Abstract
Decreased brain activity in the default mode network, particularly in the precuneus (PCU), has been consistently shown in acquired brain injury (ABI) patients. However, it is unclear whether resting‐state brain activity recovers longitudinally in ABI patients and whether functional activity restoration is associated with improvements in consciousness level. Here, resting‐state fMRI data were acquired from 23 ABI patients and 30 age‐ and gender‐matched controls with two longitudinal observations for each participant. The fMRI data were analyzed using amplitude of low‐frequency fluctuation (ALFF) to measure the fluctuation strength of local spontaneous activity, and seed‐based functional connectivity was used to measure functional relationship with the seed region in the whole brain. The level of consciousness was assessed using the Glasgow Coma Scale (GCS) and Coma Recovery Scale‐Revised (CRS‐R) on both scanning days of the patients. Interaction effect between the two groups and two scans in ALFF was observed in the PCU, which was driven by restored ALFF in the ABI, while a stable ALFF in the control group. Moreover, restoration of ALFF in the PCU correlated with improvements in both the CRS‐R and GCS. Specifically, recovery of ALFF in the PCU primarily reflected the signals of the slow‐4 frequency band (0.027–0.073 Hz). Based on the functional connectivity maps of the PCU, we observed a nonsignificant interaction effect or correlation with consciousness level. These findings suggest local activity in the PCU but possibly not its functional connectivity, is related to the longitudinal changes in behavioral responsiveness in ABI. Hum Brain Mapp 38:3579–3591, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: longitudinal recovery, ABI, ALFF, GCS, CRS‐R, resting‐state fMRI
INTRODUCTION
In patients with acquired brain injury (ABI), hypo‐functional activity primarily in the default‐mode network [Raichle et al., 2001], particularly the precuneus (PCU) and posterior cingulate cortex, has consistently been observed [Achard et al., 2012; Boly et al., 2009; Huang et al., 2014a; Norton et al., 2012; Vanhaudenhuyse et al., 2010; Wu et al., 2015]. Moreover, hypo‐metabolism in the PCU, posterior cingulate cortex and frontoparietal regions has been reported in ABI patients [Di Perri et al., 2016; Laureys et al., 2004; Nakayama et al., 2006]. These cross‐sectional functional neuroimaging studies suggest that PCU plays a central role in the neurobiological mechanisms underlying the loss of consciousness in ABI patients. Significant advances in patient care and rehabilitation have provided ABI patients with an opportunity to recover in terms of physical and mental health and regain level of consciousness. However, the underlying neuronal mechanisms of the progression to recovery of consciousness level in ABI patients remain unclear.
The findings from previous longitudinal fMRI studies in ABI patients have been inconsistent [Falletta Caravasso et al., 2016; Hillary et al., 2011; Nakamura et al., 2009; Venkatesan et al., 2015], and conclusions have been limited, possibly due to the absence of longitudinal data from matched control subjects [Falletta Caravasso et al., 2016; Nakamura et al., 2009; Venkatesan et al., 2015]. Thus, it is important to systematically investigate the potential roles of brain functional activity in tracking the recovery of consciousness level in ABI patients in a relatively large ABI sample with matched healthy controls. Moreover, cross‐sectional studies have demonstrated the clinical relevance of hypo‐activity [Vanhaudenhuyse et al., 2010; Wu et al., 2015], showing correlations between hypo‐activity and the degree of loss of consciousness. Exploration of the potential relationship between the longitudinal recovery of brain activity and the extent of improvements in clinical symptoms is lacking.
Resting‐state fMRI has emerged as a powerful tool for mapping the spontaneous functional activity of the human brain noninvasively, and it is particularly useful for clinical populations, such as ABI patients [Di Perri et al., 2016; Sharp et al., 2014]. The amplitude of low‐frequency fluctuation (ALFF, generally in the range of 0.01–0.08 Hz) [Zang et al., 2007] is an efficient index that quantifies resting‐state local spontaneous neuronal activity, reflects the regional metabolic level of glucose [Aiello et al., 2015], shows high test–retest reliability particularly suitable for longitudinal observations [Zuo et al., 2010], and has been widely adopted in clinical studies [Han et al., 2011; Hare et al., 2017; Lui et al., 2009; Meda et al., 2015] which closely correlates with clinical symptoms [Hare et al., 2017; Lui et al., 2009]. By decomposing the low‐frequency fluctuations into slow‐4 (0.027–0.073 Hz) and slow‐5 (0.01–0.027 Hz) frequency bands consistent with Buzkasi's theory [Buzsaki and Draguhn, 2004], Meda and colleagues reported that ALFF in slow‐4 and slow‐5 might play complementary roles underlying the physiologic mechanisms for psychosis [Meda et al., 2015]. In addition, ALFF in slow‐5 could detect greater functional abnormalities in amnestic mild cognitive impairment patients [Han et al., 2011]. These findings suggest that we should carefully consider the frequency‐dependent effect on intrinsic local neuronal activity.
To assess the potential correspondence between the longitudinal recovery of resting‐state brain activity and consciousness level in ABI patients, we collected two longitudinal resting‐state scans in 23 ABI patients and 30 matched controls, and evaluated the consciousness level of the ABI patients at both time points. Resting‐state brain activity was calculated using ALFF, and consciousness level was assessed using the Glasgow Coma Scale (GCS) [Teasdale and Jennett, 1974] and Coma Recovery Scale‐Revised (CRS‐R) [Giacino et al., 2004]. We hypothesized that (1) the disrupted resting‐state activity in ABI with loss of consciousness is longitudinally restored, particularly in the PCU; (2) the extent of resting‐state activity recovery in the PCU correlates with the improvement in consciousness level; and (3) slow‐4 and slow‐5 signals might show a frequency‐dependent effect on tracking longitudinal changes in CRS‐R and GCS in ABI patients. With the ALFF findings used as seeds for functional connectivity analysis, we additionally explored whether the connectivity of those regions could be used to track the restoration of consciousness level in ABI.
