2016): Age effects and sex differences in human brain white matter of young to middle‐aged adults: A DTI, NODDI, and q‐space study. Neuroimage 128:180–192. 10.1016/j.neuroimage.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen E, Brander A, Hakulinen U, Luoto T, Helminen M, Ylinen A, Ohman J (2013): Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma 30:1587–1595. http://www.ncbi.nlm.nih.gov/pubmed/23758292. [DOI] [PubMed] [Google Scholar]
- Koskinen E, Hakulinen U, Brander A, Luoto TM, Ylinen A, Ohman JE (2014): Clinical correlates of cerebral diffusion tensor imaging findings in chronic traumatic spinal cord injury. Spinal Cord 1–7. http://www.ncbi.nlm.nih.gov/pubmed/24418961. [DOI] [PubMed] [Google Scholar]
- Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD (2010): Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 1348:156–164. [DOI] [PubMed] [Google Scholar]
- Nudo R, Plautz EJ, Frost SB (2001): Role of adaptive plasticity in recovery after damage to motor cortex. Muscle Nerve 24:1000–1019. [DOI] [PubMed] [Google Scholar]
- Petersen J. a, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, Dietz V, Kollias S (2012): Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 29:1556–1566. http://www.ncbi.nlm.nih.gov/pubmed/22150011. [DOI] [PubMed] [Google Scholar]
- Ramu J, Herrera J, Grill R, Bockhorst T, Narayana P (2008): Brain fiber tract plasticity in experimental spinal cord injury: Diffusion tensor imaging. Exp Neurol 212:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJG, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJW, Barkhof F (2009): Regional DTI differences in multiple sclerosis patients. Neuroimage 44:1397–1403. [DOI] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur‐Moryosef S, Blumenfeld‐Katzir T, Assaf Y (2012): Learning in the Fast Lane: New Insights into Neuroplasticity. Neuron 73:1195–1203. 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Schallert T, Leasure JL, Kolb B (2000): Experience‐Associated Structural Events, Subependymal Cellular Proliferative Activity, and Functional Recovery After Injury to the Central Nervous System. J Cereb Blood Flow Metab 20:1513–1528. http://jcb.sagepub.com/content/20/11/1513.long. [DOI] [PubMed] [Google Scholar]
- Sen PN, Basser PJ (2005): A model for diffusion in white matter in the brain. Biophys J 89:2927–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen‐Berg H, Bannister P, De Luca M, Drobnjak I, Flitney D, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady J, Matthews P (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–S219. http://www.ncbi.nlm.nih.gov/pubmed/15501092. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Song S‐K, Sun S‐W, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. Neuroimage 17:1429–1436. http://www.sciencedirect.com/science/article/pii/S105381190291267X. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Bailey JA, Zatorre RJ, Penhune VB (2013): Early musical training and white‐matter plasticity in the corpus callosum: Evidence for a sensitive period. J Neurosci 33:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Van Wedeen J (2002): High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48:577–582. [DOI] [PubMed] [Google Scholar]
- Waring WP, Biering‐Sorensen F, Burns S, Donovan W, Graves D, Jha A, Jones L, Kirshblum S, Marino R, Mulcahey MJ, Reeves R, Scelza WM, Schmidt‐Read M, Stein A (2010): 2009 Review and Revisions of the International Standards for the Neurological Classification of. Spinal Cord Injury. J Spinal Cord Med 33:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CW, Tharmakulasingam J, Crawley A, Kideckel DM, Mikulis DJ, Bradbury CL, Green RE (2008): Use of Diffusion‐Tensor Imaging in Traumatic Spinal Cord Injury to Identify Concomitant Traumatic Brain Injury. Arch Phys Med Rehabil 89:S85–S91. 10.1016/j.apmr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due‐Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM (2010): Life‐span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20:2055–2068. [DOI] [PubMed] [Google Scholar]
- Wheeler‐Kingshott CAM, Cercignani M (2009): About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA (2009): Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex 19:224–232. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yamasaki M, Imai T (1989): Retrograde pyramidal tract degeneration in a patient with cervical haematomyelia. J Neurol Neurosurg Psychiatry 52:382–386. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1032415/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields R, Johansen‐Berg H (2013): Plasticity in Gray and White : Neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