MATERIALS AND METHODS
Participants
Fifty‐nine right‐handed participants, including 29 ABI patients and 30 healthy controls (CON), participated in the present study. The patients were recruited from the Department of Neurosurgery at Huashan Hospital, and its related rehabilitation hospitals from March 2010 to July 2014. Healthy controls were recruited from the local community from July 2015 to February 2016. The present study was approved by the Medical Research Ethics Committee and the Institutional Review Board of Huashan Hospital, and informed consent was obtained from each participant or from a legal representative. Participants were excluded if they had any of the following clinical conditions: a) a history of psychiatric or neurological illness prior to suffering from disorders of consciousness, b) a previous history of medically documented brain injury, c) a history of psychoactive drug consumption, or d) current or previous drug or alcohol abuse. The patients were diagnosed and assigned to the following four categories: fully preserved consciousness state (PC), minimally conscious state (MCS), unresponsive wakefulness syndrome/vegetative state (UWS/VS), and coma [Schnakers, 2012]. Six ABI patients were excluded for excessive head motion during one or both of the functional scans. The remaining 23 ABI patients and 30 control subjects were included in further analyses.
The 23 patients were scanned twice under stable conditions (no intubation or sedation used). The first scan was conducted at 5–344 days (81.1 ± 95.4 days) after the onset of acquired brain injury, and a time gap between the two scans ranged from 3 to 289 days (75.8 ± 93.6 days). The level of consciousness was assessed using the GCS and CRS‐R [Wu et al., 2015] on both fMRI scan days. Detailed demographic and clinical characteristics of the ABI patients, including etiology and lesion locations are listed in Supporting Information Table SI. Among the 23 ABI patients, 8 patients were in a PC state, 9 patients were in a MCS state, 4 patients were in an UWS/VS state, and the remaining 2 patients were in a COMA state at Scan 1 (Supporting Information Table SI). Seven out of the 9 MCS patients transitioned to the PC state at Scan 2, 2 out of the 4 UWS/VS patients transitioned to the PC state, and one COMA patient transitioned to the PC state and the other COMA patient transitioned to the MCS state.
Data Acquisition
All participants were scanned using a 3 T Siemens Verio scanner (Siemens Medical Systems, Erlangen, Germany) with a 12‐channel head coil at the Huashan Hospital of Fudan University. Resting‐state fMRI images were collected using an echo‐planar imaging sequence (repetition time = 2000 ms; echo time = 35 ms; flip angle = 90°; number of slices = 33; slice thickness = 4 mm; gap = 0 mm; matrix = 64 × 64). The participants were scanned with two in‐plane resolutions reflecting the different field‐of‐view selections used by the different MRI scanner operators [Wu et al., 2015], as follows: (1) in‐plane resolution = 4 × 4 mm2 for 20 ABI patients at Scan 1, 19 ABI patients at Scan 2 and all CON subjects at both scans and (2) in‐plane resolution = 3.281 × 3.281 mm2 for 3 ABI patients at Scan 1 and 4 ABI patients at Scan 2. During data acquisition, the participants lay quietly in the scanner. The resting‐state fMRI scans lasted 400 s and resulted in 200 volumes for each participant. Healthy control subjects and communicative ABI patients were instructed to keep their eyes closed during the scan. A set of T2‐weighted images was acquired from each participant to assess focal brain injury, and a senior neuroradiologist reviewed these images.
Data Preprocessing
The fMRI data were preprocessed using Analysis of Functional NeuroImages (AFNI) [Cox, 1996], Statistical Parametric Mapping (Wellcome Department of Imaging Neuroscience, University College, London), and Matlab (MathWorks). Specifically, the preprocessing steps included the removal of the first four volumes, slice timing, and head motion correction, spatial normalization using a template from the Montreal Neurological Institute, linear trend removal, and spatial smoothing with a 6‐mm Gaussian kernel [Power et al., 2014]. After correcting for motion, the subjects with minimal motion, i.e., less than one voxel, for at least 135 continuous volumes in both resting‐state scans were included in further analyses. Six ABI patients were thus excluded. Among the other 23 ABI patients and 30 CON subjects, 5 ABI patients and 1 CON subject generated truncated data, with continuous data for more than 135 volumes.
ALFF Analysis
Whole brain voxel‐wise ALFF [Zang et al., 2007; Zou et al., 2013; Zuo et al., 2010] was calculated for each subject based on the continuous fMRI data. ALFF, which quantifies local resting‐state signal fluctuations, was calculated as the integral of signal amplitude in the low‐frequency domain (0.009–0.08 Hz) [Zou et al., 2015]. The subject‐level voxelwise ALFF map was converted into a z‐score map by subtracting the mean ALFF of the whole brain and dividing by the standard deviation [Hare et al., 2017; Zang et al., 2007; Zou et al., 2010, 2013]. We decomposed the ALFF calculation in the low‐frequency range into two bands as previously described [Buzsaki and Draguhn, 2004; Han et al., 2011; Hare et al., 2017; Zuo et al., 2010], i.e., slow‐4 (ALFFs4, 0.027–0.073 Hz) and slow‐5 (ALFFs5, 0.01–0.027 Hz). Similarly, ALFFs4 and ALFFs5 maps were converted into z‐score maps.
Statistical Analysis
Linear mixed‐effects (LME) modeling was performed for the two (groups: ABI and CON) by two (scans: Scans 1 and 2) analyses using 3dLME in AFNI [Chen et al., 2013; Lu et al., 2014]. The effects of age, gender, resolution type, number of volumes remaining, and time gap between the two functional scans were controlled prior to the 3dLME analyses. We used autocorrelation function (ACF) modeling approach combined with a voxel‐wise threshold of P < 0.001 to correct for multiple comparisons [Cox et al., 2016; Eklund et al., 2016]. ACF was developed and implemented into the 3dClustSim tool to determine the cluster‐size threshold to use for a given voxel‐wise threshold. ACF was estimated using 3dFWHMx based on the preprocessed resting‐state fMRI data obtained prior to ALFF calculations. Correspondingly, a corrected significance level of P < 0.05 for the resulting statistical maps was obtained using clusters, with a minimum number of 33 voxels at an uncorrected individual voxel height threshold of P < 0.001.
Spearman's correlation coefficient was calculated to assess the relationship between the changes in ALFF in the regions showing significant interaction effect and the improvements in behavioral responsiveness, indicated as changes in the GCS and CRS‐R in all 23 patients, with the effects of age, gender, resolution type, number of volumes remaining, and time gap between the two functional scans controlled.
We further divided the ABI patients into two subgroups based on their degree of improvements in consciousness level, i.e., improved ABI (ABII, N = 12) and nonimproved ABI (ABIN, N = 11). The ABII group included patients with significant improvements in consciousness level: 1) eleven patients transitioned to a less severe diagnostic category (Supporting Information Table SI), and 2) one patient remained in the same category at both scans but showed changes in GCS and CRS‐R greater than the minimum clinical improvement in the 11 ABI patients who progressed to a less severe category, i.e., GCS improvement >1 and CRS‐R improvement >4. The ABIN group included the other 11 patients who remained in the same category without significant improvement in consciousness level. Interaction effect among the three groups (the two ABI subgroups and the control group) and between the two scans were further tested using 3dLME, with the effects of age, gender, resolution type, number of volumes remaining, and time gap between the two functional scans controlled.
The same statistical analyses were conducted separately for ALFFs4 and ALFFs5 maps as used for the ALFF maps described above.
Seed‐Based Functional Connectivity Analysis
The regions showing significant interaction effect (two groups by two scans) of ALFF were selected as “seed” to perform seed‐based resting‐state functional connectivity analysis. This analysis was adopted to further explore whether functional connectivity between the seed and different regions in the whole brain recovered with the restoration of consciousness level in the ABI patients. The same strategy performed in a previous study for data preprocessing and FC map calculation was adopted here [Wu et al., 2015]. The statistical analyses used for the ALFF maps were conducted on the connectivity maps, including (1) testing interaction effects on two participating groups and two scans; (2) if the two by two interaction effects were significant, then calculating Spearman's correlation coefficient between functional connectivity changes in the ROIs showing significant interaction effect and the improvements in the GCS and CRS‐R scores; and (3) further testing of the interaction effects on these three groups (the improved and nonimproved ABI subgroups and control group) and two scans.
RESULTS
Demographic and Clinical Characteristics
Supporting Information Tables SI and Table 1 represent the demographic and clinical features of all participants. There were no significant differences between the two groups in terms of gender ( = 0.225, P = 0.430), age (t (51) = 1.373, P = 0.176) or time gap between the two fMRI scans (t (51) = −0.536, P = 0.594). Significant improvements in consciousness level were observed in the CRS‐R (t (22) = 3.583, P = 0.0017) and GCS (t (22) = 3.691, P = 0.0013) in the ABI patients.
Table 1.
Summarized demographic and clinical characteristics of the healthy control subjects and the ABI patients
|
CON (N = 30) |
ABI (N = 23) |
P value (ABI vs. CON) |
|
|---|---|---|---|
| Gender | 19 M, 11 F | 16 M, 7 F | 0.430 |
| Age at Scan 1 (years) | 36.8 ± 9.5 | 41.1 ± 13.2 | 0.176 |
| Time gap between the two scans (days) | 85.6 ± 33.0 | 75.7 ± 93.6 | 0.594 |
| dCRS‐R | – | 5.17 ± 6.93 | – |
| dGCS | – | 2.13 ± 2.77 | – |
M: male; F: female; dCRS‐R, longitudinal changes in CRS‐R score, i.e., CRS‐R score at Scan 2 minus that at Scan 1; dGCS, longitudinal changes in GCS score.
Disrupted Local Neuronal Activity Was Partially Restored in ABI Patients
Significant group by scan interaction effects were primarily located in the PCU (Fig. 1A). Bar and error bar plots and post hoc analyses (Fig. 1B) revealed that the interaction effect in the PCU was driven by restored ALFF in the ABI group (P < 0.001), while there was no significant change in the CON group. Importantly, the ALFF of the ABI group was similar to that of CON subjects at Scan 2, suggesting that the local neuronal activity in the PCU in ABI patients approached normal. To validate that the interaction effect was not affected by varied data length among participants, we generated bar and error bar plots based on the 18 ABI and 29 CON participants with full‐length fMRI data. We observed identical interaction patterns and post hoc results in the PCU (Fig. 1C).
Figure 1.
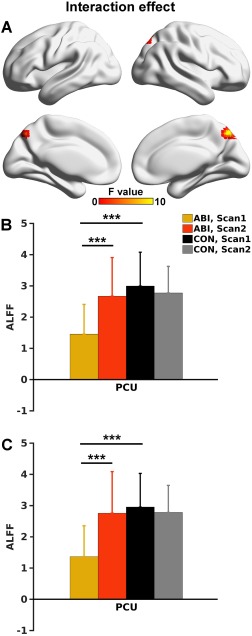
A. Group by scan interaction effect (P < 0.05 corrected) mapped on to the cortical surfaces using in‐house BrainNet viewer software (Xia et al., 2013). B. Bar and error bar plots of ALFF values in the PCU demonstrated in A. C. Bar and error bar plots of the ALFF values in the small sample (18 ABI and 29 CON participants) with full‐length fMRI data. The ROI for the bar and error bar plots were the same as in B. The error bar represents standard deviation. *** indicates P < 0.001. PCU = precuneus. [Color figure can be viewed at http://wileyonlinelibrary.com]
Relationship Between Restoration of Brain Activity and Recovery of Consciousness Level in ABI
Using Spearman's correlation analysis, the average longitudinal changes in ALFF in the PCU region showed significantly positive correlations (P < 0.05) with improvements in both the CRS‐R (Fig. 2A) and GCS (Fig. 2B) scores. There was a negligible effect of data length in the Spearman's correlation analysis. Based on the data from the 18 ABI subjects with full‐length fMRI time courses, we observed significant correlations between longitudinal changes in ALFF in the PCU and recovery in consciousness level (Fig. 2C,D).
Figure 2.
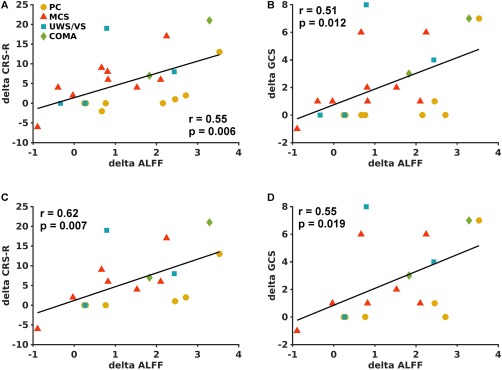
Scatter plots between ALFF changes (Scan 2–Scan 1) in the PCU ROI as shown in Fig. 1A and the corresponding changes in CRS‐R (A) and GCS (B) scores in 23 ABI patients. Scatter plots between ALFF changes in the PCU and the corresponding changes in CRS‐R (C) and GCS (D) scores in the 18 ABI patients with full length of fMRI data. The ABI patients in a fully preserved consciousness state (PC) at Scan 1 are labeled with yellow circles, patients in a minimally conscious state (MCS) at Scan 1 are labeled with red triangles, patients in a unresponsive wakefulness syndrome/vegetative state (UWS/VS) at Scan 1 are labeled in blue squares, and patients in a COMA state at Scan 1 are labeled with green diamonds. [Color figure can be viewed at http://wileyonlinelibrary.com]
Restoration of Local Neuronal Activity Was Observed in the Improved, but Not the Nonimproved, ABI Patients in the PCU
After further dividing the ABI patients into improved and nonimproved subgroups, significant group (among these two ABI subgroups and healthy controls) by scan (Scans 1 and 2) interaction effects located in the PCC/PCU and anterior part of the PCC extending to middle cingulate cortex (Fig. 3A) were identified. Bar and error bar plots and post hoc analyses (Fig. 3B) revealed that the interaction effect in the PCC/PCU was primarily driven by a restored ALFF in the ABII group (P < 0.001), while there were no significant changes in the ABIN and CON groups. The ALFF of both the ABII and ABIN groups was lower than that of the CON group at Scan 1. While the ALFF of the ABII group was similar to that of the CON subjects at Scan 2, ALFF in the ABIN group remained lower than that of the CON group.
Figure 3.
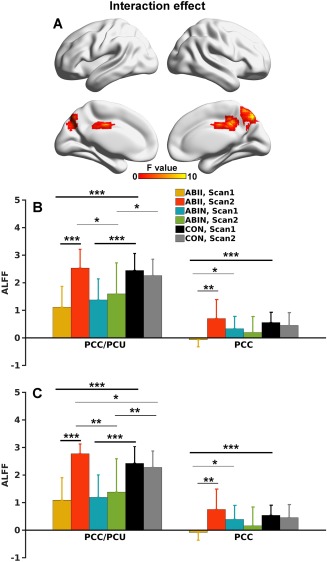
A. Group (three groups) by scan interaction effects (P < .05 corrected) mapped onto the cortical surfaces. B. Bar and error bar plots of ALFF values in the two regions demonstrated in A. C. Bar and error bar plots of the ALFF values in the small sample (10 ABII, 8 ABIN, and 29 CON participants) with full‐length fMRI data. The ROIs for the bar and error bar plots were the same as those in B. *** indicates P < .001; ** indicates P < .01; and * indicates P < .05. PCC/PCU = posterior cingulate cortex/precuneus; PCC = posterior cingulate cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
The interaction effect in the anterior PCC was primarily driven by decreased ALFF in the ABII group at Scan 1 compared to the CON group, and the restoration of ALFF values in the ABII group at Scan 2. Notably, the interaction effects were minimally affected by varied data length across subjects (Fig. 3C).
To direct compare patients who did recover consciousness versus patients who did not recover consciousness, we divided the 23 ABI patients into four subgroups based on the extent of longitudinal recovery of consciousness: (1) two patients who were in unconsciousness state at Scan 1 and stayed in unconsciousness state at Scan 2 (unC–>unC); (2) four patients who recovered consciousness at Scan 2 from unconsciousness state at Scan 1 (unC–> C); (3) nine patients who were already conscious at Scan 1 with nonimproved GCS or CRS‐R (C–>C (n)); and (4) eight patients who were already conscious at Scan 1 with improved GCS or CRS‐R (C–> C (i)). However, due to the limited sample size of each subgroup, there was only a trend (P > 0.05) that patients who recovered consciousness (unC–>C) showed large changes in ALFF in the PCU compared to the patients who did not recover consciousness (unC– unC) (Fig. 4). There was also a trend (both p values > 0.1) that patients who recovered consciousness (unC–>C) showed large changes in ALFF in the PCU compared to the patients who were already conscious and remained so (both C–> C(n) and C–> C(i)) (Fig. 4).
Figure 4.
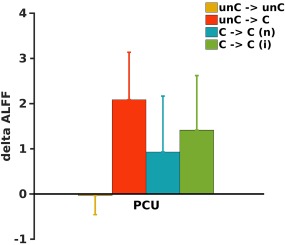
Bar and error bar plots of changes in ALFF values in the PCU ROI as shown in Fig. 1A in four subgroups of patients. unC–>unC, two patients who were in unconsciousness state at Scan 1 and stayed in unconsciousness state at Scan 2; unC–>C, four patients who recovered consciousness at Scan 2 from unconsciousness state at Scan 1; C–>C (n), nine patients who were already conscious at Scan 1 with nonimproved GCS or CRS‐R; and C–>C (i), eight patients who were already conscious at Scan 1 with improved GCS or CRS‐R. [Color figure can be viewed at http://wileyonlinelibrary.com]
Local Neuronal Activity in Slow‐4 Frequency Range Primarily Contributed to Recovery
Based on the ALFFs4 maps, two groups by two scans interaction effect was observed in the PCU, identical to the results observed for ALFF (Fig. 5A,B). However, the interaction effect based on ALFFs5 maps did not survive multiple comparison correction. Nevertheless, the average ALFFs5 values extracted from the PCU region (shown in Fig. 5A), exhibited group by scan patterns (Fig. 5C) similar to those of ALFFs4 (Fig. 5B).
Figure 5.
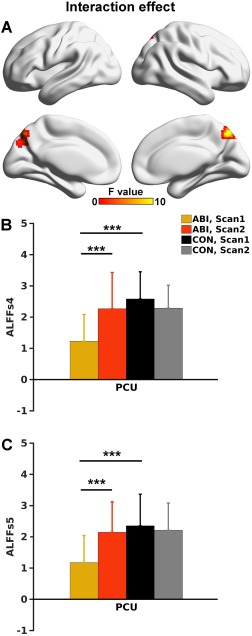
A. Group by scan interaction effect of ALFFs4 (P < .05 corrected) mapped onto the cortical surfaces. B. Bar and error bar plots of ALFFs4 values in the PCU demonstrated in A. C. Bar and error bar plots of the ALFFs5 values in the PCU ROI as shown in B. ALFFs4, ALFF calculated in the slow‐4 frequency band (0.027–0.073 Hz); ALFFs5, ALFF calculated in the slow‐5 frequency band (0.01–0.027 Hz). [Color figure can be viewed at http://wileyonlinelibrary.com]
Similar to ALFF, average changes of ALFFs4 in the PCU region (Fig. 5A) showed positive correlations with improvements in both the CRS‐R and GCS scores (Fig. 6A,B). For ALFFs5, the significance of Spearman's correlation with the changes in consciousness level was marginal (Fig. 6C,D).
Figure 6.
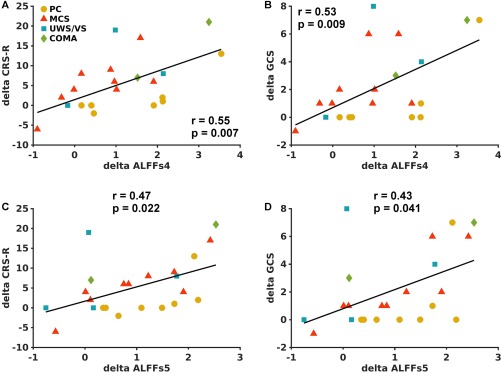
Scatter plots between ALFFs4 changes in the PCU ROI as shown in Fig. 5A and the corresponding changes in CRS‐R (A) and GCS (B) scores in 23 ABI patients. Scatter plots between ALFFs5 changes in the PCU and the corresponding changes in CRS‐R (C) and GCS (D) scores. The ABI patients were labeled as shown in Figure 2. [Color figure can be viewed at http://wileyonlinelibrary.com]
The interaction effects from the three groups by two scans in ALFFs4 maps were almost identical to those in ALFF (Fig. 7A,B, in comparison to Fig. 3A,B). Based on ALFFs5 maps, no significant interaction effect was observed after statistical correction on the whole brain voxel‐wise analysis, although bar and errorbar plots and post hoc analyses in the PCU ROI (Fig. 7A) showed patterns across groups and between scans similar to those of ALFF and ALFFs4 (Fig. 7C, in comparison to Figs. 3B and 7B).
Figure 7.
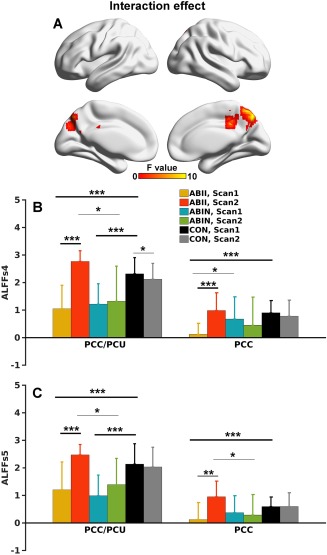
A. Group (three groups) by scan interaction effects of ALFFs4 (P < 0.05 corrected) mapped on to the cortical surfaces. B. Bar and error bar plots of ALFFs4 values in the two regions demonstrated in A. C. Bar and error bar plots of the ALFFs4 values in the two ROIs as shown in B. [Color figure can be viewed at http://wileyonlinelibrary.com]
No Significant Interaction Effect on Functional Connectivity with the PCU
With a seed placed at the PCU as shown in Figure 1A, we observed no significant two groups by two scans interaction effect based on the functional connectivity maps. Further dividing the ABI into two subgroups and testing the three groups by two scans interaction effect yielded non significant findings.
DISCUSSION
A longitudinal investigation was performed on adaptations in resting‐state activity in a cohort of ABI patients with loss of consciousness, and the results were compared to age‐ and gender‐matched healthy controls. Group by scan interaction effect was observed in the PCU, with disrupted ALFF in ABI patients compared to CON subjects in these regions at Scan 1 and restored functional activity approaching the normal level in the CON group at Scan 2. Moreover, the extent of functional restoration in the PCU correlated with the improvements in consciousness level, indicated as the GCS and CRS‐R scores. The restoration of local neuronal activity in the PCU associated with longitudinal recovery of consciousness level was primarily contributed by the signal in the slow‐4 frequency band. Together, these findings suggest that the ALFF in the PCU plays an important role in tracking the clinical improvement of the behavioral responsiveness in ABI patients.
Decreased ALFF in the PCU in ABI Patients
The decreased resting‐state local activity in the PCU was consistent with findings from a previous small‐sample cross‐sectional study on 11 ABI patients and 12 control subjects [Huang et al., 2014a]. In a similar investigation of subjects with loss of consciousness induced using anesthesia, Huang and colleagues observed a decreased amplitude of low‐frequency fluctuations in the PCU [Huang et al., 2014b]. However, these authors did not map the restoration of functional activity in the subjects after these individuals regained consciousness from anesthesia.
ALFF has been shown to significantly correlate with local glucose metabolism in healthy subjects [Aiello et al., 2015]. The observations of disrupted spontaneous activity in the PCU in ABI patients in the present study are spatially consistent with previous FDG‐PET studies indicating hypo‐metabolism in the PCU [Laureys et al., 2004; Nakayama et al., 2006; Stender et al., 2015], which further extended the notion that disruptions in ALFF reflect brain hypo‐metabolism in ABI patients. Moreover, the disruption in ALFF in the PCU closely corresponds with consistent findings based on functional connectivity methodology [Achard et al., 2012; Boly et al., 2009; Norton et al., 2012; Vanhaudenhuyse et al., 2010; Wu et al., 2015].
Longitudinal Restoration of ALFF in the PCU in ABI Patients
The present investigation represents the first longitudinal study to evaluate local neuronal activity restoration in ABI patients. Previous longitudinal fMRI studies in ABI patients have been focused on functional connectivity changes [Falletta Caravasso et al., 2016; Hillary et al., 2011; Nakamura et al., 2009; Venkatesan et al., 2015]. Nakamura and colleagues showed that functional connectivity strength throughout the whole brain longitudinally decreased in 6 ABI patients, with only one scan from control subjects [Nakamura et al., 2009]. Hillary and colleagues showed increased functional connectivity between the posterior cingulate cortex and PCU in 10 ABI patients while decreased connectivity in 10 controls [Hillary et al., 2011]. On a sample of 15 ABI patients and without data from control subjects, Falletta Caravasso and colleagues showed longitudinally increased functional connectivity between the posterior cingulate cortex and the right angular gyrus [Falletta Caravasso et al., 2016]. Venkatesan and colleagues reported increased positive connectivity between the posterior cingulate cortex and dorsomedial prefrontal cortex, and decreased negative connectivity between the posterior cingulate cortex and the frontoparietal operculum and inferior parietal lobule, based on longitudinal data from 13 ABI patients and only a baseline scan from 18 control subjects [Venkatesan et al., 2015]. Conclusions from these previous longitudinal fMRI studies were inconsistent and might be caused by the relatively small sample size (less than 20 ABI patients) and the absence of longitudinal data from matched controls [Falletta Caravasso et al., 2016; Nakamura et al., 2009; Venkatesan et al., 2015]. Here, we alternatively adopted the ALFF analytic strategy from the aspect of local activity based on a relatively large cohort including 23 ABI patients and 30 matched healthy control participants. We showed that the disrupted ALFF in the PCU reached a normal level with the restoration of consciousness level, as indicated by CRS‐R and GCS, in ABI patients, particularly for improved ABI participants, whereas for control subjects, ALFF in the PCU remained unchanged. These findings suggest that ALFF in the PCU is related to restoration of behavioral responsiveness in ABI.
Interestingly, no significant group by scan interaction effect was observed based on seed‐based functional connectivity maps with the seed placed at the PCU, while the ALFF values in the PCU could be used to track the longitudinal recovery of consciousness level in ABI patients. Intuitively, lower resting‐state signal fluctuations in the PCU might affect the seed‐based functional connectivity with this region, which was the motivation of the seed‐based analysis in the present study. The non significant finding of the group by scan interaction effect based on seed‐based functional connectivity with the PCU might reflect increased inter‐subject variation in the connectivity analysis. As shown in Supporting Information Table SI, the locations of lesion varied across the ABI patients over the whole brain, while the PCU was one of the relatively intact regions. ALFF is an index reflecting local neuronal activity thus could reveal longitudinal changes in functional activity with high sensitivity. Seed‐based functional connectivity captures the functional synchrony between the seed region and a target region [Biswal et al., 1995; Greicius et al., 2003], thus intersubject variations in both seed and target regions could result in the variation in functional connectivity. We propose that the ABI patients would gradually regain functional connectivity toward more recovery in the consciousness level, requiring more sensitive functional connectivity computational methodology, such as intranetwork and internetwork connectivity strength [Peraza et al., 2017; Zhu et al., 2017], and dynamic functional connectivity [Chang et al., 2013; Zhang et al., 2016] to track the longitudinal recovery of connectivity.
Correlation Between Restoration of ALFF in the PCU and Consciousness Level
None of the previous longitudinal fMRI studies performed a direct correlation analysis between functional activity changes and improvement in consciousness level. Here, we showed a positive correlation between increases in ALFF in the PCU and level of consciousness recovery indexed by both the GCS and CRS‐R scales. These results greatly extend previous findings based on cross‐sectional investigations of the relationship between functional connectivity strength and consciousness level in ABI patients [Wu et al., 2015]. These findings contribute to the understanding of the neural mechanisms underlying restoration of behavioral responsiveness and provide important clinical information for future treatment assessment of ABI patients. In addition to the potential clinical relevance of ALFF regarding ABI and restoration of consciousness level, these observations provide further evidence of resting‐state fMRI and ALFF as an important imaging technique and computational methodology for various psychiatric and neurological diseases.
Roles of the PCU and Posterior Cingulate Cortex in the Loss of Consciousness Level and Recovery in ABI
The PCU and posterior cingulate cortex constitute the main components of the neural network correlates of consciousness [Vogt and Laureys, 2005] and act as important components of the default mode network [Fox et al., 2005; Greicius et al., 2003; Lu et al., 2012; Raichle et al., 2001; Vincent et al., 2007] and the main hub regions of the brain [Achard et al., 2006; Buckner et al., 2009; Liang et al., 2013]. Specifically, for ABI with loss of consciousness, hypo‐activity in the PCU and posterior cingulate cortex, indexed as local fluctuation strength, metabolism and functional connectivity with other regions of the brain, have been frequently reported. Thus, the PCU and posterior cingulate cortex should be the main regions affected by the loss of consciousness. Our longitudinal recovery data further showed that the PCU, particularly the cognitive subdivision [Margulies et al., 2009), regained functional activity with the restoration of behavioral responsiveness, i.e., improvements in the GCS and CRS‐R. In addition, the PCU together with posterior cingulate cortex could track the extent of recovery, showing different patterns of longitudinal changes in improved and nonimproved ABI patients (Figs. 5 and 7).
Contributions of Slow‐4 and Slow‐5 Frequency Bands to ALFF Restoration
Buzsaki and colleagues showed a hierarchical organization of oscillation classes [Buzsaki and Draguhn, 2004; Penttonen and Buzsáki, 2003], i.e., frequency bands in the brain [Zuo et al., 2010], and suggested that fluctuations are functionally relevant, such as input selection and plasticity and consolidation of information [Buzsaki and Draguhn, 2004]. Accordingly, we subdivided the low‐frequency resting‐state fMRI signals to slow‐4 (0.027–0.073 Hz) and slow‐5 (0.01–0.027 Hz) and observed that the ALFF in the slow‐4 frequency band showed higher sensitivity in tracking the restoration of behavioral responsiveness, although ALFF in the slow‐5 frequency band showed similar patterns. Buzsaki and colleagues showed that independent frequency bands are generated by different mechanisms and serve different physiological functions, and each band might carry different dimensions of brain integration and behavioral state‐dependent [Penttonen and Buzsáki, 2003]. Thus, the findings of the present study suggested that signals in the slow‐4 frequency band might be more sensitive to track the longitudinal recovery of ABI patients. Frequency‐dependent findings in ALFF have been reported for multiple populations with brain disorders. Disrupted ALFF in the visual cortex in schizophrenia patients has been observed in the slow‐4 frequency band [Hoptman et al., 2010], while complementary roles of ALFF in slow‐4 and slow‐5 frequency bands have been shown in neural signatures of psychosis [Meda et al., 2015]. Han and colleagues observed higher sensitivity of the slow‐5 frequency band in detecting changes in amnestic mild cognitive impairment patients [Han et al., 2011], while Di Martino and colleagues showed fluctuations of response time in children with higher ALFF in the slow‐4 band [Di Martino et al., 2008]. Together, the findings from other groups and the present study suggest the presence of frequency‐dependent effects of different bands on the intrinsic brain activity and highlight investigations of potentially specific effects from different frequency bands.
LIMITATIONS AND FUTURE WORK
Potential limitations in the present study should be recognized. First, the time gap between the two longitudinal scans varies across participants. However, the time gap matched between the ABI and CON groups, and the effect of the time gap was modeled in the statistical analyses [Wu et al., 2015]. Nonetheless, a more rigorous control of the time gap between the baseline scan and follow‐up visits is preferred in future studies. In addition, a more detailed longitudinal paradigm design, including more than two longitudinal observations, is needed for further studies. Second, the radiological findings regarding lesion locations were heterogeneous in ABI patients. The fact that the location and severity of lesions generally differ across patients remains a challenge for imaging studies in acquired brain injury [Di Perri et al., 2016; Norton et al., 2012; Silva et al., 2015; Vanhaudenhuyse et al., 2010]. To overcome these obstacles, additional multi‐center collaborative studies with much larger samples are needed to examine the convergent and divergent effects from the location and severity of lesions on the disruption and recovery of brain activity and consciousness level. Nevertheless, we observed the longitudinal restoration of local neuronal activity in the PCU and the recovery of behavioral responsiveness in the ABI patients. To investigate the longitudinal recovery of consciousness, we need further compare between patients who recovered consciousness from unconsciousness state versus patients who stayed in the unconsciousness state on a larger sample. In addition, the causal relationship between the functional activity in the PCU and consciousness remains unclear. In future studies, we need to systematically investigate whether patients/animals with lesion in the PCU either in part or in full, show loss of consciousness. We could further pursue whether neurostimulation (excitatory or inhibitory) in the PCU improves the consciousness level on patients/animals with loss of consciousness.
CONCLUSIONS
In summary, these data illustrated that ALFF in the PCU could potentially track progressive brain functional changes, and these changes showed a close correlation with clinical improvements in ABI. Furthermore, the longitudinal recovery of ALFF in the PCU primarily distributed in the slow‐4 frequency band. The resting‐state local neuronal activity in the PCU might provide important clinical information for the guidance and development of rehabilitation therapy.
Supporting information
Supporting Information Table 1.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the participation of the technicians and engineers (Huashan Hospital) for their help in acquiring the MRI data, the medical staff (Huashan Hospital and its related rehabilitation hospitals) for their help in transporting patients, and all the participants for their contributions to this project. We thank National Center for Protein Sciences at Peking University in Beijing, China, for assistance with MRI data acquisition and data analyses.
Contributor Information
Qihong Zou, Email: zouqihong@pku.edu.cn.
Jia‐Hong Gao, Email: jgao@pku.edu.cn.
REFERENCES
- Achard S, Delon‐Martin C, Vertes PE, Renard F, Schenck M, Schneider F, Heinrich C, Kremer S, Bullmore ET (2012): Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci USA 109:20608–20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006): A resilient, low‐frequency, small‐world human brain functional network with highly connected association cortical hubs. J Neurosci 26:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello M, Salvatore E, Cachia A, Pappata S, Cavaliere C, Prinster A, Nicolai E, Salvatore M, Baron JC, Quarantelli M (2015): Relationship between simultaneously acquired resting‐state regional cerebral glucose metabolism and functional MRI: A PET/MR hybrid scanner study. NeuroImage 113:111–121. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Boly M, Tshibanda L, Vanhaudenhuyse A, Noirhomme Q, Schnakers C, Ledoux D, Boveroux P, Garweg C, Lambermont B, Phillips C, Luxen A, Moonen G, Bassetti C, Maquet P, Laureys S (2009): Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp 30:2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews‐Hanna JR, Sperling RA, Johnson KA (2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Chang C, Liu Z, Chen MC, Liu X, Duyn JH (2013): EEG correlates of time‐varying BOLD functional connectivity. NeuroImage 72:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, Cox RW (2013): Linear mixed‐effects modeling approach to FMRI group analysis. NeuroImage 73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Reynolds RC, Taylor PA (2016): AFNI and clustering: False positive rates redux. bioRxiv:1–15. 10.1101/065862. [DOI] [PMC free article] [PubMed]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, Petkova E, Klein DF, Castellanos FX (2008): Decomposing intra‐subject variability in children with attention‐deficit/hyperactivity disorder. Biol Psychiatry 64:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Perri C, Bahri MA, Amico E, Thibaut A, Heine L, Antonopoulos G, Charland‐Verville V, Wannez S, Gomez F, Hustinx R, Tshibanda L, Demertzi A, Soddu A, Laureys S (2016): Neural correlates of consciousness in patients who have emerged from a minimally conscious state: A cross‐sectional multimodal imaging study. Lancet Neurol 15:830–842. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci USA 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falletta Caravasso C, de Pasquale F, Ciurli P, Catani S, Formisano R, Sabatini U (2016): The default mode network connectivity predicts cognitive recovery in severe acquired brain injured patients: A longitudinal study. J Neurotrauma 33:1247–1262. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J (2004): The JFK Coma Recovery Scale‐Revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85:2020–2029. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a Network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang J, Zhao Z, Min B, Lu J, Li K, He Y, Jia J (2011): Frequency‐dependent changes in the amplitude of low‐frequency fluctuations in amnestic mild cognitive impairment: A resting‐state fMRI study. NeuroImage 55:287–295. [DOI] [PubMed] [Google Scholar]
- Hare SM, Ford JM, Ahmadi A, Damaraju E, Belger A, Bustillo J, Lee HJ, Mathalon DH, Mueller BA, Preda A, van Erp TG, Potkin SG, Calhoun VD, Turner JA, Functional Imaging Biomedical Informatics Research (2017): Modality‐dependent impact of hallucinations on low‐frequency fluctuations in Schizophrenia. Schizophr Bull 43:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, Good DC, Wylie GR (2011): Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol 82:115–123. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, Milham MP (2010): Amplitude of low‐frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr Res 117:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Dai R, Wu X, Yang Z, Liu D, Hu J, Gao L, Tang W, Mao Y, Jin Y, Wu X, Liu B, Zhang Y, Lu L, Laureys S, Weng X, Northoff G (2014a): The self and its resting state in consciousness: An investigation of the vegetative state. Hum Brain Mapp 35:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Wang Z, Zhang J, Dai R, Wu J, Li Y, Liang W, Mao Y, Yang Z, Holland G, Zhang J, Northoff G (2014b): Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum Brain Mapp 35:5368–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND (2004): Brain function in coma, vegetative state, and related disorders. Lancet Neurol 3:537–546. [DOI] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y (2013): Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci USA 110:1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Chefer S, Ross TJ, Vaupel DB, Guillem K, Rea WP, Yang Y, Peoples LL, Stein EA (2014): Abstinence from cocaine and sucrose self‐administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connect 4:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y (2012): Rat brains also have a default mode network. Proc Natl Acad Sci USA 109:3979–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, Sweeney JA, Gong Q (2009): High‐field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci USA 106:15412–15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M (2009): Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106:20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Wang Z, Ivleva EI, Poudyal G, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Schretlen DJ, Calhoun VD, Lui S, Damaraju E, Pearlson GD (2015): Frequency‐specific neural signatures of spontaneous low‐frequency resting state fluctuations in psychosis: Evidence From Bipolar‐Schizophrenia Network on Intermediate Phenotypes (B‐SNIP) Consortium. Schizophr Bull 41:1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hillary FG, Biswal BB (2009): Resting network plasticity following brain injury. PloS One 4:e8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Nakashima T, Iwama T (2006): Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: An FDG‐PET study with statistical parametric mapping analysis. J Neurol Neurosurg Psychiatry 77:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM (2012): Disruptions of functional connectivity in the default mode network of comatose patients. Neurology 78:175–181. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Buzsáki G (2003): Natural logarithmic relationship between brain oscillators. Thalamus Relat Syst 2:145–152. [Google Scholar]
- Peraza LR, Nesbitt D, Lawson RA, Duncan GW, Yarnall AJ, Khoo TK, Kaiser M, Firbank MJ, O'Brien JT, Barker RA, Brooks DJ, Burn DJ, Taylor JP (2017): Intra‐ and inter‐network functional alterations in Parkinson's disease with mild cognitive impairment. Hum Brain Mapp 38:1702–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakers C (2012): Clinical assessment of patients with disorders of consciousness. Arch Ital Biol 150:36–43. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, Leech R (2014): Network dysfunction after traumatic brain injury. Nat Rev Neurol 10:156–166. [DOI] [PubMed] [Google Scholar]
- Silva S, de Pasquale F, Vuillaume C, Riu B, Loubinoux I, Geeraerts T, Seguin T, Bounes V, Fourcade O, Demonet JF, Peran P (2015): Disruption of posteromedial large‐scale neural communication predicts recovery from coma. Neurology 85:2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender J, Kupers R, Rodell A, Thibaut A, Chatelle C, Bruno MA, Gejl M, Bernard C, Hustinx R, Laureys S, Gjedde A (2015): Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J Cereb Blood Flow Metab 35:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G, Jennett B (1974): Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, Soddu A, Perlbarg V, Ledoux D, Brichant JF, Moonen G, Maquet P, Greicius MD, Laureys S, Boly M (2010): Default network connectivity reflects the level of consciousness in non‐communicative brain‐damaged patients. Brain 133:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan UM, Dennis NA, Hillary FG (2015): Chronology and chronicity of altered resting‐state functional connectivity after traumatic brain injury. J Neurotrauma 32:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME (2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S (2005): Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog Brain Res 150:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, Zhu J, Jin Y, Wu X, Lu L, Zhang Y, Zhang Y, Dai Z, Gao JH, Weng X, Zhou L, Northoff G, Giacino JT, He Y, Yang Y (2015): Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci 35:12932–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PloS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF (2007): Altered baseline brain activity in children with ADHD revealed by resting‐state functional MRI. Brain Dev 29:83–91. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cheng W, Liu Z, Zhang K, Lei X, Yao Y, Becker B, Liu Y, Kendrick KM, Lu G, Feng J (2016): Neural, electrophysiological and anatomical basis of brain‐network variability and its characteristic changes in mental disorders. Brain 139:2307–2321. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen Q, Xia L, Beaty RE, Yang W, Tian F, Sun J, Cao G, Zhang Q, Chen X, Qiu J (2017): Common and distinct brain networks underlying verbal and visual creativity. Hum Brain Mapp 38:2094–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Miao X, Liu D, Wang DJ, Zhuo Y, Gao JH (2015): Reliability comparison of spontaneous brain activities between BOLD and CBF contrasts in eyes‐open and eyes‐closed resting states. NeuroImage 121:91–105. [DOI] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, Gao JH, Stein EA, Zang YF, Yang Y (2013): Intrinsic resting‐state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp 34:3204–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP (2010): The oscillating brain: Complex and reliable. NeuroImage 49:1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1.


